Although Operation Warp Speed (the US federal funding effort for vaccine development by pharmaceutical corporations) helped to facilitate the worldwide development of three US Food and Drug Administration (FDA) emergency-use approved COVID-19 vaccines in the USA in record time in 2020, it also resulted in rapid and large-scale vaccine dissemination before real-word safety studies outside of the original clinical trials could be done. Following widespread international use of several COVID-19 vaccines, rare, unexpected, and serious haematological complications began to be noticed by haematologists in the UK, Norway, Germany, Canada, and the USA in early 2021.
SARS-CoV-2 began spreading around the world in January, 2020. In February, 2021, following administration of two of the first COVID-19 vaccines, the ChAdOx1 nCoV-19 (Oxford–Astra Zeneca) and the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen), a small number of primarily middle-aged (range 18–79 years) women developed a novel syndrome that was termed vaccine-induced thrombocytopenia and thrombosis (VITT). The first vaccine associated with VITT, the Oxford–Astra Zeneca vaccine, uses a non-replicating chimpanzee adenovirus to deliver the spike antigen. The second vaccine associated with VITT, the Johnson & Johnson/Janssen vaccine, is a recombinant adenovirus serotype 26 vector encoding the SARS-CoV-2 spike glycoprotein. In March, 2021, a 48-year-old woman in the USA was hospitalised with thrombotic thrombocytopenia following administration of the Johnson & Johnson/Janssen vaccine. This patient resulted in the USA pausing administration of the Johnson & Johnson/Janssen vaccine while the Centers for Disease Control and Prevention, the National Institutes of Health, the US FDA, and scientists from each of the marketed COVID-19 vaccines evaluated VITT. The pause was lifted 10 days later.
Because of this new and unexpected safety concern, our group (the USA National Institute of Health funded pharmacovigilance group, the Southern Network on Adverse Reactions [SONAR]), in collaboration with haematologists at the City of Hope Comprehensive Cancer Center in Duarte, CA, USA, initiated a project to identify the first reports of COVID-19 vaccine-associated haematological complications on Twitter, one of the largest social media corporations in the world with 353 million monthly average users. Social media has become an increasingly important venue for people worldwide to discuss medical topics and can provide a method for the rapid sharing of information. We questioned if social media could be used as an early warning system for the detection of haematological complications of COVID-19 vaccinations. How reliable would Twitter posts be? Would such notifications be more noise than signal?
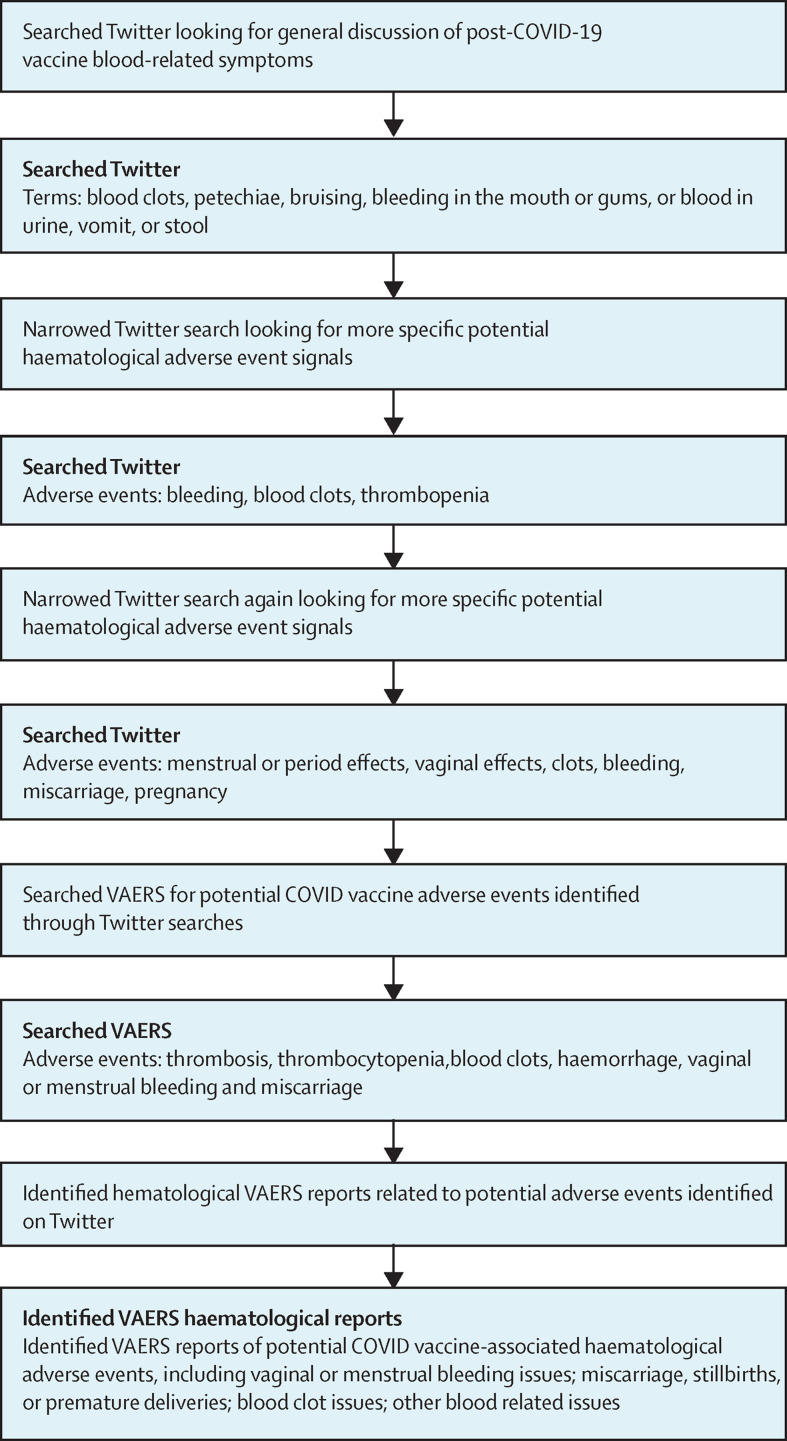
We reviewed Twitter posts for the 8-week period from Feb 1 to April 1, 2021, searching for reports of COVID-19 vaccine-associated haematological adverse events (figure ). We could not evaluate accuracy of any of the posts on this topic, but we identified 21 Twitter reports of possible haematological complications. Our group then developed an ontology of clinical terms encompassing haematological complications—key words included thrombosis, thrombocytopenia, blood clots, haemorrhage, vaginal or menstrual bleeding, and miscarriage.
Figure.
Incorporation of social media searches into early identification of COVID-19 vaccine-associated haematological complications
VAERS=Vaccine Adverse Event Reporting System.
We then queried the Centers for Disease Control and Prevention's Vaccine Adverse Event Reporting System (VAERS) database for specific reports of adverse events that mapped to these terms. This search identified 460 instances of vaginal or menstrual bleeding related adverse events (including increases in bleeding, abnormal time frame for cycles, excessive cramping, and early onset of bleeding); 459 instances of miscarriage, stillbirth delivery, or premature birth; 3880 instances of clotting related adverse events; and 3799 instances of bleeding and other haematological adverse events. Included were detailed descriptions of 12 patients with VITT, including three who died from this complication.
We initiated a prospective monitoring system for COVID-19 vaccine-associated haematological complications on the basis of the positive feedback from this pilot effort. Analysts associated with SONAR, guided by social media investigative efforts developed at The University of Alabama at Birmingham, Birmingham, AL, USA, are continuously querying Twitter posts for reports in this area. Once unique reports of haematology-associated adverse events are identified, other members of SONAR query VAERS for individual reports of related haematological side-effects, on the basis of an expanded ontology that is constantly updated following Twitter findings.
Our experience shows the importance of adapting 21st century approaches to pharmacovigilance efforts. Ongoing safety initiatives conducted by regulatory agencies and the pharmaceutical industry focus on 20th century methods that primarily evaluate VAERS and other large databases that are maintained by the US FDA, national health insurers, and government agencies. Social media, although limited by signal-to-noise considerations and an inability to verify the accuracy of patient reports of possible toxicities, provides benefits from its reach and depth. This approach provides an unparalleled mechanism to identify the first reports of potential haematology-related safety signals that might be associated with any of the numerous COVID-19 vaccines currently in use around the world.
A key limitation of 20th century database approach to vaccine safety is the estimated 1% reporting rate for adverse events to databases, such as VAERS. Social media provides an opportunity to obtain initial safety reports from a vast number of the vaccinated population. We expect that within a year, SONAR will be able to formally evaluate the costs and benefits of incorporating 21st century data approaches into worldwide vaccine safety initiatives.
For the vaccine adverse event reporting system see https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html
Acknowledgments
STR, LWK, BD, and CLB declare research funding from the National Institutes of Health. KG is the institutional primary investigator for clinical trials that received grants from Incyte, Samus, Pfizer, and Autolus. The other authors declare no competing interests. This work received support from the National Cancer Institute (1R01CA102713-01 [CLB]) and unrestricted funding from the Rick Martin Initiative on Pharmaceutical Drug Safety at the Beckman Institute and the City of Hope Comprehensive Center (CLB).