Abstract
Objectives. To identify associations between patient race and annual chlamydia screening among adolescent females.
Methods. We performed a retrospective cohort study of females aged 15 to 19 years in a 31-clinic pediatric primary care network in Pennsylvania and New Jersey from 2015 through 2019. Using mixed-effect logistic regressions, we estimated associations between annual chlamydia screening and patient (race/ethnicity, age, previous chlamydia screening and infection, insurance type) and clinic (size, setting) characteristics. We decomposed potential effects of clinician’s implicit racial bias and screening, using covariates measuring the proportion of Black patients in each clinician’s practice.
Results. There were 68 935 well visits among 37 817 females, who were 28.8% Black and 25.8% Medicaid insured. The mean annual chlamydia screening rate was 11.1%. Black females had higher odds of screening (adjusted odds ratio [AOR] = 1.67; 95% confidence interval [CI] = 1.51, 1.84) than did White females. In the clinician characteristics model, individual clinicians were more likely to screen their Black versus non-Black patients (AOR = 1.88; 95% CI = 1.65, 2.15).
Conclusions. Racial bias may affect screening practices and should be addressed in future interventions, given the critical need to increase population-level chlamydia screening.(Am J Public Health. 2022;112(1):135–143. https://doi.org/10.2105/AJPH.2021.306498)
In the United States, 2019 marked the sixth consecutive year of increasing rates of sexually transmitted infections (STIs).1 Chlamydia trachomatis, the most commonly reported bacterial disease in the United States, now has the highest prevalence ever recorded.1 Nearly half of infections occurred in individuals aged 15 to 24 years.2,3 Chlamydia infections can significantly affect quality of life and morbidity across the lifespan. Untreated chlamydia in females may lead to pelvic inflammatory disease (PID) and result in chronic pelvic pain, infertility, ectopic pregnancy, and increased susceptibility to HIV infection.4,5 Recent Centers for Disease Control and Prevention (CDC) analyses estimate that chlamydia in those aged 15 to 24 years accounted for approximately $452 000 000 in direct lifetime medical costs.6
Routine population-based screening is a key strategy to reduce the morbidity and transmission of chlamydia. The CDC, the US Preventive Services Task Force (USPSTF), and the American Academy of Pediatrics (AAP) recommend annual screening of sexually active cisgender females aged 15 to 26 years.7–9 Routine screening can lead to early identification and treatment of asymptomatic infections, thereby lowering the risk of forward transmission and PID.10 Screening also introduces a golden opportunity for clinicians to counsel adolescents regarding comprehensive sexual health, such as STI and HIV prevention, contraception, and healthy communication in relationships—all practices strongly recommended in preventative health guidelines.11,12
Unfortunately, the application of these screening guidelines has been far from universal in pediatric practices.13–17 Despite clinical practice guidelines, rates of chlamydia screening in pediatric care settings are both suboptimal and often inequitable.17 Previous research demonstrates higher lifetime risk of chlamydia and PID among Black females than among their White counterparts.18–20 However, it is unclear whether the higher burden of infection is attributable to increased prevalence alone or is exacerbated by clinician implicit bias. Implicit bias, defined as associations existing outside conscious awareness that may negatively influence clinician behavior and treatment choices,21 may lead to higher screening rates in Black females. The extent to which implicit bias could contribute to disproportionate screening, and thus detection of early asymptomatic infection, in Black and Latinx females is currently unknown.22,23 Population estimates of lifetime sexual activity for adolescent females across racial and ethnic groups are nearly equivalent (38% for White, 42% for Latinx, and 42% for Black female high school students) and do not justify differential screening practices in pediatric care settings.24
To improve routine chlamydia screening for all adolescents, there is a critical need to elucidate and ameliorate the drivers of inequitable screening practices. As national chlamydia prevalence continues to increase, understanding targets for interventions to improve universal screening of sexually active young women is a key public health task. Although previous analyses have identified higher rates of screening of Black women,17,22 it is unclear whether these clinician effects are attributable to between-clinician effects (wherein clinicians with robust screening practices may also care for a higher proportion of Black patients) or in-clinician effects (wherein implicit bias may drive individual clinicians to disproportionately screen their Black, rather than White, patients).
We assessed variability in annual chlamydia screening rates across a large and geographically diverse pediatric primary care network and determined the influence of patient race and ethnicity on screening outcomes at the patient, clinic, and individual clinician levels.
METHODS
This retrospective cohort study included females aged 15 to 19 years receiving primary care services in a 31-practice academic, pediatric, primary care network serving approximately 250 000 patients annually across urban and suburban Pennsylvania and New Jersey. Two of the urban practices receive federal Title X family-planning funding and provide additional adolescent confidential family-planning services, such as free contraception and HIV testing, in addition to routine primary care. The other 29 sites are standard pediatric primary care offices.
We included patients if they were assigned female sex at birth and attended an annual well visit during the study period of July 2015 through December 2019. We collected relevant screening data from Qlik (Radnor, PA), a commercial business intelligence platform. As part of a chlamydia screening quality improvement initiative, Qlik captured chlamydia screening data from well visits for all females aged 15 to 19 years since 2014, including screening status (screened vs not screened), demographic characteristics, clinic site, and well visit clinician.
Measures
Our outcome measure was receipt of chlamydia screening by nucleic acid amplification test (urine, vaginal, or cervical swab) in an annual well visit year, defined as the 364 days before and on the day of the annual well visit. We used the year-long measure to account for chlamydia screening that was asynchronous with the annual well visit but still fulfilled the annual screening recommendations on the day of the annual well visit. We classified individuals (n = 478) who had chlamydia screening outside the annual well visit year (i.e., in a previous 365-day period if a well visit did not occur) as not screened at their annual well visit. We determined patient- and clinic-level exposures based on previous literature documenting their potential influence on chlamydia screening.17,19,20,22
Patient Characteristics
We collected race and ethnicity by patient report or registrar assessment at visit registration; therefore, race should be interpreted as “observed race.” We calculated age as age in years at the time of visit. Sexual history is not captured as a discrete variable in the electronic health record system, and previous studies have demonstrated that proxy metrics of sexual activity (e.g., Healthcare Effectiveness Data and Information Set criteria) perform poorly in pediatric data.25,26 Thus, we used previous receipt of chlamydia screening and, separately, previous chlamydia infection as proxy metrics for sexual activity and chlamydia risk. We categorized insurance as public (Medicaid), private, none, or missing.
Clinic Characteristics
We estimated clinic size by the total number of unique clinicians over the study period and categorized them as less than 10, 10 to 19, or 20 or more clinicians. We categorized clinic setting as Title X urban, non-Title X urban, or suburban to account for collinearity between geography and Title X funding status. We derived the adolescent patient proportion (proportion of total clinic volume composed of patients aged 13 years or older) and the proportion of patients privately insured at each clinic from estimates created through Arcus Cohort Discovery (Children’s Hospital of Philadelphia, Philadelphia, PA), a proprietary tool that provides aggregate statistics describing the patient population in the source health system.
Clinician Characteristics
We categorized clinicians by training as general pediatricians, residents, adolescent medicine specialists (i.e., attending physicians or fellows), or nurse practitioners. We calculated clinicians’ years in practice at the time of each visit by health system data supplemented with National Provider Identification data when needed. The study data set had clinician information only for the day of the annual well visit; therefore, for the clinician characteristics analysis, we excluded visits where chlamydia screening was not ordered at the well visit (i.e., excluding tests ordered in the 364 days before the visit) to avoid falsely attributing the well visit clinician characteristics to chlamydia screening episodes that did not occur synchronously with the well visit. We also excluded visits where the patient was seen by resident physicians (n = 2049) because of collinearity between resident clinician type and clinician years in practice. Clinician age, sex, and race data were not available for analysis.
Statistical Analysis
Descriptive statistics summarized characteristics of patients and clinic sites. We determined annual chlamydia screening rates for each clinic by calculating the proportion of all well visits with a completed chlamydia screening in the annual well visit year. We calculated the mean annual screening rate for the study period by summing the annual screening rates across the network and dividing by 5 (the number of observation years). We determined the clinic-adjusted mean annual screening rate by first calculating the mean annual screening rate at each individual clinic, summing these, and dividing by the 31 clinics in the network. To examine associations between patient and clinic characteristics on chlamydia screening, we used mixed-effects logistic regression models and estimated odds ratios accounting for random effects of patients and clinic sites. We first conducted models assessing associations between patient factors and then separately clinic factors on the chlamydia screening outcome. We were unable to include the clinic proportions of adolescent and insured patients at each clinic in these models because of collinearity with the clinic setting variable. We intended the coefficients for race and ethnicity in the regression analyses to measure the racial/ethnic health inequities that would remain for non-White patients if clinic context and our proxy metrics for sexual activity were standardized across the sample and insurance was set to equal that of the White patient sample.27 The final multivariable model contained both patient and clinic characteristic factors with a P level of less than .2.
In the clinician characteristics model, we used the combined patient and clinic characteristic multivariable model described, further including clinician training and clinician’s years in practice. Given previous data demonstrating higher rates of screening among Black versus White adolescents,17 we aimed to assess whether patient observed race affected chlamydia screening at the level of the individual clinician. For example, data from individual clinicians who screen a high proportion of patients for chlamydia and also have a high volume of Black patients could erroneously strengthen the association between race and screening across the sample.
To examine whether race-based screening disparities were potentially related to individual clinician implicit bias (in-clinician effect) compared with the proportion of Black patients seen by each clinician (between-clinician effect), we created a parameter presenting the proportion of Black patients in each clinicians’ practice (mblack = the mean number of encounters with Black patients per clinician). To decompose the in-clinician and the between-clinician components, creating an estimated effect of clinician implicit bias on the odds of chlamydia screening, we created a new “Black” variable that represented the difference between individual patient race (0 = non-Black vs 1 = Black) and the mblack parameter (Black = patient race-mblack) using methods previously described by Gerber et al.28
We conducted all statistical analyses using Stata version 16 (StataCorp, College Station, TX) and SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
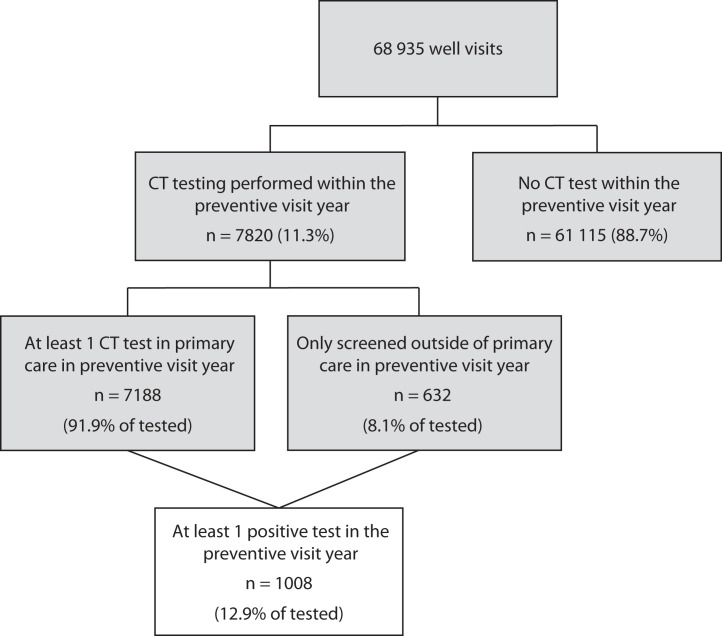
From July 1, 2015, to December 31, 2019, 37 817 females aged 15 to 19 years attended 68 935 annual well visits (37 817 females had 1 visit, 21 028 had 2 visits, 8468 had 3 visits, 1561 had 4 visits, and 61 had 5 visits). The demographic characteristics of patients by chlamydia screening status are displayed in Table 1. Demographic characteristics by each of the 31 clinic sites are displayed in Table A (available as a supplement to the online version of this article at http://www.ajph.org). Patients were 28.8% Black and 25.8% Medicaid insured, with a median age of 15 years (interquartile range = 15–16 years). Over the observation period, 11.3% of patients at well visits had a chlamydia test completed at or in the annual well visit year (Figure 1), translating to 16% (n = 6067) of patients having at least 1 chlamydia test performed during the study period. The mean annual screening rate for the study period was 11.1%, and the rate increased annually (Table B, available as a supplement to the online version of this article at http://www.ajph.org). However, screening rates between the clinics were highly variable. The clinic-adjusted mean annual screening rate was 5.6% (range = 0%–39%). The chlamydia test positivity rate for well visits where screening occurred across the study period was 12.9%, with 1008 infections identified.
TABLE 1—
Patient-Level Demographics of the Study Sample Attending Well Visits and Clinical Sites: Pennsylvania and New Jersey, 2015–2019
| Characteristic | Never Screened in Preventative Visit Year (n = 31 750; 84.0%), No. (%) or Median (IQR) | Ever Screened in Preventative Visit Year (n = 6067; 16.0%), No. (%) or Median (IQR) | Total n = 37 817, No. (%) or Median (IQR) |
| Patient characteristics | |||
| Race | |||
| Black | 6 895 (21.7) | 4 012 (66.2) | 10 907 (28.8) |
| White | 19 949 (62.9) | 1 457 (24.0) | 21 406 (56.6) |
| Other | 4 881 (15.4) | 593 (9.8) | 5 474 (14.5) |
| Missing | 25 (0.1) | 5 (0.1) | 30 (0.1) |
| Ethnicity | |||
| Non-Latinx/missing | 29 924 (94.2) | 5 741 (94.6) | 35 665 (94.3) |
| Latinx | 1 826 (5.8) | 326 (5.4) | 2 152 (5.7) |
| Age at well visit, y | 15 (15–16) | 16 (15–17) | 15 (15–16) |
| No. of well visits in study period | 2 (1–2) | 2 (1–3) | 2 (1–2) |
| Insurance type | |||
| Uninsured/missing | 2 726 (8.6) | 700 (11.5) | 3 426 (9.1) |
| Public | 6 817 (21.5) | 2 952 (48.7) | 9 769 (25.8) |
| Private | 22 207 (69.9) | 2 415 (39.8) | 24 622 (65.1) |
| Clinic characteristics | |||
| Clinic setting | |||
| Urban Title X | 2 419 (7.6) | 2 914 (48.0) | 5 333 (14.1) |
| Urban non-Title X | 4 108 (12.9) | 1 562 (25.8) | 5 670 (15.0) |
| Suburban | 25 223 (79.4) | 1 591 (26.2) | 26 814 (70.9) |
| Mean no. of clinicians by clinica | |||
| 1–9 | 7 481 (23.6) | 392 (6.5) | 7 873 (20.8) |
| 10–19 | 21 154 (66.6) | 1 948 (32.1) | 23 102 (61.1) |
| ≥ 20 | 3 115 (9.8) | 3 727 (61.4) | 6 842 (18.1) |
| Proportion of privately insured patients by clinicb | |||
| < 49% | 3 499 (11.2) | 3 744 (61.7) | 7 243 (19.2) |
| ≥ median, ≥ 50% | 28 251 (89.0) | 2 323 (38.3) | 30 574 (80.8) |
| Proportion of clinic patients who are adolescentsc | |||
| < median < 33% | 13 237 (41.7) | 4 683 (77.2) | 17 920 (47.4) |
| ≥ median ≥ 34% | 18 513 (58.3) | 1 384 (22.8) | 19 897 (52.6) |
Note. IQR = interquartile range. The sample comprised females aged 15–19 years. The sample size was n = 37 817.
Number of unique clinicians in clinics over the study period.
Proportion of patients in each clinic (entire clinic, not just sample) that were privately insured.
Proportion of patients in each clinic (entire clinic, not just sample) that were adolescents aged 13–18 years.
FIGURE 1—
Chlamydia Screening Status Across Well Visits by Females Aged 15–19 Years: Pennsylvania and New Jersey Health System-Affiliated Clinics, 2015–2019
Note. CT = Chlamydia trachomatis. Preventative care year represents the 365 days before and of the well visit. The sample size was n = 37 817 individuals.
In the patient characteristics model, Black race, Latinx ethnicity, older age, having public insurance, having had a previous chlamydia screening, and having had a previous chlamydia infection were all significantly associated with increased odds of screening in an annual well visit year. In the clinic characteristics model, larger clinic size was associated with increased odds of screening, and urban clinics (both Title X and non-Title X) had significantly higher odds of screening. In the combined multilevel patient and clinic model, Black race (adjusted odds ratio [AOR] = 1.67; 95% confidence interval [CI] = 1.51, 1.84) and Latinx ethnicity (AOR = 1.24; 95% CI = 1.07, 1.44) remained significantly associated with odds of chlamydia screening (Table 2).
TABLE 2—
Association of Patient and Clinic Characteristics and Receipt of Chlamydia Screening by Well Visit: Pennsylvania and New Jersey, 2015–2019
| Characteristic of Well Visit Patients | AOR (95% CI) |
| Patient characteristics | |
| Age | 1.23 (1.20, 1.26) |
| Race/ethnicity | |
| White (Ref) | 1 |
| Black | 1.67 (1.51, 1.84) |
| Other race | 1.09 (0.97, 1.22) |
| Latinx | 1.24 (1.07, 1.44) |
| Insurance | |
| Public (Ref) | 1 |
| Private | 0.77 (0.72, 0.83) |
| Previous chlamydia screening | 20.17 (18.53, 21.95) |
| Previous chlamydia infection | 3.84 (2.84, 5.18) |
| Clinic characteristics | |
| Clinic size | |
| < 10 clinicians (Ref) | 1 |
| 10–20 clinic clinicians | 1.77 (0.93, 3.38) |
| > 20 clinic clinicians | 9.56 (1.56, 58.46) |
| Clinic setting | |
| Suburban clinics (Ref) | 1 |
| Urban Title X | 1.74 (0.22, 14.00) |
| Urban non-Title X | 2.29 (1.40, 3.74) |
Note. AOR = adjusted odds ratio; CI = confidence interval. The table presents the results of the combined patient and clinic characteristics multilevel mixed-effects regression model. The sample size was n = 68 935 individuals.
The clinician characteristics analysis (Table 3), exploring the effects of the Black versus non-Black composition of patients in clinicians’ case mix on screening practices as a measurement of implicit racial bias, included 66 401 encounters with 630 unique clinicians. We identified significant associations between patient race (Black vs non-Black) and chlamydia screening (AOR = 1.88; 95% 95 = 1.65, 2.15), indicating that after accounting for the proportion of Black versus non-Black patients in a clinicians’ case mix and the other patient and clinic characteristics captured in the first regression model, clinicians remained significantly more likely to screen Black than White female patients, suggesting that implicit bias may play a role in screening decisions.
TABLE 3—
Associations of Patient and Clinic Characteristics and Receipt of Chlamydia Screening Accounting for Race of Patients for Each Clinician, by Well Visits: Pennsylvania and New Jersey, 2015–2019
| Characteristics of Well Visits | AOR (95% CI) |
| Patient characteristics | |
| Race/ethnicity | |
| Black racea | 1.88 (1.65, 2.15) |
| Other race | 1.26 (1.08, 1.46) |
| Latinx ethnicity | 1.18 (0.97, 1.42) |
| Age, y | 1.36 (1.31, 1.41) |
| Insurance | |
| Public (Ref) | 1 |
| Private | 0.79 (0.72, 0.87) |
| Previous chlamydia screening | 8.17 (7.29, 9.16) |
| Previous chlamydia infection | 6.48 (4.46, 9.40) |
| Clinician characteristics | |
| Proportion of Black patients per cliniciana | 11.95 (6.85, 20.87) |
| Clinician type | |
| General pediatrics attending physician (Ref) | 1 |
| Nurse practitioner | 1.48 (1.33, 1.65) |
| Adolescent medicine specialist (attending physician/fellow) | 1.93 (1.61, 2.31) |
| Clinician’s years in practice | |
| ≥ 15 y (Ref) | 1 |
| < 3 y | 1.49 (1.29, 1.71) |
| 3–14 y | 1.39 (0.26, 1.53) |
| Clinic characteristics | |
| Clinic size | |
| < 10 clinicians (Ref) | 1 |
| 10–20 clinicians | 1.58 (0.53, 4.69) |
| ≥ 20 clinicians | 10.72 (0.53, 216.99) |
| Clinic type and geography | |
| Suburban (Ref) | 1 |
| Urban Title X | 0.88 (0.03, 28.78) |
| Urban non-Title X | 2.96 (0.59, 14.80) |
Note. AOR = adjusted odds ratio; CI = confidence interval. The sample size was n = 63 221 individuals.
The difference between individual patients’ race (race: 0 = non-Black vs 1 = Black) and the composition of Black vs non-Black patients in encounter-level data (mblack) represented the estimated effect of the clinician’s implicit bias on the odds that the clinician ordered chlamydia screening (Black = race-mblack).
DISCUSSION
In a geographically diverse regional health care system, we found overall low chlamydia screening rates, high variability in screening practices across clinics, and evidence of inequitable screening practices by patient race and ethnicity. These findings emphasize the need to standardize adherence to chlamydia screening guidelines across health systems and to ensure that screening efforts are applied equitably across patient groups. Although our data were limited by a lack of a standard informatic measure of sexual activity, the 11.1% mean annual chlamydia screening rate across clinics falls far below population estimates of adolescent sexual activity, even among high school freshman.24 Health systems should adopt and promote standardized guidelines promoting equitable universal chlamydia screening in sexually active young women in accordance with USPSTF, CDC, and AAP guidelines.
Notably, we found that Black and Latinx adolescents had significantly higher odds of screening than did their White peers, after adjusting for markers of sexual activity, including increasing age, previous chlamydia screening, and previous chlamydia diagnosis. In our clinician characteristics analysis examining the impact of race on chlamydia screening practices, adjusting for clinic characteristics and accounting for the race-based case mix of clinicians, evidence of inequitable screening practices persisted, suggesting that racial bias may have influenced screening rates. Although the higher rates of screening among Black adolescents may be seen as a favorable outcome given the role of early detection and treatment of chlamydia in reducing PID, this pattern of differential screening suggests that clinicians may use race in either algorithmic or heuristic assessments of sexual health needs, rather than applying universal screening logic recommended by the AAP, CDC, and USPSTF. A recent analysis of the National Survey of Family Growth demonstrated that Black females were more likely to be asked about sexual activity, offered condoms, and offered STI screening at routine preventative health visits than were their White peers.29
The race-based differences in sexual health service delivery observed in our data are consistent with the Institute of Medicine’s definition of health inequities: care that has been “differentially allocated on the basis of social class, race, and ethnicity.”30(p123) These inequities may stem from historical and systemic racism, wherein Black females are sexualized and seen as more likely to engage in “risky” sexual behavior, despite no evidence from national data.24 Notably, these stereotypes not only may influence which tests clinicians order in a well visit but can also lead to biased conversations on sexual health that further stigmatize and marginalize Black adolescents. Further, although racial bias may have led to disproportionately higher service delivery to Black adolescents in this study, these same biases may lead to undertreatment of acute pain and criminalization of mental health in other clinical settings.21 For any health outcome and directionality, implicit racial bias is not benign.
It is also possible that the inequitable screening rates by race or ethnicity in our data could be influenced by a clinician’s desire to test those “at higher risk” according to population prevalence. Previous epidemiologic studies have consistently reported higher rates of STIs among Black and Latinx females than among their White counterparts.18,31 However, this logic may create a “chicken or the egg” phenomenon, whereby racial bias drives suboptimal routine screening rates in White females, thus leading to White females contributing to a smaller proportion of the population chlamydia prevalence rates than their Black peers. This trend would lead to biased estimates that then further influence clinician screening practices. Notably, we derived our data from the Philadelphia, Pennsylvania, metropolitan area, which has the third highest STI rates in the nation. Arguably, the entire sample thus had an increased population prevalence of chlamydia.
In a recent Journal of the American Medical Association Viewpoint “Addressing Systemic Racism Through Clinical Preventive Service Recommendations From the US Preventive Services Task Force,” the authors noted:
Across clinical preventive services, more evidence is needed to move beyond the current state of merely knowing that certain groups have higher disease prevalence and worse health outcomes to understanding effective evidence-based interventions to improve health outcomes.32(p628)
With respect to chlamydia screening, system-wide quality improvement interventions have been successful in improving chlamydia screening rates in adolescent females.33–37 However, these quality improvement efforts have largely targeted overall screening rates, not the essential task of closing the equity gap in screening practices. Without concerted efforts to target racial bias, improving screening rates in the absence of ensuring equity will not be enough. Without careful attention, these interventions can result in differential effects by race and ethnicity, as well as further distrust of the health care system and the continued emotional trauma of racism.38
Interventions that focus on standardizing and automating care delivery may uniquely hold promise for reducing disparities. One such tool is clinical decision support, including electronic nudges or “best practice alerts,” which can move clinicians closer to universal screening of sexually active youths. In addition, there is a need for an accurate and standardized collection of sexual activity data to ensure that pediatricians are screening the right youths at the right time. Lastly, whereas quality improvement efforts to standardize care may lessen disparities, they do not directly address the drivers of the inequities. Clinician training to reduce implicit bias and systemic racism in medicine is a key step toward rooting out the potential underlying causes of these health inequities. There is a critical need for implementation science research to elucidate optimal dissemination of implicit bias training across health systems.
Limitations
Our analyses have limitations. The network’s electronic health record system does not have a metric for sexual activity—a common problem in pediatric health systems. Although researchers have proposed algorithms and decision rules for identifying sexually active individuals in health system data, many of these methods perform poorly when applied to adolescent data.25,26 In our analysis, we instead used proxy markers for sexual activity, including age, previous screening, and previous infection. Importantly, although we were not able to measure sexual activity in our cohort, the mean annual 11% chlamydia screening rate falls far below the 38% of high school females estimated to be sexually active in the most recent Youth Risk Behavioral Surveillance Survey (YRBSS) data.24
Additionally, given the minimal differences in sexual activity rates for White versus Black high school students in the YRBSS data, we would not expect differences in sexual activity rates by race to explain our findings. In the available data, we were unable to reliably distinguish asymptomatic screening from symptomatic testing. Without knowledge of symptoms, our analysis categorized any test as screening, which could have inflated our estimates of screening. Our data come from a single health system, which may limit generalizability. However, this system spans 2 states, includes urban and suburban regions, and includes clinics with clinicians who have varying degrees of experience in sexual health care delivery. We had only 2 Title X–funded clinics in our analysis, both of which also provided routine primary care; thus we were unable to assess the effects of stand-alone Title X clinics on receipt of chlamydia screenings, which is an important area for future research given recent federal limitations on Title X funding. The small number of Title X–funded clinics also led to wide CIs in our estimates. Lastly, we were unable to account for chlamydia screening that may have occurred at community-based sites outside the network.
Conclusions
We identified race and ethnicity-based inequities in chlamydia screening for adolescent females across a large primary care network. Future research should focus on the combined impact of quality improvement initiatives focusing on standardization of care as well as clinician training to eliminate implicit racial bias.
ACKNOWLEDGMENTS
This research was supported by the National Institute of Mental Health (grant K23MH119976 to S. W.) and a pilot grant from PolicyLab and the Center for Pediatric Clinical Effectiveness at the Children’s Hospital of Philadelphia. This project was supported in part by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS; grant T71MC30798 Leadership Education in Adolescent Health).
We would like to thank Danielle Apple for her assistance with proofreading and formatting this article.
Note. This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, the HRSA, the HHS, or the US government.
CONFLICTS OF INTEREST
No authors have conflicts of interest to disclose.
HUMAN PARTICIPANT PROTECTION
This research was reviewed and deemed exempt by the institutional review boards of the Children’s Hospital of Philadelphia and Access Matters, as all activities were secondary analyses of data routinely required for health care purposes and did not require informed consent.
Footnotes
See also Pickett et al., p. 7.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/std/statistics/2019/default.htm
- 2.Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/std/chlamydia/stdfact-chlamydia-detailed.htm
- 3.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48(4):208–214. doi: 10.1097/OLQ.0000000000001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoenderboom BM, van Benthem BHB, van Bergen JEAM, et al. Relation between Chlamydia trachomatis infection and pelvic inflammatory disease, ectopic pregnancy and tubal factor infertility in a Dutch cohort of women previously tested for chlamydia in a chlamydia screening trial. Sex Transm Infect. 2019;95(4):300–306. doi: 10.1136/sextrans-2018-053778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med. 1996;334(21):1362–1366. doi: 10.1056/NEJM199605233342103. [DOI] [PubMed] [Google Scholar]
- 6.Chesson HW, Spicknall IH, Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48(4):215–221. doi: 10.1097/OLQ.0000000000001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Preventative Services Task Force. 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
- 8.American Academy of Pediatrics. 2021. https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/adolescent-sexual-health/Pages/STI-Screening-Guidelines.aspx
- 9.Centers for Disease Control and Prevention. 2015. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6403a1.htm
- 10.Owusu-Edusei K, Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: insights from exploratory time-series analyses. Am J Prev Med. 2010;38(6):652–657. doi: 10.1016/j.amepre.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 11.US Preventative Services Task Force. 2020. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling
- 12.Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244–e1256. doi: 10.1542/peds.2014-2299. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Chlamydia screening among females aged 15–21 years—multiple data sources, United States, 1999–2010. MMWR Suppl. 2014;63(2):80–88. [PubMed] [Google Scholar]
- 14.Généeux M, Leclerc P, Bédard L, Allard R. Upsurge of chlamydial reinfection in a large Canadian city: an indication of suboptimal chlamydia screening practices? Can J Public Health. 2010;101(5):420–424. doi: 10.1007/BF03404865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuffe KM, Newton-Levinson A, Gift TL, McFarlane M, Leichliter JS. Sexually transmitted infection testing among adolescents and young adults in the United States. J Adolesc Health. 2016;58(5):512–519. doi: 10.1016/j.jadohealth.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Shafii T, Levine D. Office-based screening for sexually transmitted infections in adolescents. Pediatrics. 2020;145(suppl 2):S219–S224. doi: 10.1542/peds.2019-2056K. [DOI] [PubMed] [Google Scholar]
- 17.Wiehe SE, Rosenman MB, Wang J, Fortenberry JD. Disparities in chlamydia testing among young women with sexually transmitted infection symptoms. Sex Transm Dis. 2010;37(12):751–755. doi: 10.1097/OLQ.0b013e3181e50044. [DOI] [PubMed] [Google Scholar]
- 18.Chambers LC, Khosropour CM, Katz DA, Dombrowski JC,, Manhart LE, Golden MR. Racial/ethnic disparities in the lifetime risk of Chlamydia trachomatis diagnosis and adverse reproductive health outcomes among women in King County, Washington. Clin Infect Dis. 2018;67(4):593–599. doi: 10.1093/cid/ciy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einwalter LA, Ritchie JM, Ault KA, Smith EM. Gonorrhea and chlamydia infection among women visiting family planning clinics: racial variation in prevalence and predictors. Perspect Sex Reprod Health. 2005;37(3):135–140. doi: 10.1363/3713505. [DOI] [PubMed] [Google Scholar]
- 20.Harling G, Subramanian S, Barnighausen T, Kawachi I. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40(7):575–581. doi: 10.1097/OLQ.0b013e31829529cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105(12):e60–e76. doi: 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiehe SE, Rosenman MB, Wang J, Katz BP, Fortenberry JD. Chlamydia screening among young women: individual- and provider-level differences in testing. Pediatrics. 2011;127(2):e336–e344. doi: 10.1542/peds.2010-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang LY, Chang MH, Burstein G, Hocevar Adkins S. Human immunodeficiency virus, chlamydia, and gonorrhea testing in New York Medicaid-enrolled adolescents. Sex Transm Dis. 2018;45(1):14–18. doi: 10.1097/OLQ.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention 2019. Morbidity and Mortality Weekly Report. August 21, 2020. Available at: https://www.cdc.gov/mmwr/volumes/69/su/pdfs/su6901-H.pdf
- 25.Berlan ED, Ireland AM, Morton S, Byron SC, Canan BD, Kelleher KJ. Variations in measurement of sexual activity based on EHR definitions. Pediatrics. 2014;133(5):e1305–e1312. doi: 10.1542/peds.2013-3232. [DOI] [PubMed] [Google Scholar]
- 26.Tao G, Walsh CM, Anderson LA, Irwin KL. Understanding sexual activity defined in the HEDIS measure of screening young women for Chlamydia trachomatis. Jt Comm J Qual Improv. 2002;28(8):435–440. doi: 10.1016/S1070-3241(02)28043-8. [DOI] [PubMed] [Google Scholar]
- 27.VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25(4):473–484. doi: 10.1097/EDE.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerber JS, Prasad PA, Localio AR, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics. 2013;131(4):677–684. doi: 10.1542/peds.2012-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townes A, Rosenberg M, Guerra-Reyes L, Murray M, Herbenick D. Inequitable experiences between Black and White women discussing sexual health with healthcare providers: findings from a U.S. probability sample. J Sex Med. 2020;17(8):1520–1528. doi: 10.1016/j.jsxm.2020.04.391. [DOI] [PubMed] [Google Scholar]
- 30.Betancourt JR, Maina AW. The Institute of Medicine report “Unequal Treatment”: implications for academic health centers. Mt Sinai J Med. 2004;71(5):314–321. [PubMed] [Google Scholar]
- 31.Stein CR, Kaufman JS, Ford CA, Leone PA, Feldblum PJ, Miller WC. Screening young adults for prevalent chlamydial infection in community settings. Ann Epidemiol. 2008;18(7):560–571. doi: 10.1016/j.annepidem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doubeni CA, Simon M, Krist AH. Addressing systemic racism through clinical preventive service recommendations from the US Preventive Services Task Force. JAMA. 2021;325(7):627. doi: 10.1001/jama.2020.26188. [DOI] [PubMed] [Google Scholar]
- 33.DiVasta AD, Trudell EK, Francis M, et al. Practice-based quality improvement collaborative to increase chlamydia screening in young women. Pediatrics. 2016;137(5):e20151082. doi: 10.1542/peds.2015-1082. [DOI] [PubMed] [Google Scholar]
- 34.Wood SM, McGeary A, Wilson M, et al. Effectiveness of a quality improvement intervention to improve rates of routine Chlamydia Trachomatis screening in female adolescents seeking primary preventive care. J Pediatr Adolesc Gynecol. 2019;32(1):32–38. doi: 10.1016/j.jpag.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guy RJ, Ali H, Liu B, et al. Efficacy of interventions to increase the uptake of chlamydia screening in primary care: a systematic review. BMC Infect Dis. 2011;11(1):211. doi: 10.1186/1471-2334-11-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafer MA, Tebb KP, Pantell RH, et al. Effect of a clinical practice improvement intervention on chlamydial screening among adolescent girls. JAMA. 2002;288(22):2846–2852. doi: 10.1001/jama.288.22.2846. [DOI] [PubMed] [Google Scholar]
- 37.Elattma A, Laves E, Taber B, Karvonen KL, Herrera MC, Bakken EH. Using provider incentives and an opt-out strategy in a successful quality initiative to increase chlamydia screening. Jt Comm J Qual Patient Saf. 2020;46(6):326–334. doi: 10.1016/j.jcjq.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasulu S, Shah SD, Schechter CB, Prine L, Rubin SE. Effectiveness of clinical decision support to enhance delivery of family planning services in primary care settings. Contraception. 2020;101(3):199–204. doi: 10.1016/j.contraception.2019.11.002. [DOI] [PubMed] [Google Scholar]