Abstract
Asthma is an inflammatory lung disorder characterized by mucus hypersecretion, cellular infiltration, and bronchial hyper-responsiveness. House dust mites (HDM) are the most prevalent cause of allergic sensitization. Canonical and noncanonical inflammasomes are multiprotein complexes that assemble in response to pathogen or danger-associated molecular patterns (PAMPs or DAMPs). Murine caspase-11 engages the noncanonical inflammasome. We addressed the role of caspase-11 in mediating host responses to HDM and subsequent allergic inflammation using caspase-11−/− mice, which lack caspase-11 while express caspase-1. We found that HDM induce caspase-11 expression in vitro. The presence of IL-4 and IL-13 promote caspase-11 expression. Additionally, caspase-11−/− macrophages show reduced release of IL-6, IL-12, and KC, and express lower levels of costimulatory molecules (e.g., CD40, CD86 and MHCII) in response to HDM stimulation. Notably, HDM sensitization of caspase-11−/− mice resulted in similar levels of IgE responses and hypothermia in response to nasal HDM challenge compared to WT. However, analysis of cell numbers and cytokines in bronchiolar alveolar lavage fluid (BALF) and histopathology of representative lung segments demonstrate altered inflammatory responses and reduced neutrophilia in the airways of the caspase-11−/− mice. These findings indicate that caspase-11 regulates airway inflammation in response to HDM exposure.
1. Introduction
Asthma is a long-term recurring inflammatory lung disorder characterized by mucus hypersecretion, cellular infiltration, chronic airway inflammation and bronchial hyper-responsiveness [5,85,100]. Different forms of asthma can coexist in some patients including atopic (allergic, Th2-dependent), and non-atopic (non-allergic, Th2-independent). These forms are initiated and regulated by multiple genetic and environmental factors, such as HDM allergens.
House dust mites, such as Dermatophagoides pteronyssinus, are an unsurpassed cause of atopic sensitization and the major causes of allergic asthma worldwide [22,92]. A battery of D. pteronyssinus allergens, proteases, bacterial lipopolysaccharide (LPS), and chitin from the mite exoskeleton are present in fecal pellets, and decaying mite materials and have potent sensitizing capacities [18,101]. Protease-dependent effects of mite allergens are associated with breaching of the epithelial layer, breaking tight junctions and stimulating protease-activated receptors. These events lead to increased epithelial permeability and production of chemokines and cytokines, which recruit antigen presenting cells (APCs) into the epithelial layers and promote airway inflammation and remodelling [7,52,53,58,66]. CD4+ Th2 cells orchestrate the HDM allergic response via production of IgE against mite allergens and stimulation of inflammation and lung remodelling [83]. It is also now recognized that the innate immune system plays a fundamental role in initiating and shaping the allergic response by programming and maintaining Th2-biased adaptive immunity in response to HDM allergens and their contaminants [47,106].
Innate immune system activation is mediated through pattern recognition receptors (PRRs), which sense PAMPs and DAMPs [54,59]. The PRR families include intracellular NOD-like receptors (NLRs) and extracellular Toll-like receptors (TLRs) [59]. Sensing of PAMPs or DAMPs by the NLR protein family, such as NLRP3 or NLRC4, mediates the assembly of inflammasomes [3]. Downstream of inflammasome signaling, IL-1β and IL-18 are cleaved by active caspase-1 which delineates the canonical inflammasome pathway [20,68,109]. In addition to canonical inflammasome activation, caspase-11 mediates non-canonical inflammasome activation in response to cytosolic LPS [61].
Caspase-11 in mice (or caspase-4/5 in humans) belongs to the family of inflammatory caspases and exhibits 46% similarity to caspase-1 [35,36,55,67,71,74,82,104]. It has been demonstrated that caspase-11−/− but not caspase-1−/− mice are resistant to septic shock mediated by injection of lethal doses of LPS [61,62]. Caspase-11 is expressed broadly in immune and non-immune cells and its expression in resting cells is low [61,104]. In contrast to other caspases that are regulated by proteolytic cleavage, caspase-11 is regulated at both transcriptional and post-translational levels. Caspase-11 expression is induced through LPS-activated TLR4 signaling via the adaptor TIR-domain-containing adaptor-inducing interferon-β (TRIF) and TRIF-dependent type I inter-feron (IFN) production [21,23,86,93,104]. In this regard, extracellular LPS and cytokines primarily serve as a priming signal for inducing the expression of caspase-11 and other inflammasome components. On the other hand, intracellular LPS triggers caspase-11-dependent inflammasome activation in the cytoplasm independently of TLR4 [12,24,46,61,62].
A previous study examined the role of caspase-11 in asthma using ovalbumin (OVA) as an allergen [111]. Sensitization of mice was achieved by intraperitoneal injection of OVA and the adjuvant potassium aluminum sulfate (alum), followed 7 days later by airway challenges with OVA. Even though this protocol results in eosinophilic inflammation and induction of Th2 cytokines, it may not fully represent the same conditions experienced by asthmatics where allergen exposure may be more frequent and for much longer periods of time [95]. HDM has been increasingly used as an allergen in mice. Repeated administration of this allergen induces asthma attacks in humans and chronic airway inflammation and tissue remodeling in mice [39]. Adjuvants such as aluminum hydroxide (alum) are known to promote the development of Th2-type responses, and alum has been extensively used in animal models of allergy and asthma [34]. Adjuvant-free sensitization protocols are also effective, but these usually require a greater number of exposures to achieve the proper sensitization and tend to elicit less robust responses to allergen challenge [17].
In this study, we use caspase-11−/− mice and an HDM-mediated allergic inflammation model to delineate the role of caspase-11 in vivo. An optimization model with WT mice was used to confirm symptoms of asthma, antibody responses and lung inflammation before experimenting with caspase-11−/− mice. We demonstrate that the inflammatory environment of asthma, specifically IL-4 and IL-13 cytokines, promote caspase-11 expression. Furthermore, caspase-11−/− macrophages produce lower amounts of KC, IL-6, and IL-12, and express lower levels of costimulatory molecules (e.g., CD40, CD86 and MHCII) in response to HDM stimulation. In vivo data show that HDM induce caspase-11 expression in WT mice. Furthermore, HDM promote differential inflammation and cellular infiltration with lessened neutrophilia in the caspase-11−/− mice. Therefore, caspase-11 promotes inflammation and neutrophil trafficking in response to HDM exposure.
2. Results
2.1. HDM promote caspase-11 expression in macrophages derived from WT mice.
Since caspase-11 is only expressed upon induction with proinflammatory stimuli [93], we tested whether HDM induce caspase-11 expression in vitro using primary bone marrow derived macrophages (BMDMs) from WT C57BL/6 mice. In contrast to freshly isolated lung macrophages, dendritic cells or epithelial cells, BMDMs are not activated or primed. We therefore expect their response will be directed only to HDM stimulation. The BMDMs were treated with different concentrations of HDM for 4 or 24 h, and caspase-11 expression was detected by Western blotting (Fig. 1A). Our data shows that the full-length (43 kDa) caspase-11 is expressed at 4 h post-HDM at all tested concentrations, and stays active up to 24 h post-treatment. Furthermore, the anti-caspase 11 antibody detected a shorter form (38 KDa) of caspase 11 in cells exposed to HDM for 24 hr suggesting that HDM could stimulate a non-canonical signaling of the CARD domain in macrophages (Fig. 1A).
Fig. 1. HDMs, IL-4 and IL-13 cytokines promote caspase-11 expression in macrophages derived from WT mice.
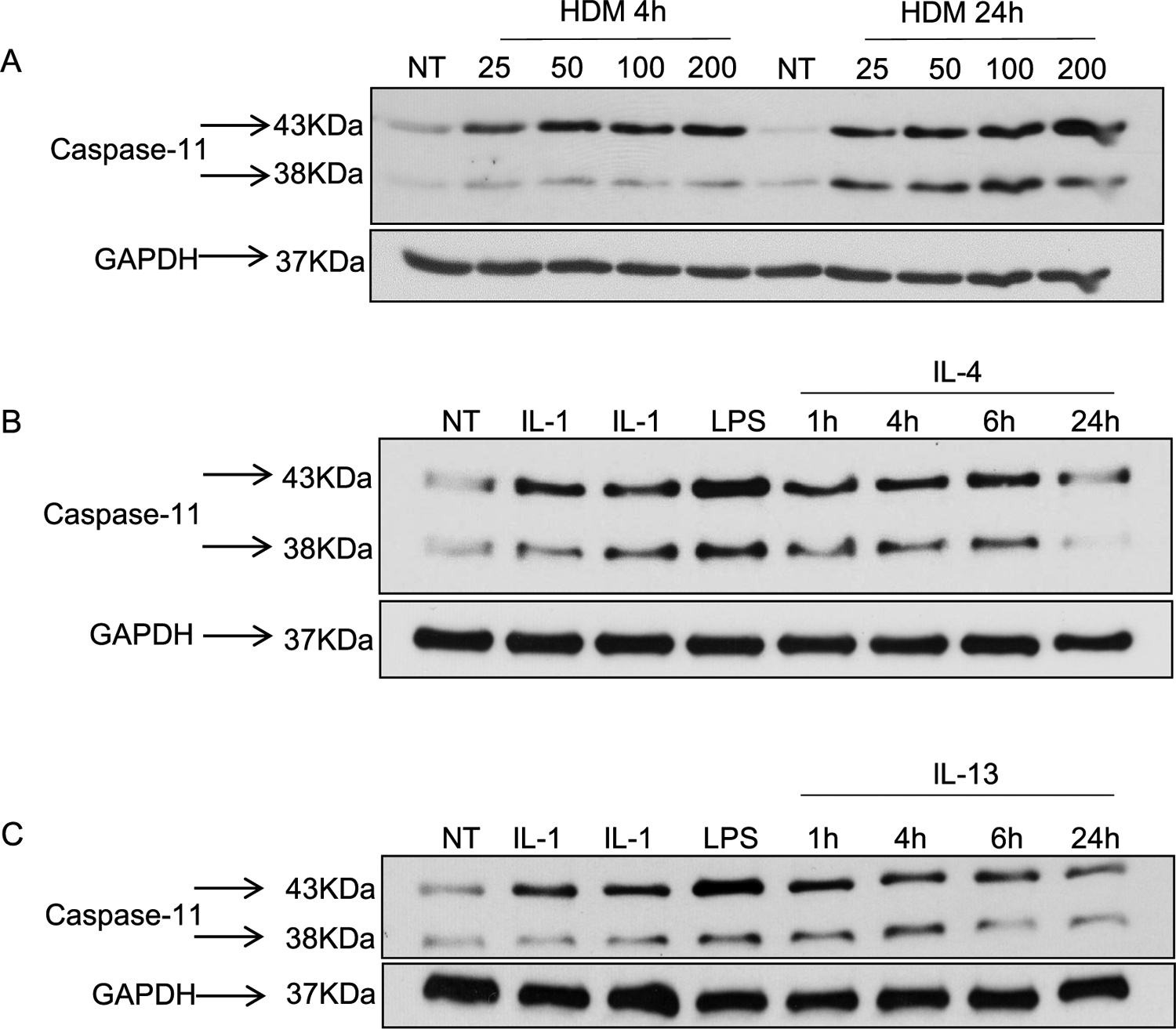
WT macrophages were plated at 2×106 cells/well. Cells were either not treated (NT) or treated with different concentrations of HDM (25, 50, 100 and 200 μg/ml) for 4 or 24 h. Caspase-11 expression was examined by Western blotting and GAPDH was used as a loading control (A). WT macrophages were treated with several inflammatory stimuli (IL-1β, IL-1α or LPS) or stimulated with different concentrations of IL-4 (5 ng/mL) (B) or IL-13 (20 ng/mL) (C) for 1, 4, 6 or 24 h and compared to NT samples. Western blot membranes were immunoblotted with capsase-11 and GAPDH was used as a loading control. Representative blots are shown from one of two independent experiments (n = 2).
2.2. Allergy-associated cytokines promote caspase-11 expression in macrophages derived from WT mice
Macrophages are categorized into classically- (M1) and alternatively-activated macrophages (M2 or AAM), which reflect the T helper (Th1/Th2) subsets [40,96]. M1 macrophages promote inflammation, whereas M2 macrophages are critical for inflammation resolution [8]. To determine if the inflammatory environment of asthma, and more specifically IL-4 and IL-13 cytokines, promote caspase-11 expression, we treated macrophages with IL-4 or IL-13 and examined caspase-11 expression by Western blotting. Our data demonstrate that macrophage treatment with IL-4 or IL-13 induces the expression of the 43 and the 38 kDa fragments of caspase-11, similar to the treatment with the inflammatory stimuli IL-1β, IL-1α and LPS (Fig. 1B & C).
Therefore, the inflammatory environment of asthma, including IL-4 and IL-13 cytokines, induces caspase-11 expression.
2.3. Macrophages derived from caspase-11−/− mice show reduced proinflammatory responses and reduced expression of costimulatory molecules in response to HDM treatment
IL-1β is a pivotal cytokine in asthma pathogenesis. Thus, it plays a central role in HDM-mediated allergic inflammation [52,53,98], and its presence often designates the activation of the inflammasome [21,45,61,86]. We determined the role of caspase-11 in the production of IL-1β by treating WT or caspase-11−/− macrophages with 100 μg/mL of HDM. In contrast to WT, caspase-11−/− macrophages released significantly less IL-1β (Fig. 2A). Therefore, HDM induced caspase-11 expression and increased IL-1β release in macrophages derived from WT mice.
Fig. 2. Caspase-11−/− macrophages show reduced release of proinflammatory cytokines and reduced expression of costimulatory molecules post-HDM treatment.
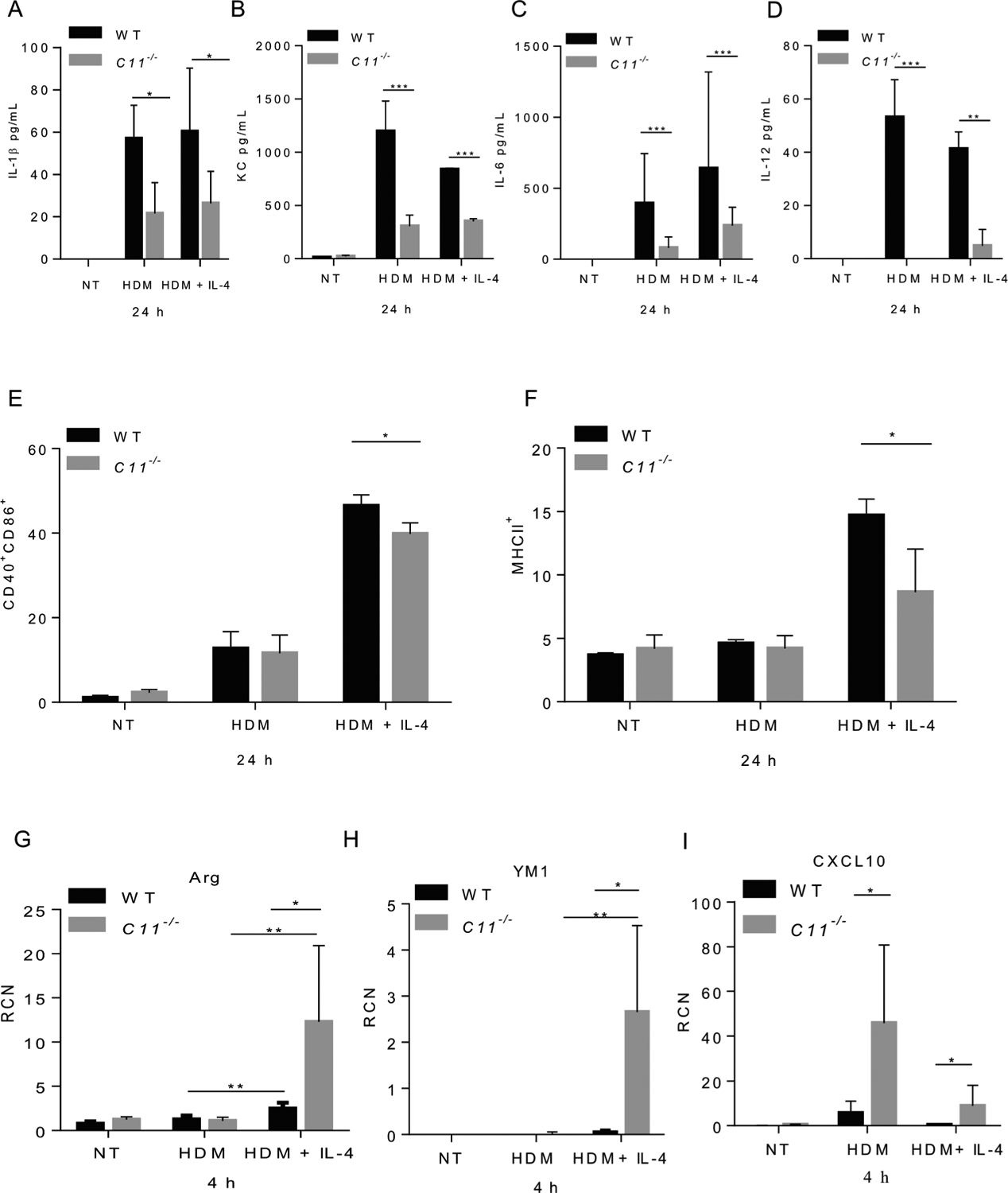
Level of IL-1β released in the supernatants of WT or caspase-11−/− macrophages treated with 100 μg/mL of HDM after 24 h (A). Data represent the mean ± SD (n = 4) obtained from four independent experiments. Multiple t-tests performed for statistical analysis, * p < 0.05. Concentrations of keratinocyte-derived protein chemokine (KC) (B), IL-6 (C), and IL-12 (D) released in supernatants from WT and caspase-11−/− macrophages either un-treated (NT), treated with HDM (100 μg/mL), pre-stimulated with IL-4 (5 ng/mL) then treated with HDM and IL-4 for 24 h. Data represent the mean ± SD (n = 4) obtained from four independent experiments. Multiple t-tests performed for statistical analysis, ** p < 0.01, *** p < 0.001. Expression of costimulatory molecules CD40 and CD86 (E), and MHCII (F) by flow cytometry 24 h post- HDM treatment. Data represent the mean ± SD (n = 3) obtained from three independent experiments. Multiple t-tests performed for statistical analysis, * p < 0.05. WT and caspase-11−/− macrophages either NT, treated with HDM (100 μg/mL), pre-stimulated with IL-4 (5 ng/mL) then treated with HDM and IL-4 for 4 h. Expression of M2 (Arg1, YM1) or M1 (CXCL10) markers by RT-qPCR 4 h post-treatment (G, H & I). RCN on the Y axis represents relative copy number. Data represent the mean ± SD (n = 3) obtained from three independent experiments. Multiple t-tests performed for statistical analysis, * p < 0.05.
HDMs elicit a strong immune response via stimulation of proinflammatory and anti-inflammatory cytokines. Increased amounts of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, are found in the sputum and BALF of individuals with asthma. These cytokines amplify inflammation, in part through the activation of NF-κB and subsequent increased expression of multiple inflammatory genes [11]. IL-4 is released and upregulated in asthma [32,60]. Despite data showing that HDM or IL-4 induced caspase-11 expression, we hypothesized that the caspase-11 response would be different in an environment containing the allergen only (which models the priming phase), or in asthma-like environment where both allergen and IL-4 are present (which represent the effector phase). To examine the role of caspase-11 in response to these environments, we treated macrophages with HDM to model the priming phase or polarized BMDMs toward M2 by stimulating macrophages with IL-4 overnight followed by treatment with IL-4 + HDM for 24 h, modeling the effector phase. Our data demonstrate that caspase-11−/− macrophages release significantly less KC, IL-6 and IL-12 at 24 h post-HDM treatment when compared to their WT counterparts (Fig. 2B, C, & D). Treatment with IL-4 in the presence of HDM yields the same trend of responses in caspase-11−/− macrophages with significant decrease in the levels of released KC, IL-6 and IL-12 (Fig. 2B, C, & D). Treatment with IL-4 + HDM induced a significant increase in the level of IL-6 in both WT and caspase-11−/− macrophages but significantly less in caspase-11−/− compared to WT macrophages (Fig. 2C). Therefore, our data demonstrate reduced production of pro-inflammatory mediators (KC, IL-6 and IL-12) from caspase-11−/− macrophages as compared to WT in response to HDM.
Macrophages are involved in antigen presentation to immune effector cells, providing two specific signals to achieve optimum activation of T cells [76]. The CD28 and CD40L molecules on T cells are known to interact, respectively, with costimulatory-molecules CD80/CD86, and CD40 on APCs [41,44]. We used flow cytometry to examine CD86 and CD40 expression, 24 h following HDM or HDM + IL-4 treatment, in WT and caspase-11−/− macrophages. Caspase-11−/− macrophages showed significantly reduced levels of CD86, CD40 and MHCII 24 h post-HDM + IL-4 stimulation (Fig. 2E & F). In asthmatic animals, the M2 phenotype of macrophages is typically associated with high level of arginase-1 and family proteins chitinase-like Ym1 [15,51,112]. Under HDM + IL-4 stimulation, caspase-11−/− macrophages expressed higher levels of arginase-1 and Ym1 but lower levels of the M1 marker Cxcl10, when compared to macrophages treated with HDM only (Fig. 2G, H & I). A significant change in the level of arginase was seen in WT or caspase-11−/− macrophages treated with HDM, or treated with HDM + IL-4 (Fig. 2G). A significant change in the level of YM1 was seen in caspase-11−/− macrophages treated with HDM, or treated with HDM + IL-4 (Fig. 2H). No significant change in the level of CXCL10 was seen between WT treated with HDM, or treated with HDM + IL-4. Moreover, no significant change in the level of CXCL10 was seen between caspase-11−/− macrophages treated with HDM, or treated with HDM + IL-4. Taken together, caspase-11−/− macrophages express lower levels of costimulatory molecules and higher levels of arginase-1 and Ym1 associated with M2 phenotype.
2.4. HDM sensitization and challenge promotes differential allergic inflammation and antibody responses in WT and caspase-11−/− mice
Recently, an acute model of HDM-mediated experimental allergic lung inflammation was used to delineate the role of caspase-1 in asthma [73]. However, mice used in these studies lack both caspase-1 and caspase-11 and thus, it is not clear if the phenotype observed is due to the lack of caspase-1, caspase-11, or both. Our results above showed altered inflammatory cytokine responses to HDM in caspase-11−/− macrophages in vitro. Thus, to study the role of caspase-11 in HDM-mediated inflammation, in vivo, WT mice were sensitized with alum + 100 μg HDM on day 0 and 7 intra-peritoneally. Mice were then challenged intranasally with HDM (25 μg/dose). Control WT mice were sensitized and challenged with phosphate-buffered saline (PBS) (Supplementary Fig. 1A). Optimization experiments were performed initially to determine the number of HDM challenges needed to provoke asthma symptoms. Self-isolation, low activity and drop in body temperature after each challenge were used as basic measurements to evaluate signs of allergic reaction in mice (Supplementary Fig. 1B). This model of HDM administration was used to ensure a robust IgE response before we sacrifice the animals [6,43,48]. Further, we aimed to mimic aspects of chronic allergic asthma including eosinophilic inflammation, airway remodelling and airway hyperresponsiveness (AHR). Our data suggest that HDM administration mediated successive and daily drop in body temperatures, which confirmed the validity of our model. Moreover, lungs derived from mice challenged with HDM showed an increase resistance to increasing concentrations of methacholine challenge (Supplementary Fig. 1C). Allergic reaction was evaluated by measuring total level of IgE in the serum and BALF of WT mice. HDM-challenged mice show elevated levels of total IgE in sera and BALF as compared to the PBS control (Supplementary Fig. 1D & E). Our results show a significant increase in the HDM-specific IgG1 and IgG2A isotypes in the serum (Supplementary Fig. 1F & G). We further demonstrate that HDM induce caspase-11 expression in vivo in WT mice (Supplementary fig. H). Thus, we successfully induced systemic and lung inflammation in HDM-sensitized and challenged WT mice.
To investigate the role of caspase-11 in mediating allergic lung inflammation, caspase-11−/− and WT mice were sensitized with alum + 100 μg HDM on day 0 and 7 intra-peritoneally. Then, mice were challenged with 15 doses of HDM intranasally (25 μg/dose) (Fig. 3A). Both WT and caspase-11−/− mice displayed low activity and self-isolation, which were consistent clinical signs of allergy. Caspase-11−/− mice showed significantly more prominent drops in body temperature on challenges 9, 13 and 14 as compared to WT mice. However, no significant difference was seen after challenge 15 which corresponds to day 35 (Fig. 3B). Further, markers of allergy were evaluated in sera and BALF from WT and caspase-11−/− mice challenged with HDM. IgE antibody is normally present at low level in the plasma and is mainly produced by plasma cells in mucosal-associated lymphoid tissues. Atopic conditions, such as asthma, elevate serum levels of total IgE and thus drive the disease. Continuous exposure to HDM allergens leads to continuous production of HDM-specific IgE as well as continued maintenance of allergic inflammation in the airways [101,102]. Therefore, we examined IgE levels in the sera of WT and caspase-11−/− mice before sensitization (day 0) and after sensitization with HDM and alum (day 14, day 28, and 24 h after the last challenge on day 35. Our data show that sera and BALF of WT and caspase-11−/− mice exhibit similar total IgE levels, (Fig. 3C, D). Sera-derived from WT and caspase-11−/− had similar levels of HDM-specific IgG1 on day 14, 28 and 35 (Fig. 3G). However, BALF derived from caspase-11−/− show significantly higher levels of total IgA on day 28 and 35 (Fig. 3F). There was no difference in HDM-specific IgA titers in the BALF on day 35 (Fig. 3E). Our data showed that BALF derived from caspase-11−/− mice contain elevated level of IgA as compared to WT. No significant differences were seen in total IgE, lung function or body temperature.
Fig. 3. HDM sensitization and challenge promotes differential allergic inflammation and antibody response in live WT and caspase-11−/− mice.
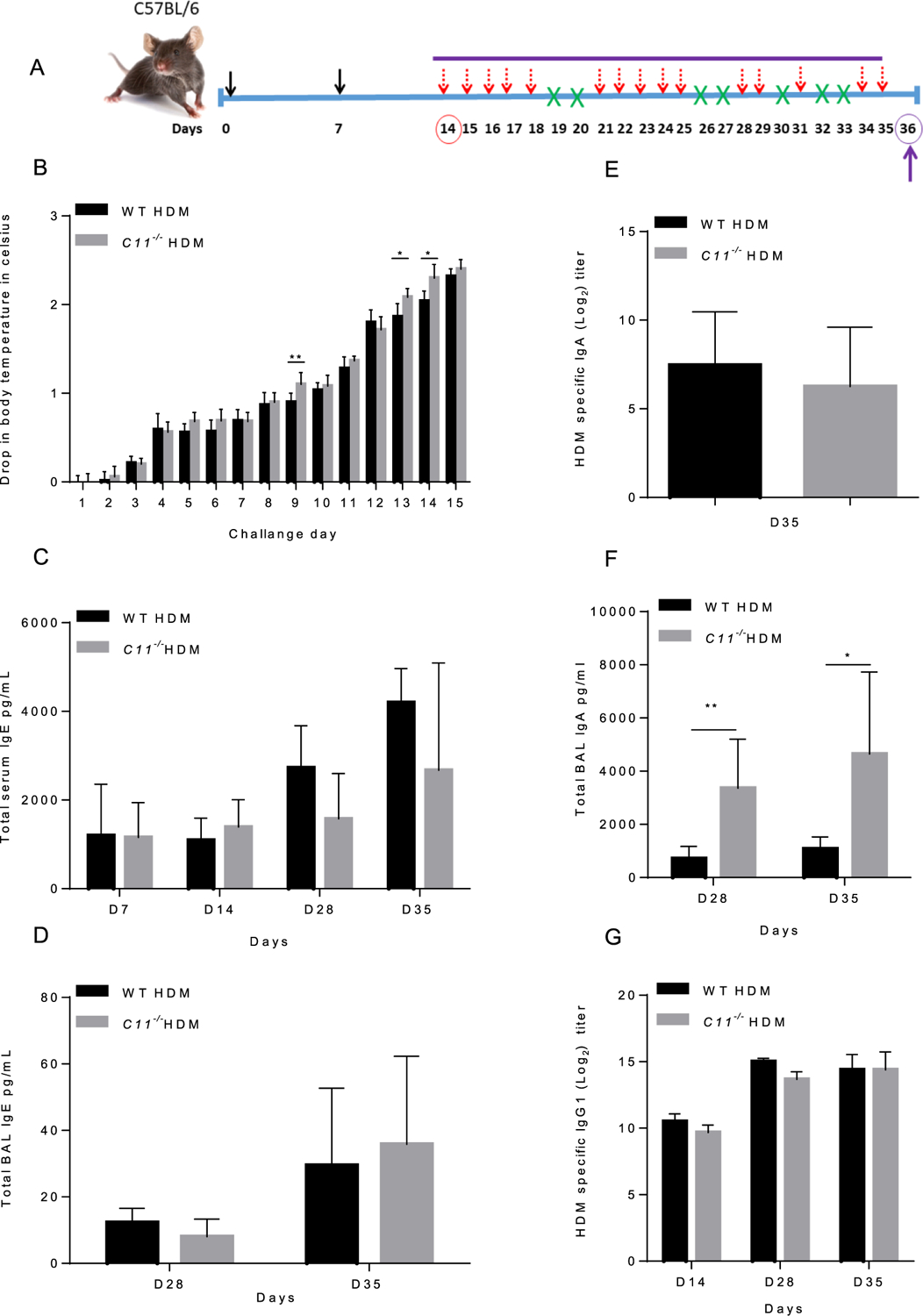
Schematic of HDM model (A), five WT and five caspase-11−/− mice were sensitized with alum + 100 μg HDM on day 0 and 7 intra-peritoneally (solid arrows). Mice were challenged with 15 doses of HDM intranasally (25 μg) as indicated (dashed arrows). Days without a challenge are indicated by (X) (A). The experiment was done twice with seven mice per group in the first in vivo experiment and 5 mice per group in the second in vivo experiment and lead to similar results. Drop in surface body temperature after intra-nasal challenges with HDM was measured before and thirty minutes after each challenge (B). Data indicates the difference in body temperature before and after each challenge. The experiment was done twice with seven mice per group in the first in vivo experiment and 5 mice per group in the second in vivo experiment and lead to similar results. Data represent the mean ± SD (n = 5) obtained from the second in vivo experiment. Two-way ANOVA performed for statistical analysis, * p < 0.05, ** p < 0.01, the experiment was performed twice. Total IgE level was quantified in serum (C) and BALF samples (D) collected before sensitization (day 0), after sensitization with HDM and alum (day 14), day 28, and 24 h after the last challenge (day 35). HDM-specific IgA was quantified on day 28 and 35 (E), and total IgA level was quantified in BALF on day 35 (F). HDM-specific IgG1 was quantified in serum samples collected before sensitization (day 0), after sensitization with HDM and alum (day 14), day 28, and 24 h after the last challenge (day 35) (G). Day 0, 14 and (28, 35) represent naïve, sensitized, and sensitized + challenged mice. The experiment was done twice with seven mice per group in the first in vivo experiment and 5 mice per group in the second in vivo experiment and lead to similar results. Data represent the mean ± SD (n = 5) obtained from the second in vivo experiment. The experiment was performed twice and lead to similar results. Multiple t-tests performed for statistical analysis, * p < 0.05, ** p < 0.01, the experiment was performed twice.
2.5. Caspase-11−/− mice show altered cellular infiltration in their BALF in response to HDM
Development of allergic responses requires activation of mast cells, basophils, and eosinophils [103]. Therefore, we examined infiltration into the BALF of WT and caspase-11−/− mice on day 35. Our data show a significant decrease in the total cellular infiltration into the BALF of caspase-11−/− mice compared to WT (Fig. 4A). BALF was also analyzed by flow cytometry. Our data demonstrate that caspase-11−/− mice exhibit a significant increase in eosinophils (CD11b+SiglecF+), total T cells (CD3+), T helper cells (CD3+CD8−) (Fig. 4F, I & K). However, no significant difference was observed in myeloid cells (CD11b+), alveolar macrophages (CD11c+F480+), neutrophils (CD11b+Ly6G+), NK cells (CD49b+), B cells (CD19+), cytotoxic T cells (CD3+CD8+) and mast cells (Ckit+) (Fig. 4B–K)) in BALF. Therefore, caspase-11−/− mice show differential cellular infiltration in their BALF compared to WT in response to HDM.
Fig. 4. WT and caspase-11−/− mice show differential cellular infiltration in their BALF in response to HDM.

Immune cells in bronchial alveolar lavage fluids (BALF) of WT or caspase-11−/− mice sensitized and challenged with HDM. Twenty-four hours following the last HDM challenge, cells were isolated by centrifugation and counted with a hemocytometer (A).The experiment was done twice with seven mice per group in the first in vivo experiment and 5 mice per group in the second in vivo experiment and lead to similar results. Data represent the mean ± SD (n = 4) per group obtained from the second in vivo experiment. Multiple t-tests performed for statistical analysis, * p < 0.05. Specific cell populations in the BALF were determined by staining cells with several markers followed by flow cytometry. Flow markers are (CD11b+), (CD11c+F480+), (CD11b+Ly6G+), (CD11b+SiglecF+), (CD49b+), (CD19+), CD3+, (CD3+CD8+), (CD3+CD8−) and (C-kit+) (B-K). The experiment was performed twice with seven mice per group in the first in vivo experiment and 5 mice per group in the second in vivo experiment. Data represent the mean ± SD (n = 9) per group pooled from the two experiments. Multiple t-tests performed for statistical analysis, * p < 0.05, ** p < 0.01, *** p < 0.001.
2.6. Caspase-11−/− mice show reduced histologic signs of lung inflammation but similar mucus production compared to WT mice
Asthma is often sustained by allergic exposure, which leads to bronchial hyper-responsiveness and acute bronchoconstriction in response to specific and non-specific triggers. HDMs cause bronchoconstriction in asthma patients and induce an inflammatory response in the lungs due to the release of cytokines, chemokines and additional mediators. Lungs of both WT and caspase-11−/− mice were similarly resistant to increasing concentrations of methacholine measured by Flexivent (Fig. 5A). To determine the effect of chronic HDM challenge on lung pathology in mice, lung tissues were collected 24 h after the last challenge. Serial lung sections were stained with hematoxylin & eosin (H&E) and Periodic Acid Schiff (PAS). HDM challenge for 35 days resulted in significantly reduced inflammatory cell infiltration in caspase-11−/− lungs as compared to WT, but similar levels of PAS staining which indicate mucus production in bronchiolar epithelium (Fig. 5B). Semi-quantitative scoring of inflammation in lung sections from caspase-11−/− mice demonstrates reduced neutrophils, macrophages and lymphocytes when compared to lung tissues from WT mice (Fig. 5C). Mucus hypersecretion was also examined by measuring the expression level of muc5ac in lungs of WT and caspase-11−/− mice by RT-qPCR (Fig. 5D). WT and caspase-11−/− mice express similar levels of muc5ac in their lungs (Fig. 5E). These data show that caspase-11−/− lungs have reduced infiltration of inflammatory cells into the lung tissue without an accompanying decrease in bronchiolar mucus production.
Fig. 5. Caspase-11−/− mice show reduced lung inflammation with similar airway resistance and mucus production.

Airway hyper-responsiveness to increasing concentrations of methacholine was measured 24 h after the last HDM challenge. Airway hyper-responsiveness was measured by recording changes in lung resistance of WT and caspase-11−/− mice (A). The experiment was performed once with five mice per genotype. Data represent the mean ± SD (n = 5) per group obtained from one experiment. Two-way ANOVA performed for statistical analysis. A linear mixed model was used to take account of the correlation among observations from the same mouse within the same cage. No significant difference was seen between caspase-11−/− and WT on the lung contraction averaged across the drug concentrations. Semi-quantitative scoring of inflammation and cellular infiltration in the lungs scored was performed in a blinded fashion by a board-certified veterinary comparative pathologist (B). Representative H&E sections showing reduced lung inflammation in caspase-11−/− mice exposed to HDM compared to WT. WT mice had more significant perivascular and peribronchiolar inflammation, while there were no statistical differences in alveolar macrophages (arrows). Inset shows a blood vessel with perivascular and vascular wall inflammation at higher magnification (C). Total magnifications are 40x for lung overviews and 200x for other images including insets. Scale bar for 40x photomicrographs = 200 μm, scale bar for 200x photomicrographs = 50 μm. Data represent the mean ± SD (n = 5) per group obtained from the second in vivo experiment. Mann Whitney test performed for statistical analysis, * P < 0.05. The experiment was done twice with seven mice per group in the first in vivo experiment and 5 mice per group in the second in vivo experiment. Representative lung images derived from WT and caspase-11−/− mice are shown from the second in vivo experiment. Lung sections derived from WT or caspase-11−/− stained with periodic acid schiff (PAS). Similar quantities of PAS-positive mucosubstances (arrows) are visible in bronchiolar epithelium in both WT and caspase-11−/− mice (D). Representative (n = 2) expression of muc5ac mRNA was measured by quantitative PCR obtained from the second in vivo experiment (E).
2.7. BALF from caspase-11−/− mice show differential levels of Th1 and Th2 cytokines compared to WT
HDM allergens initiate and sustain allergic inflammation. T cells play a role in the initiation and perpetuation of inflammation [87,108]. In particular, Th2 cells are known to be involved in controlling IgE production because of their ability to produce IL-4 and IL-13, and influence function of eosinophils through production of IL-5 [91]. In atopic individuals, allergen-specific-Th2 cytokines, particularly IL-4, IL-5, IL-9, and IL-13, orchestrate and amplify the CD4+ Th2 response. In addition, Th2 and Th1 chemokines, including CCL11 and CXCL1, trigger the extravasation and accumulation of eosinophils and neutrophils to perpetuate the allergic inflammation of the airways [90,113]. Furthermore, pro-inflammatory Th1 cytokines, such as IL-1β, IL-1α and IL-6, exacerbate lung inflammation [52,53,98,105]. Selected cytokines (TGF-β, IL-17 and IL-10) are involved in tissue remodeling [13]. Therefore, we examined levels of Th1, Th2 and Th17 cytokines in sera and BALF samples of WT and caspase-11−/− mice. In HDM-treated mice, the levels of KC, TNFα and INFγ were reduced in the BALF of caspase-11−/− mice on day 35 (Fig. 6A, B& C). The level of IL-33 (Fig. 6D), but also those of IL-4, IL-5 and IL-10 were reduced in the BALF of caspase11−/− mice (Fig. 6E, F & H). Furthermore, IL-17A levels were significantly lower in the BALF of caspase-11−/− mice (Fig. 6G). Finally, the levels of IL-13, IL-1α and IL-1β were below the detection level of the multiplex assay. Thus, our data show that caspase-11−/− mice exhibit a reduction in the level of Th1, Th2, and Th17 cytokines in BALF.
Fig. 6. BALF from caspase-11−/− mice show differential levels of Th1 and Th2 cytokines compared to WT.
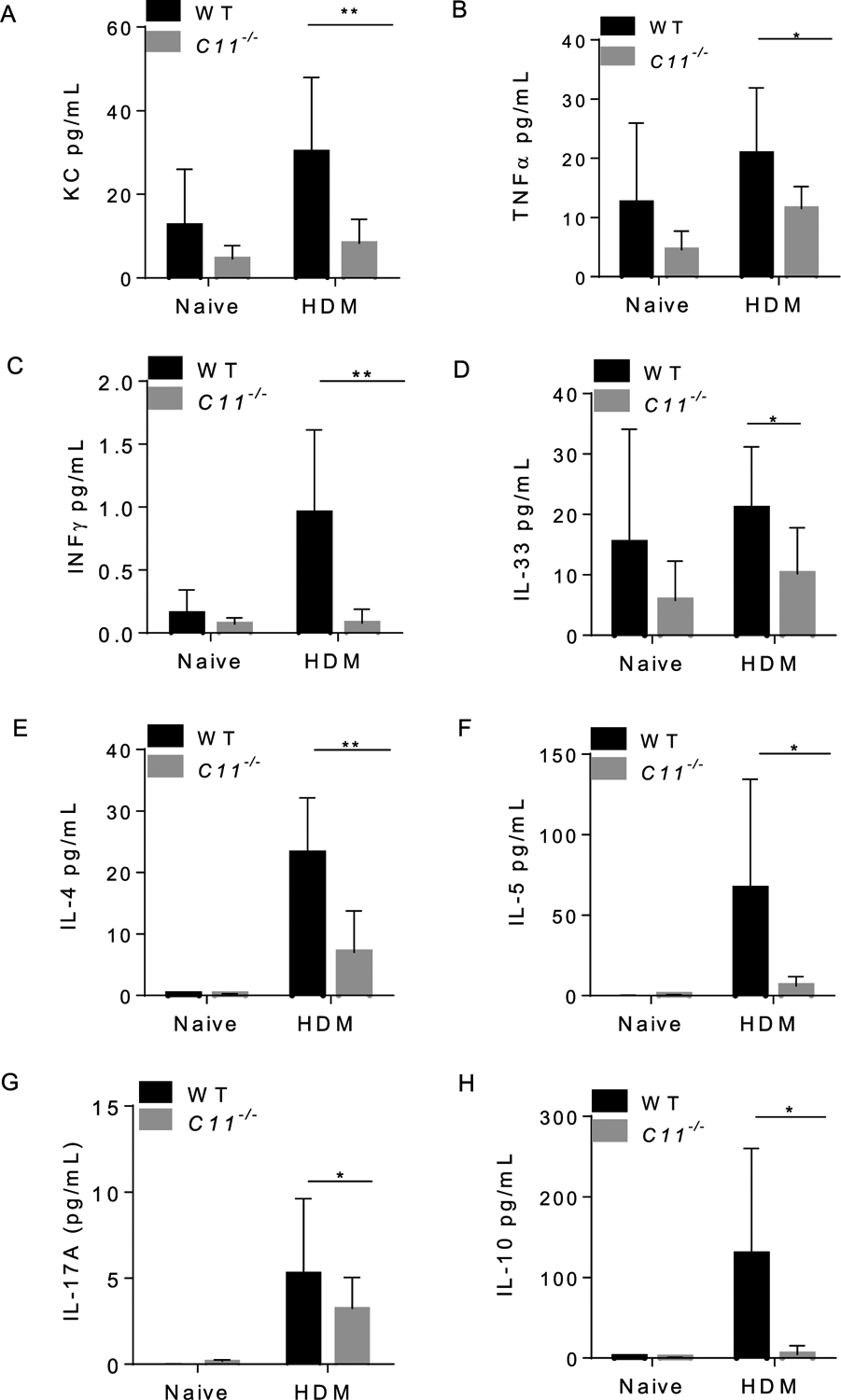
BALF samples were obtained from mice on day 35 post HDM challenge. Levels of BALF cytokines were quantified by multiplex MSD electro-chemiluminescence (OSU core facility). Day 0 represents serum obtained from naïve animals, while day 35 represents serum obtained from animals exposed to HDM for 35 days. KC (A), TNFα (B), IFNγ (C), IL-33 (D), IL-4 level (E), IL-5 (F), IL-17A (G), IL-10 level (H). Data represent the mean ± SD (n = 4) per group obtained from the second in vivo experiment. Multiple t tests performed for statistical analysis, * P < 0.05, ** p < 0.01.
3. Discussion
HDM is one of the most common aeroallergens, inducing sensitization in approximately 85% of patients with asthma [42], and playing a pivotal role in initiating and perpetuating lung inflammation in patients with asthma [50,75,89]. Clinically, there is a strong correlation between the level of HDM exposure and sensitization, which is a strong predictor for asthma [16,30]. Activation of the inflammasome in the lung can be triggered by stimuli such as HDM in the asthmatic airway [73]. However, the role of the noncanonical inflammasome in asthma has not been elucidated. Herein, we used caspase-11−/− mice and macrophages from these mice to dissect whether caspase-11 plays a regulatory role in HDM induced allergic inflammation.
The study of macrophages in allergic asthma has focused on the role of alternatively activated macrophages (M2) mediated by exposure to IL-4/IL-13. This macrophage subset positively correlated with the severity of airway inflammation in many studies [31]. Adoptive transfer of in vitro differentiated IL-4/IL-13-stimulated macrophages into the lungs of allergic mice showed that these macrophages actively contribute to the exacerbation of the disease and are not just bystanders [77]. These findings were later confirmed by a few other studies [27,64,78], showing that alternatively activated macrophage enhanced the allergic inflammatory responses in lung tissue. Our data show that caspase-11−/− macrophages treated with IL-4 expressed more markers of M2 cells than WT. Ym1 is overexpressed in asthma and aggravates lung injury [28,79]. Even though macrophages express a stronger M2 phenotype, caspase-11−/− macrophages exhibit a significant reduction in expression of co-stimulatory molecules CD86, CD40 and MHCII 24 h post-treatment. Co-stimulation by macrophages is required to enhance the release of Th2 cytokines associated with asthma and B cells that will switch to IgE production. Our in vivo data show reduced IL-4 and other Th2 cytokines in the BALF of caspase-11−/− mice. Therefore, we speculate that reduced Th2 cytokines in caspase-11−/− lungs are associated with reduced M2 phenotypes and asthma features.
In our model, caspase-11−/− and WT mice exhibit similar drop in body temperature upon allergen challenge, which is consistent with similar IgE level and similar lung functions. However, caspase-11 BALF contain a significant increased number of eosinophils despite lower IL-5 levels. It has been demonstrated that eotaxin (an important eosinophil-specific chemokine that is associated with the recruitment of eosinophils into sites of inflammation) can promote tissue eosinophilia independent of IL-5 [81]. We have tested the expression of CCL11 in lungs derived from caspase-11−/− mice challenged with HDM. Expression of CCL11 is higher in caspase-11−/− compared to WT lungs (Supplementary Fig. 1I). This finding could explain the increase in eosinophils in caspase-11−/− derived BALF while IL-5 is reduced.
Additionally, caspase-11−/− mice exhibit reduced airway inflammation and reduced cellular infiltration including neutrophils, macrophages and lymphocytes in their lungs. The reduced infiltration of cells could be due to an inherent defect in their ability to migrate to sites of inflammation due to defects in the cytoskeleton [24,70]. We have shown that capase-11−/− neutrophils are defective in migration toward chemokines such as KC in vivo [24]. Furthermore, caspase-11 promotes neutrophil directional trafficking in an acute model of gout [24]. Others have shown that splenocytes and macrophages derived from caspase-11−/− mice are defective in migration toward different chemokines in vitro and in vivo [23,70]. It was found that caspase-11−/− T cells migrate less efficiently into lymphoid tissues [14]. Modulation of actin polymerization by caspase-11 could regulate additional aspects of T cell biology, including T cell receptor (TCR) signalling [88]and hence affect their ability to migrate.
Another possibility for the reduced inflammation in caspase-11−/− mice could be associated with the increased level of total IgA in these mice. In addition to inhibition of bacterial colonization and neutralizing viruses at mucosal surfaces [19,107], IgA plays a protective role at mucosal surfaces by mediating tolerance and preventing hyper-inflammation toward allergens that can induce allergic inflammation, such as asthma [9,38]. Therefore, it is plausible that the reduced lung inflammation in caspase-11−/− is associated with the increased level of IgA, which could potentially limit binding of HDM to host cells in our experimental model.
The reduced inflammation in caspase-11−/− lungs was evident by an overall reduction in KC, Th1 (TNFα and IFNγ), Th2 (IL-33, IL-4, IL-5 and IL-10), and Th17 (IL-17A) levels in BALF. Caspase-11−/− mice also show reduced level of IL-17A, which is secreted by a distinct subset of CD4+ T helper cells and innate lymphoid cells [110]. By secreting IL-17, Th17 cells orchestrate the recruitment of neutrophil granulocytes in the lungs and their activation directly through CXCL8 production [84]. Several reports have linked IL-17A production with asthma severity [10,97]. Therefore, it is possible that the reduced number of neutrophils we found in the caspase-11−/− mice is due to lower IL-17A release.
The level of Th2 cytokines is reduced in the BALF of caspase 11−/− mice. IL-33 is of particular importance since it is a chromatin-associated nuclear cytokine from the IL-1 family [94]. IL-33 is involved in polarization of T cells towards a Th2 phenotype and must be released extra-cellularly in order to bind to the ST2 receptor. Full length (FL) IL-33 is released passively during cell necrosis or when tissues are damaged, and functions as an alarmin [25,26,49,69,72]. While proteolysis of IL-33 by caspase-1 suppresses IL-33 bioactivity [25,72,99] inflammatory proteases including cathepsin G and neutrophil elastase also cleave IL-33 full length (FL) into a shorter mature form with higher activity than IL-33 FL [69,80].
It has been shown that caspase-1−/−/caspase 11−/− mice exposed to HDM exhibit enhanced lung inflammation associated with a marked eosinophil recruitment, increased expression of IL-4, IL-5, IL-13, as well as FL and cleaved bioactive IL-33 [73]. In our caspase-11−/− mouse model, we detected decreased IL-33 in BALF and decreased Th2 cytokines associated with reduced inflammation. We demonstrated reduced IL-4, IL-5 and IL-10 in caspase-11−/− mice. The reduction of Th2 cytokines could be a consequence of the reduced IL-33 in the BALF, given the role of IL-33 in enhancing Th2 cytokine production.
On the inflammasome side, it is possible that caspase-1 is activated in vivo in the absence of caspase-11. We have seen a reduction in IL-1β level in caspase-11−/− macrophages treated with HDM in vitro. However, IL-1β level in WT and caspase-11−/− BALF derived from mice challenged with HDM on day 35 were below the detection of the multiplex. As a proinflammatory cytokine, it is possible that IL-1β level is more important at earlier time points in our model.
Even though we have not identified a direct mechanism by which caspase-11 regulates airway inflammation in response to HDM, we speculate that caspase-11−/− lungs exhibit global reduction in inflammation as evidenced by reduced chemokines and Th1, and Th2 cytokines. This reduction could impact cell recruitment into the lungs of these mice in response to HDM. Intriguingly, a new paper was published by Zaslona et al. on the role of caspase-11−/− in response to OVA and alum in which the authors demonstrated that caspase-11−/− mice show reduction in Th1, Th2 and Th17 cytokines [111]. The findings reported by Zaslona et al. are in line with our data despite the use of a different allergen, exposure times and routes of administration. Macrophages and dendritic cells experience the allergen early on and present processed fragments to naïve T cells. Following activation, T cells release cytokines that polarize immune cells and skew the environment toward asthma. It is possible that macrophages and dendritic cells show reduced ability to present the allergen to naïve T cells in vivo, which results in an overall reduction of cytokines and chemokines involved in cells polarization as well as the disease progression.
In conclusion, caspase-11 regulates lung inflammation in response to HDM during the priming and the effector phases of asthma. Our study offers several intriguing scenarios for the diverse functions of caspase-11. Expression of caspase-11 in innate immune cells following HDM exposure may be required for the antigen presentation by macrophages and dendritic cells to naïve T cells. Subsequently, insufficient antigen presentation leads to reduction in cytokines and chemokines released in the BAL fluids. We have shown that caspase-11 regulates autophagy, which is an essential process for antigen presentation by immune cells [65]. Our published work and that of others also support the notion for inherent defect in migration of caspase-11−/− cells due to defect in the actin cytoskeleton [24,70]. Furthermore, [73] showed that lack of caspase-1 is associated with increased Th2 inflammation. As a major player in IL-1β release, we speculate that that caspase-1 activation plays a role at earlier stages of the disease. However, our data suggest decreased Th2 cytokine in a longer and more chronic model of inflammation. These data suggest that caspase-11 might play a role in later stages of asthma progression which is associated with increased Th2 response. Future studies will explore these possibilities.
4. Materials and methods
4.1. Mice
C57BL/6 wt mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The caspase-11−/− mice were generously provided by Dr. Junying Yuan. (Harvard Medical School, Boston, MA, USA) [104]. All mice are in the C57BL/6 background. All mice were housed in a pathogen-free facility and experiments were conducted with approval from the Animal Care and Use Committee at The Ohio State University (Columbus, OH, USA) in accordance with NIH and OSU IACUC guidelines. Mice were maintained in a temperature-controlled facility with a strict 12h light/ dark cycle and were given free access to food and water.
4.2. Cell culture and treatment of BMDMs with HDM and inflammatory stimuli
BMDMs from WT or caspase-11−/− were derived from the femurs of five to eight-week-old mice as previously described [1,63], and grown in IMDM (Iscove’s modified Dulbecco’s medium) supplemented with L929 fibroblast cell line (ATCC)-cultured supernatant and 10% heat inactivated fetal bovine serum (HIFBS) (Gibco). Approximately 2×106 cultivated macrophages were washed twice with PBS and incubated in IMDM media during treatments. Caspase-11 induction was measured by treating WT macrophages with various inflammatory mediators including IL-1α (R&D Systems, 400-ML-005). (20 ng/mL), IL-1β (20 ng/mL) (R&D Systems, 401-ML-005), LPS (100 ng/mL) (tlrl-eklps) for 4 h. WT macrophages were stimulated with IL-4 (5 ng/mL) or IL-13 (20 ng/mL) (PeproTech, 214–14, 210–13) for 1,4,6 and 24 h in IMDM media. In some instances, macrophages were pre-treated with IL-4 overnight, and then macrophages were un-treated or treated with HDM, HDM + IL-4 for 4 or 24 h (Stallergenes Greer, XPB82D3A2.5). The vial was suspended with phosphate buffer saline to obtain 10 mg/ml stock, then 10 μl of the stock was used for each mouse which included ~ 7.85 ng of endotoxin.
4.3. Induction of HDM-mediated allergic airway inflammation
To induce HDM-driven airway inflammation, mice were injected with HDM (100 μg) adsorbed with alum (Imject™ Alum, Thermo Scientific 77161) intraperitoneally on day 0 and day 7. Isoflurane anesthetized mice were challenged with (25 μg) HDM intranasally for 15 challenges from day 14 until day 35. Twenty-four hours after the last challenge, mice were sacrificed for bronchoalveolar lavage (BAL), bronchial hyperresponsiveness, cytokines and chemokines concentration, histology and quantitative real time PCR.
4.4. Assessment of surface body temperature
Body temperature was assessed by measuring surface body temperature with the aid of infrared thermometers [Heat spy infrared thermal imaging camera (Wahl, Culver City, CA, USA)]. Mice were incubated for 10 min at room temperature, and then their body temperatures were measured. Subsequently, mice were challenged with HDM and incubated for 30 min, and their body temperatures were measured again. Then, the body temperature after the challenge was subtracted from that before the challenge for each mouse. This is shown as drop in body temperature as indicated in the figures.
4.5. Bronchoalveolar lavage (BAL) and flow cytometry of lung mononuclear cells
Bronchoalveolar lavage was performed by washing the lungs once with 0.8 ml of saline solution at room temperature. After centrifugation at 1500 RPM for 5 min at 4° C, the supernatant (cell-free BALF) was stored at −20° C for cytokine analysis. To determine the percentages of inflammatory cells infiltration in BALF, cells were suspended in FACS buffer and either unstained or single/ multiple stained with the following antibodies: anti CD3 (T-cells), anti CD8 (cytotoxic T cells), anti CD19 (B cells), anti C-kit (mast cells), anti SiglecF (eosinophils), anti CD49b (NK-cells) and anti CD11c/F480 (alveolar macrophages). Stained cells were analyzed with an Attune NxT flow cytometer (Thermo Fisher Scientific, Waltham, MA).
4.6. Assessment of HDM-specific IgG1 and IgA Ab responses and total IgA levels
Total and HDM-specific Ab responses were measured in sera and BALF by enzyme-linked immunosorbent assay (ELISA). Briefly, micro-titer plates were coated with HDM (25 μg/ml). For detection of HDM-specific IgG1 and IgA Abs, serial dilutions of serum or BALFs were added to the plates and the binding antibodies were detected with HRP-conjugated antisera (Southern Biotech Associates Inc., Birmingham, AL, USA). Biotin-conjugated rat anti-mouse IgG1 monoclonal Abs and HRP-conjugated streptavidin (BD Bioscience, San Jose, CA, ISA) were used to measure IgG1. The Ab titers were determined as the last dilutions of samples that with an absorbance of > 0.1 above that of control samples. Total IgA levels were determined by ELISA using extrapolation against IgA standards [33].
5. Total IgE quantification
Total IgE Ab levels were determined by a mouse IgE ELISA kit (Thermofischer scientific). Sample dilution and IgE detection were done according to instructions from the manufacturer.
5.1. Real-time PCR
Tissues were collected; snap frozen, and reduced to powder before adding TRIzol (Invitrogen, Carlsbad, CA, USA). RNA was isolated and cDNA was synthesized by using Superscript III (Invitrogen) and real-time PCR was performed as previously described [2,37] with the aid of specific primers. The gene of interest Ct was normalized to β-actin Ct and expressed as relative copy number (RCN) of mRNA expression (RCN = 2−ΔCt).
5.2. Lung histopathology
The right lungs from each group (n = 5 per group) were fixed with 4 % buffered formalin solution. Sections were stained with hematoxylin and eosin (H&E) for quantifying inflammation and Periodic Acid Schiff (PAS, Sigma-Aldrich) for visualizing mucosubstances and bronchiolar mucus goblet cells. Histology services were performed in the Histology Laboratory of the Comparative Pathology and Digital Imaging Shared Resource (CPDISR) of the Ohio State University Comprehensive Cancer Center. Tissues were routinely processed for histopathology on a Leica Peloris 3 Tissue Processor (Leica Biosystems, Buffalo Grove, IL), embedded in paraffin, sectioned at an approximate thickness of 4–5 μm, and batch stained with hematoxylin and eosin (H&E) on a Leica ST5020 autostainer (Leica Biosystems, Buffalo Grove, IL) using a routine and quality controlled protocol. Slides were then evaluated by a board-certified veterinary comparative pathologist (Dr. Corps) using a Nikon Eclipse Ci-L Upright Microscope (Nikon Instruments, Inc., Melville, NY). Representative photomicrographs were taken using an 18 megapixel Olympus SC180 microscope-mounted digital camera and cellSens imaging software (Olympus Life Science, Center Valley, PA). For evaluation of mucins and mucosubstances, serial histology sections taken immediately following the sections utilized for H&E were routinely stained with PAS and counterstained with hematoxylin. Semi-quantitative scoring was performed by board-certified comparative veterinary pathologist Dr. Kara N. Corps, DVM, PhD, DACVP, of the CPDISR of The Ohio State University Comprehensive Cancer Center. Dr. Corps developed the standardized scoring system based on the requirements of our project and employing standard semi-quantitative methods for the assessment of pulmonary inflammation and mucus production, with modifications of the published methods as necessary [4,29]. Several parameters were evaluated, including perivesicular, peribronchiolar and parenchymal inflammation; inflammatory cell infiltration in vascular walls; perivascular and peribronchiolar edema; alveolar macrophages distant to affected airways/blood vessels; predominance of neutrophils; and goblet cell mucus in bronchioles. Scoring scale was 0–5 with 0, 1, 2, 3, 4 and 5 assigned for no increase/within normal limits, mild (<25%), moderate (26–50%), marked (51–75%), severe (>75%), and maximal inflammation, respectively.
6. Western blotting
Protein extraction from macrophages was performed using TRIzol™ reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Briefly, after phase separation using chloroform, 100% ethanol was added to the interphase/phenol–chloroform layer to precipitate genomic DNA. Subsequently, the phenol-ethanol supernatant was mixed with isopropanol in order to precipitate out proteins. Isolated protein was then denatured in a urea-based lysis buffer. The Bradford method was used to determine protein concentrations. Equal amounts of protein were separated by 12% SDS- polyacrylamide gel (Biorad, 161–0158) and transferred to a polyvinylidene fluoride (PVDF) membrane (Biorad, 162–0177). Membranes were immunoblotted with antibodies that recognize caspase-11 (Sigma Aldrich, C1354), GAPDH (Cell Signaling Technology, 2118), Phospho-Stat6 (Tyr641) (D8S9Y) (Cell Signaling Technology, 56554), total Stat6 D3H4 (Cell Signaling Technology, 5394). Corresponding secondary antibodies conjugated with horseradish peroxidase (Cell Signaling Technology, 7074; Santa Cruz Biotechnology, sc-2006) and in combination with enhanced chemiluminescence reagent (Amersham, RPN2209,) were used to visualize protein bands
7. Cytokine measurements.
Cytokines including IL-1β, KC, TNFα, IL-4, IL-5, IL-13, IL-10, IFNγ and IL-33, were measured in serum and BALF samples using multiplex ELISA and Meso Scale Diagnostics (MSD) electro-chemiluminescence according to recommendations of the manufacturer. The level of IL-1β, IL-6, IL-12 and KC in the supernatant of treated macrophages was determined by specific sandwich ELISA following the manufacturer’s protocol (R&D) system Inc.
7.1. Statistical analysis
Figures display the mean and standard deviation (SD) as indicated in the figure legends. Comparisons between groups were conducted by multiple t-test, two-way ANOVA or linear mixed effects models were used for analysis to take account of the correlation among observations from the same animal within the same cage. Holm’s procedure was used to adjust for multiple comparisons or multiple outcomes to control for type I error at 0.05. Mice were age and sex matched, however no difference was seen in the gender. We did two independent in vivo experiments with seven and five mice per group, respectively which lead to similar results. The numbers of animal/group are indicated for each statistical analysis and this is indicated in the figure legends. Statistically significant differences were defined as *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgements
Cytokine analysis using the V-PLEX Proinflammatory Panel 1 (mouse) Kit with modification was supported by Award Number Grant from the National Center for Advancing Translational Sciences. We thank Dr. Ian Davis at the Department of Veterinary Biosciences at the Ohio State University for providing access to the Flexivent.
Funding
Studies in the Amer laboratory were supported by NIAID R01 AI24121 and NHLBI R01 HL127651-01A1. Studies in the Boyaka laboratory were supported by NIH grant R01DK101323 and R01AI145144. AAK was supported by Taawon Welfare Association, West Bank, Palestine and Bank of Palestine. KK was supported by a Cystic Fibrosis Foundation Postdoctoral Research Fellowship and by Deutsche Forschungsgemeinschaft (DFG—German Research Foundation). KH is supported by a Cure Cystic Fibrosis Columbus Training grant. AB and SE are supported by funding from the Egyptian Bureau of Education. KC and the CPDISR are supported by the Ohio State University Comprehensive Cancer Center Support Grant (CCSG) and the National Institutes of Health under grant number P30 CA016058. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
9. Ethics statement
The animal study was reviewed and approved by OSU IACUC.
CRediT authorship contribution statement
Arwa Abu Khweek: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. Marisa R. Joldrichsen: Investigation, Methodology, Data curation, Formal analysis. Eunsoo Kim: Investigation, Methodology, Visualization, Supervision. Zayed Attia: Investigation, Methodology, Formal analysis. Kathrin Krause: Investigation, Methodology, Formal analysis. Kylene Daily: Investigation, Methodology, Formal analysis. Shady Estfanous: Investigation, Methodology, Formal analysis. Kaitlin Hamilton: Investigation, Methodology, Formal analysis. Asmaa Badr: Investigation, Methodology, Formal analysis. Midhun N.K. Anne: Investigation, Methodology. Mostafa Eltobgy: Investigation, Methodology. Kara N Corps: Validation, review & editing, Data curation. Cierra Carafice: Investigation, Methodology. Xiaoli Zhang: Data curation, Formal analysis. Mikhail A. Gavrilin: Supervision, Resources, Data curation. Prosper N. Boyaka: Conceptualization, Writing – original draft, Project administration, Writing – review & editing, Resources. Amal O. Amer: Conceptualization, Writing – review & editing, Project administration, Resources.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellimm.2021.104425.
8. Availability of data and materials
All the obtained data are available in the laboratory of Dr. Amal Amer and Dr. Prosper N Boyaka.
References
- [1].Abu Khweek A, Fernandez Davila NS, Caution K, Akhter A, Abdulrahman BA, Tazi M, Hassan H, Novotny LA, Bakaletz LO, Amer AO, Biofilm-derived Legionella pneumophila evades the innate immune response in macrophages, Front. Cell. Infect. Microbiol 3 (2013) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abu Khweek A, Kanneganti A, Guttridge DD, Amer AO, The Sphingosine-1-phosphate lyase (LegS2) contributes to the restriction of legionella pneumophila in murine macrophages, PLoS ONE 11 (1) (2016), e0146410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amer A, Franchi L, Kanneganti T-D, Body-Malapel M, Özӧren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Núñez G, Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf, J. Biol. Chem 281 (46) (2006) 35217–35223. [DOI] [PubMed] [Google Scholar]
- [4].Anas AA, Yang J, Daan de Boer J, Roelofs JJTH, Hou B, de Vos AF, van der Poll T, General, but not myeloid or type II lung epithelial cell, myeloid differentiation factor 88 deficiency abrogates house dust mite induced allergic lung inflammation, Clin. Exp. Immunol 187 (2) (2017) 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arlian LG, Platts-Mills TA, The biology of dust mites and the remediation of mite allergens in allergic disease, J. Allergy Clin. Immunol 107 (3 Suppl) (2001) S406–413. [DOI] [PubMed] [Google Scholar]
- [6].Arora M, Poe SL, Oriss TB, Krishnamoorthy N, Yarlagadda M, Wenzel SE, Billiar TR, Ray A, Ray P, TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung, Mucosal Immunol. 3 (6) (2010) 578–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA, House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1, J. Immunol 169 (8) (2002) 4572–4578. [DOI] [PubMed] [Google Scholar]
- [8].Awad F, Assrawi E, Jumeau C, Georgin-Lavialle S, Cobret L, Duquesnoy P, Piterboth W, Thomas L, Stankovic-Stojanovic K, Louvrier C, Giurgea I, Grateau G, Amselem S, Karabina SA, Impact of human monocyte and macrophage polarization on NLR expression and NLRP3 inflammasome activation, PLoS ONE 12 (4) (2017), e0175336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Balzar S, Strand M, Nakano T, Wenzel SE, Subtle immunodeficiency in severe asthma: IgA and IgG2 correlate with lung function and symptoms, Int. Arch. Allergy Immunol 140 (2) (2006) 96–102. [DOI] [PubMed] [Google Scholar]
- [10].Barczyk A, Pierzchala W, Sozañska E, Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine, Respir. Med 97 (6) (2003) 726–733. [DOI] [PubMed] [Google Scholar]
- [11].Barnes PJ, The cytokine network in asthma and chronic obstructive pulmonary disease, J Clin Invest 118 (11) (2008) 3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E, Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression, J. Immunol 183 (2) (2009) 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bergeron C, Tulic MK, Hamid Q, Airway remodelling in asthma: from benchside to clinical practice, Can Respir J 17 (4) (2010) e85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bergsbaken T, Bevan MJ, Cutting edge: caspase-11 limits the response of CD8+ T cells to low-abundance and low-affinity antigens, J. Immunol 195 (1) (2015) 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bhatia S, Fei M, Yarlagadda M, Qi Z, Akira S, Saijo S, Iwakura Y, van Rooijen N, Gibson GA, St Croix CM, Ray A, Ray P, Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype, PLoS ONE 6 (1) (2011), e15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Birrell MA, Van Oosterhout AJM, Belvisi MG, Do the current house dust mite-driven models really mimic allergic asthma? Eur. Respir. J 36 (5) (2010) 1220–1221. [DOI] [PubMed] [Google Scholar]
- [17].Blyth DI, Pedrick MS, Savage TJ, Hessel EM, Fattah D, Lung inflammation and epithelial changes in a murine model of atopic asthma, Am. J. Respir. Cell Mol. Biol 14 (5) (1996) 425–438. [DOI] [PubMed] [Google Scholar]
- [18].Bordas-Le Floch V, Le Mignon M, Bussieres L, Jain K, Martelet A, Baron-Bodo V, Nony E, Mascarell L, Moingeon P, A combined transcriptome and proteome analysis extends the allergome of house dust mite Dermatophagoides species, PLoS ONE 12 (10) (2017), e0185830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boyaka PN, Inducing mucosal IgA: a challenge for vaccine adjuvants and delivery systems, J. Immunol 199 (1) (2017) 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Broz P, Dixit VM, Inflammasomes: mechanism of assembly, regulation and signalling, Nat. Rev. Immunol 16 (7) (2016) 407–420. [DOI] [PubMed] [Google Scholar]
- [21].Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM, Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1, Nature 490 (7419) (2012) 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez D de Rojas Fernandez, Virchow JC, Demoly P, Respiratory allergy caused by house dust mites: what do we really know? J. Allergy Clin. Immunol 136 (1) (2015) 38–48. [DOI] [PubMed] [Google Scholar]
- [23].Caution K, Gavrilin MA, Tazi M, Kanneganti A, Layman D, Hoque S, Krause K, Amer AO, Caspase-11 and caspase-1 differentially modulate actin polymerization via RhoA and Slingshot proteins to promote bacterial clearance, Sci. Rep 5 (2015) 18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Caution K, Young N, Robledo-Avila F, Krause K, Abu Khweek A, Hamilton K, Badr A, Vaidya A, Daily K, Gosu H, Anne MNK, Eltobgy M, Dakhlallah D, Argwal S, Estfanous S, Zhang X, Partida-Sanchez S, Gavrilin MA, Jarjour WN, Amer AO, Caspase-11 mediates neutrophil chemotaxis and extracellular trap formation during acute gouty arthritis through alteration of cofilin phosphorylation, Front. Immunol 10 (2019) 2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cayrol C, Girard J-P, The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1, Proc Natl Acad Sci U S A 106 (22) (2009) 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cayrol C, Girard JP, IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy, Curr. Opin. Immunol 31 (2014) 31–37. [DOI] [PubMed] [Google Scholar]
- [27].Chung S, Lee TJ, Reader BF, Kim JY, Lee YG, Park GY, Karpurapu M, Ballinger MN, Qian F, Rusu L, Chung HY, Unterman TG, Croce CM, Christman JW, FoxO1 regulates allergic asthmatic inflammation through regulating polarization of the macrophage inflammatory phenotype, Oncotarget 7 (14) (2016) 17532–17546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret M-C, Aubier M, Pretolani M, Elias JA, A chitinase-like protein in the lung and circulation of patients with severe asthma, N. Engl. J. Med 357 (20) (2007) 2016–2027. [DOI] [PubMed] [Google Scholar]
- [29].Clarke DL, Davis NHE, Campion CL, Foster ML, Heasman SC, Lewis AR, Anderson IK, Corkill DJ, Sleeman MA, May RD, Robinson MJ, Dectin-2 sensing of house dust mite is critical for the initiation of airway inflammation, Mucosal Immunol. 7 (3) (2014) 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].De Alba J, Raemdonck K, Dekkak A, Collins M, Wong S, Nials AT, Knowles RG, Belvisi MG, Birrell MA, House dust mite induces direct airway inflammation in vivo: implications for future disease therapy? Eur. Respir. J 35 (6) (2010) 1377–1387. [DOI] [PubMed] [Google Scholar]
- [31].Draijer C, Peters-Golden M, Alveolar macrophages in allergic asthma: the forgotten cell awakes, Curr Allergy Asthma Rep 17 (2) (2017) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dullaers M, De Bruyne R, Ramadani F, Gould HJ, Gevaert P, Lambrecht BN, The who, where, and when of IgE in allergic airway disease, J. Allergy Clin. Immunol 129 (3) (2012) 635–645. [DOI] [PubMed] [Google Scholar]
- [33].Duverger A, Jackson RJ, van Ginkel FW, Fischer R, Tafaro A, Leppla SH, Fujihashi K, Kiyono H, McGhee JR, Boyaka PN, Bacillus anthracis edema toxin acts as an adjuvant for mucosal immune responses to nasally administered vaccine antigens, J. Immunol 176 (3) (2006) 1776–1783. [DOI] [PubMed] [Google Scholar]
- [34].Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA, Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants, Nature 453 (7198) (2008) 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Faucheu C, Blanchet A-M, Collard-Dutilleul V, Lalanne J-L, Diu-Hercend A, Identification of a cysteine protease closely related to interleukin-1 beta-converting enzyme, Eur. J. Biochem 236 (1) (1996) 207–213. [DOI] [PubMed] [Google Scholar]
- [36].Faucheu C, Diu A, Chan AW, Blanchet AM, Miossec C, Hervé F, Collard-Dutilleul V, Gu Y, Aldape RA, Lippke JA, A novel human protease similar to the interleukin-1 beta converting enzyme induces apoptosis in transfected cells, EMBO J. 14 (9) (1995) 1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD, Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release, Proc Natl Acad Sci U S A 103 (1) (2006) 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gloudemans AK, Lambrecht BN, Smits HH, Potential of immunoglobulin A to prevent allergic asthma, Clin. Dev. Immunol 2013 (2013), 542091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goplen N, Karim MZ, Liang Q, Gorska MM, Rozario S, Guo L, Alam R, Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma, J. Allergy Clin. Immunol 123 (4) (2009) 925–932 e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gordon S, Alternative activation of macrophages, Nat. Rev. Immunol 3 (1) (2003) 23–35. [DOI] [PubMed] [Google Scholar]
- [41].Greenfield EA, Nguyen KA, Kuchroo VK, CD28/B7 costimulation: a review, Crit. Rev. Immunol 18 (5) (1998) 389–418. [DOI] [PubMed] [Google Scholar]
- [42].Gregory LG, Lloyd CM, Orchestrating house dust mite-associated allergy in the lung, Trends Immunol. 32 (9) (2011) 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM, Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25, Am. J. Respir. Crit. Care Med 182 (2) (2010) 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grewal IS, Flavell RA, CD40 and CD154 in cell-mediated immunity, Annu. Rev. Immunol 16 (1998) 111–135. [DOI] [PubMed] [Google Scholar]
- [45].Gurung P, Malireddi RK, Anand PK, Demon D, Vande Walle L, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD, Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens, J. Biol. Chem 287 (41) (2012) 34474–34483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA, Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock, Science 341 (6151) (2013) 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN, House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells, Nat. Med 15 (4) (2009) 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN, Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen, J. Exp. Med 207 (10) (2010) 2097–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Küchler AM, Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 30 (5) (2009) 227–233. [DOI] [PubMed] [Google Scholar]
- [50].Hatzivlassiou M, Grainge C, Kehagia V, Lau L, Howarth PH, The allergen specificity of the late asthmatic reaction, Allergy 65 (3) (2010) 355–358. [DOI] [PubMed] [Google Scholar]
- [51].Hong JY, Chung Y, Steenrod J, Chen Q, Lei J, Comstock AT, Goldsmith AM, Bentley JK, Sajjan US, Hershenson MB, Macrophage activation state determines the response to rhinovirus infection in a mouse model of allergic asthma, Respir. Res 15 (2014) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jacquet A, The role of innate immunity activation in house dust mite allergy, Trends Mol. Med 17 (10) (2011) 604–611. [DOI] [PubMed] [Google Scholar]
- [53].Jacquet A, The role of the house dust mite-induced innate immunity in development of allergic response, Int. Arch. Allergy Immunol 155 (2) (2011) 95–105. [DOI] [PubMed] [Google Scholar]
- [54].Janeway CA Jr., R. Medzhitov, Innate immune recognition, Annu. Rev. Immunol 20 (2002) 197–216. [DOI] [PubMed] [Google Scholar]
- [55].Kamens J, Paskind M, Hugunin M, Talanian RV, Allen H, Banach D, Bump N, Hackett M, Johnston CG, Li P, et al. , Identification and characterization of ICH-2, a novel member of the interleukin-1 beta-converting enzyme family of cysteine proteases, J. Biol. Chem 270 (25) (1995) 15250–15256. [DOI] [PubMed] [Google Scholar]
- [58].Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, Ishii A, Ikeda S, Okumura K, Ogawa H, Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes, Allergy 64 (9) (2009) 1366–1374. [DOI] [PubMed] [Google Scholar]
- [59].Kawai T, Akira S, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors, Nat. Immunol 11 (5) (2010) 373–384. [DOI] [PubMed] [Google Scholar]
- [60].Mackay IR, Rosen FS, Kay AB, Allergy and allergic diseases. First of two parts, N. Engl. J. Med 344 (1) (2001) 30–37. [DOI] [PubMed] [Google Scholar]
- [61].Kayagaki N, Warming S, Lamkanfi M, Walle LV, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM, Non-canonical inflammasome activation targets caspase-11, Nature 479 (7371) (2011) 117–121. [DOI] [PubMed] [Google Scholar]
- [62].Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM, Noncanonical inflammasome activation by intracellular LPS independent of TLR4, Science 341 (6151) (2013) 1246–1249. [DOI] [PubMed] [Google Scholar]
- [63].Khweek AA, Caution K, Akhter A, Abdulrahman BA, Tazi M, Hassan H, Majumdar N, Doran A, Guirado E, Schlesinger LS, Shuman H, Amer AO, A bacterial protein promotes the recognition of the Legionella pneumophila vacuole by autophagy, Eur. J. Immunol 43 (5) (2013) 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kim DY, Park BS, Hong GU, Lee BJ, Park JW, Kim SY, Ro JY, Anti-inflammatory effects of the R2 peptide, an inhibitor of transglutaminase 2, in a mouse model of allergic asthma, induced by ovalbumin, Br. J. Pharmacol 162 (1) (2011) 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Krause K, Caution K, Badr A, Hamilton K, Saleh A, Patel K, Seveau S, Hall-Stoodley L, Hegazi R, Zhang X, Gavrilin MA, Amer AO, CASP4/caspase-11 promotes autophagosome formation in response to bacterial infection, Autophagy (2018) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kubo M, Innate and adaptive type 2 immunity in lung allergic inflammation, Immunol. Rev 278 (1) (2017) 162–172. [DOI] [PubMed] [Google Scholar]
- [67].Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P, Alice in caspase land. A phylogenetic analysis of caspases from worm to man, Cell Death Differ. 9 (4) (2002) 358–361. [DOI] [PubMed] [Google Scholar]
- [68].Lamkanfi M, Dixit V, Mechanisms and functions of inflammasomes, Cell 157 (5) (2014) 1013–1022. [DOI] [PubMed] [Google Scholar]
- [69].Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C, IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G, Proc Natl Acad Sci U S A 109 (5) (2012) 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Li J, Brieher WM, Scimone ML, Kang SJ, Zhu H, Yin H, von Andrian UH, Mitchison T, Yuan J, Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization, Nat. Cell Biol 9 (3) (2007) 276–286. [DOI] [PubMed] [Google Scholar]
- [71].Lin XY, Choi MS, Porter AG, Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma, J. Biol. Chem 275 (51) (2000) 39920–39926. [DOI] [PubMed] [Google Scholar]
- [72].Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, Brumatti G, Taylor RC, Kersse K, Vandenabeele P, Lavelle EC, Martin SJ, Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases, Immunity 31 (1) (2009) 84–98. [DOI] [PubMed] [Google Scholar]
- [73].Madouri F, Guillou N, Fauconnier L, Marchiol T, Rouxel N, Chenuet P, Ledru A, Apetoh L, Ghiringhelli F, Chamaillard M, Zheng SG, Trovero F, Quesniaux VFJ, Ryffel B, Togbe D, Caspase-1 activation by NLRP3 inflammasome dampens IL-33-dependent house dust mite-induced allergic lung inflammation, J. Mol. Cell. Biol 7 (4) (2015) 351–365. [DOI] [PubMed] [Google Scholar]
- [74].Martinon F, Tschopp J, Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases, Cell 117 (5) (2004) 561–574. [DOI] [PubMed] [Google Scholar]
- [75].Maunsell K, Wraith DG, Cunnington AM, Mites and house-dust allergy in bronchial asthma, Lancet 291 (7555) (1968) 1267–1270. [DOI] [PubMed] [Google Scholar]
- [76].McAdam AJ, Schweitzer AN, Sharpe AH, The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells, Immunol. Rev 165 (1998) 231–247. [DOI] [PubMed] [Google Scholar]
- [77].Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A, Macrophages: regulators of sex differences in asthma? Am. J. Respir. Cell Mol. Biol 42 (5) (2010) 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Moreira AP, Cavassani KA, Hullinger R, Rosada RS, Fong DJ, Murray L, Hesson DP, Hogaboam CM, Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease, J. Allergy Clin. Immunol 126 (4) (2010) 712–721.e717. [DOI] [PubMed] [Google Scholar]
- [79].Moreira AP, Hogaboam CM, Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution, J. Interferon Cytokine Res 31 (6) (2011) 485–491. [DOI] [PubMed] [Google Scholar]
- [80].Morita H, Nakae S, Saito H, Matsumoto K, IL-33 in clinical practice: Size matters? J. Allergy Clin. Immunol 140 (2) (2017) 381–383. [DOI] [PubMed] [Google Scholar]
- [81].Mould AW, Matthaei KI, Young IG, Foster PS, Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice, J Clin Invest 99 (5) (1997) 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Munday NA, Vaillancourt JP, Ali A, Casano FJ, Miller DK, Molineaux SM, Yamin T-T, Yu VL, Nicholson DW, Molecular cloning and pro-apoptotic activity of ICErelII and ICErelIII, members of the ICE/CED-3 family of cysteine proteases, J. Biol. Chem 270 (26) (1995) 15870–15876. [DOI] [PubMed] [Google Scholar]
- [83].Na H, Cho M, Chung Y, Regulation of Th2 cell immunity by dendritic cells, Immune Netw 16 (1) (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA, Evidence for a cross-talk between human neutrophils and Th17 cells, Blood 115 (2) (2010) 335–343. [DOI] [PubMed] [Google Scholar]
- [85].Platts-Mills TA, Chapman MD, Dust mites: immunology, allergic disease, and environmental control, J. Allergy Clin. Immunol 80 (6) (1987) 755–775. [DOI] [PubMed] [Google Scholar]
- [86].Rathinam VK, Vanaja S, Waggoner L, Sokolovska A, Becker C, Stuart L, Leong J, Fitzgerald K, TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria, Cell 150 (3) (2012) 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ricci M, Rossi O, Bertoni M, Matucci A, The importance of Th2-like cells in the pathogenesis of airway allergic inflammation, Clin. Exp. Allergy 23 (5) (1993) 360–369. [DOI] [PubMed] [Google Scholar]
- [88].Ritter AT, Angus KL, Griffiths GM, The role of the cytoskeleton at the immunological synapse, Immunol. Rev 256 (1) (2013) 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Roche N, Chinet TC, Huchon GJ, Allergic and nonallergic interactions between house dust mite allergens and airway mucosa, Eur. Respir. J 10 (3) (1997) 719–726. [PubMed] [Google Scholar]
- [90].Romagnani S, The role of lymphocytes in allergic disease, J. Allergy Clin. Immunol 105 (3) (2000) 399–408. [DOI] [PubMed] [Google Scholar]
- [91].Romagnani S, Immunologic influences on allergy and the TH1/TH2 balance, J. Allergy Clin. Immunol 113 (3) (2004) 395–400. [DOI] [PubMed] [Google Scholar]
- [92].Sanchez-Borges M, Fernandez-Caldas E, Thomas WR, Chapman MD, Lee BW, Caraballo L, Acevedo N, Chew FT, Ansotegui IJ, Behrooz L, Phipatanakul W, Gerth van Wijk R, Pascal D, Rosario N, Ebisawa M, Geller M, Quirce S, Vrtala S, Valenta R, Ollert M, Canonica GW, Calderon MA, Barnes CS, Custovic A, Benjaponpitak S, Capriles-Hulett A, International consensus (ICON) on: clinical consequences of mite hypersensitivity, a global problem, World Allergy Organ. J 10 (1) (2017) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Schauvliege R, Vanrobaeys J, Schotte P, Beyaert R, Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1, J. Biol. Chem 277 (44) (2002) 41624–41630. [DOI] [PubMed] [Google Scholar]
- [94].Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA, IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines, Immunity 23 (5) (2005) 479–490. [DOI] [PubMed] [Google Scholar]
- [95].Shin YS, Takeda K, Gelfand EW, Understanding asthma using animal models, Allergy Asthma Immunol Res 1 (1) (2009) 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sica A, Mantovani A, Macrophage plasticity and polarization: in vivo veritas, J. Clin. Invest 122 (3) (2012) 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sun YC, Zhou QT, Yao WZ, Sputum interleukin-17 is increased and associated with airway neutrophilia in patients with severe asthma, Chin. Med. J. (Engl) 118 (11) (2005) 953–956. [PubMed] [Google Scholar]
- [98].Sundaram K, Mitra S, Gavrilin MA, Wewers MD, House dust mite allergens and the induction of monocyte interleukin 1beta production that triggers an ikappabzeta-dependent granulocyte macrophage colony-stimulating factor release from human lung epithelial cells, Am. J. Respir. Cell Mol. Biol 53 (3) (2015) 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G, Interleukin-33 is biologically active independently of caspase-1 cleavage, J. Biol. Chem 284 (29) (2009) 19420–19426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Thomas WR, House dust allergy and immunotherapy, Hum Vaccin Immunother 8 (10) (2012) 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Thomas WR, Hales BJ, Smith W-A, House dust mite allergens in asthma and allergy, Trends Mol. Med 16 (7) (2010) 321–328. [DOI] [PubMed] [Google Scholar]
- [102].Wahn U, Lau S, Bergmann R, Kulig M, Forster J, Bergmann K, Bauer CP, Guggenmoos-Holzmann I, Indoor allergen exposure is a risk factor for sensitization during the first three years of life, J. Allergy Clin. Immunol 99 (6 Pt 1) (1997) 763–769. [DOI] [PubMed] [Google Scholar]
- [103].Wambre E, James EA, Kwok WW, Characterization of CD4+ T cell subsets in allergy, Curr. Opin. Immunol 24 (6) (2012) 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wang S, Miura M, Jung Y-K, Zhu H, Li E.n., Yuan J, Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE, Cell 92 (4) (1998) 501–509. [DOI] [PubMed] [Google Scholar]
- [105].Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H, Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33, J. Exp. Med 209 (8) (2012) 1505–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Willart MA, Lambrecht BN, The danger within: endogenous danger signals, atopy and asthma, Clin. Exp. Allergy 39 (1) (2009) 12–19. [DOI] [PubMed] [Google Scholar]
- [107].Williams RC, Gibbons RJ, Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal, Science 177 (4050) (1972) 697–699. [DOI] [PubMed] [Google Scholar]
- [108].Woodfolk JA, T-cell responses to allergens, J Allergy Clin Immunol. 119(2), 2007, 280–294; quiz 295–286. [DOI] [PubMed] [Google Scholar]
- [109].Yang J, Zhao Y, Shao F, Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity, Curr. Opin. Immunol 32 (2015) 78–83. [DOI] [PubMed] [Google Scholar]
- [110].Yu S, Kim HY, Chang YJ, DeKruyff RH, Umetsu DT, Innate lymphoid cells and asthma, J. Allergy Clin. Immunol 133(4), 943–950 (2014) quiz 951. [DOI] [PubMed] [Google Scholar]
- [111].Zaslona Z, Flis E, Wilk MM, Carroll RG, Palsson-McDermott EM, Hughes MM, Diskin C, Banahan K, Ryan DG, Hooftman A, Misiak A, Kearney J, Lochnit G, Bertrams W, Greulich T, Schmeck B, McElvaney OJ, Mills KHG, Lavelle EC, Wygrecka M, Creagh EM, O’Neill LAJ, Caspase-11 promotes allergic airway inflammation, Nat. Commun 11 (1) (2020) 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Zhong B.o., Yang X, Sun Q, Liu L.i., Lan X.i., Tian J, He Q, Hou W, Liu H, Jiang C, Gao N, Lu S, Pdcd4 modulates markers of macrophage alternative activation and airway remodeling in antigen-induced pulmonary inflammation, J. Leukoc. Biol 96 (6) (2014) 1065–1075. [DOI] [PubMed] [Google Scholar]
- [113].Zimmermann N, Hershey GK, Foster PS, Rothenberg ME, Chemokines in asthma: cooperative interaction between chemokines and IL-13, J Allergy Clin Immunol 111(2), 2003, 227–242; quiz 243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the obtained data are available in the laboratory of Dr. Amal Amer and Dr. Prosper N Boyaka.


