Abstract
Objectives. To assess the association between individual-level adherence to social-distancing and personal hygiene behaviors recommended by public health experts and subsequent risk of COVID-19 diagnosis in the United States.
Methods. Data are from waves 7 through 26 (June 10, 2020–April 26, 2021) of the Understanding America Study COVID-19 survey. We used Cox models to assess the relationship between engaging in behaviors considered high risk and risk of COVID-19 diagnosis.
Results. Individuals engaging in behaviors indicating lack of adherence to social-distancing guidelines, especially those related to large gatherings or public interactions, had a significantly higher risk of COVID-19 diagnosis than did those who did not engage in these behaviors. Each additional risk behavior was associated with a 9% higher risk of COVID-19 diagnosis (hazard ratio [HR] = 1.09; 95% confidence interval [CI] = 1.05, 1.13). Results were similar after adjustment for sociodemographic characteristics and local infection rates.
Conclusions. Personal mitigation behaviors appear to influence the risk of COVID-19, even in the presence of social factors related to infection risk.
Public Health Implications. Our findings emphasize the importance of individual behaviors for preventing COVID-19, which may be relevant in contexts with low vaccination. (Am J Public Health. 2022;112(1):169–178. https://doi.org/10.2105/AJPH.2021.306565)
COVID-19 continues to be a major public health concern in the United States and worldwide. Since its recognition in late 2019 through August 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 215 million individuals globally, with more than 39 million cases in the United States alone.1 These cases have resulted in an enormous mortality toll: COVID-19 was the third-leading cause of death in the United States in 2020, and it reduced 2020 US life expectancy by more than a year.2,3 At the time of writing (August 2021), many areas of the United States are experiencing surges of cases fueled by the highly transmissible Delta variant, and the Centers for Disease Control and Prevention (CDC) has revised its guidance to again recommend indoor masking for all individuals in high-transmission areas, regardless of vaccination status.4
Stopping the pandemic still requires using all available tools, including simple behavioral modifications. Government officials and public health experts have been urging people to engage in preventive behaviors, including wearing a mask that covers the nose and mouth, staying 6 feet apart from others, avoiding crowds and poorly ventilated indoor spaces, and washing hands often with soap and water or hand sanitizer.5
Empirical evidence has shown that social-distancing and personal hygiene recommendations or mandates at aggregate levels effectively slow the spread of the virus. In the United States, county-level and state-level mask mandates have significantly reduced the growth rates of local COVID-19 cases.6–10 By the end of 2020, mask mandate policies were found to be associated with significant reductions in the growth rates of county-level daily case numbers and deaths within a month of implementation, as well as reductions in state-level cases.6,7,9,11 Studies have also found that other nonpharmaceutical policy interventions, such as quarantine of exposed individuals, social distancing, workplace closures, and restrictions on large gatherings and events, help reduce the spread of the virus.6,8,11–15 For example, reopening restaurant dining increased the growth rates of county-level cases and deaths within 41 to 80 days of reopening.6 Isolation or quarantine, social distancing, and traffic restriction policies were found to have reduced the reproduction number of the disease by up to 43%.11 Notably, the stringency of control measures was associated with greater reductions in disease proliferation.15
The effectiveness of preventive behaviors undertaken by individuals remains unclear, likely because of limitations of existing data sources. Studies on the topic have primarily been retrospective studies or case–control studies,16–20 which may be biased by inaccurate recall of past behavior. A recent study linked preventive behaviors to COVID-19 infection using prospective data, but it focused on a limited set of social-distancing behaviors, captured infections through only October 2020, and was based on a sample that may not be widely generalizable.21 Population-level studies have typically focused on large gatherings or public spaces as sources of exposure to COVID-19, but there has been less evidence about the risk of COVID-19 from smaller gatherings, which were less amenable to policy interventions.22 Although individuals who repeatedly engage in risky behaviors likely have a higher risk of COVID-19 infection than those who do not, no nationally representative research has examined the cumulative role of individual behaviors in determining the risk of COVID-19 infection.
We addressed these gaps in the literature by using the Understanding America Study (UAS) survey, one of the only nationally representative longitudinal data sources that assess changes in behaviors that affect one’s risk of COVID-19 infection over time. We hypothesized that not practicing the recommended social distancing or COVID-19–related personal hygiene behaviors would elevate the risk of COVID-19 infection and that the risk of COVID-19 infection would increase with engagement in additional numbers of risky behaviors.
METHODS
We used data from the UAS, which is conducted by the Center for Economic and Social Research at the University of Southern California. The UAS is a nationally representative Internet panel study of US adults that began in 2014 with the support of the National Institute on Aging, the Social Security Administration, and the Gates Foundation. The UAS uses address-based probability sampling to reduce coverage bias and improve representativeness, and it lends Internet-connected tablets to respondents if needed, which minimizes the effect of the digital divide on this Internet panel.23 As an already established online panel study, the UAS was able to safely continue data collection during the pandemic and ask participants about their personal experiences with COVID-19, behaviors, and social, psychological, and economic consequences of the pandemic. The UAS COVID-19 longitudinal survey began in March 2020, and subsequent surveys were conducted every 2 weeks through March 2021, at which point the survey became monthly.
For this study, we used data from waves 7 through 26 of the UAS COVID-19 survey, covering the period from June 10, 2020, to April 26, 2021, because these waves consistently asked about the set of COVID-19–related behaviors that we study. Of the 8110 respondents who participated in at least 1 of these waves, we restricted our analysis to individuals who did not report a COVID-19 diagnosis at their first interview in this period, who had at least 1 subsequent wave of follow-up, and who had complete sociodemographic and behavioral information for at least 1 wave (n = 7604). The excluded respondents were younger on average than those included in the sample but were otherwise demographically similar (Table A [available as a supplement to the online version of this article at http://www.ajph.org]). We included all observations of each respondent until COVID-19 diagnosis, receipt of at least 1 dose of a COVID-19 vaccine, loss to follow-up, or the end of our study period (n = 104 677 person-waves over 4769 person-years of follow-up). Although at the time of writing there was considerable attention to breakthrough infections among vaccinated individuals, we chose to censor individuals upon vaccination because during the study follow-up period, breakthrough infections were relatively rare and several of the behaviors analyzed in our study were considered safe for vaccinated individuals.24
Measures
The outcome of our study is COVID-19 diagnosis. At each wave, respondents reported whether they had tested positive for COVID-19 or been diagnosed by a health care professional as probably having COVID-19 since their last interview. We considered an affirmative response to either of these questions to be an incident COVID-19 diagnosis. We assigned the date of diagnosis to be the midpoint between the interview date at which the respondent reported having received a COVID-19 diagnosis and their previous interview date.
The predictor variables were a set of time-varying behaviors related to lack of adherence to public health guidance. At each wave in our study period, respondents were asked whether in the previous 7 days they did each of the following: avoided public places, gatherings, or crowds; washed hands with soap or used hand sanitizer several times a day; wore a mask or face covering; avoided eating at restaurants; avoided contact with high-risk people; had visitors at their residence; went to another’s residence; went out to a bar, club, or other place where people gather; attended a gathering with more than 10 people; had close contact (within 6 ft) with people not in the household; and attended an in-person religious service. Respondents could answer yes, no, or unsure to each of these behaviors. Because some questions asked about preventive behaviors whereas others asked about risky activities, we recoded some of these so that 1 indicated higher risk and 0 lower risk for each behavior; we coded unsure responses as 1.
To assess how the degree of adherence to public health guidelines was associated with risk of COVID-19 diagnosis, we created a summary index ranging from 0 to 11 that was equal to the count of high-risk behaviors. We also created indicators for 3 categories of risk behaviors indicating any large gathering or public interaction, any small gathering, and any lack of adherence to COVID-19–related personal hygiene guidelines. Details on the question wording for these behaviors and categorizations are provided in Table B (available as a supplement to the online version of this article at http://www.ajph.org).
Demographic covariates included sex, age group at first interview (18–44, 45–64, and ≥ 65 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic other), and educational attainment (high school or less, some college, and college or more). To proxy for workplace exposure to COVID-19, we used information on labor force participation and days worked from home in the past 7 days to create a time-varying measure of employment status, which we categorized as working from home; working outside the home; and retired, unemployed, or otherwise out of the labor force. We used information on the age of the respondent’s household members, which is updated approximately every 3 months, to create 2 binary indicators of living arrangement: living with working-aged adults (aged 18–64 years; yes = 1) and living with school-aged children (aged < 18 years; yes = 1), because these living arrangements may entail additional exposure through in-person contact with individuals from outside the household.25,26 As a measure of local infection risk, we included the natural logarithm of the state-level rate of COVID-19 cases over the 7-day period before each interview date; for respondents who did not report a state of residence, we assigned national rates. We obtained COVID-19 case rates from the CDC COVID Data Tracker.27
Statistical Analysis
We used Cox hazard models to assess the association between behaviors and risk of COVID-19 diagnosis.28 These models estimated how observable risk factors multiply a nonparametrically estimated baseline hazard that is shared across all individuals. The clock on these models is time in days since June 10, 2020, which was the first day of our study period. We structured these observations as counting-process data, meaning that for each individual, we partitioned the follow-up period into intervals that started at the interview date and ended at the next interview date or diagnosis date.28,29 Respondents entered on their first interview date and we considered them at risk until COVID-19 diagnosis, receipt of at least 1 dose of a COVID-19 vaccine, loss to follow-up, or their wave 26 interview date, which was the end of our study period.
We treated risk behaviors as time varying to capture the variation in adherence to behavioral guidelines over the course of the pandemic and to estimate short-term associations between these behaviors and COVID-19. We conducted the Schoenfeld residual test and found no evidence that the proportional hazard assumption was violated.29 We first fit a set of models assessing the bivariate relationships between the 11 behaviors and the risk of COVID-19. Second, we fit a model predicting COVID-19 from the count of 11 behaviors. Third, we fit a model predicting COVID-19 from the 3 categories of risk behaviors. We have presented both unadjusted results and results adjusted for covariates for each of these models. All analyses included weights provided by the UAS and we conducted all analyses in R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
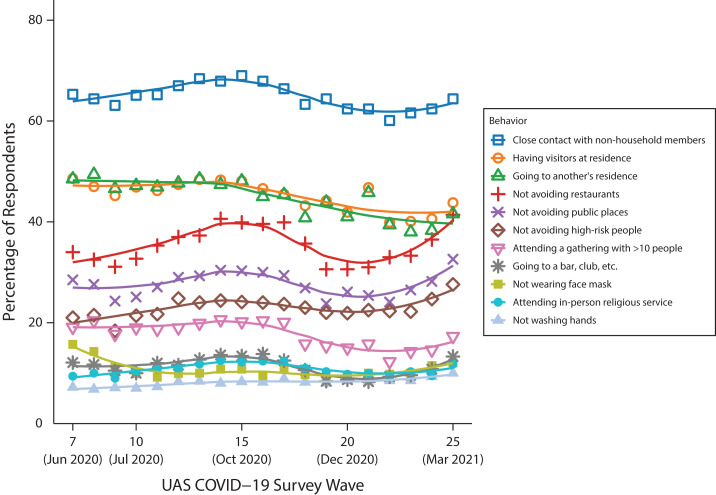
Characteristics of respondents at their first interview in our study period are displayed in Table 1. We have also presented these characteristics by COVID-19 diagnosis. Approximately 9% (748 respondents) were diagnosed with COVID-19 at some point during the study period; of these cases, 70% were diagnosed with a positive test result. Compared with those who were never diagnosed with COVID-19, those who were diagnosed were on average younger, more likely to be Hispanic, more likely to have lower educational attainment, more likely to work outside the home, and more likely to live with working-aged adults and school-aged children. In terms of behaviors, those ultimately diagnosed with COVID-19 had a greater prevalence of social behaviors and more risk behaviors on average (3.82 vs 3.03) than did those who were never diagnosed. Figure 1 displays the prevalence of each of these behaviors by UAS study wave, revealing that the adherence to behavioral guidelines fluctuated over the course of the study period.
TABLE 1—
Summary Statistics of Participants at First Interview: Understanding America Study (UAS), United States, June 10, 2020–April 26, 2021
| Percentage or Mean (SD) | ||||
| Full Sample | Never Diagnosed With COVID-19 | Diagnosed With COVID-19 During Follow-Up | CDC COVID-19 Cases | |
| Ever diagnosed with COVID-19 | 9.3 | |||
| Sex | ||||
| Female | 52.3 | 51.9 | 56.4 | 52.2 |
| Male | 47.7 | 48.1 | 43.6 | 47.8 |
| Age, y | ||||
| 18–44 | 49.3 | 49.4 | 48.4 | 84.5a |
| 45–64 | 32.1 | 31.4 | 38.8 | . . .a |
| ≥ 65 | 18.6 | 19.2 | 12.8 | 15.5 |
| Race/ethnicity | ||||
| Non-Hispanic White | 61.5 | 62.0 | 56.4 | 50.1 |
| Non-Hispanic Black | 11.9 | 12.0 | 10.7 | 11.2 |
| Hispanic | 17.1 | 16.4 | 24.5 | 29.0 |
| Non-Hispanic other | 9.5 | 9.6 | 8.4 | 9.8 |
| Educational attainment | ||||
| ≤ high school | 37.3 | 37.1 | 39.1 | |
| Some college | 28.4 | 27.7 | 34.8 | |
| ≥ college | 34.3 | 35.2 | 26.1 | |
| Employment status | ||||
| Working from home | 25.9 | 26.4 | 21.1 | |
| Working away from home | 32.0 | 31.1 | 41.1 | |
| Retired, unemployed, or out of labor force | 42.1 | 42.5 | 37.8 | |
| Living arrangement | ||||
| Living with working age adults | 67.9 | 67.3 | 74.0 | |
| Living with school age children | 33.8 | 33.0 | 41.3 | |
| State-level cases in past week (per 100 000) | 80.19 | 80.8 | 74.22 | |
| Behaviors | ||||
| Not avoiding public places | 29.2 | 28.3 | 37.8 | |
| Not washing hands | 7.3 | 7.3 | 6.6 | |
| Not wearing face mask | 14.5 | 14.6 | 12.7 | |
| Not avoiding restaurants | 34.2 | 33.2 | 44.1 | |
| Not avoiding high-risk people | 21.1 | 20.8 | 24.2 | |
| Having visitors at residence | 48.3 | 47.2 | 59.1 | |
| Going to another’s residence | 48.9 | 47.9 | 58.8 | |
| Going to a bar, club, etc. | 13.1 | 12.7 | 16.9 | |
| Attending a gathering with > 10 people | 19.5 | 18.2 | 32.5 | |
| Contact with non–household members | 64.4 | 63.5 | 73.2 | |
| Attending religious service in person | 9.8 | 9.1 | 16.0 | |
| Count of 11 risk behaviors | 3.1 (2.41) | 3.03 (2.39) | 3.82 (2.53) | |
| Any small gathering | 80.3 | 79.6 | 87.0 | |
| Any large gatheringb | 51.0 | 49.7 | 62.9 | |
| Any lack of adherence to personal hygiene behaviors | 17.5 | 17.7 | 15.7 | |
| No. of respondents | 7 604 | 6 856 | 748 | |
| No. of observations | 104 677 | 97 602 | 7 075 | |
| Person-years of follow-up | 4 769 | 4 477 | 292 | |
Note. CDC = Centers for Disease Control and Prevention. Percentages and means were based on the first observation of each respondent in this period and were calculated using weights provided by the UAS. State-level cases in the previous week (per 100 000) and CDC COVID-19 cases were from the COVID Data Tracker, updated May 13, 2021.
Source. Data are from the UAS COVID-19 survey waves 7–26.
Indicates combined age group of 18--64 years. bSmall gathering includes contact with non--household members, having visitors at residence, going to another’s residence, and not avoiding high-risk people. Large gathering includes going to a bar, club, etc.; attending a gathering with > 10 people; attending religious service in person; not avoiding public places; and not avoiding restaurants.
FIGURE 1—
Percentage of Sample Respondents Engaging in Risk Behaviors at Each Understanding America Study (UAS) COVID-19 Survey Wave in the Study Period: United States, June 10, 2020–April 26, 2021
Note. We calculated percentages using weights provided by the UAS. The points represent the percentage of respondents engaging in the behavior at each wave, and the lines represent smoothed fits of these discrete estimates.
For comparison, the rightmost column of Table 1 displays basic demographic characteristics collected by the CDC for national COVID-19 cases reported through May 13, 2021. The demographic composition of the COVID-19 cases in this study was similar to those of the national COVID-19 cases.
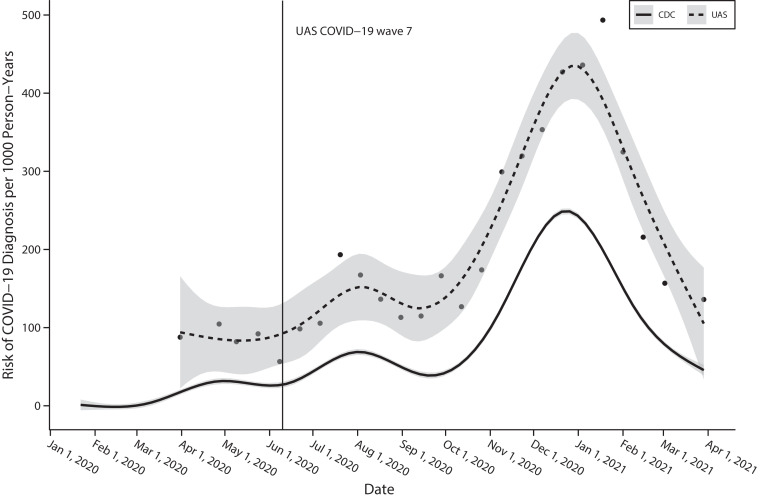
Figure 2 displays the estimated risk of COVID-19 diagnosis since the previous UAS COVID-19 wave along with the case rates reported by the CDC. Our study period of waves 7 through 26 began after the initial surge in cases, and, by contrast with the CDC data, our case data included both positive tests and doctor diagnoses but did not include cases of children younger than 18 years. Despite these differences, the cases identified in the UAS COVID-19 survey displayed the same general temporal pattern as national COVID-19 cases, beginning with the initial wave in spring 2020, a larger wave in summer 2020, and the largest wave of cases in winter 2020 through 2021.
FIGURE 2—
Estimated Risk of COVID-19 Diagnosis in the Understanding America Study (UAS) COVID-19 Survey Compared With CDC Case Data: United States, January 2020–April 2021
Note. CDC = Centers for Disease Control and Prevention. The dots represent the risk of incident COVID-19 diagnosis following each UAS wave, calculated as the number of incident diagnoses divided by the total number of person-years of exposure. The dotted line presents a smoothed fit of these discrete estimates. The vertical line indicates the beginning of wave 7, which was the first wave in our study period. The solid line represents a smoothed fit of the 7-day COVID-19 case rate reported by the CDC COVID Data Tracker. The UAS data included both positive test results and diagnoses by health care providers and were limited to adults 18 years and older. The CDC case data contained individuals of all ages.
Behavior–Diagnosis Association
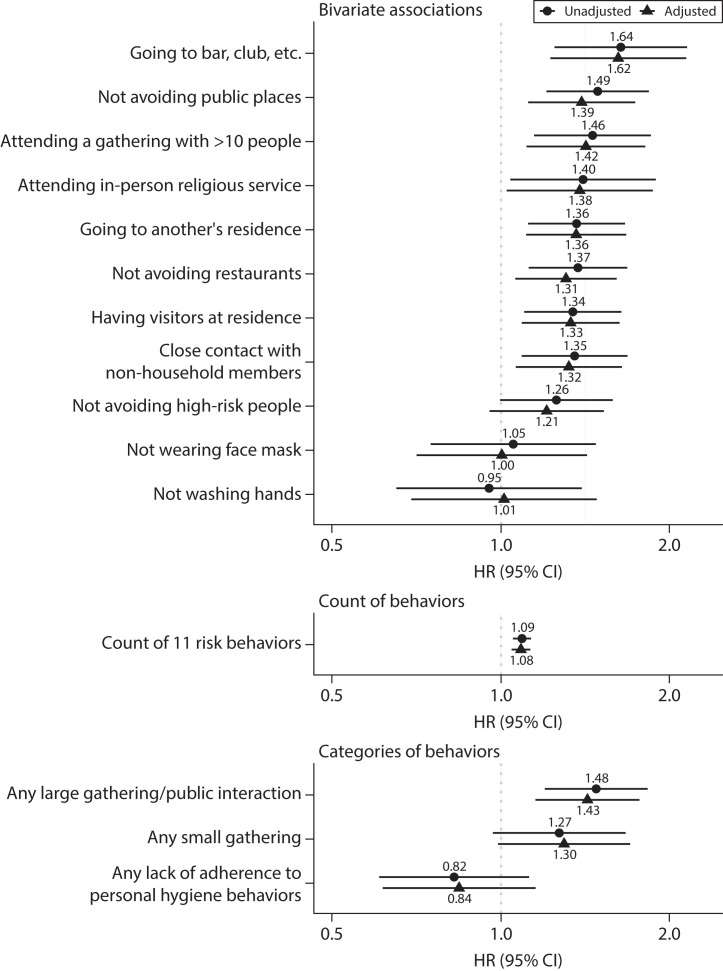
Figure 3 displays the hazard ratios (HRs) and 95% confidence intervals (CIs) from the Cox hazard models predicting COVID-19 diagnosis from relevant behaviors. Coefficients from these models are displayed in Table C (available as a supplement to the online version of this article at http://www.ajph.org). With the exception of not avoiding high-risk people, each of the behaviors indicating lack of adherence to social-distancing guidelines was significantly associated with risk of COVID-19 diagnosis in the unadjusted bivariate models. For example, going to a bar, club, or other place where people gather was associated with 64% higher risk of COVID-19 diagnosis before the next interview (HR = 1.64; 95% CI = 1.25, 2.15). Neither hand washing nor face mask wearing were significantly associated with COVID-19 diagnosis in this analysis. The model based on the count of the 11 risk behaviors suggests that each additional risk behavior is associated with 9% greater risk of COVID-19 diagnosis (HR = 1.09; 95% CI = 1.05, 1.13).
FIGURE 3—
Association Between Adherence to Personal Mitigation Behaviors and Risk of COVID-19 Diagnosis: Understanding America Study, United States, June 10, 2020–April 26, 2021
Note. CI = confidence interval; HR = hazard ratio. Results from Cox proportional hazards models of 748 COVID-19 diagnoses over 4769 person-years of exposure. Unadjusted models included the behavior alone. Adjusted models additionally included sex, age category, race/ethnicity, educational attainment, employment status, living arrangement, and the natural logarithm of the state-level 7-day COVID-19 case rate.
Last, the model including categories of risk behaviors together found that engagement in any large gathering or public interaction was more strongly associated with COVID-19 diagnosis (HR = 1.48; 95% CI = 1.20, 1.83) than was engagement in any small gathering (HR = 1.27; 95% CI = 0.97, 1.67; P < .10). Lack of adherence to either of the COVID-19–related personal hygiene behaviors was not significantly associated with COVID-19 diagnosis after controlling for social behaviors. When we included sociodemographic covariates and state COVID-19 case rates in these models, associations were generally attenuated but still suggested that individuals who recently engaged in social activities, especially large gatherings, and those who engaged in more total high-risk behaviors had an increased risk of COVID-19 diagnosis.
Supplementary Analyses
In addition to our main specification predicting COVID-19 diagnoses, we considered both stricter and more inclusive case definitions. The stricter case definition included only positive tests. The more inclusive case definition included instances in which respondents believed they had COVID-19 even if they did not get tested to confirm this suspicion, because testing was not always easily available for the general population and because individuals with mild cases may have forgone testing. Using both of these case definitions, we still found associations on the same order of magnitude as our main results (Tables D–E [available as a supplement to the online version of this article at http://www.ajph.org]). Besides the count of high-risk behaviors, we created a cumulative behavioral risk index (ranging from zero to 5 or more) to assess whether there is a dose–response or threshold effect. We found that the risk of COVID-19 appeared to increase with additional risky behaviors, especially for those who engaged in 4 or 5 or more risky behaviors compared to those who engaged in none (Table F [available as a supplement to the online version of this article at http://www.ajph.org]).
DISCUSSION
For much of the COVID-19 pandemic, nonpharmaceutical interventions, including behavioral modifications, were the only defense against COVID-19. Although vaccines are now widely available in the United States, the surge in infections fueled by the Delta variant underscores that behavioral modifications continue to be important for reducing the spread of COVID-19.4 Although there have been numerous studies examining adherence to these behavioral guidelines,30–32 there has been scant evidence to suggest that individuals who did not adhere to these guidelines actually faced a higher risk of COVID-19. In this study, we examined the association between behaviors considered high risk for COVID-19 and diagnosis of COVID-19 using a nationally representative longitudinal data source.
We found that individuals who recently engaged in widely discouraged social activities, including going to a bar or club, attending in-person religious services, attending a gathering with more than 10 people, having visitors at one’s residence, and having close contact with non–household members, had a significantly higher risk of being diagnosed with COVID-19 than did those who did not engage in these activities. Likewise, we found that individuals who did not report taking the precautions of avoiding public places or avoiding eating at restaurants had a significantly higher risk of a subsequent COVID-19 diagnosis. These findings generally persisted after controlling for a set of sociodemographic characteristics and were consistent with previous research at the population level that found that behaviors related to social distancing were associated with reduced COVID-19 infection.6,8,9,11–15
We also examined the number of high-risk behaviors, finding that each additional risk behavior was associated with an 8% to 9% higher risk of COVID-19, suggesting that individuals who engaged in multiple risk behaviors were infected more frequently than were those who engaged in fewer risk behaviors. Last, we found that individuals who engaged in at least 1 behavior related to large gatherings or public interactions had more than a 40% increased risk of contracting COVID-19 compared to those who did not engage in any of these behaviors. Even after controlling for engagement in large gatherings, those who engaged in at least 1 behavior related to small gatherings had an approximately 30% greater risk of COVID-19, a finding that was marginally significant. Although large gatherings appeared to be associated with a greater risk of COVID-19, our findings suggest that small gatherings are also important risk factors for COVID-19, consistent with previous research suggesting that small birthday gatherings were associated with household COVID-19 infections.22
We did not find the COVID-19–related personal hygiene behaviors of hand washing and mask wearing to be significantly related to COVID-19 diagnosis in this study. The UAS questionnaire simply asked whether one wore a face covering in the past week, without requiring respondents to specify frequency. Previous research indicates that this type of question wording, along with social desirability bias, may have led respondents to overreport these personal hygiene behaviors.33 Those who reported not wearing a mask in the previous week were likely a heterogeneous group, including those who avoided interactions with others entirely and thus did not need to wear a mask and those who went out but chose not to wear a mask. Moreover, the question about wearing a face covering did not ask about the type or fit of the mask worn, both of which can influence the degree of protection conferred by the mask.34 As a result of these data limitations and previous evidence documenting the effectiveness of masks,5–7,11,34 we do not make claims about the effectiveness of masks and handwashing based on our null findings.
Strengths and Limitations
Strengths of this study include a nationally representative, individual-level data source with more than 10 months of follow-up. Because adherence to risk-mitigation behaviors varied over the course of the pandemic,30–32 our study also benefited from information on numerous risk behaviors updated at regular intervals.
Despite these strengths, our study contained several limitations. First, although the UAS was designed to be nationally representative, individuals may be reluctant to participate in surveys they perceive as asking about sensitive topics, and it is possible that these individuals differed in terms of their exposure to COVID-19. Second, classification of COVID-19 diagnosis is imperfect. Respondents who had severe cases of COVID-19, were hospitalized, or died may have missed survey waves or dropped out of the survey entirely, leading us to misclassify their diagnosis date or incorrectly classify them as censored because of loss to follow-up. As with all studies of COVID-19 infection, we likely undercounted mild and asymptomatic infections because infection severity influences whether individuals get tested or seek medical care for COVID-19.35 Third, the questions about personal behaviors do not capture all relevant aspects of risk and omit important details. For example, the question about avoiding restaurants does not distinguish between outdoor and indoor dining, and the question about close contact refers only to distance but not duration. Fourth, we are unable to establish a causal relationship between these risk factors and COVID-19 infection. It is not possible with our data and study design to determine exactly how an individual contracted COVID-19.
Public Health Implications
Our findings demonstrate that even in the presence of structural factors that influence the risk of infection, such as one’s work or living situation, personal mitigation behaviors related to social distancing can influence risk of COVID-19. Although our study ended at a time when vaccines against COVID-19 were widely available for US adults, many areas had low vaccination rates, and evidence suggests that even vaccinated individuals can spread the highly transmissible Delta variant of SARS-CoV-2.4 In this context, public health messaging should keep emphasizing the importance of these personal risk-mitigation behaviors, especially for unvaccinated individuals or in places where vaccination rates are low. These findings are also important to inform guidelines during future surges of COVID-19 or future outbreaks of viruses with similar transmission dynamics.
ACKNOWLEDGMENTS
This study was supported by the National Institute on Aging (grants T32 AG000037 and P30 AG017265). The collection of the Understanding America Study (UAS) COVID-19 tracking data are supported in part by the Bill & Melinda Gates Foundation and the National Institute on Aging (grant U01AG054580).
The project described in this article relies on data from surveys administered by the UAS, which is maintained by the Center for Economic and Social Research at the University of Southern California.
Note. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the University of Southern California or the UAS.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
HUMAN PARTICIPANT PROTECTION
The UAS was approved by the University of Southern California institutional review board, and electronic informed consent was obtained from all participants.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad FB, Anderson RN. The leading causes of death in the US for 2020. JAMA. 2021;325(18):1829–1830. doi: 10.1001/jama.2021.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrasfay T, Goldman N. Association of the COVID-19 pandemic with estimated life expectancy by race/ethnicity in the United States, 2020. JAMA Netw Open. 2021;4(6):e2114520. doi: 10.1001/jamanetworkopen.2021.14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
- 5.Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/index.html
- 6.Centers for Disease Control and Prevention. Association of state-issued mask mandates and allowing on-premises restaurant dining with county-level COVID-19 case and death growth rates—United States, March 1–December 31, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(10):350–354. doi: 10.15585/mmwr.mm7010e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayodeji OJ, Ramkumar S. Effectiveness of face coverings in mitigating the COVID-19 pandemic in the United States. Int J Environ Res Public Health. 2021;18(7):3666. doi: 10.3390/ijerph18073666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margraf J, Brailovskaia J, Schneider S. Adherence to behavioral COVID-19 mitigation measures strongly predicts mortality. PLoS One. 2021;16(3):e0249392. doi: 10.1371/journal.pone.0249392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyu W, Wehby GL. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US. Health Aff (Millwood). 2020;39(8):1419–1425. doi: 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Trends in county-level COVID-19 incidence in counties with and without a mask mandate—Kansas, June 1–August 23, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(47):1777–1781. doi: 10.15585/mmwr.mm6947e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bo Y, Guo C, Lin C, et al. Effectiveness of non-pharmaceutical interventions on COVID-19 transmission in 190 countries from 23 January to 13 April 2020. Int J Infect Dis. 2021;102:247–253. doi: 10.1016/j.ijid.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Askitas N, Tatsiramos K, Verheyden B. Estimating worldwide effects of non-pharmaceutical interventions on COVID-19 incidence and population mobility patterns using a multiple-event study. Sci Rep. 2021;11(1):1972. doi: 10.1038/s41598-021-81442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai S, Ruktanonchai NW, Zhou L, et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020;585(7825):410–413. doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584(7820):257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 15.Korevaar HM, Becker AD, Miller IF, Grenfell BT, Metcalf CJE, Mina MJ.2020. https://www.medrxiv.org/content/10.1101/2020.06.30.20142877v1
- 16.Murray T. Stay-at-home orders, mobility patterns, and spread of COVID-19. Am J Public Health. 2021;111(6):1149–1156. doi: 10.2105/AJPH.2021.306209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Community and close contact exposures associated with COVID-19 among symptomatic adults ≥18 years in 11 outpatient health care facilities—United States, July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1258–1264. doi: 10.15585/mmwr.mm6936a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doung-ngern P, Suphanchaimat R, Panjangampatthana A, et al. Case–control study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis. 2020;26(11):2607–2616. doi: 10.3201/eid2611.203003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speaker SL, Doherty CM, Pfoh E, et al. Social behaviors associated with a positive COVID-19 test result. Cureus. 2021;13(2):e13064. doi: 10.7759/cureus.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenforde MW, Fisher KA, Patel MM. Identifying COVID-19 risk through observational studies to inform control measures. JAMA. 2021;325(14):1464–1465. doi: 10.1001/jama.2021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fazio RH, Ruisch BC, Moore CA, Samayoa JAG, Boggs ST, Ladanyi JT. Social distancing decreases an individual’s likelihood of contracting COVID-19. Proc Natl Acad Sci U S A. 2021;118(8):e2023131118. doi: 10.1073/pnas.2023131118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whaley CM, Cantor J, Pera M, Jena AB. Assessing the association between social gatherings and COVID-19 risk using birthdays. JAMA Intern Med. 2021;181(8):1090–1099. doi: 10.1001/jamainternmed.2021.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alattar L, Rogofsky D, Messel M.2018. https://www.ssa.gov/policy/docs/ssb/v78n2/v78n2p13.html
- 24.Christie A, Mbaeyi SA, Walensky RP. CDC interim recommendations for fully vaccinated people: an important first step. JAMA. 2021;325(15):1501–1502. doi: 10.1001/jama.2021.4367. [DOI] [PubMed] [Google Scholar]
- 25.Brandén M, Aradhya S, Kolk M, et al. Residential context and COVID-19 mortality among adults aged 70 years and older in Stockholm: a population-based, observational study using individual-level data. Lancet Healthy Longev. 2020;1(2):e80–e88. doi: 10.1016/S2666-7568(20)30016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monod M, Blenkinsop A, Xi X, et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021;371(6536):eabe8372. doi: 10.1126/science.abe8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. COVID data. 2020. https://covid.cdc.gov/covid-data-tracker
- 28.Therneau TM, Grambsch PM. The Cox model. In: Therneau TM, Grambsch PM, editors. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. pp. 39–77. [DOI] [Google Scholar]
- 29.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med. 2018;6(7):121. doi: 10.21037/atm.2018.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JK, Crimmins EM. How does age affect personal and social reactions to COVID-19: results from the national Understanding America Study. PLoS One. 2020;15(11):e0241950. doi: 10.1371/journal.pone.0241950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao E, Wu Q, Crimmins EM, Ailshire JA. Media trust and infection mitigating behaviours during the COVID-19 pandemic in the USA. BMJ Glob Health. 2020;5(10):e003323. doi: 10.1136/bmjgh-2020-003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crane MA, Shermock KM, Omer SB, Romley JA. Change in reported adherence to nonpharmaceutical interventions during the COVID-19 pandemic, April–November 2020. JAMA. 2021;325(9):883–885. doi: 10.1001/jama.2021.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmons S, McGinnity F, Belton C, Barjaková M, Lunn P. It depends on how you ask: measuring bias in population surveys of compliance with COVID-19 public health guidance. J Epidemiol Community Health. 2021;75(4):387–389. doi: 10.1136/jech-2020-215256. [DOI] [PubMed] [Google Scholar]
- 34.Howard J, Huang A, Li Z, et al. An evidence review of face masks against COVID-19. Proc Natl Acad Sci U S A. 2021;118(4):e2014564118. doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu SL, Mertens AN, Crider YS, et al. Substantial underestimation of SARS-CoV-2 infection in the United States. Nat Commun. 2020;11(1):4507. doi: 10.1038/s41467-020-18272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]