Abstract
CCR5 is a coreceptor of human immunodeficiency virus type 1 (HIV-1). Transplantation of hematopoietic stem cells homozygous for a 32-bp deletion in CCR5 resulted in a loss of detectable HIV-1 in two patients, suggesting that genetic strategies to knockout CCR5 expression would be a promising gene therapy approach for HIV-1-infected patients. In this study, we targeted CCR5 by CRISPR-Cas9 with a single-guide (sgRNA) and observed 35% indel frequency. When we expressed hCas9 and two gRNAs, the Surveyor assay showed that Cas9-mediated cleavage was increased by 10% with two sgRNAs. Genotype analysis on individual clones showed 11 of 13 carried biallelic mutations, where 4 clones had frameshift (FS) mutations. Taken together, these results indicate that the efficiency of biallelic FS mutations and the knockout of the CCR5 necessary to prevent viral replication were significantly increased with two sgRNAs. These studies demonstrate the knockout of CCR5 and the potential for translational development.
Introduction
Despite of the success of antiretroviral therapy (ART) in controlling infection and extending the life span of human immunodeficiency virus (HIV)-infected people, ART has some limitations, such as the emergence of drug-resistant strains in patients, a need for lifelong administration, and cost. Thus, alternative therapies are needed to treat HIV.
The entry of the human immunodeficiency virus type 1 (HIV-1) into target cells involves sequential binding of its surface envelope glycoprotein to cellular primary receptor CD4 and the chemokine receptor CCR5 or CXCR4.1,2–4 CCR5 is the major coreceptor used by HIV-1 and is expressed in CD4+ T cells and myeloid cells that are depleted during HIV-1 infection.5–9 A natural 32 base-pair deletion in CCR5 (CCR5Δ32) leads to a premature stop codon and a nonfunctional gene product; therefore, CCR5 is not expressed on the cell surface.10 The rare group of individuals homozygous for a naturally occurring CCR5Δ32 mutation is resistant to HIV-1 infections, with only subtle changes to their immune system.11,12 Even individuals heterozygous for the CCR5Δ32 mutation are associated with lower viral load and have delayed progressions to AIDS.13–16 Targeting CCR5 has been shown as an effective therapeutic strategy to block viral replication.10,17,18
In 2007 in Berlin, an HIV-1-infected patient with acute myeloid leukemia received an allogeneic hematopoietic stem cell (HSC) transplantation from a donor, who was homozygous for the CCR5Δ32 mutation.19,20 More than 9 year later, this individual had no signs of HIV-1 or leukemia in the absence of antiretroviral drug therapy.20,21 A second patient has undetectable virus after a similar clinical intervention.22 These clinical findings support the potential stem cell-targeted, gene therapy strategies for HIV based on the mutation of CCR5 coreceptor to inhibit viral replication observed in the Berlin patient.23–25 After transplantation of these modified HSCs, it was expected that the host CD4+ T cells and myeloid cells would become HIV-resistant and expand.26 Studies have shown that long-lived, self-renewing, multilineage HSCs and their progeny are resistant to HIV infection when CCR5 is deleted.27–32
However, primary HSC origin in bone marrow is limited in supply as they cannot effectively be renewed or expanded in vitro.33,34 These cells proliferate and differentiate into all cells in the hematopoietic compartment, including CD4+ T cells and tissue macrophages. Under normal conditions, the number of stem or progenitor cells in the peripheral blood and bone marrow is extremely low and only divide a limited number of times in vitro.35,36 Multiple studies have explored conditions to expand ex vivo sufficient HSCs required for clinical applications,37 however, maintaining stem cell pluripotency without causing differentiation has been difficult.38
In contrast, uncultured adipose-derived stem cells (ASCs) or resident mesenchymal stem cells from within the stromal vascular fraction have become an attractive source for regenerative cell therapy.39 These tissue-resident stem and regenerative cells are abundantly associated with blood vessels in adipose tissue.40 As such, adipose tissue contains a significantly greater proportion of stem cells than bone marrow (5% vs. 0.01%), resulting in greater stem cell yield from adipose tissue than from bone marrow.41 In addition, ASCs can be easily harvested from adipose tissue using minor surgical interventions such as mini-liposuction.41 Most importantly, our group and others have shown that ASCs, induced pluripotent stem cells (iPSCs), or embryonic stem cell (ESCs) could be induced to differentiate into hematopoietic cells when cultured in the hematopoietic induction medium,42–47 which can be infected with HIV.46 These studies show the potential of hematopoietic differentiated cells from stem cells especially for the treatment of patients with advanced HIV-1 infection.
Recently, CCR5 has been successfully targeted using zinc-finger nucleases, microRNA, and CRISPR-Cas9 in HSCs.27–32 However, targeting CCR5 by CRISPR-Cas9 in ASCs has not been investigated. The CRISPR-Cas9 system has been developed into an effective genome engineering tool.48–52 The application of the CRISPR-Cas9 system in genome editing requires two components: the single-guide RNA (sgRNA) and the Cas9 nuclease.53 The Cas9 protein forms a complex with the sgRNA, upon which the cognate target is identified by the complex by recognition of the protospacer adjacent motif (PAM) in the targeted region.54,55 Subsequently, the catalytic activity of Cas9 cuts the DNA between the third and fourth nucleotides upstream of the PAM56 and generates double-strand breaks (DSBs). Cleaved DSBs are rapidly repaired by either nonhomologous end joining (NHEJ) or homology-directed repair. NHEJ-mediated DNA repair is error-prone, which results in frameshift (FS) insertions or deletions (indels) and premature termination.57–59
In this study, we used the CRIPR/Cas9 system to introduce mutations in the CCR5 locus. We compared the efficiency of targeting the CCR5 locus with one gRNA alone to the results with two sgRNAs simultaneously. Our data show that two sgRNAs are more efficient than one sgRNA. Genotyping analysis of ASC clones transduced with two sgRNAs showed successful biallelic targeting, with both large in-frame (IF) deletions and FS mutations. Therefore, a high incidence of biallelic mutations of CCR5 using CRISPR-Cas9 in ASCs can be achieved with two sgRNAs, providing a valuable resource for the study of HIV-1 disease with the potential of therapeutic application.
Materials and Methods
Isolation and culture of cell lines
IRB approval was not needed for this study. HEK293T cells were cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM) (D10), supplemented with 10% fetal bovine serum (FBS; Atlanta Biological, Atlanta, GA) and 1% penicillin/streptomycin (Pen/Strep) (Cellgro, Herndon, VA) at 37°C and 5% CO2. Over the last years, freshly isolated, uncultured, autologous ASCs have become highly attractive for regenerative cell therapy.39 Adipose tissues were obtained based on the approved protocol by the Institutional Review Board of the Tulane University Health Sciences Center. ASCs were isolated from subcutaneous white adipose tissue from each donor by enzymatic digestion as described previously.43
Briefly, 50 g of tissue slurry was minced and digested with collagenase type I (Invitrogen Corp., Carlsbad, CA) for 60 min at 37°C. After being treated with red blood cell lysis buffer (BioWhittaker, Walkersville, MD), the cells were plated in D10 at 37°C and 5% CO2. The cells were passaged further upon reaching 70% confluency and used for experiments at passage 3–5. Clonal lines were generated from the sorted population by plating the sorted ASCs at low cell density in 24-well plates and screened individual colonies. The clones were expanded from those single cells in separate wells.
Generation of CRISPR-Cas9 plasmids
We used the CRISPR Design Tool (http://crispr.mit.edu/) to design the sgRNAs (Table 1) against the human CCR5 locus (Fig. 1A). The pSpCas9(BB)-2A-GFP plasmid (pX458), the multiple lentiviral expression (MuLE) Entry vectors for sgRNA expression pMuLE ENTR U6 stuffer sgRNA scaffold L1-L4 (#62128), the pMuLE ENTR U6 stuffer sgRNA scaffold R4-R3 (#62131), Cas9 expression pMuLE ENTR SV40-hCas9 L3-L2 (#62133), and destination vector pMuLE Lenti Deste eGFP (#62175) were obtained from Addgene (Watertown, MA).60,61 For cloning of the sgRNAs, complementary oligonucleotides were annealed to generate double-stranded DNA fragments with 5′ ACCG and 5′ AAAC overhangs and then ligated into the digested plasmids [pSpCas9(BB)-2A-GFP, Entry vector pMuLE ENTR U6 stuffer sgRNA scaffold L1-L4 and pMuLE ENTR U6 stuffer sgRNA scaffold R4-R3 plasmids]. The constructed plasmids were verified by sequencing analysis (Fig. 1).
Table 1.
Primer sequences used for single-guide RNA cloning, sequencing, and Surveyor assay
| Primer | Sequence (5′-3′) | Function |
|---|---|---|
| sgRNA2a for pX458 | CACCGTCATCCTCCTGACAATCGATAAACATCGATTGTCAGGAGGATGAC | Single-guide cloning |
| sgRNA1a for MulE vector | ACCGCAATGTGTCAACTCTTGACAAAACTGTCAAGAGTTGACACATTG | Single-guide cloning |
| sgRNA2a for MulE vector | ACCGTCATCCTCCTGACAATCGATAAACATCGATTGTCAGGAGGATGA | Single-guide cloning |
| hU6_Seq | GGACTATCATATGCTTACCGTAACTTGA | Primer for sequencing |
| CCR5-F | CCTGCCAAAAAATCAATGTGA | Surveyor assay/sequencing |
| CCR5-R | AGGACCAGCCCCAAGATGAC | Surveyor assay/sequencing |
| CCR2-F | GCAAATTGGGGCCCAACTCC | Surveyor assay/sequencing |
| CCR2-R | CCAAAATGTTCCTCATTATTGTGTGG | Surveyor assay/sequencing |
These oligos were annealed to make the double-stranded linker with 5′ overhangs (underlined nucleotides).
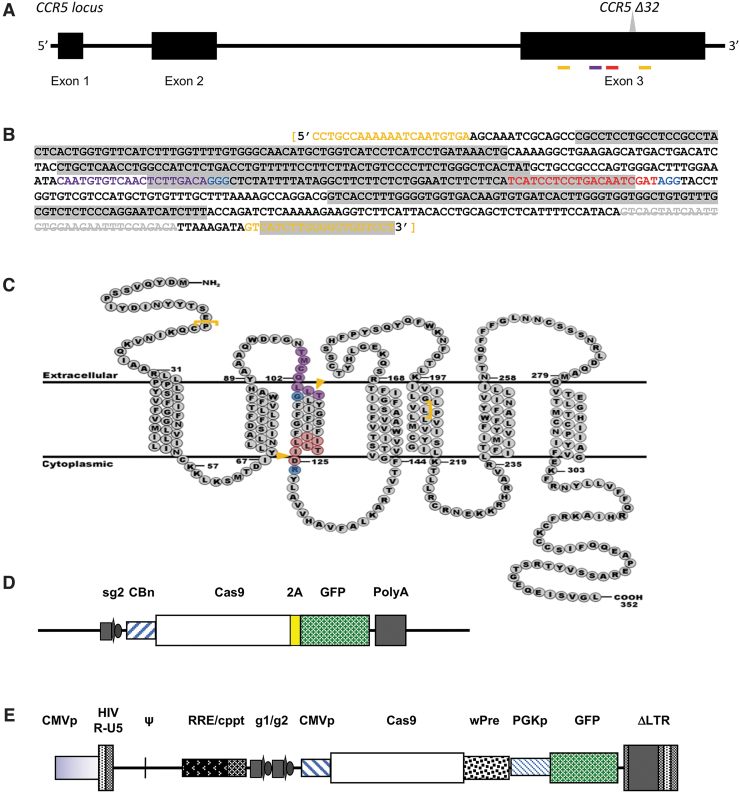
FIG. 1.
Schematic diagrams of CCR5 and the targeting vectors. (A) Schematic representation of the CCR5 loci with exons 1–3 and the Δ32 mutation. The sgRNA target Sites I and II are indicated by violet and red bars, respectively; the PCR primers encompassing the sgRNA and the Δ32 mutation are identified with gold bars. (B) The sequence of the 558 bp CCR5 amplicon showing the target sites for sgRNA1 (violet) and sgRNA2 (red), the PAMs (blue), and the PCR primers (gold). The gray-highlighted text is transmembrane domains and the white text is intracellular/extracellular domains. The Δ32 mutation is gray strikethrough. (C) A schematic diagram of the CCR5 protein structure as the seven-transmembrane receptor. The target sites for sgRNA1 (violet) and sgRNA2 (red), the PAMs (blue), and the PCR primers (gold brackets) are identified. The gold arrows indicated the predicted cutting sites. (D) Schematic diagram of pX458, the Cas9/sgRNA expression vector. The GFP is expressed with the Cas9 as a 2A fusion product, which is cleaved. The sgRNA is expressed by the U6 promoter. (E) Schematic diagram of the lentiviral vector expressing two sgRNAs, Cas9, and GFP. The cis-acting lentiviral elements (CMVp, Ψ, RRE, cPPT, wPRE, PGKp, LTRs) are also labeled. GFP, green fluorescent protein, PAM, protospacer adjacent motif; PCR, polymerase chain reaction; sgRNA, single-guide RNA.
The tetracistronic MuLE lentiviral expression vector (Fig. 1E) was generated using MultiSite Gateway-based recombination.61 MuLE Entry vectors can be recombined efficiently via the attL-attR recombination with the Gateway destination lentiviral vector. The attL-attR sites provide directional specificity to the recombination reactions and allow three MuLE Entry vectors to be simultaneously recombined into the destination vector (pMuLE Lenti Deste eGFP). The MultiSite Gateway attL-attR recombinations (LR Recombinations) were carried out as described as previously.48 Briefly, 10–20 fmoles of the four fragments were mixed Gateway LR Clonase II Plus Enzyme mix (#12538-120; Life Technologies) in 10 μL and incubated at 25°C. After 16–20 h, reaction mixtures were digested with proteinase K for 10 min at 37°C and transformed into chemically competent One Shot Mach1 Escherichia coli cells (#C8620-03; Life Technologies).
Electroporation
For the transfection of ASCs, the Neon Transfection System (Invitrogen-Life Technologies, Grand Island, NY) was used. Twenty micrograms of plasmid DNA in 5 × 105 cells was used per electroporation with pulse voltage 1400 V, pulse width 10 ms, pulse number 3, and tip type 100 μL. Post-transfection, each batch of cells was suspended with prewarmed antibiotic-free media and transferred to 100-mm tissue culture plates. After 24 h, the medium was replaced with fresh medium containing antibiotics. Cells were sorted by flow cytometry (BD FACS AriaIII) for green fluorescent protein (GFP)-positive cells 48 h post-transfection and collected for genomic DNA extraction.
Lentivirus production, concentration, titration, and transduction
Lentivirus production was performed as described previously with modifications.62 For transfection, 293T cells were plated at 1.2 × 107 cells per plate on a 10-cm Petri dish in 6 mL medium (DMEM, 4.5 g/L glucose with GlutaMAX-I) with 10% FBS without antibiotics. On the following day, the medium was replaced by 6 mL advanced DMEM (12491015; Gibco). Meanwhile, 1200 μL of transfection mix was prepared as follows: 36 μg transfer plasmid, 18 μg psPAX2 (packaging vector), and 18 μg pMD2.G (envelope vector) were mixed with CaCl2 (0.25 M final). Then, an equal volume of 2 × HBS buffer was added dropwise and gently mixed. After 15 min at room temperature, chloroquine (0.15 mM final) was added and the entire mixture was added dropwise to the plate. After a 5-h incubation at 37°C and 5% CO2, the medium was aspirated and replaced with 8 mL of advanced DMEM with 2% FBS and 1% Pen/Strep. After 72 h, the supernatant was collected at 4°C, cleared by centrifugation, and filtered (0.45 μm; Millipore). The lentiviral-supernatant was concentrated through polyethylene glycol (PEG)-mediated precipitation using Lenti-XConcentrator (Cat# 631232; Clontech) according to the manufacturer's instructions.
To determine the titer, 2.5 × 105 HEK293T cells were plated in serial dilutions of virus in the presence of 8 μg/mL polybrene. One day later, the viral supernatant was replaced with fresh media. Cells were analyzed for the percentage of GFP-positive cells by flow cytometry 2 days post-transduction. For transduction, 5 × 105 ASCs were plated per 10 cm dish in 6 mL of DMEM containing 10% FBS without antibiotics on day 1. The cells were transduced by concentrated lentivirus at an multiplicity of infection (MOI) of 15 with 8 μg/mL polybrene on day 2, and DMEM containing 10% FBS and 1% Pen/Strep was added to each dish of transduced cells on day 3. The transduced cells were split into three 10-cm dishes on day 4. Cells were replaced with fresh medium on day 6. The GFP-positive transduced cells were sorted by flow cytometry on day 8.
Surveyor assays and DNA sequencing
Genomic DNA was isolated using the Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich). A 558-bp fragment (Fig. 1B) surrounding the sgRNA target sites was amplified for 30 cycles using 200 ng genomic DNA, 10 μM forward and reverse polymerase chain reaction (PCR) primers (Table 1), and 1 × PCR Master Mix (M7502; Promega) at 95°C for 1 min, 51°C for 1 min, and 72°C for 1 min. PCR products were purified with the PureLink Quick PCR Purification Kit (K310002; Life Technologies). DNA heteroduplex formation and Surveyor nuclease digestion were performed as described previously.60 In summary, the amplicon spanning the CCR5 targeting site was denatured and reannealed before cleavage with the nuclease at 42°C for 30 min. Surveyor nuclease digestion products were run on a 4–20% gradient polyacrylamide TBE gel. The frequency of cleavage [Fcut = (b + c)/(a + b + c)] was calculated from the gels by measuring the intensity of the undigested PCR product (a) and each cleavage product (b and c).60
For sequence analysis, the purified PCR product was cloned into the pCR2.1-TOPO TA vector (TOPO TA cloning kit; Life Technologies) and sequenced. To accurately screen every allele individually, clonal lines were generated from the GFP-positive population. Then the targeted locus was PCR-amplified and cloned into a TOPO vector and 10 different bacterial clones were sequenced. Knowing there are only maximum two different results to expect from correctly isolated ASC clones, one for each allele, ASC clones showing more than two different sequencing results were excluded.
Results
Targeting CCR5 with an sgRNA
To efficiently disrupt the CCR5 allele, we designed and screened a series of sgRNAs to induce double-stranded breaks that would lead to indel mutations via NHEJ. We targeted different conserved sites in the human and rhesus macaque CCR5 gene from the beginning of the open-reading frame to the Δ32 mutation site (Fig. 1A). After removing those with high off-target potential, we selected targets with the highest efficiency scores (Table 1) and generated single targeting plasmids (Fig. 1D). In HEK293T cells, three single target guide RNAs (sgRNAs) efficiently generated indel mutations using the double-stranded Cas9 gene (data not shown); however, the double-nicking vectors did not generate any detectable indels (data not shown).
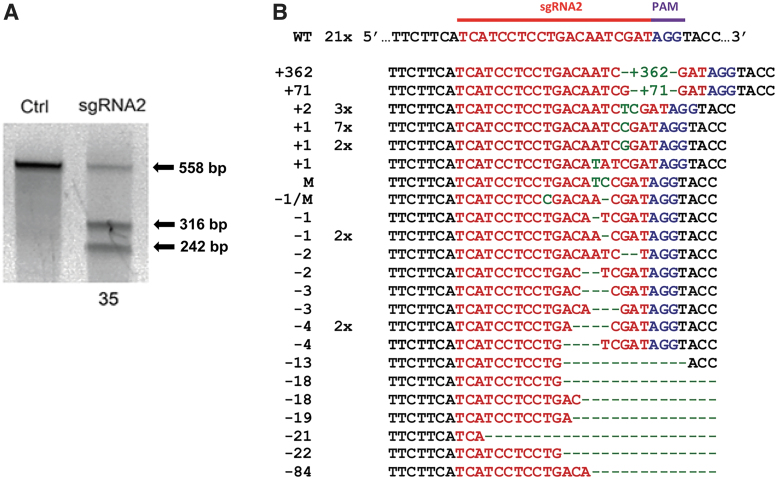
Then, ASCs were transfected with the pX458-CCR5-sgRNA2 plasmid by using the Neon Transfection System. At 48 h, GFP-positive cells expressing Cas9 (via 2A-GFP) were isolated by flow cytometry and expanded. The frequency of mutations as a measure of targeting efficiency can be determined using the Surveyor assay.60 In Figure 2A, we show the indel frequency in the CCR5 gene at the sgRNA2 site was 35% indicating that many of the cells transfected with the sgRNA generated mutations. Our goal was to disrupt CCR5. If the indel mutation caused a FS, expression of a functional CCR5-protein would be knocked out as in CCR5 Δ32 mutation. Therefore, we TOPO cloned and sequenced the 558 bp PCR product encompassing the Site I and Site II (Fig. 1B). We found that 67% of the clones contained Cas9-mediated mutations, including deletions and insertions, at or near the sgRNA2 PAM (Fig. 2B). However, only 50% of the mutations were FS mutations, where single base-pair changes or IF deletions or insertions may not impact the expression of CCR5.
FIG. 2.
Targeting CCR5 with an sgRNA using CRISPR-Cas9. (A) CCR5 gene editing with CRISPS-Cas9. The 558 bp PCR amplicon (black arrow) was generated from sorted ASCs after transfection with control (Ctrl) or sgRNA2. Surveyor assay (Sur) demonstrates cleavage of the amplicon into the expected 316 and 242 bp fragments (black arrow). The frequency of indels is 35%.60 (B) Sequencing results of TOPO cloned PCR amplicon generated with the Fwd and Rev CCR5 primers. The sequences (right column) were sorted from largest inserts (green letters) to the largest deletions (green dashes). The left column is the size of the indel. The center column is the frequency of each sequence. ASC, adipose-derived stem cell; indels, insertions or deletions; Fwd, forward; Rev, reverse.
Efficient transduction of ASCs and disruption of CCR5 with two gRNAs
While there have been many different chemical transfection strategies attempting to genetically modify stem cells, one of the current limitations is the fact that resulting efficiencies have generally been very poor, often significantly <1%.63 To improve the mutation efficiency of indels of CCR5 gene in the CRISPR-Cas9 system, we used two gRNAs of CCR5 in a single vector.
For an improved gene transfer efficiency with large vectors expressing two gRNAs of CCR5, we used a MuLE system, based on MultiSite Gateway cloning.64 This system allows for expression of multiple genes after transduction with a single lentiviral vector. The MuLE system represents a favorable experimental platform to allow multiple sgRNAs to be expressed together with Cas9 from a single viral construct.61 Furthermore, lentiviral vectors are capable of infecting a wide variety of dividing and nondividing cells. The transduction efficiency was measured by flow cytometry of GFP-positive cells. We found that GFP expression started at 24 h and reached maximum levels on day 7 up to 96% (data not shown). The transduced GFP-positive cells were separated by fluorescence-activated cell sorting (FACS).
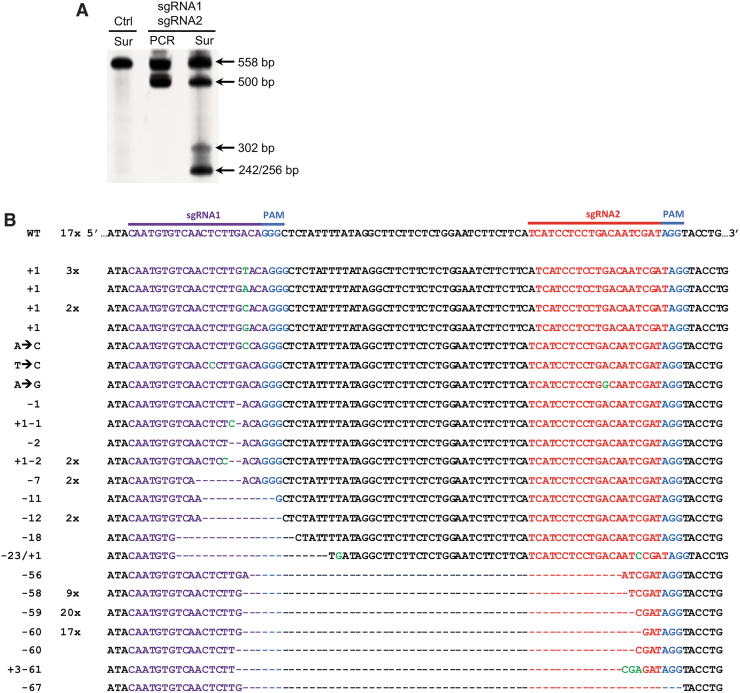
Genomic regions spanning both sgRNA1 and sgRNA2 were PCR amplified. We observed a fragment shorter than expected (558 bp) in the PCR products, indicating that the 60 bp fragment between the sgRNA1 and sgRNA2 targeting sites was deleted (Fig. 3A). The cleavage efficiency using two gRNAs was assessed via the Surveyor assay. We observed that the indel frequency in targeting cells with two gRNAs (45%) is higher than that using a sgRNA (Figs. 2A and 3A), suggesting that dual sgRNAs induced a higher targeting efficiency compared with one sgRNA. A cleavage by sgRNA1 only would result in a 256 bp and a 302 bp fragment; by sgRNA2 only would result in a 242 bp and a 316 bp fragment. The dual cleavage at both sites would lead to deletion of the 60 bp fragment and generate a 256 bp and a 242 bp fragment (Fig. 3A).
FIG. 3.
Targeting CCR5 with two guide RNAs using a tetracistronic lentiviral vector expressing CRISPR-Cas9. (A) CCR5 gene editing with CRISPS/Cas9. Surveyor assay (Sur) or standard PCR product (PCR) with the 558 bp amplicon generated from ASCs transduced with the dual-guide (sgRNA1 and sgRNA2) lentiviral vector or control (Ctrl). Arrows indicate the WT allele (558 bp), the deleted allele (∼500 bp) with the ∼60 bp deletion, and the expected 302 bp and 256/242 bp fragments from editing sgRNA1 and sgRNA1/2, respectively. (B) Sequencing results of TOPO cloned PCR amplicon generated with the Fwd and Rev CCR5 primer pairs. The sequences (right column) were sorted from largest inserts (green letters) to the largest deletions (dashes). The left column is the size of the indel. The center column is the frequency of each sequence.
To precisely characterize these CRISPR-Cas9-mediated events, PCR products from the target region of both gRNAs were cloned into a TOPO-TA vector and sequenced. Interestingly, we detected wild-type sequences (17/89 or 19%), the targeting of only the sgRNA1 (22/89 or 25%), and the targeting of both sgRNAs (50/89 or 56%), resulting in a deletion of the fragments between the two cutting sites (Fig. 3B). We did not detect any cleavage activity in only sgRNA2 when sequencing the dually targeted cells. Even with detecting an indel frequency of 81%, only 56% were FS mutations known to completely delete CCR5 activity.
Previous studies have reported off-target mutagenesis in CCR2 when CRISPR targeted CCR5 due to the high similarity of the sequence of CCR2 to that of CCR5.29,30 When analyzed by the Surveyor assay, neither CCR2 nor CCR3 had any detectible mutations. To increase the resolution of CRISPR off-target activity, CCR2 was amplified from genomic DNA of ASCs modified with a single CCR5 sgRNA, and the amplicons were cloned into TOPO TA vector and sequenced. In 50 Topo clones, we did not observe any mutations. These data demonstrate that dual targeting of the same gene increases the frequency of indel mutations, particularly large deletions between the two target sites, without off-target activity.
Genotype analysis of both alleles in dual-targeted clonal cell lines
Because only individuals homozygous for CCR5Δ32 confer resistance to HIV-1 infection and individuals heterozygous for CCR5Δ32 are associated with lower pre-AIDS viral load and delayed progressions to AIDS, we examined the mutations in each CCR5 allele from the individual clones generated by dual sgRNA targeting. When considered alleles within a diploid cell, the type and significance will determine whether the CCR5 function will be effective in blocking HIV infection. The clones that block HIV infection will require homozygotic FS mutations or extensive deletions in the CCR5 gene. The sequencing results from the isolated clones showed a variety of targeted mutation types, including deletions, insertions, and/or combination mutations (Fig. 4A–C). Therefore, we categorized the clones into three groups: those with monoallelic mutations with 1 wild-type allele and 1 indel (2/13 or 16%; Fig. 4A and Table 2), or with biallelic mutations (11/13 or 85%) either with IF/FS mutations (7/15 or 54%; Fig. 4B) or 2 FS mutations (4/13 or 31%; Fig. 4C).
FIG. 4.
Analysis of the CCR5 alleles detected in 13 clonal lines. Individual clones were isolated from cell populations transduced with both sgRNA1 and sgRNA2. The 558 bp PCR product for each allele was amplified and sequenced. (A) The clones with one mutant allele and one wild-type sequence. (B) The clones with two mutant alleles, one FS mutation, and one IF mutation. (C) The clones carrying two FS mutant alleles. FS, frameshift; IF, in-frame.
Table 2.
Specific allelic mutation in 13 expanded clonal lines
| Genotype | Clonal line No. | Editing |
Sum | |||
|---|---|---|---|---|---|---|
| Allele
1 |
Allele
2 |
|||||
| sgRNA1 | sgRNA2 | sgRNA1 | sgRNA2 | |||
| Monoallelic | 32 | Y | N | N | N | 2 |
| 34 | Y | Y | N | N | ||
| Biallelic | 5 | Y | N | Y | N | 11 |
| 6 | Y | N | Y | N | ||
| 8 | Y | N | Y | N | ||
| 10 | Y | Y | Y | Y | ||
| 11 | Y | Y | Y | N | ||
| 17 | Y | Y | Y | N | ||
| 20 | Y | Y | Y | N | ||
| 23 | Y | Y | Y | Y | ||
| 25 | Y | Y | Y | N | ||
| 27 | Y | Y | Y | Y | ||
| 33 | Y | Y | Y | N | ||
Site I: 22/26; Site II: 12/26.
We observed that 70% (24/34) of the different indel mutations were deletions, in which 24% (10/34) were deletions between the two target sites (approximately a 60 bp fragment) and 44% (14/34) were small deletions (<18 bp) in Site I and/or Site II (Table 3). In most cases, dual targeting in both Site I and Site II leads to a deletion of the intervening sequence; however, some alleles with dual targeting did not delete the intervening sequence, but only created small deletions on both sites. Although less frequent, we also observed that some indels were insertions and/or combinations. Single editing and duel editing were equally likely (Table 4). The frequency of editing in Site I (24/52 or 46%) was higher than in Site II (12/52 or 23%), with half targeting both sites and half targeting only Site I, which is consistent with the findings in Figure 3 and the previous report with dual sgRNA targeting.65 Overall, the frequency of clones homozygotic for FS mutations was 4/13 or 31%.
Table 3.
Summary of indel mutations
| Mutation type | Frequency | Rate, % | ||
|---|---|---|---|---|
| Deletion | ≥59 bp | 10 | 29 | 71 |
| ≤18 bp | 14 | 41 | ||
| Insertion | 10 | 29 | ||
Table 4.
Summary of allele types in 26 alleles of 13 clonal lines
| Allele type | Frequency | Rate, % |
|---|---|---|
| Wild type | 2 | 8 |
| Editing in one gRNA | 12 | 46 |
| Editing in dual gRNAs | 12 | 46 |
Not all dual = >60 bp deletion.
Discussion
One therapeutic strategy to control HIV-1 replication is to engineer a population of HIV-resistant immune cells that could expand in the presence of replicating virus, while nonresistant cells get depleted.23,26 Individuals with a CCR5Δ32 variant are resistant to HIV1 infection,19,20 making CCR5 an attractive target for drug and genetic intervention against HIV-1. Allogeneic HSC transplantation from a donor with homozygous CCR5Δ32 mutations19,20 resulted in a loss of detectable HIV-1 in two patients22 even more than 9 years later in the absence of antiretroviral drug therapy.20,21 These clinical data support the potential of HSC therapies based on the elimination of CCR5. Several groups had previously shown that stem cell populations such as ESCs, iPSCs, and ASCs, which normally lack expression of CCR5, are able to be induced to differentiate into cells with hematopoietic characteristics that include CCR5 expression.42–47 Therefore, CCR5 knockout in ASCs may have a potential application in HIV-1 treatment.
CRISPR-Cas9-based gene therapy strategies have been proposed for HIV-1 because of the high efficiency and limited off-target effect.66 These strategies target multiple steps in the viral life cycle,67 including the HIV-1 provirus directly and host factors.66 CRISPR technology is used to excise the complete or partial HIV genome, or coupled to transcriptional activators/repressors to regulate expression.66,68 Because of the genetic variability in the HIV-1 genome within and between patients, incomplete targeting of viral sequence predicts the emergence of viral resistance.69,70 Therefore, multiple targeting of conserved sequences in Gag, Pol, and Tat/Rev, but not in regulatory domains nor the accessory genes nef and vpr, is required to control viral replication.71,72 In addition, efficient in vivo delivery of the CRISPR-Cas9 to a sufficient portion of the latent reservoir remains a challenge.73 Among the host factors, the HIV coreceptor CCR5 is a target for drug and gene therapy against virus infection,66,67,74,75 where the genetic variability is significantly reduced.
In the present study, we used the CRISPR-Cas9 system to disrupt the CCR5 gene. Using sgRNAs targeting different sites in the CCR5 coding sequence immediately upstream of the Δ32 mutation, we observed FS mutations in 53% of the CCR5 sequences. To potentially increase the frequency, we used the MuLE system to express two sgRNAs together with Cas9 and GFP from a single viral vector.61 Based on the Surveyor mutation detection assay, two sgRNAs showed that Cas9-mediated cleavage efficiency was more efficient than with one sgRNA. In addition, the dual sgRNA CRISPR-Cas9-mediated CCR5 gene editing technology in this study shows indel frequency at 45% in stem cells, which is much higher than what we found or the 27% previously reported with one sgRNA.29 More importantly, genotyping analysis showed that up to 84% (11/13) of isolated clones carried biallelic mutations (Fig. 4 and Table 2), suggesting that the CRISPR-Cas9 technology with dual sgRNAs is very efficient for disruption of CCR5 in both alleles in ASCs.
Since small indel mutations will randomly regenerate IF mutations, our two-site strategy increased the frequency of large deletions; however, even these large deletions can regenerate IF mutations. Given the high incidence of IF mutations even with the increased indel frequency, we sequenced the individual alleles in multiple clonal lines to determine the frequency of biallelic IF and FS mutations. Although 84% of the clones were biallelic, only four of these clones (31%) had biallelic FS mutations and five clones had a biallelic FS mutation with large IF mutations. Based on T cell modeling that begins with the differentiation of HSCs into CD4 T cells and includes HIV infection and apoptosis, the frequency of complete CCR5 knockout necessary to protect a patient from viral replication is >75%, if the CD4 T cells are not effectively clearing virus.76 Therefore, even with these frequencies of indels and biallelic mutations, it may still necessary to increase the level of FS mutations in the patient's T cell compartment to prevent the progress of HIV infection, dissemination, and disease.
For delivering the constructs needed to target CCR5, we used the MuLE system to express the two sgRNAs with hCas9 and GFP.61 Generally, large vectors generate low titers and are less efficient in transducing target cells.62 We demonstrated production of 105 transducing units per milliliter (TU/mL) with a large lentiviral insert of 10.7 kb. We observed that the transduction efficiency of ASCs with the concentrated virus (107 TU/mL) was about 90% on day 8 post-transduction, but that expression of the vector (GFP) was delayed in comparison with other vector systems. Using lentiviral vectors to express Cas9, while efficiently targeting CCR5 gene in these studies, could extend the expression of the Cas9 endonuclease and increase the potential for off-target toxicity. Nonetheless, we did not observe any off-target activity against two closely related chemokine receptors, either CCR2 or CCR3. Other delivery systems may need to be explored to transiently express the Cas9/sgRNAs.
Interestingly, we observed several CCR5 indels with unique characteristics. Our sequencing strategy amplified 558 bps of genomic DNA that surrounds the targeting site and we TOPO cloned/sequenced this PCR product. This strategy provides very accurate sequence analysis of the isolated clones. In several of the clones using the two sgRNAs, we found that both Site I and Site II had indel mutations, but that the intervening sequences were still intact. This implies that the repair activity is more rapid than the CRISPR digestions and that the CRISPR endonuclease activity is maintained after the repair activity corrects the double-stranded breaks. In one clone, after transfection of an sgRNA, we identified a 362 bp insert, which matched the plasmid backbone. This demonstrates that other sequences can be used to repair the DSB and should raise additional safety concerns using CRISPR technology. In addition, our strategy will only detect relatively small indel mutations; larger deletions that eliminate the primer binding sites necessary for PCR amplification will not be detectable.77
Overall, these findings demonstrate that the CRISPR-Cas9 technology with two sgRNAs generated more mutations than an sgRNA. However, we also showed that not all mutations will cause a FS mutation and completely knockout the CCR5 gene. When analyzing both alleles in individual ASC clones, some clones were heterozygotic and contained either the wild-type CCR5 sequence or minor IF mutations that would not be expected to prevent HIV infection. Larger deletions that target functional domains may be required to ensure the absence of CCR5 surface expression. Although we did not detect any off-target activity in other chemokine receptors, limited Cas9 expression may be required to limit off-target effects.
Future studies should prove that improving the CCR5-specific CRISPR-Cas9 targeting in ASCs would provide a continuous supply of HIV-resistant progeny from HSCs. This could be a novel therapeutic approach to partly and repetitively reconstitute the immune system with HIV-resistant autologous cells avoiding the need for a complete replacement of the existing bone marrow, with all its associated risks. Further studies are required as well regarding the number of homozygotic CCR5 knockout stem and progenitor cells necessary to prevent further spreading of HIV, first in culture and later in patients in the absence of ART.
Conclusion
Our study demonstrates that a lentiviral vector expressing two sgRNA targeting CCR5 near the common Δ32 mutation increases the disruption of the CCR5 gene without off-target activity in the closely related CCR2 and CCR3 genes. In most clones, these mutations were biallelic. However, in contrast to the FS mutation present in the Δ32 allele, individual CCR5 alleles contained IF and FS mutations due to the stochastic nature of double-stranded breaks and repair mechanism. Our novel therapeutic strategy opens up the possibility to generate an autologous stem cell pool, which is homozygous for CCR5 knockout and resistant to HIV replication for the future treatment of advanced HIV-infected patients with clinical symptoms associated with a paucity of regenerative cells in all organs of the body.
Authors' Contributions
D.L. designed the targets, performed the experiments, analyzed data, and wrote the article. S.H.S. analyzed the data and edited the article. M.M.R. performed experiments and analyzed the data. R.I. conceived the project, analyzed data, and edited the article. E.U.A. conceived the project, funded the project, analyzed data, edited and wrote the article. S.E.B. conceived and supervised the project, analyzed data, wrote and edited the article. All the coauthors have reviewed and approved the article before submission. This article has only been submitted to this journal and has not been published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
These studies were funded by the Alliance for Cardiovascular Research.
References
- 1. Wu L, Gerard NP, Wyatt R, et al. . CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. DOI: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 2. Dalgleish AG, Beverley PC, Clapham PR, et al. . The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. DOI: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 3. Alkhatib G, Combadiere C, Broder CC, et al. . CC CKR5: A RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. DOI: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 4. Bleul CC, Farzan M, Choe H, et al. . The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. DOI: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 5. de Roda Husman AM, Blaak H, Brouwer M, et al. . CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J Immunol. 1999;163:4597–4603. [PubMed] [Google Scholar]
- 6. Morris L, Cilliers T, Bredell H, et al. . CCR5 is the major coreceptor used by HIV-1 subtype C isolates from patients with active tuberculosis. AIDS Res Hum Retroviruses. 2001;17:697–701. DOI: 10.1089/088922201750236979. [DOI] [PubMed] [Google Scholar]
- 7. Herrera-Carrillo E, Berkhout B. Novel AgoshRNA molecules for silencing of the CCR5 co-receptor for HIV-1 infection. PLoS One. 2017;12:e0177935. DOI: 10.1371/journal.pone.0177935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanpain C, Lee B, Vakili J, et al. . Extracellular cysteines of CCR5 are required for chemokine binding, but dispensable for HIV-1 coreceptor activity. J Biol Chem. 1999;274:18902–18908. DOI: 10.1074/jbc.274.27.18902. [DOI] [PubMed] [Google Scholar]
- 9. Scarlatti G, Tresoldi E, Bjorndal A, et al. . In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. DOI: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 10. Hutter G, Bodor J, Ledger S, et al. . CCR5 Targeted Cell Therapy for HIV and Prevention of Viral Escape. Viruses. 2015;7:4186–4203. DOI: 10.3390/v7082816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muntinghe FL, Verduijn M, Zuurman MW, et al. . CCR5 deletion protects against inflammation-associated mortality in dialysis patients. J Am Soc Nephrol. 2009;20:1641–1649. DOI: 10.1681/ASN.2008040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drozd-Dabrowska M, Ganczak M, Karpinska E. Concerns related to CCR5 gene delta 32 mutation role in hepatitis B virus infection. Przegl Epidemiol. 2017;71:571–581. [PubMed] [Google Scholar]
- 13. Samson M, Libert F, Doranz BJ, et al. . Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. DOI: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 14. Schroers R, Davis CM, Wagner HJ, et al. . Lentiviral transduction of human T-lymphocytes with a RANTES intrakine inhibits human immunodeficiency virus type 1 infection. Gene Ther. 2002;9:889–897. DOI: 10.1038/sj.gt.3301711. [DOI] [PubMed] [Google Scholar]
- 15. Liu R, Paxton WA, Choe S, et al. . Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. DOI: S0092-8674(00)80110-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 16. Lim JK, McDermott DH, Lisco A, et al. . CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J Infect Dis. 2010;201:178–185. DOI: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoxie JA, June CH. Novel cell and gene therapies for HIV. Cold Spring Harb Perspect Med. 2012;2. DOI: 10.1101/cshperspect.a007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dau B, Holodniy M. Novel targets for antiretroviral therapy: Clinical progress to date. Drugs. 2009;69:31–50. DOI: 10.2165/00003495-200969010-00003. [DOI] [PubMed] [Google Scholar]
- 19. Hutter G, Nowak D, Mossner M, et al. . Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. DOI: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 20. Hutter G, Thiel E. Allogeneic transplantation of CCR5-deficient progenitor cells in a patient with HIV infection: An update after 3 years and the search for patient no. 2. AIDS. 2011;25:273–274. DOI: 10.1097/QAD.0b013e328340fe28. [DOI] [PubMed] [Google Scholar]
- 21. Brown TR. I am the Berlin patient: A personal reflection. AIDS Res Hum Retroviruses. 2015;31:2–3. DOI: 10.1089/AID.2014.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta RK, Abdul-Jawad S, McCoy LE, et al. . HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568:244–248. DOI: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rossi JJ, June CH, Kohn DB. Genetic therapies against HIV. Nat Biotechnol. 2007;25:1444–1454. DOI: 10.1038/nbt1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braun SE, Johnson RP. Setting the stage for bench-to-bedside movement of anti-HIV RNA inhibitors-gene therapy for AIDS in macaques. Front Biosci. 2006;11:838–851. DOI: 10.2741/1841. [DOI] [PubMed] [Google Scholar]
- 25. Herrera-Carrillo E, Berkhout B. Bone marrow gene therapy for HIV/AIDS. Viruses. 2015;7:3910–3936. DOI: 10.3390/v7072804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kimpel J, Braun SE, Qiu G, et al. . Survival of the fittest: Positive selection of CD4+ T cells expressing a membrane-bound fusion inhibitor following HIV-1 infection. PLoS One. 2010;5:e12357. DOI: 10.1371/journal.pone.0012357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holt N, Wang J, Kim K, et al. . Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–847. DOI: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myburgh R, Ivic S, Pepper MS, et al. . Lentivector knockdown of CCR5 in hematopoietic stem and progenitor cells confers functional and persistent HIV-1 resistance in humanized mice. J Virol. 2015;89:6761–6772. DOI: 10.1128/JVI.00277-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu L, Yang H, Gao Y, et al. . CRISPR/Cas9-mediated CCR5 ablation in human hematopoietic stem/progenitor cells confers HIV-1 resistance in vivo. Mol Ther. 2017;25:1782–1789. DOI: 10.1016/j.ymthe.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hofer U, Henley JE, Exline CM, et al. . Pre-clinical modeling of CCR5 knockout in human hematopoietic stem cells by zinc finger nucleases using humanized mice. J Infect Dis. 2013;208(Suppl 2):S160–S164. DOI: 10.1093/infdis/jit382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mandal PK, Ferreira LM, Collins R, et al. . Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell. 2014;15:643–652. DOI: 10.1016/j.stem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu L, Wang J, Liu Y, et al. . CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019;381:1240–1247. DOI: 10.1056/NEJMoa1817426. [DOI] [PubMed] [Google Scholar]
- 33. Han SH, Kim J, Her Y, et al. . Phytosphingosine promotes megakaryocytic differentiation of myeloid leukemia cells. BMB Rep. 2015;48:691–695. DOI: 10.5483/bmbrep.2015.48.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johal KS, Lees VC, Reid AJ. Adipose-derived stem cells: Selecting for translational success. Regen Med. 2015;10:79–96. DOI: 10.2217/rme.14.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun Q, Zhang Z, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1:113–119. DOI: 10.1016/j.gendis.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhartiya D. Stem cells, progenitors & regenerative medicine: A retrospection. Indian J Med Res. 2015;141:154–161. DOI: 10.4103/0971-5916.155543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagree MS, Lopez-Vasquez L, Medin JA. Towards in vivo amplification: Overcoming hurdles in the use of hematopoietic stem cells in transplantation and gene therapy. World J Stem Cells. 2015;7:1233–1250. DOI: 10.4252/wjsc.v7.i11.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zielske SP, Braun SE. Cytokines: Value-added products in hematopoietic stem cell gene therapy. Mol Ther. 2004;10:211–219. DOI: 10.1016/j.ymthe.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 39. Winnier GE, Valenzuela N, Peters-Hall J, et al. . Isolation of adipose tissue derived regenerative cells from human subcutaneous tissue with or without the use of an enzymatic reagent. PLoS One. 2019;14:e0221457. DOI: 10.1371/journal.pone.0221457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alt EU, Winnier G, Haenel A, et al. . Towards a comprehensive understanding of UA-ADRCs (uncultured, autologous, fresh, unmodified, adipose derived regenerative cells, isolated at point of care) in regenerative medicine. Cells. 2020;9. DOI: 10.3390/cells9051097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fraser JK, Wulur I, Alfonso Z, et al. . Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. DOI: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 42. Ning H, Lei HE, Xu YD, et al. . Conversion of adipose-derived stem cells into natural killer-like cells with anti-tumor activities in nude mice. PLoS One. 2014;9:e106246. DOI: 10.1371/journal.pone.0106246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Freisinger E, Cramer C, Xia X, et al. . Characterization of hematopoietic potential of mesenchymal stem cells. J Cell Physiol. 2010;225:888–897. DOI: 10.1002/jcp.22299. [DOI] [PubMed] [Google Scholar]
- 44. Bernareggi D, Pouyanfard S, Kaufman DS. Development of innate immune cells from human pluripotent stem cells. Exp Hematol. 2019;71:13–23. DOI: 10.1016/j.exphem.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Knorr DA, Ni Z, Hermanson D, et al. . Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2013;2:274–283. DOI: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nazari-Shafti TZ, Freisinger E, Roy U, et al. . Mesenchymal stem cell derived hematopoietic cells are permissive to HIV-1 infection. Retrovirology. 2011;8:3. DOI: 10.1186/1742-4690-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang H, Minder P, Park MA, et al. . CCR5 Disruption in induced pluripotent stem cells using CRISPR/Cas9 provides selective resistance of immune cells to CCR5-tropic HIV-1 virus. Mol Ther Nucleic Acids. 2015;4:e268. DOI: 10.1038/mtna.2015.42. [DOI] [PubMed] [Google Scholar]
- 48. Jinek M, East A, Cheng A, et al. . RNA-programmed genome editing in human cells. Elife. 2013;2:e00471. DOI: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cong L, Ran FA, Cox D, et al. . Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. DOI: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mali P, Yang L, Esvelt KM, et al. . RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. DOI: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lander ES. The heroes of CRISPR. Cell. 2016;164:18–28. DOI: 10.1016/j.cell.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 52. Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;168:20–36. DOI: 10.1016/j.cell.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jinek M, Chylinski K, Fonfara I, et al. . A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. DOI: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sternberg SH, Redding S, Jinek M, et al. . DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. DOI: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jinek M, Jiang F, Taylor DW, et al. . Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343:1247997. DOI: 10.1126/science.1247997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen B, Zhang J, Wu H, et al. . Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 2013;23:720–723. DOI: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang H, Yang H, Shivalila CS, et al. . One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. DOI: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang L, Shao Y, Guan Y, et al. . Large genomic fragment deletion and functional gene cassette knock-in via Cas9 protein mediated genome editing in one-cell rodent embryos. Sci Rep. 2015;5:17517. DOI: 10.1038/srep17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakao H, Harada T, Nakao K, et al. . A possible aid in targeted insertion of large DNA elements by CRISPR/Cas in mouse zygotes. Genesis. 2016;54:65–77. DOI: 10.1002/dvg.22914. [DOI] [PubMed] [Google Scholar]
- 60. Ran FA, Hsu PD, Wright J, et al. . Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. DOI: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Albers J, Danzer C, Rechsteiner M, et al. . A versatile modular vector system for rapid combinatorial mammalian genetics. J Clin Invest. 2015;125:1603–1619. DOI: 10.1172/JCI79743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. al Yacoub N, Romanowska M, Haritonova N, et al. . Optimized production and concentration of lentiviral vectors containing large inserts. J Gene Med. 2007;9:579–584. DOI: 10.1002/jgm.1052. [DOI] [PubMed] [Google Scholar]
- 63. Moore JC, Atze K, Yeung PL, et al. . Efficient, high-throughput transfection of human embryonic stem cells. Stem Cell Res Ther. 2010;1:23. DOI: 10.1186/scrt23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Katzen F. Gateway((R)) recombinational cloning: A biological operating system. Expert Opin Drug Discov. 2007;2:571–589. DOI: 10.1517/17460441.2.4.571. [DOI] [PubMed] [Google Scholar]
- 65. Adikusuma F, Pfitzner C, Thomas PQ. Versatile single-step-assembly CRISPR/Cas9 vectors for dual gRNA expression. PLoS One. 2017;12:e0187236. DOI: 10.1371/journal.pone.0187236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saayman S, Ali SA, Morris KV, et al. . The therapeutic application of CRISPR/Cas9 technologies for HIV. Expert Opin Biol Ther. 2015;15:819–830. DOI: 10.1517/14712598.2015.1036736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yin L, Hu S, Mei S, et al. . CRISPR/Cas9 inhibits multiple steps of HIV-1 infection. Hum Gene Ther. 2018;29:1264–1276. DOI: 10.1089/hum.2018.018. [DOI] [PubMed] [Google Scholar]
- 68. Olson A, Basukala B, Lee S, et al. . Targeted chromatinization and repression of HIV-1 provirus transcription with repurposed CRISPR/Cas9. Viruses. 2020;12. DOI: 10.3390/v12101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dampier W, Sullivan NT, Chung CH, et al. . Designing broad-spectrum anti-HIV-1 gRNAs to target patient-derived variants. Sci Rep. 2017;7:14413. DOI: 10.1038/s41598-017-12612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roychoudhury P, De Silva Feelixge H, Reeves D, et al. . Viral diversity is an obligate consideration in CRISPR/Cas9 designs for targeting the HIV reservoir. BMC Biol. 2018;16:75. DOI: 10.1186/s12915-018-0544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Z, Pan Q, Gendron P, et al. . CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep. 2016;15:481–489. DOI: 10.1016/j.celrep.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 72. Wang G, Zhao N, Berkhout B, et al. . CRISPR-Cas9 can inhibit HIV-1 replication but NHEJ repair facilitates virus escape. Mol Ther. 2016;24:522–526. DOI: 10.1038/mt.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jerome KR. Disruption or excision of provirus as an approach to HIV cure. AIDS Patient Care STDS. 2016;30:551–555. DOI: 10.1089/apc.2016.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang W, Ye C, Liu J, et al. . CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection. PLoS One. 2014;9:e115987. DOI: 10.1371/journal.pone.0115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ye L, Wang J, Beyer AI, et al. . Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Delta32 mutation confers resistance to HIV infection. Proc Natl Acad Sci U S A. 2014;111:9591–9596. DOI: 10.1073/pnas.1407473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ratti V, Nanda S, Eszterhas SK, et al. . A mathematical model of HIV dynamics treated with a population of gene-edited haematopoietic progenitor cells exhibiting threshold phenomenon. Math Med Biol. 2020;37:212–242. DOI: 10.1093/imammb/dqz011. [DOI] [PubMed] [Google Scholar]
- 77. Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765–771. DOI: 10.1038/nbt.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]