Keywords: cortical collecting duct, distal convoluted tubule, epithelial Na+ channel, renal outer medullary K+ channel, with no lysine kinase 1
Abstract
We used whole cell recording to examine the renal outer medullary K+ channel (ROMK or Kir1.1) and epithelial Na+ channel (ENaC) in the late distal convoluted tubule (DCT2)/initial connecting tubule (iCNT) and in the cortical collecting duct (CCD) of kidney tubule-specific neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) knockout mice (Ks-Nedd4-2 KO) and floxed neural precursor cell-expressed developmentally downregulated 4-like (Nedd4l) mice (control). Tertiapin Q (TPNQ)-sensitive K+ currents (ROMK) were smaller in both the DCT2/iCNT and CCD of Ks-Nedd4-2 KO mice on a normal diet than in control mice. Neither high dietary salt intake nor low dietary salt intake had a significant effect on ROMK activity in the DCT2/iCNT and CCD of control and Ks-Nedd4-2 KO mice. In contrast, high dietary K+ intake (HK) increased, whereas low dietary K+ intake (LK) decreased TPNQ-sensitive K+ currents in floxed Nedd4l mice. However, the effects of dietary K+ intake on ROMK channel activity were absent in Ks-Nedd4-2 KO mice since neither HK nor LK significantly affected TPNQ-sensitive K+ currents in the DCT2/iCNT and CCD. Moreover, TPNQ-sensitive K+ currents in the DCT2/iCNT and CCD of Ks-Nedd4-2 KO mice on HK were similar to those of control mice on LK. Amiloride-sensitive Na+ currents in the DCT2/iCNT and CCD were significantly higher in Ks-Nedd4-2 KO mice than in floxed Nedd4l mice on a normal K+ diet. HK increased ENaC activity of the DCT2/iCNT only in control mice, but HK stimulated ENaC of the CCD in both control and Ks-Nedd4-2 KO mice. Moreover, the HK-induced increase in amiloride-sensitive Na+ currents was larger in Ks-Nedd4-2 KO mice than in control mice. Deletion of Nedd4-2 increased with no lysine kinase 1 expression and abolished HK-induced inhibition of with no lysine kinase 1. We conclude that deletion of Nedd4-2 increases ENaC activity but decreases ROMK activity in the aldosterone-sensitive distal nephron and that HK fails to stimulate ROMK, but robustly increases ENaC activity in the CCD of Nedd4-2-deficient mice.
NEW & NOTEWORTHY We demonstrate that renal outer medullary K+ (ROMK) channel activity is inhibited in the late distal convoluted tubule/initial connecting tubule and cortical collecting duct of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2)-deficient mice. Also, deletion of Nedd4-2 abolishes the stimulatory effect of dietary K+ intake on ROMK. The lack of high K+-induced stimulation of ROMK is associated with the absence of high K+-induced inhibition of with no lysine kinase 1.
INTRODUCTION
Renal outer medullary K+ (ROMK; Kir1.1) activity is detected in the apical membrane of the thick ascending limb (TAL) (1), late distal convoluted tubule (DCT2), connecting tubule (CNT), and cortical collecting duct (CCD) (2–8). Although ROMK immunostaining was also detected in the early distal convoluted tubule (DCT) (9), patch-clamp failed to detect its channel activity (10), suggesting that ROMK channels in the early DCT were either closed or not actively expressed in the apical membrane. Although ROMK in the TAL plays a key role in K+ recycling (11), which is essential for maintaining NaCl reabsorption via type II Na+-K+-2Cl− cotransporters (12), ROMK plays an important role in mediating K+ excretion in the aldosterone-sensitive distal nephron (ASDN) including the DCT2, CNT, and CCD (13). This notion is supported by finding that high dietary K+ intake (HK) stimulates, whereas low dietary K+ intake (LK) inhibits, ROMK channel activity (14–17), suggesting the role of ROMK in maintaining body K+ homeostasis. Indeed, deletion of ROMK1, a major ROMK isoform in the CCD, impaired renal K+ excretion during HK and caused hyperkalemia (18). ROMK-mediated K+ excretion in the ASDN is a two-step process: K+ enters the cell across the basolateral membrane of principal cells by Na+-K+-ATPase and then is secreted into the lumen through luminal ROMK channels. The driving force for mediating K+ excretion is provided by the epithelial Na+ channel (ENaC), which is colocalized with ROMK in the apical membrane of the ASDN (19–23). ENaC is composed of α-, β-, and γ-subunits (24), and the COOH-termini of β-ENaC and γ-ENaC have a PY motif that binds to the WW domain of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) (25), an E3 ubiquitin ligase (26–28), which is expressed in the ASDN (29, 30). Binding of Nedd4-2 to ENaC is essential for inducing ubiquitination of ENaC. It is well established that low salt intake (LS) or HK stimulates ENaC activity in the ASDN, whereas high salt intake (HS) or LK inhibits ENaC activity (31–33). The regulation of ENaC expression by dietary K+ or Na+ intake is most likely to require Nedd4-2 (29). For instance, both HK and LS stimulate aldosterone secretion, and aldosterone stimulates ENaC activity, in part, by inhibiting Nedd4-2 binding to the PY motif of ENaC through stimulation of serum/glucocorticoid regulated kinase 1-induced phosphorylation (34). Although the role of Nedd4-2 in regulating ENaC is well illustrated, the role of Nedd4-2 in regulation of the ROMK channel is largely unknown. Using immunostaining, a previous study demonstrated that ROMK expression was increased in the ASDN of kidney tubule-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice (35), suggesting the possibility that Nedd4-2 may play a role in regulating ROMK protein abundance. Because ROMK immunostaining intensity may not be equal to K+ channel activity, the main goal of this study was to examine whether deletion of Nedd4-2 affects baseline activity of the ROMK channel and whether regulation of ROMK by dietary K+ and Na+ intake is different in control and Nedd4-2-deficient mice.
METHODS
Animals
Male floxed neural precursor cell-expressed developmentally downregulated 4-like (Nedd4l) (Nedd4lfl/fl) mice (control) and Ks-Nedd4-2 KO mice were used in our study, and the method for generation of animals has been previously described (35–37). Briefly, mice expressing Pax8-rtTA and tet-on LC-1, which drive Pax8 and Cre expression under tetracycline-dependent induction, were crossed with Nedd4lfl/fl mice to generate inducible Ks-Nedd4-2 KO mice. For genotyping, we amplified tail DNA by the PCR method. For Nedd4l, the forward and reverse primers were 5′- TGAGCTCATTGCTTCACTTCC-3′ and5′- TTCATGCTCGAAGCCTTAGC-3′, respectively (230 bp for floxed Nedd4l and 150 bp for wild type). For Pax8-rtTA, the forward and reverse primers were 5′- CCATGTCTAGACTGGACAAGA-3′ and 5′- CTCCAGGCCACATATGATTAG-3′, respectively (a 650-bp product). For LC1-CRE, the forward and reverse primers were 5′- TCGCTGCATTACCGGTCGATGC-3′ and 5′- CCATGAGTGAACGAACCTGGTCG-3′, respectively (a 430-bp product). We fed 10- to 12-wk-old Pax8-cre-Nedd4lfl/fl mice with doxycycline (2 mg in 2% sucrose solution) for 2 wk, and mice were then kept for an additional 2 wk without doxycycline. We randomly selected three mice from each group of peers to conduct Western blot analysis to confirm the deletion of Nedd4-2. We also fed Nedd4lfl/fl mice with 2% sucrose for 2 wk as control mice. Animals were housed in the New York Medical College animal facility with lights on at 7:00 AM and lights off at 7:00 PM. Mice had unlimited access to water and rodent chow. Mice were fed with the control diet (0.8% K+ and 0.4% Na+), HK diet (5% K+ and 0.4% Na+), HK diet plus spironolactone (added in drinking water) at 40 mg/kg body wt/day for 7 days, LK diet (0.01–0.02% K+ and 0.4% Na+), HS diet (4% Na+ and 0.8% K+), or LS diet (0.01–0.02% Na+ and 0.8% K+) for 7 days. The HS (4%) diet (Cat. No. TD.92034), LS diet (Cat. No. TD.90228), HK diet (Cat. No. TD110866), and LK diet (Cat. No. TD 120441) were purchased from Envigo-Teklad Diets (Madison, WI). All procedures were reviewed and approved by the Institutional Animal Care and Use Committee.
Preparation of the DCT and CCD
We used 14- to 16-wk-old male Nedd4lfl/fl and Ks-Nedd4-2 KO mice for dissection of the renal DCT or CCD and harvest of renal tissues. Mice were euthanized by CO2 inhalation plus cervical dislocation, and the abdomen of the mice was then opened to expose the left kidney. We perfused the left kidney with 2 mL L-15 medium (Life Technology) containing collagenase type 2 (250 U/mL) and then removed the collagenase-perfused kidney. The renal cortex was separated and further cut into small pieces for additional incubation in collagenase-containing L-15 media for 40–50 min at 37°C. The tissue was then washed three times with fresh L-15 medium and transferred to an ice-cold chamber for dissection. The isolated DCT or CCD tubules were placed on a small cover glass coated with poly-l-lysine, and the cover glass was placed on a chamber mounted on an inverted microscope.
Whole Cell Recording
We measured whole cell tertiapin Q (TPNQ)-sensitive K+ currents (ROMK) and amiloride-sensitive Na+ currents (ENaC) in male control and Ks-Nedd4-2 KO mice with an Axon 200 A amplifier. The patch-clamp experiments were performed in the split-open DCT/initial CNT (iCNT) (the last 100 µm of the DCT before the start of the CNT) and CCD using a gap-free protocol. Since the diameter of the DCT2 is normally larger than the CNT, this anatomic characterization has been used to determine the end of the DCT or start of the CNT. However, it was not always obvious to identify the beginning of the CNT in some cases. Thus, it was very likely that some experiments were actually performed in the early CNT. Thus, we have referred that the study was performed in the DCT2/iCNT. The CCD was identified after the first branch of the CNT. After formation of a high-resistance seal, the membrane capacitance was monitored until the whole cell patch configuration was formed. To measure TPNQ-sensitive K+ currents, the tip of the pipette was filled with a pipette solution containing (in mM) 140 KCl, 2 MgATP, 1 EGTA, and 10 HEPES (pH 7.4). The pipette was then back filled with pipette solution containing amphotericin B (20 μg/0.1 mL). The bath solution contained (in mM) 140 KCl, 2 MgCl2, 1.8 CaCl2, and 10 HEPES (pH 7.4). ENaC currents were determined by adding amiloride (10 µΜ) in the bath solution. The pipette solution contained (in mM) 125 K-gluconate, 15 KCl, 2 MgATP, 1 EGTA, and 10 HEPES (pH 7.4), and the bath solution contained 130 Na-gluconate, 10 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, and 5 HEPES (pH 7.4). Currents were low-pass filtered at 1 kHz and digitized by an Axon interface with a sampling rate of 4 kHz (Digidata 1440 A). Data were analyzed using pClamp software system 9.0 (Axon).
Single-Channel Recording
Single-channel patch-clamp experiments were performed in the split-open CCD to examine ROMK channel activity. Single K+ channel currents were recorded with an Axon200B amplifier (Axon), low-pass filtered at 1 kHz, and digitized by an Axon interface (Digidata 1332) with a sampling rate of 4 kHz. The pipette solution for the single channel recording contained (in mM) 140 KCl, 2 MgCl2, 1 EGTA, and 10 HEPES (titrated with KOH to pH 7.4), and the bath solution contained 135 NaCl, 5 KCl, 2 MgCl2, 1.8 CaCl2, 5 glucose, and 10 HEPES (titrated with NaOH to pH 7.4). For the calculation of channel open probability (Po), we selected a channel recording that was at least 5 min long and had less than three current levels to determine the close line. We determined channel Po from the product of channel number (N) and Po (NPo), which was calculated from data samples of 60 s duration in the steady state. NPo was determined using the following equation:
where ti is the fractional open time spent at each of the observed current levels. The channel conductance was determined by measuring current amplitudes over several voltages.
Measurement of Benzamil-Induced Renal K+ Excretion
Animal were anesthetized with inactin at 100 mg/kg by peritoneal injection. Mice were placed on a heated small blanket to maintain body temperature at 37°C. The trachea was cannulated to clear any mucus that could be produced during the experiment. A carotid artery was catheterized with PE-10 tubing for blood collection; the jugular vein was also cannulated for intravenous infusion. The bladder was exposed and catheterized via a suprapubic incision with a 10-cm piece of PE-10 tubing for urine collections. After completion of surgery, isotonic saline was given intravenously for 4 h (0.25–0.3 mL/1 h and total 1.0–1.2 mL of 0.9% saline) to replace surgical fluid losses and to maintain hemodynamics. Urine collections started 1 h after infusion of 0.3 mL saline, and a total of six collections (every 30 min) were performed (2 collections before and 4 collections after benzamil at 5 mg/kg body wt). Urine K+ concentrations were measured using a dual-channel flame photometer with an internal lithium standard (Cole-Parmer Instrument, Vernon Hills, IL).
Immunoblot Analysis
Renal tissues harvested from the cortex were homogenized in buffer containing 250 mM sucrose, 50 mM Tris·HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, and 1 mM DTT supplemented with phosphatase and protease inhibitor cocktails (Sigma). Protein (40–60 µg) was separated on 4–12% (wt/vol) Tris-glycine gels (Thermo Fisher Scientific) and transferred to nitrocellulose membranes. Membranes were incubated for 1 h with LI-COR blocking buffer (PBS) and then incubated overnight at 4°C with the addition of with no lysine kinase 1 (WNK1; Novus Biologicals, dilution: 1:1,000) or Nedd4-2 antibody (Cell Signaling, dilution: 1:1,000). An Odyssey infrared imaging system (LI-COR) was used to capture images at a wavelength of 680 or 800 nm.
Materials
Inactin, amiloride, benzamil, spironolactone, and TPNQ were purchased from Sigma Aldrich (St. Louis, MO). WNK1 antibody was obtained from Novus Biologicals (Centennial, CO) and diluted at 1:1,000 for Western blots (38).
Statistical Analysis
We used software (Sigma Plot 12) for the statistical analysis. For analyzing the values between two groups, we used a t test; for comparisons of values within the same group, we used a paired t test. We used two-way ANOVA for analyzing the results of more than two groups, and a Holm–Sidak test was used as post hoc analysis. P values of <0.05 were considered statistically significant. Data are presented as means ± SE.
RESULTS
To measure ROMK channel activity in the split-open DCT2/iCNT or CCD, we used TPNQ (400 nM), a specific ROMK inhibitor (39), to measure the whole cell TPNQ-sensitive K+ currents. Figure 1A shows a whole cell gap-free recording (clamped at −40 mV) performed in the DCT2/iCNT and CCD of control mice. The addition of 400 nM TPNQ partially inhibited whole cell K+ currents and the addition of 0.1 mM Ba2+ completely inhibited whole cell K+ currents in both the DCT2/iCNT and CCD. These TPNQ-insensitive/Ba2+-sensitive K+ currents represent basolateral Kir4.1/Kir5.1 channel activity since the K+ currents were absent in Kir4.1-deficient mice (W.-H. Wang, unpublished observations). We also measured TPNQ-sensitive K+ currents in the DCT2/iCNT and CCD of Ks-Nedd4-2 KO mice (Fig. 1B). It was apparent that TPNQ-sensitive K+ currents were smaller in Ks-Nedd4-2 KO mice than in control mice. Figure 1C shows a scatterplot summarizing each value of the measurement and the mean value of TPNQ-sensitive K+ currents measured at −40 mV in control and Ks-Nedd4-2 KO mice. TPNQ-sensitive whole cell K+ currents in the DCT2/iCNT and CCD of control mice were 1,020 ± 60 pA (n = 8 patches or tubules from 5 male mice) and 460 ± 21 pA (n = 7 from 4 male mice), respectively. Deletion of Nedd4-2 decreased ROMK channel activity, and TPNQ-sensitive whole cell K+ currents in the DCT2/iCNT and CCD were 580 ± 21 pA (n = 7 from 4 male mice) and 335 ± 27 pA (n = 7 from 4 male mice), respectively. To determine whether ROMK channel Po was decreased in Ks-Nedd4-2 KO mice, we used single channel recording to examine Po of ROMK channels in the CCD of both control and Ks-Nedd4-2 KO mice (Fig. 1D). We observed that Po of ROMK channels in Ks-Nedd4-2 KO mice was 0.93 ± 0.01 (n = 4 patches) and that it was similar to the control value (0.95 ± 0.01, n = 4), suggesting that the decrease in ROMK channel activity of Ks-Nedd4-2 KO mice is most likely due to a reduction of active ROMK channel numbers in the plasma membrane. Although TPNQ-sensitive K+ currents were decreased in Ks-Nedd4-2 KO mice, we confirmed our previous finding that deletion of Nedd4-2 increased Kir4.1/Kir5.1 activity since TPNQ-insensitive and Ba2+-sensitive K+ currents were larger in Ks-Nedd4-2 KO mice than in control mice (37, 40). Thus, it is unlikely that Nedd4-2 is responsible for causing ubiquitination or degradation of ROMK because deletion of Nedd4-2 did not increase ROMK channel activity as expected, if Nedd4-2 is directly responsible for inhibiting ROMK.
Figure 1.
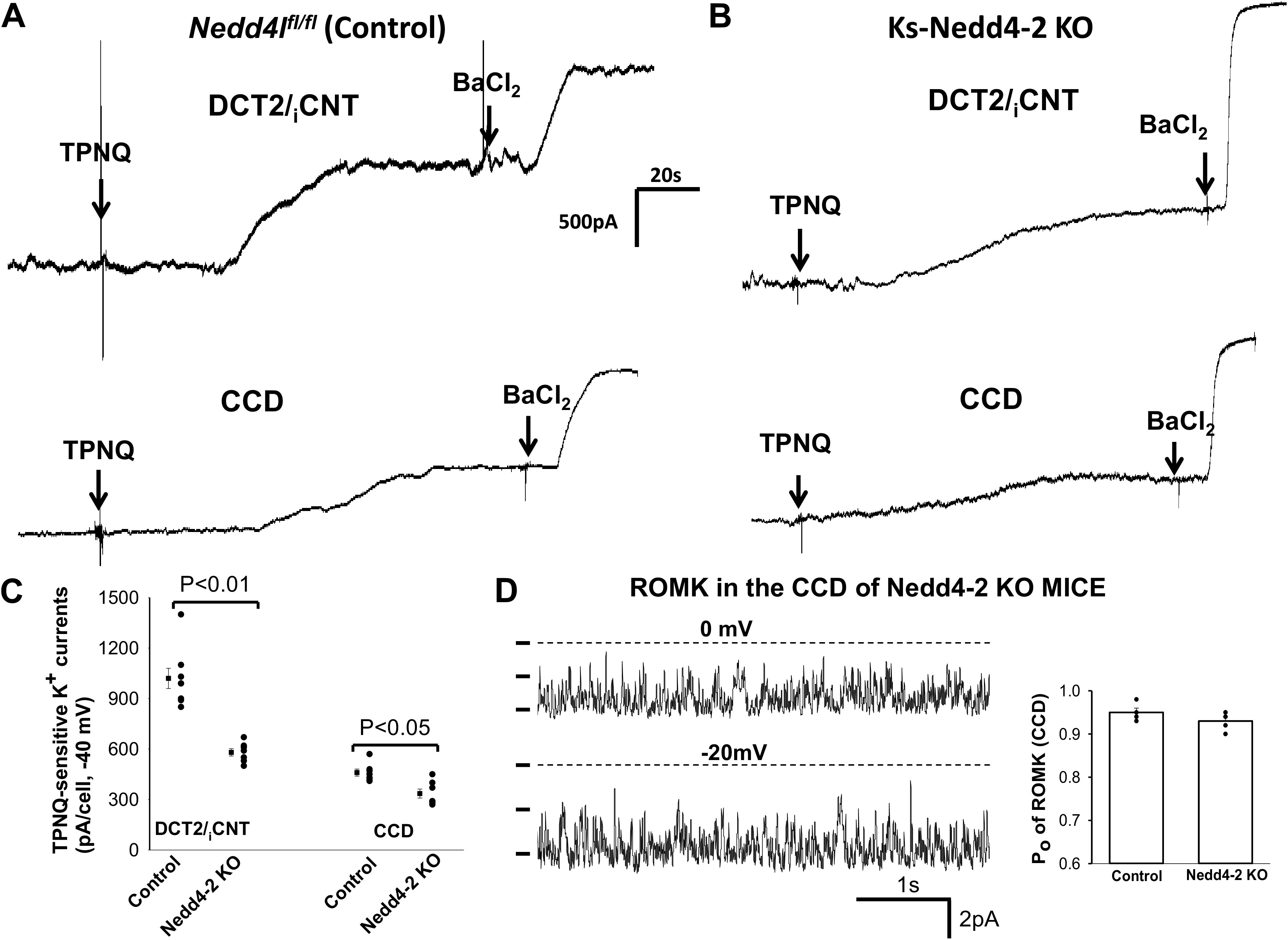
Deletion of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) decreased renal outer medullary K+ (ROMK) activity of the late distal convoluted tubule (DCT2)/initial connection tubule (iCNT) and cortical collecting duct (CCD). A and B: sets of whole cell recordings showing tertiapin Q (TPNQ; 400 nM)-sensitive K+ currents (ROMK) of the DCT2/iCNT and CCD in male control mice (Nedd4lfl/fl; A) and male kidney-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice (B). K+ currents were measured with a gap-free protocol at −40 mV, and BaCl2 (0.1 mM) was added after TPNQ-sensitive K+ currents reached the steady state to detect TPNQ-insensitive and Ba2+-sensitive K+ conductance (basolateral K+ conductance). C: scatterplot summarizing each single value and mean value (on the left of each column) of the experiments in which TPNQ-sensitive K+ currents were measured at −40 mV in the DCT2/iCNT and CCD of control mice and Ks-Nedd4-2 KO mice (n = 7 tubules). Significance was determined by a t test. D: single channel recording showing ROMK channel activity in the CCD of male Ks-Nedd4-2 KO mice on normal K+. The ROMK channel open probability (Po) of each experiment in control and Ks-Nedd4-2 KO mice is summarized as a scatter graph and the mean value is demonstrated in a bar graph (n = 4 tubules). Experiments were performed in a cell-attached patch, and the channel closed level is indicated by a dotted line. The holding potential of the membrane patch is indicated at the top of the trace. Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like.
We next examined the effect of dietary Na+ intake on ROMK channel activity in the control and Ks-Nedd4-2 KO mice because dietary Na+ intake has been shown to regulate Nedd4-2 expression in the ASDN such that HS decreases Nedd4-2 expression (29). If Nedd4-2 regulates ROMK channels in the ASDN, dietary Na+ intake should affect ROMK channel activity differently between control and Nedd4-2-deficient mice. Figure 2A shows a whole cell gap-free recording (clamped at −40 mV) performed in the DCT2/iCNT of control mice on a HS or LS diet for 7 days, and Fig. 2B shows a whole cell recording performed in the DCT2/iCNT of Ks-Nedd4-2 KO mice on a HS or LS diet for 7 days. Figure 2C is a scatterplot summarizing each value of the measurement and the mean value of TPNQ-sensitive K+ currents measured at −40 mV in control mice and in Ks-Nedd4-2 KO mice on different Na+ diets for 7 days. From the inspection of Fig. 2, it is apparent that dietary Na+ intake had no effect on TPNQ-sensitive K+ currents in the DCT2/iCNT of control mice (HS: 920 ± 43 pA, n = 5 from 3 mice; LS: 980 ± 48 pA, n = 6 from 4 mice). Moreover, like control mice, dietary Na+ intake had also no effect on ROMK channel activity in the DCT2/iCNT of Nedd4-2-deficient mice (HS: 620 ± 54 pA, n = 6 from 4 mice; LS: 610 ± 46 pA, n = 6 from 4 mice).
Figure 2.
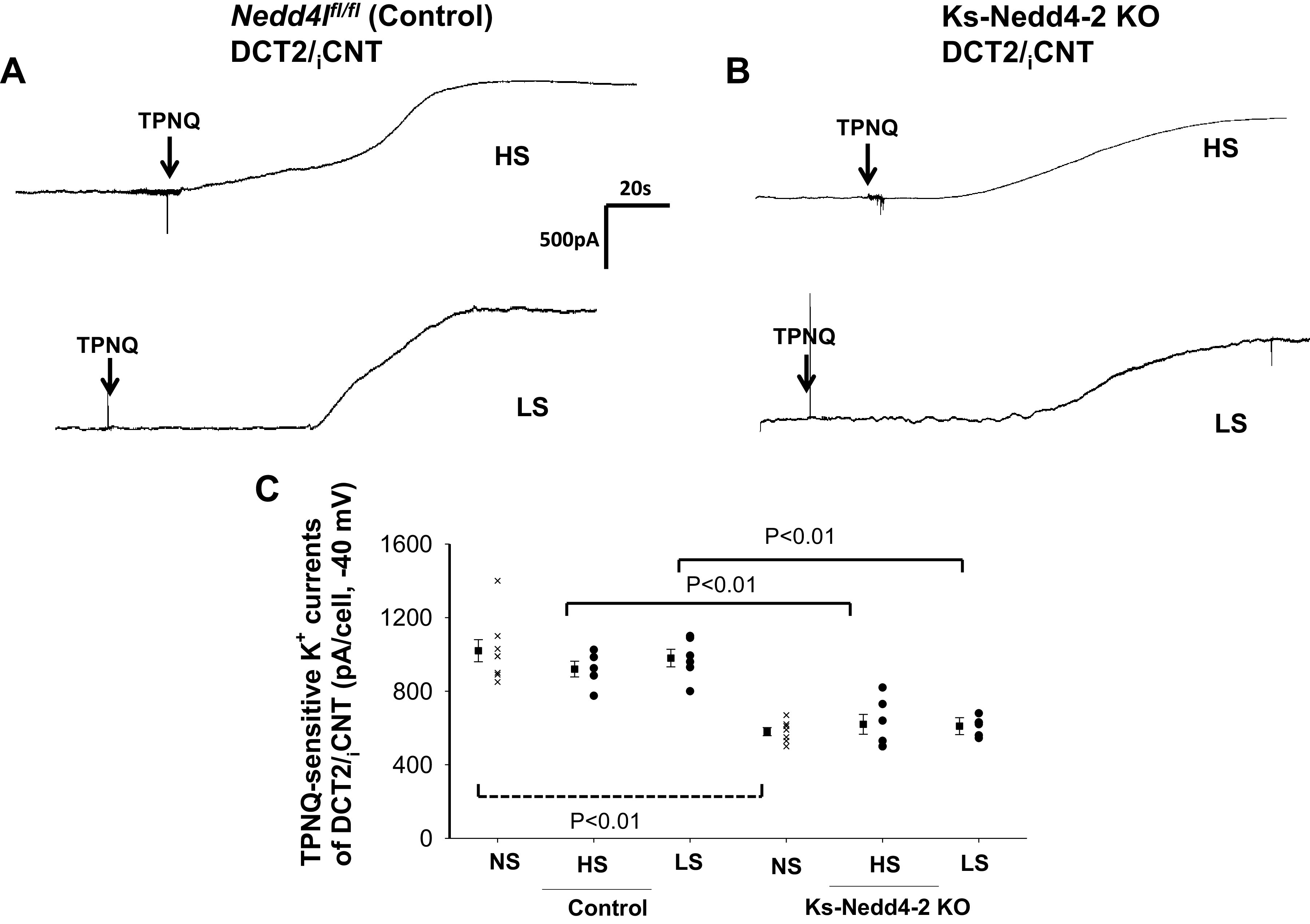
Dietary Na+ intake did not affect renal outer medullary K+ (ROMK) activity in the late distal convoluted tubule (DCT2)/initial connection tubule (iCNT). A and B: sets of whole cell recordings showing tertiapin Q (TPNQ; 400 nM)-sensitive K+ currents (ROMK) of the DCT2/iCNT in male control mice on a high-salt (HS) diet or low-salt (LS) diet for 7 days (A) and in male kidney-specific neural precursor cell-expressed developmentally downregulated protein 4-2 knockout (Ks-Nedd4-2 KO) mice on different Na+ diets (B). K+ currents were measured with a gap-free protocol at −40 mV. C: scatterplot summarizing each single value and mean value (at the left of each column) of the experiments in which TPNQ-sensitive K+ currents were measured at −40 mV in the DCT2/iCNT of control mice and Ks-Nedd4-2 KO mice on different Na+ diets for 7 days (n = 5 or 6 tubules). Results obtained in mice on normal salt (NS; shown in Fig. 1C) are also included for comparison (indicated by a dotted line). Significance was determined by two-way ANOVA. Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like.
We next examined the effect of dietary Na+ intake on ROMK channels in the CCD. Figure 3A shows a whole cell gap-free recording (clamped at −40 mV) performed in the CCD of control mice on a HS or LS diet for 7 days, and Fig. 3B shows a whole cell recording performed in the CCD of Ks-Nedd4-2 KO mice on a HS or LS diet for 7 days. Figure 3C shows a scatterplot summarizing each value of the measurement and the mean value of TPNQ-sensitive K+ currents measured at −40 mV in control mice and in Ks-Nedd4-2 KO mice on different Na+ diets for 7 days. Similar to that in the DCT2/iCNT, dietary Na+ intake had no effect on TPNQ-sensitive K+ currents in the CCD of control mice (HS: 390 ± 30 pA, n = 5 from 3 mice; LS: 440 ± 50 pA, n = 6 from 4 mice). Moreover, dietary Na+ intake did not affect ROMK channel activity in the CCD of Nedd4-2 deficient mice (HS: 320 ± 25 pA, n = 6 from 4 mice; LS: 280 ± 25 pA, n = 6 from 4 mice). However, ROMK currents in the CCD of Ks-Nedd4-2 KO mice on LS were lower than in control mice. We conclude that neither HS nor LS intake had an effect on ROMK channel activity compared with normal salt in the DCT2/iCNT and CCD of either control or Ks-Nedd4-2 KO mice.
Figure 3.
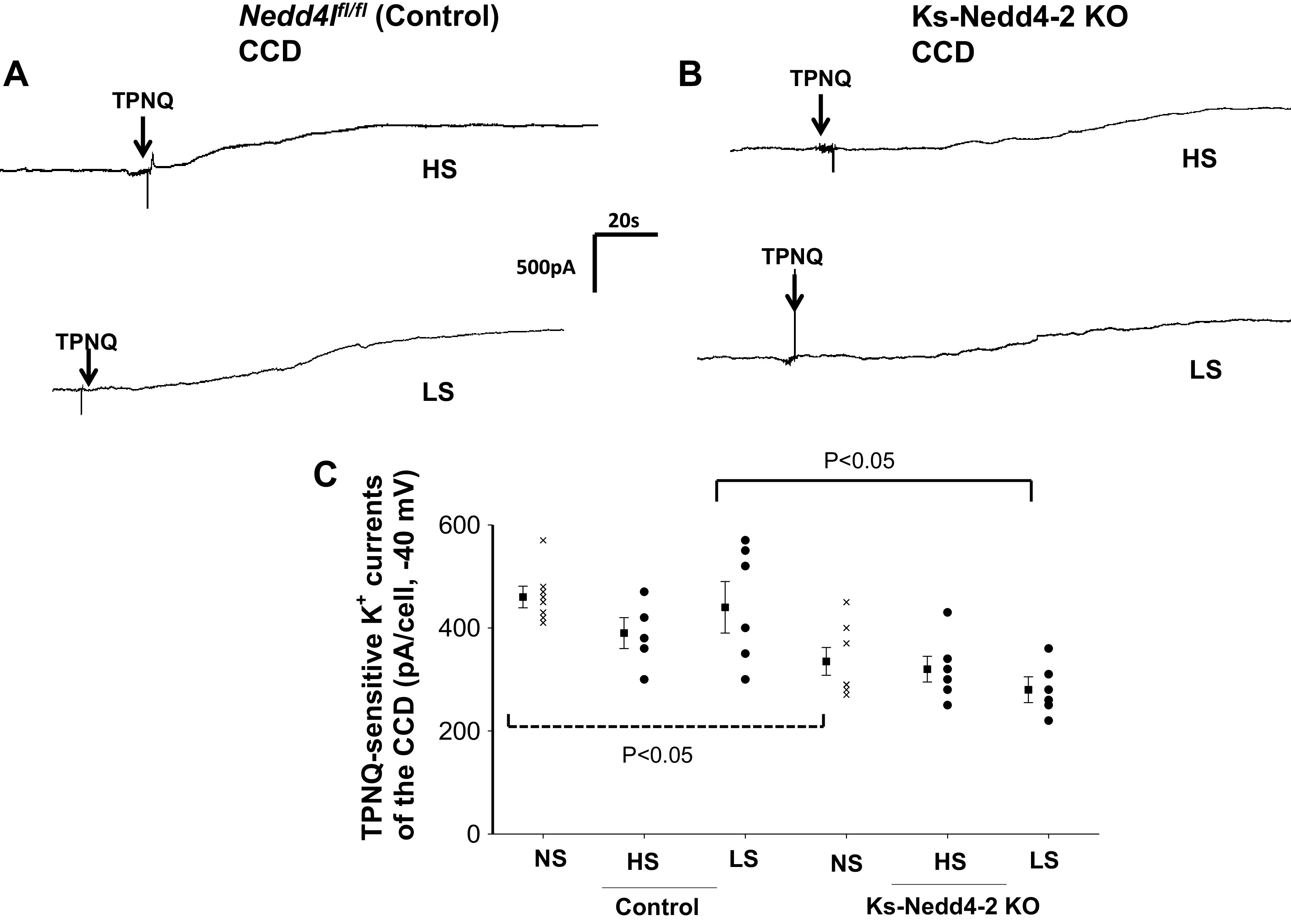
Dietary Na+ intake did not affect renal outer medullary K+ (ROMK) activity in the cortical collecting duct (CCD). A and B: sets of whole cell recordings showing tertiapin Q (TPNQ; 400 nM)-sensitive K+ currents (ROMK) of the CCD in male control mice on a high-salt (HS) diet or low-salt (LS) diet for 7 days (A) and in male kidney-specific neural precursor cell-expressed developmentally downregulated protein 4-2 knockout (Ks-Nedd4-2 KO) mice on different Na+ diets (B). K+ currents were measured with a gap-free protocol at −40 mV. C: scatterplot summarizing each single value and mean value (at the left of each column) of the experiments in which TPNQ-sensitive K+ currents were measured at −40 mV in the CCD of control mice and Ks-Nedd4-2 KO mice on different Na+ diets for 7 days (n = 5 or 6 tubules). The results obtained in mice on normal salt (NS; shown in Fig. 1C) are also included for comparison (indicated by a dotted line). Significance was determined by two-way ANOVA. Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like.
We next examined the effect of dietary K+ intake on ROMK channels in control and Ks-Nedd4-2 KO mice. We and others have demonstrated that HK stimulates, whereas LK inhibits, ROMK channel activity in the ASDN (11, 33). Figure 4A shows a whole cell gap-free recording (clamped at −40 mV) performed in the DCT2i/CNT of control mice on a HK or LK diet for 7 days, and Fig. 4B shows a whole cell recording performed in the DCT2i/CNT of Ks-Nedd4-2 KO mice on a HK or LK diet for 7 days. Figure 4C is a scatterplot summarizing each value of the measurement and the mean value of TPNQ-sensitive K+ currents measured at −40 mV in control mice and in Ks-Nedd4-2 KO mice on different K+ diets for 7 days. From the inspection of Fig. 4, it is apparent that HK increased, whereas LK decreased, TPNQ-sensitive K+ currents in the DCT2/CNT of control mice compared with normal K+ (NK) (HK: 1,520 ± 60 pA, n = 6 from 4 mice; LK: 630 ± 40 pA, n = 6 from 4 mice). However, dietary K+ intake had no effect on ROMK channel activity in the DCT2/iCNT of Nedd4-2-deficient mice compared with NK (HK: 620 ± 33 pA, n = 6 from 4 mice; LK: 600 ± 20 pA, n = 6 from 4 mice). We also examined the effect of HK or LK on ROMK channels in the CCD. Figure 5A shows a whole cell gap-free recording (at −40 mV) performed in the CCD of control mice on a HK or LK diet for 7 days, and Fig. 5B shows a whole cell recording performed in the CCD of Ks-Nedd4-2 KO mice on a HK or LK diet for 7 days. Figure 5C shows a scatterplot summarizing each value of the measurement and the mean value of TPNQ-sensitive K+ currents measured at −40 mV in control mice and in Ks-Nedd4-2 KO mice on different K+ diets for 7 days. HK increased, whereas LK decreased, TPNQ-sensitive K+ currents in the CCD of control mice compared with NK (HK: 850 ± 45 pA, n = 6 from 4 mice; LK: 280 ± 20 pA, n = 6 from 4 mice). However, dietary K+ intake had no effect on ROMK channel activity in the CCD of Nedd4-2-deficient mice compared with NK (HK: 290 ± 25 pA, n = 6 from 4 mice; LK: 260 ± 20 pA, n = 6 from 4 mice).
Figure 4.
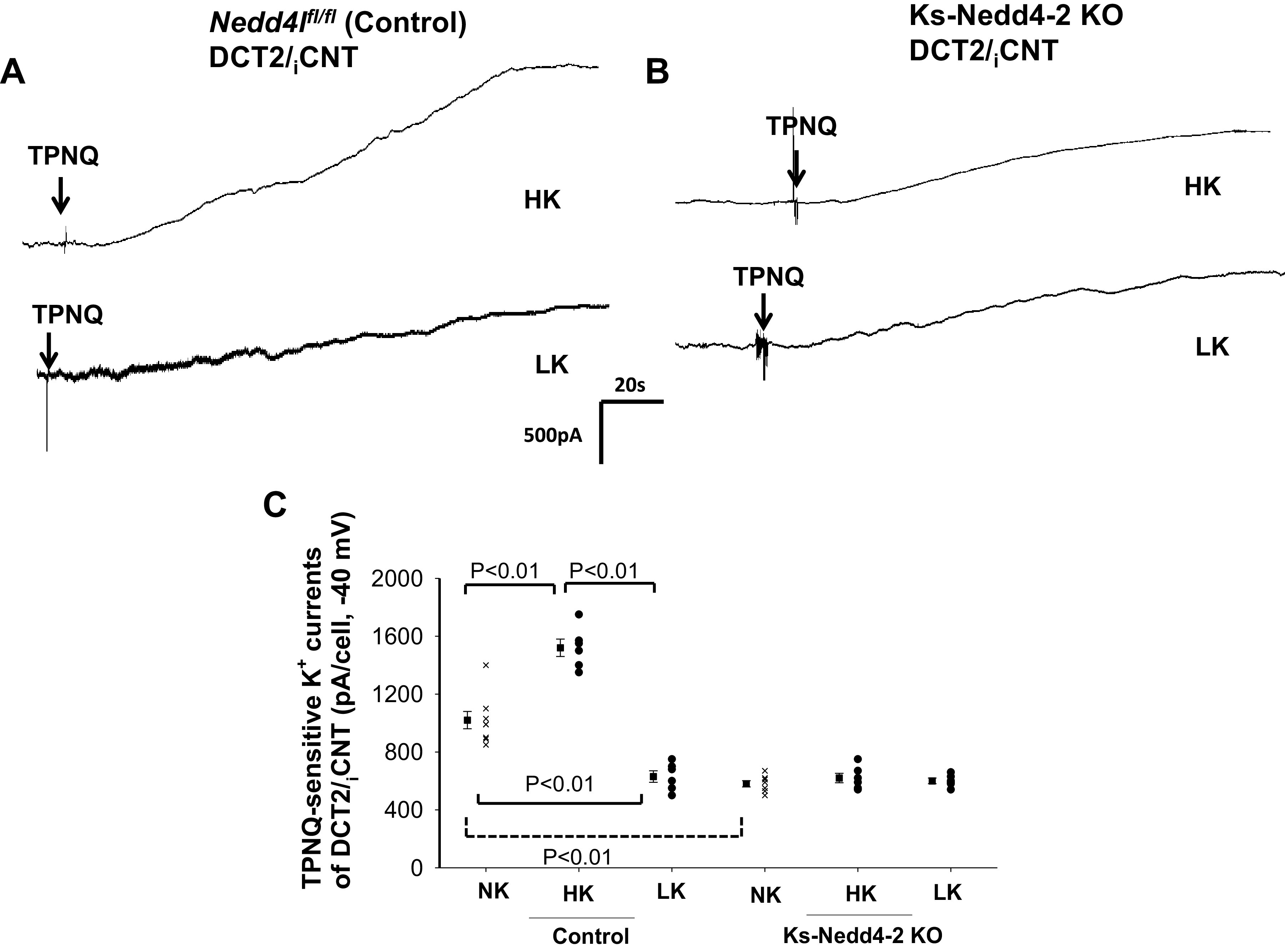
Deletion of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) abolished the effect of dietary K+ intake on renal outer medullary K+ (ROMK) channel activity in the late distal convoluted tubule (DCT2)/initial connection tubule (iCNT). A and B: sets of whole cell recordings showing tertiapin Q (TPNQ; 400 nM)-sensitive K+ currents (ROMK) of the DCT2/iCNT in male control mice on a high K+ (HK) diet or low K+ (LK) diet for 7 days (A) and in male kidney-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice on different K+ diets (B). K+ currents were measured with a gap-free protocol at −40 mV. C: scatterplot summarizing each single value and mean value (at the left of each column) of the experiments in which TPNQ-sensitive K+ currents were measured at −40 mV in the DCT2/iCNT of control mice and Ks-Nedd4-2 KO mice on different K+ diets for 7 days (n = 6 tubules). The results obtained in mice on normal K+ (NK; shown in Fig. 1C) are also included for the comparison (indicated by a dotted line). Significance was determined by two-way ANOVA. Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like.
Figure 5.
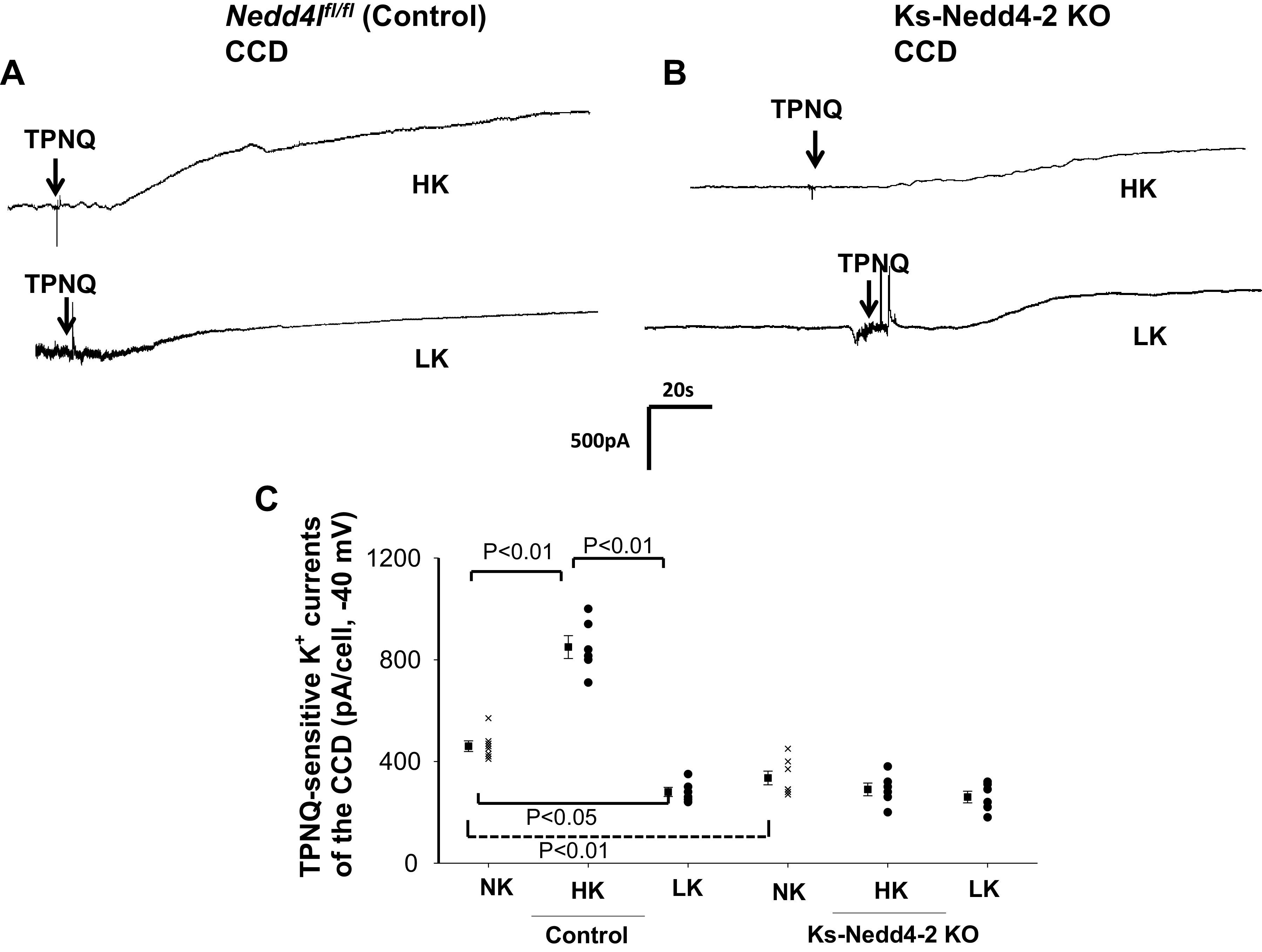
Deletion of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) abolished the effect of dietary K+ intake on renal outer medullary K+ (ROMK) channel activity in the cortical collecting duct (CCD). A and B: sets of whole cell recordings showing tertiapin Q (TPNQ; 400 nM)-sensitive K+ currents (ROMK) of the CCD in male control mice on a high K+ (HK) diet or low K+ (LK) diet for 7 days (A) and in kidney-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice on different K+ diets (B). K+ currents were measured with a gap-free protocol at −40 mV. C: scatterplot summarizing each single value and mean value (at the left of each column) of the experiments in which TPNQ-sensitive K+ currents were measured at −40 mV in the CCD of control mice and Ks-Nedd4-2 KO mice on different K+ diets for 7 days (n = 6 tubules). The results obtained in mice on normal K+ (NK; shown in Fig. 1C) are also included for comparison (indicated by a dotted line). Significance was determined by two-way ANOVA. Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like.
Although HK did not increase, whereas LK failed to decrease ROMK channel activity in Nedd4-2-deficient mice, it is unlikely that Nedd4-2 deletion was directly responsible for the lack of the dietary K+ intake-induced regulation of ROMK channels. Our reasoning is that if Nedd4-2 plays a direct role in mediating the effect of dietary K+ intake on ROMK channels, deletion of Nedd4-2 should increase ROMK channel activity during LK intake. Thus, we suspect that the lack of HK-induced stimulation of ROMK channels in Ks-Nedd4-2 KO mice might be a compensatory regulation in response to excessive high ENaC activity, which was expected to induce renal K+ wasting. To test this notion, we next used the whole cell recording technique to examine the effect of dietary K+ intake on amiloride-sensitive Na+ currents in the DCT2/iCNT of control and Ks-Nedd4-2 KO mice (male). Figure 6, A and B, shows two sets of gap-free recordings demonstrating amiloride (10 µM)-sensitive Na+ currents at −60 mV in the DCT2/iCNT measured with the perforated whole cell patch in control mice on NK or HK for 7 days and in Ks-Nedd4-2 KO mice, respectively. Figure 6C shows a scatterplot summarizing the results of experiments in which amiloride-sensitive Na+ currents were measured at -60 mV with perforated whole cell recording in the DCT2/iCNT of control and Ks-Nedd4-2 KO mice on different K+ diets (7 days). HK increased amiloride-sensitive Na+ currents from 210 ± 11 pA (n = 6 from 4 mice) to 376 ± 29 pA (n = 6 from 4 mice), whereas LK decreased Na+ currents to 87 ± 10 pA (n = 6 from 4 mice) in the DCT2/iCNT of control mice. Amiloride-sensitive Na+ currents were significantly larger (304 ± 34 pA, n = 6 from 4 mice) in Ks-Nedd4-2 KO mice on NK than in control mice. Moreover, neither HK increased (295 ± 14 pA, n = 6 from 4 mice) nor LK significantly decreased (260 ± 20 pA, n = 6 from 4 mice) amiloride-sensitive Na+ currents in Ks-Nedd4-2 KO mice compared with NK. Thus, deletion of Nedd4-2 abolished the effect of HK or LK on ENaC in the DCT2/iCNT.
Figure 6.
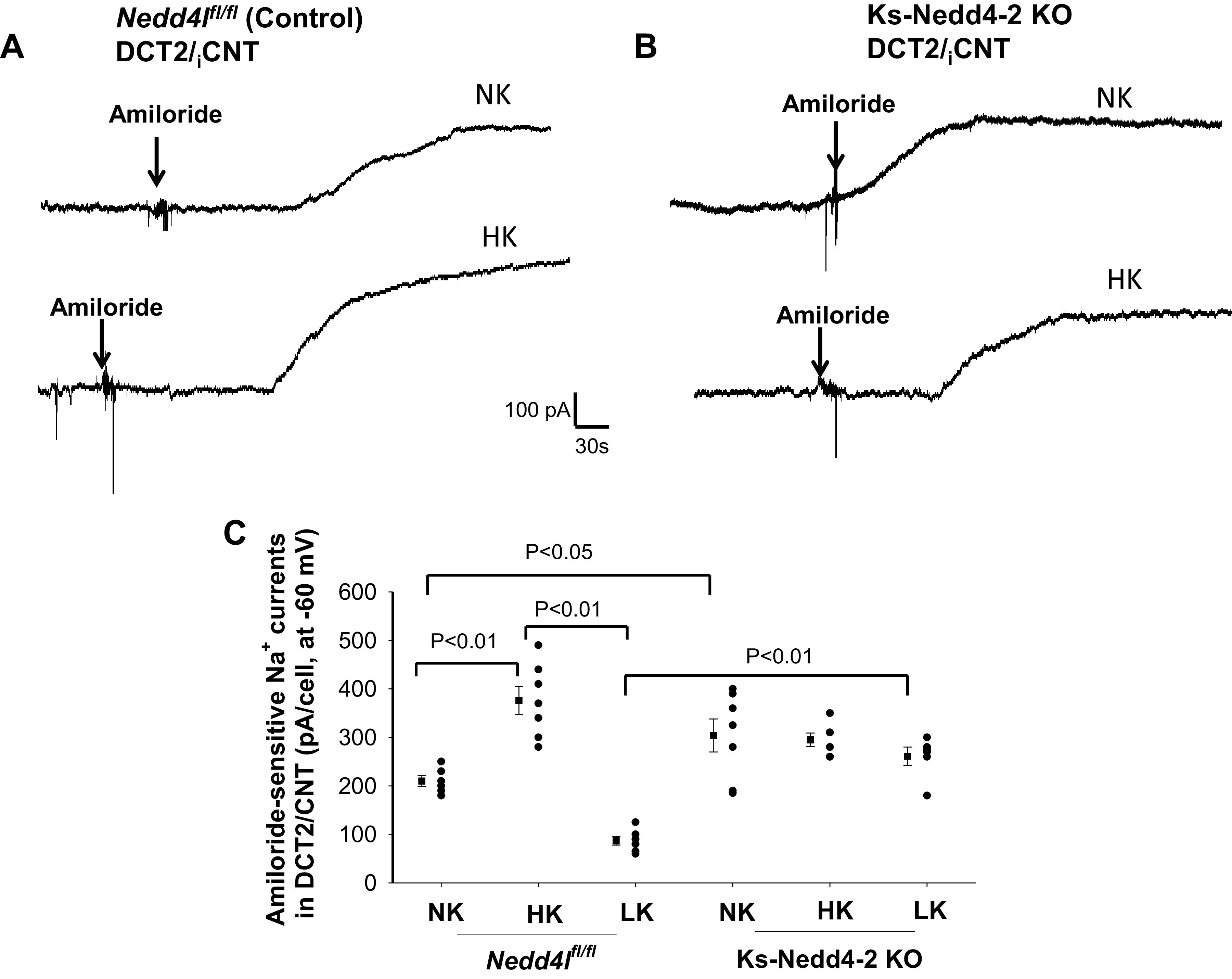
High K+ (HK) intake stimulates the epithelial Na+ channel (ENaC) of the late distal convoluted tubule (DCT2)/initial connection tubule (iCNT) only in control mice. A and B: sets of gap-free recordings showing amiloride-sensitive Na+ currents in the DCT2/iCNT measured at −60 mV with the perforated whole cell patch in male control mice (A) and in male kidney-specific neural precursor cell-expressed developmentally downregulated protein 4-2 knockout (Ks-Nedd4-2 KO) mice (B) on normal K+ (NK) and HK (7 days). C: scatterplot summarizing the results of experiments in which amiloride (10 µM)-sensitive Na+ currents were measured at −60 mV in control and Ks-Nedd4-2 KO mice on different K+ diets for 7 days (n = 6 tubules). Mean values and SEs are shown on the left of each column. An arrow indicates the addition of amiloride (10 µM). Significance was determined by two-way ANOVA. Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like.
We also examined the effect of dietary K+ intake on amiloride-sensitive Na+ currents in the CCD of control and Ks-Nedd4-2 KO mice. Figure 7, A and B, shows two sets of gap-free recordings demonstrating amiloride (10 µM)-sensitive Na+ currents at −60 mV in the CCD measured with perforated whole cell recording in control mice on NK or HK for 7 days and in Ks-Nedd4-2 KO mice, respectively. Figure 7C shows a scatterplot summarizing the results of the above experiments in which amiloride-sensitive Na+ currents of the CCD were measured at −60 mV. HK increased amiloride-sensitive Na+ currents of the CCD from 66 ± 6 pA (n = 5 from 3 mice) to 225 ± 21 pA (n = 7 from 4 mice), whereas LK decreased Na+ currents to 42 ± 5 pA (n = 5 from 3 mice) in control mice. Amiloride-sensitive Na+ currents were significantly larger (217 ± 18 pA, n = 7 from 4 mice) in Ks-Nedd4-2 KO mice on NK than in control mice. Moreover, HK further significantly increased Na+ currents to 395 ± 33 pA (n = 8 from 5 mice). However, treatment of animals with spironolactone for 7 days reduced amiloride-sensitive Na+ currents to 246 ± 23 pA (n = 4 tubules) in the CCD of Ks-Nedd4-2 KO mice on a HK diet for 7 days (Fig. 7C). Also, LK had a trend to decrease Na+ currents (not significant compared with NK) to 151 ± 15 pA (n = 7 from 5 mice) in the CCD of Ks-Nedd4-2 KO mice. Thus, HK stimulates ENaC in the CCD partially by a Nedd4-2-independent mechanism. Although deletion of Nedd4-2 increased amiloride-sensitive Na+ currents in both the DCT2/iCNT and CCD, a previous study demonstrated that plasma K+ concentrations in control and Ks-Nedd4-2 KO mice were similar (see Table 1) (36, 37). Furthermore, measurement of benzamil (5 mg/kg body wt)-sensitive renal K+ excretion in control and Ks-Nedd4-2 KO mice (n = 5) showed that benzamil-induced K+ excretion in Ks-Nedd4-2 KO mice (0.25 ± 0.01 µeq/min/100 g body wt) was similar to control (0.26 ± 0.03 µeq/min/100 g body wt; Fig. 7D).
Figure 7.
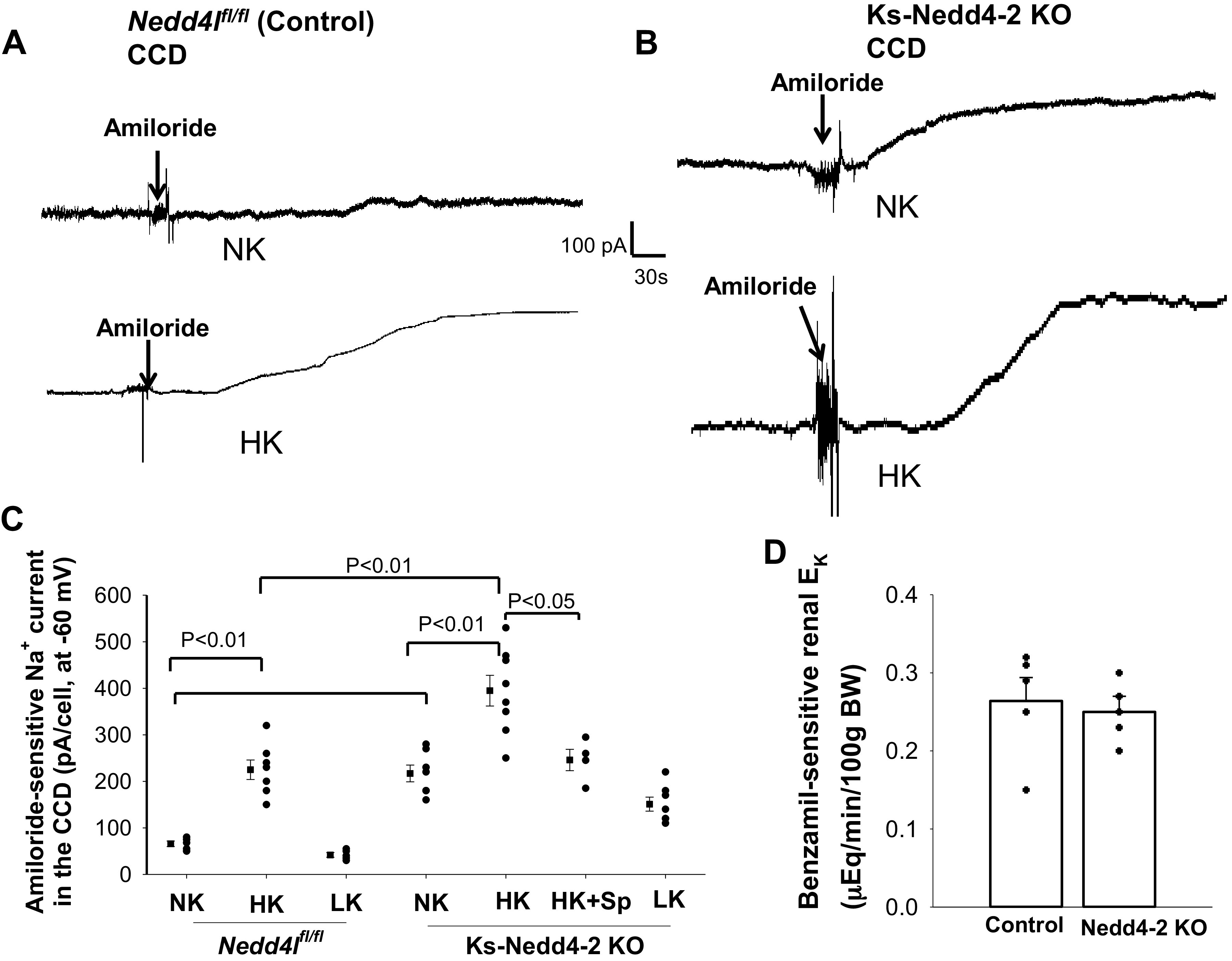
Deletion of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) enhanced high K+ (HK)-induced stimulation of the epithelial Na+ channel (ENaC) in the cortical collecting duct (CCD). A and B: sets of gap-free recordings showing amiloride-sensitive Na+ currents in the CCD measured at −60 mV with the perforated whole cell patch in male control mice (A) and in male kidney-specific neural precursor cell-expressed developmentally downregulated protein 4-2 knockout (Ks-Nedd4-2 KO) mice (B) on normal K+ (NK) and HK (7 days). C: scatterplot summarizing the results of experiments in which amiloride (10 µM)-sensitive Na+ currents were measured at −60 mV in male control mice (n = 5–7 tubules), in male Ks-Nedd4-2 KO mice on different K+ diets for 7 days (n = 5–8 tubules), and in male Ks-Nedd4-2 KO mice on HK plus spironolactone (Sp) for 7 days (n = 4 tubules). Mean values and SEs are shown on the left of each column. An arrow indicates the addition of amiloride (10 µM). Significance was determined by two-way ANOVA. Spironolactone was dissolved in ethanol and further diluted at a 1:200 ratio into drinking water. By measuring the volume of water intake, the daily dose of spironolactone for each mouse was 40 mg/kg body wt. D: bar graph (mean value) and scatterplot (each data point) summarizing benzamil (5 mg/1 kg body wt)-induced renal K+ excretion (EK) in male control and Ks-Nedd4-2 KO mice on NK (n = 5 mice). Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like.
Table 1.
Plasma K+ concentrations in control (Nedd4lfl/fl) and Ks-Nedd4-2 KO mice on different K+/Na+ diets
| Normal Diet | Low K+ (7 Days) | High K+ (7 Days) | Low Na+ (7 Days) | High Na+ (7 Days) | |
|---|---|---|---|---|---|
| Control K+ concentration, mM | 3.81 ± 0.1 | 2.93 ± 0.15* | 3.95 ± 0.1 | 3.71 ± 0.08 | 3.93 ± 0.06 |
| Nedd4-2 KO K+ concentration, mM | 3.85 ± 0.1 | 2.74 ± 0.1* | 3.71 ± 0.06 | 3.66 ± 0.12 | 3.53 ± 0.13 |
Values are represented as means ± SE. KO, knockout; Ks, kidney specific; Nedd4-2, neural precursor cell-expressed developmentally downregulated protein 4-2; Nedd4l, neural precursor cell-expressed developmentally downregulated 4-like. *Significant difference compared with the corresponding control. Data were obtained from our previous publications (36, 37).
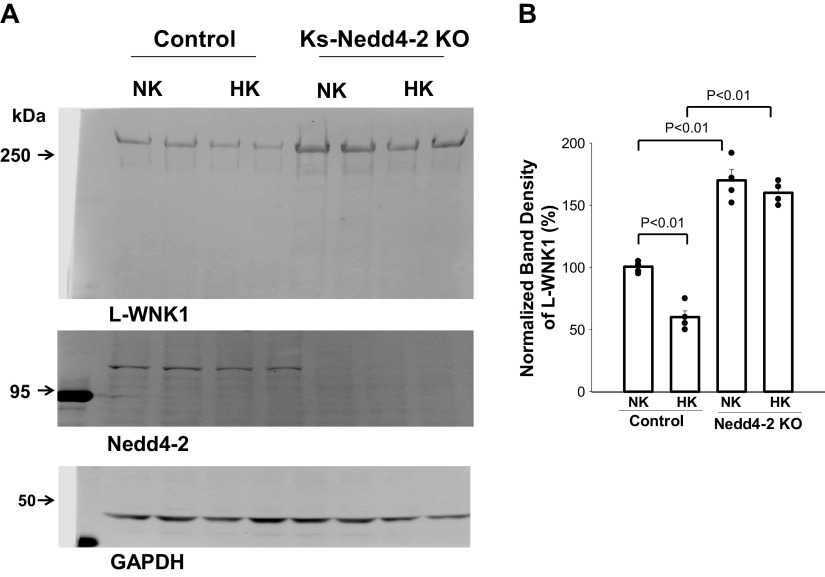
Because deletion of Nedd4-2 has been reported to increase the expression of long WNK1 (L-WNK1), which inhibits ROMK channels (41), we next examined the expression of L-WNK1 in the kidneys of control and Ks-Nedd4-2 KO mice. Figure 8 shows a Western blot showing the effect of HK on L-WNK1 expression in control and Ks-Nedd4-2 KO mice. We confirmed the previous report demonstrating that deletion of Nedd4-2 increased the expression of L-WNK1 (170 ± 9% of the control, n = 4 mice). Moreover, HK decreased L-WNK1 expression only in control mice (60 ± 6% of NK, n = 4 mice), but this effect was absent in Ks-Nedd4-2 KO mice (HK, 160 ± 6% of control mice on NK). Thus, deletion of Nedd4-2 abolished HK-induced inhibition of L-WNK1.
Figure 8.
Deletion of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) abolished the inhibitory effect of high K+ (HK) on long with no lysine kinase 1 (L-WNK1). A: Western blots showing the expression of L-WNK1 and Nedd4-2 in the kidney from male control mice and kidney-specific Nedd4-2 knockout (Ks-Nedd4-2 KO) mice on normal K+ (NK) and HK for 7 days. B: normalized band density of L-WNK1 expression summarized in a bar graph (mean value) and scatterplot (each data point) (n = 4 mice). Significance was determined by two-way ANOVA. The band density of L-WNK1 in control mice on NK was used as 100% after being adjusted with GAPDH loading, and the value was used to normalize the rest of the group.
DISCUSSION
It is now well established that thiazide-sensitive NaCl cotransporter (NCC) activity in the DCT (42), regulated by basolateral Kir4.1/Kir5.1 and intracellular Cl− (4, 43–48), and ENaC, ROMK, and large-conductance Ca2+-activated K+ channels in the ASDN work in concert to regulate renal K+ excretion (49). Although the role of Nedd4-2 in regulating NaCl cotransporter, Kir4.1, and ENaC is well demonstrated (25, 35, 40, 50), it is not understood whether Nedd4-2 regulates ROMK channels. The main finding of the present study was that deletion of Nedd4-2 decreased ROMK channel activity in the ASDN. Although our finding seems not consistent with the previous report showing that ROMK immunostaining was enhanced in the ASDN of Ks-Nedd4-2 KO mice (35), we believe that the discrepancy between the two studies was due to the different methods used in two studies. We directly measured TPNQ-sensitive K+ currents, which should reflect functional or active ROMK channel activity, whereas ROMK immunostaining represents ROMK protein levels, including active and inactive K+ channels expressed in the apical and subapical membrane. Consequently, ROMK immunostaining may not be equal to active ROMK channel numbers. The finding that ROMK channel activity was decreased in the ASDN of Ks-Nedd4-2 KO mice suggests that Nedd4-2 is unlikely to directly regulate ROMK channels through ubiquitin ligation. If Nedd4-2 could be an E3 ubiquitin ligase directly regulating ROMK channel ubiquitination, deletion of Nedd4-2 should increase rather than decrease ROMK channel activity.
The mechanism by which deletion of Nedd4-2 decreases ROMK channel activity in the ASDN is not completely understood. However, there are at least two possible mechanisms contributing to the Nedd4-2 deletion-induced decrease in ROMK channel activity in the ASDN. First, increased expression of L-WNK1 may be responsible for inhibiting ROMK channels in the ASDN. Previous studies have demonstrated that deletion of Nedd4-2 increased expression of L-WNK1 in the kidney because Nedd4-2 regulated L-WNK1 ubiquitination (35, 51). Since L-WNK1 has been shown to inhibit ROMK channels (38, 52), it is possible that Nedd4-2 deletion-induced stimulation of L-WNK1 is responsible for decreasing ROMK channel activity in the ASDN of Nedd4-2-deficient mice. In addition, we speculate that a decrease in ROMK channel activity might be due to a compensatory regulation of increased ENaC-dependent renal K+ excretion. Deletion of Nedd4-2 robustly increased expression of β-ENaC and γ-ENaC in the kidney, and patch-clamp experiments demonstrated that ENaC activity in both the DCT2 and CCD was stimulated in Ks-Nedd4-2 KO mice (37). Consequently, high ENaC activity is expected to stimulate renal K+ excretion, thereby decreasing plasma K+ concentrations. However, we and others have observed that Ks-Nedd4-2 KO mice were normakalemic despite high ENaC activity (see Table 1) (35–37, 41). Our present study also showed that inhibition of ENaC-induced renal K+ excretion is the same between control and Nedd4-2 KO mice. We reason that decreased ROMK channel activity in the ASDN may be part of a compensatory mechanism for maintaining normal plasma K+ levels in Nedd4-2-deficient mice. However, the compensatory regulation of ROMK in Ks-Nedd4-2 KO mice was not perfect because long-term HS or LK intake decreased plasma K+ concentrations in Ks-Nedd4-2 KO mice compared with control mice (37, 41). We demonstrated that renal K+ wasting induced by high ENaC activity is responsible for hypokalemia of Ks-Nedd4-2 KO mice on HS because K+ supplement prevents the development of hypokalemia (37).
The second finding of the present study was that HK-induced stimulation of ROMK channel activity was absent in Nedd4-2-deficient mice, and we confirmed that HK stimulated, whereas LK inhibited, ROMK channel expression/activity in the ASDN of control mice (39, 53, 54). The observation that HK did not decrease L-WNK1 expression in Ks-Nedd4-2 KO mice suggests that high expression of L-WNK1 may be responsible for the absence of HK-induced inhibition of ROMK channels in the ASDN. Previous studies have demonstrated that HK intake inhibited L-WNK1 expression and increased kidney-specific WNK1 (Ks-WNK1) expression and that LK intake had an opposite effect on L-WNK1 and Ks-WNK1 (38, 52, 55). It has been suggested that a decrease in L-WNK1 and an increase in Ks-WNK1 were responsible for HK-induced stimulation of ROMK channel activity. We speculate that increased expression of L-WNK1 may abrogate the stimulatory effect of HK on ROMK in the ASDN. Second, we also speculate that the lack of HK-induced stimulation of ROMK activity may be a compensatory regulation of K+ wasting in the CCD of Ks-Nedd4-2 KO mice. Because an abnormal high ENaC activity in the CCD is expected to stimulate renal K+ excretion, thereby decreasing plasma K+ concentration, a decrease in plasma K+ concentration may abolish the effect of HK on ROMK. Indeed, we have observed that plasma K+ concentrations of Ks-Nedd4-2 KO mice on HK tend to be lower than those of the corresponding control mice (see Table 1), although the difference is not significant (36).
Previous studies have demonstrated that deletion of Nedd4-2 increased the expression of ENaC in the kidney (27, 35), and we have further shown that HK stimulated the expression of all three ENaC subunits in Nedd4-2-deficient mice (36). Our present patch-clamp experiments have shown that ENaC activity in the CCD was significantly higher in Ks-Nedd4-2 KO mice on HK than in control mice. In contrast, HK did not further increase ENaC activity in the DCT2/iCNT of Ks-Nedd4-2 KO mice, suggesting that HK stimulated ENaC activity of the CCD by a Nedd4-2-independent mechanism. HK intake is expected to stimulate aldosterone release from the adrenal gland, which not only inhibits the effect of Nedd4-2 on ENaC but also stimulates ENaC expression in the ASDN (56). The finding that HK stimulated ENaC activity only in the CCD but not in the DCT2/iCNT of Nedd4-2-deficient mice suggests that aldosterone-induced stimulation of ENaC by a Nedd4-2-independent mechanism takes place mainly in the late ASDN including the CCD. This notion is also supported by the finding that ENaC activity is decreased in the CCD of Ks-Nedd4-2 KO mice on HK plus treated with mineralocorticoid receptor antagonist. Our finding is also consistent with previous observations that ENaC activity in the CCD was more dependent on mineralocorticoid receptors than in the DCT2 (21, 22).
Perspectives and Significance
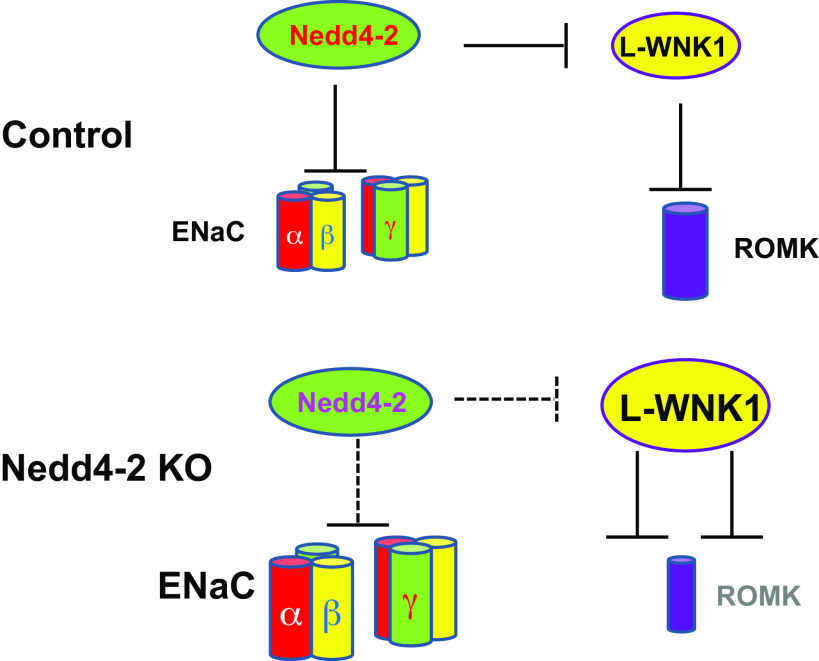
Figure 9 shows a scheme illustrating a possible mechanism by which deletion of Nedd4-2 inhibits ROMK channel activity in the ASDN. Deletion of Nedd4-2 is expected to increase expression of L-WNK1, which, in turn, inhibits ROMK channel activity in the ASDN. Inhibition in ROMK channels in the ASDN of Ks-Nedd4-2 KO mice should play a role in maintaining plasma K+ concentrations in a normal range despite high ENaC activity under control conditions. We conclude that deletion of Nedd4-2 inhibits ROMK channel activity and abolishes the stimulatory effect of HK on ROMK channels in the ASDN. Finally, HK stimulates ENaC activity in the CCD by a Nedd4-2-dependent and Nedd4-2-independent mechanism.
Figure 9.
Scheme i llustrating the mechanism by which deletion of neural precursor cell-expressed developmentally downregulated protein 4-2 (Nedd4-2) decreases renal outer medullary K+ (ROMK) channels in the aldosterone-sensitive distal nephron. Solid and dotted lines represent enhanced or diminished action, respectively. The large or small size of the subjects represents increased or decreased activity, respectively. ENaC, epithelial Na+ channel; L-WNK1, long with no lysine kinase 1.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK54983 (to W.-H.W.) and DK115366 (to D.-H.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.-H.W. conceived and designed research; D.-D.Z., J.-Y.Z., X.-P.D., and D.-H.L. performed experiments; D.-H.L. and W.-H.W. analyzed data; D.-D.Z., D.-H.L., and W.-H.W. interpreted results of experiments; D.-D.Z., D.-H.L., and W.-H.W. prepared figures; W.-H.W. drafted manuscript; W.-H.W. edited and revised manuscript; D.-D.Z., J.-Y.Z., X.-P.D., D.-H.L., and W.-H.W. approved final version of manuscript.
REFERENCES
- 1.Wang W, White S, Geibel J, Giebisch G. A potassium channel in the apical membrane of rabbit thick ascending limb of Henle's loop. Am J Physiol Renal Physiol 258: F244–F253, 1990. doi: 10.1152/ajprenal.1990.258.2.F244. [DOI] [PubMed] [Google Scholar]
- 2.Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Physiol 256: F143–F151, 1989. doi: 10.1152/ajprenal.1989.256.1.F143. [DOI] [PubMed] [Google Scholar]
- 3.Frindt G, Palmer LG. Apical potassium channels in the rat connecting tubule. Am J Physiol Renal Physiol 287: F1030–F1037, 2004. doi: 10.1152/ajprenal.00169.2004. [DOI] [PubMed] [Google Scholar]
- 4.Manis AD, Hodges MR, Staruschenko A, Palygin O. Expression, localization, and functional properties of inwardly rectifying K+ channels in the kidney. Am J Physiol Renal Physiol 318: F332–F337, 2020. doi: 10.1152/ajprenal.00523.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer LG, Choe H, Frindt G. Is the secretory K channel in the rat CCT ROMK? Am J Physiol Renal Physiol 273: F404–F410, 1997. doi: 10.1152/ajprenal.1997.273.3.F404. [DOI] [PubMed] [Google Scholar]
- 6.Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol Renal Physiol 272: F397–F404, 1997. doi: 10.1152/ajprenal.1997.272.3.F397. [DOI] [PubMed] [Google Scholar]
- 7.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Physiol 259: F494–F502, 1990. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- 8.Wu P, Gao ZX, Su XT, Ellison DH, Hadchouel J, Teulon J, Wang WH. Role of WNK4 and kidney-specific WNK1 in mediating the effect of high dietary K+ intake on ROMK channel in the distal convoluted tubule. Am J Physiol Renal Physiol 315: F223–F230, 2018. doi: 10.1152/ajprenal.00050.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA. Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011. doi: 10.1152/ajprenal.00592.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan X, Gu L, Xiao Y, Gao Z, Wu P, Zhang Y, Meng X, Wang J, Zhang D, Lin D, Wang W, Gu R. Norepinephrine-induced stimulation of Kir4.1/Kir5.1 is required for the activation of NaCl transporter in distal convoluted tubule. Hypertension 73: 112–120, 2019. doi: 10.1161/HYPERTENSIONAHA.118.11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Annu Rev Physiol 59: 413–436, 1997. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 12.Simon DB, Karet FE, Rodriguez J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K channel, ROMK. Nat Genet 14: 152–156, 1996. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 13.Giebisch G, Wang WH. Potassium transport: from clearance to channels and pumps. Kidney Int 49: 1624–1631, 1996. doi: 10.1038/ki.1996.236. [DOI] [PubMed] [Google Scholar]
- 14.Lin D, Sterling H, Yang B, Hebert SC, Giebisch G, Wang W. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol 286: F881–F892, 2004. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mennitt PA, Frindt G, Silver RB, Palmer LG. Potassium restriction downregulates ROMK expression in rat kidney. Am J Physiol Renal Physiol 278: F916–F924, 2000. doi: 10.1152/ajprenal.2000.278.6.F916. [DOI] [PubMed] [Google Scholar]
- 16.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999. doi: 10.1152/ajprenal.1999.277.5.F805. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z-J, Sun P, Xing W, Pan C, Lin D-H, Wang W-H. Decrease in dietary K intake stimulates the generation of superoxide anions in the kidney and inhibits K secretory channels in the CCD. Am J Physiol Renal Physiol 298: F1515–F1522, 2010. doi: 10.1152/ajprenal.00502.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong K, Yan Q, Lu M, Wan L, Hu H, Guo J, Boulpaep E, Wang W, Giebisch G, Hebert SC, Wang T. Romk1 knockout mice do not produce Bartter phenotype but exhibit impaired K excretion. J Biol Chem 291: 5259–5269, 2016. doi: 10.1074/jbc.M115.707877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frindt G, Palmer LG. K+ secretion in the rat kidney: Na+ channel-dependent and -independent mechanisms. Am J Physiol Renal Physiol 297: F389–F396, 2009. doi: 10.1152/ajprenal.90528.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garty H, Palmer LG. Epithelial sodium channel: function, structure, and regulation. Physiol Rev 77: 359–396, 1997. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 21.Nesterov V, Bertog M, Canonica J, Hummler E, Coleman R, Welling PA, Korbmacher C. Critical role of the mineralocorticoid receptor in aldosterone-dependent and aldosterone-independent regulation of ENaC in the distal nephron. Am J Physiol Renal Physiol 321: F257–F268, 2021. doi: 10.1152/ajprenal.00139.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu P, Gao Z, Zhang D, Duan X, Terker AS, Lin D, Ellison DH, Wang W. Effect of angiotensin II on ENaC in the distal convoluted tubule and in the cortical collecting duct of mineralocorticoid receptor deficient mice. J Am Heart Assoc 9: e014996, 2020. doi: 10.1161/JAHA.119.014996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Xu Y, Gravotta D, Frindt G, Weinstein AM, Palmer LG. ENaC and ROMK channels in the connecting tubule regulate renal K+ secretion. J Gen Physiol 153: e202112902, 2021. doi: 10.1085/jgp.202112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 25.Staub O, Dho S, Henry PC, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J 15: 2371–2380, 1996. doi: 10.1002/j.1460-2075.1996.tb00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamynina E, Staub O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na+ transport. Am J Physiol Renal Physiol 283: F377–F387, 2002. doi: 10.1152/ajprenal.00143.2002. [DOI] [PubMed] [Google Scholar]
- 27.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, Snyder PM, Staub O, Stokes JB, Yang B. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol 295: F462–F470, 2008. doi: 10.1152/ajprenal.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr Rev 23: 258–275, 2002. doi: 10.1210/edrv.23.2.0458. [DOI] [PubMed] [Google Scholar]
- 29.Loffing-Cueni D, Flores SY, Sauter D, Daidié D, Siegrist N, Meneton P, Staub O, Loffing J. Dietary sodium intake regulates the ubiquitin-protein ligase Nedd4-2 in the renal collecting system. J Am Soc Nephrol 17: 1264–1274, 2006. doi: 10.1681/ASN.2005060659. [DOI] [PubMed] [Google Scholar]
- 30.Staub O, Yeger H, Plant PJ, Kim H, Ernst SA, Rotin D. Immunolocalization of the ubiquitin-protein ligase Nedd4 in tissues expressing the epithelial Na+ channel (ENaC). Am J Physiol Cell Physiol 272: C1871–C1880, 1997. doi: 10.1152/ajpcell.1997.272.6.C1871. [DOI] [PubMed] [Google Scholar]
- 31.Frindt G, Sackin H, Palmer LG. Whole-cell currents in rat cortical collecting tubule: low-Na diet increases amiloride-sensitive conductance. Am J Physiol Renal Physiol 258: F562–F567, 1990. doi: 10.1152/ajprenal.1990.258.3.F562. [DOI] [PubMed] [Google Scholar]
- 32.Nesterov V, Dahlmann A, Krueger BK, Bertog M, Loffing J, Korbmacher C. Aldosterone-dependent and -independent regulation of the epithelial sodium channel (ENaC) in mouse distal nephron. Am J Physiol Renal Physiol 303: F1289–F1299, 2012. doi: 10.1152/ajprenal.00247.2012. [DOI] [PubMed] [Google Scholar]
- 33.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraïbi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J 20: 7052–7059, 2001. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013. doi: 10.1172/JCI61110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Duan XP, Zhang DD, Wang WH, Lin DH. Deletion of renal Nedd4-2 abolishes the effect of high K+ intake on Kir4.1/Kir5.1 and NCC activity in the distal convoluted tubule. Am J Physiol Renal Physiol 321: F1–F11, 2021. doi: 10.1152/ajprenal.00072.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang DD, Duan XP, Xiao Y, Wu P, Gao Z-X, Wang W-H, Lin D-H. Deletion of renal Nedd4-2 abolishes the effect of high sodium intake (HS) on Kir4.1, ENaC and NCC, and causeds hypokalemia during HS. Am J Physiol Renal Physiol 320: F883–F896, 2021. doi: 10.1152/ajprenal.00555.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA 103: 8558–8563, 2006. doi: 10.1073/pnas.0603109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frindt G, Shah A, Edvinsson J, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009. doi: 10.1152/ajprenal.90527.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu P, Su XT, Gao ZX, Zhang DD, Duan XP, Xiao Y, Staub O, Wang WH, Lin DH. Renal tubule Nedd4-2 deficiency stimulates Kir4.1/Kir5.1 and thiazide-sensitive NaCl cotransporter in distal convoluted tubule. J Am Soc Nephrol 31: 1226–1242, 2020. doi: 10.1681/ASN.2019090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Qusairi L, Basquin D, Roy A, Rajaram RD, Maillard MP, Subramanya AR, Staub O. Renal tubular ubiquitin-protein ligase NEDD4-2 is required for renal adaptation during long-term potassium depletion. J Am Soc Nephrol 28: 2431–2442, 2017. doi: 10.1681/ASN.2016070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 43.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang C-L, Ellison DH, Wang WH. Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017. doi: 10.1681/ASN.2016090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duan XP, Wu P, Zhang DD, Gao ZX, Xiao Y, Ray EC, Wang WH, Lin DH. Deletion of Kir5.1 abolishes the effect of high Na+ intake on Kir4.1 and Na+-Cl− cotransporter. Am J Physiol Renal Physiol 320: F1045–F1058, 2021. doi: 10.1152/ajprenal.00004.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su XT, Klett NJ, Sharma A, Allen CN, Wang WH, Yang CL, Ellison DH. Distal convoluted tubule Cl− concentration is modulated via K+ channels and transporters. Am J Physiol Renal Physiol 319: F534–F540, 2020. doi: 10.1152/ajprenal.00284.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M-X, Cuevas CA, Su X-T, Wu P, Gao Z-X, Lin D-H, McCormick JA, Yang C-L, Wang W-H, Ellison DH. Potassium intake modulates the thiazide-sensitive sodium-chloride cotransporter (NCC) activity via the Kir4.1 potassium channel. Kidney Int 93: 893–902, 2018. doi: 10.1016/j.kint.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu P, Gao ZX, Zhang DD, Su XT, Wang WH, Lin DH. Deletion of Kir5.1 impairs renal ability to excrete potassium during increased dietary potassium intake. J Am Soc Nephrol 30: 1425–1438, 2019. doi: 10.1681/ASN.2019010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C, Wang L, Zhang J, Su X-T, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1). Proc Natl Acad Sci USA 111: 11864–11869, 2014. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giebisch G, Hebert SC, Wang WH. New aspects of renal potassium transport. Pflugers Arch 446: 289–297, 2003. doi: 10.1007/s00424-003-1029-8. [DOI] [PubMed] [Google Scholar]
- 50.Wang MX, Su XT, Wu P, Gao ZX, Wang WH, Staub O, Lin DH. Kir5.1 regulates Nedd4-2-mediated ubiquitination of Kir4.1 in distal nephron. Am J Physiol Renal Physiol 315: F986–F996, 2018. doi: 10.1152/ajprenal.00059.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy A, Al-Qusairi L, Donnelly BF, Ronzaud C, Marciszyn AL, Gong F, Chang YPC, Butterworth MB, Pastor-Soler NM, Hallows KR, Staub O, Subramanya AR. Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action. J Clin Invest 125: 3433–3448, 2015. doi: 10.1172/JCI75245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sterling H, Lin DH, Gu RM, Dong K, Hebert SC, Wang WH. Inhibition of protein-tyrosine phosphatase stimulates the dynamin-dependent endocytosis of ROMK1. J Biol Chem 277: 4317–4323, 2002. doi: 10.1074/jbc.M109739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y, Bloom P, Gu R, Wang W. Protein-tyrosine phosphatase reduces the number of apical small conductance K+ channels in the rat cortical collecting duct. J Biol Chem 275: 20502–20507, 2000. doi: 10.1074/jbc.M000783200. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Wang HR, Huang CL. Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase. J Biol Chem 284: 12198–12206, 2009. doi: 10.1074/jbc.M806551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]