Abstract
Nociceptive input diminishes recovery and increases lesion area after a spinal cord injury (SCI). Recent work has linked these effects to the expansion of hemorrhage at the site of injury. The current article examines whether these adverse effects are linked to a pain-induced rise in blood pressure (BP) and/or flow. Male rats with a low-thoracic SCI were treated with noxious input (electrical stimulation [shock] or capsaicin) soon after injury. Locomotor recovery and BP were assessed throughout. Tissues were collected 3 h, 24 h, or 21 days later. Both electrical stimulation and capsaicin undermined locomotor function and increased the area of hemorrhage. Changes in BP/flow varied depending on type of noxious input, with only shock producing changes in BP. Providing behavioral control over the termination of noxious stimulation attenuated the rise in BP and hemorrhage. Pretreatment with the α-1 adrenergic receptor inverse agonist, prazosin, reduced the stimulation-induced rise in BP and hemorrhage. Prazosin also attenuated the adverse effect that noxious stimulation has on long-term recovery. Administration of the adrenergic agonist, norepinephrine 1 day after injury induced an increase in BP and disrupted locomotor function, but had little effect on hemorrhage. Further, inducing a rise in BP/flow using norepinephrine undermined long-term recovery and increased tissue loss. Mediational analyses suggest that the pain-induced rise in blood flow may foster hemorrhage after SCI. Increased BP appears to act through an independent process to adversely affect locomotor performance, tissue sparing, and long-term recovery.
Keywords: behavioral control, blood flow, capsaicin, electrical stimulation, hemorrhage, locomotor performance, nociception, norepinephrine, pain, prazosin, systolic BP
Introduction
Pain input after a spinal cord injury (SCI) is common because of the etiology of the injury, which is often associated with polytrauma. One of the goals of our laboratory at Texas A&M University is to determine the impact of this pain input soon after injury. Using two models of pain, electrical stimulation and capsaicin, we have previously found that nociceptive input increases the extent of hemorrhage, increases lesion volume, and impairs locomotor recovery when given soon after SCI.1–3 The detrimental effects of nociceptive stimulation have been linked to the upregulation of pro-inflammatory cytokines and activation of cell death pathways.4,5 The expansion of hemorrhage has been tied to progressive hemorrhagic necrosis, which is characterized by capillary fragmentation, infiltration of red blood cells, and the breakdown of the blood–spinal cord barrier.2,6 Recent work examining the emergence of pain-induced hemorrhage exacerbation has shown that only a short duration (as little as 72 sec) of electrical stimulation is needed to cause an increase in hemorrhage.7 Additionally, that study found that stimulation intensity only has to be moderate (0.5 mA) to induce a lasting impairment.
The present article explores the possibility that nociceptive input augments the area of hemorrhage because it induces a rise in BP. Previous work has suggested a link between hemorrhage expansion and changes in hemodynamic regulation after traumatic SCI.8–12 Additionally, research has shown that a rise in mean arterial BP (MAP) around the time of injury is associated with poor locomotor recovery in rats.13 Retrospective studies in humans have shown that either pre-existing hypertension or experiencing hyper- or hypotension during surgical interventions were correlated with lower motor scores and worse neurological recovery.14,15 Research has shown that aggressive hemodynamic management (i.e., treatment with fluids and vasopressors to maintain the MAP within 85–90 mm Hg for the first 7 days post-injury) is correlated with improved neurological outcomes after SCI.9,15 Understanding the effect of BP on SCI outcomes is of particular concern given recent trends showing that the average age at the time of SCI is increasing and with it the incidence of pre-exiting hypertension.16,17
In the current article, we examine the acute effect of two forms of noxious stimulation (electrical stimulation or application of the irritant capsaicin) on acute hemorrhage, cardiovascular function, and locomotor performance. Because prior work has shown that introducing behavioral control attenuates the adverse effect that noxious stimulation has on long-term recovery,3 we also assessed whether this factor influences the acute hemodynamic response and pain-induced hemorrhage. We then assessed the effect of two pharmacological manipulations, using prazosin to block the pain-induced rise in BP, and norepinephrine (NE) to artificially drive an increase in BP. These interventions sought to determine whether changes in BP are necessary and sufficient to cause hemorrhage expansion and deficits in locomotor performance.
Methods
Subjects
Adult male Sprague Dawley (200–400 g) rats were obtained from Envigo (Houston, TX). Upon arrival to the vivarium, animals were dual-housed in a room with a 12-h light–dark cycle with food and water ad libitum. Animals were acclimated to the room for at least 7 days before experimentation. Experiments were performed during the light cycle. All work was conducted in accordance with National Institutes of Health (NIH) standards for the care and use of laboratory animals and approved by the University Laboratory Animal Care Committee at Texas A&M University.18 Every effort was made to minimize suffering and limit the number of animals used.
Contusion surgery
Using the New York University (NYU) Multicenter Animal Spinal Cord Injury Study (MASCIS) device, rats received a contusion at the T11–T12 vertebral level. For the surgery, animals were anesthetized with a mixture of 5% isoflurane and medical oxygen. Concentrations of isoflurane were maintained at 2–3% isoflurane during surgery. An initial incision was made through the skin centered over the T12 vertebra. Next, two longitudinal incisions (3 cm in length) centered over the T12 vertebra were made on both sides of the spinal column. The T12 vertebra was then exposed and a laminectomy was performed. The spinal column was secured in the MASCIS device and the impactor centered on the lesion site. A 10 g weight was then dropped from a height of 12.5 mm. Rats receiving a sham operation went through all surgery steps except that the weight was not dropped. After surgery, Michel clips were used to close the skin incision. Animals then received an intraperitoneal (i.p.) of 100,000 U/kg of penicillin and 3 mL of saline to prevent infections and compensate for fluid loss. Animals were then placed in a temperature-controlled recovery room (25°C) and allowed to recover overnight (24 h) with food and water ad libitum. Bladders were voided twice daily and prior to any experimental procedures. After experimentation was finished, animals were euthanized with a lethal dose of pentobarbital (100 mg/kg; i.p.).
Locomotor performance
Before surgery, rats were acclimated on three separate days to a 99.1 (diameter) × 20.3 (deep) cm blue wading pool (Target) for 4 min. Beginning the day after contusion, locomotor performance was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor scale.19 To obtain the BBB score, the rat was placed in the observation arena and locomotor performance was assessed for 4 min by two trained observers blinded to animal's treatment condition. BBB scores were re-sampled at later time points during each experiment to examine changes in locomotor performance. For long-term recovery studies, BBB scores were analyzed for the first 7 days, at 10 days, and then once a week until day 21. BBB scores were converted using the formula described in the study by Ferguson and colleagues,20 which improves the metric properties of the scale by avoiding discontinuities and better equating the intervals between units.
Blood pressure, heart rate, and blood flow
Before surgery, rats were acclimated on three separate days to clear acrylic tubes with a black adjustable nose cone atop a far-infrared warming platform (Kent Scientific). Testing was done in a warm room (27°C) with dim lighting. Tail temperature was monitored with an infrared thermometer to ensure that temperatures reached and were maintained between 32 and 35°C. Animals were acclimated to restraint tubes for 5 min before the occlusion cuff and volume pressure recording (VPR) sensor were carefully glided up the tail and secured at the base of the tail. Animals were then acclimated to the cuffs for another 5 min before BP measurements were obtained. BP measurements, heart rate, and blood flow were obtained using the CODA® High Throughput Noninvasive BP system and data acquisition software. The program was set to automatically inflate and deflate the cuffs for 15 consecutive cycles with 5 sec between cycles. The maximum occlusion pressure was set at 250 mm Hg with a deflation time of 20 sec and a minimum volume of 15 μL. Acquisition of data lasted approximately 10 min. During acquisition, tail and body temperature were regularly monitored. If for any reason the animal did not have at least 4 acceptable BP readings and 1 heart rate reading, the animal would receive an additional 5 trials immediately following the last regular trial. After the assessment of BP, heart rate, and blood flow, the occlusion cuff and VPR sensor were taken off the tail and animals were removed from the restraint tubes. Finally, animals had their bladders voided before being placed back in their home cage. MAP was calculated using the following formula: [(2 × diastolic) + systolic)/3].
Drug preparation and administration
Prazosin (3 mg/kg) was dissolved in 30% glucose. Because of the low solubility of prazosin, hydrochloric acid was added when dissolving the drug. Once dissolved, the solution was adjusted to pH 7 and brought up to the final volume. Prazosin was delivered by an i.p. injection with a volume of 2 mL per 350 g rat.
NE (0.5 mg/kg) was dissolved in 0.9% saline and adjusted to pH 7. NE was injected subcutaneously (s.c.) with a volume of 1–2 mL into the rat's lower flank.
Capsaicin (3%) was dissolved in a solution of 7% Tween-80 and 93% saline (0.9% NaCl). Because of the low solubility of capsaicin, it had to be slightly heated and vortexed immediately before administration. Capsaicin was administered using an intradermal (ID) injection into the dorsal surface of the paw at a volume of 0.05 mL.
Noxious electrical tail stimulation
Rats were set up for noxious electrical stimulation to the tail as previously described in the study by Crown and colleagues.21 In brief, animals were restrained in opaque Plexiglas tubes with their hindlimbs hanging freely in a soundproof box. An electrode was placed ∼4 cm from the tip of the tail and secured with porous tape. Electrode gel was used to ensure that the tail maintained a good contact with the electrode surface. Electrical stimulation was given in a variable-spaced pattern (80 ms pulse, intertrial interval 0.2–3.8 sec, 60 Hz AC current). Animals received 180 stimulations at an intensity of 1.5 mA. Unshocked controls were restrained in tubes with the electrode attached for an equivalent duration, but no shock was administered.
Instrumental learning
Rats were loosely restrained in opaque Plexiglas tubes, which allowed the legs to hang freely. The leg was then set up for instrumental learning as described in Grau and colleagues.3 In brief, a thin wire electrode was placed into the tibialis anterior muscle 3.2 cm above the ankle. The other lead consisted of a stainless-steel wire looped through the skin 1.5 cm above the ankle (1.7 cm below the other lead). An 8 cm contact electrode was secured with porous tape to the plantar side of the paw. To minimize lateral leg movements, a piece of porous tape was wrapped around the leg above the ankle and taped to a bar extending across the apparatus directly under the front panel of restraining tube. Once the leg was set up, a small glass dish filled with a salt solution was placed under the animal's leg. To establish the initial resting position of the leg, three brief (100 ms) shocks were applied to the tibialis anterior muscle. The underlying salt solution was then adjusted so that the rod was submerged by 4 mm. The leg used for testing was counterbalanced across groups. To monitor leg position, an electrode was placed in the water. Whenever the contact electrode touched the salt solution, it completed a circuit that was measured by the computer.
Instrumental training lasted a total of 30 min. Master and yoked rats were always run in pairs. For animals receiving Master shock, electrical stimulation to the tibialis anterior muscle was given whenever the foot touched the underlying salt solution. Because contused animals learn very quickly, salt solution was added to the underlying dish when the master subject learned to maintain its leg above the solution for 2 consecutive min within the first 10 min of training. Solution was added in increments of 25 mL (i.e., 2 mm increase) until the contact electrode touched the underlying solution. For subjects receiving noncontingent shock (yoked), animals received shock whenever the master rat's foot touched the underlying solution, and solution level was adjusted at the same time and by the same amount. As a control, a group of animals remained unshocked. Unshocked rats had their legs set up as outlined; however, after receiving the three shocks used to set the water level, the animal received no other shocks and remained in the tubes for 30 min. At the end of training, the computer compiled data into 1 min time bins. Learning was defined as an increase in flexion duration. Flexion duration was calculated for each time bin using the following formula: (60 sec minus time in solutioni) / (Response Numberi + 1), where i is the current time bin.
ID capsaicin injection
Rats were restrained in opaque Plexiglas tubes with their hindlimbs hanging freely. Capsaicin (3%) was injected ID into the dorsal surface of the hindpaw using a 27-gauge needle. Controls received an injection of vehicle (7% Tween-80 in 23% saline). The injected paw (right versus left) was counterbalanced across subjects. To maintain consistency across treatment conditions and with past studies, capsaicin and vehicle treated animals remained in the tubes for 6 min after injection.
Hemorrhage analysis
Tissue collection
After a lethal dose of pentobarbital, a 1 cm section of spinal cord tissue centered at the lesion site was extracted and flash frozen in liquid nitrogen. Samples were stored at -80°C until processed. Tissue was processed for protein isolation using QIAzol lysis reagent according to the manufacturer's instructions.
Immunoblotting
A Bradford assay was used to quantify protein concentration of each sample. Samples were then diluted with 4 × Laemeli buffer to a final concentration of 3 μg/μL, except for Experiment 1, in which the concentration was 2 μg/μL. Western blots were run on pre-cast 26-well Criterion gels (BIORAD). Briefly, protein samples in 4 × Laemeli buffer were heated to 96°C for 10 min before 10 μL of each sample was loaded into the wells. Electrophoresis was performed at 180 V for ∼1 h and 15 min. Samples were then transferred to a polyvinylidene difluoride (PVDF) membrane for 1 h at 100 V. Membranes were incubated in blocking solution (milk) for 1 h before being placed overnight in primary antibody (hemoglobin-α, 1:1000:ADD ID) at 4°C. The next day, membranes underwent three 15-min washes with TBST before incubation for 1 h in secondary antibodies (goat anti-rabbit, 1:5000) at room temperature. Finally, blots were incubated in Enhanced Chemiluminescence (ECL) and imaged with Fluorchem HD2 (ProteinSimple, Santa Clara, CA, USA). To obtain a measure of net hemoglobin-α content, binding was summed for the monomer, dimer, and trimer.
Spectrophotometry
A 1.5 μL sample of the protein extract (before it was diluted with Laemeli buffer) was loaded into a spectrophotometer (Thermo) and a full spectrum analysis was run. Absorbance was analyzed at 420 nm for hemoglobin.22
To further assess the level of hemorrhage, samples were incubated with Drabkin's reagent. The reagent binds and converts free hemoglobin to its cyanmethemoglobin derivative, which can then be analyzed at 540 nm to quantify the total hemoglobin concentration in the protein extracts.23 The following equation was derived, using hemoglobin standards, to convert absorbance to hemoglobin concentration: μg/μL =
Histology
Animals were deeply anesthetized with pentobarbital (100 mg/kg, i.p.). After the heartbeat had terminated, rats received a transcardial perfusion with ∼300 mL of ice-cold phosphate buffered saline (PBS) (pH 7.3) followed by ∼400 mL of 4% paraformaldehyde (PFA). A 1 cm-long segment of the spinal cord, centered at the lesion site, was collected and incubated in 4% PFA for 24 h at 4°C. The tissue was rinsed with PBS before cryoprotection in a solution of 30% sucrose in PBS for at least 72 h before being embedded in Tissue-Tek O.C.T compound for cryosectioning. The tissue was sectioned coronally into 20 μm-thick sections. Every fifth section was mounted on Fisherbrand Superfrost Plus (Fisher Scientific) microscope slides for lesion analysis. All sections were stained with cresyl violet for Nissl substance and Luxol fast blue for myelin.24 Sections were imaged using light microscopy at 4 × magnification and were analyzed by blinded observers using ImageJ software. Lesion area and total cross-sectional area of the cord were determined as described in the study by Grau and colleagues.3 The lesion size was quantified as a percentage of the total section area (% area).
Experimental design
Twenty-four hours after receiving a moderate spinal cord contusion at T10–T11, male Sprague Dawley rats had their locomotor scores evaluated using the BBB locomotor scale, and their baseline (BL) BP measurements taken using a non-invasive BP cuff. Groups were balanced using BL systolic BP and BBB scores. Rat were then administered noxious input (i.e., capsaicin or electrical stimulation) and/or a drug (i.e, Nor prazosin). BP and locomotor activity were then examined at set time points. After obtaining the last BBB score and/or BP measurement, rats were euthanized and tissue was collected for hemorrhage analysis.
Statistical analysis
SPSS and jamovi were used to perform all statistical tests. Significance was set at 0.05. Post hoc comparisons were conducted using the Duncan New Multiple Range Test. For missing values, an average of the adjacent cells was used to enable statistical analysis. Mediational analyses were performed using the jAMM module within jamovi. Completely standardized effect sizes were estimated using the standard (delta) method. Similar results were obtained using the bootstrap (percent) method.
Results
Experiment 1: Examining changes 3 h after noxious input
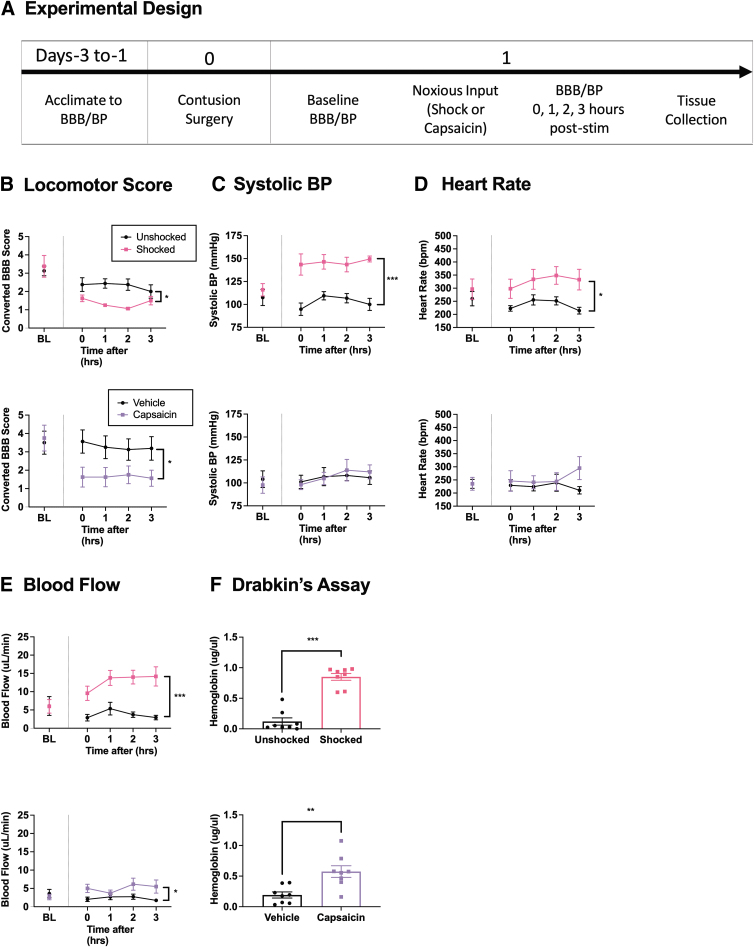
Previous research has shown that exposure to nociceptive stimulation (6 min of electrical stimulation or capsaicin) 1 day after SCI induces an acute disruption in locomotor function and hemorrhage that is evident within 3 h of treatment. To explore whether these effects are related to alterations in cardiovascular function, we assessed BP, heart rate, blood flow, and locomotor function over a period of 3 h in two cohorts of rats that had received noxious electrical stimulation/unshocked (n = 16) or capsaicin/vehicle (n = 16), and then assayed the extent of hemorrhage at the site of injury (Fig. 1A).
FIG. 1.
Locomotor performance, hemorrhage, and hemodynamics 3 h after noxious input. One day after injury, rats were exposed to noxious stimulation (shock or capsaicin) or nothing and then tested for 3 h (A). Animals exposed to noxious electrical stimulation (upper panels) or capsaicin (lower panels) 24 h after a SCI showed decreased BBB locomotor scores for up to 3 h post-stimulation (B). Electrical stimulation caused an increase in systolic BP, whereas capsaicin treatment had no effect (C). A sustained heart rate was seen after electrical stimulation, but not capsaicin treatment (D). Both types of noxious input caused an increase in blood flow (E). The amount of free hemoglobin in the spinal cord, as assessed with Drabkin's reagent, was increased in animals given noxious stimulation (F). Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significant group differences (*p < 0.05, **p < 0.01, ***p < 0.001).
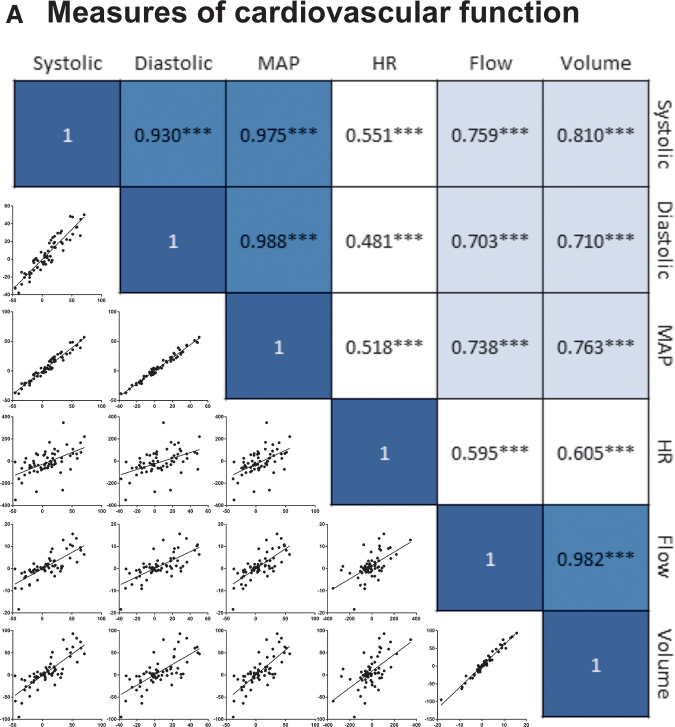
As detailed in our subsequent analyses (Experiment 9), the three measures of BP (systolic, diastolic, and mean arterial) were highly correlated (all rs > 0.93). Because the pattern of results observed across these measures were very similar, just one index (systolic BP) is presented. Likewise, blood flow and volume had a high correlation (r = 0.98) and yielded similar trends over time. For this reason, just one (flow) is illustrated.
Noxious input caused a decrease in locomotor scores within 3 h of treatment
Locomotor scores did not differ between groups prior to treatment, both Fs < 1.0, p > 0.05 (Fig. 1B). Performance was re-assessed immediately, 1, 2, and 3 h after noxious stimulation to examine early changes in locomotor recovery. Two separate repeated measures analysis of covariance (ANCOVAs) were run with BL locomotor scores as the covariate, time as a repeated measure, and either shocked/unshocked or capsaicin/vehicle as the between-subjects variables. The overall analyses revealed a main effect of shock and capsaicin treatment, with noxious stimulation showing lower locomotor scores, Fshock (1, 13) = 11.942, p = 0.0043 and Fcapsaicin (1, 13) = 9.428, p = 0.0089 (Fig. 1B). No other terms were statistically significant, Fshock < 2.732, p > 0.05 and Fcapsaicin < 1.973, p > 0.05.
Electrical stimulation induced hypertension within 3 h of treatment, while capsaicin showed no significant changes
There were no differences in BP (i.e., systolic, diastolic, and MAP) prior to noxious stimulation, all Fs < 1.0, p > 0.05 (Fig. 1C, diastolic and MAP data not shown). Cardiovascular function was assessed immediately, 1, 2, and 3 h after noxious input. For each dependent variable, separate ANCOVAs were conducted to assess the effect of capsaicin and shock treatment. In each analysis, statistical power was enhanced by employing the pre-treatment measure (BL) as a covariate. The analyses revealed a significant main effect of shock on systolic BP, F (1, 13) = 30.698, p = 0.0001 (Fig. 1C). Likewise, diastolic pressure and MAP were elevated by shock treatment, both Fs > 19.090, p < 0.0008 (data not shown). For shock, no other term was statistically significant, all Fs < 1.689, p > 0.05. In contrast to shock, capsaicin had no effect on BP, all Fs < 1.0, p > 0.05.
Electrical stimulation elevated heart rate within 3 h of treatment
Heart rate was not different between groups prior to treatment, both Fs < 1.0, p > 0.05 (Fig. 1D). Exposure to shock, F (1, 13) = 7.675, p = 0.0169, but not capsaicin, F (1, 13) < 1.0, p > 0.05, induced an elevation in heart rate. No other term was statistically significant, all Fs < 2.248, p > 0.05
Cutaneous blood flow was increased in animals exposed to noxious stimulation
Blood flow and volume did not differ prior to noxious stimulation, all Fs < 1.0, p > 0.05 (Fig. 1E, volume data not shown). Analyses of blood flow after noxious stimulation revealed a significant main effect of shock, F (1, 13) = 19.033, p = 0.0008, and capsaicin, F (1, 13) = 4.990, p = 0.0435. Shock, F (1, 13) = 22.842, p = 0.0004, but not capsaicin, F (1, 13) = 3.440, p > 0.05, had a significant effect on blood volume. No other term was statistically significant, all Fs < 2.401, p > 0.05.
Noxious input caused an increase in indices of hemorrhage within 3 h of treatment
Separate analyses of variance (ANOVAs) were used to assess the effect of shock and capsaicin treatment on hemorrhage. A Drabkin's assay revealed a greater concentration of hemoglobin in animals that received noxious input, Fshock (1, 14) = 80.100, p = 0.0001 and Fcapsaicin (1, 14) = 12.651, p = 0.0032 (Fig. 1F). Identical results were obtained when hemoglobin content was assessed by assaying peak absorbance (420 nm) for hemoglobin using spectrophotometry and quantitative Western blotting for hemoglobin-α, all Fs > 4.758, p < 0.0467 (data not shown).
Experiment 2: Examining changes 24 h after noxious input
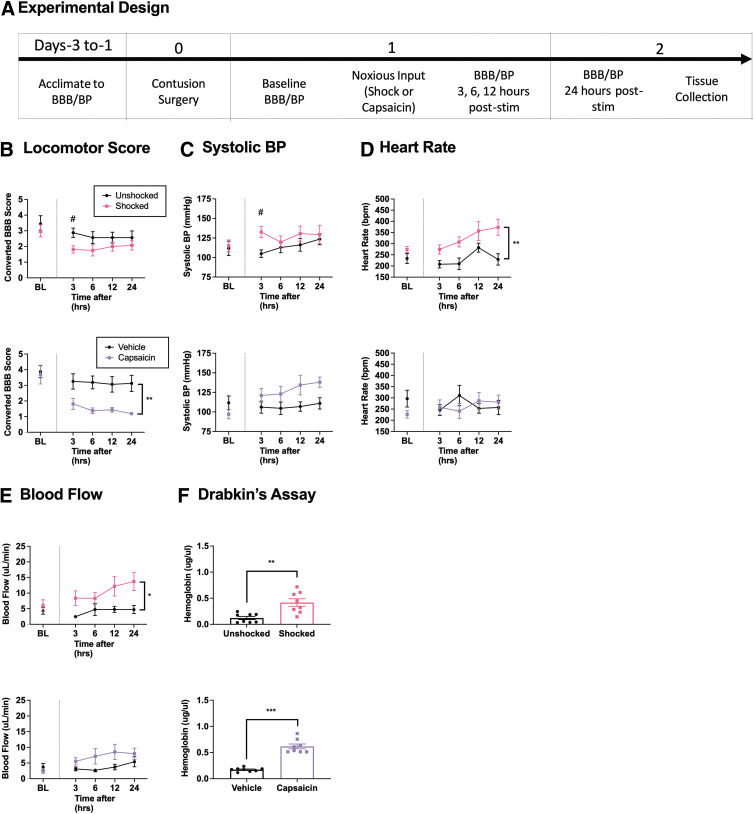
In the previous experiment, heart rate and blood flow were elevated for up to 3 h after noxious input. To examine how long these changes are maintained, we assessed locomotor and hemodynamic function over a 24-h period after nociceptive stimulation (n = 16) or capsaicin (n = 16), followed by assays for hemorrhage at the site of injury (Fig. 2A).
FIG. 2.
Locomotor performance, hemorrhage, and hemodynamics 24 h after noxious input. One day after injury, rats were exposed to noxious stimulation (shock or capsaicin) or nothing and then tested 3, 6, 12, and 24 h later (A). Application of the irritant capsaicin (lower panel) 24 h after a SCI caused a lasting disruption in locomotor performance, whereas exposure to noxious electrical stimulation (upper panel) only affected performance at 3 h (B). Electrical stimulation produced an acute rise in systolic BP at 3 h. Capsaicin appeared to produce a sustained effect, but this did not reach statistical significance (C). Only electrical stimulation induced a significant increase in heart rate that was evident over the 24 hr period of testing (D). An increase in blood flow was seen for up to 24 h after only electrical stimulation (E). Hemoglobin content was higher at the site of injury 24 h after both forms of noxious stimulation (F). Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significance differences between groups (*p < 0.05, **p < 0.01, ***p < 0.001). #Pre-planned analysis for significant differences (p < 0.05).
Locomotor recovery was impaired after noxious stimulation
Locomotor scores did not differ between groups prior to treatment, both Fs < 1.0, p > 0.05 (Fig. 2B). Performance was re-assessed 3, 6, 12, and 24 h after noxious input. Two separate repeated measures ANCOVAs were conducted with BL locomotor scores as the covariate, time as the repeated measure, and either shock/unshock or capsaicin/vehicle as the between-subjects variables. The analyses revealed a main effect of capsaicin, F (1, 13) = 12.839, p = 0.0033. No other effects were statistically significant, all Fshock < 2.193, p > 0.05 and Fcapsaicin < 1.0, p > 0.05 (Fig. 2B). Preplanned analysis of the 3-h time point using an ANCOVA revealed a significant decrease in locomotor scores in shocked animals relative to the unshocked controls, F (1, 13) = 8.797, p = 0.0109 (Fig. 2B top graph), replicating Experiment 1.
Electrical stimulation induces an acute increase in BP
All three measures of BP (systolic, diastolic, and MAP) did not differ between groups prior to treatment, all F's < 3.254, p > 0.05 (Fig. 2C, diastolic and MAP data not shown). Cardiovascular function was assessed 3, 6, 12, and 24 h after noxious stimulation. Separate repeated measures ANCOVAs were conducted on each dependent variable with the pre-treatment value (BL) serving as the covariate, time as the repeated measure, and either shock/unshock or capsaicin/vehicle as the between-subjects variables. Overall analyses revealed no significant effects of shock treatment, all Fs < 2.737, p > 0.05 (Fig. 2C). Pre-planned comparisons at the 3-h time point using an ANCOVA revealed a significant increase in systolic BP, diastolic BP, and MAP in shocked animals, all Fs > 9.054, p < 0.01, replicating Experiment 1 (Fig. 2C top graph). In capsaicin-treated animals, the difference in overall systolic BP approached statistical significance, F (1, 13) = 4.258, p = 0.0596. No other term was significant, all Fs < 2.914, p > 0.05.
Heart rate remained elevated after electrical stimulation for up to 24 h, while no changes were seen after capsaicin treatment
Heart rate did not differ between groups prior to treatment, both Fs < 3.375, p > 0.05 (Fig. 2D). Separate ANCOVAs analyzing the changes in heart rate observed over time after noxious stimulation revealed a significant main effect of shock, F (1, 13) = 12.448, p = 0.0037. No other term was significant, all Fs < 1.164, p > 0.05.
Blood flow remained elevated after electrical stimulation for up to 24 h, while no changes were seen after capsaicin treatment
There were no differences in blood flow or volume prior to treatment, all Fs < 1.160, p > 0.05 (Fig. 2E). Independent ANCOVAs examining blood flow over time after noxious stimulation revealed a significant main effect of shock, F (1, 13) = 5.342, p = 0.0379, but not capsaicin, F (1, 13) = 2.698, p = 0.1244. Both treatments failed to induce a significant change in blood volume, both Fs < 3.650, p > 0.05 (data not shown). No other term approached statistical significance, all Fs < 2.089, p > 0.05.
Indices of free hemorrhage remained elevated 24 h after noxious input
Hemorrhage was analyzed using separate ANOVAs with shock or capsaicin as the between-subjects variables. Assessment of the amount of free hemoglobin with Drabkin's reagent found a main effect of noxious input, Fshock (1,14) = 14.441, p = 0.0020 and Fcapsaicin (1, 14) = 87.341, p = 0.0001 (Fig. 2F). Analysis of peak absorbance at 420 nm, and quantitative Western blotting for hemoglobin-α, also revealed significant effect of capsaicin treatment, F (1, 14) = 11.515, p = 0.0044 and F (1, 14) = 4.758, p = 0.0457, respectively (data not shown). In contrast, exposure to shock did not have a significant effect on hemorrhage as assessed by peak absorbance or Western blotting, F (1, 14) = 1.599, p > 0.05 and F (1, 14) < 1.0, p > 0.05, respectively (data not shown).
Indices of free hemorrhage were not different after 8 days
From the results of our first two experiments, it is clear that both shock and capsaicin treatment induce hemorrhage. The temporal profile of the hemorrhage effects appears to vary depending upon pain type; although both forms of stimulation enhanced acute hemorrhage (at 3 h), only capsaicin amplified the level of hemorrhage observed across all three assays 24 h after treatment. To evaluate whether an effect of capsaicin would be evident at a later time point, we also assessed hemorrhage 8 days after treatment. This time interval was selected because it maintained a constant ratio (eightfold) between the intervals sampled. At 8 days, our three assays of hemorrhage did not detect a significant effect of capsaicin treatment, all Fs < 1.0, p > 0.05.
We also examined the relative sensitivity of our hemorrhage assays by calculating the effect size (Cohen's d) for each analysis. Across the two pain conditions (shock and capsaicin) and time points (3 and 24 h), the average effect sizes were 1.32, 3.21, and 0.53 for absorbance at 420 nm, the Drabkin's assay, and Western blotting, respectively. Because the Drabkin's assay is more sensitive and provides a quantitative assessment of hemoglobin content, we present these results in subsequent experiments.
Experiment 3: Effect of noxious stimulation 1.5 and 6 h after injury
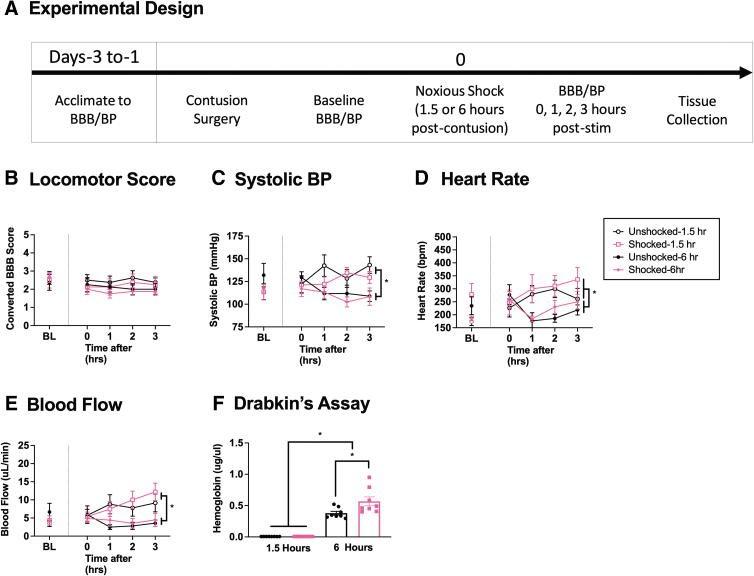
As previously reported, we found that pain input 1 day after SCI increases hemorrhage at the site of injury.2 We have not assessed the effect of pain input applied at earlier time points. The present experiment addressed this issue by exposing animals (n = 32) to noxious electrical stimulation 1.5 or 6 h after SCI. Locomotor and cardiovascular function were then assessed over a period of 3 h, followed by an assessment of hemorrhage (Fig. 3A). We expected that nociceptive stimulation would have a more robust effect when applied soon after injury.
FIG. 3.
Locomotor performance, hemorrhage, and hemodynamics 1.5 and 6 hours after injury. Contused rats were exposed to noxious electrical stimulation, or nothing, 1.5 or 6 h after injury and tested for 3 h (A). Exposure to 1.5 or 6 h of noxious electrical stimulation after injury did not affect locomotor performance (B). Animals exhibited higher systolic BP (C), heart rate (D), and blood flow (E) 1.5 h after injury relative to animals tested 6 h after injury. In no case did noxious electrical stimulation have a significant effect on hemodynamics. Electrical stimulation increased hemorrhage when applied 6 h after injury, but not when given at 1.5 h (F). Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significance differences between groups (*p < 0.05).
Electrical stimulation produced no deficits in locomotor scores
Prior to shock treatment, there were no group differences in locomotor performance, all Fs < 1.0, p > 0.05. To assess the effect of time after injury, an ANCOVA was performed with shock treatment and delay (1.5 or 6 h) as between-subject factors, time since stimulation (0–3 h) as the repeated measure, and pre-treatment BBB score (BL) as the covariate. No effect reached statistical significance, all Fs < 3.521, p > 0.05 (Fig. 3B).
Stimulation-induced changes in systolic BP varied with time and delay
There were no differences in BL BP prior to shock treatment, all Fs < 1.0, p > 0.05. Again, an ANCOVA was performed with shock treatment and delay as between-subject factors, time since stimulation as a repeated measure, and BL BP as a covariate. We found that there was a main effect of delay, F (1, 27) = 6.501, p = 0.0168, and that the effect of shock treatment depended upon both time since stimulation and the delay interval (1.5 vs. 6 h), F (3, 81) = 2.967, p = 0.0368. The interaction between time and delay was also significant, F (3, 81) = 5.203, p = 0.0025. These interactions emerged because shock treatment soon after injury had a more limited, time-dependent, effect on systolic BP (Fig. 3C). A similar pattern of results was observed for MAP and diastolic pressure, with a time-dependent effect of delay, both Fs > 5.078, p < 0.0028 (data not shown). No other term reached statistical significance, all Fs < 2.254, p > 0.05.
Stimulation did not induce a significant change in heart rate or blood flow 1.5–6 h after injury
There were no group differences in heart rate, blood flow, or blood volume prior to shock treatment, all Fs < 3.389, p > 0.05. To examine whether nociceptive stimulation affected these outcomes, independent ANCOVAs were performed using shock treatment and delay as between-subject factors, time since stimulation as the repeated measure, and the BL value as a covariate. Again, lower values were generally observed when animals were tested 6 h after injury and the magnitude of this difference increased over the 3 h period of testing, all Fs > 4.767, p < 0.0041 (Fig. 3D and E). The effect of shock treatment on heart rate approached statistical significance, F (1, 27) = 4.131, p = 0.0521. No other term was statistically significant, all Fs < 2.268, p > 0.05.
Electrical stimulation at 6 h, but not 1.5 h, enhanced hemorrhage
A Drabkin's assay revealed that both delay interval and shock treatment affected hemorrhage at the site of injury, both Fs > 6.105, p < 0.0198 (Fig. 3F). Further, there was a significant interaction between shock treatment and delay, F (1, 28) = 6.092, p = 0.0199. This interaction emerged because shock had a significant effect at 6, but not 1.5, h. Post-hoc comparisons revealed that all group differences were statistically significant (p < 0.05) with the exception of the shock and unshocked groups treated 1.5 h after injury (p > 0.05).
Electrical stimulation 4 days after injury had little effect on hemorrhage at the site of injury
Our results show that the adverse effect that noxious stimulation has on acute hemorrhage and locomotor function grows over the first 24 h after injury. Elsewhere, we have examined the effect of pain input given at longer intervals, 4 and 14 days after injury.3 By 4 days, the adverse effect on long-term behavioral recovery began to fade. To explore whether a similar pattern is observed for hemorrhage, two additional groups were exposed to shock or nothing (unshocked) 4 days after a lower thoracic injury. Nociceptive stimulation at this time point had no effect on locomotor function, F (1, 10) < 1.0, p > 0.05 (data not shown). Shock treatment 4 days after injury also failed to increase hemorrhage as assessed with the Drabkin's assay, F (1, 10) = 1.572, p > 0.05.
Experiment 4: Examining changes in response to controllable shock
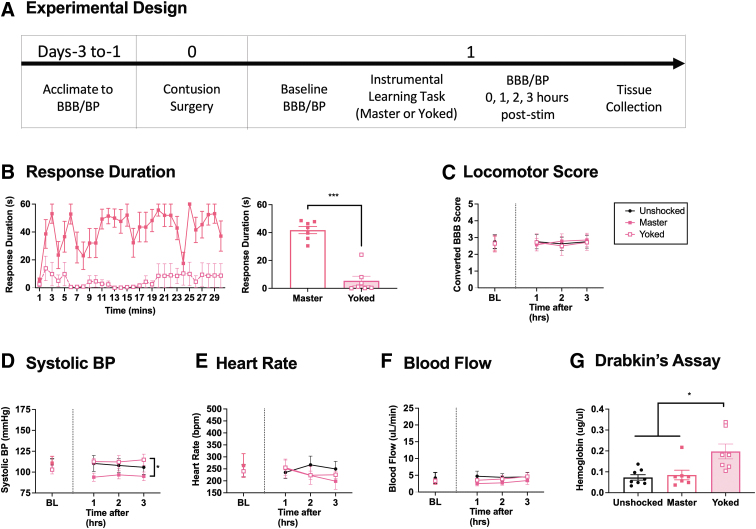
As previously reported,1,3,4 noxious stimulation disrupts locomotor function and fosters hemorrhage after a lower thoracic contusion injury.1,3,6 Other studies suggest that the adverse effect of noxious stimulation is modulated by behavioral control; exposure to uncontrollable, but not controllable, intermittent electrical stimulation impairs long-term recovery. The present experiment examines whether behavioral control also regulates the effect that noxious stimulation has on cardiovascular function and hemorrhage in contused rats (n = 30) (Fig. 4A). As in prior work, the effect of behavioral control was assessed by applying noxious electrical stimulation to the tibialis anterior muscle of one hind leg 1 day after a contusion injury.3 A behavioral contingency was instituted by applying stimulation to one group (master) whenever the leg was extended. Animals in a second group were coupled (yoked) to master rats and received the same stimulation, but independent (uncontrollable) of leg position. A third group was treated the same but never shocked (unshocked). Behavioral performance, hemodynamics, and hemorrhage were then assessed as described previously.
FIG. 4.
Locomotor performance, hemorrhage, and hemodynamics 3 h after instrumental learning. One day after a contusion injury, rats were exposed to 30 min of controllable (master) or uncontrollable (yoked) electrical stimulation (or nothing) and were tested for 3 h (A). Animals given controllable stimulation (masters) exhibited an increase in response duration (B). Training did not have an acute effect on locomotor performance (C). Systolic BP was reduced in the master animals (D). No differences were observed in heart rate and blood flow (E, F). Hemorrhage was elevated only in the yoked subjects (G). Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significance differences between groups (*p < 0.05, ***p < 0.001).
Controllable stimulation increased leg flexion over time
Master rats, which received electrical stimulation whenever the leg was extended, learned to maintain the stimulated leg in a flexed position (Fig. 4B). This was not observed in rats given stimulation independent of leg position (yoked). Performance during training was analyzed using repeated measures ANOVA with training condition as the between-subjects variable and time as the repeated measure. The analysis showed that the main effects of time and training, and their interaction, were statistically significant, all Fs > 1.998, p < 0.0001. Post-hoc comparisons of the group means confirmed that the master group differed from the other two, p < 0.05. No other group comparison was significant, p > 0.05.
As previously reported, the behavioral difference that emerges with training does not reflect a failure to respond; electrical stimulation elicited a behavioral (flexion) response in both master [mean response rate (+ SE) = 31.96 (8.46)] and yoked [mean (+ SE) = 33.41 (8.47)] animals, but only produced an increase in flexion duration (our index of learning) in master rats.3 An ANOVA confirmed that stimulation elicited an increase in the rate of responding, F (2, 27) = 131.929, p < 0.0001. Post-hoc comparisons of the group means showed that the master and yoked groups differed from the unshocked group, p < 0.05, but not from each other, p > 0.05 (data not shown).
Locomotor scores at 3 h were not different between master and yoked groups
Locomotor scores did not differ between groups prior to treatment, F (2, 27) < 1.0, p > 0.05. Locomotor function was re-assessed 1, 2, and 3 h after behavioral training. A repeated measures ANCOVA with baseline locomotor scores as the covariate showed that neither the main effect of training condition nor its interaction with time were significant, both Fs < 1.0, p > 0.05 (Fig. 4C)
Animals that experienced controllable stimulation had lower BP than those given uncontrollable shock
BP did not differ between groups prior to treatment, all Fs (2, 27) < 1.0, p > 0.05. Cardiovascular function was assessed 1, 2, and 3 h after training. For each dependent variable, an ANCOVA was conducted with time as the repeated measure, training condition as the between-subjects variable, and the pre-treatment value (BL) as the covariate. Overall systolic BP differed after training, F (2, 26) = 3.436, p = 0.0474 (Fig. 4D). No other term was statistically significant, all Fs < 1.0, p > 0.05. Post-hoc comparisons of the group means showed that animals with behavioral control (master) exhibited lower BP after training, relative to rats given uncontrollable shock (yoked), p < 0.05. No other group comparison was statistically different, p > 0.05. Identical results were found for diastolic BP and MAP, both Fs (2, 26) > 3.523, p < 0.0443 (data not shown).
Heart rate and blood flow was not different between shock conditions
Heart rate, blood flow, and volume did not differ prior to treatment, all Fs < 1.0, p > 0.05. Training had no effect on these indices of cardiovascular function, all Fs < 1.456, p > 0.05 (Fig. 4E and F, volume data not shown).
Uncontrollable stimulation increased hemorrhage within 3 h
Three hours after training, animals given uncontrollable stimulation (yoked) exhibited greater hemorrhage (Fig. 4G). An ANOVA conducted on the data collected using the Drabkin's assay revealed a significant effect of training condition, F (2, 19) = 7.610, p = 0.0037. Post-hoc comparisons showed that the animals in the yoked group differed from the other two, p < 0.05. No other group comparison was statistically significant, p > 0.05.
Experiment 5: Short-term effects of attenuating the changes in BP produced by electrical stimulation after SCI
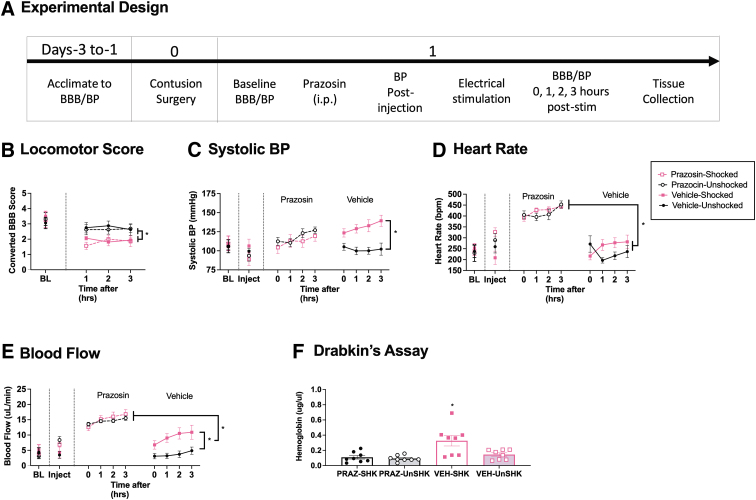
Our results suggest exposure to noxious electrical stimulation may expand the area of hemorrhage because it drives a rise in BP. Pain could have this effect by enhancing stress/arousal, increasing NE release, which would engage α-1 adrenergic receptors on vascular smooth muscle and induce a rise in BP.25 We explore this possibility by testing whether an α-1 adrenergic receptor antagonist (prazosin) blocks the nociception-induced hemorrhage after SCI. We chose to test prazosin because this drug is routinely used to treat high BP after SCI in humans.26 If the pain-induced rise in BP is causally related to the expansion of hemorrhage, pre-treatment with prazosin should attenuate this effect. For this study, rats (n = 32) received an i.p. injection of prazosin (3 mg/kg) or the vehicle (30% glucose) (Fig. 5A). Six minutes later, BP was re-assessed. Thirty minutes after drug treatment, animals were given 6 min of electrical stimulation (shocked) or nothing (unshocked). Locomotor performance, BP, and hemorrhage where then assessed as described previously.
FIG. 5.
Locomotor performance, hemorrhage, and hemodynamics 3 h after prazosin and shock. Contused rats were given prazosin or vehicle, followed by electrical stimulation or nothing, and were tested for 3 h (A). Electrical stimulation decreased locomotor performance (B). Shock treatment increased systolic BP, heart rate, and blood flow in vehicle-treated, but not prazosin-treated animals (C–E). Prazosin per se increased heart rate and blood flow compared with vehicle-treated groups (D, E). The amount of free hemoglobin in the cord was increased in vehicle-treated shocked animals, but not in prazosin-treated shocked subjects (F). Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significance differences between groups (*p < 0.05).
Prazosin did not block the decrease in locomotor behavior seen after nociceptive stimulation
Locomotor scores did not differ between groups prior to drug treatment, F < 1.0, p > 0.05. Exposure to shock produced an acute disruption in locomotor performance that was evident over the 3 h of testing (Fig. 5B). Administration of prazosin had no effect. A repeated measures ANCOVA with shock and drug as the between-subjects variable, BL locomotor scores as the covariate, and time as the repeated measure, revealed a main effect of shock, F (1, 27) = 20.852, p = 0.0001. No other group difference was statistically significant, all Fs < 1.0, p > 0.05 (Fig. 5B).
Prazosin blocked the increase in systolic BP seen after electrical stimulation, but also increased BP in unshocked controls
BP did not differ prior to treatment, all Fs < 1.0, p > 0.05. As reported, exposure to shock induced a rise in systolic BP in the vehicle-treated rats that was evident across the 3 h of testing. This effect was blocked by pre-treatment with prazosin (Fig. 5C). In addition, it appears that prazosin treated animals exhibited a compensatory rise in BP over time. A similar pattern of results was observed for diastolic BP and MAP (data not shown). The data were analyzed using independent ANCOVA, with shock as the between-subjects variable, BL BP as a covariate, and time as a repeated measure. In each case, there was a significant shock × drug treatment interaction, all Fs > 5.470, p < 0.0270. In addition, for diastolic pressure and MAP, the effect of shock treatment became larger over time, yielding a three-way interaction among time, shock, and drug treatment, both Fs > 4.013, p < 0.0102. No other term was statistically significant, all Fs < 3.978, p > 0.05. Post-hoc comparisons of the group means showed that both the vehicle-treated shocked group, and the prazosin-treated unshocked group, differed from the vehicle-treated unshocked animals, p < 0.05. For systolic BP, there was also a significant difference between the prazosin- and vehicle-treated shocked groups, p < 0.05. No other group comparison was significant, p > 0.05.
Prazosin augmented other indices of cardiovascular function and attenuated the effect of shock
Heart rate, blood flow, and blood volume did not differ prior to treatment, all Fs < 1.284, p > 0.05. After prazosin treatment, the relaxation in vascular smooth muscles led to an increase in blood flow (Fig. 5E) and a compensatory rise in heart rate (Fig. 5D). Shock induced a rise in blood flow in vehicle-treated, but not prazosin-treated, rats. An ANCOVA examining heart rate revealed a main effect of drug treatment, F (1, 27) = 222.531, p < 0.0001, and a shock × time interaction, F (3, 81) = 4.478, p = 0.0052. For both flow and volume, the main effects of drug and shock treatment, all Fs > 6.946, p < 0.0137, and the shock × drug treatment interaction, both Fs > 6.579, p < 0.0162, were statistically significant. For these two measures, post-hoc comparisons of the group means showed that vehicle-treated unshocked group differed from the other three, p < 0.05. Prazosin also increased flow in both the shocked and unshocked groups relative to the vehicle-treated shocked group, p < 0.05. For heart rate, the two prazosin-treated groups differed from the vehicle controls, p < 0.05. No other group comparison was statistically significant, p > 0.05.
Prazosin blocked the expansion of the hemorrhage at 3 h
Hemorrhage was analyzed using an ANOVA with shock and drug as the between-subjects variables. Assessment of the amount of free hemoglobin with Drabkin's reagent revealed a main effect of drug and shock treatment, both Fs > 6.565, p < 0.0161, as well as a shock × drug treatment interaction, F (1, 29) = 4.554, p = 0.0417 (Fig. 5F). Post-hoc comparisons showed that the vehicle-treated shocked group differed from the other three, p < 0.05. No other group comparison was significant, p > 0.05.
Experiment 6: Long-term effects of attenuating the changes in BP produced by electrical stimulation after SCI
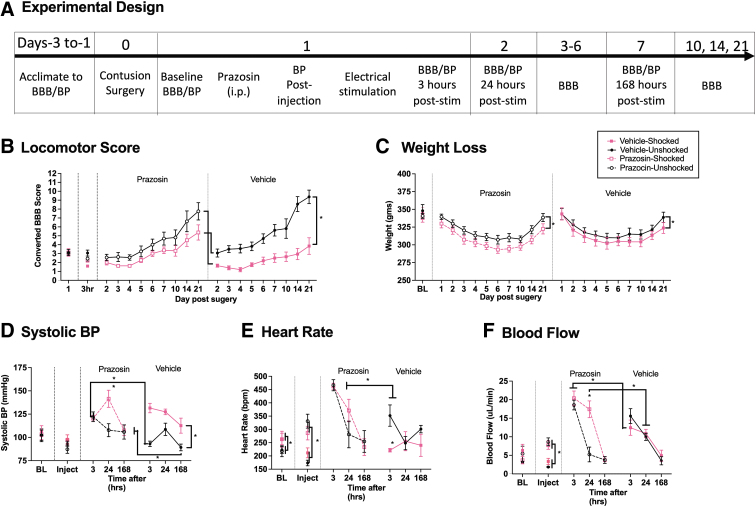
We found that pre-treatment with prazosin attenuated shock-induced hemorrhage in contused rats. Given that other treatments known to block hemorrhage (i.e., lidocaine) have been associated with improved long-term outcomes,1 we tested whether pre-treatment with prazosin reduces the adverse effect that noxious electrical stimulation has on long-term recovery, assessed over a period of 21 days after injury. Rats (n = 32) were treated as in Experiment 5, except that BP was assessed just once (at 3 h) after noxious stimulation (Fig. 6A). In a subset of rats (n = 4), BP was re-assessed 1 and 8 days later. Two rats in the vehicle-shocked group died within the first week (i.e., days 5 and 7). To assure an equal number at the end of testing, two animals were folded into this condition.
FIG. 6.
Long-term locomotor performance, weight, and hemodynamics after prazosin and shock. Contused rats were given prazosin or vehicle, followed by electrical stimulation or nothing, and recovery was observed over 21 days (A). Exposure to noxious electrical stimulation disrupted long-term recovery of stepping in vehicle-treated, but not prazosin-treated rats (B). Electrical stimulation decreased weight gain compared with unshocked controls independent of drug treatment (C). Prazosin increased hypertension, heart rate, and blood flow at 3 h after stimulation compared with vehicle-unshocked controls (D–F). Prazosin-shocked rats exhibited an increase in BP and blood flow at 24 h post-stimulation relative to prazosin-unshocked animals (D, F). Additionally, blood flow was significantly higher in prazosin-shocked subjects than in vehicle-shocked subjects at 3 h (F). Vehicle-treated shocked animals exhibited increased hypertension for up to 7 days (D). Further, heart rate and blood flow were elevated in vehicle-shocked subjects for up to 3 hours, compared with the vehicle-unshocked group (E, F). Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significance differences between groups (*p < 0.05).
A single dose of prazosin before electrical stimulation improved long-term locomotor recovery
There were no group differences in locomotor performance 1 day after SCI prior to drug/shock treatment, all Fs < 1.0, p > 1.0. Locomotor scores were re-assessed 3 h after treatment, daily over the next week, and at 10, 14, and 21 days (Fig. 6B). Shock impaired long-term recovery in vehicle-treated rats. Pre-treatment with prazosin attenuated this effect. An ANCOVA was used to assess the effect of shock and drug treatment (between-subject factors), with day as a repeated measure and the baseline (Day 1) locomotor score serving as the covariate. As observed previously,3 there was some mortality in the vehicle-treated shocked group, with two animals dying at the end of the 1st week. No mortalities were observed in the other groups. We handled the missing scores in two ways: By (1) replacing the missing values with the group mean; and (2) performing an independent analysis of the week 1 data. Both analyses yielded an identical pattern of statistical significance. There was a significant effect of shock treatment, both Fs > 28.321, p < 0.0001, and a significant drug × shock treatment interaction, both Fs > 4.552, p < 0.0415. The latter emerged because prazosin reduced the effect of shock treatment and undermined performance in the unshocked controls. The repeated factors revealed that the effect shock treatment varied across days, F (9, 261) = 4.734, p < 0.0001 and that locomotor performance improved over the 21-day period of testing, F (9, 261) = 4.734, p < 0.0001.
Prazosin did not attenuate shock-induced weight loss
There were no group differences in weight prior to treatment, all Fs < 1.0, p > 0.05. An ANCOVA was used to analyze weight over days after treatment, with pre-treatment (day 1) weight serving as the covariate. The analysis revealed a main effect of shock treatment, F (1, 29) = 11.930, p = 0.0017 (Fig. 6C). There was also a significant effect of day and a day × shock treatment interaction, both Fs > 5.257, p < 0.0001. No other term was statistically significant, all Fs < 1.479, p > 0.05. Post hoc comparisons of the group means showed that the two shocked groups differed from the prazosin unshocked group, p < 0.05. In addition, the vehicle shocked and unshocked groups differed, p < 0.05. No other group comparison was significant, p > 0.05.
Prazosin lowered BP at 3 h after electrical stimulation, but not at 24 h
BP did not differ prior to drug/shock, all Fs < 1.0, p > 0.05. Cardiovascular function was reassessed after drug treatment and again 3 h after animals received shock or nothing (unshocked). Additional tests were performed 1 and 7 days later in half of the animals (n = 4). A limited sample was taken on days 1 and 7 in case the assessment of BP affected long-term recovery (no effect was observed, p > 0.05). To evaluate the effect of drug treatment per se, an ANCOVA was performed on the post-injection values, with BL BP as the covariate. Prazosin had no effect, all Fs < 2.128, p > 0.05 (Fig. 6D). An ANCOVA performed on the post-shock scores, with drug and shock treatment serving as between-subjects factors and BL BP as the covariate, revealed a significant effect of drug treatment and a drug × shock treatment interaction, on all three measures of BP, all Fs > 9.213, p < 0.0050. For diastolic pressure and MAP, there was also a main effect of drug treatment, both Fs > 7.373, p < 0.0110. F (1, 29), p = 0.0386 (data not shown).
Additional ANCOVAs were performed on the subset of animals tested 24 and 168 h after drug/shock treatment. For systolic BP, we found a main effect of shock (F [1, 12] = 5.738, p = 0.0338) (Fig. 6D). The repeated factors showed that the effect of shock treatment varied across days and depended upon drug treatment, both F > 4.852, p < 0.0479. The three-way interaction emerged because prazosin attenuated the effect of shock treatment at 168 h, but not at 24 h. MAP also yielded a significant effect of shock treatment, F (1, 12) = 5.279, p < 0.0404 (data not shown). No other term was statistically significant.
Prazosin had a time-dependent effect on heart rate, blood flow, and volume
There were no group differences in heart rate, blood flow, or volume prior to drug/shock treatment, all Fs < 3.790, p > 0.05. Three hours after shock treatment, vehicle-treated shocked animals exhibited a higher heart rate, blood flow, and volume than the unshocked controls (Fig 6E and F, volume data not shown). Prazosin per se increased blood flow/volume and brought about a compensatory increase in heart rate (Fig. 6E and F). Shock had no effect on these measures of cardiovascular function in prazosin-treated animals. On all three measures, the main effects of shock and drug treatment and the shock × drug treatment interaction were statistically significant, all Fs > 6.098, p < 0.0197. Post-hoc comparisons for blood volume showed that the vehicle unshocked group differed from the other three, p < 0.05. For blood flow and heart rate, there was also a significant difference between the vehicle-treated shocked group and both of the prazosin-treated groups, p < 0.05. No other group differences were statistically significant, p > 0.05.
Experiment 7: Short-term effects of pharmacologically increasing BP after SCI
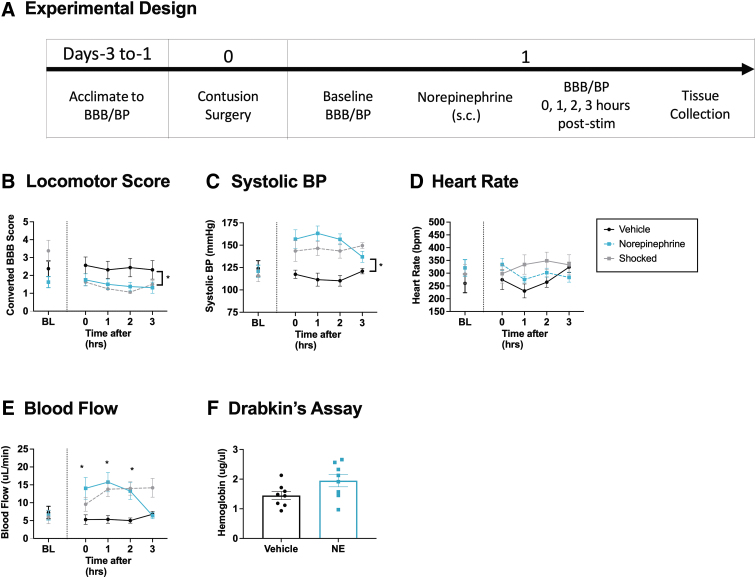
We have shown that noxious electrical stimulation induces a rise in both BP and hemorrhage. In addition, attenuating the pain-induced rise in BP with prazosin reduced hemorrhage and improved long-term recovery. The findings suggest that the adverse effect of pain input after SCI may be related to its effect on cardiovascular function. Here, we examined whether inducing a rise in BP is sufficient to drive hemorrhage and undermine locomotor performance soon after injury (n = 16), Fig. 7A. BP was experimentally increased using the nonselective adrenergic agonist NE, which is often used in humans to counter hypotension after SCI. The dosage selected (0.5 mg/kg, s.c.) was based on pilot data demonstrating that it induces an increase in BP comparable to that produced by noxious electrical stimulation. Higher doses (e.g., 1–2 mg/kg) were often lethal in contused rats, and for this reason, not tested. Locomotor function and hemodynamics were assessed over a period of 3 h, and hemorrhage assessed, as described.
FIG. 7.
Locomotor performance, hemorrhage, and hemodynamics 3 h after norepinephrine (NE) treatment. One day after injury, rats were treated with NE or vehicle and tested for 3 h (A). Animals given NE (0.5 mg/kg) 24 h after a SCI exhibited a significant decline in locomotor performance (B). NE induced an increase in systolic BP comparable with that observed after shock treatment (C). No changes in heart rate were seen after NE treatment (D). NE caused an increase in blood flow (E). The amount of free hemoglobin in the cord was not significantly different after NE treatment (F). To illustrate how the magnitude of these effects compared with noxious electrical stimulation, we have re-plotted that data here. Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significance differences between groups (*p < 0.05).
NE impaired locomotor performance
Locomotor scores did not differ between groups prior to drug treatment, F (1, 14) = 1.707, p < 0.05 (Fig. 7B). Performance was reassessed immediately, 1, 2, and 3 h after injection. An ANCOVA was used to assess the effect of drug treatment over time with BL BBB score serving as the covariate. Although the main effect of NE treatment was not statistically significant, F (1, 13) = 1.786, p = 0.2044, the drug × time interaction approached significance, F (3, 39) = 2.534, p = 0.0709. Trend analysis showed that the linear component of this interaction was statistically significant, F (1, 39) = 5.0961, p < 0.05. Neither the quadratic nor the cubic term approached significance, both Fs < 1.0, p > 0.05.
NE significantly increased systolic BP
BP did not differ between groups prior to drug treatment, all Fs < 1.0, p > 0.05 (Fig. 7C). After NE administration, BP was augmented across the 3 h of testing. To illustrate how the magnitude of this effect compared with the rise in BP observed after noxious electrical stimulation, we have re-plotted that data (from Experiment 1). The effect of NE treatment on BP was analyzed using an ANCOVA, with drug treatment as a between-subjects variable, time as a repeated factor, and BL BP as a covariate. Treatment with NE produced a significant rise in systolic BP, F (1, 13) = 16.345, p = 0.0014, and the magnitude of this effect varied with time, F (3, 39) = 4.287, p = 0.0104. An identical pattern of results was observed for diastolic pressure and MAP, with both yielding a significant main effect of drug treatment, both Fs > 12.397, p < 0.0023, and a drug × time interaction, both Fs > 4.983, p < 0.0051 (data not shown).
Heart rate, blood flow, and volume
Other indices of cardiovascular function (heart rate, blood flow, and blood volume) did not differ prior to drug treatment, all Fs < 1.596, p > 0.05 (Fig. 7D and E, volume data not shown). Treatment with NE induced an increase in blood flow and volume, both Fs > 17.153, p < 0.0012, and the magnitude of these effects varied with time, both Fs > 3.636, p < 0.0209. Treatment with NE did not have a significant effect on heart rate, all Fs < 1.0, p > 0.05.
NE did not cause an expansion of the hemorrhage
Treatment with NE appears to have induced a slight increase in hemorrhage. This effect approached statistical significance, F (1, 14) = 4.096, p = 0.0625 (Fig. 7F). Likewise, our other indices of hemorrhage (absorbance at 420 nm and Western blotting) approached a statistical significance effect of NE treatment, both Fs < 4.150, p > 0.0610 (data not shown).
We also evaluated the effect of a lower dose of NE (0.1 mg/kg). It also induced a significant rise in BP, volume, and flow, all Fs > 14.776, p < 0.0020 (data not shown). Again, however, NE 1 day after injury failed to enhance hemorrhage, all Fs < 1.0, p > 0.05 (data not shown).
Experiment 8: Long-term effects of pharmacologically increasing the BP after SCI
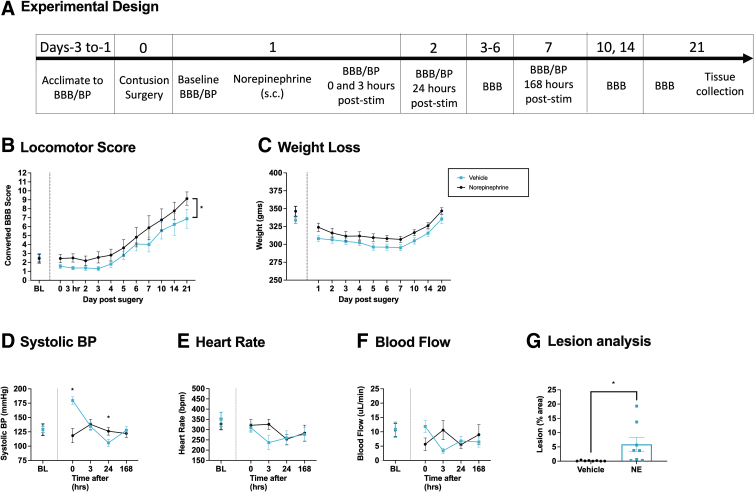
In the previous experiment, we showed that pre-treatment with NE induces a rise in BP and flow comparable with that produced by shock. NE treatment also caused an acute disruption in locomotor performance. To examine whether pharmacologically inducing a rise in BP has a lasting effect, we administered NE (0.5 mg/kg) 1 day after injury and monitored recovery over 21 days (n = 32, Fig. 8A).
FIG. 8.
Long-term locomotor performance, weight, tissue sparing, and hemodynamics after NE treatment. One day after injury, rats were treated with NE or vehicle, and long-term recovery was assessed over 21 days (A). Animals injected with NE (0.5 mg/kg) 24 h after a SCI showed significantly lower BBB locomotor scores across the 21-day recovery period (B). No changes in weight loss were seen between groups (C). NE increased systolic BP immediately after treatment (D). Drug treatment had no effect on heart rate (E). NE induced an acute increase in blood flow (F). Histological analyses revealed that NE-treated rats showed greater lesion volume (G). Error bars represent standard error of the mean (SEM) (n = 8). Asterisks indicate significance differences between groups (*p < 0.05).
Locomotor recovery was reduced in subjects treated with NE
Locomotor scores did not differ prior to drug treatment, F (1, 14) < 1.0, p > 0.05 (Fig. 8B). Performance was re-assessed daily from days 2–7 and on days 10, 14, and 21 after injury. A repeated measures ANCOVA with drug treatment as the between-subjects variable, BL locomotor scores as a covariate, and time as a repeated measure found a main effect of drug and time, both Fs > 4.692, p < 0.0375. No other effects were statistically significant, all Fs < 1.0, p > 0.05.
NE did not impact weight loss after SCI
Prior to drug treatment, there were no group differences in weight, F (1, 14) = 2.369, p > 0.05. (Fig. 8C). A repeated measures ANCOVA revealed a main effect of days, F (8, 104) = 4.467, p < 0.0001, but no effect of drug treatment, both Fs < 1.0, p > 0.05.
NE increased BP
Systolic BP did not differ prior to drug treatment, F (1, 14) = 1.043, p > 0.05 (Fig. 8D). BP was assessed immediately and at 3, 24, and 168 h post-NE treatment. A repeated measures ANCOVA with drug treatment as the between-subjects variable, BL systolic BP as a covariate, and time as a repeated measure found a main effect of drug, F (1, 13) = 6.206, p = 0.0270, and an interaction between drug and time, F (3, 39) = 9.802, p = 0.0001. No other effects were statistically different, all Fs < 1.708, p > 0.05. Post-hoc analysis of the interaction revealed that NE induced a significant rise immediately after the injection, but lowered BP the next day. NE also induced a time-dependent rise in MAP, F (3, 39) = 9.694, p = 0.0001, but not diastolic pressure, F < 1.0, p > 0.05 (data not shown).
NE acutely increased blood flow and volume but had no effect on heart rate
Heart rate, blood flow, and volume did not differ prior to drug treatment, all Fs < 1.0, p > 0.05. These indices of cardiovascular function were reassessed 0, 3, 24, and 168 h after NE treatment. For each dependent variable, a ANCOVA was performed using the BL value as the covariate. For blood flow and volume, there was a significant drug × time interaction, F (3, 39) > 4.129, p < 0.0123 (Fig. 8F). NE did not have a significant effect on heart rate all Fs < 1.130, p > 0.05 (Fig. 8E). No other term approached statistical significance, all Fs < 1.0, p > 0.05.
A single dose of NE soon after injury increased tissue loss at the site of injury
The extent of tissue loss at the site of injury was assessed at the end of the recovery period (Fig. 8G). Treatment with NE increased lesion size, F (1, 14) = 5.458, p = 0.0349.
Experiment 9: Mediational modeling
Statistical modeling techniques were used to explore whether pain-induced alterations in cardiovascular function contribute to hemorrhage or the acute disruption in locomotor function after SCI. The data from Experiments 1 and 2 were used to perform these analyses (n = 64). To obtain a common measure of cardiovascular function, we computed the change observed 3 h after treatment (relative to BL). Likewise, a common measure of locomotor function was obtained by calculating the difference between performances at 3 h relative to baseline. This sample yields a large effect size for the impact of pain input on hemorrhage (Cohen's d = 1.787) and locomotor function (Cohen's d = 0.969).
The effect of pain input on cardiovascular function can be characterized in terms of three measures: systolic BP, heart rate, and flow
Our first set of analyses examined the relationship between alternative measures of cardiovascular function to determine the degree to which the changes observed soon after (0–3 h) nociceptive stimulation are interrelated. A correlation heat map (Fig. 9A) revealed three clusters. As expected, systolic, diastolic, and MAP were highly correlated (shaded in dark blue), with correlation coefficients (r) of 0.930–0.988, p < 0.0001. Likewise, there was a strong relation between volume and flow, r = 0.982, p < 0.0001. These two clusters were themselves related, with r values ranging from 0.703 to 0.810, p < 0.0001 (shaded in light blue). Heart rate had a moderate correlation with the other measures, with r values ranging from 0.481 to 0.605, p < 0.0001, which accounted for approximately one third of the variance (23.1–36.6%) shaded in white. Based on these preliminary observations, a principal component analysis was performed (using a promax rotation and a fixed number of components [3]). The analysis generated three clusters: (1) measures of BP (systolic, diastolic and MAP); (2) flow and volume; and (3) heart rate. Components 1 and 2 accounted for 48.26% and 33.59% of the variance, respectively. Heart rate accounted for 16.84%. Of the three measures of BP, systolic generated the strongest η2 and was selected to represent this factor. Likewise, because pain input had a more reliable effect on blood flow across the eight experiments (relative to volume), it was used to represent the second factor.
FIG. 9.
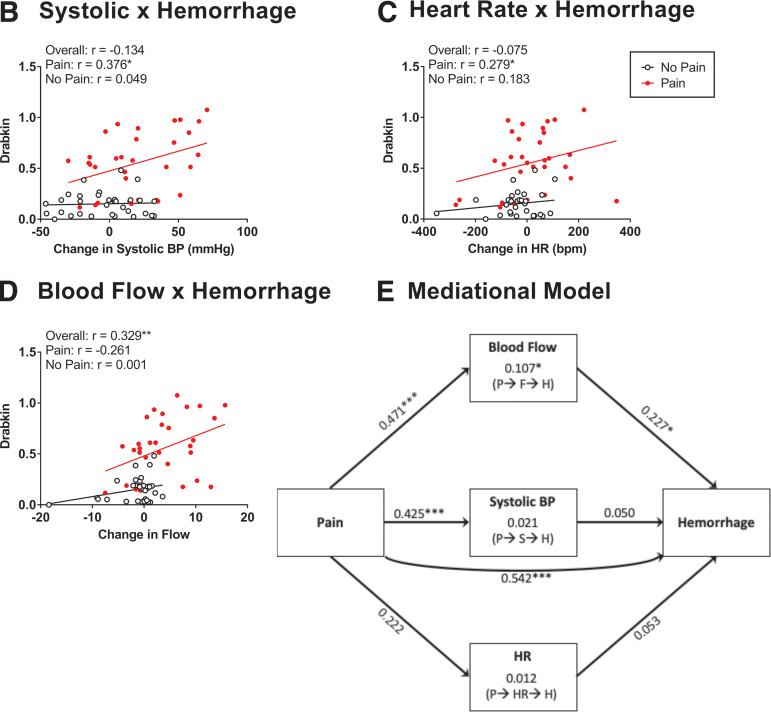
Relationship between hemodynamic changes and hemorrhage development. Correlational heat map showing the relationship between alternative measures of cardiovascular function (A). The three measures of blood pressure (BP) (systolic, diastolic, and MAP) were highly correlated (light green) (dark blue). Likewise, there was a strong correlation between blood flow and volume. Blood flow and volume were moderately related to the three indices of BP (light blue), whereas a weaker relationship was observed with heart rate (HR) (white). There was a significant relationship between systolic BP and hemorrhage (B) and between HR and hemorrhage (C) for animals receiving pain input. Independent of pain treatment (overall), an increase in blood flow predicted greater hemorrhage (D). A mediational analysis showed that pain input drove an increase in both blood flow and systolic BP, but only the former predicted greater hemorrhage (E). There was also a significant direct effect of pain input on hemorrhage that arose independent of these indices of cardiovascular function. The values along each line indicate the standardized effect sizes (beta values). Indirect effects are indicated within the center boxes and the path is indicated below in parentheses for pain (P), flow (F), systolic (S), heart rate (HR), and hemorrhage (H). Asterisks indicate significance differences between groups (*p < 0.05, ***p < 0.001).
Systolic BP and flow were correlated with hemorrhage
We then evaluated the relationship between the alternative measures of cardiovascular function and hemorrhage (Fig. 9B–D). Hemorrhage was estimated based on the Drabkin's assay because it provides a quantitative measure and accounted for the largest proportion of variance (relative to spectrophotometry at 420 nm or Western blotting). When all animals (pain treated and no pain) were considered, we found a significant relationship between flow and hemorrhage, r = 0.329, p = 0.0078. A weaker non-significant relation was found when systolic BP or heart rate were used to predict hemorrhage, with r values of -0.134 to -0.075, p > 0.05. When only pain treated animals were considered, we found that hemorrhage was predicted by systolic BP and blood flow, both rs < 0.376, p < 0.0340.
The effect of pain input on hemorrhage is partially mediated by its impact on blood flow
A mediational analysis (Fig. 9E) was conducted to explore the role of systolic BP, heart rate, and blood flow in pain-induced hemorrhage. The analysis revealed both a direct effect of pain input on hemorrhage, z = 4.953, p < 0.0001, and an indirect effect of flow, z = 2.012, p = 0.0442. Neither systolic BP nor heart rate had a significant indirect effect, both zs < 0.557, p > 0.05. Flow had a mediational role because it was engaged by pain input, z = 4.271, p < 0.0001, and fostered hemorrhage, z = 2.227, p = 0.0225. Although pain input affected systolic BP, z = 3.752, p = 0.0002, systolic BP did not drive hemorrhage, z = 0.514, p > 0.05. Pain input had only a weak effect on heart rate, z = 1.821, p = 0.0686, and an increase in heart rate did not drive hemorrhage, z = 0.586, p > 0.05.
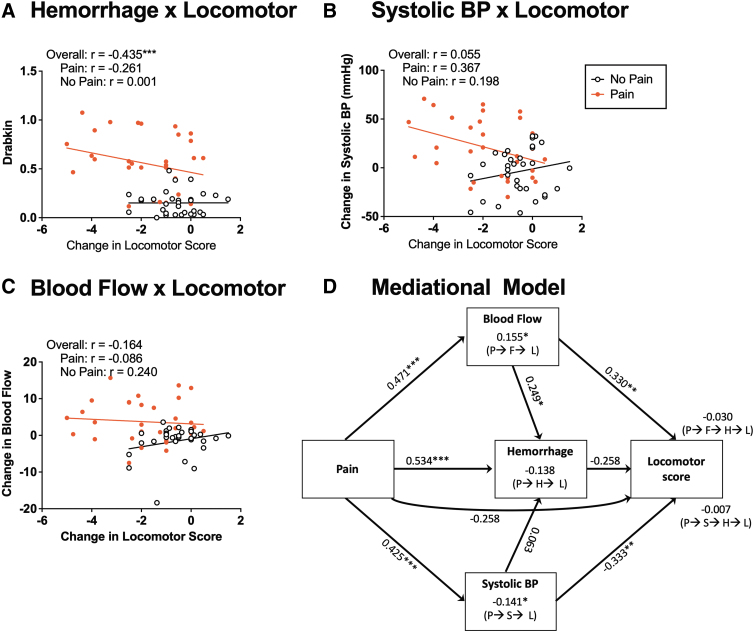
The acute disruption in locomotor function is related to pain-induced hemorrhage
Next, we examined whether the acute decline in locomotor function was related to pain-induced hemorrhage (Fig. 10A). We found that the change in locomotor function (based on BBB scores) was inversely related to the amount of hemorrhage detected, r = -0.435, p = 0.0003. This inverse relation was driven by the increase in hemorrhage observed in animals that experienced nociceptive stimulation. Importantly, capsaicin and electrical stimulation had nearly identical effects.
FIG. 10.
Relationship among hemodynamic changes, hemorrhage, and locomotor function. There was an overall effect of hemorrhage on locomotor recovery (A). Systolic BP was significantly correlated with locomotor recovery in animals receiving pain input (B). Blood flow was not correlated with locomotor recovery (C). A mediational analysis showed that pain (P) input drove blood flow (F), hemorrhage (H), and systolic BP (S). Increased blood flow also drove hemorrhage. Both blood flow and systolic BP affected locomotor performance (L), but in opposite directions. The decline in locomotor performance was moderately predicted by hemorrhage and a direct effect of pain input, both ps < 0.075. The values along each line indicate the standardized effect sizes (beta values). Indirect effects are indicated above the labels set in parentheses indicating the path. (D). Asterisks indicate significance differences between groups (*p < 0.05, **p < 0.01, ***p < 0.001).
An increase in systolic BP and flow predicts the decline in locomotor performance
Finally, we examined how alterations in BP related to the acute decline in locomotor function (Fig. 10B and C). Somewhat surprisingly, opposing trends were observed in animals exposed to nociceptive stimulation (pain) or nothing (no pain), leading to a weak overall relation to locomotor performance, with r values that ranged from -0.164 to 0.156, p > 0.05. Separate analyses on pain-treated and vehicle/unshocked animals revealed a significant negative correlation for systolic BP, r = -0.367, p = 0.0388, for pain-treated rats. No other relationship approached statistical significance, with absolute r values <0.261, p > 0.05.
Systolic BP and blood flow mediate the acute effect of pain on locomotor performance
A mediational analysis was then performed, with the change in locomotor performance as the dependent variable, and systolic BP, blood flow, and hemorrhage serving as mediators. In this model (Fig. 10D), we assumed that changes in cardiovascular function could impact locomotor performance directly or by means of an indirect path mediated by hemorrhage. Neither indirect path played a statistically significant role, both zs < -1.388, p > 0.05. Pain input did drive systolic BP, flow, and hemorrhage, all zs > 3.752, p < 0.0002. The decline in locomotor function was only moderately driven by acute hemorrhage, z = -1.808, p = 0.0707, and for this reason, the indirect effect of pain input mediated by hemorrhage was not significant, z = -1.699, p = 0.0893. Likewise, the direct effect of pain input on locomotor performance did not reach statistical significance, z = -1.784, p = 0.0745. Both systolic BP and flow impacted locomotor function, albeit in opposite ways, z = -3.010, p = 0.0026 and z = 2.782, p = 0.0054, respectively. These relationships enabled an indirect effect of pain input on locomotor function, mediated by systolic BP, z = -2.348, p = 0.0189, and flow, z = 2.331, p = 0.0198. We also assessed an alternative model that assumed that systolic BP and/or blood flow moderated, rather than mediated, the relationships among pain, hemorrhage, and locomotor performance. No component was statistically significant, all absolute z values <1.176, p > 0.05.
Taken together, the mediational analyses suggest that a pain-induced rise in systolic BP does not fuel hemorrhage but can adversely affect locomotor performance by means of an independent mechanism. In contrast, an increase blood flow appears to have divergent effects, partially mediating the effect of pain on hemorrhage and, at the same time, engaging an alternative process that promotes locomotor function.
Discussion
The present study explored whether pain input after SCI increases the area of hemorrhage because it induces a rise in BP. Statistical analyses revealed that the effect of noxious stimulation on cardiovascular function could be characterized in terms of three variables: systolic BP, heart rate, and blood flow. Electrical stimulation given 24 h after injury induced hemorrhage and an acute elevation (0–3 h after treatment) on all three measures of cardiovascular function. Electrical stimulation also had a lasting effect on both heart rate and blood flow that was evident when animals were re-tested 6–24 h later. Electrical stimulation given closer to injury (i.e., 1.5 or 6 h), did not augment systolic BP, heart rate, or blood flow, although it did increase hemorrhage at 6 h. Prior work has shown that providing behavioral control over nociceptive stimulation attenuates its adverse effect on long-term recovery after SCI.3 Here, we found that only uncontrollable stimulation (yoked) enhanced hemorrhage and that having behavioral control (master) attenuated systolic BP. Treatment with capsaicin caused an increase in hemorrhage but had a more limited effect on cardiovascular function, inducing an acute rise in blood flow and heart rate but not in systolic BP.
To explore whether a pain-induced rise in BP plays an essential (necessary) role in the induction of hemorrhage, we pre-treated animals with the α-1 adrenergic agonist prazosin, which is used to treat high BP after SCI in humans.26 Because the drug relaxes vascular smooth muscles, it caused a rise in blood flow and a compensatory increase in heart rate. Importantly, exposure to electrical stimulation had no effect on cardiovascular function in prazosin-treated animals, and prazosin attenuated shock-induced hemorrhage. When assayed over a longer period (21 days), we found that vehicle-treated shocked animals showed poor locomotor recovery and that this effect was attenuated by prazosin.
Next, we examined whether pharmacologically inducing a rise in BP with the adrenergic agonist NE is sufficient to induce hemorrhage and impair long-term recovery after SCI. NE induced an acute rise in systolic BP and blood flow comparable with that induced by noxious electrical stimulation. NE also produced an acute disruption in locomotor performance. NE treatment did not enhance the area of hemorrhage. Elsewhere,27 we have further analyzed the acute effect of NE across a range of doses and injection regimes and observed a similar pattern of results. To assess whether NE has a lasting effect on locomotor performance, we evaluated animals over a 3-week period. NE treatment induced an acute rise in systolic BP/blood flow and a long-term disruption in locomotor performance. Histological analyses revealed greater tissue loss in NE-treated animals.
Finally, statistical analyses were conducted to explore whether alterations in cardiovascular function mediate the effect of pain input on hemorrhage and/or locomotor performance. The analyses revealed that pain input has both a direct and an indirect effect on hemorrhage, with the latter driven by an increase in blood flow. Although pain input increased systolic BP and heart rate, neither were linked to hemorrhage. Analyses aimed at predicting the decline in locomotor performance showed that this effect was driven by a rise in BP independent of hemorrhage. This outcome, which was derived from our analyses of the effect of pain input, is consistent with the effect of NE, which drove a rise in systolic BP and adversely affected locomotor performance but did not increase hemorrhage. Interestingly, the analyses suggested that an increase in blood flow can influence locomotor performance in two ways: fueling an increase in hemorrhage that undermines performance, as well as having an alternative (direct) effect that promotes function. The latter is consistent with the idea that bouts of hypotension must be avoided after injury to maintain blood flow to the injured tissue.13,28–30 It is also worth noting that prazosin per se drove a rise in blood flow, but did not amplify hemorrhage and only weakly affected long-term recovery (relative to the vehicle-treated group). These observations suggest that an increase in blood flow alone is not sufficient to drive hemorrhage and that this consequence must depend on an interaction with another process.
Our results are consistent with other studies examining BP in anesthetized animals with SCI and clinical data with humans, which show that maintaining BP within the normative range (MAP between 85 and 90 mmHg) can improve spinal cord blood flow and oxygenation and mitigate hemorrhage.13,28–30 Additionally, regulating BP has been associated with improved neurological recovery in humans.31–33 Our study expands this research by experimentally manipulating BP in awake animals after SCI, allowing us to examine the impact of BP on both hemorrhage and locomotor recovery. The results support the general view that BP needs to be regulated after injury and suggest that pain input can drive a rise in cardiovascular function that can (via an increase in blood flow) foster hemorrhage and (via an increase in systolic BP and hemorrhage) undermine locomotor function. These observations suggest that blunting pikes in cardiovascular function can have therapeutic benefits and that caution should be taken when attempting to pharmacologically enhance BP with NE.
Noxious stimulation and BP
It is interesting that the two forms of nociceptive input (electrical stimulation and capsaicin) had somewhat distinct cardiovascular consequences, which are presumably linked to their temporal profiles (intermittent vs. continuous) and the fiber types engaged. Capsaicin has a more selective effect, acting on TRPV1 positive C-fibers, whereas intense electrical stimulation activates both Adα and C-fibers. Electrical stimulation may induce a stronger cardiovascular response because it engages Adα fibers and is applied in a phasic manner. Further, electrical stimulation can induce a form of Pavlovian conditioning wherein environmental cues (the conditioned stimulus) are associated with shock (the unconditioned stimulus). As a consequence of this learning, re-exposure to these cues can trigger a conditioned cardiovascular response.28 This may explain why previously shocked animals continued to exhibit an increase in heart rate and blood flow when placed in the apparatus used for testing cardiovascular function, which requires restraint in opaque Plexiglas tubes that resemble those where shock was given.
Whereas both shock and capsaicin produced robust hemorrhage and undermined locomotor function, only the former had a robust effect on cardiovascular function. The capsaicin results imply that pain input can drive hemorrhage independent of its effect on BP, a conclusion that was supported by our mediational analysis.
Decreasing BP with prazosin treatment after SCI
Management of BP after SCI is important, especially in people with cervical injuries who are prone to develop a condition known as autonomic dysreflexia.29 During an episode of autonomic dysreflexia, SCI patients experience a sudden and severe increase in BP that can be deadly if left untreated.29 Although the dysregulation after thoracolumbar injuries is not as fatal, 13–35% of people with this injury will experience bradycardia.30 A number of drugs have been used for treating autonomic dysreflexia and bradycardia after SCI; however, few controlled trials have been conducted.31 The one drug currently approved for the management of autonomic dysreflexia is prazosin. After SCI, prazosin has been shown to reduce the occurrence and severity of autonomic dysreflexia episodes, help with the relaxation of the bladder, and decrease motoneuron excitation by modulating calcium-mediated currents.26,32,33
We found that prazosin given prior to noxious stimulation attenuated hemorrhage and improved long-term recovery. Further, although two animals in the vehicle-shocked group died during the recovery period, no animal died in the prazosin-treated condition. In addition, prazosin increased blood flow, which our mediational analyses suggest may improve locomotor function.
Increasing BP with NE after SCI
NE is frequently used to restore BP after SCI in humans. In the present study, a high dose of NE was used to increase BP to a level comparable to that observed after exposure to noxious electrical stimulation. At issue was whether this would be sufficient to drive hemorrhage. Encouraged by the marginal effect observed, further work has been conducted to assess the effect of NE over a wider range of doses and under conditions that maintain a prolonged rise in systolic BP. We have also tested the effect of NE given in conjunction with electrical stimulation.27 We again found that the NE-induced rise in systolic BP is accompanied by a decline in locomotor performance. However, in no case did NE treatment increase the area of hemorrhage at the site of injury. Our results suggest that a drug-induced rise in systolic BP can adversely affect behavioral function and that this effect is mediated by a process independent of hemorrhage. The results imply that caution is warranted when using NE to re-establish BP after SCI and that care must be taken to avoid bouts of hypertension.
Although we did not observe increased hemorrhage when NE is given 1 day after injury, other work suggests that this drug treatment can promote hemorrhage when applied 30 min after injury in anesthetized pigs.9 Again, the findings suggest caution regarding the use of this drug in controlling BP after SCI. Further work is needed to detail the circumstances under which a NE-induced rise in BP adversely affects spinal cord function, the processes that mediate these effects, and how the consequence of drug treatment vary with time since injury.
Pain-induced hemorrhage after SCI depends upon communication with brain systems
The observation that alterations in cardiovascular function can impact spinal cord function after SCI is consistent with the broader literature implicating an important role for systemic processes.34,35 Indeed, work in our laboratory has shown that pain-induced hemorrhage after SCI depends upon systems beyond the peripheral input and damaged spinal cord. Our work on this topic was motivated by earlier research showing that descending fibers can dampen neural excitability in response to pain input, exerting a protective effect that attenuates the development of maladaptive plasticity.36–38 Given this, we posited that removing brain input, by means of a thoracic (T2) transection would amplify pain-induced hemorrhage in animals that have received a lower (T9–10) thoracic injury.39 Contrary to our hypothesis, disrupting communication with the brain blocked pain-induced hemorrhage. Identical results were obtained when the rostral spinal cord was temporarily anesthetized with lidocaine and when rats were anesthetized with pentobarbital.1,40 Interestingly, pentobarbital anesthesia also blocked the pain-induced rise in BP/flow. The observations imply that afferent ascending fibers engage brain processes that fuel hemorrhage and tissue loss after injury. These effects may be in part the result of a brain-induced rise in BP and flow. One brain region that could be of interest is the nucleus tractus solitarius of the medulla. This region has been implicated in autonomic dysreflexia and is an important regulator of cardiopulmonary reflexes in response to nociceptive stimulation.41,42
We have framed the current work in terms of pain input, demonstrating that acute noxious stimulation fuels the spread of secondary injury and impairs long-term recovery. Elsewhere, we have shown that noxious stimulation also fosters the development of spasticity and the development of chronic pain weeks after injury.3,5 Although this work has clearly shown that engaging pain (nociceptive) fibers soon after injury has an adverse effect, and that this depends on brain systems, evidence suggests that perceived/psychological pain is not the driving force, because systemic administration of the analgesic morphine at a dose that abolishes behavioral reactivity to noxious stimulation does not attenuate nociception-induced hemorrhage or its adverse effect on behavioral recovery.1,43 This is consistent with the idea that lower-level processes within the brainstem fuel tissue loss after injury.
Implications
These results highlight the importance of managing BP soon after SCI, especially within the context of polytraumatic injuries. Active measures should be taken to ensure that BP is maintained within the normal range after SCI. Prazosin may help to reduce early hemorrhage by reducing hypertension. Morphine should be avoided, as it has been previously shown to detrimentally impact recovery after SCI and does not attenuate pain-induced hemorrhage.1,43 This study also indicates that early hemorrhage affects long-term recovery. Therefore, treatments such as lidocaine, which have been shown to reduce hemorrhage expansion, should be employed soon after injury.
Funding Information
Work on this project was supported by the National Institute of Neurological Disorders and Stroke (NS104422), and the Office of the Assistant Secretary of Defense for Health Affairs, through the Spinal Cord Injury Research Program under Award No. W81XWH-18-1-0807.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Turtle, J.D., Strain, M.M., Aceves, M., Huang, Y.J., Reynolds, J.A., Hook, M.A., and Grau, J.W. (2017). Pain input impairs recovery after spinal cord injury: treatment with lidocaine. J. Neurotrauma 34, 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turtle, J.D., Henwood, M.K., Strain, M.M., Huang, Y.J., Miranda, R.C., and Grau, J.W. (2019). Engaging pain fibers after a spinal cord injury fosters hemorrhage and expands the area of secondary injury. Exp. Neurol. 311, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grau, J.W., Washburn, S.N., Hook, M.A., Ferguson, A.R., Crown, E.D., Garcia, G., Bolding, K.A., and Miranda, R.C. (2004). Uncontrollable stimulation undermines recovery after spinal cord injury. J. Neurotrauma 21, 1795–1817. [DOI] [PubMed] [Google Scholar]
- 4. Turtle, J.D., Strain, M.M., Reynolds, J.A., Huang, Y.J., Lee, K.H., Henwood, M.K., Garraway, S.M., and Grau, J.W. (2018). Pain input after spinal cord injury (SCI) undermines long-term recovery and engages signal pathways that promote cell death. Front. Syst. Neurosci. 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garraway, S.M., Turtle, J.D., Huie, J.R., Lee, K.H., Hook, M.A., Woller, S.A., and Grau, J.W. (2011). Intermittent noxious stimulation following spinal cord contusion injury impairs locomotor recovery and reduces spinal brain-derived neurotrophic factor-tropomyosin-receptor kinase signaling in adult rats. Neuroscience 199, 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simard, J.M., Woo, S.K., Aarabi, B. and Gerzanich, V. (2013). The Sur1-Trpm4 channel in spinal cord injury. J. Spine, Suppl. 4, 002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strain, M.M., Hook, M.A., Reynolds, J.D., Huang, Y.J., Henwood, M.K., and Grau, J.W. (2019). A brief period of moderate noxious stimulation induces hemorrhage and impairs locomotor recovery after spinal cord injury. Physiol. Behav. 212, 112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Santamaria, A.J., Benavides, F.D., Padgett, K.R., Guada, L.G., Nunez-Gomez, Y., Solano, J.P., and Guest, J.D. (2019). Dichotomous locomotor recoveries are predicted by acute changes in segmental blood flow after thoracic spinal contusion injuries in pigs. J. Neurotrauma 36, 1399–1415. [DOI] [PubMed] [Google Scholar]
- 9. Williams, A.M., Manouchehri, N., Erskine, E., Tauh, K., So, K., Shortt, K., Webster, M., Fisk, S., Billingsley, A., Munro, A., Tigchelaar, S., Streijger, F., Kim, K.T., Kwon, B.K., and West, C.R. (2020). Cardio-centric hemodynamic management improves spinal cord oxygenation and mitigates hemorrhage in acute spinal cord injury. Nat. Commun. 11, 5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bingham, W.G., Goldman, H., Friedman, S.J., Murphy, S., Yashon, D. and Hunt, W.E. (1975). Blood flow in normal and injured monkey spinal cord. J. Neurosurg 43, 162–171. [DOI] [PubMed] [Google Scholar]
- 11. Hall, E.D., and Wolf, D.L. (1987). Post-traumatic spinal cord ischemia: relationship to injury severity and physiological parameters. Cent. Nerv. Syst. Trauma 4, 15–25. [DOI] [PubMed] [Google Scholar]
- 12. Fassbender, J.M., Whittemore, S.R., and Hagg, T. (2011). Targeting microvasculature for neuroprotection after SCI. Neurotherapeutics 8, 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielson, J.L., Paquette, J., Liu, A.W., Guandique, C.F., Tovar, C.A., Inoue, T., Irvine, K.A., Gensel, J.C., Kloke, J., Petrossian, T.C., Lum, P.Y., Carlsson, G.E., Manley, G.T., Young, W., Beattie, M.S., Bresnahan, J.C., and Ferguson, A.R. (2015). Topological data analysis for discovery in preclinical spinal cord injury and traumatic brain injury. Nat. Commun. 6, 8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kepler, C.K., Schroeder, G.D., Martin, N.D., Vaccaro, A.R., Cohen, M., and Weinstein, M.S. (2015). The effect of preexisting hypertension on early neurologic results of patients with an acute spinal cord injury. Spinal Cord 53, 763–766. [DOI] [PubMed] [Google Scholar]
- 15. Ehsanian, R., Haefeli, J., Quach, N., Kosarchuk, J., Torres, D., Stuck, E.D., Endo, J., Crew, J.D., Dirlikov, B., Bresnahan, J.C., Beattie, M.S., Ferguson, A.R., and McKenna, S.L. (2020). Exploration of surgical blood pressure management and expected motor recovery in individuals with traumatic spinal cord injury. Spinal Cord 58, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeVivo, M.J., and Chen, Y. (2011). Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch. Phys. Med. Rehabil. 92, 332–338. [DOI] [PubMed] [Google Scholar]
- 17. Furlan, J.C., Krassioukov, A.V., and Fehlings, M.G. (2005). The effects of gender on clinical and neurological outcomes after acute cervical spinal cord injury. J. Neurotrauma 22, 368–381. [DOI] [PubMed] [Google Scholar]
- 18. National Research Council. (2011). Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press. 10.17226/12910. [DOI]
- 19. Basso, D.M., Beattie, M.S., and Bresnahan, J.C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21. [DOI] [PubMed] [Google Scholar]
- 20. Ferguson, A.R., Hook, M.A., Garcia, G., Bresnahan, J.C., Beattie, M.S., and Grau, J.W. (2004). A simple post hoc transformation that improves the metric properties of the BBB scale for rats with moderate to severe spinal cord injury. J. Neurotrauma 21, 1601–1613. [DOI] [PubMed] [Google Scholar]
- 21. Crown, E.D., Ferguson, A.R., Joynes, R.L., and Grau, J.W. (2002). Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behav. Neurosci. 116, 1032–1051. [DOI] [PubMed] [Google Scholar]
- 22. Prahl, S. (1999). Optical Absorption of Hemoglobin. Oregon Medical Laser Center: https://omlc.org/spectra/hemoglobin/index.html. (Last accessed January 4, 2019).
- 23. Drabkin, D.L. and Austin, J.H. (1935). Spectrophotometric studies. II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J. Biol. Chem. 112, 51–65. [Google Scholar]
- 24. Behrmann, D.L., Bresnahan, J.C., Beattie, M.S., and Shah, B.R. (1992). Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J. Neurotrauma 9, 197–217. [DOI] [PubMed] [Google Scholar]
- 25. Reid, J.L., Hamilton, C.A., and Hannah, J.A. (1983). Peripheral alpha 1- and alpha 2-adrenoreceptor mechanisms in blood pressure control. Chest 83, 302–304. [PubMed] [Google Scholar]
- 26. Krum, H., Louis, W.J., Brown, D.J., and Howes, L.G. (1992). A study of the alpha-1 adrenoceptor blocker prazosin in the prophylactic management of autonomic dysreflexia in high spinal cord injury patients. Clin. Auton. Res. 2, 83–88. [DOI] [PubMed] [Google Scholar]
- 27. Johnston, D.T., Lout, E., Baine, R.E. and Grau, J.W. (2021). Susceptibility of the spinal cord to pain and hypertension after Injury. J. Neurotrauma 38, A1–A132. [Google Scholar]
- 28. Cohen, D.H., and Obrist, P.A. (1975). Interactions between behavior and the cardiovascular system. Circ. Res. 37, 693–706. [DOI] [PubMed] [Google Scholar]
- 29. Eldahan, K.C., and Rabchevsky, A.G. (2018). Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton. Neurosci. 209, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grigorean, V.T., Sandu, A.M., Popescu, M., Iacobini, M.A., Stoian, R., Neascu, C., Strambu, V., and Popa, F. (2009). Cardiac dysfunctions following spinal cord injury. J. Med. Life 2, 133–145. [PMC free article] [PubMed] [Google Scholar]
- 31. Krassioukov, A., Warburton, D.E., Teasell, R., Eng, J.J., and Spinal Cord Injury Rehabilitation Evidence Research Team (2009). A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch. Phys. Med. Rehabil. 90, 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rank, M.M., Murray, K.C., Stephens, M.J., D'Amico, J., Gorassini, M.A., and Bennett, D.J. (2011). Adrenergic receptors modulate motoneuron excitability, sensory synaptic transmission and muscle spasms after chronic spinal cord injury. J. Neurophysiol. 105, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kontani, H., and Hayashi, K. (1997). Urinary bladder response to hypogastric nerve stimulation after bilateral resection of the pelvic nerve or spinal cord injury in rats. Int. J. Urol. 4, 394–400. [DOI] [PubMed] [Google Scholar]
- 34. Sweis, R., and Biller, J. (2017). Systemic complications of spinal cord injury. Curr. Neurol. Neurosci. Rep. 17, 8. [DOI] [PubMed] [Google Scholar]
- 35. Donnelly, D.J., and Popovich, P.G. (2008). Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 209, 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Washburn, S.N., Patton, B.C., Ferguson, A.R., Hudson, K.L., and Grau, J.W. (2007). Exposure to intermittent nociceptive stimulation under pentobarbital anesthesia disrupts spinal cord function in rats. Psychopharmacology (Berl) 192, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang, Y.J., and Grau, J.W. (2018). Ionic plasticity and pain: The loss of descending serotonergic fibers after spinal cord injury transforms how GABA affects pain. Exp. Neurol. 306, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crown, E.D., and Grau, J.W. (2005). Evidence that descending serotonergic systems protect spinal cord plasticity against the disruptive effect of uncontrollable stimulation. Exp. Neurol. 196, 164–176. [DOI] [PubMed] [Google Scholar]
- 39. Reynolds, J.A., Henwood, M.K., Turtle, J.D., Baine, R.E., Johnston, D.T., and Grau, J.W. (2019). Brain-dependent processes fuel pain-induced hemorrhage after spinal cord injury. Front. Syst. Neurosci. 13, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis, J.A., Bopp, A.C., Henwood, M.K., Baine, R.E., Cox, C.C., and Grau, J.W. (2020). Pharmacological transection of brain–spinal cord communication blocks pain-induced hemorrhage and locomotor deficits after spinal cord injury in rats. J. Neurotrauma 37, 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang, K., Duan, S., Wen, X., Wang, W., Fang, S., Qi, D., Huan, X., Wang, L., and He, Z. (2017). Angiotensin II system in the nucleus tractus solitarii contributes to autonomic dysreflexia in rats with spinal cord injury. PLoS One 12, e0181495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foreman, R.D. (1999). Mechanisms of cardiac pain. Annu. Rev. Physiol. 61, 143–167. [DOI] [PubMed] [Google Scholar]
- 43. Hook, M.A., Liu, G.T., Washburn, S.N., Ferguson, A.R., Bopp, A.C., Huie, J.R., and Grau, J.W. (2007). The impact of morphine after a spinal cord injury. Behav. Brain Res. 179, 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]