Summary
Background
Abatacept was well tolerated by patients with early diffuse cutaneous systemic sclerosis in a phase 2, double-blind randomised trial, with potential efficacy at 12 months. We report here the results of an open-label extension for 6 months.
Methods
Patients (aged ≥18 years) with diffuse cutaneous systemic sclerosis of less than 3 years’ duration from their first non-Raynaud’s symptom were enrolled into the ASSET trial (A Study of Subcutaneous Abatacept to Treat DiffuseCutaneous Systemic Sclerosis), which is a double-blind trial at 22 sites in Canada, the UK, and the USA. Aftercompletion of 12 months of treatment with either abatacept or placebo, patients received a further 6 months ofabatacept (125 mg subcutaneous every week) in an open-label extension. The primary endpoint of the double-blind trial was modified Rodnan Skin Score (mRSS) at 12 months, which was reassessed at 18 months in the open-label extension. The primary analysis included all participants who completed the double-blind trial and received at least one dose of open-label treatment (modified intention to treat). This trial is registered with ClinicalTrials.gov, NCT02161406.
Findings
Between Sept 22, 2014, and March 15, 2017, 88 participants were randomly allocated in the double-blind trial either abatacept (n=44) or placebo (44); 32 patients from each treatment group completed the 6-month open-labelextension. Among patients assigned abatacept, a mean improvement from baseline in mRSS was noted at 12 months (−6·6 [SD 6·4]), with further improvement seen during the open-label extension period (−9·8 [8·1] at month 18). Participants assigned placebo had a mean improvement from baseline in mRSS at 12 months (−3·7 [SD 7·6]), with a further improvement at month 18 (−6·3 [9·3]). Infections during the open-label extension phase occurred in nine patients in the placebo–abatacept group (12 adverse events, one serious adverse event) and in 11 patients in theabatacept–abatacept group (14 adverse events, one serious adverse event). Two deaths occurred during the 12-month double-blind period in the abatacept group, which were related to scleroderma renal crisis; no deaths were recorded during the open-label extension.
Interpretation
During the 6-month open-label extension, no new safety signals for abatacept were identified in the treatment of diffuse cutaneous systemic sclerosis. Clinically meaningful improvements in mRSS and other outcome measures were observed in both the abatacept and placebo groups when patients transitioned to open-label treatment. These data support further studies of abatacept in diffuse cutaneous systemic sclerosis.
Funding
Bristol-Myers Squibb and National Institutes of Health.
Introduction
Systemic sclerosis is an immune-mediated connective tissue disease characterised by inflammation and fibrosis of the skin and internal organs.1 Participants with diffuse cutaneous systemic sclerosis have high mortality rates, particularly those who experience progressive skin fibrosis.2 Autologous haematopoietic stem-cell transplantation shows survival benefits in early diffuse cutaneous systemic sclerosis but can be associated with substantial toxicities and costs and is usually reserved for individuals with worsening internal organ involvement.3,4 Nintedanib has been approved for the treatment of systemic sclerosis-associated interstitial lung disease, but options remain scarce for disease-modifying therapies aimed at treatment of overall disease, including skin involvement.5,6
Several studies implicate activated T cells in the pathogenesis of early diffuse cutaneous systemic sclerosis, particularly with respect to cutaneous disease.7 Skin biopsy samples from patients with early diffuse cutaneous systemic sclerosis are enriched with an inflammatory infiltrate consisting of activated T cells and macrophages in perivascular regions.8–10 Abatacept is a cytotoxic T-lymphocyte-associated antigen 4 immuno globulin fusion protein that blocks T-cell co-stimulation. Animal models that mimic the early inflammatory skin changes seen in diffuse cutaneous systemic sclerosis show that abatacept can prevent and induce the regression of dermal fibrosis.11 In addition to decreasing T-cell activation, abatacept might mediate its anti-fibrotic effects by preventing the differentiation of circulating fibrocytes into myofibroblasts or fibroblasts.12
A 6-month, placebo-controlled pilot study of ten participants with diffuse cutaneous systemic sclerosis showed that abatacept was well tolerated, with potential use for treatment of skin tightening.13 In a EUSTAR observational study that included 27 patients, abatacept had a good safety profile, improving inflammatory arthritis and function in patients with systemic sclerosis treated in routine care.14 In view of these preclinical and clinical data, we did the ASSET trial (A Study of Subcutaneous Abatacept to Treat Diffuse Cutaneous Systemic Sclerosis), which is a phase 2, double-blind, placebo-controlled trial of weekly subcutaneous abatacept over 12 months in patients with early diffuse cutaneous systemic sclerosis (≤36 months of disease).15 ASSET showed numerical, but not statistically signi ficant, improvements with abatacept in the primary endpoint of mean change from baseline to month 12 of modified Rodnan Skin Score (mRSS).16 Moreover, abatacept was safe and showed significant and clinically meaningful changes in secon dary outcome measures, including the Health Assessment Questionnaire disability index (HAQ-DI) and the composite index the American College of Rheumatology (ACR) Combined Response Index in diffuse cutan eous Systemic Sclerosis (CRISS).17 At completion of the 12-month double-blind phase, all participants were eligible to transition to open-label treat ment with weekly subcutaneous abatacept for an additional 6 months. Here, we describe safety and exploratory efficacy outcomes up to month 18, including a 6-month open-label exten sion period.
Methods
Study design
ASSET is an investigator-initiated, phase 2, double-blind randomised trial with an open-label extension phase done at 22 sites in Canada, the UK, and the USA. Eligible participants (aged ≥18 years) fulfilled the 2013 ACR and European Union League Against Rheumatism classification criteria for systemic sclerosis18 with diffuse cutaneous involvement as defined by LeRoy and Medsger.19 Further inclusion criteria were either disease duration of 18 months or less from the time of first non-Raynaud’s symptoms and an mRSS of 10–35 at the time of screening, or disease duration of 36 months or less but more than 18 months, an mRSS of 15–45, and evidence of active disease at the screening visit compared with the last clinic visit in the previous 6 months. Active disease was defined as an increase in mRSS of 3 or more units, involvement of one new body area with an increase in mRSS of 2 or more units, involvement of two new body areas with an increase in mRSS of 1 or more unit, or presence of one or more tendon friction rubs.
If the participant completed the 12-month double-blind trial, they were offered the chance to join the 6-month open-label extension. If the participant worsened with respect to their systemic sclerosis for 3 months or longer during the 12-month study, and escape therapy was indicated (as determined by the clinician), study medication was withdrawn. However, if the participant agreed to continue study follow-up (up to month 12), participated in visits and procedures, and agreed to blood and tissue collection, they were offered the chance to participate in the open-label phase.
Each participating site obtained approval from their local institutional review board or ethics committee. The study design and participant inclusion and exclusion criteria have been previously published and are available in the appendix (pp 1–2); the study protocol is available from the corresponding author.15 All participants provided written informed consent before any study procedures. The study was done in accordance with the Declaration of Helsinki.
Randomisation and masking
Participants were randomly assigned (1:1) either abatacept or matching placebo. Randomisation was stratified by duration of diffuse cutaneous systemic sclerosis (≤18 months vs>18 to ≤36 months). The Data Coordinating Center (DCC) at the University of Michigan prepared the randomisation schedule, using computer-generated block randomisation (random block sizes of two and four [known only by the DCC]). Study staff (including research pharmacists, outcomes assessors, and investigators analysing data) and participants were unaware of treatment assignments.
Procedures
During the double-blind phase, patients received either 125 mg subcutaneous abatacept weekly or matching placebo (both provided by Bristol-Myers Squibb, Manati, PR, USA). Escape therapy with non-biological immunomodulatory agents was permitted at month 6 for par ticipants with worsening diffuse cutaneous systemic scler osis. Partici pants in both the abatacept and placebo groups who completed 12 months in the double-blind phase of the trialtransitioned to open-label treatment with 125 mg subcutaneous abatacept weekly for up to 6 months.
Participants were assessed for adverse events, had a physical examination, and were analysed by mRSS at baseline and months 1, 3, 6, 9, and 12 during the double-blind phase, then at months 14, 16, and 18 during the open-label phase. Scores on the HAQ-DI (range 0–3), Scleroderma-HAQ (S-HAQ) visual analogue scale (VAS), Patient-Reported Outcomes Measurement Information System (PROMIS)-29,20 University of California at Los Angeles Scleroderma Clinical Trial Consortium (UCLA) gastrointestinal tract (GIT) 2.0,21 and patient and physician global assessments of overall disease by VAS (range 0–10; higher score denotes worse symptoms) were obtained at baseline and months 3, 6, 12 and 18. Pulmonary function tests were done at baseline and months 6, 12, and 18. Systemic sclerosis-specific autoantibodies were assessed by immunofluorescence for anti-centromere. Ten other antibodies were evaluated by protein and RNA immunoprecipitation.
Outcomes
The primary efficacy endpoint for the double-blind phase of the trial was change from baseline in mRSS at month 12, as previously reported.15 Exploratory efficacy endpoints for the open-label extension included changes from baseline to month 18 in mRSS, % predicted forced vital capacity (FVC), HAQ-DI, patient and physician global assessments, and ACR CRISS. We also included S-HAQ VAS, PROMIS-29, and UCLA GIT 2.0 questionnaires as exploratory outcomes. Finally, we calculated the percen tage of participants fulfilling a novel consensus-driven definition of low disease activity for patients with moderate-to-severe diffuse cutaneous systemic sclerosis who met all three of the following criteria: mRSS 10 or lower, HAQ-DI 0·75 or lower, and patient global assessment 3 or lower (on a scale of 0–10).22 We also assessed the proportion of participants who achieved minimal clinically important differences in mRSS (decrease of ≥5 units),23 % predicted FVC (increase >3%),24 HAQ-DI (decrease >0·14),25 and ACR CRISS (≥0·6).17 Safety was assessed by the number of participants with at least one adverse event, adverse event leading to withdrawal, or serious adverse event. The number of serious adverse events was reported by system organ class.
Statistical analysis
Descriptive statistics for safety and exploratory efficacy endpoints by original randomised treatment group are provided separately for the double-blind and open-label treatment phases. This approach, which includes all measures without censoring for escape therapy (the principle approach in the primary double-blind analyses), allows for more interpretable conclusions about the effect of continued abatacept in participants allocated abatacept and the early abatacept experience in participants allocated placebo. All randomised participants who received at least one dose of double-blind or open-label abatacept (modified intention-to-treat population) were included in double-blind and open-label analyses, respectively. Summary statistics (eg, mean [SD]) of observed data (ie, with no imputation for missing data) were calculated to allow a simple consistent approach to investigate treatment-specific estimates between the double-blind and open-label extension phases. Estimates using this complete case-analysis approach are valid under a strict missing data assumption (missing completely at random), which does not reflect that dropouts could be attributable to scant tolerability or efficacy. SAS version 9.4 was used for all statistical analyses.
This trial is registered with ClinicalTrials.gov, NCT02161406.
Role of the funding source
The industry funder (Bristol-Myers Squibb) provided study medication and matching placebo but had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The academic funder (National Institutes of Health) funded the biomarker analysis and had no role in the study. Data were stored at the University of Michigan. The manuscript was reviewed by the industry funder before final submission, but publication of this Article was not contin gent on approval by the funder. The corres ponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
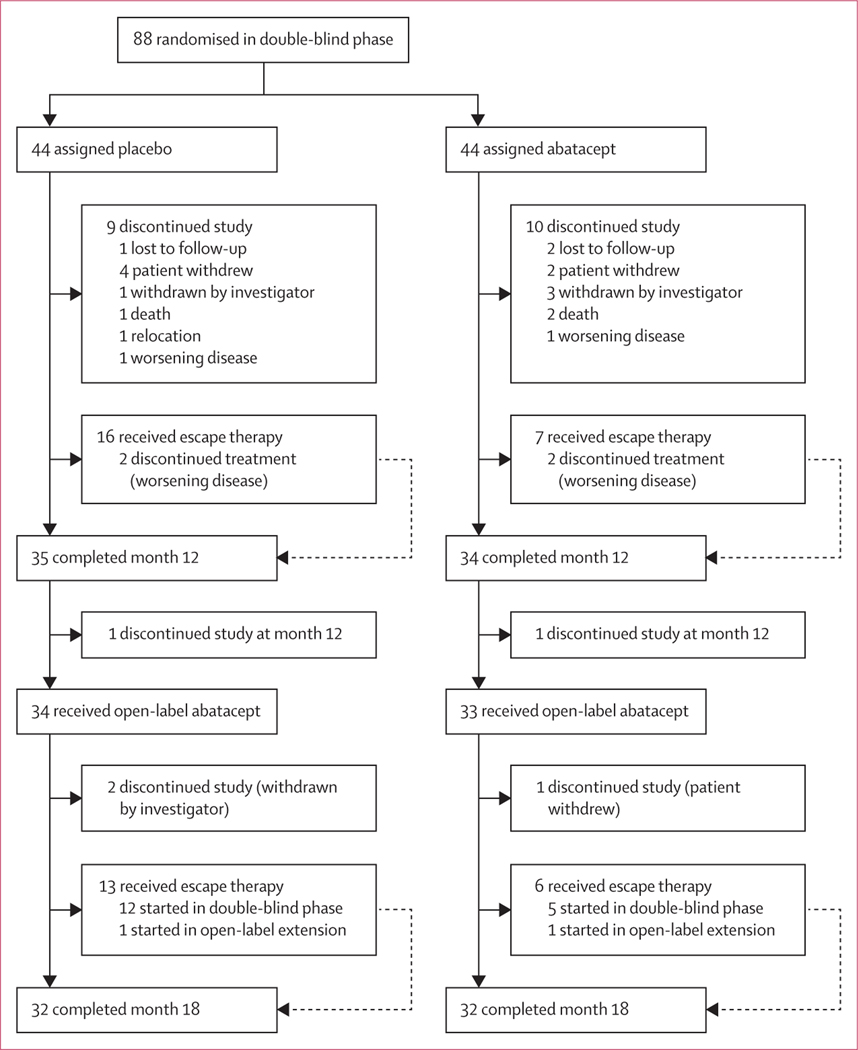
Between Sept 22, 2014, and March 15, 2017, 88 participants were randomly allocated either placebo (n=44) or abatacept (n=44) during the double-blind phase of the trial. 34 (77%) participants assigned placebo and 33 (75%) assigned abatacept completed the 12-month study period and transitioned to open-label treatment for another 6 months. 32 participants in each group (placebo–abatacept and abatacept–abatacept) completed the month 18 assessments (figure 1). During the open-label extension, three participants discontinued the study (two originally assigned placebo and one originally assigned abatacept). Escape therapy was received by 13 participants originally assigned placebo (12 who started escape therapy during the double-blind phase and one who started during the open-label extension) and six participants originally assigned abatacept (five who started escape therapy during the double-blind phase and one who started during the open-label extension; appendix p 4). Baseline characteristics were similar between participants who were randomly assigned in the double-blind phase and those who transitioned to open-label treatment (table 1).
Figure 1:
Disposition of study participants
Table 1:
Baseline demographics and disease characteristics
| Double-blind phase |
Open-label extension |
|||
|---|---|---|---|---|
| Placebo (n=44) | Abatacept (n=44) | Placebo–abatacept (n=34) | Abatacept– abatacept (n=33) | |
| Age, years | 51 (47–58) | 52 (42–56) | 53 (47–59) | 50 (42–56) |
| Sex | ||||
| Female | 35 (80%) | 31 (70%) | 27 (79%) | 27 (82%) |
| Male | 9 (20%) | 13 (30%) | 7 (21%) | 6 (18%) |
| Ethnic origin | ||||
| White | 37 (84%) | 35 (80%) | 30 (88%) | 27 (82%) |
| Not Hispanic or Latino | 36 (82%) | 40 (91%) | 29 (85%) | 32 (97%) |
| Disease duration, years* | 1·3 (1·0–2·2) | 1·5 (0·9–2·5) | 1·3 (1·0–2·1) | 1·7 (0·9–2·4) |
| Disease duration ≤18 months | 27 (61%) | 26 (59%) | 22 (65%) | 18 (55%) |
| Modified Rodnan Skin Score | 21·6 (7·3) | 23·3 (7·9) | 21·1 (6·6) | 23·4 (8·5) |
| Forced vital capacity, % predicted | 86·5% (16·6) | 84·2% (13·5) | 88·5% (16·5) | 85·9% (11·3) |
| Diffusion capacity of carbon monoxide, % predicted† | 76·4% (18·4) | 79·6% (18·1) | 78·7% (19·2)‡ | 84·3% (16·0) |
| Patient global assessment§ | 4·3 (2·6)¶ | 3·9 (2·2)‡ | 4·1 (2·6) | 3·4 (2·0) |
| Health Assessment Questionnaire disability index|| | 1·0 (0·7) | 1·1 (0·7) | 0·9 (0·7) | 1·0 (0·7) |
| Physician global assessment§ | 4·8 (1·7)¶ | 4·8 (1·7) | 4·7 (1·7)¶ | 4·6 (1·7) |
| Tender joint count | 5·4 (7·1) | 3·6 (5·7) | 4·1 (6·1) | 4·2 (6·4) |
| Any tender joint | 28 (64%) | 21 (48%) | 19 (56%) | 15 (45%) |
| Swollen joint count | 3·9 (5·8) | 3·6 (5·6) | 3·9 (6·5) | 3·0 (4·9) |
| Any swollen joint | 21 (48%) | 21 (48%) | 13 (38%) | 14 (42%) |
| Large joint contractures | 32 (74%)‡ | 31 (70%) | 26 (76%) | 24 (73%) |
| Friction rub | 13 (30%) | 19 (43%) | 12 (35%) | 14 (42%) |
| Erythrocyte sedimentation rate, mm/h |
17·6 (15·8) | 17·9 (15·2)‡ | 17·2 (16·5) | 15·1 (11·2)‡ |
| High-sensitivity C-reactive protein, mg/dL | 1·0 (1·4) | 1·1 (1·2) | 0·9 (1·5) | 0·9 (0·9) |
| Anti-centromere positive | 1 (2%)¶ | 3 (7%)‡ | 1 (3%)¶ | 2 (6%)‡ |
| Anti-RNA polymerase 3 positive | 17 (40%)¶ | 22 (51%)‡ | 13 (41%)¶ | 19 (59%)‡ |
| Anti-topoisomerase positive | 7 (17%)¶ | 9 (21%)‡ | 4 (13%)¶ | 6 (19%)‡ |
| Use of prednisone | 5 (11%) | 7 (16%) | 2 (6%) | 7 (21%) |
Data are median (IQR), mean (SD), or n (%).
Disease onset was defined as first non-Raynaud’s sign or symptoms.
Corrected for haemoglobin.
Data missing for one patient.
Theoretical range 0–10.
Data missing for two patients.
Theoretical range 0–3.
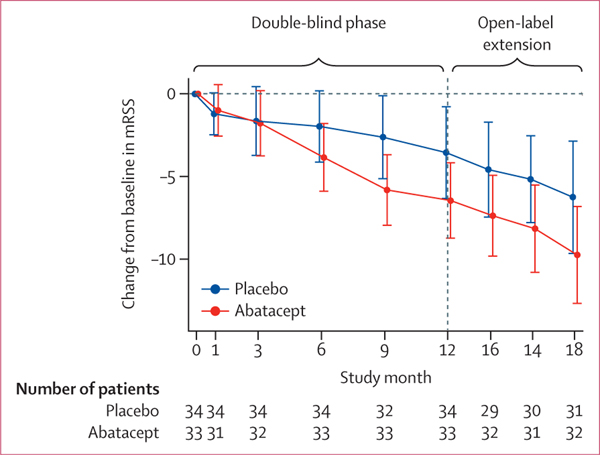
Among patients assigned abatacept, a mean improvement from baseline in mRSS was noted at 12 months (−6·6 [SD 6·4]), with further improvement seen during the open-label extension (−9·8 [8·1] at month 18; figure 2, table 2). Participants assigned placebo had a mean improvement from baseline in mRSS at 12 months (−3·7 [SD 7·6]), with a further improvement at month 18 (−6·3 [9·3]). The proportion of participants who had a 5-unit or greater improvement in mRSS at month 12 was 68% (23 of 34) among those assigned abatacept and 50% (19 of 38) among those assigned placebo. After 6 months of open-label treatment, this proportion had risen to 72% (23 of 32) in the abatacept–abatacept group and 65% (20 of 31) in the placebo–abatacept group (appendix p 3).
Figure 2: Change in mRSS from baseline to month 18 in the modified intention-to-treat population.
Data are mean (95% CI). mRSS=modified Rodnan Skin Score.
Table 2:
Change from baseline to month 12 (double-blind phase) or month 18 (including open-label extension) in modified Rodnan Skin Score and exploratory endpoints (intention-to-treat population; observed data)
| Double-blind phase, month 12 |
Open-label extension, month 18 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n=44) | Abatacept (n=44) | Placebo–abatacept (n=34) | Abatacept–abatacept (n=33) | |||||
|
|
|
|||||||
| n | Change from baseline | n | Change from baseline | n | Change from baseline | n | Change from baseline | |
| Modified Rodnan Skin Score | 38 | −3·7 (7·6) | 34 | −6·6 (6·4) | 31 | −6·3 (9·3) | 32 | −9·8 (8·1) |
| Health Assessment Questionnaire disability index* | 36 | 0·09 (0·43) | 33 | −0·09 (0·46) | 28 | 0·04 (0·47) | 32 | −0·13 (0·43) |
| Scleroderma Health Assessment Questionnaire, visual analogue scale | ||||||||
| Overall* | 35 | 13·5 (33·8) | 34 | −8·6 (34·1) | 28 | −6·2 (37·0) | 31 | −18·2 (36·7) |
| Breathing* | 35 | 16·1 (32·0) | 34 | −0·6 (35·2) | 28 | 8·8 (32·0) | 31 | 3·5 (38·5) |
| Raynaud’s* | 35 | −2·4 (36·8) | 34 | 7·2 (34·2) | 28 | 4·0 (42·2) | 31 | −9·3 (37·7) |
| Digital ulcers* | 35 | 9·3 (42·8) | 34 | −2·7 (27·0) | 28 | 3·7 (37·0) | 31 | −0·1 (22·7) |
| Gastrointestinal* | 36 | 5·2 (37·3) | 34 | 8·4 (36·8) | 28 | 14·4 (30·3) | 32 | 15·2 (46·7) |
| Physician global assessment, visual analogue scale* | 35 | −0·3 (1·9) | 34 | −1·4 (1·5) | 27 | −1·0 (2·0) | 32 | −1·3 (2·1) |
| Patient global assessment, visual analogue scale* | 35 | −0·4 (3·3) | 33 | 0·0 (2·2) | 29 | −0·6 (3·3) | 31 | −0·4 (2·1) |
| Forced vital capacity, % predicted† | 37 | −2·7% (5·5) | 32 | −1·6% (8·0) | 29 | −0·3% (6·3) | 30 | 0·9% (9·9) |
| Forced vital capacity, mL | 37 | −113·5 (202·9) | 32 | −74·4 (308·7) | 29 | −38·6 (243·9) | 30 | 1·7 (389·7) |
| Diffusion capacity of carbon monoxide, % predicted†‡ | 33 | −2·1% (10·8) | 30 | 0·7% (12·5) | 27 | −2·4% (11·7) | 29 | 0·9% (11·9) |
| Tender joint count* | 38 | −0·9 (6·5) | 34 | −1·1 (8·2 | 31 | −1·6 (3·9) | 32 | −2·5 (6·4) |
| Swollen joint count* | 38 | −1·5 (4·1) | 34 | −0·7 (3·9) | 31 | −1·5 (4·4) | 32 | −1·7 (4·2) |
| PROMIS-29 | ||||||||
| Physical function† | 35 | 0·1 (4·0) | 34 | −1·8 (3·6) | 29 | −1·3 (4·7) | 30 | −1·6 (4·7) |
| Anxiety* | 35 | −1·3 (10·1) | 34 | −4·0 (9·1) | 29 | −5·3 (9·0) | 30 | −1·5 (8·7) |
| Depression* | 35 | −0·8 (7·9) | 34 | 0·6 (7·5) | 29 | −2·9 (7·2) | 30 | 1·4 (5·9) |
| Fatigue* | 36 | −1·4 (6·7) | 34 | −1·1 (7·7) | 29 | −2·6 (7·6) | 31 | −2·5 (8·2) |
| Sleep disturbance* | 36 | −0·3 (3·6) | 34 | 0·0 (2·9) | 29 | −0·5 (4·3) | 31 | −0·4 (3·9) |
| Pain interference* | 36 | −0·8 (7·6) | 34 | −4·1 (8·4) | 28 | −1·3 (8·0) | 31 | −2·9 (6·9) |
| Social roles† | 36 | −1·5 (5·0) | 34 | −0·8 (7·1) | 28 | 0·5 (8·1) | 31 | 0·0 (8·4) |
| Pain intensity* | 36 | −0·2 (1·7) | 34 | −0·7 (2·5) | 28 | −0·7 (1·9) | 31 | −1·2 (1·9) |
| UCLA GIT 2.0, total score* | 36 | 0·0 (0·3) | 34 | 0·1 (0·3) | 28 | 0·2 (0·4) | 30 | 0·1 (0·3) |
| ACR CRISS† | 30 | 0·03 (0·00–0·93) | 29 | 0·81 (0·00–1·00) | 26 | 0·5 (0·01–0·99) | 29 | 0·99 (0·06–1·00) |
Data are mean (SD) or median (IQR). n=patients with available data for measurement. PROMIS-29=Patient-Reported Outcomes Measurement Information System-29. UCLA GIT 2.0=University of California at Los Angeles Scleroderma Clinical Trial Consortium gastrointestinal tract 2.0. ACR CRISS=American College of Rheumatology Combined Response Index in diffuse cutaneous Systemic Sclerosis.
A negative value indicates improvement.
A positive value indicates improvement.
Corrected for haemoglobin.
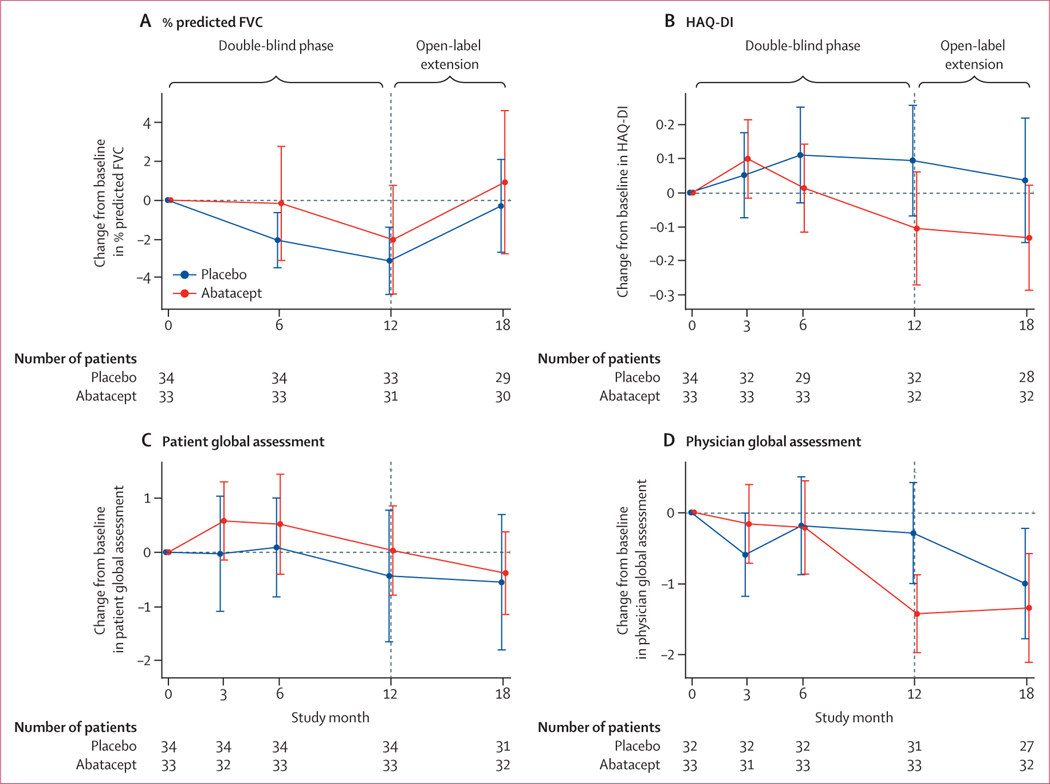
% predicted FVC declined in both groups during the double-blind trial; at 12 months, the mean change from baseline was −2·7% (SD 5·5) among those assigned placebo and −1·6% (8·0) among those assigned abatacept owever, during the open-label extension both treatment groups saw improvements in % predicted FVC such that the mean change from baseline to month 18 was small (−0·3% [SD 6·3] in the placebo–abatacept group and 0·9% [9·9] in the abatacept–abatacept group; figure 3A, table 2). A minimal clinically important difference for % predicted FVC was attained at month 12 by 19% (six of 32) of patients assigned abatacept and by 16% (six of 37) of patients assigned placebo, which increased to 30% (nine of 30) of the placebo–abatacept group and 28% (eight of 29) of the abatacept–abatacept group at month 18 (appendix p 3).
Figure 3: Change in exploratory endpoints from baseline to month 18 in the modified intention-to-treat population.
Data are mean (95% CI).
Participants assigned abatacept during the double-blind phase had an improvement in disability score at month 12 (mean HAQ-DI −0·09 [SD 0·46]), whereas the disability score in those assigned placebo worsened (0·09 [0·43]). Disability scores did not change by much in both groups during the open-label extension, with mean HAQ-DI of −0·13 (SD 0·43) in the abatacept–abatacept group and 0·04 (0·47) in the placebo–abatacept group (figure 3B, table 2). The proportion of participants who achieved the minimal clinically important difference for HAQ-DI at month 12 was 79% (26 of 33) of those assigned abatacept and 67% (24 of 36) of those assigned placebo; these proportions did not change by much in both groups during the open-label extension (appendix p 3).
Patient global assessment VAS scores for participants assigned abatacept initially worsened and then returned to the baseline score during the double-blind phase (figure 3C), with a mean score at month 12 of 0·0 (SD 2·2) in those assigned abatacept versus −0·4 (3·3) in those assigned placebo. Improvements in scores continued during the open-label extension (mean score −0·4 [SD 2·1] in the abatacept–abatacept group and −0·6 [3·3] in the placebo–abatacept group; table 2). By contrast, physician global assessment VAS scores improved during the double-blind phase among patients assigned abatacept (mean score −1·4 [SD 1·5]) compared with those assigned placebo (−0·3 [1·9]; table 2). During the open-label extension, physi cian global assessment VAS scores were maintained in the abatacept–abatacept group (mean score −1·3 [SD 2·1]) and improved in the placebo– abatacept group (−1·0 [2·0]; figure 3D).
The median ACR CRISS score was significantly greater at month 12 in patients assigned abatacept compared with those assigned placebo; both groups showed improvements in ACR CRISS scores during the open-label treatment period (table 2). An ACR CRISS score of 0·60 or higher was achieved at month 12 in 55% (18 of 33) of participants assigned abatacept compared with 36% (13 of 36) of those assigned placebo; in the open-label extension, 66% (19 of 29) of patients in the abatacept–abatacept group versus 50% (13 of 26) of those in the placebo–abatacept group achieved an ACR CRISS score of 0·60 or higher (appendix p 3). Physical function and anxiety, as measured by PROMIS-29 questionnaires, also improved more in the abatacept group at month 12, followed by improvements in both groups in the open- label extension (table 2). Low disease activity criteria were fulfilled at month 12 in five (15%) of 34 patients assigned abatacept and in six (16%) of 38 patients assigned placebo, increasing at month 18 to ten (31%) of 32 patients in the abatacept–abatacept group compared with four (13%) of 31 patients in the placebo–abatacept group (appendix p 3).
Abatacept was well tolerated throughout the study, with no new safety signals noted during the open-label extension. In general, during the double-blind phase, adverse events, infectious adverse events, adverse events leading to study withdrawal, and serious adverse events were more frequent among patients assigned placebo than among those assigned abatacept; in the open-label extension, these adverse events occurred in fewer participants in both groups than in the double-blind phase (table 3). During the open-label extension, treatment-emergent adverse events that led to study drug discontinuation included a serious adverse event of ventricular fibrillation with cardiac arrest in one partici pant in the placebo–abatacept group and two adverse events of tachy cardia (in one participant) and an upper respiratory infection and night sweats (in one participant) in the abatacept–abatacept group. Infections occurred during the open-label extension in nine patients (12 events) in the placebo–abatacept group and 11 patients (14 events) in the abatacept–abatacept group, and one event in each group was considered serious: one participant in the placebo–abatacept group had an infected Bartholin’s cyst and one participant in the abatacept–abatacept group had cellulitis (table 3). Other serious adverse events included one participant in the placebo–abatacept group who had ventricular fibrillation with cardiac arrest (which led to study discontinuation) and participants in the abatacept–abatacept group with gastric antral vascular ectasia related to underlying systemic sclerosis (n=1), pan creatitis (1), and pregnancy (1). Regarding adverse events of special interest during the open-label extension, seven participants had a decrease in haemo globin greater than 2 g/dL, five in the placebo–abatacept group (two of whom had a drop that resulted in haemoglobin <8 g/dL) and two in the abatacept–abatacept group. Two deaths occurred during the 12-month double-blind phase in the abatacept group, which were related to scleroderma renal crisis; no deaths were reported during the openlabel extension.
Table 3:
Adverse events (safety population)
| Double-blind phase |
Open-label extension |
|||
|---|---|---|---|---|
| Placebo (n=44) | Abatacept (n=44) | Placebo–abatacept (n=34) | Abatacept–abatacept (n=33) | |
| One or more adverse event | 40 (91%) | 35 (80%) | 23 (68%) | 25 (76%) |
| One or more infectious adverse event | 25 (57%) | 19 (43%) | 9 (26%) | 11 (33%) |
| Adverse events leading to withdrawal | 6 (14%) | 5 (11%) | 1 (3%) | 2 (6%) |
| One or more serious adverse event | 12 (27%) | 9 (20%) | 2 (6%) | 4 (12%) |
| Infections and infestations (serious adverse events) | 2/2 | 2/2 | 1/1 | 1/1 |
| Cellulitis | 0 | 1 | 0 | 1 |
| Mastoiditis | 0 | 1 | 0 | 0 |
| Paronychia | 1 | 0 | 0 | 0 |
| Pneumonia | 1 | 0 | 0 | 0 |
| Infected Bartholin’s cyst | 0 | 0 | 1 | 0 |
| Cardiac disorders (serious adverse events) | 6/3 | 2/2 | 1/1 | 0 |
| Atrial flutter with conduction defects | 1 | 0 | 0 | 0 |
| Cardiac arrest | 1 | 0 | 0 | 0 |
| Congestive heart failure | 1 | 0 | 0 | 0 |
| Myocardial infarction or acute coronary syndrome | 1 | 1 | 0 | 0 |
| Pulmonary arterial hypertension | 1 | 1 | 0 | 0 |
| Pericardial effusion | 0 | 1 | 0 | 0 |
| Worsening atrioventricular block | 1 | 0 | 0 | 0 |
| Ventricular fibrillation cardiac arrest | 0 | 0 | 1 | 0 |
| Gastrointestinal disorders (serious adverse events) | 6/6 | 3/2 | 0 | 2/2 |
| Anaemia | 1 | 0 | 0 | 0 |
| Cholecystitis | 1 | 0 | 0 | 0 |
| Dysphagia | 1 | 1 | 0 | 0 |
| Erosive oesophagitis | 1 | 0 | 0 | 0 |
| Gastric antral vascular ectasia | 1 | 0 | 0 | 1 |
| Gastric antral vascular ectasia with anaemia | 1 | 0 | 0 | 0 |
| Melaena | 0 | 1 | 0 | 0 |
| Pseudo-obstruction | 0 | 1 | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 1 |
| Gynaecological events (serious adverse events) | 0 | 0 | 0 | 1/1 |
| Pregnancy | 0 | 0 | 0 | 1 |
| Malignant disorders (serious adverse events) | 1/1 | 1/1 | 0 | 0 |
| Basal-cell skin carcinoma | 1 | 0 | 0 | 0 |
| Squamous-cell skin carcinoma | 0 | 1 | 0 | 0 |
| Respiratory disorders (serious adverse events) | 0 | 1/1 | 0 | 0 |
| Respiratory failure | 0 | 1 | 0 | 0 |
| Renal disorders (serious adverse events) | 1/1 | 3/3 | 0 | 0 |
| Scleroderma renal crisis | 1 | 3 | 0 | 0 |
| Vascular disorders (serious adverse events) | 1/1 | 0 | 0 | 0 |
| Digital ischaemia | 1 | 0 | 0 | 0 |
| Mental disorders (serious adverse events) | 1/1 | 0 | 0 | 0 |
| Depression with suicidal ideation | 1 | 0 | 0 | 0 |
Data are either number of adverse events (%), number of serious adverse events by system organ class/number of patients with at least one serious adverse event in that class, or number of serious adverse events for preferred terms within a system organ class. Percentages of adverse events are reported separately for the 12-month double-blind phase and the 6-month open-label extension.
Discussion
The findings of a 6-month open-label extension of the ASSET phase 2 randomised trial showed that abatacept is safe in participants with early diffuse cutaneous systemic sclerosis for up to 18 months and suggest preliminary efficacy for various outcome measures. Infectious adverse events were less frequent in participants initially treated with abatacept than in those treated with placebo during the double-blind phase of the trial. Moreover, lower rather than higher proportions of participants had infectious complications in both groups on transitioning to open-label treatment. Although the primary endpoint of change in mRSS from baseline to month 12 was not statistically different with abatacept compared with placebo, exploratory analyses suggested potential disease-modifying effects of abatacept in participants with systemic sclerosis.
The treatment of early diffuse cutaneous systemic sclerosis, including skin involvement, remains a challenge. Current options include mycophenolate mofetil, methotrexate, and cyclophosphamide.6 These three drugs stabilised or showed some improvement in systemic sclerosis in well controlled trials.26,27 Improvements in skin involvement in those studies were modest and, similar to other immunosuppressive and immunomodulatory treatments, all three drugs can be complicated by serious or severe toxic effects, including cytopenias, infections, and gastrointestinal events. Haematopoietic stem-cell transplantation also leads to improvements in skin fibrosis and prevention of pulmonary deterioration or improvement, but this procedure has high associated risks and costs and requires specialty multidisciplinary management.3,4
Similar to findings of other clinical trials, at 12 months in the ASSET trial, mRSS showed numerical improve ment in patients assigned abatacept compared with those assigned placebo, but with substantial individual hetero geneity. This improvement occurred despite enrichment strategies (such as short disease duration and requirement for worsening skin disease) that were included in the trial design. In the double-blind phase of the trial, skin gene expression signatures affected change in outcome measures over 12 months,15 highlighting molecular heterogeneity in early disease. Data for explora tory endpoints in the double-blind and open-label extension phases of the ASSET trial provide confidence for a potential disease-modifying effect of abatacept in participants with early diffuse cutaneous systemic sclerosis. For example, patients in both groups showed numerical improvements in % predicted FVC during the open-label extension. % predicted FVC is an objective outcome measure and is now considered by many clinicians to be a surrogate measure for systemic sclerosis-associated interstitial lung disease. Furthermore, the ACR CRISS score improved more at month 12 in patients assigned abatacept than in those assigned placebo, with continued improvements in both groups at month 18. This finding should be interpreted with caution since ACR CRISS was developed using patient-level data at 12 months. Ongoing incorporation of ACR CRISS at different timepoints will provide further validity of this outcome measure. HAQ-DI and patient and physician global assessment scores also improved in both groups during open-label treatment with abatacept. Additionally, we calculated the proportion of participants achieving a minimal clinically important difference and investigated the idea of low disease activity in this cohort with early diffuse cutaneous systemic sclerosis. The percentage of participants who had low disease activity at month 12 was similar between groups, but at month 18 it was more than twice as high in the abatacept–abatacept group compared with the placebo–abatacept group after open-label treatment. This finding might indicate that a longer duration of treatment is necessary to meet the threshold for low disease activity in diffuse cutaneous systemic sclerosis, and this should be validated in other studies.
Abatacept was well tolerated in participants with early diffuse cutaneous systemic sclerosis with a safety profile that was better than placebo during the double-blind phase of the study. In particular, abatacept does not seem to increase the risk for infectious complications. The two deaths that occurred in the double-blind period in the abatacept group were related to scleroderma renal crisis, a severe complication that can affect up to 25% of participants with early diffuse cutaneous systemic sclerosis;28 no participants died during open-label treatment with abatacept.
Many novel agents are currently being assessed for treatment of skin tightening in participants with early diffuse cutaneous systemic sclerosis.24,29,30 Our results are similar to those from other phase 2 clinical trials, such that clinically important but statistically insignificant improvements in mRSS were noted during the doubleblind trial, followed by further improvements in the open-label extension.31,32 In a study of tocilizumab versus placebo,32 the overall improvement in mRSS from baseline to the end of the open-label extension at week 96 was mean −9·4 (SD 5·6 [95% CI −8·9 to −2·4]) for the placebo–tocilizumab group and −9·1 (8·7 [−12·5 to −5·6]) for the tocilizumab–tocilizumab group, whereas in a study of riociguat versus placebo,33 the improvement in mRSS from baseline to the end of the open-label extension at week 52 was mean −3·96 (SD 5·43) in the placebo–riociguat group and −3·02 (5·51) in the riociguat–riociugat group.34 Our results (mean −6·3 [SD 9·3] in the placebo–abatacept group and −9·8 [8·1] in the abatacept–abatacept group from baseline to month 18) were more closely aligned with those of the tocilizumab versus placebo study32 because the participant populations were more similar. However, all these trials showed improvement in mRSS during an open-label extension, which could be a result of the natural history of early diffuse cutaneous systemic sclerosis. 50% (32 of 64) of participants in our trial were positive for anti-RNA polymerase 3, which might have played a part in this improvement. Likewise, stabilisation in % predicted FVC was seen when comparing baseline values with those at the end of the open-label extension period in both the tocilizumab versus placebo trial32 and in our trial. However, infectious adverse events were numerically more common in patients treated with tocilizumab than placebo in that study,32 whereas they were numerically less common in patients treated with abatacept compared with placebo in our trial, which could reflect enhanced trial design. In fact, in the phase 3 trial of tocilizumab,35 the number of infectious adverse events was numerically smaller with tocilizumab compared with placebo. Other biological agents (eg, belimumab,36 fresolimumab,37 and ritux imab)38 have been assessed for the treatment of early diffuse cutaneous systemic sclerosis in small single-centre studies, but larger studies are necessary for more definitive results.
The 6-month extension to our study had some limitations, particularly with respect to its open-label uncontrolled nature. First, it is possible that survivor bias affected our study results, since participants who completed 12 months of double-blind treatment and entered the open-label extension were probably more responsive to treatment or had less severe disease (ie, only those doing well continue from the end of the double-blind phase through to the end of the open-label extension). Second, we used an alternate approach in tabular summaries to maximise the sample size for estimates and because the differences in sample size (figure 1) between the double-blind and open-label extension populations, and in missing outcome data between the 12-month timepoint and the 18-month timepoint, were very small. Moreover, our study was not powered for formal statistical comparison of the two treatment arms, and all results from the open-label period must be considered exploratory. Third, missing data were unavoidable, despite rigorous monitoring during the clinical trial. Finally, the number of participants was small, and caution should be exercised before inferring too much from the low rates (eg, of infections) during the open-label extension.
Our study has several strengths. The first is sole participation of centres with substantial clinical trial experience in systemic sclerosis. Second, the discontin uation rate during the open-label extension was low, with only three (4%) of 67 participants discontinuing the study. Third, we obtained further information on sensitivity to change in ACR CRISS over an 18-month period, which is especially useful because this outcome measure is now being used as the primary endpoint for several clinical trials in early diffuse cutaneous systemic sclerosis. Finally, we provide information about use of a novel low disease activity definition in clinical trials of diffuse cutaneous systemic sclerosis.
In summary, the results of this 6-month open-label extension support those of the 12-month double-blind phase, in that abatacept seems to be safe in participants with early diffuse cutaneous systemic sclerosis. Exploratory outcome measures during the open-label extension, including the composite ACR CRISS score, indicate that abatacept might promote overall global improvement in these participants. A phase 3 clinical trial is necessary to definitively assess the safety and efficacy of abatacept in this participant population.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed with the terms “systemic sclerosis” OR“scleroderma” and a combination of “systemic sclerosis” AND“ CTLA4”, “abatacept”, “modified Rodnan skin score”, “clinicaltrials”, or “interstitial lung disease”. Activated T cells areimplicated in the pathogenesis of early systemic sclerosis,particularly with respect to cutaneous disease. Animal models that mimic the early inflammatory skin changes seen in systemic sclerosis show that abatacept can prevent and induce the regression of dermal fibrosis. In addition to decreasing T-cell activation, abatacept might mediate its antifibrotic effects by preventing the differentiation of circulating fibrocytes into myofibroblasts or fibroblasts. A pilot trial and recent analysis from an observational cohort showed beneficial effects of abatacept on skin, joints, and disability.
Added value of this study
We did a 6-month open-label extension of a 12-month, phase2, randomised double-blind trial in patients with early diffuse cutaneous systemic sclerosis treated with either abatacept or placebo. A clinically significant (albeit not statistically significant) improvement of skin sclerosis, and a clinically relevant improvement in disability and a new composite index, were seen with abatacept over the 18-month period. The safety profile was consistent with complications of systemic sclerosis and with the safety profile of abatacept.
Implications of all the available evidence
In view of the paucity of disease-modifying treatment options for patients with early systemic sclerosis, combined with the high morbidity and mortality associated with this disease, data from our trial provide hope for a potential future treatment. These data should be further investigated in a phase 3 randomised trial before definitive conclusions can be made about the risks and benefits of abatacept.
Acknowledgments
This investigator-initiated clinical trial was funded by Bristol-Myers Squibb and the National Institutes of Health (National Institute of Allergy and Infectious Diseases Clinical and Autoimmunity Center of Excellence, grant 5-UM1-AI-110557 to the University of Michigan; and National Institute of Arthritis and Musculoskeletal and Skin Diseases, grants K24-AR-063120 and R01-AR-07047 to DK). We thank the patients who participated in this trial and the research coordinators for their hard work and contributions.
Declaration of interests
LC is on the advisory board and steering committee for Bristol-Myers Squibb, during the conduct of the study; the advisory board for Eicos and Mitsubishi Tanabe, outside of the submitted work; the data safety monitoring board for Reata, outside of the submitted work; and reports grants from and is on the advisory board for Boehringer Ingelheim, outside of the submitted work. CS reports statistical consultant fees from Eicos, outside of the submitted work. SRJ reports clinical trial support from Bayer, Corbus, GlaxoSmithKline, Bristol-Myers Squibb, and Ikaria, outside of the submitted work; and clinical trial support from and is on the advisory board for Boehringer Ingelheim, outside of the submitted work. CPD reports grants and personal fees from GlaxoSmithKline, Bristol-Myers Squibb, Inventiva, and CSL Behring, during the conduct of the study; and personal fees from Roche, Horizon, Sanofi, Boehringer Ingelheim, and Galapagos, during the conduct of the study. JAM reports clinical trial support from Bristol-Myers Squibb, during the conduct of the study; personal fees from Eicos, outside of the submitted work; personal fees, consulting fees, and clinical trial support from Boehringer Ingelheim, outside of the submitted work; and clinical trial support from Corbus and Kadmon, outside of the submitted work. VDS reports grants, is a site principal investigator for a trial in scleroderma, and is on the advisory board for Corbus and Bayer, during the conduct of the study; grants and is on a steering committee for a trial in scleroderma for CSL Behring, during the conduct of the study; is on the advisory board for Forbius, during the conduct of the study; reports grants and consultancy, advisory board, and site principal investigator for a trial in scleroderma and lung disease forBoehringer Ingelheim, outside of the submitted work; is site principal investigator for a scleroderma and Raynaud’s trial and is on a steering committee for Eisocs, outside of the submitted work; and reports grants from Reata, outside of the submitted work. RL reports grants from Bristol-Myers Squibb, during the conduct of the study; personal fees from Bristol-Myers Squibb, Formation, Sanofi, Biocon, Boehringer-Mannheim, Merck, and Genentech/Roche, outside of the submitted work; and grants from Corbus, Formation, Elpidera, Regeneron, Pfizer, and Kiniksa, outside of the submitted work. RWS reports grants from Bristol-Myers Squibb, GlaxoSmithKline, Corbus, Boehringer Ingelheim, EMD Serono, Actelion, Roche, and Sanofi, during the conduct of the study. SK reports clinical trial support from the University of California at Los Angeles Division of Rheumatology, during the conduct of the study. RTD reports personal fees from Formation, Eicos, and Corbus, outside of the submitted work. JEP reports grants and honoraria for consulting from Bristol-Myers Squibb and Boehringer Ingelheim, during the conduct of the study; and grants from Merck, during the conduct of the study. JKG reports grants from the University of Michigan, during the conduct of the study; and grants from Eicos and Cumberland Pharmaceuticals, outside of the submitted work. MDM reports personal fees from Medtelligence, Actelion Pharma, Astellas, and Mitsubishi-Tanabe, outside of the submitted work; grants from Bayer, Reata, Sanofi, Corbus, and GlaxoSmithKline, outside of the submitted work; and grants and personal fees from Boehringer Ingelheim, Eicos, and Galapagos Pharma, outside of the submitted work. EJB reports grants from the National Institutes of Health (NIH), outside of the submitted work; grants and personal fees from Boehringer Ingelheim and Eicos, outside of the submitted work; and grants from Pfizer, outside of the submitted work. YA reports grants and personal fees from Inventiva and Sanofi, during the conduct of the study; and personal fees from Bayer, Bristol-Myers Squibb, Chemomab, Curzion, and Roche, during the conduct of the study. MM-C has received consulting fees or honorarium from Inventiva, Bayer, Boehringer, CSL Behring, Corbus, Galapagos, Mitsubishi, Samsung, Regeneron, Acceleron, MSD, Chemomab, Lilly, Pfizer, and Roche, outside of the submitted work. MLW reports grants from NIH, during the conduct of the study; grants and personal fees from Bristol-Myers Squibb, during the conduct of the study; grants and personal fees from Celdara Medical, outside of the submitted work; personal fees from Acceleron, AbbVie, and Boehringer Ingelheim, outside of the submitted work; and grants and personal fees from Corbus, outside of the submitted work. OD reports personal fees from AbbVie, Actelion, Acceleron Pharma, Amgen, AnaMar, Baecon Discovery, and Blade Therapeutics, outside of the submitted work; personal fees from Catenion, Competitive Drug Development, CSL Behring, ChemomAb, Curzion Pharmaceuticals, Ergonex, Galapagos, Glenmark Pharmaceuticals, GlaxoSmithKline, Inventiva, Italfarmaco, iQone, iQvia, Lilly, Medac, Medscape, MSD, Novartis, Pfizer, Roche, Sanofi, Target BioScience, and UCB, outside of the submitted work; grants and personal fees from Bayer, Boehringer Ingelheim, and Mitsubishi Tanabe, outside of the submitted work; and has a patent (mir-29 for the treatment of systemic sclerosis) issued to US8247389, EP2331143. VN reports clinical trial support from Bristol-Myers Squibb, during the conduct of the study. DAF reports grants from NIH (National Institute of Allergy and Infectious Diseases Clinical and Autoimmunity Center of Excellence), Immune Tolerance Network, Bristol-Myers Squibb, and Pfizer, outside of the submitted work; and personal fees from CSL Behring, outside of the submitted work. DEF reports grants from Bristol-Myers Squibb, during the conduct of the study; grants and personal fees from Acetlion, Amgen, Bristol-Myers Squibb, Corbus, Galapagos, GlaxoSmithKline, NIH, Novartis, and Pfizer, outside of the submitted work; grants from Sanofi and Genentech/Roche, outside of the submitted work; and fees for Continuing Medical Education (CME) presentations, outside of the submitted work. DK reports grants and personal fees from Bristol-Myers Squibb, during the conduct of the study; grants from NIH, Immune Tolerance Network, and Bayer, outside of the submitted work; grants from Bristol-Myers Squibb, Horizon, and Pfizer, outside of the submitted work; personal fees from Acceleron, Acetlion, Amgen, Blade Therapeutics, Boehringer Ingelheim, CSL Behring, Corbus, Cytori, Galapagos, Genentech/Roche, GlaxoSmithKline, Horizon, Merck, Mitsubishi Tanabe, Regeneron, Sanofi-Aventis, United Therapeutics, and Eicos, outside of the submitted work; CME support from Impact PH, outside of the submitted work; holds stock in Eicos; and declares personal fees from and is Chief Medical Officer for CiviBioPharma/Eicos, outside of the submitted work. All other authors declare no competing interests.
Footnotes
Data sharing
Deidentified data are available from the corresponding author. Interested researchers are encouraged to complete a 1-page proposal highlighting the objectives, planned analyses, and data elements needed for their proposed analysis. (The whole dictionary can be obtained from the corresponding author on request). The proposal will be reviewed by the steering committee and, if approved, data will be shared in the mutually agreed format. The study protocol is available from the corresponding author.
References
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390: 1685–99. [DOI] [PubMed] [Google Scholar]
- 2.Wu W, Jordan S, Graf N, et al. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann Rheum Dis 2019; 78: 648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan KM, Goldmuntz EA, Keyes-Elstein L, et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N Engl J Med 2018; 378: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Laar JM, Farge D, Sont JK, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 2014; 311: 2490–98. [DOI] [PubMed] [Google Scholar]
- 5.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–28. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez-Codina A, Walker KM, Pope JE, Scleroderma Algorithm Group. Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheumatol 2018; 70: 1820–28. [DOI] [PubMed] [Google Scholar]
- 7.Skaug B, Khanna D, Swindell WR, et al. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann Rheum Dis 2020; 79: 379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischmajer R, Perlish JS, Reeves JR. Cellular infiltrates in scleroderma skin. Arthritis Rheumatol 1977; 20: 975–84. [DOI] [PubMed] [Google Scholar]
- 9.Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017; 76: 1327–39. [DOI] [PubMed] [Google Scholar]
- 10.Roumm AD, Whiteside TL, Medsger TA Jr, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis; quantification, subtyping, and clinical correlations. Arthritis Rheumatol 1984; 27: 645–53. [DOI] [PubMed] [Google Scholar]
- 11.Ponsoye M, Frantz C, Ruzehaji N, et al. Treatment with abatacept prevents experimental dermal fibrosis and induces regression of established inflammation-driven fibrosis. Ann Rheum Dis 2016; 75: 2142–49. [DOI] [PubMed] [Google Scholar]
- 12.Cutolo M, Soldano S, Montagna P, et al. Effects of CTLA4-Ig treatment on circulating fibrocytes and skin fibroblasts from the same systemic sclerosis patients: an in vitro assay. Arthritis Res Ther 2018; 20: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakravarty EF, Martyanov V, Fiorentino D, et al. Gene expression changes reflect clinical response in a placebo-controlled randomized trial of abatacept in patients with diffuse cutaneous systemic sclerosis. Arthritis Res Ther 2015; 17: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elhai M, Meunier M, Matucci-Cerinic M, et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann Rheum Dis 2013; 72: 1217–20. [DOI] [PubMed] [Google Scholar]
- 15.Khanna D, Spino C, Johnson S, et al. Abatacept in early diffuse cutaneous systemic sclerosis: results of a phase II investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol 2020; 72: 125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medsger TA Jr, Benedek TG. History of skin thickness assessment and the Rodnan skin thickness scoring method in systemic sclerosis. J Scleroderma Relat Disord 2019; 4: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna D, Berrocal VJ, Giannini EH, et al. The American College of Rheumatology provisional composite response index for clinical trials in early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol 2016; 68: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Hoogen F, Khanna D, Fransen J, et al. 2013. classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol 2013; 65: 2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeRoy EC, Medsger TA Jr. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001; 28: 1573–76. [PubMed] [Google Scholar]
- 20.Kwakkenbos L, Thombs BD, Khanna D, et al. Performance of the Patient-Reported Outcomes Measurement Information System-29 in scleroderma: a Scleroderma Patient-centered Intervention Network Cohort Study. Rheumatology (Oxford) 2017; 56: 1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khanna D, Furst DE, Maranian P, et al. Minimally important differences of the UCLA Scleroderma Clinical Trial Consortium gastrointestinal tract instrument. J Rheumatol 2011; 38: 1920–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaraja V, Matucci-Cerinic M, Furst DE, et al. Current and future outlook on disease modification and defining low disease activity in systemic sclerosis. Arthritis Rheumatol 2020; 72: 1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna D, Clements PJ, Volkmann ER, et al. Minimal clinically important differences for the modified Rodnan Skin Score: results from the Scleroderma Lung Studies (SLS-I and SLS-II). Arthritis Res Ther 2019; 21: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kafaja S, Clements PJ, Wilhalme H, et al. Reliability and minimal clinically important differences of forced vital capacity: results from the Scleroderma Lung Studies (SLS-I and SLS-II). Am J Respir Crit Care Med 2018;197: 644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna D, Furst DE, Hays RD, et al. Minimally important difference in diffuse systemic sclerosis: results from the D-penicillamine study. Ann Rheum Dis 2006; 65: 1325–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namas R, Tashkin DP, Furst DE, et al. Efficacy of mycophenolate mofetil and oral cyclophosphamide on skin thickness: post hoc analyses from two randomized placebo-controlled trials. Arthritis Care Res (Hoboken) 2018; 70: 439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pope JE, Bellamy N, Seibold JR, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheumatol 2001; 44: 1351–58. [DOI] [PubMed] [Google Scholar]
- 28.Hudson M. Scleroderma renal crisis. Curr Opin Rheumatol 2015; 27: 549–54. [DOI] [PubMed] [Google Scholar]
- 29.Chung MP, Chung L. Drugs in phase I and phase II clinical trials for systemic sclerosis. Expert Opin Investig Drugs 2020; 29: 349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aringer M, Denton C. Systemic sclerosis phase III clinical trials: hope on the horizon? J Scleroderma Relat Disord 2018; 3: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanna D, Denton CP, Jahreis A, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 2016; 387: 2630–40. [DOI] [PubMed] [Google Scholar]
- 32.Khanna D, Denton CP, Lin CJF, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis 2018; 77: 212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanna D, Allanore Y, Denton CP, et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis (RISE-SSc): randomised, double-blind, placebo-controlled multicentre trial. Ann Rheum Dis 2020; 79: 618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khanna D, Pope J, Matucci-Cerinic M, et al. OP0249 Long-term extension results of RISE-SSc, a randomized trial of riociguat in patients with early diffuse cutaneous systemic sclerosis (DCSSC). Ann Rheum Dis 2020; 79 (suppl 1): 156–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanna D, Lin CJF, Furst DE, et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2020; 8: 963–74. [DOI] [PubMed] [Google Scholar]
- 36.Gordon JK, Martyanov V, Franks JM, et al. Belimumab for the treatment of early diffuse systemic sclerosis: results of a randomized, double-blind, placebo-controlled, pilot trial.Arthritis Rheumatol 2018; 70: 308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice LM, Padilla CM, McLaughlin SR, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest 2015; 125: 2795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sircar G, Goswami RP, Sircar D, Ghosh A, Ghosh P. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology (Oxford) 2018; 57: 2106–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.