Abstract
Type-I hypersensitivity is commonly characterized by increased levels of antigen-specific immunoglobulin (Ig) E. Therefore, it is important for clinical and research investigators to reliably measure serum levels of IgE in allergic patients and animal models. While current ELISA-based methods are simple and commonly performed for the detection of allergen-specific IgE using serum or plasma, they may produce misleading results. This is in part due to decreased sensitivity for IgE in the presence of other Ig isotypes in the same sample, such as IgG, that are typically more abundant than IgE. When assessment of multiple Ig isotypes is necessary, performing optimized assays for individual isotypes requires high sample volumes. Here we describe an approach to increase the sensitivity for IgE detection while conserving the sample volume needed. This method not only improves the accuracy of serum IgE measurements but also allows simultaneous analysis of other allergen-specific immunoglobulins.
Keywords: Adsorption, allergy, enzyme-linked immunosorbent assay (ELISA), immunoglobulins, protein-G
1. Introduction
Allergic sensitization results from inappropriate recognition and subsequent presentation of normally benign substances as harmful antigens. This process is mediated by the major histocompatibility complex II (MHC-II), a receptor complex expressed by professional antigen presenting cells such as dendritic cells and macrophages. MHC-II forms a complex with a processed antigen peptide and presents them to CD4+ T cells, which in turn induce isotype class switching in B cells for antigen-specific immunoglobulin (Ig) E production [1]. The produced IgE bind to the Fcε receptors on mast cells and basophils for rapid release of histamine, cytokines, and other inflammatory factors upon re-exposure to the allergen, causing swelling and redness commonly associated with type-I immediate hypersensitivity reactions [2]. Because serum IgE concentrations are normally very low, testing allergic individuals for elevated levels of IgE for particular antigens is useful in identifying offending allergens. This test is commonly practiced in clinics for diagnosis of in addition to skin prick tests [1].
However, detection of other Ig isotypes, especially IgG and IgA, can also provide important information for understanding the development and progression of allergy. While IgG is classically associated with delayed type hypersensitivity reactions, IgG1, IgG2a, and IgG2b subclasses have been shown to potentially contribute to or independently cause anaphylaxis in experimental studies and possibly allergic individuals [3]. In contrast, an increase of serum antigen specific IgG4 has been implicated in allergy resolution [4]. Additionally, individuals with decreased IgA are thought to be more susceptible to development to various allergies [5]. While the precise roles of these other Igs in allergy development are still elusive, IgE levels alone may not provide a complete measure of humoral responses in allergic individuals.
The enzyme-linked immunosorbent assay (ELISA) has become a widely used method to detect a variety of Ig isotypes over the past decades. However, the assay may produce biased and unreliable outcomes if the presence of allergen-specific IgG is predominant and competes for the same antigen against a relatively small amount of allergen-specific IgE in the same serum sample [6] (Fig. 1). Additionally, when multiple Ig isotype levels are to be measured in smaller animals such as mice, it is often not feasible to dedicate a large volume of serum the assays. Therefore, the current ELISA approach may not be optimal in some cases, especially when the Ig isotype of interest is present in low abundance.
Fig 1. Potential masking of IgE by IgG during ELISA.
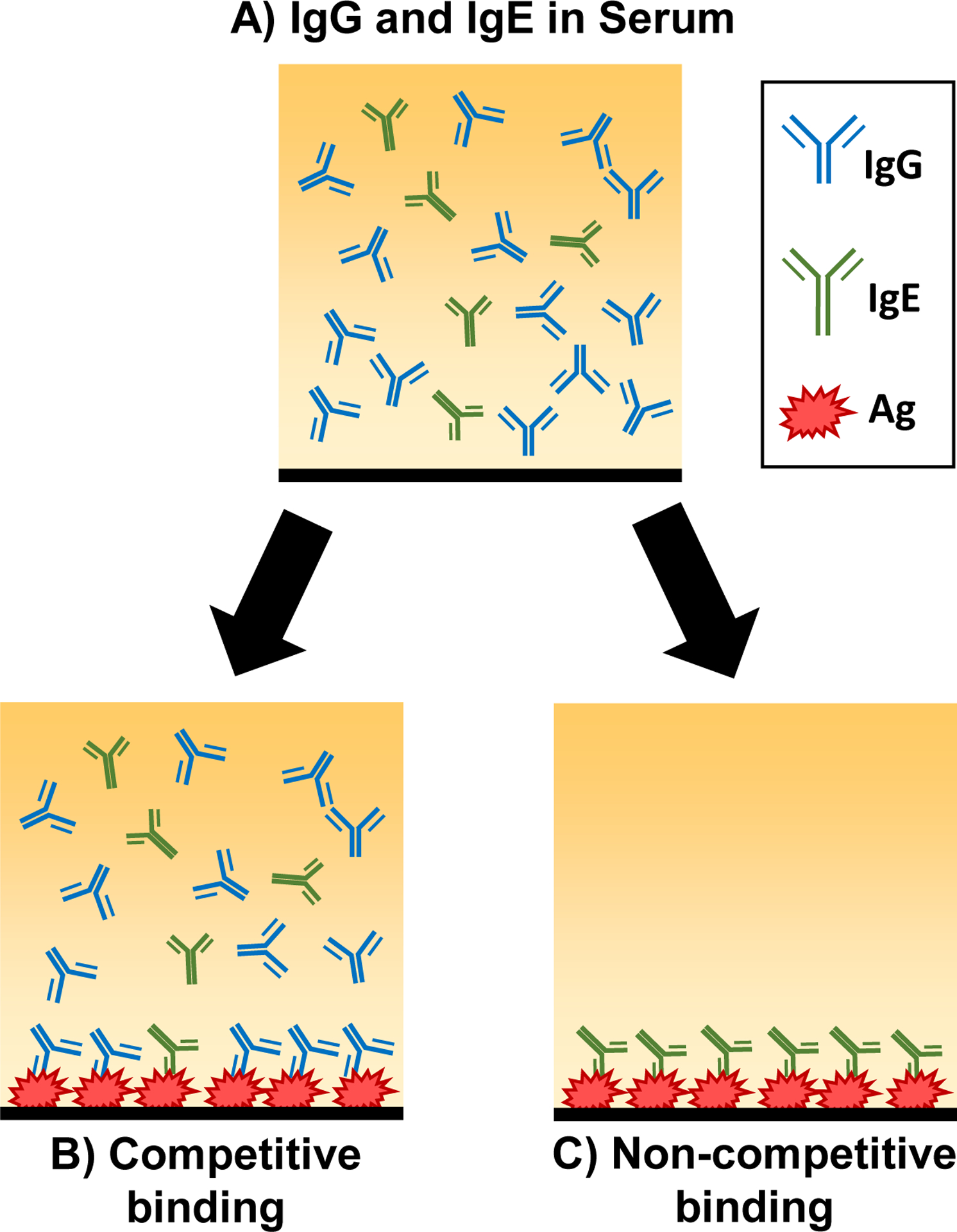
IgE is present in a relatively low amount in serum samples compared to IgG (A). When untreated serum is used for ELISA, antigen-specific IgG competes for the antigen binding sites (B). Pretreatment of serum samples with protein G adsorption removes the IgG, allowing antigen-specific IgE to bind to the antigen (C).
Here, we outline a method to separate Ig isotypes from a single serum sample by adsorbing total IgG using magnetic protein G beads to provide a more accurate measure of IgE in the sample by ELISA (Fig. 2). Since each serum sample is highly diluted prior to the separation process, only 6 μL serum is required to individually assay multiple Igs. Moreover, the adsorbed IgG can be subsequently eluted and used for detection of total or allergen-specific IgG using a standard ELISA protocol with minor modifications.
Fig 2. Separation of protein G beads with a magnet.

After vortexing, protein G beads are well-suspended in the solution (A). With a magnet applied to the side of the tube, the beads become easily separated from the solution (B). Remove the solution with a pipet and prepare the beads as described in Section 3.1.1.
2. Materials
Use ultrapure water (18.2 Ω) and analytical grade reagents. Prepare and store all reagents at 4 °C unless indicated otherwise. Do not add sodium azide to buffers.
2.1. Preparation of Serum Samples
Phosphate buffered saline (PBS): 137 mM NaCl (8 g), 2.7 mM KCl (0.2 g), 10.14 mM Na2HPO4 (1.44 g), 1.76 mM KH2PO4 (0.24 g), pH 7.4. Dissolve 8 g NaCl, 0,2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 to 800 mL water. Adjust pH to 7.4 and add water to a final volume of 1 L. Store at room temperature.
Assay buffer: 0.05% (v/v) Tween-20 and 0.5% (w/v) bovine serum albumin (BSA) in phosphate-buffered saline (PBS), pH 7.4. Add 500 μL of Tween-20 and 5 g of BSA in 1 L of PBS.
0.5-mL Microfuge tubes.
Magnetic protein G beads (see Note 1).
Magnet.
Vortexer.
Tris buffer: 1 M Tris-HCl, pH 7.5. Dissolve 12.1 g Tris in 80 mL of H2O. Adjust pH and add water to a final volume 100 mL.
2.2. Antigen-Specific IgE/IgG ELISA
High-binding 96-well ELISA plate or strips: clear, flat-bottom.
Coating buffer: 44.0 mM NaHCO3, 6.04 mM Na2CO3, pH 9.6. Add 3.7 g NaHCO3 and 0.64 g Na2CO3 to 1 L of water.
100x Target antigen stock solution: 2 mg/mL in the coating buffer for coating ELISA plate (see Note 2). Dissolve 10 mg target antigen in 5 mL the coating buffer (see Note 3).
10-mL Syringe.
0.2-μm Syringe filter.
PBS: See Section 2.1, Item 1.
Wash buffer: 0.05% Tween-20. Add 500 μL of Tween-20 to 1 L of PBS.
Assay buffer: See section 2.1, Item 2.
Species-specific biotinylated secondary antibody.
Horse radish peroxidase-conjugated avidin (HRP-avidin).
3,3’,5,5’-tetramethylbenzidine (TMB) Substrate solution: Commercially available stabilized TMB solution.
Stop solution: 2N H2SO4.
Micropipettors: multichannel pipettors optional.
Pipet tips.
Plate sealers.
Plate rocker or orbital shaker.
Microplate reader capable of reading at 450 nm
3. Methods
3.1. Preparation of Serum Samples
This section describes a method to separate IgG from serum samples to be used for antigen-specific IgE detection by ELISA. To avoid freezing and thawing of the samples, this brief procedure should be performed when an ELISA plate has been coated and ready to be used, or when a pre-coated ELISA plate is used.
3.1.1. Adsorption of IgG from Diluted Samples
If frozen aliquots of serum samples are being used, thaw quickly and keep on ice until diluted in the assay buffer.
Dilute 6 μL of each serum sample with 234 μL of the assay buffer to a total volume of 240 μL and set aside (see Note 4).
Vortex magnetic protein G beads in the original container for 1 min and then immediately transfer 50 μL of the beads into a 0.5-mL microfuge tubes. Place a magnet on the side of the tube and wait briefly for beads to aggregate on the tube wall (Fig. 2). Discard the liquid, leaving only the beads in the tube.
Add the diluted serum from Step 2 to the beads from Step 3 and close the lid tightly. Vortex briefly for 3 sec and place the tube on a rocker to keep beads agitated in suspension for 10 min at room temperature.
Place the magnet on the side of the tube and collect the supernatant (Fig 3A), which is now devoid of IgG (Fig. 3B). Transfer the supernatant in a clean tube and save for allergen-specific IgE ELISA described in Section 3.3 (see Note 5). If performing IgG detection, immediately proceed to Section 3.1.2 to elute IgG adsorbed by the beads (see Note 6).
Fig 3. Adsorption of total immunoglobulin G.

A diluted serum sample is added to washed magnetic protein-G beads. The beads bind to IgG and are subsequently pelleted with a magnet (A). The remaining supernatant, which contains IgE but not IgG (B), is collected and used for total or antigen-specific IgE ELISA (B). Bead-bound IgG is immediately eluted with a low-pH buffer, neutralized, and used for total or antigen-specific IgG ELISA. (C).
3.1.2. Elution of IgG from Magnetic Beads
Immediately add 200 μL of wash buffer to the beads from Section 3.1.1, Step 5. Gently mix by pipetting to avoid foaming.
Place the magnet to the side of the tube and discard the wash buffer. Immediately add 25 μL of elution buffer to the beads and gently mix by pipetting. Incubate for 2 min at room temperature.
Neutralize the elution buffer with 25 μL of Tris buffer and mix well (see Note 7). Adjust the volume to 240 μL with the assay buffer and collect the IgG-containing supernatant in a clean microfuge tube (Fig. 3C). Keep on ice until proceeding to antigen-specific IgG ELISA described in Section 3.4.
3.2. Detection of Antigen-Specific IgE or IgG with ELISA
3.2.1. Preparation of ELISA Plate with an Antigen
If a non-sterile 96-well ELISA plate or 8-well ELISA strips are used, sterilize under ultraviolet light in a biological safety cabinet (see Note 8).
Dilute the 100x antigen stock solution with coating buffer to make 1x antigen coating solution. For 1x antigen coating solution enough for one 96-well plate, dilute 100 μL of the 100x antigen stock solution in 10 mL of the coating buffer. Sterile filter the 1x antigen solution using a 0.2-μm filter attached to a syringe.
Pipet 100 μL of the 1x antigen coating solution to all wells. Seal the plate and incubate on a rocker with gentle agitation overnight at 4 °C.
Remove the antigen coating solution and rinse wells with 300 μL of the wash buffer 3 times, 1 min each. Blot dry between the rinses by tapping the inverted plate on an absorbent surface, such as a stack of paper towels (see Note 9).
Place 200 μL assay buffer in each well to block non-specific binding. Seal the plate and incubate on a rocker for 2 h at room temperature.
Remove the assay buffer from the wells and place 100 μL of prepared samples from Section 3.1.1 (for IgE) or Section 3.1.2 (for IgG) in their designated wells (see Note 10). Seal plate and incubate overnight at 4 °C.
3.2.2. Colorimetric Reaction and Plate Reading
Aspirate serum samples and wash wells with 300 μL of wash buffer 3 times, 1 min each. Blot dry between the rinses as described in Section 3.2.1, Step 4. Place 100 μL of a biotinylated secondary antibody at a working concentration in each well (see Note 11). Seal the plate and incubate on a rocker with gentle agitation for 2 h at room temperature.
Remove the secondary antibody solution and rinse wells with wash buffer 4 times, 1 min each. Blot dry between the rinses. Add 100 μL of HRP-avidin at a working concentration to each well (see Note 11). Seal the plate, protect from light, and incubate on a rocker with gentle agitation for 1 h at room temperature.
Remove HRP-avidin and rinse the wells with the wash buffer 3 times, 1 min each.
Place 100 μL of TMB substrate solution (see Note 11) to each well and incubate on a rocker for 20–30 min, protected from light (see Note 12).
Stop the color reaction with 100 μL of the stop solution and immediately read the absorbance at 450 nm on a microplate reader. If possible, additionally read with a reference wavelength at 540–570 nm (see Note 13). Example of results are shown in Fig. 4.
Fig 4. Comparison of allergen-specific IgE and IgG1 ELISAs using adsorbed and unadsorbed serum samples.

Male C57BL6/J mice were orally sensitized to a cow’s milk protein, β-lactoglobulin. Allergen-specific IgE and IgG1 ELISAs comparing the sera from sham control and sensitized mice were performed without or with the IgG adsorption pretreatment. The difference in the IgE levels between the control and sensitized mice was more evident with the pretreatment (A) while the relative differences observed for IgG1 with the eluted sera remained unchanged (B). Mean ± SEM, n=5. OD: optical density.
4. Notes
This protocol describes the use of magnetic protein G beads, which bind to all IgG isotypes with high affinity. However, other protein bead options, such as protein A beads, that show variable affinities to other antibody isotypes are also available.
An important factor in coating the plate adequately with a given antigen is the pH of the buffer used. The pH of the buffer facilitates the attachment of the antigen to the microplate and thus and has a significant effect on the strength of the color reaction. The most common buffer used is a bicarbonate buffer (pH 9.6), although PBS (pH 7.4) is occasionally used. If the color reaction is weak, adjusting the pH of the coating buffer may facilitate better binding of some antigens. Optimal coating conditions should ultimately be determined for specific antigens used by individual laboratories.
The antigen concentration of 2 μg/mL (using the 1x stock solution) has been optimized for the detection of IgE against a purified bovine milk whey protein, β-lactoglobulin (Bos d 5) [8]. Optimal coating concentrations of other antigens may vary and therefore need to be determined by individual laboratories. Other investigators have used 20 μg/mL to coat plates with bovine whey proteins [9] and up to 50 μg/mL to coat plates with shrimp and peanut extract [6].
While 6 μL of serum is a sufficient volume for the detection of IgE against β-lactoglobulin in our orally-sensitized mouse model of milk allergy, a greater amount of sera may be necessary depending on the mouse model being investigated. The optimal serum concentration should ultimately be determined by each laboratory.
These aliquots may be kept on ice until the IgG elution procedure is completed. Avoid repetitive freezing and thawing of the samples.
If adsorbed IgG needs to be eluted from the beads, immediately proceed to Section 3.1.2 and add wash buffer to the remaining beads. Do not leave the beads to dry.
With a short incubation time, a repeating pipettor is a convenient tool to add neutralization buffer quickly.
We perform ELISA plate coating under a sterile condition to avoid potential microbial growth during the coating and sample incubation processes.
Care should be taken to remove as much of the wash buffer as possible without completely drying out the wells. Firmly tapping the plate 3–4 times on a stack of paper towels laid on a laboratory bench should be sufficient.
The supernatants from the protein G adsorption should yield enough (approximately 200 μL) to run each sample in duplicates. While the post-adsorption supernatant can also be used to detect serum IgA it should not be used for IgM ELISA since this isotype is partially absorbed by protein G.
Use working concentrations of secondary antibodies and HRP-avidin as suggested by the suppliers. However, optimal concentrations for these reagents to detect each Ig should ultimately be determined by individual laboratories.
For an initial reaction, periodically inspect the development of the yellow reaction product to avoid color saturation.
The reference wavelength can help to correct for optical imperfections in the wells, and the reading can be subtracted from the measurements at 450 nm.
Acknowledgments:
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under grant numbers P20GM103442 and 5P20GM113123.
References:
- 1.Galli SJ, Tsai M (2012). IgE and mast cells in allergic disease. Nat Med, 18(5), 693–704. 10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder HW Jr, Cavacini L (2010) Structure and function of immunoglobulins. J Allergy Clin Immunol 125(2 Suppl 2):S41–S52 doi: 10.1016/j.jaci.2009.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelman FD, Khodoun MV, Strait R (2016) Human IgE-independent systemic anaphylaxis J All Clin Immunol 137(6):1674–1680 doi: 10.1016/j.jaci.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gocki J, Zbigniew B (2016) Role of immunoglobulin G antibodies in diagnosis of food allergy. Postepy Dermatol Alergol 33(4): 253–256 doi: 10.5114/ada.2016.61600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yel L (2010) Selective IgA deficiency. J Clin Immunol 30(1):10–16 doi: 10.1007/s10875-009-9357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehrer SB, Reish R, Fernandes J, et al. (2004) Enhancement of murine IgE antibody detection by IgG removal. J Immunol Methods 284(1–2):1–6 doi: 10.1016/j.jim.2003.08.017 [DOI] [PubMed] [Google Scholar]
- 7.Choe W, Durgannavar TA, Chung SJ (2016) Fc-Binding Ligands of Immunoglobulin G: An overview of high affinity proteins and peptides. Materials (Basel) 9(12):994 doi: 10.3390/ma9120994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith NA, Germundson DL, Combs CK, et al. (2019) Astrogliosis associated with behavioral abnormality in a non-anaphylactic mouse model of cow’s milk allergy. Front Cell Neurosci 13:320 doi: 10.3389/fncel.2019.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germundson DL, Smith NA, Vendsel LP, et al. (2018) Oral sensitization to whey proteins induces age- and sex-dependent behavioral abnormality and neuroinflammatory responses in a mouse model of food allergy: a potential role of mast cells. J Neuroinflammation 15:120 doi: 10.1186/s12974-018-1146-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


