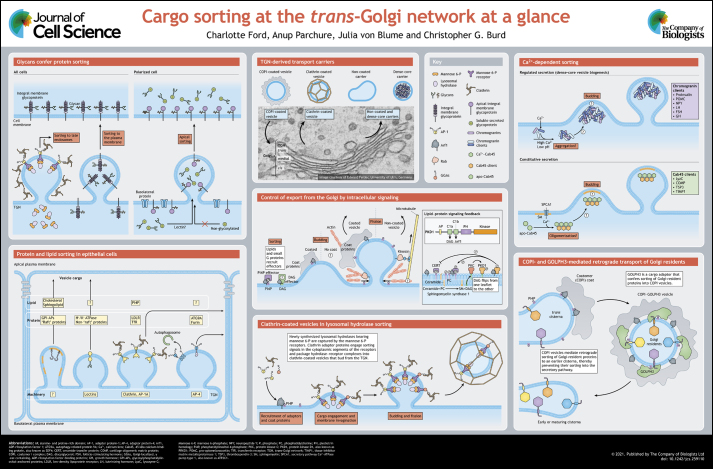
ABSTRACT
The Golgi functions principally in the biogenesis and trafficking of glycoproteins and lipids. It is compartmentalized into multiple flattened adherent membrane sacs termed cisternae, which each contain a distinct repertoire of resident proteins, principally enzymes that modify newly synthesized proteins and lipids sequentially as they traffic through the stack of Golgi cisternae. Upon reaching the final compartments of the Golgi, the trans cisterna and trans-Golgi network (TGN), processed glycoproteins and lipids are packaged into coated and non-coated transport carriers derived from the trans Golgi and TGN. The cargoes of clathrin-coated vesicles are chiefly residents of endo-lysosomal organelles, while uncoated carriers ferry cargo to the cell surface. There are outstanding questions regarding the mechanisms of protein and lipid sorting within the Golgi for export to different organelles. Nonetheless, conceptual advances have begun to define the key molecular features of cargo clients and the mechanisms underlying their sorting into distinct export pathways, which we have collated in this Cell Science at a Glance article and the accompanying poster.
KEY WORDS: Golgi, Secretion, Clathrin, Glycoprotein, Calcium, Epithelial cells, Lipids
Summary: An illustrated framework of the conceptual mainstays of Golgi sorting and export.
Introduction
The Golgi is an organelle of the eukaryotic endomembrane system that functions principally in the biogenesis and intracellular sorting of newly synthesized glycoproteins and lipids, herein termed ‘biosynthetic cargo’. It is composed of three or more flattened membrane sacs termed the cis, medial and trans cisternae, which adhere to each other in a polarized orientation to form the Golgi stack (Klumperman, 2011). The terminal Golgi compartment is a network of convoluted membranes termed the trans-Golgi network (TGN). Newly synthesized proteins and lipids produced in the endoplasmic reticulum (ER) are directed to the cis Golgi, where they enter the Golgi stack and are trafficked in the anterograde direction to the medial and trans cisternae and the TGN (Li et al., 2019). Within the Golgi stack, cargo-processing enzymes, primarily glycosyltransferases that attach carbohydrate moieties to biosynthetic cargo, reside in one or two cisternae. This arrangement establishes a processing pathway whereby sequentially acting Golgi-resident enzymes appropriately modify cargo proteins, via reactions that include elaboration of glycan chains on proteins and lipids, sulfation of proteins and proteolysis (Stanley, 2011; Stone et al., 2009; Tan and Gleeson, 2019; Thomas, 2002) (see Box 1).
Box 1. How is secretory cargo transported through the Golgi?
The mechanism(s) by which secretory cargo is transported through the stack of Golgi cisternae has been a contentious topic of debate for decades. Two models currently dominate the debate: the cisterna maturation model and the stable compartments model (Lujan and Campelo, 2021; Rabouille and Klumperman, 2005). A key feature of the cisterna maturation model is that secretory cargo remains within a single Golgi cisterna that matures from an early compartment (for example the cis cisterna) into a later compartment (the medial and trans cisternae) by the removal of existing cisterna-resident proteins and their replacement with residents of the subsequent cisterna. Transport vesicles are posited to mediate trafficking of Golgi residents (such as glycosyltransferases) in the retrograde direction, that is, from late to early cisternae. In the stable compartments model, secretory cargo is trafficked via transport vesicles that bud from an early cisterna and fuse with the subsequent cisterna.
COPI-coated vesicles are derived from Golgi cisternae, and the cargo that they contain – Golgi residents or secreted cargo proteins – has been used to distinguish between the two models. Whereas the cisterna maturation model posits that Golgi residents are the major cargo of COPI-coated vesicles, the stable compartments model posits that secretory cargo is the major component. It is now firmly established that ER and Golgi residents are abundant integral membrane proteins of COPI-coated vesicles (Adolf et al., 2019; Gommel et al., 1999; Martínez-Menárguez et al., 2001), although some studies have reported that such vesicles also contain anterograde secretory cargo (Malsam et al., 2005; Orci et al., 1997). Some secretory cargoes (such as algal scales of algae and some collagens) are too large to be accommodated by COPI-coated vesicles (which are 40–80 nm in diameter), and they are observed to transit the Golgi stack while remaining within cisternae (Bonfanti et al., 1998; Melkonian et al., 1991). Cisterna maturation has been observed to occur in yeast cells, suggesting that cisterna maturation is the major if not sole mechanism of anterograde transport through the Golgi of this organism (Losev et al., 2006; Matsuura-Tokita et al., 2006). In favor of the stable compartments model, normal rates of secretion have been observed for mammalian cell lines in which transfer of secretory cargo could transit successive cisternae via vesicles (Dunlop et al., 2017; Lavieu et al., 2013). Ultimately, elucidation of the mode of intra-Golgi transport may require observation of both anterograde and retrograde protein trafficking in living cells.
Once biosynthetic cargo processing is complete, cargo is exported from the Golgi by coated vesicles and pleiomorphic uncoated vesicles, and subsequently transported to the plasma membrane, endosome or other Golgi compartments (De Matteis and Luini, 2008). Cargo sorting and processing in the Golgi is essential for the generation of bioactive proteins (such as hormones and cell surface receptors), organelle biogenesis, cell polarization and lipid homeostasis. Organismal physiology depends critically on these Golgi-mediated cargo-sorting processes as their disruption can contribute to a wide range of disorders, including neurodegenerative diseases, diabetes and cancer (Liu et al., 2021). This Cell Science at a Glance article and the accompanying poster summarize the conceptual advances made toward understanding mechanisms of cargo sorting and export from the trans Golgi and TGN (referred to hereafter as the trans/TGN) during secretion. While most of the cargo-sorting mechanisms presented here apply generally to all eukaryotic cells, the poster focuses on research of mammalian cell types.
Lysosomal hydrolase sorting in clathrin-coated vesicles
Clathrin-coated vesicles (CCVs) traffic integral membrane proteins, predominantly precursors of lysosomal resident enzymes and proteins required for lysosome biogenesis, from the trans/TGN to organelles of the endo-lysosome system (see poster). The CCV coat is composed of two protein shells that are associated with the cytoplasmic leaflet of the vesicle membrane; the inner layer comprises a network of integral membrane proteins and peripheral cargo adaptor proteins that provide a platform for the assembly of clathrin triskelia into the second, outer layer (Wood and Smith, 2021). The best-characterized CCV cargo adaptors are those of the heterotetrameric clathrin adaptor protein family: AP-1, AP-2 and AP-3. These adaptors are comprised of four subunits: two large (one of α, γ, δ or ε and one β) subunits, a medium (μ) subunit and a small (σ) subunit (Sanger et al., 2019). For example, the AP-1 adaptor complex (which consists of β1 γ, μ1 and σ1 subunits) responsible for sorting at the trans/TGN has two γ (γ1 and γ2), two μ1 (μ1A and μ1B, also known in mammals as AP1M1 and AP1M2, respectively) and three σ1 (σ1A, σ1B and σ1C) isoforms, which are implicated in cell type-specific sorting functions of AP-1 (discussed below; Mattera et al., 2011). AP-1 binds two core trans/TGN membrane components, activated Arf1 GTPase (i.e. the GTP-bound form) and the signaling lipid phosphatidylinositol 4-phosphate (PI4P) (Kirchhausen et al., 2014). Additional CCV-associated proteins complement AP-1-mediated sorting by recognizing overlapping but distinct cargo clients. Of these, the Golgi-localized γ-ear-containing ADP-ribosylation factor-binding (GGA) proteins GGA1, GGA2 and GGA3 have prominent roles in lysosome biogenesis through the trafficking of mannose 6-phosphate (mannose 6-P) receptors (discussed below), with a principal role for GGA2 (Uemura and Waguri, 2020). Like AP-1, GGA proteins recognize sorting signals in the cytoplasmic segments of cargo proteins that conform to the sequence motif YXXΦ or [DE]XXXL[LI] (where X is any amino acid and Φ is a bulky hydrophobic amino acid) (Doray et al., 2007; Ohno et al., 1998, 1995; Puertollano et al., 2001; Zhu et al., 2001). Whereas AP-1 is present in two types of Golgi-derived CCVs, those containing only AP-1 and those containing both AP-1 and GGA proteins, GGA2 is specific to only AP-1-containing CCVs (Hirst et al., 2012).
Retrograde sorting of Golgi residents
The vesicle coat protein complex coatomer I (COPI) is the core component of Golgi retrograde pathways. COPI localizes to the rims of Golgi cisternae, whose edges are surrounded by vesicles, suggesting that these represent dynamic sites of protein sorting and export (Orci et al., 1997). Retrograde sorting of some trans/TGN-resident Golgi proteins is initiated by recruitment of COPI from the cytosol to Golgi membranes by Arf1-GTP and GOLPH3, to which COPI binds (Donaldson et al., 1992; Orci et al., 1993; Palmer et al., 1993; Serafini et al., 1991; Tu et al., 2012) (see poster). On Golgi membranes, COPI assembles into a polymeric membrane coat that contains binding sites for retrograde sorting signals present in the cytoplasmic segments of integral membrane client cargo proteins (Cosson and Letourneur, 1994; Letourneur et al., 1994). Cargo is captured by this nascent vesicle coat during the budding process, and once budded from the cisterna, the COPI coat dissociates and the vesicle fuses with an earlier, maturing cisterna, thus maintaining Golgi cisterna composition (Popoff et al., 2011).
In addition to direct capture of cargo by the COPI coat, the peripheral membrane protein GOLPH3 associates with COPI on Golgi membranes, and these interactions are necessary for retention of many residents of the early Golgi cisternae (Eckert et al., 2014; Tu et al., 2012, 2008). Loss of GOLPH3 increases the rate at which these Golgi residents are degraded in the lysosome, resulting in decreased levels of these proteins in the Golgi, perturbations to protein glycosylation and complex sphingolipid homeostasis (Chang et al., 2013; Rizzo et al., 2021; Wood et al., 2012), and ultimately secretion (Dippold et al., 2009; Rahajeng et al., 2019; Xing et al., 2016). GOLPH3 contains an evolutionarily conserved pattern of arginine residues (two or three within the first 12 amino acids of the N termini) that confers binding to coatomer (COPI) and is required for retention of Golgi residents (Eckert et al., 2014; Tu et al., 2012). Based on these observations, GOLPH3 has been proposed to be a cargo-selective adaptor for the COPI vesicle coat via coincident recognition of PI4P, COPI and sequence motifs – LXX[RK] and [FL][LIV]XX[RK] – present in many residents of early Golgi compartments (such as the cis or medial cisternae) (Chang et al., 2013; Dippold et al., 2009; Eckert et al., 2014; Rizzo et al., 2021; Tu et al., 2012; Welch et al., 2021; Wood et al., 2009). Alternatively, GOLPH3 and COPI might prevent packaging of Golgi residents into anterograde intra-Golgi transport vesicles to maintain Golgi residence. GOLPH3 expression is increased in cells derived from a wide variety of human tumors, reportedly contributing to cellular transformation through enhanced mitogenic signaling caused by altered sphingolipid metabolism (Farber-Katz et al., 2014; Rizzo et al., 2021; Scott et al., 2009).
Ca2+-dependent constitutive and regulated pathways
The mechanisms for sorting of constitutively secreted soluble proteins and those destined for regulated secretion remain poorly understood, mostly because of a lack of an identified sorting receptor. Nevertheless, Ca2+ has emerged as a critical regulator in these processes (see poster).
In the constitutive secretion pathway, Ca2+ is pumped into the trans/TGN lumen, resulting in Ca2+-dependent protein aggregation that facilitates cargo sorting via SPCA1 (also known as ATP2C1), a trans/TGN-localized Ca2+ ATPase (von Blume et al., 2011). Conversely, knockdown of SPCA1 reduces Ca2+ levels in the TGN lumen and results in decreased secretion of soluble cargoes (Deng et al., 2018; von Blume et al., 2011). The Golgi-resident protein Cab45 (also known as SDF4) couples Ca2+ concentration to secretory activity as it oligomerizes in the presence of Ca2+ (Crevenna et al., 2016; von Blume et al., 2012) and binds to soluble secretory proteins (including lysozyme C and cartilage oligomeric matrix protein) (von Blume et al., 2012). Thus, sorting of constitutively secreted soluble cargo is regulated by Ca2+, although further studies are necessary to understand how Cab45 oligomers drive sorting.
In the regulated secretory pathway, secretory cargo is retained within cytoplasmic vesicles, including dense-core vesicles (DCVs), which fuse with the plasma membrane upon stimulation (see poster). Regulated secretion is crucial for the physiology of specialized secretory cells, such as pancreatic β-cells, cells of the neuroendocrine system and the immune system. Members of the granin family of proteins, including chromogranin A (CHGA or CgA), chromogranin B (CHGB or CgB) and secretogranin II (SCG2 or SgII), are thought to play a role in DCV biogenesis via Ca2+-dependent aggregation (Beuret et al., 2004; Colomer et al., 1996; Gerdes et al., 1989; Huh et al., 2003; Kim et al., 2001; Yoo, 1995). Biochemical studies of crude extracts derived from pituitary or adrenal glands and purified proteins have demonstrated that chromogranins can aggregate at acidic pH and high (millimolar) Ca2+ concentrations (Colomer et al., 1996; Gerdes et al., 1989; Yoo, 1995). This has led to the ‘sorting for entry’ (also termed ‘sorting by aggregation’) hypothesis, whereby chromogranins aggregate within the TGN milieu and, in the process, co-aggregate other secretory granule-destined cargoes, resulting in active sorting (Tooze, 1998).
Trafficking in epithelial cells
The plasma membrane of polarized cells is itself polarized. In the case of epithelial cells, the apical plasma membrane faces the external environment, and the basolateral domain faces neighboring cells and the growth substratum, with intercellular tight junctions preventing diffusion of proteins and lipids between the two domains (see poster) (Cao et al., 2012; Stoops and Caplan, 2014). A longstanding goal regarding trafficking in polarized cells is to elucidate the mechanisms by which newly synthesized lipids and proteins are targeted to the correct plasma membrane domains and to understand the physiology of polarized cells in these terms.
At least four distinct Golgi-to-plasma membrane trafficking pathways have been described for polarized epithelial cells based on distinct sorting requirements for different cargo proteins (see poster) (Weisz and Fölsch, 2020). For basolateral trafficking, the most straightforward sorting mechanisms involve the recognition of tyrosine- or dileucine-based sorting motifs by AP-1, which mediates polarized sorting at the trans/TGN into CCVs (see above) (Caceres et al., 2019; Deborde et al., 2008; Gravotta et al., 2012). Some types of epithelial cells, including Madin–Darby canine kidney (MDCK)-derived cell lines that are commonly used for polarized cell sorting studies, express two forms of AP-1, AP-1A and AP-1B (Fölsch et al., 1999; Ohno et al., 1999), which differ solely in their distinct medium subunits (μ1A and μ1B). Curiously, AP-1A and AP-1B recognize tyrosine- or dileucine-based motifs (see above) with partly overlapping cargo-recognition preferences and so cooperate and compensate to accomplish basolateral sorting (Gravotta et al., 2012; Guo et al., 2013). Despite functional overlap, AP-1A localizes prominently to the trans/TGN and AP-1B to the recycling endosome (Fölsch, 2015). The role of AP-1 exclusively in basolateral trafficking has been challenged by recent reports showing that loss of AP-1 broadly effects both basolateral and apical protein targeting, highlighting the requirement for enhanced scrutiny when limited cargoes or cell types are employed (Caceres et al., 2019; Gravotta et al., 2019). One further heterotetrameric adaptor complex, AP-4, functions as a cargo adaptor for non-clathrin-coated vesicles at the trans/TGN, with a role in basolateral sorting that is apparently redundant to AP-1 (Simmen et al., 2002). Although little is known about the role of AP-4 in basolateral sorting at the trans/TGN, only AP-4 mediates sorting of the autophagy factor ATG9A from the trans/TGN to the nascent autophagosome (Davies et al., 2018; De Pace et al., 2018; Mattera et al., 2017). Additionally, several integral membrane proteins, notably the amyloid precursor (APP) and related proteins (likely APLP1 and APLP2), along with ATG9A, accumulate in the trans/TGN in AP-4-deficient cell lines (Burgos et al., 2010; Mattera et al., 2020), suggesting that they are AP-4 clients. Mutations in the genes encoding each of the AP-4 subunits cause a hereditary spastic paraplegia referred to as AP-4-deficiency syndrome, likely due to perturbed autophagy in neurons (Moreno-De-Luca et al., 2011; Tüysüz et al., 2014; Verkerk et al., 2009).
The apical plasma membrane of many types of epithelial cells is enriched in cholesterol and glycosphingolipids, such as galactosylceramide sulfate (also known as sulfatide) and Forssman glycolipid (Hansson et al., 1986; Koichi et al., 1974; Nichols et al., 1987), that are synthesized in the Golgi. Pioneering studies of lipid trafficking in MDCK cells employed synthetic fluorescent sphingolipid analogs that could be tracked and visualized in cells (van 't Hof and van Meer, 1990; van der Bijl et al., 1996; van Genderen and van Meer, 1995; van IJzendoorn and Hoekstra, 1998; van IJzendoorn et al., 1997), revealing that vesicles containing these fluorescent lipids are trafficked directly to the apical plasma membrane (van IJzendoorn et al., 2020).
In all cell types, sphingolipids reside exclusively in the exofacial and lumenal membrane leaflets of cellular membranes (Lorent et al., 2020; Verkleij et al., 1973), so it is not obvious how their identities and organization in the lumenal leaflet could be coupled to budding of secretory vesicles, which typically requires cytoplasmic factors. The ‘raft’ hypothesis postulates that phase condensation of cholesterol and sphingolipids within membrane bilayers forms a patch and/or domain of locally ordered lipids [the liquid-ordered (Lo) domain], termed a ‘lipid raft’ (Simons and Van Meer, 1988). Proteins bearing a glycosylphosphatidylinositol anchor (GPI-APs) and some palmitoylated integral membrane proteins co-segregate with ‘raft’ lipids in model membranes (Friedrichson and Kurzchalia, 1998; Levental et al., 2010; Varma and Mayor, 1998), and structural features of the membrane-spanning segments of model and native proteins have been identified whose segregation correlates with lipid rafts, with efficient targeting to the plasma membrane (Lorent et al., 2017; Sharpe et al., 2010; Yurtsever and Lorent, 2020). In addition, glycosylation of proteins has been shown to contribute to their clustering within these domains and export from the Golgi within apically targeted transport vesicles (see below) (Lebreton et al., 2019). Though it is obvious that the physicochemical properties of Golgi membranes are harnessed for the concentration and export of secretory products, it is now appreciated that vesicle-sized sphingolipid–cholesterol condensates are not present in biological membranes due to their molecular complexity (Anderson and Jacobson, 2002; Veatch and Keller, 2005). Nevertheless, lipid-based protein sorting mechanisms represent an active area of debate (Levental et al., 2020).
Role of glycans in cargo sorting
Glycosylation of cargo proteins plays a role in sorting at the trans/TGN. Two distinct glycosylation patterns exist on proteins: N-linked and O-linked. N-linked glycosylation is initiated in the ER and involves the covalent attachment of glycosidic linkages to the amide side chain of asparagine residues (Vagin et al., 2009). In contrast, O-linked glycosylation takes place in the Golgi by linkage of glycosyl chains to serine or threonine residues (Potter et al., 2006).
One of the best-characterized TGN sorting mechanisms, the sorting of lysosomal hydrolases by mannose 6-P receptors, relies on glycosylation (see poster). The N-linked glycosyl chains on lysosomal hydrolases are sequentially modified by two Golgi-resident enzymes, N-acetylglucosamine 1-phosphotransferase and N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase, to generate the mannose 6-P moiety (Kornfeld and Mellman, 1989). Mannose 6-P-tagged lysosomal hydrolases are then recognized by the mannose 6-P receptor and sorted into CCVs (Brown and Farquhar, 1984; Friend and Farquhar, 1967; Geuze et al., 1985; Hoflack and Kornfeld, 1985a,b; Lemansky et al., 1987). These vesicles deliver cargo–mannose 6-P complexes to the late endosome and/or lysosome, where the acidic lumenal pH drives dissociation of the enzymes from mannose 6-P receptors, allowing for their recycling back to the trans/TGN (Griffiths et al., 1988). Deficiency of N-acetylglucosamine 1-phosphotransferase leads to the lysosomal storage disorder mucolipidosis type 2 (Kornfeld and Mellman, 1989), causes secretion of lysosomal hydrolases (Gelfman et al., 2007; Vogel et al., 2009) and results in the formation of lysosomes filled with undigested substrates (Otomo et al., 2011).
N- and O-linked glycosylation have also been shown to contribute to apical sorting of both membrane-anchored and soluble proteins in polarized cells (see poster). Treatment of cells with inhibitors of early steps in glycan addition, expression of glycosylation-deficient mutant proteins and studies of glycosylation gain-of-function mutations have revealed a strong correlation between N-glycosylation and apical sorting of secreted proteins (Hendriks et al., 2004; Scheiffele et al., 1995; Urban et al., 1987; Vagin et al., 2009). There is limited data to delineate the role of O-linked glycosylation in protein sorting (Lebreton et al., 2019; Potter et al., 2006), although it has been shown to promote efficient exit of cargoes from the Golgi of HeLa cells (Sun et al., 2020). How these glycosylation signals promote sorting and exit at the trans/TGN remains unclear. Lectins, including VIP36 (also known as LMAN2) and galectin-3, have been implicated as putative glycan-sorting receptors (Delacour et al., 2006; Hara-Kuge et al., 2002); however, it is not clear to what extent endogenous levels of these proteins can contribute to sorting (Fullekrug et al., 1999).
Regulation of cargo export
Intracellular signaling pathways control numerous aspects of trans/TGN functions, including protein sorting, transport carrier formation and lipid homeostasis (Farhan and Rabouille, 2011) (see poster).
GTPase-mediated TGN export
G proteins such as small GTPases act as molecular switches that facilitate the formation and transport of trans/TGN-derived carriers. Effectors generally interact with the GTP-bound form of the respective GTPase to induce a specific downstream signaling cascade that modulates cargo recognition, vesicular budding, membrane curvature, fission and transport of the vesicle to the cell surface on cytoskeletal tracks (Hutagalung and Novick, 2011).
The best-described GTPases regulating membrane trafficking are the Arf, Rab and Rho subfamily members of the Ras superfamily, which serve as membrane-associated platforms that recruit specific effectors to the trans/TGN, facilitating vesicle formation (Anitei and Hoflack, 2012; Bankaitis et al., 2012). These effectors include clathrin adaptor-associated proteins and F-actin, as well as microtubule-associated proteins that modulate membrane bending for budding and fission, and motors that transport the vesicles to their final destination (Anitei et al., 2010). Specifically, members of the Rab GTPase family are involved in TGN carrier budding, fission and movements of vesicles via cytoskeletal networks (both F-actin and microtubules). Rab6 connects myosin and kinesin motors to promote vesicular movement from the TGN to the cell periphery or focal adhesions (Eisler et al., 2018; Fourriere et al., 2019; Grigoriev et al., 2007, 2011; Hutagalung and Novick, 2011; Jasmin et al., 1992).
A role for heterotrimeric G proteins in trans/TGN carrier formation has also been described (Jamora et al., 1999; Pimplikar and Simons, 1994) but remains poorly understood. Heterotrimeric G proteins are associated with G-protein-coupled receptors (GPCRs), and it has been speculated that cargo exit from the Golgi is regulated by yet-to-be-identified GPCRs (Di Martino et al., 2019).
Protein kinase signaling and cargo export
Protein kinases and phosphatases have been associated with secretory carrier formation at the trans/TGN (Mayinger, 2011). AP-1 recruitment to the membrane is coupled with protein phosphatase 2A-mediated dephosphorylation of the µ1 subunit of AP-1, promoting clathrin assembly. Once on the membrane, the AP-1 µ1 subunit is phosphorylated, which induces a conformational change and increases the binding to sorting signals in the cytosolic tails of cargo proteins (see poster). This process is a well-established example of how cyclical phosphorylation and dephosphorylation of AP-1 regulate its function from membrane recruitment until release into the cytosol (Ghosh and Kornfeld, 2003).
Protein kinase D (PKD, of which there are three mammalian isoforms with a similar modular structure, known as PRKD1, PRKD2 and PRKD3) is a key regulator of TGN lipid homeostasis (von Blume and Hausser, 2019) and TGN carrier fission (Bard and Malhotra, 2006). The C1a domain of PKD binds to diacylglycerol (DAG) (Baron and Malhotra, 2002), whereas the C1b domain binds to Arf1 (Pusapati et al., 2010) to tightly associate PKD with the TGN membrane (see poster). Fission of some secretory carriers from the TGN requires catalytic activation of PKD by protein kinase C (PKC)-mediated phosphorylation (Añel and Malhotra, 2005). Although several models describing how PKD could promote carrier fission have been proposed, the mechanism is still unknown (Campelo and Malhotra, 2012). Moreover, relevant substrates that influence fission have not yet been identified (Wakana and Campelo, 2021). Involvement of PKA–cyclic AMP signaling in export from the TGN has also been described, but the relevant substrates are also unknown (Pimplikar and Simons, 1994).
Lipid-based signaling and cargo export
Rapid changes in particular lipids at the trans/TGN membranes play a significant role in cargo sorting, budding and fission (von Blume and Hausser, 2019). Lipid effectors are proteins that recognize these lipids and are recruited from the cytosol to the TGN membrane. Such lipids include phosphatidylserine (PS), phosphatidic acid (PA) and, in particular, PI4P. PI4P generated by phosphatidylinositol 4-kinase-IIIβ (encoded by PI4KB) recruits sorting factors such as AP-1, GGAs and GOLPH3 to TGN membranes (Bankaitis et al., 2012). It is also critical for determining TGN membrane composition by regulating non-vesicular lipid transfer of sphingolipids and cholesterol via ER–TGN contact sites by CERT (also known as CERT1) and OSBP, respectively (Hanada et al., 2003; Mesmin et al., 2013). CERT and OSBP activities are regulated by PKD phosphorylation, facilitating the crosstalk between lipid metabolism and protein signaling at the trans/TGN (Wang et al., 2003) (see poster).
Perspective
A great deal is known about the features of cargo proteins that confer their sorting and trafficking from the Golgi to other organelles, yet much remains to be learned about the mechanisms that produce Golgi-derived specific transport carriers (see Box 2 for outstanding key questions). A large body of work suggests that in contrast to canonical, coat protein-driven budding mechanisms that are induced by cytoplasmic coat proteins, secretory carrier formation may be directed partially by the cargo itself. Aggregation of lumenal cargo proteins is controlled in part by the surrounding milieu, including pH and Ca2+ concentration, and can account for cargo sorting. Such aggregates could themselves provide a substrate around which membrane is wrapped to form a secretory carrier. In this scenario, association of cargo aggregates with the lumenal membrane leaflet could involve the lipid-binding activity of cargo proteins or, alternatively, integral membrane receptors that would capture cargo aggregates. Similarly, condensation of sphingolipids and cholesterol in the membrane, along with proteins with affinity for these condensates, likely explains sorting of membrane components such as ‘raft’ lipids and proteins, yet here too the mechanism(s) underlying carrier formation is unknown. Future insights into carrier formation may come from efforts to understand how the actin- and microtubule-based cytoskeletons influence Golgi membrane dynamics, which may initiate carrier budding and/or contribute to carrier fission.
Box 2. Open questions.
How are the activities of Arf and Rab GTPase family members orchestrated to confer cargo sorting, carrier formation and transport?
In general, Arf GTPases control the recruitment to Golgi membranes of cytosolic factors that aid in cargo sorting and formation of a subset of Golgi-derived transport carriers. Rab and Rho family GTPases, in general, link vesicle formation and transport to the cytoskeleton (Mizuno-Yamasaki et al., 2012). How the activities of all these GTPases and crosstalk between their signaling pathways are coordinated is key for understanding the role of the Golgi in secretion.
What are the mechanisms of vesicle fission from the TGN?
The mechanisms that mediate fission of transport carriers from Golgi compartments are largely unknown. Prominent roles for the actin and microtubule-based cytoskeleton (Chakrabarti et al., 2021; Efimov et al., 2007), Arf1 (Beck et al., 2011), dynamin (Cao et al., 2005), PKD (Liljedahl et al., 2001) and CtBP3 (also known as BARS and CTBP1; Bonazzi et al., 2005) have been proposed to regulate and/or mediate fission, but the underlying mechanisms are yet to be elucidated.
How do carriers acquire the appropriate SNARE molecules to mediate vesicle fusion with the appropriate target membrane?
Unique combinations of SNARE molecules on the carrier and target membrane mediate fusion of the transport carrier with a particular target organelle; it is unknown how the appropriate SNAREs are loaded into transport carriers (Südhof and Rothman, 2009).
How do changes in lipid composition of the TGN influence cargo sorting and trafficking?
It is likely that transfer of sterol and sphingolipid precursors from the ER to the trans/TGN, as well as processing and/or synthesis of these lipids and others (for example, PI4P) within the TGN, is tightly coordinated with carrier formation, functioning not only to provide a reservoir of membrane for the nascent vesicle but also for spatiotemporal control of cargo sorting and membrane budding required for TGN export (Wakana and Campelo, 2021).
What are the mechanisms that underpin cargo sorting and budding of non-coated vesicles?
In the paradigm of coated vesicle biogenesis, the vesicle is formed by coat and accessory proteins that capture cargo and shape the membrane (Tan and Gleeson, 2019). It is largely unclear how any of these processes occur for non-coated carriers.
Acknowledgements
We are grateful to colleagues for discussions and critical reading of the manuscript. The authors would like to thank Edward Felder (University of Ulm, Germany) for the collaboration to produce the electron micrograph shown in the poster.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Cell science at a glance
Individual poster panels are available for downloading at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.259110#supplementary-data
References
- Adolf, F., Rhiel, M., Hessling, B., Gao, Q., Hellwig, A., Béthune, J. and Wieland, F. T. (2019). Proteomic profiling of mammalian COPII and COPI vesicles. Cell Rep. 26, 250-265.e5. 10.1016/j.celrep.2018.12.041 [DOI] [PubMed] [Google Scholar]
- Anderson, R. G. W. and Jacobson, K. (2002). A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821. 10.1126/science.1068886 [DOI] [PubMed] [Google Scholar]
- Añel, A. M. D. and Malhotra, V. (2005). PKCη is required for β1γ2/β3γ2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J. Cell Biol. 169, 83-91. 10.1083/jcb.200412089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitei, M. and Hoflack, B. (2012). Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat. Cell Biol. 14, 11-19. 10.1038/ncb2409 [DOI] [PubMed] [Google Scholar]
- Anitei, M., Stange, C., Parshina, I., Baust, T., Schenck, A., Raposo, G., Kirchhausen, T. and Hoflack, B. (2010). Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN. Nat. Cell Biol. 12, 330-340. 10.1038/ncb2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis, V. A., Garcia-Mata, R. and Mousley, C. J. (2012). Golgi membrane dynamics and lipid metabolism. Curr. Biol. 22, R414-R424. 10.1016/j.cub.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard, F. and Malhotra, V. (2006). The formation of TGN-to-plasma-membrane transport carriers. Annu. Rev. Cell Dev. Biol. 22, 439-455. 10.1146/annurev.cellbio.21.012704.133126 [DOI] [PubMed] [Google Scholar]
- Baron, C. L. and Malhotra, V. (2002). Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325-328. 10.1126/science.1066759 [DOI] [PubMed] [Google Scholar]
- Beck, R., Prinz, S., Diestelkötter-Bachert, P., Röhling, S., Adolf, F., Hoehner, K., Welsch, S., Ronchi, P., Brügger, B., Briggs, J. A.et al. (2011). Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. J. Cell Biol. 194, 765-777. 10.1083/jcb.201011027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuret, N., Stettler, H., Renold, A., Rutishauser, J. and Spiess, M. (2004). Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J. Biol. Chem. 279, 20242-20249. 10.1074/jbc.M310613200 [DOI] [PubMed] [Google Scholar]
- Bonazzi, M., Spanò, S., Turacchio, G., Cericola, C., Valente, C., Colanzi, A., Kweon, H. S., Hsu, V. W., Polishchuck, E. V., Polishchuck, R. S.et al. (2005). CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 7, 570-580. 10.1038/ncb1260 [DOI] [PubMed] [Google Scholar]
- Bonfanti, L., Mironov, A. A., Jr., Martínez-Menárguez, J. A., Martella, O., Fusella, A., Baldassarre, M., Buccione, R., Geuze, H. J., Mironov, A. A. and Luini, A. (1998). Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell 95, 993-1003. 10.1016/S0092-8674(00)81723-7 [DOI] [PubMed] [Google Scholar]
- Brown, W. J. and Farquhar, M. G. (1984). Accumulation of coated vesicles bearing mannose 6-phosphate receptors for lysosomal enzymes in the Golgi region of I-cell fibroblasts. Proc. Natl. Acad. Sci. USA 81, 5135-5139. 10.1073/pnas.81.16.5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos, P. V., Mardones, G. A., Rojas, A. L., daSilva, L. L. P., Prabhu, Y., Hurley, J. H. and Bonifacino, J. S. (2010). Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev. Cell 18, 425-436. 10.1016/j.devcel.2010.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres, P. S., Gravotta, D., Zager, P. J., Dephoure, N. and Rodriguez-Boulan, E. (2019). Quantitative proteomics of MDCK cells identify unrecognized roles of clathrin adaptor AP-1 in polarized distribution of surface proteins. Proc. Natl. Acad. Sci. USA 116, 11796-11805. 10.1073/pnas.1821076116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo, F. and Malhotra, V. (2012). Membrane fission: the biogenesis of transport carriers. Annu. Rev. Biochem. 81, 407-427. 10.1146/annurev-biochem-051710-094912 [DOI] [PubMed] [Google Scholar]
- Cao, H., Weller, S., Orth, J. D., Chen, J., Huang, B., Chen, J.-L., Stamnes, M. and McNiven, M. A. (2005). Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat. Cell Biol. 7, 483-492. 10.1038/ncb1246 [DOI] [PubMed] [Google Scholar]
- Cao, X., Surma, M. A. and Simons, K. (2012). Polarized sorting and trafficking in epithelial cells. Cell Res. 22, 793-805. 10.1038/cr.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti, R., Lee, M. and Higgs, H. N. (2021). Multiple roles for actin in secretory and endocytic pathways. Curr. Biol. 31, R603-R618. 10.1016/j.cub.2021.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W.-L., Chang, C.-W., Chang, Y.-Y., Sung, H.-H., Lin, M.-D., Chang, S.-C., Chen, C.-H., Huang, C.-W., Tung, K.-S. and Chou, T.-B. (2013). The Drosophila GOLPH3 homolog regulates the biosynthesis of heparan sulfate proteoglycans by modulating the retrograde trafficking of exostosins. Development 140, 2798-2807. 10.1242/dev.087171 [DOI] [PubMed] [Google Scholar]
- Colomer, V., Kicska, G. A. and Rindler, M. J. (1996). Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J. Biol. Chem. 271, 48-55. 10.1074/jbc.271.1.48 [DOI] [PubMed] [Google Scholar]
- Cosson, P. and Letourneur, F. (1994). Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263, 1629-1631. 10.1126/science.8128252 [DOI] [PubMed] [Google Scholar]
- Crevenna, A. H., Blank, B., Maiser, A., Emin, D., Prescher, J., Beck, G., Kienzle, C., Bartnik, K., Habermann, B., Pakdel, M.et al. (2016). Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J. Cell Biol. 213, 305-314. 10.1083/jcb.201601089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, A. K., Itzhak, D. N., Edgar, J. R., Archuleta, T. L., Hirst, J., Jackson, L. P., Robinson, M. S. and Borner, G. H. H. (2018). AP-4 vesicles contribute to spatial control of autophagy via RUSC-dependent peripheral delivery of ATG9A. Nat. Commun. 9, 3958. 10.1038/s41467-018-06172-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis, M. A. and Luini, A. (2008). Exiting the Golgi complex. Nat. Re. Mol. Cell Biol. 9, 273-284. 10.1038/nrm2378 [DOI] [PubMed] [Google Scholar]
- De Pace, R., Skirzewski, M., Damme, M., Mattera, R., Mercurio, J., Foster, A. M., Cuitino, L., Jarnik, M., Hoffmann, V., Morris, H. D.et al. (2018). Altered distribution of ATG9A and accumulation of axonal aggregates in neurons from a mouse model of AP-4 deficiency syndrome. PLoS Genet. 14, e1007363. 10.1371/journal.pgen.1007363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborde, S., Perret, E., Gravotta, D., Deora, A., Salvarezza, S., Schreiner, R. and Rodriguez-Boulan, E. (2008). Clathrin is a key regulator of basolateral polarity. Nature 452, 719-723. 10.1038/nature06828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour, D., Cramm-Behrens, C. I., Drobecq, H., Le Bivic, A., Naim, H. Y. and Jacob, R. (2006). Requirement for galectin-3 in apical protein sorting. Curr. Biol. 16, 408-414. 10.1016/j.cub.2005.12.046 [DOI] [PubMed] [Google Scholar]
- Deng, Y., Pakdel, M., Blank, B., Sundberg, E. L., Burd, C. G. and von Blume, J. (2018). Activity of the SPCA1 calcium pump couples sphingomyelin synthesis to sorting of secretory proteins in the trans-Golgi network. Dev. Cell 47, 464-478.e8. 10.1016/j.devcel.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino, R., Sticco, L. and Luini, A. (2019). Regulation of cargo export and sorting at the trans-Golgi network. FEBS Lett. 593, 2306-2318. 10.1002/1873-3468.13572 [DOI] [PubMed] [Google Scholar]
- Dippold, H. C., Ng, M. M., Farber-Katz, S. E., Lee, S.-K., Kerr, M. L., Peterman, M. C., Sim, R., Wiharto, P. A., Galbraith, K. A., Madhavarapu, S.et al. (2009). GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell 139, 337-351. 10.1016/j.cell.2009.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, J. G., Cassel, D., Kahn, R. A. and Klausner, R. D. (1992). ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc. Natl. Acad. Sci. USA 89, 6408-6412. 10.1073/pnas.89.14.6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray, B., Lee, I., Knisely, J., Bu, G. and Kornfeld, S. (2007). The γ/σ1 and α/σ2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell 18, 1887-1896. 10.1091/mbc.e07-01-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop, M. H., Ernst, A. M., Schroeder, L. K., Toomre, D. K., Lavieu, G. and Rothman, J. E. (2017). Land-locked mammalian Golgi reveals cargo transport between stable cisternae. Nat. Commun. 8, 432. 10.1038/s41467-017-00570-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, E. S. P., Reckmann, I., Hellwig, A., Röhling, S., El-Battari, A., Wieland, F. T. and Popoff, V. (2014). Golgi phosphoprotein 3 triggers signal-mediated incorporation of glycosyltransferases into coatomer-coated (COPI) vesicles. J. Biol. Chem. 289, 31319-31329. 10.1074/jbc.M114.608182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov, A., Kharitonov, A., Efimova, N., Loncarek, J., Miller, P. M., Andreyeva, N., Gleeson, P., Galjart, N., Maia, A. R. R., McLeod, I. X.et al. (2007). Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917-930. 10.1016/j.devcel.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisler, S. A., Curado, F., Link, G., Schulz, S., Noack, M., Steinke, M., Olayioye, M. A. and Hausser, A. (2018). A Rho signaling network links microtubules to PKD controlled carrier transport to focal adhesions. eLife 7, e35907. 10.7554/eLife.35907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber-Katz, S. E., Dippold, H. C., Buschman, M. D., Peterman, M. C., Xing, M., Noakes, C. J., Tat, J., Ng, M. M., Rahajeng, J., Cowan, D. M.et al. (2014). DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3. Cell 156, 413-427. 10.1016/j.cell.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhan, H. and Rabouille, C. (2011). Signalling to and from the secretory pathway. J. Cell Sci. 124, 171-180. 10.1242/jcs.076455 [DOI] [PubMed] [Google Scholar]
- Fölsch, H. (2015). Role of the epithelial cell-specific clathrin adaptor complex AP-1B in cell polarity. Cell. Logist. 5, e1074331. 10.1080/21592799.2015.1074331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch, H., Ohno, H., Bonifacino, J. S. and Mellman, I. (1999). A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99, 189-198. 10.1016/S0092-8674(00)81650-5 [DOI] [PubMed] [Google Scholar]
- Fourriere, L., Kasri, A., Gareil, N., Bardin, S., Bousquet, H., Pereira, D., Perez, F., Goud, B., Boncompain, G. and Miserey-Lenkei, S. (2019). RAB6 and microtubules restrict protein secretion to focal adhesions. J. Cell Biol. 218, 2215-2231. 10.1083/jcb.201805002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichson, T. and Kurzchalia, T. V. (1998). Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 394, 802-805. 10.1038/29570 [DOI] [PubMed] [Google Scholar]
- Friend, D. S. and Farquhar, M. G. (1967). Functions of coated vesicles during protein absorption in the rat vas deferens. J. Cell Biol. 35, 357-376. 10.1083/jcb.35.2.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug, J., Scheiffele, P. and Simons, K. (1999). VIP36 localisation to the early secretory pathway. J. Cell Sci. 112, 2813-2821. 10.1242/jcs.112.17.2813 [DOI] [PubMed] [Google Scholar]
- Gelfman, C. M., Vogel, P., Issa, T. M., Turner, C. A., Lee, W.-S., Kornfeld, S. and Rice, D. S. (2007). Mice lacking α/β subunits of GlcNAc-1-phosphotransferase exhibit growth retardation, retinal degeneration, and secretory cell lesions. Invest. Ophthalmol. Vis. Sci. 48, 5221-5228. 10.1167/iovs.07-0452 [DOI] [PubMed] [Google Scholar]
- Gerdes, H. H., Rosa, P., Phillips, E., Baeuerle, P. A., Frank, R., Argos, P. and Huttner, W. B. (1989). The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J. Biol. Chem. 264, 12009-12015. 10.1016/S0021-9258(18)80167-3 [DOI] [PubMed] [Google Scholar]
- Geuze, H. J., Slot, J. W., Strous, G. J., Hasilik, A. and von Figura, K. (1985). Possible pathways for lysosomal enzyme delivery. J. Cell Biol. 101, 2253-2262. 10.1083/jcb.101.6.2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, P. and Kornfeld, S. (2003). Phosphorylation-induced conformational changes regulate GGAs 1 and 3 function at the trans-Golgi network. J. Biol. Chem. 278, 14543-14549. 10.1074/jbc.M212543200 [DOI] [PubMed] [Google Scholar]
- Gommel, D., Orci, L., Emig, E. M., Hannah, M. J., Ravazzola, M., Nickel, W., Helms, J. B., Wieland, F. T. and Sohn, K. (1999). p24 and p23, the major transmembrane proteins of COPI-coated transport vesicles, form hetero-oligomeric complexes and cycle between the organelles of the early secretory pathway. FEBS Lett. 447, 179-185. 10.1016/S0014-5793(99)00246-X [DOI] [PubMed] [Google Scholar]
- Gravotta, D., Carvajal-Gonzalez, J. M., Mattera, R., Deborde, S., Banfelder, J. R., Bonifacino, J. S. and Rodriguez-Boulan, E. (2012). The clathrin adaptor AP-1A mediates basolateral polarity. Dev. Cell 22, 811-823. 10.1016/j.devcel.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta, D., Perez Bay, A., Jonker, C. T. H., Zager, P. J., Benedicto, I., Schreiner, R., Caceres, P. S. and Rodriguez-Boulan, E. (2019). Clathrin and clathrin adaptor AP-1 control apical trafficking of megalin in the biosynthetic and recycling routes. Mol. Biol. Cell 30, 1716-1728. 10.1091/mbc.E18-12-0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, G., Hoflack, B., Simons, K., Mellman, I. and Kornfeld, S. (1988). The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell 52, 329-341. 10.1016/S0092-8674(88)80026-6 [DOI] [PubMed] [Google Scholar]
- Grigoriev, I., Splinter, D., Keijzer, N., Wulf, P. S., Demmers, J., Ohtsuka, T., Modesti, M., Maly, I. V., Grosveld, F., Hoogenraad, C. C.et al. (2007). Rab6 regulates transport and targeting of exocytotic carriers. Dev. Cell 13, 305-314. 10.1016/j.devcel.2007.06.010 [DOI] [PubMed] [Google Scholar]
- Grigoriev, I., Yu, K. L., Martinez-Sanchez, E., Serra-Marques, A., Smal, I., Meijering, E., Demmers, J., Peränen, J., Pasterkamp, R. J., van der Sluijs, P.et al. (2011). Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr. Biol. 21, 967-974. 10.1016/j.cub.2011.04.030 [DOI] [PubMed] [Google Scholar]
- Guo, X., Mattera, R., Ren, X., Chen, Y., Retamal, C., González, A. and Bonifacino, J. S. (2013). The adaptor protein-1 μ1B subunit expands the repertoire of basolateral sorting signal recognition in epithelial cells. Dev. Cell 27, 353-366. 10.1016/j.devcel.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada, K., Kumagai, K., Yasuda, S., Miura, Y., Kawano, M., Fukasawa, M. and Nishijima, M. (2003). Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803-809. 10.1038/nature02188 [DOI] [PubMed] [Google Scholar]
- Hansson, G. C., Simons, K. and van Meer, G. (1986). Two strains of the Madin-Darby canine kidney (MDCK) cell line have distinct glycosphingolipid compositions. EMBO J. 5, 483-489. 10.1002/j.1460-2075.1986.tb04237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Kuge, S., Ohkura, T., Ideo, H., Shimada, O., Atsumi, S. and Yamashita, K. (2002). Involvement of VIP36 in intracellular transport and secretion of glycoproteins in polarized Madin-Darby canine kidney (MDCK) cells. J. Biol. Chem. 277, 16332-16339. 10.1074/jbc.M112188200 [DOI] [PubMed] [Google Scholar]
- Hendriks, G., Koudijs, M., van Balkom, B. W. M., Oorschot, V., Klumperman, J., Deen, P. M. T. and van der Sluijs, P. (2004). Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J. Biol. Chem. 279, 2975-2983. 10.1074/jbc.M310767200 [DOI] [PubMed] [Google Scholar]
- Hirst, J., Borner, G. H. H., Antrobus, R., Peden, A. A., Hodson, N. A., Sahlender, D. A. and Robinson, M. S. (2012). Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr. Biol. 22, 1711-1716. 10.1016/j.cub.2012.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoflack, B. and Kornfeld, S. (1985a). Lysosomal enzyme binding to mouse P388D1 macrophage membranes lacking the 215-kDa mannose 6-phosphate receptor: evidence for the existence of a second mannose 6-phosphate receptor. Proc. Natl. Acad. Sci. USA 82, 4428-4432. 10.1073/pnas.82.13.4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoflack, B. and Kornfeld, S. (1985b). Purification and characterization of a cation-dependent mannose 6-phosphate receptor from murine P388D1 macrophages and bovine liver. J. Biol. Chem. 260, 12008-12014. 10.1016/S0021-9258(17)38977-9 [DOI] [PubMed] [Google Scholar]
- Huh, Y. H., Jeon, S. H. and Yoo, S. H. (2003). Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J. Biol. Chem. 278, 40581-40589. 10.1074/jbc.M304942200 [DOI] [PubMed] [Google Scholar]
- Hutagalung, A. H. and Novick, P. J. (2011). Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119-149. 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora, C., Yamanouye, N., Van Lint, J., Laudenslager, J., Vandenheede, J. R., Faulkner, D. J. and Malhotra, V. (1999). Gβγ-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98, 59-68. 10.1016/S0092-8674(00)80606-6 [DOI] [PubMed] [Google Scholar]
- Jasmin, B. J., Goud, B., Camus, G. and Cartaud, J. (1992). The low molecular weight guanosine triphosphate-binding protein Rab6p associates with distinct post-Golgi vesicles in Torpedo marmorata electrocytes. Neuroscience 49, 849-855. 10.1016/0306-4522(92)90361-5 [DOI] [PubMed] [Google Scholar]
- Kim, T., Tao-Cheng, J.-H., Eiden, L. E. and Loh, Y. P. (2001). Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell 106, 499-509. 10.1016/S0092-8674(01)00459-7 [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T., Owen, D. and Harrison, S. C. (2014). Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 6, a016725. 10.1101/cshperspect.a016725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman, J. (2011). Architecture of the mammalian Golgi. Cold Spring Harb. Perspect. Biol. 3, a005181. 10.1101/cshperspect.a005181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koichi, K., Michiya, F. and Makoto, N. (1974). Lipid components of two different regions of an intestinal epithelial cell membrane of mouse. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 369, 222-233. 10.1016/0005-2760(74)90253-7 [DOI] [PubMed] [Google Scholar]
- Kornfeld, S. and Mellman, I. (1989). The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5, 483-525. 10.1146/annurev.cb.05.110189.002411 [DOI] [PubMed] [Google Scholar]
- Lavieu, G., Zheng, H. and Rothman, J. E. (2013). Stapled Golgi cisternae remain in place as cargo passes through the stack. eLife 2, e00558. 10.7554/eLife.00558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton, S., Paladino, S. and Zurzolo, C. (2019). Clustering in the Golgi apparatus governs sorting and function of GPI-APs in polarized epithelial cells. FEBS Lett. 593, 2351-2365. 10.1002/1873-3468.13573 [DOI] [PubMed] [Google Scholar]
- Lemansky, P., Hasilik, A., von Figura, K., Helmy, S., Fishman, J., Fine, R. E., Kedersha, N. L. and Rome, L. H. (1987). Lysosomal enzyme precursors in coated vesicles derived from the exocytic and endocytic pathways. J. Cell Biol. 104, 1743-1748. 10.1083/jcb.104.6.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur, F., Gaynor, E. C., Hennecke, S., Démollière, C., Duden, R., Emr, S. D., Riezman, H. and Cosson, P. (1994). Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell 79, 1199-1207. 10.1016/0092-8674(94)90011-6 [DOI] [PubMed] [Google Scholar]
- Levental, I., Lingwood, D., Grzybek, M., Coskun, Ü. and Simons, K. (2010). Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl Acad. Sci. USA 107, 22050-22054. 10.1073/pnas.1016184107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental, I., Levental, K. R. and Heberle, F. A. (2020). Lipid rafts: controversies resolved, mysteries remain. Trends Cell Biol. 30, 341-353. 10.1016/j.tcb.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Ahat, E. and Wang, Y. (2019). Golgi structure and function in health, stress, and diseases. In The Golgi Apparatus and Centriole: Functions, Interactions and Role in Disease (ed. Kloc M.), pp. 441-485. Cham: Springer International Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedahl, M., Maeda, Y., Colanzi, A., Ayala, I., Van Lint, J. and Malhotra, V. (2001). Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell 104, 409-420. 10.1016/S0092-8674(01)00228-8 [DOI] [PubMed] [Google Scholar]
- Liu, J., Huang, Y., Li, T., Jiang, Z., Zeng, L. and Hu, Z. (2021). The role of the Golgi apparatus in disease (Review). Int. J. Mol. Med. 47, 38. 10.3892/ijmm.2021.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorent, J. H., Diaz-Rohrer, B., Lin, X., Spring, K., Gorfe, A. A., Levental, K. R. and Levental, I. (2017). Structural determinants and functional consequences of protein affinity for membrane rafts. Nat. Commun. 8, 1219. 10.1038/s41467-017-01328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorent, J. H., Levental, K. R., Ganesan, L., Rivera-Longsworth, G., Sezgin, E., Doktorova, M., Lyman, E. and Levental, I. (2020). Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 16, 644-652. 10.1038/s41589-020-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev, E., Reinke, C. A., Jellen, J., Strongin, D. E., Bevis, B. J. and Glick, B. S. (2006). Golgi maturation visualized in living yeast. Nature 441, 1002-1006. 10.1038/nature04717 [DOI] [PubMed] [Google Scholar]
- Lujan, P. and Campelo, F. (2021). Should I stay or should I go? Golgi membrane spatial organization for protein sorting and retention. Arch. Biochem. Biophys. 707, 108921. 10.1016/j.abb.2021.108921 [DOI] [PubMed] [Google Scholar]
- Malsam, J., Satoh, A., Pelletier, L. and Warren, G. (2005). Golgin tethers define subpopulations of COPI vesicles. Science 307, 1095-1098. 10.1126/science.1108061 [DOI] [PubMed] [Google Scholar]
- Martínez-Menárguez, J. A., Prekeris, R., Oorschot, V. M. J., Scheller, R., Slot, J. W., Geuze, H. J. and Klumperman, J. (2001). Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J. Cell Biol. 155, 1213-1224. 10.1083/jcb.200108029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura-Tokita, K., Takeuchi, M., Ichihara, A., Mikuriya, K. and Nakano, A. (2006). Live imaging of yeast Golgi cisternal maturation. Nature 441, 1007-1010. 10.1038/nature04737 [DOI] [PubMed] [Google Scholar]
- Mattera, R., Boehm, M., Chaudhuri, R., Prabhu, Y. and Bonifacino, J. S. (2011). Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J. Biol. Chem. 286, 2022-2030. 10.1074/jbc.M110.197178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera, R., Park, S. Y., De Pace, R., Guardia, C. M. and Bonifacino, J. S. (2017). AP-4 mediates export of ATG9A from the trans-Golgi network to promote autophagosome formation. Proc. Natl Acad. Sci. USA 114, E10697. 10.1073/pnas.1717327114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera, R., Williamson, C. D., Ren, X. and Bonifacino, J. S. (2020). The FTS-Hook-FHIP (FHF) complex interacts with AP-4 to mediate perinuclear distribution of AP-4 and its cargo ATG9A. Mol. Biol. Cell 31, 963-979. 10.1091/mbc.E19-11-0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayinger, P. (2011). Signaling at the Golgi. Cold Spring Harb. Perspect. Biol. 3, a005314. 10.1101/cshperspect.a005314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian, M., Becker, B. and Becker, D. (1991). Scale formation in algae. J. Electron Microsc. Tech. 17, 165-178. 10.1002/jemt.1060170205 [DOI] [PubMed] [Google Scholar]
- Mesmin, B., Bigay, J., Moser von Filseck, J., Lacas-Gervais, S., Drin, G. and Antonny, B. (2013). A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 155, 830-843. 10.1016/j.cell.2013.09.056 [DOI] [PubMed] [Google Scholar]
- Mizuno-Yamasaki, E., Rivera-Molina, F. and Novick, P. (2012). GTPase networks in membrane traffic. Annu. Rev. Biochem. 81, 637-659. 10.1146/annurev-biochem-052810-093700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-De-Luca, A., Helmers, S. L., Mao, H., Burns, T. G., Melton, A. M. A., Schmidt, K. R., Fernhoff, P. M., Ledbetter, D. H. and Martin, C. L. (2011). Adaptor protein complex-4 (AP-4) deficiency causes a novel autosomal recessive cerebral palsy syndrome with microcephaly and intellectual disability. J. Med. Genet. 48, 141-144. 10.1136/jmg.2010.082263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, G. E., Shiraishi, T., Allietta, M., Tillack, T. W. and Young, W. W. (1987). Polarity of the Forssman glycolipid in MDCK epithelial cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 930, 154-166. 10.1016/0167-4889(87)90027-9 [DOI] [PubMed] [Google Scholar]
- Ohno, H., Stewart, J., Fournier, M.-C., Bosshart, H., Rhee, I., Miyatake, S., Saito, T., Gallusser, A., Kirchhausen, T. and Bonifacino, J. S. (1995). Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872-1875. 10.1126/science.7569928 [DOI] [PubMed] [Google Scholar]
- Ohno, H., Aguilar, R. C., Yeh, D., Taura, D., Saito, T. and Bonifacino, J. S. (1998). The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273, 25915-25921. 10.1074/jbc.273.40.25915 [DOI] [PubMed] [Google Scholar]
- Ohno, H., Tomemori, T., Nakatsu, F., Okazaki, Y., Aguilar, R. C., Foelsch, H., Mellman, I., Saito, T., Shirasawa, T. and Bonifacino, J. S. (1999). μ1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 449, 215-220. 10.1016/S0014-5793(99)00432-9 [DOI] [PubMed] [Google Scholar]
- Orci, L., Palmer, D. J., Ravazzola, M., Perrelet, A., Amherdt, M. and Rothman, J. E. (1993). Budding from Golgi membranes requires the coatomer complex of non-clathrin coat proteins. Nature 362, 648-652. 10.1038/362648a0 [DOI] [PubMed] [Google Scholar]
- Orci, L., Stamnes, M., Ravazzola, M., Amherdt, M., Perrelet, A., Söllner, T. H. and Rothman, J. E. (1997). Bidirectional transport by distinct populations of COPI-coated vesicles. Cell 90, 335-349. 10.1016/S0092-8674(00)80341-4 [DOI] [PubMed] [Google Scholar]
- Otomo, T., Higaki, K., Nanba, E., Ozono, K. and Sakai, N. (2011). Lysosomal storage causes cellular dysfunction in mucolipidosis II skin fibroblasts. J. Biol. Chem. 286, 35283-35290. 10.1074/jbc.M111.267930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, D. J., Helms, J. B., Beckers, C. J., Orci, L. and Rothman, J. E. (1993). Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J. Biol. Chem. 268, 12083-12089. 10.1016/S0021-9258(19)50311-8 [DOI] [PubMed] [Google Scholar]
- Pimplikar, S. W. and Simons, K. (1994). Activators of protein kinase A stimulate apical but not basolateral transport in epithelial Madin-Darby canine kidney cells. J. Biol. Chem. 269, 19054-19059. 10.1016/S0021-9258(17)32273-1 [DOI] [PubMed] [Google Scholar]
- Popoff, V., Adolf, F., Brugger, B. and Wieland, F. (2011). COPI budding within the Golgi stack. Cold Spring Harb. Perspect. Biol. 3, a005231. 10.1101/cshperspect.a005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, B. A., Hughey, R. P. and Weisz, O. A. (2006). Role of N- and O-glycans in polarized biosynthetic sorting. Am. J. Physiol. Cell Physiol. 290, C1-C10. 10.1152/ajpcell.00333.2005 [DOI] [PubMed] [Google Scholar]
- Puertollano, R., Aguilar, R. C., Gorshkova, I., Crouch, R. J. and Bonifacino, J. S. (2001). Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science 292, 1712-1716. 10.1126/science.1060750 [DOI] [PubMed] [Google Scholar]
- Pusapati, G. V., Krndija, D., Armacki, M., von Wichert, G., von Blume, J., Malhotra, V., Adler, G. and Seufferlein, T. (2010). Role of the second cysteine-rich domain and Pro275 in protein kinase D2 interaction with ADP-ribosylation factor 1, trans-Golgi network recruitment, and protein transport. Mol. Biol. Cell 21, 1011-1022. 10.1091/mbc.e09-09-0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille, C. and Klumperman, J. (2005). The maturing role of COPI vesicles in intra-Golgi transport. Nat. Rev. Mol Cell Biol. 6, 812-817. 10.1038/nrm1735 [DOI] [PubMed] [Google Scholar]
- Rahajeng, J., Kuna, R. S., Makowski, S. L., Tran, T. T. T., Buschman, M. D., Li, S., Cheng, N., Ng, M. M. and Field, S. J. (2019). Efficient Golgi forward trafficking requires GOLPH3-Driven, PI4P–dependent membrane curvature. Dev. Cell 50, 573-585.e5. 10.1016/j.devcel.2019.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, R., Russo, D., Kurokawa, K., Sahu, P., Lombardi, B., Supino, D., Zhukovsky, M. A., Vocat, A., Pothukuchi, P., Kunnathully, V.et al. (2021). Golgi maturation-dependent glycoenzyme recycling controls glycosphingolipid biosynthesis and cell growth via GOLPH3. EMBO J. 40, e107238. 10.15252/embj.2020107238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger, A., Hirst, J., Davies, A. K. and Robinson, M. S. (2019). Adaptor protein complexes and disease at a glance. J. Cell Sci. 132, jcs222992. 10.1242/jcs.222992 [DOI] [PubMed] [Google Scholar]
- Scheiffele, P., Peränen, J. and Simons, K. (1995). N-glycans as apical sorting signals in epithelial cells. Nature 378, 96-98. 10.1038/378096a0 [DOI] [PubMed] [Google Scholar]
- Scott, K. L., Kabbarah, O., Liang, M.-C., Ivanova, E., Anagnostou, V., Wu, J., Dhakal, S., Wu, M., Chen, S., Feinberg, T.et al. (2009). GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 459, 1085-1090. 10.1038/nature08109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini, T., Orci, L., Amherdt, M., Brunner, M., Kahn, R. A. and Rothmant, J. E. (1991). ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell 67, 239-253. 10.1016/0092-8674(91)90176-Y [DOI] [PubMed] [Google Scholar]
- Sharpe, H. J., Stevens, T. J. and Munro, S. (2010). A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158-169. 10.1016/j.cell.2010.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen, T., Höning, S., Icking, A., Tikkanen, R. and Hunziker, W. (2002). AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 4, 154-159. 10.1038/ncb745 [DOI] [PubMed] [Google Scholar]
- Simons, K. and Van Meer, G. (1988). Lipid sorting in epithelial cells. Biochemistry 27, 6197-6202. 10.1021/bi00417a001 [DOI] [PubMed] [Google Scholar]
- Stanley, P. (2011). Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 3, a005199. 10.1101/cshperspect.a005199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, M. J., Chuang, S., Hou, X., Shoham, M. and Zhu, J. Z. (2009). Tyrosine sulfation: an increasingly recognised post-translational modification of secreted proteins. New Biotechnol. 25, 299-317. 10.1016/j.nbt.2009.03.011 [DOI] [PubMed] [Google Scholar]
- Stoops, E. H. and Caplan, M. J. (2014). Trafficking to the apical and basolateral membranes in polarized epithelial cells. J. Am. Soc. Nephrol. 25, 1375-1386. 10.1681/ASN.2013080883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof, T. C. and Rothman, J. E. (2009). Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474-477. 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Tie, H. C., Chen, B. and Lu, L. (2020). Glycans function as a Golgi export signal to promote the constitutive exocytic trafficking. J. Biol. Chem. 295, 14750-14762. 10.1074/jbc.RA120.014476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, J. Z. A. and Gleeson, P. A. (2019). Cargo sorting at the trans-Golgi network for shunting into specific transport routes: role of arf small G proteins and adaptor complexes. Cells 8, 531. 10.3390/cells8060531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G. (2002). Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol Cell Biol. 3, 753-766. 10.1038/nrm934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze, S. A. (1998). Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1404, 231-244. 10.1016/S0167-4889(98)00059-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, L., Tai, W. C., Chen, L. and Banfield, D. K. (2008). Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science 321, 404-407. 10.1126/science.1159411 [DOI] [PubMed] [Google Scholar]
- Tu, L., Chen, L. and Banfield, D. K. (2012). A conserved N-terminal arginine-motif in GOLPH3-family proteins mediates binding to coatomer. Traffic 13, 1496-1507. 10.1111/j.1600-0854.2012.01403.x [DOI] [PubMed] [Google Scholar]
- Tüysüz, B., Bilguvar, K., Koçer, N., Yalçınkaya, C., Çağlayan, O., Gül, E., Şahin, S., Çomu, S. and Günel, M. (2014). Autosomal recessive spastic tetraplegia caused by AP4M1 and AP4B1 gene mutation: expansion of the facial and neuroimaging features. Am. J. Med. Genet. A 164, 1677-1685. 10.1002/ajmg.a.36514 [DOI] [PubMed] [Google Scholar]
- Uemura, T. and Waguri, S. (2020). Emerging roles of Golgi/endosome-localizing monomeric clathrin adaptors GGAs. Anat. Sci. Int. 95, 12-21. 10.1007/s12565-019-00505-2 [DOI] [PubMed] [Google Scholar]
- Urban, J., Parczyk, K., Leutz, A., Kayne, M. and Kondor-Koch, C. (1987). Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J. Cell Biol. 105, 2735-2743. 10.1083/jcb.105.6.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin, O., Kraut, J. A. and Sachs, G. (2009). Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am. J. Physiol. Renal Physiol. 296, F459-F469. 10.1152/ajprenal.90340.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Hof, W. and van Meer, G. (1990). Generation of lipid polarity in intestinal epithelial (Caco-2) cells: sphingolipid synthesis in the Golgi complex and sorting before vesicular traffic to the plasma membrane. J. Cell Biol. 111, 977-986. 10.1083/jcb.111.3.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bijl, P., Lopes-Cardozo, M. and van Meer, G. (1996). Sorting of newly synthesized galactosphingolipids to the two surface domains of epithelial cells. J. Cell Biol. 132, 813-821. 10.1083/jcb.132.5.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen, I. and van Meer, G. (1995). Differential targeting of glucosylceramide and galactosylceramide analogues after synthesis but not during transcytosis in Madin-Darby canine kidney cells. J. Cell Biol. 131, 645-654. 10.1083/jcb.131.3.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn, S. C. D. and Hoekstra, D. (1998). (Glyco)sphingolipids are sorted in sub-apical compartments in HepG2 cells: a role for non-Golgi-related intracellular sites in the polarized distribution of (glyco)sphingolipids. J. Cell Biol. 142, 683-696. 10.1083/jcb.142.3.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn, S. C. D., Zegers, M. M. P., Kok, J. W. and Hoekstra, D. (1997). Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J. Cell Biol. 137, 347-357. 10.1083/jcb.137.2.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn, S. C. D., Agnetti, J. and Gassama-Diagne, A. (2020). Mechanisms behind the polarized distribution of lipids in epithelial cells. Biochim. Biophys. Acta (BBA) Biomembr. 1862, 183145. 10.1016/j.bbamem.2019.183145 [DOI] [PubMed] [Google Scholar]
- Varma, R. and Mayor, S. (1998). GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394, 798-801. 10.1038/29563 [DOI] [PubMed] [Google Scholar]
- Veatch, S. L. and Keller, S. L. (2005). Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1746, 172-185. 10.1016/j.bbamcr.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Verkerk, A. J. M. H., Schot, R., Dumee, B., Schellekens, K., Swagemakers, S., Bertoli-Avella, A. M., Lequin, M. H., Dudink, J., Govaert, P., van Zwol, A. L.et al. (2009). Mutation in the AP4M1 gene provides a model for neuroaxonal injury in cerebral palsy. Am. J. Hum. Genet. 85, 40-52. 10.1016/j.ajhg.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij, A. J., Zwaal, R. F. A., Roelofsen, B., Comfurius, P., Kastelijn, D. and van Deenen, L. L. M. (1973). The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta (BBA) Biomembr. 323, 178-193. 10.1016/0005-2736(73)90143-0 [DOI] [PubMed] [Google Scholar]
- Vogel, P., Payne, B. J., Read, R., Lee, W.-S., Gelfman, C. M. and Kornfeld, S. (2009). Comparative pathology of murine mucolipidosis types II and IIIC. Vet. Pathol. 46, 313-324. 10.1354/vp.46-2-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Blume, J. and Hausser, A. (2019). Lipid-dependent coupling of secretory cargo sorting and trafficking at the trans-Golgi network. FEBS Lett. 593, 2412-2427. 10.1002/1873-3468.13552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Blume, J., Alleaume, A.-M., Cantero-Recasens, G., Curwin, A., Carreras-Sureda, A., Zimmermann, T., van Galen, J., Wakana, Y., Valverde, M. A. and Malhotra, V. (2011). ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell 20, 652-662. 10.1016/j.devcel.2011.03.014 [DOI] [PubMed] [Google Scholar]
- von Blume, J., Alleaume, A.-M., Kienzle, C., Carreras-Sureda, A., Valverde, M. and Malhotra, V. (2012). Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol. 199, 1057-1066. 10.1083/jcb.201207180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana, Y. and Campelo, F. (2021). The PKD-dependent biogenesis of TGN-to-plasma membrane transport carriers. Cells 10, 1618. 10.3390/cells10071618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. J., Wang, J., Sun, H. Q., Martinez, M., Sun, Y. X., Macia, E., Kirchhausen, T., Albanesi, J. P., Roth, M. G. and Yin, H. L. (2003). Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299-310. 10.1016/S0092-8674(03)00603-2 [DOI] [PubMed] [Google Scholar]
- Weisz, O. A. and Fölsch, H. (2020). Molecular mechanisms of apical and basolateral sorting in polarized epithelial cells. In Basic Epithelial Ion Transport Principles and Function: Ion Channels and Transporters of Epithelia in Health and Disease - Vol. 1 (ed. Hamilton K. L. and Devor D. C.), pp. 135-158. Cham: Springer International Publishing. [Google Scholar]
- Welch, L. G., Peak-Chew, S.-Y., Begum, F., Stevens, T. J. and Munro, S. (2021). GOLPH3 and GOLPH3L are broad-spectrum COPI adaptors for sorting into intra-Golgi transport vesicles. J. Cell Biol. 220, e202106115. 10.1083/jcb.202106115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, K. M. and Smith, C. J. (2021). Clathrin: the molecular shape shifter. Biochem. J. 478, 3099-3123. 10.1042/BCJ20200740 [DOI] [PubMed] [Google Scholar]
- Wood, C. S., Schmitz, K. R., Bessman, N. J., Setty, T. G., Ferguson, K. M. and Burd, C. G. (2009). PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J. Cell Biol. 187, 967-975. 10.1083/jcb.200909063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, C. S., Hung, C.-S., Huoh, Y.-S., Mousley, C. J., Stefan, C. J., Bankaitis, V., Ferguson, K. M. and Burd, C. G. (2012). Local control of phosphatidylinositol 4-phosphate signaling in the Golgi apparatus by Vps74 and Sac1 phosphoinositide phosphatase. Mol. Biol. Cell 23, 2527-2536. 10.1091/mbc.e12-01-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, M., Peterman, M. C., Davis, R. L., Oegema, K., Shiau, A. K. and Field, S. J. (2016). GOLPH3 drives cell migration by promoting Golgi reorientation and directional trafficking to the leading edge. Mol. Biol. Cell 27, 3828-3840. 10.1091/mbc.E16-01-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, S. H. (1995). pH- and Ca2+-induced conformational change and aggregation of chromogranin B. Comparison with chromogranin A and implication in secretory vesicle biogenesis. J. Biol. Chem. 270, 12578-12583. 10.1074/jbc.270.21.12578 [DOI] [PubMed] [Google Scholar]
- Yurtsever, D. and Lorent, J. H. (2020). Structural modifications controlling membrane raft partitioning and curvature in human and viral proteins. J. Phys. Chem. B 124, 7574-7585. 10.1021/acs.jpcb.0c03435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., Drake, M. T. and Kornfeld, S. (2001). Adaptor protein 1-dependent clathrin coat assembly on synthetic liposomes and Golgi membranes. Methods Enzymol. 329, 379-387. 10.1016/S0076-6879(01)29099-5 [DOI] [PubMed] [Google Scholar]