PURPOSE
CAPTIVATE (NCT02910583), a randomized phase II study, evaluates minimal residual disease (MRD)-guided treatment discontinuation following completion of first-line ibrutinib plus venetoclax treatment in patients with chronic lymphocytic leukemia (CLL).
METHODS
Previously untreated CLL patients age < 70 years received three cycles of ibrutinib and then 12 cycles of combined ibrutinib plus venetoclax. Patients in the MRD cohort who met the stringent random assignment criteria for confirmed undetectable MRD (Confirmed uMRD) were randomly assigned 1:1 to double-blind placebo or ibrutinib; patients without Confirmed uMRD (uMRD Not Confirmed) were randomly assigned 1:1 to open-label ibrutinib or ibrutinib plus venetoclax. Primary end point was 1-year disease-free survival (DFS) rate with placebo versus ibrutinib in the Confirmed uMRD population. Secondary end points included response rates, uMRD, and safety.
RESULTS
One hundred sixty-four patients initiated three cycles of ibrutinib lead-in. After 12 cycles of ibrutinib plus venetoclax, best uMRD response rates were 75% (peripheral blood) and 68% (bone marrow). Patients with Confirmed uMRD were randomly assigned to receive placebo (n = 43) or ibrutinib (n = 43); patients with uMRD Not Confirmed were randomly assigned to ibrutinib (n = 31) or ibrutinib plus venetoclax (n = 32). Median follow-up was 31.3 months. One-year DFS rate was not significantly different between placebo (95%) and ibrutinib (100%; arm difference: 4.7% [95% CI, –1.6 to 10.9]; P = .15) in the Confirmed uMRD population. After ibrutinib lead-in tumor debulking, 36 of 40 patients (90%) with high tumor lysis syndrome risk at baseline shifted to medium or low tumor lysis syndrome risk categories. Adverse events were most frequent during the first 6 months of ibrutinib plus venetoclax and generally decreased over time.
CONCLUSION
The 1-year DFS rate of 95% in placebo-randomly assigned patients with Confirmed uMRD suggests the potential for fixed-duration treatment with this all-oral, once-daily, chemotherapy-free regimen in first-line CLL.
INTRODUCTION
Targeted therapies that antagonize B-cell receptor signaling by inhibiting the Bruton tyrosine kinase (BTK) pathway and restore apoptosis by inhibiting the antiapoptotic protein B-cell lymphoma 2 (BCL-2) have remarkably improved outcomes for patients with chronic lymphocytic leukemia (CLL).1 Ibrutinib, a once-daily BTK inhibitor, is the only targeted therapy to demonstrate both improved progression-free survival (PFS) and overall survival (OS) over standard chemotherapy and/or chemoimmunotherapy regimens in randomized phase III studies in previously untreated CLL or small lymphocytic lymphoma (SLL; RESONATE-2; ECOG1912).2,3 Venetoclax is an oral BCL-2 inhibitor approved for the treatment of CLL and SLL as a single agent or combined with anti-CD20 monoclonal antibodies (rituximab or obinutuzumab).4 Venetoclax provides deep responses with undetectable minimal residual disease (uMRD) rates in bone marrow (BM) of 16% with single-agent venetoclax in relapsed or refractory CLL5-8 and 57% with venetoclax plus obinutuzumab in previously untreated CLL.9 Continuous ibrutinib affords survival benefit, but there is increasing desire for convenient, all-oral, time-limited treatment options that may be safely administered in the outpatient setting.
CONTEXT
Key Objective
Continuous single-agent ibrutinib affords survival benefit in the first-line treatment of chronic lymphocytic leukemia (CLL), but there is increasing desire for convenient, all-oral, time-limited treatment options that may be safely administered in the outpatient setting. This randomized phase II study evaluated minimal residual disease (MRD)-guided treatment discontinuation following combination treatment with ibrutinib plus venetoclax in patients with previously untreated CLL.
Knowledge Generated
One-year disease-free survival rates after random assignment were not significantly different between placebo- and ibrutinib-randomly assigned patients with confirmed undetectable MRD following 12 cycles of combined ibrutinib plus venetoclax. Progression-free survival rates were ≥ 95% across all MRD-guided randomized treatment arms.
Relevance (J.W. Friedberg)
These results show the potential for fixed-duration treatment with ibrutinib plus venetoclax, using MRD guidance. Ongoing randomized trials are comparing fixed-duration therapy to continuous Bruton tyrosine kinase inhibitor therapy in patients with CLL.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Ibrutinib plus venetoclax provides synergistic and complementary antitumor activity beyond peripheral blood (PB) and BM compartments.10-14 In preclinical CLL models, combined ibrutinib plus venetoclax resulted in greater antitumor activity than either agent alone.10,11,14 Ibrutinib and venetoclax have complementary mechanisms of action: ibrutinib inhibition of BTK enhances dependence of CLL cells on BCL-2 through reductions in MCL-1 and BCL-XL.10,11 In addition to inhibiting CLL cell proliferation and survival, ibrutinib mobilizes CLL cells from protective microenvironment niches and disease compartments into circulation by blocking retention signals, rendering cells more susceptible to apoptosis, which is notably accelerated by addition of venetoclax.10,12,14,15 Recent clinical studies with ibrutinib plus venetoclax demonstrated high uMRD rates in both PB and BM in patients with CLL or SLL.16-20
With time-limited therapies, uMRD is an important end point that appears predictive of durable efficacy outcomes. In patients treated with chemoimmunotherapy, such as fludarabine, cyclophosphamide, and rituximab (FCR), uMRD status correlated with longer PFS and OS, regardless of depth of clinical response per International Workshop on CLL (iwCLL) criteria.21-23 uMRD at the end of combination venetoclax and rituximab in relapsed or refractory CLL was also predictive of longer PFS.24 Increasing MRD clearance with targeted combinations is anticipated to lead to longer PFS and potentially OS.
In CAPTIVATE, we investigated combined ibrutinib plus venetoclax in first-line treatment of CLL or SLL. We report primary analysis results from the CAPTIVATE MRD cohort evaluating disease-free survival (DFS) and treatment-free remission.
METHODS
Study Design and Patients
CAPTIVATE is a multicenter, international, randomized, phase II study conducted at 35 sites (Data Supplement, online only). The study comprised two cohorts: the MRD cohort and a separate fixed-duration (FD) cohort (enrolled after the MRD cohort and to be reported subsequently). The study was conducted in accordance with International Conference on Harmonisation guidelines for Good Clinical Practice and principles of the Declaration of Helsinki. The Protocol (online only) was approved by institutional review boards or independent ethics committees of all participating institutions. All patients provided written informed consent. This study is registered with ClinicalTrials.gov (NCT02910583).
Eligible patients were age ≥ 18 to < 70 years with previously untreated CLL or SLL requiring treatment per iwCLL criteria25 and had measurable nodal disease by computed tomography; Eastern Cooperative Oncology Group performance status 0-1; and adequate hepatic, renal, and hematologic function. Patients with known allergy to xanthine oxidase inhibitors and/or rasburicase were excluded because of requirement for tumor lysis syndrome (TLS) prophylaxis per venetoclax prescribing information.4
Treatment and Random Assignment
Patients in the MRD cohort received treatment during two phases: a prerandomization phase followed by an MRD-guided randomization phase (Data Supplement). During the prerandomization phase, patients received single-agent oral ibrutinib (420 mg once daily) lead-in for three cycles followed by ibrutinib plus oral venetoclax (target dose 400 mg once daily after standard 5-week ramp-up, with TLS prophylaxis and monitoring per venetoclax prescribing information)4 for 12 cycles. Each cycle was 28 days. TLS risk categories were based on tumor burden (Data Supplement).4 Patients in the high-risk category for TLS (≥ 1 lymph node lesion ≥ 10 cm in longest diameter, or ≥ 1 lesion ≥ 5 cm plus circulating lymphocytes > 25 × 109/L) were hospitalized during the first 24-48 hours of venetoclax treatment for more rigorous TLS monitoring and prophylaxis.4 Hospitalization was also recommended for patients with medium TLS risk and creatinine clearance < 80 mL/min.4
Patients who completed the three-cycle ibrutinib lead-in and then 12 cycles of ibrutinib plus venetoclax continued one additional cycle of ibrutinib plus venetoclax, during which MRD status was confirmed and tumor response was assessed; eligible patients were then randomly assigned to subsequent treatment according to MRD status (stratified by immunoglobulin heavy variable [IGHV] gene mutation status). Patients with Confirmed uMRD (defined as < 1 CLL cell per 10,000 leukocytes, serially over ≥ 2 assessments ≥ 3 months apart, and in both PB and BM) (Data Supplement) were randomly assigned 1:1 to double-blinded treatment with placebo or ibrutinib until confirmed MRD relapse (increase to ≥ 1 CLL cell per 100 leukocytes, confirmed on two separate occasions) or disease progression. Patients who did not meet the strict Confirmed uMRD definition (ie, uMRD Not Confirmed population) were randomly assigned 1:1 to open-label treatment with single-agent ibrutinib or continued ibrutinib plus venetoclax (maximum 2 years overall duration for venetoclax) until disease progression or unacceptable toxicity.
Outcomes and Assessments
The primary end point was 1-year DFS rate in the Confirmed uMRD population. One-year DFS was defined as absence of MRD relapse, progression, or death at least 1 year after random assignment. Secondary end points were uMRD rates in PB and BM, overall response rate per investigator assessment using 2008 iwCLL criteria,25,26 complete response (CR) rate (including CR with incomplete BM recovery [CRi]), duration of response, TLS risk category reduction (proportion of patients at high risk for TLS after ibrutinib lead-in v baseline), PFS, OS, pharmacokinetics of ibrutinib and venetoclax in combination, and safety and tolerability (Data Supplement).
Assessments included MRD status by flow cytometry and clinical response using physical examination, laboratory evaluations, and radiographic evaluation (Data Supplement). Safety was evaluated at every visit. TLS risk per US prescribing information for venetoclax4 was assessed at baseline and before venetoclax initiation, and limited pharmacokinetic sampling was performed (Data Supplement).
Statistical Analysis
Sample size was calculated based on the primary end point (1-year DFS rate) with placebo versus ibrutinib in patients with Confirmed uMRD. Assuming a 40% uMRD rate at random assignment, enrollment of 150 patients would ensure random assignment of 60 patients with Confirmed uMRD. This would provide approximately 80% power to detect a 30% difference for continued ibrutinib versus placebo at a two-sided significance level of 0.05.
Efficacy and safety were evaluated in all patients who received ≥ 1 dose of study treatment. Time-to-event end points, including 1-year DFS rates, were estimated using the Kaplan-Meier method. Between-arm difference in 1-year DFS rates was tested by Z test with standard error of each arm computed based on Greenwood's formula. Pharmacokinetics were evaluated using noncompartmental analysis. Other efficacy end points and adverse events (AEs) were summarized descriptively. 95% CIs for response rates were estimated based on the exact binomial distribution.
RESULTS
Patient Characteristics
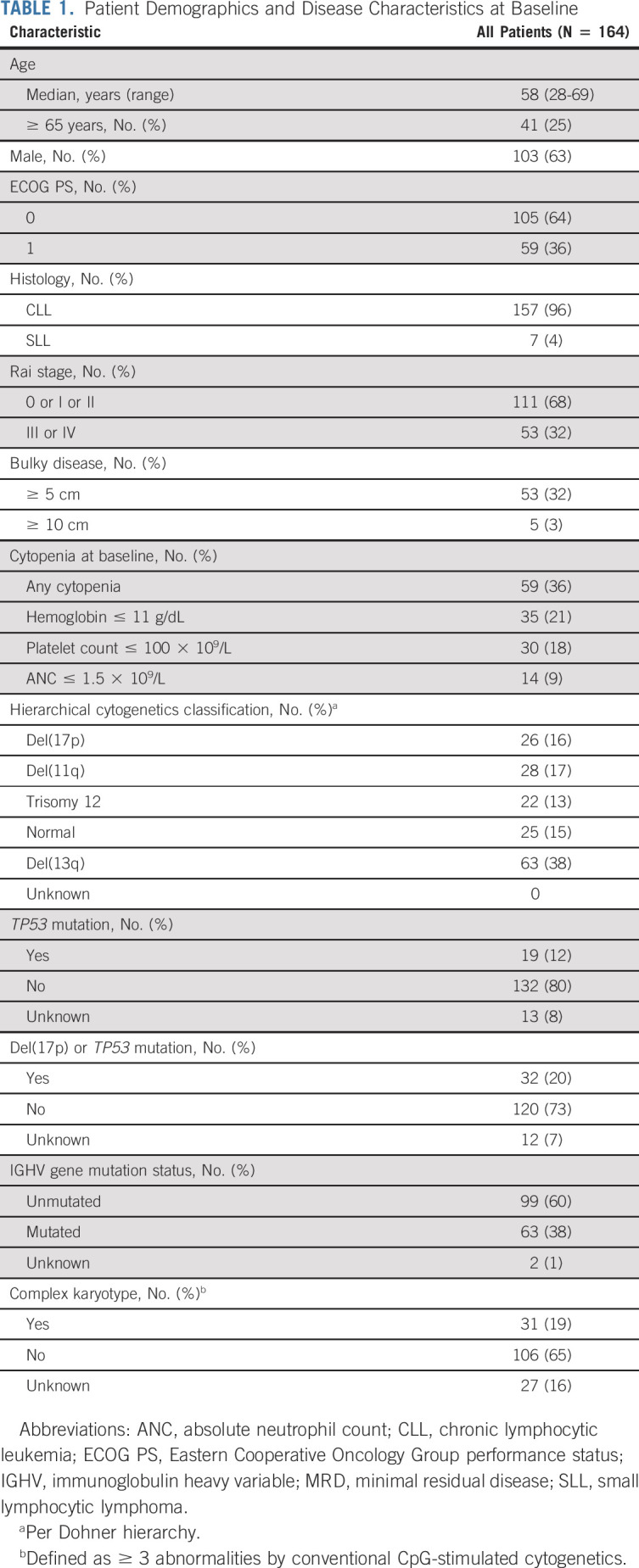
Among 164 enrolled patients, median age was 58 years (range, 28-69 years). Most patients had high-risk disease features, including del(17p) (16%), del(11q) (17%), del(17p) or TP53 mutation (20%), complex karyotype (19%), TP53 mutation (12%), and unmutated IGHV gene (60%; Table 1). Baseline characteristics by MRD-guided randomized treatment arm are shown in the Data Supplement.
TABLE 1.
Patient Demographics and Disease Characteristics at Baseline
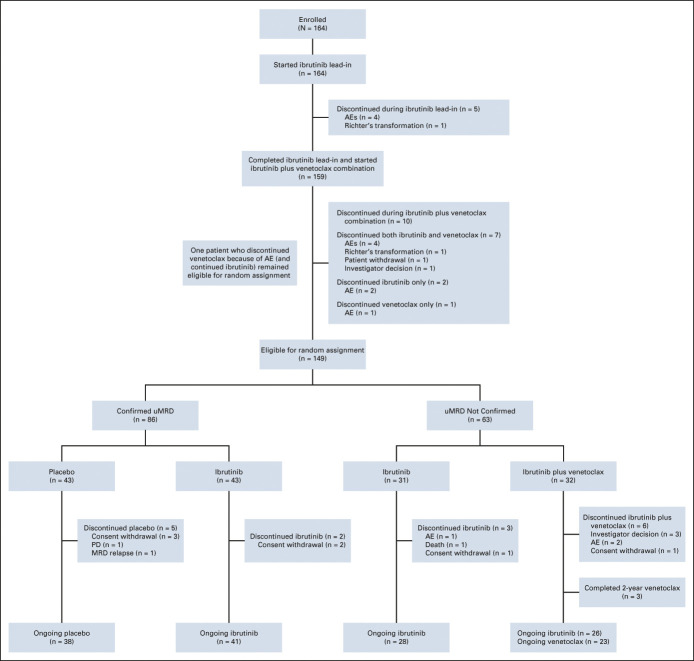
Prerandomization Phase: Disposition and Efficacy
All patients initiated ibrutinib lead-in and 159 received ibrutinib plus venetoclax (Fig 1). Overall, 148 of 164 patients (90%) completed planned prerandomization treatment (Fig 1); see the Data Supplement for all progressive disease events. Fifteen patients were ineligible for random assignment because of discontinuation of one or both study drugs (Fig 1).
FIG 1.
Patient flow and disposition. AEs, adverse events; MRD, minimal residual disease; PD, progressive disease; uMRD, undetectable minimal residual disease.
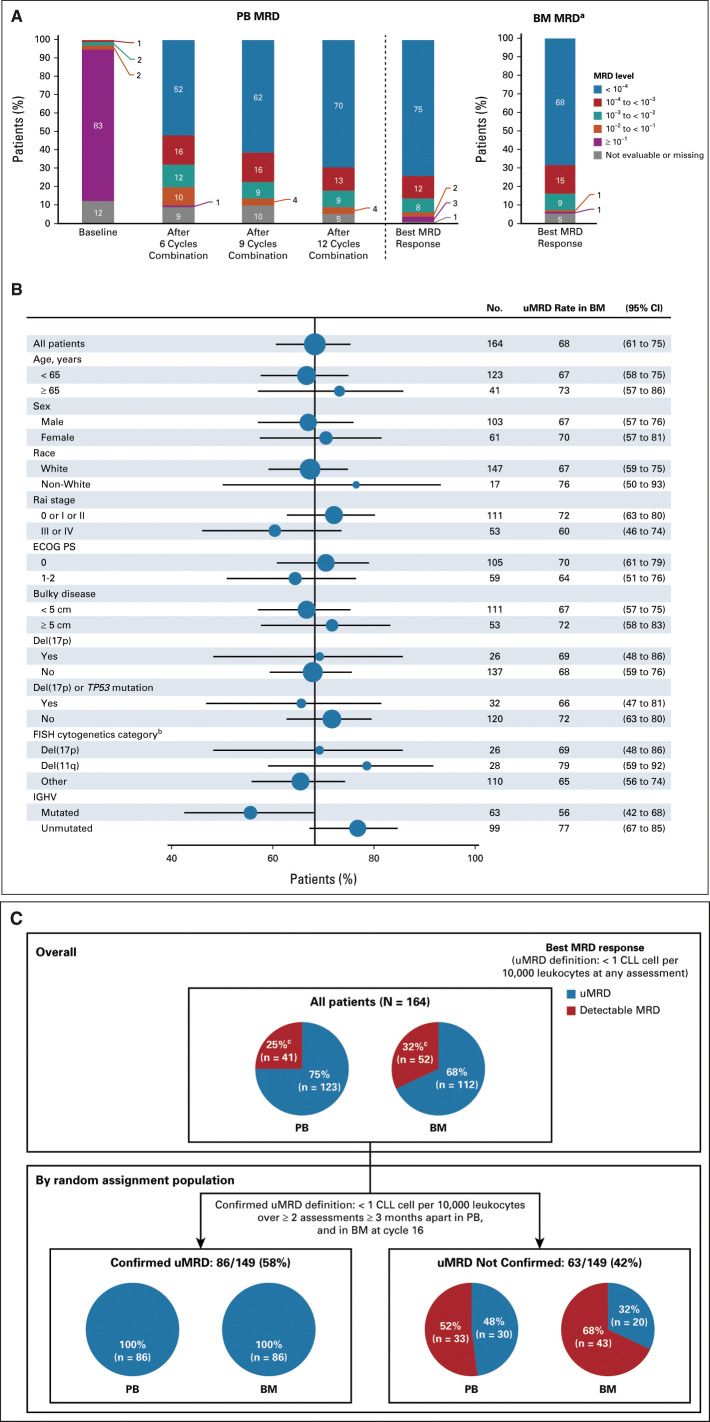
During the prerandomization phase, 75% (123 of 164) and 68% (112 of 164) of all-treated patients achieved a best MRD response of uMRD in PB and BM, respectively (75% [123 of 163] and 72% [112 of 156] of evaluable patients, respectively) (Fig 2A). Among patients with uMRD in PB prerandomization with matched BM sample, 94% had uMRD in both compartments. High uMRD rates in BM ranging from 56% to 79% were observed across patient subgroups based on clinical and biologic features, including those with high-risk disease features (Fig 2B). Response was achieved in 159 of 164 patients (overall response rate, 97%; 95% CI, 93 to 99) with CR including CRi in 76 of 164 (46%; 95% CI, 39 to 54). In patients with best response of CR including CRi, 83% (63 of 76) achieved PB uMRD and 80% (61 of 76) achieved BM uMRD; in patients with a best response of partial response (PR) or nodular PR, corresponding rates were 72% (60 of 83) and 61% (51 of 83), respectively.
FIG 2.
MRD response during the prerandomization phase. (A) MRD levels serially over time in PB and best MRD response in PB and BM. (B) Forest plot of undetectable MRD in BM across patient subgroups by baseline characteristics. (C) Best MRD response of the prerandomization phase in all-treated patients and according to Confirmed uMRD status in patients eligible for random assignment. aBM MRD assessment was scheduled after completion of 12 cycles of combination treatment (cycle 16). bPer Dohner hierarchy. cPatients without available samples (PB, n = 1; BM, n = 8) were counted as having detectable MRD. BM, bone marrow; CLL, chronic lymphocytic leukemia; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; IGHV, immunoglobulin heavy variable; MRD, minimal residual disease; PB, peripheral blood; uMRD, undetectable minimal residual disease.
Among 149 patients eligible for random assignment, 86 patients met Confirmed uMRD criteria for random assignment (best uMRD rates of 100% in both PB and BM prerandomization) (Fig 2C). The remaining 63 patients not meeting strict criteria of Confirmed uMRD for random assignment (Data Supplement) still achieved best uMRD rates of 48% (30 of 63) in PB and 32% (20 of 63) in BM prerandomization (Fig 2C).
Tumor debulking with three cycles of ibrutinib27 (reductions in lymph node diameter and absolute lymphocyte count) (Data Supplement) led to substantial reduction in TLS risk category. Together, 36 of 40 patients (90%) with high TLS risk at baseline shifted to medium or low TLS risk categories after ibrutinib lead-in and 4 of 164 (2%) remained at high TLS risk (Fig 3); no patients with medium or low TLS risk shifted to high-risk category. The proportion of patients with hospitalization indicated for TLS monitoring decreased from 47% (77 of 164) at baseline to 18% (30 of 164) after ibrutinib lead-in. Overall, 131 of 159 patients (82%) initiated venetoclax without hospitalization. Rasburicase was used per investigator discretion for treatment of hyperuricemia in 1 of 164 (1%) patients and as TLS prophylaxis in 10 of 164 (6%) patients.
FIG 3.
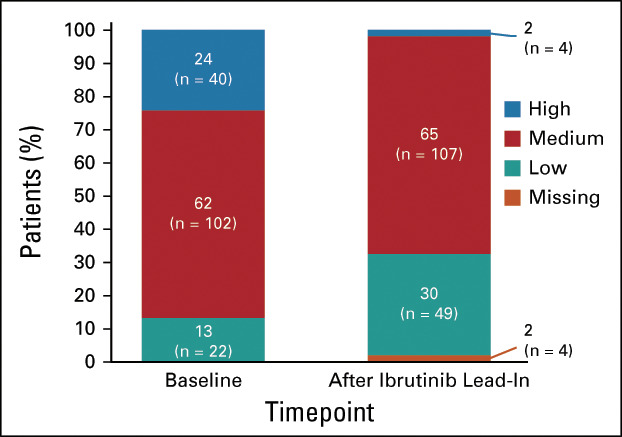
Impact of single-agent ibrutinib lead-in on TLS risk category. Shown are TLS risk categories at baseline and after ibrutinib lead-in in the prerandomization phase. TLS, tumor lysis syndrome.
Prerandomization Phase: Safety and Pharmacokinetics
Median treatment duration in the prerandomization phase was 14.7 months (range, 0.5-22.7 months) (Data Supplement). The most common treatment-emergent AEs among 164 patients treated during the prerandomization phase were diarrhea (71%, n = 116), nausea (45%, n = 74), and neutropenia (43%, n = 70) (Data Supplement). Most diarrhea events and all nausea events were grade 1 or 2. The most common grade 3 or 4 AEs were neutropenia (35%, n = 58), hypertension (8%, n = 13), thrombocytopenia (5%, n = 9), and diarrhea (5%, n = 8) (Data Supplement). No fatal AEs occurred. Serious AEs of any grade occurred in 35 patients (21%) (Data Supplement). Atrial fibrillation of any grade occurred in 12 patients (7%) and was grade ≥ 3 in three (2%). Major hemorrhage occurred in two patients (1%). Grade ≥ 3 infections occurred in 14 patients (9%) and febrile neutropenia occurred in three (2%). No clinical TLS occurred. Laboratory TLS per Howard criteria28 occurred in one patient categorized as low risk for TLS who did not receive Protocol-specified oral hydration and allopurinol; abnormalities spontaneously resolved without dose modification, clinical sequelae, or hospitalization.
AEs led to dose reductions of ibrutinib in 24 patients (15%) and venetoclax in 16 patients (10%) before random assignment. AEs led to discontinuation of ibrutinib in 10 patients (6%) and venetoclax in six patients (4%). Two patients with grade 3 or 4 cardiac arrest discontinued both ibrutinib and venetoclax; no other AEs led to discontinuation of either study drug in > 1 patient, and no patients discontinued because of infections. Diarrhea, neutropenia, and thrombocytopenia were rarely associated with dose reductions (1%-4% of patients) or discontinuations. Forty-eight patients (29%) received neutrophil growth factor at investigator discretion per local standards of care.
There was no change in ibrutinib mean plasma area under the curve (n = 112) when coadministered with venetoclax (660 ng·h/mL) versus that observed during single-agent ibrutinib lead-in (677 ng·h/mL). Venetoclax mean plasma area under the curve (n = 151) was higher when coadministered with ibrutinib (58.6 µg·h/mL) than that previously reported for single-agent venetoclax 400 mg/d (32.8 µg·h/mL), but was within the range observed in doses previously studied (150-800 mg/d).4 Pharmacokinetic-safety analyses revealed no association between exposure and AEs (data not shown).
Randomized Phase: Efficacy and Safety in Confirmed uMRD Population
The primary end point of DFS rate 1 year after random assignment was not significantly different for patients with Confirmed uMRD randomly assigned to placebo (95%) versus ibrutinib (100%; arm difference: 4.7% [95% CI, –1.6 to 10.9]; P = .15) (Fig 4). With a median follow-up of 31.3 months (16.6 months after random assignment), there were three (7%) DFS events (disease progression, n = 2; MRD relapse, n = 1) in the placebo arm and no events in the ibrutinib arm. No additional DFS events were observed with additional follow-up (Data Supplement). Estimated 30-month PFS rates (from first dose of study treatment) were 95% (95% CI, 83 to 99) with placebo and 100% (95% CI, 100 to 100) with ibrutinib (Data Supplement). Modest CR including CRi rate improvements (5%-9%) were observed postrandomization in both arms (Data Supplement). At random assignment, uMRD in PB and BM was 100%; at 12 cycles postrandomization, uMRD was 84% (36 of 43) in PB and 81% (35 of 43) in BM with placebo, and 77% (33 of 43) in both PB and BM with ibrutinib.
FIG 4.
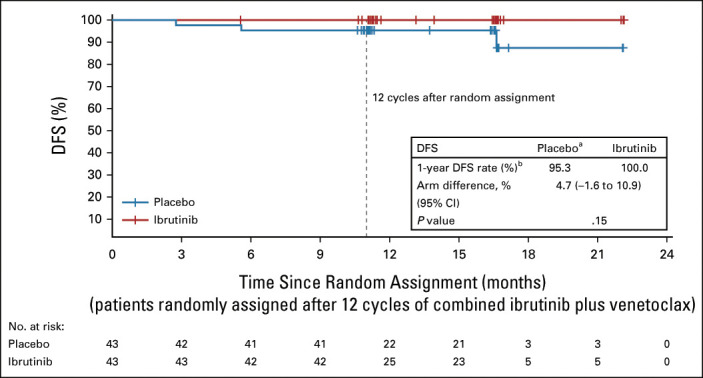
Disease-free survival. Kaplan-Meier estimates of DFS by randomized treatment arm in the Confirmed uMRD population. Tick marks indicate patients with censored data. Patients who did not experience a DFS event were censored by the last MRD sample date with a valid result or the date of last adequate disease assessment after random assignment, whichever occurred earlier. aThe three DFS events in the placebo arm were disease progression in two patients and MRD relapse in one patient; of the two patients with disease progression, after primary analysis one was confirmed to have partial response (not progression), for a total of two DFS events. bAt 12 cycles after random assignment. DFS, disease-free survival; MRD, minimal residual disease; uMRD, undetectable minimal residual disease.
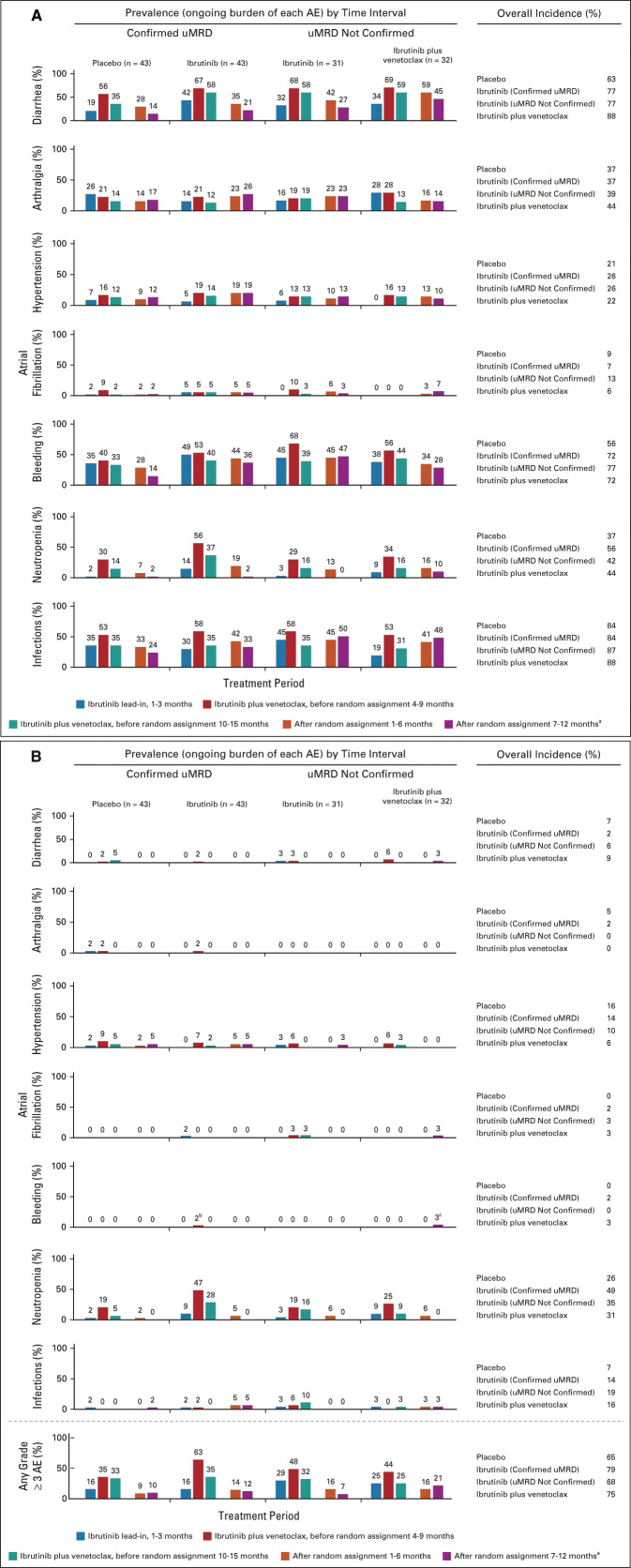
Median treatment duration across the overall study period was 31.3 months (range, 19.4-37.0 months) in the placebo arm and 31.3 months (range, 20.3-39.8 months) in the ibrutinib arm (Data Supplement). AEs of any grade generally decreased in prevalence over time after random assignment (Fig 5A). Prevalence of grade ≥ 3 AEs also decreased before and after random assignment, with a greater reduction in the placebo arm (Fig 5B). Diarrhea and neutropenia were less prevalent with placebo versus ibrutinib before and after random assignment and decreased over time in both arms (Figs 5A and 5B).
FIG 5.
Rates over time of AEs of clinical interest. (A) Prevalence of AEs of clinical interest over time by randomized treatment arm for any-grade AEs and (B) grade ≥ 3 AEs. aAt postrandomization 7-12 months, Confirmed uMRD population: placebo (n = 42), ibrutinib (n = 42); uMRD Not Confirmed population: ibrutinib (n = 30), ibrutinib plus venetoclax (n = 29). bGrade 3 menorrhagia in one patient. cGrade 3 retroperitoneal hemorrhage in one patient. AEs, adverse events; uMRD, undetectable minimal residual disease.
Randomized Phase: Efficacy and Safety in uMRD Not Confirmed Population
Estimated 30-month PFS rates in the uMRD Not Confirmed population were 95% (95% CI, 71 to 99) with ibrutinib and 97% (95% CI, 79 to 100) with ibrutinib plus venetoclax (Data Supplement). Approximately half of the patients who achieved a best response of PR prerandomization converted to CR including CRi with further ibrutinib (8 of 15) or ibrutinib plus venetoclax (10 of 24) (Data Supplement). With randomized treatment, the proportion of patients with best response of uMRD remained relatively unchanged at 45% in PB but improved from 32% to 42% in BM with ibrutinib, and from 50% to 69% in PB and from 31% to 66% in BM with ibrutinib plus venetoclax (Fig 6).
FIG 6.
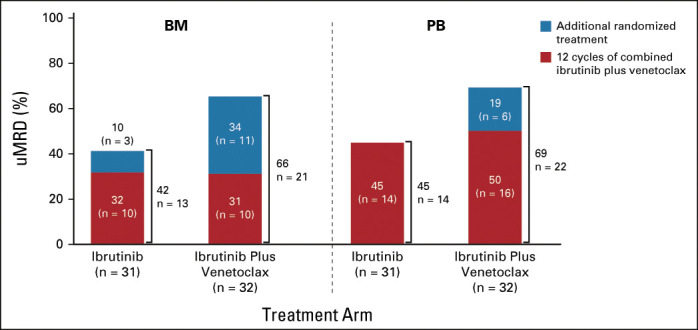
Best overall uMRD rates by randomized treatment arm in the uMRD Not Confirmed population. BM, bone marrow; PB, peripheral blood; uMRD, undetectable minimal residual disease.
Median treatment duration across the overall study period was 31.2 months (range, 17.8-36.6 months) in the ibrutinib arm and 29.2 months (range, 15.4-36.9 months) in the ibrutinib plus venetoclax arm (Data Supplement). In the uMRD Not Confirmed population, the prevalence of grade ≥ 3 AEs was higher with ibrutinib plus venetoclax than with ibrutinib during months 7-12 postrandomization (Fig 5B). The prevalence of infections increased postrandomization in both arms (Fig 5A). One grade 5 AE (sudden cardiac death) occurred in the ibrutinib arm during cycle 32.
DISCUSSION
First-line treatment with three cycles of single-agent ibrutinib followed by 12 cycles of combined ibrutinib plus venetoclax provided deep remissions, as evidenced by attainment of uMRD in over two-thirds of patients with CLL or SLL, including high BM uMRD rates in those with high-risk disease features, such as del(17p) or TP53 mutation, del(11q), and unmutated IGHV gene. Overall, best uMRD rates were high in both BM (68%) and PB (75%), and compartmental concordance was 94%. These findings are consistent with preclinical synergistic antitumor activity between ibrutinib and venetoclax.10,11,14
Given the lack of information on PFS outcomes after FD treatment with ibrutinib or venetoclax at the time of study design, a strict definition of Confirmed uMRD was used to ensure equipoise for placebo-randomly assigned patients. For patients who achieved Confirmed uMRD in PB and BM with ibrutinib plus venetoclax prerandomization, rates of DFS 1 year after random assignment to placebo or ibrutinib were comparable at 95% and 100%, respectively, suggesting that ibrutinib can be discontinued in the setting of a deep response with minimal risk of early relapse during the first year after discontinuation. The proportion of patients with uMRD in PB at 12 cycles postrandomization was comparable with placebo or ibrutinib; the majority (84%) of placebo patients still had uMRD through the first year after discontinuing active CLL treatment, supporting the potential for durable, treatment-free remissions with FD ibrutinib plus venetoclax.
PFS rates at 30 months were ≥ 95% across all four treatment arms in this population of young, fit patients. Taking patient population differences into account, and pending confirmation in the phase III setting, these rates appear to compare favorably with 3-year PFS rates reported with FCR (73%), continuous ibrutinib combined with rituximab (89%), and FD venetoclax plus obinutuzumab (82%).3,9 Longer follow-up and results from the CAPTIVATE FD cohort and ongoing randomized phase III study comparing FD ibrutinib plus venetoclax to chlorambucil plus obinutuzumab (GLOW, NCT03462719) will help answer important remaining questions regarding time-limited treatment with this promising combination, including durability, efficacy in patients with high-risk genomic features, and characteristics of patients most suitable to receive time-limited versus continuous treatment with ibrutinib. Observed high CR and uMRD rates may translate into even longer-term PFS.21
uMRD rates are consistent with those previously reported with ibrutinib plus venetoclax (61% in BM after 12 cycles) in first-line CLL,29 and higher than those reported with single-agent ibrutinib (≤ 10% in both PB and BM)30,31 or single-agent venetoclax (PB, 27%; BM, 16% in relapsed or refractory patients).5-8 uMRD rates also compare favorably with first-line CLL treatments, such as ibrutinib plus obinutuzumab (PB, 30%; BM, 20%),32 FCR (PB, 59%-63%; BM, 43%),3,21-23 venetoclax plus obinutuzumab (PB, 76%; BM, 57%),9 and ibrutinib plus venetoclax plus obinutuzumab (67% in both PB and BM),33 particularly for MRD eradication in BM. The results from the postrandomization phase add to the body of evidence correlating depth of response (uMRD and CR) with survival outcomes, with similar changes in uMRD and CR status in patients receiving further treatment with placebo or ibrutinib after 12 cycles of ibrutinib plus venetoclax. Greater improvements in uMRD rates are seen in patients with uMRD Not Confirmed status receiving further treatment with ibrutinib plus venetoclax. Additional follow-up will elucidate the impact of further treatment with ibrutinib or ibrutinib plus venetoclax on long-term outcomes, such as PFS.
Reductions in TLS risk category through effective debulking before venetoclax initiation have the potential to improve convenience for patients, caregivers, and health care providers and increase the ease of venetoclax administration. In the CLL14 study, no TLS was noted during venetoclax initiation after initial treatment with obinutuzumab; however, TLS occurred during obinutuzumab lead-in.34 Three cycles of single-agent ibrutinib reduced TLS risk category in 90% of patients with high baseline TLS risk and only 2% remained categorized with high risk before initiation of venetoclax ramp-up. TLS risk category reduction was primarily attributable to rapid reductions in lymph node bulk with single-agent ibrutinib as predicted by earlier studies.27,29 Consequently, the frequency with which hospitalization was indicated for TLS monitoring decreased by more than half (from 47% to 18%) after ibrutinib lead-in. Moreover, rasburicase use for TLS prophylaxis (6%) was lower than that reported with single-agent venetoclax (27%-45%).35,36
The safety profile of ibrutinib plus venetoclax was consistent with known AEs for each agent alone, with no new safety signals observed. Diarrhea and neutropenia infrequently led to dose modifications or treatment discontinuation. Rates of grade ≥ 3 diarrhea were somewhat higher than expected with ibrutinib alone but were similarly low (≤ 5%) as with continuous ibrutinib plus rituximab or FCR,3 venetoclax plus obinutuzumab,9 or ibrutinib plus venetoclax plus obinutuzumab.33 Rates of grade ≥ 3 neutropenia (35%) were higher than continuous ibrutinib plus rituximab (26%)3 but lower than FCR (45%),3 venetoclax plus obinutuzumab (53%),9 or ibrutinib plus venetoclax plus obinutuzumab (56%).33 AEs led to discontinuation of ibrutinib and/or venetoclax in 7% of patients prerandomization, compared with 11% and 24% with ibrutinib plus rituximab and FCR, respectively, in ECOG19123 and 16% with venetoclax plus obinutuzumab in CLL14.9
Frequencies of new or ongoing AEs were generally highest during the first 6 months of prerandomization treatment with ibrutinib plus venetoclax and then decreased over time irrespective of randomized arms. A slightly higher prevalence of infections was observed postrandomization in the uMRD Not Confirmed population than in the Confirmed uMRD population, suggesting that prevalence of infections may be influenced by disease status. Postrandomization treatment with ibrutinib plus venetoclax was associated with higher prevalence of grade ≥ 3 AEs, any-grade neutropenia, and any-grade diarrhea than placebo or continued ibrutinib. Thus, the benefit of improved uMRD and CR rates with continued ibrutinib plus venetoclax needs to be weighed against the risk of increased or ongoing toxicities.
In conclusion, the ibrutinib plus venetoclax combination for first-line treatment of patients with CLL or SLL represents an all-oral, once-daily, chemotherapy-free regimen that provides high rates of uMRD in both BM and PB. The 1-year DFS rate of 95% in patients with Confirmed uMRD randomly assigned to placebo following 12 cycles of combined ibrutinib plus venetoclax and 30-month PFS rates of ≥ 95% across MRD-guided randomized treatment arms suggest the potential for FD treatment with this combination, which may provide physicians with the ability to continue to select ibrutinib-based therapy (continuous or fixed) in the outpatient setting while considering patient preferences and treatment goals. Fixed duration is being evaluated in the younger population of patients in the CAPTIVATE FD cohort and in a complementary elderly or unfit population in the ongoing phase III GLOW study.
ACKNOWLEDGMENT
The authors thank all the patients who participated in this study and their supportive families, as well as the investigators and clinical research staff from the study centers; Kristin Russell, BS, of Pharmacyclics LLC, an AbbVie Company, for her contributions to clinical trial management; and Melanie Sweetlove, MSc, for medical writing support, which was funded by Pharmacyclics LLC, an AbbVie Company.
William G. Wierda
Consulting or Advisory Role: Sanofi
Research Funding: GlaxoSmithKline/Novartis, AbbVie, Genentech, Pharmacyclics, Acerta Pharma, Gilead Sciences, Janssen, Juno Therapeutics, Kite, a Gilead Company, Oncternal Therapeutics, Loxo, Xencor, miRagen, Sunesis Pharmaceuticals, Cyclacel
John N. Allan
Honoraria: AstraZeneca, AbbVie, Pharmacyclics/Janssen, BeiGene
Consulting/Advisory Role: AbbVie/Genentech, Pharmacyclics, Ascentage Pharma, BeiGene, Janssen Oncology, Epizyme, AstraZeneca, TG Therapeutics, ADC Therapeutics
Research Funding: Genentech, Janssen, Celgene, TG Therapeutics
Tanya Siddiqi
Consulting/Advisory Role: Juno Therapeutics, AstraZeneca, BeiGene, Celgene, Pharmacyclics, Bristol Myers Squibb/Celgene, Pharmacyclics/Janssen
Speakers Bureau: Pharmacyclics/Janssen, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene
Research Funding: Juno Therapeutics (Inst), Kite, a Gilead Company (Inst), Acerta Pharma (Inst), TG Therapeutics (Inst), BeiGene (Inst), Pharmacyclics (Inst), Celgene (Inst), Oncternal Therapeutics (Inst)
Thomas J. Kipps
Employment: Moores Cancer Center
Stock and Other Ownership Interests: Oncternal Therapeutics
Honoraria: Pharmacyclics, AbbVie, Janssen, Genentech, Gilead Sciences, DAVAOncology, AstraZeneca
Consulting/Advisory Role: AbbVie, Pharmacyclics, Genentech, Janssen, DAVAOncology
Speakers Bureau: Verastem/Pharmacyclics, Pharmacyclics/Janssen, AbbVie/Genentech, Gilead Sciences, DAVA Pharmaceuticals
Research Funding: Pharmacyclics/Janssen (Inst), Breast Cancer Research Foundation (Inst), Oncternal Therapeutics (Inst), Leukemia and Lymphoma Society (Inst), California Institute for Regenerative Medicine (CIRM) (Inst), National Cancer Institute (Inst), NIH (Inst)
Patents, Royalties, Other Intellectual Property: Cirmtuzumab was developed by Thomas J. Kipps in the Thomas J. Kipps laboratory and licensed by the University of California to Oncternal Therapeutics, Inc, which provided stock options and research funding to the Thomas J. Kipps laboratory. (Inst)
Travel, Accommodations, Expenses: AbbVie/Pharmacyclics, Genentech/Roche, Janssen, Gilead Sciences, Celgene, DAVAOncology, Breast Cancer Research Foundation, TG Therapeutics, Verastem, AstraZeneca
Stephen Opat
Honoraria: AbbVie, AstraZeneca, Janssen, Roche, Gilead Sciences, Mundipharma, Takeda, Merck, and CSL Behring
Consulting/Advisory Role: AbbVie, AstraZeneca, Janssen, Celgene, Novartis, Gilead Sciences, Takeda, Merck, Mundipharma, CSL Behring
Research Funding: AstraZeneca (Inst), BeiGene (Inst), Roche (Inst), AbbVie (Inst), Gilead Sciences (Inst), Takeda (Inst), Pharmacyclics (Inst), Janssen (Inst), Celgene (Inst), Merck (Inst), Epizyme (Inst)
Travel, Accommodations, Expenses: Roche
Alessandra Tedeschi
Consulting/Advisory Role: Janssen, BeiGene, AstraZeneca, AbbVie
Speakers Bureau: AbbVie, AstraZeneca, Janssen, BeiGene
Xavier C. Badoux
Honoraria: Janssen/Pharmacyclics, AbbVie
Bryone J. Kuss
Stock and Other Ownership Interests: Commonwealth Serum Laboratories (CSL)
Honoraria: Roche, Janssen Oncology, AstraZeneca, AbbVie, Merck, Mundipharma, Takeda, Sandoz
Consulting/Advisory Role: AbbVie, Janssen Oncology, Kyowa Kirin International, AstraZeneca
Speakers Bureau: AstraZeneca, Janssen-Ortho
Sharon Jackson
Honoraria: AbbVie NZ Ltd
Consulting/Advisory Role: AbbVie NZ
Speakers Bureau: AbbVie NZ
Travel, Accommodations, Expenses: Roche NZ
Carol Moreno
Consulting/Advisory Role: Janssen Oncology, Abbott/AbbVie, AstraZeneca, BieGene
Speakers Bureau: Janssen Oncology
Research Funding: AbbVie
Ryan Jacobs
Consulting/Advisory Role: AstraZeneca, Janssen Oncology, Secura Bio, Genentech, Adaptive Biotechnologies, ADC Therapeutics, TG Therapeutics
Speakers Bureau: Pharmacyclics, Janssen Oncology, AbbVie, TG Therapeutics, AstraZeneca
Research Funding: TeneoBio (Inst), Pharmacyclics (Inst), TG Therapeutics (Inst), MEI Pharma (Inst)
John M. Pagel
Consulting/Advisory Role: Gilead Sciences, AstraZeneca, Actinium Pharmaceuticals, BeiGene, Loxo, MEI Pharma, TG Therapeutics, MorphoSys, Epizyme
Ian Flinn
Consulting/advisory role: AbbVie (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Verastem (Inst), Roche (Inst), Gilead Sciences (Inst), Kite, a Gilead Company (Inst), Janssen (Inst), BeiGene (Inst), Takeda (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Nurix (Inst), Shanghai Yingli Pharmaceuticals (Inst), Genentech (Inst), Great Point Partners (Inst), Iksuda Therapeutics (Inst), Novartis (Inst), Pharmacyclics (Inst), Century Therapeutics (Inst), Hutchison MediPharma (Inst), Servier (Inst), Vincerx (Inst)
Research Funding: Acerta Pharma (Inst), Agios (Inst), Calithera Biosciences (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Kite, a Gilead Company (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), Forma Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Takeda (Inst), Teva (Inst), Verastem (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Seattle Genetics (Inst), IGM Biosciences (Inst), Loxo (Inst), Rhizen Pharmaceuticals (Inst), Triact Therapeutics (Inst)
Yvonne Pak
Employment: BridgeBio Pharma, AbbVie
Stock and Other Ownership Interests: BridgeBio Pharma, AbbVie
Cathy Zhou
Employment: Abbvie/Pharmacyclics
Stock and Other Ownership Interests: Abbvie/Pharmacyclics
Edith Szafer-Glusman
Stock and Other Ownership Interests: AbbVie
Joi Ninomoto
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
James P. Dean
Employment: Pharmacyclics
Stock and Other Ownership Interests: AbbVie
Danelle F. James
Employment: Abbvie/Pharmacyclics
Leadership: Abbvie/Pharmacyclics
Stock and Other Ownership Interests: AbbVie/Pharmacyclics
Patents, Royalties, Other Intellectual Property: AbbVie/Pharmacyclics
Travel, Accommodations, Expenses: Abbvie/Pharmacyclics
Paolo Ghia
Honoraria: AbbVie, BeiGene, Janssen Oncology, Gilead Sciences, Juno Therapeutics, Sunesis Pharmaceuticals, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, Juno/Celgene/Bristol Myers Squibb, MSD, Lilly, Roche
Consulting/Advisory Role: AbbVie, BieGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, MSD, Lilly, Roche
Research Funding: AbbVie, Janssen Oncology, Gilead Sciences, Sunesis Pharmaceuticals, Novartis, AstraZeneca
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, BeiGene, Pharmacyclics
Consulting/Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag, AbbVie
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the All-Virtual 62nd American Society of Hematology (ASH) Annual Meeting & Exposition, December 5-8, 2020.
SUPPORT
Supported by Pharmacyclics LLC, an AbbVie Company.
CLINICAL TRIAL INFORMATION
P.G. and C.S.T. contributed equally to the study and are cosenior authors.
DATA SHARING STATEMENT
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: William G. Wierda, Thomas J. Kipps, Cathy Zhou, Joi Ninomoto, Danelle F. James, Paolo Ghia
Provision of study material or patients: Tanya Siddiqi, Thomas J. Kipps, Stephen Opat, Alessandra Tedeschi, Sharon Jackson, John M. Pagel
Collection and assembly of data: William G. Wierda, John N. Allan, Tanya Siddiqi, Thomas J. Kipps, Alessandra Tedeschi, Xavier C. Badoux, Bryone J. Kuss, Sharon Jackson, Carol Moreno, Ryan Jacobs, John M. Pagel, Ian Flinn, Cathy Zhou, Edith Szafer-Glusman, Joi Ninomoto, James P. Dean, Paolo Ghia, Constantine S. Tam
Data analysis and interpretation: William G. Wierda, John N. Allan, Tanya Siddiqi, Thomas J. Kipps, Stephen Opat, Xavier C. Badoux, Bryone J. Kuss, Sharon Jackson, Carol Moreno, John M. Pagel, Ian Flinn, Yvonne Pak, Cathy Zhou, Joi Ninomoto, James P. Dean, Danelle F. James, Paolo Ghia
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
William G. Wierda
Consulting or Advisory Role: Sanofi
Research Funding: GlaxoSmithKline/Novartis, AbbVie, Genentech, Pharmacyclics, Acerta Pharma, Gilead Sciences, Janssen, Juno Therapeutics, Kite, a Gilead Company, Oncternal Therapeutics, Loxo, Xencor, miRagen, Sunesis Pharmaceuticals, Cyclacel
John N. Allan
Honoraria: AstraZeneca, AbbVie, Pharmacyclics/Janssen, BeiGene
Consulting/Advisory Role: AbbVie/Genentech, Pharmacyclics, Ascentage Pharma, BeiGene, Janssen Oncology, Epizyme, AstraZeneca, TG Therapeutics, ADC Therapeutics
Research Funding: Genentech, Janssen, Celgene, TG Therapeutics
Tanya Siddiqi
Consulting/Advisory Role: Juno Therapeutics, AstraZeneca, BeiGene, Celgene, Pharmacyclics, Bristol Myers Squibb/Celgene, Pharmacyclics/Janssen
Speakers Bureau: Pharmacyclics/Janssen, AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene
Research Funding: Juno Therapeutics (Inst), Kite, a Gilead Company (Inst), Acerta Pharma (Inst), TG Therapeutics (Inst), BeiGene (Inst), Pharmacyclics (Inst), Celgene (Inst), Oncternal Therapeutics (Inst)
Thomas J. Kipps
Employment: Moores Cancer Center
Stock and Other Ownership Interests: Oncternal Therapeutics
Honoraria: Pharmacyclics, AbbVie, Janssen, Genentech, Gilead Sciences, DAVAOncology, AstraZeneca
Consulting/Advisory Role: AbbVie, Pharmacyclics, Genentech, Janssen, DAVAOncology
Speakers Bureau: Verastem/Pharmacyclics, Pharmacyclics/Janssen, AbbVie/Genentech, Gilead Sciences, DAVA Pharmaceuticals
Research Funding: Pharmacyclics/Janssen (Inst), Breast Cancer Research Foundation (Inst), Oncternal Therapeutics (Inst), Leukemia and Lymphoma Society (Inst), California Institute for Regenerative Medicine (CIRM) (Inst), National Cancer Institute (Inst), NIH (Inst)
Patents, Royalties, Other Intellectual Property: Cirmtuzumab was developed by Thomas J. Kipps in the Thomas J. Kipps laboratory and licensed by the University of California to Oncternal Therapeutics, Inc, which provided stock options and research funding to the Thomas J. Kipps laboratory. (Inst)
Travel, Accommodations, Expenses: AbbVie/Pharmacyclics, Genentech/Roche, Janssen, Gilead Sciences, Celgene, DAVAOncology, Breast Cancer Research Foundation, TG Therapeutics, Verastem, AstraZeneca
Stephen Opat
Honoraria: AbbVie, AstraZeneca, Janssen, Roche, Gilead Sciences, Mundipharma, Takeda, Merck, and CSL Behring
Consulting/Advisory Role: AbbVie, AstraZeneca, Janssen, Celgene, Novartis, Gilead Sciences, Takeda, Merck, Mundipharma, CSL Behring
Research Funding: AstraZeneca (Inst), BeiGene (Inst), Roche (Inst), AbbVie (Inst), Gilead Sciences (Inst), Takeda (Inst), Pharmacyclics (Inst), Janssen (Inst), Celgene (Inst), Merck (Inst), Epizyme (Inst)
Travel, Accommodations, Expenses: Roche
Alessandra Tedeschi
Consulting/Advisory Role: Janssen, BeiGene, AstraZeneca, AbbVie
Speakers Bureau: AbbVie, AstraZeneca, Janssen, BeiGene
Xavier C. Badoux
Honoraria: Janssen/Pharmacyclics, AbbVie
Bryone J. Kuss
Stock and Other Ownership Interests: Commonwealth Serum Laboratories (CSL)
Honoraria: Roche, Janssen Oncology, AstraZeneca, AbbVie, Merck, Mundipharma, Takeda, Sandoz
Consulting/Advisory Role: AbbVie, Janssen Oncology, Kyowa Kirin International, AstraZeneca
Speakers Bureau: AstraZeneca, Janssen-Ortho
Sharon Jackson
Honoraria: AbbVie NZ Ltd
Consulting/Advisory Role: AbbVie NZ
Speakers Bureau: AbbVie NZ
Travel, Accommodations, Expenses: Roche NZ
Carol Moreno
Consulting/Advisory Role: Janssen Oncology, Abbott/AbbVie, AstraZeneca, BieGene
Speakers Bureau: Janssen Oncology
Research Funding: AbbVie
Ryan Jacobs
Consulting/Advisory Role: AstraZeneca, Janssen Oncology, Secura Bio, Genentech, Adaptive Biotechnologies, ADC Therapeutics, TG Therapeutics
Speakers Bureau: Pharmacyclics, Janssen Oncology, AbbVie, TG Therapeutics, AstraZeneca
Research Funding: TeneoBio (Inst), Pharmacyclics (Inst), TG Therapeutics (Inst), MEI Pharma (Inst)
John M. Pagel
Consulting/Advisory Role: Gilead Sciences, AstraZeneca, Actinium Pharmaceuticals, BeiGene, Loxo, MEI Pharma, TG Therapeutics, MorphoSys, Epizyme
Ian Flinn
Consulting/advisory role: AbbVie (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Verastem (Inst), Roche (Inst), Gilead Sciences (Inst), Kite, a Gilead Company (Inst), Janssen (Inst), BeiGene (Inst), Takeda (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Nurix (Inst), Shanghai Yingli Pharmaceuticals (Inst), Genentech (Inst), Great Point Partners (Inst), Iksuda Therapeutics (Inst), Novartis (Inst), Pharmacyclics (Inst), Century Therapeutics (Inst), Hutchison MediPharma (Inst), Servier (Inst), Vincerx (Inst)
Research Funding: Acerta Pharma (Inst), Agios (Inst), Calithera Biosciences (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Kite, a Gilead Company (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), Forma Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Takeda (Inst), Teva (Inst), Verastem (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), Seattle Genetics (Inst), IGM Biosciences (Inst), Loxo (Inst), Rhizen Pharmaceuticals (Inst), Triact Therapeutics (Inst)
Yvonne Pak
Employment: BridgeBio Pharma, AbbVie
Stock and Other Ownership Interests: BridgeBio Pharma, AbbVie
Cathy Zhou
Employment: Abbvie/Pharmacyclics
Stock and Other Ownership Interests: Abbvie/Pharmacyclics
Edith Szafer-Glusman
Stock and Other Ownership Interests: AbbVie
Joi Ninomoto
Employment: AbbVie
Stock and Other Ownership Interests: AbbVie
James P. Dean
Employment: Pharmacyclics
Stock and Other Ownership Interests: AbbVie
Danelle F. James
Employment: Abbvie/Pharmacyclics
Leadership: Abbvie/Pharmacyclics
Stock and Other Ownership Interests: AbbVie/Pharmacyclics
Patents, Royalties, Other Intellectual Property: AbbVie/Pharmacyclics
Travel, Accommodations, Expenses: Abbvie/Pharmacyclics
Paolo Ghia
Honoraria: AbbVie, BeiGene, Janssen Oncology, Gilead Sciences, Juno Therapeutics, Sunesis Pharmaceuticals, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, Juno/Celgene/Bristol Myers Squibb, MSD, Lilly, Roche
Consulting/Advisory Role: AbbVie, BieGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics, ArQule, Adaptive Biotechnologies, Dynamo Therapeutics, MEI Pharma, Acerta Pharma/AstraZeneca, MSD, Lilly, Roche
Research Funding: AbbVie, Janssen Oncology, Gilead Sciences, Sunesis Pharmaceuticals, Novartis, AstraZeneca
Constantine S. Tam
Honoraria: Janssen-Cilag, AbbVie, Novartis, BeiGene, Pharmacyclics
Consulting/Advisory Role: Janssen, Loxo, Roche, BeiGene, AbbVie
Research Funding: Janssen-Cilag, AbbVie
No other potential conflicts of interest were reported.
REFERENCES
- 1.Burger JA: Treatment of chronic lymphocytic leukemia. N Engl J Med 383:460-473, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Barr PM, Robak T, et al. : Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 34:787-798, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanafelt TD, Wang XV, Kay NE, et al. : Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 381:432-443, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VENCLEXTA (Venetoclax Tablets) for Oral Use [Package Insert]. South San Francisco, CA, Genentech USA, 2020 [Google Scholar]
- 5.Roberts AW, Davids MS, Pagel JM, et al. : Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 374:311-322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stilgenbauer S, Eichhorst B, Schetelig J, et al. : Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: Results from the full population of a phase II pivotal trial. J Clin Oncol 36:1973-1980, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Stilgenbauer S, Eichhorst B, Schetelig J, et al. : Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol 17:768-778, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Roberts AW, Ma S, Kipps TJ, et al. : Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood 134:111-122, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Sawaf O, Zhang C, Tandon M, et al. : Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 21:1188-1200, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. : Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res 21:3705-3715, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng J, Isik E, Fernandes SM, et al. : Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 31:2075-2084, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman SE, Gordon AL, Hertlein E, et al. : Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 117:6287-6296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YLL, Franzen C, Wang S, et al. : Ibrutinib and venetoclax target distinct subpopulation of CLL cells: Rationale for drug combination and implication of minimal residual disease eradication. 61st ASH Annual Meeting & Exposition, Orlando, FL, December 7-10, 2019
- 14.Slinger E, Balasubramanian S, Leverson JD, et al. : Combinatorial treatment of chronic lymphocytic leukemia with ibrutinib and venetoclax is superior to treatment with single agents in the TCL1 mouse model, Blood 130:3018, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrd JC, Furman RR, Coutre SE, et al. : Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 369:32-42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillmen P, Rawstron AC, Brock K, et al. : Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: The CLARITY study. J Clin Oncol 37:2722-2729, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieman CU, Dubois J, Kersting S, et al. : Venetoclax and ibrutinib for patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL) – 15-month safety, response and MRD evaluation: third interim analysis from the phase II Vision HO141 trial. 61st ASH Annual Meeting & Exposition, Orlando, FL, December 7-10, 2019
- 18.Jain N, Keating MJ, Thompson PA, et al. : Combined ibrutinib and venetoclax for first-line treatment for patients with chronic lymphocytic leukemia (CLL). 61st ASH Annual Meeting & Exposition, Orlando, FL, December 7-10, 2019
- 19.Jain N, Keating MJ, Thompson PA, et al. : Combined ibrutinib and venetoclax in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). 61st ASH Annual Meeting & Exposition, Orlando, FL, December 7-10, 2019
- 20.Thompson PA, Keating MJ, Jain N, et al. : Venetoclax added to ibrutinib in high-risk CLL achieves a high rate of undetectable minimal residual disease [RTS1]. 61st ASH Annual Meeting & Exposition, Orlando, FL, December 7-10, 2019
- 21.Thompson PA, Tam CS, O'Brien SM, et al. : Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood 127:303-309, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottcher S, Ritgen M, Fischer K, et al. : Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: A multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol 30:980-988, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Strati P, Keating MJ, O'Brien SM, et al. : Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood 123:3727-3732, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kater AP, Seymour JF, Hillmen P, et al. : Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: Post-treatment follow-up of the MURANO phase III study. J Clin Oncol 37:269-277, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Hallek M, Cheson BD, Catovsky D, et al. : Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the international Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111:5446-5456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallek M, Cheson B, Catavsky D: Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase in peripheral blood lymphocytes. Blood 119:5348, 2012 [Google Scholar]
- 27.Wierda WG, Byrd JC, O'Brien S, et al. : Tumour debulking and reduction in predicted risk of tumour lysis syndrome with single-agent ibrutinib in patients with chronic lymphocytic leukaemia. Br J Haematol 186:184-188, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Howard SC, Jones DP, Pui CH: The tumor lysis syndrome. N Engl J Med 364:1844-1854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain N, Keating M, Thompson P, et al. : Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med 380:2095-2103, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woyach JA, Ruppert AS, Heerema NA, et al. : Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 379:2517-2528, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn IE, Farooqui MZH, Tian X, et al. : Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 131:2357-2366, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno C, Greil R, Demirkan F, et al. : Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:43-56, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Rogers KA, Huang Y, Ruppert AS, et al. : Phase II study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naïve and relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 38:3626-3637, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer K, Al-Sawaf O, Bahlo J, et al. : Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 380:2225-2236, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Davids MS, Hallek M, Wierda W, et al. : Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 24:4371, 2018 [DOI] [PubMed] [Google Scholar]
- 36.Roeker LE, Fox CP, Eyre TA, et al. : Tumor lysis, adverse events, and dose adjustments in 297 venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res 25:4264-4270, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for access to individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.