Abstract
Two-dimensional (2D) nanomaterials are an emerging class of biomaterials with remarkable potential for biomedical applications. The planar topography of these nanomaterials confers unique physical, chemical, electronic and optical properties, making them attractive candidates for therapeutic delivery, biosensing, bioimaging, regenerative medicine, and additive manufacturing strategies. The high surface-to-volume ratio of 2D nanomaterials promotes enhanced interactions with biomolecules and cells. A range of 2D nanomaterials, including transition metal dichalcogenides (TMDs), layered double hydroxides (LDHs), layered silicates (nanoclays), 2D metal carbides and nitrides (MXenes), metal-organic framework (MOFs), covalent organic frameworks (COFs) and polymer nanosheets have been investigated for their potential in biomedical applications. Here, we will critically evaluate recent advances of 2D nanomaterial strategies in biomedical engineering and discuss emerging approaches and current limitations associated with these nanomaterials. Due to their unique physical, chemical, and biological properties, this new class of nanomaterials has the potential to become a platform technology in regenerative medicine and other biomedical applications.
Keywords: nanomaterials, two dimensional (2D), bioengineering, regenerative medicine, drug delivery
Graphical Abstract
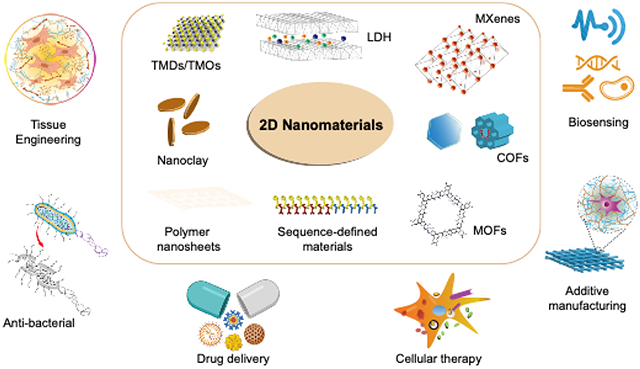
1. Introduction
The experimental discovery of graphene by Boehm and co-workers in 1962 1 and the subsequent identification of single layered graphene by Geim and Novoselov in 20042 paved the way for an elevated interest in two-dimensional (2D) nanomaterials3. 2D nanomaterials are ultrathin class of layered materials with high anisotropic and planar architecture4–8. The planar topography of 2D nanomaterials imparts desirable characteristics for biomedical applications, including a very high surface-to-volume ratio, excellent functionalization capabilities, good mechanical properties, and inherent optical activity for various biomedical applications. Compared to 1D or 3D nanomaterials, 2D nanomaterials have the highest specific surface area, resulting in high number of surface atoms compared to volume atoms. The presence of large number of surface atom provide high surface energy and can provide a high number of surface anchoring sites. This unique characteristics, results in enhanced interactions of 2D nanomaterials with biological moieties including cells, cellular components and biomolecules. The high surface area of these 2D nanomaterials have been explored for various biomedical applications including regenerative medicine, additive manufacturing, cancer thereputics as well as biosensing.
2D nanomaterials can be brodly classified into three major categories - inorganic, organic, or hybrid 2D nanomaterials. Monolayers of carbon atoms (graphene and its analogs)9–11, transition metal dichalcogenides (TMDs)12–15, transition metal oxides (TMOs)16, 2D metal carbides and nitrides (MXenes)17,18, and monoelemental 2D semiconductors, layered silicate (nanoclay)19, and layered double hydroxides (LDHs)20 constitute the family of inorganic 2D nanomaterials. Polymer nanosheets21–23, covalent organic frameworks (COFs)24–27, and sequence-defined 2D nanomaterials28 derived from DNA/RNA, proteins, peptides, and peptoids comprise organic 2D nanomaterials. A third hybrid class of 2D nanomaterial is characterized by the presence of both organic and inorganic groups, called metal-organic frameworks (MOFs)29–32.
2D nanomaterials interact with cells at the nano-scale while offering large surface areas for interaction, thus providing a unique bridge between nano- and micro-scale systems. Specifically, 2D nanomaterials find applications in bio-integrated soft electronics for non-invasive in situ monitoring of physiological variables33,34, soft cell culture platforms promoting cell alignment in vivo35–37, electrochemical sensing38, biosensing39, therapeutic delivery19,40, regenerative medicine41 and diagnostic imaging42–45 (Figure 1C) . For example, TMDs have attracted interest due to their semiconducting characteristics46–48.These 2D nanomaterials exhibit piezoelectric effects and could respond to voltage changes associated with strain gradients (flexoelectric effects), suggesting promise for biosensing49–52. Since physiological bone repair is associated with piezoelectric and flexoelectric voltage changes through strain gradients within the bone extracellular matrix, TMDs hold promise for bone regeneration applications49,53. TMOs like MnO2 and TiO2 have been used as targeted drug delivery vehicles and as a component of nanocomposite hydrogels to mimic the ECM of articular cartilage54,55 due to controlled release of adsorped drug arising from the high specific surface area and high modulus values, respectively. Some of the 2D nanomaterials have been exploited for excellent photothermal (PTT) and photodynamic therapy (PDT) therapies in cancer and bioimaging56,57. For example, 2D black phosphorus possess an PTT response in the near-infrared region. These characteristics can be successfully exploited for the controlled delivery of therapeutic moieties and imaging applications58,59.
Figure 1. 2D nanomaterials in biomedical engineering.
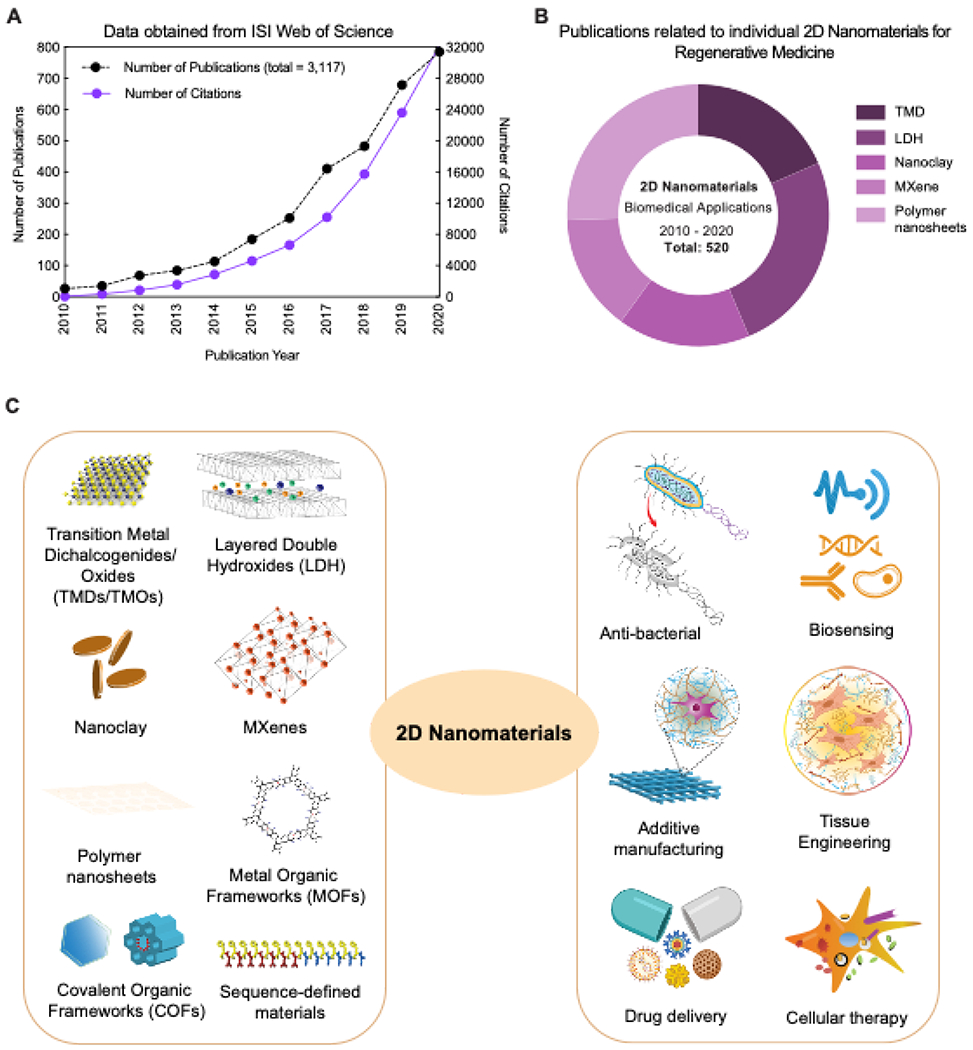
(A) Exponential increase in the field of 2D nanomaterials as evident from number of publications in past decade (2010-2020). Data obtained from ISI Web of Science using “2D nanomaterials” and “Biomedical applications” (obtained till December 2020). (B) Contribution of individual 2D nanomaterials for biomedical applications in the last decade. Data obtained from ISI Web of Science using “2D nanomaterials” AND “Biomedical applications” OR “TMD”, “LDH”, “Nanoclay”, “MXene”, “Polymer nanosheets” (obtained November 2020). (C) Different types of 2D nanomaterials for various biomedical applications.
2D nanomaterials possess a single sheet of covalently-bonded atoms stacked on top of one another to form an ultrathin plate-like structure with a high aspect ratio (usually >10) and high in-plane stiffness8,60. 2D layered materials, such as LDH and nanoclays, are characterized by the presence of solid sheets that are held together by weak Van der Waals forces61,62. These nanolayers can be used to sequester biological molecules for sustained and prolong delivery via tuning Van der Waals interactions 63–66. Mechanical properties of 2D nanomaterials depend on crystallinity and the presence of defects such as dislocations, grain boundaries, and vacancies, among others67,68. Crystallinity of the structure determines elastic properties,46,67 an important consideration for biomedical applications especially for tissue regeneration. Lysosomal degradation of LDHs and nanoclay releases bioactive dissolution products that can regulate cellular functions post-internalization. For example, synthetic nanoclay (Laponite®) can induce osteogenic differentiation of stem cells in absence of exogenous growth factors69. In addition, these nanoclay also have an ability to sequester and release stem cell secretome to promote angiogenesis and regeneration in myocardial tissue70,71. Moreover, LDHs have been shown to improve chondrogenic differentiation of tonsil-derived mesenchymal stem cells (MSCs) by sequesting and releasing therapeutic molecules72. These examples show the bioactivity of 2D nanomaterials and its ability to sequester and release therapeutic molecules to drive cellular functions.
Advancements in 2D nanomaterial research for biomedical applications has occurred at a relatively slow pace as compared to other nanomaterials. Owing to the lack of systematic biocompatibility evaluation, there have been conflicting reports regarding their toxicity. As a result, utilization of 2D nanomaterials for biomedical applications is approached with cautious optimism, especially for those involving long-term therapeutic strategies for tissue regeneration. Even so, translational research involving 2D nanomaterials has expanded rapidly in the last 10 years 11,73–75 (Figure 1A–B) .
This review will focus on recent development of 2D nanomaterials for biomedical applications. Since there are multiple excellent reviews available on graphene-based nanomaterials for biomedical applications9,10,75,76, we have limited our discussion to the other classes of 2D nanomaterials. We present a critical evaluation of recent developments (2015-2021) in 2D nanomaterials for regenerative medicine, and therapeutic delivery along with a brief discussion on their applications in cancer therapy and biosensing. We will critically discuss emerging approaches and current limitations associated with these nanomaterials. We will also highlight some of the new class 2D nanomaterials that have potential biomedical applications.
2. Synthesis and characteristics of 2D nanomaterials
2D nanomaterials can be synthesized by a variety of techniques that can be broadly classified into top-down and bottom-up methods (Figure 4A). The top-down process of fabrication usually involves breaking down the bulk material into smaller units until they reach the desired nanoscale range. Examples of top-down techniques to synthesize 2D nanomaterials include mechanical compression, exfoliation and nanolithography77. While top-down approaches are relatively inexpensive and simple, nanomaterials synthesized using this approach often exhibit a very high polydispersity , presence of impurities , rough edges and surface defects that may pose toxicity concerns in biological systems. Alternativley, the bottom-up approaches rely on precise control over the growth kinetics of thermal decomposition or chemical reduction of a suitable precursor. Some commonly used bottom-up fabrication strategies include organic ligand-assisted growth, template confined synthesis, polyol methods, seeded growth, hydro/solvo-thermal methods, nanoparticle assembly and biological template mediated synthesis78. These techniques result in 2D nanomaterials with highly uniform size distribution and high purity, however, the yield is very low and the reaction conditions are harsh, requiring extensive optimization and precise control which can be challenging.
Figure 4. Biological interaction of 2D nanomaterials.
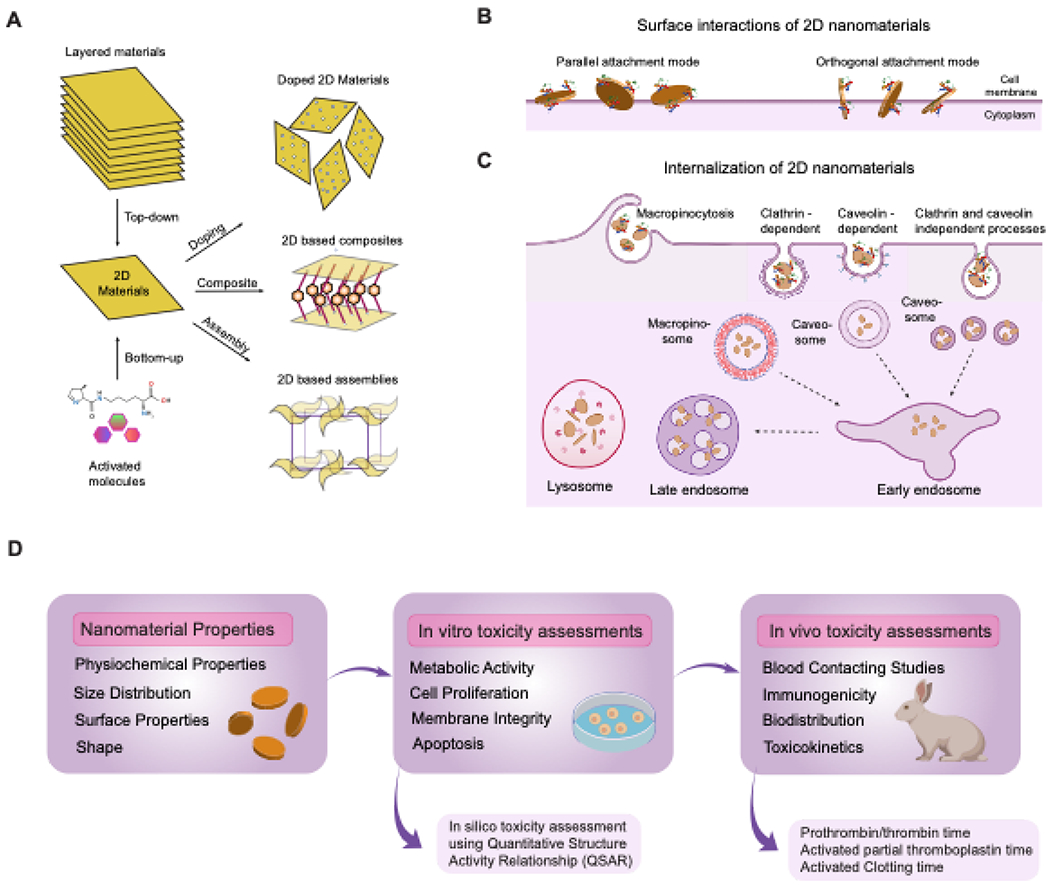
(A) Top down and bottom up fabrication approach of 2D nanomaterials.(B) Mechanism of interaction and (C) internalization of 2D nanomaterials in cells. The surface charge and size of these nanomaterials determines their mode of interaction. Once internalized, these 2D nanomaterials are prone to accumulate in the lysosome. (D) In vitro and in vivo evaluation of 2D nanomaterials.
2.1. Transition metal dichalcogenides (TMDs) and transition metal oxides (TMOs)
TMDs are 2D layer materials with the general formula MX2. They can be classified as semiconductors, metals, semi metals, or superconductors based on their composition, tuned according to the required crystal structure for various applications13. Unlike graphene, which possesses a single layer of carbon atom, TMDs consist of a sandwich structure14 with the central transition metal layer (e.g., Mo, W, Nb) positioned between two layers of chalcogenides (e.g., S, Se, Te)(Figure 2A). TMDs can be prepared by mechanical cleavage of the bulk material,5 electrochemical Li-intercalation and exfoliation,79 sonication in solvents,80 chemical vapor deposition, and other methods81,82. The two commonly observed structural phases of TMDs are transition metal atoms in the trigonal prismatic (2H) or octahedral coordination (1T). The thermodynamically stable phase is determined by the combination of the metal and chalcogen atom. The diverse chemical composition and structural phases of TMDs results in a broad range of electronic properties beneficialfor regenerating electrically active tissues. TMDs also possess very high strength and can endure strains up to 10% before breaking. The three-fold symmetry of TMD allows for easier strain engineering by application of lower strains15. The morphology and ultrathin structure of TMDs imparts a large specific area usable for tagging therapeutic molecules and allowing increased cell-substrate interactions83. Unlike other 2D nanomaterials, TMDs do not possess many functional groups on the surface84 and hence require ligand conjugation before use in any application85.
Figure 2. Characteristics of various 2D nanomaterials.
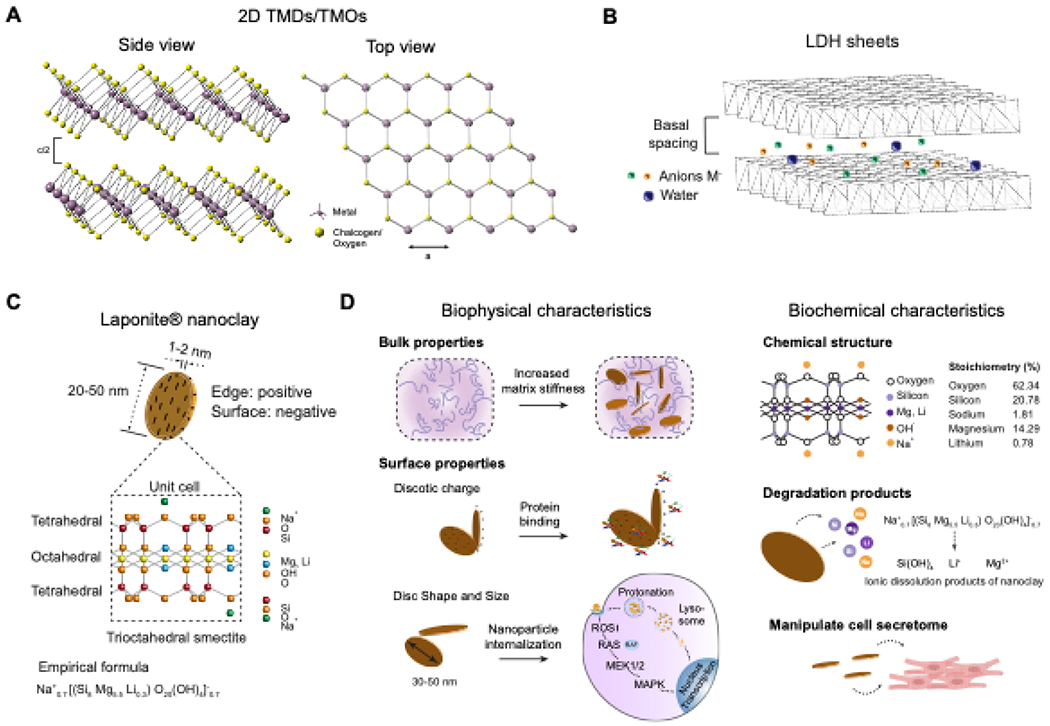
(A) Schematic representation of top and side view of the atomic framework of TMDs and TMOs. (B) Schematic representation of the LDHs atomic structure. (C) Atomic structure of nanoclay showcasing the tetrahedral and octahedral arrangement of atoms which imparts a dual charge nature to the nanomaterials. (D) Schematic representation of biophysical and biochemical characteristics of nanoclay.
2D TMOs are single or multilayered metal oxides wherein the transition metal’s-electron is strongly polarized by the oxygen atom which consequently results in the unique surface properties arising from the correlated d-electron behaviour. The strong polarizability arising from the oxygen atom enables the planar TMOs to exhibit large non-linear and non-uniform distribution of charges(64). This kind of charge distribution results in lattices showing electrostatic screening over sclaes of 1-100 nm resulting in exceptional local surface and interfacial properties16. The fundamental properties of TMOs are governed by the nature of the transition metal cation and its oxidation state resulting in tunable stability and electrical band gap characteristics. In fact, just by varying the stoichiometries of the transition metal, the same TMOs layer can exhibit different electrical behaviour86. TMOs such as MoO387, Ga2O3 88and WO3 89can be found as naturally occurring layered crystals in hydrated or anhydrous forms. These crystals can be exfoliated using liquid or gas exfoliation methods to yield 2D TMO nanosheets. Other TMOs such as those based on titanium and zinc oxides, perovskites are not naturally occurring and are fabricated by exfoliation of their bulk counterparts or by layer-by-layer deposition techniques. Synthetically fabricated TMOs require stabilization with functional groups or charged motifs in order to prevent the 2D layered arrangement from collapsing into more energetically favourable non-specific 2D planar arrangements90.
2.2. Layered double hydroxides (LDHs)
LDHs have been the most widely investigated 2D nanomaterials in regenerative medicine because of their good biocompatibility and low cytotoxicity91,92. LDHs are a class of inorganic layered structures with the general formula [M2+1–xM3+x(OH)2]x+[Ap−x/p]x+·mH2O, where M2+ and M3+ represent divalent and trivalent metal cations, respectively, at the octahedral positions93. Their structure consists of a cationic brucite-like layer and an exchangeable anionic interlayer (Figure 2B). The synthesis of LDHs occurs through three primary approaches: co-precipitation (direct), ion-exchange (indirect), and reconstruction/rehydration (memory-effect)94. The co-precipitation method involves the slow addition of the intercalating ion solution to a bulk solution of the divalent and trivalent metal cations at a fixed ratio. This promotes the formation of hexa-aqua metal complexes that form the brucite-like layers characteristic of LDHs95. The co-precipitation method is superior to other methods due to its ability to produce LDH composites with sizes in the nano range96. The anion exchange route involves the formation of LDH using the direct method followed by an intercalating ion exchange (host anion) in an inert atmosphere97. The anion exchange route is preferred over the co-precipitation method in situations where hybrid LDHs consisting of very large host anions are required or when there is a requirement to intercalate therapeutic molecules between the layers for various biomedical applications. The reconstruction route of LDH synthesis utilizes calcined LDHs that recover their structure after rehydration under an inert nitrogen atmosphere. This method has been utilized for incorporating salicylic acid, phenylalanine, and terephthalate anions into the interlayer spaces of LDHs98. The presence of the anionic interlayers makes it possible to encapsulate various therapeutic moieties with a negative charge via a simple anion exchange-like process99. As a consequence, LDHs have been extensively investigated for drug delivery applications since they exhibit a very high drug loading density, good bioavailability and reduced side effects92,100.
2.3. Synthetic nanoclay
2D nanoclays are layered mineral materials with at least one dimension in the order of 1-100 nm. To overcome the inhomogeneities associated with naturally-derived nanoclays, synthetic mineral clays have been explored for biomedical applications. Synthetic nanoclays are synthesized from inorganic mineral salts with highly controlled composition and properties. These silicates have a general formula of (Ca, Na, H)(Al, Mg, Fe, Zn)2(Si, Al)4O10(OH)2-xH2O, where x represents the amount of water101. They feature one or more phyllosilicate minerals composed of a silicate crystal with different elemental composition and sizes. The basic building blocks of nanoclays consists of octahedral and tetrahedral sheets. The specific arrangement and composition of these layers is used to categorize them into different families.
Commonly reported types of nanoclay are montmorillonite, laponite, kaolinite, sepiolite, halloysite, and hectorite. Of these, laponite, montmorillonite (MMT), and halloysite nanotubes (HNTs) are most widely utilized for biomedical applications. Laponite® nanoclay (Laponite is a trademark of BYK Additives Ltd) is widely used for biomedical applications19. It is a synthetic trioctahedral smectite with the empirical formula (Na+0.7[(Si8Mg5.5Li0.3)O20(OH)4]−0.7. Laponite nanoclay has a disc-like geometry with a diameter of 20-50 nm and 1-2 nm thickness. Due to its unique size and composition, laponite nanodisks exhibit dual charge distribution with a negative charge on the faces and a positive charge along the edges (Figure 2C). Laponite nanoclay has been extensively used as a rheological modifier to improve the structure, property, and performance of various products. Due to the intrinsic hydrophilic property and high surface-to-volume ratio, laponite can facilitate physical interactions with numerous biomolecules, making it an attractive material in regenerative medicine, therapeutic delivery, and additive manufacturing. By utilizing nanoclay in existing scaffolds, essential features such as drug entrapment, sustained release profiles, and targeted release have been introduced to regenerate various tissues successfully. MMT is an example of a natural nanoclay, wherein Mg2+ cations replace the Al3+ cation in the octahedral layer102. Similar to laponite, MMT nanoclay is widely used as a drug carrier and as an additive to improve the performance of various biomaterials. Due to its high specific area and ability to be exfoliated into layers with thickness in the nanometer range, MMTs are good candidates for the preparation of polymer-nanoclay composites103. HNT nanoclays have a hollow tubular structure with outer and inner diameters of 50-80 nm and 10-15 nm, respectively, and a length of approximately 1μm104. A packing disorder caused by interactions between the water and alumina/silica layers causes the HNTs to curve and roll up, forming multilayer tubes. As a result, the exposed aluminol and siloxane groups on the surface of HNTs can form hydrogen bonds with various biomaterials. This allows for the fabrication of unique support matrices for controlled drug release, nanocomposite fabrication, and novel nanotemplating strategies for regenerative medicine.
2.4. Metal carbides and nitrides (MXenes)
MXenes are a relatively new class of 2D nanomaterials characterized by an atom-thin layer of transition metal carbides, nitrides, or carbonitrides105. MXenes have become increasingly popular for supercapacitor design, flexible energy storage devices, chemical catalysis, and opto-electronic devices. These materials have a general formula of Mn+1XnTx and are synthesized by selective etching of the atomic layers of their MAX phases. MAX phases can consist of ternary carbides and nitrides, reliable solutions as well as ordered double transition metal structures (Figure 3C). Wet etching and delamination methods are most widely used for the generation of MXenes from their MAX precursors106. Wet etching techniques can be carried out using hydrofluoric acid (HF) or a mixture of a strong acid and fluoride salt at temperatures between 21°C to 55°C. The structure and size of the MXene can be controlled by varying the etching time and HF concentration 107. MXenes derived from aluminum with higher ‘n’ in Mn+1XnTx are synthesized using wet etching because they require stronger etching conditions and longer etching times. Etching in the presence of a fluoride salt is carried out in a mixture of HCl and LiF, which reacts to form HF in situ and selectively etches the A layer of MAX phases. A mixture of HF and LiCl can also be used to obtain a similar etching pattern, suggesting the presence of protons and fluoride ions drive this process 18. The use of metal halide ions in the etching process leads to an increase in the interlayer distance between the MXene sheets due to cation intercalation. This allows for easy delamination to produce single flake or few-layered flake structures without the need for any additional processing steps. Interlayer interactions in multilayered MXenes are two to six-fold stronger than in other 2D nanomaterials such as graphene and bulk MoS2. To pull apart these strongly-bonded layers, most MXenes are produced by a process of delamination via intercalation with different metal cations in aqueous solutions, followed by delamination using mechanical vibration at a neutral pH108. This yields a stable aqueous colloidal suspension of MXenes, which do not aggregate due to the negative zeta potential and can thus, withstand an aqueous biological environment with reduced cytotoxicity 6,109.
Figure 3. Characteristics of various 2D nanomaterials.
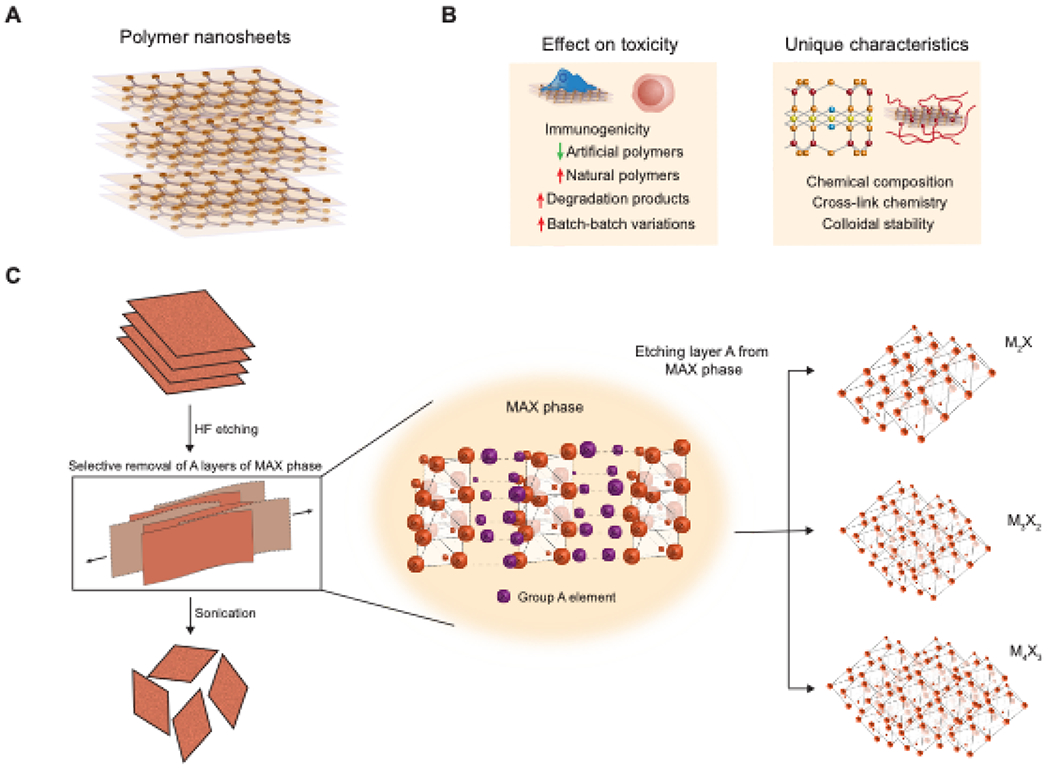
(A) Atomic structure of polymer nanosheets with lattice-defined interaction between sheets. (B) Factors affecting toxicity and unique characteristics of polymer nanosheets. (C) Schematic representation of MXenes synthesis. Evolution in the formation of MXenes on the atomic scale and its various different configurations are shown.
2.5. Polymer nanosheets
Polymers have readily replacing metallic components of medical devices, thus allowing for extended device lifespans. In recent years, polymers are also gathering momentum for their use on the micro- and nano-scale. Owing to their ultra-thin structure and large surface area, polymeric nanosheets are widely studied and applied in biomedical fields21,110,111. Polymer nanosheets are synthesized when ultra-thin polymeric films are exfoliated from a substrate as its sacrificial layer is dissolved (Figure 3A). The resulting deposition of the exfoliated nanosheets can be on solid substrates or via a liquid-air or liquid-liquid contact. Polymer phase separation, layer-by-layer (LbL) deposition, spin coating, self-assembled monolayers (SAMs), and micropatteming are some of the widely used methods for synthesizing polymer nanosheets. These methods are defined by the control over nanosheet chemistry and architecture or by the step at which the nanosheets are exfoliated from the substrate. Polymer nanosheets can be easily synthesized using a wide range of techniques that do not require any specialized modifications. In an early study, gold nanoparticles were entrapped between alternating monolayers of poly(allylamine hydrochloride) (PAH) and poly(sodium 4-styrenesulphonate) (PSS), which combined spin coating and LbL techniques for an efficient nanosheet system112. Recently, pyrrole nanosheets synthesized by intercalating pyrrole moieties between FeOCl layers and polymerized in situ have been shown to have flexible structures, binding sites for biological and other organic surfaces, and photothermal activity in the second NIR window (1000–1350 nm)113. In 2019, green synthesis of polymer nanosheets was demonstrated114,115. For example, laser inkjet printing and spin coating was combined for exfoliating layers of a graphene/Au hybrid antenna onto PLA nanosheets114,115. In another study, exfoliation of polydopamine nanosheet was obtained by employing the oxidizing agent (KMnO4) to obtain MnOx/PDA nanosheets114,115.
3. Physiochemical properties of 2D nanomaterials
3.1. Physical and chemistry characteristics
The recent interest in 2D nanomaterials for biological applications is due to their unique properties, such as high surface-to-volume ratio, mechanical flexibility, and tunable interfacial chemistry. Fabrication of these 2D nanomaterials can be carried out either through top-down or bottom-up processes (Figure 3A). While the bottom-up process yields materials with very high quality and very few atomic defects. However, due to harsh reaction conditions, these nanomaterials are unsuitable for biomedical applications. To bypass these limitations, the top-down approach is typically used. Here, a bulk layered crystal is broken down into smaller nano-scale layers using mechanical or chemical exfoliation methods116,117. While the yield of 2D nanostructures from these methods is high, it results in significant surface defects and high polydispersity, which may contribute to toxicity.
2D nanomaterials exhibit point defects, lattice vacancies, and line defects. Materials with sp2-bonded structures resembling graphene such as MoS2, WS2, and WSe2 show Stone-Wales (SW) defects arising from non-canonical rotations about the sp2 bonded atoms33,. Lattice vacancies may arise when an atom is missing from the 2D lattice, while line defects occur at the boundaries of different lattice orientations. The net sum of these atomic alterations is the effective increase in the surface reactivity that often manifests in toxicity and mechanical failure63,118,119.
2D nanomaterials are rigid when stretched in-plane but exhibit a rippling or wrinkling effect when subjected to out-of-plane bending or folding120. While such an effect may compromise the electronic and barrier properties of the nanomaterial, the increase in the effective surface area allows cells to strongly attach and spread freely over the underlying substrate composed of nanogrooves and nanoridges. Mechanical distortion of the 2D lattice can also modulate the local electronic structure and directly influence chemical reactivity. In summary, mechanical perturbations of an imperfect 2D lattice could increase chemical reactivity and aid greatly towards surface functionalization120,121.
Mechanical strain gradients observed in 2D nanomaterials can influence the interaction of stem cells with the ECM and affect their differentiation potential. Strain gradients can also drive electrical polarization (flexoelectricity), which can be exploited to regenerate electrically active tissues such as bone, neurons, and cardiac tissue, and also fine-tune intermolecular interactions using precisely controlled lattice deformations122.
Microscale interactions of 2D nanomaterials are influenced by surface topography and can be used to drive surface adhesion of cells123,124. Anisotropic architectures such as aligned nanogrooves and nanoridges mimic the fibrillar structure of the ECM protein Collagen I and promote cell attachment. These patterned architectures can also be used to control the cell shape and differentiation characteristics of stem cells125. The physical interaction of 2D nanomaterials with the biological interface remains an active area of research to optimize applications in regenerative medicine.
3.2. Nano-bio interface and cellular interactions
The cellular uptake of 2D nanomaterials is dependent on their size, geometry, and surface properties126–128. 2D nanomaterials can interact with the cell membrane by two modes (Figure 4B). In the near-perpendicular membrane penetration mode, a hydrophobic attraction between lipid tails in the membrane and nanomaterial surface acts as driving forces for interaction. The preferential uptake mechanism includes orthogonal piercing of the plasma membrane by rough edges (asperity) or corner sites, followed by embedding in the lipid bilayer. This orthogonal conformation minimizes the interactive free energy and increases the overall entropy, and therefore is the thermodynamically preferred mechanism of entry. An alternative uptake strategy for 2D nanomaterials is the parallel attachment mode, with hydrophilicity and specific attraction between membrane bilayer and nanomaterial surfaces as the major driving force. The choice between perpendicular or parallel attachment modes depends on the lateral size of the 2D nanomaterial; nanomaterials with lateral dimensions comparable to the dimension of the plasma membrane adopt the parallel entry mode, while those with larger lateral dimensions adopt the near-perpendicular penetration route126,128. The internalization of 2D nanomaterials into cells is an energy-dependent process (Figure 4C). For example, protein-coated graphene oxide nanosheets (PCGO) with larger lateral sizes were preferentially internalized by phagocytosis, whereas smaller PCGOs were internalized by a clathrin-mediated endocytosis process129.
The cell membrane is a critical transport barrier encountered by 2D nanomaterials. The nature of nanomaterial-cell membrane interactions will dictate the uptake efficiency and cytotoxicity of the nanomaterial. The parallel attachment mode has been associated with enhanced cell viability by reducing cellular communication with the environment. Transmembrane attachment of 2D nanomaterials induces rearrangements of the actin cytoskeleton underlying the cell membrane in murine macrophages, with potential implications in determining nanomaterial immunogenicity The membrane attachment mode of 2D nanomaterials also has broader implications in encapsulation, manipulation, and monitoring of cells128.
4. Biocompatibility of 2D nanomaterials
2D nanomaterial toxicity arises from differences in synthesis and post-processing steps, resulting in changes in the final material composition and degradation characteristics, leading to significant effects at the nano-bio interface118,130,131,132. Tables 1–6 outline studies showing that the extent of toxicity is directly proportional to the reactivity of the constituent atoms of the different types of 2D nanomaterials.
Table 1:
Overview of the cytotoxicity of TMDs
| 2D nanomaterial | Synthesis | Sizes (lateral dimension) | Functionalization | Cell viability | Maximum cone. tested | Tested on | Assays used | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| MoS2(1T) | Chemical intercalation (n-butyllithium) | 50-500 nm, | - | ≥95% | 100μg/ml | HeLa , RAW 264.7 HMDM | Flow cytometry using FITC-annexin V and PI stain | Drug delivery for combined photothermal and chemotherapy for cancer | 301 |
| VS2 | Chemical exfoliation (butyllithium) | 100nm-1μm, | - | IC50 ~25μg/ml | 200μg/ml(≥20 % viability) | A549 | MTT and WST-8 assay | Biosensing | 137 |
| VTe2 | Chemical exfoliation (butyllithium) | 100nm-1μm, | IC50 >3μg/ml | 200μg/ml(≥5% viability) | A549 | MTT and WST-8 assay | Biosensing | 137 | |
| MoS2 (2H) | Liquid-phase exfoliation (Sulfuric acid) | ~30-50nm, 5-7nm thickness | Hyaluronic acid decorate by polyethyleneimine | ≥95% | 200 μg/ml | A549, MCF-7 | CCK-8 | Nanotheranostic for stimulated drug delivery to reduce chemotherapy resistance | 302 |
| MoS2 | Liquid-phase exfoliation (Sulfuric acid) | ~80nm, 4-6nm thickness | chitosan | ≥80% | 400 μg/ml | KB and Pane-1 cells | CCK-8 | NIR triggered photothermally stimulated drug delivery | 303 |
| MoS2 | Ultrasound sonication and Tris stabilization | 30-100nm | Bovine serum Albumin | ≥80% | 200μg/ml | SKBR3, MCF10A, MCF7 | ApoLive-Glo | NIR triggered delivery of doxorubicin | 304 |
| VS2 | VCl4 with sulfur dissolved in oleylamine and 1-octadecene | 35nm | PEG | ≥80% | 200μg/ml | 4T1 | MTT assay, calcein-AM/PI staining | Paramagnetism and NIR-based cancer theranostics | 305 |
Table 6:
Overview of the cytotoxicity of polymer nanosheets
| 2D nanomaterial | Synthesis | Sizes | Functionalization | Cell viability | Maximum conc. tested | Tested on | Assays used | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| APTES-COFs | Sonication based-Liquid exfoliation | 120-150nm width | PEG-curcumin. DOX(0-2.5μg/ml) | ~80% (preloading fo DOX). <30% (max DOX loaded) | DOX loaded 3000kDa PEG (most toxic) | HeLA | MTT assay | In vivo fluorescent tracking and targeted doxorubicin delivery | 320 |
| Porphyrin-based COF nanodots | Sonication based-Liquid exfoliation | 2nm (uncoated)- 3.46nm (coated) thickness, 9.46nm hydrodynamic diameter | PEG | <80% | 200μg/ml | MDA-MB-231, Raw 264.7, L929, HeLA | MTT assay, Calcein AM-PI, Flow cytometry using FITC-annexin V and PI stain | ROS generating photodynamic agent for anti-cancer therapy | 321 |
| Phloroglucinol COFs | Solvothermal process | 200nm length | - | IC50 – 5-10μg/ml | 100μg/ml | Hep G2, HCT 116, A549, MIA PaCa-2 | MTT assay | ROS generation induced apoptosis for cancer therapy | 322 |
| Porphyrinic COFs | Co-solvent precipitation and aging | 150-200nm diameter | >80% | 200μg/ml | HeLA | MTT assay | Combination of photothermal and photodynamic therapy for cancer | 323 | |
| Copper-MOFs | Co-precipitation method | 100nm diameter | Folic acid | >80% | - | HeKA, HDF | MTT, LDH assay | Targeted drug delivery for cancer | 324 |
| Copper-MOFs | Co-precipitation method | 285nm diameter | - | >80% | - | HeKA, HDF | MTT, LDH assay | Diabetic wound healing by copper ion release | 325 |
| Cyclodextrin-based-MOFs | Solvothermal process | 500nm diameter | - | >90% | 250μg/ml | C6 | MTT assay | Antioxidant and anticancer activity | 326 |
| Zeolite imidazolate MOFs | Hydrothermal method | 200-300nm width | - | >85% | - | MG63 | CCK-8 assay, Actin/DAPI staining | Osteogenic and antibacterial activity | 254 |
| MnOx/Polydopamine nanoparticles | Facile oxidation method | 54nm, diameter | - | >90% | 200μg/ml | L929 | CCK-8 assay | MRI guided multimodal photothermal tumor therapy | 115 |
| Peptoid nanofibers (Biot-GGG(Prop)FSS TKT-CONH2) | Peptide synthesizer | 15-30nm width, 6-12nm height | - | ≥95% | - | hNSC | Calcein- AM/EtHD staining | Drug delivery and cell therapy | 327 |
| Peptoid macrocycles | Peptide synthesizer | 6nm size | - | >80% | 100μg/ml | U2OS, A549 cells | CellTiter-Fluor Cell Viability Assay | Inhibition of B-catenin TCF interaction in prostate cancer | 328 |
| Black Phosphorus nanosheets | Liquid exfoliation method | 225.8 ± 4.0 nm width, 3.8-4.5 nm thickness | - | ≥95% | 400μg/ml | Hek293 | MTT assay | Antioxidative activity to treat kidney injury | 329 |
| Black Phosphorus nanovesicles | Liquid exfoliation method | 15-100nm diameter | Poly(propylene sulphide, PEG | >85% | 150μg/ml | 4T1, MCF-7 | CCK-8 assay, Calcein-AM/EtHD staining, Flow cytometry using FITC-annexin V and PI stain | NIR-ROS triggered immunoadjuvant delivery for Photothermal immunotherapy | 330 |
The extent of toxicity of layered 2D materials is also influenced by exfoliation and lateral dimensions. For certain layered materials such as TMDs (especially MoS2 and black phosphorus), a higher exfoliation state corresponds to higher toxicity due to many active edge sites and an increase in surface area133. Thinner and smaller-sized 2D nanomaterials elicit a greater toxicity response due to the increased effective surface area. Other factors, such as the crystal structure of the material101, surface functionality114, chemical transformation118, and structural form119, also influence toxicity118,130,134,135. However, the lack of more comprehensive material characterization and toxicological studies makes it difficult to assess the hazard potential of 2D nanomaterials precisely (Figure 4D).
4.1. Transition metal dichalcogenides (TMDs) and transition metal oxides (TMOs)
Several reports discuss the biological response of TMDs119,136,137. WS2 and MoS2 exhibited lower cytotoxicity than WSe2 on A549 lung carcinoma epithelial cells136. Studies on the ditelluride forms of vanadium, niobium, and tantalum showed that NbTe2 and TaTe2 had lower cytotoxicity than VTe2119,137. While ditelluride forms of TMDs were more toxic than sulfide forms in A549 cells, for example VSe2 and VTe2 show higher toxicity than VS2. These findings indicate that the choice of the chalcogen atom plays a significant role in determining the toxicological response of cells to TMDs.
Highly exfoliated MoS2 exhibits higher cytotoxicity than non-exfoliated MoS2 due to increased surface area and a number of active edges119,138. However, well-exfoliated stable dispersions of MoS2 show lower cytotoxicity than dispersions of aggregated MoS2 in multiple cell types, suggesting that the degree of exfoliation is a critical factor. Tungsten sulfide nanotubes and molybdenum sulfide nanoparticles have shown good cell viability139 at concentrations up to 300 μg/ml, whereas carbon nanotubes or graphene show exceedingly high cytotoxicity at concentrations as low as 100μg/ml, suggesting that the former TMDs provide superior biocompatibility. In another study, cytocompatibility via the half inhibitor concentration (IC50) of MoS2 shown to be~400 μg/mL140. The primary mechanisms of MoS2 nanosheets uptake were clathrin and macropinocytosis. Long-term studies indicate that cells readily proliferated over a course of 7 days in presence of various concentration of MoS2, indicating cytocompatibility even at higher concentrations (>IC50). Recent report have demonstrated that MoS2 nanosheets degrade in phosphate buffer solution (PBS) and release molybdenum (Mo) ions, which lead to cellular toxicity49. However, the study did not investigate the effect of Mo ions on cells any further than viability testing49. Similarly, the constituent transition metals in MoS2 nanosheets (Mo and S) as dissociation products have not been studied at the cell- and tissue-level, although MoS2 has been shown to degrade within physiological environments. Characterization of the extent of cytotoxicity and the potential for mutagenic effects due to acute and prolonged exposure of MoS2 and their degradation products is therefore needed in order to inform precise exposure guidelines for specific tissue types.
Toxicity in 2D TMOs can be attributed to the exposed crystal facet at the edge sites, defect ratio and chemical composition, with trends similar to that observed in TMDs141. V2O5 142and MoO37 have been reported to be slightly genotoxic. 2D MoO3 nanosheets have been known to induce the activation of caspase pathway in MCF-7 breast cancer cell line thus leading to apoptosis143. Similar toxicity has also been reported for 2D MnO2 in MCF-7 cells143 . In vivo toxicity evaluation of 2D TiO2 nanosheets has shown low toxicity following intraperitoneal injection in mice models. However, prolonged exposure to TiO2 nanosheets led to particle accumulation resulting in minor abnormalities144.
4.2. Layered double hydroxides (LDHs)
Endocytosis of LDHs relies on nanomaterial size and can interfere with cellular functions145. The cytotoxicity observed is mostly attributed to the release of reactive oxygen species (ROS), membrane damage, and induction of the pro-inflammatory cytokine IL-8146,147. Physico-chemical properties such as the shape, size, and composition of the LDH also plays a critical role in determining cytotoxicity105 .Mg-based LDHs are less cytotoxic than their Zn counterparts, prone to release greater levels of the enzyme lactate dehydrogenase and induce hemolysis Size-dependent cytotoxicity of LDHs is observed, with smaller LDH particles (50 nm) more toxic than larger particles (100 nm and above) The chemical stability of the LDH and the type of anion intercalated between LDH sheets also influence cytotoxicity, with Cl− promoting faster degradation of the material and showing less cytotoxicity, while CO3− possesses greater solution stability and causes increased cytotoxicity through accumulation and retention within cells146.
In vivo biocompatibility of LDHs was performed by implanting intramuscular tablets composed of chloride ions intercalated into Mg-Al and Zn-AL LDHs in abdominal walls of rats147. Four weeks after implantation, neither LDH showed any signs of inflammatory reactions or fibrous capsule formation. Instead, Mg-Al LDH promoted the formation of Collagen type I invaginations around its fragments. At the same time, the Zn-Al LDH aided the deposition of Collage type III networks, thus corroborating the biocompatibility of LDHs and their potential use for biomedical applications.
4.3. Synthetic nanoclay
Nanoclays are considered environmentally friendly and biocompatible, making them attractive for a range of biomedical applications . Oral administration of MMT nanoclay is well tolerated by Sprague-Dawley rats at concentrations as high as 5700 mg/kg148. MMT nanoclay did not induce DNA damage or micronucleus induction in Chinese hamster ovary (CHO) cells even at a concentration of 1mg/ml, indicating a significantbiocompatibility. In vitro toxicity testing of various other nanoclays such as sepiolite and HNTs has also shown high cytocompatibility up to a concentration of 500 μg/ml149. Laponite nanoclay was found to be well tolerated by human mesenchymal stem cells (hMSCs) and pre-osteoblasts at concentrations as high as 4-5 mg/ml69. Compared to other 2D nanomaterials, nanoclay IC50 values are 5-10 fold higher, indicating incredibly high cytocompatibility. However, nanoclays may still induce cytotoxicity at higher concentrations. Pristine nanoclays have been shown to be toxic to HepG2 and SK-MEL 28 cells by the activation of apoptosis through caspase 3/7 in a concentration-dependent manner150. Other mechanisms of cytotoxicity induction by nanoclays include sequestration and depletion of essential components in tissue culture media, causing detrimental effects to cells151. The formation of a protein corona and, therefore, sequestration of proteins is also an important indicator of nanoclay cytotoxicity152. Thus, applications must take into account the concentration-dependent toxicity of 2D nanoclays.
4.4. Metal carbides and nitrides (MXenes)
While MXenes have shown superior properties compared to organic materials with similar structures, their toxicological evaluation remains to be addressed in depth. Treatment of zebrafish embryos with different concentrations of the MXene Ti3C2Tx showed no significant neurotoxicity or changes in locomotion assessment assays153. No significant cytotoxicity or teratogenicity was observed with Ti3C2Tx treatments up to a concentration of 100 μM for shorter periods; however, longer incubation times over 96 h revealed the formation of larger aggregates that disrupted cell membranes resulting in cell death.
4.5. Polymer nanosheets
Polymeric nanosheets maintain properties of bulk polymers while providing a nano-scale size advantage. This binary characteristic has been critical in designing these materials for applications in therapeutic delivery, imaging, and theranostics. As with other 2D nanomaterials, the cytotoxic responses of polymer nanosheets can be influenced by factors such as lateral size, shape, chemical composition, surface functionalization and charge, concentration, and colloidal stability. Most polymeric nanosheets are synthesized from artificial polymers, which offer a low likelihood of immunogenicity and batch-to-batch variations, unlike those derived from natural polymers (Figure 3B). Consequently, they are highly biocompatible. Ultra-fine and porous PDLLA-PS nanosheets have been shown to mimic the physiological organization of basement membranes23. In another study, thermally crosslinked poly (propylene fumarate) (PPF) nanosheets have shown negligible inflammatory responses and toxicity when implanted in vivo, in contrast to non-crosslinked PPF nanosheets154. Polymer coating of otherwise toxic 2D nanomaterials such as MoS2 and graphene has been a popular strategy to improve biocompatibility155–158. Because of this, most reports describing pristine polymer nanosheets for various biomedical applications rarely perform extensive toxicity testing.
5. Biomedical applications of 2D nanomaterials
5.1. Regenerative medicine
2D nanomaterials have caught the attention of biomedical researchers due to their large surface area, tunable chemistry and unique mechanical properties. By being able to interact with cells at the nano-scale and still offering very large surface area for interactions with therapeutic molecules, this nanomaterial class provides a unique bridge between nano- and micro-scale systems. Among different 2D nanomaterials, nanoclays, and LDHs have been used extensively for regenerative applications owing to their higher biocompatibility and favorable interactions with the biological milieu19,159. In fact, some nanoclays and LDHs are able to themselves function as drivers of cellular fate due to their ability to degrade and release biologically relevant ions in a sustained manner69,160. The layered structure also promotes encapsulation of various types of drugs that can be released in a sustained manner161 .TMDs and MXenes have gather momentum for tissue engineering applications because of their unique mechanical properties and potential electrical conductivity, in addition to their large surface area and ease of functionalization. Their utilization as reinforcing materials for mechanically weak scaffolds has been particularly been very useful for the regeneration of load bearing tissues and imparting antimicrobial effects162–164. In the following sections, we will discuss the different classes of 2D nanomaterials for regenerative medicine and drug delivery.
5.1.1. Transition metal dichalcogenides (TMDs) and transition metal oxides (TMOs)
Exfoliated TMD nanosheets have been explored for biosensing, drug delivery, diagnostic imaging, and photothermal and photoacoustic therapy12. More recently, their affinity for microbial cells has expanded their use as an anti-microbial additive to existing scaffolds12,165. TMDs such as MoS2, WS2, and WSe2 prevent the growth of different bacterial strains by generating reactive oxygen species or by direct nanoparticle-mediated oxidation of the cellular machinery of bacteria165 (Figure 5A). For example, chemically exfoliated MoS2 nanosheets show a more significant anti-bacterial activity compared to raw MoS2166. In addition to providing anti-microbial properties, polyacrylonitrile nanofibers containing 40% MoS2 seeded with bone marrow stem cells (BMSCs) showed that the chemical and physical cues provided by MoS2 promote osteogenic differentiation167. A recent study also demonstrated that MoS2 can be readily internalized by cells and impairs cellular motility and wound healing ability by directly affecting cell surface integrin receptors 140.
Figure 5. 2D nanomaterials for tissue regeneration.
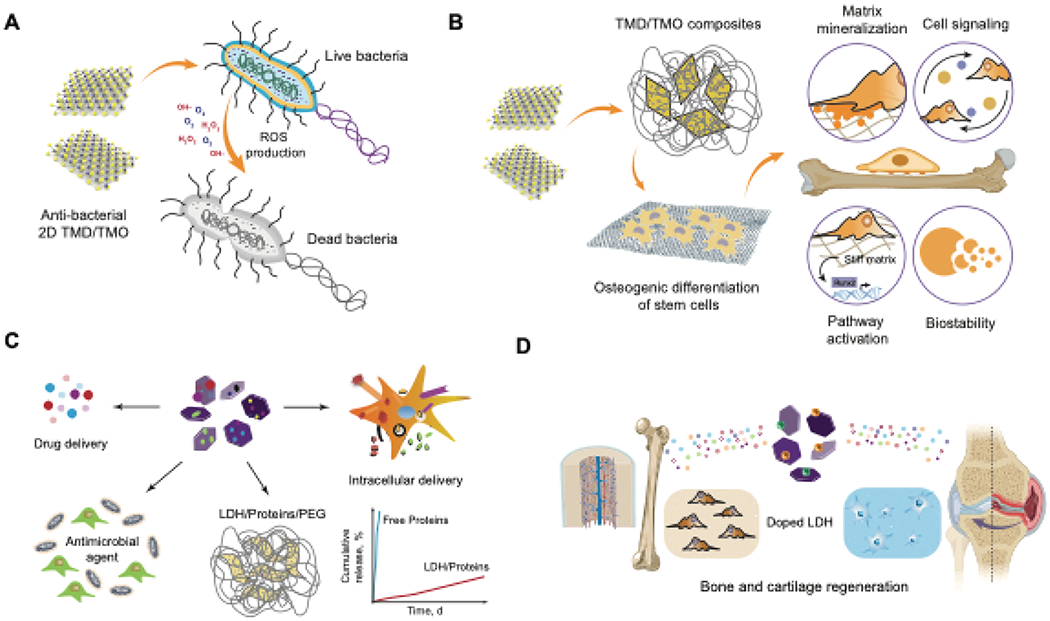
(A) Schematic representation of the anti-bacterial mechanism of TMDs and TMOs. When internalized, a burst of ROS generation within the bacterial cell results in apoptosis.(B) TMD-based nanocomposites are capable of inducing differentiation in stem cells by various mechanisms. (C) LDH nanosheets are able to sequester small molecule drugs and large proteins, resulting in sustained delivery. (D) Therapeutic loaded LDHs directing stem cell differentiation.
Recently, composite scaffolds consisting of 3D-printed akermanite (AKT, Ca2MgSi2O7) with hydrothermally-deposited MoS2 were used for photothermal therapy and regeneration of ablated bone tissue areas. AKT-MoS2 composite scaffolds induced greater expression levels of osteogenic markers runt-related transcription factor 2 (RunX2), osteopontin (OPN), and osteocalcin (OCN) when compared to AKT scaffolds alone168. These examples highlight the potential for MoS2 as an additive to support bone tissue regeneration. Similarly, WS2 nanotubes have been used as reinforcing materials to improve the compressive and flexural moduli of polypropylene fumarate (PPF) composites for bone tissue engineering169. These findings show that TMDs in conventional polymeric scaffolds promote greater mechanical strength of biodegradable implants for high loading applications in orthopedic tissue engineering (Figure 5B). The current drawbacks for the use of TMD nanocomposites in regenerative medicine include the lack of information on the effects of TMD on physical properties of nanocomposite as well as its biological response.
TMO nanosheets based on Ti have been used to improve cell attachment and spreading170. Rat bone marrow stem cells (rBMSCs) seeded on Ti-based nanosheets exhibited increased osteogenic differentiation as compared to TCPS controls. Implants coated with Ti nanosheets have shown improved osteointegration with surrounding tissues by upregulation of α-integrin receptor mediated cell adhesion to the implant170.
5.1.2. Layered double hydroxides (LDHs)
Early reports suggested that LDHs had no discernible cytotoxicity and that the efficiency of delivery was dependent on loading concentration. LDHs also serve as a reservoir for drug cargo and prevent non-specific release due to their hydroxide layers171. Furthermore, metallic Mg substrates coated with a layer of highly oriented Mg-Fe-CO3 LDHs promoted a greater degree of cell adhesion and proliferation compared to plain Mg substrates, which is attributable to the absence of Mg+-induced toxicity in highly oriented LDHs. Over a 48-h release period, LDH-coated Mg substrates show a lower release of free Mg+ ions, which improved cytocompatibility. Additionally, LDH coatings have also been shown to improve the hydrophilicity of implants172 (Figure 5C).
LDHs have been utilized in conjunction with thermoresponsive gels to deliver therapeutic molecules. Thermoresponsive poly(N-isopropylacrylamide) gels containing MgAl-LDH have been used to deliver siRNA into primary chondrocytes173. These gels led to enhanced uptake of siRNA resulting in an 82-98% reduction in target gene expression levels, thus beneficial for treating cartilaginous tissue degeneration using RNA interference technology. Additionally, LDHs show good cell viability at concentrations less than 250μg/ml174,175. Mg-Al LDHs loaded with antibiotics and incorporated into PLGA films (5% w/w) displayed a prolonged drug release profile at appropriate therapeutic concentrations and prolonged anti-microbial activity176. This ability for sustained release has also been utilized for the delivery of NSAIDs100,177. PEG hydrogels are extensively used as scaffolds for regenerative medicine applications but require functionalization with a bioactive molecule via a complex multi-step reaction. To overcome this limitation, bioactive nanocomposite PEG-hydrogel crosslinked with a multifunctional polydopamine-layered double hydroxide crosslinker improved the bioactivity and mechanical strength of the hydrogel and also promoted the osteogenic differentiation of mesenchymal stem cells178.
More recently, several reports used layered double hydroxides containing nanocomposite scaffolds for bone tissue engineering applications179,180,181,182,183. Mg-Al LDH/chitosan porous scaffolds loaded with pifithrin-α (PFT-α) steadily releases PFT-α, which inhibits p53 activity and subsequently decreases bone resorption while also promoting bone formation of human bone marrow stem cells (hBMSCs)179,180. Polymer nanocomposite scaffolds loaded with LDH were reported to enhance the regeneration of bone defects in rabbit models181. LDHs polypeptide-thermogel composites have also been utilized for promoting chondrogenic differentiation of tonsil-derived mesenchymal stem cells through a steady release of kartogenin, an inducer of chondrogenic differentiation182. Carbonate-intercalated LDH networks have been used as initiator species to promote the self-assembly of lactone monomers derived from copolymer hybrids183. This approach is a safe, non-toxic method to formulate polymerized structures containing porous networks that can be exploited for tissue engineering applications (Figure 5D). Despite the excellent biocompatibility of LDHs, their applications for biomedical applications remain limited. More detailed investigations of their in vivo behavior and effects on stem cell differentiation would be critical in establishing these materials as a new frontier for regenerative medicine.
5.1.3. Synthetic nanoclay
2D nanoclays have shown high biocompatibility and degradation characteristics that lead to non-toxic by-products184, which are essential for biomedical applications (Figure 6A). 2D nanoclay (Laponite) is shown to induce growth factor-free osteogenic differentiation of human mesenchymal stem cells (hMSCs)69,185,186. A recent study describing the effect of 2D nanoclay on the transcriptome of hMSCs showed upregulation of transcriptional programs for osteochondroal differentiation, including genes important for cytoskeletal rearrangements, cell migration, and differentiation of stem cells185. This study highlights the potential for nanoclay to be used in tissue-engineering applications for bone and cartilage regeneration. Another in vitro studies showed higher levels of alkaline phosphatase (ALP) and calcium deposition associated with osteogenic differentiation of hMSCs cultured on laponite bioceramics187. In vivo implantation of laponite bioceramics into pigs subjected to a large bone defect led to complete healing of the defect, in contrast, to control animals. These studies highlight the potential of nanoclay in regenerative medicine.
Figure 6. 2D nanomaterials for tissue regeneration.
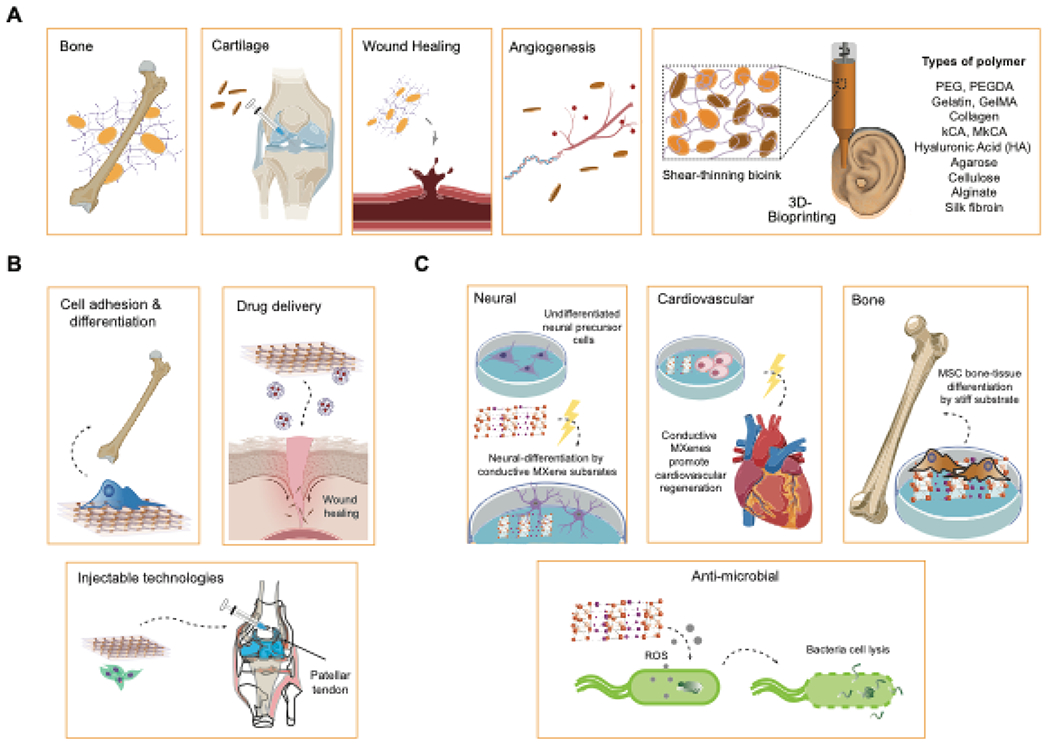
(A) Various biomedical applications of nanoclay. (B) Applications of polymer nanosheets for regenerating various tissues. (C) Applications of MXenes in biomedical engineering.
Nanoclay incorporation into polymer-based hydrogels or scaffolds exhibits enhanced cell adhesion, proliferation, and differentiation while simultaneously providing better mechanical and rheological properties. For example, the stimuli-responsive polymer poly (N-isopropylacrylamide) (PNIPA), used in biomedical applications due to its clear volume phase transition, has low swelling, optical, and mechanical properties above its lower critical solution temperature (LCST). These aspects can be improved by grafting PNIPA chains to uniform dispersions of exfoliated inorganic clay, thus increasing its utility in biomedical strategies. In another example, MMT nanoplatelets were incorporated into poly L-lactic acid (PLLA) scaffolds to improve mechanical stiffness, porosity, and biodegradability188, resulting in a 40% increase in the tensile modulus of the nanocomposite scaffold. This improvement is attributed to the lowering of the glass transition temperature and crystallinity by the addition of modified MMT nanoplatelets. Therefore, nanoclays are useful additives to improve the mechanical and rheological properties of biomaterials.
Polymer clay nanocomposites (PCNs) composed of MMT improve mechanical properties and enhance osteogenic differentiation of cells in bone tissue engineering applications189. Specifically, structural modification of MMT by the integration of the unnatural amino acids (±)-2-aminopimelic acid, 5-aminovaleric acid, and DL-2-aminocaprylic acid into a chitosan-polyglycolic acid scaffold showed osteoblast biocompatibility and potential as a mechanical cue to promote bone formation. Addition of MMT to silk-based scaffolds improved their bioactivity and provided osteogenic stimuli for hMSCs188. Doping of electrospun fibrous PLGA scaffold with up to 5% laponite for osteogenic differentiation of hMSCs showed improved attachment and mechanical stability190 along with the sequestration of osteogenic proteins191. PLGA nanofibers incorporated with laponite nanodisks were found to enhance attachment, proliferation, and osteogenic differentiation of hMSCs by releasing Si and Mg2+ ions191. The self-organizing nature of Laponite in solution has also been exploited to form pristine hydrogels that act as a sustained delivery reservoir for bone morphogenetic protein 2 (BMP-2) in ectopic bone formation192. These qualities extend to other biomedical applications such as regeneration of the retina and the periosteal layer of bone. Polyurethane nanocomposites composed of 5% montmorillonite clay support the attachment, growth, and proliferation of ARPE-19 retinal pigment epithelial cells. In vivo implantation under the retina and suprachoroidal space of Brown Norway rats and subsequent histological analysis showed no inflammatory responses or acute ocular toxicity40.
Drug delivery systems also capitalize on the incorporation of nanoclay. A sustained release profile of the drug pyrene was achieved by combining laponite clay with (α-acetal-poly (ethylene glycol)-block-[poly (2-(N, N-dimethylamino) ethyl methacrylate)] (Acetal-PEG-b-PAMA), which improved dispersion stability of the nanoclay by increasing ionic strength193. Laponite nanosilicates loaded with the secretome of MSCs was incorporated into GelMA hydrogels (0.8% w/v) and showed a significant increase in human vascular endothelial cell (HUVEC) proliferation and formation of a HUVEC-characteristic lumen70. Cardiomyocytes (CM) cultured in the presence of the MSC secretome-loaded laponite-containing GelMA showed healthy morphology, as evidenced by F-actin and nuclei staining. In another study, hydroxyl groups on the surface of MMT clay have been used to intercalate positively charged drugs such as ibuprofen for delivery to target sites.194 One-dimensional halloysite clay nanotubes employed for delivering active molecules showed 50-100 times longer release rates than microcrystal-based release systems195. These examples suggest that nanoclays improve the retention of therapeutics for sustained drug delivery applications.
The shear-thinning effect imparted by nanosilicate addition allows the design of minimally invasive injectable hydrogels while maintaining their structural integrity post-injection. Application of shear stress allows the preformed hydrogels to be injected into the target site; when shear stress is removed, the gels quickly self-heal196. Furthermore, encapsulation of cells within the network of shear-thinning hydrogels protects the cells from high shear forces experienced during injection, thus improving the outcome of cell-based therapeutics and also allowing the hydrogels to be utilized as bioinks for 3D bioprinting197–199.
5.1.4. Metal carbides and nitrides (MXenes)
MXenes such as Ti3C2 exhibit improved anti-bacterial activities compared to other related 2D nanomaterials. MXenes promote the formation of ROS and selectively cause oxidative stress of neoplastic cells, making them useful anticancer agents 200,201. MXenes have also been employed for the regeneration of neuronal and bone tissue due to their electrically conductive nature and high tensile strength. The first discovered MXene, Ti3C2, showed superior electronic conductivity (10,000 S/cm) and volumetric capacitance (1500 F/cm3), exceeding that of graphene (60-100 F/cm3), which makes it a useful candidate for the regeneration of electrically active tissues such as cardiac and neural tissues202. Ti3C2 nanosheets have been used to culture neuronal cells as they exhibit good biocompatibility, promote neurite outgrowth, and facilitate synapse formation. These films successfully support the attachment and proliferation of progenitor neurons202.
MXene nanosheets composed of Ti3C2Tz have also been reported to reinforce components for PLLA membranes to guide bone regeneration203 (Figure 6C). Incorporation of 1wt.% of Ti3C2Tz in PLLA membranes significantly improved the mechanical strength of membranes by increasing the ultimate tensile strength and Young’s modulus. MXene-reinforced PLLA membranes also showed improved biocompatibility by promoting greater attachment of pre-osteoblasts, increased proliferation, and higher alkaline phosphatase activity of cells seeded on the nanocomposite.
5.1.5. Polymer nanosheets
Polymer nanosheets have gained prominence in tissue engineering due to their high flexibility, adhesiveness, and transparency. Polymer nanosheets can easily be fabricated from biocompatible, biodegradable, and low-immunogenic components like polysaccharides. Free-standing polysaccharide nanosheets derived from chitosan and sodium alginate have been reported as an alternative to fibrin sutures for the repair of large size visceral pleural injuries204. The polysaccharide nanosheets also helped to selectively accumulate blood clots at specified sites along with the interface of the nanosheets, which supported the migration of fibroblasts and promoted subsequent angiogenesis without causing any hemolysis of blood cells. Using the LbL technique by repeated dipping of the substrate between the anionic and cationic solutions, the number of polymer layers was increased, resulting in a thicker nanosheet (75 nm) with higher mechanical strength that could withstand pleural pressures within the physiological range.
Polymer nanosheets serve as flexible systems for cell adhesion and proliferation since they can be easily handled in liquid form. Polylactic acid (PLA) nanofilms exemplify this property on silicon substrates, which promoted cell adhesion, spreading, and enhanced endochondral ossification markers in MSCs205. Thus, these nanofilms have the potential to be used as MSC-loaded injectable scaffolds for bone and tendon injury repair. Retinal pigmental epithelial (RPE) cells seeded onto micropattemed PLGA nanosheets that contained magnetic nanoparticles showed higher proliferation rates and greater resemblance to the natural hexagonal morphology of RPE cells as compared to those seeded on smooth PLGA nanosheets206. A very high cell viability rate (over 80%) was observed with nanosheets having dimensions in the range of 300-500μm. Polydopamine (PDA) nanosheets consisting of various polymer brushes grafted onto the surface have also shown increased attachment and proliferation of mouse embryonic fibroblasts (MEFs)22.
The use of 2D polymer nanosheets in conjunction with three-dimensional scaffolds has been exploited to control the differentiation of MSCs for successful stem cell therapy by providing topographic cues, cell adhesion capabilities, and improved release of bioactive factors (Figure 6B). For example, topographic cues on micro-grooved PLGA nanosheets that mimic the periosteal layer of bone promoted hMSC alignment and resulted in the upregulation of osteogenic markers207. Biocompatible and porous polymer nanosheets consisting of a 1:1 polymer blend of biodegradable poly (D, L-lactic) acid (PDLLA) and polystyrene promoted cell adhesion of murine skeletal myoblast cells and enhanced cellular protein secretion, which resulted in the formation of a rolled tube that mimicked the 3D tubular structure of blood vessels23. Poly(L-lactic) acid (PLLA) nanosheets have been utilized for drug delivery methods by secreting bone morphogenetic protein-2 (BMP2) in a sustained release manner over 57 days, which greatly enhanced bone regeneration in mice with critically-sized calvarial defects208. These examples demonstrate the utility of polymer nanosheets in applications designed to direct stem cell fate and differentiation.
5.2. Cancer therapeutics
Utilization of 2D nanomaterials in the treatment of cancer is primarily advantageous due to physiochemical properties offering unique targeting capabilities, therapeutic binding ability, and sustained drug release209. 2D nanomaterials have previously been shown to effectively accumulate in tumors due to their enhanced permeability and retention (EPR) effect, which is an added benefit attributed to both the size of this class of biomaterial and other unique physiochemical properties often found in 2D nanomaterials210. These nanomaterials also provide a large surface area for loading chemotherapeutics via covalent or non-covalent interations, depending on surface functionalization and charged properties211. Further, 2D nanomaterial properties can be harnessed for diagnostic purposes along with their therapeutic capabilities which is the basis for the theranostic field of study. Many 2D nanomaterials have a strong capability to absorb wavelengths in the NIR range, imparting properties beneficial for harnessing the photothermal effect. Thus, emerging techniques utilizing 2D nanomaterials for identification and targeted destruction of tumors have heavily focused on harnessing photothermal properties of materials such as TMDs, MXenes, MOFs, and LDHs.
5.2.1. Transition metal dichalcogenides (TMDs) and transition metal oxides (TMOs)
Photothermal capabilities of 2D nanomaterials can cause the absorption of external optical energy (such as NIR light) which generate heat for tumor ablation or even drug release. This technique of hyperthermia causes a local rise in temperature to 40-45°C subsequently causing cell death in the tumor being irradiated. Both TMDs and TMOs provide unique physiochemical properties that are beneficial for chemotherapy applications, such as their large surface area for drug loading capabilities, and their ability to act as anti-cancer agents through cytotoxicity by producing reactive oxygen species212. Further, TMDs such as MoS2 have unique electrical and physiochemical properties that provide the added benefit of photoacoustic imaging in addition to improved NIR absorbance213 (Figure 7A). Photoacoustic imaging is noninvasive and depends on light-adsorption by a sample which creates mechanical vibrations that generate transmittable, acoustic waves214. This imaging technique allows for deeper tissue penetration in areas where it would be difficult to image hard to reach tumors with other optical imaging technologies, making TMD theranostic strategies an attractive alternative215. Hexagonal MoS2 nanosheets can provide photothermal modulation140, and provide photoacoustic signaling due to their high optical absorbance213. Along with high optical absorbance, 2D TMDs have good photostability and photothermal conversion efficiency making them able to sustain their intrinsic properties under NIR irradiation without requiring additional components216.These nanomaterials are also highly efficient for therapeutic binding and release because of their 2D structure with a high surface-area-to-mass ratio161. Further, in the low pH tumor environment and under NIR stimulus, MoS2 nanosheets have been shown to have greater therapeutic release of anti-cancer drugs such as doxorubicin due to the increased solubility of the drug at low pH and increased molecular motion from temperature increase217. A recent study has also shown that the NIR activable nature of MoS2 nanosheets can be incorporated into polymer hydrogel scaffolds to form in-situ gelling hydrogels 218. The addition of defect rich MoS2 nanosheets improved the hydrogel mechanical stiffness and drug loading capacity for NIR triggered release of doxorubicin.
Figure 7. 2D nanomaterials in cancer therapy.
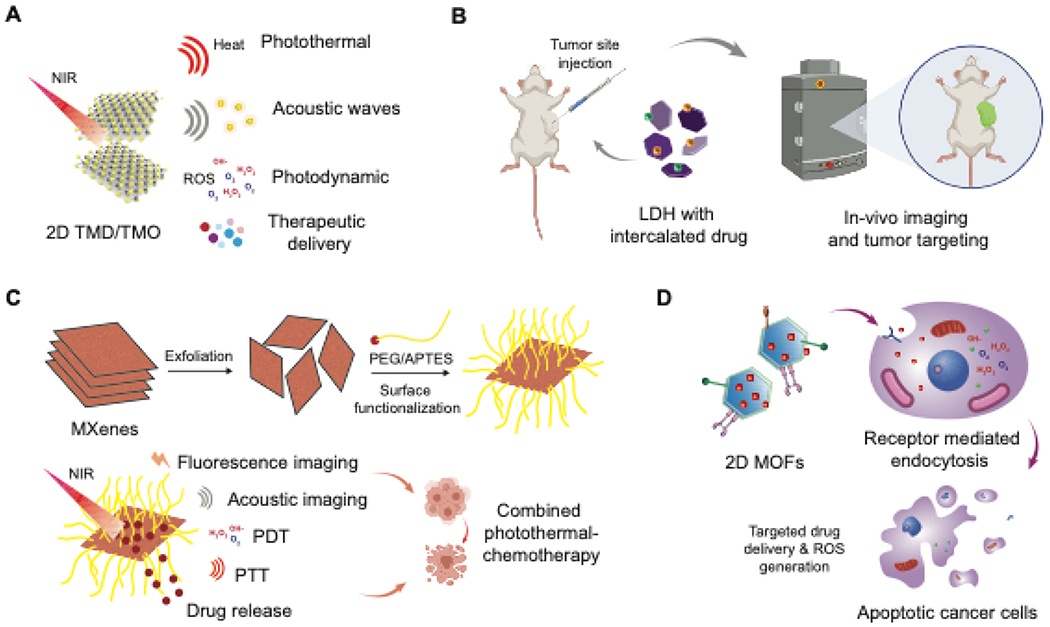
(A) Different application modalities of TMDsand TMOs for cancer therapeutics. (B) Application of LDHs for tumor-targeting and imaging applications. (C) Surface functionalization of MXenes and NIR mediated PDT/PTT effect exhibited by MXenes. (D) Applications of 2D MOFs for targeting cancer cells to cause oxidative stress resulting in apoptosis.
5.2.2. Layered double hydroxides (LDHs)
LDHs are attractive 2D nanomaterials for cancer therapeutic delivery due to their ability to bind anionic drugs (Figure 7B). Their high charge density allow for high drug loading efficiency, and with the addition of rare earth elements, pH dependent drug release capabilities are achieved to ensure appropriate drug release within the tumor microenvironment 219. LDHs require doping to achieve additional MRI contrast capabilities, which one group has achieved by doping with Mn2+ and Fe3+ ions for their cancer imaging and drug delivery platform220. The addition of these ions imparts paramagnetic properties due to their high magnetic moment and extended electron spin relation time, which provide the desired contrast signals221. To achieve NIR optical absorption, copper ions (Cu2+) have been used to dope LDH nanosheets to achieve photothermal therapy capabilities in addition to providing MRI contrast signals222. Chemotherapy strategies involving LDH rely on the inherent anti-cancer activity of LDH nanosheets, high drug loading capabilities, and positive charge for easy internalization into cancer cells20. LDH nanosheets have their own inherent ability to generate ROS production in cancer cells via the Ca2+/calmodulin pathway223. Further, their ability to engage in an anion exchange with drug molecules allows for easy incorporation of cancer therapeutics in between LDH layers224.
5.2.3. Synthetic nanoclay
Like LDHs, synthetic nanoclay have previously been utilized for anti-cancer therapeutics for both their large surface area to volume ratio, their high charge density, and pH dependent durg release capabilities. Synthetic nanoclay offer unique electrostatic interactions, and have the ability to both load high quantities of anti-cancer drugs and provide sustained release due to their surface charge225. The unique surface charges of nanoclay also impart pH sensitivity, offering effective delivery and sustained release of anti-cancer therapeutics such as doxorubicin and methotrexate in the tumor microenvironment in a pH-dependent manner226. Further, these nanomaterials are capable of functionalization with ligands such as polyethylene glycol-linked (PEG-FA), which can impart targeted delivery to cancer cells over expressing folic acid receptors227. For added theranostic capabilities, as nanoclay do not offer NIR absorbance capabilities on their own, nanoclay composites have incorporated gold nanoparticles to provide CT contrast agents which allow for tumor imaging228.
5.2.4. Metal carbides and nitrides (MXenes)
MXenes also possess good theranostic properties. MXene materials have previously been utilized along TMDs in nanocomposites designed to provide photoacoustic properties215. MXenes are useful alone and in nanocomposites with TMDs because they have good NIR absorbance and a high surface-to-volume ratio allowing for excellent therapeutic loading capabilities (Figure 6C). Their ability to absorb NIR light imparts their ability to provide photothermal ablation to tumors229. Doping of manganese oxide nanoparticles on Ta4C3-SP nanosheets has been shown to provide both photoacoustic imaging capabilities (due to MnO2 component) and photothermal tumor ablation upon NIR laser application17.Further, MXenes can have better hydrophilicity (when functionalized with hydroxyl groups) compared to previously discussed 2D nanomaterials, allowing for the adsorption of cations and positively charged therapeutic molecules230. This aspect of MXene functionalization is another primary attractive attribute for this 2D nanomaterial, as functionalization of other 2D nanomaterials such as graphene or MoS2 TMDs have proven more difficult than with MXenes231. Additional multifunctionalization of MXenes can be done to provide magnetic resonance imaging (MRI) signaling capabilities, such as is the case in one study which grew superparamagnetic iron oxide nanoparticles on the surface of 2D Ta4C3 MXenes232. Multifunctionalization with amino groups can provide biosensing behavior, as one study found Ti3C2 MXenes biofunctionalized with carcinoembryonic antigen to detect and immobilize this cancer receptor231.
5.2.5. Polymer nanosheets
Polymeric nanosheets have been less utilized for cancer therapeutic applications, though some 2D polymer-based nanomaterials have recently been shown to absorbe NIR light and provide photothermal (PTT) capabilities. Of note, polypyrrole has been shown to achieve tumor ablation along side photothermal imaging for cancer theranostics. Alongside other non-2D nanomaterials such as polyaniline, poly(3,4-ethylene dioxythiophene):poly(4-styrenesulfonate) (PEDOT:PSS), and dopamine-melanin, these polymers are considered conducting233. 2D ultrathin polypyrrole shows good biocompatibility while also supporting tumor ablation abilities in the second NIR window (1000-1350 nm), by specifically exhibiting absorption at 1064 nm113.
5.3. Biosensing and Bioimaging
The sheet like structure of 2D nanomaterials and broad range of properties such as high surface area, high electrical conductivity and excellent thermal conductivity allow for their applications in the biosensing and bioimaging fields (Figure 8). 2D nanomaterials possess good biocompatibility as compared to graphene and can easily be dispersed in aqueous environments, making them suitable for biosensing and bioimaging applications. 2D nanomaterials can also interact with specific biomolecules leading to spontaneous surface biofunctionalization improving biocompatibility. Transtion metal based 2D nanomaterials like TMDs and TMOs are excellent candidates for biosensing and bioimaging applications due to their good biocompatibility and fluorescence quenching abilities56. While other classes of 2D nanomaterials like nanoclay and LDHs do not possess intrinsic electronic properties for biosensing applications, their ability to sequester molecules has been used to efficiently localize large amount of biomolecules and maintain their activity for prolonged read out times234.
Figure 8. 2D nanomaterials for biosensing applications.
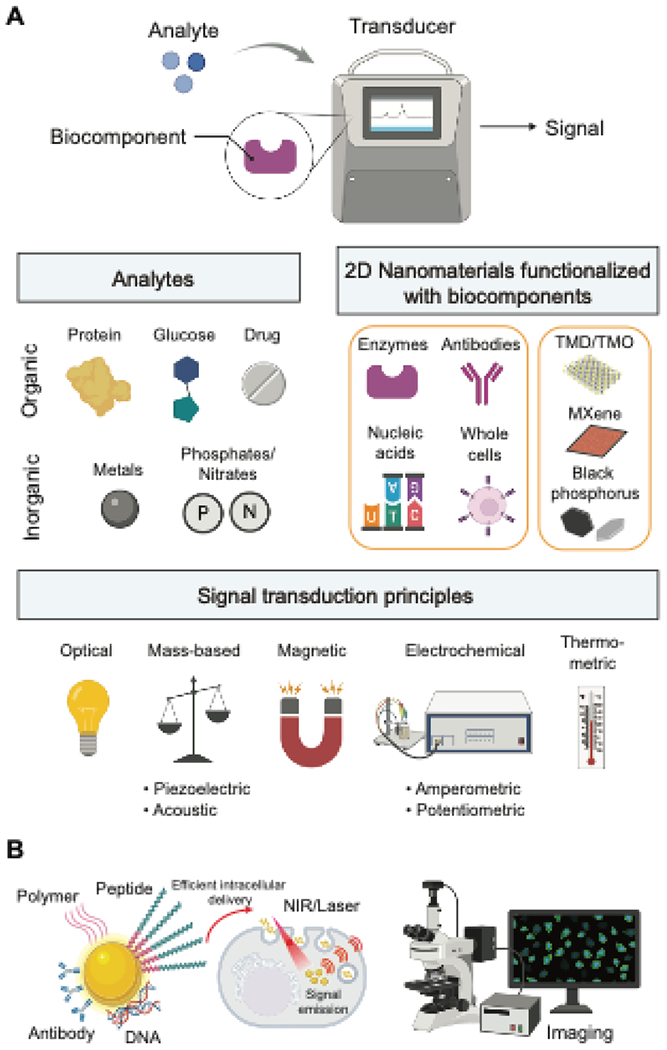
(A)Principle of biosesensing using 2D nanomaterials. (B) Range of analytes detecteable using 2D nanomaterials. (C) Functional capabilities of 2D nanomaterials. (D) Signal transduction principles achieved while designing 2D nanomaterial based biosensors.
5.3.1. Transition metal dichalcogenides (TMDs) and transition metal oxides (TMOs)
2D nanomaterials such as boron nitride (BN), TMDs, TMOs , MXenes and black phosphorus (BP) have been extensively applied in the field of biosensing due to their excellent optical and electronic properties. Such nanomaterials have been used to design a plethora of biosensors which are based on principles like electrochemistry, fluorescence, surface enhanced Raman scattering (SERS), surface plasmon resonanace (SPR) and colorimetry (Figure 7D). For electrochemical biosensors, 2D nanomaterials having high electrical conductivity, fast transfer kinetics and high surface area for functionalization have been used39. These include biosensors based on 2D layered materials such as phosphorene, MoS2 and WS2. For example, MoS2 based biosensors have been used for detection of glucose and selective detection of dopamine in mixtures containingascorbic and uric acid235 (Figure 7B). Surface modified WS2 nanosheets have been reported for enzyme sensing applications. Black phosphorus has also been explored for biosensing applications due to its high carrier mobility and thickness depedent band-gap236.
TMOs have also been reported for biosening and biomaging applications due to their unique physicochemical, optical and electronic properties16. TMOs can also interact directly with biomolecules causing changes in their structure or degradation. MnO2 nanosheet modified upconversion nanoparticle system have been reported for simultaneous sensing and fluorescent detection of the reducing agent GSH237. The strong quenching effect exhibited by MnO2 nanosheets towards hydroxycoumarin has also been used to design MnO2 fluorescent probes to detect ascorbic acid in rat brain238. Recently, a multimodal application of MnO2 nanosheets was reported239. They demonstrated that MnO2 nanosheets could act as DNA nanocarriers to deliver the cargo to target cells, get reduced by intracellular GSH and release Mn2+ ions that could be utilized as a contrast agent for MRI.
5.3.2. Synthetic nanoclay and layered double hydroxides (LDHs)
Biosensors based on clays like LDH and nanoclays are also popular for healthcare applications. Transducers that have been coated with a layer of LDH can effectively immobilize of biomolecules onto the surface while still maintaining their activity, stability and increased accessibility to the analyte. To this effect, cationic LDHs having good mechanical and thermal stabilities have been extensively used for modifying electrode surfaces240. Biosensors modified with Ni/Al LDH have been reported for the sensing of glucose and lactate at concentrations upto 0.04 mM241. While a majority of sensors modified with LDH usually operate on the principe of electrochemical sensing, there have been reports of fluorescence based sensors as well. For example, enhanced green fluoresecent protein (EGFP) was successfully immobilized on the surface of Mg/Al LDHs by layer-by-layer assembly. The immobilized EGFP retained its secondary structure within the layers of the LDH and showed differential responses in fluorescence depeding on the pH of the environment and could also detect small biomolecules like protoporphyrin242. Similar to LDHs, graphene based biosensors modified with 2D nanoclays such as montmorillonite and laponite have also been successfully reported for glyphosate243 and glucose biosensing applications244.
5.3.3. Metal carbides and nitrides (MXenes)
MXenes have emerged as excellent 2D nanomaterials for biosensing applications. Properties such as low diffusion barrier and high ion transport coupled with their inherent biocompatibility and large surface area make these materials excellent candidate for biosensing applications245. MXene based electrochemical biosensors have been reported for the detection of blood glucose levels246, hydrogen peroxide 247 and other small biomolecules such as NADH, ascorbic acid, dopamine and uric acid with very high sensitivity and selectivity248. Detection of cells using MXenes has also been achieved using Ti3C2 MXene due to the ease of micromaching and printing MXene based geometries that have a large contact area for cells249.
5.1.5. Polymer nanosheets
Biosensing applications based on polymer nanosheets have been restricted to usage of conducting polymers (CPs) such as polyaniline (PANi), polypyrrole(PPy) and poly(3,4-ethylenedioxythiophene) (PEDOT) which have unique electrical and optical properties. CPs exhibit high biocompatibility and low impedance with cells which makes them attractive candidates for biosensing applications. Their ease of fabrication, functionalization and ability to be used as modifiers to improve other biosensing substartes using simple deposition techniques as drop casting and electrochemical deposition also adds to the advantages of using CP nanosheets for biosensing applications 250.
6. Emerging 2D nanomaterials
6.1. Metal-organic frameworks (MOFs)
Metal-organic frameworks (MOFs) are a new class of organic/inorganic hybrid materials consisting of a porous crystalline structure formed by linking metal ions or clusters (nodes) to negatively charged organic ligands251 (Figure 8A). MOFs are fabricated using a number of techniques that can be broadly classified into top-down and bottom-up methods. The top-down approach relies on perturbing the layers of multilayered MOFs to form single-layered 2D MOF nanosheets252. Layered-structure MOFs exhibit strong coordination bonding within the layers but possess weak Van der Waals interactions or hydrogen bonding between the layers, which can be easily broken by applying mechanical force. Commonly used methods include sonication exfoliation, sonication with Li intercalation, shaking treatment, spontaneous exfoliation, freeze-thaw exfoliation, ball milling followed by sonication, exfoliation, and chemical exfoliation.
While the top-down approach is efficient in producing pure, highly crystalline, and non-layered 2D MOF nanosheets, the production rate and yield from these methods remains very low for practical applications. The stabilization of the exfoliated nanosheets against restacking/aggregation also remains a considerable challenge. In contrast, the bottom-up approach involves techniques that can directly synthesize 2D MOF nanosheets from metal ions and organic linkers by blocking growth along the vertical direction31. Techniques that fall under this approach include interfacial synthesis, surface-assisted synthesis, three-layer synthesis, modulated, and sonication synthesis. However, it remains a challenge to selectively block and grow single-layered MOFs using these techniques, with only limited success being reported for the modulated synthesis.
6.1.1. Regenerative Medicine
Due to their tunable nature, high porosity, large surface area, controllable surface functionalization, and biodegradability, 2D MOF nanosheets have been extensively investigated as nanocarriers for drug delivery31,253. While most applications have been targeted towards the delivery of chemotherapeutic drugs for cancer, there have been some investigations that utilize MOFs for regenerative applications254. Porous titanium surfaces coated with nano-scale Zn-based MOF films composed of zeolitic imidazolate framework-8 (ZIF-8) have been shown to possess improved biocompatibility and osteogenic differentiation-inducing properties such as increasing ALP activity, matrix mineralization, and expression of osteogenic markers, making them a useful additive for bone tissue engineering. Copper-based MOF nanoparticles conjugated with folic acid have been shown to promote wound healing in diabetic mice by inducing angiogenesis255. The copper-based MOFs also promoted collagen deposition and re-epithelialization. However, MOFs in their 2D nanosheet form remain largely unexplored for various tissue regenerative applications despite their useful qualities.
6.1.2. Cancer Therapeutics
Metal organic frameworks (MOFs) have a tunable porous framework that can be utilized as a vehicle for therapeutic delivery (Figure 7D). However, MOFs require modifications and fuctionalization to ensure tumor targeting256. To combat this, studies have successfully utilized polydopamine (PDA), which itself is a photoacoustic agent, to functionalize MOFs to ensure they are targeted and retained in tumors257. To improve uptake by cancer cells, studies have utilized protective gelatin layers to harness the proton sponge effect to improve the efficiency of Cu-MOFs loaded with methotrexate in the killing of human breast adenocarcinoma cells258.The release of therapeutics from MOFs in the tumor environment occurs by degradation of the nanomaterial at low pH. Further, MOFs have been shown to cause toxic reactive oxygen species (ROS) generation, adding to their utility for tumor killing applications259. Interestingly, MOFs have been readily used in an emerging field investigating chemodynamic therapy, which harnesses the high hydrogen peroxide levels of the tumor environment to induce Fenton reactions which are highly effective against cancer cells29. This process occurs when cations catalyze hydrogen peroxide in this environment to OH radicals that subsequently interrupt key biological processes in cancer cells, effectively harnessing the natural tumor environment to work against itself260. In this way, MOFs have been dubbed “nanozymes” as they behave an enzyme-like material that increases the production of hydrogen peroxide in the tumor microenvironment that create Fenton reactions for chemodynamic therapy applications261.
6.1.3. Biosensing and Bioimaging
The porous nature of MOFs combined with the large surface area for adsorption or encapsulation has resulted in the ability of combining these nanomaterials with various biomolecules for biosensing applications. MOFs have been used in conjunction with antibodies, nucleic acids, enzymes, ions and dyes. Nucleic acids have been conjugated over the the surface of MOFs using coordination chemistry or covalent conjugation strategies. Stable constructs composed of DNA/MOFs fabricated via ultrasonic mixing have been reported262. Targeted miRNA detection was demonstrated by adsorbing dsDNA on the surface of MOFs that were pre-loaded with electroactive dyes that would be released upon target hybridization263. Electrochemical detection of the small biomolecules such as adenosine have been reported using ZnNi MOFs bound with aptamers264. Highly sensitive detection of cancer cells overexpressing the folic acid receptor have also been carried out using folic acid conjugated UiO-66 MOFs265. The good biocompatibility exhibited by MOFs has also allowed their utilization for intracellular imaging applications. MOF based bioimaging applications utilizing the principles of electrochemical luminescence, SERS, MRI and photoelectrical chemistry have been reported. The porous nature and large surface area of MOFs also allows for binding of a great number of inorganic and organic fluorescent guest molecules to fabricate nanocomposites for in vitro and in vivo bioimaging266. Rhodamine B (RhB) loaded on zeolitic imidazolate frameworks (ZIF) such as ZIF-90 have shown promising results for intracellular ATP sensing and imaging267. Zirconium based MOFs such as nUiO-67 carrying rhodamine 6G and RhB and have been reported for imaging FL83B and HepG2 cells and as fluoresecent indicators in the yellow-green and red emission spectrums respectively.Al- and Fe- based MOFs have been utilized with a wide variety of guest materials such as methylene blue, carbon dots, and upconversion nanoparticles (UCNPs)30,267.
6.2. Covalent organic frameworks (COFs)
Covalent organic frameworks (COFs) are anew class of 2D and 3D porous, crystalline materials interconnected by organic linker molecules held together by covalent atoms such as boron, oxygen, and carbon to form a regular framework24 (Figure 8A). 2D COFs are defined by a framework that is restricted to two-dimensional layers26,268. As a result, the crystal structure resembles graphene or hexagonal boron nitride with stacked layers held together by London dispersion forces instead of covalent bonds. The topology of individual layers of 2D COFs is primarily determined by the structure of the connector and linker moieties, usually forming a hexagonal pattern due to the D3h symmetry of the linker molecules. These layers are then stacked to form a regular crystal lattice. Such geometrical arrangement results in the formation of straight channels that are orthogonal to the individual layers, forming a highly ordered porous structure251. The synthesis of COFs is carried out by solvothermal processes involving boronate ester, imine, or reversible boroxine anhydride condensation, and ionothermal, microwave mediated, interfacial, mechanochemical, or room temperature synthesis. The combination of highly reversible condensation reactions and in situ tautomerization result in the high chemical stability and crystalline structures of these 2D nanomaterials25,26. A tunable pore structure and unique photoelectric properties also add to the potential of COFs as controlled theranostic vehicles for various biomedical applications26,269.
6.2.1. Regenerative medicine
The dynamic nature of the covalent linkages observed in 2D COFs suggests a potential as biodegradable stimuli-responsive materials for drug delivery26,270. pH-responsive COFs that exhibit protonation-deprotonation of constituent triazine groups have also been reported269. The surface area of COFs, as measured by N2 adsorption experiments, far surpasses those of well-established highly porous materials such as mesoporous silica and carbon-based structures. Besides, the presence of aromatic compounds in the structure of COFs allows the loading of cyclic monomers through π-π stacking interactions. 2D COFs exist as spherical nanoparticles (~50 nm) in aqueous environment and show good biocompatibility270. COFs have been extensively used for drug delivery but their usage has been limited to stable small molecule drugs for cancer therapeutics and synthetic aptamers mimicking growth factor receptors for biosensing and boimaging applications. The loading of guest molecules into COFs mostly takes place during the later stages of synthesis, which may not represent an ideal environment to maintain the biological structure and activity of sensitive biomolecules such as growth factors, mRNA and DNA. Further optimization of the synthesis process and exploration of milder reaction conditions to fabricate COFs without compromising on the yield and structure would help in expanding the usage of these nanomaterial to deliver biomolecules for tissue regeneration.
6.2.2. Cancer therapeutics
The highly porous nature of COFs has been utilized photocarrier agents for the loading and delivery of anticancer agents such 5-FU271, quercetin272 and doxorubicin27 to tumor cells. Photodynamic (PDT) and photothermal (PTT) therapy has also been demonstrated using COFs directly functionalized or loaded with sensitizing agents such as LZU-1-BODIPY-2273 and indocyanine green274. COFs have also been explored for their intrinsic photothermal capabilities for anti-cancer therapy. Mi et.al have reported a water soluble PEG modified Py-BPy+.-COF that exhibited high absorption in the NIR range. The authors have demonstrated high photothermal effect of this COF in A549 lung carcinoma cells evidenced by rapid temperature elevation resulting in tumor growth inhibition275. Combinatorial cancer therapy has also been reported using 2D COFs. Wang et.al demonstrated that cyanine mediated exfoliatin of the boronate ester based 2D COF produced an cyanine-COF nanocomposite with photothermal activity in the NIR range. The loading of doxorubicin in the cyanine-COF nanocomposite resulted in a simultaneous PTT and chemotherapeutic effects on human breast tumor xenografts in nude mice276. Simultaneous PTT and PDT effects were also reported with COFs by Dong et.al. The 2D COF based nanocomposite VONc@COF-Por system consisted of a covalently conjugated porphyrinic photosensitizer and napthalocyanine as the phothermal agent loaded via π-π interactions. This system showed higher ROS generation and ~56% greater photothermal conversion inhibiting the growth of MCF-7 cells both, in vitro and in vivo277.
6.2.3. Biosensing and bioimaging
COFs are widely used for biosensing and bioimaging applications due to their excellent photoelectrical properties and ability to bind to form hydrogen bonds with biomolecules. 2D COFs imine based COFs such as p-COF and Py-M-COF have been reported for electrochemical sensing of BSA and DNA in aqueous solutions278, epidermal growth factor receptor (EGFR) aptamer279 and aptasensor based detection of enrofloxacin and ampicillin at femtogram levels279 respectively. Fluorescence based detection of single and double stranded DNA has also been reported using 2D COFs such as TPA280 and EB-TFB COFs281 respectively. COFS have also been widely investigated for bioimaging applications. Triazine based COFs synthesized using a microwave assisted strategy have shown a 6 fold increase in photoluminescence when exposed to aqueous environment. These TTA-DFP-COF nanosheets have been intemalzed by clathrin mediated endocytosis and accumulated in the nucleus of HeFa cells without any detrimental effects for nuclear staining282. 2D COFs have also been explored as host material to improve the fluorescence yield of dyes. A 2D COF based nanoprobe, TpASH-NPHS system was designed for intracellular enzyme resistant imaging of endogenous hydrogen sulfide (H2S) in live cells, deep tumor tissue and mouse models of cirrhosis283.
6.3. Sequence-defined 2D nanomaterials
Sequence-defined 2D nanomaterials derived from biological polymers such as DNA,284 proteins,285 peptides286, and peptoids287 have become active targets of the investigation for bioactive scaffolds due to their properties of self-assembly and molecular recognition120. The molecular recognition exhibited by these class of materials is highly selective and sequence-dependent, thus making it possible to fabricate 2D nanostructures with highly tunable functionalities and surface properties. Such sequence-defined 2D nanomaterials have been reported for various applications such as controlled templates for the synthesis of Au nanoparticles,284 bioinspired catalysts,288 enhancers of retroviral transduction efficiencies,289 and as antibody-mimetic materials290.
6.3.1. DNA-based 2D nanomaterials
DNA based 2D nanomaterials, particularly those assembled using the DNA origami technique, have attracted attention due to their unique structural features and self-recognition properties. Specifically, the assembly of a 2D crystalline DNA-origami array from two complementary DNA origami tiles attracted attention from the scientific community43 (Figure 8B). During the formation of such structures, the sequence-controlled complementarity plays a critical role in forming the crystalline 2D nanostructure291,292. The use of such 2D nanostructures derived from DNA can develop cell substrates with distinct topological patterns. The nanotopography plays a crucial role in regulating cell adhesion, morphology, expansion, and self-renewal capacity, for example, through modulation of integrin-mediated ECM signaling. Incorporating such 2D nanostructures into scaffolds as topographical cues can provide a greater level of tunability over directed differentiation of stem cells for regenerating specific tissues. Though DNA-based 2D nanomaterials show great promise in advancing the precision of tissue regenerative strategies, their application remains limited due to the high degree of uncertainty in predicting the DNA sequences that would result in the desired 2D nanostructure.
Current techniques for the application of these DNA-based nanostructures rely heavily on computational calculations and simulations to determine whether desired structures can be generated from the available sequences43,291. Additional problems can arise due to the low thermal and chemical stabilities of large DNA molecules, the restriction to four base pairs limiting the potential functionalities that can be generated, and difficulties introducing surface functionalities without disturbing the 2D structure292,293.
6.3.2. Protein-based 2D nanomaterials
Proteins can be programmed at the genetic level to assemble into molecular machines to carry out cellular functions (Figure 8C). Proteins are made up by a random arrangement of 20 amino acids, which can provide a wide range of functionalities for various high-level biological applications. The self-assembly of proteins into 2D nanostructures is driven by the discovery of in silico tools to model the interactions involved in the assembly process and the advent of making protein building blocks using metal coordination294. Metal coordination is the preferred method for designing protein-based 2D nanomaterials because the bonding provides strength, directionality, and reversibility to the assembly process. As a proof-of-concept for designing biologically significant protein nanosheets, scientists have successfully demonstrated the self-assembly of proteins to resemble the structure of the monomeric redox protein, cytochrome cb562, while retaining their structural stability and function285. While there have been significant advances in the field of protein self-assembly for designing 2D nanomaterials, their applications in regenerative medicine to design scaffolds with intrinsic growth factor activity is restricted by the lack of analytical tools that can precisely predict the nature of interactions of the complex protein building blocks and insufficient tools to manipulate these interactions295.
6.3.3. Peptide-based 2D nanomaterials
Peptide-based 2D nanomaterials are more widely investigated than protein-based nanostructures for regenerative medicine due to the reduced complexity of short amino acid sequences28,296. Despite their small size, these peptide-based nanostructuresexhibit molecular recognition similar to their protein-based counterparts. They can also self-assemble into 2D nanomaterials through the formation of a-helices and ß-sheets (Figure 8C). For example, collagen mimetic peptides can self-assemble into 2D nanosheets through a layered packing of the peptide triple helices. To prevent the formation of the fibrillar morphology, common for collagen-based materials, specific structural features that preferentially cause self-assembly have been incorporated into nanosheets instead of classical fibrillar forms. Furthermore, by tuning the length of peptides and terminal functionalities, nanosheet thickness can be varied286. This study established the potential application of peptide-based 2D nanomaterials for designing scaffolds that can provide both mechanical and chemical cues to stem cells to promote their differentiation into specific lineages. Despite significant advances in understanding the mechanism of peptide synthesis and self-assembly, there has been a lack of meaningful translation of peptide-based 2D nanomaterials into regenerative applications. This stems from the lack of analytical tools to study the complex folding between the peptide backbones, lack of predictive tools to study the self-assembly of peptides, and their low chemical, biological and thermal stability.
6.3.4. Peptoid-based 2D nanomaterials
Peptoids (poly-N-substituted glycine) are a relatively new class of sequence-defined materials that exhibit unique structural, and functional features (Figure 8C). This class of peptidomimetics is characterized by the presence of the side chain on the nitrogen atom of the peptide backbone rather than the a-carbon. Peptoids are synthetic molecules that can mimic both the structural and functional features of both proteins and peptides, thus bridging the gap between synthetic polymers and biopolymers297.
In contrast to proteins and peptides, peptoids exhibit exceptionally high thermal and chemical stabilities resulting from the absence of intra- and inter-molecular backbone hydrogen bonds28,298. The assembly of peptoids into 2D nanomaterials can occur by two processes: interface-assisted compression and solvent-induced spontaneous crystallization. In the interface-assisted compression process, amphiphilic peptoids consisting of both polar and non-polar residues form an initial globular phase that converts into extended 2D ordered nanosheets at a water-oil or water-air interface. The ordering of the peptoids is dependent on the hydrophobic interactions, and the monolayer formation is influenced by temperature299.
To alleviate the need for a water-oil or water-air interface, the solvent-induced spontaneous crystallization process was created300. In this process, amphiphilic peptoids are presented in solution as amorphous particles which crystallize to form elongated nanoribbons before finally transforming into highly crystalline nanomembranes. The presence of collective π-π interactions leads to the formation of nanomembranes exhibiting high chemical and thermal stabilities120. Advantages of using peptoid-based 2D nanomaterials include ease of tuning surface characteristics, the introduction of functionalities using simple cocrystallization approaches, and accessible synthetic routes.
7. Conclusions and outlook
Two-dimensional nanomaterials have steadily garnered increasing interest in the biomedical field with applications spanning therapeutic delivery, biosensing, bioimaging, regenerative medicine, and additive manufacturing strategies. Their benefits include large surface areas, facile synthesis procedures, and ease of functionalization. Some 2D nanomaterials such as nanoclays and LDHs can serve as therapeutic modalities even in their native forms since they are composed of physiologically significant ions and are able to sequester biological molecules and release them gradually over a long period. While there have been significant developments in using 2D nanomaterials as photothermal agents for cancer therapy, biosensing/imaging applications and anti-microbial coatings, their use in regenerative medicine remains to be explored more thoroughly. A major hurdle to this is the restricited control over the size of the nanomaterial. This need for monodisperse size distribution has a great impact on the toxicity of the nanomaterial while simuataneously impacting in vitro effects.
While emerging 2D nanomaterials such as MOFs , COFs and biological template based 2D nanomaterials have been demonstrated to behave as intelligent nanostructures that are multifunctional, adapt to the local environment and are programmable, there yet exists a gap in knowledge that hinders their utilization at the maximum potential. This gap in knowledge is mainly attributable to the lack of significant long-term in vitro and in vivo toxicological evaluations and biological stability . Lack of sufficient characterization of these materials also introduces a level of uncertainty regarding their interactions with cells. Rigorous physicochemical characterization, followed by extensive toxicological evaluation of 2D nanomaterials in different cell lines in vitro and subsequent in vivo studies, would help in extending applications towards regenerative therapies.
Combining the high-throughput power of OMICS techniques such as transcriptomics and proteomics can provide a holistic view of the cellular processes being affected sequentially as a result of nanomaterial treatment at both the gene and protein levels. This could potentially help in better predicting the interaction of 2D nanomaterials with cells at and potentially leveraging the findings to fine tune their design to address specific biomedical challenges or their toxicity. With such a holistic understanding, it would be easier to design hybrid, multimodal materials for more complex applications that require a greater range of stimuli responsiveness. This underutilized class of nanomaterials could potentially emerge as the gold standard for bioengineering strategies that involve precise modulation of cellular behavior for successful biomedical applications.
Figure 9. Emerging 2D nanomaterials.
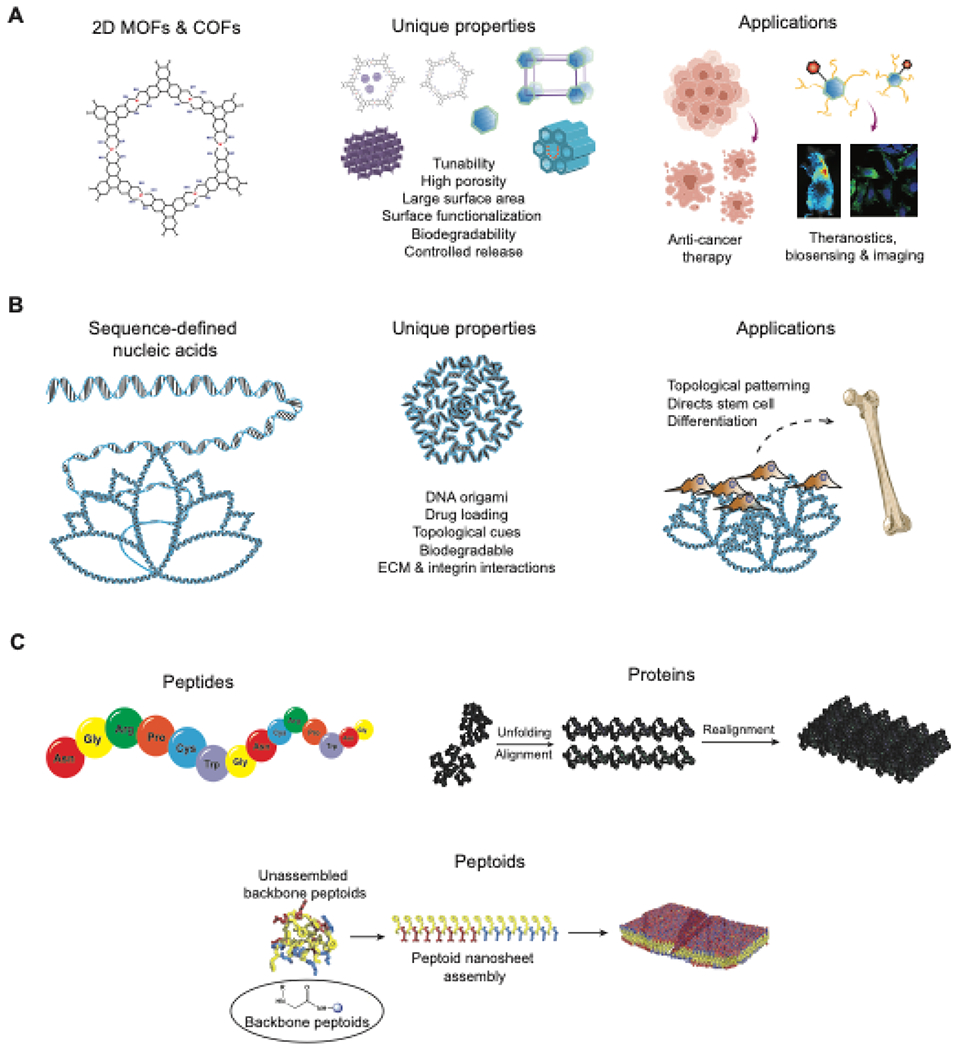
Schematic representation of the structure, properties and biomedical applications of new and emerging 2D nanomaterials (A) 2D covalent and metal organic frameworks, (B) sequence-defined nucleic acid, and (C) sequence defined 2D nanomaterials based on peptide, protein and peptoid.
Table 2:
Overview of the cytotoxicity of LDHs
| 2D nanomaterial | Synthesis | Sizes | Functionalization | Cell viability | Maximum cone. tested | Tested on | Assays used | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Mg-Fe LDHs | Hydrothermal process | 300nm diameter, 10nm thickness | Minocycline | >90% | - | MC3T3-E1, HUVEC, Human gingival fibroblasts | Calcein-AM/PI staining | Broad spectrum antibacterial properties due to ROS generation | 306 |
| Tb3+-doped LDHs | Solvothermal process | 100-200nm diameter | β-cyclodextrin containing hyperbranched polyglycerols | >90% | 120μg/ml | L929 | CCK-8 assay | 307 | |
| Mg-Al LDHs | Hydrothermal process | ~115nm diameter, 321nm post modification | nNH2-SiO2 coated, mannose conjugated, siRNA loaded | >90% uncoated >80% (100 μg/ml for coated) | 400μg/ml | U2OS | MTT assay | Targeted siRNA delivery for enhanced cancer therapy | 308 |
| NiTi-layered double hydroxides | Hydrothermal process | ~2μm length, ~30nm thickness | gold nanorods-modified butyrate-inserted | >90% unmodified | - | RBE, MG63, 4TI, HIBEpic | Alamar blue assay, JC-1 assay, Calcein AM-PI | NIR triggered localized chemothermal tumor therapy | 309 |
| Mg-Al LDHs | Co-precipitation method | 200±15nm diameter, 15±5nm thickness | - | ≥80% | 500μg/ml | A549, L-132, HeLa, HOS | MTT assay | Targeted drug delivery for cancer in vitro and in vivo | 174 |
| Mg-Al-CO3 LDHs | Hydrothermal process | 20nm diameter | - | >80% | 200μg/ml | NSC-34 | MTT assay | In vitro gene transfection for possible gene delivery applications | 310 |
Table 3:
Overview of the cytotoxicity of 2D nanoclays
| 2D nanomaterial | Synthesis | Sizes | Functionalization | Cell viability | Maximum conc. tested | Tested on | Assays used | Applications | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Silicate nanoparticles | Dissociation in deionized water | 25-50nm diameter, 11.5nm thickness | - | >75% | 100μg/ml | hMSCs | MTT assay | 185 | |
| Copper/manganese silicate nanospheres | Hydrothermal process | 25nm size, 10nm thickness | Cancer cell membrane coated | >60% | 200μg/ml | NHDF cells | CCK-8 assay | Synergistic therapy with increased ROS production for hypoxia relief and glutathione reduction | 311 |
| Montmorillonite | Intercalation | - | Cetylpyridinium chloride | >70% | - | NIH-3T3, Ca9-22 cells | WST-1 assay | Antimicrobial activity due to release of cetylpyridinium chloride | 312 |
| Allophane | H2O2 purification | ~5nm diameter | - | >70% | 316μg/ml | A549 cells | WST-1 assay | 313 | |
| Kaolinite nanotubes | DMSO-assisted ultrasonic irradiation | 50-600nm length, 10-50nm diameter | 5-fluorouracil | >95% | 100μg/ml | L929 cells | MTT assay | Controlled release of 5-fluorouracil for chemotherapy applications | 314 |
| Halloysite | - | ~300nm length | Octadecyltri methoxysilane | >90% | 250μg/ml | A549 cells | MTT assay | 315 |
Table 4:
Overview of the cytotoxicity of MXenes
| 2D nanomaterial | Synthesis | Sizes | Functionalization | Cell viability | Maximum conc. tested | Tested on | Assays used | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Tantalum carbide MXenes | Multi-step exfoliation method | 100nm, width | MnOx | ≥95% | 400μg/ml | 4T1 | CCK-8 assay | Local delivery of engineered retinal pigment epithelial monolayer | 17 |
| Titania carbide Mxenes | HF-HCl etching | 1 nm thickness | - | >80% | - | Primary cortical neurons | Calcein-AM/EtHD staining | Far NIR triggered photothermal therapy for cancer theranostics | 202 |
| Niobium carbide MXenes | Liquid exfoliation method | ~150nm width, 0.3-0.8nm thickness | PVP40 | ≥85% | 200μg/ml | 4T1, U87 | CCK-8 assay | NIR triggered high efficiency tumor ablation for cancer therapy | 316 |
| Titania carbide Mxenes | HF-HCl etching | ~185nm width, ~1.44 nm thickness | Glucose oxidase and iron nanopartic les, PEG | ≥95% | 800μg/ml | 4T1 | CCK-8 assay | Mimic the structural characteristics of cellular basement membranes for evaluating regenerative therapeutics | 317 |
| Titanium carbide MXenes | Multi-step exfoliation method | ~227nm, diameter, ~2.8nm thickness | MnOx | ≥95% | 100μg/ml | 4T1 | CCK-8 assay | Controlled therapeutic release with kinetic flexibility | 318 |
Table 5:
Overview of the cytotoxicity of polymer nanosheets
| 2D nanomaterial | Synthesis | Sizes (technique used) | Functionalization | Cell viability | Maximum conc. tested | Tested on | Assays used | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PLGA nanosheets | Spin coating and microcontact printing technique | 174nm, thickness | - | >80% | - | RPE-J | Calcein-AM/EtHD staining | Local delivery of engineered retinal pigment epithelial monolayer | 21 |
| Polypyrrole nanosheets | Space- confined liftoff technique | 100-200nm width, 1-2nm thickness | - | >80% | 300μg/ml | MDA-MB-231 | Calcein-AM/EtHD staining | Far NIR triggered photothermal therapy for cancer theranostics | 113 |
| PLGA nanospheres | Oil-in-water emulsion solvent evaporation method | 102.8±35.7 nm diameter | Black phosphorus quantum dots | >80% | 100μg/ml | Human skin fbroblasts, MCF7, B16 melanoma | CCK-8 assay, Calcein AM-PI | NIR triggered high efficiency tumor ablation for cancer therapy | 58 |
| PDLLA-PS nanosheets | polymer phase separation and gravure coating | 70-350nm, thickness | Fibronectin | >90% | - | C2C12 | Calcein-AM/EtHD staining | Mimic the structural characteristics of cellular basement membranes for evaluating regenerative therapeutics | 23 |
| PEI-b-PCL nanosheets | Layer Multiplying Coextrusion | 52-85nm thickness | Polyethylene oxide | >90% | - | NIH/3T3 | WST-1 | Controlled therapeutic release with kinetic flexibility | 319 |
Acknowledgments
A.K.G. acknowledges financial support from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health (NIH), Director’s New Innovator Award (DP2 EB026265), National Science Foundation (NSF) Award (CBET 1705852), and Presiden’s Excellence Fund (X-Grants) from Texas A&M University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency. All of the schematics (Figures) are designed by the authors and some of the icons in the Figures were created with www.Biorender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Boehm H-P, et al. , Zeitschrift fur anorganische und allgemeine Chemie (1962) 316 (3-4), 119 [Google Scholar]
- 2.Novoselov KS, et al. , science (2004) 306 (5696), 666. [DOI] [PubMed] [Google Scholar]
- 3.Geim AK, and Novoselov KS, The rise of graphene. In Nanoscience and technology: a collection of reviews from nature journals, World Scientific; (2010), pp 11 [Google Scholar]
- 4.Chimene D, et al. , Advanced Materials (2015) 27 (45), 7261. [DOI] [PubMed] [Google Scholar]
- 5.Coleman JN, et al. , Science (2011) 331, 568. [DOI] [PubMed] [Google Scholar]
- 6.Khan K, et al. , J. Mater. Chem. C (2020) 8 (2), 387 [Google Scholar]
- 7.Wang Z, et al. , Chem.Soc.Rev. (2016) 45 (6), 1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, ACS Nano (2015) 9 (10), 9451. [DOI] [PubMed] [Google Scholar]
- 9.Ghosal K, and Sarkar K, ACS Biomater. Sci. Eng (2018) 4 (8), 2653. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. , Nanosccde (2012) 4 (13), 3833 [Google Scholar]
- 11.Zhu C, et al. , 2D Mater. (2015) 2 (3), 032004 [Google Scholar]
- 12.Agarwal V, and Chatterjee K, Nanoscale (2018) 10 (35), 16365. [DOI] [PubMed] [Google Scholar]
- 13.Chhowalla M, et al. , Nat. Chem. (2013) 5 (4), 263. [DOI] [PubMed] [Google Scholar]
- 14.Lv R, et al. ,Acc. Chem. Res (2015) 48 (1), 56. [DOI] [PubMed] [Google Scholar]
- 15.Manzeli S, et al. , Nat. Rev. Mater (2017) 2 (8) [Google Scholar]
- 16.Kalantar-zadeh K et al. , Appl. Mater. Today (2016) 5, 73 [Google Scholar]
- 17.Dai C, et al. , ACS Nano (2017) 11 (12), 12696. [DOI] [PubMed] [Google Scholar]
- 18.Hong Ng VM, et al. , J. Mater. Chem. A (2017) 5 (7), 3039 [Google Scholar]
- 19.Gaharwar AK, et al. ,Adv. Mater (2019) 31 (23), 1900332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen J, et al. , Mater. Des (2021) 198, 109298 [Google Scholar]
- 21.Fujie T, et al. , (2014) 26 (11), 1699. [DOI] [PubMed] [Google Scholar]
- 22.Hafner D, et al. ,Adv. Mater. (2016) 28 (7), 1489. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki S, et al. , (2016) 1 (6), 1600064 [Google Scholar]
- 24.Bhunia S, et al. , Adv. Funct. Mater (2020) 30 (27), 2002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma RK, et al. , Mater. Horiz (2020) 7 (2), 411 [Google Scholar]
- 26.Zhao F, et al. , Nanomaterials (2017) 8(1), 15 [Google Scholar]
- 27.Zhao K, et al. , Microporous Mesoporous Mater (2021)311, 110713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CL, et al. , ACS Nano (2016) 10 (5), 5314. [DOI] [PubMed] [Google Scholar]
- 29.Zhong Y, et al. , Dalton Trans (2020) 49 (32), 11045. [DOI] [PubMed] [Google Scholar]
- 30.Liu M, et al. , Chin.J.Chem (2021) 39 (2), 473 [Google Scholar]
- 31.Zhao M, et al. , Chem.Soc.Rev (2018) 47 (16), 6267. [DOI] [PubMed] [Google Scholar]
- 32.Arun Kumar S, et al. , WIREs Nanomedicine and Nanobiotechnology (2021), e1674. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, et al. , Adv. Mater. Technol (2017) 2 (9), 1700053 [Google Scholar]
- 34.Yang Y, et al. , Nano Res (2017) 10(5), 1560 [Google Scholar]
- 35.Kang ES, et al. , Biomater. Res (2018) 22, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suhito IR, et al. , Biochem. Biophys. Res. Commun (2017) 493 (1), 578. [DOI] [PubMed] [Google Scholar]
- 37.Lutolf MP, et al. , Nature (2009) 462 (7272), 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasir MZM, and Pumera M, Trends Anal. Chem (2019) 121, 115696 [Google Scholar]
- 39.Su S, et al. , Trends Anal. Chem. (2019) 119, 115610 [Google Scholar]
- 40.Da Silva GR, et al. , J. Mater. Sci. Mater. Med (2013) 24 (5), 1309. [DOI] [PubMed] [Google Scholar]
- 41.Yin F, et al. , Coord. Chem. Rev (2017) 347, 77 [Google Scholar]
- 42.Meng Z, et al. , Chem.Rev (2019) 119 (1), 478. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, et al. ,Angew. Chem. Int. Ed (2011) 50 (1), 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anichini C, et al. , Chem. Soc. Rev (2018) 47 (13), 4860. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan TN, et al. , Adv. Mater. (2012) 24 (22), 2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan C, et al. , Chem.Rev (2017) 117 (9), 6225. [DOI] [PubMed] [Google Scholar]
- 47.Choi W, et al. , Mater. Today (2017) 20 (3), 116 [Google Scholar]
- 48.Mak KF, and Shan J, Nat. Photon (2016) 10 (4), 216 [Google Scholar]
- 49.Yang Y, et al. , Joule (2018) 2 (6), 1075 [Google Scholar]
- 50.Zhang J, and Meguid SA, Semicond. Sci. Technol (2017) 32 (4), 043006 [Google Scholar]
- 51.Duerloo K-AN et al. , J. Phys. Chem. Lett (2012) 3 (19), 2871 [Google Scholar]
- 52.Kannan PK et al. , Nanoscale (2015) 7 (32), 13293. [DOI] [PubMed] [Google Scholar]
- 53.Jacob J, et al. , Inflamm. Regen (2018) 38, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao Y, et al. , Int. J. Nanomedicine (2016) 11. 1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu K, et al. , J. Mater. Chem. B (2016) 4 (10). 1797. [DOI] [PubMed] [Google Scholar]
- 56.Wen W, et al. , Mater. Today (2018) 21 (2). 164 [Google Scholar]
- 57.Cheng L, et al. , Adv. Mater (2020) 32 (13). 1902333. [DOI] [PubMed] [Google Scholar]
- 58.Shao J, et al. , Nat. Commun (2016) 7. 12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu Y, et al. , ACS Appl. Mater. Interfaces (2019) 11 (24). 21399. [DOI] [PubMed] [Google Scholar]
- 60.Qian X, et al. , Mater. Horiz (2017) 4 (5). 800 [Google Scholar]
- 61.Zhan H, et al. , Nanoscale (2019) 11 (28). 13181. [DOI] [PubMed] [Google Scholar]
- 62.Chin JS, et al. , Acta Biomater (2019) 90. 60. [DOI] [PubMed] [Google Scholar]
- 63.Kenry, and Lim CT, ChemNanoMat (2017) 3 (1). 5 [Google Scholar]
- 64.Tu Z, et al. , Adv. Mater (2018). e1706709. [DOI] [PubMed] [Google Scholar]
- 65.Zhao F, et al. , Small (2011) 7 (10). 1322. [DOI] [PubMed] [Google Scholar]
- 66.Arun Kumar S, et al. , Adv. Funct. Mater (2020). 2003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Androulidakis C, et al. , 2D Mater (2018) 5 (3). 032005 [Google Scholar]
- 68.Zhang C, et al. , Acta Biomater (2018) 66. 141. [DOI] [PubMed] [Google Scholar]
- 69.Gaharwar AK, et al. , Adv. Mater (2013) 25 (24). 3329. [DOI] [PubMed] [Google Scholar]
- 70.Waters R, et al. , Nanoscale (2016) 8 (14). 7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deo KA, et al. , Encyclopedia of Tissue Engineering and Regenerative Medicine (2019) [Google Scholar]
- 72.Lee SS, et al. , ACS Appl. Mater. Interfaces (2017) 9 (49). 42668. [DOI] [PubMed] [Google Scholar]
- 73.Luo Y, et al. , Trends Biotechnol (2018) 36 (11). 1145. [DOI] [PubMed] [Google Scholar]
- 74.Yadav V, et al. , Small (2019) 15 (1). 1803706. [DOI] [PubMed] [Google Scholar]
- 75.Bullock CJ, and Bussy C, Adv. Mater (2019) 6 (11). 1900229 [Google Scholar]
- 76.Goenka S, et al. , J. Control. Release (2014) 173. 75. [DOI] [PubMed] [Google Scholar]
- 77.Fan Z, et al. , Chem.Sci (2015) 6 (1). 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corma A, et al. , Nat. Chem (2013) 5 (9). 775. [DOI] [PubMed] [Google Scholar]
- 79.Murphy DW, et al. , Inorg. Chem (1976) 15. 17 [Google Scholar]
- 80.Zhou KG, et al. , Angew. Chem. Int. Ed (2011) 50 (46). 10839. [DOI] [PubMed] [Google Scholar]
- 81.Chen J, et al. , Adv. Sci (2016) 3 (8). 1500033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brent JR, et al. Prog. Mater. Sci (2017) 89. 411 [Google Scholar]
- 83.Yadav V, et al. , Small (2019) 15 (1). e1803706. [DOI] [PubMed] [Google Scholar]
- 84.Tan C, and Zhang H, Chem.Soc.Rev (2015) 44 (9). 2713. [DOI] [PubMed] [Google Scholar]
- 85.Chou SS, et al. , J. Am. Chem. Soc (2013) 135 (12). 4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Q-H, et al. , Int. Rev. Phys. Chem (2009) 28 (4). 517 [Google Scholar]
- 87.Alsaif MMYA, et al. , Adv. Funct. Mater (2016) 26 (1). 91 [Google Scholar]
- 88.Feng W, et al. , J. Mater. Chem. C (2014) 2 (17). 3254 [Google Scholar]
- 89.Kalantar-zadeh K, et al. , Chem Mater (2010) 22 (19). 5660 [Google Scholar]
- 90.Yang T, et al. , Adv. Mater. Interfaces (2019) 6 (1). 1801160 [Google Scholar]
- 91.Wu Y, et al. , Nanoscale (2015) 7 (25). 11102. [DOI] [PubMed] [Google Scholar]
- 92.Evans DG, and Duan X, Chem.Commun (2006) (5). 485. [DOI] [PubMed] [Google Scholar]
- 93.Kim TH, et al. , Dalton Trans (2014) 43 (27). 10430. [DOI] [PubMed] [Google Scholar]
- 94.Mishra G, et al. , Appl. Clay Sci (2018) 153. 172 [Google Scholar]
- 95.Silva CG, et al. , J. Am. Chem. Soc (2009) 131. 13833. [DOI] [PubMed] [Google Scholar]
- 96.Chen C, et al. , Adv. Funct. Mater (2012) 22. 780 [Google Scholar]
- 97.Kooli F, and Jones W, J. Mat. Sc. Lett (1997) 16. 27 [Google Scholar]
- 98.Rives V, et al. , Appl. Clay Sci (2014) 88-89. 239 [Google Scholar]
- 99.Tagaya H, et al. , Chem.Mater (1993) 5. 1431 [Google Scholar]
- 100.Rives V, et al. , J. Control. Release (2013) 169 (1-2). 28. [DOI] [PubMed] [Google Scholar]
- 101.Saba N, et al. , Recent advances in nanoclay/natural fibers hybrid composites 2016. pp. 1 [Google Scholar]
- 102.Paul DR, and Robeson LM, Polymer (2008) 49 (15). 3187 [Google Scholar]
- 103.Nguyen QT, and Baird DG, Adv.Polym.Technol (2006) 25 (4). 270 [Google Scholar]
- 104.Satish S, et al. , Nanobiomedicine (2019) 6. 1849543519863625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barsoum MW, Prog. Solid State Chem (2000) 28. 201 [Google Scholar]
- 106.Luo J, et al. , InfoMat (2020) 2 (6). 1057 [Google Scholar]
- 107.Munir S, et al. , ACS Omega (2020) 5 (41). 26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mashtalir O, et al. , Nat. Commun (2013) 4 (1), 1716. [DOI] [PubMed] [Google Scholar]
- 109.Natu V, et al. , J.Phys.Chem.C (2018) 122 (48), 27745 [Google Scholar]
- 110.Imani R, et al. , RSC Adv (2016) 6 (81), 77818 [Google Scholar]
- 111.Fujie T,Polym. J (2016) 48 (7), 773 [Google Scholar]
- 112.Jiang C, et al. , Nat. Mater (2004) 3 (10), 721. [DOI] [PubMed] [Google Scholar]
- 113.Wang X, et al. , Nano Lett (2018) 18 (4), 2217. [DOI] [PubMed] [Google Scholar]
- 114.Tetsu Y, et al. , n/a (n/a), 1901143 [Google Scholar]
- 115.Chen Y, et al. , (2019)4 (37), 11156 [Google Scholar]
- 116.Tao H, et al. , Phys. Chem. Chem. Phys (2017) 19 (2), 921. [DOI] [PubMed] [Google Scholar]
- 117.Niu L, et al. , Small (2016) 12 (3), 272. [DOI] [PubMed] [Google Scholar]
- 118.Martin C, et al. , Chem Commun (2019) 55 (39), 5540. [DOI] [PubMed] [Google Scholar]
- 119.Guiney LM, et al. , ACS Nano (2018) 12 (7), 6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mu P, et al. , Nano-Struct. Nano-Objects (2018) 15, 153 [Google Scholar]
- 121.Guo Y, et al. , Chem.Soc.Rev (2015) 44 (3), 637. [DOI] [PubMed] [Google Scholar]
- 122.Marino A, et al. , ACS Appl. Mater. Interfaces (2017) 9 (21), 17663. [DOI] [PubMed] [Google Scholar]
- 123.Lim JY, and Donahue HJ, Tissue. Eng (2007) 13 (8), 1879. [DOI] [PubMed] [Google Scholar]
- 124.Bettinger CJ, et al. , Angew. Chem. Int. Ed (2009) 48 (30), 5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen W, et al. , ACS Nano (2012) 6, 4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang S, et al. , ACS Nano (2015) 9, 8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo Z, et al. , Chem.Phys.Chem (2019) 20 (19), 241731342629 [Google Scholar]
- 128.Yi X, and Gao H, Nanoscale (2015) 7 (12), 5457. [DOI] [PubMed] [Google Scholar]
- 129.Mu Q, et al. , ACS Appl. Mater. Interfaces (2012) 4 (4), 2259. [DOI] [PubMed] [Google Scholar]
- 130.Nath NCD, et al. , Biomedical Applications of Graphene and 2D Nanomaterials (2019), 165 [Google Scholar]
- 131.Nel A, et al. , Acc. Chem. Res (2013) 46, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu M, et al. , Acc. Chem. Res (2013) 46, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Latiff NM, et al. , Chem. Eur.J (2015) 21 (40), 13991. [DOI] [PubMed] [Google Scholar]
- 134.Tan E, et al. , Bioconjug. Chem (2019) 30 (9), 2287. [DOI] [PubMed] [Google Scholar]
- 135.Fojtů M, et al. , Environ. Sci. Nano (2017) 4 (8), 1617 [Google Scholar]
- 136.Teo WZ, et al. , Chem.Eur.J( 2014) 20 (31), 9627. [DOI] [PubMed] [Google Scholar]
- 137.Latiff NM, et al. , Chem.Eur.J (2017) 23 (3), 684. [DOI] [PubMed] [Google Scholar]
- 138.Chng EL, et al. , Nanoscale (2014) 6 (23), 14412. [DOI] [PubMed] [Google Scholar]
- 139.Rashkow JT, et al. , Nanomed (2015) 10, 693 [Google Scholar]
- 140.Carrow JK, et al. , Proc. Natl. Acad. Sci. USA (2020) 117 (24), 13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kalantar-zadeh K et al. , Adv. Fnnct. Mater (2015) 25 (32), 5086 [Google Scholar]
- 142.Altamirano-Lozano M, et al. , Teratog. Carcinog. Mutagen (1999) 19 (4), 243. [DOI] [PubMed] [Google Scholar]
- 143.Anh Tran T, et al. , ACS Appl. Mater. Interfaces (2014) 6 (4), 2980. [DOI] [PubMed] [Google Scholar]
- 144.Song S-S, et al. , RSC Adv (2014) 4 (80), 42598 [Google Scholar]
- 145.Chung H-E, et al. , Appl Clay Sci (2012) 65-66, 24 [Google Scholar]
- 146.Choi S-J, and Choy J-H, J.Mater.Chem (2011) 21 (15), 5547 [Google Scholar]
- 147.Cunha VR, et al. , Sci. Rep (2016) 6, 30547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li PR, et al. , ACS Appl. Mater. Interfaces (2010) 2 (6), 1608. [DOI] [PubMed] [Google Scholar]
- 149.Vergaro V, et al. , Biomacromolecules (2010) 11, 820. [DOI] [PubMed] [Google Scholar]
- 150.Janer G, et al. , Nanotoxicology (2014) 8 (3), 279. [DOI] [PubMed] [Google Scholar]
- 151.Lordan S, et al. , J.Appl. Toxicol (2011) 31 (1), 27. [DOI] [PubMed] [Google Scholar]
- 152.Lordan S, and Higginbotham CL, Cell Biol Int (2012) 36 (1), 57. [DOI] [PubMed] [Google Scholar]
- 153.Nasrallah GK, et al. , Environ. Sci. Nano (2018) 5 (4), 1002 [Google Scholar]
- 154.Ma C, et al. , Biomed. Mater (2019) 14 (4), 045002. [DOI] [PubMed] [Google Scholar]
- 155.Bracaglia LG, et al. , J.Biomed.Mater.Res. A (2019) 107 (3), 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Golabchi A, et al. , Biomaterials (2019) 225, 119519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yuan Z, et al. , Biomaterials (2019) 217, 119290. [DOI] [PubMed] [Google Scholar]
- 158.Gao F, et al. , Mater. Sci. Eng., C (2019) 104, 109947. [DOI] [PubMed] [Google Scholar]
- 159.Chimene D et al. , Adv. Mater (2015) 27 (45), 7261. [DOI] [PubMed] [Google Scholar]
- 160.Cheng S, et al. , Bioact. Mater (2021) 6 (1), 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li BL, et al. , Mater. Horiz (2020) 7 (2), 455 [Google Scholar]
- 162.Yang B, et al. , Adv. Mater (2018) 30 (10), 1705611 [Google Scholar]
- 163.Feng P, et al. , Appl. Mater. Today (2019) 17, 216 [Google Scholar]
- 164.Zheng J, et al. , J. Mater. Sci (2020) 55 (17). 7226 [Google Scholar]
- 165.Sun W, and Wu FG. Chem. Asian. J (2018) 13 (22), 3378. [DOI] [PubMed] [Google Scholar]
- 166.Yang X, et al. , Nanoscale (2014) 6 (17), 10126. [DOI] [PubMed] [Google Scholar]
- 167.Wu S, et al. , ACS Appl. Nano Mater (2017) 1 (1). 337 [Google Scholar]
- 168.Wang X, et al. , NPG Asia Mater (2017) 9 (4). e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lalwani G, et al. , Acta Biomater (2013) 9 (9). 8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marinucci L, et al. , Int J Oral Maxillofac Implants (2006) 21 (5) [PubMed] [Google Scholar]
- 171.Choy JH, et al. , Angew. Chem. Int. Ed (2000) 39, 4042 [Google Scholar]
- 172.Lin J-K, et al. , J. Mater. Chem (2011) 21 (13). 5011 [Google Scholar]
- 173.Yang HY, et al. , Acta Biomater (2015) 23, 214. [DOI] [PubMed] [Google Scholar]
- 174.Choi SJ, et al. , J. Inorg. Biochem (2009) 103 (3). 463. [DOI] [PubMed] [Google Scholar]
- 175.Chimene D, et al. , Adv. Mater (2015) 27 (45). 7261. [DOI] [PubMed] [Google Scholar]
- 176.Chakraborti M, et al. , J. Mater. Sci. Mater. Med (2012) 23 (7). 1705. [DOI] [PubMed] [Google Scholar]
- 177.Gu Z, et al. , Biomaterials (2010) 31 (20), 5455. [DOI] [PubMed] [Google Scholar]
- 178.Huang H, et al. , Macromol. Biosci (2016) 16 (7). 1019. [DOI] [PubMed] [Google Scholar]
- 179.Chen YX, et al. , Nanoscale (2017) 9 (20), 6765. [DOI] [PubMed] [Google Scholar]
- 180.Chen YX, et al. , J. Mater. Chem. B (2017) 5 (12), 2245. [DOI] [PubMed] [Google Scholar]
- 181.Fayyazbakhsh F, et al. , Mater. Sci. Eng. C Mater. Biol. Appl (2017) 76, 701. [DOI] [PubMed] [Google Scholar]
- 182.Lee SS, et al. , ACS Appl. Mater. Interfaces (2017) 9 (49), 42668. [DOI] [PubMed] [Google Scholar]
- 183.Zhou T, et al. , Appl. Clay Sci (2018) 153, 246 [Google Scholar]
- 184.Shi P, et al. , Adv. Healthc. Mater (2018) 7 (15). e1800331. [DOI] [PubMed] [Google Scholar]
- 185.Carrow JK, et al. , Proc. Natl. Acad. Sci. USA (2018) 115 (17), E3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mihaila SM, et al. , Biomaterials (2014) 35 (33), 9087. [DOI] [PubMed] [Google Scholar]
- 187.Wang C, et al. , PloS one (2014) 9 (6), e99585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mieszawska AJ, et al. , Acta Biomater (2011) 7 (8). 3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Katti KS, et al. , Philos. Trans. A Math. Phys. Eng. Sci (2010) 368 (1917). 1963. [DOI] [PubMed] [Google Scholar]
- 190.Nitya G, et al. , J. Mater. Sci. Mater. Med (2012) 23 (7), 1749. [DOI] [PubMed] [Google Scholar]
- 191.Wang S, et al. , J. Mater. Chem (2012) 22 (44). 23357 [Google Scholar]
- 192.Gibbs DM, et al. , Biomaterials (2016) 99, 16. [DOI] [PubMed] [Google Scholar]
- 193.Takahashi T, et al. , J. Control. Release (2005) 107 (3), 408. [DOI] [PubMed] [Google Scholar]
- 194.Zheng JP, et al. , Appl. Clay Sci (2007) 36 (4). 297 [Google Scholar]
- 195.Yuan P, et al. , Appl. Clay Sci (2015) 112-113, 75 [Google Scholar]
- 196.Thakur A, et al. , Nanoscale (2016) 8 (24), 12362. [DOI] [PubMed] [Google Scholar]
- 197.Wilson SA, et al. , ACS Appl. Mater. Interfaces (2017) 9 (50). 43449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Deo KA, et al. , Tissue Eng. Part A (2020) 26 (5-6), 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Peak CW, et al. , Adv. Healthc. Mater (2019) 8 (11). 1801553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Szuplewska A. et al. , Mater. Sci. Eng. C (2019) 98. 874. [DOI] [PubMed] [Google Scholar]
- 201.Jastrzebska A, et al. , ACS Sustainable Chem. Eng (2020) 8 (21). 7942 [Google Scholar]
- 202.Driscoll N, et al. , ACS nano (2018) 12 (10), 10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen K, et al. , Mater. Lett (2018) 229, 114 [Google Scholar]
- 204.Fujie T, et al. , Adv. Funct. Mater (2009) 19 (16). 2560 [Google Scholar]
- 205.Pensabene V, et al. , Acta Biomater (2011) 7 (7). 2883. [DOI] [PubMed] [Google Scholar]
- 206.Fujie T, et al. , Biomaterials (2015) 53, 86. [DOI] [PubMed] [Google Scholar]
- 207.Shi X, et al. , Adv. Mater (2014) 26 (20). 3290. [DOI] [PubMed] [Google Scholar]
- 208.Huang KC, et al. , Acta Biomater (2017) 59. 12. [DOI] [PubMed] [Google Scholar]
- 209.Li M, et al. , Sci. China Chem (2018) 61 (10). 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li R, et al. , Nanotheranostics (2017) 1 (4). 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Azadmanjiri J, et al. , ACS Appl. Nano Mater (2020) 3 (4). 3116 [Google Scholar]
- 212.Ghosh D, et al. , In Nanobiomedicine, Springer Singapore; (2020), pp 107 [Google Scholar]
- 213.Chen J, et al. , Adv. Funct. Mater (2016) 26 (47), 8715 [Google Scholar]
- 214.Wetsel GC, J. Opt. Soc. Am (1980) 70 (5), 471 [Google Scholar]
- 215.Fu Q, et al. , Adv. Mater (2019) 31 (6), 1805875. [DOI] [PubMed] [Google Scholar]
- 216.Gong L, et al. , J. Mater. Chem. B (2017) 5 (10), 1873. [DOI] [PubMed] [Google Scholar]
- 217.Yang H, et al. , Chem.Eng. J (2018) 351, 548 [Google Scholar]
- 218.Hung Pang Lee, et al. , Adv. Mater. (2021) [Google Scholar]
- 219.Hakeem A, et al. , J. Mater. Chem. B (2018) 6 (36), 5768. [DOI] [PubMed] [Google Scholar]
- 220.Huang G, et al. , J. Mater. Chem. B (2017) 5 (20), 3629. [DOI] [PubMed] [Google Scholar]
- 221.Huang G, et al. , Nanoscale (2014) 6 (17), 10404. [DOI] [PubMed] [Google Scholar]
- 222.Li B, et al. , Biomaterials (2018) 177, 40. [DOI] [PubMed] [Google Scholar]
- 223.Bhattacharjee A, et al. , Appl. Clay Sci (2019) 168, 31 [Google Scholar]
- 224.Barahuie F, et al. , J. Solid State Chem (2015) 221, 21 [Google Scholar]
- 225.Khatoon N, et al. , J. Mater. Chem. B (2020) 8 (33), 7335. [DOI] [PubMed] [Google Scholar]
- 226.Zheng L, et al. , Mater. Sci. Eng., C (2019) 99, 1407. [DOI] [PubMed] [Google Scholar]
- 227.Wu Y, et al. , Bioconjug. Chem (2020) 31 (10), 2404. [DOI] [PubMed] [Google Scholar]
- 228.Zhuang Y, et al. , ACS Biomater. Sci. Eng (2017) 3 (3), 431. [DOI] [PubMed] [Google Scholar]
- 229.Han X, et al. , Adv. Healthc. Mater (2018) 7 (9), 1701394. [DOI] [PubMed] [Google Scholar]
- 230.Liu G, et al. , ACS Appl. Mater. Interfaces (2017) 9 (46), 40077. [DOI] [PubMed] [Google Scholar]
- 231.Kumar S, et al. , Biosens. Bioelectron (2018) 121, 243. [DOI] [PubMed] [Google Scholar]
- 232.Liu Z, et al. , Theranostics (2018) 8 (6), 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhou J, et al. , Biomaterials (2013) 34 (37), 9584. [DOI] [PubMed] [Google Scholar]
- 234.Mousty C, Appl. Clay Sci (2004) 27 (3), 159 [Google Scholar]
- 235.Wu S, et al. , Small (2012) 8 (14), 2264. [DOI] [PubMed] [Google Scholar]
- 236.Li X, et al. , Electrochem. Commun (2018) 86, 68 [Google Scholar]
- 237.Yao C, et al. , Sens. Actuators. B (2017) 252, 30 [Google Scholar]
- 238.Zhai W, et al. , Anal. Chem (2014) 86 (24), 12206. [DOI] [PubMed] [Google Scholar]
- 239.Zhao Z, et al. , J. Am. Chem. Soc (2014) 136 (32), 11220. [DOI] [PubMed] [Google Scholar]
- 240.Shan D, et al. , Anal. Chem (2003) 75 (15), 3872. [DOI] [PubMed] [Google Scholar]
- 241.Gualandi I, et al. , Sensors (2020) 20 (12), 3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang P, et al. , J. Mater. Chem. B (2017) 5 (1), 160. [DOI] [PubMed] [Google Scholar]
- 243.Sok V, and Fragoso A, Microchim. Acta (2019) 186 (8), 569. [DOI] [PubMed] [Google Scholar]
- 244.Shan D, et al. , Biosens. Bioelectron (2010) 25 (6), 1427. [DOI] [PubMed] [Google Scholar]
- 245.Sinha A, et al. , Trends Anal. Chem (2018) 105, 424 [Google Scholar]
- 246.Rakhi RB, et al. , Sci. Rep (2016) 6 (1), 36422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang F, et al. , J. Electrochem. Soc (2014) 162 (1), B16 [Google Scholar]
- 248.Lorencova L, et al. , Sens. Actuators, B (2018) 263, 360 [Google Scholar]
- 249.Xu B, et al. , Adv. Mater (2016) 28 (17), 3333. [DOI] [PubMed] [Google Scholar]
- 250.Oh W-K, et al. , Polym. Rev (2013) 53 (3), 407 [Google Scholar]
- 251.Arun Kumar S, et al. , Wiley Interdiscip. Rev.Nanomed.Nanobiotechnol (2020), e167433137846 [Google Scholar]
- 252.Howarth AJ, et al. , Nat. Rev. Mater (2016) 1 (3) [Google Scholar]
- 253.He C, et al. , J. Am. Chem. Soc (2014) 136 (14), 5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chen J, et al. , J. Biomed. Mater. Res. A (2017) 105 (3), 834. [DOI] [PubMed] [Google Scholar]
- 255.Xiao J, et al. , ACS Nano (2018) 12 (2), 1023. [DOI] [PubMed] [Google Scholar]
- 256.Cai W, et al. , ACS Appl. Mater. Interfaces (2017) 9 (3), 2040. [DOI] [PubMed] [Google Scholar]
- 257.Zhang Y, et al. , ACS Appl. Mater. Interfaces (2018) 10 (48), 41035. [DOI] [PubMed] [Google Scholar]
- 258.Nezhad-Mokhtari P, et al. , J.Drug Deliv. Sci. Technol (2019) 50, 174 [Google Scholar]
- 259.Lian X, et al. , Angew. Chem. Int. Ed (2018) 57 (20), 5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wan X, et al. , Angew. Chem. Int. Ed (2019) 58 (40), 14134. [DOI] [PubMed] [Google Scholar]
- 261.Ling P, et al. , ACS Appl. Mater. Interfaces (2020) 12 (15), 17185. [DOI] [PubMed] [Google Scholar]
- 262.Wang S, et al. , J. Am. Chem. Soc (2019) 141 (6), 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chang J, et al. , Anal. Chem (2019) 91 (5), 3604. [DOI] [PubMed] [Google Scholar]
- 264.Lv M, et al. , Biosens. Bioelectron (2021) 176, 112947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Afreen S, et al. , J. Mater. Chem. B (2020) 8 (7), 1338. [DOI] [PubMed] [Google Scholar]
- 266.Sava Gallis DF, et al. , ACS Appl. Mater. Interfaces (2017) 9 (27), 22268. [DOI] [PubMed] [Google Scholar]
- 267.Zhao Y-T, et al. , ACS Appl. Mater. Interfaces (2020) 12 (42), 47840. [DOI] [PubMed] [Google Scholar]
- 268.Lyle SJ, et al. , Trends Chem (2019) 1 (2), 172 [Google Scholar]
- 269.Scicluna MC, and Vella-Zarb L, ACS Appl. Nano Mater (2020) 3 (4), 3097 [Google Scholar]
- 270.Bai L, et al. , Chem.Commun (2016) 52 (22), 4128. [DOI] [PubMed] [Google Scholar]
- 271.Hashemzadeh H, and Raissi H, J. Phys. D Appl. Phys (2018) 51 (34), 345401 [Google Scholar]
- 272.Vyas VS, et al. , Adv. Mater (2016) 28 (39), 8749. [DOI] [PubMed] [Google Scholar]
- 273.Guan Q, et al. , Iscience (2019) 14, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gan S, et al. , Adv. Funct. Mater (2019) 29 (46), 1902757 [Google Scholar]
- 275.Mi Z, et al. , J. Am. Chem. Soc (2019) 141 (36), 14433. [DOI] [PubMed] [Google Scholar]
- 276.Wang K, et al. , ACS Appl. Mater. Interfaces (2019) 11 (43), 39503. [DOI] [PubMed] [Google Scholar]
- 277.Guan Q, et al. , ACS nano (2019) 13 (11), 13304. [DOI] [PubMed] [Google Scholar]
- 278.Wang P, et al. , Chin.J.Chem (2014) 32 (9), 838 [Google Scholar]
- 279.Wang M, et al. , Biosens. Bioelectron (2019) 132, 8. [DOI] [PubMed] [Google Scholar]
- 280.Peng Y, et al. , J. Am. Chem. Soc (2017) 139 (25), 8698. [DOI] [PubMed] [Google Scholar]
- 281.Chandra S, et al. , J. Am. Chem. Soc (2013) 135 (47), 17853. [DOI] [PubMed] [Google Scholar]
- 282.Das G, et al. , Chem.Sci (2018) 9 (44), 8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang P, et al. , Chem.Sci (2018) 9 (44), 8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wang P, et al. , J. Am. Chem. Soc (2016) 138 (24), 7733. [DOI] [PubMed] [Google Scholar]
- 285.Brodin JD, et al. , Nat. Chem (2012) 4 (5), 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Jiang T, et al. , J. Am. Chem. Soc (2014) 136 (11), 4300. [DOI] [PubMed] [Google Scholar]
- 287.Jiao F, et al. , Adv. Funct. Mater (2016) 26 (48), 8960 [Google Scholar]
- 288.Jang HS, et al. , Nat. Commun (2014) 5, 3665. [DOI] [PubMed] [Google Scholar]
- 289.Dai B, et al. , Proc. Natl. Acad. Sci. USA (2015) 11225535392 [Google Scholar]
- 290.Olivier GK, et al. , ACS Nano (2013) 7, 9276. [DOI] [PubMed] [Google Scholar]
- 291.Hong F, et al. , Chem.Rev (2017) 117 (20), 12584. [DOI] [PubMed] [Google Scholar]
- 292.Tørring T, et al. , Chem. Soc. Rev (2011) 40, 5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pinheiro AV, et al. , Nat. Nanotechnol (2011) 6 (12), 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Suzuki Y, et al. , Nature (2016) 533 (7603), 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Drobnak I, et al. , Designed Protein Origami (2016), pp 7. [DOI] [PubMed] [Google Scholar]
- 296.Ulijn RV, and Smith AM, Chem.Soc.Rev (2008) 37 (4), 664. [DOI] [PubMed] [Google Scholar]
- 297.Sun J, and Zuckermann RN, ACS Nano (2013) 7, 4715. [DOI] [PubMed] [Google Scholar]
- 298.Robertson EJ, et al. , Acc. Chem. Res (2016) 49 (3), 379. [DOI] [PubMed] [Google Scholar]
- 299.Robertson EJ, et al. , Proc. Natl. Acad. Sci. USA (2014) 111, 13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jin H, et al. , Nat. Commun (2016) 7, 12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kurapati R, et al. , Adv. Mater (2016) 28 (29), 6052. [DOI] [PubMed] [Google Scholar]
- 302.Dong X, et al. , ACS Appl. Mater. Interfaces (2018) 10 (4), 4271. [DOI] [PubMed] [Google Scholar]
- 303.Yin W, et al. , ACS Nano (2014) 8, 6922. [DOI] [PubMed] [Google Scholar]
- 304.Li Z, and Wong SL, Mater. Sci. Eng. C Mater. Biol. Appl (2017) 70 (Pt 2), 1095. [DOI] [PubMed] [Google Scholar]
- 305.Chen Y, et al. , Angew. Chem. Int. Ed. Engl (2017) 56 (42), 12991. [DOI] [PubMed] [Google Scholar]
- 306.Wang D, et al. , Adv. Mater. Interfaces (2019) 6 (17), 1900514 [Google Scholar]
- 307.Deng F, et al. , Mater. Sci. Eng. C Mater. Biol. Appl (2019) 104, 109976. [DOI] [PubMed] [Google Scholar]
- 308.Li L, et al. , Nanomed-Nanotechnol (2018) 14, 2355 [Google Scholar]
- 309.Wang D, et al. , Adv. Sci (2018) 5 (4), 1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li S, et al. , J. Mater. Chem. B (2013) 1 (1), 61. [DOI] [PubMed] [Google Scholar]
- 311.Liu C, et al. , ACS Nano (2019) 13 (4), 4267. [DOI] [PubMed] [Google Scholar]
- 312.Naoe T, et al. , J. Prosthodont. Res (2020) [DOI] [PubMed] [Google Scholar]
- 313.Toyota Y, et al. , Appl. Clay Sci (2017) 135, 485 [Google Scholar]
- 314.Abukhadra MR, and Allah AF, Inorg. Chem. Commun (2019) 103, 30 [Google Scholar]
- 315.Rozhina E, et al. , Appl. Clay Sci (2020) 185, 105371 [Google Scholar]
- 316.Lin H, et al. , J. Am. Chem. Soc (2017) 139 (45), 16235. [DOI] [PubMed] [Google Scholar]
- 317.Liang R, et al. , ACS Appl. Mater. Interfaces (2019) 11 (46), 42917. [DOI] [PubMed] [Google Scholar]
- 318.Dai C, et al. , Chem.Mater (2017) 29 (20), 8637 [Google Scholar]
- 319.Cao PF, et al. , Small (2018) 14 (22), e1800115. [DOI] [PubMed] [Google Scholar]
- 320.Zhang G, et al. , Nat. Commun (2018) 9 (1), 2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhang Y, et al. , Biomaterials (2019) 223, 119462. [DOI] [PubMed] [Google Scholar]
- 322.Bhanja P, et al. , ACS Appl. Mater. Interfaces (2017) 9 (37), 31411. [DOI] [PubMed] [Google Scholar]
- 323.Shi Y, et al. , ACS Appl. Mater. Interfaces (2019) 11 (13), 12321. [DOI] [PubMed] [Google Scholar]
- 324.Masood F, Mater. Sci. Eng. ,C Mater. Biol. Appl (2016) 60, 569. [DOI] [PubMed] [Google Scholar]
- 325.Xiao J, et al. , Adv. Funct. Mater (2017) 27 (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Ke F, et al. , J. Mater. Chem (2019) 54 (14), 10420 [Google Scholar]
- 327.Gelain F, et al. , ACS Nano (2011) 5, 1845. [DOI] [PubMed] [Google Scholar]
- 328.Schneider JA, et al. , Nat. Commun (2018) 9 (1), 4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hou J, et al. , Nano Lett (2020) 20 (2), 1447. [DOI] [PubMed] [Google Scholar]
- 330.Li Z, et al. , Adv. Funct. Mater (2019) 30 (3), 1905758 [Google Scholar]


