Abstract
A study investigating the use of a nonviable Lactobacillus acidophilus (NVL: Culbac; TransAgra, Storm Lake, IA) and a mixed prebiotic (MP) blend (beet pulp, fructooligosaccharide (FOS), mannanoligosaccharide (MOS), inulin, and kelp) was done to evaluate changes in fecal microbiota, fermentative end products, and gut immune health in healthy female and male adult Beagle dogs (n = 24; 5.74 ± 2.18 yr; 9.30 ± 1.32 kg). The study protocol was first approved by the facility’s Institutional Animal Care and Use Committee (Summit Ridge Farms; Susquehanna, PA) and followed throughout. Each of four test diets (control, NVL, MP, and MP + NVL [formulated to crude protein 25%, crude fat 14%, crude fiber 10% as-fed]) was fed once daily to maintain body weight for 21 d in a randomized-crossover design (four treatment periods and four washout periods). Fecal samples were collected on days 0 and 21 only for immunoglobulin A (IgA) and microbiota evaluation (16S rRNA V4 region and qPCR for Escherichia coli and Bifidobacterium), and fecal fermentative end-products and fecal pH were assessed only on day 21. Over the test periods, apparent total tract nutrient digestibility and stool quality were assessed. Data were analyzed by ANOVA (SAS v9.4, Cary, NC) or Kruskal–Wallis for between-diet effects, and paired t-test or Wilcoxon for time effects. Statistical significance was set at P ≤ 0.05. Apparent total tract nutrient digestibility revealed feeding MP-containing diets resulted in lower (P < 0.05) crude protein and fat digestibility vs. control and NVL diets. When dogs were fed MP, they had lower (P < 0.05) fecal pH compared with control and NVL diets, whereas fecal pH was lower in (P < 0.05) MP + NVL- vs. NVL-fed dogs. Fecal E. coli was (P < 0.05) lower at day 21 vs. day 0 when dogs were fed MP. Fecal Fusobacterium spp. was lower (P < 0.05) in both MP diets vs. control. Fecal Lactobacillus spp. increased (P < 0.05) from baseline with MP. Both diets with MP elicited greater (P < 0.05) fecal acetate and propionate concentration vs. control diet. At day 21, fecal IgA was greater (P < 0.05) in MP and MP + NVL compared with NVL diet. Only when dogs were fed MP did they have increased (P < 0.05) fecal IgA from day 21 vs. day 0. The MP + NVL diet decreased (P < 0.05) fecal isovalerate, isobutyrate, phenol, and indole vs. control. Overall, the MP elicited the most changes on microbiota, fermentative end-products, and IgA. Further investigation into NVL’s gut health benefits is warranted.
Keywords: digestibility, dog, microbiome, nutrition, postbiotics, prebiotics
Introduction
Pet humanization has made for higher demand for nutrition beyond the basic requirements of the animal. Specifically, digestive health has been an area often studied to bring these added benefits. Pre- and probiotic ingredients are often at the forefront in delivering gut health benefits. Prebiotics are defined as carbohydrates that resist hydrolytic/enzymatic digestion and absorption in the small intestine, stimulate the growth and/or activity of beneficial gut microorganisms, and are fermented in the large bowel to promote fermentation end-product production (Gibson et al., 2017). Conversely, probiotics are viable bacteria that when consumed in adequate concentration confer a health benefit to the host (Hill et al., 2014). Indeed, many common fiber ingredients in pet food including beet pulp, fructooligosaccharide (FOS), mannanoligosaccharide (MOS), and inulin have been studied extensively for their application in pet foods on nutrient digestibility, fecal fermentative end-products, and fecal microbiota (Fahey et al., 1990; Swanson et al., 2002b; Flickinger et al., 2003; Middelbos et al., 2007a). However, pet food formulations are often complex in that they can contain multiple fermentable fiber sources and prebiotics that collectively are delivering the stimulus for gut health.
Previous studies feeding viable Lactobacillus acidophilus to healthy dogs have produced mixed results. Healthy adult dogs when fed L. acidophilus strain DSM13241 via oil mixture coated on extruded kibble vs. control resulted in numerous changes in immune parameters including increased serum neutrophils, monocytes and serum immunoglobulin G, as well as reduced serum nitric oxide concentrations and red blood cell fragility (Baillon et al., 2004). Healthy dogs administered FOS and a combination of FOS + L. acidophilus strain NCFM via gelatin capsule resulted in lower fecal protein metabolites and increases in fecal bifidobacteria and lactobacilli, whereas L. acidophilus strain NCFM alone showed minimal changes in fecal outcomes (Swanson et al., 2002a). German Shorthaired Pointers with nonspecific dietary sensitivity exhibited improvements in fecal consistency, fecal DM, and stool frequency when provided L. acidophilus strain DSM 13241; however, no changes were observed in fecal microbiota (Pascher et al., 2008).
Indeed, a majority of the evidence around dietary bacterial species are administered as viable bacterial cells (i.e., probiotics). The requirement of a probiotic needing to be viable, however, poses challenges to pet food manufacturers due to processing conditions of high heat, moisture, and pressure. To date, no studies in healthy dogs have investigated the potential gut health benefits of using a nonviable bacteria in dried extruded dog kibble. Interestingly, unpublished data from the manufacturer show that a stabilized, nonviable L. acidophilus (NVL; Culbac; TransAgra: Storm Lake, IA) fermentation product elicited improvements in feed conversion (lower feed to body weight gain ratio), lower physiological stress responses (e.g., heat stress, co-mingling stress, and transport stress), modulated immune responses in gut-associated lymphoid tissues and increased IgA, and maintained gut morphology in swine, poultry, dairy, and beef cattle. More recent studies in production animals utilizing bacterial-derived metabolites, often labeled as “postbiotics”, have shown similar results. Broilers fed Lactobacillus plantarum-derived metabolites exhibited greater villi length in duodenum, jejunum, and ileum, compared with negative and positive controls, and upregulation of gut barrier function genes (Humam et al., 2019, 2021). Similarly, investigations on heat-treated, nonviable Bifidobacterium longum strain CECT-7347 exhibited an ability in vitro to protect against oxidative stress damage, inhibit colonization of bacteria through innate immune activation, and reduce the acute inflammatory response (Martorell et al., 2021). In a fermentation model of the human gut, a heat-treated preparation from Limosilactobacillus fermentum CNCM MA65/4E-1b elicited a prebiotic-like effect by stimulating the growth of various Bifidobacterium species (Warda et al., 2021).
Here our objectives of this study were to (1) evaluate the inclusion of a nonviable L. acidophilus to dog diets and its impacts on fecal microbiota, fecal fermentative end-products, and fecal IgA, and (2) determine if there is an additive effect on fecal outcomes when adding an NVL in combination with mixed prebiotics (MPs). We hypothesized that some changes in fecal outcomes would be observed with NVL feeding, including increases in fecal short-chain fatty acids (SCFA) and fermentative-bacterial phyla and genera, decreases in proteolytic fermentation end-products, and increased fecal IgA; however, these changes would be more robust in combination with MPs.
Materials and Methods
Animals and diets
Twenty-four (12 males and 12 females) adult spayed and neutered Beagle dogs (age: 5.74 ± 2.18 yr; BW: 9.30 ± 1.32 kg) were utilized in a 168-d, four-dietary treatment, randomized crossover design. All animal use was first approved by the animal facility’s Institutional Animal Care and Use Committee (Summit Ridge Farms; Susquehanna, PA). All dogs were deemed healthy before the study by physical examination and exhibited normal physiologic ranges on serum chemistries before, during, and at the end of the study. All dogs were individually housed in a temperature-controlled room on a 12 h light: 12 h dark cycle. Dogs were fed once per day to maintain body weight throughout the study. Body weights were assessed weekly to determine whether adjustments were needed to each dog’s individual food allowance. However, over the 5 d digestibility collections, further adjustments were not made an each dog continue to receive their same individual amount of food throughout this period. All dogs began the study receiving the control diet for 21 d and then were randomized to one of four dietary treatment groups for the 21 d treatment period: control, NVL, MPs, and MP + NVL. The four washout and four treatment periods were alternated for a total of 168 d to complete the factorial, resulting in n = 24 per dietary treatment. During the treatment phase, dogs were acclimated to the diet for 14 d followed by 5 d of total fecal collections for apparent total tract nutrient digestibility. Fecal scores were also assessed during the 5 d total fecal collection. Body weights were taken weekly, and food intake was assessed daily.
The NVL ingredient is a fermentate derived from L. acidophilus. Table 1 shows the chemical composition of the test diets. The NVL, MP, and MP + NVL diets were formulated at the expense of cellulose and pea starch to target isonitrogenous, isoenergetic, and isofibrous concentrations across all diets. All test diets were formulated to meet requirements set by American Association of Feed Control Officials for adult maintenance (AAFCO, 2017).
Table 1.
Ingredient and chemical composition of control, nonviable Lactobacillus acidophilus (NVL), mixed prebiotics (MP), and MP + NVL diets
| Ingredient | Amount | |||
|---|---|---|---|---|
| %, as-is | ||||
| Control | NVL | MP | MP + NVL | |
| Mechanically deboned chicken | 18.95 | 18.95 | 18.95 | 18.95 |
| Chicken meal | 18.76 | 18.76 | 18.76 | 18.76 |
| Brown rice | 18.47 | 18.47 | 18.47 | 18.47 |
| Barley | 16.45 | 16.45 | 16.45 | 16.45 |
| Pea starch | 10.32 | 10.07 | 8.43 | 8.18 |
| Chicken fat | 5.67 | 5.67 | 5.67 | 5.67 |
| Prebiotic package* | — | — | 5.23 | 5.23 |
| Powdered cellulose | 3.98 | 3.98 | 0.65 | 0.65 |
| Liquid digest | 1.72 | 1.72 | 1.72 | 1.72 |
| Pea protein | 1.68 | 1.68 | 1.68 | 1.68 |
| Fish meal | 1.47 | 1.47 | 1.47 | 1.47 |
| Powdered digest | 0.86 | 0.86 | 0.86 | 0.86 |
| Salt | 0.49 | 0.49 | 0.49 | 0.49 |
| Potassium chloride | 0.41 | 0.41 | 0.41 | 0.41 |
| NVL1 | — | 0.25 | — | 0.25 |
| Vitamin premix | 0.23 | 0.23 | 0.23 | 0.23 |
| Mineral premix | 0.23 | 0.23 | 0.23 | 0.23 |
| Choline chloride | 0.15 | 0.15 | 0.15 | 0.15 |
| Methionine (DL) | 0.09 | 0.09 | 0.09 | 0.09 |
| Mixed tocopherols | 0.06 | 0.06 | 0.06 | 0.06 |
| Analyzed composition | ||||
| Dry matter, % | 92 | 92 | 92 | 92 |
| Metabolizable energy, kcal/g | 3.56 | 3.59 | 3.53 | 3.45 |
| %, DM | ||||
| Organic matter | 94 | 93 | 93 | 93 |
| Crude fat | 15.7 | 15.5 | 14.0 | 12.8 |
| Crude protein | 27.2 | 27.7 | 29.6 | 27.2 |
| Total dietary fiber | 11.1 | 10.2 | 10.7 | 10.9 |
1Nonviable Lactobacillus acidophilus, TransAgra Inc., Storm Lake, IA.
*Overall target inclusion of prebiotic package was 5.23% including Beet pulp, La Budde, Bay City, MI; FOS, Cosucra groupe, Warcoing, Luxembourgh, Belgium; MOS, Alltech, Nicholasville, KY; Inulin, Beneo, Manheim, Germany; Kelp, Tasco, Dartmouth, Nova Scotia, Canada.
All test diets were formulated at the expense of pea starch and cellulose to target equal protein, fat, and fiber across all diets.
Fecal collection and consistency scoring
During the fecal collection phase of each period, all feces were collected, weighed, scored, and frozen at −20 °C until analyses. Fecal samples were scored on a system as previously done by Freel et al. (2021). Briefly, fecal samples were scored three times daily over the 5-d total fecal collection according to a five-point scale where 0 = none, 1 = watery diarrhea, 1.5 = diarrhea, 2 = moist, no form, 2.5 = moist, some form, 3 = moist, formed, 3.5 = well-formed, sticky, 4 = well-formed, 4.5 = hard, dry, and 5 = hard, dry, crumbly. At the beginning and end of each test period, fresh fecal samples (within 15 min of defecation) were collected. Each collection was done over the first or last 3 d of the feeding period to ensure a sample was obtained from each dog. Dogs were not switched to the next diet until a fresh fecal sample was obtained. Collections for fecal immunoglobulin A (IgA) and 16S microbiota analyses were obtained at the beginning and end of each test period, while samples for fecal fermentative end products, pH, and DM were obtained only at the end of each test period. Due to feasibility of collection and variability in fecal IgA and microbiota, we chose these outcomes for multiple timepoint collections.
All fresh fecal samples were immediately measured for pH with a meter (Denver Instrument, Bohemia, NY) equipped with an electrode (Beckman Instruments Inc., Fullerton, CA). Two aliquots of fresh fecal sample were collected for fecal IgA and microbiota and stored at −70 °C until analysis. For fecal SCFA and branched-chain fatty acids (BCFA) concentrations, a fresh fecal sample was mixed with 2 N hydrochloric acid in a 1:1 (weight:weight) ratio and stored at −20 °C until analysis. Approximately 3–5 g of fresh fecal sample was collected in duplicate and stored at −20 °C until fecal phenol and indole analyses. The remaining fresh fecal sample was used for DM determination, where the sample was dried at 105 °C for 2 d.
Apparent total tract nutrient digestibility
At the end of each digestibility collection, individual fecal samples were shipped to a contract analytical laboratory (Eurofins US, Des Moines, Iowa). The test diets and all feces collected were analyzed according to the Association of Official Analytical Chemists (AOAC) approved analytical methodology for the following: moisture, fat, protein, fiber, ash, and calories (AOAC 930.15, AOAC 954.02, AOAC 990.03, AOAC 992.15, AOAC 991.43) (AOAC, 2006).
The digestibility calculations for nutrients and energy were: apparent total tract nutrient digestibility (%) = [nutrient intake (g/d) – fecal output (g/d)]/nutrient intake (g/d) × 100%.
Fecal fermentative end-products
All fecal fermentation end products were analyzed as described previously (Lin et al., 2019). Briefly, fecal SCFA (acetate, propionate, and butyrate), and BCFA (valerate, isovalerate, and isobutyrate) concentrations were determined by gas chromatography according to Erwin et al. (1961). During analyses, a gas chromatograph (Hewlett-Packard 5890A series II, Palo Alto, CA) and a glass column (180 cm × 4 mm i.d.) packed with 10% SP to 1,200/1% H3PO4 on 80/100 mesh Chromosorb WAW (Supelco Inc., Bellefonte, PA) were used. Nitrogen was the carrier gas with a flow rate of 75 mL/min. Temperatures of the oven, detector, and injector were 125, 175, and 180 °C, respectively. Fecal phenol and indole concentrations were evaluated by gas chromatography according to Flickinger et al. (2003).
DNA extraction and sequencing of 16S rRNA genes
Extraction of DNA, sequencing of 16S rRNA genes, and quantitative polymerase chain reaction (qPCR) analysis was carried out according to Pilla et al (2020). Briefly, a MoBio Power soil DNA isolation kit (MoBio Laboratories; Carlsbad, CA) was used to extract DNA from fecal samples according to manufacturer’s instructions. Illumina sequencing was performed at the MR DNA laboratory (Shallowater, TX) as previously described (Minamoto et al., 2015; Kasiraj et al., 2016; Honneffer et al., 2017; Isaiah et al., 2017). Briefly, the primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) to 806R (5′-GGACTACVSGGGTATCTAAT-3″) on the V4 region of 16S rRNA bacterial genes were amplified. For the PCR reaction, a single-step 30 cycle PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, Hilden, Germany) under the following conditions: 94 °C for 3 min, followed by 28 cycles (five cycles used on PCR products) of 94 °C for 30 s, 53 °C for 40 s, and 72 °C for 1 min, after which a final elongation step at 72 °C for 5 min was performed. The Illumina TruSeq DNA’s protocol was used to create a DNA library, and Illumina MiSeq was utilized for sequencing according the manufacturer’s guidelines. Analysis of sequences was performed using QIIME 2 31 2018.8 pipeline as previously described (Marsilio et al., 2019; Park et al., 2020). The operational taxonomic unit (OTU) table was created using DADA2, (Callahan et al., 2016) and rarefied to 19,200 sequences per sample based on the lowest read depth in all samples for even depth of analysis.
Alpha diversity metrics were assessed by Chao1 (richness), observed OTUs (species richness), and Shannon diversity (evenness). Beta diversity (diversity between samples) was evaluated with the phylogeny-based weighted UniFrac distance metric and plots were visualized using principal coordinate analysis (Lozupone et al., 2005) Analysis of similarity test within PRIMER 6 software package (PRIMER‐E Ltd., Luton, UK) was used to analyze significant differences in microbial communities between time points.
Quantitative PCR analysis
Quantitative PCR assays were performed for total bacteria, Escherichia coli, Lactobacillus, and Bifidobacterium as previously described (AlShawaqfeh et al., 2017; Gavazza et al., 2018). Briefly, SYBR green-based reaction mixtures were used for qPCR reactions. The total reaction volume was 10 μL. The reaction mix consisted of the following: 5 μL SsoFast EvaGreen supermix (Bio-Rad Laboratories, CA), 0.4 μL of forward and reverse primer (final concentration: 400 nM), 2.6 μL of PCR water, and 2 μL of normalized DNA (final concentration: 5 ng/μL). Conditions for PCR reaction were as follows: initial denaturation at 98 °C for 2 min, then 40 cycles with denaturation at 98 °C for 3 s and annealing for 3 s. Acceptable data were considered when within the range of the lowest standard, which was between 30 and 32 cycles. After amplification, melt curve analysis was conducted using the following conditions: 95 °C for 1 min, 55 °C for 1 min and increasing incremental steps of 0.5 °C for 80 cycles for 5 s each. All samples were run in duplicate. Data were expressed as the log amount of DNA for each bacterial group/10 ng of isolated total DNA.
Fecal IgA extraction and quantification analysis
Fecal IgA was extracted and quantified according to Tress et al (2006). Fecal IgA collection tubes (Sarstedt Ag & Co.; Numbrecht, Germany) intended for collection of fecal samples from human patients were used for collection of canine fecal samples. The exact amount of feces added was determined by weighing the filled tube and subtracting the weight of the empty tube. All samples were frozen until further analysis. Validated sandwich enzyme-linked immunosorbent assay plates that contained 96 flat-bottom wells (Combiplate 8, Labsystems Oy; Atlsinki, Finland) were coated with affinity-purified goat anti-dog IgA (Fc region) antibodies (Goat anti-dog IgA (Fc) antibodies, Nordic Immunochemicals, Tilberg, The Netherlands) that served as capture antibody. Plates were read at a wavelength of 450 nm with an automated platereader (Labsystems Multiskan Ascent; VWR, Plainview, NY). Standard curves were calculated by use of a four-parameter curve fit.
Statistical analysis
Average food intake, body weight, fecal pH, apparent total tract nutrient digestibility, fecal scores, fecal pH, and fecal fermentative end-products were analyzed by ANOVA using Mixed models in SAS (version 9.4; SAS Institute, Cary, NC), where diet was considered a fixed effect and dog as a random effect. The univariate procedure was used to evaluate normality and heterogeneity of variance. Significant differences between means were identified by using a Tukey’s post hoc adjustment and values are expressed as means ± pooled SEM.
For fecal microbiota and IgA, normality was first assessed using the Shapiro–Wilk test. A paired t-test or nonparametric Wilcoxon-Signed rank test were used for within diet time effect (i.e., day 21 vs. day 0), and an ANOVA or nonparametric Kruskal–Wallis tests for within-diet time effects and between diet differences at days 0 and 21. Adjustments for multiple comparisons were done using the Benjamini and Hochberg’s false discovery rate at each taxonomic level, alpha diversity parameters, and qPCR data using GraphPad Prism version 8.2.1 for Windows (GraphPad Software, San Diego, CA) (Benjamini and Hochberg, 1995). All fecal microbiota and IgA data are presented as means ± standard errors. Statistical significance was set at P ≤ 0.05 and q ≤ 0.10.
Results
Animal characteristics, food intake, and apparent total tract nutrient digestibility
Table 2 shows body weight, food intake, and apparent total tract nutrient digestibility data. Body weight and food intake were similar across all dietary treatments throughout the study. Dry matter digestibility was not impacted by dietary treatments; however, crude protein and crude fat digestibility were lower (P < 0.05) when dogs consumed the MP and MP + NVL diets. When dogs consumed the MP + NVL diet, they had lower (P < 0.05) energy digestibility, compared with feeding NVL, whereas no changes were observed with other diets.
Table 2.
Average daily food and energy intake, body weight, and apparent total tract apparent nutrient digestibility in adult dogs fed control, nonviable Lactobacillus acidophilus (NVL), mixed prebiotics (MP), and MP + NVL diets
| Dietary treatments | ||||||
|---|---|---|---|---|---|---|
| Item | Control | NVL | MP | MP + NVL | SEM | P-value |
| Food intake, g/d | 266 | 237 | 256 | 267 | 10.1 | 0.10 |
| Energy intake, kcal/d | 945 | 849 | 942 | 883 | 35.7 | 0.13 |
| BW, kg | 9.42 | 9.33 | 9.42 | 9.26 | 0.23 | 0.37 |
| Digestibility, % | ||||||
| DM | 87.2 | 87.8 | 87.5 | 86.3 | 0.45 | 0.22 |
| CP | 90.0a | 90.4a | 88.3b | 87.2b | 0.42 | 0.01 |
| Crude fat | 95.2a | 95.1a | 93.8b | 92.9b | 0.24 | 0.01 |
| Energy | 90.2ab | 90.9a | 90.5ab | 89.3b | 0.35 | 0.04 |
abDenotes significant (P < 0.05) difference between diets.
All values are means and pooled SEM.
Stool quality and fecal fermentation end-products
Fecal consistency remained ideal across all diets (Table 3); however, when dogs were fed MP and MP + NVL diets, they had lower (P < 0.05) fecal scores and fecal DM percent compared with feeding control and NVL diets. Fecal pH was lower (P < 0.05) when dogs were fed the MP diet compared with control and NVL feeding, while MP + NVL feeding gave lower (P < 0.05) fecal pH compared with NVL feeding. Fecal SCFA acetate and propionate were greater (P < 0.05) when dogs were fed the MP and MP + NVL diets, compared with control and NVL feeding. No significant diet effect was observed in fecal butyrate concentrations. Total fecal SCFA (acetate + propionate + butyrate) were also greater (P < 0.05) when dogs were fed MP and MP + NVL diets versus control and NVL diets.
Table 3.
Fecal characteristics, short-chain fatty acid (SCFA), branched-chain fatty acid (BCFA), phenol, and indole concentrations in adult dogs fed test diets control, nonviable Lactobacillus acidophilus (NVL), mixed prebiotics (MP), and MP + NVL diets
| Dietary treatments | ||||||
|---|---|---|---|---|---|---|
| Item | Control | NVL | MP | MP + NVL | SEM | P-value |
| Fecal pH | 6.20ab | 6.42a | 5.70c | 5.97bc | 0.48 | 0.01 |
| Fecal score1 | 3.45a | 3.42a | 3.31b | 3.32b | 0.05 | 0.01 |
| Fecal DM, % | 33.7a | 34.7a | 29.0b | 28.6b | 0.62 | 0.01 |
| Fecal fermentative end-products, µmol/g DM | ||||||
| Acetate | 221.11b | 230.75b | 346.97a | 371.43a | 9.43 | 0.01 |
| Propionate | 135.85b | 146.32b | 207.14a | 215.19a | 9.62 | 0.01 |
| Butyrate | 42.02 | 37.43 | 31.48 | 32.21 | 2.56 | 0.05 |
| Total SCFA2 | 398.98b | 414.50b | 585.59a | 618.83a | 16.0 | 0.01 |
| Isobutyrate | 4.37a | 4.41a | 3.80ab | 2.81b | 0.31 | 0.01 |
| Isovalerate | 6.63a | 6.83a | 5.88ab | 4.57b | 0.45 | 0.01 |
| Valerate | 0.93b | 0.89b | 1.31a | 1.38a | 0.10 | 0.01 |
| Total BCFA3 | 11.93ab | 12.13a | 11.00ab | 8.77b | 0.80 | 0.03 |
| Phenol | 0.63a | 0.67a | 0.44ab | 0.14b | 0.09 | 0.01 |
| Indole | 1.04a | 1.02a | 0.60ab | 0.23b | 0.10 | 0.01 |
1Fecal scores: 1 = watery diarrhea, 1.5 = diarrhea, 2 = moist, no form, 2.5 = moist, some form, 3 = moist, formed, 3.5 = well-formed, sticky, 4 = well-formed, 4.5 = hard, dry, and 5 = hard, dry, crumbly.
2Total short chain fatty acids (SCFA) = acetate + propionate + butyrate.
3Total branched-chain fatty acid (BCFA) = isobutyrate + isovalerate + valerate.
All values are means and pooled SEM. abDenotes significant (P < 0.05) difference between diets.
Markers of proteolyticfermentation, including isobutyrate, isovalerate, valerate, and phenolic and indolic compounds, were measured. Interestingly, fecal isobutyrate and isovalerate were lower (P < 0.05) when dogs were fed MP + NVL diet compared with other dietary treatments. Fecal valerate concentrations were greater (P < 0.05) when dogs were fed prebiotics and MP + NVL diets compared with both control and NVL diets. Total BCFA (isobutyrate + isovalerate + valerate) fecal concentrations were only lower (P < 0.05) when dogs were fed MP + NVL compared with NVL feeding. Similarly, when dogs were fed MP + NVL they exhibited lower (P < 0.05) fecal phenolic and indolic compound concentrations compared with feeding control and NVL.
Fecal microbiota and IgA
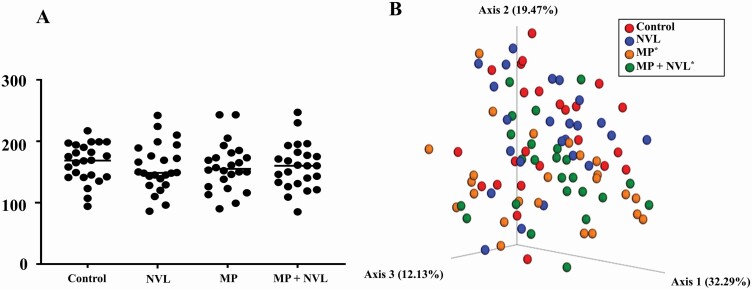
Both α diversity, which measures species richness and evenness within a sample, and β diversity were measured at both days 0 and 21 of each test period (Figure 1A and B). No differences in α diversity were observed at day 0 or day 21 between dietary treatments (A). Similarly, at day 0 no differences were observed in β diversity between dietary treatments (data not shown). However, at day 21 β diversity as measured by weighted unifrac distances (B) revealed that when dogs consumed MP and MP + NVL, they were more similar (P < 0.05) to each other compared with control and NVL diets.
Figure 1.
Fecal microbial communities of dogs fed control, nonviable Lactobacillus acidophilus (NVL), mixed prebiotics (MP), and MP + NVL diets. Alpha diversity by median values of observed operational taxonomic units (OTUs) was not impacted by diet at day 21 (A). Unweighted Unifrac distances displayed as a principal coordinate analysis (PCoA) plot suggest MP-containing diets resulted in changes in the microbiota (P < 0.05) compared with control and MP at day 21. No differences were observed between control and NVL, and between MP and MP + NVL (B).
For fecal microbiota, the predominant phylum present in all dogs throughout the study was Firmicutes (56.4% to 59.1% at day 0 and 61.2% to 72.9% at day 21), followed by Fusobacteria (20.9% to 22.3% at day 0 and 10.2% to 22.7% at day 21), Bacteroidetes (12.1% to 14.3% at day 0 and 9.16% to 10.2% at day 21), Proteobacteria (4.75% to 6.38% at day 0 and 4.66% to 5.95% at day 21), and Actinobacteria (1.82% to 2.48% at day 0 and 2.25 to 2.64% at day 21). At the genus level, Fusobacterium (20.9% to 22.3% at day 0 and 10.2% to 22.7% at day 21), Lactobacillus (8.44% to 14.9% at day 0 and 12.0% to 18.6% at day 21), and Bacteroides (7.37% to 8.55% at day 0 and 5.19% to 6.26% at day 21) were most common (Table 4).
Table 4.
Fecal operational taxonomic unit relative abundances of adult dogs fed test diets control, nonviable Lactobacillus acidophilus (NVL), mixed prebiotics (MP), and MP + NVL
| Dietary treatments | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | NVL | MP | MP + NVL | P-values between diets | ||||||||||
| Taxonomy | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 |
| Actinobacteria1 | 1.88 ± 0.25 | 2.25 ± 0.31 | 0.43 | 1.82 ± 0.26 | 2.64 ± 0.34* | 0.03 | 1.97 ± 0.28 | 2.48 ± 0.39 | 0.21 | 2.48 ± 0.37 | 2.62 ± 0.34 | 0.22 | 0.73 | 0.78 |
| Collinsella 2 | 1.69 ± 0.22 | 1.78 ± 0.22 | 0.78 | 1.59 ± 0.25 | 2.31 ± 0.30 | 0.11 | 1.76 ± 0.26 | 2.08 ± 0.28 | 0.27 | 2.24 ± 0.34 | 2.22 ± 0.26 | 0.65 | 0.66 | 0.61 |
| Bacteroidetes | 14.3 ± 1.65 | 9.17 ± 0.92* | <0.01 | 13.5 ± 1.40 | 9.53 ± 1.07* | 0.02 | 12.1 ± 1.27 | 9.16 ± 1.05* | 0.02 | 13.3 ± 1.27 | 10.2 ± 1.14* | 0.01 | 0.60 | 0.94 |
| Bacteroides | 8.55 ± 1.00 | 5.58 ± 0.64* | <0.01 | 8.25 ± 0.88 | 6.26 ± 0.79 | 0.11 | 7.37 ± 0.86 | 5.19 ± 0.64* | 0.03 | 8.30 ± 0.88 | 6.00 ± 0.66* | 0.01 | 0.81 | 0.77 |
| Prevotella | 1.97 ± 0.45 | 0.81 ± 0.21b* | <0.01 | 1.44 ± 0.30 | 0.75 ± 0.19b | 0.06 | 1.12 ± 0.33 | 1.84 ± 0.30a* | <0.01 | 1.59 ± 0.33 | 1.69 ± 0.30a | 0.39 | 0.50 | <0.01 |
| Firmicutes | 56.4 ± 2.87 | 61.2 ± 2.85b | 0.27 | 56.9 ± 2.43 | 62.5 ± 2.79b | 0.15 | 59.1 ± 2.57 | 72.9 ± 2.58a* | <0.01 | 57.0 ± 3.08 | 69.1 ± 2.20a* | <0.01 | 0.83 | <0.01 |
| Lactobacillus | 14.9 ± 3.26 | 13.8 ± 3.02 | 0.99 | 9.40 ± 2.01 | 12.0 ± 3.20 | 0.30 | 8.44 ± 1.87 | 18.6 ± 3.43* | 0.01 | 10.7 ± 3.36 | 13.2 ± 3.10 | 0.33 | 0.30 | 0.46 |
| Streptococcus | 3.10 ± 0.54 | 4.76 ± 0.92 | 0.35 | 4.90 ± 0.96 | 3.37 ± 0.78 | 0.06 | 5.13 ± 1.16 | 5.18 ± 1.42 | 0.84 | 2.81 ± 0.52 | 5.78 ± 0.88* | <0.01 | 0.25 | 0.12 |
| Turicibacter | 0.89 ± 0.36 | 0.93 ± 0.23 | 0.26 | 1.19 ± 0.24 | 1.65 ± 0.32 | 0.18 | 1.19 ± 0.33 | 2.04 ± 0.67 | 0.31 | 0.73 ± 0.18 | 2.74 ± 0.66* | <0.01 | 0.14 | 0.37 |
| Blautia | 4.01 ± 0.48 | 4.47 ± 0.47 | 0.42 | 4.63 ± 0.38 | 5.62 ± 0.63 | 0.33 | 5.00 ± 0.51 | 5.68 ± 0.50 | 0.41 | 5.02 ± 0.43 | 5.72 ± 0.48 | 0.09 | 0.23 | 0.27 |
| Dorea | 0.66 ± 0.16 | 0.88 ± 0.15 | 0.29 | 0.82 ± 0.15 | 1.04 ± 0.16 | 0.27 | 0.90 ± 0.17 | 0.71 ± 0.13 | 0.26 | 1.12 ± 0.15 | 0.85 ± 0.12 | 0.33 | 0.20 | 0.55 |
| Ruminococcus | 2.70 ± 0.21 | 2.99 ± 0.26 | 0.28 | 3.51 ± 0.24 | 3.43 ± 0.31 | 0.63 | 3.44 ± 0.34 | 3.95 ± 0.36 | 0.08 | 3.22 ± 0.34 | 3.81 ± 0.29 | 0.23 | 0.16 | 0.26 |
| Faecalibacterium | 1.18 ± 0.17 | 0.72 ± 0.16 | 0.14 | 1.16 ± 0.18 | 0.74 ± 0.12 | 0.16 | 0.90 ± 0.21 | 1.27 ± 0.22 | 0.07 | 0.98 ± 0.19 | 1.29 ± 0.22 | 0.26 | 0.34 | 0.08 |
| Megamonas | 2.48 ± 0.33 | 1.81 ± 0.30 | 0.17 | 2.99 ± 0.46 | 1.77 ± 0.25 | 0.11 | 3.55 ± 0.52 | 2.31 ± 0.27* | 0.02 | 3.07 ± 0.44 | 2.18 ± 0.18 | 0.07 | 0.63 | 0.23 |
| Phascolarctobacterium | 0.67 ± 0.12 | 0.35 ± 0.06* | 0.02 | 0.69 ± 0.12 | 0.42 ± 0.08 | 0.16 | 0.60 ± 0.14 | 0.49 ± 0.12 | 0.51 | 0.58 ± 0.12 | 0.34 ± 0.07* | 0.02 | 0.91 | 0.85 |
| Allobaculum | 1.29 ± 0.25 | 1.10 ± 0.15 | 0.77 | 1.32 ± 0.22 | 1.25 ± 0.20 | 0.98 | 1.52 ± 0.22 | 0.89 ± 0.18* | <0.01 | 1.40 ± 0.25 | 0.81 ± 0.14 | 0.01 | 0.80 | 0.20 |
| Catenibacterium | 6.35 ± 0.76 | 7.54 ± 0.93 | 0.18 | 7.34 ± 0.82 | 6.14 ± 0.73 | 0.28 | 7.05 ± 0.80 | 9.24 ± 1.11 | 0.01 | 7.47 ± 0.95 | 10.5 ± 1.27 | 0.09 | 0.75 | 0.07 |
| Eubacterium | 2.22 ± 0.35 | 2.37 ± 0.33 | 0.66 | 2.28 ± 0.34 | 3.05 ± 0.45 | 0.11 | 2.94 ± 0.39 | 3.87 ± 0.52 | 0.21 | 2.44 ± 0.33 | 3.84 ± 0.49* | <0.01 | 0.53 | 0.08 |
| Fusobacteria | 20.9 ± 1.92 | 22.7 ± 2.80a | 0.36 | 22.3 ± 1.51 | 19.9 ± 1.96a | 0.32 | 22.1 ± 1.93 | 10.3 ± 1.30b* | <0.01 | 21.2 ± 2.40 | 12.0 ± 1.65b* | <0.01 | 0.75 | <0.01 |
| Proteobacteria | 6.38 ± 0.82 | 4.66 ± 0.59 | 0.04 | 5.24 ± 0.62 | 5.31 ± 0.65 | 0.94 | 4.75 ± 0.56 | 5.10 ± 0.85 | 0.92 | 5.93 ± 0.68 | 5.95 ± 0.80 | 0.81 | 0.51 | 0.64 |
| Sutterella | 2.40 ± 0.28 | 1.40 ± 0.18* | <0.01 | 1.87 ± 0.23 | 1.44 ± 0.17 | 0.23 | 1.61 ± 0.25 | 1.51 ± 0.28 | 0.56 | 2.08 ± 0.25 | 1.50 ± 0.17 | 0.09 | 0.18 | 0.87 |
| Helicobacter | 0.28 ± 0.10 | 0.16 ± 0.08 | 0.08 | 0.23 ± 0.10 | 0.12 ± 0.04 | 0.08 | 0.14 ± 0.06 | 0.18 ± 0.06 | 0.61 | 0.20 ± 0.08 | 0.39 ± 0.14 | 0.22 | 0.18 | 0.38 |
1Phylum taxonomic level.
2Genus taxonomic level.
abDenotes significant (P < 0.05) difference between diets on day 21.
*Significant difference (P < 0.05) between days 21 and 0 within diet.
All values are expressed as means ± standard errors.
Relative abundances of OTUs at the phylum and genus taxonomic levels at day 0 showed no differences between diets. At the phylum level, when dogs were fed prebiotics and MP + NVL they exhibited a robust increase (P < 0.05) in fecal Firmicutes and decrease (P < 0.05) in fecal Fusobacteria from days 0 to 21. Both MP- and MP + NVL feeding resulted in greater (P < 0.05) Firmicutes and lower (P < 0.05) Fusobacteria compared with control and NVL feeding. Fecal Bacteroidetes decreased (P < 0.05) from days 0 to 21 when dogs were fed control, NVL, MP, and MP + NVL; however, no differences were noted between dietary treatments. Only NVL feeding increased Actinobacteria from days 0 to 21.
At the genus taxonomic level, fecal Bacteroides decreased (P < 0.05) from days 0 to 21 when dogs were fed control, MP, and MP + NVL diets. When dogs were fed control and NVL diets, they exhibited a decrease (P < 0.05), while MP feeding increased (P < 0.05) fecal relative abundances of Prevotella from days 0 to 21. Feeding both MP and MP + NVL resulted in greater (P < 0.05) Prevotella compared with control and NVL feeding at day 21.
Within Firmicutes, an increase (P < 0.05) in fecal relative abundances of Lactobacillus and a decrease (P < 0.05) in fecal Megamonas and Allobaculum from days 0 to 21 was evident when dogs were fed MP. Fecal relative abundances of Streptococcus, Turcibacter, and Eubacterium increased (P < 0.05) from days 0 to 21 when dogs were fed MP + NVL. When dogs were fed control and MP + NVL, they exhibited a decrease (P < 0.05) in fecal relative abundance of Phascolarctobacterium. Changes within the phylum Fusobacteria were solely due to the genus Fusobacterium. Within Proteobacteria, only Sutterella exhibited a decrease (P < 0.05) when dogs were fed control diet.
Fecal IgA, a marker of gut immune function, and qPCR of E. coli and Bifidobacterium spp. are shown in Table 5. Fecal IgA concentrations were greater (P < 0.05) at day 21 vs. day 0 when dogs were fed MP. At day 21, when dogs were fed NVL, lower (P < 0.05) fecal IgA was observed compared with when dogs were fed control, MP, and MP + NVL. When dogs were fed MP, fecal E. coli was lower (P < 0.05) at day 21 vs. day 0. No differences were observed in fecal Bifidobacterium spp.
Table 5.
Fecal qPCR (expressed as log DNA per 10 ng total DNA) for Escherichia coli and Bifidobacteria spp. and fecal immunoglobulin A (IgA; expressed as mg/g DM) concentration in adult dogs fed test diets control, nonviable Lactobacillus acidophilus (NVL), mixed prebiotics (MP), and MP + NVL
| Dietary treatments | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | NVL | MP | MP + NVL | P-values between diets | ||||||||||
| Item | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 | P-values day 0 vs. day 21 | Day 0 | Day 21 |
| Escherichia coli | 0.40 ± 0.04 | 0.45 ± 0.03 | 0.11 | 0.45 ± 0.03 | 0.43 ± 0.04 | 0.83 | 0.42 ± 0.03 | 0.33 ± 0.03* | <0.01 | 0.39 ± 0.04 | 0.42 ± 0.03 | 0.21 | 0.73 | 0.06 |
| Bifidobacterium spp | 0.24 ± 0.01 | 0.27 ± 0.02 | 0.20 | 0.25 ± 0.01 | 0.28 ± 0.02 | 0.08 | 0.23 ± 0.01 | 0.27 ± 0.02 | 0.11 | 0.25 ± 0.01 | 0.31 ± 0.03 | 0.09 | 0.62 | 0.53 |
| Immunoglobulin A | 37.8 ± 4.64 | 35.4 ± 4.61ab | 0.68 | 32.9 ± 4.12 | 24.8 ± 3.71b | 0.15 | 24.9 ± 3.25 | 43.6 ± 4.04a* | <0.01 | 30.4 ± 3.38 | 37.5 ± 2.46a | 0.08 | 0.11 | <0.01 |
*Difference (P < 0.05) between days 21 and 0 within diet.
abDenotes significant (P < 0.05) difference between diets on day 21.
All values are means ± standard deviations.
Discussion
Delivering health benefits beyond the basic nutrient requirements of the animal is a continual demand in the pet food industry. Both pre- and probiotic ingredients are dietary strategies that deliver benefits to gut health. Detriments to viability of probiotics in response to manufacturing and storage conditions have been acknowledged in both the production animal and human nutrition areas (de Lange et al., 2010; Kechagia et al., 2013). In the case of dry extruded pet food, application of probiotic ingredients presents numerous challenges to ensuring viability of bacterial strains due to the heat, pressure, and moisture that extrusion brings. Therefore, ingredients that can be offered in dry mixes and not be impacted by extrusion are intriguing. To our knowledge, no studies in healthy dogs have investigated markers of gut health with feeding a nonviable bacteria probiotic species (L. acidophilus; NVL). Here, we investigated the use of NVL; an MP blend containing beet pulp, MOS, FOS, inulin, and kelp; and the combination of both on stool quality, apparent total tract nutrient digestibility, fecal fermentative end-products, fecal microbiota, and fecal IgA in healthy adult Beagle dogs. We hypothesized that the MP package would result in more robust changes in fermentative end products, microbiota, and fecal IgA; however, NVL would provide some added benefits in combination with the MP package.
Feeding NVL did not result in any significant changes to nutrient digestibility in this study. The inclusion rate investigated here is similar in concentration to previous studies in dogs (Swanson et al., 2002a; Baillon et al., 2004). Furthermore, internal vendor data suggested improvements in feed to gain and gut mucosal integrity in livestock species which was used as the basis for inclusion in the current dog study. Previous studies that have investigated viable L. acidophilus with FOS supplemented to healthy dogs via a gelatin capsule exhibited a significant lowering of fecal protein metabolites and increases in Bifidobacterium and Lactobacillus spp. (Swanson et al., 2002a). However, these outcomes were not observed when L. acidophilus was fed alone (Swanson et al., 2002a). A dose-dependent response to viable L. acidophilus was evident in pigs, with improvements growth performance, nutrient digestibility, and increases in Bifidobacterium spp. and E. coli (Lan et al., 2017). Furthermore, supplementing four times greater the inclusion in the current study (1% L. acidophilus in water) resulted in improvements in growth performance, immune function, and cecal microbial populations (Zhang et al., 2021). In terms of companion animal nutrition, perhaps more investigation looking at effects of higher inclusion doses (1% or greater) and viable vs. nonviable forms are warranted to better understand effectiveness in dogs and cats.
Both diets that contained the MP package had detriments in crude protein and crude fat, while the MP + NVL diet had lower energy apparent total tract nutrient digestibility. Indeed, FOS and MOS have been investigated at concentrations comparable to what was observed in our study with no detriments to apparent total tract nutrient digestibility (Swanson et al., 2002; Grieshop et al., 2004; Pawar et al., 2017). However, it has been observed that feeding dogs varying combinations of cellulose, FOS, and yeast-cell wall can result in lower crude protein, fat, and energy digestibility compared with diets containing 2.7% beet pulp and cellulose (Middelbos et al., 2007a). Although there is no published research in dogs on nutrient digestibility, microalgae sources such as kelp contain substantial amounts of alginate oligosaccharides, which may contribute to fermentation in the large bowel and impact nutrient digestibility. In terms of beet pulp, a more recent study found greater acid hydrolyzed fat and crude protein digestibility in adult dogs fed a high-cellulose diet (10.3% inclusion as-is) vs. a high-beet pulp diet (16.6% inclusion as is) when total dietary fiber was similar (Detweiler et al., 2019). Other studies observed detriments in crude protein apparent total tract nutrient digestibility when dogs were fed cellulose versus beet pulp in similar concentrations (Kröger et al., 2017; Middelbos et al., 2007b). Our results of lower crude protein nutrient digestibility in the MP and MP + NVL diets are most likely due to the increased oligosaccharide concentrations in the diet, which increased large bowel fermentation. This in turn causes greater bacterial nitrogen accumulation in the large bowel and decreases crude protein digestibility (Hesta et al., 2003; Middelbos et al., 2007b, Silvio et al., 2000).
When dogs were fed MP and MP + NVL diets, they exhibited increases in fecal acetate, propionate, and total SCFA, as well as a concomitant decrease in fecal pH compared with control and NVL. Indeed, inclusion of beet pulp to dog diets at levels comparable in this study have also elicited increased fecal SCFA, whereas dietary FOS and MOS combinations at similar dietary concentrations found no appreciable change (Swanson et al., 2002a). However, recent evidence has suggested that the fermentability of yeast cell wall extracts can be linked their degree of solubility (Theodoro et al., 2019). Fructans with lower degrees of polymerization will have higher fermentability in the proximal large bowel, whereas longer degree of polymerization fructans such as inulin will ferment longer. Overall, the combination of beet pulp, FOS, inulin, MOS, and kelp seemed to provide sufficient fermentable substrate to promote increases in fecal SCFA concentrations.
Interestingly, fecal isobutyrate and isovalerate, which are fermentative end-products of protein fermentation, were lower in feces of when dogs were fed the MP + NVL compared with the non-MP diets. Fecal valerate was increased in feces when dogs were fed both MP-containing diets. Both FOS and MOS when fed at <0.5% of the diet have elicited decreases in fecal protein catabolites (Swanson et al., 2002b). Healthy dogs consuming a basal diet of 3.5% beet pulp and supplemental FOS and viable L. acidophilus in combination had lower fecal isovalerate, total BCFA, and total indole compared with control (Swanson et al., 2002a). Here, our data suggest that the addition of NVL, in combination with the MP package, influenced bacterial fermentation to lower phenol, indole, and BCFA fecal concentrations. In reference to our observed lack of changes in fermentation end-products from nitrogen with NVL alone, perhaps the inclusion of NVL was influencing gut nitrogen metabolism in a similar way to prebiotics, resulting in an additive effect when combined with the MP diet. In livestock species, probiotic supplementation is often added to improve immune and gut health and lower ammonia excretion (Lan et al., 2017). In this current study, NVL alone was not enough of a stimulus to impact gut nitrogen metabolism, but perhaps the combination of the prebiotics and NVL provided enough carbohydrate substrate for gut bacteria to metabolize resulting in lower phenol and indole. This also warrants investigating multiple inclusion levels (e.g., 0.25, 0.5, 1, and 1.25% of the diet) of NVL alone to confirm whether or not higher inclusions result in similar outcomes observed in livestock species.
Indeed, dogs consuming diets containing MP resulted in greater changes in the fecal microbiota, while NVL alone exhibited minimal modulation of the fecal microbiota. Consumption of MP-containing diets promoted α-diversity, which is a measure of species richness and evenness. β-diversity, which is a measure of similarities between samples, revealed that when dogs consumed the MP-containing diets they had microbiota more similar to each other than when they did not consuming prebiotics. The major phyla present in all dogs in this study were Firmicutes, Fusobacteria, Bacteroides, and Proteobacteria, which is similar to previous studies (Middelbos et al., 2010; Panasevich et al., 2015; Deng and Swanson, 2015; Lin et al., 2019). However, the relative abundances of these phyla were different from past studies. Our study shows Firmicutes as the predominant phylum at (56.4% to 57.1% at days 0 and 61.2 to 72.9% at day 21), followed by Fusobacteria (20.9% to 22.3% at day 0 and 10.2% to 22.7% at day 21), Bacteroidetes (12.1% to 14.3% at day 0 and 9.16% to 10.2% at day 21), Proteobacteria (4.75% to 6.38% at day 0 and 4.66% to 5.95% at day 21), and Actinobacteria (1.82% to 2.48% at day 0 and 2.25% to 2.64% at day 21). Differences in variable regions amplified on 16S rRNA, dog breed, and dietary treatments may explain changes in relative abundances (Deng and Swanson, 2015).
Interestingly, diets containing the MP package elicited the most profound changes on the fecal microbiome at the phylum level. Differences between diets at day 21 and changes from day 0 within treatment were most apparent at the phylum taxonomic level. Fecal Fusobacteria was significantly lowered from baseline, while Firmicutes increased when dogs were fed MP. This is consistent with dogs consuming moderately fermentable fibers like potato fiber and beet pulp where an increase in the ratio of fecal Firmicutes to Fusobacteria was observed (Middelbos et al., 2010; Panasevich et al., 2015). Changes and diversity within the Firmicutes phylum are consistent with diets containing fermentable carbohydrate, which matched our observations of increases in fecal SCFA concentrations (Middelbos et al., 2010). Whether lower fecal Fusobacteria is beneficial is not fully understood. Indeed, feeding of fermentable fiber and fractions of yeast-ingredients have lowered fecal Fusobacteria (Middelbos et al., 2010; Panasevich et al., 2015; Lin et al., 2019). Previous studies have revealed both higher and lower fecal concentrations in dogs and humans with inflammatory bowel disease compared to healthy counterparts (Vázquez-Baeza et al., 2016; AlShawaqfeh et al., 2017). At the genus taxonomic level for both 16S sequencing and qPCR, results revealed changes in microbiota over time and between diets. Within Firmicutes, Lactobacillus spp. increased over time within the MP diet feeding period, while feeding the NVL and MP + NVL diets resulted in no appreciable change in the genus. In agreement with our results, feeding FOS as a supplement to dogs has elicited increases in fecal Lactobacillus spp. and decreases in Clostridium perfringens (Swanson et al., 2002a) compared with no supplementation. Our results revealed a more robust change in the genus Prevotella with both MP and MP + NVL feeding, which is in agreement with our observed changes in fecal SCFA. Previous evidence in dogs fed a high protein low carbohydrate diet revealed lower concentrations of this genus compared with dogs fed a low protein high carbohydrate diet (Li et al., 2017). Prevotella has been associated with greater carbohydrate metabolism, is found in higher concentrations in plant-based diets and is associated with improvements in glucose metabolism in humans (De Filippo et al., 2010; Kovatcheva-Datchary et al., 2015). Furthermore, dogs with inflammatory bowel disease have been found to have lower concentrations of the genus Prevotella (Suchodolski et al., 2012; Minamoto et al., 2019).
We performed qPCR on genera not amplified in the 16S amplification. Specifically, when dogs were fed the MP diet, we found a significant lowering of fecal E. coli from days 0 to 21. Escherichia coli is commonly known as a pathogenic bacterium and is commonly considered as an indicator of improved gut health and a prebiotic effect when decreased. Furthermore, it is in the class of Gamma-Proteobacteria that is overrepresented in fecal samples of dogs with chronic enteropathies (Minamoto et al., 2019; Pilla and Suchodolski, 2019). Overall, changes in fecal microbiota were predominantly driven by MP and are in agreement with the observed changes in fecal fermentative end-product concentrations.
Immunoglobulin A is an essential defense against allergens and foreign antigens. Secretion of IgA from Peyer’s patches is often used as an indicator of mucosal immunity (Norris and Gershwin, 2003; Zaine et al., 2011). Studies using combinations of MOS and FOS have shown an increase in fecal and systemic IgA, and peripheral immune markers in healthy adult dogs (Swanson et al., 2002b; Grieshop et al., 2004). No evidence of immune modulation has been observed with dietary kelp in companion animals. However, in pigs fed alginate oligosaccharide, jejunal concentrations of IgA were increased compared with control; although, this change was not observed in duodenum or ileal regions (Wan et al., 2018). Interestingly, in a study that compared diets containing fermentable fiber from beet pulp vs. soybean meal diet in healthy dogs, dogs fed soybean meal had increased fecal IgA, compared with the beet pulp diet (Maria et al., 2017). Therefore, it seems most plausible that the changes in fecal IgA are mainly driven by the oligosaccharide fractions from FOS, inulin, MOS, and kelp in the MP package rather than beet pulp.
In conclusion, the majority of our observed changes were a result of dogs consuming the MP-containing diets. Indeed, feeding of prebiotics resulted in depressed apparent total tract nutrient digestibility, most likely due to increased fermentability of the MP-containing diets. The observed increased concentrations in fecal SCFA with the MP-containing diets seems to align with our changes in the fecal microbiota. Specifically, when dogs were fed MP they had increased fecal relative abundances of Firmicutes and decreased relative abundances of Fusobacteria, as well as genera within these phyla (i.e., Prevotella, Lactobacillus, and E. coli), which is a consistent response with fermentable fiber intake. The MP diet also promoted increased fecal IgA concentrations, which we attribute to the sources of oligosaccharide in the diet from FOS, inulin, MOS, and kelp. The inclusion of NVL had minimal influence on most gut health outcomes; however, markers of fecal bacterial protein fermentation were differentially altered when NVL was consumed with MP. Specifically, isovalerate, isobutyrate, and putrefactive compounds phenol and indoles seemed to be lowest when MP were fed in combination with NVL, suggesting perhaps that this combination lessens bacterial and dietary nitrogen from entering the large bowel. Overall, whether NVL can elicit similar changes as MP should be evaluated as a titration trial where an effective dose can be established. Future studies that look at this, as well as more challenged models (i.e., animals with inflammatory bowel disease), are warranted to evaluate the efficacy of NVL in companion animal diets.
Acknowledgment
This study was jointly funded by TransAgra International Inc. and Blue Buffalo Company Ltd.
Glossary
Abbreviations
- AOAC
Association of Official American Chemists
- BCFA
branched-chain fatty acids
- FOS
fructooligosaccharide
- IgA
immunoglobulin A
- MP
mixed prebiotic
- MOS
mannanoligosaccharide
- OTU
operational taxonomic units
- PCoA
principal coordinate analysis
- qPCR
quantitative polymerase chain reaction
- SCFA
short-chain fatty acids
Conflict of interest statement
MRP, LD, and NZF are employed by Blue Buffalo, Ltd who formulated and manufactured the foods. RQ is employed by TransAgra Internation Inc. who has a trademark on NVL used in this study.
Literature Cited
- AlShawaqfeh, M. K., Wajid B., Minamoto Y., Markel M., Lidbury J. A., Steiner J. M., Serpedin E., and Suchodolski J. S.. . 2017. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 93:fix136. doi: 10.1093/femsec/fix136. [DOI] [PubMed] [Google Scholar]
- Association of American Feed Control Officials (AAFCO). 2017. Official publication. Oxford, IN: AAFCO. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). 2006. Official methods of analysis. 17th ed. Gaithersburg, MD: Association of Official Analytical Chemists. [Google Scholar]
- Baillon, M. L., Marshall-Jones Z. V., and Butterwick R. F.. . 2004. Effects of probiotic Lactobacillus acidophilus strain DSM13241 in healthy adult dogs. Am. J. Vet. Res. 65:338–343. doi: 10.2460/ajvr.2004.65.338. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y., and Hochberg Y.. . 1995. Controlling the false discovery rate. A practical and powerful approach to multiple testing. J. R. Stat. Soc. B Methodol. 57:289–300. doi.org/ 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Callahan, B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J., and Holmes S. P.. . 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13: 581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo, C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J. B., Massart S., Collini S., Pieraccini G., and Lionetti P.. . 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange, C. F. M., Pluske J., Gong J., and Nyachoti C. M.. . 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 134:124–134. doi: 10.1016/j.livsci.2010.06.117. [DOI] [Google Scholar]
- Deng, P., and Swanson K. S.. . 2015. Gut microbiota of humans, dogs and cats: current knowledge and future opportunities and challenges. Br. J. Nutr. 113(Suppl.):S6–17. doi: 10.1017/S0007114514002943. [DOI] [PubMed] [Google Scholar]
- Detweiler, K. B., He F., Mangian H. F., Davenport G. M., and de Godoy M. R. C.. . 2019. Effects of high inclusion of soybean hulls on apparent total tract macronutrient digestibility, fecal quality, and fecal fermentative end-product concentrations in extruded diets of adult dogs. J. Anim. Sci. 97:1027–1035. doi: 10.1093/jas/skz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin, E. S., Marco G. J., and Emery E. M.. . 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6 [DOI] [Google Scholar]
- Fahey, G. C., Jr, Merchen N. R., Corbin J. E., Hamilton A. K., Serbe K. A., and Hirakawa D. A.. . 1990. Dietary fiber for dogs: II. Iso-total dietary fiber (TDF) additions of divergent fiber sources to dog diets and their effects on nutrient intake, digestibility, metabolizable energy and digesta mean retention time. J. Anim. Sci. 68:4229–4235. doi: 10.2527/1990.68124229x. [DOI] [PubMed] [Google Scholar]
- Flickinger, E. A., Schreijen E. M., Patil A. R., Hussein H. S., Grieshop C. M., Merchen N. R., and G. C.Fahey, Jr. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi: 10.2527/2003.8182008x. [DOI] [PubMed] [Google Scholar]
- Freel, T. A., McComb A., and Koutsos E. A.. . 2021. Digestibility and safety of dry black soldier fly larvae meal and black soldier fly larvae oil in dogs. J. Anim. Sci. 99:skab047. doi: 10.1093/jas/skab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazza, A., Rossi G., Lubas G., Cerquetella M., Minamoto Y., and Suchodolski J. S.. . 2018. Faecal microbiota in dogs with multicentric lymphoma. Vet. Comp. Oncol. 16:E169–E175. doi: 10.1111/vco.12367. [DOI] [PubMed] [Google Scholar]
- Gibson, G. R., Hutkins R., Sanders M. E., Prescott S. L., Reimer R. A., Salminen S. J., Scott K., Stanton C., Swanson K. S., Cani P. D., . et al. 2017. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Grieshop, C. M., Flickinger E. A., Bruce K. J., Patil A. R., Czarnecki-Maulden G. L., and G. C.Fahey, Jr. 2004. Gastrointestinal and immunological responses of senior dogs to chicory and mannan-oligosaccharides. Arch. Anim. Nutr. 58:483–493. doi: 10.1080/00039420400019977. [DOI] [PubMed] [Google Scholar]
- Hesta, M., Roosen W., Janssens G. P., Millet S., and De Wilde R.. . 2003. Prebiotics affect nutrient digestibility but not faecal ammonia in dogs fed increased dietary protein levels. Br. J. Nutr. 90:1007–1014. doi: 10.1079/bjn2003988. [DOI] [PubMed] [Google Scholar]
- Hill, C., Guarner F., Reid G., Gibson G. R., Merenstein D. J., Pot B., Morelli L., Canani R. B., Flint H. J., Salminen S., . et al. 2014. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Honneffer, J. B., Steiner J. M., Lidbury J. A., and Suchodolski J. S.. . 2017. Variation of the microbiota and metabolome along the canine gastrointestinal tract. Metabolomics. 13:1–20. doi: 10.1007/s11306-017-1165-3.27980501 [DOI] [Google Scholar]
- Humam, A. M., Loh T. C., Foo H. L., Izuddin W. I., Zulkifli I., Samsudin A. A., and Mustapha N. M.. . 2021. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 100:100908. doi: 10.1016/j.psj.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humam, A. M., Loh T. C., Foo H. L., Samsudin A. A., Mustapha N. M., Zulkifli I., and Izuddin W. I.. . 2019. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals (Basel). 9:644. doi: 10.3390/ani9090644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaiah, A., Parambeth J. C., Steiner J. M., Lidbury J. A., and Suchodolski J. S.. . 2017. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 45:50–58. doi: 10.1016/j.anaerobe.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Kasiraj, A. C., Harmoinen J., Isaiah A., Westermarck E., Steiner J. M., Spillmann T., and Suchodolski J. S.. . 2016. The effects of feeding and withholding food on the canine small intestinal microbiota. FEMS Microbiol. Ecol. 92:fiw085. doi: 10.1093/femsec/fiw085. [DOI] [PubMed] [Google Scholar]
- Kechagia, M., Basoulis D., Konstantopoulou S., Dimitriadi D., Gyftopoulou K., Skarmoutsou N., and Fakiri E. M.. . 2013. Health benefits of probiotics: a review. ISRN Nutr. 2013:481651. doi: 10.5402/2013/481651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatcheva-Datchary, P., Nilsson A., Akrami R., Lee Y. S., De Vadder F., Arora T., Hallen A., Martens E., Björck I., and Bäckhed F.. . 2015. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kröger, S., Vahjen W., and Zentek J.. . 2017. Influence of lignocellulose and low or high levels of sugar beet pulp on nutrient digestibility and the fecal microbiota in dogs. J. Anim. Sci. 95:1598–1605. doi: 10.2527/jas.2016.0873. [DOI] [PubMed] [Google Scholar]
- Lan, R., Koo J., and Kim I.. . 2017. Effects of Lactobacillus acidophilus supplementation on growth performance, nutrient digestibility, fecal microbial and noxious gas emission in weaning pigs. J. Sci. Food Agric. 97:1310–1315. doi: 10.1002/jsfa.7866. [DOI] [PubMed] [Google Scholar]
- Li, Q., Lauber C. L., Czarnecki-Maulden G., Pan Y., and Hannah S. S.. . 2017. Effects of the dietary protein and carbohydrate ratio on gut microbiomes in dogs of different body conditions. mBio. 8:e01703-16. doi: 10.1128/mBio.01703-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C-Y., Alexander C., Steelman A. J., Warzecha C. M., Godoy M. R. C., and Swanson K. S.. . 2019. Effects of a Saccharomyces cerevisiae fermentation product on fecal characteristics, nutrient digestibility, fecal fermentative end-products, fecal microbial populations, immune function, and diet palatability in adult dogs. J. Anim. Sci. 97:1586–1599. doi: 10.1093/jas/skz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone, C., and Knight R.. . 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria, A. P. J., Ayane L., Putarov T. C., Loureiro B. A., Neto B. P., Casagrande M. F., Gomes M. O. S., Glória M. B. A., and Carciofi A. C.. . 2017. The effect of age and carbohydrate and protein sources on digestibility, fecal microbiota, fermentation products, fecal IgA, and immunological blood parameters in dogs. J. Anim. Sci. 95:2452–2466. doi: 10.2527/jas.2016.1302. [DOI] [PubMed] [Google Scholar]
- Marsilio, S., Pilla R., Sarawichitr B., Chow B., Hill S. L., Ackermann M. R., Estep J. S., Lidbury J. A., Steiner J. M., and Suchodolski J. S.. . 2019. Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma. Sci. Rep. 9:19208. doi: 10.1038/s41598-019-55691-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell, P., Alvarez B., Llopis S., Navarro V., Ortiz P., Gonzalez N., Balaguer F., Rojas A., Chenoll E., Ramón D., and Tortajada M.. . 2021. Heat-Treated Bifidobacterium longum CECT-7347: a whole-cell postbiotic with antioxidant, anti-inflammatory, and gut-barrier protection properties. Antioxidants (Basel). 10:536. doi: 10.3390/antiox10040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbos, I. S., Godoy M. R., Fastinger N. D., and G. C.Fahey, Jr. 2007a. A dose-response evaluation of spray-dried yeast cell wall supplementation of diets fed to adult dogs: effects on nutrient digestibility, immune indices, and fecal microbial populations. J. Anim. Sci. 85:3022–3032. doi: 10.2527/jas.2007-0079. [DOI] [PubMed] [Google Scholar]
- Middelbos, I. S., Fastinger N. D., and G. C.Fahey, Jr. 2007b. Evaluation of fermentable oligosaccharides in diets fed to dogs in comparison to fiber standards. J. Anim. Sci. 85: 3033–3044. doi: 10.2527/jas.2007-0080. [DOI] [PubMed] [Google Scholar]
- Middelbos, I. S., Vester Boler B. M., Qu A., White B. A., Swanson K. S., and G. C.Fahey, Jr. 2010. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS One 5:e9768. doi: 10.1371/journal.pone.0009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto, Y., Otoni C. C., Steelman S. M., Büyükleblebici O., Steiner J. M., Jergens A. E., and Suchodolski J. S.. . 2015. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 6:33–47. doi: 10.1080/19490976.2014.997612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamoto, Y., Minamoto T., Isaiah A., Sattasathuchana P., Buono A., Rangachari V. R., McNeely I. H., Lidbury J., Steiner J. M., and Suchodolski J. S.. . 2019. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 33:1608–1618. doi: 10.1111/jvim.15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, C. R., and Gershwin L. J.. . 2003. Evaluation of systemic and secretory IgA concentrations and immunohistochemical stains for IgA-containing B cells in mucosal tissues of an Irish setter with selective IgA deficiency. J. Am. Anim. Hosp. Assoc. 39:247–250. doi: 10.5326/0390247. [DOI] [PubMed] [Google Scholar]
- Panasevich, M. R., Kerr K. R., Dilger R. N., G. C.Fahey, Jr, Guérin-Deremaux L., Lynch G. L., Wils D., Suchodolski J. S., Steer J. M., Dowd S. E., . et al. 2015. Modulation of the faecal microbiome of healthy adult dogs by inclusion of potato fibre in the diet. Br. J. Nutr. 113:125–133. doi: 10.1017/S0007114514003274. [DOI] [PubMed] [Google Scholar]
- Park, M. J., Pilla R., Panta A., Pandey S., Sarawichitr B., Suchodolski J., and Sohrabji F.. . 2020. Reproductive senescence and ischemic stroke remodel the gut microbiome and modulate the effects of estrogen treatment in female rats. Transl. Stroke Res. 11:812–830. doi: 10.1007/s12975-019-00760-5. [DOI] [PubMed] [Google Scholar]
- Pascher, M., Hellweg P., Khol-Parisini A., and Zentek J.. . 2008. Effects of a probiotic Lactobacillus acidophilus strain on feed tolerance in dogs with non-specific dietary sensitivity. Arch. Anim. Nutr. 62:107–116. doi: 10.1080/17450390801892583. [DOI] [PubMed] [Google Scholar]
- Pawar, M. M., Pattanaik A. K., Sinha D. K., Goswami T. K., and Sharma K.. . 2017. Effect of dietary mannanoligosaccharide supplementation on nutrient digestibility, hindgut fermentation, immune response and antioxidant indices in dogs. J. Anim. Sci. Technol. 59:11. doi: 10.1186/s40781-017-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla, R., Gaschen F. P., Barr J. W., Olson E., Honneffer J., Guard B. C., Blake A. B., Villanueva D., Khattab M. R., AlShawaqfeh M. K., . et al. 2020. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 34: 1853–1866. doi: 10.1111/jvim.15871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla, R., and Suchodolski J. S.. . 2019. The role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 6:498. doi: 10.3389/fvets.2019.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvio, J., Harmon D. L., Gross K. L., and McLeod K. R.. . 2000. Influence of fiber fermentability on nutrient digestion in the dog. Nutrition 16:289–295. doi: 10.1016/s0899-9007(99)00298-1. [DOI] [PubMed] [Google Scholar]
- Suchodolski, J. S., Dowd S. E., Wilke V., Steiner J. M., and Jergens A. E.. . 2012. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One 7:e39333. doi: 10.1371/journal.pone.0039333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, K. S., Grieshop C. M., Flickinger E. A., Bauer L. L., Chow J., Wolf B. W., Garleb K. A., and G. C.Fahey, Jr. 2002a. Fructooligosaccharides and Lactobacillus acidophilus modify gut microbial populations, total tract nutrient digestibilities and fecal protein catabolite concentrations in healthy adult dogs. J. Nutr. 132:3721–3731. doi: 10.1093/jn/132.12.3721. [DOI] [PubMed] [Google Scholar]
- Swanson, K. S., Grieshop C. M., Flickinger E. A., Bauer L. L., Healy H., Dawson K. A., Merchen N. R., and Fahey G. C. Jr. 2002b. Supplemental fructooligosaccharides and mannnanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 132:980–989. doi: 10.1093/jn/132.5.980. [DOI] [PubMed] [Google Scholar]
- Theodoro, S. S., Putarov T. C., Tiemi C., Volpe L. M., de Oliveira C. A. F., Glória M. B. A., and Carciofi A. C.. . 2019. Effects of the solubility of yeast cell wall preparations on their potential prebiotic properties in dogs. PLoS One 14:e0225659. doi: 10.1371/journal.pone.0225659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tress, U., Suchodolski J. S., Williams D. A., and Steiner J. M.. . 2006. Development of a fecal sample collection strategy for extraction and quantification of fecal immunoglobulin A in dogs. Am. J. Vet. Res. 67:1756–1759. doi: 10.2460/ajvr.67.10.1756. [DOI] [PubMed] [Google Scholar]
- Vázquez-Baeza, Y., Hyde E. R., Suchodolski J. S., and Knight R.. . 2016. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 1:16177. doi: 10.1038/nmicrobiol.2016.177. [DOI] [PubMed] [Google Scholar]
- Wan, J., Zhang J., Chen D., Yu B., Mao X., Zheng P., Yu J., Luo J., and He J.. . 2018. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J. Anim. Sci. Biotechnol. 9:58. doi: 10.1186/s40104-018-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warda, A. K., Clooney A. G., Ryan F., de Almeida Bettio P. H., Di Benedetto G., Ross R. P., and Hill C.. . 2021. A postbiotic consisting of heat-treated lactobacilli has a bifidogenic effect in pure culture and in human fermented faecal communities. Appl. Environ. Microbiol. 87:e02459–20. doi: 10.1128/AEM.02459-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaine, L., Ferreira C., Gomes M. d. e. O., Monti M., Tortola L., Vasconcellos R. S., and Carciofi A. C.. . 2011. Faecal IgA concentration is influenced by age in dogs. Br. J. Nutr. 106 (Suppl. 1):S183–S186. doi: 10.1017/S0007114511000559. [DOI] [PubMed] [Google Scholar]
- Zhang, L., Zhang R., Jia H., Zhu Z., Li H., and Ma Y.. . 2021. Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens. Open Life Sci. 16:311–322. doi: 10.1515/biol-2021-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]