Abstract
Serum bactericidal assays (SBAs) for Group B meningococci are considered the methods of choice for the evaluation of functional antimeningococcal antibodies. Many investigators regard SBAs as time- and labor-intensive. Variations in SBA protocols among different laboratories make interpretation of results difficult. Here we describe a fluorescence-based serum bactericidal assay (fSBA) and compare the results obtained with the fSBA to the results obtained with a more conventional SBA. The results generated by both assays were dependent upon the surviving bacteria after incubation, and the assay mixtures contained identical components. Differences between assays lie in how the surviving bacteria are quantified. The fSBA described in the paper uses the fluorescent dye alamarBlue (M. V. Lancaster and R. D. Fields, U.S. patent 5501959, March 1996). The fluorescent signals generated in the fSBA correlate to the oxidative respiration of surviving bacteria. Viable bacteria were detected between 6 and 8 h directly from reaction mixtures in 96-well plates by the fSBA, whereas colonies isolated on semisolid media could be counted after 24 h of incubation. The bactericidal titers generated by both assays were nearly identical. The fSBA described here can be used as an assay for the screening of large quantities of individual sera as complement sources or as a method for the detection of functional antibodies directed against group B Neisseria meningitidis in both human and mouse antisera.
Serum bactericidal assays (SBAs) that are antibody dependent and complement mediated have been described for group B Neisseria meningitidis (4, 9, 10, 15, 21, 30, 40, 41) and other pathogenic microorganisms (5, 14, 18, 19, 23). Complement-mediated antibody-dependent serum bactericidal activity has not been demonstrated as an absolute correlate to protection from group B N. meningitidis, although many vaccine clinical trials and immunogenicity studies rely upon results from an enzyme-linked immunosorbent assay (ELISA) and SBA as indicators of protection (2, 3, 12, 13, 15, 22, 24, 25, 28, 29, 31–33, 37, 39). ELISA-based assays are important for detection of antigen-specific antibody but are not predictive of functional characteristics. The SBA measures a single functional characteristic of a particular, species-specific subclass of immunoglobulins. A successful SBA relies upon conditions in which antibody recognizes surface-exposed antigens and binds to complement (activation via the classical pathway), resulting in bacteriolysis and death of the target organism. Functional attributes such as whether such an antibody also functions as an opsonin or whether it inhibits bacterial colonization, invasion, or attachment cannot be predicted from the results generated by an SBA. The SBA is considered the assay of choice for measurement of functional antimeningococcal antibody in vitro. The results generated by an SBA are dependent on the source and quantity of the complement, the test serum, and the target strain, as well as expression of the target antigens. Unquestionably, SBA is considered labor-intensive and not amenable for the evaluation of large numbers of serum samples (e.g., from efficacy or postlicensing surveillance studies), and SBAs have been difficult to standardize among laboratories. The major problem with traditional SBAs lies within the techniques that involve the plating and counting of target bacteria. Here we describe for the first time a fluorescence-based SBA (fSBA) that has the potential to replace other currently accepted SBA protocols.
The fSBA described here demonstrates a novel application for the use of alamarBlue (17) in a complement-mediated, antibody-dependent bactericidal assay for the detection of group B N. meningitidis. fSBA uses the reduction-oxidation (redox) indicator in alamarBlue to detect the surviving bacteria after SBA components are allowed to react in microtiter plates. The manufacturer describes alamarBlue as a stable nontoxic, noncarcinogenic, fluorescent, and colorimetric indicator of cellular respiration. According to the manufacturer, the specific indicator incorporated into alamarBlue exhibits both a fluorescence change and a colorimetric change under the appropriate range of cellular metabolic reductions in viable prokaryotic and eukaryotic cells. The manufacturers also state that alamarBlue may be a substitute for molecular oxygen for any of the oxidoreductases in the electron transport system. alamarBlue has also been compared to prokaryotic and eukaryotic cell proliferation indicators such as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Thiazol blue), 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl]-2H-tetrazolium-5 carboxanilide (6, 27), and 3H thymidine (1). alamarBlue has also been incorporated into antimicrobial drug susceptibility tests against gram-negative microorganisms (26) and gram-positive microorganisms (S. Jenkins, J. Lewis, and J. Kihara, abstr. 91st Gen. Meet. Am. Soc. Microbiol. 1991, abstr. C-158, p. 368, 1991) as well as Mycobacterium tuberculosis (38).
MATERIALS AND METHODS
Complement source.
Thirty-eight human serum specimens were evaluated as a complement source in a group B N. meningitidis-specific SBA. Human serum (approximately 10 ml) was obtained by venipuncture from healthy adult volunteers and placed into tubes that did not contain anticoagulants. Individuals who provided serum samples that were found to be an adequate complement source were bled again within a 24-h period. For the second bleed 1 U (approximately 500 ml) of blood was collected in sterile collection bags without anticoagulant. On the day of collection, whole-blood samples (10 ml and 1 U) were allowed to clot on ice for approximately 7 h and serum was separated by centrifugation (15 min, 2,000 × g), aliquoted, and frozen at −70°C.
Bacterial strains, media, and growth conditions.
The N. meningitidis strains chosen for this study represent a diversity of strains that cause most epidemic disease worldwide (Table 1). All strains used in the assays were grown under identical conditions. Cells were grown on GC agar plates (Difco Laboratories, Detroit, Mich.) with Kellogg's supplement (dextrose, 4 g/liter; glutamine, 0.1 g/liter; cocarboxylase, 0.2 mg/liter; ferric nitrate, 0.5 g/liter) (GCK agar) (16), and the plates were incubated overnight at 36°C in 5% CO2. A portion of the plated overnight culture was used to inoculate a prewarmed, triple-baffled nephelo culture flask (Bellco, Vineland, N.J.) that contained a modified version (MFK medium) of Franz medium (8) (glutamic acid, 1.3 g/liter; cysteine, 0.02 g/liter; sodium phosphate dibasic hepta hydrate, 10 g/liter; potassium chloride, 0.09 g/liter; sodium chloride, 6.0 g/liter; ammonium chloride, 1.25 g/liter; and yeast dialysate, 40 ml/liter [0.2%], plus Kellogg's supplement). Overnight cultures were added to liquid medium to a final concentration of 20 to 30 Klett units, as measured in a Klett-Sumerson photometer at 660 nm (or an optical density of 0.1 at a wavelength of 650 nm). The cultures were incubated at 36°C with shaking at 150 rpm. Working stock cultures were grown to the mid-logarithmic phase (approximately 2 to 4 h), and the cells were removed for use in the assay (>120 Klett units or an optical density of 0.8 at a wavelength of 650 nm).
TABLE 1.
Group B N. meningitidis target strains
| N. meningitidis strain | Capsule:serotype:subserotypea |
|---|---|
| H44/76 | B:15:P1.7,16 |
| 2996 | B:2b:P1.5,2 |
| 880049 | B:4,15:P1.4 |
| H355 | B:15:P1.15 |
| M982 | B:9:P1.3 |
| 8529 | B:15:P1.3 |
| 870227 | B:4:P1.10 |
Source: National Institute of Public Health and the Environment, Bilthoven, The Netherlands.
Complement screening by colony counting and fluorescence.
Reaction mixtures and serial dilutions were prepared in sterile 96-well microtiter plates (Falcon; Becton Dickinson, Franklin Lakes, N.J.). Dilutions of cells and test serum were made in phosphate-buffered saline containing 0.5 mM magnesium chloride and 0.15 mM calcium chloride (PCM). Target cells were diluted in PCM to a concentration of approximately 1,000 to 3,000 CFU per 10 μl. To determine the bactericidal activity of the complement source, sets of assay mixtures containing target cells (10 μl), PCM (30 μl), and 10 μl of test serum or heat-inactivated (56°C for 30 min) test serum were prepared in triplicate. One set of reaction mixtures (that with the numbers of CFU introduced into the assay mixture) was immediately diluted with 200 μl of PCM prior to incubation (to minimize serum-bacterium interactions), and 50-μl aliquots were removed and plated onto GCK agar plates (time zero) (T0). The other two sets of reaction mixtures were allowed to react at 36°C in 5% CO2 for 30 min (T30) after slight agitation. After incubation, one set of reactions was terminated by adding 200 μl of PCM to each well, followed by the removal of duplicate 50-μl aliquots per well. These aliquots were then plated onto GCK agar plates, and the plates were incubated at 36°C in 5% CO2 for at least 18 h. The remaining set of reactions was terminated by the addition of 200 μl of MFK medium containing 10 μl of alamarBlue (Trek Diagnostic Systems, Westlake, Ohio) and 0.7% molten (melted and cooled to 42°C) SeaPlaque low-melting-point agarose (FMC Bioproducts, Rockland, Maine). The reaction mixtures were covered with sterile plate covers, allowed to solidify, and then incubated at 36°C in 5% CO2. For initial complement-screening experiments, human serum was considered a candidate as a complement source if (i) the average number of target CFU at T30 was ≥90% of the average number of target CFU in wells that contained reactions at T0 and (ii) the average number of target CFU at T30 in wells that contained normal test serum was ≥90% of the average number of CFU in wells that contained reactions with heat-inactivated serum. For subsequent screening experiments, serum was considered an adequate complement source if the average arbitrary fluorescence units (AFUs) generated from T30 reaction wells with normal test serum was ≥90% of the average number of AFUs obtained from reaction wells with heat-inactivated serum.
Assay conditions for SBA and fSBA.
Target cells were removed from the working stock culture and diluted to a concentration of 1,000 to 3,000 CFU per 10 μl immediately prior to addition to the reaction mixtures. The reaction mixtures that contained target cells (10 μl), test serum (5 μl, neat or diluted), and PCM (25 μl), to which 10 μl of complement (which lacked significant bactericidal activity in screening experiments) was added, were incubated at 36°C in 5% CO2 for 30 min after slight agitation. After incubation, the reactions were terminated by adding either 200 μl of PCM or 200 μl of MFK medium containing 10 μl of alamarBlue (Trek Diagnostic Systems) and 0.7% molten (melted and cooled to 42°C) SeaPlaque low-melting-point agarose (FMC Bioproducts). Aliquots (50 μl) from the reaction wells diluted with PCM were plated onto solid GCK agar medium, and the plates were incubated at 36°C in 5% CO2 for at least 18 h. The reaction wells in the fSBA were allowed to solidify and covered with sterile plate covers, and the plates were incubated between 6 and 8 h at 36°C in 5% CO2.
Instrumentation.
Fluorescence was detected with a Cytofluor 4000 96-well plate reader (Perceptive Biosystems, Framingham, Mass.) (excitation wavelength, 530 nm; emission wavelength, 590 nm). AFUs were determined as the average fluorescence of three consecutive measurements per well at a gain of approximately 50. Fluorescence was detected from the bottom of each reaction well in 96-well plates at specified time points (30-min intervals or between 360 and 420 min) after introduction of 200 μl of alamarBlue-agarose additive and incubation in the temperature-controlled chamber of the Cytofluor 4000 reader set at 36°C.
Interpretation of results.
Complement-mediated antibody-dependent bactericidal titers for both SBA and fSBA were expressed as the reciprocal of the highest dilution of test serum that killed ≥50% of the target cells introduced into the assays (BC50 titer). For the fSBA, BC50 titers were determined by comparing the number of AFUs for wells with test serum to the number of AFUs for wells with concentrations of 50% total target cells after incubation. The number of AFUs obtained for control wells without viable cells was considered the background fluorescence and was subtracted from the number of AFUs obtained for wells with viable bacteria.
Control mouse antiserum.
Heat-killed whole cells (56°C for 30 min) of each target strain were used to immunize groups of 4- to 6-week-old Swiss Webster mice (Taconic Labs, Germantown, N.Y.). Each group of mice received approximately 103 heat-killed whole cells in 100 μl of phosphate-buffered saline. Heat-killed cells were emulsified with incomplete Freund's adjuvant (1:1; vol/vol), and the mixture was injected subcutaneously. Mice were given two injections over a 4-week period and were bled 2 weeks after the second injection. Positive control sera for each target strain were pooled, aliquoted, and stored at −20°C. The negative control sera included in each assay were either normal mouse serum or sera from preimmunized mice.
RESULTS
Screening of human complement for bactericidal activity against seven strains of group B N. meningitidis.
Our method of complement screening includes the criterion described by McQuillen et al. (21). Specifically, complement must not display any significant bactericidal killing of a target strain of bacteria. In particular, 90% of the target cells must survive conditions of an SBA with active and heat-inactivated complement (from T0 to T30). Our goal for the development of an fSBA was to be able to rapidly identify human sera as appropriate complement sources for multiple target strains in a complement-mediated antibody-dependent bactericidal assay. Results from our initial screening experiment identified two human serum samples as complement sources for target strains 2996 and H44/76 (no significant killing between plates at T0 and plates at T30 and no significant killing compared to that for heat-inactivated controls). These complement sources were used in preliminary experiments in which fSBA was compared to SBA. Subsequent screening of 38 human serum samples in an fBSA identified 34 serum samples which could be used as complement sources for strain 2996. Of the 34 serum samples screened, only 11 (28.9%) were considered good complement sources for all seven target strains (Table 2).
TABLE 2.
Bactericidal activity of human serum against target strains
| Target strain | No. of serum samples without bactericidal activity/total no. of serum samples testa (% of totalb) |
|---|---|
| 2996 | 34/38 (89.5) |
| M982 | 29/34 (85.3) |
| H44/76 | 24/25 (96.0) |
| 880049 | 15/25 (60.0) |
| H355 | 11/15 (73.3) |
| 539 | 11/11 (100.0) |
| 870227 | 11/11 (100.0) |
Number of individual human serum samples that did not demonstrate significant bactericidal activity (those with AFUs for active complement at T30 greater than or equal to AFUs for inactive complement).
Percentage of individual serum samples that can be used as a complement source among all serum samples tested in complement screening experiments.
Target cells generate fluorescent signals that correlate with viability.
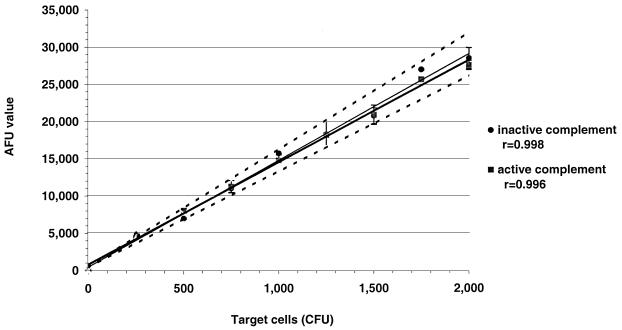
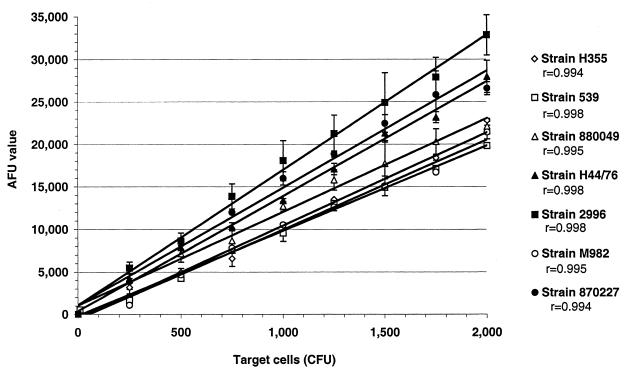
The basic principle behind the SBA and fSBA is to be able to detect and quantify viable bacteria after reactions with active complement and antibody. CFU from 0 to 2 × 103 were used to represent the surviving bacteria in an SBA. Bacteria were allowed to react with either active or inactive complement without exogenous antibody to ensure that the fluorescent signals generated were a result only of incubation with active or inactive complement alone. AFUs were measured 24 h after addition of 200 μl of MFK medium with 17 μl of alamarBlue and low-melting-point agarose to reaction wells. Results demonstrate a linear relationship between AFUs and target cell concentrations for both active and heat-inactivated complement (Fig. 1) (r > 0.99). Additionally, the AFUs generated for each concentration of target cells incubated with active complement target did not deviate more than 10% from the AFUs generated with respective concentrations of cells incubated with inactive complement (Fig. 1). These results verify the lack of bactericidal activity in the complement source. Similar results that demonstrated linear relationships of viable cell concentration and fluorescence were obtained with the six remaining target strains (Fig. 2; plots for inactive complements are not shown).
FIG. 1.
AFUs obtained with a series of concentrations of target cells (strain H44/76, ranging from 0 to approximately 2,000 CFU) in reaction wells incubated with active (■) or inactive (⧫) complement. The area within dashed lines represents AFUs ±10% of the AFUs obtained in reaction wells with cells and inactive complement.
FIG. 2.
Average ± standard deviation (bars) AFUs obtained from a series of concentrations of cells of seven target strains (◊, H355; □, 539, ▵, 880049; ▴, H44/76; ■, 2996; ○, M982; ●, 870227) ranging from 0 to approximately 2,000 CFU per well for duplicate reaction mixtures incubated with active complement. Each line represents the linear relationship between the target cell concentration and AFU (r values range between 0.994 and 0.998).
Optimum incubation time for group B N. meningitidis-specific fSBA.
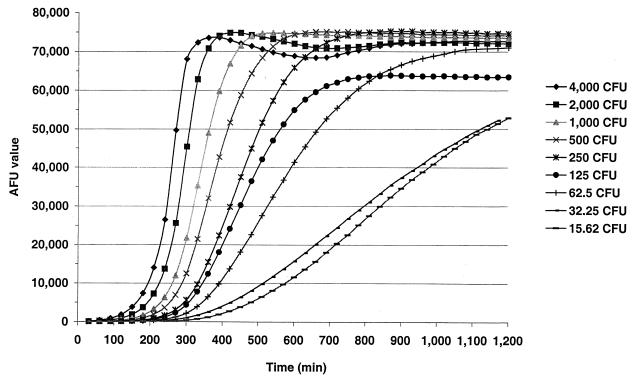
Bactericidal assays dependent upon counting of colonies are limited to those with incubation times and plating techniques that yield visibly discernible colonies on or in semisolid media. For most strains of N. meningitidis, colonies are not visible until after 18 h of incubation under optimum conditions. Even after 18 h no assurance can be given that colonies arise from individual viable cells (or aggregates). To determine the optimum incubation time for fSBA, target strain H44/76 at concentrations between 16 and 4,000 CFU per 10 μl was incubated in reaction wells with 10 μl of complement, 30 μl of PCM, and 200 μl of MFK medium that contained 17 μl of alamarBlue and molten low-melting-point agarose. The AFUs were then measured every 30 min for each reaction well over a 24-h time period (Fig. 3). The results demonstrate that concentrations of target cells between 250 and 4,000 CFU per reaction well (geometric mean, ≅1,500 CFU) yield the greatest range of AFUs at between 200 and 600 min (mean time, ≅390 ± 30 min). For subsequent optimized fSBA experiments, AFUs were determined between 360 and 420 min after the addition of alamarBlue-MFK medium-agarose additive and incubation at 36°C in 5% CO2 for reactions with 1,000 to 3,000 CFU per well. The optimum incubation conditions were found to be similar for all seven target strains (data not shown).
FIG. 3.
AFUs obtained from reaction wells with specific concentrations of target strain H44/76 (ranging from ∼16 to 4,000 CFU per well) and active complement over time.
Optimum concentration of alamarBlue for use in fSBA.
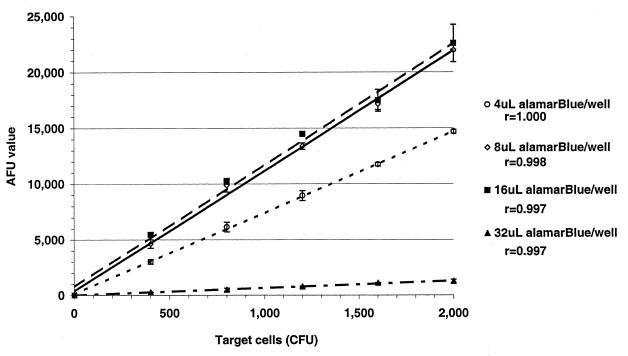
Initially, 17 μl of alamarBlue was used per reaction mixture to detect fluorescence (manufacturer's recommendation). Reaction mixtures similar to those described above were used with volumes of 4, 8, 16, and 32 μl of alamarBlue in the 200-μl MFK medium-agarose additive and incubated over a 420-min period. The results demonstrate that assay mixtures with 4 and 32 μl of alamarBlue generated AFUs significantly lower than those generated by mixtures with 8 and 16 μl (Fig. 4). Repeat assays that compared the results obtained for reaction wells with 10 μl of alamarBlue demonstrated AFUs not significantly different from those obtained by assays that incorporated 17 μl of alamarBlue (data not shown). In all subsequent experiments we chose for economic reasons to use 10 μl of alamarBlue per reaction well.
FIG. 4.
Average ± standard deviation (bars) AFUs obtained from duplicate reaction wells with target strain H44/76 (100% = ∼1,500 CFU) incubated with active complement and 4 μl (○), 8 μl (◊), 16 μl (■), and 32 μl (▴) of alamarBlue per well.
Sensitivity of an fSBA and correlation with SBA.
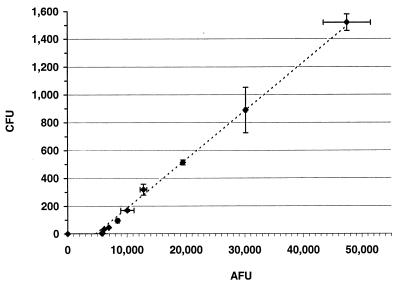
Wells that contained dilutions of target cells (range, <2 to ∼1,500 CFU) generated fluorescent signals that ranged from ∼6,000 to ∼50,000 AFU (background corrected) (Fig. 5). The AFUs correlated (r = 0.993) with the number of CFU obtained by plating aliquots from duplicate reaction wells. Both the fSBA and the SBA could detect as few as 33 ± 4 (∼3.7%) total used cells per well in an assay. Important differences between the two types of assays were (i) that data obtained from the fSBA were generated over a 7-h incubation period, whereas those obtained from the SBA were generated over a 24-h incubation period, and (ii) that the fSBA enumerates a greater range of surviving target cells without having to rely upon plating of proportions of reaction mixtures.
FIG. 5.
Linear relationship (dashed line) between average ± standard deviation (bars) AFUs and number of surviving bacteria (average ± standard deviation [bars] number of CFU) obtained in an fSBA with target strain 2996 (r = 0.993).
Detection of complement-mediated antibody-dependent bactericidal activity of mouse antiserum.
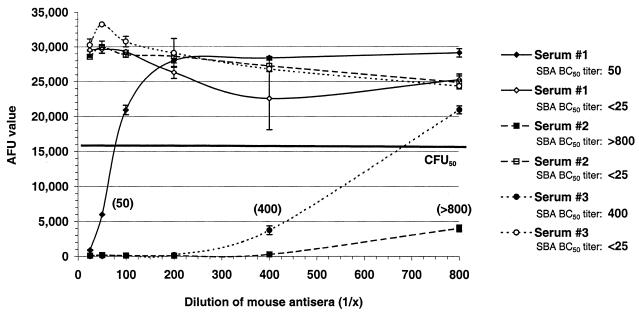
Three mouse serum samples with known BC50 titers (determined by an SBA) were incorporated into an fSBA with active or inactive complement (56°C for 30 min). The AFUs generated from reaction wells with serum and active complement demonstrate a direct relationship between the serum dilution and the number of AFU generated. This relationship was abolished in duplicate reaction wells that contained inactive complement (Fig. 6).
FIG. 6.
Bactericidal activities of three mouse serum samples in an fSBA with active complement (closed symbols) and inactive complement (open symbols) against target strain 2996. fSBA BC50 titers (in parentheses) are expressed as the reciprocal of the highest dilution of antiserum that yields AFUs greater than or equal to the AFUs obtained in reaction wells with 50% target cells incubated with normal mouse serum (the line marked CFU50, which indicates ∼15,500 AFU).
Bactericidal titers of mouse serum from an fSBA correlate to titers obtained in an SBA.
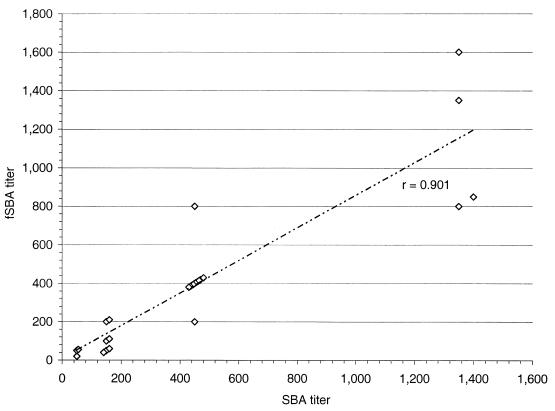
To determine the relationship between the BC50 titers obtained in an SBA and the titers obtained in an fSBA, serial dilutions of 27 mouse serum samples with known BC50 titers to selected target strains by SBA were tested in an optimized fSBA. The results of the fSBA demonstrated a loss of antibody-dependent bactericidal activity in assays that incorporated heat-inactivated complement and a direct correlation (r = 0.901) between the BC50 titers obtained in SBAs (Fig. 7).
FIG. 7.
Correlation of BC50 titers from 27 serum samples from mice tested in an SBA and an fSBA. Plots (▴) compare titers from each assay and demonstrate a linear correlation (r = 0.901).
DISCUSSION
Complement-mediated antibody-specific bactericidal activity has been described as the activation of complement by antibody bound to cell surface target antigens, resulting in bacteriolysis and cell death. Goldschneider et al. (11) and others (35, 36) have demonstrated the important role that bactericidal antibodies play in protection against meningococci. Case studies that involve individuals with terminal complement component deficiencies demonstrate the critical function that terminal serum complement components play in meningococal infections (7, 20, 34). The ability to detect functional bactericidal antibody in any in vitro assay is important for the evaluation of protective meningococcal antibody. Assays currently used for the detection of bactericidal antibody are not standardized, are labor-intensive, and are cumbersome. Results obtained from different laboratories are difficult to interpret and compare due to the nature of the variables among assays, including the complement source, target strain, cell concentration, medium, and growth conditions. More investigation is needed to standardize a universally accepted SBA for the detection of group B N. meningitidis. An important step toward the standardization of SBA protocols includes the identification of a suitable complement source.
The fSBA described in this paper can easily be used to screen large numbers of individual serum samples in antibody-dependent bactericidal assays. The fSBA is relatively easy to perform, and results are generated more rapidly than they are by a colony-counting SBA. The fSBA eliminates the labor-intensive steps of plating of reaction mixtures and counting of numbers of individual CFU, which are critical for interpretation of the results of traditional SBAs. The assay allows direct comparisons between normal and inactivated complement to be made. The fSBA is amenable to the detection of bactericidal activity in the serum of agammaglobulinemic patients or serum deficient for complement factors. The ability of the fSBA to quantitatively detect viable organisms is an attractive feature over current SBA methods, in that a more accurate description of serum killing potential can be derived. Direct quantitation of the surviving bacteria by fluorescence reduces the assay-to-assay variability often encountered in current SBA protocols. Agglutinating bacteria, plating methods, or dilution errors are not factors that affect fSBA. The relative ease of performance of fSBA makes complement screening and evaluation more efficient. The 96-well format makes fSBA an appealing assay in the sense that it can be adapted to robotic systems similar to those used for ELISA. It is conceivable that in a semiautomated system fSBA throughput would increase and data acquisition and analysis could be standardized.
Studies are in progress to investigate the adaptability of fSBA to automation and to other pathogenic organisms. Fluorescence-based bactericidal assays that incorporate group A, C, Y, and W135 N. meningitidis, Moraxella catarrhalis, nontypeable Haemophilus, and Neisseria gonorrhoeae as target strains have been adapted to fSBAs in our laboratory (unpublished data). The use of alamarBlue for an fSBA allows investigators to evaluate the importance of serum bactericidal activity as protection against pathogenic bacteria without the limitation that traditional SBAs possess. Assays such as fSBA may elucidate the correlates to protection in animal models, vaccine efficacy trials, postmarketing surveillance studies, and epidemiological studies.
REFERENCES
- 1.Ahmed S A, Gogal R M, Jr, Walsh J E. A new rapid and simple nonradioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 2.Bjune G, Gronnesby J K, Hoiby E A, Closs O, Nokleby H. Results of an efficacy trial with an outer membrane vesicle vaccine against systemic serogroup B meningococcal disease in Norway. NIPH Ann (Oslo) 1991;14:125–132. [PubMed] [Google Scholar]
- 3.Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W, et al. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Chilean National Committee for Meningococcal Disease. Vaccine. 1995;13:821–829. doi: 10.1016/0264-410x(94)00037-n. [DOI] [PubMed] [Google Scholar]
- 4.Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers A B, Quentin-Millet M J. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993;11:1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 5.Elkins C, Carbonetti N H, Varela V A, Stirewalt D, Klapper D G, Sparling P F. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol Microbiol. 1992;6:2617–2628. doi: 10.1111/j.1365-2958.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 6.Fields R D, Lancaster M V. Dual attribute continuous monitoring of cell proliferation/cytotoxicity. Am Biotechnol Lab. 1993;11:48–50. [PubMed] [Google Scholar]
- 7.Figueroa J E, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frantz I D. Growth requirements of the meningococcus. J Bacteriol. 1942;43:757–761. doi: 10.1128/jb.43.6.757-761.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frasch C E, Chapman S S. Classification of Neisseria meningitidis group B into distinct serotypes. I. Serological typing by a microbactericidal method. Infect Immun. 1972;5:98–102. doi: 10.1128/iai.5.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasch C E, Robbins J D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978;147:629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golovina L I, Kuvakina V I, Alliluev A P, Basnak'ian I A, Sivko R I. Immune bacteriolysis reaction in the assessment of immunological effectiveness of serogroup B meningococcal vaccine. Klin Lab Diagn. 1995;1:26–29. . (In Russian.) [PubMed] [Google Scholar]
- 13.Gorringe A R, Borrow R, Fox A J, Robinson A. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine. 1995;13:1207–1212. doi: 10.1016/0264-410x(95)00055-6. [DOI] [PubMed] [Google Scholar]
- 14.Green B A, Quinn-Dey T, Zlotnick G W. Biologic activities of antibody to a peptidoglycan-associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H. influenzae type b. Infect Immun. 1987;55:2878–2883. doi: 10.1128/iai.55.12.2878-2883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoiby E A, Rosenqvist E, Froholm L O, Bjune G, Feiring B, Nokleby H, Ronnild E. Bactericidal antibodies after vaccination with the Norwegian meningococcal serogroup B outer membrane vesicle vaccine: a brief survey. NIPH Ann (Oslo) 1991;14:147–156. [PubMed] [Google Scholar]
- 16.Kellogg D S, Peacock W L, Deacon W E. Neisseria gonorrhoeae. I. Virulance genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster, M. V., and R. D. Fields. March 1996. U.S. patent 5501959.
- 18.Mandrell R E, Azmi F H, Granoff D M. Complement-mediated bactericidal activity of human antibodies to poly alpha 2→8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 19.Maslanka S E, Gheesling L L, Libutti D E, Donaldson K B, Harakeh H S, Dykes J K, Arhin F F, Devi S J, Frasch C E, Huang J C, Kriz-Kuzemenska P, Lemmon R D, Lorange M, Peeters C C, Quataert S, Tai J Y, Carlone G M. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews N, Stark J M, Harper P S, Doran J, Jones D M. Recurrent meningococcal infections associated with a functional deficiency of the C8 component of human complement. Clin Exp Immunol. 1980;39:53–59. [PMC free article] [PubMed] [Google Scholar]
- 21.McQuillen D P, Gulati S, Rice P A. Complement-mediated bacterial killing assays. Methods Enzymol. 1994;236:137–147. doi: 10.1016/0076-6879(94)36013-8. [DOI] [PubMed] [Google Scholar]
- 22.Milagres L G, Ramos S R, Sacchi C T, Melles C E, Vieira V S, Sato H, Brito G S, Moraes J C, Frasch C E. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect Immun. 1994;62:4419–4424. doi: 10.1128/iai.62.10.4419-4424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mountzouros K T, Kimura A, Cowell J L. A bactericidal monoclonal antibody specific for the lipooligosaccharide of Bordetella pertussis reduces colonization of the respiratory tract of mice after aerosol infection with B. pertussis. Infect Immun. 1992;60:5316–5318. doi: 10.1128/iai.60.12.5316-5318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naess L M, Aarvak T, Aase A, Oftung F, Hoiby E A, Sandin R, Michaelsen T E. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine. 1999;17:754–764. doi: 10.1016/s0264-410x(98)00259-x. [DOI] [PubMed] [Google Scholar]
- 25.Naess L M, Rosenqvist E, Hoiby E A, Michaelsen T E. Quantitation of IgG subclass antibody responses after immunization with a group B meningococcal outer membrane vesicle vaccine, using monoclonal mouse-human chimeric antibodies as standards. J Immunol Methods. 1996;196:41–49. doi: 10.1016/0022-1759(96)00108-1. [DOI] [PubMed] [Google Scholar]
- 26.Novak S M, Hindler J, Bruckner D A. Reliability of two novel methods, Alamar and E test, for detection of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:3056–3057. doi: 10.1128/jcm.31.11.3056-3057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page B, Page M, Noel C. A new fluorometric assay for cytotoxicity measurement in vitro. Int J Oncol. 1993;3:473–476. [PubMed] [Google Scholar]
- 28.Perkins B A, Jonsdottir K, Briem H, Griffiths E, Plikaytis B D, Hoiby E A, Rosenqvist E, Holst J, Nokleby H, Sotolongo F, Sierra G, Campa H C, Carlone G M, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger J D, Broome C V. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;117:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 29.Pollard A J, Galassini R, van der Voort E M, Booy R, Langford P, Nadel S, Ison C, Kroll J S, Poolman J, Levin M. Humoral immune responses to Neisseria meningitidis in children. Infect Immun. 1999;67:2441–2451. doi: 10.1128/iai.67.5.2441-2451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poolman J T, Wientjes F B, Hopman C T, Zanan H C. Influence of the length of lipopolysaccharide molecules on the surface exposure of class 1 and class 2 outer membrane proteins of Neisseria meningitidis 2996 (B:2b:P1.2) In: Schoolnik G K, editor. The pathogenic neisseriae. Washington, D.C.: American Society for Microbiology; 1985. pp. 562–570. [Google Scholar]
- 31.Rosenqvist E, Harthug S, Froholm L O, Hoiby E A, Bovre K, Zollinger W D. Antibody responses to serogroup B meningococcal outer membrane antigens after vaccination and infection. J Clin Microbiol. 1988;26:1543–1548. doi: 10.1128/jcm.26.8.1543-1548.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenqvist E, Hoiby E A, Bjune G, Bryn K, Closs O, Feiring B, Klem A, Nokleby H, Frolm L O. Human antibody responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine: results from ELISA studies. NIPH Ann (Oslo) 1991;14:169–181. [PubMed] [Google Scholar]
- 33.Rosenqvist E, Hoiby E A, Wedege E, Bryn K, Kolberg J, Klem A, Ronnild E, Bjune G, Nokleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross S C, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine. 1984;63:243–273. [PubMed] [Google Scholar]
- 35.Silverthorne H. The bactericidal power of blood and protection against the meningococcal infection. J Immunol. 1937;33:51–56. [Google Scholar]
- 36.Thomas L, Dingle J H. Investigations of meningococcal infection. III. The bactericidal action of normal and immune serums for the meningococcus. J Clin Invest. 1943;22:375–385. doi: 10.1172/JCI101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Voort E R, van der Ley P, van der Biezen J, George S, Tunnela O, van Dijken H, Kuipers B, Poolman J. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect Immun. 1996;64:2745–2751. doi: 10.1128/iai.64.7.2745-2751.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yajko D M, Madej J J, Lancaster M V, Sanders C A, Cawthon V L, Gee B, Babst A, Hadley W K. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol. 1995;33:2324–2327. doi: 10.1128/jcm.33.9.2324-2327.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zollinger W D, Boslego J, Froholm L O, Ray J S, Moran E E, Brandt B L. Human bactericidal antibody response to meningococcal outer membrane protein vaccines. Antonie Leeuwenhoek. 1987;53:403–411. doi: 10.1007/BF00415494. [DOI] [PubMed] [Google Scholar]
- 40.Zollinger W D, Brandt B L, Tramont E C, Dobek A S. Immune response to Neisseria meningitidis. In: Rose N R, Friedman H, Fahey J L, editors. Manual of clinical Laboratory immunology. 3rd ed. Washington, D.C.: American Society for Microbiology; 1986. pp. 446–453. [Google Scholar]
- 41.Zollinger W D, Mandrell R E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]