Abstract
Hydrogels have been widely investigated for localized, sustained gene delivery due to the similarity of their physical properties to native extracellular matrix and their ability to be formed under mild conditions amenable to the incorporation of bioactive molecules. The objective of this study was to develop bioactive hydrogels composed of macromolecules capable of enhancing the efficiency of nonviral vectors. Hybrid hydrogels were prepared by simultaneous enzymatic and Michael-type addition crosslinking of reduced fibrinogen and an acrylated amphiphilic block copolymer, Tetronic T904, in the presence of dithiothreitol and thrombin. T904/fibrin hydrogels degraded by surface erosion in the presence of plasmin and provided sustained release of jetPEI/pGFP polyplex vectors up to an order of magnitude longer than pure fibrin gel control. In addition, the rate of gel degradation and time course of polyplex vector release were readily controlled by varying the T904/fibrinogen ratio in the gel composition. When added to transfected neuroblastoma (N2A) cells, both native T904 itself and hydrogel degradation products significantly increased polyplex transfection efficiency with minimal effect on cell viability. N2A cells encapsulated in small fibrin clusters were covered by or suspended within polyplex-loaded hydrogels. Cells progressively degraded and invaded the hybrid hydrogels, exhibiting increasing gene expression over two weeks and then diminishing but persistent gene expression for over one month. In conclusion, these results demonstrate that T904/fibrin hybrid hydrogels can be promising tissue engineering scaffolds that provide local, controlled release of nonviral vectors in combination with the generation of bioactive gel degradation products that actively enhance vector efficiency.
Keywords: nonviral, poloxamine, hydrogel, gene delivery, controlled release, biosynthetic
1. Introduction
Gene therapy offers the potential to stimulate tissue regeneration and treat disease through the overexpression or silencing of target genes. However, safe and effective vector delivery remains a critical challenge. One promising strategy has been inductive tissue engineering through localized release of non-viral vectors from polymeric scaffolds (Bonadio, 2000; Salvay and Shea, 2006; Storrie and Mooney, 2006). This approach overcomes barriers to systemic delivery such as serum aggregation and clearance by the reticuloendothelial system and avoids the risks of immunogenicity and insertional mutagenesis associated with viral vectors (Kay et al., 2001; Al-Dosari and Gao, 2009).
Hydrogels are particularly attractive scaffolds for gene delivery because of their capability for minimally invasive delivery and in situ gelation under mild conditions compatible with inclusion of living cells and bioactive molecules, as well as provision of mechanical properties consistent with a variety of soft tissues (Drury and Mooney, 2003; Lin and Anseth, 2009). Naturally-derived hydrogels intrinsically support cell adhesion and growth and can release entrapped vectors in response to cellular enzymatic activity (Dang and Leong, 2006). Although effective in a variety of in vitro and in vivo models, their limitations include relatively low mechanical properties, difficulty in controlling degradation/release rates, and in some cases inefficient vector entrapment leading to burst release (Cohen-Sacks et al., 2004; des Rieux et al., 2009; Scherer et al., 2002). Fully synthetic hydrogels provide superior control over chemical and physical properties, but lack intrinsic bioactivity and responsiveness to cellular activity. Vector release occurs through a combination of diffusion and hydrolytic degradation, requiring careful tailoring of network properties to obtain controlled release (Quick and Anseth, 2003). Recently, much attention has focused on hybrid or biosynthetic hydrogels composed of both naturally-derived and synthetic components with the goal of integrating the beneficial characteristics of each (Jia and Kiick, 2008; Kopecek, 2007). Examples investigated for gene delivery include polyethylene glycol (PEG) / hyaluronic acid interpenetrating networks and PEG crosslinked with protease-sensitive synthetic peptides derived from the sequence of natural extracellular matrix substrates (Shepard et al., 2010; Wieland et al., 2007; Lei and Segura, 2009; Lei et al., 2010, 2010). Despite significant progress, controlling hydrogel degradation rate / vector release kinetics and improving transfection efficiency remain challenges.
One potential strategy for improving the efficacy of local gene delivery is the creation of scaffolds composed of materials that possess the ability to enhance the transfection efficiency of nonviral vectors. Towards this end, the objective of this study was to develop and characterize a novel hybrid hydrogel composed of the naturally-derived protein fibrinogen (FgN) and the synthetic poloxamine Tetronic® T904 (T904) for localized, sustained delivery of nonviral vectors. Fibrin has an extensive clinical history as a tissue sealant and provides the hybrid hydrogel with the capability to support cell adhesion and cell-mediated enzymatic degradation (Spotnitz, 2010). T904 is a 4-arm, amphiphilic block copolymer composed of poly(ethylene oxide) [PEO] and poly(propylene oxide) [PPO]. Several studies have shown that PEO/PPO block copolymers, including T904, can improve transfection efficiency of polyplex vectors through increased endocytosis, nuclear entry and transcriptional activity (Astafieva et al., 1996; Prokop et al., 2002; Sriadibhatla et al., 2006; Yang et al., 2008; Zhang et al., 2013). In the present study, acrylated T904 and FgN were crosslinked in the presence of thrombin and dithiothreitol by simultaneous enzymatic and Michael-type addition reactions. The resulting hydrogels were enzymatically degradable via surface erosion, provided sustained vector release at rates controlled by the balance between naturally-derived and synthetic components, and generated degradation products capable of increasing polyplex transfection. Cells were able to invade polyplex-loaded 3D T904/FgN hydrogels, exhibiting sustained transfection for up to 4 weeks in 3D culture.
2. Materials and Methods
2.1. Polyplex Formation and Characterization
2.1.1. Plasmid amplification and purification
The Monster Green Fluorescent Protein phMGFP Vector (pGFP) (Promega, Madison, WI) was transformed into Escherichia coli DH5α (Invitrogen, Carlsbad, CA), amplified in the presence of ampicillin, and purified using the Qiagen Mega Plus Prep kit (Qiagen, Valencia, CA). Plasmid concentration was determined by UV spectrophotometry using Take 3 (Biotek, Winooski, VT).
2.1.2. Polyplex formation
To optimize particle size and complex stability after lyophilization, polyplexes were formed by mixing pGFP (10 μg) with jetPEI (Polyplus-transfection, Illkirch, France) at N/P ratio of 5/1 in 5 % sucrose. The polyplex solutions were thoroughly mixed by light vortexing and incubated at room temperature for 25 minutes. Polyplexes or naked pGFP control solutions were frozen overnight at −80°C, lyophilized for 24 hours, and reconstituted in 100 μL water or gel precursors. The particle size of fresh and lyophilized polyplexes were measured by dynamic laser light scattering (ZetaPlus, Brookhaven, Worcestershire, UK). DNA recovery and integrity after complexation and lyophilization were characterized by DNA quantitation and electrophoresis. Fresh and lyophilized polyplexes were decomplexed by mixing with 15 mg/ml sodium tripolyphosphate (STP) for 4 hours. DNA recovery was measured by Picogreen assay (Invitrogen) relative to native pDNA control. To confirm pDNA integrity, STP-treated fresh and lyophilized polyplexes and pDNA controls were electrophoresed on a 1% (w/v) agarose gel for 90min at 80 V, stained with ethidium bromide (0.5 μg/ml) for 30 min, and imaged on a Fluorchem SP UV illuminator (ProteinSimple, Santa Clara, CA).
2.2. Hydrogel Preparation and Characterization
2.2.1. Hydrogel preparation
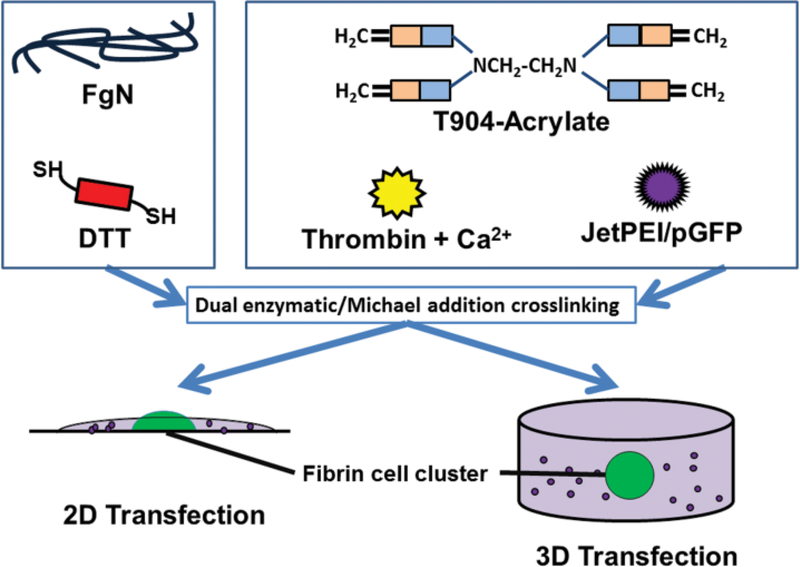
Tetronic® 904 (T904) was generously donated by BASF (Florham Park, NJ). Human fibrinogen (FgN), human alpha thrombin, and human plasmin were purchased from Enzyme Research Laboratories (South Bend, IN). T904 was reacted with acryloyl chloride as previously described (Cho et al., 2012) and 91% acrylation was determined by 1H-NMR. Varying compositions of T904/fibrin hybrid hydrogels were prepared by dual enzymatic and Michael-type addition crosslinking (Figure 1). Fibrinogen (FgN) was first reduced by mixing for 1 hour with varying amounts of DTT required to give a final 1:1 acrylate:thiol ratio within the hydrogel. Lyophilized polyplex or pGFP was reconstituted in a solution of T904-acrylate, 2.5 mM CaCl2, and 0.1U thrombin in 20 mM sodium citrate buffer. The reduced FgN/DTT solution was added to the acrylated T904 solution, mixed by gentle micropipetting, and 25 μL aliquots (2.5 μg DNA/gel) added to decapitated 1 mL syringes or 12 well plates. Samples were incubated at room temperature for 2 hours followed by 37°C for 2 hours for crosslinking.
Figure 1.
Preparation of T904/fibrin hybrid hydrogel containing jetPEI/pGFP polyplexes by dual enzymatic and Michael addition reactions. Fibrinogen (FgN) was first reduced with DTT and then combined with a solution containing T904-acrylate, thrombin, calcium chloride, and lyophilized polyplex. For the 2D transfection model, polyplex-loaded hydrogels were formed on top of the fibrin/cell clusters. For 3D cell transfection, fibrin/cell clusters were positioned at the center of polyplex-loaded hydrogel.
2.2.2. Scanning Electron Microscopy
3% fibrin, 3% T904/2% FgN, and 5% T904/2% FgN gels containing lyophilized jetPEI/pGFP were prepared as described above. Hydrogels were flash frozen with liquid nitrogen, bisected vertically to expose the inside of the hydrogel, and imaged with a Hitachi S-3400N Fully Automated VP SEM.
2.2.3. Swelling and mass loss
3% T904/2% FgN hydrogels (25 μL) with pGFP or polyplex were crosslinked and incubated in 1 mL 50 mM PBS (pH 7.2) containing 100 nM plasmin (Smith et al., 2007). At 4 (pGFP) and 24 (polyplex) hour intervals, samples were collected, photographed, and wet/dry weights measured. Mass swelling ratio was calculated as the ratio of wet weight to dry weight. Percent mass loss was calculated as (dry weighttime x – dry weighttime 0) / dry weighttime0.
2.2.4. Cytocompatibility
Cytocompatibility testing was performed for 3% fibrin gels and 3% T904/2% FgN gels. Gel extracts were prepared by incubating 25 μL gels in 1 ml media for 24 hours at 37°C while shaking at 300 RPM. Gel degradation products were prepared from 25 μL gels by incubating gels in 100 μL media with 100 nM plasmin for 24 hours at 37°C on a plate mixer at 300 rpm. Both gel extract and gel degradation product solutions were filtered through 0.2 μm membrane filter to remove large particles. N2A neuroblastoma cells (N2A) (ATCC, Manassas, VA) were routinely cultured in Eagle’s Minimum Essential Medium (EMEM, ATCC) with 10% FCS (Hyclone, Logan, UT) and 1% penicillin and streptomycin (Mediatech, Manassas, VA). N2A cells were plated at a density of 1 × 105 cells/mL in 12 well plates and cultured overnight. 100 μL of gel extracts, gel degradation products, or fresh medium (control) was added to each well. Cells were incubated an additional 48 hours and viability was assessed by MTT assay.
2.3. Vector loading and release from hybrid hydrogels
Polyplexes and pGFP (2.5 μg DNA) were lyophilized and incorporated within 3% fibrin gels or hybrid hydrogels (25 μL) composed of 2% FgN and varying concentrations of acrylated T904 and DTT. To measure initial loading efficiency, gels were incubated in PBS containing 0.01% trypsin for 2 days and then pH 12 TE buffer for 1 day at 37°C on a 300 rpm plate mixer for complete degradation. To measure release kinetics, gels were incubated in 1mL 50 mM PBS containing 100nM plasmin, which was collected and replenished at 4, 8, 24 hours and every other day for 2 weeks. Solutions from both loading and release studies were treated with 15 mg/ml sodium tripoly phosphate (STP) for 30 minutes and recovered DNA quantitated by Picogreen assay. Loading efficiency was calculated as the percentage of initial DNA recovered during accelerated degradation. DNA release was calculated as a percentage of loaded DNA determined by loading efficiency.
2.4. Transfection with hydrogel degradation products
The effect of gel degradation products on transfection efficiency and cytotoxicity was assessed by conventional 2D transfection. Hydrogels (3% T904/2% FgN) were made as previously described in 1 mL syringes with 25 μL aliquots and rapidly degraded by 48 hours total incubation in 50 μL PBS containing 2 μM plasmin supplemented by an additional 50 μL pH 12 TE buffer for the second 24 hours. N2A cells were seeded in 12 well plates (1.5 X 105 cells, n=3) and cultured for 24 hours to reach 70–80% confluence. Cells were then transfected with jetPEI/pGFP (N/P = 5/1) using 0.5 μg DNA per well in fresh medium containing 5% FCS. After 4 hours, media was replaced again and cells were incubated with soluble T904 (10 μM), 4-arm polyethylene glycol (PEG, 10 kDa, Nektar, San Francisco, CA) (10 μM), or gel degradation products containing 10–50 μM T904. After 48 hours, cells were washed with PBS, trypsinized, centrifuged at 1500 rpm for 3 minutes, and fixed with 1% formaldehyde and assayed by flow cytometry in a Guava easyCyte flow cytometer (Millipore, Billerica, MA, USA)with 5000 counts per sample. Cell viability in parallel cultures was determined by MTT assay.
2.5. Transfection of cells encapsulated within hydrogels
A gel-within-gel cell culture model (Figure 1) was used to investigate the bioactivity of released polyplexes and duration of transfection of invading cells. N2A cells (1 X 105 cells) were first entrapped within 4 μL fibrin clots (3% FgN, 0.1 U thrombin, 2.5 mM CaCl2 in 20mM sodium citrate buffer, 15 minutes for crosslinking). In the 2D model, 3.5% T904/2% FgN hybrid hydrogels (3 μg pGFP/30 μL) were crosslinked on top of N2A-containing fibrin clots on the surface of tissue culture plates for 15 minutes at room temperature and 15 minutes at 37 °C. For the 3D model, identical gels were crosslinked in decapitated 1 mL syringes after N2A-containing fibrin clots were positioned in the center of the solution volume. Hybrid hydrogels were cultured in 2 mL media containing 5% FCS and digitally imaged using an inverted epifluorescent microscope (Zeiss Axiovert 200) at predetermined time points.
2.6. Statistical Analysis
Results were analyzed using ANOVA with Tukey’s test for multiple comparisons (significance p<0.05). Sample sizes were n=3/group and all experiments were performed in triplicate unless otherwise stated.
3. Results
3.1. Polyplex formation and characterization
The effect of lyophilization on polyplex particle size and DNA stability following decomplexation by STP was first assessed. The hydrodynamic diameter of jetPEI/pGFP was reduced from 182.2±6.7 nm when freshly prepared to 123.8±5.3 nm following lyophilization. Polyplexes generated essentially no signal in Picogreen assay, indicative of efficient pDNA condensation by jetPEI. Incubation of polyplexes with 15 mg/mL STP for 4 hours resulted in recovery of 93.3% and 85.2% initially loaded DNA for fresh and lyophilized polyplexes respectively. Fresh and lyophilized polyplexes exhibited complete gel retardation (data not shown). After treatment with STP, intact pDNA that migrated as a single band comparable to untreated pDNA control was recovered. Gel extracts and degradation products were cytocompatible with no significant differences detected for either 3% T904 / 2% FgN or 3% fibrin gels relative to medium-only controls (data not shown).
3.2. Hydrogel preparation and characterization
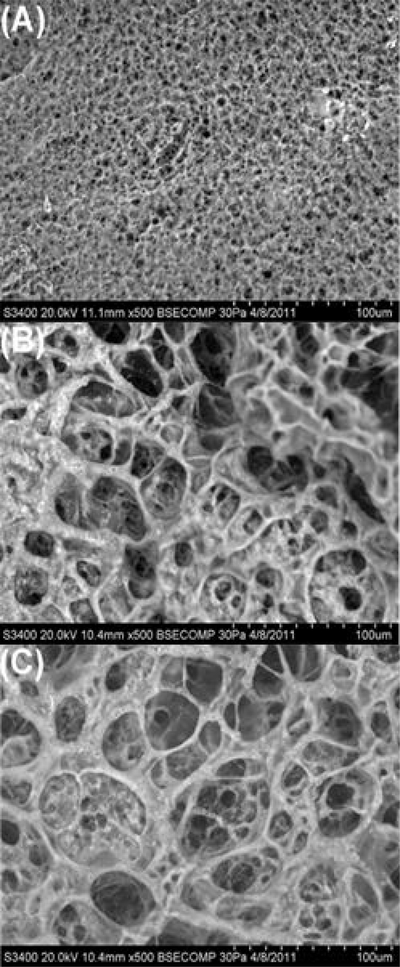
Scanning electron micrographs showed that T904/fibrin hydrogels exhibited an overall fibrillar structure, although the fibril thickness and pore size were much larger than that of the pure enzymatically crosslinked fibrin hydrogel (Figure 2). Increasing the T904 content from 3 to 5% did not substantially affect gel structure.
Figure 2.
SEM micrographs of 3% fibrin hydrogel (A), 3% T904/2% FgN hydrogel (B), and 5% T904/2% FgN hydrogel (C). All hydrogels contained lyophilized jetPEI/pGFP polyplexes prepared in 5% sucrose. Gel cross-sections were imaged at 500X magnification. Scale bar = 100 μm.
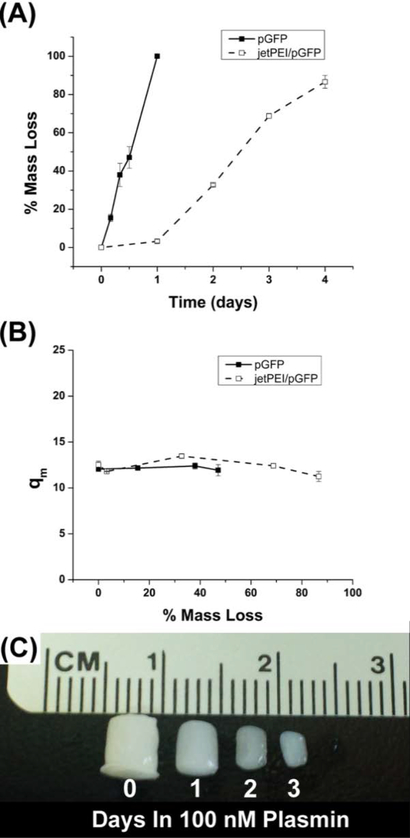
The mass loss of polyplex-loaded 3% T904/2% FgN hydrogels incubated in 100 nM plasmin was much slower than hydrogels with pGFP (Figure 3A). pGFP-loaded hydrogels lost 38% of original mass after 8 hours while those with polyplex only achieved 32.7% mass loss after 2 days. The mass swelling ratio of pGFP- and polyplex-loaded 3% T904/2% FgN hybrid hydrogels was monitored during degradation (Figure 3B). Swelling was plotted as a function of mass loss rather than time due to the substantial difference in degradation rate. The initial swelling ratio did not significantly differ between the two gel types and remained stable for both during mass loss. Figure 3C shows representative images of 100 μl 3% T904/2% FgN gels prepared using the syringe method at 0, 1, 2, and 3 days incubation in 100 nM plasmin. Gels maintained their cylindrical geometry as their volume decreased.
Figure 3.
The mass loss (A) and mass swelling ratio (B) of 3% T904/2% FgN hydrogels containing pGFP or jetPEI/pGFP polyplexes. Degradation of 3% T904/2% FgN hydrogel after 0, 1, 2, and 3 days incubation in PBS containing 100 nM plasmin (C).
3.3. Vector loading and release from hybrid hydrogels
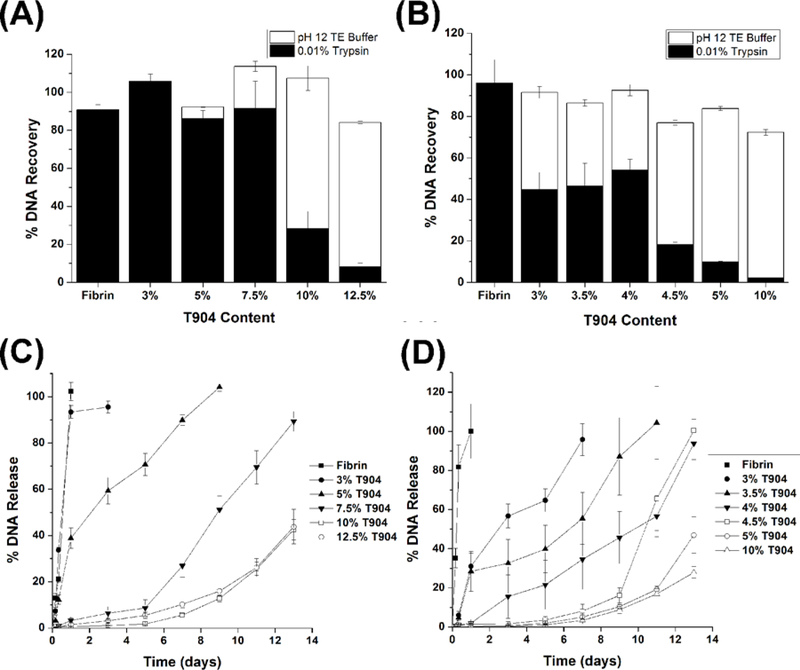
Loading efficiencies for pGFP and polyplex vectors (Figures 4 A and B, respectively) were determined after accelerated enzymatic (trypsin) and hydrolytic (pH 12 TE) degradation. All gels displayed high total loading efficiency (approximately 80% or greater). For pGFP-loaded gels, high levels of DNA recovery (>80%) were achieved by enzymatic degradation for fibrin gels and hybrid gels with lower range of T904 concentration (3–7.5%). At 10 and 12.5% T904, the vast majority of DNA was only recoverable after hydrolytic degradation. Polyplex-loaded gels exhibited a similar pattern of decreasing fraction of enzymatically recoverable DNA with increasing T904 concentration; although the range of T904 concentration tested (3–5%) was narrower due to the reduced degradation relative to pGFP-loaded gels. In addition, the fraction of enzymatically recoverable DNA from hybrid hydrogels was substantially lower for polyplexes than pGFP.
Figure 4.
Loading efficiency of pGFP (A) and jetPEI/pGFP polyplexes (B) in 3% fibrin and hybrid hydrogels containing 2% fibrinogen with varying concentrations of T904-acrylate. The total loading efficiency was obtained from combining the amounts of pDNA recovered by enzymatic degradation (0.01% trypsin) and hydrolytic degradation (pH 12 TE buffer). Release of pGFP (C) and jetPEI/pGFP polyplexes (D) from hybrid hydrogels containing 2% fibrinogen and varying concentrations of T904-acrylate during incubation in 100 nM plasmin.
Release profiles for pGFP- and polyplex-loaded fibrin and various hybrid hydrogels during incubation in 100 nM plasmin are shown in Figure 4 C and D, respectively. Complete degradation and vector release from fibrin only gels occurred within 1 day. The release profiles for both vectors from hybrid hydrogels were varied over a broad time scale by changing the balance between synthetic and naturally-derived components within the gels, with increasing T904 concentration slowing gel degradation and vector release. Approximately linear release profiles and 100% total release were obtained at relatively lower T904 concentrations (3 and 5% for pGFP and 3–4% for polyplex). At higher T904 concentrations, release was substantially reduced during the first week of incubation, and then increased during the second week. Gels with relatively high T904 concentrations were still present at the end of 2 weeks and had not reached complete degradation / release. In contrast to release in the presence of plasmin, very little release of pGFP (<2%) from 5% T904/2% FgN gels was observed during incubation in PBS for up to 7 days (data not shown).
3.4. Transfection with hydrogel degradation products
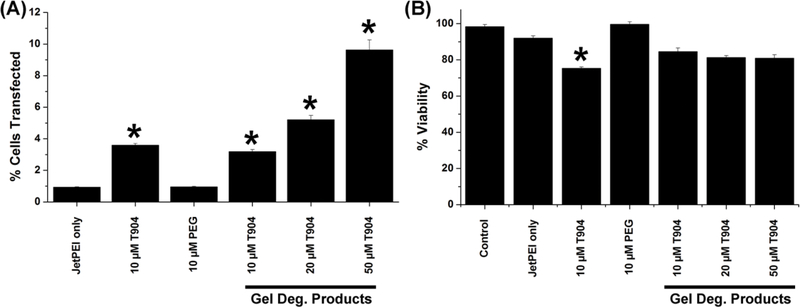
The ability of hydrogel degradation products to increase the transfection efficiency of the polyplex was assessed by in vitro transfection using freshly prepared jetPEI/pGFP and rapidly degraded 3% T904/ 2% FgN hydrogels (Figure 5A). A relatively low amount of DNA (0.5 μg) was used per well in order to approximate the limited amount of vector accessible to cells when homogeneously dispersed in a 3D network, as opposed to 2D transfection where sedimentation effectively concentrates vectors at the cell surface. Exposure to 10 μM unmodified T904 significantly increased transfection efficiency, while 4-arm PEG at comparable concentration had no significant effect. Cells cultured with gel degradation products displayed a dose-dependent increase in transfection efficiency with increasing T904 concentration, with all groups showing significantly increased expression relative to jetPEI transfection alone. High cell viability was maintained in all groups, with only 10 μM native T904 showing a significant decrease relative to jetPEI transfection alone (Figure 5B).
Figure 5.
Transfection efficiency (A) of jetPEI/pGFP complexes alone and with 10 μM T904 or 4-arm PEG and gel degradation products containing 10–50 μM T904 was assessed by flow cytometry and expressed as mean fluorescence units. Corresponding cell viability (B) was measured by MTT assay. (*P<0.05 relative to jetPEI/pGFP alone)
3.5. Transfection with polyplex-loaded hydrogels
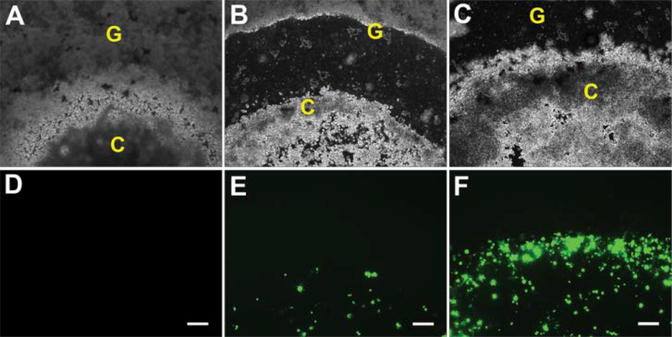
In the 2D transfection model, fibrin clots containing N2A cells in tissue culture plates were overlaid with polyplex-loaded hybrid hydrogels. The fibrin clots were initially visible as opaque regions within the gel. By 3 days, a region of gel degradation was visible surrounding the clots as N2A cells began to proliferate and migrate through the surrounding gel (Figure 6). GFP expression by transfected cells was clearly visible at 3 days and substantially increased by 7 days. N2A cells were able to eventually fully degrade the hybrid hydrogels and reach confluence upon the tissue culture plate.
Figure 6.
GFP expression by transfected N2A cells in 3.5% T904/2% FgN hydrogels in 2D model. Transfected cells were visualized at 1 day (A,D), 3 days (B,E), and 7 days (C,F) with phase contrast (A-C) and fluorescence microscopy (D-E). Original magnifications, 200X. Scale bar represents 200 μm.
C: Fibrin cluster, G: 3.5% T904/2% FgN hydrogels (3 μg pGFP/gel).
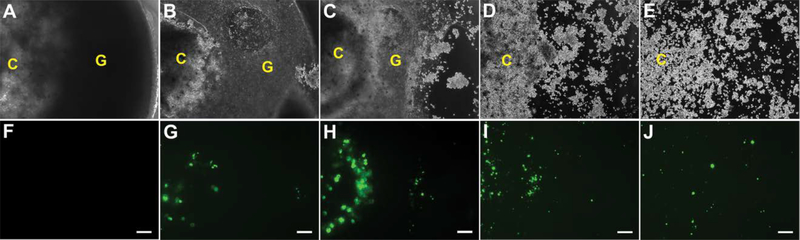
In the 3D model, N2A cells entrapped within fibrin clots were suspended within polyplex-loaded hydrogels. In contrast to the 2D transfection model, no zone of degradation was observed in the 3D transfection model between the expanding cell cluster and the surrounding hybrid hydrogel (Figure 7). Instead, N2A cells appeared to individually migrate through the hydrogels. GFP transfection was clearly visible at 7 days and progressively increased up to 15 days, at which point the gels fragmented from extensive degradation. After 15 days, cells migrating out of the degrading gels adhered and grew on the underlying well plate, continuing to exhibit transfection up to 27 days.
Figure 7.
GFP expression by transfected N2A cells in 3.5% T904/2% FgN hydrogels in 3D model. Transfected cells were visualized at 3 days (A,F), 7 days (B,G), 11 days (C,H), 15 days (D,I), and 27 days (E,J) with phase contrast (A-E) and fluorescence microscopy (F-J)). Original magnifications, 200X. Scale bar represents 200 μm.
C: Fibrin cluster, G: 3.5% T904/2% FgN hydrogels (3 μg pGFP/gel).
4. Discussion
Localized transfection through scaffold-mediated release of nonviral vectors provides an attractive alternative to the delivery of recombinant growth factors, which have a short half-life in situ and often require super-physiological doses for therapeutic efficacy (Storrie and Mooney, 2006; Salvay and Shea, 2006). Although successful gene delivery from hybrid hydrogels has been achieved both in vitro and in vivo, the hydrogel compositions used have generally been adapted from other tissue engineering applications on the basis of their biocompatibility and/or favourable cellular interactions rather than specifically selected/designed for gene delivery (Des Rieux et al., 2009; Lei et al., 2011). Several recent studies have highlighted the potential impact of scaffold properties such as mechanics and degradation rate on non-viral gene delivery (Kong et al., 2005; Shepard et al., 2010; Gojgini et al., 2011). However, few studies have examined the potential opportunity to enhance the effectiveness of scaffold-mediated gene delivery through incorporating materials that, when released in their soluble form during degradation, can actively enhance the efficacy of released non-viral vectors.
In this study, hybrid hydrogels were successfully formed through dual enzymatic and covalent crosslinking, creating interpenetrating polymer network hydrogels of fibrin and T904. In contrast to pure fibrin gels prepared solely by enzymatic crosslinking which exhibit contraction and a residual sol fraction not tightly associated with the gel, hybrid networks formed with the same volume and geometry as the original precursor solution (data not shown). Two important observations support the critical role of dual enzymatic/covalent crosslinking. First, in the absence of thrombin, a minimum 12.5% w/v T904 concentration was required to achieve stable gelation, whereas inclusion of thrombin allowed efficient gelation with as little as 0.5% T904. Second, enzymatically crosslinked fibrin gels completely degraded after a 24-h exposure to 100 nM plasmin, while incorporation of acrylated T904 and DTT decreased degradation rate in a dose-dependent manner, as shown in release studies.
Hybrid gels exhibited a fibrillar structure more macroporous than native fibrin, likely due to protein/polymer interactions. For example, addition of soluble polyethylene glycol to protein solutions is a well-known method to induce protein precipitation (Atha and Ingham, 1981). The rapid crosslinking in our hybrid hydrogels prevents such macroscopic precipitation, instead likely producing micro-scale phase separation that results in increased fibril size and gel porosity. Unlike pure Tetronic hydrogels (Cho et al., 2012) and most other purely synthetic hydrogels containing ester linkages that undergo bulk degradation and exhibit increased swelling during degradation (Metters and Anseth, 2000), the maintenance of stable swelling ratio by the T904/fibrin hydrogel during degradation is most consistent with degradation by surface erosion (Shah et al., 2008). While both polyplex and plasmid incorporated hydrogels were enzymatically degradable, incorporation of polyplex reduced the rate of mass loss in hybrid gels, suggesting that polyplexes interact with the hydrogel network in a manner that decreases its susceptibility to enzymatic degradation. One potential explanation is that fibrinogen possesses a net negative charge in physiological buffer (Wasilewska and Adamczyk, 2011) and may participate in noncovalent, electrostatic interactions with positively charged polyplexes that reduce its accessibility to the enzyme.
While not specifically tailored for gene delivery, the Seliktar and Suggs research groups have extensively investigated similar hybrid materials formed by photo-initiated or thrombin-mediated crosslinking of different PEGylated fibrinogen derivatives (Almany and Seliktar, 2005; Liu et al., 2006). In both cases, the PEG/fibrinogen ratio was a critical variable in controlling enzymatic degradation, cell-mediated remodeling, and release of incorporated growth factors (Zhang et al., 2010; Dikovsky et al., 2006; Drinnan et al., 2010). We hypothesized that our hybrid hydrogel may behave in a similar manner, allowing control over the release kinetics of incorporated nonviral vectors through variation of T904/fibrinogen ratio. Degradation and vector release from these hybrid gels are likely attributable to a combination of the enzymatic degradation of fibrin and the hydrolysis of degradable ester linkages resulting from covalent reactions between T904 acrylate groups and thiols in fibrinogen and DTT. A noticeable inflection point occurs in both the loading efficiency data for trypsin recovery and the release data around 7.5–10% T904 for pGFP-loaded hydrogels and 4.5% T904 for polyplex-loaded hydrogels. These particular compositions likely represent a critical ratio between the naturally-derived and synthetic components, below which their release behavior is primarily governed by enzymatic degradation of the fibrin component and above which the synthetic component begins to predominate and release becomes increasingly dependent on at least partial hydrolytic degradation. T904/fibrin hydrogels demonstrated several advantages as matrices for gene delivery including high loading efficiency, the absence of initial burst release, and extended release up to an order of magnitude longer than pure fibrin controls, which was less than 1 day under the conditions used in this study. In addition, the rate of gel degradation and time course of vector release were readily controlled by varying the ratio of naturally derived to synthetic components, increasing as the ratio of synthetic/natural-derived composition increased.
After proteolytic degradation of fibrin and cleavage of ester linkages formed during thiol/acrylate crosslinking by hydrolysis or esterase enzymes, one of the expected degradation products of T904/fibrin gels will be native T904. When added to transfected cells, both native T904 and gel degradation products significantly increased polyplex transfection efficiency with minimal effects on cell viability. This outcome, previously reported with Pluronics (Yang et al., 2008; Sriadibhatla et al., 2006; Zhang et al., 2013), appears to be unique to amphiphilic copolymers as multi-arm PEG that was also tested did not affect transfection. Therefore, while the degradation products of most hydrogels are generally regarded as bio-inert with respect to vector activity, these studies suggest that T904/fibrin hydrogels offer a new approach to increasing the effectiveness of nonviral gene delivery by generating soluble degradation products that can actively increase the bioactivity of released vectors.
Transfection of cells in 3D using polyplex-loaded hydrogels is critical to validate the activity of polyplexes following lyophilization, reconstitution in gel precursors, and distribution in the 3D environment of the hydrogel. Two primary models exist for in vitro transfection using hydrogel matrices: co-encapsulation of cells with vector in the gel precursor and initial separation of cells from the vector prior to gelation by using a gel-within-gel procedure that more closely mimics invasion of hydrogels in vivo by endogenous cells from surrounding tissue (Herbert et al., 1996; Halstenberg et al., 2002). While co-encapsulation of cells and polyplex in gel precursors provides a means for fast detection of transfection (Wieland et al., 2007), results may be confounded by internalization of polyplex by cells prior to gelation. In addition, homogenously dispersed cells may demonstrate limited migration and only have access to polyplexes initially distributed in close proximity to the cells (Lei and Segura, 2009). In contrast, separation of polyplex from cells prior to hydrogel polymerization ensures that all vector internalization occurs as a result of cell invasion or hydrogel degradation (Lei et al., 2009; Kong et al., 2008). For the T904/fibrin hydrogels used here, cells seeded in fibrin clots gained access to jetPEI/pGFP incorporated within surrounding hybrid gels by release through enzyme mediated surface erosion or coming into contact with encapsulated polyplexes during cell migration. Proteolytic activity of N2A cells was confirmed by the ability of conditioned media from N2A cells to fully degrade fibrin hydrogels, which was inhibited by addition of aprotinin, suggesting a key role for plasmin in cell-mediated gel degradation (data not shown). Previous reports from Shea’s lab indicated a critical role of cell migration and matrix degradation for in vitro hydrogel transfection, particularly in the case of gel-within-gel models (Shepard et al., 2010). Transection of N2A cells by gel degradation as well as cell invasion were both observed in the current study, the former more prominent in the 2D model and the latter in the 3D model. Qualitative observations of transfection efficiency of cells in 3D were comparable or superior to other investigations using similar gel-in-gel models (Shepard et al., 2010; Lei and Segura, 2009).
5. Conclusion
Improving control of release kinetics and transfection efficiency are critical hurdles to the successful application of nonviral gene delivery in tissue engineering. By crosslinking fibrin with Tetronic T904, hybrid hydrogels were created that possess variable susceptibility to proteolytic degradation and provide controlled release of nonviral vectors over varying time frames through manipulation of the ratio of T904/fibrinogen composition. Unlike other relatively inert synthetic polymers such as PEG, the incorporation of amphiphilic T904 allowed the gels to degrade into soluble products that significantly increase polyplex transfection efficiency. T904/fibrin hydrogels efficiently transfected invading cells with expression extending to approximately one month, suggesting these matrices may be applicable for gene delivery in a variety of soft tissue applications.
6. Acknowledgments
This project was supported by grants from the National Center for Research Resources (5P20RR021949-04) and the National Institute of General Medical Sciences (8 P 20 GM103444-04) from the National Institutes of Health. We gratefully acknowledge the assistance of Dr. Joan Hudson and the SC Biomat Histology and Imaging Core Facility for assistance with SEM imaging. We thank Dr. Alexey Vertegel for access to his particle size analyzer.
Footnotes
7. Disclosure statement
No competing financial interests exist.
8. References
- Al-Dosari MS, Gao X. 2009; Nonviral gene delivery: principle, limitations, and recent progress, AAPS J, 11: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almany L, Seliktar D. 2005; Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures, Biomaterials, 26: 2467–2477. [DOI] [PubMed] [Google Scholar]
- Astafieva I, Maksimova I, Lukanidin E, et al. 1996; Enhancement of the polycation-mediated DNA uptake and cell transfection with Pluronic P85 block copolymer, FEBS lett, 389: 278–280. [DOI] [PubMed] [Google Scholar]
- Atha DH, Ingham KC. 1981; Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume., J Biol Chem, 256: 12108–12117. [PubMed] [Google Scholar]
- Bonadio J 2000; Tissue engineering via local gene delivery, J Mol Med, 78: 303–311. [DOI] [PubMed] [Google Scholar]
- Cho E, Lee JS, Webb K. 2012; Formulation and characterization of poloxamine-based hydrogels as tissue sealants, Acta Biomater; 8: 2223–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Sacks H, Elazar V, Gao J, et al. 2004; Delivery and expression of pDNA embedded in collagen matrices, J Controlled Release, 95: 309–320. [DOI] [PubMed] [Google Scholar]
- Dang JM, Leong KW. 2006; Natural polymers for gene delivery and tissue engineering, Adv Drug Deliv Rev, 58: 487–499. [DOI] [PubMed] [Google Scholar]
- Des Rieux A, Shikanov A, Shea LD. 2009; Fibrin hydrogels for non-viral vector delivery in vitro, J Controlled Release, 136: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikovsky D, Bianco-Peled H, Seliktar D. 2006; The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration, Biomaterials, 27: 1496–1506. [DOI] [PubMed] [Google Scholar]
- Drinnan CT, Zhang G, Alexander M a., et al. 2010; Multimodal release of transforming growth factor-β1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels, J Controlled Release; 147: 180–186. [DOI] [PubMed] [Google Scholar]
- Drury JL, Mooney DJ. 2003; Hydrogels for tissue engineering: scaffold design variables and applications, Biomaterials, 24: 4337–4351. [DOI] [PubMed] [Google Scholar]
- Gojgini S, Tokatlian T, Segura T. 2011; Utilizing cell-matrix interactions to modulate gene transfer to stem cells inside hyaluronic acid hydrogels, Mol Pharm, 8: 1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstenberg S, Panitch A, Rizzi S, et al. 2002; Biologically engineered protein-graft-poly(ethylene glycol) hydrogels: a cell adhesive and plasmin-degradable biosynthetic material for tissue repair, Biomacromolecules, 3: 710–723. [DOI] [PubMed] [Google Scholar]
- Herbert CB, Bittner GD, Hubbell J a. 1996; Effects of fibinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels., J Comp Neurol, 365: 380–391. [DOI] [PubMed] [Google Scholar]
- Jia X, Kiick K. 2008; Hybrid multicomponent hydrogels for tissue engineering, Macromol Biosci, 9: 140–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MA, Naldini L, Glorioso JC. 2001; Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics, Nat Med, 7: 33–40. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Liu J, Riddle K, et al. 2005; Non-viral gene delivery regulated by stiffness of cell adhesion substrates, Nat Mater, 4: 460–464. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Kim ES, Huang Y, et al. 2008; Design of Biodegradable Hydrogel for the Local and Sustained Delivery of Angiogenic Plasmid DNA, Pharm Res, 25: 1230–1238. [DOI] [PubMed] [Google Scholar]
- Kopecek J 2007; Hydrogel biomaterials: a smart future?, Biomaterials, 28: 5185–5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Padmashali RM, Andreadis ST. 2009; Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels, Biomaterials; 30: 3790–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Segura T. 2009; DNA delivery from matrix metalloproteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells, Biomaterials, 30: 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Huang S, Sharif-Kashani P, et al. 2010; Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds, Biomaterials, 31: 9106–9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Rahim M, Ng Q, et al. 2011; Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery, J Controlled Release; 153: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Anseth K. 2009; PEG hydrogels for the controlled release of biomolecules in regenerative medicine, Pharm Res, 26: 631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Collins SF, Suggs LJ. 2006; Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells, Biomaterials, 27: 6004–6014. [DOI] [PubMed] [Google Scholar]
- Metters A, Anseth K. 2000; Fundamental studies of a novel, biodegradable PEG-b-PLA hydrogel, Polymer, 41: 3993–4004. [Google Scholar]
- Prokop A, Kozlov E, Moore W, et al. 2002; Maximizing the in vivo efficiency of gene transfer by means of nonviral polymeric gene delivery vehicles, J Pharm Sci, 91: 67–76. [DOI] [PubMed] [Google Scholar]
- Quick DJ, Anseth KS. 2003; Gene delivery in tissue engineering: a photopolymer platform to coencapsulate cells and plasmid DNA, Pharm Res, 20: 1730–1737. [DOI] [PubMed] [Google Scholar]
- Salvay DM, Shea LD. 2006; Inductive tissue engineering with protein and DNA-releasing scaffolds., Mol Biosyst, 2: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer F, Schillinger U, Putz U, et al. 2002; Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo, J Gene Med, 4: 634–643. [DOI] [PubMed] [Google Scholar]
- Shah DN, Recktenwall-Work SM, Anseth KS. 2008; The effect of bioactive hydrogels on the secretion of extracellular matrix molecules by valvular interstitial cells, Biomaterials, 29: 2060–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JA, Huang A, Shikanov A, et al. 2010; Balancing cell migration with matrix degradation enhances gene delivery to cells cultured three-dimensionally within hydrogels, J Controlled Release; 146: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Chen A, Ernst LA, et al. 2007; Immobilization of aprotinin to fibrinogen as a novel method of controlling degradation of fibrin gels. Bioconj Chem 18: 695–701. [DOI] [PubMed] [Google Scholar]
- Spotnitz WD. 2010; Fibrin sealant: past, present, and future: a brief review, World J Surg, 34: 632–634. [DOI] [PubMed] [Google Scholar]
- Sriadibhatla S, Yang Z, Gebhart C, et al. 2006; Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells, Mol Ther, 13: 804–813. [DOI] [PubMed] [Google Scholar]
- Storrie H, Mooney DJ. 2006; Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering, Adv Drug Deliv Rev, 58: 500–514. [DOI] [PubMed] [Google Scholar]
- Wasilewska M, Adamczyk Z. 2011; Fibrinogen adsorption on mica studied by AFM and in situ streaming potential measurements., Langmuir, 27: 686–696. [DOI] [PubMed] [Google Scholar]
- Wieland JA, Houchin-Ray TL, Shea LD. 2007; Non-viral vector delivery from PEG-hyaluronic acid hydrogels, J Controlled Release; 120: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sahay G, Sriadibhatla S, et al. 2008; Amphiphilic block copolymers enhance cellular uptake and nuclear entry of polyplex-delivered DNA., Bioconjug Chem, 19: 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Drinnan CT, Geuss LR, et al. 2010; Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix., Acta Biomater, 6: 3395–3403. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bae S, Lee JS, et al. 2013; Efficacy and mechanismof poloxamine-assisted polyplex transfection. J GeneMed 15: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]