Abstract
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death in patients with refractory epilepsy. A proposed risk marker for SUDEP is the duration of post-ictal generalized EEG suppression (PGES). The mechanisms underlying PGES are unknown. Serotonin (5-HT) has been implicated in SUDEP pathophysiology. Seizures suppress activity of 5-HT neurons in the dorsal raphe nucleus (DRN). We hypothesized that suppression of DRN 5-HT neuron activity contributes to PGES and increasing 5-HT neurotransmission or stimulating the DRN before a seizure would decrease PGES duration. Adult C57BL/6J and Pet1-Cre mice received EEG/EMG electrodes, a bipolar stimulating/recording electrode in the right basolateral amygdala, and either a microdialysis guide cannula or an injection of adeno-associated virus (AAV) allowing expression of channelrhodopsin2 plus an optic fiber into the DRN. Systemic application of the selective 5-HT reuptake inhibitor citalopram (20 mg/kg) decreased PGES duration from seizures induced during wake (n = 23) and non-rapid eye movement (NREM) sleep (n = 13) whereas fluoxetine (10 mg/kg) pretreatment decreased PGES duration following seizures induced from wake (n = 11), but not NREM sleep (n = 9). Focal chemical (n = 6) or optogenetic (n = 8) stimulation of the DRN reduced PGES duration following seizures in kindled mice induced during wake. During PGES, animals exhibited immobility and suppression of EEG activity that was reduced by citalopram pretreatment. These results suggest 5-HT and the DRN may regulate PGES.
Keywords: SUDEP, PGES, epilepsy, seizure, mortality, serotonin
Introduction
Epilepsy is a highly prevalent neurological disease characterized by recurrent, spontaneous, and often unpredictable seizures (Fisher et al., 2014). It is estimated that one in twenty-six Americans will develop epilepsy during their lifetime (Hesdorffer et al., 2011). Approximately one-third of these patients will not achieve seizure freedom with currently available therapies (Chen et al., 2018; Kwan and Brodie, 2000), putting them at greater risk for sudden unexpected death in epilepsy (SUDEP). SUDEP is the leading cause of death in individuals with uncontrolled seizures (Devinsky et al., 2016) and is second only to stroke in years of potential life lost due to neurological disease (Thurman et al., 2014). While cardiac (Auerbach et al., 2013; Richerson, 2013), respiratory (Richerson, 2013; Richerson and Buchanan, 2011), and arousal impairment (Buchanan, 2019; Richerson, 2013) are involved, the exact etiology of SUDEP is unknown. Serotonin (5-HT) has been implicated in SUDEP (Petrucci et al., 2020) and seizure-induced death (Buchanan et al., 2014; Faingold et al., 2014) due to its role modulating seizures (Bagdy et al., 2007; Petrucci, et al., 2020), breathing (Buchanan et al., 2015; Richerson, 2004), and sleep/wake and arousal (Buchanan, 2019; Purnell et al., 2018). Administration of 5-HT enhancing drugs reduces seizure frequency in epilepsy patients (Ceulemans et al., 2012; Favale et al., 2003; Favale et al., 1995). This is recapitulated in rodent seizure models including genetically epilepsy prone rats (GEPR) (Dailey et al., 1992), EL mice (Imaizumi et al., 1959; Kabuto et al., 1994), and audiogenic seizure prone DBA mice (Tupal and Faingold, 2006). Conversely, chemical 5-HT depletion increases seizure susceptibility and frequency in the pilocarpine and kainic acid seizure model (Maia et al., 2017; Trindade-Filho et al., 2008).
A potential risk marker for SUDEP is the duration of post-ictal generalized EEG suppression (PGES), a period of low amplitude, low frequency electrographic activity following some seizures (Lhatoo et al., 2010; Okanari et al., 2017). Patients are more likely to be immobile, unresponsive, and require resuscitative measures during PGES (Kuo et al., 2016; Semmelroch et al., 2012). However, PGES remains poorly understood and its origin is unknown. Recently, a study in humans observed that interictal serum 5-HT levels are inversely correlated with PGES duration (Murugesan et al., 2018). While serum 5-HT does not directly relate to parenchymal 5-HT, it does support involvement 5-HT or 5-HT producing neurons in PGES. Here we examine the effect of increasing 5-HT tone on PGES.
There are two major populations of 5-HT neurons within the brain: a rostral group and a caudal group (Dahlstroem and Fuxe, 1964). The dorsal raphe nucleus (DRN) is a major component of the rostral group (Hornung, 2010). 5-HT neurons within the DRN send projections to regions associated with sleep/wake regulation (Monti, 2010; Steininger et al., 1997) and arousal (Vertes and Kocsis, 1994). Moreover, SUDEP is thought to be more likely to occur during sleep (Lamberts et al., 2012) and it is known that DRN 5-HT neurons fire fastest during wake, slow down during non-rapid eye movement (NREM) sleep, and are nearly quiescent during rapid eye movement (REM sleep) (McGinty et al., 1973). DRN firing is also depressed by seizures (Zhan et al., 2016). Therefore, we hypothesized that seizure-induced depression of the DRN 5-HT network may contribute to PGES and that increasing 5-HT tone via 5-HT enhancing drugs or DRN stimulation would reduce PGES.
There are very few animal studies examining PGES. Here we establish amygdala kindling as a reliable model of PGES and examine the role of 5-HT on behavior and EEG characteristics during PGES. We tested our hypothesis in amygdala kindled mice by increasing 5-HT tone via (1) systemic administration of selective serotonin reuptake inhibitors (SSRIs), (2) focal stimulation of the DRN with acidosis, and (3) optogenetic stimulation of the DRN prior to seizure induction. Because SUDEP is likely to occur during sleep and 5-HT is regulated in a sleep/wake dependent manner (Lamberts, et al., 2012; McGinty and Harper, 1976; Purnell, et al., 2018), when possible seizures were induced during different sleep/wake states.
Experimental Procedures and Methods
Animals
All procedures and experiments were approved by and performed in compliance with the Institutional Animal Care and Use Committee at the University of Iowa Carver College of Medicine. Animal pain and distress was minimized during experimental procedures. Adult (6–20 week) male and female C57BL/6J and Pet1-Cre mice were used for these studies. Pet1-Cre mice (B6.Cg-Tg[Fev-cre]1Esd/J) (Scott et al., 2005) were originally obtained from Jackson Labs (Bar Harbor, ME), subsequently crossed to C57BL/6J, and maintained in our animal care facilities at University of Iowa. Pet1-Cre mice express Cre-recombinase exclusively in Pet1 expressing neurons, enabling Cre-dependent targeting to 5-HT neurons (Hendricks et al., 1999). Mice were housed in 12:12 light:dark cycle (6:00 AM to 6:00 PM or 12:00 PM to 12:00 AM) with food and water available ad libitum. Experiments were performed 2–6 hrs after the start of the light phase and were spaced at least two days apart to avoid confound of repeated stimulations.
Surgical procedures
General
Surgical procedures were performed under anesthesia (isoflurane gas 2–5% induction; 0.5–2% maintenance) using aseptic technique. Surgical plane of anesthesia was verified via toe pinch every 15 min and anesthesia adjusted as needed. Mice were secured into a stereotaxic apparatus (51730; Stoelting Co.; Wood Dale, IL), their heads were shaved and cleaned with betadine and 70% ethanol, and a ∼2.0 cm longitudinal incision was made in the scalp. Throughout surgery and initial recovery, a heating pad regulated the animal’s body temperature (Physiosuite; Kent Scientific; Torrington, CT). For pain management, animals received meloxicam (2.0 mg/kg; i.p.) pre-operatively and at 24 and 48 hrs post-operatively. Animals recovered for at least seven days before being studied.
EEG/EMG headmount and bipolar stimulating/recording electrode implantation
Six holes were drilled into the skull using a 0.5 mm drill bit. The holes were positioned as follows: two 2 mm ±2 mm anterior to bregma, two 2 mm ±2 mm anterior to lambda, one overlying the right basolateral amygdala (in mm from bregma with skull level: AP: −1.3, ML: −2.8, DV: −4.7), and one equidistant between the left holes. Unless specified, holes were made approximately 1.5 mm lateral to midline. Small screws (1.0 mm thread, 4.1 mm length, 0.8 mm shaft diameter; #80404; Vigor Optical; Carlstadt, NJ) soldered to stainless steel wires (793000; A-M Systems; Carlsborg, WA) were threaded into four holes to serve as EEG electrodes. A ground screw without wire was threaded in the hole between the left EEG electrodes. Then a polyimide coated, bipolar twisted pair stimulating/recording electrode (MS333–3-BIU-SPC; Plastics One, Inc; Roanoke, VA) with the distal 0.5 mm of insulation removed was implanted into the right basolateral amygdala. The implant was secured with thick cyanoacrylate (PT-33; Pacer Technology – ZAP; Ontario, CA) and dental acrylic before the stainless steel ground wire was soldered to the ground screw.
After insertion of additional implants as described below, the wires attached to the EEG screws were twisted and soldered to wires protruding from a six-pin socket (853–41-006–30-001000; Millmax Co.; Oyster Bay, NY). EMG electrodes on the headmount were inserted bilaterally into the nuchal muscles. Dental acrylic (Lang Dental; Wheeling, IL) was applied over the EEG wires to increase stability and decrease electrical noise. The overlying skin was closed with 5–0 nylon suture (668G; Ethilon, Inc.; Somerville, NJ).
Microdialysis cannula implantation
C57BL/6J mice undergoing chemical stimulation of the DRN were implanted with a guide cannula (CMAP000138 CMA7; CMA, Inc; Kista, Sweden) into the DRN (AP: −4.6, ML: 0, DV: - 3.0) during the EEG/EMG headmount and amygdala electrode surgery (Smith et al., 2018). Guide cannula were secured with thick cyanoacrylate and dental acrylic. Identical metal-free cannula (CMA8010773; CMA, Inc) were implanted for maximal electroshock (MES) experiments to prevent electrical propagation along metal components.
Adeno-associated virus administration and optic fiber insertion
Pet1-Cre mice and WT littermates were instrumented for EEG/EMG recording and amygdala kindling as described. After insertion of epidural screws, an adeno-associated virus (AAV) containing channelrhodopsin2 (ChR2; rAAV5-EF1a-DIO-hChR2[H134R]-mCherry; University of North Carolina Gene Therapy Center Vector Core; Chapel Hill, NC; Titer 1.8×1012 vg/ml), was injected into the DRN using either a 2 μl Hamilton syringe (6545901; Hamilton Company; Reno, NV) or an autoinjector (70–4507; Harvard Apparatus; Holliston, MA) at a rate of 0.5 μl over 10 min and allowed to diffuse for another 10 min. A polyimide optical fiber (MFC_200/240–0.22_4mm_ZF1.25_FLT; Doric Lenses; Québec, Canada) was then implanted into the DRN and secured with thick cyanoacrylate and dental acrylic. Animals receiving viral vectors were not tested for at least 28 days following surgery to allow transfection (Paterna et al., 2004).
Seizure models
Amygdala kindling
Amygdala kindling was utilized because it induces a hyperexcitable brain and shares many features of human epilepsy (Kamphuis et al., 1988; McNamara, 1995). For example, kindling induces increases in extracellular glutamate around the epileptogenic zone (Ueda and Tsuru, 1994), which is seen prior to and during seizures in epilepsy patients (During and Spencer, 1993). Structural changes, such as increases in mossy fiber sprouting within the hippocampus, also occur in kindled animals and epilepsy patients (McNamara, 1995; Sutula et al., 1989; Sutula et al., 1988). Although kindling is an inducible seizure model, spontaneous seizures (McNamara et al., 1980; Pinel and Rovner, 1978) and, more rarely, death (Pinel and Rovner, 1978) can occur, making it a particularly relevant model for studying epilepsy and facets related to SUDEP. Consequently, experiments took place within a plethysmography chamber to record breathing in the event of seizure-induced death.
A fast-kindling paradigm was utilized for amygdala kindling as described previously (Blumenfeld et al., 2009; Santoro et al., 2010). An EEG/EMG preamplifier (8202-SL; Pinnacle Technology; Lawrence, KS) and a 3-channel electrode cable (335–340/3; Plastics One) were attached to the animal’s headmount and connected to a data conditioning amplifier (LP511AC; AstroNova; West Warwick, RI), a stimulating/recording switch (SRS-13C0113G; AstroNova), and a pulse stimulator (Model 2100; A-M Systems). To determine the amygdala afterdischarge threshold, 20 μA incremental currents were administered every two minutes until after-discharges were recorded. The threshold stimulus was applied twice daily at least 1 hr apart (80–500 μA range, 1 s train of 1 ms biphasic square wave pulses at 60 Hz) until the animal had three consecutive Racine grade 4 generalized seizures as rated on a modified Racine scale: 1, behavioral arrest; 2, automatisms, drooling, whisker twitches, body jerks; 3, unilateral limb myoclonus; 4, bilateral myoclonus, loss of posture, wild running; 5, status epilepticus; 6, death (Buchanan, et al., 2014; Racine, 1972).
Maximal electroshock seizure induction
C57BL/6J mice (control group: n = 6, 3 male, 3 female; acidosis group: n = 6, 2 male, 4 female) were acclimated to the recording apparatus and ear-clip electrodes (modified, toothless, stainless steel alligator clips coated with saline-moistened gauze) for at least 1 hr per day on two consecutive days. On trial days the mice were plugged into a Rodent Shocker pulse generator (D-79232; Harvard Apparatus). After a 30 min period, a single 50 mA (0.2 s, 60 Hz sine wave pulses) stimulus was administered during wake to induce a maximal hindlimb extension seizure (Buchanan, et al., 2014; Hajek and Buchanan, 2016; Purnell et al., 2017). Mortality, seizure duration, and extension-to-flexion (E/F) ratios were determined post-hoc using EEG/EMG monitoring and video assessments. E/F ratios were calculated as the time the mouse spends with hind limb extension (> 90°) divided by hind limb flexion (≤ 90°). Higher E/F ratios correlate with widespread propagation of epileptiform activity and are used as an indicator of seizure severity (Anderson et al., 1986).
Experimental procedures
Systemic Drug Administration
Adult male and female C57BL/6J mice were instrumented for EEG/EMG recording and amygdala kindling. After surgical recovery and completion of kindling, mice received an i.p. injection of citalopram (20 mg/kg; wake: n = 23, male = 12, female = 11; NREM: n = 13, male = 8, female = 5), fluoxetine (10 mg/kg; wake: n = 11, male = 6, female = 5; NREM: n = 9, male = 4, female = 5), or saline (wake: n = 30, male = 15, female = 15; NREM: n = 19, male = 12, female = 7) 30–60 min prior to induction of an seizure during wake or NREM sleep. This stimulation window was based on prior studies and drug kinetics (Fredricson Overo, 1982; Holladay et al., 1998; Leander, 1992; Tupal and Faingold, 2006). Citalopram hydrobromide and fluoxetine hydrochloride were obtained from Tocris Biosciences through Bio-Techne (Minneapolis, MN). Dosages were based upon the lab’s previous experience with the drugs and the primary literature (Buchanan, et al., 2014). Drugs were administered in consistent volumes (0.15 – 0.2 ml). SSRI administration can produce disruptions in NREM and REM sleep in both animals and humans (Wilson and Argyropoulos, 2005). Under normal conditions, DRN 5-HT neurons fire slowly during NREM and are nearly quiescent during REM, therefore our manipulations increasing 5-HT neurotransmission may have precluded sleep induction (McGinty and Harper, 1976). Our mice were able to achieve NREM sleep with some difficulty, but induction of seizures during REM was prevented.
Reverse microdialysis for delivery of normal and acidified aCSF
During reverse microdialysis trials mice were attached to cabling for EEG/EMG recording and the dummy cannula was replaced with either a metal dialysis probe for kindling experiments (pores <6 kDa, tip length 1 mm, tip diameter 240 μm; CMAP000082; CMA7; CMA Inc; n = 6, 2 male, 4 female) or a metal-free dialysis probe for the MES experiments (CMA8010771; CMA7; CMA Inc.; pores <6 kDa; tip length 1 mm; tip diameter 240 μm; control group: n = 6, 3 male, 3 female; acidosis group: n = 6, 2 male, 4 female).
Normal artificial cerebrospinal fluid (aCSF) (bubbled with 5% CO2 / 21% O2 / balance N2; pH 7.4) was dialyzed using a syringe pump (45 μl/min; 70–3005, Harvard Apparatus) throughout the 20 min habituation period. The dialysate was then changed to aCSF bubbled with 25% CO2 / 21% O2 / balance N2 (pH 6.8) or another syringe with normal aCSF for 10 min prior to seizure induction as described (Dias et al., 2008; Li et al., 2013; Smith, et al., 2018). Gas tanks were obtained from Praxair (Cedar Rapids, IA). The composition of the aCSF (Smith, et al., 2018) was as follows (in mM): 152 Na+, 3.0 K+, 2.1 Mg2+, 2.2 Ca2+, 131 Cl−, and 26 HCO3−. The Ca2+ was added after the aCSF was warmed to 37 °C and equilibrated with CO2.
Optogenetic manipulation of 5-HT neurons
Pet1-Cre mice and their WT counterparts were instrumented for EEG/EMG and amygdala kindling. Mice were injected with AAV-ChR2 (Pet1-Cre: n = 8, 3 male, 5 female; WT: n = 7, 3 male, 4 female) and instrumented with an optic fiber (4 mm, 240 μm diameter, 0.22 NA; MFC_200/240–0.22_4mm_ZF1.25_FLT; Doric Lenses) in the DRN. On the day of trials, light power (∼12.5 mW to account for 25% loss) was tested with a digital optical power and energy meter (P0014083; Thor Labs; Dachau, Germany). Mice were placed into the recording chamber and plugged into the EEG/EMG lead, the amygdala electrode, and an optical fiber patch cord (MFC_200/240/900–0.22_0.6m_FC-ZF1.25[F]; Doric Lenses). Baseline EEG was recorded for ∼5 min. Animals received light stimulation (473 nm, 4 Hz, 10 mW, 2% duty, 10 s ON / 10 s OFF; Opto Engine, LLC.; Midvale, UT) or no light stimulation 10 min prior to seizure induction during wake. Laser vs no laser trials were randomized to account for number of seizure inductions. Optogenetic manipulation prevented animals from falling into a deep sleep, precluding trials during NREM and REM. Wake was confirmed offline by EEG/EMG analysis and video.
Post-ictal immobility determination
Post-ictal immobility was quantified following seizures in kindled C57BL/6J mice through video analysis by two reviewers (A.N.P and K.M.V). Each reviewer recorded latency to the first twitch, paw movement (unilateral, no change in body position), and body movement (bilateral limb movement, body position change, preening) after seizure termination. We utilized an interrater reliability threshold of ≥90%. Both reviewers re-evaluated video for data points with <90% interrater reliability until consensus was reached. Reviewer scores were then averaged to produce final quantification. Post-ictal immobility was assessed in mice that received i.p. citalopram (wake: n = 23, 12 male,11 female; NREM: n = 13, 8 male, 5 female), fluoxetine (wake: n = 11, 6 male, 5 female; NREM: n = 10, 5 male, 5 female), or saline (n = 55; 32 male, 23 female) 30–60 min prior seizure induction. Video was not analyzed in mice whose limbs were obscured.
Data collection and analysis
EEG/EMG data acquisition
Mice were attached to a preamplifier (8202-SL; Pinnacle Technology) connected a commutator (8204; Pinnacle Technology) and digital conditioning amplifier (Model 440 Instrumentation Amplifier; Brownlee Precision; San Jose, CA). The EEG signals were amplified 50,000x and band-pass filtered from 0.3 to 200 Hz. The EMG signals were amplified 50,000x and band-pass filtered from 10 to 1000 Hz. Data were digitized with an analog-to-digital converter (PCI-6221 or NI-USB-6009; National Instruments; Austin, TX) at 1000 Hz. The data were then compiled using custom MATLAB software.
Sleep state determination
Sleep state was determined on-line and verified post-hoc using a standard approach based on the EEG/EMG frequency characteristics as described previously (Buchanan and Richerson, 2010; Buchanan, et al., 2015; Franken et al., 1998; Smith, et al., 2018): Wake: low-amplitude, high-frequency (7–13 Hz) EEG with high EMG power; NREM sleep: high-amplitude, low-frequency (0.5–4 Hz) EEG with moderate to low EMG power and lack of voluntary motor activity. Video was recorded to assist with vigilance state determination and for verification post-hoc. It is not possible to be blind to vigilance state on-line, but offline analysis was performed blind to vigilance state.
EEG frequency band activity analysis
EEG data for frequency analysis was extracted from mice in the aforementioned experiments (1–12 Hz: wake: n = 31, 17 male, 14 female; NREM: n = 19, 12 male, 7 female; 12–30 Hz: wake: n = 26, 12 male, 11 female; NREM: n = 13, 8 male, 4 female). The frequency bands of interest were: delta (δ), 1 – 4 Hz; theta (θ), 4 – 8 Hz; alpha (α), 8 – 12 Hz; beta (β), 12 – 30 Hz. EEG signals were first trimmed to a total length of 500 s (1000 Hz sample rate; 200 s before and 300 s after the seizure ended). The time-frequency information was then extracted via variable wavelet cycles using EEGLAB toolbox. Post-ictal time-frequency power was smoothed using a moving 5 s window. The baseline 95% confidence interval for frequency bands was calculated using the average power across the 200 s pre-seizure period. Results were compared to the corresponding lower limit of 95% confidence interval. A frequency band was considered recovered when power across the selected frequencies stayed above the lower limit for ≥ 5 s.
PGES determination
There are discrepancies amongst experts in PGES identification (Theeranaew et al., 2018). PGES duration was assessed automatically here using a custom MATLAB (Mathworks) script and event-related spectral perturbation (ERSP) time-frequency spectral decompositions of the preictal, ictal, and post-ictal period in the MATLAB EEGlab toolbox (Swartz Center for Computational Neuroscience, San Diego, CA). Variable wavelet cycles were used to extract power-frequency parameters from the EEG signal. PGES duration was calculated in a moving window (5 s). The mean EEG power of pre-ictal baseline was subtracted from the mean of the moving window until post-ictal EEG power exceeded 3 standard deviations of baseline EEG power for at least 5 s. ERSP power values are expressed in decibel (dBs) as a measure of relative intensity compared to a baseline pre-ictal period (100s). See Roach and Mathalon (2008) for review. Accuracy of the analysis was verified by examining the duration of negative dB values within the spectral plots.
Plethysmography
Visualization of respiratory parameters was achieved via a high-sensitivity/ultra-low-pressure transducer (DC002NDR5; Honeywell International, Minneapolis, MN) attached to the recording chamber. Flow regulators ensured that a constant and consistent flow of RA permeated the chamber for the duration of the recording session (0.41 L/min). Analog signal from the pressure transducer was digitized (PCI-6221; National Instruments) and displayed on a computer monitor in real time using a custom MATLAB acquisition program.
Tissue processing
Intracardiac perfusion
At the completion of experiments, animals were anesthetized with an overdose of ketamine/xylazine (i.p., 50–75 mg/kg; 5–7.5 mg/kg). Depth of anesthesia was confirmed by toe pinch prior to making an abdominal incision. The diaphragm was cut to expose the heart, the right atrium was punctured, and a 25 gauge needle was inserted into the left ventricle. Chilled (4°C) phosphate buffered saline (PBS) was perfused (∼17 ml/min) until blood was flushed from the circulation. After, chilled 4% paraformaldehyde (PFA) was perfused to fix the tissue. The brains were extracted and placed into 4% PFA at 4°C for 24 hrs. Brains were then cryoprotected in 30% sucrose until the tissue was saturated enough to sink in the solution. Brains were then embedded in Tissue-Tek optimum cutting temperature compound (VWR International; Radnor, PA) and stored at −80°C until they were sectioned on a cryostat (30 μm; Leica Biosystems; Buffalo Grove, IL).
Histology
Cell bodies were visualized with Nissl staining to verify placement of cannulae and electrodes. Verification of AAV transfection and location was confirmed via immunohistochemistry. Primary and secondary antibodies were diluted with 1x PBS-T containing 1:50 normal horse serum and 0.05% NaN3 to permeabilize tissue membranes, block nonspecific binding, and prevent bacterial contamination. Tissue was washed 4–6 times in 1x PBS prior to being bathed in primary antibodies overnight (mouse anti-TpOH; 1:30,000; MAB5278; EMD Millipore; Burlington, MA, rabbit anti-DsRed; 1:1000; 632496; Takara Bio Inc.; Kusatsu, Japan). Tissue was then washed in 1x PBS 4–6 times before a 2 hr exposure to secondary antibodies (Alexa 488 donkey anti-mouse, 1:500, 715545150; Cy3 donkey anti-rabbit, 1:500, 712165153; Jackson Immunoresearch; West Grove, PA). Anatomic landmarks were identified with the aid of a mouse brain atlas (Franklin and Paxinos, 2013). Fluorescent images were obtained utilizing confocal microscopy and post-processed in OlyVIA software (Olympus Life Science; Center Valley, PA).
Statistical analyses
Normality of the data was assessed via a Shapiro-Wilk normality test. Statistical significance was computed using Mann-Whitney U tests, Wilcoxon matched pairs signed rank tests, or paired t-tests. Mortality data was compared using a chi-squared goodness-of-fit contingency test. Data were analyzed using Graphpad Prism (San Diego, CA) and represented as box-and-whisker plots with the box depicting the median and the whiskers depicting the 5th-95th percentiles. Threshold for statistical significance was set at p < 0.05 for all comparisons. Post-hoc power analyses were performed using G*Power (open source, Heinrich Heine University Düsseldorf) and to confirm ≥0.80 β power for experiments.
Results
Induction of seizures in amygdala kindled mice consistently produced PGES
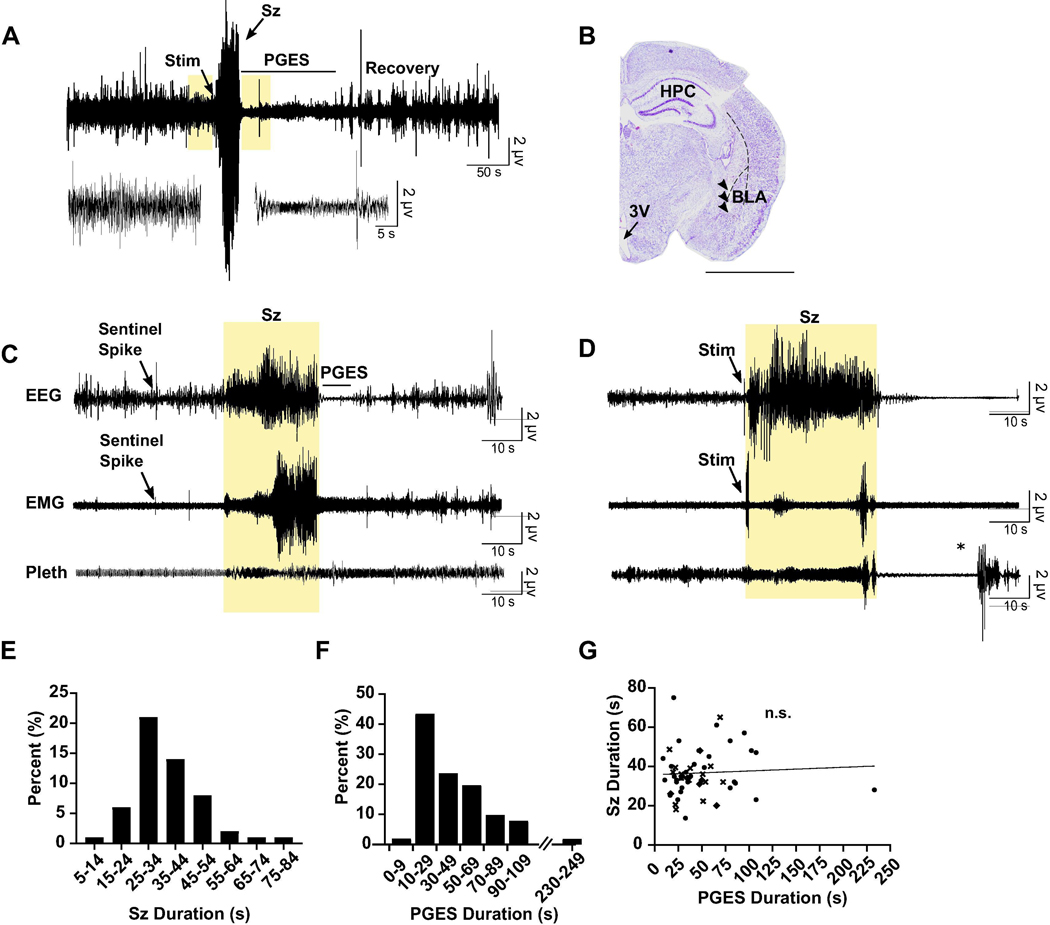
Electrical stimulation of the amygdala in kindled C57BL/6J mice elicited Racine grade 4 seizures that were followed by PGES (Fig. 1A, B). Spontaneous seizures occurred in a subset of our kindled mice. These seizures elicited a motor phenotype similar to the induced seizures (Pinel and Rovner, 1978): seizures began with behavioral arrest that transitioned into bilateral myoclonus and wild running. A recorded spontaneous seizure from one of our kindled mice is presented in Fig. 1C. Experimenters also observed spontaneous motor seizures in home cages, although these were not recorded. On rare occasions animals were found dead-in-pen with full hind-limb extension, potentially indicative of a fatal seizure. Rarely, induced seizures in our kindled mice also resulted in death (Fig. 1D). Unlike non-fatal induced seizures, fatal seizures involved progression from bilateral myoclonus to full hind-limb extension. During this time the mouse gasped until terminal apnea occurred. As the seizure ended, EEG suppression and artifact arising from ventricular escape beats could be detected until terminal asystole occurred (Levine et al., 2020). The mean non-fatal seizure duration in the kindled mice was 36.64 ± 11.83 s. Seizures ranged from 13.60–75.00 s with most (71.58%) seizures lasting between 25–45 s (Fig. 1E). The mean PGES duration was 47.14 ± 36.26 s with a range between 8.75–233.10 s. Most (81.4%) mice exhibited PGES between 20–60 s (n = 55, 32 male, 23 female; Fig. 1F). There was no correlation between seizure duration and PGES duration, indicating that effects on PGES were not simply due to a decrease in seizure length (Fig. 1G).
Figure 1.
PGES was consistently observed following seizures in amygdala kindled mice. (A) Top: 500 s raw EEG trace depicting seizure stimulation (Stim), seizure (Sz), PGES (duration indicated by horizontal line), and gradual EEG recovery in a C57BL/6J mouse. Scale bars: horizontal, 50 s; vertical, 2 μV. Bottom traces correspond to boxes on top trace and demonstrate EEG at baseline and during PGES. Scale bars: horizontal, 5 s; vertical 2 μV. (B) Nissl stained coronal hemi-section demonstrating electrode tract (arrowheads) in the basolateral amygdala. HPC, hippocampus; 3V, third ventricle; BLA, basolateral amygdala. Scale bar: 3 mm. (C) 150 s raw EEG (top), EMG (middle), and plethysmography (Pleth; bottom) traces depicting a spontaneous seizure (highlighted) emerging from non-rapid eye movement (NREM) in a kindled mouse. PGES duration is indicated by horizontal line. Scale bars: horizontal, 10 s; vertical 2 μV. (D) 150 s raw EEG (top), EMG (middle), and plethysmography (bottom) traces depicting a seizure stimulation and seizure (highlighted) that led to death in a kindled mouse. Scale bars: horizontal, 10 s; vertical 2 μV. *, Artifact from opening chamber. (E,F) Average seizure durations (E) and PGES durations (F) in the amygdala kindled mice. (G) Linear regression analysis of seizure duration in amygdala kindled mice versus PGES duration. Symbols represent different groups of animals.
Systemic application of SSRIs decreased PGES duration following seizures in amygdala kindled mice
To determine whether increasing 5-HT could reduce PGES duration, the SSRIs citalopram and fluoxetine, or vehicle, were systemically administered (i.p.) to mice prior to seizures induced during wake or NREM. Compared to saline treatment, citalopram (20 mg/kg) administration prior to seizure induction reduced PGES duration from seizures induced during wake (52.33 ± 8.336 s vs 18.54 ± 2.345 s; n = 30, 23; p < 0.05; Fig. 2A, E) and NREM sleep (37.16 ± 17.18 s vs 20.00 ± 7.84 s, n = 13; p < 0.05; Fig. 2A, F). Citalopram also reduced the duration of seizures induced during wake (38.43 ± 2.234 s vs 30.72 ± 2.704 s; n = 30, 23; p < 0.05; Fig. 2C), but not NREM sleep (34.59 ± 2.578 s vs 32.26 ± 3.274 s; n = 18, 14; Fig. 2C). Pretreatment with fluoxetine (10 mg/kg) significantly reduced PGES following seizures induced during wake (52.33 ± 45.66 s vs 28.25 ± 13.31 s, n = 11; p < 0.05; Fig. 2B, G), but not seizures induced during NREM (52.33 ± 8.336 s vs 27.97 ± 4.96 s; n = 30, 11; Fig. 2B, H). The duration of seizures induced during wake (38.43 ± 2.234 s vs 35.91 ± 2.04 s; n = 30, 11; Fig. 2D) and NREM (34.59 ± 2.57 s vs 36.22 ± 2.53 s; n = 18, 9; Fig. 2D) was unaffected by fluoxetine administration.
Figure 2.
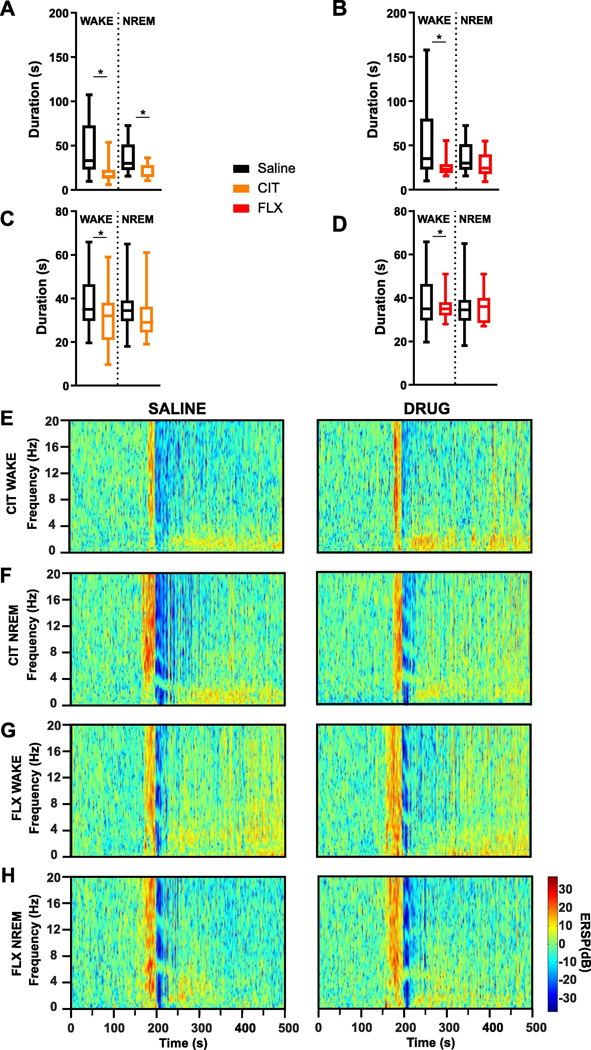
Systemic application of SSRIs reduced PGES following seizures in amygdala kindled mice. (A,B) PGES duration and seizure duration (C,D) following seizures induced during wake and non-rapid eye movement (NREM) in mice pre-treated with citalopram (A,C; CIT; 20 mg/kg, i.p.) or fluoxetine (B,D; FLX; 10 mg/kg, i.p.). *, p < 0.05. (E-H) Representative spectral plots demonstrating PGES duration following amygdala stimulation in kindled mice that received (E,F) CIT or (G,H) FLX prior to seizures induced during wake (E,G) or NREM (F,H).Y-axis: event related spectral perturbation (ERSP) power in decibels (dB).
Stimulation of DRN with acidosis reduced PGES following seizures in amygdala kindled mice
To determine whether stimulation of DRN neurons prior to a seizure could reduce PGES duration, adult male and female C57BL/6J mice were instrumented with a guide cannula aimed at the DRN. 5-HT neurons within the DRN are chemosensitive and respond robustly to decreases in pH (Richerson, 2004), therefore, to stimulate DRN 5-HT neurons we focally administered normal or acidified aCSF through the guide cannula for 10 min prior to seizure induction. Pre-seizure perfusion of acidified aCSF to the DRN reduced PGES duration following seizures induced during wake (43.75 ± 6.73 s vs 25.79 ± 4.95 s; n = 6; p < 0.05; paired t-test; Fig. 3A, D). There was no effect on seizure duration (Fig. 3B). Application of acidified aCSF to the DRN in this manner during NREM sleep causes arousal (Smith, et al., 2018), so the effect of DRN stimulation on seizures induced during sleep could not be tested.
Figure 3.
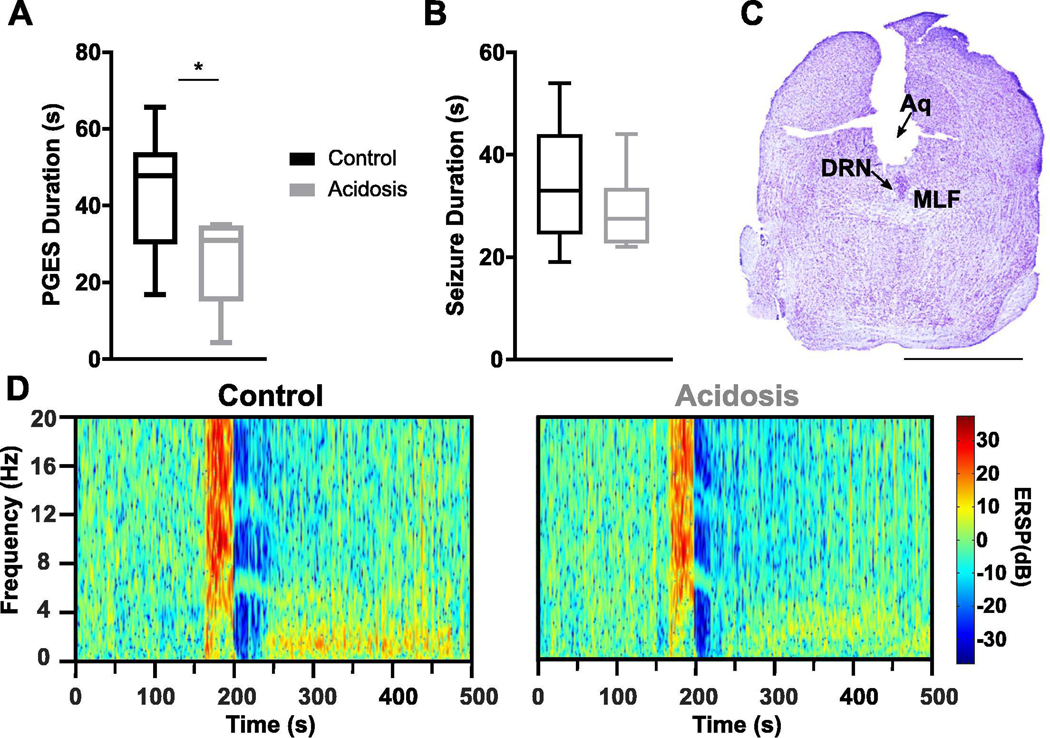
Focal chemical stimulation of the dorsal raphe nucleus (DRN) reduced PGES following seizures in amygdala kindled mice. (A,B) PGES duration (A) and seizure duration (B) following perfusion of normal (pH 7.4) or acidified (pH 6.8) artificial cerebrospinal fluid (aCSF) prior to seizure induction during wake. *, p < 0.05. (C) Nissl stained coronal section at the level of the midbrain depicting position of the cannula targeted toward the DRN. Aq, aqueduct; DRN, dorsal raphe nucleus; MLF, medial longitudinal fasciculus; scale bar: 2.5 mm. (D) Representative spectral plots of PGES duration following perfusion of normal (left) or acidified (right) aCSF prior to seizure induction.
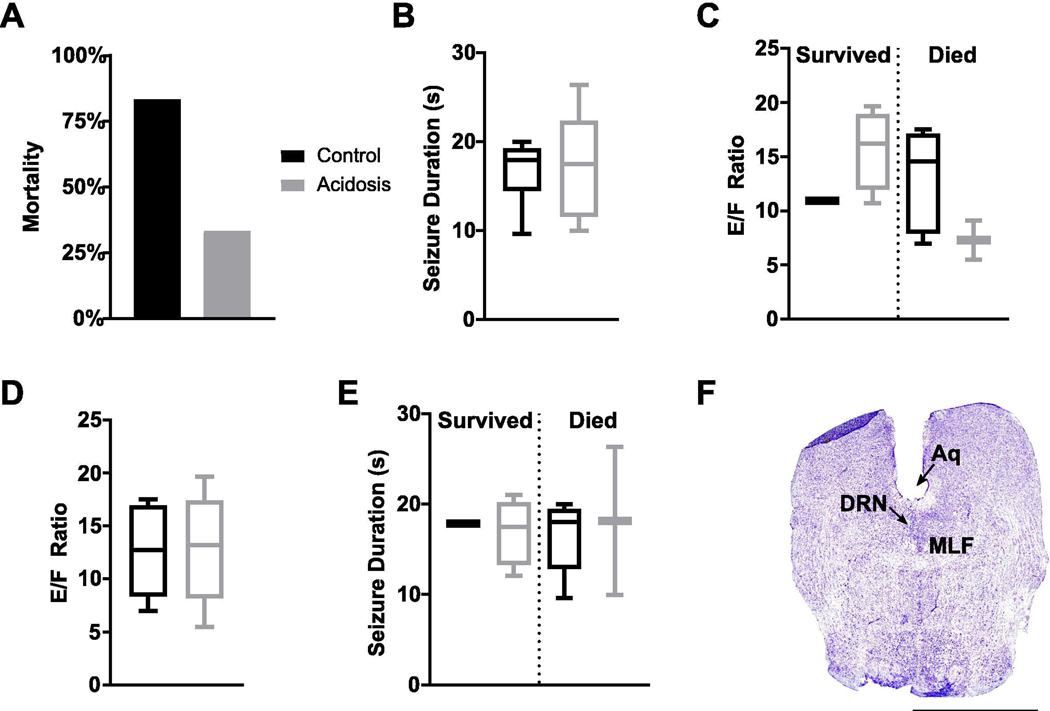
Stimulation of DRN with acidosis may influence mortality following MES seizures
While stimulation of DRN with acidosis reduced PGES duration following seizures in amygdala kindled mice, there is not consistent mortality in the model. To determine whether stimulation of DRN neurons with acidosis could also reduce mortality, DRN neurons were stimulated with acidified aCSF for 10 min prior to seizure induction via MES, a model with frequent seizure-induced mortality (Buchanan, et al., 2014; Hajek and Buchanan, 2016; Kruse et al., 2019; Li and Buchanan, 2019; Purnell, et al., 2017). Perfusion of acidified aCSF into the DRN prior to MES seizures trended toward reducing mortality (4 survived vs 2 died; acidosis: n = 6; normal: n = 6; p = 0.078; Fig. 4A). Application of acidified aCSF to the DRN had no effect on seizure duration (Fig. 4B) and seizure duration did not differ between animals that survived versus died (independent t-test) (Fig. 4C). Seizure severity was similarly unaffected and did not differ between animals that survived versus died (Fig. 4D, E).
Figure 4.
Focal chemical stimulation of the dorsal raphe nucleus (DRN) does not affect seizure duration or severity. (A,B) Seizure-induced mortality (A) and seizure duration (B) from seizures induced following perfusion of normal (pH 7.4) or acidified artificial cerebrospinal fluid (aCSF; pH 6.8) into the DRN. (C) Seizure duration in mice that survived vs died from the MES seizure. (D) Seizure severity (E/F ratio) following pre-seizure perfusion of acidified aCSF. (E) Seizure severity in mice that survived vs died from the MES seizure. (F) Nissl stained coronal section demonstrating cannula targeted toward the DRN. Aq, aqueduct; DRN, dorsal raphe nucleus; MLF, medial longitudinal fasciculus; scale bar: 2.5 mm.
Optogenetic stimulation of DRN 5-HT neurons reduced PGES following seizures in amygdala kindled mice
Kindled Pet1-Cre or WT mice that received an injection of AAV-ChR2 into the DRN were subjected to laser light stimulation or no laser light stimulation for 10 min prior to induction of a seizure during wake. Application of laser light to DRN 5-HT neurons prior to seizure induction during wake decreased PGES (100.40 ± 26.82 s vs 37.57 ± 13.22 s; n = 8; p < 0.05; Wilcoxon matched pairs signed rank test; Fig. 5A, F) without affecting seizure duration (Pet1-Cre: 29.57 ± 5.54 s vs 29.61 ± 2.83 s; n = 8; WT: 22.14 ± 1.89 s vs 23.14 ± 1.62 s; n = 7; Fig. 5B). Viral transfection in Pet1-Cre mice was successful and limited to our 5-HT cell populations of interest (Fig. 5C–E). PGES duration and seizure duration did not differ between Pet1-Cre mice and their WT littermates (Fig. 5A, B, F). There was no ChR2 expression in WT mice (Fig. 5E).
Figure 5.
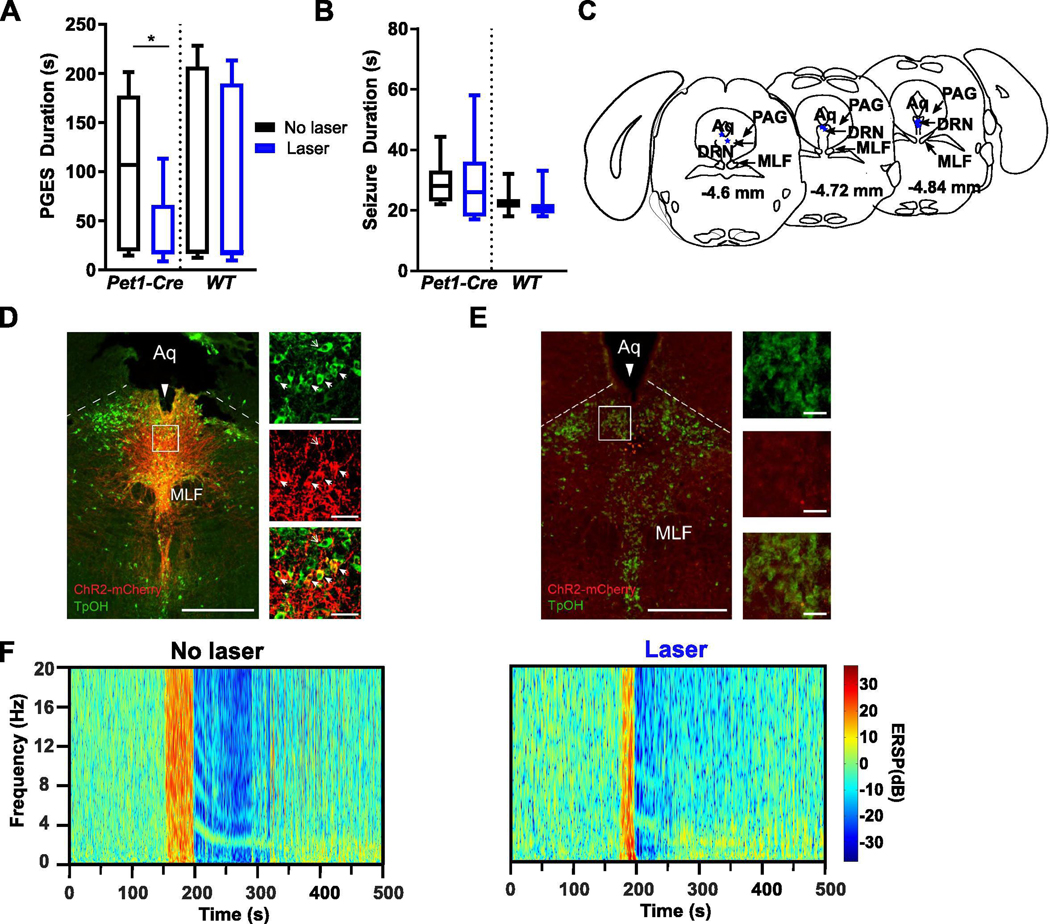
Optogenetic stimulation of the dorsal raphe nucleus (DRN) reduced PGES following seizures in amygdala kindled mice. (A, B) Duration of PGES (A) and seizures (B) following laser stimulation (blue; 473 nm; 4 Hz, 10 mW, 2% duty; 10 s on / 10 s off) or no stimulation (black) in DRN neurons prior to seizure induction during wake in Pet1-Cre (n = 7) and WT (n = 8) mice injected with AAV-ChR2 into the DRN. *, p < 0.05. (C) Depiction of optic fiber positioning within the DRN in Pet1-Cre mice injected with AAV-ChR2. Aq, aqueduct; DRN, dorsal raphe nucleus; MLF, medial longitudinal fasciculus; PAG, periaqueductal gray. (D,E) Immunostained coronal section depicting optical fiber tracts (long solid arrow), ChR2 expression (red), serotonergic (5-HT) cell bodies (green/non-solid small arrow), and co-expression (yellow/solid small arrows) within the DRN of a Pet1-Cre (D) and WT (E) mouse that received an injection of AAV-ChR2 into DRN. Box indicates location of magnified cells on right (top, 5-HT cells; middle, ChR2; bottom, merge). Aq, aqueduct; MLF, medial longitudinal fasciculus; PAG, periaqueductal gray. Scale bar: 500 μm, 50 μm. (F) Representative spectral plots of PGES duration following either no laser (left) or laser stimulation (right) of DRN 5-HT neurons prior to seizure induction in a Pet1-Cre mouse injected with AAV-ChR2.
PGES in amygdala kindled mice was associated with immobility that could be prevented by pretreatment with citalopram.
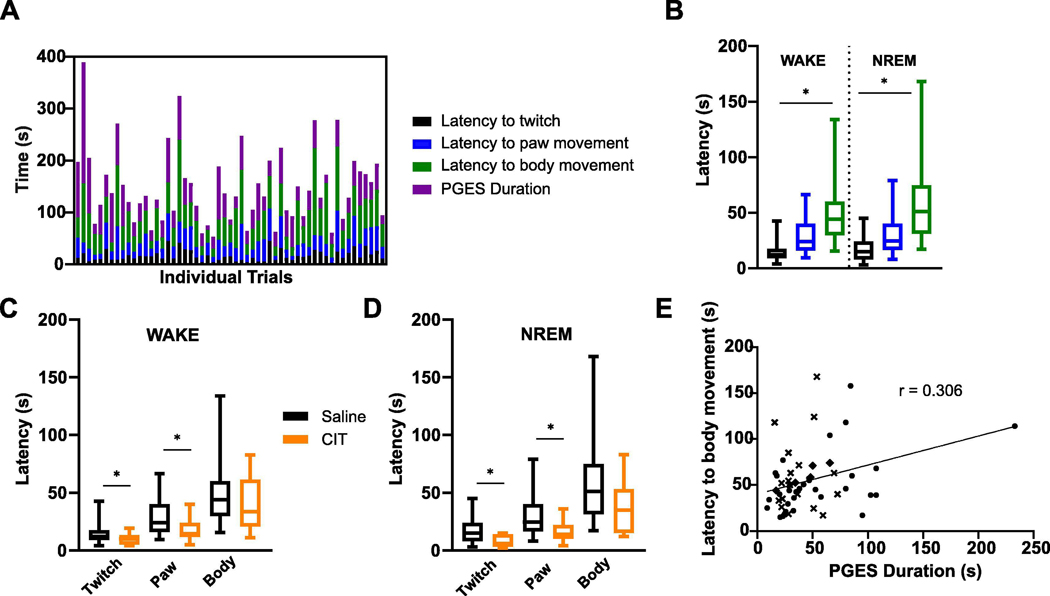
During PGES in amygdala kindled mice, there was immobility that dissipated in a stepwise manner. Mice were immobile at the onset of PGES. This gradually progressed to a body twitch, then unilateral paw movements, and then finally bilateral effort to reposition the body or resumption of preening behavior (n = 55; Fig. 6A). The stepwise return of mobility was observed during PGES following seizures induced during wake (twitch: 15.08 ± 1.81 s vs paw movement: 28.40 ± 2.89 s vs body movement: 51.27 ± 5.95 s; n = 31; p < 0.05) and NREM sleep (twitch: 16.78 ± 2.52 s vs paw movement: 29.69 ± 4.74 s vs body movement: 60.17 ± 9.60 s; n = 18; p < 0.05) with no difference in the latency to each phase (Fig. 6B).
Figure 6.
PGES was associated with immobility that could be reduced by administration of citalopram (CIT; i.p., 20 mg/kg). (A) Latency to observation of first twitch (black), paw movement (blue), and body movement (green) after seizure termination. Duration of PGES (purple) is plotted for each trial. Columns depict a different animal/trial (n = 55). (B) Mean latencies to first observed twitch (black), paw movement (blue), and body movement (green) after seizures induced during wake (n = 31) and non-rapid eye movement (NREM) (n = 19). *, p < 0.05. (C,D) Latency to observation of first twitch, paw movement, and body movement after seizures induced during wake (C; n = 23) and NREM (D; n = 13) in mice pretreated with saline (black) or CIT (orange). *, p < 0.05. (E) Linear regression between PGES duration and latency to body movement following seizures induced during wake (n = 31). *, p < 0.05. Symbols represent different groups.
Pretreatment with citalopram (i.p., 20 mg/kg) 30–60 min before seizure induction during wake (twitch: 15.08 ± 1.81 s vs 9.591 ± 4.56 s; paw movement: 28.40 ± 2.89 s vs 18.55 ± 10.11 s; body movement: 51.27 ± 5.94 s vs 39.19 ± 2.73 s; n = 31, 22; p < 0.05) and NREM (twitch: 16.78 ± 2.52 s vs 7.27 ± 1.48 s; paw movement: 29.69 ± 4.74 s vs 16.00 ± 2.85 s; body movement: 60.17 ± 9.60 s vs 36.82 ± 6.49 s; n = 18, 11; p < 0.05) sleep reduced the latency to first twitch and paw movement (Fig. 6C, D). Fluoxetine administration prior to seizures induced during wake (twitch: 15.08 ± 1.81 s vs 11.91 ± 1.80 s; paw movement: 28.40 ± 2.89 s vs 25.50 ± 3.43 s; body movement: 51.27 ± 5.94 s vs 48.48 ± 6.31 s; n = 31, 11) and NREM (twitch: 16.78 ± 2.52 s vs 15.28 ± 2.96 s; paw movement: 29.69 ± 4.74 s vs 35.61 ± 6.80 s; body movement: 60.17 ± 9.60 s vs 52.00 ± 7.23 s; n = 18, 11) did not reduce latency to twitch, paw movement, or body movement during PGES. There was a positive correlation observed between latency to body movement and PGES duration in seizures induced during wake (n = 31; p < 0.05; r = 0.306; Fig. 6E), but not NREM (n = 18; r = 0.09).
Citalopram reduced latency to return of EEG activity to baseline during PGES.
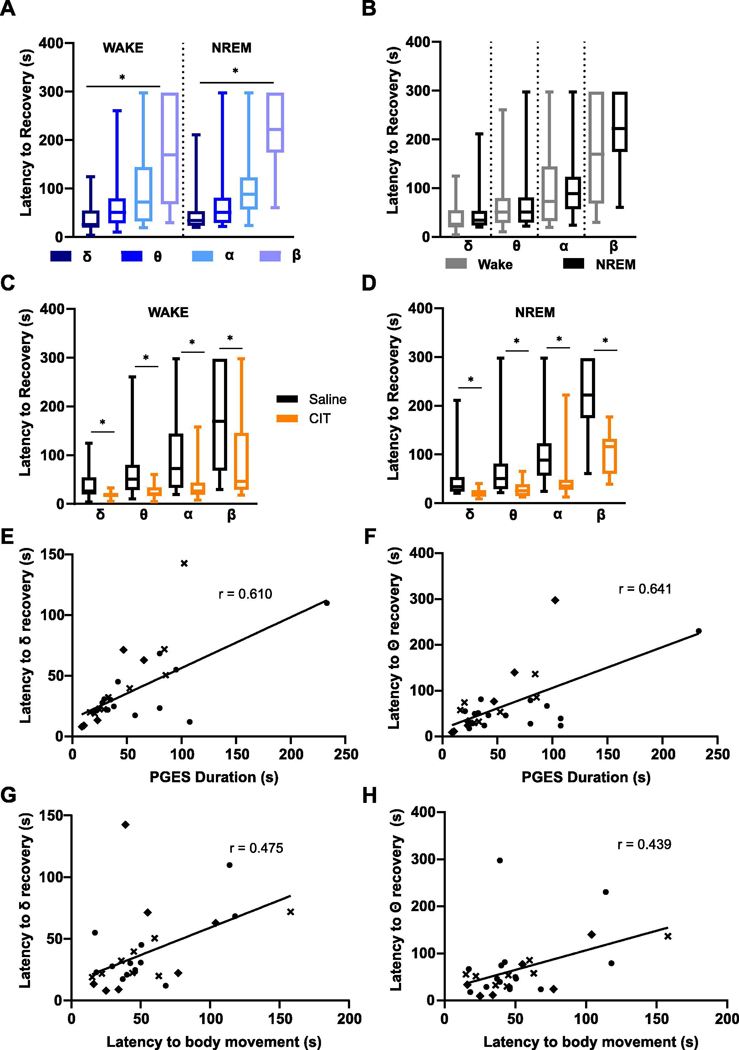
EEG bands re-emerged during PGES with the slower, low frequency bands recovering before the high frequency bands (Fig. 7A). This pattern was observed during PGES following seizures induced during wake (δ: 38.24 ± 31.20 s; θ: 67.60 ± 63.02 s; α: 102.70 ± 82.43 s; β: 183.30 ± 104.50 s; p < 0.05) or NREM (δ: 46.37 ± 42.75 s; θ: 73.41 ± 74.76 s; α: 115.1 ± 91.53 s; β: 223.60 ± 78.13 s; p < 0.05) sleep (Fig. 7B). Pretreatment with citalopram (i.p., 20 mg/kg) 30–60 min before seizure induction during wake (δ: 18.27 ± 7.60 s; θ: 24.25 ± 14.15 s; α: 37.50 ± 36.63 s; β: 87.80 ± 81.49 s; δ, θ, α, wake: n = 23; β, wake: n = 12; p < 0.05) and NREM (δ: 20.25 ± 9.613 s; θ: 28.09 ± 15.50 s; α: 50.03 ± 53.68 s; β: 102.9 ± 49.37 s; δ, θ, α, NREM: n = 13; β, NREM: n = 7; p < 0.05) reduced the latency of all frequencies to return to baseline activity (Fig. 7C, D). Pretreatment with fluoxetine did not affect the latency of EEG rhythms returning to baseline activity following seizures induced during wake, but did following NREM (δ: 31.63 ± 3.73 s; θ: 39.28 ± 5.92 s; α: 73.34 ± 15.58 s; β: 148.3 ± 23.78 s; δ, θ, α, β wake: n = 11; δ: 24.47 ± 5.33 s; θ: 33.05 ± 4.49 s; α: 59.72 ± 20.73 s; β: 106.2 ± 32.57 s; NREM: n = 9; p < 0.05). Latency to resumption of baseline activity in the δ and θ frequency bands correlated most strongly with PGES duration in seizures induced during wake (δ: r = 0.610; θ: r = 0.641; α: r = 0.442; β: r = 0.478; p < 0.05). Similarly, latency to full body movement also correlated with return to baseline activity of the δ and θ frequency bands in seizures induced from wake (δ: r = 0.47; θ: r = 0.44; p < 0.05). However, these correlations were not observed for seizures induced from NREM (δ: r = 0.044; θ: r = 0.094).
Figure 7.
Resumption of baseline EEG activity occurred in a stepwise manner during PGES and could be accelerated with citalopram pretreatment. (A,B) Recovery of EEG rhythms to baseline during PGES following seizures induced during (A) wake (δ, θ, α: n = 31; β: wake: n = 26) and (B) non-rapid eye movement (NREM) (δ, θ, α: n = 19; β: n = 13). *, p < 0.05. (C,D) Effect of citalopram pretreatment (CIT; i.p., 20 mg/kg) 30–60 min following seizures induced during (C) wake (δ, θ, α: n = 23; β: n = 12) and (D) NREM (δ, θ, α: n = 13, β: n = 7) on recovery of EEG rhythms to baseline during PGES. *, p < 0.05. (E,F) Linear regression analysis of the relationship between recovery of δ (E, r = 0.610) and θ (F, r = 0.641) with PGES duration following seizures induced during wake. *, p < 0.05. (G,H) Linear regression of the relationship between recovery of δ (G, r = 0.475) and θ (H, r = 0.439) with latency to body movement during PGES. *, p < 0.05. delta (δ), 1 – 4 Hz; theta (θ), 4 – 8 Hz; alpha (α), 8 – 12 Hz; beta (β), 12 – 30 Hz. Symbols represent different groups.
Discussion
Prolonged PGES duration may portend increased risk of SUDEP. Understanding methods of shortening PGES duration may translate to methods to reduce the likelihood of SUDEP. Here we found that stimulating 5-HT neurotransmission at the DRN reduced PGES duration.
PGES is common following seizures in humans. It is most common with generalized seizures (Surges et al., 2011), whether they are primary generalized (Poh et al., 2012) or focal to bilateral tonic clonic seizures (Marchi et al., 2019), but can also be seen with focal seizures (Lhatoo, et al., 2010). Similarly, PGES has been observed in animal models following kindled seizures (Buterbaugh, 1987), MES seizures (Hajek and Buchanan, 2016), and pentylenetetrazol induced seizures (Luttjohann et al., 2009; Mirski and Fisher, 1994). PGES preceded all cases of SUDEP with adequate data in the multi-center MORTEMUS study and early research in humans suggests that PGES duration exceeding 50 s increases SUDEP risk (Lhatoo, et al., 2010).
PGES often follows nocturnal (Alexandre et al., 2015; Latreille et al., 2017; Okanari, et al., 2017) and generalized tonic clonic seizures (Lhatoo, et al., 2010; Surges, et al., 2011), which are independent risk factors for SUDEP (Lamberts, et al., 2012). PGES has also been associated with peri-ictal tachycardia and hypoxemia in pediatric patients (Moseley et al., 2013). Increases in sympathetic tone and decreases in parasympathetic tone observed during PGES may also precipitate fatal cardiac arrhythmias (Bozorgi et al., 2013; Moseley et al., 2013; Poh, et al., 2012). In turn, cardiac dysfunction could further precipitate PGES by reducing blood flow and oxygenation to the brain (Bozorgi and Lhatoo, 2013).
It should be noted that while some studies observed a positive correlation between PGES and SUDEP (Lhatoo, et al., 2010), other studies have not recapitulated this finding (Lamberts et al., 2013; Surges, et al., 2011). Moreover, despite suppression of scalp EEG activity, subcortical activity may persist during PGES (Bateman et al., 2019). However, motor artifact (oral tonicity, facial automatisms) and proximity of electrodes to the skull can cause detection of gamma activity during PGES (Marchi, et al., 2019; McGonigal et al., 2019; Okanari, et al., 2017). It is possible that small movements may have contributed to these findings, but it is likely that not all brain regions are equally suppressed during PGES. Faster recovery may occur in subcortical structures that are beyond detection on scalp EEG. Continued PGES research in animal models may provide insight into such questions that highly variable human studies cannot.
Parallels are often drawn between PGES and spreading depression (SD), a self-propagating wave of depolarization that can result from disturbances in ion homeostasis during seizures (Kramer et al., 2016). SD causes suppression of the EEG as the affected neurons become refractory to excitation (Pietrobon and Moskowitz, 2014). However, while PGES abruptly begins at seizure termination and has an acute phase, SD can occur after seizure termination (Aiba and Noebels, 2015) and reverberate about the seizure focus for 4 ½ minutes (Koroleva and Bures, 1979). SD may also be observed without the presence of PGES (Aiba and Noebels, 2015). More research is needed to disentangle these two processes, but it is plausible that PGES is consequent to innate seizure cessation mechanisms, whereas SD may result from the direct effect of the seizure on neuronal microenvironments.
Impairment of arousal is also relevant to PGES. SUDEP victims are often found in a prone position. In a systemic review of SUDEP and body position, 73.3% of 253 recorded SUDEP cases were discovered prone (Liebenthal et al., 2015). Seizures with PGES exhibit longer post-ictal immobility (Asadollahi et al., 2018; Seyal et al., 2013) which may increase risk of rebreathing CO2 and exacerbate unconsciousness. This immobility was recapitulated in our mice, which progressively recovered mobility from twitches to paw movements to body movements following seizures. Here pretreatment with citalopram not only shortened PGES duration, but shortened latency to first twitch and paw movement. It is difficult to correlate post-ictal movement in a mouse to an epilepsy patient; however, it may provide an adequate approximation for when life-saving responses to stimuli may reoccur.
Pretreatment with fluoxetine reduced PGES duration only following seizures induced during wake and did not shorten latency to twitch, paw, or body movements. Fluoxetine and citalopram both interact with serotonin transporters, although the (S)-enantiomer of citalopram binds much more efficiently than fluoxetine (Owens et al., 2001), potentially contributing to divergent results. It is also possible that the concentration of fluoxetine was lower in animals with seizures induced earlier, contributing to differences in SSRI efficacy (Malagie et al., 1995). However, our 30–60 min stimulation window fell well below the half-life of citalopram (1.5 hrs) (Fredricson Overo, 1982) and fluoxetine (2.61 hrs) (Holladay, et al., 1998) in mice. In other animal studies,10 mg/kg of fluoxetine reduced the dose of anti-epileptic drug needed to protect mice against MES seizures (Leander, 1992). However, in another study only 15–25 mg/kg fluoxetine reduced the incidence of respiratory arrest after audiogenic seizures in DBA/2 mice (Tupal and Faingold, 2006). Therefore, a higher dose may have potentiated the effect of fluoxetine on PGES duration and reduced PGES duration from NREM as citalopram did.
Reduced arousal, stupor, and unconsciousness are frequently associated with generalized seizures (Blumenfeld, 2012; Englot et al., 2010) and are associated with PGES (Kuo, et al., 2016). An inability to arouse to CO2 or other stimuli during the postictal period may be one mechanism by which PGES contributes to seizure mortality. We hypothesize that PGES may be an electrographic marker of impaired arousal consequent to ictal 5-HT neurotransmission dysfunction. Here administration of SSRIs prior to a seizure decreased PGES duration in amygdala kindled mice. Similarly, pre-seizure application of acidified aCSF to the DRN or optogenetic stimulation of DRN 5-HT neurons decreased PGES duration in amygdala kindled mice. In the MES model, studies have demonstrated that PGES duration and mortality is greater following seizures induced during NREM sleep (Hajek and Buchanan, 2016; Purnell, et al., 2017). We observed a trend indicating that pre-seizure acidosis of the DRN may reduce mortality following MES seizures induced from wake. Our sample was underpowered therefore it is possible that pre-seizure DRN acidosis may reduce mortality with a larger cohort. Application of acidified aCSF to the DRN precluded induction of seizures during sleep, but it is likely that seizure induction during NREM/REM could increase mortality despite DRN acidosis due to decreases in 5-HT activity during sleep (McGinty and Harper, 1976). We did not observe an increase in PGES following seizures induced in NREM; however, this may be due to differing seizure propagation and affected regions between the MES and kindling model.
Stimulation of the DRN with laser light or acidosis could have unintentionally affected nearby neurons in the PAG or median raphe nucleus (MRN). The MRN modulates firing from the medial septum and can alter θ rhythm activity, thus stimulation of the MRN could be responsible for EEG changes (Vertes and Kocsis, 1997). Transfection of AAV-ChR2 was seen within our mice, however, the distance between the DRN and MRN (∼1 mm) likely prevented sufficient light power from reaching transfected neurons (Yizhar et al., 2011). Similarly, the affected area of tissue acidosis was approximately 550 μm, (0.69 mm3 assuming spherical distribution) and likely did not affect MRN neurons (Li et al., 1999). Chemical stimulation of DRN 5-HT neurons could have resulted in some acidosis of the PAG. But, we did not observe any effects of stimulating the PAG such as vocalizations and irregular breathing during the pretreatment period (Faull et al., 2019).
In humans, PGES is often followed by δ slowing (Fisher and Engel, 2010; Kaibara and Blume, 1988) that eventually transitions into θ as the EEG begins to return to normal (Fisher and Engel, 2010; So and Blume, 2010). PGES duration correlated most strongly with the recovery latency of the δ and θ bands. Cortical δ slow waves are often associated with reduced consciousness and responsiveness to stimuli (Blumenfeld, 2012). Disruption of brainstem ascending arousal system structures by seizures may lead to depressed cortical activity and detection of cortical slow waves (Blumenfeld, 2012). It is possible that the emergence of slow waves following PGES indicates resumption of some subcortical activity, but continued suppression of cortical activity. The DRN is a major component of the ascending arousal system that sends 5-HT projections to the cortex (Vertes, 1991). Stimulation of the DRN with acidosis may have reduced PGES by increasing activity of its projections to the cortex, thus restoring reciprocal interactions between cortical-subcortical networks and terminating PGES.
The θ rhythm is primarily generated in the hippocampus (Adamantidis et al., 2019; Lubenov and Siapas, 2009) and is driven by GABAergic neurons in the medial septum firing at 4–8 Hz (Colgin, 2016). It is plausible that as EEG recovery continues following PGES, the emergence of θ waves may be an EEG indicator of continued post-ictal stupor and confusion. Persistence of the θ rhythm following waking is associated with disorientation, reduced vigilance, and reduced arousal in humans (Ferrara et al., 2006). We also observed a correlation between the recovery of the δ and θ frequency bands and latency to full body movement in our mice. During wakefulness, hippocampal θ activity is associated with locomotion, spatial navigation, and locomotion speed (Bender et al., 2015; Slawinska and Kasicki, 1998). Therefore, increases in θ activity may have preceded or followed movement initiated by the mice as they recovered from PGES.
Here we demonstrate that PGES occurs consistently following seizures in kindled animals and that increasing 5-HT activity can reduce PGES duration and immobility following PGES. Our results support recent work implicating 5-HT in SUDEP and SUDEP risk factors such as breathing and arousal (Richerson, 2013). It is possible that decreases in 5-HT (influenced by time-of-day, sleep state, and having epilepsy) increases risk of cardiorespiratory dysregulation and impairment of arousal which could consequently increase risk for SUDEP following a seizure (Buchanan, 2019; Petrucci, et al., 2020). PGES could result from seizure-induced depression of subcortical arousal network activity to the cortex. The DRN may be integral because seizures reduce firing of DRN 5-HT neurons (Zhan, et al., 2016) and, under normal conditions, the DRN sends 5-HT projections to the hippocampus, the pedunculopontine tegmental nucleus, the basal forebrain, the cortex, and other wake promoting structures (Monti, 2010; Vertes, 1991). While the DRN is likely not the only structure involved in the generation of PGES, it is evident that increasing 5-HT activity prior to a seizure reduces PGES and post-ictal immobility. More work will be needed to understand the precise location and mechanism of action of 5-HT in modulating PGES. Ultimately, it is our hope that enhancing knowledge of PGES will aid in identifying high-risk epilepsy patients and reduce seizure-induced mortality.
Highlights.
PGES may be a risk indicator for SUDEP, but its mechanisms are unknown.
5-HT enhancing drugs and stimulation of DRN 5-HT neurons reduced PGES duration.
Citalopram pretreatment reduced post-ictal immobility associated with PGES.
EEG recovery following PGES occurred in an organized manner with lower frequencies recovering first.
Reducing PGES duration by enhancing 5-HT neurotransmission may reduce SUDEP risk.
Acknowledgements:
This work was supported by a Post-Comprehensive Exam Fellowship from the Graduate College at the University of Iowa, a Pre-Doctoral Fellowship from the American Epilepsy Society (with funding from LivaNova, PLC), and NIH/NINDS F31 NS113479 to A.N.P., a Summer Fellowship from the Iowa Center for Research by Undergraduates to K.M.V., and NIH/NINDS R01 NS095842 and the Beth L. Tross Epilepsy Professorship from the Carver College of Medicine at the University of Iowa to G.F.B. Funding sources did not influence study design, data collection, analysis, interpretation, preparation of the manuscript, or the decision to publish.
Abbreviations:
- 3V
third ventricle
- 5-HT
5-hydroxytryptamine, serotonin
- AAV
adeno-associated virus
- aCSF
artificial cerebrospinal fluid
- AP
anterior-posterior
- Aq
aqueduct
- BLA
basolateral amygdala
- ChR2
channelrhodopsin2
- CIT
citalopram
- Cy
cyanine
- dB
decibel
- DRN
dorsal raphe nucleus
- Ds
discosoma
- DV
dorsal-ventral
- EEG
electroencephalogram
- E/F ratio
extension-to-flexion ratio
- EMG
electromyogram
- ERSP
event-related spectral perturbation
- FLX
fluoxetine
- GEPR
genetic epilepsy prone rats
- GFP
green fluorescent protein
- HPC
hippocampus
- i.p.
intraperitoneally
- MES
maximal electroshock
- ML
medial-lateral
- MLF
medial longitudinal fasciculus
- MRN
median raphe nucleus
- NREM
non-rapid eye movement
- PAG
periaqueductal gray
- PBS
phosphate buffered saline
- PGES
post-ictal generalized EEG suppression
- Pleth
plethysmography
- SD
spreading depression
- REM
rapid eye movement
- SSRI
selective serotonin reuptake inhibitor
- SUDEP
sudden unexpected death in epilepsy
- Sz
seizure
- TpOH
tryptophan hydroxylase
- WT
wild type
Footnotes
Conflict of interest statement: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis AR, Gutierrez Herrera C, Gent TC (2019), Oscillating circuitries in the sleeping brain. Nat Rev Neurosci 20:746–762. [DOI] [PubMed] [Google Scholar]
- Aiba I, Noebels JL (2015), Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci Transl Med 7:282ra246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre V, Mercedes B, Valton L, Maillard L, Bartolomei F, Szurhaj W, Hirsch E, Marchal C, et al. (2015), Risk factors of postictal generalized EEG suppression in generalized convulsive seizures. Neurology 85:1598–1603. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Howard RA, Woodbury DM (1986), Correlation between effects of acute acetazolamide administration to mice on electroshock seizure threshold and maximal electroshock seizure pattern, and on carbonic anhydrase activity in subcellular fractions of brain. Epilepsia 27:504–509. [DOI] [PubMed] [Google Scholar]
- Asadollahi M, Noorbakhsh M, Simani L, Ramezani M, Gharagozli K (2018), Two predictors of postictal generalized EEG suppression: Tonic phase duration and postictal immobility period. Seizure 61:135–138. [DOI] [PubMed] [Google Scholar]
- Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, Ogiwara I, Yamakawa K, Meisler MH, et al. (2013), Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One 8:e77843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Kecskemeti V, Riba P, Jakus R (2007), Serotonin and epilepsy. J Neurochem 100:857–873. [DOI] [PubMed] [Google Scholar]
- Bateman LM, Mendiratta A, Liou JY, Smith EJ, Bazil CW, Choi H, McKhann GM, Pack A, et al. (2019), Postictal clinical and electroencephalographic activity following intracranially recorded bilateral tonic-clonic seizures. Epilepsia 60:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender F, Gorbati M, Cadavieco MC, Denisova N, Gao X, Holman C, Korotkova T, Ponomarenko A (2015), Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat Commun 6:8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H (2012), Impaired consciousness in epilepsy. Lancet Neurol 11:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Lampert A, Klein JP, Mission J, Chen MC, Rivera M, Dib-Hajj S, Brennan AR, et al. (2009), Role of hippocampal sodium channel Nav1.6 in kindling epileptogenesis. Epilepsia 50:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorgi A, Chung S, Kaffashi F, Loparo KA, Sahoo S, Zhang GQ, Kaiboriboon K, Lhatoo SD (2013), Significant postictal hypotension: expanding the spectrum of seizure-induced autonomic dysregulation. Epilepsia 54:e127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorgi A, Lhatoo SD (2013), Seizures, Cerebral Shutdown, and SUDEP. Epilepsy Curr 13:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF (2019), Impaired CO2-Induced Arousal in SIDS and SUDEP. Trends Neurosci 42:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Murray NM, Hajek MA, Richerson GB (2014), Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 592:4395–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB (2010), Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A 107:16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Smith HR, MacAskill A, Richerson GB (2015), 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol 114:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buterbaugh GG (1987), Postictal Events in Amygdala-Kindled Female Rats with and without Estradiol Replacement. Exp Neurol 95:697–713. [DOI] [PubMed] [Google Scholar]
- Ceulemans B, Boel M, Leyssens K, Van Rossem C, Neels P, Jorens PG, Lagae L (2012), Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 53:1131–1139. [DOI] [PubMed] [Google Scholar]
- Chen Z, Brodie MJ, Liew D, Kwan P (2018), Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol 75:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL (2016), Rhythms of the hippocampal network. Nat Rev Neurosci 17:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K (1964), Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta physiologica Scandinavica Supplementum:SUPPL 232:231–255. [PubMed] [Google Scholar]
- Dailey JW, Yan QS, Mishra PK, Burger RL, Jobe PC (1992), Effects of Fluoxetine on Convulsions and on Brain-Serotonin as Detected by Microdialysis in Genetically Epilepsy-Prone Rats. J Pharmacol Exp Ther 260:533–540. [PubMed] [Google Scholar]
- Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G (2016), Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 15:1075–1088. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E (2008), Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol (1985) 105:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Spencer DD (1993), Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341:1607–1610. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Yang L, Hamid H, Danielson N, Bai X, Marfeo A, Yu L, Gordon A, et al. (2010), Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain 133:3764–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL, Kommajosyula SP, Long X, Plath K, Randall M (2014), Serotonin and sudden death: differential effects of serotonergic drugs on seizure-induced respiratory arrest in DBA/1 mice. Epilepsy Behav 37:198–203. [DOI] [PubMed] [Google Scholar]
- Faull OK, Subramanian HH, Ezra M, Pattinson KTS (2019), The midbrain periaqueductal gray as an integrative and interoceptive neural structure for breathing. Neurosci Biobehav Rev 98:135–144. [DOI] [PubMed] [Google Scholar]
- Favale E, Audenino D, Cocito L, Albano C (2003), The anticonvulsant effect of citalopram as an indirect evidence of serotonergic impairment in human epileptogenesis. Seizure-Eur J Epilep 12:316–318. [DOI] [PubMed] [Google Scholar]
- Favale E, Rubino V, Mainardi P, Lunardi G, Albano C (1995), Anticonvulsant effect of fluoxetine in humans. Neurology 45:1926–1927. [DOI] [PubMed] [Google Scholar]
- Ferrara M, Curcio G, Fratello F, Moroni F, Marzano C, Pellicciari MC, Gennaro LD (2006), The electroencephalographic substratum of the awakening. Behav Brain Res 167:237–244. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr., Forsgren L, et al. (2014), ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55:475–482. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Engel JJ Jr. (2010), Definition of the postictal state: when does it start and end? Epilepsy Behav 19:100–104. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M (1998), Genetic variation in EEG activity during sleep in inbred mice. Am J Physiol 275:R1127–1137. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (2013) Paxinos and Franklin’s The mouse brain in stereotaxic coordinates. Elsevier. [Google Scholar]
- Fredricson Overo K (1982), Kinetics of citalopram in test animals; drug exposure in safety studies. Prog Neuropsychopharmacol Biol Psychiatry 6:297–309. [DOI] [PubMed] [Google Scholar]
- Hajek MA, Buchanan GF (2016), Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J Neurophysiol 115:2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES (1999), The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci 19:10348–10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, Walczak TS, Beghi E, et al. (2011), Combined analysis of risk factors for SUDEP. Epilepsia 52:1150–1159. [DOI] [PubMed] [Google Scholar]
- Holladay JW, Dewey MJ, Yoo SD (1998), Pharmacokinetics and antidepressant activity of fluoxetine in transgenic mice with elevated serum alpha-1-acid glycoprotein levels. Drug Metab Dispos 26:20–24. [PubMed] [Google Scholar]
- Hornung J-P (2010) The Neuronatomy of the Serotonergic System. In: Handbook of the Behavioral Neurobiology of Serotonin, vol., pp. 51–64. [Google Scholar]
- Imaizumi K, Ito S, Kutukake G, Takizawa T, Fujiwara K, Tutikawa K (1959), Epilepsy Like Anomaly of Mice. Exp Anim 8:6–10. [Google Scholar]
- Kabuto H, Yokoi I, Takei M, Kurimoto T, Mori A (1994), The anticonvulsant effect of citalopram on El mice, and the levels of tryptophan and tyrosine and their metabolites in the brain. Neurochem Res 19:463–467. [DOI] [PubMed] [Google Scholar]
- Kaibara M, Blume WT (1988), The postictal electroencephalogram. Electroencephalogr Clin Neurophysiol 70:99–104. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Lopes da Silva FH, Wadman WJ (1988), Changes in local evoked potentials in the rat hippocampus (CA1) during kindling epileptogenesis. Brain Res 440:205–215. [DOI] [PubMed] [Google Scholar]
- Koroleva VI, Bures J (1979), Circulation of cortical spreading depression around electrically stimulated areas and epileptic foci in the neocortex of rats. Brain Res 173:209–215. [DOI] [PubMed] [Google Scholar]
- Kramer DR, Fujii T, Ohiorhenuan I, Liu CY (2016), Cortical spreading depolarization: Pathophysiology, implications, and future directions. J Clin Neurosci 24:22–27. [DOI] [PubMed] [Google Scholar]
- Kruse SW, Dayton KG, Purnell BS, Rosner JI, Buchanan GF (2019), Effect of monoamine reuptake inhibition and alpha1 blockade on respiratory arrest and death following electroshock-induced seizures in mice. Epilepsia 60:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Zhao W, Li CS, Kennedy JD, Seyal M (2016), Postictal immobility and generalized EEG suppression are associated with the severity of respiratory dysfunction. Epilepsia 57:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ (2000), Early identification of refractory epilepsy. N Engl J Med 342:314–319. [DOI] [PubMed] [Google Scholar]
- Lamberts RJ, Gaitatzis A, Sander JW, Elger CE, Surges R, Thijs RD (2013), Postictal generalized EEG suppression: an inconsistent finding in people with multiple seizures. Neurology 81:1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW (2012), Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 53:253–257. [DOI] [PubMed] [Google Scholar]
- Latreille V, Abdennadher M, Dworetzky BA, Ramel J, White D, Katz E, Zarowski M, Kothare S, et al. (2017), Nocturnal seizures are associated with more severe hypoxemia and increased risk of postictal generalized EEG suppression. Epilepsia 58:e127–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leander JD (1992), Fluoxetine, a selective serotonin-uptake inhibitor, enhances the anticonvulsant effects of phenytoin, carbamazepine, and ameltolide (LY201116). Epilepsia 33:573–576. [DOI] [PubMed] [Google Scholar]
- Levine AT, Born HA, Landstrom AP, Larson S, Lee WL, Dao AT, Wehrens XH, Lai YC, et al. (2020), Cardiac dysregulation following intrahippocampal kainate-induced status epilepticus. Sci Rep 10:4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM (2010), An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 68:787–796. [DOI] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE (1999), CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol 87:910–919. [DOI] [PubMed] [Google Scholar]
- Li N, Li A, Nattie E (2013), Focal microdialysis of CO(2) in the perifornical-hypothalamic area increases ventilation during wakefulness but not NREM sleep. Respir Physiol Neurobiol 185:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Buchanan GF (2019), Scurrying to Understand Sudden Expected Death in Epilepsy: Insights From Animal Models. Epilepsy Curr 19:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenthal JA, Wu S, Rose S, Ebersole JS, Tao JX (2015), Association of prone position with sudden unexpected death in epilepsy. Neurology 84:703–709. [DOI] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG (2009), Hippocampal theta oscillations are travelling waves. Nature 459:534–539. [DOI] [PubMed] [Google Scholar]
- Luttjohann A, Fabene PF, van Luijtelaar G (2009), A revised Racine’s scale for PTZ-induced seizures in rats. Physiol Behav 98:579–586. [DOI] [PubMed] [Google Scholar]
- Maia GH, Brazete CS, Soares JI, Luz LL, Lukoyanov NV (2017), Serotonin depletion increases seizure susceptibility and worsens neuropathological outcomes in kainate model of epilepsy. Brain Res Bull 134:109–120. [DOI] [PubMed] [Google Scholar]
- Malagie I, Trillat AC, Jacquot C, Gardier AM (1995), Effects of acute fluoxetine on extracellular serotonin levels in the raphe: an in vivo microdialysis study. Eur J Pharmacol 286:213–217. [DOI] [PubMed] [Google Scholar]
- Marchi A, Giusiano B, King M, Lagarde S, Trebuchon-Dafonseca A, Bernard C, Rheims S, Bartolomei F, et al. (2019), Postictal electroencephalographic (EEG) suppression: A stereo-EEG study of 100 focal to bilateral tonic-clonic seizures. Epilepsia 60:63–73. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM (1976), Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res 101:569–575. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM, Fairbanks MK (1973) 5-Ht-Containing Neurons: Unit Activity in Behaving Cats. In: Serotonin and Behavior, vol. (Barchas J, Usdin E, eds), pp. 267–279. Academic Press. [Google Scholar]
- McGonigal A, Marquis P, Medina S, Bartolomei F, Rheims S, Bernard C, Benar C (2019), Postictal stereo-EEG changes following bilateral tonic-clonic seizures. Epilepsia 60:1743–1745. [DOI] [PubMed] [Google Scholar]
- McNamara JO (1995), Analyses of the molecular basis of kindling development. Psychiatry Clin Neurosci 49:S175–178. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Byrne MC, Dasheiff RM, Fitz JG (1980), The kindling model of epilepsy: a review. Prog Neurobiol 15:139–159. [DOI] [PubMed] [Google Scholar]
- Mirski MA, Fisher RS (1994), Electrical stimulation of the mammillary nuclei increases seizure threshold to pentylenetetrazol in rats. Epilepsia 35:1309–1316. [DOI] [PubMed] [Google Scholar]
- Monti JM (2010), The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep Med Rev 14:307–317. [DOI] [PubMed] [Google Scholar]
- Moseley B, Bateman L, Millichap JJ, Wirrell E, Panayiotopoulos CP (2013), Autonomic epileptic seizures, autonomic effects of seizures, and SUDEP. Epilepsy Behav 26:375–385. [DOI] [PubMed] [Google Scholar]
- Moseley BD, So E, Wirrell EC, Nelson C, Lee RW, Mandrekar J, Britton JW (2013), Characteristics of postictal generalized EEG suppression in children. Epilepsy Res 106:123–127. [DOI] [PubMed] [Google Scholar]
- Murugesan A, Rani MRS, Hampson J, Zonjy B, Lacuey N, Faingold CL, Friedman D, Devinsky O, et al. (2018), Serum serotonin levels in patients with epileptic seizures. Epilepsia 59:e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanari K, Otsubo H, Kouzmitcheva E, Rangrej J, Baba S, Ochi A, Okanishi T, Homma Y, et al. (2017), Ictal Symmetric Tonic Extension Posturing and Postictal Generalized EEG Suppression Arising From Sleep in Children With Epilepsy. Pediatr Neurol 76:54–59. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB (2001), Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry 50:345–350. [DOI] [PubMed] [Google Scholar]
- Paterna JC, Feldon J, Bueler H (2004), Transduction profiles of recombinant adeno-associated virus vectors derived from serotypes 2 and 5 in the nigrostriatal system of rats. J Virol 78:6808–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucci AN, Joyal KG, Purnell BS, Buchanan GF (2020), Serotonin and sudden unexpected death in epilepsy. Exp Neurol 325:113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA (2014), Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci 15:379–393. [DOI] [PubMed] [Google Scholar]
- Pinel JP, Rovner LI (1978), Experimental epileptogenesis: kindling-induced epilepsy in rats. Exp Neurol 58:190–202. [DOI] [PubMed] [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Madsen JR, Picard RW (2012), Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 78:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell BS, Hajek MA, Buchanan GF (2017), Time-of-day influences on respiratory sequelae following maximal electroshock-induced seizures in mice. J Neurophysiol 118:2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell BS, Thijs RD, Buchanan GF (2018), Dead in the Night: Sleep-Wake and Time-Of-Day Influences on Sudden Unexpected Death in Epilepsy. Front Neurol 9:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ (1972), Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294. [DOI] [PubMed] [Google Scholar]
- Richerson GB (2004), Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5:449–461. [DOI] [PubMed] [Google Scholar]
- Richerson GB (2013), Serotonin: The Anti-SuddenDeathAmine? Epilepsy Curr 13:241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Buchanan GF (2011), The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia 52 Suppl 1:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH (2008), Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull 34:907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Lee JY, Englot DJ, Gildersleeve S, Piskorowski RA, Siegelbaum SA, Winawer MR, Blumenfeld H (2010), Increased seizure severity and seizure-related death in mice lacking HCN1 channels. Epilepsia 51:1624–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, et al. (2005), A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A 102:16472–16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmelroch M, Elwes RD, Lozsadi DA, Nashef L (2012), Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia 53:e21–24. [DOI] [PubMed] [Google Scholar]
- Seyal M, Bateman LM, Li CS (2013), Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia 54:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U, Kasicki S (1998), The frequency of rat’s hippocampal theta rhythm is related to the speed of locomotion. Brain Res 796:327–331. [DOI] [PubMed] [Google Scholar]
- Smith HR, Leibold NK, Rappoport DA, Ginapp CM, Purnell BS, Bode NM, Alberico SL, Kim YC, et al. (2018), Dorsal Raphe Serotonin Neurons Mediate CO2-Induced Arousal from Sleep. J Neurosci 38:1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So NK, Blume WT (2010), The postictal EEG. Epilepsy Behav 19:121–126. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Wainer BH, Blakely RD, Rye DB (1997), Serotonergic dorsal raphe nucleus projections to the cholinergic and noncholinergic neurons of the pedunculopontine tegmental region: a light and electron microscopic anterograde tracing and immunohistochemical study. J Comp Neurol 382:302–322. [DOI] [PubMed] [Google Scholar]
- Surges R, Strzelczyk A, Scott CA, Walker MC, Sander JW (2011), Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav 21:271–274. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L (1989), Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 26:321–330. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G (1988), Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science 239:1147–1150. [DOI] [PubMed] [Google Scholar]
- Theeranaew W, McDonald J, Zonjy B, Kaffashi F, Moseley BD, Friedman D, So E, Tao J, et al. (2018), Automated Detection of Postictal Generalized EEG Suppression. IEEE Trans Biomed Eng 65:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Hesdorffer DC, French JA (2014), Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia 55:1479–1485. [DOI] [PubMed] [Google Scholar]
- Trindade-Filho EM, de Castro-Neto EF, de ACR, Lima E, Scorza FA, Amado D, Naffah-Mazzacoratti Mda G, Cavalheiro EA (2008), Serotonin depletion effects on the pilocarpine model of epilepsy. Epilepsy Res 82:194–199. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL (2006), Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia 47:21–26. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Tsuru N (1994), Bilateral seizure-related changes of extracellular glutamate concentration in hippocampi during development of amygdaloid kindling. Epilepsy Res 18:85–88. [DOI] [PubMed] [Google Scholar]
- Vertes RP (1991), A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313:643–668. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B (1994), Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol 340:11–26. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B (1997), Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81:893–926. [DOI] [PubMed] [Google Scholar]
- Wilson S, Argyropoulos S (2005), Antidepressants and sleep: a qualitative review of the literature. Drugs 65:927–947. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011), Optogenetics in neural systems. Neuron 71:9–34. [DOI] [PubMed] [Google Scholar]
- Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, Serout F, Gummadavelli A, et al. (2016), Impaired Serotonergic Brainstem Function during and after Seizures. J Neurosci 36:2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]