Abstract
Background:
Growing evidence exists about the fetal and environmental origins of hypertension, but mainly limited to single-exposure studies. The exposome has been proposed as a more holistic approach by studying many exposures simultaneously.
Objectives:
This study aims to evaluate the association between a wide range of prenatal and postnatal exposures and blood pressure (BP) in children.
Methods:
Systolic and diastolic BP were measured among 1277 children from the European HELIX cohort at age 6–11 years. Prenatal (n=89) and postnatal (n=128) exposures include air pollution, built environment, meteorology, natural spaces, traffic, noise, chemicals, and lifestyles. Two methods adjusted for confounders were applied: an exposome-wide association study considering the exposures independently, and the deletion-substitution-addition algorithm considering all the exposures simultaneously.
Results:
Decreases in systolic BP were observed with facility density (β[95% CI] mmHG change for an interquartile-range increase in exposure: −1.7[−2.5;−0.8]), maternal concentrations of polychlorinated biphenyl 118 (−1.4[−2.6;−0.2]) and child concentrations of dichlorodiphenyldichloroethylene (DDE: −1.6[−2.4;−0.7]), hexachlorobenzene (−1.5[−2.4;−0.6]), and mono-benzyl phthalate (−0.7[−1.3;−0.1]), while increases in systolic BP were observed with outdoor temperature during pregnancy (1.6[0.2;2.9]), high fish intake during pregnancy (2.0[0.4;3.5]), maternal cotinine concentrations (1.2[−0.3;2.8]), and child perfluorooctanoate concentrations (0.9[0.1;1.6]). Decreases in diastolic BP were observed with outdoor temperature at examination (−1.4[−2.3;−0.5]) and child DDE concentrations (−1.1[−1.9;−0.3]) while increases in diastolic BP were observed with maternal bisphenol-A concentrations (0.7[0.1;1.4]), high fish intake during pregnancy (1.2[−0.2;2.7]), and child copper concentrations (0.9[0.3;1.6]).
Conclusion:
This study suggests that early-life exposure to several chemicals, as well as built environment and meteorological factors, may affect BP in children.
Keywords: Environment, Blood pressure, Children, Cohort, Exposome, Epidemiology, Chemicals
Condensed Abstract
There is growing evidence about the fetal and the environmental origins of hypertension but few studies have investigated the effect of early-life environmental exposures on blood pressure (BP) in children. Using a unique and systematic exposome approach covering hundreds of exposures, this study highlights that the early-life environment is associated with blood pressure in children. The identification of modifiable risk factors could be translated into prevention practices to reduce exposure from the early-life that could be effective to prevent cardiovascular disease later in life.
Introduction
Cardiovascular diseases are one of the leading causes of death and high blood pressure (BP) is a major contributing factor(1). Until recently, prevention and control of hypertension mainly concerned adults, but studies now report that children with elevated blood pressure are more likely to become hypertensive adults, showing the importance of blood pressure control earlier in life(2).
There are a number of well-known risk factors for high blood pressure and hypertension such as obesity, physical inactivity, high sodium intake, tobacco and alcohol consumption, low socioeconomic status, and psychosocial stressors(1). Also, there is emerging evidence that environmental exposures may be important risk factors for high blood pressure(3). There is moderate to strong evidence that exposure to air pollution, cold temperature, and noise increase BP in adults(4–6). On the other hand, beneficial effect of the built environment, such as living in a walkable environment or close to green spaces, have been associated to lower BP(7). The evidence regarding exposure to chemicals is weaker.
According to recent reviews, environmental risk factors in children are similar to those in adults: moderate to strong evidence exists regarding the increase in BP with exposure to tobacco smoke during pregnancy(8) and to air pollution and noise during childhood(5, 9), while the evidence about chemical exposure(9) is weaker. Existing studies were limited by considering single, or a restricted number of environmental exposures at a time, without taking into account the wide range of other exposures to which an individual is simultaneously exposed.
In recent years, a more comprehensive approach has been advocated in the field of environmental epidemiology through the development of the exposome concept(10). It aims to evaluate all environmental exposures of an individual to better understand the role of environmental factors – which may act in synergy – on multi-factorial and chronic diseases(11). This approach limits publication bias resulting from selective reporting of associations and limits the finding of false positive associations when appropriate methods are used(11).
The fetal life is the starting point of the lifecourse exposome and is of particular interest since it corresponds to a period of rapid development which, if disturbed, may have long-term health effects(11). Characterising the early-life exposome is particularly relevant to study blood pressure in children regarding the growing evidence on the developmental origins of hypertension(12). To date, this innovative exposome concept has not been applied to blood pressure.
This study aims to evaluate the association between a wide range of prenatal and postnatal environmental exposures and blood pressure in children.
Methods
Study population
The study population is based on the Human Early-Life Exposome (HELIX) project which pooled data of six longitudinal-based European birth cohorts: BiB (Born in Bradford; United Kingdom), EDEN (Étude des Déterminants pré et postnatals du développement et de la santé de l’ENfant; France), INMA (INfancia y Medio Ambiente; Spain), KANC (Kaunus Cohort; Lithuania), MoBa (Norwegian Mother and Child Cohort Study; Norway)(13), and Rhea (Mother-Child Cohort in Crete, Greece)(14). They included a total of 32,000 mother–child pairs among which a sub-cohort of 1,301 children (approximately 200 children in each cohort) was selected according to the following criteria of eligibility: a) age 6–11 years, b) stored pregnancy blood and urine samples available, c) complete address history, and d) no serious health problems that may affect the clinical testing or the child safety. These 1,301 mother-child pairs were followed in 2014–2015 for a clinical examination, a computer-assisted interview with the mother and the collection of additional biological samples. Data collection was standardized and performed by trained staff. A full description of the subcohort methods and participants can be found elsewhere(15).
Assessment of exposures
A wide range of exposures were evaluated in HELIX to define the early-life exposome during two time periods, the prenatal pregnancy period and childhood (6–11 years). We assessed exposures in three main parts of the exposome: outdoor exposures, chemical exposures measured through biomarkers, and lifestyle factors. In the present study, 89 prenatal exposures and 128 postnatal exposures were included (Table 1).
Table 1.
List of exposures retained in the present study.
| Pregnancy period (89 exposures) | Childhood (6–11 years; 128 exposures) | |
|---|---|---|
|
| ||
| Outdoor exposures | ||
|
| ||
| Air pollution | PM10 (preg.), PM2.5 (preg.), PMabsorbance (preg.), NO2 (preg.) | PM10 (day), PM2.5 (day), PMabsorbance (day), NO2 (day), PM10 (year), PM2.5 (year), PMabsorbance (year), NO2 (year) |
| Built environment | Accessibility (bus lines 300m), Accessibility (bus stops 300m), Building density (300m), Connectivity (300m), Facility density (300m), Facility richness (300m), Land use (300m), Population density, Walkability | Accessibility (bus lines 300m), Accessibility (bus stops 300m), Building density (300m), Connectivity (300m), Facility density (300m), Facility richness (300m), Land use (300m), Population density, Walkability, Accessibility (bus lines 300m - School), Accessibility (bus stops 300m - School), Building density (300m - School), Connectivity (300m - School), Facility density (300m - School), Facility richness (300m - School), Land use (300m - School), Population (School) |
| Meteorological* | Humidity (preg.), Pressure (preg.), Temperature (preg.) | Humidity (day), Pressure (day), Temperature (day), UV-Vit. D (day) |
| Natural spaces | Blue spaces (300m), Green spaces (300m), NDVI (100m) | Blue spaces (300m), Green spaces (300m), NDVI (100m), Blue spaces (300m - School), Green spaces (300m - School), NDVI (100m - School) |
| Traffic | Inverse distance to nearest road, Road traffic load (100 m), Traffic density on nearest road | Inverse distance to nearest road, Road traffic load (100 m), Traffic density on nearest road, Traffic load of major roads (100 m), Traffic load of major roads (100 m - school) |
| Noise* | Traffic noise (24h) | Traffic noise (24h), Traffic noise (night), Traffic noise (24h - school) |
| Water Disinfection by product | Brominated trihalometanes, Chloroform, Trihalometanes | Not assessed |
| Indoor air | Not assessed | Indoor PMabsorbance, Indoor benzene, Indoor NO2, Indoor PM2.5, Indoor BTEX |
|
| ||
| Chemicals | ||
|
| ||
| Organochlorine compounds | DDE, DDT, HCB, PCB 118, PCB 138, PCB 153, PCB 170, PCB 180, PCBs (sum) | DDE, DDT, HCB, PCB 118, PCB 138, PCB 153, PCB 170, PCB 180, PCBs (sum) |
| Brominated flame retardants | PBDE 47, PBDE 153 | PBDE 47, PBDE 153 |
| Per- and polyfluoroalkyl substances | PFHxS, PFNA, PFOA, PFOS, PFUNDA | PFHxS, PFNA, PFOA, PFOS, PFUNDA |
| Metals | Arsenic, Cadmium, Cobalt, Cesium, Copper, Mercury, Manganese, Molybdenum, Lead, Thallium | Arsenic, Cadmium, Cobalt, Cesium, Copper, Mercury, Manganese, Molybdenum, Lead, Thallium |
| Phthalate metabolites | MBzP, MECPP, MEHHP, MEHP, MEOHP, MEP, MiBP, OH-MiNP, oxo-MiNP, DEHP (sum of metabolites) | MBzP, MECPP, MEHHP, MEHP, MEOHP, MEP, MiBP, OH-MiNP, oxo-MiNP, DEHP (sum of metabolites) |
| Phenols | BUPA, ETPA, MEPA, PRPA, BPA OXBE, TRCS | BUPA, ETPA, MEPA, PRPA, BPA OXBE, TRCS |
| Organophosphate pesticides | DEP, DETP, DMP, DMTP | DEP, DETP, DMP, DMTP, DMDTP |
|
| ||
| Lifestyles | ||
|
| ||
| Smoking | Maternal smoking (active and passive), Cigarette number, Urinary cotinine | Parental smoking, Passive smoking, Urinary cotinine |
| Diet | Alcohol consumption, Cereals intake, Dairy intake, Fastfood intake, Fish and seafood intake, Fruits intake, Legumes intake, Meat intake, Vegetables intake, | Breastfeeding duration (days), Bakery products intake, Soda intake, Breakfast cereals intake, Caffeinated drinks, Dairy products intake, Fastfood intake, Organic food intake, Processed meat intake, Readymade food intake, Bread intake, Cereals intake, Fish and seafood intake, Fruits intake, Added fat intake, Meat intake, Potatoes intake, Sweets intake, Vegetables intake, Yogurt intake, KIDMED score |
| Supplementation | Folic acid supplementation | Not assessed |
| Physical activity | Moderate physical activity (t3), Vigorous physical activity (t3) | Moderate and vigorous physical activity, Sedentary behaviour |
| Sleep | Not assessed | Sleep duration |
| Allergens | Not assessed | Cat at home, Dog at home, Other pets at home |
| Social and economic capital | Not assessed | Family affluence score, Social contact, House crowding, Social participation |
BPA, Bisphenol-A; BTEX, Benzene, toluene, ethylbenzene and xylenes; BUPA, Butyl-paraben; DDE, Dichlorodiphenyldichloroethylene; DDT, Dichlorodiphenyltrichloroethane; DEHP, Di-EthylhexylPhthalate; DEP, Diethyl phosphate; DETP, Diethyl thiophosphate; DMDTP, Dimethyl dithiophosphate; DMP, Dimethyl phosphate; DMTP, Dimethyl thiophosphate; ETPA, Ethyl-paraben; HCB, Hexachlorobenzene; KIDMED, Mediterranean diet in children; MBzP, Mono benzyl phthalate; MECPP, Mono-2-ethyl 5-carboxypentyl phthalate; MEHHP, Mono-2-ethyl-5-hydroxyhexyl phthalate; MEHP, Mono-2-ethylhexyl phthalate; MEOHP, Mono-2-ethyl-5-oxohexyl phthalate; MEP, Monoethyl phthalate; MEPA, Methyl-paraben; MiBP, Mono-iso-butyl phthalate; NDVI, Normalized difference vegetation index; NO2, Nitrogen dioxide; OH-MiNP, Mono-4-methyl-7-hydroxyoctyl phthalate; OXBE, Oxybenzone; oxo-MiNP, Mono-4-methyl-7-oxooctyl phthalate; PBDE, Polybrominated diphenyl ether; PCB, Polychlorobiphenyls; PFHxS, Perfluorohexane sulfonate; PFNA, Perfluorononanoate; PFOA, Perfluorooctanoate; PFOS, Perfluorooctane sulfonate; PFUNDA, Perfluoroundecanoate; PM, Particulate matter; Preg, Pregnancy; PRPA, Propyl-paraben; t3, 3rd trimester of pregnancy; TRCS, Triclosan; UV-Vit. D, Vitamin-D dose from ultraviolet.
Traffic noise at night is not available for the pregnancy period because of missing information in 3 cohorts (non existing maps at the time of recruitment). UV-Vit D dose is not available for the pregnancy period due to measurement errors.
Details on exposure assessment are provided in Online Appendix 1. In summary, assessment of outdoor exposures was based on the home address for the pregnancy period and on the home and school addresses for the childhood period (6–11 years) (see Online Appendix 1, Part 1). It includes air pollution, measures of the built environment, meteorological conditions, natural spaces, road traffic, and noise. Additional exposures assessed by predictive modeling from home address and questionnaire include water disinfection by products (for pregnancy only) and indoor pollutants (for childhood period only) (see Online Appendix 1, Part 2).
Exposure to environmental chemical contaminants was assessed through determination of concentrations in biological samples from the mother and the child (see Online Appendix 1, Part 3)(16). The chemicals list was based on concern for child health and include organochlorine compounds (polychlorinated biphenyls [PCBs] and organochlorine pesticides), polybrominated diphenyl ethers (PBDEs), per- and polyfluoroalkyl substances (PFASs), metals, phthalate metabolites, phenols, organophosphate pesticide metabolites, and cotinine. When appropriate, the concentrations were adjusted for lipids or creatinine.
Lifestyle factors were collected by questionnaire and included smoking habits, diet, physical activity, allergens, sleep, and socioeconomic status (see Online Appendix 1, Part 4).
The correlations between exposures are described elsewhere (17).
Blood pressure measurements
Blood pressure was measured during the clinical examination using a standardized protocol: after 5 minutes of rest in sitting position, 3 consecutive measurements were taken by oscillometric device (OMRON 705-CPII) with one-minute time intervals between them, in a predefined posture and in preference in the right arm. Adequate cuff sizes were chosen with respect to each child’s arm length and circumference. Systolic (SBP) and diastolic (DBP) blood pressures from each measurement have been recorded and the mean of the second and the third measurements was calculated and used in further analysis. Because of some device errors leading to unreliable values, we excluded 24 subjects and thus had a final population of n=1277 (98%) children.
Statistical analyses
For each variable, the optimal transformation to approach normality was applied. Missing data for all confounders and exposures were imputed using the method of chained equations(18). A total of 20 imputed datasets were generated and used in all the analyses mentioned hereafter; Rubin’s rules were used to aggregate the results(18).
The statistical methods were identified a priori through a series of simulation studies mimicking as closely as possible the situation expected with Helix data(19); two approaches were retained. First, exposome-wide association study (ExWAS) analyses were used to perform exposure-by-exposure estimation of the association with SBP and with DBP, using multivariate linear regressions and applying a Bonferroni-type correction to control for multiple testing(20). Then, the iterative model search ‘deletion-substitution-addition’ (DSA) algorithm – a variable selection method– was used in order to identify the exposures that jointly affect blood pressure(19, 21). DSA uses cross-validation and was shown to provide a lower proportion of false positive associations than ExWAS(19, 21). The DSA was applied 50 times and all the exposures that were selected in at least 5% of them were included in a multi-exposure linear regression model in order to obtain estimates of effect for each exposure adjusted for the others. We consider the multi-exposure model as our main analysis.
Both statistical methods (ExWAS and DSA) were performed separately for the prenatal and the postnatal exposomes and were adjusted for a common set of confounders identified from a Directed Acyclic Graph (see Online Appendix 2): the cohort of inclusion, maternal age (continuous in years), maternal educational level (low, middle, high), self-reported maternal pre-pregnancy body mass index (continuous in kg/m2), parity (nulliparous, primiparous, multiparous), parental country of birth (none, one or both parents born in the country of inclusion), child age (continuous in years), child sex (boy, girl), and child height (continuous in cm).
More details on data pre-processing, imputation process and statistical analyses are provided in Online Appendix 2. Description of exposure levels, missing rate and transformation used are provided in Online Appendix 3. All analyses were run under R version 3.4.0.
Sensitivity analyses
Five sets of sensitivity analyses were performed, the first one being done for the full exposome (ExWAS) the others performed on the exposures included in the multi-exposures model only. They included: 1) using age- sex- and height-specific z-score of blood pressure determined by existing charts(22), 2) refitting the multi-exposure models excluding subjects with missing data for a specific exposure, 3) stratifying the multi-exposure models by cohort and testing for heterogeneity (i.e., I-square), 4) adjusting models for birth weight as a potential mediator, 5) exploring non-linearity of the associations using restrictive cubic splines.
Results
Study population
At the time of delivery, pregnant women were 31 years old on average, were of high educational level (52%), were native of the cohort country (84%) and most of them already had a child (54%) (Table 2). At birth, the newborns’ birthweight was on average 3380g and less than 5% of them were born preterm. At the time of examination, children were around 8 years old on average (range from 6.5 to 11), 72% of them were of normal weight and around 10% could be classified as pre-hypertensive or hypertensive according to existing charts(22).
Table 2.
Description of the study population
| Parental characteristics | N (%) or mean ± sd | Child characteristics at birth | N (%) or mean ± sd |
|---|---|---|---|
| Cohort of inclusion | Year of birth | ||
| BIB, UK | 202 (15.8) | 2003 | 53 (4.2) |
| EDEN, France | 198 (15.5) | 2004 | 104 (8.1) |
| INMA, Spain | 221 (17.3) | 2005 | 238 (18.6) |
| KANC, Lithuania | 203 (15.9) | 2006 | 243 (19.0) |
| MOBA, Norway | 254 (19.9) | 2007 | 269 (21.1) |
| RHEA, Greece | 199 (15.6) | 2008 | 361 (28.3) |
| Family native from the country of the cohort | 2009 | 9 (0.7) | |
| Both native parents | 1053 (84.2) | Sex | |
| One native parent | 64 (5.1) | Male | 697 (54.6) |
| No native parent | 133 (10.6) | Female | 580 (45.4) |
| Maternal education level | Gestational duration (weeks) | 39.6 ± 1.7 | |
| Low | 170 (13.7) | <37 | 62 (4.9) |
| Middle | 428 (34.6) | ≥37 | 1204 (95.1) |
| High | 639 (51.7) | Birthweight (g) | 3380 ± 506 |
| Paternal education level | <2500 | 47 (3.7) | |
| Low | 207 (17.4) | 2500–3500 | 701 (55.4) |
| Middle | 470 (39.4) | 3500–4000 | 387 (30.6) |
| High | 515 (43.2) | ≥4000 | 131 (10.3) |
| Maternal age at inclusion (years) | 31.0 ± 4.9 | Ethnicity | |
| Paternal age at inclusion | 33.9 ± 5.3 | Caucasian | 1151 (90.2) |
| Parity | Other | 125 (9.8) | |
| Nulliparous | 572 (45.8) | Child characteristics at examination | |
| Primiparous | 455 (36.4) | Age (years) | 8.0 ± 1.6 |
| Multiparous | 222 (17.8) | Body mass index * | 16.9 ± 2.5 |
| Maternal pre-pregnancy body mass index | 24.9 ± 5.0 | Thinness | 99 (7.8) |
| Underweight (<18.5 kg/m2) | 49 (3.9) | Normal weight | 919 (72.4) |
| Normal weight (18.5–25 kg/m2) | 712 (56.7) | Overweight | 183 (14.4) |
| Overweight (25–30 kg/m2) | 309 (24.6) | Obese | 69 (5.4) |
| Obese (>30 kg/m2) | 186 (14.8) | Height (cm) | 129 ± 10.5 |
| Maternal weight-gain (kg) | 13.6 ± 6.2 | Weight (kg) | 28.5 ± 7.7 |
| Marital status | Systolic blood pressure (mmHg) | 99.4 ± 10.9 | |
| Living with the father | 1195 (94.5) | Diastolic blood pressure (mmHg) | 58.2 ± 9.2 |
| Living alone | 39 (3.1) | z-score of systolic blood pressure | −0.01 ± 0.82 |
| Other situation | 30 (2.4) | z-score of diastolic blood pressure | −0.06 ± 0.96 |
| Type of residence during pregnancy | Blood pressure categories † | ||
| Urban | 827 (87.4) | Normotensive | 1138 (89.1) |
| Rural | 119 (12.6) | Pre-hypertensive | 52 (4.1) |
| Hypertensive | 87 (6.8) |
According to the WHO growth reference for children aged between 5–19 years: http://www.who.int/growthref/who2007_bmi_for_age/en/
Children with z-score of SBP or DBP between the 90th and the 95th percentiles (or if BP exceeds 120/80 mmHg) were classified as pre-hypertensive and those above the 95th percentile were classified as hypertensive.
Associations between single exposures and blood pressure in children
Exposures studied individually in association with SBP and DBP through the ExWAS method are visually presented in Figure 1 and the corresponding estimates and 95% confidence intervals (CI) are provided in Online Tables 4.1 and 4.2.
Figure 1. Environmental-wide association study between prenatal and postnatal exposome and systolic and diastolic blood pressure (n=1277 children).
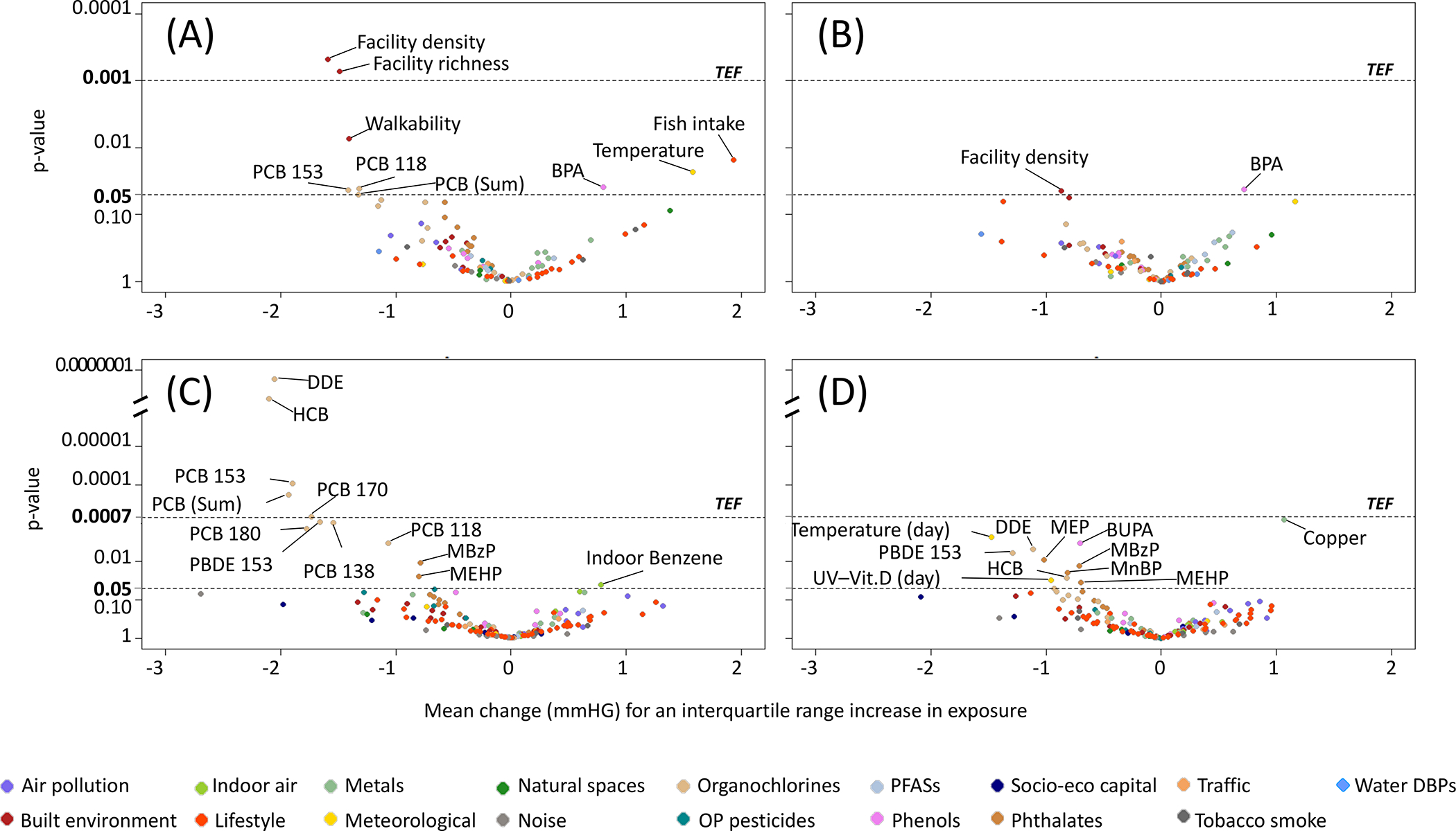
Volcano plots showing the results of the environmental-wide association studies (ExWAS) between (A) prenatal exposome and systolic blood pressure, (B) prenatal exposome and diastolic blood pressure, (C) postnatal exposome and systolic blood pressure, and (D) postnatal exposome and diastolic blood pressure. All models are adjusted for cohort, maternal age, maternal education level, maternal pre-pregnancy body mass index, parity, parental country of birth, child age, child sex, child height.
BPA, Bisphenol-A; BUPA, Butyl-paraben; DBPs, Disinfection-by-product; DDE, 4,4’dichlorodiphenyldichloroethylene; HCB, Hexachlorobenzene; MBzP, Mono benzyl phthalate; MEHP, Mono-2-ethylhexyl phthalate; MEP, Monoethyl phthalate; MnBP, Mono-n-butyl phthalate; OP, Organophosphate; PBDE, Polybrominated diphenyl ether; PCB, Polychlorobiphenyls; PFASs, Perfluoroalkyl substances; UV-Vit. D, Vitamin-D dose from ultraviolet; TEF, Threshold for effective number of tests (i.e., p-value correction for multiple testing).
Nine out of the 89 prenatal exposures showed associations with SBP and two of these, namely facility density and facility richness (both markers of the built environment) remained negatively associated with SBP after correction for multiple testing. In the childhood exposome, 12 out of the 128 exposures showed associations with SBP, and 5 of these, namely dichlorodiphenyldichloroethylene (DDE), hexachlorobenzene (HCB), PCB 153, PCB 170 and the sum of PCBs (all being organochlorine compounds) remained negatively associated with SBP after correction for multiple testing.
Two prenatal exposures and 11 postnatal exposures were associated with DBP but none of them remain associated after correction for multiple testing.
Associations between simultaneous exposures and blood pressure in children
After applying the DSA method in order to study the associations between multiple exposures simultaneously and SBP, 5 prenatal and 4 postnatal exposures were selected respectively in the prenatal and postnatal multiple-exposure models (Table 3). A lower SBP was observed for higher facility density during pregnancy (β[95% CI] for an IQR increase in exposure =−1.7[−2.5;−0.8]; i.e., a 1.7 mmHG decrease in mean SBP is observed for a 49.5 unity/km2 increase in the number of facilities), maternal concentrations of polychlorinated biphenyl 118 (−1.4[−2.6;−0.2]) and child concentrations of DDE (−1.6[−2.4;−0.7]), HCB (−1.5[−2.4;−0.6]), and mono-benzyl phthalate (MBzP: −0.7[−1.3;−0.1]). A higher SBP was observed with high fish intake during pregnancy (2.0[0.4;3.5]) and with higher outdoor temperature during pregnancy (1.6[0.2;2.9]), maternal cotinine concentrations (1.2[−0.3;2.8]), and child perfluorooctanoate concentrations (PFOA: 0.9[0.1;1.6]).
Table 3.
Mean change (mmHG) in systolic and diastolic blood pressure in association with maternal (89 candidate exposures) and child (128 candidate exposures) exposures selected into the multiple-exposure models*; n=1277 mother child pairs.
| Exposures |
Systolic blood pressure (SBP) |
Diastolic blood pressure (DBP) |
|||||
|---|---|---|---|---|---|---|---|
| Interquartile range | Frequency (%) of selection † | Adjusted beta [95% CI] ‡ |
p-value | Frequency (%) of selection † | Adjusted beta [95% CI] ‡ |
p-value | |
| Pregnancy period | |||||||
| Facility density (300m) | 49.5 unity/km2 | 98 | −1.7 [−2.5; −0.8] | 0.0003 | 0 | ||
| Polychlorobiphenyl 118 (PCB 118) | 2.8 ng/g lipids | 56 | −1.4 [−2.6; −0.2] | 0.0262 | 0 | ||
| Fish and seafood intake | - | 50 | - | 0.0414 | 10 | - | 0.1032 |
| <2 times/week vs. 2–4 times/week | - | - | 1.0 [−0.5; 2.5] | 0.1799 | - | 1.4 [−0.1; 2.8] | 0.0657 |
| >4 times/week vs. 2–4 times/week | - | - | 2.0 [0.4; 3.5] | 0.0121 | - | 1.2 [−0.2; 2.7] | 0.0961 |
| Temperature (av. pregnancy) | 5.8°C | 34 | 1.6 [0.2; 2.9] | 0.0240 | 0 | ||
| Cotinine (μg/L) | - | 8 | - | 0.1211 | 0 | ||
| 18.4–50 vs. <18.4 | - | - | −0.8 [−2.5; 2.8] | 0.3784 | - | ||
| >50 vs. <18.4 | - | - | 1.2 [−0.3; 2.8] | 0.1138 | - | ||
| Bisphenol-A (BPA) | 4.9 μg/g creat | 2 | 6 | 0.7 [0.0; 1.4] | 0.0488 | ||
| Childhood period | |||||||
| Hexachlorobenzene (HCB) | 5.1 ng/g lipids | 100 | −1.5 [−2.4; −0.6] | 0.0018 | 0 | ||
| Dichlorodiphenyldichloroethylene (DDE) | 34.0 ng/g lipids | 18 | −1.6 [−2.4; −0.7] | 0.0004 | 6 | −1.1 [−1.9; −0.3] | 0.0052 |
| Mono benzyl phthalate (MBzP) | 5.5 μg/g creat | 6 | −0.7 [−1.3; −0.1] | 0.0189 | 0 | ||
| Perfluorooctanoate (PFOA) | 0.8 μg/L | 6 | 0.9 [0.1; 1.6] | 0.0213 | 0 | ||
| Temperature (daily average) | 10.8°C | 0 | 28 | −1.4 [−2.3; −0.5] | 0.0037 | ||
| Copper | 186 μg/L | 0 | 18 | 0.9 [0.3; 1.6] | 0.0036 | ||
The selection of exposures was determined by the DSA (Deletion-Substitution-Addition) algorithm. DSA selections were performed separately for the prenatal and the childhood exposome, and for SBP and DBP.
Frequency of selection of each exposure from the 50 DSA ran. Only the exposures selected in at least 5% of the DSA were included in the multiple exposure model.
Beta coefficient represents the change in blood pressure (expressed in mmHg) for an interquartile-range increase in exposure level. These coefficients are adjusted for the other exposures and for cohort, maternal age, maternal education level, maternal pre-pregnancy body mass index, parity, parental country of birth, child age, child sex, and child height.
In association with DBP, 2 prenatal and 3 postnatal exposures were selected in the multi-exposure models (Table 3). We observed a lower DBP for higher outdoor temperature at examination (−1.4[−2.3;−0.5]) and child DDE concentrations (−1.1[−1.9;−0.3]) while we observed higher DBP for higher maternal bisphenol-A concentrations (BPA: 0.7[0.1;1.4]), low and high fish intake during pregnancy (1.4 [−0.1; 2.8] and 1.2 [−0.2;2.7], respectively), and higher child copper concentrations (0.9[0.3;1.6]).
Overall, the estimates obtained in the multiple-exposure models were similar to the ones obtained in the single exposure models (i.e., ExWAS, see Online Appendix 4). However, changes in estimates and narrower confidence intervals were observed in the multiple-exposure model for childhood concentrations of PFOA (β single vs multiple-exposure model = 0.4 vs 0.9), DDE (2.1 vs 1.6) and HCB (2.1 vs 1.5) in association with SBP, suggesting residual confounding in the single exposure models.
Sensitivity analyses
Similar findings were observed using z-score of BP, adjusting models for birth weight or restricting analyses to complete cases (see Online Appendix 5, Part 1–3). Most of the results were consistent across cohorts but some heterogeneity can be highlighted even if the number of subject per cohort is quite small: 1) the association between the average temperature during pregnancy and child SBP was driven by the BIB cohort, 2) the association between prenatal BPA and DBP was mainly driven by BIB, 3) the association between child PFOA and SBP was not observed in KANC nor in RHEA, and 4) the effect of child MBzP level was associated with an increase in SBP in EDEN and RHEA but a decrease in BIB, INMA, KANC and MOBA (see Online Appendix 5, Part 4). Linearity of the risk was observed for all exposures except for prenatal levels of BPA (see Online Appendix 5, Part 5).
Discussion
This study is the first to simultaneously consider the possible effects of exposure to hundreds of environmental factors during early-life on blood pressure in children. Several environmental exposures were associated with an increase (fish intake, bisphenol-A, cotinine and temperature during pregnancy; perfluorooctanoate and copper at age 6–11) or a decrease (facility density, polychlorinated biphenyl 118 during pregnancy; dichlorodiphenyldichloroethylene, hexachlorobenzene, mono-benzyl phthalate and temperature at age 6–11) in BP.
Blood pressure is regulated by various mechanisms involving several biological systems as potential target, in particular the renal, cardiovascular, and endocrine systems. Evidence exists regarding the long-term effect of fetal events on blood pressure (fetal programming; e.g., maternal undernutrition) while blood pressure is also affected by current factors (acute effect; e.g., temperature)(3, 12). From the extensive experimental studies available on the fetal origins of hypertension – mainly concerning maternal undernutrition – several hypotheses have been advanced to explain this delayed effect including renal dysfunction (e.g., nephron number and the renal renin-angiotensin system), vascular dysfunction, placental dysfunction, hypothalamic–pituitary–adrenal axis programming, oxidative stress, sodium homeostasis, and epigenetic modifications(12).
We observed that measures of the built environment, notably the facility density where the mother was living during pregnancy, were associated with lower SBP in offspring. Urban design factors, such as the density of facilities in a particular area (e.g., shops, restaurants, parks, or transportation hubs), determine how people use and move around the city and promote physical activity and social contact(7). Good evidence exists regarding the effects of urban and transport planning on cardiovascular health and highly walkable environments were associated with lower blood pressure in adult populations(7). To our knowledge, no study on the effect of the built environment exists in pregnant women and their offspring. We could hypothesis that the beneficial effect observed results from higher physical activity during pregnancy, a condition known to optimize fetal weight (i.e., reduces fat accumulation and maintains muscle mass)(23).
We observed that an increase in outdoor temperature (average of the day before the clinical examination) was associated with a decrease in SBP. Outdoor temperature is among the known environmental factors that affect BP in adult, as reported in a recent meta-analysis(6), but also in children(24).
For organochlorine compounds, we observed that exposure to PCBs during pregnancy and to PCBs, DDE, and HCB during childhood were inversely associated with SBP. PCBs and organochlorine pesticides are persistent organic pollutants that accumulate through the food chain and to which people are chronically exposed, especially through diet, despite they were banned decades ago(25). One study performed in 427 children from the RHEA cohort reported an increase in SBP and DBP at 4 years in association with prenatal exposure to DDE and HCB but not PCBs(26). Another study reported higher DBP but not SBP at 7–9 years old with exposure to PCBs (but not DDE nor HCB) measured one year earlier(27). We suspect that reverse causality may explain the associations we observed, especially for the associations observed cross-sectionally in childhood. Indeed, these compounds are highly lipophilic and children with a higher fat mass are expected to store more of these compounds in fat tissues and thus have lower circulating blood levels for a given exposure(28). Therefore, circulating level of POPs may imperfectly reflect the total body burden, to an extent varying according to the child adiposity or BMI, which may confound the associations with BMI-related health outcomes such as blood pressure.
While we did not observe association between exposure to PFASs (either during pregnancy or childhood) in the single exposure models, exposure to PFOA during childhood was associated with higher SBP after co-adjustment for organochlorine compounds (i.e., DDE and HCB). PFASs are chemicals present in various consumer products (e.g., cookware coatings, food packing materials, waterproof textiles) to which humans are exposed though diet but also indoor air and dust(25). Few studies on the effects of PFASs on blood pressure exist. One study performed in children from the INMA cohort reported no association between prenatal exposure to PFASs and BP at 4 and 7 years old(29), as did one cross-sectional study performed during childhood (12–18 years old)(30). However, none of them have taken into account the level of exposure to organochlorine pesticides.
In our study population, exposure to some phthalates during childhood was associated with lower SBP and, in a lesser extent, with lower DBP. Phthalates are chemicals mainly used as plasticizers and to which people are exposed through diet but also through cosmetics use or medical devices(25). They are suspected to adversely affect cardiovascular health but evidence from previous studies is limited, in particular regarding blood pressure in children(9). One study performed among 379 children enrolled in the INMA cohort also reported a decrease in SBP (but not DBP) at 4 and 7 years old with exposure during pregnancy(31). In contrast, two cross-sectional studies performed in 8–19 years old US children reported higher SBP and DBP with higher phthalates exposure(32, 33). Since phthalates are PPARγ agonist, they may act through the modulation of the renin-angiotensin system (34, 35).
Exposure to BPA during pregnancy, but not during childhood, was associated with higher DBP and to a lesser extent with higher SBP. BPA is a chemical present in various consumer products (e.g., plastic and canned food containers, toys, and cashier’s receipt) in countries with no restrictions on use(25). In children, BPA exposure during pregnancy but not during childhood was associated with higher DBP at 4 years old in 486 Korean children(36), but not in 500 children from the RHEA cohort(37). Animal studies have reported that BPA alters nephrogenesis during embryonic development, has endocrine disturbance properties (has estrogenic activity and disrupt the thyroid axis), induces oxidative stress and inflammation, and implies epigenetic changes(38).
Regarding exposure to metals, higher DBP were observed with increasing circulating copper level in children; others metals were not statistically significantly associated with BP. Copper is a trace element essential for the body and for which deficiency is known to adversely affect cardiovascular health, while findings are inconsistent regarding higher copper levels(39). Epidemiological studies between copper exposure and BP are very limited in number. One study performed among 581 children (6–19 years old) enrolled in the NHANES study also reported higher SBP and a trend for higher DBP with increasing serum copper level(40).
Low and high fish intake during pregnancy (less and more than 2 to 4 times a week) were associated with an increase in SBP in children. To our knowledge, no previous studies were published on the effect of fish intake during pregnancy and offspring BP. It is well known that omega-3 fatty acids in fish are beneficial for cardiovascular health, but there is a debate regarding a possible opposite effect in the case of contamination of fish by chemicals that could reduce any positive effect of omega-3 fatty acids, or entail nonlinear associations(41). Indeed, fish intake is a major source of exposure to metals and lipophilic compounds and may thus be a proxy of exposure to a mixture of compounds suspected to affect BP (e.g., mercury, arsenic, cadmium, lead), which, each taken individually, were positively but not statistically significantly associated with BP in children (Online Appendix 4, Part 1).
We did not observe statistically significant associations for some of the environmental exposures known to affect BP such as air pollution, noise and tobacco exposures(3, 5, 9). We do note that higher BP supporting previous findings were suggested in the ExWAS analyses (e.g., p<0.10 for NO2 and PM absorbance during childhood) and the DSA selection (prenatal cotinine selected and positively associated with SBP). Similarly, we did not find statistically significant associations between individual behaviours such as unhealthy diet component and physical activity and BP in children, even if some point estimates are in the expected direction (i.e., higher SBP and DBP with fast-food intake and sedentary behaviour of the child).
Strengths and limitations
Our study had several strengths, including its multicentric design with mother-child pairs of 6 European countries, the use of standardized protocols to measure both BP and the majority of exposures, and the use of advanced statistical methods to deal with the exposome context. The broad number of exposures simultaneously assessed both during pregnancy and the childhood period, represents a step forward over previous approaches and is also a strength of the study, although we note that only a very small part of the exposome is covered. Limitations of this study include exposure misclassification, which is expected to be differential across exposures (e.g., stronger for the least persistent chemicals and for exposures based on modelling of outdoor levels), the relative small sample size given the large number of exposures investigated, and the cross-sectional design for the postnatal exposome. While we made effort to balance sensitivity and specificity basing our statistical analysis on a previous simulation study(19), we acknowledge that the present study remains at risk of false positives due to its agnostic approach and false negatives that may result from a lack of power.
Conclusion
Using a unique and systematic exposome approach that avoids the issue of selective reporting and reduces co-exposure confounding, this study highlights that early life exposures such as measures of the built environment, meteorological conditions, fish intake, and chemicals are potentially important in the development of blood pressure in children. Although some of the reported associations go in the same direction than previous research, the novel findings should be replicated in future studies. The clinical impact of the associations reported remains to be evaluated but the unique strength of the longitudinal cohorts should be fostered to implement follow-ups that allow the investigation of long term health implications.
Supplementary Material
Central illustration. Early-life environmental exposures and blood pressure in children.
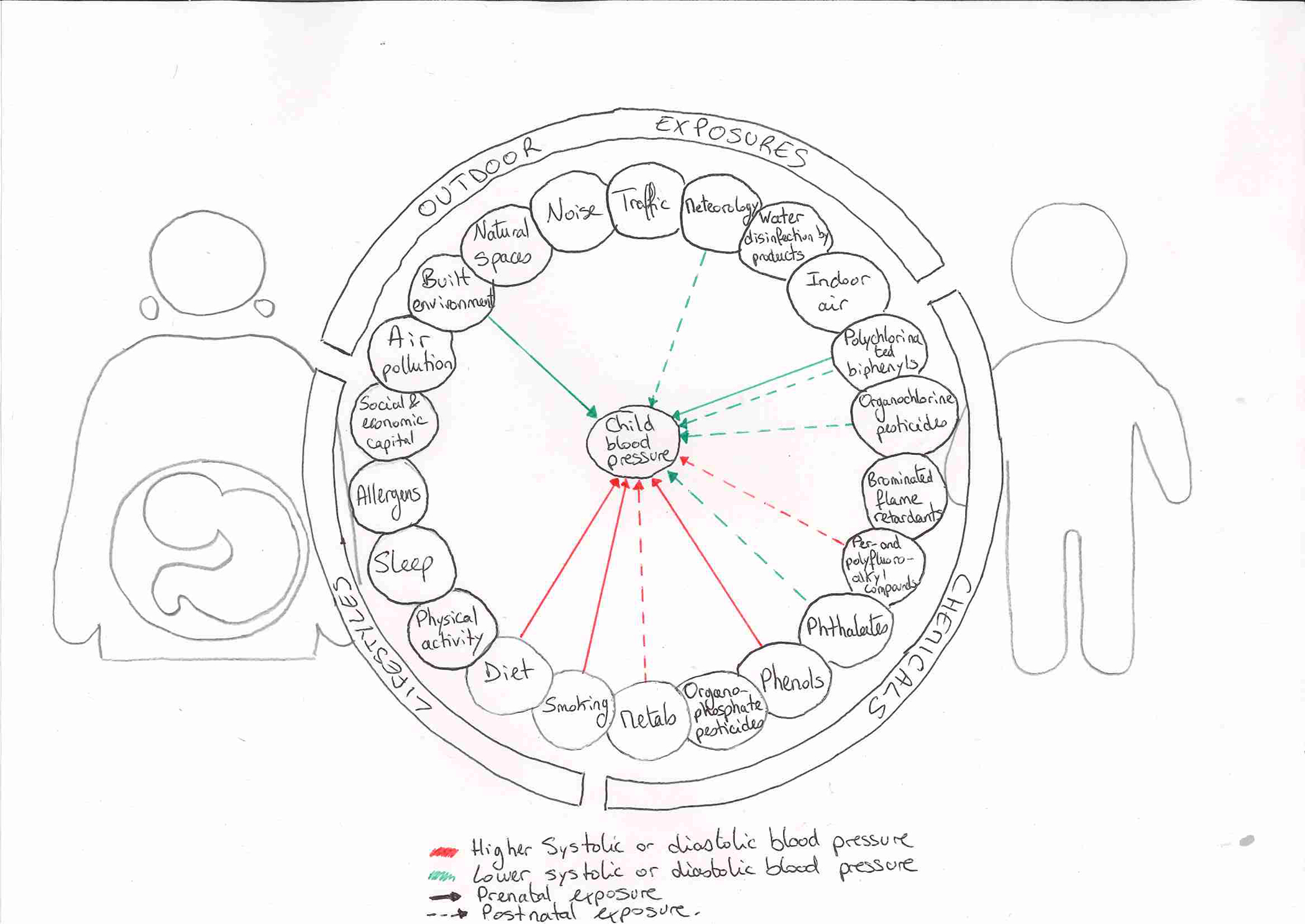
The illustration shows the overall framework of the exposome and summarizes the associations observed between early-life environmental exposures and blood pressure in 6–11 years old children. Within the longitudinal European HELIX mother-child cohort (n=1,277), this study covered a wide-range of environmental exposures assessed during pregnancy and in childhood including outdoor exposures, chemicals, and lifestyles. Decreases (green line) and increases (red line) in systolic or diastolic blood pressure have been observed in association with either prenatal (solid line) or postnatal (dotted line) exposures including several chemicals, as well as with fish intake, markers of the built environment, and meteorological factors. Using a unique and systematic exposome approach covering hundreds of exposures, this study highlights that early-life environmental exposures may affect blood pressure in children.
Clinical Perspectives.
Competency in Medical Knowledge:
This study highlights that early life exposures, including during the fetal life, are potentially important in the development of blood pressure in children.
Competency in Patient Care:
The identification of modifiable risk factors could be translated into prevention practices to reduce exposure from the early-life. This could be effective to prevent cardiovascular disease later in life.
Translational Outlook 1:
Although the evidence about the role of the environment on cardiovascular health is growing, additional studies with prospective design are needed to strengthen the findings.
Translation Outlook 2:
Follow-ups of the existing longitudinal cohorts should allow the investigation of the long term health implications.
Acknowledgment
ISGlobal is a member of the Agency for the Research Centres of Catalonia (CERCA) Programme, Generalitat de Catalunya. We are grateful to all the participating children, parents, practitioners and researchers in the six countries who took part in this study. We further thank Muireann Coen, Sonia Brishoual, Angelique Serre, Michele Grosdenier, Prof Frederic Millot, Elodie Migault, Manuela Boue, Sandy Bertin, Veronique Ferrand-Rigalleau, Céline Leger, Noella Gorry, Silvia Fochs, Nuria Pey, Cecilia Persavente, Susana Gross, Georgia Chalkiadaki, Danai Feida, Eirini Michalaki, Mariza Kampouri, Anny Kyriklaki, Minas Iakovidis, Maria Fasoulaki, Ingvild Essen, Heidi Marie Nordheim, and the Yorkshire Water.
Funding
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-206) under grant agreement no 308333 – the HELIX project. CW received funding from the Fondation de France (00069251, France). MC received funding from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness) (MS16/00128). INMA data collections were supported by grants from the Instituto de Salud Carlos III, CIBERESP, and the Generalitat de Catalunya- CIRIT (Spain). KANC was funded by the grant of the Lithuanian Agency for Science Innovation and Technology (6-04-2014_31V-66). The Norwegian Mother and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no N01-ES-75558), and NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1). The Rhea project was financially supported by European projects, and the Greek Ministry of Health (Program of Prevention of obesity and neurodevelopmental disorders in preschool children, in Heraklion district, Crete, Greece: 2011–2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–15). The work was also supported by MICINN [MTM2015-68140-R] and Centro Nacional de Genotipado- CEGEN- PRB2- ISCIII (Spain). This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Collaboration for Applied Health Research and Care (CLAHRC) for Yorkshire and Humber (UK). Core support for Born in Bradford is also provided by the Wellcome Trust (WT101597MA, UK).
Abbreviations list
- BP
Blood pressure
- DBP
Diastolic blood pressure
- DSA
Deletion-substitution-addition algorithm
- ExWAS
Exposome-wide association study
- HELIX
Human Early-Life Exposome
- KIDMED
Mediterranean diet in children
- SBP
Systolic blood pressure
- TEF
Threshold for effective number of test
Footnotes
Competing interests
The authors declare that they have no competing interests.
Short tweet: The first exposome study on blood pressure in children suggests the importance of early-life environmental exposures. @ISGlobalorg @Cha_Warembourg
References
- 1.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Lurbe E, Cifkova R, Cruickshank JK, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J. Hypertens. 2009;27:1719–42. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J. Clin. Hypertens. (Greenwich). 2011;13:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang B-Y, Qian Z, Howard SW, et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ. Pollut. 2018;235:576–588. [DOI] [PubMed] [Google Scholar]
- 5.van Kempen E, Casas M, Pershagen G, Foraster M. WHO environmental noise guidelines for the European region: A systematic review on environmental noise and cardiovascular and metabolic effects: A summary. Int. J. Environ. Res. Public Health 2018;15:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Li C, Guo Y, et al. Environmental ambient temperature and blood pressure in adults: A systematic review and meta-analysis. Sci. Total Environ. 2017;575:276–286. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwenhuijsen MJ. Influence of urban and transport planning and the city environment on cardiovascular disease. Nat. Rev. Cardiol. 2018;15:432–438. [DOI] [PubMed] [Google Scholar]
- 8.Banderali G, Martelli A, Landi M, et al. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med 2015;13:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders AP, Saland JM, Wright RO, Satlin L. Perinatal and childhood exposure to environmental chemicals and blood pressure in children: a review of literature 2007–2017. Pediatr. Res. 2018;84:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wild CP. The exposome: from concept to utility. Int. J. Epidemiol. 2012;41:24–32. [DOI] [PubMed] [Google Scholar]
- 11.Siroux V, Agier L, Slama R. The exposome concept: a challenge and a potential driver for environmental health research. Eur. Respir. Rev. 2016;25:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton JS, Cooke C-L, Davidge ST. In Utero Origins of Hypertension: Mechanisms and Targets for Therapy. Physiol. Rev. 2016;96:549–603. [DOI] [PubMed] [Google Scholar]
- 13.Magnus P, Birke C, Vejrup K, et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol 2016;45:382–388. [DOI] [PubMed] [Google Scholar]
- 14.Vrijheid M, Slama R, Robinson O, et al. The human early-life exposome (HELIX): project rationale and design. Environ. Health Perspect. 2014;122:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitre L, de Bont J, Casas M, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open 2018;8:e021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haug LS, Sakhi AK, Cequier E, et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ. Int 2018;121:751–763. [DOI] [PubMed] [Google Scholar]
- 17.Tamayo-Uria I, Maitre L, Thomsen C, et al. The early-life exposome: Description and patterns in six European countries. Environ. Int 2019;123:189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 19.Agier L, Portengen L, Chadeau-Hyam M, et al. A Systematic Comparison of Linear Regression-Based Statistical Methods to Assess Exposome-Health Associations. Environ. Health Perspect. 2016;124:1848–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M-X, Yeung JMY, Cherny SS, Sham PC. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012;131:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinisi SE, van der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics. Stat. Appl. Genet. Mol. Biol. 2004;3:Article18. [DOI] [PubMed] [Google Scholar]
- 22.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–76. [PubMed] [Google Scholar]
- 23.Reyes LM, Davenport MH. Exercise as a therapeutic intervention to optimize fetal weight. Pharmacol. Res. 2018;132:160–167. [DOI] [PubMed] [Google Scholar]
- 24.Miersch A, Vogel M, Gausche R, et al. Influence of seasonal variation on blood pressure measurements in children, adolescents and young adults. Pediatr. Nephrol. 2013;28:2343–2349. [DOI] [PubMed] [Google Scholar]
- 25.Mitro SD, Johnson T, Zota AR. Cumulative Chemical Exposures During Pregnancy and Early Development. Curr. Environ. Heal. reports 2015;2:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vafeiadi M, Georgiou V, Chalkiadaki G, et al. Association of Prenatal Exposure to Persistent Organic Pollutants with Obesity and Cardiometabolic Traits in Early Childhood: The Rhea Mother-Child Cohort (Crete, Greece). Environ. Health Perspect. 2015;123:1015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HA, Park SH, Hong YS, Ha EH, Park H. The Effect of Exposure to Persistent Organic Pollutants on Metabolic Health among KOREAN Children during a 1-Year Follow-Up. Int. J. Environ. Res. Public Health 2016;13:pii: E270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dirinck E, Dirtu AC, Jorens PG, Malarvannan G, Covaci A, Van Gaal LF. Pivotal Role for the Visceral Fat Compartment in the Release of Persistent Organic Pollutants During Weight Loss. J. Clin. Endocrinol. Metab. 2015;100:4463–4471. [DOI] [PubMed] [Google Scholar]
- 29.Manzano-Salgado CB, Casas M, Lopez-Espinosa M-J, et al. Prenatal Exposure to Perfluoroalkyl Substances and Cardiometabolic Risk in Children from the Spanish INMA Birth Cohort Study. Environ. Health Perspect. 2017;125:097018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geiger SD, Xiao J, Ducatman A, Frisbee S, Innes K, Shankar A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere 2014;98:78–83. [DOI] [PubMed] [Google Scholar]
- 31.Valvi D, Casas M, Romaguera D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ. Health Perspect. 2015;123:1022–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. J. Pediatr. 2013;163:747–53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trasande L, Attina TM. Association of Exposure to Di-2-Ethylhexylphthalate Replacements With Increased Blood Pressure in Children and AdolescentsNovelty and Significance. Hypertension 2015;66:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bility MT, Thompson JT, McKee RH, et al. Activation of Mouse and Human Peroxisome Proliferator-Activated Receptors (PPARs) by Phthalate Monoesters. Toxicol. Sci. 2004;82:170–182. [DOI] [PubMed] [Google Scholar]
- 35.Rőszer T, Ricote M. PPARs in the Renal Regulation of Systemic Blood Pressure. PPAR Res. 2010:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae S, Lim Y-H, Lee YA, Shin CH, Oh S-Y, Hong Y-C. Maternal Urinary Bisphenol A Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4Novelty and Significance. Hypertension 2017;69:367–374. [DOI] [PubMed] [Google Scholar]
- 37.Vafeiadi M, Roumeliotaki T, Myridakis A, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ. Res. 2016;146:379–387. [DOI] [PubMed] [Google Scholar]
- 38.Nuñez P, Fernandez T, García-Arévalo M, et al. Effects of bisphenol A treatment during pregnancy on kidney development in mice: a stereological and histopathological study. J. Dev. Orig. Health Dis. 2017:1–7. [DOI] [PubMed] [Google Scholar]
- 39.Bost M, Houdart S, Oberli M, Kalonji E, Huneau J-F, Margaritis I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016;35:107–115. [DOI] [PubMed] [Google Scholar]
- 40.Zang X, Huang H, Zhuang Z, et al. The association between serum copper concentrations and cardiovascular disease risk factors in children and adolescents in NHANES. Environ. Sci. Pollut. Res. 2018;25:16951–16958. [DOI] [PubMed] [Google Scholar]
- 41.Choi AL, Cordier S, Weihe P, Grandjean P. Negative Confounding in the Evaluation of Toxicity: The Case of Methylmercury in Fish and Seafood. Crit. Rev. Toxicol. 2008;38:877–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


