Abstract
Purpose: To characterize the effects of timolol and latanoprost on calculated ocular perfusion pressure (OPP) in a multicenter, prospective, crossover-design study.
Methods: Nonglaucomatous volunteers were evaluated at baseline, after 1 week of timolol 0.5% dosed twice daily, and after 1 week of latanoprost 0.005% dosed nightly (randomized treatment order; 6-week washout period). Pneumatonometric intraocular pressure (IOP) and brachial blood pressure (BP) were evaluated at each visit. Using 3 commonly used equations, OPP was calculated based on IOP and BP. The OPPs at each visit were compared by using linear mixed-effects models.
Results: This analysis includes 121 participants (242 eyes; 75% female, 87% White, mean age 55 years). Mean OPP (standard deviation) calculated with mean arterial pressure was 46.8 (8.1) mmHg at baseline, 48.5 (7.9) mmHg with timolol (P = 0.005), and 49.6 mmHg (8.2) with latanoprost (P < 0.001). When compared with baseline, OPP calculated with diastolic BP was significantly increased with both timolol (1.3 mmHg) and latanoprost (3.1 mmHg). The OPP calculated with systolic BP was increased with latanoprost (2.8 mmHg) but decreased with timolol (−1.3 mmHg). Timolol reduced systolic BP by 3.2 mmHg. Compared with timolol, latanoprost conferred greater increases in OPP calculated with both systolic and diastolic BP compared with baseline; however, the difference in treatment effects on OPP calculated with mean arterial pressure was not significantly different (P = 0.068).
Conclusion: In this crossover study of nonglaucomatous volunteers, latanoprost increased OPP. However, timolol's benefit to OPP may be limited in part because it reduced systolic BP.
Clinical Trial Registration number: NCT01677507.
Keywords: ocular perfusion pressure, timolol, latanoprost
Introduction
Glaucoma is an optic neuropathy characterized by atrophy of the optic nerve head (ONH) and damage to retinal ganglion cells, resulting in visual field loss.1 Clinical trials have identified risk factors for glaucoma, including older age, increased intraocular pressure (IOP), thinner cornea, lower corneal hysteresis, optic disk hemorrhage, beta-zone peripapillary atrophy, systemic hypotension, and low ocular perfusion pressure (OPP).2
The OPP is an estimate of the perfusion of the ONH, and it is calculated by using equations incorporating systemic blood pressure (BP) and IOP.3–5 Increased BP allows for increased perfusion of the ONH, whereas increased IOP decreases OPP.3 Low OPP increases the risk of ONH and retinal ganglion cell damage and is a well-characterized risk factor for the development and progression of glaucoma.3–9 Unlike IOP, which is measured routinely in clinical practice, ophthalmologists rarely utilize OPP in glaucoma screening and evaluation.10
Although studies have investigated the effects of topical treatments on IOP, relatively little is known about their effects on OPP.11 Latanoprost, a prostaglandin analogue that increases the rate of uveoscleral outflow,12,13 and timolol, a topical beta-blocker that reduces the rate of aqueous humor production,14,15 are commonly used topical treatments that decrease IOP. The reduction of IOP should, in turn, increase OPP. However, as a beta-blocker, timolol (which can be systemically absorbed when applied topically to the eye) can reduce systemic BP,16 which could decrease OPP. Further research regarding the effects of timolol and latanoprost on OPP will inform the potential use of OPP as a clinical tool.
With this multicenter, prospective, crossover study, we compare the effects of timolol and latanoprost on OPP in the eyes of nonglaucomatous volunteers. We hypothesized that both latanoprost and timolol increase OPP. However, latanoprost is associated with greater reductions in IOP than timolol,17,18 and timolol may also reduce systemic BP.16 Thus, we further hypothesized that, compared with timolol, latanoprost will confer greater increases in OPP.
Methods
Study design and sample
This study presents data from the Eye Dynamics and Engineering Network (EDEN) Consortium. This is a multicenter, prospective study designed to evaluate aqueous humor dynamics in healthy volunteers, which is registered in ClinicalTrials.gov. The 3 centers were the University of Michigan, Mayo Clinic, and the University of Nebraska Medical Center. Power analysis was performed. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study. This study was approved by the Institutional Review Boards at each participating institution and conformed to the Declaration of Helsinki.
At the screening visit, subjects were interviewed and received a clinical examination, including an ophthalmologic evaluation to assess whether they met study criteria. The inclusion criteria were: age ≥40 years; healthy eyes with crystalline lenses; open angles; and ability to participate on site during the trial period. Exclusion criteria included any type of glaucoma; current use of glaucoma medication; ocular trauma, surgery, or infection; chronic eye disease; and contraindications to timolol (eg, severe asthma or chronic obstructive pulmonary disease). Participants with hypertension and those who use systemic medications that may affect IOP, such as beta-blockers, alpha-adrenergic agonists, calcium channel blockers, etc., were allowed to participate if they were on a stable regimen for at least 30 days before the baseline visit.
Participants were examined 3 times: at baseline, after 1 week of timolol 0.5% twice daily both eyes, and after 1 week of latanoprost 0.005% nightly both eyes. The treatment order was randomized with a crossover design and a 6-week intervening washout period. Participants were in- structed on how to self-administer eye drops for the 1-week treatments, including instruction to close eyelids for a short time after instilling.
Measurements
Intraocular pressure
The IOP measurements were made by pneumatonometry (Model 30 Classic, Reichert, Depew, NY) under topical anesthesia. The IOP was measured in duplicate in each eye with a third measurement taken if the difference was greater than 2 mmHg, and the mean value was recorded. The right eye was measured before the left, with both measurements taken in the morning, typically between 9 AM and 12:30 PM.
Systemic BP
Systolic (SBP) and diastolic BP (DBP) were measured in a seated position according to the PhenX toolkit protocol.19,20 The mean of 3 measurements was recorded, with each measurement taken in the morning.
Calculated OPP
OPP was calculated according to the following formulas, which have been commonly used in the prior studies.3–5 First, mean OPP (MOPP) was calculated based on participants' mean arterial pressure: MOPP = ⅔ [DBP + ⅓ (SBP − DBP)] − IOP. Second, systolic OPP (SOPP) was calculated as: SBP − IOP. Third, diastolic OPP (DOPP) was calculated by the formula: DBP − IOP. Although prior studies of OPP have typically selected 1 or 2 of these equations, we present all 3 OPPs for ease of comparison with prior studies.
Ocular characteristics
Ophthalmologic measures assessed at baseline included axial length measured by either IOLMaster (Zeiss, Dublin, CA) or Pacscan Series 300 (Sonomed, Lake Success, NY), corneal hysteresis measured by the Ocular Response Analyzer® (Reichert Technologies, Depew, NY), central corneal thickness (CCT) measured by ultrasound pachymetry (DGH Pachette, DGH Technology, Inc., Exton, PA; or Pacscan Series 300; Sonomed, New Hyde Park, NY), episcleral venous pressure measured by episcleral venomanometry (Eyetech Ltd., Boca Raton, FL), and refractive error.
Other participant characteristics
At baseline, body mass index (BMI), neck circumference, systemic disease, lifetime tobacco use, medical history, and systemic medications were assessed by using the PhenX Toolkit (www.phenxtoolkit.org).21
Statistical analysis
Analyses were performed by using Stata Statistical Software: Release 15 (StataCorp, College Station, TX) and R (R Core Team, Vienna, Austria). Two-sided p-values are presented for all statistical tests, and P < 0.05 was designated as statistically significant. Both eyes of each participant were included in the analysis. Means and standard deviations (SDs) are reported for IOP, SBP, DBP, MOPP, SOPP, and DOPP at baseline, with timolol, and with latanoprost. Linear mixed-effects models with unstructured covariance matrices and generalized least squares estimators, which adjust for inter-eye correlation, were used to compare mean IOP, SBP, DBP, MOPP, SOPP, and DOPP between baseline, and treatment with timolol and latanoprost.22
An exploratory multivariable analysis was conducted to detect associations between participant baseline characteristics and the magnitude of the effects of timolol and latanoprost on MOPP. MOPP was selected as our outcome variable, because it has been most commonly utilized in studies of OPP.3 Separate linear mixed-effects models were constructed with the effect of timolol or latanoprost on MOPP (compared with baseline) as the dependent variable. All models were adjusted for order of treatment, baseline MOPP, age, sex, and ethnicity.
Separate models were fitted to assess different categories of predictors: (1) health history (BMI, history of smoking, diabetes, hypertension, hypercholesterolemia, thrombotic disorders, depression, and cancer); (2) medication use (antihypertensive, diabetes medication, statins, steroids, thyroid medications, and psychotropic drugs); and (3) baseline ophthalmologic factors (axial length, CCT, corneal hysteresis, episcleral venous pressure, and spherical equivalent). Bonferroni adjustment was utilized to account for multiple testing.
Literature search
A search of the published literature was conducted to identify English language, peer-reviewed studies characterizing OPP under treatment with timolol or latanoprost, or both. The PubMed database was searched by using the following search strategy: ((timolol) OR (latanoprost)) AND (“ocular perfusion”). Inclusion criteria were: treatment using timolol and or latanoprost, reporting of OPP (mean, systolic, and diastolic) as an outcome, study of human subjects, and randomized controlled trial or crossover trial design. When our search identified systematic review articles, we also searched the studies included in the systematic reviews.
Results
Participant characteristics at baseline
Of the 135 participants who consented to the study, 14 were excluded from the analysis due to either: (1) missing data on IOP and BP at baseline and visits 2 and 3; (2) participant received neither latanoprost nor timolol due to withdrawal from the study, or (3) participant was found to be using topical steroid medication during the trial period. Of the 242 eyes included in this analysis, data were available for 218 eyes treated with timolol and 226 eyes treated with latanoprost.
The 121 study participants were 75% female and 87% White, with ages ranging from 40 to 81 years (mean ± SD, 55 ± 8.8 years). There were no significant differences in baseline characteristics between those included and excluded, or between those randomized to timolol first and latanoprost first. For the ocular characteristics, mean axial length (SD) was 23.9 (1.2) mm for the right eye (OD), and 23.8 (1.2) mm for the left eye (OS); CCT was 554 (38) μm for OD, and 554 (38) μm for OS; and corneal hysteresis was 10.7 (1.5) for OD, and 10.8 (1.6) for OS. Details of participant demographic information and baseline characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics of 121 Non-Glaucomatous Participants (242 Eyes) Aged 40–81 Years
| Variable | % | Proportion |
|---|---|---|
| Female | 75 | (91/121) |
| White | 87 | (105/121) |
| Black | 9 | (11/121) |
| Other Race | 4 | (5/121) |
| Known Family History of Glaucoma | 26 | (31/121) |
| Smoking (>100 lifetime cigarettes) | 34 | (33/97) |
| Diabetes | 4 | (5/121) |
| Hypercholesterolemia | 20 | (24/121) |
| Hypertension | 22 | (27/121) |
| Variable | Mean | SD |
| Age (years) | 55.1 | 8.8 |
SD, standard deviation.
Intraocular pressure
Using pneumatonometry to measure IOP, mean (SD) IOP was 15.8 (3.0) mmHg at baseline, 13.8 (2.5) mmHg with timolol, and 13.0 (2.1) mmHg with latanoprost (Table 2). Timolol reduced IOP by 2.0 mmHg [95% confidence interval (CI): 1.6, 2.4; P < 0.001] and latanoprost reduced IOP by 2.8 mmHg (95% CI: 2.4, 3.2; P < 0.001) compared with baseline. The IOP with timolol treatment was 0.8 mmHg higher than IOP with latanoprost treatment (95% CI: 0.4, 1.2; P < 0.001).
Table 2.
Blood Pressure, Intraocular Pressure, and Ocular Perfusion Pressure at Baseline, with 1-Week Timolol Treatment, and with 1-Week Latanoprost Treatment in n = 121 Nonglaucomatous Volunteers
| |
Baseline |
Timolol |
Latanoprost |
|---|---|---|---|
| Measure | Mean (SD); units: mmHg | ||
| SBP | 124.4 (14.7) | 120.8 (13.7)a | 123.1(13.0) |
| DBP | 77.1 (10.3) | 75.9 (10.5) | 77.6 (9.7) |
| IOP | 15.8 (3.0) | 13.8 (2.5)a | 13.0 (2.1)a |
| MOPP | 46.8 (8.1) | 48.5 (7.9)a | 49.6 (8.2)a |
| SOPP | 108.5 (14.4) | 106.9 (13.7)a | 110.2 (13.1)a |
| DOPP | 61.3 (10.8) | 62.0 (10.8)a | 64.7 (10.0)a |
MOPP = ⅔ [DBP + ⅓ (SBP − DBP)] − IOP. SOPP: (SBP − IOP). DOPP: (DBP − IOP).
Statistically significant difference (P < 0.05) from baseline in linear mixed-effect models.
DBP, diastolic blood pressure; DOPP, diastolic ocular perfusion pressure; IOP, intraocular pressure; MOPP, mean ocular perfusion pressure; SBP, systolic blood pressure; SOPP, systolic ocular perfusion pressure.
Systemic BP
Mean (SD) brachial SBP and DBP at each study visit are shown in Table 2. Timolol reduced SBP by 3.2 mmHg (95% CI: 0.2, 6.2; P = 0.037) compared with baseline. The differences between SBP with timolol versus latanoprost (P = 0.20) or latanoprost versus baseline (P = 0.44) were not statistically significant. In linear mixed-effects models, there was no significant difference in DBP between the study visits for either timolol or latanoprost.
Calculated OPP
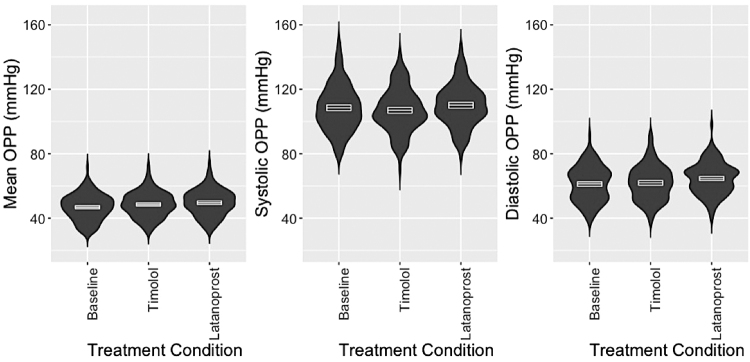
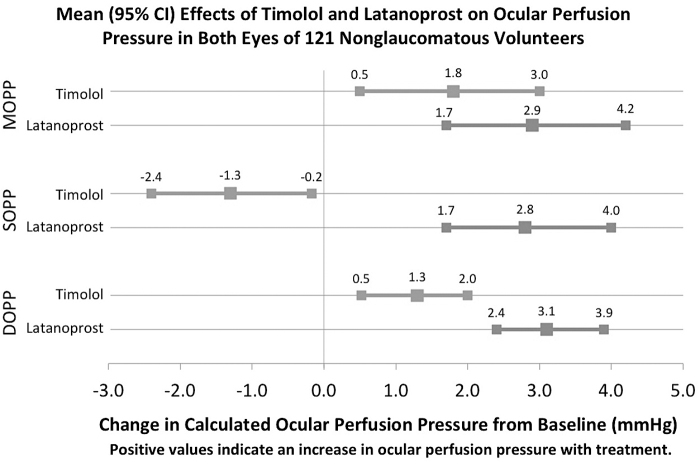
The OPP was calculated by using IOP and systemic BP. There was large overlap in the distributions of MOPP, SOPP, and DOPP by treatment, as depicted in Fig. 1. The means and SD of MOPP, SOPP, and DOPP are shown in Table 2, and the mean effect sizes of timolol and latanoprost on MOPP, SOPP, and DOPP are shown in Fig. 2. In linear mixed-effects models, latanoprost increased MOPP (mean 2.9 mmHg), SOPP (2.8 mmHg), and DOPP (3.1 mmHg). Timolol increased MOPP (1.8 mmHg) and DOPP (1.3), but it decreased SOPP (−1.3 mmHg). The changes in SOPP (P < 0.001) and DOPP (P < 0.001) were significantly different with latanoprost compared with timolol, but the difference in treatment effects on MOPP was not significantly different (P = 0.068).
FIG. 1.
Distribution of OPP among nonglaucomatous participants at baseline, with 1-week timolol treatment, and with 1-week latanoprost treatment, combined right and left eyes. Mean OPP = ⅔ [DBP + ⅓ (SBP − DBP)] − IOP. Systolic OPP = (SBP − IOP). Diastolic OPP = (DBP − IOP). The filled areas represent the kernel density distributions. White lines and boxes within each distribution represent the mean and 95-percent confidence interval for the mean. DBP, diastolic blood pressure; IOP, intraocular pressure; MOPP, mean ocular perfusion pressure; OPP, ocular perfusion pressure; SBP, systolic blood pressure.
FIG. 2.
The effects of timolol and latanoprost on OPP in linear mixed-effects models of both eyes of 121 nonglaucomatous volunteers. MOPP = ⅔ [DBP + ⅓ (SBP − DBP)] − IOP. SOPP: (SBP − IOP). DOPP: (DBP − IOP). DOPP, diastolic ocular perfusion pressure; SOPP, systolic ocular perfusion pressure.
There was no significant difference in the effect of timolol or latanoprost on OPP by treatment order. Participants at the Mayo Clinic had slightly greater increases in MOPP by both timolol (0.95 mmHg, P = 0.001) and latanoprost (1.2 mmHg, P < 0.001) compared with the other 2 study sites. Otherwise, the effects of timolol and latanoprost on SOPP and DOPP did not vary by treatment site.
Multivariable models
Separate multivariable linear mixed-effects models with generalized least squares estimators were fitted to assess whether participant characteristics were associated with the magnitudes of the effects of timolol and latanoprost on MOPP. All models were adjusted for age, sex, ethnicity, treatment order, and baseline MOPP. The results are presented in Table 3. In all 3 separate models (health conditions, medication use, and ocular factors), higher baseline MOPP was associated with a 0.05–0.10 mmHg greater increase in MOPP with timolol (P ≤ 0.005 for each). This association was not found for latanoprost.
Table 3.
Multivariable Linear Mixed-Effects Models of Change in Ocular Perfusion Pressure (Dependent Variable) and Patient Characteristics (Predictor Variables) After Treatment with Timolol or Latanoprost
| Timolol | Latanoprost | |
|---|---|---|
| Independent variable a | β (95% CI) | β (95% CI) b |
| Health history model | ||
| Body mass index | −0.01 (−0.06, 0.05) | −0.03 (−0.1, 0.04) |
| Cigarette smoking | 0.79 (0.16, 1.43)c | 0.04 (−0.79, 0.88) |
| Diabetes | −1.48 (−3.09, 0.14) | −0.62 (−2.72, 1.47) |
| Hypertension | −0.35 (−1.17, 0.46) | −0.23 (−1.3, 0.84) |
| Hypercholesterolemia | −0.54 (−1.23, 0.15) | 0.12 (−0.81, 1.05) |
| Depression | −1.63 (−2.51, −0.74)d | −0.42 (−1.74, 0.91) |
| Cancer | −0.06 (−1.06, 0.94) | −0.46 (−1.54, 0.62) |
| Thrombotic disorder | 2.34 (0.23, 4.46)c | −0.59 (−3.28, 2.11) |
| Medication use model | ||
| Psychotropic medication | −0.56 (−1.17, 0.06) | −1.24 (−1.98, −0.51)d |
| Antihypertensive | −0.33 (−1.04, 0.38) | −0.69 (−1.55, 0.18) |
| Diabetes medication | −3.88 (−5.59, −2.16)d | −1.99 (−4.04, 0.06) |
| Statin | 0.16 (−0.6, 0.92) | 0.16 (−0.76, 1.09) |
| Steroid | −1.84 (−3.42, −0.27)c | −2.43 (−4.38, −0.47)c |
| Thyroid medication | −0.30 (−1.19, 0.6) | −0.44 (−1.51, 0.63) |
| Ocular factors model | ||
| Episcleral venous pressure | −0.07 (−0.30, 0.15) | −0.19 (−0.40, 0.03) |
| Central corneal thickness | 0.01 (−0.01, 0.02) | 0.002 (−0.01, 0.01) |
| Corneal hysteresis | −0.05 (−0.31, 0.21) | −0.09 (−0.34, 0.16) |
| Axial length | 0.24 (−0.28, 78) | 0.29 (−0.07, 0.43) |
| Spherical equivalents | 0.10 (−0.14, 0.35) | 0.18 (−0.24, 0.35) |
Estimates for binary variables (ie, medical conditions and medication use) are compared with a reference group who did not have a given medical condition or did not use a given type of medication.
Positive β coefficients indicate larger increases in OPP. Models were adjusted for age, sex, ethnicity, order of treatment, and baseline OPP.
Indicates β coefficient is statistically significantly different from 0 (P < 0.05) before Bonferroni adjustment.
β coefficient is statistically significantly different from 0 (P < 0.05) with Bonferroni adjustment.
95% CI, 95-percent confidence interval; OPP, ocular perfusion pressure.
History of thrombotic disorders (estimate: 2.3 mmHg; 95% CI: 0.2, 4.5; P = 0.030) and lifetime use of >100 cigarettes (estimate: 0.8 mmHg; 95% CI: 0.2, 1.4; P = 0.014) were associated with a greater increase in MOPP on timolol treatment. Mean increase in MOPP by timolol was smaller in those who used diabetes medications (estimate: −3.9 mmHg; 95% CI: −2.2, −5.6; P < 0.001), steroid medications (estimate: −1.8 mmHg; 95% CI: −0.3, −3.4; P = 0.022), and had a history of depression (estimate: −1.6 mmHg; 95% CI: −0.7, −2.5; P < 0.001). The associations with the use of diabetes medication use and history of depression were significant after Bonferroni adjustment.
The mean increase in MOPP by latanoprost was smaller in those who used psychotropic medications (estimate: −1.2 mmHg; 95% CI: −0.5, −2.0; P = 0.001) and steroid medications (estimate: −2.4 mmHg; 95% CI: −0.5, −4.4; P = 0.015). The association with the use of psychotropic medications was significant after Bonferroni adjustment. Of note, in this cohort, 33 participants reported using psychotropic medications, 20 reported history of depression, and only 3 reported using diabetes medications. Altogether, after adjusting for multiple comparisons, the use of diabetes medications and history of depression were each associated with lesser benefits of timolol on MOPP; and the use of psychotropic medications was associated with lesser benefits of latanoprost on MOPP.
Comparison with prior studies
The PubMed search strategy for studies characterizing OPP under treatment with timolol or latanoprost returned 64 studies, 39 of which were excluded after title and abstract review. Of the remaining 25 studies, 10 were excluded after full-text review because they only used timolol or latanoprost as part of a combination treatment (n = 3), did not report sufficient data on OPP results (n = 2), did not report calculated OPP (n = 1 estimating OPP by oculo-oscillodynamography; n = 1 estimating OPP by scanning laser flowmetry), were conducted in animals (n = 1), or were systematic reviews (n = 2). Review of the references of the identified systematic reviews11,22 yielded one additional study meeting our inclusion criteria.23 Altogether, we identified 16 studies meeting our inclusion criteria.23–38 Due to high clinical heterogeneity (ie, different patient populations) and high methodological heterogeneity (ie, differing treatment lengths), meta-analysis was not performed. Study characteristics, treatment conditions, and OPPs (MOPP, SOPP, and/or DOPP) are presented in Table 4.
Table 4.
Prior Studies in Patients (with Ocular Hypertension, Normal Tension Glaucoma, or Primary Open-Angle Glaucoma) Characterizing Ocular Perfusion Pressure with Timolol or Latanoprost Treatment
| Studya | Year | Study populationb | n | Treatment condition | Treatment length | OPP typec | OPP (mean) | SD |
|---|---|---|---|---|---|---|---|---|
| Kolli et al. (present study in controls) | 2021 | CTRL | 121 | Baseline | 1 week | Mean | 46.8 | 8.1 |
| Timolol | 48.5 | 7.9 | ||||||
| Latanoprost | 49.6 | 8.2 | ||||||
| Baseline | Systolic | 108.5 | 14.4 | |||||
| Timolol | 106.9 | 13.7 | ||||||
| Latanoprost | 110.2 | 13.1 | ||||||
| Baseline | Diastolic | 61.3 | 10.8 | |||||
| Timolol | 62.0 | 10.8 | ||||||
| Latanoprost | 64.7 | 10.0 | ||||||
| Seibold et al.24 | 2017 | POAG/OHT | 30 | Baseline | 4 weeks | Mean | 39.5 | 4.9 |
| Timolol | 40.7 | 4.4 | ||||||
| Lee et al.25 | 2016 | NTG | 44 | Baseline | 4 weeks | Mean | 47.9 | 7.3 |
| Latanoprost | 47.5 | 7.2 | ||||||
| Baseline | Diastolic | 63.2 | 9.1 | |||||
| Latanoprost | 62.8 | 10.1 | ||||||
| Liu et al.26 | 2016 | POAG/OHT | 25 | Baseline | 4 weeks | Mean | 52.8 | 8.0 |
| Timolol | 54.8 | 7.5 | ||||||
| Rossetti et al.27 | 2015 | POAG/OHT | 99 | Baseline | 4 weeks | Diastolic | 62.4 | 11.1 |
| Timolol and Latanoprost | 62.8 | 6.9 | ||||||
| Baseline | Systolic | 111.8 | 15.3 | |||||
| Timolol and Latanoprost | 120.2 | 15.7 | ||||||
| Oddone et al.28 | 2015 | POAG/OHT | 32 | Baseline | 8 weeks | Diastolic | 59.8 | 9.1 |
| Timolol | 59.5 | 7.7 | ||||||
| Baseline | Systolic | 109.2 | 15.7 | |||||
| Timolol | 105.2 | 11.4 | ||||||
| Quaranta et al.29 | 2012 | POAG/OHT | 28 | Baseline | 8 weeks | Mean | 54.9 | 6.1 |
| Timolol | 48.0 | 9.0 | ||||||
| Konstas et al.30 | 2009 | POAG/OHT | 29 | Baseline | 8 weeks | Mean | 36.1 | 6.9 |
| Timolol | 43.2 | 5.5 | ||||||
| Quaranta et al.31 | 2008 | POAG/OHT | 27 | Baseline | 6 weeks | Diastolic | 47.4 | 3.32 |
| Latanoprost | 55.9 | 2.48 | ||||||
| Koz et al.32 | 2007 | POAG/OHT | 51 | Baseline | 6 months | Mean | 33.5 | 3.2 |
| Latanoprost | 39.9 | 3.1 | ||||||
| Quaranta et al.33 | 2006 | POAG/OHT | 27 | Baseline | 6 weeks | Diastolic | 50.7 | 5.9 |
| Timolol | 53.0 | 5.5 | ||||||
| Latanoprost | 56.4 | 4.9 | ||||||
| Gherghel et al.34 | 2006 | POAG/OHT | 51 | Baseline | 6 months | Mean | 39.0 | 7.9 |
| Latanoprost | 50.4 | 7.5 | ||||||
| Fuchsjäger-Mayrl et al.35 | 2005 | POAG/OHT | 70 | Baseline | 6 months | Mean | 39.3 | 7.4 |
| Timolol | 41 | 8.3 | ||||||
| Inan et al.23 | 2003 | POAG/OHT | 20 | Baseline | 3 months | Mean | 40.7 | 5 |
| Latanoprost | 46.6 | 2.9 | ||||||
| Harris et al.36 | 2003 | NTG | 20 | Baseline | 4 weeks | Mean | 48 | 9 |
| Timolol | 48 | 7 | ||||||
| Liu et al.37 | 2002 | NTG | 28 | Baseline | 4 weeks | Mean | 45.7 | 4.3 |
| Latanoprost | 49.3 | 5.9 | ||||||
| Drance et al.38 | 1998 | NTG | 27 | Baseline | 3 weeks | Mean | 49.5 | 6.7 |
| Timolol | 50.9 | 5.7 | ||||||
| Latanoprost | 53.2 | 7.3 |
For studies that reported OPP values at multiple times during the day, we present values from the morning measurement in this table.
Study population: CTRL: Healthy volunteers, POAG/OHT, NTG.
Mean OPP = ⅔ [DBP + ⅓ (SBP − DBP)] − IOP. Systolic OPP = (SBP − IOP). Diastolic OPP = (DBP − IOP).
NTG, normal tension glaucoma; POAG/OHT, primary open-angle glaucoma and ocular hypertension.
Discussion
The growing interest regarding OPP may fill a gap in knowledge of vascular perfusion of the posterior segment. Many prior clinical research tools to assess vascular perfusion of the ONH have been limited by low usability, burdensome examiner training requirements, or low reproducibility.3,39 As there is no widely used clinical instrument that provides such a measure of ONH perfusion, OPP is calculated by using 2 commonly measured clinical factors: IOP and BP. Although calculated OPP cannot account for autoregulation and dysregulation in the eye, it is a readily calculable estimate of ONH blood flow.
Based on the calculation, decreased IOP results in increased calculated OPP. Conversely, decreased BP results in decreased calculated OPP. Both timolol and latanoprost reduce IOP12–15; thus, we expected that both of these medications would increase OPP. However, topical beta-blockers can have systemic effects, including reduced BP.16 Consequently, we hypothesized that timolol's ability to increase OPP may be limited by its effect on systemic BP, consistent with prior evidence that prostaglandin analogues may be more effective than other classes of medication at increasing OPP.11
As hypothesized, both timolol and latanoprost significantly increased calculated MOPP and DOPP in this crossover design study of non-glaucomatous volunteers. However, latanoprost increased SOPP, whereas timolol significantly decreased SOPP as well as SBP. The decrease in SBP conferred by timolol indicates that there was likely some systemic absorption of timolol. Compared with timolol, the increases in both SOPP and DOPP conferred by latanoprost were significantly greater, but it is unknown whether the differences of 2–3 mmHg are clinically significant. As previously reported,17,18 latanoprost was slightly more effective in reducing IOP compared with timolol. This, in combination with timolol's ability to reduce BP, may contribute to latanoprost's theoretical effect to increase OPP compared with timolol.
Several limitations of calculated OPP as a clinical measure should be noted. First, BP in the brachial artery may not correlate precisely with ocular arterial BP. Moreover, the relationship between brachial BP and ocular arterial BP may vary based on the position in which the subject is measured (ie, supine vs. upright).40 Because BP was assessed in the seated position at each study visit and within-person differences in OPP were the primary outcome of interest, we do not expect that our results are influenced by body position. Second, calculated OPP does not account for autoregulatory changes in BP and IOP that function to optimize ocular blood flow.16,41 Third, circadian fluctuations of BP and IOP may be important in the pathogenesis of glaucoma,3,42 and these are not captured when OPP is assessed at a single time. Future interventional research should measure both IOP and systemic BP at multiple times throughout the day to determine the effects of topical medication on circadian fluctuations in calculated OPP. Fourth, because OPP is calculated based on IOP, it is difficult to distinguish whether clinical outcomes associated with increased OPP could be explained by decreases in IOP. Nevertheless, calculated OPP is a potentially useful tool to easily estimate ONH perfusion based on commonly assessed clinical measures (BP and IOP).
The results of prior studies characterizing OPP under treatment with timolol or latanoprost are summarized in Table 4. Our findings of a mean benefit of timolol and latanoprost to OPP are consistent with these previous, smaller studies of OPP.11,16,31,33,38 Prior studies have not presented all 3 calculated values for MOPP, SOPP, or DOPP as outcome measures. We calculated all 3 versions of OPP in healthy volunteers to provide results comparable to prior studies that selected only certain calculations of OPP in patients who had glaucoma. Prior studies in various glaucoma cases had small sample sizes and strict exclusion criteria that limited their generalizability. Our study includes participants with hypertension and diabetes and those who use antihypertensive medication, which is more reflective of the general population who are at risk for glaucoma.
The magnitudes of the effects of timolol and latanoprost on IOP in healthy controls tended to be slightly lower than in prior studies conducted in patients with glaucoma (Table 4). This was expected since the participants in this study were nonglaucomatous volunteers whose baseline IOPs were in the normal range. The effect of timolol on systemic BP in the present study is consistent with findings of prior studies.31,33,38 Timolol's ability to reduce BP, even when administered topically, may contribute to the smaller effect size of timolol compared with latanoprost. Altogether, patients undergoing treatment for glaucoma may not experience a clinically significant increase in OPP even when their IOP is substantially lowered.
As illustrated in Fig. 1, participants had a wide range of OPPs and there was large overlap among OPPs at the 3 study visits. This overlap reflects the small incidental effect of pharmacologically changing OPP using topical IOP-reducing drugs alone. In severe cases of glaucoma with low OPP, topical reduction of IOP may be insufficient, and laser, surgical management, or even reduction of antihypertensive medication may be indicated in some cases in consultation with the prescribing physician.41
Prior studies of OPP have used cutoffs (eg, 40 or 50 mmHg for MOPP) to dichotomize participants into high versus low OPP, rather than assessing OPP as a continuous measure.5,6 Thus, further research will be needed to assess the minimal clinically important difference for OPP reduction to confer clinical benefits (eg, visual field stabilization).43 Consequently, whether the effect sizes reported in the present study are clinically significant remains unknown. Currently, OPP is rarely used in clinical practice. This may be due to lack of BP measurement in the routine workflow, lack of familiarity with OPP as a clinical parameter, and/or the need for further research on the clinical utility of OPP. Further interventional research in patients with glaucoma is needed to determine whether monitoring of OPP provides clinically significant benefits (eg, slowing of visual field progression) over the current standard of care.
Although the pathogenesis of glaucoma remains unclear, vascular factors and ischemia may play a significant role in the disease.44,45 History of thrombotic disorders and lifetime use of >100 cigarettes were associated with greater increases in OPP with timolol. However, these associations were not robust to Bonferroni adjustment. In prior studies, several pro-thrombotic factors46,47 and smoking48 have been associated with glaucoma. Further research will be needed to assess whether the benefits of timolol may be greater in those with risk factors for micro-vascular damage near the ONH (eg, those who smoke or have thrombotic disorders). After Bonferroni adjustment, history of depression and use of diabetes medications were associated with lesser increases in OPP with timolol, and use of psychotropic medications was associated with lesser increases in OPP by latanoprost.
High IOP remains the major treatable clinical risk factor for glaucoma.49 In addition, large clinical and epidemiological studies such as the Early Manifest Glaucoma Trial and the Los Angeles Latino Eye Study (LALES) found that lower OPP is associated with increased glaucoma risk irrespective of baseline IOP.5,6 In the cross-sectional population-based LALES, a calculated MOPP of 50 mmHg or less was associated with a 3.6 times greater odds of incident open-angle glaucoma compared with a reference group with MOPP of 61–80 mmHg.6 Moreover, the Barbados Eye Study, a 9-year longitudinal study, demonstrated that calculated MOPP of 40 mmHg or less was associated with a risk ratio of 2.6 for developing open-angle glaucoma.50 Moreover, results of both LALES and the Barbados Eye Study demonstrated a significant association of higher SOPP and DOPP with greater incidence of glaucoma.6,50 Altogether, there is strong and growing evidence that IOP alone does not fully account for a person's risk of developing glaucoma.
Our study results need to be viewed with regard to several limitations. First, this study was conducted with nonglaucomatous volunteers. As such, the generalizability of the results to patients with glaucoma is uncertain. Nevertheless, the present study does not exclude those with hypertension, diabetes, and subjects who take a wide array of systemic medications. These conditions and medications (which have been considered exclusion criteria in previous studies of OPP) are prevalent in those with glaucoma and those at risk for incident glaucoma. Second, this study implemented 1-week treatment periods, which may have different effects than more prolonged use. However, this difference is likely minimal given that both timolol and latanoprost can achieve significant IOP-lowering effects within the first day of use.51 Third, the study sample was 87% White, which does limit the generalizability of our findings to other racial or ethnic groups. Fourth, this study calculated OPP by using 3 different methods, which is a surrogate for true physiologic OPP. It is possible that calculated OPP is different from true physiologic OPP, and future studies using direct measures of ocular perfusion—such as Doppler optical coherence tomography52,53—could potentially yield different results. This is an important area for future research, as calculated OPP does not capture autoregulation of IOP or BP in the eye. Though crude, calculated OPP is a convenient estimate of true OPP and has been associated with glaucoma risk. Further, the balance of IOP and BP to maintain a stable OPP relies on a complex local autoregulatory mechanism in the eye,7,54 which may not be captured when using brachial BP to calculate OPP. Nevertheless, large epidemiological studies that have associated OPP with glaucoma progression have been based on calculated OPP.3–5,50
Lastly, this study uses pneumotonometry to measure IOP, rather than Goldmann applanation tonometry. Despite differences in IOP measurements when assessed with penumotonometry versus Goldmann applanation tonometry, prior research has reported high correlation between IOP values elicited from these 2 methods.55,56 Because our main outcome is related to changes in OPP, rather than levels of OPP, different tonometry methods for measurement of IOP would likely yield a similar result and would not be expected to affect the conclusion.
This study also has several notable strengths. This is the largest study of the effects of topical timolol and latanoprost on OPP in subjects without glaucoma and implements a prospective study design. In contrast, several prior studies were underpowered to detect significant differences.11 The current study includes participants with diabetes, hypertension, and those who take anti-hypertensive medications, which is more reflective of the population at risk of developing glaucoma. For example, most prior studies (Table 4) have excluded those who have taken anti-hypertensive medications (eg, systemic beta blockers) because these may modify the effect of topical beta-blockers, such as timolol. However, this study is designed to be reflective of the general population that may be at risk of incident glaucoma, many of whom take systemic antihypertensive medications, including beta-blockers. Our crossover design with randomized treatment order allowed the calculation of within-participant effects of timolol and latanoprost on OPP. Further, the crossover design allows each participant to serve as their own control, limiting confounding by characteristics such as age, medical history, medication use, or ocular parameters.
Conclusion
Timolol and latanoprost conferred statistically significant increases in OPP in this crossover study of volunteers without glaucoma, but timolol's benefit to OPP may be limited because it also significantly reduced systemic SBP. Topical drugs that confer clinically significant benefits to IOP may not necessarily benefit OPP to the same extent. Thus, the utilization of OPP in assessing response to glaucoma treatment could provide benefits over-relying on IOP alone.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by NEI EY022124. Additional support was provided from Research to Prevent Blindness Unrestricted Grant (University of Michigan; Mayo Clinic), from the Core Center for Vision Research (P30 EY007003), and from the Michigan Institute for Clinical & Health Research for support with the REDCap database (CTSA: UL1TR000433). PhenX is funded by the National Institutes of Health (NIH) Genomic Resource Grant (U41HG007050) from the National Human Genome Research Institute (NHGRI) with current or prior funding support from the National Institute on Drug Abuse (NIDA), Office of Behavioral and Social Sciences Research (OBSSR), National Institute of Mental Health (NIMH), National Heart, Lung, and Blood Institute (NHLBI), National Institute on Minority Health and Health Disparities (NIMHD), and Tobacco Regulatory Science Program (TRSP). PhenX Toolkit version 4.
References
- 1. Weinreb, R.N., Aung, T., and Medeiros, F.A.. The pathophysiology and treatment of glaucoma: a review. JAMA. 311:1901–1911, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blumberg, D., Skaat, A., and Liebmann, J.M.. Chapter 5- Emerging risk factors for glaucoma onset and progression. In: Bagetta, G., Nucci, C., eds. Progress in Brain Research. Vol. 221. New Trends in Basic and Clinical Research of Glaucoma: A Neurodegenerative Disease of the Visual System, Part B. Waltham, MA: Elsevier; 2015; pp. 81–101. DOI: 10.1016/bs.pbr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 3. Costa, V.P., Harris, A., Anderson, D., et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 92:e252–e266, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Tham, Y.-C., Lim, S.-H., Gupta, P., Aung, T., Wong, T.Y., and Cheng, C.-Y.. Inter-relationship between ocular perfusion pressure, blood pressure, intraocular pressure profiles and primary open-angle glaucoma: the Singapore Epidemiology of Eye Diseases study. Br. J. Ophthalmol. 102:1402–1406, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Leske, M.C., Heijl, A., Hyman, L., Bengtsson, B., Dong, L., and Yang, Z.. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 114:1965–1972, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Memarzadeh, F., Ying-Lai, M., Chung, J., Azen, S.P., Varma, R., and Group LALES.. Blood pressure, perfusion pressure, and open-angle glaucoma: the Los Angeles Latino Eye Study. Invest. Ophthalmol. Vis. Sci. 51:2872–2877, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cherecheanu, A.P., Garhofer, G., Schmidl, D., Werkmeister, R., and Schmetterer, L.. Ocular perfusion pressure and ocular blood flow in glaucoma. Curr. Opin. Pharmacol. 13:36–42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samsudin, A., Isaacs, N., Tai, M.-L.S., Ramli, N., Mimiwati, Z., and Choo, M.M.. Ocular perfusion pressure and ophthalmic artery flow in patients with normal tension glaucoma. BMC Ophthalmol. 16:39, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng, Y., Wong, T.Y., Mitchell, P., Friedman, D.S., He, M., and Aung, T.. Distribution of ocular perfusion pressure and its relationship with open-angle glaucoma: the Singapore Malay Eye Study. Invest. Ophthalmol. Vis. Sci. 51:3399, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Karmel, M. The “New” Pressure for Glaucoma Specialists: Ocular Perfusion Arrives. American Academy of Ophthalmology. Published January 1, 2011. https://www.aao.org/eyenet/article/new-pressure-glaucoma-specialists-ocular-perfusion (accessed February 28, 2021).
- 11. Rennie, G., Wilkinson, A., White, A., Ruospo, M., Teixeira-Pinto, A., and Strippoli, G.. Topical medical therapy and ocular perfusion pressure in open angle glaucoma: a systematic review and meta-analysis. Curr. Med. Res. Opin. 35:1421–1431, 2019. [DOI] [PubMed] [Google Scholar]
- 12. Toris, C.B., Camras, C.B., and Yablonski, M.E.. Effects of PhXA41, a new prostaglandin F2α analog, on aqueous humor dynamics in human eyes. Ophthalmology. 100:1297–1304, 1993. [DOI] [PubMed] [Google Scholar]
- 13. Ziai, N. The effects on aqueous dynamics of PhXA41, a new prostaglandin F2α analogue, after topical application in normal and ocular hypertensive human eyes. Arch. Ophthalmol. 111:1351, 1993. [DOI] [PubMed] [Google Scholar]
- 14. Brubaker, R.F., Nagataki, S., and Bourne, W.M.. Effect of chronically administered timolol on aqueous humor flow in patients with glaucoma. Ophthalmology. 89:280–283, 1982. [DOI] [PubMed] [Google Scholar]
- 15. Schenker, H.I., Yablonski, M.E., Podos, S.M., and Linder, L.. Fluorophotometric study of epinephrine and timolol in human subjects. Arch. Ophthalmol. 99:1212–1216, 1981. [DOI] [PubMed] [Google Scholar]
- 16. Costagliola, C., Parmeggiani, F., Virgili, G., et al. Circadian changes of intraocular pressure and ocular perfusion pressure after timolol or latanoprost in Caucasians with normal-tension glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 246:389–396, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Li, T., Lindsley, K., Rouse, B., et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 123:129–140, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hou, P., Gao, P., Yang, Q., Zheng, F., and Peng, K.. Effect of latanoprost on intraocular pressure, visual acuity and C-reactive protein. Saudi J. Biol. Sci. 27:1569–1572, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. PhenX Toolkit: Protocols. https://www.phenxtoolkit.org/protocols/view/40301 (accessed February 28, 2021).
- 20. Dyer, A.R., Liu, K., Walsh, M., Kiefe, C., Jacobs, D.R., and Bild, D.E.. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J. Hum. Hypertens. 13:13–21, 1999. [DOI] [PubMed] [Google Scholar]
- 21. Phenx toolkit. PhenX Toolkit. http://www.phenxtoolkit.org/ (accessed August 17, 2021).
- 22. Greve, E.L., Rulo, A.H., Drance, S.M., Crichton, A.C., Mills, R.P., and Hoyng, P.F.. Reduced intraocular pressure and increased ocular perfusion pressure in normal tension glaucoma: a review of short-term studies with three dose regimens of latanoprost treatment. Surv. Ophthalmol. 41 Suppl 2:S89–S92, 1997. [DOI] [PubMed] [Google Scholar]
- 23. Inan, U.U., Ermis, S.S., Yücel, A., and Oztürk, F.. The effects of latanoprost and brimonidine on blood flow velocity of the retrobulbar vessels: a 3-month clinical trial. Acta Ophthalmol. Scand. 81:155–160, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Seibold, L.K., DeWitt, P.E., Kroehl, M.E., and Kahook, M.Y.. The 24-hour effects of brinzolamide/brimonidine fixed combination and timolol on intraocular pressure and ocular perfusion pressure. J. Ocul. Pharmacol. Ther. 33:161–169, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee, N.Y., Park, H.-Y.L., and Park, C.K.. Comparison of the effects of dorzolamide/timolol fixed combination versus latanoprost on intraocular pressure and ocular perfusion pressure in patients with normal-tension glaucoma: a randomized, crossover clinical trial. PLoS One. 11:e0146680, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu, J.H.K., Slight, J.R., Vittitow, J.L., Scassellati Sforzolini, B., and Weinreb, R.N.. Efficacy of latanoprostene bunod 0.024% compared with timolol 0.5% in lowering intraocular pressure over 24 hours. Am. J. Ophthalmol. 169:249–257, 2016. [DOI] [PubMed] [Google Scholar]
- 27. Rossetti, L., Sacchi, M., Karabatsas, C.H., et al. Comparison of the effects of bimatoprost and a fixed combination of latanoprost and timolol on 24-hour blood and ocular perfusion pressures: the results of a randomized trial. BMC Ophthalmol. 15:7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oddone, F., Rossetti, L., Tanga, L., et al. Effects of topical bimatoprost 0.01% and timolol 0.5% on circadian IOP, blood pressure and perfusion pressure in patients with glaucoma or ocular hypertension: a randomized, double masked, placebo-controlled clinical trial. PLoS One. 10:e0140601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quaranta, L., Katsanos, A., Floriani, I., Riva, I., Russo, A., and Konstas, A.G.P.. Circadian intraocular pressure and blood pressure reduction with timolol 0.5% solution and timogel 0.1% in patients with primary open-angle glaucoma. J. Clin. Pharmacol. 52:1552–1557, 2012. [DOI] [PubMed] [Google Scholar]
- 30. Konstas, A.G.P., Pikilidou, M.I., Tsironi, S., et al. 24-hour intraocular pressure and blood pressure levels with latanoprost/timolol fixed combination versus timolol. Curr. Eye Res. 34:369–377, 2009. [DOI] [PubMed] [Google Scholar]
- 31. Quaranta, L., Miglior, S., Floriani, I., Pizzolante, T., and Konstas, A.G.P.. Effects of the timolol-dorzolamide fixed combination and latanoprost on circadian diastolic ocular perfusion pressure in glaucoma. Invest. Ophthalmol. Vis. Sci. 49:4226–4231, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Koz, O.G., Ozsoy, A., Yarangumeli, A., Kose, S.K., and Kural, G.. Comparison of the effects of travoprost, latanoprost and bimatoprost on ocular circulation: a 6-month clinical trial. Acta Ophthalmol. Scand. 85:838–843, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Quaranta, L., Gandolfo, F., Turano, R., et al. Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Invest. Ophthalmol. Vis. Sci. 47:2917–2923, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Gherghel, D., Hosking, S.L., Cunliffe, I.A., and Armstrong, R.A.. First-line therapy with latanoprost 0.005% results in improved ocular circulation in newly diagnosed primary open-angle glaucoma patients: a prospective, 6-month, open-label study. Eye (Lond). 22:363–369, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Fuchsjäger-Mayrl, G., Wally, B., Rainer, G., et al. Effect of dorzolamide and timolol on ocular blood flow in patients with primary open angle glaucoma and ocular hypertension. Br. J. Ophthalmol. 89:1293–1297, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harris, A., Migliardi, R., Rechtman, E., Cole, C.N., Yee, A.B., and Garzozi, H.J.. Comparative analysis of the effects of dorzolamide and latanoprost on ocular hemodynamics in normal tension glaucoma patients. Eur. J. Ophthalmol. 13:24–31, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Liu, C.J.-L., Ko, Y.-C., Cheng, C.-Y., et al. Changes in intraocular pressure and ocular perfusion pressure after latanoprost 0.005% or brimonidine tartrate 0.2% in normal-tension glaucoma patients. Ophthalmology. 109:2241–2247, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Drance, S.M., Crichton, A., and Mills, R.P.. Comparison of the effect of latanoprost 0.005% and timolol 0.5% on the calculated ocular perfusion pressure in patients with normal-tension glaucoma. Am. J. Ophthalmol. 125:585–592, 1998. [DOI] [PubMed] [Google Scholar]
- 39. Kaufmann, C., Bachmann, L.M., Robert, Y.C., and Thiel, M.A.. Ocular pulse amplitude in healthy subjects as measured by dynamic contour tonometry. Arch Ophthalmol. 124:1104–1108, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Caprioli, J., Coleman, A.L., and Blood Flow in Glaucoma Discussion.. Blood pressure, perfusion pressure, and glaucoma. Am. J. Ophthalmol. 149:704–712, 2010. [DOI] [PubMed] [Google Scholar]
- 41. Topouzis, F., and Founti, P.. Weighing in ocular perfusion pressure in managing glaucoma. Open Ophthalmol. J. 3:43–45, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matlach, J., Bender, S., König, J., Binder, H., Pfeiffer, N., and Hoffmann, E.M.. Investigation of intraocular pressure fluctuation as a risk factor of glaucoma progression. Clin. Ophthalmol. 13:9–16, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kolli, A., Daniel-Wayman, S., and Newman-Casey, P.A.. The minimal clinically important difference in glaucoma medication adherence: interviews of glaucoma experts. Ophthalmic Res. 64:524–528, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chung, H.S., Harris, A., Evans, D.W., Kagemann, L., Garzozi, H.J., and Martin, B.. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv. Ophthalmol. 43:S43–S50, 1999. [DOI] [PubMed] [Google Scholar]
- 45. Moore, D., Harris, A., WuDunn, D., Kheradiya, N., and Siesky, B.. Dysfunctional regulation of ocular blood flow: a risk factor for glaucoma? Clin. Ophthalmol. 2:849–861, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yin, X., Li, J., Zhang, B., and Lu, P.. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 97:652–659, 2019. [DOI] [PubMed] [Google Scholar]
- 47. Li, S., Gao, Y., Shao, M., Tang, B., Cao, W., and Sun, X.. Association between coagulation function and patients with primary angle closure glaucoma: a 5-year retrospective case–control study. BMJ Open. 7, e016719, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pérez-de-Arcelus, M., Toledo, E., Martínez-González, M.Á., Martín-Calvo, N., Fernández-Montero, A., and Moreno-Montañés, J. Smoking and incidence of glaucoma. Medicine (Baltimore). 96, e5761, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramdas, W.D., Wolfs, R.C.W., Hofman, A., de Jong, P.T.V.M., Vingerling, J.R., and Jansonius, N.M.. Ocular perfusion pressure and the incidence of glaucoma: real effect or artifact? The Rotterdam Study. Invest. Ophthalmol. Vis. Sci. 52:6875–6881, 2011. [DOI] [PubMed] [Google Scholar]
- 50. Leske, M.C., Wu, S.-.Y., Hennis, A., Honkanen, R., and Nemesure, B.. Risk factors for incident open-angle glaucoma. Ophthalmology. 115:85–93, 2008. [DOI] [PubMed] [Google Scholar]
- 51. Lai, J.S.M., Chua, J.K.H., Leung, A.T.S., and Lam, D.S.C.. Latanoprost versus timolol gel to prevent ocular hypertension after phacoemulsification and intraocular lens implantation. J. Cataract Refract. Surg. 26:386–391, 2000. [DOI] [PubMed] [Google Scholar]
- 52. Puchner, S., Schmidl, D., Ginner, L., et al. Changes in retinal blood flow in response to an experimental increase in IOP in healthy participants as assessed with Doppler optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 61:33, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leitgeb, R.A., Werkmeister, R.M., Blatter, C., and Schmetterer, L.. Doppler optical coherence tomography. Prog. Retin. Eye Res. 41:26–43, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Best, M., Gerstein, D., Wald, N., Rabinovitz, A.Z., and Hiller, G.H.. Autoregulation of ocular blood flow. Arch. Ophthalmol. 89:143–148, 1973. [DOI] [PubMed] [Google Scholar]
- 55. Molina, N., Milla, E., Bitrian, E., Larena, C., and Martínez, L.. Comparación del tonómetro de Goldmann, neumotonómetro de contacto y el efecto del grosor corneal [Comparison of Goldmann tonometry, pneumotonometry and the effect of the central corneal thickness]. Arch. Soc. Esp. Oftalmol. 85:325–328, 2010. [DOI] [PubMed] [Google Scholar]
- 56. Zadok, D., Tran, D.B., Twa, M., Carpenter, M., and Schanzlin, D.J.. Pneumotonometry versus Goldmann tonometry after laser in situ keratomileusis for myopia. J. Cataract Refract. Surg. 25:1344–1348, 1999. [DOI] [PubMed] [Google Scholar]