Abstract
Context
The PDX1 gene encodes pancreatic and duodenal homeobox, a critical transcription factor for pancreatic β-cell differentiation and maintenance of mature β-cells. Heterozygous loss-of-function mutations cause PDX1-MODY (MODY4).
Case Description
Our patient is an 18-year-old lean man who developed diabetes at 16 years of age. Given his early-onset age and leanness, we performed genetic testing. Targeted next-generation sequencing and subsequent Sanger sequencing detected a novel heterozygous frameshift mutation (NM_00209.4:c.218delT. NP_000200.1: p.Leu73Profs*50) in the PDX1 transactivation domain that resulted in loss-of-function and was validated by an in vitro functional study. The proband and his 56-year-old father, who had the same mutation, both showed markedly reduced insulin and gastric inhibitory polypeptide (GIP) secretion compared with the dizygotic twin sister, who was negative for the mutation and had normal glucose tolerance. The proband responded well to sitagliptin, suggesting its utility as a treatment option. Notably, the proband and his father showed intriguing phenotypic differences: the proband had been lean for his entire life but developed early-onset diabetes requiring an antihyperglycemic agent. In contrast, his father was overweight, developed diabetes much later in life, and did not require medication, suggesting the oligogenic nature of PDX1-MODY. A review of all reported cases of PDX1-MODY also showed heterogeneous phenotypes regarding onset age, obesity, and treatment, even in the presence of the same mutation.
Conclusions
We identified the first Japanese family with PDX1-MODY. The similarities and differences found among the cases highlight the wide phenotypic spectrum of PDX1-MODY.
Keywords: PDX1, MODY, maturity-onset diabetes of the young type 4
Pancreatic and duodenal homeobox 1 (PDX1), encoded by the gene PDX1, is an essential factor for β-cell maturation, survival, and function. Homozygous null mutations of PDX1 result in pancreatic agenesis [1]; heterozygous loss-of-function mutations can cause maturity-onset diabetes of the young 4 (MODY4) [2].
Although MODY has been traditionally regarded as a monogenic form of diabetes, recent studies have found that some forms of MODY, including HNF1B-MODY and NEUROD1-MODY, are in fact oligogenic, with low–moderate penetrance [3, 4]. Their clinical presentation can differ even in the same family with the same mutation, suggesting that genetic and environmental background play an important role in determining the phenotype of certain MODYs. However, it remains unclear whether the phenotype of PDX1-MODY also varies. Here, we report a novel heterozygous PDX1 frameshift mutation in a Japanese family with PDX1-MODY and discuss intriguing phenotypic similarities and differences between the proband and his father, who share the same mutation. We also review the clinical characteristics of all reported cases of PDX1-MODY to highlight the phenotypic spectrum of PDX1-MODY. In addition, we also provide an in vitro expression analysis of the PDX1 mutation and discuss its possible effect on glucose-dependent insulinotropic polypeptide (GIP) secretion.
Materials and Methods
Clinical Measurements
Plasma GIP and glucagon-like peptide-1 (GLP-1) concentrations were evaluated by enzyme-linked immunosorbent assay (ELISA) using the human active GIP ELISA kit (IBL Co Ltd, Fujioka, Japan) and human active GLP-1 ELISA kit (Millipore, Billerica, USA), respectively. Plasma glucagon levels were measured by a glucagon ELISA kit (Mercodia, Uppsala, Sweden). Other laboratory measurements were performed by standard assays.
Genetic Analysis of MODY Genes
Targeted next-generation sequencing of HNF4A, GCK, HNF1A, PDX1, HNF1B, NEUROD1, KLF11, CEL, PAX4, INS, BLK, KCNJ11, ABCC8, and APPL1 was performed using Illumina MiSeq (Illumina, San Diego, CA, USA). The outputs were processed in accordance with the GATK Best Practice Workflow (https://gatk.broadinstitute.org/, accessed August 16, 2021). Large genomic rearrangements of HNF4A, GCK, HNF1A, PDX1, HNF1B, NEUROD1, KLF11, CEL, PAX4, and INS also were examined by Salsa Multiplex (SALSA MLPA Probemix P241-E1 MODY Mix 1 and P357 MODY Mix 2, MRC-Holland, Amsterdam, Netherlands).
Functional Analysis of Wild-Type and Mutant PDX1
HEK293 cells grown in Dulbecco’s modified Eagle medium (Gibco BRL, Gaithersburg, MD, USA) supplemented with 5% heat-inactivated fetal calf serum at 1.0 × 105 cells per well of a 6-well plate (Catalog no. 353046, Corning, USA) were transfected using Lipofectamine 3000 (Catalog no. L3000015, Thermo Fisher Scientific, MA, USA) and 2 µg of DNA including 0.25 µg of pCMV6b-PDX1 Wild or/and pCMV6b-PDX1 Mutant (p.Leu73Profs*50), 0.50 µg of the reporter gene pGL3 containing human insulin promoter, and 25 ng of pRL-SV40 to control for efficiency of transfection. After 30 hours, the transactivation activity of the wild and the mutant PDX1 proteins was measured using the Dual-Luciferase Reporter Assay System (Catalog no. E1910, Promega, Madison, WI, USA) according to the manufacture’s protocol. Normalized luciferase activities were calculated as the ratio of firefly luciferase activities to renilla luciferase activity. Data represent mean ± SD of 4 wells per condition.
Results
Case Description
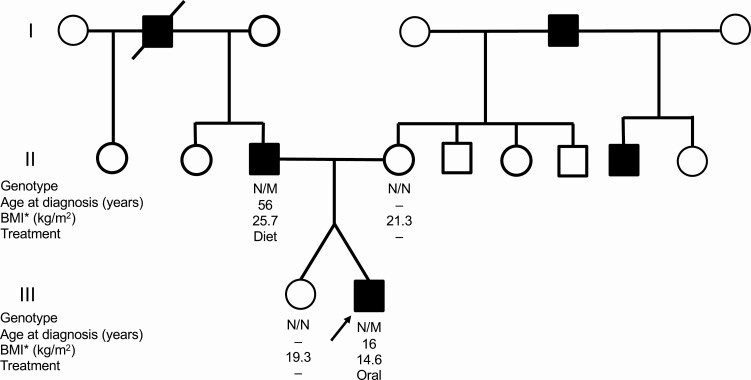
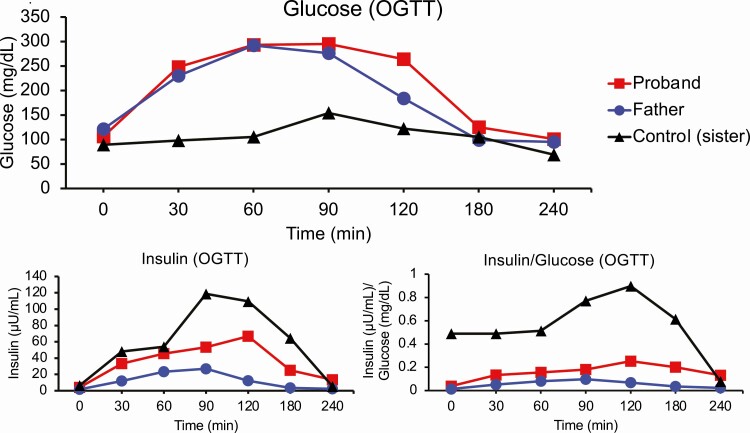
The proband was an 18-year-old lean Japanese man referred to our hospital for the management of early-onset diabetes that was diagnosed at the age of 16 years. He had no history of obesity or ketoacidosis. He was born at 37 weeks gestational age; his birth weight was 2016 g. He had been lean for his entire life; his body mass index (BMI) was 15.0 kg/m2 at presentation (weight: 41.6 kg, height: 166.7 cm). In contrast, his father was overweight (BMI: 25.0 kg/m2) and had impaired glucose tolerance at age 55 years, which subsequently progressed to diabetes at age 56 (Table 1). The father then recalled excess food intake and irregular dietary habits as possible contributors to the exacerbation of glycemic control. The proband’s mother and his dizygotic twin sister had no remarkable medical history, birth history, or developmental history. His mother had no history of gestational diabetes. His twin sister’s birth weight was 2536 g at 37 weeks of gestation. A pedigree chart is shown in Figure 1. The clinical measurements of the proband and his family members are presented in Table 1. Islet autoantibodies (GAD, IA-2) were negative in the proband as well as in the other family members. On the 75-g oral glucose tolerance test (OGTT), both the proband and his father showed glucose intolerance and markedly reduced insulin secretion (Figure 2), with insulinogenic indices of 0.21 and 0.09, respectively. His sister and mother showed normal glucose tolerance (Supplementary Figure 1) [5]. Intriguingly, the proband and his father showed reduced GIP secretion compared to the dizygotic sister (Supplementary Figure 2) [5]. In contrast, the proband and his father showed no consistent change in GLP-1 or glucagon levels. For incretin measurements, the proband underwent another OGTT with a shorter duration (measured time: 0, 30, 60, and 120 minutes). Incretin measurement was not performed for his mother. No pancreatic structural abnormalities were observed in the proband or his father. While the proband and his father had good glycemic control, the proband required oral hyperglycemic agents, but his father did not. The proband’s hemoglobin A1c (HbA1c) level was 6.8% (51 mmol/mol) without medication and 6.0% to 6.5% (42-48 mmol/mol) on monotherapy with sitagliptin 25 to 50 mg or gliclazide 20 to 40 mg. The father’s HbA1c level without medication was 6.3% to 6.5% (45-48 mmol/mol).
Table 1.
Clinical characteristics
| Proband (at age 18-20) | Father (at age 55-57) | Dizygotic twin sister (at age 20) | Mother (at age 53) | |
|---|---|---|---|---|
| PDX1 mutation(c.218delT) | + | + | – | – |
| Glucose tolerance | Diabetes | Diabetes | Normal | Normal |
| Age of diabetes diagnosis, years | 16 | 56 | – | – |
| Height, cm | 166.5 | 168.5 | 151 | 148.7 |
| Body weight, kg | 40.4 | 73.1 | 44 | 47.2 |
| Body mass index, kg/m2 | 14.6 | 25.7 | 19.3 | 21.3 |
| HbA1c, % (mmol/mol) | 6.0–6.8 (42–51) | 6.3–6.5 (45–48) | 5.3 (34) | 5.6 (38) |
| Fasting blood glucose, mg/dL (mmol/L) | 106–120 (5.6–6.7) | 121–128 (6.7–7.1) | 89 (4.9) | 91 (5.1) |
| 2-hour postprandial blood glucose on OGTT, mg/dL (mmol/L) | 246–264 (13.7–14.7) | 184–271 (10.2–15.1) | 122 (6.8) | 128 (7.1) |
| Treatment | Sitagliptin or gliclazide | Diet | – | – |
Abbreviation: OGTT, oral glucose tolerance test (75 g)
Figure 1.
Pedigree chart of the family. The proband is indicated by an arrowhead. Individuals with diabetes are indicated by filled symbols (square, male; circle, female). Genotype: N, normal allele; M, mutant allele (c.218delT). Abbreviations: BMI, body mass index; Diet, diet therapy without medication; Oral, oral antihyperglycemic agent (gliclazide or sitagliptin).
Figure 2.
75-g oral glucose tolerance test (OGTT). Plasma glucose (mg/dL), insulin (µL/mL), and insulin (µL/mL) to glucose (mg/dL) ratio of the mutation-positive individuals (proband and his father), and the mutation-negative control (proband’s dizygotic twin sister) during the 75-g OGTT. Red line: proband (mutation-positive); blue line: father (mutation-positive); black line: dizygotic sister (mutation-negative).
Identification of PDX1 Frameshift Mutation and its Functional Analysis
Given the proband’s early-onset diabetes and lean phenotype, we suspected MODY. DNA was extracted from the peripheral blood mononuclear cells of the proband and his father, dizygotic twin sister, and his mother. Sequencing analysis identified a novel heterozygous frameshift mutation in the transactivation domain of exon 1 of PDX1 (NM_00209.4:c.218delT. NP_000200.1: p.Leu73Profs*50). Subsequent Sanger sequencing confirmed the mutation in the proband and also found the same mutation in his father but not in his mother or dizygotic twin sister (Supplementary Figure 3) [5]. Based on the American College of Medical Genetics and Genomics Standards and Guidelines [6], the present variant was classified as pathogenic due to PVS1 (frameshift in a gene where loss-of-function is a known mechanism of disease) + PS3 (in vivo functional studies supportive of a damaging effect) + PM1 (a mutational hot spot and/or critical and well-established functional domain) + PM2 (absent from controls of Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium). Multiplex ligation-dependent probe amplification did not detect any copy number variation in the proband.
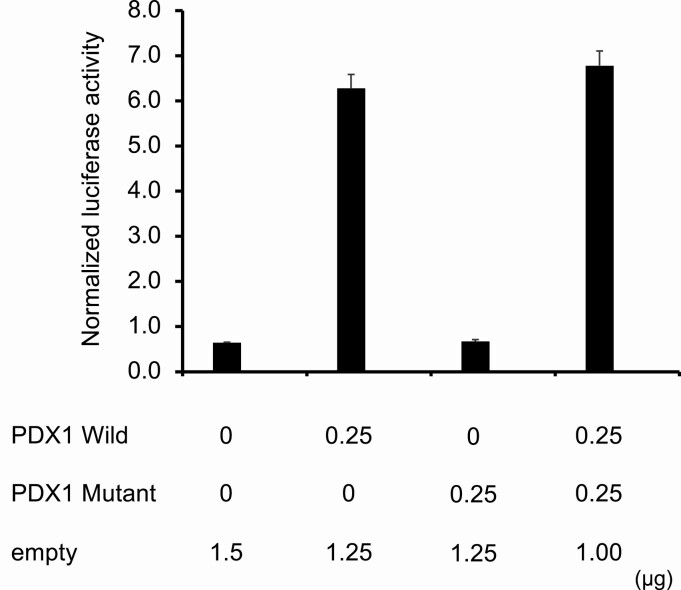
Transfection studies showed that wild-type PDX1 was able to stimulate transcription of a firefly luciferase reporter gene linked to the PDX1 binding site of the human insulin gene by 9.7-fold. In contrast, mutant-type PDX1 (p.Leu73Profs*50) was inactive. There was no inhibition of PDX1 activity when the cells were transfected with equivalent amounts of wild-type and mutant-type PDX1 expression vectors, indicating that mutant PDX1 does not function in a dominant-negative manner (Figure 3).
Figure 3.
Transcriptional activation by wild-type and mutant PDX1. The relative luciferase activity (firefly/renilla) of 0.25 µg of pCMV6b-PDX1 Wild (PDX1 Wild) or/and pCMV6b-PDX1-Mutant (PDX1 Mutant) proteins was measured in HEK293 cells. Normalized (relative) luciferase activities were as follows: Empty (pCMV6b only): 0.65 ± 0.01, PDX1 Wild: 6.28 ± 0.30, PDX1 Mutant: 0.67 ± 0.04, Wild + PDX1 Mutant: 6.78 ± 0.33. Data are shown as mean ± SD (n = 4). n.s. = not significant.
Discussion
Phenotypic Similarities
For the OGTT, the proband and his father showed markedly reduced insulin secretion and overt diabetes, compared to his dizygotic twin sister and his mother, who were negative for the mutation and had normal glucose tolerance. Interestingly, the proband and his father showed reduced GIP secretion compared to his sister (control). These findings are consistent with those of previous studies, which reported that PDX1 is required for GIP expression in cell lines and mice [7]. The decreased GIP levels likely contributed to impaired insulin secretion, as GIP promotes early-phase insulin release [8]. No consistent change was observed for GLP-1 or glucagon levels. To the best of our knowledge, the present study is the first to illustrate a reduction of GIP secretion in PDX1-MODY. We also found that the proband responded well to sitagliptin, suggesting dipeptidyl peptidase-4 inhibitors as possible treatment options for PDX1-MODY patients.
Phenotypic Differences
While sharing β-cell dysfunction, the proband and his father also showed intriguing phenotypic differences. Whereas the proband had been lean for his entire life (BMI was 15.0 kg/m2 at presentation) and developed early-onset diabetes at 16 years of age, his father was overweight and showed a milder form of glucose intolerance. The father initially presented with impaired glucose tolerance at 55 years and did not meet the diagnostic criteria for diabetes but subsequently developed the disease at 56 years. Given that the father had been obese and that he experienced excessive eating and drinking between the ages of 55 and 56, it is likely that both increased insulin resistance and impaired β-cell function contributed to his development of diabetes.
The difference in the manifestation of the disease between the proband and his father could be partly due to additional genetic factors inherited from the proband’s mother or environmental factors. Regarding the additional genetic components, his mother, who was negative for the PDX1 mutation, was nonobese but showed reduced insulin secretion (Supplementary Figure 1) [5] and had a family history of diabetes, suggesting the presence of genetic predispositions to β-cell dysfunction. In addition, plasma insulin levels of his mother and the dizygotic sister peaked at 120 minutes and 90 minutes, respectively, during the 75-g OGTT. Considering that plasma insulin levels usually peak at 30 minutes and rarely after 60 minutes in glucose tolerant subjects [9], these delayed peaks may also indicate other genetic defects. Thus, the proband might have inherited these additional genetic predispositions from his mother, which acted in concert with the PDX1 mutation inherited from his father. Environmental factors might also have contributed to the phenotypic differences, given the father’s history of dietary indiscretion and obesity.
Comprehensive Literature Review: Highlighting the Wide Phenotypic Spectrum of PDX1-MODY (MODY4)
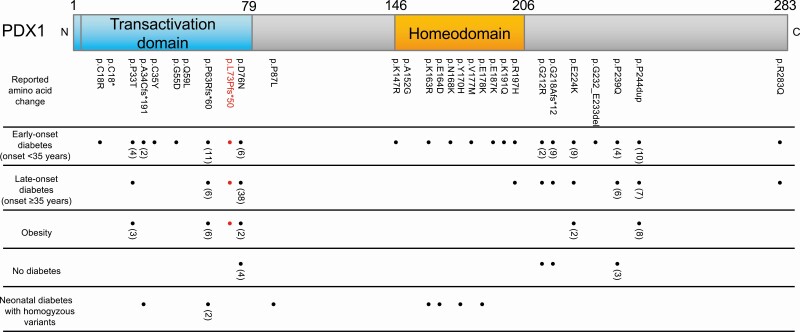
The notion that some forms of MODY traditionally classified as monogenic are actually oligogenic is not speculation [10]. Recent studies have shown that certain forms of MODY such as HNF1B-MODY and NEUROD1-MODY are oligogenic, with different clinical phenotypes even in the same family with the same causative mutation [3, 4, 11]. We also revealed that the small heterodimer partner (SHP) pathogenic variant, which coexists with an HNF1A pathogenic variant, modifies the age of diagnosis, illustrating its oligogenic nature [10, 12]. However, there has been no comprehensive review of PDX1-MODY, and it is unclear whether PDX1-MODY also presents with a wide range of phenotypes. To clarify the phenotypic spectrum and genotype-phenotype correlations of PDX1-MODY, especially in terms of onset age, obesity, and treatment, we performed a literature review of all previous studies in the PubMed database up to July 1, 2021. Only papers published in English were included. The search terms were PDX1-MODY OR MODY4 as well as PDX1 AND diabetes. Patients with type 2 diabetes diagnoses were excluded. We also reviewed the Human Gene Mutation Database (HGMD) Professional RELEASE 2019.4. A total of 31 pathogenic PDX1 mutations were identified [1, 13-38]. Each of the reported variants and the corresponding phenotypes is summarized in Table 2; Figure 4 provides a schematic representation of the genotype-phenotype relationships. Pathogenic mutations were observed to be enriched in the transactivation domain and the homeodomain (Figure 4). This is reasonable given that both domains are crucial components of PDX1: the transactivation domain contains binding sites for other proteins such as transcription coregulators, and the homeodomain plays key roles in the DNA binding site and protein-protein interaction [39]. As for PDX1-MODY, previous studies reported various phenotypes associated with heterozygous PDX1 variants regarding age at diagnosis (ranging from 8 to 65 years), obesity, and treatment (ranging from diet to insulin) (Table 2). In clear contrast, homozygous PDX1 variants exhibited the homogeneous phenotype of neonatal diabetes with pancreatic agenesis. These findings strongly suggest that the impact and consequences associated with PDX1 heterozygous variants could be modified by genetic and environmental background and highlight the need to revisit the definition of PDX1-MODY as a simple monogenic disorder, it being rather an oligogenic disease with a wide phenotypic spectrum. These findings warrant future investigations to identify the phenotype-modifying factors of PDX1-MODY.
Table 2.
Genetic and clinical characteristics of all reported PDX1-MODY cases
| Nucleotide change (NM_000209.3) | Amino acid change (NP_000200.1) | Heterozygous variants | Homozygous variants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early-onset diabetes (onset ≤ 35 years) | Late-onset diabetes (onset > 35 years) | Obesity | No diabetes | Neonatal diabetes | ||||||||
| Number of patients | Mean age of diagnosis | Treatment | Number of patients | Mean age of diagnosis | Treatment | References | ||||||
| 1 | c.52T>C | p.C18R | 1 | 13.0 | Diet:1 | 1 | 65.0 | Ins:1 | · | · | – | 13 |
| 2 | c.54C>A | p.C18* | 1 | 8.0 | · | – | – | – | · | · | – | 14 |
| 3 | c.97C>A | p.P33T | 8 | 19.7 | Diet:2 Ins:2 | 1 | 46.0 | Ins:1 | + (3) | + (1) | – | 15, 16 |
| 4 | c.98dupC | p.A34Cfs*191 | 2 | 18.0 | – | – | – | – | · | · | + (1) | 14 |
| 5 | c.104G>A | p.C35Y | 1 | · | · | – | – | – | · | · | – | 17 |
| 6 | c.164G>A | p.G55D | 1 | 14.0 | Ins:1 | – | – | – | · | · | – | 18 |
| 7 | c.176A>T | p.Q59L | – | – | – | · | · | · | · | · | – | 19 |
| 8 | c188delC | p.P63Rfs*60 | 11 | 18.1 | Diet:3, Oral:4, Ins:5 | 6 | 45.7 | Diet:2, Oral:4 | + (6) | – | + (2) | 1, 2, 20–22 |
| 9 | c.218delT | p.L73Pfs*50 | 1 | 16.0 | Oral:1 | 1 | 56.0 | Diet:1 | + (1) | – | – | our case |
| 10 | c.226G>A | p.D76N | 6 | 24.2 | Diet:1, Oral:1 | 38 | 47.4 | Diet:2, Oral:4, Ins:4 | +(2) | + (4) | – | 13 |
| 11 | c.260C>T | p.P87L | – | – | – | – | – | – | · | · | + (1) | 14 |
| 12 | c.440G>A | p.K147R | 1 | · | · | – | – | – | · | · | – | 23 |
| 13 | c.455C>G | p.A152G | – | – | – | – | – | – | · | · | + (1) | 24 |
| 14 | c.463C>A | p.R155S | 1 | 14.0 | Oral:1 | 2 | 42.5 | Oral:2 | + (2) | – | – | 25 |
| 15 | c.488A>G | p.K163R | 1 | 18.0 | Ins:1 | – | – | – | · | · | + (1) | 26 |
| 16 | c.492G>T | p.E164D | – | – | – | – | – | – | · | · | + (1) | 27 |
| 17 | c.499T>G | p.F167V | – | – | – | – | – | – | – | – | + (1) | 28 |
| 18 | c.504C>G | p.N168K | 1 | · | · | – | – | – | · | · | – | 29 |
| 19 | c.508T>C | p.Y170H | – | – | – | – | – | – | · | · | + (1) | 30 |
| 20 | c.529G>A | p.V177M | 1 | 26.0 | Ins:1 | – | – | – | · | · | – | 31 |
| 21 | c.532G>A | p.E178K | – | – | – | – | – | – | · | · | + (1) | 27 |
| 22 | c.559G>A | p.E187K | 1 | · | · | – | – | – | · | · | – | 32 |
| 23 | c.571A>C | p.K191Q | 1 | · | · | – | – | – | · | · | – | 33 |
| 24 | c.590G>A | p.R197H | 1 | 35.0 | Oral:1 | 1 | 45.0 | Oral:1 | · | · | – | 13 |
| 25 | c.634G>A | p.G212R | 2 | 23.0 | · | 1 | 40.0 | · | + (1) | – | 23 | |
| 26 | c.651delT | p.G218Afs*12 | – | · | – | – | – | · | · | – | 34 | |
| 27 | c.670G>A | p.E224K | 9 | 24.8 | · | 1 | 40.0 | · | + (2) | · | – | 31, 36, 36 |
| 28 | c.694_697del GGCGinsAGCT | p.G232_E233del insSer* | 1 | 26.0 | Ins:1 | – | – | – | – | · | – | 37 |
| 29 | c.716C>A | p.P239Q | 4 | 20.5 | · | 6 | 56.7 | · | · | + (3) | – | 23 |
| 30 | c.726_728dup GCC | p.P244dup | 10 | 34.0 | · | 7 | 44.1 | · | +(8) | · | – | 19 |
| 31 | c.848G>A | p.R283Q | 1 | · | · | 1 | · | · | · | · | – | 38 |
diet: diet therapy; oral: oral antidiabetic agent; Ins: insulin therapy
Figure 4.
Diagram of PDX1 protein structure and reported amino acid changes as well as their phenotypes. The mutations and the phenotypes of the present cases are highlighted in red. Note that all phenotypes (“early-onset diabetes (onset < 35 years),” “late-onset diabetes (onset ≥ 35 years),” “obesity,” and “no diabetes”) except for “neonatal diabetes with homozygous variants” correspond to heterozygous variants. Obesity was defined as a body mass index of ≥ 25 kg/m2. “No diabetes” is defined as the state of not having developed diabetes by the age of ≥ 50.
In conclusion, we found a novel heterozygous PDX1 frameshift mutation in the first Japanese family to be identified with PDX1-MODY (MODY4). Mutant, truncated PDX1 protein is likely to act through haploinsufficiency, as it did not exert a dominant-negative effect in an overexpression experiment. As for the clinical presentations, the proband and his father, who had overt diabetes and carried the same mutation, showed markedly reduced insulin secretion as well as reduced GIP secretion compared with the mutation-negative family member. The proband responded well to sitagliptin, suggesting dipeptidyl peptidase-4 inhibitors as a treatment option for PDX1-MODY patients. Intriguingly, both men had notable phenotypic differences regarding onset age, obesity, and treatment requirements, suggesting that PDX1-MODY is an oligogenic rather than a monogenic form of diabetes. The phenotypic similarities and differences of the present cases, along with the heterogeneous phenotypes of previously reported cases, highlight the wide phenotypic spectrum of PDX1-MODY.
Acknowledgments
We thank H. Ikeda for his assistance with collecting clinical information. The authors also thank H. Furuta, H. Tsuchida, J. Kawada, and M. Kato for their technical assistance and M. Yato, Y. Ogiso, and M. Nozu for their secretarial assistance.
Financial Support : This work was supported by a Health and Labor Science Research Grant for Research on Rare and Intractable Diseases from the Japanese Ministry of Health, Labor and Welfare and a Grant-in-Aid for Scientific Research from the Japanese Ministry of Science, Education, Sports, Culture and Technology, and a Research Grant from Japan Diabetes Foundation and Costco Wholesale Japan Ltd. Satoshi Yoshiji is supported by the Japan Society for the Promotion of Science (DC1).
Author Contributions : All authors have read and approved the final manuscript. S.Y., Y.H., S.K., M.E., K.H., and A.H. collected and analyzed the data. S.Y. and Y.H. wrote the first draft of the manuscript. S.Y., Y.H., S.K., M.E., Y.H., Y.K., M.A., K.I., S.H., K.H., D.Y., and A.H. contributed to the final version of the manuscript.
Ethical Approval : All clinical examinations and genetic testing conformed to the provisions of the Declaration of Helsinki and were approved by the local ethics committee of Kitano Hospital and Gifu University Hospital. The study protocol was approved by the Institutional Review Board of Kitano Hospital (No.1807005) and Gifu University (No. 25-153).
Patient Consent : Written informed consent was obtained from all participants for the publication of this study.
Glossary
Abbreviations
- GIP
gastric inhibitory polypeptide
- GLP-1
glucagon-like peptide-1
- HbA1c
glycosylated hemoglobin A1c
- OGTT
oral glucose tolerance test
- PDX1
pancreatic and duodenal homeobox 1
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15(1):106-110. [DOI] [PubMed] [Google Scholar]
- 2. Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17(2):138-139. [DOI] [PubMed] [Google Scholar]
- 3. Horikawa Y, Enya M. Genetic Dissection and Clinical Features of MODY6 (NEUROD1-MODY). Curr Diab Rep. 2019;19(3):12. [DOI] [PubMed] [Google Scholar]
- 4. Aarthy R, Aston-Mourney K, Mikocka-Walus A, et al. Clinical features, complications and treatment of rarer forms of maturity-onset diabetes of the young (MODY) - A review. J Diabetes Complications. 2021;35(1):107640. [DOI] [PubMed] [Google Scholar]
- 5. Yoshiji S, Horikawa H, Kubota S, et al. Identification of the First Japanese Family with PDX1-MODY (MODY4): A Novel PDX1 Frameshift Mutation, Clinical Characteristics, and Implications. Dryad Digital Repository. Deposited September 18, 2021. https://doiorg/105061/dryadvdncjsxvs [DOI] [PMC free article] [PubMed]
- 6. Richards S, Aziz N, Bale S, et al. ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jepeal LI, Fujitani Y, Boylan MO, Wilson CN, Wright CV, Wolfe MM. Cell-specific expression of glucose-dependent-insulinotropic polypeptide is regulated by the transcription factor PDX-1. Endocrinology. 2005;146(1):383-391. [DOI] [PubMed] [Google Scholar]
- 8. Lewis JT, Dayanandan B, Habener JF, Kieffer TJ. Glucose-dependent insulinotropic polypeptide confers early phase insulin release to oral glucose in rats: demonstration by a receptor antagonist. Endocrinology. 2000;141(10):3710-3716. [DOI] [PubMed] [Google Scholar]
- 9. Hayashi T, Boyko EJ, Sato KK, et al. Patterns of insulin concentration during the OGTT predict the risk of type 2 diabetes in Japanese Americans. Diabetes Care. 2013;36(5):1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Locke JM, Saint-Martin C, Laver TW, et al. The Common HNF1A Variant I27L Is a Modifier of Age at Diabetes Diagnosis in Individuals With HNF1A-MODY. Diabetes. 2018;67(9):1903-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horikawa Y. Maturity-onset diabetes of the young as a model for elucidating the multifactorial origin of type 2 diabetes mellitus. J Diabetes Investig. 2018;9(4):704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tonooka N, Tomura H, Takahashi Y, et al. High frequency of mutations in the HNF-1alpha gene in non-obese patients with diabetes of youth in Japanese and identification of a case of digenic inheritance. Diabetologia. 2002;45(12):1709-1712. [DOI] [PubMed] [Google Scholar]
- 13. Macfarlane WM, Frayling TM, Ellard S, et al. Missense mutations in the insulin promoter factor-1 gene predispose to type 2 diabetes. J Clin Invest. 1999;104(9):R33-R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Franco E, Shaw-Smith C, Flanagan SE, et al. Biallelic PDX1 (insulin promoter factor 1) mutations causing neonatal diabetes without exocrine pancreatic insufficiency. Diabet Med. 2013;30(5):e197-e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gragnoli C, Stanojevic V, Gorini A, Von Preussenthal GM, Thomas MK, Habener JF. IPF-1/MODY4 gene missense mutation in an Italian family with type 2 and gestational diabetes. Metabolism. 2005;54(8):983-988. [DOI] [PubMed] [Google Scholar]
- 16. Hildebrand JM, Lo B, Tomei S, et al. A family harboring an MLKL loss of function variant implicates impaired necroptosis in diabetes. Cell Death Dis. 2021;12(4):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Retterer K, Juusola J, Cho MT, et al. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2016;18(7):696-704. [DOI] [PubMed] [Google Scholar]
- 18. Anık A, Çatlı G, Abacı A, et al. Molecular diagnosis of maturity-onset diabetes of the young (MODY) in Turkish children by using targeted next-generation sequencing. J Pediatr Endocirnol Metab. 2015;28(11-12):1265-1271. [DOI] [PubMed] [Google Scholar]
- 19. Hani EH, Stoffers DA, Chèvre JC, et al. Defective mutations in the insulin promoter factor-1 (IPF-1) gene in late-onset type 2 diabetes mellitus. J Clin Invest. 1999;104(9):R41-R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fajans SS, Bell GI, Paz VP, et al. Obesity and hyperinsulinemia in a family with pancreatic agenesis and MODY caused by the IPF1 mutation Pro63fsX60. Transl Res. 2010;156(1):7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas IH, Saini NK, Adhikari A, et al. Neonatal diabetes mellitus with pancreatic agenesis in an infant with homozygous IPF-1 Pro63fsX60 mutation. Pediatr Diabetes. 2009;10(7):492-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caetano LA, Santana LS, Costa-Riquetto AD, et al. PDX1 -MODY and dorsal pancreatic agenesis: new phenotype of a rare disease. Clin Genet. 2018;93(2):382-386. [DOI] [PubMed] [Google Scholar]
- 23. Weng J, Macfarlane WM, Lehto M, et al. Functional consequences of mutations in the MODY4 gene (IPF1) and coexistence with MODY3 mutations. Diabetologia. 2001;44(2):249-258. [DOI] [PubMed] [Google Scholar]
- 24. Mohan V, Radha V, Nguyen TT, et al. Comprehensive genomic analysis identifies pathogenic variants in maturity-onset diabetes of the young (MODY) patients in South India. BMC Med Genet. 2018;19(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng M, Xiao X, Zhou L, Wang T. First Case Report of Maturity-Onset Diabetes of the Young Type 4 Pedigree in a Chinese Family. Front Endocrinol (Lausanne). 2019;10:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulkarni A, Sharma VK, Nabi F. PDX1 Gene Mutation with Permanent Neonatal Diabetes Mellitus with Annular Pancreas, Duodenal Atresia, Hypoplastic Gall Bladder and Exocrine Pancreatic Insufficiency. Indian Pediatr. 2017;54(12):1052-1053. [DOI] [PubMed] [Google Scholar]
- 27. Schwitzgebel VM, Mamin A, Brun T, et al. Agenesis of human pancreas due to decreased half-life of insulin promoter factor 1. J Clin Endocrinol Metab. 2003;88(9):4398-4406. [DOI] [PubMed] [Google Scholar]
- 28. Sahebi L, Niknafs N, Dalili H, et al. Iranian neonatal diabetes mellitus due to mutation in PDX1 gene: a case report. J Med Case Rep. 2019;13(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson SR, Ellis JJ, Leo PJ, et al. Comprehensive genetic screening: The prevalence of maturity-onset diabetes of the young gene variants in a population-based childhood diabetes cohort. Pediatr Diabetes. 2019;20(1):57-64. [DOI] [PubMed] [Google Scholar]
- 30. Ellard S, Lango Allen H, De Franco E, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia. 2013;56(9):1958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chapla A, Mruthyunjaya MD, Asha HS, et al. Maturity onset diabetes of the young in India - a distinctive mutation pattern identified through targeted next-generation sequencing. Clin Endocrinol (Oxf). 2015;82(4):533-542. [DOI] [PubMed] [Google Scholar]
- 32. Flannick J, Beer NL, Bick AG, et al. Assessing the phenotypic effects in the general population of rare variants in genes for a dominant Mendelian form of diabetes. Nat Genet. 2013;45(11):1380-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brahm AJ, Wang G, Wang J, et al. Genetic Confirmation Rate in Clinically Suspected Maturity-Onset Diabetes of the Young. Can J Diabetes. 2016;40(6):555-560. [DOI] [PubMed] [Google Scholar]
- 34. Steinthorsdottir V, Thorleifsson G, Sulem P, et al. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46(3):294-298. [DOI] [PubMed] [Google Scholar]
- 35. Cockburn BN, Bermano G, Boodram LL, et al. Insulin promoter factor-1 mutations and diabetes in Trinidad: identification of a novel diabetes-associated mutation (E224K) in an Indo-Trinidadian family. J Clin Endocrinol Metab. 2004;89(2):971-978. [DOI] [PubMed] [Google Scholar]
- 36. Doddabelavangala Mruthyunjaya M, Chapla A, Hesarghatta Shyamasunder A, et al. Comprehensive Maturity Onset Diabetes of the Young (MODY) Gene Screening in Pregnant Women with Diabetes in India. Plos One. 2017;12(1):e0168656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mangrum C, Rush E, Shivaswamy V. Genetically Targeted Dipeptidyl Peptidase-4 Inhibitor Use in a Patient with a Novel Mutation of MODY type 4. Clin Med Insights Endocrinol Diabetes. 2015;8:83-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zubkova N, Burumkulova F, Plechanova M, et al. High frequency of pathogenic and rare sequence variants in diabetes-related genes among Russian patients with diabetes in pregnancy. Acta Diabetol. 2019;56(4):413-420. [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Sterr M, Ansarullah, et al. Point mutations in the PDX1 transactivation domain impair human β-cell development and function. Mol Metab. 2019;24:80-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.