Dear Editor,
Patients with cancer are at increased risk for severe COVID-19 and have reduced humoral immune responses after SARS-CoV-2 infection and dual-dose BNT162b2 vaccination [1,2]. Recently, it was observed that the third dose of BNT162b2 is able to elicit higher SARS-CoV-2 anti-receptor-binding domain (RBD) IgG titres in solid-organ transplant patients and adults aged ≥60 years than two doses [3,4]. Initial reports with limited sample size also indicated that the third BNT162b2 vaccination dose would be beneficial for patients with solid tumour receiving active anti-neoplastic treatment [[5], [6], [7]]. The present study (B-VOICE: EudraCT 2021-000300-38), which is the first large-scale prospective study on humoral responses after a third dose six months post primo vaccination in patients with cancer, confirms that most patients with cancer will elicit a higher humoral response after the third dose of BNT162b2. Quantitative analysis of IgG-antibodies against the SARS-CoV-2 RBD antigen by enzyme-linked immunosorbent assay was performed before the first dose, 28 days and six months after the second dose and 28 days after third BNT162b2 dose in 141 patients with oncohaematological malignacies (Suppl. Table 1). From the 200 initial participants that were included in the B-VOICE study [2], 141 participants were evaluable after the third dose. Drop-out was due to demise (20 participants, 10.0%), postponed vaccination due to illness or disease progression (8 participants, 4.0%), vaccination outside study protocol (5 participants, 2.5%) and consent withdrawal (26 participants, 13.0%).
All subjects were assigned to a cohort based on the treatment type receiving at the time of the third dose administration. Subjects with a solid tumour were divided into four treatment cohorts: chemotherapy, immunotherapy, targeted/hormonal therapy and chemotherapy + immunotherapy. A differentiation for patients with haematological malignancies was made between patients receiving rituximab and patients who have had undergone haematopoietic stem cell transplantation more than one year ago. An additional cohort was created for 7 subjects with haematological malignancy and 13 subjects with solid tumours that were no longer receiving active therapy at the time of the third dose. A third BNT162b2 vaccination dose was administered at 183 ± 10 days (6 months) for 97% and 169–200 days for 3% of the subjects, after administration of the second BNT162b2 dose. Log-transformed antibody titres were compared using a random intercept linear mixed model, including time, treatment at the time of sampling and the interaction between time and treatment at the time of sampling.
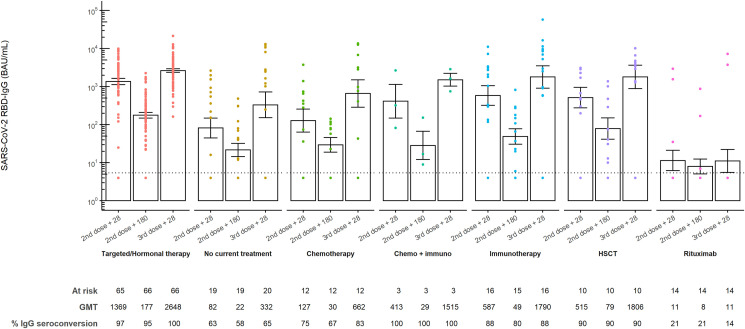
Anti-RBD IgG titres had waned significantly at six months post-second dose (GMT 65.8BAU/mL [95% CI 48.0–90.2] versus GMT 386.2BAU/mL [95% CI 253.3–588.7], p < 0.0001). Administration of a third dose of BNT162b2 induced significantly higher anti-RBD IgG titres 28 days post-third dose than 28 days post-second dose (GMT 936.5BAU/mL [95% CI 600.5–1460.4], versus GMT 386.2BAU/mL [95% CI 253.3–588.7], p < 0.0001). In comparison to other treatment cohorts, significantly lower anti-RBD IgG titres 28 days after the second dose and 28 days after the third dose could be observed in the rituximab cohort, with only a few additional seroconverted patients after the third dose (Fig. 1 ).
Fig. 1.
SARS-CoV-2 anti-RBD IgG antibody titres before and after a third dose BNT162b2 in patients with cancer. SARS-CoV-2 anti-receptor-binding domain (RBD) IgG antibody titres 28 days and six months after second BNT162b2 dose and 28 days after third BNT162b2 mRNA COVID-19 vaccine in the different treatment cohorts. Subjects were assigned to treatment cohorts based on the therapy received at the time of the third dose. The height of each bar represents the geometric mean titre (GMT). All samples were analysed using an enzyme-linked immunosorbent assay (ELISA) for the quantitative detection of IgG-class antibodies to RBD (BAU/mL). The dotted line indicates the lower limit of quantification (LLQ) of 5.4 BAU/mL. Values below this detection limit were imputed to half the LLQ. Ι bars indicate standard errors.
A significant decrease in anti-RBD IgG titres at six months post-second BNT162b2 dose was observed in all treatment cohorts. Although waning anti-RBD IgG levels after primo-vaccination are also observed in healthy controls [8], the percentual decrease in anti-RBD IgG titres at six months after primo-vaccination is much lower than the decrease reported in patients with cancer. Considering the investigation that patients with cancer have reduced humoral immune responses after dual-dose BNT162b2 vaccination compared to healthy controls [2], this observation highlights the importance of prioritising the administration of a third vaccination dose in patients with cancer. The significant increase in antibody response upon a third dose is an important observation indicating the successful generation of memory B-cells after primo vaccination [9]. However, in patients receiving rituximab, a third dose hardly induced any humoral immune response as rituximab depletes B-cells [10]. Hence, the role of a third dose remains debatable in this population, although adaptive cellular immunity after vaccination might play a role in protecting these patients against SARS-CoV-2 [10]. In conclusion, most patients after haematopoietic stem cell transplantation or with solid tumours, including those under active anti-cancer treatment, will benefit from the third dose of the BNT16b2 vaccine. However, as a true serological correlate of protection is not yet established, future research on post-vaccine antibody durability should be coupled to the measurement of B-cell and T-cell responses over time.
Funding
This work was supported by the Belgian Government through Sciensano [COVID-19_SC004, COVID-19_SC059, COVID-19_SC061].
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The University Hospital of Antwerp received funding for the project from the Belgian Government through Sciensano [COVID-19_SC004, COVID-19_SC059, COVID-19_SC061]. Yana Debie, Dr. Timon Vandamme (MD), Prof. Dr. Peter A. van Dam (MD) and Prof. Dr. Marc Peeters (MD) are employed at the funded institution. Dr. Maria E. Goossens (MD) is employed at the funding institution.
Acknowledgements
The authors kindly thank the B-VOICE patients for participation, the nursing staff members at the Day Care Unit of the Antwerp University Hospital, the staff members of the Biobank Antwerp and all recruiting physicians. The authors are grateful to Dr. Ella Roelant (Clinical Trial Center (CTC), CRC Antwerp, Antwerp University Hospital, University of Antwerp; StatUa, Center for Statistics, University of Antwerp) for statistical support, and Lise Verbruggen, MSc (Multidisciplinary Oncological Centre Antwerp (MOCA), Antwerp University Hospital), Dr. Greetje Vanhoutte (Multidisciplinary Oncological Centre Antwerp (MOCA), Antwerp University Hospital) and Laure-Anne Teuwen, MD (Multidisciplinary Oncological Centre Antwerp (MOCA), Antwerp University Hospital) for data-analysis and writing. The authors also thank Dr. Isabelle Desombere (SD Infectious Diseases in Humans, Service Immune response, Sciensano) and her team for performing serological analyses. The authors are thankful to Dr. Pieter Pannus (SD Infectious Diseases in Humans, Observational Clinical Trials, Sciensano for critical revision of the manuscript. In addition, the authors thank the B-VOICE team for patient inclusion and sample collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.12.025.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.van Dam P., Huizing M., Roelant E., Hotterbeekx A., De Winter F., Kumar-Singh S., et al. Immunoglobin G/total antibody testing for SARS-CoV-2: a prospective cohort study of ambulatory patients and health care workers in two Belgian oncology units comparing three commercial tests. Eur J Cancer. 2021;148:328–339. doi: 10.1016/j.ejca.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeters M., Verbruggen L., Teuwen L., Vanhoutte G., Vande Kerckhove S., Peeters B., et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6(5):100274. doi: 10.1016/j.esmoop.2021.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eliakim-Raz N., Leibovici-Weisman Y., Stemmer A., Ness A., Awwad M., Ghantous N., et al. Antibody titers before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in adults aged ≥60 years. JAMA. 2021;326(21):2203–2204. doi: 10.1001/jama.2021.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gounant V., Ferré V.M., Soussi G., Charpentier C., Flament H., Fidouh N., et al. Efficacy of SARS-CoV-2 vaccine in thoracic cancer patients: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. J Thorac Oncol: Off Publ Int Assoc Study Lung Canc. 2021 doi: 10.1016/j.jtho.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shroff R.T., Chalasani P., Wei R., Pennington D., Quirk G., Schoenle M., et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med. 2021;27(11):2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rottenberg Y., Grinshpun A., Ben-Dov I.Z., Oiknine Djian E., Wolf D.G., Kadouri L. Assessment of response to a third dose of the SARS-CoV-2 BNT162b2 mRNA vaccine in patients with solid tumors undergoing active treatment. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciabattini A., Pastore G., Fiorino F., Polvere J., Lucchesi S., Pettine E., et al. Evidence of SARS-CoV-2-specific memory B cells six months after vaccination with the BNT162b2 mRNA vaccine. Front Immunol. 2021;12(3751) doi: 10.3389/fimmu.2021.740708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benucci M., Damiani A., Infantino M., Manfredi M., Grossi V., Lari B., et al. Presence of specific T cell response after SARS-CoV-2 vaccination in rheumatoid arthritis patients receiving rituximab. Immunol Res. 2021;69(4):309–311. doi: 10.1007/s12026-021-09212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.