Abstract
Nicotinamide adenine dinucleotide (NAD+), an essential co-factor for mitochondrial function, declines with aging, which may lead to impaired physical performance. Nicotinamide riboside (NR), a NAD+ precursor, restores cellular NAD+ levels. Here we examined the impacts of short term NR supplementation on physical performance in middle-aged mice and impacts on mouse and human muscle stem cells. We treated fifteen-month-old male C57BL/6J mice with NR at 300 mg/kg/day (NR3), 600 mg/kg/day (NR6), or placebo (PLB), n=8 per group, and assessed changes in physical performance, muscle histology, and NAD+ content after 4 weeks of treatment. We found that NR increased total NAD+ in muscle tissue (NR3 p=0.01; NR6 p=0.004, both versus PLB), enhanced treadmill endurance and open field activity, and prevented decline in grip strength. Histologic analysis revealed NR treated mice exhibited enlarged slow twitch fibers (NR6 versus PLB p=0.014; NR3 p=0.16) and a trend towards more slow fibers (NR3 p=0.14; NR6 p=0.22). We next carried out experiments to characterize NR impacts on mitochondrial activity and cellular energetics in vitro. We observed that NR boosted basal and maximal cellular aerobic and anaerobic respiration in both mouse and human myoblasts and human myotubes. Additionally, NR treatment improved the differentiating capacity of myoblasts and increased myotube size and fusion index upon stimulation of these progenitors to form multinucleated myotubes. These findings support a role for NR in improving cellular energetics and functional capacity in mice, which support the translation of this work into clinical settings as a strategy for improving and/or maintaining healthspan during aging.
Keywords: muscle, vitamin B3, niacin, aging, mitochondria, functional capacity
1.0. Introduction
Sarcopenia affects as many as 30% of older individuals in community dwelling and long-term care populations [1], and will increasingly impact society as the number of individuals over the age of 65 rapidly grows. Hallmarks of sarcopenia include loss of muscle mass and function [1], and although exercise can mitigate these declines, participant rates remain as low as 8–12% in those over the age of 65 [2–4]. Thus, pharmacologic supplements and/or alternatives to exercise could help meet this oncoming healthcare challenge. Nicotinamide riboside (NR), a nicotinamide adenine dinucleotide (NAD+) precursor, is showing promise as a pleiotropic healthspan agent [5], which may mitigate the age-associated decline of NAD+ and its impacts across multiple tissues [6–8].
Boosting NAD+ with NR has also yielded benefits for skeletal muscle tissue in multiple mouse models including natural aging, obesity, muscular dystrophy, and mitochondrial dysfunction models [9–13]. These enhancements include increased fiber cross sectional area, muscle stem cell content, and recovery from injury [9, 10, 12, 13]. NR supplementation also increases physical performance, particularly treadmill endurance, across these various models [9, 10, 12, 13]. The addition of NR also exerts beneficial impacts upon muscle stem cells. Zhang et al. reported NR supplementation increased muscle stem cell populations and response to injury [13]. Zhang et al. also found improved mitochondrial activity in isolated stem cells including membrane potential and basal respiration [13]. Additionally, treatment of mouse C2C12 myoblast cells with NR increases mitochondrial maximal respiration [12]. Together, these findings have led to speculation that NAD+ plays an important role in muscle stem cell differentiation [11].
Interestingly, the benefits of NR for physical performance have been observed in aged animals [13], and younger animals with a genetic impairment [9, 10, 12], but not in younger wild-type animals [10, 13, 14]. In this study, we investigate for the first time whether NR supplementation boosts physical performance in middle-aged, wild type animals. Furthermore, given the apparent role of muscle stem cells in mediating the beneficial response to NR, our investigation also examined the impacts of NR treatment on mouse derived and, for the first time, human derived stem cells. Our findings reveal NR increases physical performance and muscle fiber size in vivo, as well as both aerobic and aerobic respiration and myofiber size in vitro, suggesting a role for the use of NR for the treatment of sarcopenia.
2.0. Materials and Methods
2.1. Animals
All studies and experimental protocols were approved by and in compliance with the institutional animal care and use committee (IACUC) guidelines of the University at Buffalo and the Veteran Affairs Western New York. Twenty-four C57BL/6J mice were bred and aged in house. At 15 months of age, mice were sorted into groups based upon body weight and treadmill performance. Mice were supplemented with AIN93G chow containing 0, 3, or 6 grams of nicotinamide riboside (Chromadex, Los Angeles, CA, USA) per kg, formulated to provide 0, 300, or 600 mg of nicotinamide riboside / kg mouse weight / day (n=8), respectively (Dyets Inc., Bethlehem, PA), the route of administration for other studies [9, 13]. The specialized chow and water were provided ad libitum, and mice were housed in large animal cages containing 2 or 3 mice per cage, with lighting provided on a 12 hour on / 12 hour off cycle. Additionally, ten 7-month old C567BL/6J male mice were used to acquire tissues for comparison. Body weight and food consumption via weight food hoppers were measured on a weekly basis.
2.2. Quantitative magnetic resonance (qMR)
We measured both body fat percentage and lean mass using a magnetic resonance minispec LF65 Whole Body Composition Analyzer (Bruker, Germany). Two scans were performed per animal before and after the 4-week NR treatment. Body fat mass and lean mass data were derived from the magnetic resonance scans using a standard curve generated from lean chicken muscle and lard.
2.3. Physical performance assessments
We utilized a series of physical performance assessments as we have described previously and that included grip strength, treadmill endurance, open field activity, and overnight activity [15–17]. Mice were acclimated to physical performance equipment one month prior to baseline. Baseline and endpoint tests were carried out for each behavioral assessment by the same investigator who was blind to the treatment of the mice. All experiments were carried out during lighted hours and at the same time of day between baseline and endpoint. Grip strength was assessed on a grip force meter (Columbus Instruments, Columbus OH, USA) as maximal force generated in the best 3 of 5 trials. For each trial the mouse is held by the tail and placed on a force meter and pulled parallel to the ground until loss of grip. Mice were given 10 seconds rest between trials. Treadmill endurance was acquired as the best of two assessments given on consecutive days on a mouse treadmill (Columbus Instruments) set with no inclination (flat - 0°). For each trial the belt accelerates from 5 to 35 meters/minute over 60 minutes, and the time on belt was recorded for each mouse until exhaustion defined as 10 visits to a shock pad or 20 total shocks (shocks at 54V, 0.72mA). Open field activity was assessed using an open field arena with infrared photobeam arrays (MedAssociates Inc., Fairfax VT, USA). Each arena was 40 cm × 40 cm × 35 cm, and the total distance covered in 30 minutes was automatically calculated by system software. Overnight activity was determined using wireless mouse activity wheels (MedAssociates). Mice were individually housed and wheels were placed in home cages, and total distance was determined automatically by system software starting at 5:00 PM local time and ending 24 hours later.
2.4. Quantitation of intramuscular NAD+ levels
Approximately 50 mg of gastrocnemius muscle tissue from the three 15-month old experimental mouse groups and a cohort of 7-month old male mice was homogenized using a bullet blender (NextAdvance, Troy, NY, USA) in a lysis buffer that was included in a NAD+ colorimetric assay kit (Biovision Milpitas, CA, USA). NAD+ was subsequently assessed using the kit according to the manufacturer’s instructions.
2.5. Muscle histology
NADH histological analysis of gastrocnemius muscle was performed as described previously [15]. Briefly, 10 μm frozen muscle sections were placed in a solution containing 1 mg/ml Nitro Blue Tetrazolium (VWR #TCD0844), 1 mg/ml NADH (Sigma, St. Louis, MO), and 0.2 M Tris-HCl buffer at pH 7.4 for 45 minutes at 37° C. Sections were immersed in a succession of acetone baths (30%, 60%, 90%, 60%, 30% v/v), rinsed in distilled water for 1 minute, and dehydrated by progressively immersing for 1 minute each in ethanol (95%, 100%, 100% v/v) and subsequently xylene (2 × 100% v/v), before finally mounting on a cover slip with Cytoseal (Fisher #23–244257). An investigator, blinded to the identity of the mice, identified and tallied fiber types and also measured cross sectional area (CSA) of the fibers using Motic software (Motic, Hong Kong).
2.6. Mitochondrial biomass
Total DNA was isolated from anterior tibialis muscle using a Qiagen Tissue Quick mini-prep kit (Qiagen, Germantown, MD). Primers were designed to amplify mitochondrial DNA (forward: 5’- CCGCAAGGGAAAGATGAAAGA-3’, reverse: 5’-TCGTTTGGTTTCGGGGTTTC-3’) and nuclear DNA (hexokinase gene, forward: 5’-CCCTGTCATGTCCCTTTGTT-3’, reverse: 5’GCCACCAGCTCAGTTAAAGG-3’) and then amplified using quantitative PCR (LightCycler 2.0, Roche).
2.7. Western blot analysis
Approximately 50 mg of gastrocnemius muscle were homogenized in ice cold extraction buffer (20 mM HEPES, 1 mM EDTA, 5 mM EGTA, 1.5 mM NaVO4, 10 mM MgCl2, 50 mM glycerolphosphate, 2 mM DTT, 10 mM NaF, 1% Triton X-100, 100 μM PMSF, pH 7.4), and protein concentration was determined using Bradford assay. Proteins were resolved by protein electrophoresis by applying 30 μg of each sample onto a 10 % Biorad gel (Ready Gel Tris-Hcl -101–1394). Assessment of western blot expression was performed using overnight incubation of sample with antibodies as follows: Sirt1 (Millipore: Cat# 07–131; Lot# 2956795), PGC-1α (Novus Biologicals: Cat# NBP1–04676; Lot# G-5), Sirt3 (Cell Signaling Technology: Cat# 5490; Lot# 2), Mitochondrial complexes I–V (Abcam: Cat# ab110413; Lot# N1912), and GAPDH (Abcam, Cat# ab181602; Lot# GR194633-12). Secondary antibodies include anti-rabbit (Sigma: Cat# A0545; Lot# 017M4850V) and anti-mouse (Abcam: Cat# ab6789; Lot# GR3257574-1): Band intensities were quantified using ImageJ software (NIH).
2.8. Creation of mouse myoblast cell lines
Mouse skeletal muscle myoblasts were isolated from the hindlimb of C57BL6 mice at the age of 12 months according to our previously published protocol [18]. The cells were cultured in proliferation medium composed of high glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Grand Island, NY), 20% fetal bovine serum (FBS, Atlanta Biologicals, Flowery Branch, GA), 10% horse serum (HS, Gibco), 0.5% chicken embryo extract (CEE, Accurate Chemical and Scientific, Westbury, NY), 2.5 ng/ml bFGF (ORF Genetics, Iceland), 10 μg/ml gentamycin (Gibco), and 1% Antibiotic-Antimitotic (AA, Gibco), and 2.5 μg/ml Plasmocin prophylactic (Invitrogen, San Diego, CA). Differentiation medium (DM) contained DMEM with high glucose, 5% HS and 1% AA. The mouse myoblasts were differentiated to multinucleated myotubes by seeding them at the high density of 50k cells/cm2 and changing the media to differentiation media composed of high glucose DMEM supplemented with 5% HS and 1% AA for 5 days. To assess the effects of NR on the myoblasts or myotubes, the cells were treated with proliferation or differentiation media supplemented with NR at 0.5 mM concentration for 24 hours.
2.9. Human myoblast cell lines:
Human skeletal muscle myoblasts from 25 year old female, 75 year old female, and 68 year old male donors (Cook Myosite Inc., Pittsburgh, PA) were cultured in human skeletal muscle growth medium (HSGM) composed of low glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Grand Island, NY) supplemented with 10% (v/v) fetal bovine serum (FBS, Athlanta Biologicals, Flowery Branch, GA), 10 ng/ml Epidermal Growth Factor (EGF, Lonza, Allendale, NJ), 1 ng/ml Fibroblastic Growth Factor (FGF, Isokine, Kópavogur, Iceland), 10 μg/ml insulin (Sigma-Aldrich, St. Louis, MO), 50 μg/ml fetuin (Sigma-Aldrich), 0.2 ug/ml dexamethasone (VEDCO, Saint Joseph Missouri), 10 μg/ml gentamycin (Gibco), 1% Antibiotic-Antimitotic (AA, Gibco, Grand Island, NY), and 2.5 μg/ml Plasmocin prophylactic (Invitrogen, San Diego, CA). To differentiate human myoblasts to multinucleated skeletal muscle myotubes, the cells were seeded at the high density of 50k cells/cm2, and two days after seeding the media was changed to differentiation media composed of high glucose DMEM supplemented with 10 μg/ml insulin, 500 μg/ml Bovine Serum Albumin (BSA, VWR International, Radnor, PA), 10 ng/ml EGF, 10 μg/ml gentamycin, 1% AA, and 2.5 μg/ml Plasmocin prophylactic for 7 days.
2.10. Cellular energetics analysis
Human and mouse myoblast and myotube cellular energetics were determined using the XFe24 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA). The cells were seeded in a Seahorse 24-well tissue culture plate in an optimized concentration of approximately 7,000 cells per well for both mouse and human derived muscle stem cells. To evaluate the impact of NR on mitochondrial respiration, NR was added at a concentration of 0.5 mM to the cells 24 hours prior to measurement for myoblasts and 24 and 72 hours prior to measurement in mature myotubes. Basal oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were detected in the presence of physiological concentrations of 10 mM glucose, 1 mM pyruvate and 2 mM glutamine. Next, mitochondrial ATP dependent respiration, uncoupled respiration, and non-mitochondrial respiration were assessed after the addition of 1 μM oligomycin, 1.5 μM carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP) and the combination of 0.5 μM each of antimycin A and rotenone, respectively. All experiments were performed using 5 wells per treatment and repeated for the three donors.
2.11. Immunostaining:
The cells were fixed in 10% natural buffered formalin for 10 min at room temperature (RT), permeabilized with 0.1% (v/v) triton X-100 in PBS for 10 min at RT, and blocked with the blocking buffer composed of 5% (v/v) goat serum and 0.01% (w/v) triton X-100 in PBS at RT for 1 h. The fixed cells were then incubated overnight at 4 °C with the following primary antibodies diluted in blocking buffer, myosin heavy chain (MYHC, 1:1000 dilution, Millipore, Billerica, MA), Myogenin (MYOG, 1:200 dilution, Cell Signaling, Danver, MA). Subsequently, the cells were stained with Alexa Fluor 594 or 488 conjugated goat anti-mouse or goat anti-rabbit antibodies (Thermofisher Scientific) at 1:200 dilution in blocking buffer for 1hr. To visualize the nuclei, the cells were counter stained with Hoechst 33342 nuclear dye (Thermo Fisher Scientific) at 1:500 dilution in PBS for 5 min. The images were taken using Zeiss Axio Observer Z1 (LSM 510; Zeiss, Oberkochen, Germany) equipped with digital camera (ORCA-ER C4742-80; Hamamatsu, Bridgewater, NJ). The intensity of the signal, myotube diameter, and myotube area were quantified using ImageJ software. Fusion index was measured according to the formula .
2.12. Statistics
For in vivo experiments, the statistical analysis was performed using XLStat statistical software (Addinsoft, New York, NY). Where applicable, groups were compared using one-way (treatment) or two-way ANOVA (treatment × baseline-endpoint comparisons), followed by Tukey’s post-hoc analysis to examine between and within group comparisons. All data were screened for outliers using a Grubbs outlier test with alpha equal to 0.05. The statistical significance of cell culture experiments was measured by one-way ANOVA with Fisher’s least significant difference post-hoc analysis using Minitab software version 18 and two tailed unpaired Student’s t-test using GraphPad software. Each in vitro experiment was repeated for three independent donors to ensure repeatability across different donors. All data are presented as mean ± standard deviation and the cut-off for significant comparisons was p < 0.05.
3.0. Results and Discussion
3.1. One month of NR supplementation increases skeletal muscle NAD+ levels in middle-aged mice.
NR supplementation is a promising intervention for skeletal muscle decline, and we initially set out to determine if a short 4-week treatment course affects body weight and body composition in middle-aged mice (15-months of age, which are approximately equivalent to 50 year old humans [19]). Interestingly, our data reveal that our higher dose of NR (NR600) at 600 mg/kg/day significantly reduced body weight in mice (p=0.0304, Figure 1A), while our lower dose (NR300) and placebo (PLB) treated mice did not exhibit significant weight loss (p=0.10 and 0.08, respectively, Figure 1A). Although the loss of body weight was modest in this study, there was no report of weight differences in normal weight mice in four other studies [9, 20–22]. However, these findings involved young lean mice, and NR did reduce body weight in high fat diet fed mice [9, 22]. This raises the possibility that the effect of NR on body weight may be masked by a floor effect in young mice, whereas we were able to observe some impact of the higher dose in our middle-aged mice. Interestingly, despite the observed weight losses, we did not observe significant decline in either body fat % or lean mass % in any of the groups (Figure 1B). Although NR reportedly reduced body fat in high fat fed mice [9], the middle aged mice in this study may not have had sufficient body fat to observe this effect in the time frame of our study.
Figure 1. Nicotinamide riboside supplementation decreases body weight and increases muscle NAD+ content in middle-aged mice.
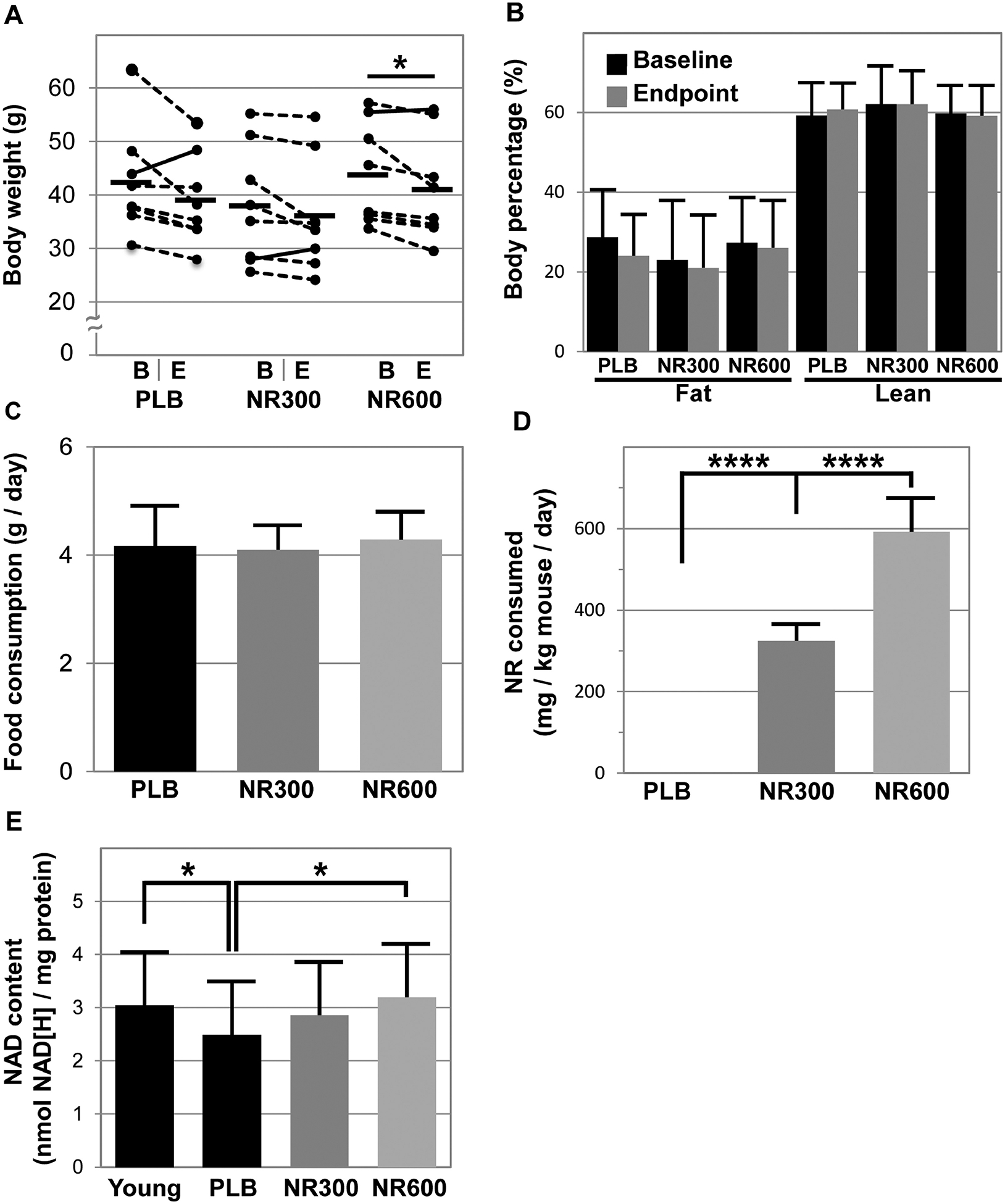
Fifteen-month-old C57BL/6J male mice were given placebo (PLB), or NR at 300 (NR300) or 600 (NR600) mg/kg/day in chow for four weeks (n=8). Body weight (A) and body composition (B) via qMR were measured at baseline (“B”) and at endpoint (“E”). Daily food consumption (C) was calculated from weekly measurements of food hopper weight and was further used to calculate total NR consumed (D). After the treatment, gastrocnemius muscles were harvested rom these mice and young 7-month old mice (n=10) and extracts were analyzed for NAD+ content using a colorimetric assay (E). A “*” denotes p < 0.05 and “****” denotes p < 0.0001.
Additionally, NR supplementation did not appear to alter food consumption, with mice in all groups consuming roughly 4 g of chow per day (Figure 1C). Based upon the NR content in the chow, we calculated the mice consumed the expected dose of approximately 300 and 600 mg/kg per day for the NR300 and NR600 groups, respectively (Figure 1D). Furthermore, we set out to determine whether gastrocnemius muscle NAD+ content was increased (Figure 1E). We first identified younger mice (7-months old) have greater muscle NAD+ content than the middle-aged mice (15-months) used in this study (young: 3.04 ± 0.51 nmol NAD[H]/mg protein versus 15-month PLB: 2.49 ± 0.21 nmol NAD[H]/mg protein, p=0.047). NAD+ content was greater in NR600 than PLB (NR600: 3.2 ± 0.50 nmol NAD[H]/mg protein, p=0.012 versus PLB), while the content in the muscles of NR300 was not statistically different from any of the other groups (NR300: 2.86 ± 0.38 nmol NAD[H]/mg protein, p=0.33 versus PLB). In comparison, Canto et al. demonstrated 400 mg/kg/day given for just one week or for six weeks significantly increased NAD+ content in muscles of 2-month old mice [9]. Given the findings by Canto et al, it is unclear why the NR300 group did not exhibit an increase in NAD+ content in the muscles of our mice, although we do note that Frederick et al. did not observe an increase in muscle NAD+ levels in 6-month old mice given 400 mg/kg/day for 6 weeks [23].
3.2. One month of NR increases treadmill endurance and open field activity as well as maintains grip strength.
NR was previously shown to increase treadmill performance in wild-type aged mice (≥ 24 months), but not in young mice (< 6 months) [9, 10, 13]. Here we investigated whether our short treatment regimen increases treadmill performance in middle-aged mice (15 months of age) as well as other aspects of physical performance. Following 4 weeks of NR treatment we observed increases in both the NR300 mice (baseline: 28.4 ± 10.2 min, endpoint: 37.7 ± 12.5 min, p=0.0055) and NR600 mice (baseline: 25.6 ± 7.3 min, endpoint: 33.8 ± 9.3 min, p=0.0311), while the PLB group did not significantly improve (baseline: 26.2 ± 7.0 min, endpoint: 30.2 ± 10.3 min, p=0.4970) (Figure 2A). We note that the improved performance of the NR600 mice was not significantly different than that seen in the NR300 mice. These data suggest a limit to the response of increasing NR dose. Younger mice with a baseline greater physical performance and higher levels of NAD+ than older mice may not exhibit as much improvement in treadmill performance with NR supplementation [9, 10, 13] than do middle-aged and older mice [13]. However, the NR supplementation did improve treadmill performance in young mice in models of muscular dystrophy [12], mitochondrial diseases [10], nicotinamide phosphoribosyltransferase (NAMPT) knock-out [23], and high fat diet feeding [9], although it was unclear if the lower muscle NAD+ levels observed in obese mice were statistically significant [9]. Together, our data and the reported literature suggest that NAD+ enhancement appears to augment performance in middle-age to older animals, but supplementation may not yield benefits in younger animals.
Figure 2. Four weeks of NR supplementation enhances physical performance.
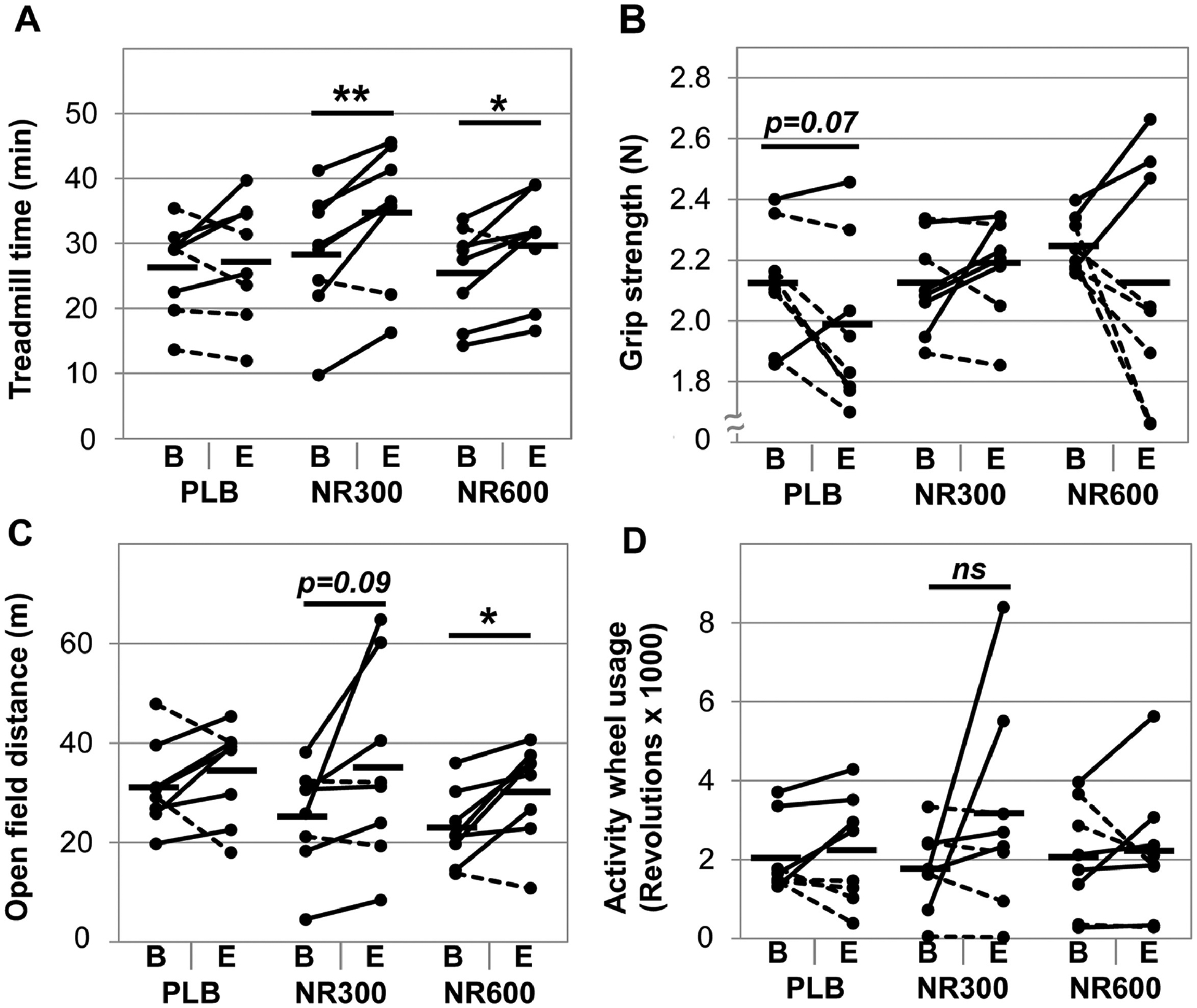
Physical performance was assessed at baseline (B) and at endpoint (E) after 4 weeks of NR supplementation. The assessments included treadmill endurance as the device accelerates from 5 to 35 m/min over 60 minutes (A), grip strength as the best 3 of 5 trials (B), total activity wheel usage over a 24-hour period (C), and total distance covered in an open field arena over a 30-minute period (D). A “*” denotes p < 0.05, “**” denotes p<0.01, and “ns” denotes a non-significant comparison, n=8 per group.
Furthermore, we set out to determine if short-term treatment with NR affects other aspects of physical performance including strength and activity levels. We found that NR, regardless of dose, did not affect grip strength (Figure 2B). Six weeks of NR treatment was previously shown to enhance grip strength in aged mice, but not in young mice [13]. Grip strength was also not improved in 22-month old mice treated for one week with nicotinamide mononucleotide (NMN), which also increases NAD+ [24]. Consequently, our mice may not have been old enough or treated long enough to experience the strength benefits of NR. Interestingly, we did observe improvement in open field activity in the NR600 group (baseline: 22.7 ± 0.8 m, endpoint: 30.5 ± 1.0 m, p=0.0175), while the NR300 group was trending (p=0.0871, Figure 2C). We did not observe an increase in activity wheel usage by the mice treated with NR (Figure 2D). One possibility for this discrepancy is that while both assessments capture self-motivated activity in mice, the open-field involves both motivation and exploration, which may require greater cognitive contribution. Although this possibility is outside the scope of this experiment, NR appears to improve cognition in aged mice and in mouse models of Alzheimer’s Disease [25–27].
3.3. NR supplemented mice exhibit greater slow twitch fiber cross sectional area, but no difference in mitochondrial biomass.
To investigate the impacts of NR supplementation on skeletal muscle, we performed histological analysis on gastrocnemius muscle using NADH staining. NR600 treated mice exhibited larger slow twitch dark stained fibers (PLB: 2001.5 ± 460.0 μm2; NR300: 2490.7 ± 676.1 μm2, p=0.25 versus PLB, NR600: 2782.3 ± 632.7 μm2, p=0.041 versus PLB, Figure 3A,B). However, we did not detect any differences between placebo and NR treated mice in the cross sectional area (CSA) of the faster twitch light stained fibers. While the benefits of NR supplementation on fiber size were reported previously in dystrophic mice [12] and NAMPT knockout mice [23], to our knowledge our study provides the first characterization of the impacts of NR on specific fiber types. Interestingly, despite the increase in the size of slower twitch aerobic fibers, we did not identify differences in fiber distribution (Figure 3C) or mitochondrial biomass (Figure 3D). Although these findings are contrary to other studies demonstrating that NR supplementation increases mitochondrial biomass [28], this may be due to the shorter treatment period of our study compared to these others. Additionally, no increase in biomass was observed by Cerutti et al. during a 4-week treatment with NR in a mouse model of mitochondrial disease [10], which may exhibit a different response to NR than do wild-type mice. We then examined underlying muscle expression levels of pro-mitochondrial regulators PGC-1α and SIRT1 and SIRT3 as well as the expression of mitochondrial complexes I–V (Figure 3E). Surprisingly, we found that none of these markers were differentially expressed, although Sirt1 and mitochondrial complexes I and II trended lower in NR600 treated mice (p = 0.22, 0.11, and 0.17, respectively). Although Cerutti et al. reported higher complex I, II, and V mRNA expression in young mice treated four 4 weeks with NR, they did not observe increased protein levels [10]. Increased mitochondrial complex content was observed with NR treatment of muscular dystrophy model (MDX) mice [12], which may suggest that our failure to see changes in these proteins may be due to a ceiling effect and that the mice may need to be of more advanced age or a genetic alteration to see differences due to treatment.
Figure 3. NR supplementation increases slower twitch fiber size, but did not enhance mitochondrial biomass.
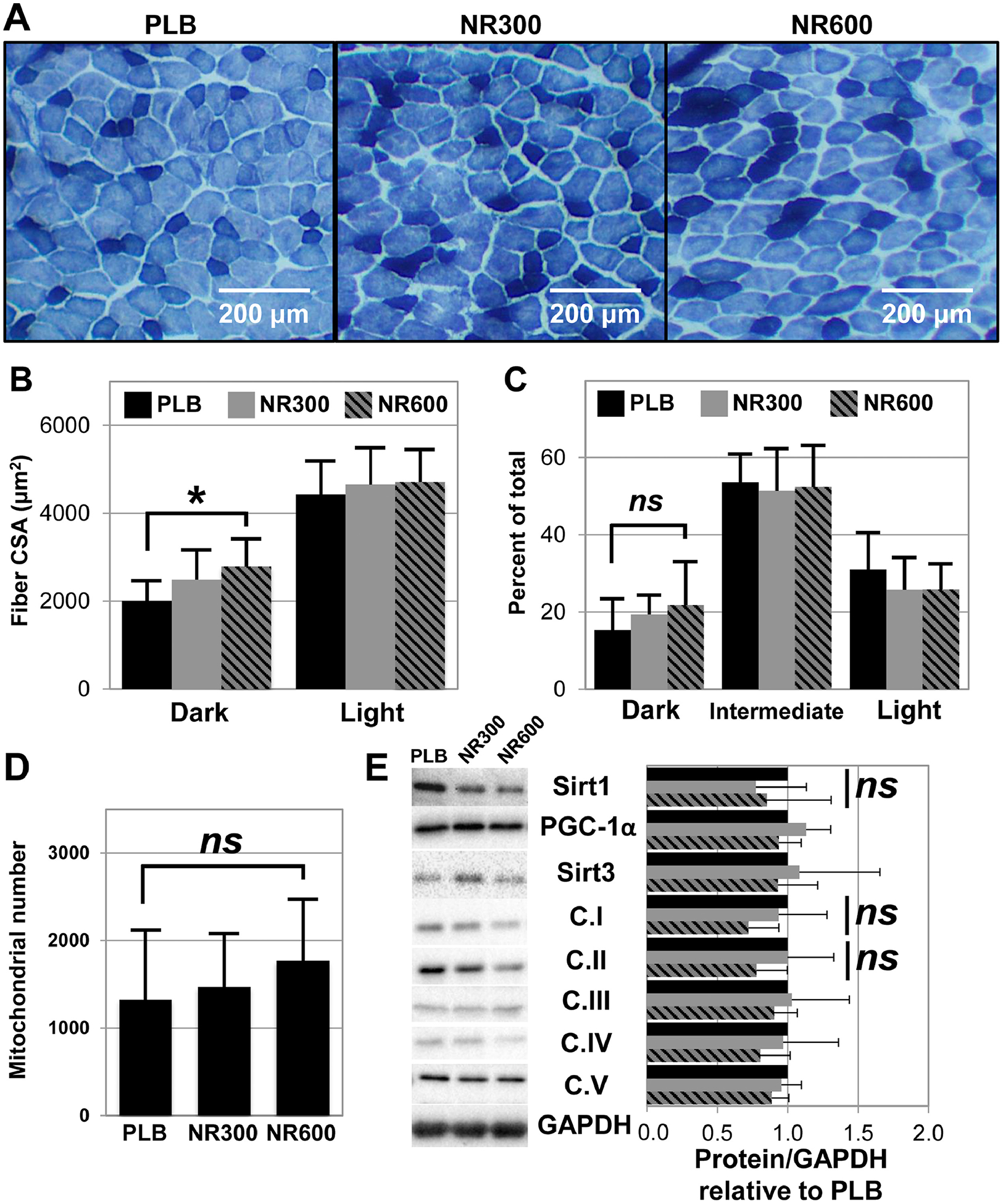
Gastrocnemius muscle was harvested from the mice following 4 weeks of treatment and analyzed using NADH staining (A). Microscopic images were than analyzed for differences in muscle fiber cross sectional area (B) and the number of dark, intermediate, and light stained fibers (C) using ImageJ software. DNA extracts were also prepared from the tibialis anterior muscle and used to determine differences in mitochondrial number using qPCR (D). Finally, protein extracts were generated from the harvested gastrocnemius muscle and probed for Sirt1, Pgc-1α, Sirt3, and the mitochondrial complexes with the appropriate antibodies via western blot (E). A “*” denotes p < 0.05 and “ns” denotes a non-significant comparison, n=8 per group.
3.4. NR boosts aerobic and anaerobic cellular energetic activity in both mouse and human muscle stem cells.
We next set out to explore the possibility that the improved physical performance was due to improved muscle differentiating potential and energy utilization. We examined whether NR administration alters the energetics of mouse derived myogenic progenitors in vitro and found that NR treatment for 24 hours shifts the cells into a higher energetic state, increasing both basal aerobic (oxygen consumption rate – OCR, p=0.0241, Figures 4A,B) and anaerobic respiration (extracellular acidification rate - ECAR, p=0.0060, Figures 4A,C). Next we treated the cells with oligomycin (which inhibits complex V in mitochondria and decreases electron flow through the electron transport chain resulting in reduction of mitochondrial respiration), FCCP (which is an uncoupling agent that collapses the proton gradient so the electrons flow through the electron transport chain), and finally a mixture of Rotenone and Antimycin A (which blocks complex I and complex III and shuts down the mitochondria) and subsequently measured the OCR and ECAR after each injection (Figures 4B,C). We calculated basal, maximal, and reserve values for both aerobic and anaerobic respiration accordingly (Figures 4D–G) and found that NR increases respiration of these mouse progenitors cells, including aerobic (basal: Veh: 229.7 ± 64.2 pmol/min versus NR: 365.9 ± 55.5 pmol/min, p=0.0019, and maximal: Veh: 536.3 ± 178.4 pmol/min versus NR: 906.2 ± 209.8 pmol/min, p=0.0055, Figure 4F) and anaerobic (basal: Veh: 19.3 ± 3.9 mpH/min versus NR: 26.0 ± 3.3 mpH/min, p=0.0069, and maximal: Veh: 46.0 ± 9.5 mpH/min versus NR: 59.1 ± 8.1 mpH/min, p=0.0221, Figure 4G). NR increases aerobic reserve (p = 0.0102, Figure 4F), but not anaerobic reserve (p = 0.0806, Figure 4G).
Figure 4. NR enhances aerobic and anaerobic respiration in both mouse and human muscle stem cells.
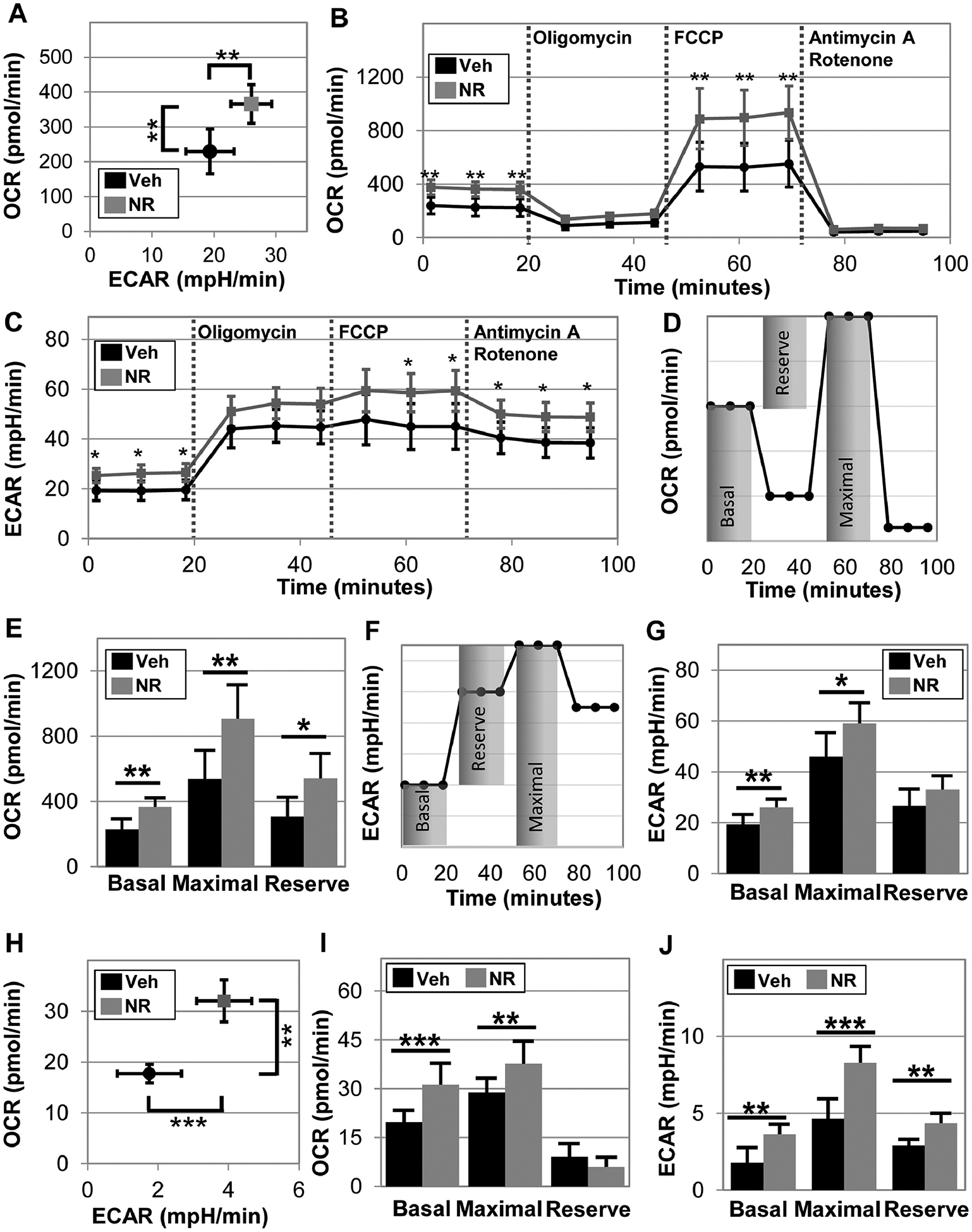
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured in mouse derived stem cells using a seahorse extracellular flux analyzer following 24 hours of 0.5 mM NR treatment (A). In the same experiment, Oligomycin, FCCP, Antimycin A and Rotenone were applied in the assay to determine maximal and reserve respiration of the mouse stem cells (B) and maximal and reserve ECAR (C). The schematics for calculation of basal, maximal, and reserve values for OCR (D) and ECAR (E). Next, this methodology was applied to human derived myoblast. OCR vs. ECAR graph suggests that NR treatment shifts human myoblasts toward a more energetic phenotype (H). Basal, maximal, and the reserve (difference between basal and maximal) aerobic (I) and anaerobic (J) respiration were determined following 24 hours of NR treatment. Data represents the mean of 4 to 5 wells per condition, which each experiment performed for three different donors to ensure repeatability. Statistical significance indicated by “*” p < 0.05, “**” p < 0.01, and “***” p < 0.001.
We next examined whether human derived myogenic progenitors also exhibit the same response to NR, regardless of age or sex, by using muscle stem cells derived from both old and young and male and females. We found that again NR induces a more energetic phenotype (Figure 4H) and markedly increases both basal and maximal aerobic respiration (basal: veh: 19.1 ± 3.6 pmol/min versus NR: 31.2 ± 6.6 pmol/min, p=0.0002, and maximal: veh: 28.9 ± 4.3 pmol/min versus NR: 37.7 ± 6.9 pmol/min, p=0.0079, Figure 4I). Furthermore, NR boosts both basal and maximal anaerobic respiration (basal: veh: 1.8 ± 1.0 mpH/min versus NR: 3.6 ± 0.7 mpH/min, p=0.0018, and maximal: veh: 4.7 ± 1.3 mpH/min versus NR: 8.3 ± 1.1 mpH/min, p=0.0005, Figure 4J), in addition to NR treated cells exhibiting a greater glycolytic reserve (veh: 2.9 ± 0.4 mpH/min versus NR: 4.4 ± 0.6 mpH/min, p = 0.0020, Figure 4J). NR did not affect population doubling time in myoblasts (NR: 43 ± 9 hours versus veh: 39 ± 5 hours, p=0.4, Figure S1), suggesting the increase in cellular energetics is not due to differences in cell numbers. In line with our observations that NR supplementation did not increase mitochondrial biomass in vivo, we did not observe a significant change in the mitochondrial membrane potential by staining for TMRM and Mitotracker nor a significant change in the mitochondrial superoxide by staining for MitoSOX (Figure S2) when treating myogenic progenitors in vitro.
3.5. Treatment of myoblasts with NR augments myotube formation during differentiation.
Given the increase in human myoblast energetics in response to NR treatment (Figure 4G–H) and previous reports on the improvement of mouse muscle regeneration upon NR administration [13], we next examined if NR affects human myoblast differentiation into skeletal muscle myotubes. We differentiated human derived muscle stem cells, with or without NR at 0.5 mM for 7 days and assessed myotube formation by immunostaining for myosin heavy chain and desmin intermediate filaments, both markers of skeletal muscle differentiation (Figure 5A&B). NR induced a marked increase of myotube diameter (NR: 49.6 ± 6.5 μm versus Veh: 42.2 ± 2.3 μm, p=0.0412, Figure 5C), fusion index (NR: 67.2 ± 2.1% versus Veh: 62.2 ± 2.8%, p=0.0127, Figure 5D), and expression of myosin heavy chain (p=0.001, N=5, Figure 5E) and desmin (p=0.01, N=10, Figure 5E). These results suggest that NAD+ augmentation is important in the differentiation of human myogenic progenitors to skeletal muscle and marks the first report on the impacts of NR treatment of human muscle progenitors. Previous studies on mouse myogenic progenitors testify to the importance of NAD+ levels in SIRT1 transcriptional activity and metabolic reprogramming of myogenic progenitors to self-renew and differentiate [9, 11, 13, 29, 30]. Additionally, cytosolic [NAD+]/[NADH] ratio decreases during myogenic differentiation, and that decrease in NAD+ levels is essential to myogenic differentiation by modulating the deacetylase activity of SIRT1 [31].
Figure 5. NR boosts size and fusion in differentiating myoblasts.
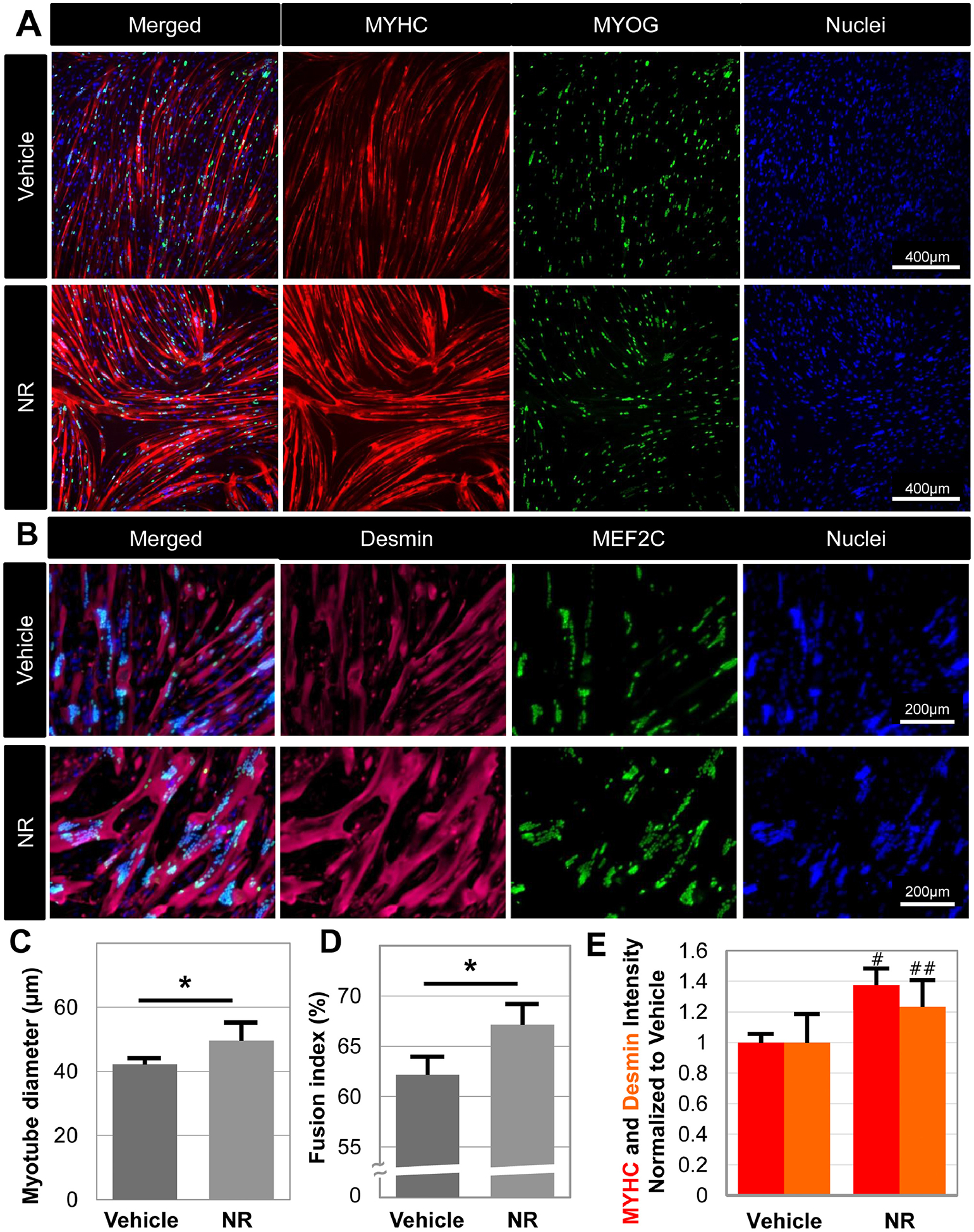
Human derived myoblasts were differentiated in the presence or absence of 0.5 mM NR for 7 days, and then immunohistochemistry assays were employed to visualize the expression of MYHC and MyoG (A) and desmin and Mef2C (B) in myotubes. Myotube diameter (C), fusion index (D), and the intensity of MYHC and Desmin (E) were measured using ImageJ software. Fusion index represents the percentage of nuclei observed within muscle fibers relative to all identified nuclei. Data represents the mean of 4 to 5 wells per condition, with each experiment performed for three different donors to ensure repeatability. Statistical significance indicated by “*” p < 0.05.
NAD+ may also be essential to genome stability as the DNA repair enzyme PARP1 requires NAD+ as a substrate to catalyze the formation of poly(ADP-ribose) polymers at DNA damage sites [11]. Myoblast fusion into multinucleated myotubes is associated with cellular stress and DNA strand breaks, and differentiating myoblasts activate DNA repair mechanisms to stabilize the genome and fuse successfully [32, 33]. Therefore, we speculate that NR supplementation improves the myogenic differentiation of human myoblasts by providing PARP-1 with more NAD+ substrate to fortify the DNA repair mechanisms that are necessary in the differentiation process, in addition to improving cellular energetics that provide the ATP required for these processes. This notion is further supported by others who have demonstrated that NAD+ supplementation improves DNA repair and synthesis [34, 35].
3.6. NR treatment of mature myotubes boosts cellular energetics, but not mitochondrial membrane potential or mitochondrial associated oxidative stress.
Mitochondrial number and activity is greatly increased upon myogenic differentiation, and the levels of oxidative phosphorylation are reported to be higher in multinucleated myotubes [29, 36]. Thus, we investigated whether NR treatment alters the mitochondrial function of mature myotubes. We differentiated human myoblasts into myotubes for 10 days, during which the cells were treated with NR for the last 3 days, only the last day, or remained untreated (vehicle control) (Figure 6A). Then, we investigated changes in mitochondrial membrane potential by TMRM staining and mitochondrial associated oxidative stress using MitoSOX staining (Figure S3), but found that neither was significantly altered upon NR treatment. These findings agree with the in vivo observations showing no changes in mitochondrial biomass due to NR treatment. However, similar to our NR treated myoblast cells (Figure 4), three days of NR treatment increased cellular energetics of the mature myotubes (Figure 6B, C). Three days of NR treatment markedly increased OCR (p = 0.0069 3 days versus Veh, Figure 6B), whereas one day did not. Similarly, both maximal respiration and aerobic reserve also increased following 3 days of treatment (max respiration, p=0.0004 3 days versus Veh; aerobic reserve, p=0.0001 3 days versus Veh, Figure 6B). In contrast to undifferentiated myoblasts, only reserve ECAR increased after three days of NR treatment (p = 0.027 3 days versus Veh). We speculate that the smaller effect of NR on anaerobic respiration of myotubes versus myoblasts may be due to the limited contribution of glycolysis and more pronounced contribution of oxidative phosphorylation in ATP production in myotubes [36].
Figure 6. NR increases both aerobic and anaerobic respiration in mature myoblasts.
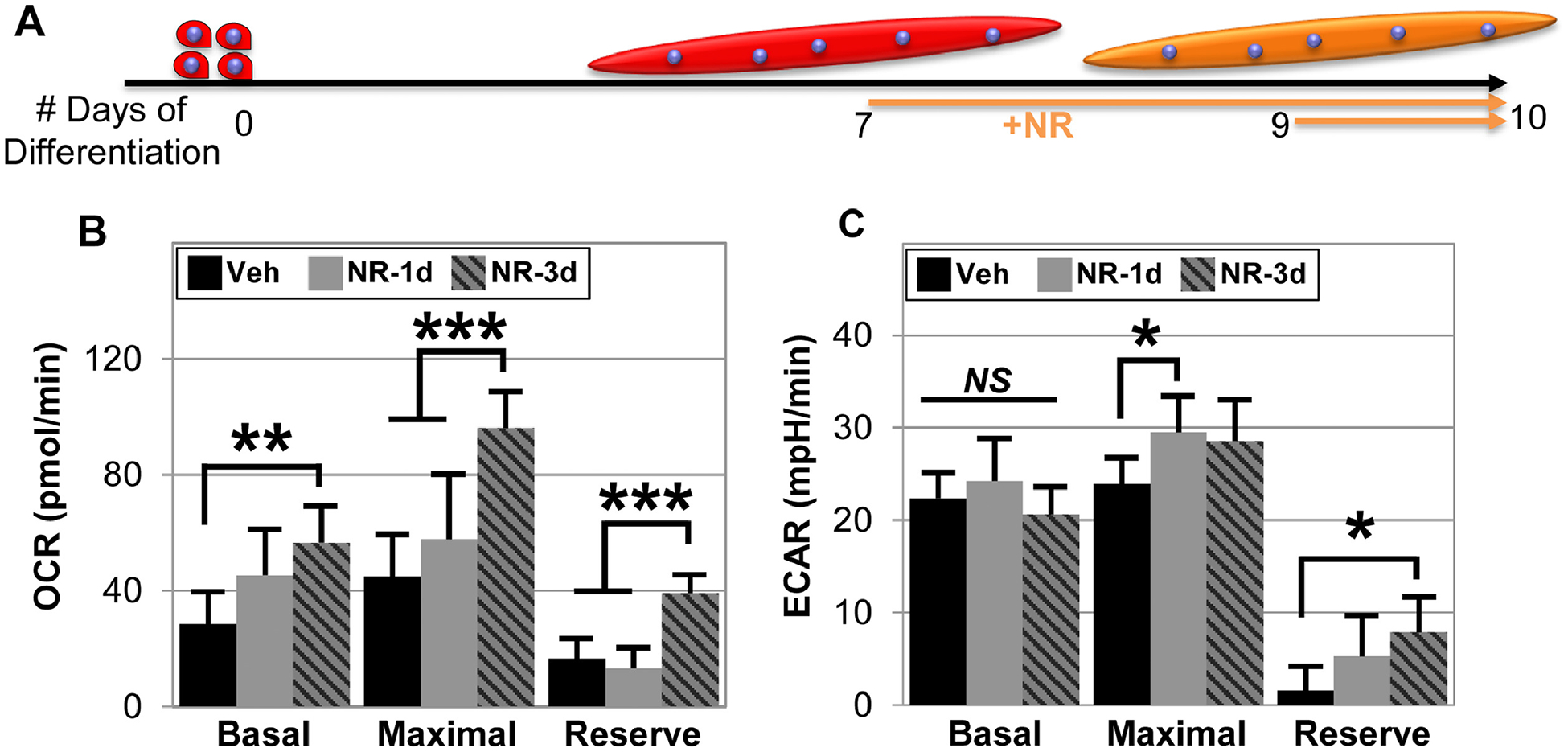
Schematic of how human derived myoblast were differentiated for 10 days into myotubes, while they were treated with 0.5 mM NR for the last 72 or 24 hours (A). The methodology explained in Figure 4 was used to measure aerobic respiration (B) and anaerobic respiration (C) using Seahorse extracellular flux analyzer. Data represents the mean of 4 to 5 wells per condition, with each experiment performed for three different donors to ensure repeatability. Statistical significance indicated by “*” p < 0.05, “**” p < 0.01, “***” p < 0.001, and “ns” indicating non-significance.
4.0. Conclusions
This study demonstrates that short term NR supplementation (4 weeks) increased muscle NAD+ content, treadmill performance, and open field activity of middle-aged mice. Interestingly, this treatment protocol increased the size of aerobic muscle fibers, but did not enhance mitochondrial biomass. NR treatment of both mouse and human derived myogenic progenitors boosted both aerobic and anaerobic, basal and maximal respiration. Additionally, the addition of NR improved the differentiation of myogenic progenitors toward multinucleated myotubes along with greater myofiber size, fusion index, and the expression of differentiation markers myosin heavy chain and desmin. When NR was added to mature myotubes, it increased both basal and maximal aerobic cellular respiration. These data suggest NR improves physical performance via increases in cellular energetics, and thus suggests that NR supplementation may be useful in enhancing muscle metabolism and physical performance.
Supplementary Material
Highlights.
Four weeks of NR supplementation enhances muscular performance and open field activity in middle aged mice.
NR increases slow twitch fiber cross sectional area, but four weeks of supplementation was not sufficient to increase mitochondrial biomass.
NR treatment improves the differentiating capacity of both mouse and human myogenic progenitors to form multinucleated myofibers in vitro.
The addition of NR boosts both aerobic and anaerobic respiration in mouse and human myogenic progenitors as well as in human myotubes, in vitro.
Acknowledgements
The authors express special thanks to Jill Madden for administrative assistance and helpful feedback in the preparation of this manuscript. The authors also wish to thank the University at Buffalo and the research service of the VA Western New York Healthcare System. Finally, we thank Chromadex for kindly gifting NR powder for use in this study.
Funding
This work was funded by Veteran Affairs Biomedical Laboratory Research and Development grant BX004369, National Institutes of Health grants (K07 AG060266 and NIH R56 AG065561), and the Indian Trail Foundation grant to Dr. Bruce Troen and a grant from the National Institutes of Health (R01 HL086582) to Stelios T. Andreadis.
Abbreviations:
- NR
nicotinamide riboside
- NAD+
nicotinamide adenine dinucleotide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Marty E, et al. , A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone, 2017. 105: p. 276–286. [DOI] [PubMed] [Google Scholar]
- 2.de Rezende LF, et al. , Sedentary behavior and health outcomes among older adults: a systematic review. BMC Public Health, 2014. 14: p. 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wullems JA, et al. , A review of the assessment and prevalence of sedentarism in older adults, its physiology/health impact and non-exercise mobility counter-measures. Biogerontology, 2016. 17(3): p. 547–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease, C. and Prevention, State-specific prevalence of no leisure-time physical activity among adults with and without doctor-diagnosed arthritis--United States, 2009. MMWR Morb Mortal Wkly Rep, 2011. 60(48): p. 1641–5. [PubMed] [Google Scholar]
- 5.Johnson S and Imai SI, NAD (+) biosynthesis, aging, and disease. F1000Res, 2018. 7: p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement J, et al. , The Plasma NAD(+) Metabolome Is Dysregulated in “Normal” Aging. Rejuvenation Res, 2019. 22(2): p. 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massudi H, et al. , Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One, 2012. 7(7): p. e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu XH, et al. , In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci USA, 2015. 112(9): p. 2876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canto C, et al. , The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab, 2012. 15(6): p. 838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerutti R, et al. , NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab, 2014. 19(6): p. 1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goody MF and Henry CA, A need for NAD+ in muscle development, homeostasis, and aging. Skelet Muscle, 2018. 8(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu D, et al. , NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med, 2016. 8(361): p. 361ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, et al. , NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science, 2016. 352(6292): p. 1436–43. [DOI] [PubMed] [Google Scholar]
- 14.Kourtzidis IA, et al. , The NAD(+) precursor nicotinamide riboside decreases exercise performance in rats. J Int Soc Sports Nutr, 2016. 13: p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seldeen KL, et al. , High Intensity Interval Training Improves Physical Performance and Frailty in Aged Mice. J Gerontol A Biol Sci Med Sci, 2018. 73(4): p. 429–437. [DOI] [PubMed] [Google Scholar]
- 16.Seldeen KL, et al. , High intensity interval training improves physical performance in aged female mice: A comparison of mouse frailty assessment tools. Mech Ageing Dev, 2019. 180: p. 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seldeen KL, et al. , Short Session High Intensity Interval Training and Treadmill Assessment in Aged Mice. J Vis Exp, 2019(144). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahini A, et al. , Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Research, 2018. 30: p. 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flurkey K, Currer JM, Harrison DE, Mouse models in aging research, in Faculty Research 2000–20009, B.S Fox JG, Davisson MT, Newcomer CE, Quimby FW, Smith AL, Editor. 2007, Elsevier. [Google Scholar]
- 20.Crisol BM, et al. , Nicotinamide riboside induces a thermogenic response in lean mice. Life Sci, 2018. 211: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, et al. , Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet. Mol Nutr Food Res, 2017. 61(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trammell SA, et al. , Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci Rep, 2016. 6: p. 26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frederick DW, et al. , Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab, 2016. 24(2): p. 269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes AP, et al. , Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 2013. 155(7): p. 1624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong B, et al. , Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging, 2013. 34(6): p. 1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou Y, et al. , NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci USA, 2018. 115(8): p. E1876–E1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie X, et al. , Nicotinamide ribose ameliorates cognitive impairment of aged and Alzheimer’s disease model mice. Metab Brain Dis, 2019. 34(1): p. 353–366. [DOI] [PubMed] [Google Scholar]
- 28.Mouchiroud L, et al. , The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell, 2013. 154(2): p. 430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abreu P, Bioenergetics mechanisms regulating muscle stem cell self-renewal commitment and function. Biomed Pharmacother, 2018. 103: p. 463–472. [DOI] [PubMed] [Google Scholar]
- 30.Ryall JG, et al. , The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell, 2015. 16(2): p. 171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulco M, et al. , Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell, 2003. 12(1): p. 51–62. [DOI] [PubMed] [Google Scholar]
- 32.Al-Khalaf MH, et al. , Temporal activation of XRCC1-mediated DNA repair is essential for muscle differentiation. Cell Discov, 2016. 2: p. 15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dibenedetto S, et al. , Enhanced Energetic State and Protection from Oxidative Stress in Human Myoblasts Overexpressing BMI1. Stem Cell Reports, 2017. 9(2): p. 528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai P, et al. , PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab, 2011. 13(4): p. 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sims JL, Berger SJ, and Berger NA, Effects of nicotinamide on NAD and poly(ADP-ribose) metabolism in DNA-damaged human lymphocytes. J Supramol Struct Cell Biochem, 1981. 16(3): p. 281–8. [DOI] [PubMed] [Google Scholar]
- 36.Fortini P, et al. , Coordinated Metabolic Changes and Modulation of Autophagy during Myogenesis. Front Physiol, 2016. 7: p. 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


