Abstract
Plasmodium falciparum Malaria and Epstein-Barr Virus (EBV) infection are risk factors in the development of Burkitt’s lymphoma. In Indonesia, 100% of the population is persistently infected with EBV early in life and at risk of developing EBV-linked cancers. Currently, 10.7 million people in Indonesia are living in Malaria-endemic areas. This cross-sectional study was initiated to investigate how acute Malaria dysregulates immune control over latent EBV infection. Using blood and plasma samples of 68 patients with acute Malaria and 27 healthy controls, we measured the level of parasitemia for each plasmodium type (P. falciparum, P. vivax, and mixed) by microscopy and rapid test. The level of 4 regulatory cytokines was determined by quantitative ELISA and the level of circulating EBV genome by real-time PCR targeting the single copy EBNA-1 sequence. All Plasmodium-infected cases had high-level parasitemia (>1000 parasites/ul blood) except for one case. EBV-DNA levels were significantly more elevated in P. falciparum and P. vivax infections (P<0.05) compared to controls. EBV-DNA levels were not related to age, gender, Malaria symptoms, or plasmodium type. TNF-α and IL-10 levels were increased in Malaria cases versus controls, but IFN-γ and TGF- β levels were comparable between the groups. Only TNF-α levels in P. falciparum cases showed a clear correlation with elevated EBV DNA levels (R2 = 0.8915). This is the first study addressing the relation between EBV (re)activation and cytokine responses during acute Malaria, revealing a clear correlation between pro-inflammatory cytokine TNF-α and EBV-DNA levels, specifically in P. falciparum cases, suggesting this cytokine to be key in dysregulating EBV homeostasis during acute P. falciparum Malaria.
Introduction
Epstein-Barr Virus (EBV) is one of the most common viruses infecting mankind and persists for life in its host after the first contact. EBV primarily infects and reproduces in B-lymphocytes and epithelial cells located in the oro-nasal cavity and surrounding lymphoid tissues and circulates in a latent form in quiescent memory B-cells [1–3]. A small number of latency associated EBV gene products is essential for EBV genome maintenance and survival of infected cells, which have an inherent capacity to transform infected cells into proliferating malignant cells [1, 4]. Usually, EBV infection is under tight immune control and does not cause health problems but in certain populations and under defined conditions (cellular stress or co-factors) EBV can cause serious diseases that vary from self-limiting acute infectious mononucleosis (kissing disease) to chronic severe EBV infection, lymphoid and epithelial malignancies as well as autoimmune diseases [2, 5–7]. It is well known that disturbances in the immune response may dysregulate EBV homeostasis with chronic and potential oncogenic consequences [6–8]. EBV was first identified in cells of Burkitt Lymphoma (BL), an endemic cancer among sub-Saharan children, that is triggered by co-infection with Malaria parasites [reviewed in 8, 9]. BL is the most common cancer in children living in Malaria endemic regions in sub-Sahara Africa and Papua New Guinea [9, 10], but is also observed in children and adults with uncontrolled HIV infection [9]. During acute Malaria EBV infected memory B-cells can interact with Malaria parasites, particularly with the CIDR1α domain of P. falciparum Erythrocyte Membrane Protein 1 (PfEMP1), causing unregulated activation of EBV+ B-cells, lymphoproliferations and potentially leading to BL [11–13]. Children with acute Malaria have elevated EBV-DNA levels in the circulation which may return to normal upon resolving the Malaria infection [14].
In Indonesia, with around 270 million population that is 100% positive for EBV, about 10,7 million people are still living in Malaria endemic areas [15]. Children in Indonesia are exposed to EBV at early age with high dose of EBV via saliva (pre-chewed food). Later in life chronic exposure to EBV carcinogens, such as formalin, tobacco additives, herbal drugs/oils, butyrate acid (dried meat) and nitrosamine (dried salty fish) are common, which can trigger aberrant and pathogenic EBV activity and malignancy [16–18].
The immune system is a highly regulated and balanced system with neutrophils, macrophages, and NK cells acting against protozoan parasites by innate and adaptive immune responses. Innate immune cells together with dendritic cells play a vital role in the induction of T- and B-cell mediated adaptive immune responses by producing different pro-inflammatory (IL-1β, IL-6, IL-8, IL-12, IL-17, IFN-γ, and TNF-α) and anti-inflammatory (TGF-β, IL-4, IL-5, IL-10, and IL-13) cytokines that cause clinical symptoms, together resulting in parasite eradication and ultimately return to immune homeostasis [13, 19, 20]. These anti-parasite responses may affect the delicate immune balance between EBV and its host [6, 7, 11, 14, 21].
The aim of this research was to investigate how acute Malaria dysregulates EBV homeostasis and what cytokines would be involved in a Malaria-endemic population in Indonesia. Previous studies in Eastern-Africa indicated that Malaria affects EBV homeostasis in children and pregnant women [14, 21, 22] showing increased EBV-DNA loads in plasma of malaria cases compared to regional matched controls. There appeared to be a direct correlation between increases in plasma EBV viral load and progression of endemic BL, associated with increasing of P. falciparum antibody titers [10, 23]. To our knowledge, no prior study has described the role of inflammatory cytokines in the interaction between EBV and individual Malaria parasites P. vivax and P. falciparum during episodes of acute Malaria.
Materials and methods
Sample collection and Malaria parasite analysis
All necessary clearances and specific approval for this study have been obtained from the Health Research Ethics Committee of Faculty of Medicine Universitas Airlangga, Surabaya (protocol No. 278/EC/KEPK/FKUA/2020) and written informed consents were taken from all the patients at the time of sample collection. Venous blood samples were collected from Malaria cases on Sumba Island in East Nusa Tenggara,—a classified high endemic region in Indonesia [15]. Malaria cases presented with a spectrum of symptoms, including fever, headaches, nausea, paleness and conjunctival pallor. Experienced health workers visited each of the suspect malaria patients at their homes in different villages and then examined the patients on site with a Rapid Diagnostic Test (RDT; see details below). When the RDT result was positive, the patient was referred to the nearest public health center in the district (such as Public Health Centre Kori and Public Health Melolo on Sumba Island) for follow-up with clinical and microscopic blood-smear examination of the malaria status by an expert parasitologist and to obtain their questionnaire and blood plasma. The plasma of malaria patients was placed in a cool box with dry ice and shipped to the Institute of Tropical Disease, University of Airlangga, Surabaya. Upon arrival, the plasma was immediately aliquoted and frozen at –80°C. When being used, plasma samples were thawed and stored on melting ice or in a refrigerator at +2°-8°C. Healthy control samples were obtained from local residents in Surabaya (East-Java) not suffering from Malaria or other acute or chronic diseases, HIV or sexually transmitted diseases. A total of 95 plasma samples from either confirmed Malaria patients with positive parasites of P. falciparum (n = 26), P. vivax (n = 28), and mixed (P. falciparum and P. vivax) (n = 14), or healthy controls (n = 27) were used in this study. Of the 68 Malaria cases, 42 cases were male and 26 were female, whereas of the controls 12 were male and 15 were female. The mean age of malaria cases was 20.2 years (range 4–78) and for the healthy controls this was 29.5 (range 20–50). All samples were aliquoted and stored at -20°C until use. The diagnosis of Malaria was confirmed by demonstrating the presence of plasmodial parasite infection in fresh blood (finger prick) using a test for Malaria antigen detection, i.c. “Rapid Diagnostic Test” (RDT) [CareStartTM Malaria Pf/PAN (HRP2/pLDH) Ag Combo RDT, lot.nr. RMRM-01071, ACCESSBIO, Somerset, NJ, USA]. All cases with a positive RDT were followed-up and confirmed in the regional health center(s) on Sumba Island by further blood examination using thick smear microscopy by expert parasitologists to confirm the parasite species and to quantify the proportion of infected red blood cells in relation to a predetermined number of white blood cells (WBC), according to WHO-2010 guidelines [24]. Briefly, a small blood drop was used for preparing thick smears on a glass slide for laboratory examination using oil-immersion microscopy. Giemsa-stained thick blood smears were visualized under the light microscope for the identification of various species of Malaria parasites. Parasites were counted for every 500 WBCs in each blood smear which is inferred from the number of WBC per μL of blood automatically calculated using blood cell counters or assumed at a fixed value of 8,000 cells/μL, according to the WHO-2010 guidelines [24]. The final number of parasites per μL of blood was calculated as the formula: [(counted parasites/500WBC) x counted or assumed WBC/μL] [25]. Parasitemia level were categorized as 4 groups, such as Group 1 with + = 1–10 parasites per 100 oil-immersion thick film fields, Group 2 with ++ = 11–100 parasites per 100 oil-immersion thick film fields, Group 3 with +++ = 1–10 parasites per single oil-immersion thick film field, Group 4 with ++++ = more than 10 parasites per single thick film field.
Plasma EBV-DNA quantification
Plasma samples were processed for molecular analyses by Real Time PCR (RT-PCR) at the Institute of Tropical Disease, Universitas Airlangga, Surabaya. Total DNA was extracted using the QIAamp DNA Mini Kit (cat. nos. 51304, Qiagen, Germany) and analysed by the Epstein-Barr Virus (EBV) RT-PCR kit using external standard ISEX calibration and UNG-dUTP contamination control (Geneproof, EBV/ISEX/100, Czech Republic). This PCR is targeting the single copy DNA sequence encoding EBNA1 ensuring exact EBV genome quantification. The ISEX-sample consisted of 50 μl sample DNA eluate spiked with 5 μl Internal Standard (IS). For each PCR run, 10 μl of ISEX-sample or 10 μl of Calibrator/Positive Control or water were added into individual PCR tubes containing 30 μl of dUTP nucleotide MasterMix with uracil-N-glycosylase (UNG) for elimination of PCR product carry-over. The final reaction mix volume was 40 μl. Amplification was done in a RotorGene thermal cycler (Qiagen). Cycling conditions for the first step included the one hold step at 37˚C for 2 min followed by one hold step at 95˚C for 10 min for UNG inactivation. The cycling conditions for second step included 45 cycles of an initial denaturation at 95˚C for 5 second followed by 45 cycles of 40 second annealing at 60˚C and 20 second at 72˚C for final extension. Data were analysed and quality controlled by Rotor-Gene ScreenClust HRM Software (Qiagen). The interpretation of positive EBV viral load could be seen in the FAM and HEX channels as valid results. The formula of Sample Concentration (copies/μl) x Elution Volume (μl) / Isolation Volume (ml) was used to calculate the virus concentration in copies/ml.
Plasma cytokine quantification
In each plasma sample, four different cytokines were quantified by capture-ELISA technique using commercial assays: Human TNF-α by RAB0476-1KT, Lot. No. 1125F0193 (Millipore, Sigma-Aldrich, Missouri-USA), human TGF-β 1 by RAB0460-1KT, Lot. No. 0127F0188, (Millipore, Sigma-Aldrich, Missouri-USA), human IL-10 by E0102Hu (Bioassay Technology Laboratory (BT-Lab), Shanghai-China) and human IFN-γ by E0105Hu (Bioassay Technology Laboratory, Shanghai-China), according to the manufacturer’s protocols.
Statistical analysis
Data collection and statistical analysis was done using Excel and Graphpad Prism version 8.0 software. The comparison between EBV genome and Malaria parasites levels in a population was done by unpaired student t-test, the correlation between gender or age and EBV-DNA load or cytokine levels was analysed for all Malaria subgroups and controls by one-way ANOVA and linear regression, respectively, and the correlation between EBV-DNA loads and individual cytokine levels was analysed by the R-square (Pearson) method.
Results
Parasitemia level and symptoms
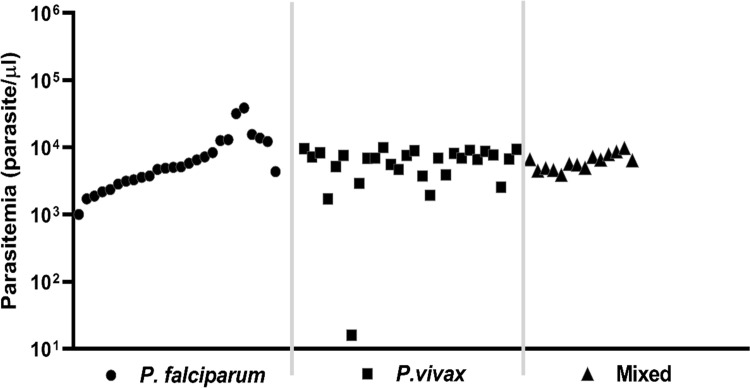
All Malaria plasma samples (n = 68) were obtained from symptomatic patients living on Sumba Island, a malaria-endemic region in Indonesia [15] and were categorized as having group-4+ high-parasitemia according to WHO-2010 criteria, with more than 10 parasites per single thick film microscopic field (>1000 parasites/ul), except for one case (Fig 1). By expert microscopic analysis of thick blood smears, samples were characterized on site for plasmodium subtype, yielding specimens with P. falciparum (n = 26), P. vivax (n = 28), and mixed (P. falciparum and P. vivax; n = 14) infections. Highest parasite levels were found in 7/26 P. falciparum cases (>10,000 parasites/μl). Symptoms of Malaria cases are detailed in S1 Fig and overall show no significant differences between the different parasite groups (P = 0.0838), except that incidence of conjunctiva pallor was less in P. falciparum (50%) compared to P. vivax or Mixed cases (both 100%) and severe headaches were more reported in P. falciparum compared to P. vivax cases (100% vs 46%). Male/female ratios were comparable between Malaria cases and controls (M/F ratio: 42/26 and 12/15, respectively; P >0.3). Healthy controls (n = 27) were collected in the city of Surabaya, East-Java and did not have a recent inflammatory disease history and showed no signs of illness at the time of sampling.
Fig 1. Parasitemia levels of Malaria cases infected with P. falciparum (n = 26), P. vivax (n = 28) and Mixed parasites (n = 14).
EBV genome by quantitative PCR (Q-PCR)
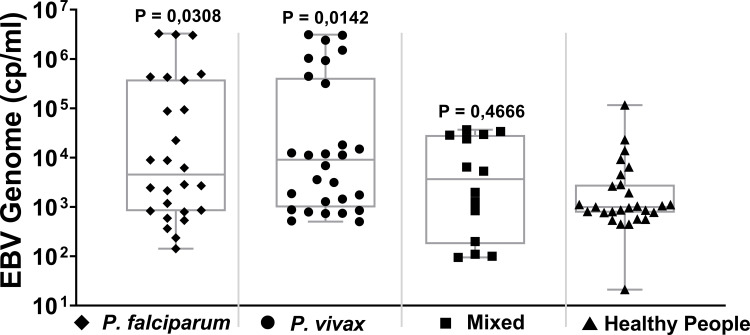
A total of 68 Malaria cases and 27 healthy controls could be analyzed for EBV-DNA genome level in blood plasma using a commercial EBNA1-targeted quantitative PCR (Fig 2). Compared to the healthy controls (mean EBV DNA level = 7,2 x103 copies/ml; SD = 2,2 x104), cases of P. falciparum (4.4 x105 copies/ml; SD = 9,9 x105) and P. vivax infection (4,6 x105 copies/ml; SD = 9,1 x105) had significantly higher mean EBV DNA levels (P = 0.0308 and 0.0142, respectively; Unpaired t-test). In Malaria patients with Mixed infection the mean EBV-DNA level was elevated compared to controls but did not reach significance (mean level = 1,2 x104 copies/ml; SD = 1,4 x104; P = 0,4666). For all patients analysed per defined malaria subgroup there was no correlation between gender or age and the level of EBV-DNA in plasma as defined by one-sided ANOVA and linear regression analysis, respectively (S2 and S3 Figs). In the healthy controls, nine individuals (33,3%) had substantially elevated EBV-DNA levels without apparent symptoms (Fig 2), but there was no relation between gender or age with EBV-DNA levels.
Fig 2. Quantitative EBV-DNA genome levels (copies/ml) in blood plasma in 3 groups of Malaria cases (P. falciparum, P. vivax and Mixed) and regional controls.
Box-limits represent the 95% confidence interval and the line represents the median level of EBV-DNA. Statistical analysis was done by one-Way ANOVA (cases versus controls).
Parasitemia versus EBV genome levels
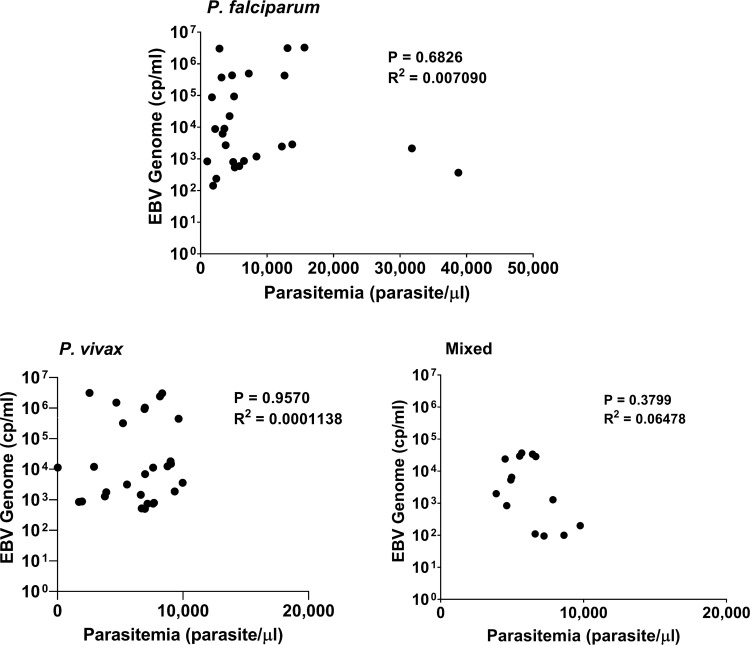
For comparison of whole blood parasitemia levels with EBV-DNA load in plasma, we grouped the Malaria cases into 3 subgroups of low (<1000/ul), intermediate (1000–10.000/ul) and high (>10.000/ul) level. No significant relation was found between parasitemia level and EBV-DNA load for the 3 subgroups of Malaria cases (P = 0,6826; P = 0,9570; P = 0,3799, respectively) (Fig 3A–3C).
Fig 3.
Comparison of Parasitemia versus EBV genome levels in Malaria cases; (a) P. falciparum, (b) P. vivax, (c) Mixed. Statistical analysis (R2) was done by linear regression.
Cytokine levels in plasma by quantitative ELISA
TNF-α
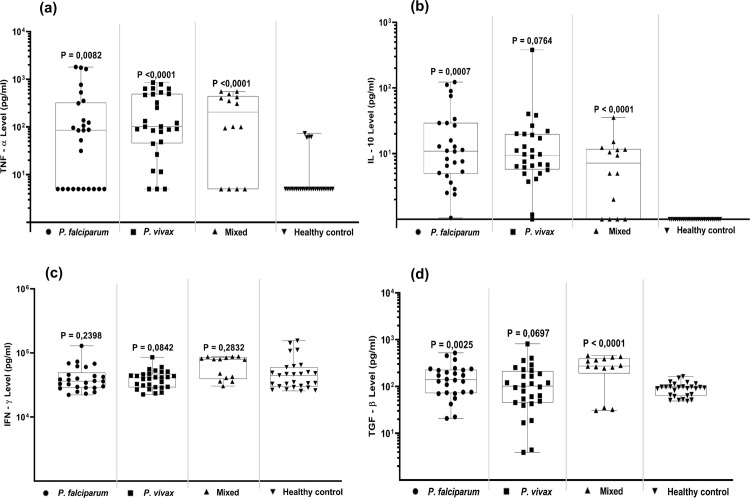
The plasma TNF-α cytokine level was significantly increased in most Malaria case in all 3 Malaria groups, with P < 0,05 overall compared to the healthy controls (P = 0,0082, P = <0,0001, P = <0,0001, respectively; Fig 4A). The increase of TNF-α levels was seen in both male and female Malaria cases, but there was no relation with age. Some healthy controls (N = 4) showed increased TNF-α levels, but this was not related to age, gender or any specific symptoms nor to elevated EBV-DNA levels (S4 Fig).
Fig 4.
Quantitative cytokine levels in Malaria cases and controls; (a) TNF-α, (b) IL-10, (c) IFN-γ and (d) TGF-β. Statistical analysis was done by One-Way ANOVA for cases versus controls.
IL-10
A low and variable but significantly increased anti-inflammatory IL-10 cytokine response was found in P. falciparum and mixed infection cases with P < 0,05 compared to the healthy controls, whereas P. vivax cases showed elevated levels as well (P = 0.0764). In the healthy controls IL-10 levels were nearly undetectable (Fig 4B).
IFN-γ
Pro-inflammatory IFN-γ cytokine levels were considerably elevated in all groups, but otherwise not significantly different between Malaria cases and healthy controls (P = 0,2398; P = 0,0842; P = 0,2832, respectively) (Fig 4C). IFN-γ levels were not related to age or gender in any of the groups.
TGF-β
Increased levels of anti-inflammatory cytokine TGF-β were detectable in all 3 Malaria groups compared to healthy controls, reaching significance in P. falciparum and mixed cases (p = 0.0025 and p <0.0001, respectively) and near significance in the P. vivax cases (p = 0.0697; Fig 4D).
All cytokine data were analysed for gender and age influences, but no significant relations were found that would influence the above data. The apparent correlation between age and cytokine levels in females with mixed Malaria infection is due to the small number of cases (N = 4) and wide spread of datapoints (S4 Fig).
EBV genome versus cytokine levels
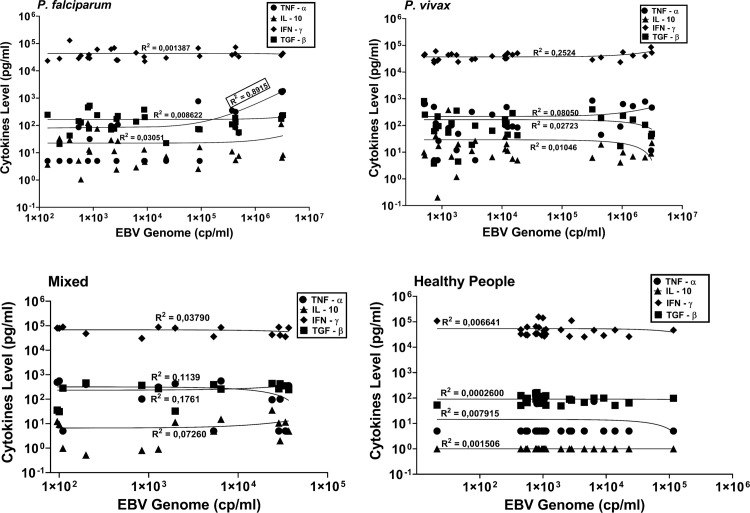
To study the potential influence of Malaria-induced altered cytokine responses on EBV homeostasis we further analysed the relation between plasma cytokine levels and EBV-DNA load in Malaria cases and controls (Fig 5A–5D). We observed a clear correlation between TNF-α levels and EBV-DNA load in Malaria cases involving P. falciparum (R2 = 0.8915), but not in the P. vivax or mixed Malaria cases (R2 = 0.08050 and R2 = 0.1137, respectively) nor in the healthy controls (R2 = 0.007915). The levels of other cytokines did not show any correlation (R2 <0.3) with EBV-DNA load in either Malaria or control samples.
Fig 5.
Comparison of circulating EBV genome levels and cytokine levels in (a) P. falciparum cases, (b) P. vivax cases, (c) Mixed cases and (d) healthy controls. Statistical analysis was done by linear regression.
Discussion
All areas of eastern Indonesia including Papua, East Nusa Tenggara, and Maluku Islands are still at high risk of Malaria, whereas subclinical Malaria may circulate in 9.4% of East-Java local people as well [10, 15, 26]. Malaria patients in this study originated from Sumba Island, part of East Nusa Tenggara, whereas controls were from Surabaya, the capital of East-Java. Malaria cases presented with characteristic symptoms and showed high-parasitemia by on-site blood-smear microscopy (Fig 1). Malaria was confirmed by a rapid Plasmodium antigen test and expert analysis identified three types of infections, namely P. falciparum, P. vivax or mixed. EBV infection in Indonesia occurs at early age, establishing a lifelong persistent infection in B lymphocytes in nearly 100% of individuals. EBV infection is generally benign but has a high risk for reactivation and contributes to more serious diseases including cancer [5, 8, 16]. Studies from sub-Sahara Africa have revealed that severe Malaria can trigger the onset of BL, which is preceded by a dysregulated immune balance and an EBV-driven oncogenic event in B-cells involving chromosomal translocation of cMYC, a key event in BL formation [8–12, 14, 21–23, 27–29]. Although BL has been described in Malaria high-incidence regions in the Indonesian archipelago, such as Papua New Guinea [10], little is known about prevalence and interactions between Malaria and EBV infection in other parts of Indonesia. Most studies on interaction between EBV and Malaria parasites have focussed on P. falciparum infections, whereas P. vivax and EBV is much less studied [27–30].
It is well known that symptomatic Malaria associates with cytokine disbalances, leading to suboptimal immunity and triggering EBV reactivation from latency [11, 12, 19–23, 27–29]. Importantly, Malaria (especially, involving infection with P. falciparum) is associated with increased risk for B-cell malignancies, even in non-endemic countries, although a role of EBV was not always investigated [8, 9, 13, 31]. However, to our knowledge no reports have described Malaria-associated cytokine responses in combination with markers of EBV reactivation, such as circulating EBV-DNA.
In this research, we noted that circulating EBV DNA levels in apparently healthy Surabaya (East-Java) controls (Mean = 7,2 x103 genome copies/ml) were higher and more variable than previously described for Indonesia, i.c. Jakarta hospital-staff (Mean = 5 x 103 DNA copies/ml) or Yogyakarta healthy blood donors (Mean = 3.5x 103 copies/ml), which all were elevated compared to Dutch healthy blood donors (< 2 x103 copies/ml) [32–34]. This is in line with previous observations in Kenya, where otherwise healthy people living in malaria-endemic region (Kisumu) had higher EBV-DNA load compared to healthy US-based controls [35]. This observation might reflect general life conditions, being more strenuous in East-Java and Sub-Sahara Africa, or relate to presence of subclinical Malaria, both potentially affecting EBV homeostasis [8, 17, 26]. Importantly, stress-induced corticoid hormones as well as DNA damaging agents/conditions are known to induce EBV lytic cycle and disturb EBV homeostasis [2, 18, 36, 37]. Interestingly, the production/consumption of nitrosamine-containing salty fish recently increased in East-Java during prolonged periods of dry season [17, 38]. Therefore, it may be not surprising that the levels of plasma EBV-DNA in the Surabaya controls are rather high, with occasional individuals experiencing clear EBV reactivation [28, 35]. However, even control subjects with highest EBV-DNA levels did not have any apparent symptoms. These elevated EBV-DNA levels in Indonesian control subjects suggest subclinical EBV reactivation may be common due to local lifestyle, environmental and physical conditions, as previously found in malaria-endemic regions in Africa [26, 35].
Little data are available on EBV-DNA loads during acute Malaria infection in adults. One study from Kenya showed increased EBV-DNA levels associated with acute Malaria in pregnant women [22], whereas most studies addressed Malaria and EBV in African young children at risk of developing BL [14, 21, 27–29]. In our study, we observed significantly elevated mean EBV-DNA loads in Malaria cases compared to regional controls (Fig 2), -despite increased EBV-DNA levels in these controls-, but no relation was found between type of parasite or level of parasitemia and the amount of circulating EBV-DNA in plasma (Fig 3). The elevated EBV-DNA levels were observed both in male and female Malaria cases but did not relate to age (S3 Fig).
Acute malaria infection is associated with a range of pro- and anti-inflammatory cytokine responses [19, 20, 39]. TNF-α and IFN-γ are pro-inflammatory cytokines that stimulate the production of Nitric Oxide (NO) by macrophages and relate to T-cell responses mediating parasite clearance, but also relate to the severity of symptoms. On the other hand, anti-inflammatory cytokines, like TGF-β and IL-10 are related to cell and tissue repair and establishing immune homeostasis [39, 40]. In the present study (Fig 4), we found that healthy controls had very low IL-10 levels, whereas Malaria cases had significant higher levels of IL-10. Low IL-10 levels associate with high stress (emotional or physiological) which may be apply to the present study population (see arguments above) [41]. Elevated IL-10 levels were observed in the Malaria cases, irrespective of subtype, which may reflect reduced T cell responses and increased B-cell activity, with IL-10 serving as endocrine growth factor [42, 43]. TGF-β is associated with repair responses and modifies B-lymphocytes into immunoglobulin-secreting cells and T lymphocytes into cytokine-producing helper cells, thus dampening the creation of cytotoxic T effector cells and natural killer cells [44]. High TGF-β levels correlate with less pathological conditions of Malaria, despite higher parasitemia level [45]. In this research, we found that Malaria cases, irrespective of the subtype, had a broad range of TGF-β responses, but the mean level did not differ significantly from the healthy controls. IFN-γ is considered to control Malaria disease in the early infection blood stage [19, 20]. Elevated levels of circulating IFN-γ are observed in depressed people and persons using anti-depressant drugs, suggesting IFN-γ plays a role in stress-related neuropathology [46]. In this study we found that healthy controls had elevated IFN-γ levels, possibly reflecting stress-health influences in Surabaya. Malaria patients had similar elevated IFN-γ levels as observed in the controls, ruling-out possible parasite-related abnormalities in the IFN-γ response. Above average of TNF-α levels are linked to high deaths rate in children [47, 48]. Disproportionate TNF-α production relates to the severity of Malaria and may serve as a prognostic factor [49]. Our study seems to confirm these findings, since we observed significantly elevated mean TNF-α levels in all Malaria cases compared to the healthy controls, irrespective of the type of Plasmodium infection, age or gender (Figs 4A and S4). Although the levels of TNF-α varied considerably between individual cases, both male and female cases showed increased mean TNF-α levels. The only clear correlation between Malaria-related inflammatory cytokine levels and EBV reactivation, as reflected by increased EBV-DNA levels, was found for TNF-α (R2 = 0.8915) in persons infected with P. falciparum, but not in P. vivax or mixed-infection cases (Fig 5). This confirms the special interaction between P. falciparum and EBV in memory B-cells, as revealed in recent molecular studies [11, 12, 21, 29, 31]. These studies have shown that the P. falciparum membrane protein PfEMP1 directly triggers EBV-carrying memory B-cells to establish a pro-inflammatory response and induces B-cell activation/maturation associating with genome-editing activities, thus creating a risk for malignant outcome. EBV-infected B-cells may produce TNF-α directly and high levels of TNF-α are associated with more severe Malaria [47–51]. However, whether EBV and TNF-α together or independently form the driving force in Malaria disease severity (in Indonesia) remains to be studied further. Thus, elevated TNF-α levels may serve as specific indicator for aberrant inflammatory responses in Malaria patients, particularly in individuals infected with P. falciparum, that have increased risk for deregulated EBV homeostasis and subsequent risk of developing EBV-related disease [12, 21, 27, 28, 52]. Such inflammation-related deregulated EBV immune balance is reflected not only by elevated EBV-DNA loads in plasma but also by aberrant anti-EBV antibody responses in the co-infected host [14, 53–55].
Conclusion
This cross-sectional study in a Malaria-endemic region in Indonesia reveals that Malaria parasite co-infection dysregulates the immune system, associating with increased EBV-DNA levels in the circulation, indicative of EBV reactivation. The elevated levels of IL-10, IFN-γ and TGF-β, being not significantly different from regional controls indicates an already pre-existing immune dysregulation due to environmental influences in this Indonesian population, which may affect the normally well-balanced EBV latent carriership. The high levels of pro-inflammatory TNF-α, correlating with increasing circulating EBV-DNA loads especially in P. falciparum infected cases, suggests that P. falciparum Malaria co-infection causes a impairment of the immune system resulting in systemic reactivation of EBV with potential pathological consequences. Further virological and cancer-registry studies are needed in this population to analyse the suggested association between P. falciparum Malaria, EBV and TNF-α in causing chronic or malignant EBV-related disease.
Supporting information
(TIF)
Box-plot shows 95% confidence interval and median levels and differences between male and female groups were defined by One-Way ANOVA.
(TIF)
Statistical analysis was done by linear regression.
(TIF)
Statistical analysis was done by linear regression.
(TIF)
Acknowledgments
We would like to thank: Heny Arwati Ph.D., Dept. Parasitology; Dr. Budi Utomo, Dept. Public Health Sciences-Preventive Medicine; Prof. Indah S. Tantular, Dept. Parasitology and Prof. Maria L. I. Lusida, Institute of Tropical Disease, at Universitas Airlangga, Indonesia for their insight and expertise.
Data Availability
All relevant data are contained within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Thorley-Lawson DA. Epstein-Barr Virus: Introducing the virus. Curr Top Microbiol Immunol.2015;390(1):151–209. doi: 10.1007/978-3-319-22822-8_8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odumade OA, Hogquist KA, Balfour HH. Progress and problems in understanding and managing primary Epstein-Barr Virus infections. Clin Microbiol Rev. 2011; 24(1):193–209. doi: 10.1128/CMR.00044-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt’s lymphoma Epstein-Barr Virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci USA. 2004; 101(1):239–44. doi: 10.1073/pnas.2237267100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middeldorp JM, Brink AATP, Van den Brule AJC, Meijer CJLM. Pathogenic roles for Epstein-Barr Virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol. 2003; 45(1):1–36. doi: 10.1016/s1040-8428(02)00078-1 . [DOI] [PubMed] [Google Scholar]
- 5.Kutok JL and Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol. 2006; 1:375–404. doi: 10.1146/annurev.pathol.1.110304.100209 . [DOI] [PubMed] [Google Scholar]
- 6.Chijioke O, Azzi T, Nadal D, Münz C. Innate immune responses against Epstein Barr virus infection. J Leukoc Biol 2013; 94(6):1185–1190. doi: 10.1189/jlb.0313173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein- Barr Virus-induced disease. Annu Rev Immunol. 2015; 33:787–821. doi: 10.1146/annurev-immunol-032414-112326 . [DOI] [PubMed] [Google Scholar]
- 8.Thorley-Lawson DA, Allday MJ. The curious case of the tumour virus: 50 years of Burkitt’s lymphoma. Nat Rev Microbiol. 2008; 6(12):913–24 doi: 10.1038/nrmicro2015 . [DOI] [PubMed] [Google Scholar]
- 9.Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, et al. Burkitt’s lymphoma. Lancet. 2012; 379(9822):1234–44. doi: 10.1016/S0140-6736(11)61177-X . [DOI] [PubMed] [Google Scholar]
- 10.Lavu E, Morewaya J, Maraka R, Kiromat M, Ripa P, Vince J. Burkitt lymphoma in Papua New Guinea—40 years on. Ann Trop Paediatr. 2005; 25(3):191–7 doi: 10.1179/146532805X58120 . [DOI] [PubMed] [Google Scholar]
- 11.Chêne A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI, et al. A molecular link between Malaria and Epstein-Barr Virus reactivation. PLoS Pathog. 2007; 3(6):e80. doi: 10.1371/journal.ppat.0030080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorley-Lawson D, Deitsch KW, Duca KA, Torgbor C. The Link between Plasmodium falciparum Malaria and Endemic Burkitt’s Lymphoma—New Insight into a 50-Year-Old Enigma. PLoS Pathog. 2016; 12(1):e1005331. doi: 10.1371/journal.ppat.1005331 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Tong H, Brindley PJ, Meyer CG, Velavan TP. Parasite Infection, Carcinogenesis and Human Malignancy. EBioMedicine. 2017; 15:12–23. doi: 10.1016/j.ebiom.2016.11.034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piriou E, Asito AS, Sumba PO, Fiore N, Middeldorp JM, Moormann AM, et al. Early age at time of primary Epstein-Barr Virus infection results in poorly controlled viral infection in infants from western Kenya: Clues to the etiology of endemic Burkitt lymphoma. J Infect Dis. 2012; 205(6):906–13. doi: 10.1093/infdis/jir872 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitohang V, Sariwati E, Fajariyani SB, Hwang D, Kurnia B, Hapsari RK, et al. Malaria elimination in Indonesia: halfway there. Lancet Glob Health. 2018; 6(6):e604–e606. doi: 10.1016/S2214-109X(18)30198-0 . [DOI] [PubMed] [Google Scholar]
- 16.Adham M, Kurniawan AN, Muhtadi AI, Roezin A, Hermani B, Gondhowiardjo S, et al. Nasopharyngeal carcinoma in Indonesia: Epidemiology, incidence, signs, and symptoms at presentation. Chin J Cancer. 2012; 31(4):185–9. doi: 10.5732/cjc.011.10328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasim M, Tan’im T, Pebriyani U, & Aprillya E. Konsumsi Ikan Asin dan Daging Asap dengan Kejadian Karsinoma Nasofaring. 2020. Jurnal Ilmiah Kesehatan Sandi Husada, 9(1), 62–71. doi: 10.35816/jiskh.v11i1.220 [DOI] [Google Scholar]
- 18.Aguayo F, Boccardo E, Corvalán A, Calaf GM, Blanco R. Interplay between Epstein-Barr virus infection and environmental xenobiotic exposure in cancer. Infect Agent Cancer. 2021; 16(1):50. doi: 10.1186/s13027-021-00391-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moormann AM, Nixon CE, Forconi CS. Immune effector mechanisms in malaria: An update focusing on human immunity. Parasite Immunol. 2019; 41(8):e12628. doi: 10.1111/pim.12628 . [DOI] [PubMed] [Google Scholar]
- 20.Muñoz-Carrillo JL, Contreras-Cordero JF, Gutiérrez-Coronado O, Villalobos-Gutiérrez PT, Ramos-Gracia LG, Hernández-Reyes VE. Cytokine Profiling Plays a Crucial Role in Activating Immune System to Clear Infectious Pathogens. In Book: Immune Response Activation and Immunomodulation. 2019. doi: 10.5772/intechopen.80843 [DOI] [Google Scholar]
- 21.Wilmore JR, Asito AS, Wei C, Piriou E, Sumba PO, Sanz I, et al. AID expression in peripheral blood of children living in a Malaria holoendemic region is associated with changes in B cell subsets and Epstein-Barr Virus. Int J Cancer. 2015; 136(6):1371–80 doi: 10.1002/ijc.29127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daud II, Ogolla S, Amolo AS, Namuyenga E, Simbiri K, Bukusi EA, et al. Plasmodium falciparum Infection is Associated with Epstein–Barr Virus Reactivation in Pregnant Women Living in Malaria Holoendemic Area of Western Kenya. Matern Child Health J. 2015; 19(3):606–14. doi: 10.1007/s10995-014-1546-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham BS, Lynch DT. Cancer, Burkitt Lymphoma. StatPearls Treasure Island (FL): StatPearls; 2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538148/. [Google Scholar]
- 24.World Health Organization. Basic Malaria microscopy-training manual. 2010. World Health Organization. [Google Scholar]
- 25.Alves-Junior ER, Gomes LT, Ribatski-Silva D, Mendes CRJ, Leal-Santos FA, Simões LR, et al. Assumed white blood cell count of 8,000 cells/ml overestimates Malaria parasite density in the Brazilian amazon. PLoS One. 2014; 9(4):e94193. doi: 10.1371/journal.pone.0094193 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arwati H, Yotopranoto S, Rohmah EA, Siafruddin D. Submicroscopic malaria cases play role in local transmission in Trenggalek district, East Java Province, Indonesia. Malaria J 2018;17(2):1–6. doi: 10.1186/s12936-017-2147-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynaldi A, Schlub TE, Chelimo K, Sumba PO, Piriou E, Ogolla S, et al. Impact of Plasmodium falciparum Coinfection on Longitudinal Epstein-Barr Virus Kinetics in Kenyan Children. J Infect Dis. 2016; 213(6):985–91. doi: 10.1093/infdis/jiv525 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moormann AM, Bailey JA. Malaria: how this parasitic infection aids and abets EBV-associated Burkitt lymphomagenesis. Curr Opin Virol. 2016; 20:78–84. doi: 10.1016/j.coviro.2016.09.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana M del P, Smith-Togobo C, Moormann A, Hviid L. Endemic Burkitt lymphoma–an aggressive childhood cancer linked to Plasmodium falciparum exposure, but not to exposure to other Malaria parasites. APMIS. 2020; 128(2):129–135. doi: 10.1111/apm.13018 . [DOI] [PubMed] [Google Scholar]
- 30.Antonelli LR, Junqueira C, Vinetz JM, Golenbock DT, Ferreira MU, Gazzinelli RT. The immunology of Plasmodium vivax Malaria. Immunol Rev. 2020; 293(1):163–189. doi: 10.1111/imr.12816 . [DOI] [PubMed] [Google Scholar]
- 31.Wyss K, Granath F, Wångdahl A, Djärv T, Fored M, Naucler P, et al. Malaria and risk of lymphoid neoplasms and other cancer: a nationwide population-based cohort study. BMC Med. 2020; 18(1):296. doi: 10.1186/s12916-020-01759-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adham M, Greijer AE, Verkuijlen SAWM, Juwana H, Fleig S, Rachmadi L, et al. Epstein-Barr Virus DNA load in nasopharyngeal brushings and whole blood in nasopharyngeal carcinoma patients before and after treatment. Clin Cancer Res. 2013; 19(8):2175–86. doi: 10.1158/1078-0432.CCR-12-2897 . [DOI] [PubMed] [Google Scholar]
- 33.Stevens SJC, Pronk I, Middeldorp JM. Toward standardization of Epstein-Barr Virus DNA load monitoring: Unfractionated whole blood as preferred clinical specimen. J Clin Microbiol. 2001; 39(4):1211–6. doi: 10.1128/JCM.39.4.1211-1216.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens SJC, Verkuijlen SAWM, Hariwiyanto B, Harijadi Fachiroh J, Paramita DK, et al. Diagnostic value of measuring Epstein-Barr Virus (EBV) DNA load and carcinoma-specific viral mRNA in relation to anti-EBV immunoglobulin A (IgA) and IgG antibody levels in blood of nasopharyngeal carcinoma patients from Indonesia. J Clin Microbiol. 2005; 43(7):3066–73. doi: 10.1128/JCM.43.7.3066-3073.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moormann AM, Chelimo K, Sumba OP, Lutzke ML, Ploutz-Snyder R, Newton D, et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005; 191(8):1233–8. doi: 10.1086/428910 . [DOI] [PubMed] [Google Scholar]
- 36.Inoue Y, Yazawa A, Li D, Du J, Jin Y, Chen Y, et al. Epstein-Barr Virus antibody titer and its association with the domain scores from the World Health Organization’s Quality of Life questionnaire: Findings from Rural Hainan Province, China. Am J Hum Biol. 2014. doi: 10.1002/ajhb.22478 24327424. [DOI] [PubMed] [Google Scholar]
- 37.Godbout JP, and Glaser R. Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006. doi: 10.1007/s11481-006-9036-0 18040814. [DOI] [PubMed] [Google Scholar]
- 38.Newswire—Bisnis.com. 2018. Access internet 2021. Available from: https://surabaya.bisnis.com/read/20180517/531/796232/produksi-ikan-asin-probolinggo-meningkat-drastis.
- 39.Yui K, Inoue SI. Host-pathogen interaction in the tissue environment during Plasmodium blood-stage infection. Parasite Immunol. 2021;43(2):e12763. doi: 10.1111/pim.12763 . [DOI] [PubMed] [Google Scholar]
- 40.Dobbs KR, Crabtree JN, Dent AE. Innate immunity to malaria-The role of monocytes. Immunol Rev. 2020; 293(1):8–24. doi: 10.1111/imr.12830 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. 2013; 8(3):e58488. doi: 10.1371/journal.pone.0058488 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugosson E, Montgomery SM, Premji Z, Troye-Blomberg M, Björkman A. Higher IL-10 levels are associated with less effective clearance of Plasmodium falciparum parasites. Parasite Immunol. 2004; 26(3):111–7. doi: 10.1111/j.0141-9838.2004.00678.x . [DOI] [PubMed] [Google Scholar]
- 43.Kumar R, Ng S, Engwerda C. The role of IL-10 in Malaria: A double edged sword. Front Immunol. 2019; 10:229. doi: 10.3389/fimmu.2019.00229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenisch C, Parschalk B, Burgmann H, Looareesuwan S, Graninger W. Decreased serum levels of TGF-β in patients with acute Plasmodium falciparum Malaria. J Clin Immunol. 1995; 15(2):69–73. doi: 10.1007/BF01541734 . [DOI] [PubMed] [Google Scholar]
- 45.Drewry LL, Harty JT. Balancing in a black box: Potential immunomodulatory roles for TGF-β signaling during blood-stage Malaria. Virulence. 2020; 11(1):159–169. doi: 10.1080/21505594.2020.1726569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litteljohn D, Nelson E, Hayley S. IFN-γ differentially modulates memory-related processes under basal and chronic stressor conditions. Front Cell Neurosci. 2014; 8:391. doi: 10.3389/fncel.2014.00391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leão L, Puty B, Dolabela MF, Povoa MM, Né YGDS, Eiró LG, et al. Association of cerebral Malaria and TNF-α levels: A systematic review. BMC Infect Dis. 2020; 20(1):442. doi: 10.1186/s12879-020-05107-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perera MK, Herath NP, Pathirana SL, Phone-Kyaw M, Alles HK, Mendis KN, et al. Association of high plasma TNF-alpha levels and TNF-alpha/IL-10 ratios with TNF2 allele in severe P. falciparum malaria patients in Sri Lanka. Pathog Glob Health. 2013; 107(1):21–9. doi: 10.1179/2047773212Y.0000000069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinra P, Dutta V. Serum TNF alpha levels: A prognostic marker for assessment of severity of Malaria. Trop Biomed 2013. 30(4):645–653. . [PubMed] [Google Scholar]
- 50.Dang VD, Hilgenberg E, Ries S, Shen P, Fillatreau S. From the regulatory functions of B cells to the identification of cytokine-producing plasma cell subsets. Curr Opin Immunol. 2014; 28:77–83. doi: 10.1016/j.coi.2014.02.009 . [DOI] [PubMed] [Google Scholar]
- 51.Klein SC, Kube D, Abts H, Diehl V, Tesch H. Promotion of IL8, IL10, TNF-alpha and TNF-beta production by EBV infection. Leuk Res. 1996; 20(8):633–6. doi: 10.1016/0145-2126(96)00029-x . [DOI] [PubMed] [Google Scholar]
- 52.Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA. A Multifactorial Role for P. falciparum Malaria in Endemic Burkitt’s Lymphoma Pathogenesis. PLoS Pathog. 2014; 10(5):e1004170. doi: 10.1371/journal.ppat.1004170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orem J, Sandin S, Mbidde E, Mangen FW, Middeldorp J, Weiderpass E. Epstein-Barr Virus viral load and serology in childhood non-Hodgkin’s lymphoma and chronic inflammatory conditions in Uganda: implications for disease risk and characteristics. J Med Virol. 2014; 86(10):1796–803. doi: 10.1002/jmv.23988 . [DOI] [PubMed] [Google Scholar]
- 54.Sd Sanjosé, Ramon B, Tabitha S, Sandra V, Alexandra N, Lenka F, et al. Epstein-Barr virus infection and risk of lymphoma: immunoblot analysis of antibody responses against EBV-related proteins in a large series of lymphoma subjects and matched controls. Int J Cancer. 2007; 121(8):1806–12. doi: 10.1002/ijc.22857 . [DOI] [PubMed] [Google Scholar]
- 55.Patel SS, Sweta S, Chinmoy S, Ujjala G, and Hemant V. A three year Seroepidemiological and molecular study of Epstein -Barr virus infection among different age groups with hematological malignancies in a Tertiary care centre of North India (2017–2019). J Family Med Prim Care. 2021; 10(1):373–377. doi: 10.4103/jfmpc.jfmpc_1594_20 . [DOI] [PMC free article] [PubMed] [Google Scholar]