Abstract
Purpose: Despite numerous recent advances in retinal gene therapy using adeno-associated viruses (AAVs) as delivery vectors, there remains a crucial need to identify viral vectors with the ability to transduce specific retinal cell types and that have a larger carrying capacity than AAV. In this study, we evaluate the retinal tropism of 2 chimeric helper-dependent adenoviruses (HDAds), helper-dependent adenovirus serotype 5 (HDAd5)/3 and HDAd5/35, both ex vivo using human retinal explants and in vivo using rats.
Methods: We transduced cultured human retinal explants with HDAd5/3 and HDAd5/35 carrying an eGFP vector and evaluated tropism and transduction efficiency using immunohistochemistry. To assess in vivo transduction efficiency, subretinal injections were performed in wild-type Sprague-Dawley rats. For both explants and subretinal injections, we delivered 10 μL (1 × 106 vector genomes/mL) and assessed tropism at 7- and 14-days post-transduction, respectively.
Results: HDAd5/3 and HDAd5/35 both transduced human retinal ganglion cells (RGCs) and Müller cells, but not photoreceptors, in human retinal explants. However, subretinal injections in albino rats resulted in transduction of the retinal pigmented epithelium only, highlighting species-specific differences in retinal tropism and the value of a human explant model when testing vectors for eventual human gene therapy.
Conclusions: Chimeric HDAds are promising candidates for the delivery of large genes, multiple genes, or neuroprotective factors to Müller cells and RGCs. These vectors may have utility for targeted therapy of neurodegenerative diseases primarily involving retinal ganglion or Müller cell types, such as glaucoma or macular telangiectasia type 2.
Keywords: chimeric helper-dependent adenovirus, tropism, human retinal explants
Introduction
Despite recent advances in retinal gene therapy using adeno-associated viruses (AAVs),1–3 there remains a crucial need for viral vectors targeting specific retinal cell types that have a larger carrying capacity than AAVs. For example, many retinal degenerative diseases (eg, ABCA4-associated Stargardt disease and USH2A-associated retinitis pigmentosa) are caused by mutations in genes that are too large to fit into a single AAV vector (maximum packaging capacity of up to 8.7 kb for some serotypes4). Although AAVs have shown the propensity to transduce multiple retinal cells types,5–7 they have primarily been used to target photoreceptors. However, other cell types are the primary site of neurodegeneration in ocular conditions such as glaucoma (retinal ganglion cells [RGCs]) or macular telangiectasia type 2 (Mac-Tel2; Müller cells) and will require vectors with tropism targeted for those cell types.
Recently, we showed that helper-dependent adenovirus serotype 5 (HDAd5), which has a large packing capacity (up to ∼35 kb), is capable of transducing explanted human retinal photoreceptors but preferentially targets Müller cells and elicits a harmful inflammatory reaction when delivered subretinally in rats.8 As such, we sought to test alternate helper-dependent adenoviruses (HDAds) that may be effective in transducing the retina and are less prone to inflammation. In this study, we evaluated the tropism and transduction efficiency of 2 chimeric HDAds, HDAd5/3 and HDAd5/35, in an ex vivo human explant model and in wild-type rats. We compared these results with our prior study evaluating tropism and transduction efficiency of different AAVs9,10 as well as HDAd5.8
Methods
Ethics statement
Human donor eyes were obtained from the Iowa Lions Eye Bank after full consent of the donors' families and in accordance with the Declaration of Helsinki. All rat experiments were conducted with the approval of the University of Iowa Animal Care and Use Committee (no. 1031317) and were consistent with the ARVO Statement for the Use of Animals in Vision Research.
Chimeric HDAds
Chimeric HDAds with the adenovirus 5 backbone and either an Ad3 or Ad35 fiber carrying eGFP under the control of the cytomegalovirus (CMVp) promoter were purchased from Creative Biolabs (Shirley, NY): HDAd5/3-CMVp-eGFP and HDAd5/35-CMVp-eGFP (Supplementary Figure S1).
Culture and viral transduction of human retinal explants
To characterize the tropism of chimeric HDAd5/3 and HDAd5/35 in human retina, we utilized our previously described approach of transducing cultured human retinal explants from fresh human donor eyes.8,10 HDAd5/3 and HDAd5/35 were each evaluated in duplicate (ie, 2 explants per virus per independent donor eye; Table 1). We pipetted 10 μL [1 × 106 vector genomes/mL (vg/mL)] of virus directly beneath each explant on the photoreceptor side to create a “bleb,” mimicking a subretinal injection, as previously described.8,10 Treating explants in this manner ensures that the retina is exposed to virus similar to that which would occur in an in vivo injection, as opposed to simply bathing the virus in media containing the virus. Treated explants were cultured for 7 days with media changes occurring every other day.
Table 1.
Human Donor Information
| Sex | Age | Cause of death | Hours after death to culture |
|---|---|---|---|
| Female | 86 | Heart and respiratory failure | 5 |
| Male | 69 | acute hypoxic respiratory failure | 7 |
Subretinal injections of chimeric HDAds in rat retinae
Two-month-old CD Sprague-Dawley IGS rats (Cat No. 001; Charles River Laboratories, Wilmington, MA) were injected subretinally, as previously detailed.8,9 Animals were injected with 10 μL of either HDAd5/3 or HDAd5/35 (1 × 106 vg/mL), and successful subretinal injections were confirmed by the presence of a subretinal bleb assessed by fundus examination. Animals were examined and sacrificed at 14 days (N = 6 independent eyes injected across 3 male and 3 female rats for each HDAd5/3 and HDAd5/35) postinjection. Fundus images were obtained before sacrifice using the Micron IV fundus camera with image-guided optical coherence tomography (Phoenix Laboratories, Pleasanton, CA).
Immunohistochemistry of retinal explants and injected rat retinae
Retinal explants were rinsed, fixed, embedded in 4% low-melting temperature agarose (Research Products International Corp., Mount Prospect, IL) and sectioned using a vibrating tissue slicer (Leica VT1000 S Vibratome, Leica Microsystems, Wetzlar, Germany) and labeled as described previously8,10 with the following primary antibodies: rabbit anti-recoverin (Cat no. AB5585; MilliporeSigma, Burlington, MA; used at a dilution of 1:1,000) and rabbit anti-Calretinin (Cat no. ab203055; Abcam, Cambridge, United Kingdom; used at a dilution of 1:200). Sections were subsequently incubated with goat anti-rabbit Alexa Fluor 546 (Cat no. A-11035; Thermo Fisher Scientific, Waltham, MA; used at a dilution of 1:1,000) and 4′,6-Diamidino-2-phenylindole dihydrochloride (Cat no. 10236276001; DAPI; MilliporeSigma; used at a dilution of 1:5,000). Chimeric HDAd-driven eGFP was visualized without antibody labeling.
Guided by limbal suture denoting injection site, rat eyes were enucleated and processed for cryosection as described previously.8,9 Eyes were sectioned at a thickness of 7 μm and labeled with rabbit anti-recoverin as described earlier. Labeled human retinal explant and rat retinal sections were imaged using a Leica TCS SPE DMi8 inverted confocal microscope system (Leica Microsystems).
Results
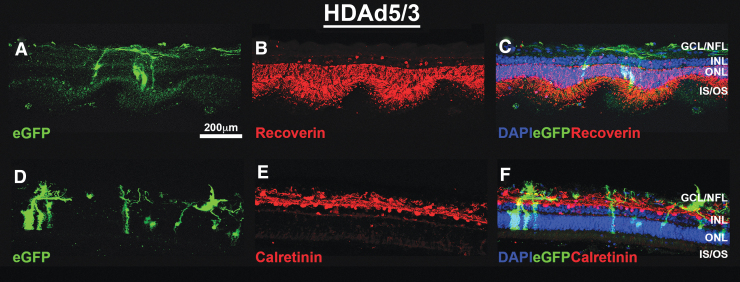
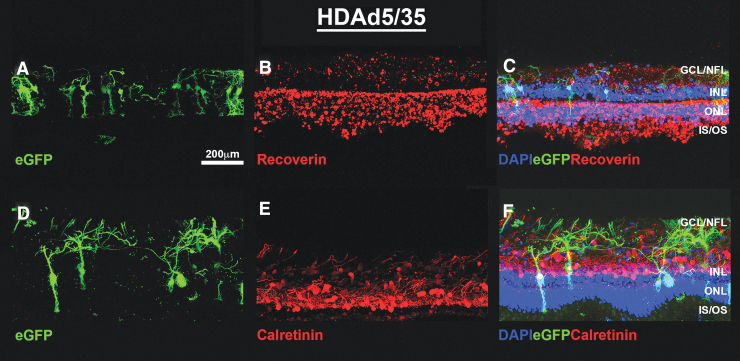
In human explants, both HDAd5/3 (Fig. 1A, D) and HDAd5/35 (Fig. 2A, D) transduce, and drive robust eGFP expression in both Müller cells and RGCs. However, no photoreceptor-specific tropism was observed for either vector in the human donor retinas tested. The vector was well tolerated, as evidenced by overall structural integrity of the retina and assessed by the expression of the photoreceptor-specific calcium-binding protein, recoverin (Figs. 1B and 2B) and the calcium homeostasis protein and amacrine cell marker, calretinin (Figs. 1E and 2E). Vector-treated explants did not differ morphologically from untransduced, control explants (Supplementary Figure S2).
FIG. 1.
HDAd5/3-CMVp-eGFP successfully transduces Müller cells and the ganglion cell/nerve fiber layers in cultured human retinal explants. Representative (N = 2 explants treated in duplicate per virus from 2 independent human donor eyes) fluorescent confocal micrographs showing eGFP (A, D; green) expression at 7-days post-transduction with HDAd5/3-CMVp-eGFP. Explants were immunocytochemically labeled with anti-Recoverin (B; RCVRN; red) or anti-Calretinin (E; red). Cell nuclei are counterstained with DAPI (blue). Merged (C, F) images showing all 3 fluorescent channels are also included. Scale bar in (A) = 200 μm. Abbreviations of retinal layers: GCL and GCL/NFL, INL, ONL, and photoreceptor IS/OS. GCL, ganglion cell layer; HDAd5, helper-dependent adenovirus serotype 5; INL, inner nuclear layer; IS/OS, inner segments and outer segments; NFL, nerve fiber layer; ONL, outer nuclear layer; RCVRN, Recoverin.
FIG. 2.
HDAd5/35-CMVp-eGFP successfully transduces Müller cells and the ganglion cell/nerve fiber layers in cultured human retinal explants. Representative (N = 2 explants treated in duplicate per virus from 2 independent human donor eyes) fluorescent confocal micrographs showing eGFP (A, D; green) expression at 7 days post-transduction with HDAd5/3-CMVp-eGFP. Explants were immunocytochemically labeled with anti-Recoverin (B; RCVRN; red) or anti-Calretinin (E; red). Cell nuclei are counterstained with DAPI (blue). Merged (C, F) images showing all 3 fluorescent channels are also included. Scale bar in (A) = 200 μm. Abbreviations of retinal layers: GCL/NFL, INL, ONL, and photoreceptor IS/OS.
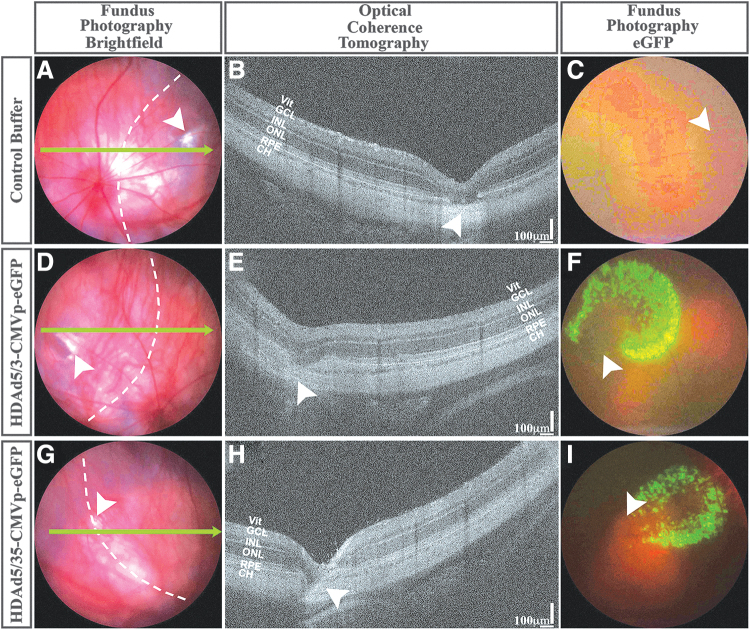
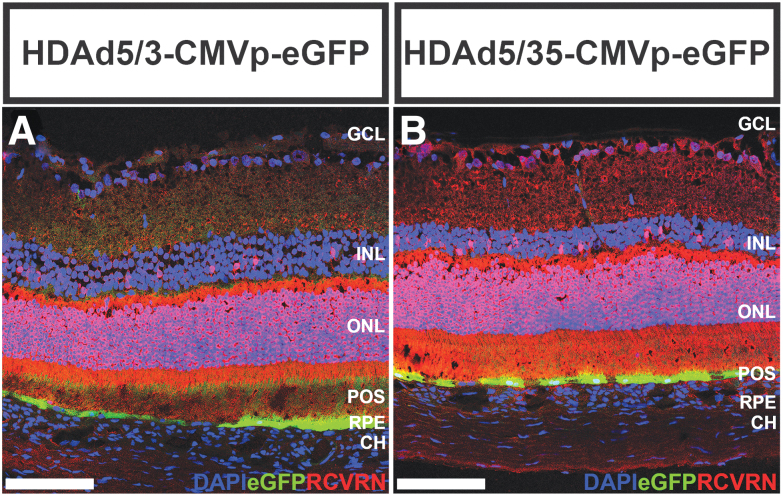
To evaluate HDAd5/3- and HDAd5/35-mediated tropism in vivo, we performed subretinal injections of each in wild-type Sprague-Dawley rats (Fig. 3). At 2-weeks postinjection, subretinal injection sites were easily identifiable, and there was no vitreous or retinal inflammation compared with buffer-injected control eyes on examination or imaging (Fig. 3E, H vs. 3B). Fluorescent fundoscopy revealed stippled eGFP expression (Fig. 3F, I), which corresponded to localized transduction of the retinal pigmented epithelium (RPE) when cryosections of chimeric HDAd-injected retinas were examined with immunohistochemistry (Fig. 4). No eGFP expression was detected in Müller cells or RGCs in the rat retinas (Fig. 4), in contrast to our studies of human retinal explants (Figs. 1 and 2). Additionally, there was no retinal inflammation observed immunohistochemically following subretinal delivery of HDAd5/3 or HDAd5/35 (Supplementary Figure S3).
FIG. 3.
Subretinal injection of chimeric HDAds in rat eyes. Representative brightfield (A, D, G) and eGFP fluorescent (C, F, I) fundus photographs and corresponding in vivo OCT line scans (B, E, H; denoted by bright green arrows in A, D, G) are shown for transscleral subretinal injections of control buffer (A–C), HDAd5/3-CMVp-eGFP (D–F) and HDAd5/35-CMVp-eGFP (G–I). Injection sites are denoted by white arrowheads. Scale bars = 100 μm in (B), (E), and (H). Abbreviations of retinal layers: Vit, GCL, INL, ONL, RPE, and CH. CH, Choroid; OCT, optical coherence tomography; RPE, retinal pigmented epithelium; HDAds, helper-dependent adenoviruses; Vit, Vitreous.
FIG. 4.
Chimeric HDAds only transduce the RPE after subretinal injection in rats. Representative [out of a total N = 6 (3 males and 3 females per vector) independently injected animals for each HDAd5/3 and HDAd5/35] fluorescent confocal micrographs showing eGFP (green) expression and labeling with anti-Recoverin (RCVRN; red) at 1-week postsubretinal injection of HDAd5/3-CMVp-eGFP (A) or HDAd5/35-CMVp-eGFP (B). Cell nuclei are labeled with DAPI (blue). Scale bars = 100 μm. Abbreviations of retinal layers: GCL, INL, ONL, RPE, and CH.
Discussion
In this study, we demonstrate that 2 chimeric HDAds (HDAd5/3 and HDAd5/35) both drive strong eGFP expression in RGCs and Müller cells, but not in photoreceptor cells of cultured human retinae. When delivered subretinally in rats, however, each chimeric vector only transduced the RPE. These results highlight the value of human explant models when testing vectors for eventual human gene therapy and emphasize the importance of considering species-specific differences across experimental models.
HDAds are an attractive target for retinal gene therapy due to their large carrying capacity, but compared with AAV, relatively little is known regarding the tropism and transduction of various HDAd subtypes. For cellular entry, HDAd5 primarily targets the coxsackievirus adenovirus receptor, which is not expressed in photoreceptor outer segments of mice.11 We previously showed that HDAd5 can transduce human photoreceptors; however, in rats the vector primarily transduced Müller cells and incited severe inflammation,8 limiting its potential for eventual clinical applications. In mice, chimeric adenoviruses HDAd5/3 and HDAd5/35 transduce photoreceptors,11 presumably because their fibers (Ad3 or Ad35 substituted for Ad5 fiber) target CD46.12 In human explants, however, we did not observe photoreceptor transduction with either HDAd5/3 or HDAd5/35; similarly, no photoreceptor transduction was seen when these vectors were injected subretinally in rats. These results further underscore the value of a human explant model when assessing vector tropism for eventual human gene therapy.8,10
Although HDAd5/3 and HDAd5/35 do not transduce photoreceptors, these chimeric vectors may have value given their specific tropism for RGCs and Müller cells and their strong transduction of these cell types. It is notable that HDAd5/3 and HDAd5/35 both transduce RGCs and Müller cells more robustly than any of the 7 different AAV serotypes we tested in prior publications.10 The ability of HDAd5/3 and HDAd5/35 to target RGCs and Müller cells make them attractive potential vectors for neurodegenerative diseases affecting these cell types. For example, glaucoma is a common and heritable13,14 ocular condition that results in RGC injury and loss. About 5% of glaucoma is caused by mutation of single genes,15 and some genes, including LTBP2 (5.5 kb), a cause of primary congenital glaucoma,16 are too large for packaging into AAV. In addition to glaucoma, RGC loss is a shared feature of other optic neuropathies, including optic neuritis (eg, due to demyelinating disorders), ischemic optic neuropathy, and inherited optic neuropathies (eg, Leber hereditary optic neuropathy), for which neuroprotective strategies are being explored to mitigate RGC cell death.17 Given their tropism and strong transduction of RGCs, HDAd5/3 and HDAd5/35 may be potential vectors to deliver neuroprotective agents such as brain-derived neurotrophic factor.18,19 Similarly, MacTel2 is a retinal degenerative disease thought to be due to Müller cell degeneration.20,21 Neuroprotective agents, such as ciliary neurotrophic factor are being explored for MacTel2,22,23 and chimeric HDAds may also be promising vectors for Müller cell-specific delivery.
Our study is limited by relatively small numbers of explants and animals tested. In addition, we only performed subretinal injections in vivo, so tropism and transduction efficiency for viral vectors may vary depending upon the route of ocular delivery,9 and there may be differences if these chimeric HDAds are delivered intravitreally or suprachoroidally. We previously showed that subretinal injection of HDAd5 caused a severe inflammatory reaction in the rat retina that was titer dependent.8 In this study, neither HDAd5/3 nor HDAd5/35 caused retinal inflammation (data not shown), but due to limitations in manufacturing these vectors, the titers delivered were lower than that used for HDAd5 (5 × 1010 vs. 1 × 106), and whether higher titers will elicit more inflammation is currently unknown.
Supplementary Material
Acknowledgments
The authors sincerely thank the Retina Research Foundation for their support of this study and Miss Katie M. Sheehan for her work in acquiring cryosections of injected rat eyes.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Pilot Grant from Retina Research Foundation (Wiley), Houston, Texas; The University of Iowa Institute for Vision Research, University of Iowa, Iowa City, Iowa; Research to Prevent Blindness, New York City, New York; National Institutes of Health Grant (NIH; Bethesda, MD, USA): P30 EY025580.
Supplementary Material
References
- 1. Bainbridge, J.W., Mehat, M.S., Sundaram, V., et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N. Engl. J. Med. 372:1887–1897, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bainbridge, J.W., Smith, A.J., Barker, S.S., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358:2231–2239, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Russell, S., Bennett, J., Wellman, J.A., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390:849–860, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu, Z., Yang, H., and Colosi, P.. Effect of genome size on AAV vector packaging. Mol. Ther. 18:80–86, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Batabyal, S., Gajjeraman, S., Pradhan, S., et al. Sensitization of ON-bipolar cells with ambient light activatable multi-characteristic opsin rescues vision in mice. Gene. Ther. 28:162–176, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lu, Q., Ganjawala, T.H., Ivanova, E., et al. AAV-mediated transduction and targeting of retinal bipolar cells with improved mGluR6 promoters in rodents and primates. Gene. Ther. 23:680–689, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vandenberghe, L.H., and Auricchio, A.. Novel adeno-associated viral vectors for retinal gene therapy. Gene. Ther. 19:162–168, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Han, I.C., Burnight, E.R., Ulferts, M.J., et al. Helper-dependent adenovirus transduces the human and rat retina but elicits an inflammatory reaction when delivered subretinally in rats. Hum. Gene. Ther. 30:1371–1384, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han, I.C., Cheng, J.L., Burnight, E.R., et al. Retinal tropism and transduction of adeno-associated virus varies by serotype and route of delivery (intravitreal, subretinal, or suprachoroidal) in rats. Hum. Gene. Ther. 31:1288–1299, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiley, L.A., Burnight, E.R., Kaalberg, E.E., et al. Assessment of adeno-associated virus serotype tropism in human retinal explants. Hum. Gene. Ther. 29:424–436, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mallam, J.N., Hurwitz, M.Y., Mahoney, T., et al. Efficient gene transfer into retinal cells using adenoviral vectors: dependence on receptor expression. Invest. Ophthalmol Vis. Sci. 45:1680–1687, 2004. [DOI] [PubMed] [Google Scholar]
- 12. Gaggar, A., Shayakhmetov, D.M., and Lieber, A.. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408–1412, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Polubriaginof, F.C.G., Vanguri, R., Quinnies, K., et al. Disease heritability inferred from familial relationships reported in medical records. Cell 173:1692–1704 e1611, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang, K., Gaitsch, H., Poon, H., et al. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet. 49:1319–1325, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fingert, J.H. Primary open-angle glaucoma genes. Eye (Lond) 25:587–595, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rauf, B., Irum, B., Khan, S.Y., et al. Novel mutations in LTBP2 identified in familial cases of primary congenital glaucoma. Mol. Vis. 26:14–25, 2020. [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson, A.M., and Di Polo, A.. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene. Ther. 19:127–136, 2012. [DOI] [PubMed] [Google Scholar]
- 18. Fudalej, E., Justyniarska, M., Kasarello, K., et al. Neuroprotective factors of the retina and their role in promoting survival of retinal ganglion cells: a review. Ophthalmic Res. 64:345–355, 2021. [DOI] [PubMed] [Google Scholar]
- 19. Chitranshi, N., Dheer, Y., Abbasi, M., et al. Glaucoma pathogenesis and neurotrophins: focus on the molecular and genetic basis for therapeutic prospects. Curr. Neuropharmacol. 16:1018–1035, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charbel Issa, P., Gillies, M.C., Chew, E.Y., et al. Macular telangiectasia type 2. Prog. Retin. Eye. Res. 34:49–77, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powner, M.B., Gillies, M.C., Zhu, M., et al. Loss of Muller's cells and photoreceptors in macular telangiectasia type 2. Ophthalmology 120:2344–2352, 2013. [DOI] [PubMed] [Google Scholar]
- 22. Chew, E.Y., Clemons, T.E., Peto, T., et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. Am. J. Ophthalmol. 159:659–666 e651, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chew, E.Y., Clemons, T.E., Jaffe, G.J., et al. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology 126:540–549, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.