Abstract
Background
Tuberculosis (TB) and COVID-19 pandemics are both diseases of public health threat globally. Both diseases are caused by pathogens that infect mainly the respiratory system, and are involved in airborne transmission; they also share some clinical signs and symptoms. We, therefore, took advantage of collected sputum samples at the early stage of COVID-19 outbreak in Ghana to conduct differential diagnoses of long-standing endemic respiratory illness, particularly tuberculosis.
Methodology
Sputum samples collected through the enhanced national surveys from suspected COVID-19 patients and contact tracing cases were analyzed for TB. The sputum samples were processed using Cepheid’s GeneXpert MTB/RIF assay in pools of 4 samples to determine the presence of Mycobacterium tuberculosis complex. Positive pools were then decoupled and analyzed individually. Details of positive TB samples were forwarded to the NTP for appropriate case management.
Results
Seven-hundred and seventy-four sputum samples were analyzed for Mycobacterium tuberculosis in both suspected COVID-19 cases (679/774, 87.7%) and their contacts (95/774, 12.3%). A total of 111 (14.3%) were diagnosed with SARS CoV-2 infection and six (0.8%) out of the 774 individuals tested positive for pulmonary tuberculosis: five (83.3%) males and one female (16.7%). Drug susceptibility analysis identified 1 (16.7%) rifampicin-resistant tuberculosis case. Out of the six TB positive cases, 2 (33.3%) tested positive for COVID-19 indicating a coinfection. Stratifying by demography, three out of the six (50%) were from the Ayawaso West District. All positive cases received appropriate treatment at the respective sub-district according to the national guidelines.
Conclusion
Our findings highlight the need for differential diagnosis among COVID-19 suspected cases and regular active TB surveillance in TB endemic settings.
Introduction
The novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory coronavirus 2 (SARS CoV-2) was first reported in the Wuhan suburb of Hubei province in China in early December 2019 and has since spread to all regions of the world. It was officially declared a pandemic by the World Health Organization (WHO) in March 2020 [1, 2]. Headache, fever, cough, muscle aches, tight chest or wheezing, and dyspnoea are symptoms presented by coronavirus [3]. However, these symptoms are shared by other respiratory tract infections (RTIs) including tuberculosis, pneumonia, bronchiolitis, acute bronchitis.
Tuberculosis (TB), caused by the Mycobacterium tuberculosis complex (MTBC) is a disease of antiquity but still remains a global health emergency [4, 5]. In 2019, an estimated 10 million people contracted TB with 1.3 million deaths accounting for the highest number of deaths caused by a single infectious disease in that year [5]. Ghana, with a TB incidence rate of 148/100,000 population per year, is ranked the 19th most TB-burdened country in Africa by WHO and among the 48 most burdened TB countries in the world [6].
Undiagnosed/misdiagnosed TB is one of the major problems facing the control of TB in Ghana. Available data indicate only a third of the TB cases are reported for appropriate care. Such late/low case reports increase community transmission [5–7]. It has been portended low case detection would be exacerbated by the COVID-19 pandemic through the diversion of resources, a shift of leadership focus, stress on healthcare personnel, quarantine of healthcare personnel, and potential stigma/fear of COVID-19 [8]. There is therefore the need to find ways to maximize diagnosis of TB amidst the COVID-19 pandemic.
Ghana’s first two COVID-19 cases were diagnosed on the 12th of March 2020 from individuals who had just arrived in the country by the Virology Department of the Noguchi Memorial Institute for Medical Research, (NMIMR), University of Ghana (UG) [9]. The NMIMR became the national coordinating center for COVID-19 diagnosis, using either sputum or oropharyngeal swab. Thus, we took advantage of sputum samples being transported to NMIMR by the enhanced active case finding employed by the national response team to offer a very good avenue for early case detection for TB and other respiratory infections which might be confused with COVID-19.
Methods
Sputum collection
Samples used for this study formed part of the enhanced national COVID-19 surveillance conducted by the Ghana health service. Sputum collected from suspected COVID-19 patients were transported to NMIMR within 5 hours of collection for COVID-19 testing. Only good quality mucopurulent sputa from patients with clinical manifestations such as cough and fever were enrolled in this study. The sputum samples were collected following national and WHO recommended guidelines to prevent the spread of COVID-19. Briefly, field staff were provided with appropriate PPE ie, lab coat, nose mask, face mask, gloves and were supplied with hand sanitizers and advised to practice frequent hand washing after each sampling while ensuring social distancing.
MTBC detection
Sputum samples were processed using Cepheid’s GeneXpert MTB/Rif Assay [10]. This is an RT-PCR-based assay used to detect the presence of the MTBC and resistance to rifampicin in sputum samples. Samples were analyzed in pools of four and following Cepheid’s GeneXpert MTB/Rif assay manufacturer’s protocol. A negative test (MTB NOT DETECTED) meant all samples in that pool were negative for tuberculosis. A positive test (MTB DETECTED) was however decoupled and the four samples in the pool ran individually to identify the specific sample(s) positive for tuberculosis. Results were communicated to the NTP for patient follow-up on the and commencement of anti-TB drug therapy.
Culture of MTBC positive samples and characterization
GeneXpert positive sputum samples were decontaminated by the 5% oxalate method and inoculated on two pairs of Lowenstein-Jensen media slants supplemented with glycerol and pyruvate, respectively [11]. The inoculated tubes were incubated at 37°C and observed weekly for growth for a period of 12 weeks. Isolates were confirmed as members of the MTBC by PCR targeting the insertion sequence IS6110 [12].
Data analysis
Chi-square test, Fischer’s exact test, or logistic regression where appropriate was used to test for statistical significance. P = values <0.05 were regarded as statistically significant at a 95% confidence level. All statistical analyses were carried out in Stata v 14.2. The GIS co-ordinates of the residential location of the cases were used to construct a graphical plot for visualization of the spread of the cases in the Greater Accra Region of Ghana using ArcMap employed in ArcGIS v10.1 [13].
Ethical consideration
Efforts to reduce the incidence of tuberculosis to the barest minimum by 2030 may not be achieved by the upsurge of COVID-19 cases which has slowed down some of the TB control activities. To ensure early case detection of TB for efficient management and help curb the continuous spread of the pathogen, the National Tuberculosis control Program (NTP) of the Ghana Health Service together with the national coordinator of COVID-19 laboratories as a matter of emergency requested the Bacteriology department of the Noguchi Memorial Institute for Medical Research to undertake this study. Participants’ data were anonymized. All diagnosed cases were located and put on therapy per national guidelines.
Results
General characteristics of participants
We obtained and analyzed 774 samples from both suspected COVID-19 cases (679/774, 87.7%) and their contacts (95/774, 12.3%). Of the participants with data on gender (N = 753), 400/753 (53.1%) were males and 353/753 (46.9%) were females. All the participants came from 30 districts within 6 regions in Southern Ghana (Table 1) with the majority coming from Ayawaso (204/714, 28.6%) and Adenta Municipal (115/714, 16.1%). Sixty participants had no data on location. Nationality data was available for 765 participants of which 752 (98.3%) were Ghanaians with the remaining 13 (1.7%) being foreigners from Nigeria (n = 5), UK (n = 5), China (n = 1), France (n = 1) and Norway (n = 1).
Table 1. Geographical location of study participants.
| Number | Region | District | Frequency N (%) |
|---|---|---|---|
| 1 | Greater Accra | Ayawaso | 204 (28.6) |
| 2 | Greater Accra | Adenta_Municipal | 115 (16.1) |
| 3 | Greater Accra | Ashaiman_Municipal | 79 (11.1) |
| 4 | Greater Accra | Tema_Metropolis | 75 (10.5) |
| 5 | Greater Accra | Ga_South_Municipal | 41 (5.7) |
| 6 | Greater Accra | Osu_Klottey | 36 (5.0) |
| 7 | Greater Accra | Ledzokuku_Krowor_Municipal (Kpeshie) | 30 (4.2) |
| 8 | Greater Accra | Ablekumah | 24 (3.4) |
| 9 | Greater Accra | Ga_East_Municipal | 16 (2.2) |
| 10 | Greater Accra | Dangme_West_District | 15 (2.1) |
| 11 | Greater Accra | Ga_Central | 10 (1.4) |
| 12 | Central | Mfantseman_Municipal_District | 10 (1.4) |
| 13 | Central | Awutu_Senya_East_District | 9 (1.3) |
| 14 | Eastern | Lower_Manya_Krobo_District | 8 (1.1) |
| 15 | Eastern | Akwatia_District | 6 (0.8) |
| 16 | Eastern | Akuapim_North_Municipal_District | 5 (0.7) |
| 17 | Central | Dangme_East_District | 4 (0.6) |
| 18 | Central | Gomoa_West_District | 4 (0.6) |
| 19 | Eastern | Suhum_Kraboa_Coaltar_district | 3 (0.4) |
| 20 | Eastern | Asuogyaman_Municipal_District | 3 (0.4) |
| 21 | Central | Cape_Coast_Municipal_District | 3 (0.4) |
| 22 | Upper West | Sissala_East_Municipal_District | 2 (0.3) |
| 23 | Western | Secondi_Takoradi_Municipal_District | 2 (0.3) |
| 24 | Volta | Ketu_South_Municipal_District | 2 (0.3) |
| 25 | Eastern | New_Juaben_North_District | 2 (0.3) |
| 26 | Eastern | Nsawam_Adoagyiri_Municipal_District | 2 (0.3) |
| 27 | Central | Komenda_Edina_Eguafo_Abirem_Municipal_D | 1 (0.1) |
| 28 | Eastern | Ayensuano_District | 1 (0.1) |
| 29 | Western | Ellembelle_District | 1 (0.1) |
| 30 | Central | Twifu_Atii_Morkwaa_District | 1 (0.1) |
SARS-CoV2 and tuberculosis infection
One hundred and eleven participants (14.3%) were confirmed by RT-PCR as having SARS-CoV-2 infection. A significant number of the COVID-19 cases were from the suspected cases (106/111, 95.5%) rather than the contact tracing group (5/111, 4.5%) p = 0.007. We observed no statistical difference (p = 0.077) between males (67/400, 16.7%) and females (43/353, 12.2%) who tested positive for SARS- CoV2. We found 6 (0.8%) out of the 774 participants to be positive for Mycobacterium tuberculosis infection from both the suspected COVID-19 cases (4/675, 0.6%) and contact tracing group (2/93, 2.1%) with no significant difference between the two groups (p = 0.161). Two (2/111, 1.8%) of the individuals positive for SARS-CoV-2 were found to be additionally positive for TB and were among the suspected COVID-19 group. The two contacts that were TB positive had no signs and symptoms of TB.
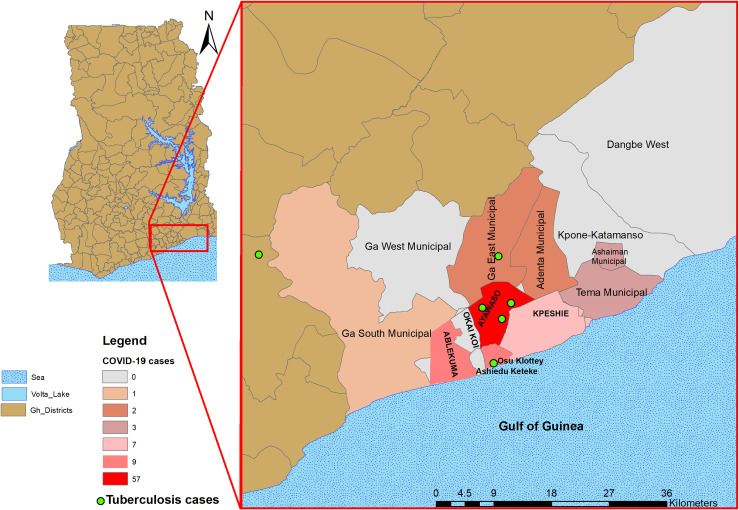
The majority of the positive COVID-19 cases (57/104, 54.8%) were from the Ayawaso district followed by the Ablekuma and Osu Klottey districts each recording 9/104 (8.6%) positive cases (Fig 1). COVID-19 cases were found to be significantly higher among those of foreign origin compared to the Ghanaian locals (11/13, 84.6% vs. 97/752, 12.9% p<0.001). Compared to the Ghanaian locals, foreigners were 37 times more likely to be positive for COVID-19 (OR = 37, CI: 8–170). All 6 TB cases identified were, however among only Ghanaian locals mainly from the Ayawaso district (3/6, 50.0%) (Fig 1).
Fig 1. Geographical density of COVID-19 and distribution of TB cases within the sampling period.
Culture results
Out of the six GeneXpert positive samples, only four (4/6, 66.6%) had adequate sputum to proceed to culture stage. A culture positivity rate of 50% (2/4) was recorded. These two isolates were confirmed part of the Mycobacterium tuberculosis complex by IS6110 PCR with one identified by spoligotyping to belong to lineage 4.
Discussion
Several studies and a recent prevalence study conducted in Ghana confirms the WHO estimates that only a third of Ghana’s TB cases are reported formally for treatment [14]. Moreover, there are claims of reactivation of several respiratory diseases of the lung after infection with COVID-19. There is even the claim that active-TB disease could develop from latent infection after infection with SARS CoV-2 as evidenced in some patients with no history of TB [15]. The NTP of Ghana together with the NMIMR conducted differential TB diagnosis using sputum samples sent to the NMIMR for COVID- 19 diagnosis to ensure early case detection of TB for efficient management.
As evidenced in this study, we identified six (0.8%) TB patients among 774 suspected COVID-19 individuals with 2 being co-infected with both the MTBC and SARS CoV-2. The quality of sputum received was generally very poor (more salivary than mucopurulent) and could account for the low number of TB coinfection detected. This was expected as the samples were not primarily taken for TB diagnosis and individuals with no cough symptoms generally produce low quality sputum.
Two of the TB positive cases in this study were found as a result of the enhanced contact tracing scheme put in place by the government of Ghana to detect COVID-19 cases early to reduce community transmission. These two samples respectively showed high and medium bacterial load when analyzed with GeneXpert MTB/RIF assay indicating that there could be a high chance of community spread of TB with such high bacterial loads detected [16]. Interestingly, these two TB positive samples from contact tracing were both from the Ayawaso sub-district which our previous studies have confirmed to be a hotspot for active TB transmission [17].
This further stresses the need to conduct frequent community screening of individuals in known hotspot communities such as Ayawaso and Ablekuma sub-districts where active TB transmission has been shown to be taking place [17]. There is therefore the need for active community case search to ensure early active TB case detection to ensure efficient management to cut any potential spread.
Our findings also indicated that differential diagnoses for COVID-19 suspected patients should be encouraged as symptoms and signs of the disease are highly synonymous with other respiratory viral and bacterial infections [18]. Although the disease can have symptoms such as gastrointestinal and neurologic complications, the most common symptoms include cough, fever, and shortness of breath which overlap with symptoms common to influenza, pneumococci, and MTBC infections [19, 20]. We were limited with the data we received from participants and so was unable to carry out analysis on other comorbidities as well as other confounding variables.
The onset of COVID-19 pandemic saw a sharp decline in the number of TB tests being requested at diagnostic facilities across Ghana, including the NMIMR due to the fear of getting infected with the virus either or the stigma of being diagnosed as COVID-19 positive. This resulted in fewer cases being recorded; in the months between March and July (5 months) when the highest coronavirus cases were recorded in Ghana. Only forty-one [41] samples were received for primary diagnosis of TB compared to ninety-four [94] samples being received in the months of January and February; 2 months period. The low numbers recorded can be attributed to the reduced attention to TB as most of the health workers were primarily focused on combating COVID-19 to the neglect of other important infectious diseases [8]. A modelling study by WHO pointed to a 25% reduction in expected TB detection for 3 months as a result of COVID-19 pandemic thus derailing the Sustainable Development Goals (SDGs) of ending the TB epidemic by 2030 and WHO’s targets of 90% reduction in TB incidence and 95% death reduction by 2030 [5].
Conclusion
The National Tuberculosis Control Programme (NTP) is adopting strategies to improve access to TB diagnosis and care with strategies such as early screening tools at OPDs, and ready access to TB treatment for home-based TB care. However, more needs to be done in the area of community education and engagement campaigns to improve early case detection and compliance to therapy to reduce negative outcomes of TB disease in Ghana. This pilot study also provided valuable information to the NTP and the health service on the need for differential diagnosis as well as strengthening surveillance of long-standing endemic infectious diseases amid the pandemic. More studies will however need to be conducted to understand better if latent TB infection progressed to active TB with COVID-19 infection or a relapse of a previous TB infection upon COVID-19 infection and the impact of co-infection on treatment outcome.
Acknowledgments
We want to express our sincere gratitude to all individuals involved in the sample collection process and personnel of the Ghana Health Service.
Data Availability
All relevant data are within the paper.
Funding Statement
Authors are part of The Pan-African Network for Rapid Research, Response, Relief and Preparedness for Infectious Diseases Epidemics (PANDORA-ID-NET) Consortium (https://www.pandora-id.net/) funded EDCTP Reg/Grant RIA2016E-1609 from the European and Developing Countries Clinical Trials Partnership (EDCTP2) programme, which is supported under Horizon 2020, the European Union’s Framework Programme for Research and Innovation. Dorothy Yeboah-Manu is a GSK-EDCTP Senior Fellow (TMA.2017.GSF.1942-TB-DM).
References
- 1.Lu H, Stratton CW, Tang Y. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92(4):401–2. doi: 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corona virus disease (COVID-19) pandemic [Internet]. WHO; 2020. Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19#:~:text=WHO%20announced%20COVID-19,on%2011%20March%202020.
- 3.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enos M, Sitienei J, Ong’ang’o J, Mungai B, Kamene M, Wambugu J, et al. Kenya tuberculosis prevalence survey 2016: Challenges and opportunities of ending TB in Kenya. PloS One. 2018;13(12):e0209098. doi: 10.1371/journal.pone.0209098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO: Global TB progress at risk [Internet]. World Health Organization; 2020 [cited 2020 Nov 23]. Available from: https://www.who.int/news/item/14-10-2020-who-global-tb-progress-at-risk#:~:text=The%20WHO%20End%20TB%20Strategy,35%25%20reduction%20in%20TB%20deaths.
- 6.Global Tuberculosis Report [Internet]. World Health Organization; 2019. Available from: https://www.who.int/teams/global-tuberculosis-programme/data
- 7.Global Tuberculosis Report. World Health Organization [Internet]. WHO; 2018 [cited 2020 Nov 23]. Available from: http://www.who.int/tb/publications/global_report/en/
- 8.Alene KA, Wangdi K, Clements AC. Impact of the COVID-19 pandemic on tuberculosis control: an overview. Trop Med Infect Dis. 2020;5(3):123. doi: 10.3390/tropicalmed5030123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghana confirms two cases of COVID-19 [Internet]. Ghana Health Service; 2020. Available from: https://ghanahealthservice.org/covid19/downloads/covid_19_first_confirmed_GH.pdf
- 10.Ahmed ST. GeneXpert MTB/RIF assay–A major milestone for diagnosing Mycobacterium tuberculosis and rifampicin-resistant cases in pulmonary and extrapulmonary specimens. Med J Babylon. 2019;16(4):297–301. [Google Scholar]
- 11.Yeboah-Manu D, Bodmer T, Mensah-Quainoo E, Owusu S, Ofori-Adjei D, Pluschke G. Evaluation of decontamination methods and growth media for primary isolation of Mycobacterium ulcerans from surgical specimens. J Clin Microbiol. 2004;42(12):5875–6. doi: 10.1128/JCM.42.12.5875-5876.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeboah-Manu D, Yates MD, Wilson SM. Application of a simple multiplex PCR to aid in routine work of the mycobacterium reference laboratory. J Clin Microbiol. 2001;39(11):4166–8. doi: 10.1128/JCM.39.11.4166-4168.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price MH. Mastering ArcGIS McGraw-Hill; New York, NY, USA; 2010. [Google Scholar]
- 14.Asare P, Asante-Poku A, Prah DA, Borrell S, Osei-Wusu S, Otchere ID, et al. Reduced transmission of Mycobacterium africanum compared to Mycobacterium tuberculosis in urban West Africa. Int J Infect Dis. 2018;73:30–42. doi: 10.1016/j.ijid.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tadolini M, Codecasa LR, García-García J-M, Blanc F-X, Borisov S, Alffenaar J-W, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020; doi: 10.1183/13993003.01398-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fradejas I, Ontañón B, Muñoz-Gallego I, Ramírez-Vela M, López-Roa P. The value of xpert MTB/RIF-generated CT values for predicting the smear status of patients with pulmonary tuberculosis. J Clin Tuberc Mycobact Dis. 2018;13:9–12. doi: 10.1016/j.jctube.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asare P, Otchere ID, Bedeley E, Brites D, Loiseau C, Baddoo NA, et al. Whole genome sequencing and spatial analysis identifies recent tuberculosis transmission hotspots in Ghana. Front Med. 2020;7:161. doi: 10.3389/fmed.2020.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Follin P, Lindqvist A, Nyström K, Lindh M. A variety of respiratory viruses found in symptomatic travellers returning from countries with ongoing spread of the new influenza A (H1N1) v virus strain. Eurosurveillance. 2009;14(24):19242. doi: 10.2807/ese.14.24.19242-en [DOI] [PubMed] [Google Scholar]
- 19.Yi-Chi W, Ching-Sung C, Yu-Jiun C. The outbreak of COVID-19. An overview. J Chi Med Asso. 2020;83(3):217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19). Atlanta: [Internet]. CDC; 2020 [cited 2020 Dec 29]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/about/symptoms.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.