Significance
The nine-member transglutaminase protein family includes five known autoantigens. Because of the frequent roles of transglutaminases in autoimmunity, we decided to explore whether the remaining members might also constitute autoantigens, but in as-yet-unexplained disorders. We turned to TGM1, and since this member is primarily expressed in squamous epithelia, we focused on skin disorders. By screening a broad range of acquired skin disorders, we identified TGM1 to be a major autoantigen in the severe blistering disease paraneoplastic pemphigus. This study illustrates a gene-centric approach to biomarker discovery—starting from a putative autoantigen to search for its corresponding disease—that may prove generally applicable for studies of autoimmunity.
Keywords: autoimmunity, biomarkers, paraneoplastic, autoantibodies, transglutaminase
Abstract
Autoantigen discovery is a critical challenge for the understanding and diagnosis of autoimmune diseases. While autoantibody markers in current clinical use have been identified through studies focused on individual disorders, we postulated that a reverse approach starting with a putative autoantigen to explore multiple disorders might hold promise. We here targeted the epidermal protein transglutaminase 1 (TGM1) as a member of a protein family prone to autoimmune attack. By screening sera from patients with various acquired skin disorders, we identified seropositive subjects with the blistering mucocutaneous disease paraneoplastic pemphigus. Validation in further subjects confirmed TGM1 autoantibodies as a 55% sensitive and 100% specific marker for paraneoplastic pemphigus. This gene-centric approach leverages the wealth of data available for human genes and may prove generally applicable for biomarker discovery in autoimmune diseases.
The identification of immune targets is crucial to the understanding of autoimmune disease mechanisms and can spur developments in clinical diagnostics and treatment. Autoantibodies in tissue-specific autoimmune disorders typically target proteins that are specifically expressed in the affected tissue and oftentimes also possess key functions. Autoimmune diseases may therefore present as phenocopies of monogenic disorders affecting the same proteins, as exemplified by the familial and acquired forms of epidermolysis bullosa caused either by mutations in the collagen VII type 1 alpha chain gene (COL7A1) or by autoantibodies targeting the corresponding protein (1, 2). Thus far targets of autoimmune attack have typically been identified by screening for immunoreactivity in individual disorders. Given the abundant and growing information about gene expression patterns across tissues, we reasoned that a reverse approach, starting with a candidate autoantigen, might serve to reveal a corresponding disease. To explore this possibility, we turned to the nine-member transglutaminase protein family, among which five clinically significant autoantigens have been identified (Table 1). Tissue transglutaminase 2 (TGM2) is the major autoantigen in celiac disease (3) and is widely used for diagnostics. Gluten sensitivity frequently involves skin manifestations and, in rare cases, also symptoms from the nervous system. Dermatitis herpetiformis is associated with autoantibodies targeting epidermal transglutaminase 3 (TGM3) (4), while gluten-related cerebellar ataxia and peripheral neuropathy is linked with autoantibodies against transglutaminase 6 (TGM6) (5). Extending beyond gluten sensitivity, acquired factor XIII-deficient hemophilia is caused by autoantibodies targeting the catalytic alpha-subunit of coagulation factor FXIII (6), and the prostate-specific TGM4 is a major autoantigen in male patients with autoimmune polyendocrine syndrome type 1 (APS1) (7, 8). Given this range of autoantigens in the transglutaminase gene family, we were interested to learn whether some of the remaining members also might represent targets in as yet unexplained autoimmune disorders. We focused our attention on transglutaminase 1 (TGM1), which is predominantly expressed in squamous epithelia, having an integral function in the assembly of the cornified cell envelope (9, 10). Individuals with autosomal recessive congenital ichthyosis due to TGM1 gene mutations (Mendelian Inheritance in Man number 242300) suffer from lifelong severe scaling of the skin (11), suggesting a crucial role for skin barrier integrity. On this basis, we wanted to determine whether TGM1 constituted an autoantigen in acquired skin disease.
Table 1.
The transglutaminase protein family
| Gene | Main functions | Main sites of expression/activity | Monogenic disorders (MIM#) | Autoimmune disorders |
| TGM1 | Epithelial barrier integrity; cornified envelope | Stratified squamous epithelia | Ichthyosis, congenital, autosomal recessive 1 (242300) | — |
| TGM2 | Apoptosis signaling; extracellular matrix stabilization | Widespread | — | Celiac disease |
| TGM3 | Epithelial barrier integrity; cornified envelope; hair shaft stabilization | Stratified squamous epithelia | Uncombable hair syndrome 2 (617251) | Dermatitis herpetiformis |
| TGM4 | Sperm capacitation; semen coagulation | Prostate epithelium/semen | — | Autoimmune polyendocrine syndrome type 1 |
| TGM5 | Epithelial barrier integrity; cornified envelope | Stratified squamous epithelia | Peeling skin syndrome 2 (609796) | — |
| TGM6 | Unknown | Not well-defined | Spinocerebellar ataxia 35 (613908) | Gluten associated neuropathy and ataxia |
| TGM7 | Unknown | Not well-defined | — | — |
| F13A1 | Blood clotting; wound healing | Blood plasma; widespread | Factor XIIIA deficiency (613225) | Autoimmune FXIII deficient hemophilia |
| EPB42 | Erythrocyte cell membrane integrity | Erythrocytes | Spherocytosis, type 5 (612690) | — |
MIM#, Mendelian Inheritance in Man number.
Results
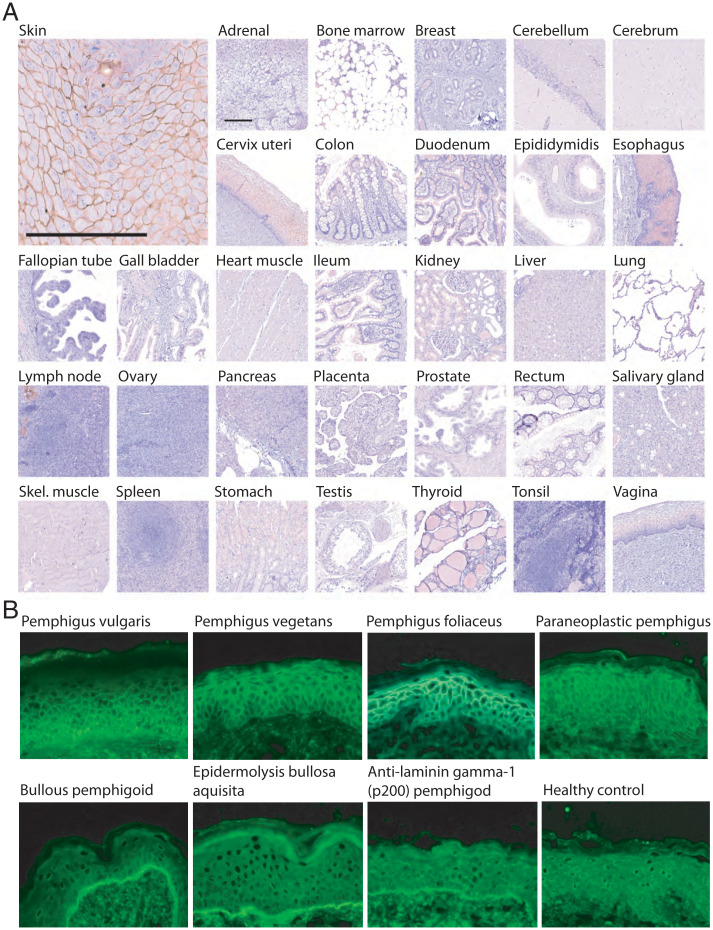
We pursued TGM1 as a candidate autoantigen in skin disease. Autoimmune bullous skin disorders appeared particularly promising since they feature autoantibodies against different components of epidermis (12, 13). For further direction, we compared the tissue-localization of TGM1 with the binding pattern of serum autoantibodies from patients with different forms of autoimmune bullous disorders. We first used affinity-purified rabbit TGM1 antibodies to perform immunohistochemistry of a human tissue panel. A distinctive intercellular staining pattern was observed in squamous epithelia, including the epidermis, esophagus, vagina, and cervix uteri, while remaining tissues showed only very faint or no staining (Fig. 1A). The results were consistent with the available consensus data set for TGM1 at http://www.proteinatlas.org, which combines data from several independent investigations of mRNA expression in human tissues (14). We next investigated how the tissue localization of TGM1 compared to the pattern of autoantibody reactivity in different bullous disorders, as detected using indirect immunofluorescence technique on normal human skin tissue sections (Fig. 1B and SI Appendix, Fig. S1). Sera from patients with intraepithelial blistering disorders—pemphigus vulgaris, pemphigus vegetans, pemphigus foliaceus, and paraneoplastic pemphigus—all displayed an intercellular pattern that was compatible with the TGM1 staining. Conversely, the autoantibody reactivity of sera from patients with subepithelial bullous disorders—bullous pemphigoid, epidermolysis bullosa acquisita, and anti-laminin gamma-1 (p200) pemphigoid—all displayed a linear pattern along the basal lamina that was clearly distinct from the TGM1 staining. The tissue distribution of TGM1 was thus consistent with the autoantibody reactivity of the pemphigus group of disorders but not with that of pemphigoid. The expression pattern of TGM1 was also similar to that of known autoantigens in pemphigus, such as desmoglein 3 (SI Appendix, Figs. S2, S3, and S4).
Fig. 1.
Anti-TGM1 colocalize with pemphigus serum autoantibodies in the cell-to-cell boarders of squamous epithelia. (A) TGM1 immunohistochemistry of multiple human formalin-fixed tissues, showing distinct intercellular staining of skin and other squamous epithelia. Scale bars, 200 µm. (B) Indirect immunofluorescence on normal human skin, revealing patterns of serum autoantibody reactivity in different autoimmune bullous skin disorders. The TGM1 staining pattern was consistent with the intercellular pattern observed for patients with different forms of intraepithelial blistering skin disorders—pemphigus vulgaris, pemphigus vegetans, pemphigus foliaceus, and paraneoplastic pemphigus—while it was distinct from the linear pattern of subepithelial bullous skin disorders—bullous pemphigoid, epidermolysis bullosa acquisita, and anti-laminin gamma-1 (p200) pemphigoid. Magnification 400×.
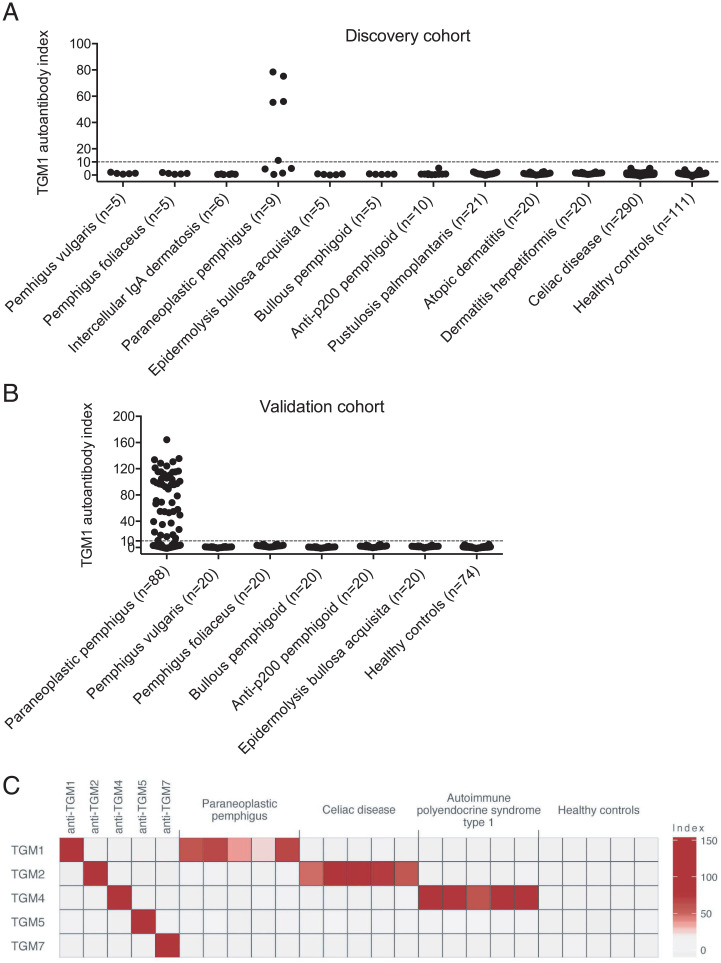
We next used a radio-ligand binding assay to perform an explorative screen for TGM1 autoantibodies in sera from patients with various acquired skin disorders. We assembled a cohort with the aim to include all major types of pemphigus and to also have broad representation of other forms of autoimmune and idiopathic skin disorders. The discovery cohort comprised patients with pemphigus vulgaris (n = 5), pemphigus foliaceus (n = 5), intercellular immunoglobulin A (IgA) dermatosis (n = 6), paraneoplastic pemphigus (n = 9), epidermolysis bullosa acquisita (n = 5), bullous pemphigoid (n = 5), anti-p200 pemphigoid (n = 10), pustulosis palmoplantaris (n = 21), atopic dermatitis (n = 20), dermatitis herpetiformis (n = 21), as well as control subjects with celiac disease (n = 290) and blood donors (n = 111). Among the 507 investigated sera, five demonstrated immunoreactivity against TGM1—all of which were from patients diagnosed with the severe mucocutaneous disease paraneoplastic pemphigus (Fig. 2A). The clinical histories, histopathology, and serological results of the five patients positive for TGM1 autoantibodies are summarized in the SI Appendix.
Fig. 2.
Discovery and validation of TGM1 autoantibodies in paraneoplastic pemphigus. (A) Explorative screen for TGM1 autoantibodies across multiple autoimmune and idiopathic skin disorders and controls, identifying TGM1 autoantibodies in sera from five patients with paraneoplastic pemphigus. (B) Validation in a replication cohort of 88 patients with paraneoplastic pemphigus and controls. TGM1 autoantibodies were detected in sera from 53 out of 97 (55%) patients with paraneoplastic pemphigus in the discovery and replication cohorts combined. Cutoff = index value 10. (C) The target specificity of serum autoantibodies was studied against a panel of transglutaminases in five subjects with paraneoplastic pemphigus as compared to controls, revealing specific reactivity toward TGM1 in paraneoplastic pemphigus.
To verify the role of TGM1 autoantibodies in this disease, we investigated a replication cohort of 88 patients with paraneoplastic pemphigus together with additional control groups: pemphigus vulgaris (n = 20), pemphigus foliaceus (n = 20), bullous pemphigoid (n = 20), anti-laminin gamma-1 (p200) pemphigoid (n = 20), epidermolysis bullosa acquisita (n = 20), and healthy controls (n = 78). Autoantibodies against TGM1 were detected in sera from 48 out of 88 patients with paraneoplastic pemphigus in the replication cohort, while all other sera were negative (Fig. 2B). Altogether TGM1 autoantibodies were present in sera from 53 out of 97 (55%) patients with paraneoplastic pemphigus while absent in all other disease categories and healthy controls (n = 652) in the discovery and replication cohorts combined, suggesting TGM1 autoantibodies were a highly specific biomarker for this disease. We also investigated the added diagnostic value of including TGM1 autoantibodies alongside the established autoantibodies against DSG1 and DSG3, in terms of increased sensitivity for paraneoplastic pemphigus. Autoantibody results for TGM1, DSG1, and DSG3 were available for 87 of the studied patients with paraneoplastic pemphigus. Among these, 67% were positive for autoantibodies against one or both desmoglein proteins. By adding TGM1 autoantibodies, the combined diagnostic sensitivity was further increased to 78% (SI Appendix, Fig S5).
We next sought to determine whether TGM1 autoantibody-positive patients with paraneoplastic pemphigus differed from the TGM1 autoantibody-negative group in their clinical phenotypes or any other aspect. To this end, we assessed associations between TGM1 autoantibody status and available data on age, sex, mucocutaneous manifestations, respiratory complications, associated neoplasms, and previously obtained autoantibody data using Fisher’s exact test (SI Appendix, Tables S1 and S2). For many of the collected phenotypes, the number of individuals were too small for meaningful statistical association testing. It was possible to conclude though that TGM1 autoantibodies appeared at even rates in women and men and that they were present at all age categories. There were no significant associations with the characteristics of mucocutaneous manifestations (erythema, blister, or erosion) or the sites of these lesions (trunk, head, extremity, oral, ocular, and genital). No associations were found with bronchiolitis obliterans or other respiratory complications seen among the patients. Furthermore, TGM1 autoantibodies appeared unrelated to the type of concomitant neoplasm. In all, there was no evidence that TGM1 autoantibody-positive patients differed from the remaining patients with paraneoplastic pemphigus in regards to underlying neoplasms or the clinical presentation.
Patients with dermatitis herpetiformis display autoantibody reactivity against both TGM3 and TGM2 (4), whereas patients with APS1 and TGM4 autoantibodies do not show cross-reactivity against TGM2 (7). To verify that TGM1 was the primary target of transglutaminase autoantibodies in paraneoplastic pemphigus, we studied autoantibody reactivity against a panel of full-length transglutaminase proteins. Five patients with paraneoplastic pemphigus who had all been previously found positive for TGM1 autoantibodies were investigated, and TGM2 autoantibody-positive patients with celiac disease (n = 5), TGM4 autoantibody-positive patients with APS1 (n = 5), and healthy blood donors (n = 5) were included as controls. Paraneoplastic pemphigus sera specifically reacted against TGM1, while celiac disease and APS1 patient sera only showed binding to TGM2 and TGM4, respectively (Fig. 2C). Transglutaminase autoantibodies in paraneoplastic pemphigus thus appeared to be highly specific for TGM1.
Discussion
With this report of TGM1 autoantibodies in paraneoplastic pemphigus we demonstrate an unconventional approach to biomarker discovery, where we started with a putative autoantigen to explore a range of disorders as guided by the expression profile and function of the investigated protein. There are reasons to expect that this gene-centric approach to biomarker discovery will be increasingly applicable for studies of autoimmune diseases. Large-scale projects such as the Human Protein Atlas, The Human Cell Atlas, and the Genotype-Tissue Expression (GTEx) project are generating a wealth of data for all human genes at the level of DNA, RNA, and protein. These resources can help selecting attractive candidate autoantigens to be screened for their corresponding disorders. The Online Mendelian Inheritance Man (OMIM) database, listing all known human monogenic disorders and the involved genes, also represents a valuable resource in this context, since autoimmune disorders often mimic the phenotype of monogenic diseases. Genes with limited tissue distribution are as a group more frequently targeted in autoimmune disease and lend themselves well for this gene-centric approach. It is also likely that future autoantigens may be identified in protein families already implicated in autoimmune disease, such as the cytochrome p450 superfamily of enzymes that includes 21-hydroxylase (CYP21A2) in Addison’s disease (15) and side-chain cleavage enzyme (CYP11A1) in ovarian insufficiency (16), the aquaporin family that includes aquaporin 4 in neuromyelitis optica (17), the collecting duct-specific aquaporin 2 in interstitial nephritis (18), and—not the least—the transglutaminase family.
With TGM1 included, six out of nine transglutaminases have been implicated as autoantigens in different autoimmune conditions (3–7). This unparalleled range of autoantigens in the same protein family raises questions on their unique predisposition to become targeted by the immune system. Insights from celiac disease point toward a role of the enzymatic activity of transglutaminases, where TGM2-mediated modification is crucial in potentiating the immunogenicity of gliadin present in wheat (19, 20). There are pertinent parallels in this respect to paraneoplastic pemphigus, where the previously described autoantigens enveloplakin and periplakin (21, 22) are substrates for TGM1 (23, 24). Similarities are also found in prostate autoimmunity in APS1 and its mouse model, where the two dominating autoimmune targets are TGM4 (7) and the major substrate of this enzyme—semenogelin (25). The autoantibody response in paraneoplastic pemphigus appears to be broader than that of other autoimmune bullous disorders, with now several identified squamous epithelial autoantigens in this disease (21, 22, 26–28). Follow-up investigation in paraneoplastic pemphigus has shown that the autoantibody response can broaden over time (29). Further studies are needed to determine if TGM1-mediated modifications of squamous epithelial proteins are involved in this process.
Paraneoplastic pemphigus is a severe autoimmune bullous disease characterized by polymorphic mucocutaneus lesions that appear in association with a neoplastic disease (30). The clinical presentation is variable and may resemble pemphigus vulgaris, erythema multiforme, or lichen planus (30, 31). Malignant lymphoma is the most commonly associated neoplastic disease, as seen in almost half of the patients, but many other malignant and benign neoplasms have been reported (31). In many cases, the underlying neoplastic disease remains undiagnosed at the time of presentation of the mucocutaneous manifestations. It is therefore urgent to establish the correct diagnosis so that investigations for occult malignancies can be initiated without delay. Autoantibody analysis constitutes a central part of the diagnostic assessment. Indirect immunofluorescence analysis of normal human skin sections forms the basis of the serological screening and has successively been extended with antigen-specific tests (13, 21, 22, 26–28, 31–33). We found TGM1 autoantibodies to be 55% sensitive and 100% specific for paraneoplastic pemphigus, as investigated in a broad group of patients with clinically relevant differential diagnoses. Given the high specificity of TGM1 autoantibodies, TGM1 autoantibody analysis may improve the diagnostic precision for paraneoplastic pemphigus alongside the established serological assays. Future studies combining TGM1 autoantibodies with currently available serological tests in a clinically relevant setting will be important to determine the added diagnostic value of TGM1 autoantibodies.
Materials and Methods
Study Subjects.
Serum samples from patients with autoimmune bullous skin diseases were obtained from a previously described cohort at Kurume University in Japan, which included patients who had visited Kurume University Hospital or been referred for serological tests from other hospitals in Japan, Korea, the United States, and European countries over a period of more than 20 y (31, 34). All patients had undergone rigorous serological testing to secure the respective diagnoses. The study was approved by the Ethical Committee of Kurume University and by the Ethical Review Board in Stockholm. Sera were also included from patients with dermatitis herpetiformis from Hungary (35), patients with atopic dermatitis from Sweden (36), patients with pustulosis palmoplantaris from Sweden (37), patients with celiac disease from Sweden (38), patients with APS1 from Finland, and healthy blood donors from Sweden, with approval from the Ethical committee at Heim Pál Children’s Hospital in Budapest, the Ethical Committee at Uppsala University Hospital, the Ethical Committee at Lund University, and the Ethical Review Board Stockholm, respectively. Consent was obtained according to the ethical approvals for the different study populations.
TGM1 Immunohistochemistry.
The expression pattern of TGM1 protein was characterized across a broad panel of human tissues. Tissue microarrays (TMAs) containing multiple formalin-fixed paraffin-embedded human tissue samples were constructed as previously described (39) and used for immunohistochemistry using a TGM1-specific rabbit antibody (Atlas antibodies, HPA040171). The TMAs were first incubated with Ultra V block (TA-125-UB, Thermo Fisher Scientific, Inc.) for 5 min, followed by anti-TGM1 at 1:1,200 dilution for 30 min, and thereafter with labeled horseradish peroxidase-polymer for 30 min. The TMAs were next incubated with 3,3′-diaminobenzidine (DAB) solution for 2 × 5 min, counterstained in Mayer’s hematoxylin (01820, Histolab) for 5 min using the Autostainer XL (Leica), rinsed in lithium carbonate water (diluted 1:5 from saturated solution) for 1 min, dehydrated in graded ethanol, and lastly coverslipped (PERTEX, Histolab) using an automated glass coverslipper (CV5030, Leica). The TMAs were scanned using the automated scanning system Aperio XT (Aperio Technologies).
Indirect Immunofluorescence.
Fresh frozen normal human skin samples were stained with sera from patients with different autoimmune bullous skin disorders or healthy controls, according to previously described methodology (12, 40).
Transglutaminase Radio-Ligand Binding Assays.
TGM1 autoantibodies were measured using a radio-ligand binding assay. Human TGM1 complementary DNA (cDNA) (Origene, SC122560) was cloned into a pTnTTM expression vector (L5610, Promega), which was used for in vitro transcription and translation in the presence of 35S methionine (Promega TNT Systems) following the manufacturer’s protocol (Promega TNT Systems). Radiolabeled TGM1 protein (30,000 counts per minute [CPM]) was immunoprecipitated with patient or control sera (2.5 µL) in 96 well filtration plates (Millipore) using protein A Sepharose (nProtein A Sepharose 4 Fast Flow, GE Health Care). All sera were analyzed in duplicate. Positive standards were included in each plate: either a polyclonal TGM1 antibody (Atlas antibodies, HPA040171) in the discovery analysis or serum from a patient with paraneoplastic pemphigus and confirmed immunoreactivity against TGM1 in the validation analysis. Negative standard, represented by 4% bovine serum albumin (BSA), was also included in each plate. Radioactivity was measured using a liquid scintillation counter (Wallac 1450 MicroBeta PerkinElmer). Autoantibody index values were calculated according to the following: (sample value-negative standard)/(positive standard-negative standard) × 100. The upper limit of the normal range was defined as an index value of 10.
The target specificity of TGM1 autoantibodies was investigated using radio-ligand binding assays for TGM1 (Origene, SC122560), TGM2 (kindly provided by Åke Lernmark at Lund University), TGM4 (Origene, SC303287), TGM5 (Origene, SC308078), and TGM7 (Origene, SC305704). Patient and control sera were added in a volume of 0.625 µL per well, and otherwise followed the same procedure as described above. Polyclonal antibodies against TGM1 (Zedira, A018), TGM2 (Zedira, A014), TGM4 (Zedira, A022), TGM5 (Novus Biologicals, NPB1-54329), and TGM7 (Zedira, A040) were included as positive standards, each in a volume of 0.625 µL per well. Z-scores were calculated based on the mean (µ) and SD () of the healthy controls, according the following: Z = (x-µ)/. Index values were thereafter calculated as described above.
Supplementary Material
Acknowledgments
We thank Dr. Cindy Wong for critical review of the manuscript and Dr. Åke Lernmark for kindly providing the cDNA plasmid encoding human TGM2. Funding was provided by the Swedish Research Council, the Alice and Knut Wallenberg Foundation, the Swedish Society for Medical Research, the Göran Gustafsson Foundation, the Novo Nordisk foundation, the Japan Agency for Medical Research and Development (AMED), and the Hungarian Research Fund.
Footnotes
Competing interest statement: N.L., T.H., and O.K. are preparing to file a patent related to the use of transglutaminase 1 autoantibodies as a diagnostic marker.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100687118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Woodley D. T., et al. , Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N. Engl. J. Med. 310, 1007–1013 (1984). [DOI] [PubMed] [Google Scholar]
- 2.Hovnanian A., et al. , Genetic linkage of recessive dystrophic epidermolysis bullosa to the type VII collagen gene. J. Clin. Invest. 90, 1032–1036 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieterich W., et al. , Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 3, 797–801 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Sárdy M., Kárpáti S., Merkl B., Paulsson M., Smyth N., Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J. Exp. Med. 195, 747–757 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadjivassiliou M., et al. , Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann. Neurol. 64, 332–343 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Franchini M., Frattini F., Crestani S., Bonfanti C., Acquired FXIII inhibitors: A systematic review. J. Thromb. Thrombolysis 36, 109–114 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Landegren N., et al. , Transglutaminase 4 as a prostate autoantigen in male subfertility. Sci. Transl. Med. 7, 292ra101 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Husebye E. S., Anderson M. S., Kämpe O., Autoimmune polyendocrine syndromes. N. Engl. J. Med. 378, 1132–1141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kárpáti S., et al. , Transglutaminases in autoimmune and inherited skin diseases: The phenomena of epitope spreading and functional compensation. Exp. Dermatol. 27, 807–814 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Lorand L., Iismaa S. E., Transglutaminase diseases: From biochemistry to the bedside. FASEB J. 33, 3–12 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Huber M., et al. , Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science 267, 525–528 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Beutner E. H., Jordon R. E., Chorzelski T. P., The immunopathology of pemphigus and bullous pemphigoid. J. Invest. Dermatol. 51, 63–80 (1968). [PubMed] [Google Scholar]
- 13.Ishii K., Importance of serological tests in diagnosis of autoimmune blistering diseases. J. Dermatol. 42, 3–10 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Uhlén M., et al. , Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Winqvist O., Karlsson F. A., Kämpe O., 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet 339, 1559–1562 (1992). [DOI] [PubMed] [Google Scholar]
- 16.Winqvist O., Gustafsson J., Rorsman F., Karlsson F. A., Kämpe O., Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison’s disease. J. Clin. Invest. 92, 2377–2385 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennon V. A., Kryzer T. J., Pittock S. J., Verkman A. S., Hinson S. R., IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 202, 473–477 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landegren N., et al. , Autoantibodies targeting a collecting duct-specific water channel in tubulointerstitial nephritis. J. Am. Soc. Nephrol. 27, 3220–3228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Wal Y., et al. , Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161, 1585–1588 (1998). [PubMed] [Google Scholar]
- 20.Molberg O., et al. , Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 (1998). [DOI] [PubMed] [Google Scholar]
- 21.Mahoney M. G., Aho S., Uitto J., Stanley J. R., The members of the plakin family of proteins recognized by paraneoplastic pemphigus antibodies include periplakin. J. Invest. Dermatol. 111, 308–313 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Kim S. C., Kwon Y. D., Lee I. J., Chang S. N., Lee T. G., cDNA cloning of the 210-kDa paraneoplastic pemphigus antigen reveals that envoplakin is a component of the antigen complex. J. Invest. Dermatol. 109, 365–369 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Yamane M., Sugimura K., Kawasaki H., Tatsukawa H., Hitomi K., Analysis on transglutaminase 1 and its substrates using specific substrate peptide in cultured keratinocytes. Biochem. Biophys. Res. Commun. 478, 343–348 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Candi E., Schmidt R., Melino G., The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 6, 328–340 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Hou Y., et al. , An aberrant prostate antigen-specific immune response causes prostatitis in mice and is associated with chronic prostatitis in humans. J. Clin. Invest. 119, 2031–2041 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amagai M., Nishikawa T., Nousari H. C., Anhalt G. J., Hashimoto T., Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J. Clin. Invest. 102, 775–782 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiyokawa C., et al. , Envoplakin and periplakin are components of the paraneoplastic pemphigus antigen complex. J. Invest. Dermatol. 111, 1236–1238 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Schepens I., et al. , The protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in human. PLoS One 5, e12250 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen G. M., et al. , Lichenoid dermatitis in paraneoplastic pemphigus: A pathogenic trigger of epitope spreading? Arch. Dermatol. 136, 652–656 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Anhalt G. J., et al. , Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N. Engl. J. Med. 323, 1729–1735 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Ohzono A., et al. , Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br. J. Dermatol. 173, 1447–1452 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Koulu L., Kusumi A., Steinberg M. S., Klaus-Kovtun V., Stanley J. R., Human autoantibodies against a desmosomal core protein in pemphigus foliaceus. J. Exp. Med. 160, 1509–1518 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amagai M., Klaus-Kovtun V., Stanley J. R., Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell 67, 869–877 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto T., et al. , Summary of results of serological tests and diagnoses for 4774 cases of various autoimmune bullous diseases consulted to Kurume University. Br. J. Dermatol. 175, 953–965 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Dahlbom I., et al. , Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J. Pediatr. Gastroenterol. Nutr. 50, 140–146 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Bradley M., et al. , Characterization by phenotype of families with atopic dermatitis. Acta Derm. Venereol. 80, 106–110 (2000). [PubMed] [Google Scholar]
- 37.Hagforsen E., Awder M., Lefvert A. K., Nordlind K., Michaëlsson G., Palmoplantar pustulosis: An autoimmune disease precipitated by smoking? Acta Derm. Venereol. 82, 341–346 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Agardh D., Borulf S., Lernmark A., Ivarsson S. A., Tissue transglutaminase immunoglobulin isotypes in children with untreated and treated celiac disease. J. Pediatr. Gastroenterol. Nutr. 36, 77–82 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Kampf C., Olsson I., Ryberg U., Sjöstedt E., Pontén F., Production of tissue microarrays, immunohistochemistry staining and digitalization within the human protein atlas. J. Vis. Exp. 63, 3620 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto T., Ogawa M. M., Konohana A., Nishikawa T., Detection of pemphigus vulgaris and pemphigus foliaceus antigens by immunoblot analysis using different antigen sources. J. Invest. Dermatol. 94, 327–331 (1990). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.