ABSTRACT
Objectives:
The aim of this article is to identify whether natural irrigants are better than synthetic conventional irrigants for smear layer removal and to analyze their influence on mechanical and chemical radicular dentin properties.
Materials and Methods:
The last electronic search was performed on June 2020 through five databases, limited to articles either published or accepted for publication in the English language using the following keywords: “Natural extracts” or “Fruit and plant extracts” and “Smear layer removal.”
Results:
According to the inclusion criteria, 36 articles were included. Most studies revealed that apple or apple cider vinegars, grape seed extract, citrus aurantifolia, 5–10% glycolic acid, and 0.5–1% phytic acid effectively removed the smear layer better or similar to synthetic conventional agents.
Conclusion:
Natural irrigants are effective smear layer removing agents with the least deteriorated effect on mechanical and chemical radicular dentin properties compared with synthetic agents.
KEYWORDS: Apple vinegar, fruit extract smear layer removal, glycolic acid, phytic acid, plant extract smear layer removal
INTRODUCTION
Smear layer was defined as an amorphous layer consisting of inorganic dentin debris and organic tissues, including vital and necrotic pulp tissues, bacterial cells, and blood cells.[1] The existence of smear layer may block the canal irregularities and prohibit the penetration of root canal disinfectants to eliminate the hidden microorganisms.[2] Moreover, it can compromise the sealing ability and adaptation of root canal obturating materials.[3]
Different chemotherapeutic agents were used for smear layer removal, including sodium hypochlorite (NaOCl), ethylenediaminetetraacetic acid (EDTA), alternative use of EDTA associated with NaOCl, synthetic acids (citric and acetic acids),[3] and organic acids (maleic acid and glycolic acid (GA)).[4,5] NaOCl is the gold standard tissue-dissolving agent. It failed to completely remove the smear layer and only dissolved its organic component.[3,6] Although all chemical chelators like EDTA and malic acid are effective for smear layer removal, they may induce certain toxicity to periapical tissues and render tooth weakening by removing the calcium ions and reducing the microhardness of radicular dentin.[1,7] Recently, some authors focussed on the use of natural extracts as biological smear layer removing agents to reduce the risk factors on tissue reaction and mechanical properties of radicular dentin.[7]
The objectives of this review were to identify whether natural irrigants are better than synthetic conventional irrigants for smear layer removal and to analyze their influence on the mechanical and chemical radicular dentin properties. The research question was addressed in terms of PICOS format (problem, interventions, comparators, outcomes, and study designs) as follows: P—smear layer removal and mechanical and chemical radicular dentin properties, I—natural irrigants (fruit and plant extracts), C—synthetic conventional irrigants, O—efficacy, and S—in-vitro studies.
MATERIALS AND METHODS
LITERATURE SEARCH STRATEGY
The articles’ selection was based on an electronic search using MEDLINE via PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Scopus (http://www.scopus.com), Web of Science (https://www.webofknowledge.com), Google Scholar (http://scholar.google.com), and Saudi Dental Library (https://kau.deepknowledge.io/KAU). Date limit was set from 2000 to 2020. Manual search was done through peer-reviewed journals, including “Journal of Endodontics” and “International Endodontic Journal” from 1990 to 2020. The initial search was performed using keywords “Natural extracts” and “Endodontic irrigants.” The last search was carried out on June 2, 2020, as filter was performed using the following keywords: “Fruit and plant extracts” or “Apple vinegar” or “Grape extract” or “Glycolic acid” or “Phytic acid” and “Smear layer removal.”
STUDIES SELECTION
The inclusion criteria were in-vitro studies that compared the impact of natural (fruit or plant extracts) irrigants/chelators with synthetic conventional irrigants/chelators on smear layer removal and their effect on the mechanical and chemical properties of radicular dentin, including microhardness, roughness, strength, calcium ions concentration/release, and erosion. Full-text articles either published or accepted for publication in the English language were included.
The exclusion criteria comprised articles that evaluated other properties of fruit or plant extracts (e.g., antimicrobial activity and cytotoxicity) or articles that tested other natural irrigants/chelators for smear layer removal than the targeted solutions.
SCREENING
Literature search results were imported to EndNote library (X7 version, Thomson Reuters, New York, NY, USA). After de-duplication, all researchers independently screened the titles and abstracts of the articles for the relevance and existence of eligibility criteria. Discrepancies in the screening of titles/abstracts and articles in full text were resolved by discussion.
DATA CHARTING
A charting table was developed to record the key information of the included studies based on the review question. The data were retrieved by one reviewer (S.T.A.Z.) and reviewed by others (H.A.B. and A.A.M.S.). Data of charting table included the following: the tested irrigating solutions (intervention and comparators); solutions’ concentration; final rinse if present; application time; properties evaluated; and the main findings.
RESULTS
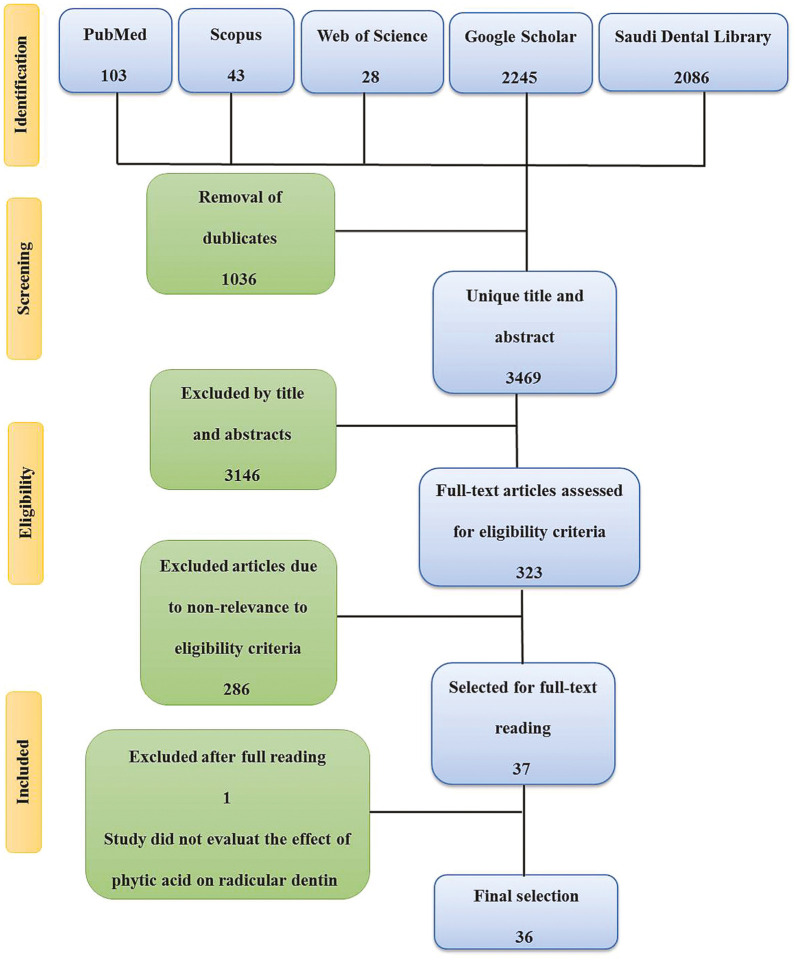
The flow chart was designed based on the guidelines of PRISMA extension for scoping reviews,[8] as shown in Figure 1. A total of 3469 citations have been collected from electronic databases after duplicates’ elimination. About 3146 were excluded based on title and abstract, whereas 323 articles in full text were retrieved and then tested for eligibility. After full-text reading, a total of 36 studies met the inclusion criteria.
Figure 1.
Flow chart of the study according to PRISMA extension for scoping reviews
Among the selected articles, several types of fruit and plant extracts were used, including apple vinegar (10 studies),[9,10,11,12,13,14,15,16,17,18] apple cider vinegar (4 studies),[19,20,21,22] pomegranate and grape vinegars (1 study),[20] grape seed extract (GSE) (6 studies),[23,24,25,26,27,28]Citrus aurantifolia (2 studies),[29,30] white vinegar (1 study),[31] GA (3 studies),[4,32,33] and phytic acid (10 studies)[34,35,36,37,38,39,40,41,42,43] [Table 1].
Table 1.
Articles included on natural extracts ordered chronologically for each solution
| Serial no. | References | Year | Interventions and comparators | Evaluated properties | Conclusion |
|---|---|---|---|---|---|
| 1 | Spanó et al.[18] | 2009 | Apple vinegar, 15% EDTA, 5% malic acid, 5% acetic acid, 10% citric acid, and 10% sodium citrate | Smear layer removal and calcium ions concentration | 15% EDTA and 10% citric acid were more effective than the other solutions in removing the smear layer. 15% EDTA exhibited the highest concentration of calcium ions in the solution, followed by 10% citric acid, then apple vinegar, 5% acetic acid and 5% malic acid, and 10% sodium citrate exhibited the lowest value. |
| 2 | Candeiro et al.[12] | 2011 | Apple vinegar, apple vinegar + 17% EDTA, 1% NaOCl +17% EDTA and saline | Smear layer removal | Apple vinegar alone or associated with 17% EDTA showed greater smear layer removal from middle than apical third. Apple vinegar associated with EDTA demonstrated the greatest removal of smear layer from middle and apical regions, compared with 1% NaOCl +17% EDTA and saline. |
| 3 | Cruz-Filho et al.[13] | 2011 | Apple vinegar, 15% EDTA, 5% malic acid, 5% acetic acid, 10% citric acid and 10% sodium citrate | Microhardness | 15% EDTA and citric acid caused overall the significant greatest (sharp) decrease in dentin microhardness. Apple vinegar, acetic acid, and malic acid caused intermediate reduction, with no significant difference between them. |
| 4 | Rodrigues et al.[16] | 2013 | Apple vinegar, EDTA, SmearClear, and saline with or without ultrasonic activation. | Smear layer removal | 17% EDTA and SmearClear were more effective when compared with apple vinegar. |
| 5 | Kirchhoff et al.[14] | 2014 | Apple vinegar, 17% EDTA, 5% malic acid, 5% acetic acid, and distilled water | Smear layer removal and calcium ions concentration | Surfaces treated with apple vinegar showed less smear layer than those treated with 17% EDTA. Apple vinegar, 5% acetic acid, and 5% malic acid exhibited similar results. EDTA provided higher concentration of calcium ions in the solution followed by malic acid, apple vinegar, and acetic acid, respectively. |
| 6 | Safwat et al.[17] | 2017 | Apple vinegar (1–3 min), 17% EDTA (1–3 min) and saline | Smear layer removal and dentin calcium content | Apple vinegar (1 min) recorded the lowest smear layer score than EDTA, at the apical region. No significant difference was found between apple vinegar-1 min, 17% EDTA-3 min, and apple vinegar-3 min. Apple vinegar 1 min recorded the highest mean calcium content followed by EDTA 3 and 1 min, respectively, whereas the least value was recorded by apple vinegar 3 min. |
| 7 | Ali et al.[11] | 2019 | 95% apple vinegar, 5% NaOCl, 100% ginger oil, and saline | Smear layer removal | Apple vinegar showed the best smear layer removing agent for the three root canal levels (with no significant difference among them), followed by NaOCl with low mean values for both middle and coronal thirds. |
| 8 | Moness Ali and Raab[15] | 2019 | 5% apple vinegar, 2.5% NaOCl + 17% EDTA as final flush, diluted apple vinegar, and distilled water | Smear layer removal | 5% apple vinegar was significantly more effective in smear layer removal. The diluted apple vinegar was equally effective to 5% apple vinegar and 2.5% NaOCl associated with 17% EDTA at coronal and middle regions. |
| 9 | Abdelghany et al.[9] | 2020 | Apple vinegar, 17% EDTA, and saline | Smear layer removal | No significant difference in the removal of smear layer produced by EDTA and apple vinegar at all root canal levels. |
| 10 | Ali et al.[10] | 2020 | Apple vinegar and 17% EDTA | Surface roughness Push-out of sealer |
17% EDTA induced higher surface roughness and push-out bond strength than apple vinegar. |
| 11 | Abraham et al.[18] | 2018 | Apple cider vinegar, 15% EDTA, and 0.2% chitosan | Calcium ions concentration | Apple cider vinegar was associated with greatest calcium content, followed by chitosan and EDTA, respectively. |
| 12 | Mittal et al.[22] | 2018 | Apple cider vinegar, 15% EDTA, and 0.2% chitosan diluted with 1% acetic acid. | Smear layer removal Calcium ions concentration |
Chitosan displayed better elimination of smear layer in middle and apical thirds, followed by apple cider vinegar and EDTA, respectively. The smear layer was removed from middle third more than apical third. Apple vinegar exhibited highest calcium ions concentration followed by chitosan and EDTA, respectively. |
| 13 | Akbulut et al.[20] | 2019 | Apple cider vinegar, pomegranate vinegar, grape vinegar, 2.5% NaOCl, 2% CHX, and octenidine hydrochloride (OCT) | Surface roughness and microhardness | Pomegranate, apple cider, and grape vinegars presented higher roughness values than that obtained by NaOCl, CHX, and OCT, and all tested vinegars were nearly similar to each other. No significant reduction in microhardness of dentin displayed after 15 min of treatment with any of the tested irrigants; however, they reduced the microhardness after 30 min of treatment. All of the irrigants displayed similar microhardness values. |
| 14 | Altaf et al.[21] | 2019 | Apple cider vinegar and 15% EDTA | Smear layer removal | No significant difference was determined between EDTA and apple vinegar in mean smear layer scores. |
| 15 | Cecchin et al.[24] | 2015 | 6.5% grape seed extract (GSE), 2.5% NaOCl, 2% CHX, and QMix for 40 min, all solutions followed by 17% EDTA for 3 min | Flexural strength and ultimate tensile strength (UTS) | NaOCl and QMix significantly decreased the flexural and ultimate tensile strength. There was no significant difference between GSE and 2% CHX, where they did not alter the flexural strength. However, GSE improved the UTS. |
| 16 | Cecchin et al.[25] | 2017 | 6.5% GSE, 6% NaOCl, 6% Ca[OCl]2, and distilled water for 30 min followed by 5 mL 17% EDTA for 1 min | Flexural strength, tensile strength, and fracture resistance | NaOCl significantly decreased all dentin strengths. No significant difference was found between Ca[OCl]2, GSE, and control groups. |
| 17 | Margono et al.[23] | 2017 | GSE (3.25%, 6.5%, and 13%) and 17% EDTA | Smear layer removal | All concentrations of grape seed extract were effective in smear layer removal, but they were not as good as 17% EDTA. |
| 18 | Sugiono et al.[27] | 2018 | GSE, NaOCl, and Aqua Bidest | Flexural strength | The significantly higher flexural strength obtained by GSE with no significant difference with Aqua Bidest. NaOCl induced significantly lowest flexural strength. |
| 19 | Sayed[26] | 2019 | 5.25% NaOCl + 13% GSE compared with 5.25% NaOCl + 17% EDTA | Microhardness and erosion | NaOCl associated with EDTA caused significant high percent reduction than that obtained by NaOCl associated with GSE. NaOCl associated with EDTA induced moderate-to-severe erosion, while 5.25% NaOCl + 13% GSE induced mild erosion. |
| 20 | Taffarel et al.[28] | 2019 | 6.5% grape seed extract, 2% CHX, 6% NaOCl, 6% Ca[OCl]2, QMix, and distilled water used 5 mL of each solution for 30 min followed by 5 mL 17% EDTA for 1 min | Microhardness | All solutions maintained the dentin microhardness similar to that of control samples, with no significant difference among all solutions. |
| 21 | Bolhari et al.[29] | 2012 | C. aurantifolia, 17% EDTA, alcoholic CA extract, and distilled water | Smear layer removal | 17% EDTA cleaned the middle and coronal parts and partially cleaned the apical part. CA extract partially cleaned the coronal and middle parts and did not remove the smear layer from the apical part. Alcoholic CA extract was not effective at the three levels. |
| 22 | Chhabra et al.[30] | 2015 | C. aurantifolia + S. mukorrossi (1:1) or (2:1) and 17% EDTA. | Smear layer removal | 17% EDTA was more effective, however, combination of C. aurantifolia and S. mukorrossi enhanced effective smear layer removal. 2:1 concentration was more effective, particularly with ultrasonic agitation. |
| 23 | Palaniswamy et al.[31] | 2016 | White vinegar (5% pure acetic acid) and 17% EDTA | Smear layer removal | EDTA was more effective in removing smear layer than white vinegar (containing 5% acetic acid). However, the results were not statistically significant. |
| 24 | Dal Bello et al.[33] | 2019 | 5%, 10%, and 17% GA, 17% EDTA, 10% citric acid and distilled water | Smear layer removal microhardness, roughness, mineral contents | All concentrations of GA was equally effective to EDTA and citric acid in removing smear layer. 5–10% GA showed no significant changes in microhardness similar to that of EDTA and distilled water. 17% GA induced the lowest microhardness and highest roughness. No chemical changes obtained by the used solutions. |
| 25 | Barcellos et al.[4] | 2020 | 17% GA (pH 1.2 and 5) and EDTA | Smear layer removal. Dentin erosion, microhardness, flexural strength (FS), mineral contents, and apatite/collagen ratio | GA demonstrated the ability to remove smear layer and produce dentin erosion similar to EDTA. EDTA and GA significantly reduced dentin microhardness with no significant difference regardless of the pH. EDTA and 5 pH GA reduced the FS than 1.2 pH GA, with no significant difference among all groups. No alternation in the distribution of dentin mineral content was determined by both solutions; however, they reduced the apatite/collagen ratio and the lowest value was obtained with GA. |
| 26 | Dal Bello et al.[32] | 2020 | GA (5%, 10% and 17%), 10% citric acid, and 17% EDTA for 1 min | Flexural strength and apatite/collagen ratio | All solutions did not significantly alter the flexural strength. All solutions reduced the apatite/collagen ratio. The lowest value obtained with increased concentration of GA. |
| 27 | Nassar et al.[38] | 2015 | 1% phytic acid (IP6) (pH 1.3) (applied for 30 s, or 1 min), 17% EDTA, and distilled water (control) | Smear layer removal | 1% IP6 and 17% EDTA showed the same ability to eliminate smear layer from the middle third; however, they showed less effectiveness at the apical third. |
| 28 | Nikhil et al.[39] | 2016 | 1% phytic acid (IP6) (pH 3.2), 0.2% chitosan (pH 3.2), and 17% EDTA, all applied for 3 min | Microhardness | 1% IP6 produced less percentage reduction in microhardness compared with 17% EDTA. No significant difference between 1% IP6 and 0.2% chitosan in microhardness. At the apical level, the percentage reduction in microhardness was lower than that in the coronal and middle levels, with no significant difference among them. |
| 29 | Shetty et al.[41] | 2016 | 1% phytic acid (IP6), 17% EDTA, etidronate, Er:YSGG laser, and 1% distilled water (control), all applied for 5 min and preceded by 5% NaOCl solution applied for 5 min | Calcium loss | 1% IP6 produced more calcium loss compared to 17% EDTA, and the results were significantly different. |
| 30 | Jagzap et al.[35] | 2017 | Phytic acid (IP6), 17% EDTA (both 1 mL/min) and Q-mix | Smear layer removal | 17% EDTA was significantly more effective than IP6 applied for 1 min. No significant difference was observed between IP6 and Q-mix. |
| 31 | Puvvada et al.[40] | 2017 | Pure 1% phytic acid (IP6) and 1% IP6 associated with 5% NaOCl | Chelated calcium ions | 1% IP6 associated with NaOCl showed more calcium chelating potential compared with 1% IP6 when used alone. |
| 32 | Sumathi[42] | 2017 | 1% Phytic acid (IP6), 18% etidronic acid, and 17% EDTA, all applied for 1, 3, and 5 min | Smear layer removal and erosion | 1% IP6 was effective in eliminating smear layer from coronal and middle thirds, at all application times similar to that of 17% EDTA and greater than the effect of 18% etidronic acid. Whereas it was ineffective to clean the apical third. 1% IP6 showed the least significant dentin erosion while 17% EDTA exhibited the highest erosion value. The erosion effect increased with increasing the contact time. |
| 33 | Kalçay and Tinaz[36] | 2018 | 0.5% and 1% phytic acid (IP6), 17% EDTA, and distilled water (control) (for 1 min) followed by 5% NaOCl solution | Smear layer removal and erosion | 1% IP6 with 1 min application time was the most effective smear layer removing agent, followed by 0.5% IP6 and 17% EDTA, with a significant difference among them at the coronal and apical root canal levels. No significant difference was found between 0.5% IP6 and 17% EDTA in their ability to remove smear layer from the coronal and middle thirds. The highest erosion was induced by 1% IP6 followed by 0.5% IP6 and 17% EDTA, at the middle and apical levels, with a significant difference among the tested groups at the middle level. No significant difference was found among 1% IP6, 0.5% IP6, and 17% EDTA at the coronal third. |
| 34 | Afshan et al.[34] | 2020 | 1% Phytic acid (IP6) (pH 3), 17% EDTA, and saline, all solutions applied for 1, 3, and 5 min | Smear layer removal and erosion | 17% EDTA was significantly better than IP6 at all levels of the root canal regarding smear layer removal. 1% IP6 (pH 3) induced significantly less erosion than 17% EDTA, at all root canal levels. IP6 and 17% EDTA showed no erosion and moderate erosion, respectively, after 1 min application time. IP6 and 17% EDTA showed moderate erosion after 3 min application time, while they showed moderate and sever erosion after 5 min application time, respectively. |
| 35 | Muana et al.[43] | 2020 | 1% Phytic acid (IP6) (pH 1.2), 17% EDTA (pH 7.5), 10% citric acid (pH 1.67), 37% phosphoric acid (pH < 1), and distilled water (control), all applied for 1 min | Microhardness and surface roughness | 1% IP6 produced significantly reduced microhardness compared with EDTA and citric acid, respectively. No significant difference was determined between IP6 and phosphoric acid in microhardness. IP6 significantly increased surface roughness compared with EDTA and citric acid, respectively. No significant difference was found between 1% IP6 and 37% phosphoric acid in surface roughness. |
| 36 | Naeem et al.[37] | In press | 0.5%, 1%, 1.5% phytic acid (IP6) and 17% EDTA, all applied for 5 min followed by 2.5% NaOCl for 5 min | Microhardness | 17% EDTA showed the highest percentage decrease in microhardness followed by 1.5% IP6 and 1% IP6. The lowest percentage decrease was observed in 0.5% IP6. No significant difference was displayed in percentage decrease in microhardness among 0.5%, 1%, and 1.5% IP6. |
SMEAR LAYER REMOVAL
From 21 studies, 13 studies[4,9,11,12,14,15,17,21,22,33,36,38,42] indicated that the natural extracts had better or similar effectiveness, compared with synthetic conventional agents such as EDTA or NaOCl, while the other 8 studies[16,18,23,29,30,31,34,35] showed better effectiveness of EDTA.
Fruit extract
Apple vinegar-apple cider vinegar determined better smear layer removal in six studies[11,12,14,15,17,22] and no significant difference in two studies,[9,21] compared with chemical agents including EDTA, NaOCl, acetic acid, citric acid, or malic acid. However, they determined lower effect than EDTA,[16,18] chitosan,[22] and SmearClear.[16]
When compared with EDTA, one study showed that different concentrations of GSE (13%, 6.5%, and 3.25%) were effective to clean the apical third[23] and two studies showed the effectiveness of C. aurantifolia (CAu) to partially clean the coronal and middle thirds.[29,30] However, both solutions were not as good as 17% EDTA. The effect of CAu increased when mixed with Sapindus mukorossi (2:1 concentration), particularly with ultrasonic agitation.[30]
Plant extract
White vinegar showed less chelating action[31]; however, GA was equally effective at different concentrations (5%, 10%, and 17%)[33] or different pH values (1.2 and 5)[4] to 17% EDTA or 10% citric. When compared with EDTA, 0.5–1% phytic acid (IP6) determined better effect in one study,[36] equal effect in two studies,[38,42] and less effect in two studies.[34,35] It had similar effect to Q-mix.[35] Two studies showed ineffectiveness of IP6 to clean the apical region.[38,42]
EFFECT OF NATURAL IRRIGANTS ON MECHANICAL AND CHEMICAL DENTIN PROPERTIES
From a total of 24 studies, 15 studies demonstrated no alteration in strength or microhardness,[24,25,28,32] less detrimental effect on calcium ions (Ca2+) loss, erosion or microhardness,[13,14,17,18,26,34,37,39,42] or even positive effect on roughness or flexural strength,[27,33] compared with synthetic conventional solutions such as NaOCl, EDTA, or citric acid.
Roughness, microhardness, and dentin strength
Apple vinegar determined lower surface roughness than 15–17% EDTA[10] or 10% citric acid,[13] whereas pomegranate, apple cider, and grape vinegars presented higher roughness values, which were nearly similar to each other, than that obtained by NaOCl and CHX.[20] All these used solutions did not alter the dentin microhardness, when used for 15 min, whereas they reduced the microhardness after 30 min of treatment.[20]
GSE maintained the dentin microhardness[28] and strength[24,25,27] with no significant difference with the untreated group. However, when preceded with 5.25% NaOCl, it induced reduction in microhardness lower than that obtained by 17% EDTA.[26]
Regarding GA, 17% GA reduced micro-hardness and increased the roughness of the dentin surface in two studies,[4,33] whereas one study showed no significant alternation in flexural dentin strength induced by 5–17% GA[32] versus 17% EDTA or 10% citric acid. With regard to IP6, 0.5–1% applied for 3 or 5 min had less detrimental effect on dentin microhardness than 17% EDTA[37,39] or similar to 0.2% chitosan.[39]
Calcium ions concentration/mineral content
Apple vinegar recorded Ca2+ concentration similar to that of 5% acetic acid and smaller than 17% EDTA in two studies,[14,18] but greater than 0.2% chitosan and 15% EDTA, respectively, in one study.[22]
When compared with EDTA, apple vinegar rendered the highest calcium dentin content in one study,[17] whereas 1% IP6 produced more calcium loss[41] particularly when associated with NaOCl.[40]
In one study, there were no chemical changes after using different concentrations of GA (5%, 10%, and 17%), 17% EDTA, or citric acid.[33] However, other two studies showed reduction in apatite/collagen ratio with the lowest significant value obtained with increasing GA concentration (17%).[4,32]
Erosion
When compared with 17% EDTA, GSE[26] as well as 1% IP6 at any application time (1, 3, or 5 min)[34,42] induced the least significant dentin erosion. Only one study showed that the highest significant erosion was induced in the middle region by 0.5–1% IP6, with no significant difference at the cervical region.[36]
DISCUSSION
Based on the current literature review, most studies found that apple vinegar, GSE, CAu, 5–10% GA, and 0.5–1% IP6 effectively removed the smear layer better or similar to synthetic agents. Natural irrigants had low risk factors on radicular dentin microhardness, roughness, strength, Ca2+ release, and erosion, especially when used for a short period of time.
Most of the included studies reported the efficiency of apple vinegar than EDTA,[14,17,22] 1–5% NaOCl,[11,12] 2.5% NaOCl associated with 17% EDTA as final flush,[15] and 10% sodium citrate,[18] or even no significant difference compared with chemical chelators.[9,21] However, the chelating action was reduced with diluted apple vinegar.[15]
Although GSE proved its efficiency with different concentrations (3.25%, 6.5%, and 13%) and CAu exhibited partially clean coronal and middle parts,[29] their effects were not as good as 17% EDTA.[23]
The smear layer removing ability of vinegars is multifactorial.[44] The different effects of multiple types of vinegars may be attributed to their different compositions and pH values. Apple vinegar and apple cider vinegar are two types of vinegars extracted from apple fruit. Both contain acetic (main component), malic, citric, formic, lactic, and succinic acids with lesser alcohol and more acetic acid in apple vinegar.[45] The chelating effect of apple vinegar could be affected by its acidic pH (2.71).[46] However, GSE contains weak acid (about 74–78% proanthocyanidin (PA)).[23]
C. aurantifolia is a genus citrus fruit, resembles orange leaves, and contains 6–8% citric acid and 2% potassium citrate.[29,30] Due to its high surface tension, it failed to penetrate into the apical region. S. mukorossi is an Indian fruit containing saponin. The mixture of CAu and S. mukorossi improved the chelating effect of citric acid by the surfactant action of saponin that lowered the surface tension of CAu and promoted better penetration.[29,30] However, white vinegar yields from food fermentation, such as sugar, beets, and potatoes, mainly consist of 5% acetic acid with pH of 2.4 and showed no-to-moderate smear layer removal.[31]
GA[4,33] and IP6[36,38] are natural plant extracts, recently used as smear layer removing agent. Compared with 17% EDTA, 5–17% GA exhibited similar effect.[4,33] GA is a hydroxyacetic acid derived from sugar cane and sweet vegetables. Its chelating efficiency may be attributed to its acidic pH (1.2 and 5) and surface tension.[4]
With regard to IP6, variable results were obtained, ranging from better effect,[36] similar effect,[38,42] or lower effect[34,35] compared with 17% EDTA. IP6 is a natural plant compound extracted from cereals, legumes, oil seeds, nuts, and rice bran. It has six reactive phosphate groups with unique binding affinity to certain dietary minerals such as calcium.[47] Its chelating effectiveness may be attributed to its negatively charged phosphate groups that have strong chelating capacity to divalent cations such as Ca+2.[48] The concentration of the solution also has impact role, as 1% IP6 was more effective than 0.5%.[36] The conflicting results of IP6 could be pH-dependent factor.
Furthermore, the action of chelator claims to be a time-dependent process, in which IP6 applied for 3 min exhibited more open dentinal tubules with less debris, compared with 1 min application time.[34] However, no statistical significant difference was reported between apple vinegar applied for 1 or 3 min and 17% EDTA applied for 3 min in their smear layer removing ability.[17]
Regarding the chelating effect at different root canal levels, it has been reported that either natural extract chelators including apple vinegar, GSE, CAu, and IP6 or chemical chelators like EDTA were ineffective to clean the apical third.[13,23,29,34,35,38] This might be due to the presence of sclerotic dentin in the apical region,[49] which impairs the irrigant’s flow.
The removal of smear layer has a significant influence on chemo-mechanical dentin properties. Although both chemical and natural chelators exhibited reduction in dentin microhardness, it seems that the natural chelators have less impact on reducing radicular dentin microhardness when compared with synthetic ones, especially when associated with NaOCl.[26,50] It has been reported that GSE did not alter the dentin microhardness[28] or strength[24,25,27] with no significant difference versus the untreated dentin or even produced higher flexural strength compared with NaOCl.[27] However, when the irrigating process was preceded with 5.25% NaOCl, GSE-induced reduction in microhardness value is significantly lower than that obtained by 17% EDTA.[26] This action could be attributed to the great PAs content in GSE. Moreover, PAs have been found to strengthen the mechanical dentin properties as they prevent the biodegradation and breakdown of collagen fibers and limit its enzymatic degradation and matrix metalloprotease enzyme.[51,52]
In contrast, apple vinegar[13] and IP6[37,39] produced lesser reduction in dentin microhardness, but GA provided greater reduction[33] than EDTA and citric acid.[32] On the contrary, one study showed the greatest microhardness reduction and the highest surface roughness produced by 1% IP6 than 17% EDTA and 10% citric acid, respectively.[43]
The dentin mechanical properties seem to be influenced by several factors. Concerning the pH factor, it has been reported that more acidic pH of IP6 (1.2)[43] can induce aggressive dentin effect compared with IP6 with less acidic pH (3.2)[39] or EDTA with neutral pH.[43] Moreover, the use of IP6 for prolonged contact time (5 min)[37] produced more percentage reduction in microhardness compared with shorter contact time (3 min).[39] In addition, 30 min application of apple cider, pomegranate, and grape vinegars significantly reduced the dentin microhardness compared with 15 min contact time.[20]
Regarding the chelator’s concentration, it has been reported that the higher a solution’s concentration, the greater the demineralizing action.[53] About 17% GA[33] and 1% IP6[37] produced significant microhardness reduction compared with their lower concentrations (5–10%) and (0.5%), respectively.
The solution’s surface tension accounts for its mechanical effect on dentin. The solution with low surface tension can easily penetrate into the dentin surface, allow efficient smear layer removal with greater dentin softening associated with more denaturation of collagen fibril, extract dentin mineral, increase Ca2+ concentration in the surrounding solution, and allow changes in the apatite/collagen ratio.[4,33] With increase in GA concentration (17%), its surface tension was decreased, which allows its better penetration to dentinal tubules and reduces dentin microhardness.[32]
The higher surface roughness is a benefit value to improve the adhesion of obturating material to dentin by creating micromechanical bonds. The high concentration and acidic pH of the chelator have an influence on the surface roughness. This fact was confirmed by Dal Bello et al.,[33] in which the surface roughness increased by 17% versus 5–10% GA. Moreover, the more acidic pH of IP6 (1.2) significantly increased the surface roughness compared with EDTA with neutral pH.[43]
The changes in microhardness and surface roughness are also affected by Ca2+ removal from the dentin surface by the irrigant, which is affected by attractive and repulsive forces between the metal ions. It was suggested that due to the ionization of carboxylic group of EDTA molecule, hydrogen atoms are released in the surrounding environment and compete with dentin calcium ions.[13] In contrast, acetic acid of apple vinegar is a weak acid with low concentration of H+ ions that may be responsible for inefficient calcium removal.[14] These results are confirmed by three studies, in which the apple vinegar recorded low amount of chelated Ca2+ in solution[14,18] or the highest dentin calcium content[17] compared with EDTA.
On the contrary, IP6 caused more calcium loss from radicular dentin compared with EDTA[41] without much altering dentin microhardness.[37,39] It seems that the preceding use of NaOCl could improve IP6 performance. The use of NaOCl dissolves the organic portion of smear layer, allows acids to dissolve the inorganic portion, penetrates into dentinal tubules, and decalcifies them.[54]
The smear layer removal has an impact on disintegration of intertubular and peritubular dentin that promotes canal wall erosion, which, in turn, may jeopardize the sealing ability of the obturating material and allow greater bacterial penetration with further tooth weakening.[42] The erosive ability of the chelator could be related to pH and application time. IP6, with weak acidic pH (3), considered a weak chelating agent with no-to-moderate erosive ability when applied for 1 and 3 min, respectively.[34] However, 1% IP6 showed moderate-to-severe erosion when applied for 1 min in association with 5% NaOCl.[36] The use of NaOCl after chelator may be the cause of further dentin erosion.[55]
GSE produced mild erosion when compared with moderate–severe erosion induced with 17% EDTA.[26,36] The mild erosive effect of GSE could be related to the PAs constituent that inhibits the degradation of intertubular and peritubular dentin. It was suggested that the excessive dentin erosion induced progressive opening of dentinal tubules.
CONCLUSION
Under the circumstances of this review, most of the included studies revealed that apple or apple cider vinegars, GSE, CAu mixed with S. mukorossi, 5–10% GA, and 0.5–1% IP6 effectively removed the smear layer better or similar to synthetic conventional agents. Natural irrigants/chelators had limited risk factors on radicular dentin microhardness, roughness, strength, calcium ions release, and erosion, particularly when used for a short time and with low concentration. All root canal irrigants, either natural (interventions) or chemical (comparators), failed to completely clean the apical root canal region.
LIMITATIONS
The first is despite the fact that the major databases were used for the literature search, papers that were not listed in these sources may have been neglected. The second limitation concern is the lack of critical appraisal of included study validity, with all evidence treated as equally valid.
STRENGTH
The current literature review is valuable to map the efficacy of natural irrigants versus synthetic ones for smear layer removal in endodontics.
FINANCIAL SUPPORT AND SPONSORSHIP
Autosupport by authors themselves.
CONFLICTS OF INTEREST
There was no conflict of interest.
AUTHORS’ CONTRIBUTIONS
The authors equally contributed to the current study.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
Not applicable.
PATIENT DECLARATION OF CONSENT
Not applicable.
DATA AVAILABILITY STATEMENT
Data used in the current article are available for all readers.
ACKNOWLEDGEMENTS
None.
REFERENCES
- 1.Hülsmann M. Effects of mechanical instrumentation and chemical irrigation on the root canal dentin and surrounding tissues. Endod Top. 2013;29:55–86. [Google Scholar]
- 2.Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: A systematic review and meta-analysis. J Endod. 2007;33:96–105. doi: 10.1016/j.joen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Violich DR, Chandler NP. The smear layer in endodontics—A review. Int Endod J. 2010;43:2–15. doi: 10.1111/j.1365-2591.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 4.Barcellos DPDC, Farina AP, Barcellos R, Souza MA, Borba M, Bedran-Russo AK, et al. Effect of a new irrigant solution containing glycolic acid on smear layer removal and chemical/mechanical properties of dentin. Sci Rep. 2020;10:7313. doi: 10.1038/s41598-020-64450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballal NV, Kandian S, Mala K, Bhat KS, Acharya S. Comparison of the efficacy of maleic acid and ethylenediaminetetraacetic acid in smear layer removal from instrumented human root canal: A scanning electron microscopic study. J Endod. 2009;35:1573–6. doi: 10.1016/j.joen.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Zehnder M, Kosicki D, Luder H, Sener B, Waltimo T. Tissue-dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:756–62. doi: 10.1067/moe.2002.128961. [DOI] [PubMed] [Google Scholar]
- 7.Ballal NV, Mala K, Bhat KS. Evaluation of the effect of maleic acid and ethylenediaminetetraacetic acid on the microhardness and surface roughness of human root canal dentin. J Endod. 2010;36:1385–8. doi: 10.1016/j.joen.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169:467–73. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 9.Abdelghany ME, Kamel WH, Bastawy HA. Efficacy of apple vinegar as final irrigating solution in removing smear layer using XP-Endo Finisher file. Al-Azhar Dent J Girls. 2020;7:95–103. [Google Scholar]
- 10.Ali AE, Fawzy MI, Bastawy HA. Evaluation of intraradicular surface roughness following final irrigation by apple vinegar and its correlation with resin sealer bond strength. Al-Azhar Dent J Girls. 2020;7:79–88. [Google Scholar]
- 11.Ali LA, Toma IS, Saeed RK. Comparative evaluation of a new endodontic irrigation solution—Apple vinegar, ginger oil and sodium hypochlorite to remove the smear layer by scanning electron microscope study. J Duhok Univ. 2019;22:30–7. [Google Scholar]
- 12.Candeiro GTdM, Matos IBd, Costa CFEd, Fonteles CSR, Vale MSd. A comparative scanning electron microscopy evaluation of smear layer removal with apple vinegar and sodium hypochlorite associated with EDTA. J Appl Oral Sci. 2011;19:639–43. doi: 10.1590/S1678-77572011000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz-Filho AM, Sousa-Neto MD, Savioli RN, Silva RG, Vansan LP, Pécora JD. Effect of chelating solutions on the microhardness of root canal lumen dentin. J Endod. 2011;37:358–62. doi: 10.1016/j.joen.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff AL, Viapiana R, Miranda CE, Sousa Neto MD, Cruz Filho AM. Comparison of the apple vinegar with other chelating solutions on smear layer and calcium ions removal from the root canal. Indian J Dent Res. 2014;25:370–4. doi: 10.4103/0970-9290.138341. [DOI] [PubMed] [Google Scholar]
- 15.Moness Ali AM, Raab WH. Smear layer removal efficiency using apple vinegar: An in vitro scanning electron microscope study. Am J Dent. 2019;32:21–7. [PubMed] [Google Scholar]
- 16.Rodrigues CT, Bernardineli N, DuarTe MAH, Bramante CM, de Andrade FB. Evaluation of EDTA, apple vinegar and SmearClear with and without ultrasonic activation on smear layer removal in different root canal levels. Dent Press Endod. 2013;3:43–8. [Google Scholar]
- 17.Safwat H, Nour El Deen M, Bastawy H. Evaluation of smear layer removal and calcium ions concentration in intraradicular dentin treated with apple vinegar (SEM study) Al-Azhar Dent J Girls. 2017;4:279–87. [Google Scholar]
- 18.Spanó JC, Silva RG, Guedes DF, Sousa-Neto MD, Estrela C, Pécora JD. Atomic absorption spectrometry and scanning electron microscopy evaluation of concentration of calcium ions and smear layer removal with root canal chelators. J Endod. 2009;35:727–30. doi: 10.1016/j.joen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Abraham AM, Yendrembam B, Kumari S, Mittal A, Dhaundiyal A. Effectiveness of different final irrigation solutions on quantification of calcium ions from the root canal wall: An in vitro study. Ind J Contemp Dent. 2018;6:28–33. [Google Scholar]
- 20.Akbulut MB, Guneser MB, Eldeniz AU. Effects of fruit vinegars on root dentin microhardness and roughness. J Conserv Dent. 2019;22:97–101. doi: 10.4103/JCD.JCD_394_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altaf A, Basher A, Mir N. Assessment of efficacy of two root canal irrigation solutions on smear layer removal during root canal therapy: A comparative study. J Adv Med Dent Sci Res. 2019;7:173–5. [Google Scholar]
- 22.Mittal A, Dadu S, Yendrembam B, Abraham A, Singh NS, Garg P. Comparison of new irrigating solutions on smear layer removal and calcium ions chelation from the root canal: An in vitro study. Endodontology. 2018;30:55. [Google Scholar]
- 23.Margono A, Angellina AN, Suprastiwi E. The effect of grape seed extraction irrigation solution towards cleanliness the smear layer on apical third of the root canal wall. J Int Dent Med Res. 2017;10:244–7. [Google Scholar]
- 24.Cecchin D, Farina AP, Souza MA, Albarello LL, Schneider AP, Vidal CM, et al. Evaluation of antimicrobial effectiveness and dentine mechanical properties after use of chemical and natural auxiliary irrigants. J Dent. 2015;43:695–702. doi: 10.1016/j.jdent.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Cecchin D, Soares Giaretta V, Granella Cadorin B, Albino Souza M, Vidal CMP, Paula Farina A. Effect of synthetic and natural-derived novel endodontic irrigant solutions on mechanical properties of human dentin. J Mater Sci Mater Med. 2017;28:141. doi: 10.1007/s10856-017-5960-1. [DOI] [PubMed] [Google Scholar]
- 26.Sayed YSM. Evaluation of dentin microhardness and push out bond strength of resin sealer to dentin after irrigation with sodium hypochlorite followed by either grape seed extract or EDTA: A randomized in-vitro study. CU Thesis. 2019:1–21. [Google Scholar]
- 27.Sugiono R, Meidyawati R, Asrianti D. Effect of grape seed extract solution on the flexural strength of root canal dentin. J Phys Conf Ser. 2018;1073:032003. [Google Scholar]
- 28.Taffarel C, Bonatto FD, do Carmo Bonfante F, Palhano HS, Vidal CdMP, Cecchin D, et al. Effect of chemical and natural irrigant solutions on microhardness of root dentin—An in vitro study. Braz J Oral Sci. 2018;17:e18409–e17. [Google Scholar]
- 29.Bolhari B, Sharifian MR, Aminsobhani M, Monsef Esfehani HR, Tavakolian P. Assessing the efficacy of Citrus aurantifolia extract on smear layer removal with scanning electron microscope. Iran Endod J. 2012;7:88–97. [PMC free article] [PubMed] [Google Scholar]
- 30.Chhabra N, Gyanani H, Kamatagi L. Smear layer removal efficacy of combination of herbal extracts in two different ratios either alone or supplemented with sonic agitation: An in vitro scanning electron microscope study. J Conserv Dent. 2015;18:374–8. doi: 10.4103/0972-0707.164035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palaniswamy U, Kaushik M, Surender LR, Prashar N, Arya S, Pasari S. A SEM evaluation of smear layer removal using two rotary instrument systems with EDTA and vinegar as a root canal irrigant. J Restor Dent. 2016;4:17–21. [Google Scholar]
- 32.Dal Bello YD, Farina AP, Souza MA, Cecchin D. Glycolic acid: Characterization of a new final irrigant and effects on flexural strength and structural integrity of dentin. Mater Sci Eng C Mater Biol Appl. 2020;106:110283. doi: 10.1016/j.msec.2019.110283. [DOI] [PubMed] [Google Scholar]
- 33.Dal Bello YD, Porsch HF, Farina AP, Souza MA, Silva EJNL, Bedran-Russo AK, et al. Glycolic acid as the final irrigant in endodontics: Mechanical and cytotoxic effects. Mater Sci Eng C Mater Biol Appl. 2019;100:323–9. doi: 10.1016/j.msec.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Afshan Z, Jat SA, Khan JA, Hasan A, Rehman Qazi FU. Erosive potential of 1% phytic acid on radicular dentine at different time intervals. Eur Endod J. 2020;5:28–34. doi: 10.14744/eej.2019.02411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jagzap JB, Patil SS, Gade VJ, Chandhok DJ, Upagade MA, Thakur DA. Effectiveness of three different irrigants—17% ethylenediaminetetraacetic acid, Q-MIX, and phytic acid in smear layer removal: A comparative scanning electron microscope study. Contemp Clin Dent. 2017;8:459–63. doi: 10.4103/ccd.ccd_524_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalçay M, Tinaz AC. Effects of different concentrations of phytic acid on smear layer removal and erosion. Atatürk Üniversitesi Diş Hekimliği Fakültesi Dergisi. 2018;28:341–7. [Google Scholar]
- 37.Naeem MM, Abdallah AM, Kamar AA, Leheta NA. Effect of phytic acid (IP6) versus ethylene diamine tetra acetic acid (EDTA) on dentin microhardness (in vitro study) Alex Dent J [Google Scholar]
- 38.Nassar M, Hiraishi N, Tamura Y, Otsuki M, Aoki K, Tagami J. Phytic acid: An alternative root canal chelating agent. J Endod. 2015;41:242–7. doi: 10.1016/j.joen.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 39.Nikhil V, Jaiswal S, Bansal P, Arora R, Raj S, Malhotra P. Effect of phytic acid, ethylenediaminetetraacetic acid, and chitosan solutions on microhardness of the human radicular dentin. J Conserv Dent. 2016;19:179–83. doi: 10.4103/0972-0707.178705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puvvada S, Prasanna Latha D, Jayalakshmi K. Comparative assessment of chelating and antimicrobial efficacy of phytic acid alone and in combination with other irrigants. Int J Appl Dent Sci. 2017;3:19–22. [Google Scholar]
- 41.Shetty K, Satish S, Krishna RK, Reza KM, Basavanagowda Patil S. Effect of EDTA, etidronic acid, phytic acid and Er:YSGG laser on calcium loss of root dentin using atomic absorption spectrophotometer. An in vitro study. J Appl Dent Med Sci. 2016;2:18–24. [Google Scholar]
- 42.Sumathi K. A Comparative Evaluation of Efficacy of Phytic Acid, Etidronic Acid and EDTA on Smear Layer Removal and Dentin Erosion at Different Time Intervals Using Scanning Electron Microscope: An In Vitro study. Chennai: Tamil Nadu Government Dental College and Hospital; 2017. [Google Scholar]
- 43.Muana HL, Nassar M, Dargham A, Hiraishi N, Tagami J. Effect of smear layer removal agents on the microhardness and roughness of radicular dentin. Saud Dent J. 2020 doi: 10.1016/j.sdentj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samad A, Azlan A, Ismail A. Therapeutic effects of vinegar: A review. Curr Opin Food Sci. 2016;8:56–61. [Google Scholar]
- 45.Budak NH, Aykin E, Seydim AC, Greene AK, Guzel-Seydim ZB. Functional properties of vinegar. J Food Sci. 2014;79:R757–64. doi: 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- 46.Zandim DL, Corrêa FO, Sampaio JE, Rossa Júnior C. The influence of vinegars on exposure of dentinal tubules: A SEM evaluation. Braz Oral Res. 2004;18:63–8. doi: 10.1590/s1806-83242004000100012. [DOI] [PubMed] [Google Scholar]
- 47.Abdulwaliyu I, Arekemase SO, Adudu JA, Batari ML, Egbule MN, Okoduwa SIR. Investigation of the medicinal significance of phytic acid as an indispensable anti-nutrient in diseases. Clin Nutr Exp. 2019;28:42–61. [Google Scholar]
- 48.Kim NH, Rhee MS. Phytic acid and sodium chloride show marked synergistic bactericidal effects against nonadapted and acid-adapted Escherichia coli O157:H7 strains. Appl Environ Microbiol. 2016;82:1040–9. doi: 10.1128/AEM.03307-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paqué F, Luder HU, Sener B, Zehnder M. Tubular sclerosis rather than the smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. Int Endod J. 2006;39:18–25. doi: 10.1111/j.1365-2591.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- 50.Sayin TC, Serper A, Cehreli ZC, Otlu HG. The effect of EDTA, EGTA, EDTAC, and tetracycline-HCl with and without subsequent NaOCl treatment on the microhardness of root canal dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:418–24. doi: 10.1016/j.tripleo.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Balalaie A, Rezvani MB, Mohammadi Basir M. Dual function of proanthocyanidins as both MMP inhibitor and crosslinker in dentin biomodification: A literature review. Dent Mater J. 2018;37:173–82. doi: 10.4012/dmj.2017-062. [DOI] [PubMed] [Google Scholar]
- 52.Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, et al. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J Dent. 2010;38:908–15. doi: 10.1016/j.jdent.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reis C, De-Deus G, Leal F, Azevedo E, Coutinho-Filho T, Paciornik S. Strong effect on dentin after the use of high concentrations of citric acid: An assessment with co-site optical microscopy and ESEM. Dent Mater. 2008;24:1608–15. doi: 10.1016/j.dental.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 54.Marending M, Luder HU, Brunner TJ, Knecht S, Stark WJ, Zehnder M. Effect of sodium hypochlorite on human root dentine—Mechanical, chemical and structural evaluation. Int Endod J. 2007;40:786–93. doi: 10.1111/j.1365-2591.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 55.Qian W, Shen Y, Haapasalo M. Quantitative analysis of the effect of irrigant solution sequences on dentin erosion. J Endod. 2011;37:1437–41. doi: 10.1016/j.joen.2011.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in the current article are available for all readers.