ABSTRACT
Aim:
The COVID-19 pandemic has strained the world’s healthcare systems. Studies have identified how the COVID-19 infections are linked to several co-morbidities such as hypertension, diabetes, cardiovascular disease, renal and pulmonary disease. It is known that periodontal disease (PD) shares the same risk factors. Moreover, both diseases are characterized by an exaggerated immune response. The aim of the study was to investigate the available evidence of a potential association between PD and the risk of COVID-19 complications and mortality.
Materials and Methods:
MEDLINE/PubMed, EMBASE, Scopus, and ProQuest were searched. Studies that assess the association between PD and the risk of COVID-19 complications and mortality were eligible for inclusion. Two independent reviewers performed the selection of articles and data extraction. The New Castle Ottawa Scale was used to assess the quality of the selected studies, and the GRADE system was used to evaluate the level of confidence to support the conclusions.
Results:
Only two studies met the eligibility criteria. One study had a low risk of bias, whereas the other had a high risk of bias.
Conclusion:
The level of confidence in the available evidence is very low. A close association between periodontitis and the risk of COVID-19 complications and mortality can neither be supported nor refuted.
KEYWORDS: Complications, COVID-19, mortality, periodontitis, SARS-COV-2
INTRODUCTION
COVID-19 is an infectious disease caused by the coronavirus SARS-CoV-2. This disease was declared as a pandemic on May 11, 2020 by the World Health Organization (WHO).[1] It is estimated that more than 4.5 million people were infected during the first 3 months. Meanwhile, this infectious disease continues spreading quickly around the world.[2] The SARS-CoV-2 is transmitted mainly through respiratory droplets during near face-to-face contact. This infection can spread through asymptomatic, pre-symptomatic, or symptomatic carriers.[3]
The COVID-19 manifestations include asymptomatic carriers and sometimes a fulminant disease progression characterized by sepsis and acute respiratory insufficiency. About 5% of all patients with COVID-19 and 20% of hospitalized patients experience severe symptoms requiring intensive care.[4] Between 17% and 35% of the hospitalized patients with COVID-19 are treated more frequently in an intensive care unit (ICU) due to hypoxemic respiratory insufficiency. For patients treated in ICU due to COVID-19, it is estimated that between 29% and 91% require invasive mechanical ventilation.[5,6,7,8,9] Hospitalized patients, besides having respiratory insufficiency, can develop acute renal insufficiency (9%), hepatic dysfunction (19%), septic shock (6%), and coagulation dysfunction or hemorrhage (10–25%).[4] Regarding the mortality, in a cohort of 3988 patients in critical conditions due to SARS-CoV-2 infection, the estimated rates of mortality per 1000 patient-days were 27 (CI of 95%, 26–29) in the hospital and 12 (CI of 95%, 11–12) in the ICU. In the subset of the first 1715 patients, the mortality was 48.8% in ICU and 53.4% in-hospital conditions.[10] COVID-19 is more prevalent in patients who suffer from co-morbidities such as hypertension (present in 48–57% of the patients), diabetes (17–34%), cardiovascular disease (21–28%), chronic pulmonary disease (4–10%), chronic renal disease (3–13%), malignant neoplasm (6–8%), and chronic hepatic disease (<5%).[4]
Periodontitis or periodontal disease (PD) is a chronic inflammatory disease caused by microorganisms and characterized by the progressive destruction of the support apparatus of the tooth that can lead to tooth loss.[11] Recent studies suggest that PD could be associated with COVID-19 complications and mortality.[11,12] PD and COVID-19 present two common characteristics: (1) risk factors such as hypertension, diabetes, cardiovascular disease, and renal and pulmonary disease[4,13,14,15,16,17] and (2) exaggerated immune response characterized by a high release of interferons, interleukins (ILs), tumor necrosis factor (TNF), chemokines, and tissue damage in the more severe presentation of these two diseases.[18,19,20,21,22]
Due to the importance and prevalence of both diseases nowadays, the purpose of this review was to evaluate the available evidence about a potential association between PD and the risk of COVID-19 complications.
MATERIALS AND METHODS
REGISTRATION AND PROTOCOL
The protocol for this systematic review was registered in the database of PROSPERO with the code CRD42021244289 (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=244289).
This review was written and conducted according to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses).[23]
FOCUSSED QUESTION
What is the association between PD and the risk of COVID-19 complications and mortality in adults?
PECO question: Population, exposure, comparison, outcome[24]
Population: Adults with COVID-19
Exposure: Adults with a diagnosis of PD
Comparison: Adults without a diagnosis of PD
Outcome: Mortality and complications due to COVID-19 such as hospitalization, admission to the ICU, and need for assisted ventilation.
SEARCH STRATEGIES
The bibliographic search was carried out in several electronic databases such as PubMed, Scopus, EMBASE, and Proquest. The final search date in all the databases was July 23, 2021. There was no time limitation. Hand searches were completed as well. Pre-publication manuscripts were retrieved from preprint servers: bioRxiv, medRxiv, and Google Scholar.
All references were managed using reference management software (RefWorks), and duplicate results were eliminated.
The search strategy considered the following terms: (“periodontitis” OR “periodontal” OR “periodontal disease” OR “periodontal disease risk” OR “periodontal bacteria” OR “periodontal pocket” OR “apical periodontitis” OR “gingivitis” OR “bleeding gum” OR “gum disease” OR “gingival disease” OR “gingival inflammation”) AND (“coronavirus” OR “coronavirus covid-19” OR “novel coronavirus” OR “SARS-CoV” OR “sars cov-2” OR “covid-19” OR “SARS-CoV-2” OR “SARS-CoV” OR “MERS-CoV” OR “2019-nCoV”) [Table 1].
Table 1.
Search strategies with uniterms and Boolean operators employed according to different electronic databases
| MedLine/PubMed (23.07.2021) |
|---|
| n= 106 |
| (“periodontitis”[All Fields] OR “periodontal”[All Fields] OR “periodontal disease”[All Fields] OR “periodontal disease risk”[All Fields] OR “periodontal bacteria”[All Fields] OR “periodontal pocket”[All Fields] OR “apical periodontitis”[All Fields] OR “gingivitis”[All Fields] OR “bleeding gum”[All Fields] OR “gum disease”[All Fields] OR “gingival disease”[All Fields] OR “gingival inflammation”[All Fields]) AND (“coronavirus”[All Fields] OR “coronavirus covid-19”[All Fields] OR “novel coronavirus”[All Fields] OR “SARS-CoV”[All Fields] OR “sars cov 2”[All Fields] OR “covid-19”[All Fields] OR “sars cov 2”[All Fields] OR “SARS-CoV”[All Fields] OR “MERS-CoV”[All Fields] OR “2019-nCoV”[All Fields]) Filters: Publication year from 2007 to 2021 |
| EMBASE(23.07.2021) |
| n= 152 |
| (“periodontitis”/exp OR “periodontitides”:ti,ab,kw OR “pericementitis”:ti,ab,kw OR “pericementitides”:ti,ab,kw OR “periodontal disease”/exp OR “disease, periodontal”:ti,ab,kw OR “diseases, periodontal”:ti,ab,kw OR “periodontal diseases”:ti,ab,kw OR “parodontosis”:ti,ab,kw OR “parodontoses”:ti,ab,kw OR “pyorrhea alveolaris”/exp OR “gingivitis”/exp OR “gingivitides”:ti,ab,kw) AND (“coronavirus”/exp OR “coronavirus covid-19”/exp OR “novel coronavirus”/exp OR “SARS-CoV”/exp OR “sars cov-2”/exp OR “covid-19”/exp OR “SARS-CoV-2”/exp OR “SARS-CoV”/exp OR “MERS-CoV”/exp OR “2019-nCoV”/exp) |
| SCOPUS (23.07.2021) |
| n= 156 |
| TITLE-ABS-KEY((periodontitis OR periodontitides OR pericementitis OR pericementitides OR periodontal OR “Diease, Periodontal” OR “Diseases, Periodontal” OR “Periodontal Disease” OR parodontosis OR parodontes OR “Pyorhea Alveolaris” OR gingivitis OR gingivitides) AND (coronavirus OR “coronavirus covid-19” OR “novel coronavirus” OR “SARS-CoV” OR “sars cov-2” OR “covid-19” OR “SARS-CoV-2” OR “SARS-CoV” OR “MERS-CoV” OR “2019-nCoV”)) |
| PROQUEST (23.07.2021) |
| n=17 |
| (“periodontitis” OR “periodontal” OR “periodontal disease” OR “periodontal disease risk” OR “periodontal bacteria” OR “periodontal pocket” OR “apical periodontitis” OR “gingivitis” OR “bleeding gum” OR “gum disease” OR “gingival disease” OR “gingival inflammation”) AND (“coronavirus” OR “coronavirus covid-19” OR “novel coronavirus” OR “SARS-CoV” OR “sars cov 2” OR “covid-19” OR “sars cov 2” OR “SARS-CoV” OR “MERS-CoV” OR “2019-nCoV”) Filters:subjet periodontitis |
INCLUSION CRITERIA
Case–control studies and cross-sectional studies were considered.
EXCLUSION CRITERIA
Interventional studies (RCTs), case series, case reports, pilot studies, editorials, reviews, animal studies, and studies that do not consider co-morbidities were excluded.
STUDY SELECTION
The initial selection of the articles was carried out independently by two reviewers (D.A.K.E.-E. and O.A.C.-L.) according to the title and abstract. If the abstract was not available, the full text was obtained and evaluated. The full text of all articles was compiled, and the inclusion criteria were verified. Any disagreement was solved by consensus or by the decision of a third author (C.F.C.-R.). The articles that did not meet the selection criteria were excluded from the review, and reasons for exclusion were registered. The description of the selected studies can be seen in Tables 2 and 3.
Table 2.
General description of included studies
| Authors, year of publication, country where the study was carried out, and journal where it was published | Objective | Study design | Sample | Exposure diagnosis: periodontitis | Condition outcome: complications and mortality from COVID-19 | Association measurement | Confusion covariates | Main findings |
|---|---|---|---|---|---|---|---|---|
| 1. Marouf et al. 2021 Qatar Journal of Clinical Periodontology | The objective of this case– control study was to investigate the association of periodontitis with COVID-19 complications. | Case–control | 568 patients diagnosed with COVID-19 who are part of the national electronic medical records of Hamad Medical Corporation (HMC) of the state of Qatar of which 258 presented periodontitis. | The periodontal status was studied from bitewing and panoramic radiographs (OPG) from the patient records.[28] | Condition of complications and mortality from COVID-19 | Association between PD and complications by COVID-19: | Age, sex, smoking, diabetes, co-morbidities, body mass index | Frequency of periodontitis: 45.42% Case group: patients with complications from COVID-19 |
| Case group: Cases were defined as patients with complications from COVID-19, such as admission to ICU, need for assisted ventilation, or death. | The percentage of bone loss was obtained from the most affected tooth using the criteria of the classification of periodontal and peri-implant diseases. | People with at least one of the following criteria: | Any complication (OR = 3.67, 95% CI 1.46–9.27) | 7.04% Control group: COVID-19 patients discharged without major complications 92.957% | ||||
| The cases consisted of 40 patients of which 33 had periodontitis and 7 did not present periodontitis. | The patients were classified according to periodontitis stages[29]: | 1. Death 2. Admission to ICU 3. Need for assisted ventilation | Mortality (OR = 8.81, 95% CI 1.00–77.7) Admission to ICU (OR = 3.54, 95% CI 1.39–9.05) | The study findings showed a strong association between periodontitis and an increased risk of complications from COVID-19, | ||||
| Control group: Controls were defined as COVID-19 patients discharged without major complications. | • Initial periodontitis or periodontally healthy (stages 0-1): bone loss less than the coronal third of the root length (15%) on OPG radiographs, or ≤2 mm on bitewing radiographs. | Need for assisted ventilation (OR = 4.57, 95% CI 1.19–17.4) | including admission to ICU, the need for assisted ventilation, and death after performing adjusted regression models for potential confounding variables. | |||||
| The controls consisted of 528 of which 225 presented periodontitis. Women: 258 Males: 310 | • Periodontitis (stages 2–4): bone loss greater than the coronal third of the root length (>15%) on OPG, or> 2 mm on bitewing radiographs. | |||||||
| 2. Larvin et al. 2020 Reino Unido Frontiers in Medicine | The objective of this study was to quantify the impact of PD on hospital admission and mortality during the COVID-19 pandemic. | Nested case–control | 13,502 patients tested for COVID-19 (part of UK Biobank database) | The status of PD was determined by the presence of self-reported oral health indicators such as: | Condition of complications and mortality from COVID-19 | Association between PD (gum pain or bleeding) and hospitalization of patients with COVID-19: | Age, sex, ethnicity, family income, body mass index, smoking history, cancer, | Participants with gum pain 365 (2.4%) Participants with bleeding gums |
| Case group: Defined as participants with a positive self- reported history of PD. | bleeding gums, painful gums, and loose teeth. These were used as surrogates for PD, since they have demonstrated their validity in the absence of a clinical diagnosis.[30] | People with at least one of the following criteria: | Hospital admission (OR = 0.91, 95% CI 0.12–2.04) Association between PD (gum pain or bleeding) and mortality in patients with COVID-19: Mortality (OR = 1.71, 95% CI 1.05–2.72) | hypertension, angina pectoris, heart attack, diabetes, myocardial infarction, | 1,329 (8.7%) Participants with tooth loss 406 (2.7%) Participants without self-report of PD 11,153 (84.1%) | |||
| Control group: Defined as participants with no self-reported history of PD. | Self-reported painful and bleeding gums were associated with mild-to-moderate PD, whereas loose teeth indicated severe PD. | 1. Hospital admission 2. Mortality | Association between PD (tooth loss) and hospitalization of patients with COVID-19: | fulminant infarction, peripheral artery disease, heart failure, atrial fibrillation, respiratory disease | Participants with a positive result of COVID-19 1,616 (10.5%) | |||
| Hospital admission (OR = 0.90, 95% CI 0.16–10.63) | Participants with a negative COVID-19 result 11,637 | |||||||
| Association between PD (tooth loss) and mortality in patients with COVID-19: Mortality (OR = 1.85, 95% CI 0.92–2.72) | The study findings showed an increased risk of mortality after COVID-19 infection in people with PD, determined by the presence of oral health indicators. While PD may not increase the risk of COVID-19 infection directly, it can increase the risk of death. |
Table 3.
Description of outcomes, exposures, and odds ratio results of the relationship between PD and the risk of complications and mortality from COVID-19
| Reference | Country | Design study | Size sample | Outcomes | Exposures | Follow up (a)/(b)/(c)/(d) | Odds ratio results | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Identify | Case | Identify | Exposed | COR (95% CI) | AOR* (95% CI) | |||||
| Marouf et al. (2021)[11] | Qatar | Case–control | 568 patients diagnosed with COVID-19: | Patient with confirmed COVID-19 diagnosis according to positive PCR test for SARS-CoV-2: | All complitations (n=40) | Periodontal condition by radiographic bone loss detection. | Periodontitis (stages 2–4): Radiographic bone level coronal 1/3 >15% (OPGs) or >2 mm (bitewing X-ray). | 303/7/225/33 | AC: 6.34 (2.79–14.61) | 3.67 (1.46–9.27) |
| 258 women and 310 men | Case with complication (n=40; mean age=53.5) Control (n=528; mean age=41.5) | Death (n=14) | Exposed Unexposed with health or initial (stages 0-1): Bone level coronal 1/3 ≤15% (OPGs) or ≤2 mm (bitewing X-ray). | 303/1/225/13 | D: 17.5 (2.27–134.8) | 8.81 (1.00–77.7) | ||||
| ICU admission (n=36) | 303/7/225/29 | ICU: 5.57 (2.40–12.9) | 3.54 (1.39–9.05) | |||||||
| Need for assisted ventilation (n=20) | 303/3/225/17 | NAV: 7.31 (2.21–26.3) | 4.57 (1.19–17.4) | |||||||
| Larvin et al. (2020)[12] | UK | Nested case–control | 13,253 participants of nested case–control data: | Patient with COVID-19 test result: | Positive test (n= 1,616) | Periodontal condition by self-reported oral health indicators. | Painful and bleeding gums: “mild to moderate PD” | 9,815/1,338/1,4 69/225 | PT: 1.07 (0.70–1.63) | 1.10 (0.72–1.69) |
| 6,802 women and 6,451 men | Case with COVID-19-positive cases (n=1,616; mean age=53.5) Control (n=11,637; mean age=41.5) | Hospital admission (n= 4,083) | Exposed Unexposed with no self-reported history of PD. | Loose teeth indicated: “severe PD” | 2,723/665/386/99 | HA: 0.99 (0.13–3.02) | 0.91 (0.12–2.94) | |||
| Death (n=644) | 292/247/37/28 | D: 1.60 (1.03–2.42) | 1.71 (1.05–2.72) | |||||||
| 9,815/1,338/353/53 | PT: 0.99 (0.73–1.38) | 1.15 (0.84–1.59) | ||||||||
| 2,723/665/101/26 | HA: 0.88 (0.16–10.04) | 0.90 (0.16–10.63) | ||||||||
| 292/247/11/16 | D: 1.34 (0.71–2.76) | 1.85 (0.92–2.72) | ||||||||
(a) control unexposed, (b) case unexposed, (c) control exposed, (d) case exposed, COR = crude odds ratio, AOR = adjusted odds ratio, AC = any complication, CI = confidence interval, PD = periodontal disease, (*) adjusted to principal covariables by age, sex, diabetes, co-morbidity, smoking behavior, D = death, ICU = intensive care unit, NAV = need for assisted ventilation, PT = positive test, HA = hospital admission
EVALUATION OF THE STUDY QUALITY
The researchers (D.A.K.E.-E., O.A.C.-L. and C.F.C.-R.) also performed the quality assessment of all the studies; the information was compared, and a consensus was made. To assess the quality of the selected studies, the Quality Access Scale—Newcastle-Ottawa Scale instrument was used.[17,25] This scale assigns a maximum of 9 points to each study: 4 for exposure selection and assessment, 2 for comparability, and 3 for outcome assessment. If a study receives a score of ≥ 6, it is considered a publication with a low risk of bias [Table 4].
Table 4.
Qualitative evaluation of case–control studies according to the Newcastle-Ottawa scale: Quality Access Scale for case–control studies
| Selection | Comparability | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author | Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Total |
| Marouf et al. (2021)[11] | * | * | * | * | * | * | * | 7/9 | |
| Larvin et al. (2020)[12] | * | * | * | * | * | 5/9 | |||
QUALITY OF EVIDENCE OF THE PRESENT STUDY—GRADE SYSTEM
The GRADE system[26] was used to evaluate the certainty of the evidence. It considers five items that may downgrade its certainty: risk of bias, inconsistency, indirect evidence, imprecision, and publication bias. The evidence certainty levels may be scored as follows: high, moderate, low, and very low [Table 5].
Table 5.
Evaluation of evidence quality using GRADE system for the studies related to the association between PD and the risk of COVID-19 complications and mortality
| Certainty assessment | ||||||
|---|---|---|---|---|---|---|
| No. of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence |
| 2 case–control studies | Serious | Not serious | Not serious | Serious | Publication bias strongly suspected all plausible residual confounding would reduce the demonstrated association | OOOOO VERY LOW |
RESULTS
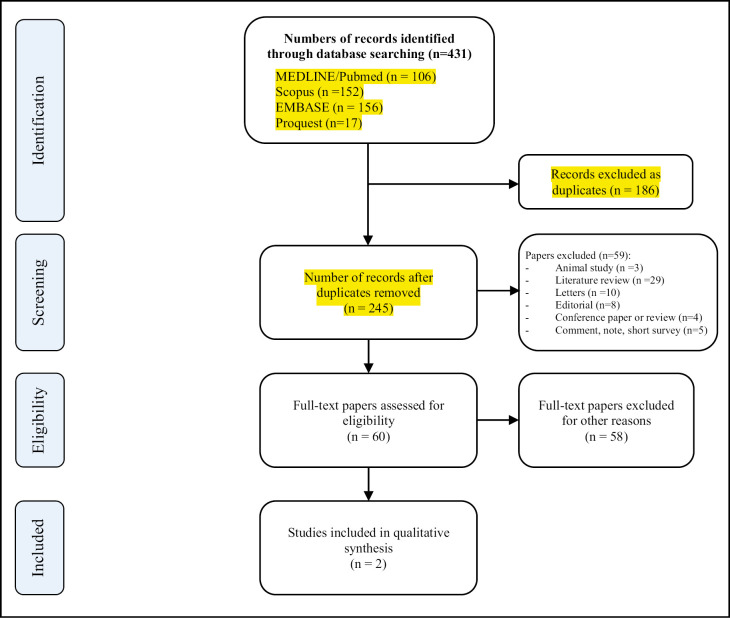
The electronic search procedure is presented in Figure 1. A total of 303 studies were initially identified from the electronic databases. After that, 158 duplicate studies were eliminated. Then, titles and abstracts were evaluated, and 143 articles were excluded. No additional studies were found from the hand search of pre-publication manuscripts. Finally, only two articles were included.
Figure 1.
Summary of the selection process of the studies related to the association between periodontitis and the risk of complications and mortality for COVID-19
One of the two included studies was published in a dental journal[11] and the other in a medical journal.[12] The sample size varied between 568 patients from Qatar[11] and 13,502 from the UK,[12] with a similar proportion in female and male participants. Regarding the methodological aspects, one was a retrospective case–control[11] and the other a nested case–control.[12] Both studies used national database records. Table 2 provides a summary of the general characteristics of the two included studies.
The characteristics of the study groups were extracted and calculated from the results study tables according to types of exposure of periodontal variables. The cases included definitions such as mortality,[11,12] need for ventilation assistance,[11] ICU,[11] and hospital admission.[12] All periodontal variables were indirectly classified through bone loss as suggested from radiographs[11] or participants’ self-reports of bleeding and tooth loss.[12] Inferential results on the association of COVID-19 and periodontitis were given in crude and adjusted odds ratios. More information on the studies’ results can be found in Table 3.
The two selected studies had a case–control design; therefore, the critical appraisal verification was carried out concerning eight items for this design (Newcastle-Ottawa Scale). All the items had a score value of one, except the item “comparability of cases and controls based on the design and analysis,” which presented a double score value. Larvin et al.’s[12] studies obtained a score of 5, corresponding to a low-quality evidence publication and a high risk of bias. In contrast, Marouf et al.’s[11] studies obtained a score of 7, which gives the article a high evidence quality value. None of the studies had a score for the item “non-response rate.” Larvin et al.’s study did not receive any score on adequate case definition, nor ascertainment of exposure [Table 4].
The evaluation for quality of evidence using the GRADE system for studies related to the association between PD and the risk of complications and mortality COVID-19 resulted in very low overall certainty [Table 5].
DISCUSSION
This systematic review aimed to explore the most recent scientific evidence on the association between PD and the risk of COVID-19 complications and mortality. The current literature on COVID-19 is increasing exponentially. Due to its pandemic nature, it seems important to investigate other possible risk factors. PD has recently been reported as a potential risk factor for higher mortality from COVID-19.[11,12] The reports indicate that the connection is based on the oral viral load or by cytokines, which have a fundamental role in the immune-inflammatory response to infections, including periodontal pathogens.[7,22,23] Although currently these are only hypothetical mechanisms, they present a plausible biological explanation. Previous studies hinted at a probable relation between them. Therefore, the present systematic review aimed to review the available evidence on the association between PD and the risk of COVID-19 complications and mortality.
Only two observational studies met the eligibility criteria of this systematic review, and these were two national multicenter studies from Qatar[11] and the UK.[12] Although these studies do not represent patients from different parts of the world, national records provide epidemiological value on diseases and/or conditions in each country with a lower selection bias. According to the Oxford Center for Evidence-Based Medicine (CEBM), the quality of the evidence concerning the type of study indicates a level 3b for both investigations.[27] The reason for excluding most of the articles was mainly the deficiency or absence of information related to the exposure of periodontitis and results.
Considering the results of the included studies, one of them found a positive association between periodontitis and COVID-19.[11] This study showed statistically significant adjusted results of high probabilities and confidence intervals (CIs) of total complications due to COVID-19 [OR = 3.67 (95% CI: 1.46–9.27)], which included death [OR = 8.81 (95% CI: 1.00–77.7)], hospital admission to ICU [OR = 3.54 (95% CI: 1.39–9.05)], assisted ventilation [OR = 4.57 (95% CI: 1.19–17.4)], and clinical parameters of blood levels of white blood cells, D-dimer, and C-reactive protein (CRP) (P < 0.01). The adjusted data controlled probable confounding factors such as smoking, co-morbidities, and individual characteristics such as sex/gender and age. Nonetheless, the study is compromised by potential inherent biases due to the retrospective nature of the design and how PD was diagnosed. The methodological evaluation of PD measurement used the concept of radiographic bone loss percentage[28] and its grouping according to periodontal stages.[29] The absence of clinical parameters such as probing depth (PD), clinical attachment level (CAL), bleeding on probing (BOP), plaque index (PI), and tooth loss limits the diagnostic interpretation of PD.
Further, they evaluated biomarkers such as D-dimer, CRP, HbA1c, vitamin D, white blood cells, and lymphocytes. This information is of interest to indirectly evaluate PD and the link between PD and COVID-19 complications as they present immunological, microbiological, and biochemical expressions. However, the coexistence of both diseases linked to the production of inflammatory biomarkers is a source of potential bias for data interpretation.
The negative association of variables from the study carried out in the UK is likely a consequence of its solid sample size of 13,253 participants.[12] The sample is based on a cohort of national data records considered representative of the country. This is an important methodological feature when selecting controls. The statistics were not conclusive concerning PD and COVID-19 as the result of the applied multivariate regression was less than 1, except the probability of mortality in cases of bleeding or painful gums [OR = 1.71; 95% CI (1.05–2.72)]. As in the study mentioned before, confounding factors and socioeconomic variables and ethnicity were controlled. The study authors reported underestimating cases as a relevant weakness; this could be explained by the short period the patients stayed in the hospital or the number of deaths. In that sense, the PD cases could have been underestimated in the selection. Perhaps, the most relevant weakness of the study is related to the diagnosis of PD, which was self-reported by the participants regarding pain and/or bleeding of the gums and tooth loss. The reliability of the data is biased in that sense as no clinical/radiological evaluation was performed. Finally, the information did not filter previous periodontal treatments, which could have underestimated the identification of PD.
The risk of bias found using the Newcastle-Ottawa scale showed that even though the study by Marouf et al. had weakness in the way PD had been determined, the case definition was extracted from valid records.[28,29] In contrast, the study by Larvin et al. used self-report to determine the cases, which does not represent a reliable source for an adequate clinical case definition, nor is it a reliable method of ascertainment of cases and controls.[12,30] As the non-response rate had not been specified, none of the studies received any score in this item.
Regarding the possible theoretical explanation between PD and the risk of COVID-19 complications and mortality, there is evidence that supports this hypothesis, such as the association between periodontitis and other systemic diseases such as diabetes, cardiovascular disease, adverse pregnancy outcomes, rheumatoid arthritis, chronic obstructive pulmonary disease, inflammatory bowel disease, and chronic kidney disease.[31,32,33,34] COVID-19 virus infects human cells by binding the viral spike protein (S) with angiotensin-converting enzyme 2 (ACE 2), a cellular receptor highly expressed in type II pneumocytes, macrophages, endothelial cells, and other lung cells.[35,36,37] This binding triggers the production of inflammatory cytokines capable of inducing intravascular coagulopathy and thrombotic complications in pulmonary blood vessels.[35,36,38,39] Although it mainly affects the lungs, the SARS-CoV-2 virus also affects the cardiovascular system. The vasculature is affected in COVID-19, both directly by the SARS-CoV-2 virus and indirectly due to a storm of systemic inflammatory cytokines. This includes the role of vascular endothelium in the recruitment of inflammatory leukocytes where they contribute to the tissue damage and the release of cytokines, which are key drivers of acute respiratory distress syndrome, in disseminated intravascular coagulation and cardiovascular complications in COVID-19.[40]
In patients who develop the most severe forms of COVID-19, there is a high incidence of macro- and microvascular thrombosis, prolonged hospitalization, and risk of death. This is attributed to a “cytokine storm,” with elevated serum levels of inflammatory cytokines (IL-6, IL-1, IFN-γ) and endothelial activation markers such as von Willebrand factor, coagulation factor 8, and P-soluble selectin, resulting in endothelialitis and microvascular thrombosis.[41,42,43] Del Valle et al.[44] identified high serum levels of IL-6 and TNF-α when the progression of the disease required the patient to be hospitalized. Moreover, it has been suggested that age-related drop in host’s immunity (immunosenescence) combined with a low-grade chronic systemic inflammation predisposes patients to further complications when infected with SARS-CoV-2.[45,46] In addition to severe injury to local tissues, there are systemic consequences such as direct lung injury, acute respiratory distress syndrome, multiple organ failure, and unfavorable prognosis.[47,48,49] Likewise, it has been reported that in severe cases of COVID-19, the leading cause of death is respiratory failure associated with excessive inflammation.[50]
It has been described that the aspiration of periodontal pathogens could increase the severity of the infection of the lungs by SARS-CoV-2.[51] Besides, it has been reported that aspiration of saliva with a high viral load can transport the virus to the lower respiratory tract, increasing the risk of developing more severe forms of the disease.[52] On the contrary, it is mentioned that inflamed periodontal tissues would act as a reservoir of pro-inflammatory cytokines (TNF-α, IL-1 α, IL-1 β and IL-6) that could be systemically disseminated through the blood circulation, exacerbating the pre-existing systemic inflammation.[53,54,55] Furthermore, the infection of alveolar epithelial cells by SARS-COV-2 generates an active acute inflammatory response to macrophages, B and T lymphocytes for the release of pro-inflammatory cytokines. These inflammatory factors induce large amounts of inflammatory exudate and red blood cells to enter the pulmonary alveoli, resulting in dyspnea, respiratory failure, and death.[56,57,58] This would explain the findings of a significant presence of microthrombi and vascular injury in post-mortem reports of patients with COVID-19.[59]
Another association mechanism between periodontitis and COVID-19 is the effect of prolonged exposure to Porphyromonas gingivalis lipopolysaccharides and other periodontal pathogens. These bacterial products produce accelerated senescence of different types of cells, including the pulmonary epithelial cells. This effect facilitates the entry and promotes the viral replication of SARS-CoV-2.[60] In contrast, Shivshankar et al.[61] showed that senescent cells promote bacterial adhesion to lung cells, eventually resulting in increased susceptibility to bacterial-induced pneumonia in older adults.
Additionally, PD has been associated with various factors and co-morbidities such as diabetes, obesity, advanced age, and high blood pressure; likewise, these factors have also been associated with the progression or severity of COVID-19. Since inflammatory factors seem to play an important role affecting systemically both PD and COVID-19, it is possible to think that periodontal status can be considered a risk indicator for complications of COVID-19.[62,63] However, in view of the statement that PD and COVID-19 present two common characteristics: (1) risk factors such as hypertension, diabetes, cardiovascular disease, and renal and pulmonary disease[4,13,14,15,16,17] and (2) exaggerated immune response characterized by a high release of interferons, ILs, TNF, chemokines, and tissue damage in the more severe presentation of these two diseases,[18,19,20,21,22] one wonders how a potential association between PD and the risk of COVID-19 complications could/should be interpreted.
The available evidence does not conclusively support an association between periodontitis and the risk of complications and mortality from COVID-19. However, the findings obtained by the studies included in this review suggest that this association may be feasible. More research is needed to minimize methodological and clinical heterogeneity between studies, particularly regarding the characteristics of the samples and the definition of the most relevant results. Additionally, various biological mechanisms could be considered as factors supporting the association between both diseases.
In addition, both periodontitis and the risk of complications such as hospitalization, ICU admission, the need for assisted ventilation, and mortality from COVID-19 are public health concerns in this pandemic. These findings could also suggest the need for additional actions to implement prevention measures and promote oral health in people who suffer from periodontitis and have risk factors such as hypertension, diabetes, cardiovascular disease, kidney disease, and lung disease.
CONCLUSION
The level of confidence in the available evidence is very low. A close association between periodontitis and the risk of COVID-19 complications and mortality can neither be supported nor refuted.
FINANCIAL SUPPORT AND SPONSORSHIP
The authors declare that this review was carried out with our own resources.
CONFLICTS OF INTEREST
None to declare.
AUTHORS CONTRIBUTIONS
All authors equally contributed to the paper.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
Not applicable.
PATIENT DECLARATION OF CONSENT
Not applicable.
DATA AVAILABILITY STATEMENT
Not applicable.
ACKNOWLEDGEMENTS
We thank the Social Responsibility team of the San Juan Bautista Private University, Academic Program of Stomatology, Lima e Ica, Peru, for their constant support in the preparation of this manuscript.
REFERENCES
- 1.World Health Organisation. WHO announces COVID-19 outbreak a pandemic. 2020. [Last accessed Apr 23, 2021]. Available from: https://www.euro.who.int/en/healthtopics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic .
- 2.Yap M, Debenham L, Kew T, Chatterjee SR, Allotey J, Stallings E, et al. PregCOV-19 Consortium. Clinical manifestations, prevalence, risk factors, outcomes, transmission, diagnosis and treatment of COVID-19 in pregnancy and postpartum: A living systematic review protocol. BMJ Open. 2020;10:e041868. doi: 10.1136/bmjopen-2020-041868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ COVID-19 Systematic Urgent Review Group Effort (SURGE) Study Authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet. 2020;395:1973–87. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. J Am Med Assoc. 2020;324:782–93. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. The Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. ISARIC4C Investigators. Features of 20133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. Br Med J. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. J Am Med Assoc. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. J Am Med Assoc. 2020;323:2195–8. doi: 10.1001/jama.2020.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. COVID-19 Lombardy ICU Network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–55. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marouf N, Cai W, Said KN, Daas H, Diab H, Chinta VR, et al. Association between periodontitis and severity of COVID-19 infection: A case–control study. J Clin Periodontol. 2021;48:483–91. doi: 10.1111/jcpe.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larvin H, Wilmott S, Wu J, Kang J. The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front Med (Lausanne) 2020;7:604980. doi: 10.3389/fmed.2020.604980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, et al. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 14.Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45:138–49. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 15.Froum SJ, Hengjeerajaras P, Liu KY, Maketone P, Patel V, Shi Y. The link between periodontitis/peri-implantitis and cardiovascular disease: A systematic literature review. Int J Periodont Restorative Dent. 2020;40:e229–33. doi: 10.11607/prd.4591. [DOI] [PubMed] [Google Scholar]
- 16.Deschamps-Lenhardt S, Martin-Cabezas R, Hannedouche T, Huck O. Association between periodontitis and chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019;25:385–402. doi: 10.1111/odi.12834. [DOI] [PubMed] [Google Scholar]
- 17.Gomes-Filho IS, Cruz SSD, Trindade SC, Passos-Soares JS, Carvalho-Filho PC, Figueiredo ACMG, et al. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis. 2020;26:439–46. doi: 10.1111/odi.13228. [DOI] [PubMed] [Google Scholar]
- 18.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180:1152–4. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Shen C, Li J, Yuan J, Wei J, Huang F, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146:119–127.e4. doi: 10.1016/j.jaci.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huck O, Buduneli N, Bravo D. Inflammatory mediators in periodontal pathogenesis. Mediators Inflamm. 2019;2019:2610184. doi: 10.1155/2019/2610184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dyke TE, Sima C. Understanding resolution of inflammation in periodontal diseases: Is chronic inflammatory periodontitis a failure to resolve? Periodontol 2000. 2020;82:205–13. doi: 10.1111/prd.12317. [DOI] [PubMed] [Google Scholar]
- 22.Sahni V, Gupta S. COVID-19 & periodontitis: The cytokine connection. Med Hypotheses. 2020;144:109908. doi: 10.1016/j.mehy.2020.109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Br Med J. 2009;339:b2535. [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–31. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 26.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. Br Med J. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.OCEBM Levels of Evidence Working Group. Oxford Center for Evidence Based Medicine. [Last accessed Apr 2, 2021]. The Oxford Levels of Evidence 2. Available from: https://www.cebm.net/index.aspx?o=5653 .
- 28.Hellén-Halme K, Lith A, Shi XQ. Reliability of marginal bone level measurements on digital panoramic and digital intraoral radiographs. Oral Radiol. 2020;36:135–40. doi: 10.1007/s11282-019-00387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl. 1):159–72. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 30.Abbood HM, Hinz J, Cherukara G, Macfarlane TV. Validity of self-reported periodontal disease: A systematic review and meta-analysis. J Periodontol. 2016;87:1474–83. doi: 10.1902/jop.2016.160196. [DOI] [PubMed] [Google Scholar]
- 31.Chapple IL, Wilson NH. Manifesto for a paradigm shift: Periodontal health for a better life. Br Dent J. 2014;216:159–62. doi: 10.1038/sj.bdj.2014.97. [DOI] [PubMed] [Google Scholar]
- 32.Payne JB, Golub LM, Thiele GM, Mikuls TR. The link between periodontitis and rheumatoid arthritis: A periodontist’s perspective. Curr Oral Health Rep. 2015;2:20–9. doi: 10.1007/s40496-014-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanz M, Kornman K Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on periodontitis and systemic diseases. J Periodontol. 2013;84:S164–9. doi: 10.1902/jop.2013.1340016. [DOI] [PubMed] [Google Scholar]
- 34.Tonetti MS, Van Dyke TE Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on periodontitis and systemic diseases. J Periodontol. 2013;84:S24–9. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 35.Huertas A, Montani D, Savale L, Pichon J, Tu L, Parent F, et al. Endothelial cell dysfunction: A major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020;56:2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colling ME, Kanthi Y. COVID-19-associated coagulopathy: An exploration of mechanisms. Vasc Med. 2020;25:471–8. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the controversy: Interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020;56:2003006. doi: 10.1183/13993003.03006-2020. 10.1183/13993003.03006-2020. PMID: 32883678; PMCID: PMC7474149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–45. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He L, Ding Y, Zhang Q, Che X, He Y, Shen H, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–97. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177–84. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Yale IMPACT Team. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–9. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–82. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–43. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domingues R, Lippi A, Setz C, Outeiro TF, Krisko A. SARS-CoV-2, immunosenescence and inflammaging: Partners in the COVID-19 crime. Aging (Albany NY) 2020;12:18778–89. doi: 10.18632/aging.103989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aquino-Martinez R, Hernández-Vigueras S. Severe COVID-19 lung infection in older people and periodontitis. J Clin Med. 2021;10:279. doi: 10.3390/jcm10020279. Published January 14, 2021. doi: 10.3390/jcm10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80:607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–7. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–9. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi Y, Watanabe N, Kamio N, Kobayashi R, Iinuma T, Imai K. Aspiration of periodontopathic bacteria due to poor oral hygiene potentially contributes to the aggravation of COVID-19. J Oral Sci. 2020;63:1–3. doi: 10.2334/josnusd.20-0388. [DOI] [PubMed] [Google Scholar]
- 52.Yoon JG, Yoon J, Song JY, Yoon SY, Lim CS, Seong H, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35:e195. doi: 10.3346/jkms.2020.35.e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourgeois D, Inquimbert C, Ottolenghi L, Carrouel F. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease—Is there cause for consideration? Microorganisms. 2019;7:424. doi: 10.3390/microorganisms7100424. Published October 9, 2019. doi: 10.3390/microorganisms7100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reis C, DA Costa AV, Guimarães JT, Tuna D, Braga AC, Pacheco JJ, et al. Clinical improvement following therapy for periodontitis: Association with a decrease in IL-1 and IL-6. Exp Ther Med. 2014;8:323–7. doi: 10.3892/etm.2014.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konkel JE, O’Boyle C, Krishnan S. Distal consequences of oral inflammation. Front Immunol. 2019;10:1403. doi: 10.3389/fimmu.2019.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–39. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim CO, Huh AJ, Han SH, Kim JM. Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch Gerontol Geriatr. 2012;54:e35–41. doi: 10.1016/j.archger.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Shivshankar P, Boyd AR, Le Saux CJ, Yeh IT, Orihuela CJ. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell. 2011;10:798–806. doi: 10.1111/j.1474-9726.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitones-Rubio V, Chávez-Cortez EG, Hurtado-Camarena A, González-Rascón A, Serafín-Higuera N. Is periodontal disease a risk factor for severe COVID-19 illness? Med Hypotheses. 2020;144:109969. doi: 10.1016/j.mehy.2020.109969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–9. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.