Short abstract
We present a rare case of an ETMR with co‐occurrence of DICER1 and H3 K27M mutations.
Keywords: C19MC, DICER1, embryonal tumor with multilayered rosettes (ETMRs), H3K27M
1. CLINICAL HISTORY AND IMAGING
A 2‐year‐old girl, previously healthy, presented with weakness in both legs for more than half a month and vomiting for 2 days. Cranial MRI showed a nodular mass located in the posterior cranial fossa. The lesion showed heterogeneously isointense T2‐weighted and hypointense T1‐weighted image signals with heterogeneous contrast enhancement and supratentorial hydrocephalus (Figure 1). During the operation, the cerebellar vermis was obviously swollen. Pink irregular tumor tissue could be seen under the vermis. The tumor was large, soft, and rich in blood supply. The tumor was successfully totally resected (Box 1).
FIGURE 1.
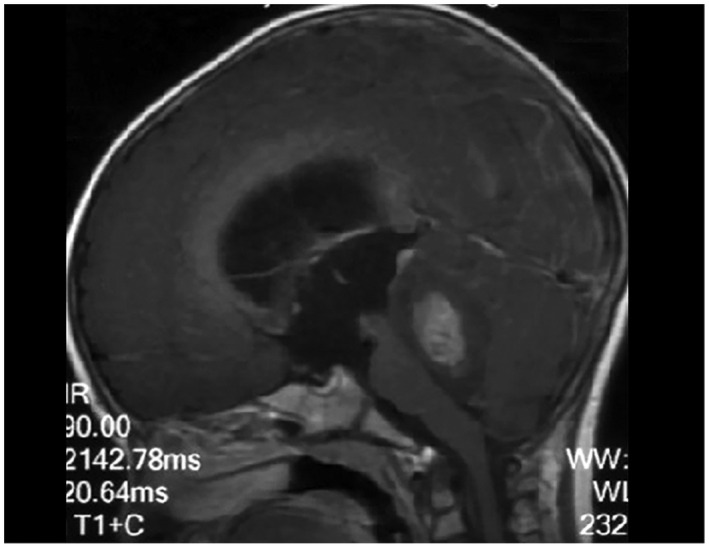
Cranial sagittal T1‐weighted MRI with contrast enhancement showed a nodular mass located in the posterior cranial fossa with heterogeneous contrast enhancement
BOX 1. Slide scan.
Access the whole slide scan at http://image.upmc.edu:8080/NeuroPathology/BPA/BPA-21-06-133.svs/view.apml?
2. FINDINGS
H&E stained sections revealed small blue malignant tumor cells which were scattered, clustered, and unevenly distributed in a background of neuropil, with multilayered rosettes with pseudostratified cells and a well‐defined lumen (Figure 2A,B). Some of the tumor cells were arranged in a nest‐like pattern, and some had a clear cytoplasm. Tumor cells had small, round to polygonal nuclei and scant cytoplasm. Mitoses were abundant, with patchy necrosis and microvascular proliferation (Figure 2C). Immunohistochemistry showed the tumor cells to be strongly and diffusely positive for synaptophysin (Figure 2D), LIN28A (Figure 2E), and H3 K27M (Figure 2F). The tumor cells were focally positive for GFAP and Olig‐2, but negative for EMA. Histone H3K27 trimethylation was lost (Figure 2G), while INI1 protein was retained. The Ki‐67 proliferation index was 80%. There was no evidence of the amplification of the 19q13.42 locus by FISH analysis. NGS revealed that the tumor harbored mutations in DICER1 (c.5113G>A, p.E1705K mutation and c.3747delA, p.K1249Nfs*9 mutation, with mutant allele frequencies of 37.8% and 48.3% respectively consistent with a somatic and a germline mutation). A HIST1H3B (H3.1) K27M mutation (with a mutant allele frequency of 45.1%) was also identified. What is your diagnosis?
FIGURE 2.
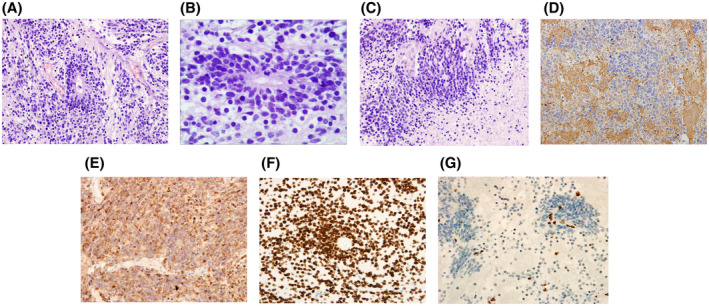
H&E staining showed tumor cells were scattered and clustered in a background of neuropil, with multilayered rosettes (A, B), with patchy necrosis and microvascular proliferation (C). Immunohistochemistry showed the tumor cells to be strongly and diffusely positive for synaptophysin (D), LIN28A (E), and mutated H3 K27M (F). Histone H3K27 trimethylation was lost (G). H&E, hematoxylin and eosin; MRI, magnetic resonance imaging
3. FINAL DIAGNOSIS
Embryonal tumor with multilayered rosettes, with DICER1 alteration (CNS WHO grade 4), harboring H3 K27M mutation.
4. DISCUSSION
Embryonal tumors with multilayered rosettes (ETMR), occurring mostly in children under the age of 2 years, are aggressive pediatric embryonal brain tumors with a universally poor prognosis. ETMRs can present with three different histological patterns: one characterized by abundant neuropil and true rosettes, as seen in our case; one resembling ependymoblastoma and one medulloepithelioma, as described in the 2016 World Health Organization (WHO) classification of CNS tumors. Genetically, ETMRs are characterized by amplification and fusion of a microRNA (miRNA) cluster on chromosome 19 (C19MC), at the 19q13.42 locus, which represents the genetic hallmark of ETMR, found in approximately 90% of the tumors, classified as “ETMR, C19MC‐altered [1]. Recently, Uro‐Coste and colleagues described infants with cerebellar tumors that histologically resembled ETMR, had diffuse LIN28 immunopositivity and DICER1 mutations, but lacked C19MC alterations [2]. Further studies reported ETMR without C19MC amplification frequently had germline and somatic mutations in DICER1 or amplification of miR‐17‐92 (also known as MIR17HG) [3]. The term "embryonal tumor with multilayered rosettes, with DICER1 alteration (CNS WHO grade 4)” has been suggested for the tumors showing the morphology of ETMR, and DICER1 alterations in the upcoming 2021 WHO classification. Co‐occurrence of DICER1 and H3K27M mutations in ETMRs to the best of our knowledge has not been reported to date.
The DICER1 gene, located at 14q32.2, encodes a component of the microRNA biogenesis machinery. DICER1 mutations are the second most common genetic event in ETMRs, besides C19MC altered [1]. In these tumors, biallelic mutations affect DICER1, with one inactivating germline mutation and one somatic mutation in the RNase III domain. Mutations in the RNase IIIb domain of DICER1 have been shown to interfere with its ability to process RNAs. Hypothesized mechanisms of DICER1‐related tumorigenesis include miRNAs from the 5′ strand of hairpin‐shaped precursor miRNA molecules resulting in altered 5p to 3p miRNA expression ratios, and the loss of tumor suppressor miRNAs of 5p origin leading to the upregulation of their target oncogenes [1].
Histone H3 K27M mutations, including H3 variants H3.3 (H3F3A) K27M and H3.1 (HIST1H3B/C) K27M mutations, have been identified in the majority of diffuse midline gliomas in children and are associated with a dismal prognosis. In tumor cells harboring the H3 K27M mutation, PRC2 may bind more avidly to K27M on chromatin, leading to reduced chromatin dynamics and a defect in H3 K27 trimethylation. H3 K27M‐mutant diffuse gliomas of the cerebellum have been previously reported. In addition, H3 K27M mutation has been reported rarely in a variety of tumors that are not diffuse midline gliomas, such as pilocytic astrocytoma, ganglion cell tumors, and ependymomas mostly occurring in the midline, posterior fossa and/or spinal cord. H3 K27M‐mutant tumors with the morphological appearance of an ETMR and harboring DICER1 mutations, to the best of our knowledge, have not been reported to date.
To conclude, we present a rare case of an ETMR with co‐occurrence of DICER1 and H3 K27M mutations. The patient received two cycles of chemotherapy (CPT‐11+ temozolomide) after the operation. Four months from surgery, she has no evidence of tumor recurrence. The prognosis of this patient remains guarded.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethics committee of Xuanwu Hospital, Capital Medical University, Beijing, China, and was conducted in full compliance with all principles of the Helsinki Declaration.
Funding information
This work was supported by the Beijing Nova Program (Z201100006820149) and the Beijing Hospitals Authority Clinical Medicine Development of Special Funding (ZYLX202113)
DATA AVAILABILITY STATEMENT
I confirm that my article contains a Data Availability Statement even if no data is available (list of sample statements) unless my article type does not require one (e.g., Editorials, Corrections, Book Reviews, etc.).
REFERENCES
- 1. Lambo S, von Hoff K, Korshunov A, Pfister SM, Kool M. ETMR: a tumor entity in its infancy. Acta Neuropathol. 2020;140(3):249–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uro‐Coste E, Masliah‐Planchon J, Siegfried A, Blanluet M, Lambo S, Kool M, et al. ETMR‐like infantile cerebellar embryonal tumors in the extended morphologic spectrum of DICER1‐related tumors. Acta Neuropathol. 2019;137(1):175–7. [DOI] [PubMed] [Google Scholar]
- 3. Lambo S, Gröbner SN, Rausch T, Waszak SM, Schmidt C, Gorthi A, et al. The molecular landscape of ETMR at diagnosis and relapse. Nature. 2019;576(7786):274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
I confirm that my article contains a Data Availability Statement even if no data is available (list of sample statements) unless my article type does not require one (e.g., Editorials, Corrections, Book Reviews, etc.).


