Significance
Epileptic encephalopathy (EE) is a devastating neurologic disorder characterized by early-onset seizures with severe cognitive and psychomotor impairments. EE is associated with dominant mutations in the KCNQ2 gene which encodes the Kv7.2 subunit of Kv7 potassium channels. Previously, we reported that multiple EE mutations in the intracellular calmodulin-binding domain of Kv7.2 decreased surface expression of axonal Kv7 channels critical for suppressing neuronal excitability. Here, we generated conditional knockin mice carrying one of these mutations, M547V. These mice displayed spontaneous seizures, cognitive impairment, neurodegeneration, and reactive astrogliosis, implicating abnormal Kv7 surface expression as a key etiology of KCNQ2-associated EE.
Keywords: KCNQ2, seizures, epilepsy
Abstract
Epileptic encephalopathy (EE) is characterized by seizures that respond poorly to antiseizure drugs, psychomotor delay, and cognitive and behavioral impairments. One of the frequently mutated genes in EE is KCNQ2, which encodes the Kv7.2 subunit of voltage-gated Kv7 potassium channels. Kv7 channels composed of Kv7.2 and Kv7.3 are enriched at the axonal surface, where they potently suppress neuronal excitability. Previously, we reported that the de novo dominant EE mutation M546V in human Kv7.2 blocks calmodulin binding to Kv7.2 and axonal surface expression of Kv7 channels via their intracellular retention. However, whether these pathogenic mechanisms underlie epileptic seizures and behavioral comorbidities remains unknown. Here, we report conditional transgenic cKcnq2+/M547V mice, in which expression of mouse Kv7.2-M547V (equivalent to human Kv7.2-M546V) is induced in forebrain excitatory pyramidal neurons and astrocytes. These mice display early mortality, spontaneous seizures, enhanced seizure susceptibility, memory impairment, and repetitive behaviors. Furthermore, hippocampal pathology shows widespread neurodegeneration and reactive astrocytes. This study demonstrates that the impairment in axonal surface expression of Kv7 channels is associated with epileptic seizures, cognitive and behavioral deficits, and neuronal loss in KCNQ2-related EE.
Epileptic encephalopathies (EEs) are a collection of heterogeneous disorders in which early-onset severe seizures contribute to developmental delay and progressive cognitive and behavioral impairments (1). Current treatments for EEs have limited efficacy in alleviating seizures and comorbidities (2), posing an urgent need to understand the etiology of EEs and find new therapeutic targets. Recent discoveries of epilepsy-related genes in multiple laboratories and through the large Epi4K, EpiPM, and EuroEPINOMICS-RES consortia have identified a diverse array of proteins that may contribute to epileptogenesis (3–5). Among them, dominant variants associated with benign familial neonatal epilepsy (BFNE) and EE have been found in KCNQ2 and KCNQ3 genes, which encode the Kv7.2 and Kv7.3 subunits of voltage-gated potassium (K+) channel subfamily Q (Kv7) (https://www.rikee.org; ClinVar Database, National Center for Biotechnology Information [NCBI]).
Neuronal Kv7 channels are mostly heterotetrameric channels of Kv7.2 and Kv7.3 subunits (6), which have overlapping distribution in the central nervous system including the cerebral cortex and hippocampus (7). In neurons, they are preferentially localized to the axonal plasma membrane with the highest concentration at the axonal initial segment (AIS) (7, 8), where the action potential (AP) initiates (9). They give rise to the slowly activating and noninactivating outward K+ current termed M current (IM) (6). Because they open at subthreshold potentials (6), IM potently suppresses AP firing (6, 10, 11), underscoring their critical roles in reducing neuronal excitability. By contrast, activation of Gq-coupled receptors, including muscarinic acetylcholine receptors, inhibits IM by depleting the lipid cofactor PIP2, resulting in enhanced AP firing (12).
To date, 193 dominant variants in KCNQ2 and 2 variants in KCNQ3 have been identified in patients with EE (https://www.rikee.org; ClinVar Database, NCBI). EE variants are clustered at the functional domains of Kv7.2 important for voltage-dependent opening of Kv7 channels (13) and typically decrease the function of heterotetrameric channels by 20 to 75% (13–17). EE variants are also enriched at helices A and B in the intracellular C-terminal tail of Kv7.2 (13, 14, 16), which mediate calmodulin (CaM) binding critical for axonal enrichment of Kv7 channels (18). Among these variants, a mutation of methionine at amino acid position 546 to valine (M546V) was found in a male patient who displayed drug-resistant neonatal tonic-clonic seizures and later developed profound intellectual and language disability, spasticity, and autistic behavior (15). While this mutation in helix B abolishes current expression of homomeric but not heteromeric channels in heterologous cells (14, 17), it severely reduces CaM binding and axonal surface expression of heteromeric channels in cultured hippocampal neurons (14). This mutation also induces ubiquitination and proteasomal degradation of Kv7.2, whereas the presence of Kv7.3 blocks this degradation and accumulates ubiquitinated Kv7.2 (14). However, whether these pathogenic mechanisms underlie epileptic seizures and behavioral deficits in EE remains unknown.
In this study, we investigated the contribution of the EE variant M546V by generating conditional transgenic mice in which heterozygous expression of mouse Kv7.2-M547V was induced in forebrain excitatory pyramidal neurons. M546 in the human Kv7.2 is conserved in the mouse Kv7.2 at amino acid position 547. These mice showed widespread neurodegeneration and reactive astrogliosis in the hippocampus and cortex, and displayed spontaneous seizures and cognitive deficit, providing a causal link between M546V-mediated disruption of axonal surface expression of Kv7 channels and KCNQ2-associated EE.
Results
cKcnq2+/M547V Mice Have Reduced Viability.
We used CRISPR-Cas–mediated genome engineering to generate a conditional knockin (KI) mouse line in the C57BL/6J background for the murine Kcnq2 gene (GenBank accession no. NM_010611.3) containing the mutation M547V, which corresponds to M546V in the long isoform of human Kv7.2 (GenBank accession no. NM_172107.4: c.1636A>G) and M518V in the short isoform (GenBank accession no. Y15065.1) and is located at the CaM contact site in helix B (Fig. 1A) (14, 17). This Kcnq2-M547Vfl/fl line contains a floxed-STOP cassette upstream of Kcnq2-M547V-ires-EGFP at the Gt(ROSA)26Sor locus (Fig. 1B). Upon removal of an upstream floxed-STOP cassette by Cre recombinase, this line expresses both Kcnq2-M547V and enhanced green fluorescent protein (EGFP) (Fig. 1B and SI Appendix, Fig. S1).
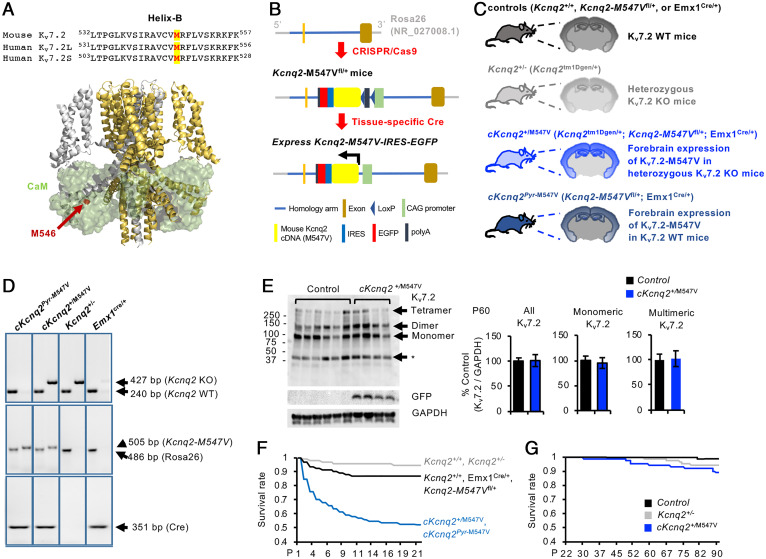
Fig. 1.
Conditional cKcnq2+/M547V mice display increased early mortality. (A, Top) Sequence alignment of helix B in which M547 in the mouse Kv7.2 (NP_034741.2), M546V in the human Kv7.2 long isoform (Kv7.2 L, NP_742105.1), and M518V in the human Kv7.2 short isoform (Kv7.2 S, NP_004509.2) are labeled in red and highlighted in yellow. (A, Bottom) The Cα atom of M546V is highlighted in red in one subunit of the cryo-EM structure of human Kv7.2 (ribbons) in complex with four CaM subunits (transparent green surfaces). (B) Schematic diagram (not to scale) showing the targeting strategy for conditional expression of Kv7.2-M547V in mice. In Kcnq2-M547Vfl/fl mice, the CAG-LoxP-Stop-LoxP mouse Kcnq2-M547V cDNA (NM_010611.3)-IRES-EGFP-polyA cassette was cloned into intron 1 of the ROSA26 allele by CRISPR-Cas–mediated genome engineering. Upon removal of an upstream floxed-STOP cassette by Cre recombinase, this line expresses the Kcnq2-M547V and EGFP transgene. (C) Schematic representation of coronal brain sections of the progenies that result from breeding conditional Kcnq2-M547Vfl/fl mice, heterozygous KO Kcnq2+/− mice (Kcnq2tm1Dgen/+), and the Emx1-ires-cre mice (Emx1cre/cre) that express Cre recombinase in forebrain excitatory pyramidal neurons from embryonic day 10.5. The cKcnq2+/M547V mice (Kcnq2-M547Vfl/+: Kcnq2tm1Dgen/+: Emx1cre/+) express Kcnq2-M547V-ires-EGFP in the forebrain excitatory pyramidal neurons of Kcnq2+/− mice, whereas cKcnq2pyr-M547V mice express this transgene in WT Kcnq2+/+ mice. (D) Representative example of a genotyping PCR using mouse tail genomic DNA as template. (E) Western blots of Kv7.2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the hippocampal homogenates (soluble S1 fractions) of control mice (Emx1cre/+) and cKcnq2+/M547V mice at P60. The identities of bands (asterisk) below 50 kDa are unclear. The cKcnq2+/M547V mice displayed similar hippocampal protein expression of Kv7.2 compared with control mice. The total number of mice used for quantification at P60: control (n = 6 including 3 males and 3 females) and cKcnq2+/M547V (n = 4 including 2 males and 2 females). Data are shown as mean ± SEM. Two-tailed Student’s t test was performed. Western blot quantification of Kv7.2 at P30 and P120 is shown in SI Appendix, Figs. S3 and S4. (F) Kaplan–Meier survival plots for all mice used in this study from P0 to P21. Out of 407 cKcnq2pyr-M547V and cKcnq2+/M547V mice, only 213 survived, including 128 cKcnq2pyr-M547V mice and 83 cKcnq2+/M547V mice. By contrast, 356 out of 409 control mice survived. Out of 282 Kcnq2+/+ and Kcnq2+/− mice, 267 survived, including 106 Kcnq2+/− mice and 161 Kcnq2+/+ mice. (G) Kaplan–Meier survival plots for control (n = 356), Kcnq2+/− (n = 106), and cKcnq2+/M547V (n = 83) from P22 to P90.
We chose this strategy because expression of this human EE mutant Kv7.2 causes cell death in HEK293T cells and hippocampal neuronal culture (14), and thus we anticipated high mortality of the KI progeny produced by homologous recombination of Kcnq2-M547V into the genomic Kcnq2 sequence. This strategy also allows us to express the Kcnq2-M547V-ires-EGFP transgene in heterozygous Kcnq2 knockout (KO) mice to induce heterozygous KI of Kcnq2-M547V (designated as cKcnq2+/M547V) or in wild-type (WT) mice in case heterozygous KI leads to high mortality (designated as cKcnq2pyr-M547V) (Fig. 1C).
We crossed Kcnq2-M547Vfl/fl mice to heterozygous Kcnq2 KO mice (Kcnq2tm1Dgen/+) (19) and Emx1-ires-cre (Emx1cre/cre) mice, which express Cre during neurogenesis in excitatory pyramidal neurons in the hippocampus and cortex (20), where endogenous Kv7.2 and Kv7.3 are highly expressed (7). The resulting progenies are cKcnq2+/M547V (Kcnq2-M547Vfl/+:Kcnq2tm1Dgen/+:Emx1cre/+), cKcnq2pyr-M547V (Kcnq2-M547Vfl/+:Emx1cre/+), Kcnq2+/− (Kcnq2tm1Dgen/+), and the WT control mice (Kcnq2+/+, Kcnq2-M547Vfl/+, and Emx1cre/+) (Fig. 1C). Their genotypes were confirmed by PCR of tail genomic DNA (Fig. 1D). Although a single copy of Kcnq2-M547V-ires-EGFP is expected to express in cKcnq2+/M547V mice, Kcnq2 messenger RNA level in cKcnq2+/M547V mice was 1.94-fold higher than that in control Emx1cre/+ mice (SI Appendix, Fig. S2), most likely due to the CAG promoter–driven expression of the transgene (Fig. 1B). Compared with control mice, the protein level of Kv7.2 in the hippocampi of cKcnq2+/M547V mice was twofold higher at postnatal day (P) 1, 45% lower at P30, and comparable at P60 and P120 (Fig. 1E and SI Appendix, Figs. S3, S4, and S5A).
Kaplan–Meier survival plots indicate that ∼40% of cKcnq2+/M547V mice died by P10 (Fig. 1F) despite the expected Mendelian inheritance at birth (SI Appendix, Fig. S5C). By contrast, Kcnq2+/− and control mice had minimal death (Fig. 1F). By P21, cKcnq2+/M547V mice also displayed abnormal behaviors including continuous jumping and digging in their home cage (Movies S1 and S2). Some showed motionless and rigid postures (Movie S3). Curiously, the high mortality of cKcnq2+/M547V mice was not observed from P21 (Fig. 1G). There were no obvious differences in gross brain appearance or size between genotypes at P30 and P120 (SI Appendix, Fig. S6 A and B). The surviving cKcnq2+/M547V males weighed less than control males at P120, but this genotype difference was not observed in females (SI Appendix, Fig. S6C).
cKcnq2+/M547V Mice Display Spontaneous Seizures and Extreme Seizure Sensitivity to Kainic Acid.
The patient with the de novo M546V mutation displayed drug-resistant neonatal tonic-clonic seizures (15). To test if spontaneous seizures occur in cKcnq2+/M547V mice, we performed simultaneous video monitoring of behavioral seizures and electroencephalogram (EEG) recording at P60 to P85 (Fig. 2 A–C). All cKcnq2+/M547V males displayed recurrent spontaneous behavioral seizures followed by death during recovery from electrode implantation surgery, precluding EEG recordings. Of six cKcnq2+/M547V females, three survived for EEG recording. Two mice demonstrated spontaneous electrographic seizures that coincided with stage 4 and 7 seizures (Fig. 2 B and C and Movies S4 and S5). By contrast, Kcnq2+/+ females did not show electrographic and behavioral seizures (Fig. 2A).
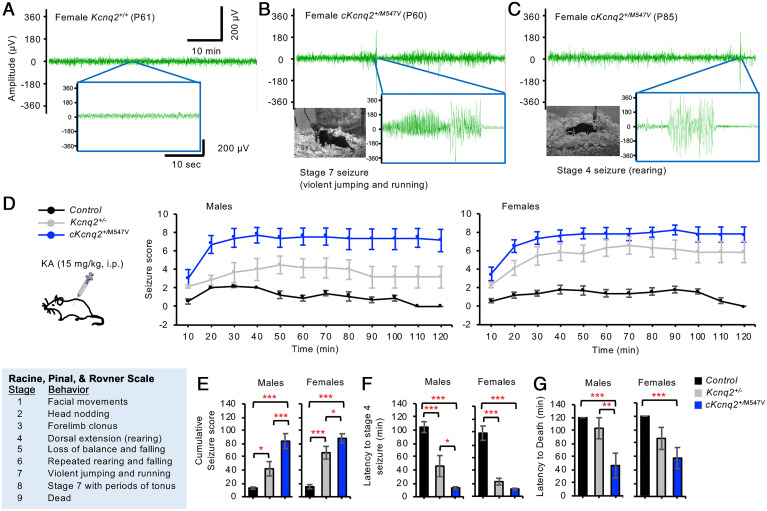
Fig. 2.
Conditional cKcnq2M547V/+ mice showed spontaneous seizures and extreme seizure sensitivity to KA. (A–C) The control (Kcnq2+/+) and cKcnq2+/M547V mice were subjected to a simultaneous video and EEG monitoring. (A) A female control Kcnq2+/+ mouse at P61 showed no electrographic or behavioral seizures. The baseline activity showed the mean peak amplitude of 28.0 ± 0.4 µV and a frequency of 0.26 ± 0.10 Hz. (B) A cKcnq2+/M547V female mouse at P60 displayed spontaneous electrographic seizures that coincided with stage 7 behavior seizures. The mean peak amplitudes were 121.7 ± 2.2 µV during stage 7 seizure and 28.6 ± 1.10 µV during the baseline. (C) Another cKcnq2+/M547V female mouse at P85 displayed spontaneous electrographic seizures that coincided with stage 4 behavioral seizures with the mean peak amplitude of 103.1 ± 4.9 µV. Interestingly, the baseline activity of this mouse showed a larger peak amplitude (31.5 ± 0.5 µV, P < 0.05) and a higher frequency (2.73 ± 0.36 Hz, P < 0.005) than the Kcnq2+/+ female in B. The behavioral seizures of the cKcnq2+/M547V female mice in B and C are shown in Movies S4 and S5. (D–G) The control (Kcnq2+/+, Kcnq2-M547Vfl/+, Emx1cre/+), Kcnq2+/−, and cKcnq2+/M547V mice (P120 to P180) were injected with a low dose of KA (15 mg/kg, i.p.) and their behavioral seizures were rated with a modified Racine, Pinal, and Rovner scale at each 10-min interval. (D and E) Average seizure scores (D) and cumulative seizure scores (E) per mouse over the first 2 h after KA injection. (F) Latency to stage 4 seizure. (G) Latency to death. Data are shown as mean ± SEM. The number of male mice used: control (n = 10), Kcnq2+/− (n = 7), and cKcnq2+/M547V (n = 8). The number of female mice used: control (n = 10), Kcnq2+/− (n = 8), and cKcnq2+/M547V (n = 7). Post hoc Tukey test results are shown here (*P < 0.05, **P < 0.01, ***P < 0.005). SI Appendix, Table S1 shows two-way ANOVA test results for control and with genotype as one factor and sex as the other.
Although Kcnq2+/− mice do not have spontaneous seizures (19, 21), they display enhanced sensitivity to seizures induced by pentylenetetrazole (21) and kainic acid (KA) (22). To compare seizure propensity of cKcnq2+/M547V mice with that of Kcnq2+/− mice, the mice (P120 to P180) received systemic injection of KA to induce seizures that arise from the hippocampus (23). A lower dose of KA (15 mg/kg, intraperitoneal [i.p.]) was used to reduce mortality (22). Control mice displayed only transient stage 2 seizures, whereas cKcnq2+/M547V mice showed stage 8 seizures by 40 min post-KA injection (Fig. 2D) and displayed a significantly higher cumulative seizure score and shorter latency to stage 4 seizure and death than control mice regardless of sex (Fig. 2 E–G and SI Appendix, Table S1). Kcnq2+/− males and females reached stage 4 and 6 seizures, respectively, by 40 min post-KA injection (Fig. 2D) and displayed a lower cumulative seizure score than cKcnq2+/M547V mice (Fig. 2E). These results indicate that cKcnq2+/M547V mice had significantly higher seizure susceptibility than Kcnq2+/− mice.
cKcnq2+/M547V Mice Display Cognitive Deficits and Abnormal Behaviors.
The patient with the M546V mutation had profound intellectual disability, spasticity, and autistic behavior (15). To test if these comorbidities occur in cKcnq2+/M547V mice, we performed six behavioral tests at P120 during the dark phase (Fig. 3A). There was no gross abnormality in motor coordination of cKcnq2+/M547V mice as indicated by their similar latency to fall from the rotarod compared with control mice (Fig. 3B and SI Appendix, Table S2). In the open-field test, cKcnq2+/M547V males traveled a longer distance in the entire open-field arena than control males (Fig. 3C and SI Appendix, Table S2), indicative of hyperactivity. Both cKcnq2+/M547V males and females displayed higher numbers of entries into the center of the arena, spent more time, and traveled a longer distance within the center than control mice (Fig. 3C and SI Appendix, Table S2), suggesting enhanced exploratory behavior or reduced anxiety-like behavior.
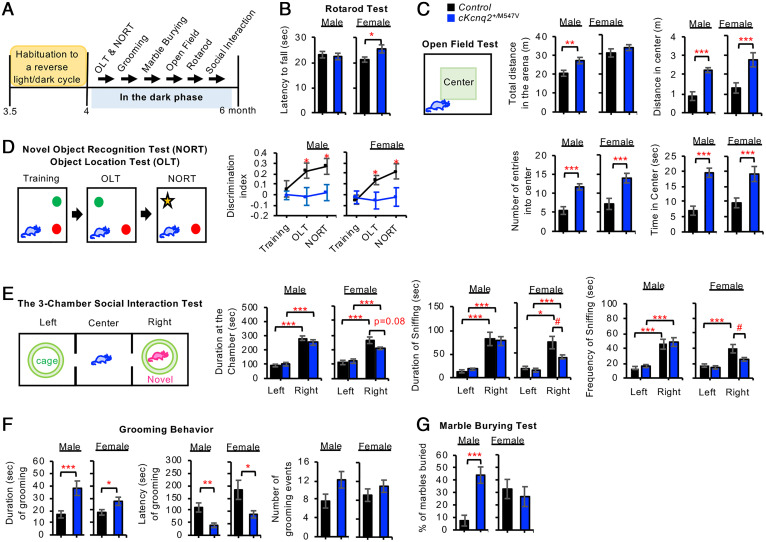
Fig. 3.
Conditional cKcnq2M547V/+ mice displayed reduced cognition and abnormal behaviors reminiscent of autism. (A) Control and cKcnq2+/M547V mice (P120) were subjected to a battery of behavioral tests in the dark phase with a test interval of >2 d. (B) Latency to fall in the rotarod test. (C) The open-field test was performed to record the total distance traveled throughout the open-field arena, entries into the center zone, duration spent within the center zone, and distance traveled within the center zone. (D) The time spent exploring both novel and familiar objects was recorded in the OLT and NORT to calculate the discrimination index as (Timenovel − Timefamiliar)/(Timenovel + Timefamiliar). (E) In the “sociability” examination of the three-chamber social interaction test, a novel stranger mouse (age-matched C57BL/6J) was placed inside the wire cage of the right chamber, and the test mouse was introduced into the center chamber and allowed to explore for 10 min. The time spent in the left and right chambers, as well as the duration, latency, and frequency of sniffing behavior, was quantified. (F) Quantification of the grooming events in their home cages without the bedding. (G) Quantification of the percentage (%) of the marbles buried in the marble-burying test. Data shown represent the mean ± SEM. The number of male mice used: control (n = 8, Kcnq2-M547Vfl/+) and cKcnq2+/M547V (n = 11). The number of female mice used: control (n = 8, Kcnq2-M547Vfl/+) and cKcnq2+/M547V (n = 11). Data are shown as mean ± SEM. Two-tailed Student’s t test results are shown in B, C, F, and G, whereas post hoc Tukey test results are shown in D and E (#P < 0.05, *P < 0.05, **P < 0.01, ***P < 0.005). SI Appendix, Table S2 shows two-way ANOVA test results for all behavioral tests except the social interaction tests with genotype as one factor and sex as the other. SI Appendix, Table S3 shows two-way ANOVA test results for the social interaction tests per sex with chamber as one factor and genotype as the other.
To test if cKcnq2+/M547V mice display cognitive deficits, we performed the object-location task (OLT) and novel object–recognition task (NORT). The OLT evaluates hippocampus-dependent spatial memory, whereas the NORT tests nonspatial memory of object identity (24). During the OLT, control mice spent significantly more time with the moved object, whereas cKcnq2+/M547V mice did not (Fig. 3D and SI Appendix, Table S2). During the NORT, control but not cKcnq2+/M547V mice spent significantly more time exploring the novel object (Fig. 3D and SI Appendix, Table S2). These results indicate that cKcnq2+/M547V mice showed impaired object-recognition memory and hippocampus-dependent spatial memory.
We next tested if cKcnq2+/M547V mice display autism-like behaviors including social avoidance and repetitive behaviors. In the three-chamber social interaction test (25), both genotypes spent significantly more time on the side of the chamber containing a novel social target mouse as compared with the empty side (Fig. 3E and SI Appendix, Table S3). While there were no genotype differences in males for sniffing duration and frequency, cKcnq2+/M547V females sniffed the novel mouse for a shorter duration and frequency than control females (Fig. 3E and SI Appendix, Table S3), indicative of reduced sociability. When another novel mouse was placed in the empty side (SI Appendix, Fig. S7), both genotypes spent more time sniffing the new mouse than the familiar mouse (SI Appendix, Fig. S7 and Table S4), suggesting that social preference of the novel mouse is similar between cKcnq2+/M547V and control mice.
Compared with control mice, cKcnq2+/M547V mice displayed a longer duration of a grooming event and shorter latency of grooming (Fig. 3F and SI Appendix, Table S2), indicating increased repetitive behaviors (26). In addition, sex differences were observed for latency of grooming (SI Appendix, Table S2). In a marble-burying test (27), cKcnq2+/M547V males buried four times as many marbles as control males, but this genotype difference was not observed in females (Fig. 3G and SI Appendix, Table S2), indicating that cKcnq2+/M547V males displayed enhanced repetitive and compulsive-like behaviors.
cKcnq2+/M547V Mice Display Neurodegeneration and Reactive Astrogliosis.
To understand the pathologic basis for spontaneous seizures and behavioral deficits of cKcnq2+/M547V mice, we next performed immunostaining with verified anti-Kv7.2 antibodies (SI Appendix, Fig. S8). The hippocampi and cortices of cKcnq2+/M547V mice showed GFP expression at P30, P60, and P120, whereas those of control mice did not (SI Appendix, Fig. S1), indicating Cre-dependent expression of the Kcnq2-M547V-ires-EGFP transgene in forebrain pyramidal neurons (Fig. 1B). In the hippocampi of control mice at P120, we observed strong immunolabeling of Kv7.2 in the apical dendrites of CA1 neurons (Fig. 4A). In cKcnq2+/M547V mice, Kv7.2 immunostaining was jagged in the stratum radiatum but was overall similar to control mice throughout the hippocampi (Fig. 4A and SI Appendix, Fig. S9 A–C). Interestingly, Kv7.2 and ankyrin-G expression at the AIS was weaker in the cortical pyramidal neurons of cKcnq2+/M547V mice compared with control mice (SI Appendix, Fig. S9D).
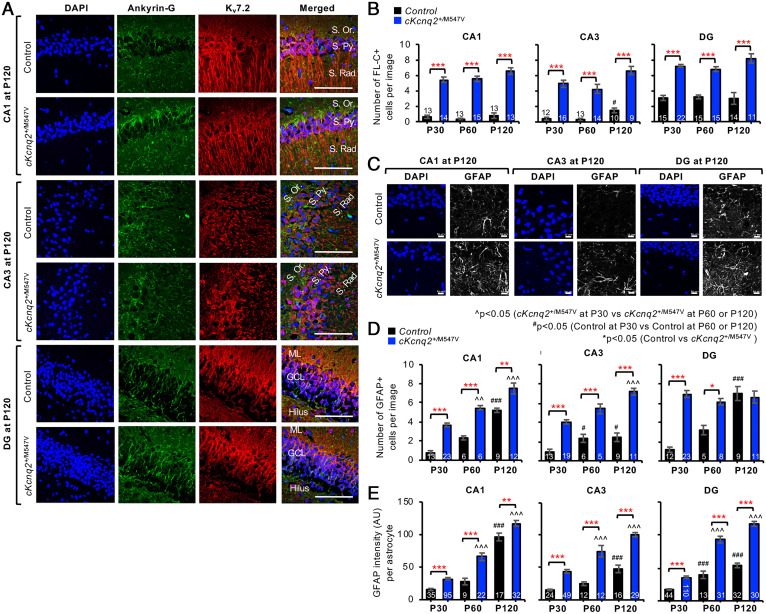
Fig. 4.
Conditional cKcnq2M547V/+ mice displayed neurodegeneration and reactive astrogliosis in their dorsal hippocampi. Coronal brain cryosections of cKcnq2+/M547V and control mice (Kcnq2-M547Vfl/+) at P120 were subjected to immunostaining for Kv7.2, ankyrin-G (A), EGFP, and GFAP (astrocyte marker) (C–E), or Fluoro-Jade C (FL-C) staining (B). Sections were counterstained with DAPI (nuclear marker). (A) Representative fluorescence images of Kv7.2, ankyrin-G, and DAPI in the hippocampal CA1 region. (Scale bars, 100 μm.) Hippocampal pyramidal cell layers: stratum pyramidale (S. Py.), stratum oriens (S. Or.), and stratum radiatum (S. Rad.). Dentate gyrus (DG) cell layers: granule cell layer (GCL), molecular layer (ML), and hilus. (B) Quantification of the number of FL-C–positive degenerating neurons in the hippocampi of control and cKcnq2+/M547V mice within the image size (160.04 × 160.04 µm). The numbers of images analyzed are indicated in the bar graphs. The number of mice used: n = 1 female mouse per genotype at P30 and P60; and n = 2 female mice per genotype at P120. Representative images of FL-C staining are shown in SI Appendix, Fig. S14. (C) Representative fluorescence images of EGFP, GFAP, and DAPI in the hippocampi of control and cKcnq2+/M547V mice. (Scale bars, 10 μm.) (D) Quantification of the number of GFAP-positive cells within the image size (101.61 × 101.61 µm) of the hippocampi of control and cKcnq2+/M547V mice. The numbers of images analyzed are indicated in the bar graphs. (E) The mean background-subtracted GFAP fluorescence intensities (arbitrary unit; AU) per GFAP-positive cell in control and cKcnq2+/M547V mice. The numbers of GFAP-positive cells analyzed are indicated in the bar graphs. The number of mice and the number of images used are the same as in D. Data are shown as mean ± SEM. Post hoc Tukey test results are shown: *P < 0.05, **P < 0.01, ***P < 0.005 (control vs. cKcnq2+/M547V); #P < 0.05, ###P < 0.005 (P30 vs. P60 or P120 in control mice); P < 0.01, P < 0.005 (P30 vs. P60 or P120 in cKcnq2+/M547V mice).
Surprisingly, Kv7.2 proteins were also detected as large perinuclear puncta in some glial fibrillary acidic protein (GFAP)–positive cells in cKcnq2+/M547V mice (SI Appendix, Fig. S12), which colocalize with GFP but not neuronal marker NeuN (SI Appendix, Fig. S13). Although the original study on the Emx1-cre line reports Cre expression in primary neocortical astrocyte culture but not in the cortex in vivo (20), the presence of Kv7.2 and GFP in astrocytes of cKcnq2+/M547V mice suggests unexpected Cre activity in off-target cells of the widely used Emx1-Cre strain.
We have previously shown that the analogous M546V mutation in human Kv7.2 induces not only ubiquitination but also severe impairments in axonal surface expression of Kv7.2/Kv7.3 channels and their intracellular retention (14). Intracellular accumulation of polyubiquitinated proteins in the endoplasmic reticulum (ER) has been shown to induce cell death (28). To test if expression of Kv7.2-M547V induces neuronal death in vivo, brain cryosections were subjected to staining with Fluoro-Jade C, a fluorescein-derived fluorochrome which specifically binds to degenerating neurons (29). Fluoro-Jade C staining revealed a larger number of degenerating hippocampal neurons in cKcnq2+/M547V mice than in control mice in P30, P60, and P120 (Fig. 4B and SI Appendix, Fig. S14).
Reactive astrocytes mediate neuroinflammation by secreting inflammatory factors and function as phagocytes for degenerated axons and apoptotic neurons in brain injury and neurodegenerative diseases (30). Compared with control mice, cKcnq2+/M547V hippocampi showed significant age-dependent increases in the number of astrocytes and GFAP expression per astrocyte (Fig. 4 C–E and SI Appendix, Figs. S10–S12), indicative of the activation and significant presence of reactive astrocytes (30).
Discussion
Contribution of the Forebrain Expression of Kv7.2-M547V to Epileptic Seizures in Mice.
Most children with BFNE and EE mutations in KCNQ2 and KCNQ3 have mild epileptic seizures that are remitted later in life or managed by antiseizure drugs (31). A subset of children has a more severe disease phenotype including refractory seizures (31). One such mutation is M546V, located in the CaM-binding helix B of Kv7.2 (15). We show that cKcnq2+/M547V mice displayed spontaneous generalized tonic-clonic seizures as early as P60 (Fig. 2 A–C) and continuous jumping and prolonged rigidity by P21 (Movies S1–S3). Since jumping and rigidity often occur with generalized tonic-clonic convulsions in mouse models of status epilepticus (32), these mice might have displayed subtle behavioral seizures that were not scored by our analyses.
Due to a mild 25 to 40% reduction in current density of heteromeric Kv7 channels, haploinsufficiency is proposed to mediate BFNE caused by KCNQ2 variants such as A306T and Y284C (31). However, heterozygous KI mice expressing these variants and Kcnq2+/− mice do not show spontaneous seizures (19, 21, 33–35). Furthermore, cKcnq2+/M547V mice are more susceptible to KA-induced seizures than Kcnq2+/− mice (Fig. 2 D–G). These differences suggest that the seizure phenotypes in cKcnq2+/M547V mice do not occur as a simple consequence of the loss of one KCNQ2 allele.
Although the M546V variant does not reduce current density of heteromeric Kv7.2/Kv7.3 channels in Xenopus oocytes (14, 17), it severely decreases axonal surface expression in cultured hippocampal neurons by >70% (14), suggesting that a dominant-negative suppression of axonal Kv7 channels may underlie EE. In support of this idea, a recurrent EE mutation, T274M, in the pore loop exerts a dominant-negative effect by decreasing current density of homomeric channels by 70 to 80% (17) and its heterozygous KI mice develop transient generalized spontaneous seizures from P20 (36). Furthermore, conditional homozygous deletion of the Kcnq2 gene from forebrain excitatory pyramidal neurons or genetic suppression of Kv7 current in the first postnatal weeks by overexpressing the Kv7.2-containing dominant-negative pore mutation G279S induces spontaneous seizure in mice (11, 37, 38). Thus, we speculate that a significant loss of Kv7 current early in development may contribute to the development of spontaneous seizures in EE.
In cKcnq2+/M547V mice, the expression of the Kcnq2-M547V-ires-EGFP transgene is driven by the CAG promoter from the Rosa locus in Cre-expressing forebrain glutamatergic neurons, whereas the expression of WT Kcnq2 is driven by the native promoter (Fig. 1 B and C), and Kcnq2 transcript levels are 1.94-fold higher than those in control mice (SI Appendix, Fig. S2). This is a different genotype from the human patient containing a heterozygous M546V mutation. The cKcnq2+/M547V mice also show the unexpected off-target expression of the transgene in some astrocytes (SI Appendix, Fig. S12), although its contribution to seizure and behavioral phenotypes is unclear. However, hippocampal Kv7.2 protein levels were comparable between control and cKcnq2+/M547V mice at P60 and P120 (Fig. 1E and SI Appendix, Fig. S4), when seizures and behaviors were examined (Figs. 2 and 3), suggesting that heightened seizure susceptibility and abnormal behaviors are likely due to impaired axonal expression of Kv7 channels.
In cKcnq2+/M547V mice born at the expected Mendelian ratio (SI Appendix, Fig. S5C), we observed postnatal death (Fig. 1F), which is considerably earlier than homozygous KI mice of BFNE variants (34) and conditional homozygous Kv7.2 KO mice (11). This is relevant to KCNQ2-associated EE because most patients appear normal at birth but develop severe seizures and clinical encephalopathy within the first days of life (31). We speculate that neonatal seizures may likely cause the early postnatal death in cKcnq2+/M547V mice, as the higher expression of Kv7.2-M547V at birth (SI Appendix, Fig. S5) is expected to induce forebrain hyperexcitability via its dominant-negative suppression of Kv7 channels. In KCNQ2-associated EE, seizure frequency diminishes with age (31). Curiously, cKcnq2+/M547V mice that had survived by P21 showed a low risk of mortality (Fig. 1G), and displayed reduced Kv7.2 expression at P30 (SI Appendix, Fig. S3). Although the mechanism underlying this transient decrease in Kv7.2 is unknown, our findings suggest that the extent of Kv7.2-M547V expression in the early postnatal period may contribute to early mortality.
Effects of the Forebrain Expression of Kv7.2-M547V on Cognition and Behavior of Mice.
The patient with the M546V mutation had profound intellectual disability and autistic behavior (15). Similarly, cKcnq2+/M547V mice display cognitive impairments (Fig. 3). They performed poorly on the OLT (Fig. 3D), which tests hippocampus-dependent spatial memory (24). Similar hippocampus-dependent spatial memory deficits were reported in the heterozygous T274M KI mice (36) and transgenic mice overexpressing dominant-negative Kv7.2-G279S (37). The cKcnq2+/M547V mice also showed deficits in nonspatial memory of object identity in NORT (Fig. 3D), which relies on the perirhinal cortex and to a lesser extent the hippocampus (24, 39). Neuronal hyperexcitability and degeneration in the hippocampus and cortex (Figs. 2 and 4 and SI Appendix, Figs. S9–S13) may underlie these memory deficits in cKcnq2+/M547V mice.
We also observed locomotor hyperactivity of cKcnq2+/M547V males (Fig. 3C) similar to the transgenic males overexpressing dominant-negative Kv7.2-G279S (37). Hippocampal sclerosis in these mice (Fig. 4C) (37) may underlie their locomotor hyperactivity given that hippocampal lesion and damage result in heightened locomotion in mice (40). Although the open-field test performed under aversive bright light is widely used to examine anxiety in mice (41), increased entry and duration of cKcnq2+/M547V mice to the exposed center in the dark (Fig. 3C) suggests their enhanced exploratory behavior in novel environments. Interestingly, the heterozygous T274M KI mice do not exhibit changes in anxiety, exploration, and repetitive behaviors (36). These behavioral differences suggest that the M546V mutation may have a different pathophysiology from the T274M mutation.
Comparison between cKcnq2+/M547V and Kcnq2+/− mice revealed that both genotypes show enhanced repetitive behaviors (Fig. 3 F and G) (22), which is one of the core clinical symptoms of autism (42). Since cKcnq2+/M547V mice have heterozygous forebrain expression of Kv7.2-M547V in Kcnq2+/− mice (Fig. 1B) and repetitive grooming behavior is mediated by GABAergic output from the striatum (43), we speculate that enhanced repetitive behaviors in cKcnq2+/M547V mice may likely be due to the heterozygous loss of Kcnq2 in the striatum. In the three-chamber social interaction test, only cKcnq2+/M547V females showed a mild reduction in sociability (Fig. 3E), which might be contributed by the hyperactivity and damage in the hippocampus and cortex (Figs. 2 and 4 and SI Appendix, Figs. S9–S13), important for social approach and cognition (44, 45).
Neuropathology in Mouse Forebrain Induced by Kv7.2-M547V.
The present study provides evidence that the expression of EE mutant Kv7.2-M547V induces neurodegeneration and reactive astrogliosis in the hippocampus and cortex of mice (Fig. 4). These findings are consistent with the MRI of a patient with the M546V mutation, which showed substantial brain lesions and neuronal loss indicated by small frontal lobes, thinned splenium of the corpus callosum, ventriculomegaly, and increased cerebral spinal fluid space (15). This patient also showed intractable seizures (15). In mesial temporal lobe epilepsy, hippocampal sclerosis is closely associated with drug-resistant seizures, progressive cognitive decline, high risk of mortality, and sudden unexpected death in epilepsy (46). Although epileptic seizures are thought to cause cognitive and behavioral impairments in EE (1, 47), our findings suggest that severe seizure phenotypes, reduced viability, and cognitive deficits seen in cKcnq2+/M547V mice may be due to neurodegeneration and neuroinflammation in addition to hyperexcitability in the hippocampus and cortex (Figs. 2–4 and SI Appendix, Figs. S9–S14).
It is noteworthy that neurodegeneration and neuroinflammation were observed in cKcnq2+/M547V mice and mice overexpressing Kv7.2-G279S but not in mice heterozygous for Kv7.2-T274M, even though all three mutations cause dominant-negative suppression of Kv7 channels (36–38). These differences may be related to transgene promoter effects in vivo (exogenous promoter vs. native promoter) and different “mutant over WT” expression ratios, which dictate the extent of dominant-negative suppression of tetrameric K+ channels (48). However, these differences also highlight the complexity and heterogeneity of the pathophysiology associated with each mutation. Whereas the T274M mutation induces dominant-negative current suppression of human Kv7.2 channels (17), the analogous mutation to M547V in the human Kv7.2 short isoform (M518V) not only blocks their current and protein expression by increasing ubiquitination but also induces dominant-negative suppression of axonal Kv7 channels by retaining them intracellularly (14). These unique effects on Kv7.2 expression may contribute to neurodegeneration.
It is unclear how expression of Kv7.2-M547V results in neurodegeneration in vivo. The M547V-induced severe reduction in axonal Kv7 channels in the forebrain excitatory neurons and subsequent increase in their excitability may lead to excitotoxicity induced by excessive release of glutamate. The same mutation is also expected to induce ubiquitination and proteasome degradation of Kv7.2, whereas the presence of Kv7.3 blocks this degradation and induces intracellular accumulation of ubiquitinated Kv7.2 (14). Since impairment of the ubiquitin-proteasome system and ER stress response can activate programmed cell death (28), we speculate that intracellular retention of Kv7.2-M547V proteins in the ER and their continuous clearance could saturate the capacity of the ER and proteasome, leading to apoptosis. Future studies shall test these hypotheses to identify the mechanisms underlying neurodegeneration and its contribution to refractory seizures associated with this EE variant.
Materials and Methods
Generation of Conditional Forebrain Heterozygous KI of Kv7.2-M547V in Mice.
The transgenic mice for conditional expression of the mouse Kcnq2 gene containing the M547V mutation (ATG to GTG) (designated as Kcnq2-M547Vfl/+) on the C57BL/6J genetic background were generated at Cyagen Biosciences by CRISPR-Cas–mediated genome engineering (49). In these mice, the cassette CAG-LoxP-Stop-LoxP-Kcnq2-M547V-IRES-EGFP-polyA was cloned into intron 1 of the Gt(ROSA)26Sor locus on mouse chromosome 6 (GenBank accession no. NR_027008.1) in a reverse direction. This cassette was flanked by 2.5- and 4.5-kb homology arms, which were generated by PCR using a bacterial artificial chromosome (BAC) clone from the C57BL/6J library as template. To induce heterozygous expression of Kcnq2-M547V-IRES-EGFP in the forebrain pyramidal neurons via Cre-mediated removal of an upstream floxed-STOP cassette (designated as cKcnq2+/M547V mice), Kcnq2-M547Vfl/fl mice were crossed to heterozygous Kcnq2 KO Kcnq2tm1Dgen/+ mice (designated as Kcnq2+/−; Jax.org stock 005830) and Emx1cre/cre mice (Jax.org stock 005628). Both Kcnq2tm1Dgen/+ and Emx1cre/cre mice were in the C57BL/6J background (19, 20). The control mice used in these studies were Kcnq2+/+, Kcnq2-M547Vfl/+, Kcnq2-M547Vfl/fl, Emx1cre/+, and Emx1cre/cre mice.
Video-ECoG Monitoring in Freely Moving Mice.
To examine spontaneous seizures, mice at P51 to P108 were subjected to a video EEG monitoring system (Pinnacle Technology) from 2:00 to 4:30 PM as described (50). The electrical signal was band pass–filtered from 1 to 100 Hz and digitized at 200 Hz. EEG epileptiform activity was identified by repetitive occurrence, large amplitude, and sharp morphology compared with baseline EEG activity (50).
Kainate-Induced Seizures.
Behavioral seizures were induced in mice (P120 to P180) with KA (15 mg/kg, i.p.; Abcam) and monitored using a modified Racine, Pinal, and Rovner scale as described (22).
Behavioral Studies.
Behavioral tests with a test interval of >2 d were started at P120 as described (22) from least to most invasive assays in the following order: object location and novel object recognition, self-grooming, marble burying, open field, rotarod, and three-chamber social interaction tests.
Immunohistochemistry of Mouse Brain Cryosections.
Coronal brain cryosections (20-µm-thick) were immunostained with antibodies for Kv7.2 (Synaptic Systems), ankyrin-G, GFAP (NeuroMab), and GFP (Abcam) or stained for Fluoro-Jade C (Biosensis) and DAPI (Invitrogen) after permeabilization. Confocal fluorescent images (1-μm optical distance) were analyzed by the ImageJ program (NIH) and our algorithm “ANIMA.”
Statistical Analysis.
Data are reported as mean ± SEM. OriginPro v9.5 (OriginLab) was used to perform statistical analyses. Comparisons between two groups were conducted with the Student’s two-tailed t test. Behavioral data were also analyzed using two-way ANOVA. Tukey tests were used to establish post hoc pairwise differences between means. A P value < 0.05 was considered statistically significant.
A detailed description of each method is provided in SI Appendix.
Supplementary Material
Acknowledgments
This research was supported by the NIH under Awards R01 NS083402, R01 NS100019, and R01 NS097610 (to H.J.C.) and R01 NS105825 and R03 NS103029 (to C.A.C.-H.), and R21 NS104293 and R21 NS109894 (to J.S.R.) from the National Institute of Neurological Disorders and Stroke.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021265118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information. The source data sets that were generated and analyzed during the current study and presented as main Figures and supplementary figures are available in the Figshare repository (DOI: 10.6084/m9.figshare.17124350).
References
- 1.Berg A. T., et al. , Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 51, 676–685 (2010). [DOI] [PubMed] [Google Scholar]
- 2.McTague A., Howell K. B., Cross J. H., Kurian M. A., Scheffer I. E., The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol. 15, 304–316 (2016). [DOI] [PubMed] [Google Scholar]
- 3.EpiPM Consortium, A roadmap for precision medicine in the epilepsies. Lancet Neurol. 14, 1219–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen A. S., et al. , Epi4K Consortium; Epilepsy Phenome/Genome Project, De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noebels J., Pathway-driven discovery of epilepsy genes. Nat. Neurosci. 18, 344–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown D. A., Passmore G. M., Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 156, 1185–1195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Z., et al. , A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. Neurosci. 26, 2599–2613 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung H. J., Jan Y. N., Jan L. Y., Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc. Natl. Acad. Sci. U.S.A. 103, 8870–8875 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett V., Lorenzo D. N., An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr. Top. Membr. 77, 143–184 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Shah M. M., Migliore M., Valencia I., Cooper E. C., Brown D. A., Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 105, 7869–7874 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soh H., Pant R., LoTurco J. J., Tzingounis A. V., Conditional deletions of epilepsy-associated KCNQ2 and KCNQ3 channels from cerebral cortex cause differential effects on neuronal excitability. J. Neurosci. 34, 5311–5321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene D. L., Hoshi N., Modulation of Kv7 channels and excitability in the brain. Cell. Mol. Life Sci. 74, 495–508 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J., et al. , Identifying mutation hotspots reveals pathogenetic mechanisms of KCNQ2 epileptic encephalopathy. Sci. Rep. 10, 4756 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E. C., et al. , Reduced axonal surface expression and phosphoinositide sensitivity in Kv7 channels disrupts their function to inhibit neuronal excitability in Kcnq2 epileptic encephalopathy. Neurobiol. Dis. 118, 76–93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weckhuysen S., et al. , KCNQ2 encephalopathy: Emerging phenotype of a neonatal epileptic encephalopathy. Ann. Neurol. 71, 15–25 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Millichap J. J., et al. , KCNQ2 encephalopathy: Features, mutational hot spots, and ezogabine treatment of 11 patients. Neurol. Genet. 2, e96 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orhan G., et al. , Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Ann. Neurol. 75, 382–394 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Cavaretta J. P., et al. , Polarized axonal surface expression of neuronal KCNQ potassium channels is regulated by calmodulin interaction with KCNQ2 subunit. PLoS One 9, e103655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzingounis A. V., Nicoll R. A., Contribution of KCNQ2 and KCNQ3 to the medium and slow afterhyperpolarization currents. Proc. Natl. Acad. Sci. U.S.A. 105, 19974–19979 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorski J. A., et al. , Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe H., et al. , Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J. Neurochem. 75, 28–33 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Kim E. C., et al. , Heterozygous loss of epilepsy gene KCNQ2 alters social, repetitive and exploratory behaviors. Genes Brain Behav. 19, e12599 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Ari Y., Cossart R., Kainate, a double agent that generates seizures: Two decades of progress. Trends Neurosci. 23, 580–587 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Denninger J. K., Smith B. M., Kirby E. D., Novel object recognition and object location behavioral testing in mice on a budget. J. Vis. Exp. 141, e58593 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moy S. S., et al. , Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Silverman J. L., Yang M., Lord C., Crawley J. N., Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 11, 490–502 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angoa-Pérez M., Kane M. J., Briggs D. I., Francescutti D. M., Kuhn D. M., Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 82, 50978 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyedmers J., Mogk A., Bukau B., Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11, 777–788 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Schmued L. C., Stowers C. C., Scallet A. C., Xu L., Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 1035, 24–31 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Liddelow S. A., Barres B. A., Reactive astrocytes: Production, function, and therapeutic potential. Immunity 46, 957–967 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Miceli F., et al. , “KCNQ2-related disorders” in GeneReviews®, Adam M. P. et al., Eds. (University of Washington, Seattle, WA, 2010; updated 2018). https://www.ncbi.nlm.nih.gov/books/NBK32534/. [PubMed] [Google Scholar]
- 32.Sharma S., Puttachary S., Thippeswamy A., Kanthasamy A. G., Thippeswamy T., Status epilepticus: Behavioral and electroencephalography seizure correlates in kainate experimental models. Front. Neurol. 9, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otto J. F., et al. , Electroconvulsive seizure thresholds and kindling acquisition rates are altered in mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions. Epilepsia 50, 1752–1759 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Singh N. A., et al. , Mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions show seizures and neuronal plasticity without synaptic reorganization. J. Physiol. 586, 3405–3423 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomonoh Y., et al. , The kick-in system: A novel rapid knock-in strategy. PLoS One 9, e88549 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milh M., et al. , A knock-in mouse model for KCNQ2-related epileptic encephalopathy displays spontaneous generalized seizures and cognitive impairment. Epilepsia 61, 868–878 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters H. C., Hu H., Pongs O., Storm J. F., Isbrandt D., Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 8, 51–60 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Marguet S. L., et al. , Treatment during a vulnerable developmental period rescues a genetic epilepsy. Nat. Med. 21, 1436–1444 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Warburton E. C., Brown M. W., Neural circuitry for rat recognition memory. Behav. Brain Res. 285, 131–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson S. M., Berkowitz L. E., Clark B. J., Behavioral and neural subsystems of rodent exploration. Learn. Motiv. 61, 3–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey K. R., Crawley J. N., “Anxiety-related behaviors in mice” in Methods of Behavioral Analysis in Neuroscience, Buccafusco J. J., Ed. (CRC Press/Routledge/Taylor & Francis Group, Boca Raton, FL, 2009), pp. 77–101. [Google Scholar]
- 42.Vorstman J. A. S., et al. , Autism genetics: Opportunities and challenges for clinical translation. Nat. Rev. Genet. 18, 362–376 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Zhang K., Hill K., Labak S., Blatt G. J., Soghomonian J. J., Loss of glutamic acid decarboxylase (Gad67) in Gpr88-expressing neurons induces learning and social behavior deficits in mice. Neuroscience 275, 238–247 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Okuyama T., Kitamura T., Roy D. S., Itohara S., Tonegawa S., Ventral CA1 neurons store social memory. Science 353, 1536–1541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murugan M., et al. , Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malmgren K., Thom M., Hippocampal sclerosis—Origins and imaging. Epilepsia 53 (suppl. 4), 19–33 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Hermann B., Seidenberg M., Epilepsy and cognition. Epilepsy Curr. 7, 1–6 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minassian N. A., Lin M. C., Papazian D. M., Altered Kv3.3 channel gating in early-onset spinocerebellar ataxia type 13. J. Physiol. 590, 1599–1614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall B., et al. , Genome editing in mice using CRISPR/Cas9 technology. Curr. Protoc. Cell Biol. 81, e57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casalia M. L., Howard M. A., Baraban S. C., Persistent seizure control in epileptic mice transplanted with gamma-aminobutyric acid progenitors. Ann. Neurol. 82, 530–542 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information. The source data sets that were generated and analyzed during the current study and presented as main Figures and supplementary figures are available in the Figshare repository (DOI: 10.6084/m9.figshare.17124350).