Summary
Intravacuolar bacterial pathogens establish intracellular niches by constructing membrane-encompassed compartments. The vacuoles surrounding the bacteria are remarkably stable, facilitating microbial replication and preventing exposure to host cytoplasmically-localized innate immune sensing mechanisms. To maintain integrity of the membrane compartment, the pathogen is armed with defensive weapons that prevent loss of vacuole integrity and potential exposure to host innate signaling. In some cases, the microbial components that maintain vacuolar integrity have been identified, but the basis for why the compartment degrades in their absence is unclear. In this review, we point out that lessons from the microbial-programmed degradation of the vacuole by the cytoplasmically-localized Shigella flexneri provide critical insights into how degradation of pathogen vacuoles occurs. We propose that in the absence of bacterial-encoded guard proteins, aberrant trafficking of host membrane-associated components results in a dysfunctional pathogen compartment. As a consequence, the vacuole is poisoned and replication is terminated.
Introduction
Bacteria that grow intracellularly within a vacuolar compartment establish intimate contact with specific membrane compartments[1]. This allows the pathogen to construct a niche that is often sequestered from degradative pathways and protected from host cell cytoplasmic innate immune surveillance mechanisms[2]. Establishing this close relationship with a partner organelle during bacterial uptake provides an attractive model for how pathogens control the biogenesis of the replication site and prevent encounter with host antimicrobial strategies. Less appreciated, however, is that once the pathogen-containing compartment is established, it remains susceptible to continued attack, indicating that the microbe must maintain the integrity of the membrane surrounding the replication site. The most dramatic consequence of post-establishment assault can be witnessed in primary macrophages, in which a damaged replication vacuole exposes the microorganism to cytosol-resident interferon-regulated proteins that promote degradation of the microbe and liberation of components that activate the inflammatory response [2]. To protect against this degradation, we propose that intravacuolar pathogens produce “guards” that prevent the host cell antimicrobial response from causing vacuolar dysfunction.
Much of the work in the field has been directed toward explaining how intravacuolar pathogens avoid attack by the host lysosomal compartment. One of the most attractive models for how pathogens avoid degradative compartments was forwarded by Hackstadt and co-workers to explain construction of Chlamydia trachomatis replication niche [3]. Shortly after contact with the host cell, C. trachomatis was proposed to directly interface with the host secretory system, bypassing any interaction with the endo-lysosomal network. This, in turn, was envisioned to drive the microbe-containing compartment into a partnership with the Golgi apparatus that participates in the anterograde exocytic pathway. The C. trachomatis model provides an excellent description of how other intravacuolar organisms establish an intracellular niche[4]. In fact, even organisms thought to replicate within an acidic lysosomal compartment, such as Salmonella typhimurium, construct vacuoles with unique properties distinct from endolysosomal routing, consistent with internalization in a fashion that bypasses or modifies the default endolysosomal pathway[5].
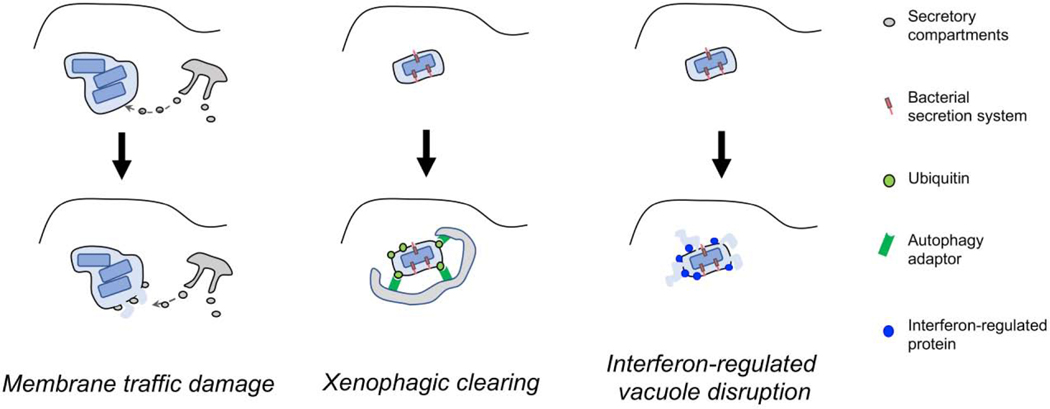
While the membrane hijacking model was being formed, evidence from studies on Salmonella argued that establishing intimate contact with a partner organelle is not sufficient to support luxurious intracellular growth, as the vacuole must protect itself from attack once established [6]. Therefore, we propose here that in addition to early events involved in establishing proper routing of the replication compartment, there exist microbial proteins that block processes directed toward disrupting vacuole integrity. These host cell processes are either intrinsic to highly conserved secretion pathways, are part of the autophagic clearing pathway known as xenophagy, or prevent an effective interferon response, all of which can target compartments that have avoided initial trafficking into the lysosomal network (Figure 1).
Figure 1. Host cell attacks on the replication vacuole.
Membrane traffic damage: Membrane traffic from host cells directed at the pathogen-containing compartment results in destabilization of the vacuolar membrane. The process that allows the host cell to recognize the vacuole is unknown. Xenophagic clearing. Replication compartments marked as derived from pathogens are recognized by the host as foreign. One strategy used is to ubiquitinate the vacuole, allowing recognition by autophagy adaptors followed by clearing, in a process called xenophagy. Specialized bacterial secretion systems can mark the replication vacuole as foreign. Interferon-regulated vacuole disruption. In response to pathogen attack, interferons are release, inducing expression of interferon-stimulated genes (ISGs). A subset of ISGs are believed to recognize pathogen compartments as foreign disrupting the compartment and releasing the pathogen into the cytosol. An interferon-dependent attack on the pathogen then occurs to clear of the infected cell.
In this review, we will analyze events that act to antagonize maintenance of intravacuolar replication. The events described involve pathways that are conserved from simple eukaryotes to humans, so the interferon-driven destruction of pathogen vacuoles will not be discussed (Figure 1) [7]. We will present evidence for guards and provide a hypothesis for how the guards act. In contrast to intravacuolar pathogens that encode vacuole guards, pathogens that grow in the host cell cytosol intentionally drive vacuole degradation to allow access to the replication site. Therefore, to gain insight into how the host cell attacks compartment integrity, events that lead to degradation of the Shigella flexneri-containing compartment will be discussed.
Pathogen-driven vacuole disruption by Shigella provides a model for host restriction of the pathogen-containing compartment.
Shigella flexneri, the causative agent of human bacillary dysentery, grows within the cytoplasm of host colonic epithelial cells after uptake into a pathogen-containing compartment. The membrane compartment surrounding the bacterium must be disrupted for replication to occur. Deciphering the mechanism of Shigella-containing vacuole rupture has been challenging because vacuole escape occurs rapidly after entry, and the events leading to cytoplasmic entry do not appear to correspond to known models for vacuole destruction, such as described for Listeria. Although entry of S. flexneri into the cytoplasm of colonic epithelial cells requires a type III secretion system (TTSS), there is no clear identification of a bacterial phospholipase that leads to this rupture. Therefore, translocated bacterial factors may act in concert with host proteins to disrupt the membrane. The fact that the pathogen encodes no vacuole guards potentially make this an excellent model for how host-driven disruption of pathogen vacuoles occurs.
There is strong evidence that membranous material derived from the endocytic-recycling compartment is involved in destabilizing the Shigella-containing vacuole. In an siRNA screen targeting membrane trafficking components, host proteins regulating endosomal and recycling traffic were identified required for efficient rupture of the Shigella-containing vacuole [8]. Notable host factors included early endosomal markers Rab5 and EEA1, sorting nexins SNX1 and SNX2, and recycling endosomal markers Rab4 and Rab11 [8]. This surprising result indicates that membrane traffic associated with homeostatic biogenesis of organelles can provide factors that either directly disrupt a non-self membrane compartment, or that collaborate with bacterial proteins to cause membrane lesions.
Rab11 recruitment to the vacuole is dependent on the Shigella T3SS substrate IpgD, which is a PI(4,5)P2 phosphatase that generates PI(5)P [8]. In the absence of IpgD or in Rab11-depleted cells, rupture of the vacuole is delayed. Consistent with this result, Rab5 and Rab11-positive macropinosomes are observed to accumulate at the Shigella-containing vacuole before rupture. The formation of these macropinosomes is dependent on the bacterial effector IgpB1, which stimulates cytoskeletal regulators at the time of entry to form these macropinosome [9]. As loss of macropinosome formation reduces vacuole rupture, fusion and fission events between the macropinosomes and the vacuole promote bacterial egress into the cytosol. Furthermore, these results argue that recruitment of the endocytic-recycling compartments to the vacuole is dependent on the phosphoinositide composition of the vacuole as remodeled by IpgD. Therefore, the composition of lipids in the membrane must be critical for controlling events that cause disruption of the pathogen-containing compartment [8]\
Evidence that vacuole guards maintain proper membrane composition to protect against loss of vacuole integrity.
A. Salmonella Typhimurium SifA protein.
Salmonella typhimurium is a facultative intracellular bacterium that can cause gastroenteritis and enteric fever. In epithelial cell lines, S. typhimurium manipulates the host cytoskeleton to form abundant tubule aggregates known as Salmonella-induced filaments (SIFs). These SIFs emanate outward from the Salmonella-containing vacuole (SCV) in the cytoplasm. SIFs link Salmonella to both the endocytic and exocytic trafficking pathways[10]. SIF biogenesis is dependent on the SPI2 T3SS and seven secreted bacterial substrates: SifA, SseJ, SseL, PipB2, SopD2, SseF, and SseG. Deletion mutants of any of the seven bacterial proteins produce an aberrant SIF morphology and altered SIF frequency [11,12].
The TTSS effector SifA was first identified as the main driver of SIF formation, as challenge of HeLa cells with a ΔsifA mutant is unable to induce SIFs and mouse infections are severely attenuated. The most striking property of a ΔsifA mutant is that the Salmonella-containing vacuole (SCV) loses integrity and bacteria enter the cytosol, mimicking the normal pathogenesis of Shigella [6]. Loss of integrity is observed even during growth in primary macrophages, in which SIF formation has never been described. In fact, the analysis of mutants provides evidence that maintenance of SCV integrity, and not SIF formation, is the primary function of SifA during disease. In contrast to the situation with Shigella, however, exposure of the bacteria to the macrophage cytosol is a lethal event for Salmonella, as it either activates a pyroptotic pathway leading to gasdermin D activation or results in autophagic clearing of the bacteria[13,14].
In a further parallel to the early steps in Shigella pathogenesis, galectin-3, a well accepted marker for compartment disruption, is recruited to the vacuole of the ΔsifA mutant vacuole as consequence of exposure to normally lumenally-exposed carbohydrates [15]. This aberrant exposure of galectin ligands appears tightly linked to a failure to establish the proper lipid composition of the SCV. Evidence supporting the importance of membrane lipid content in preventing the vacuole rupture phenotype is based on the fact that loss of SCV integrity in the absence of SifA depends on the TTSS effector SseJ, which has phospholipase and glycerophosphlipid:cholesterol acyltransferase activity [16]. A ΔsifAΔsseJ double mutant can prevent exposure of a ΔsifA mutant to the cytosol, although the vacuole of the double mutant is clearly dysfunctional because bacterial replication is blocked [16]. Therefore, in the absence of SifA, a lipid that is a substrate of SseJ presumably accumulates on the SCV. As a result, SseJ either degrades the SCV, or the action of SseJ produces a toxic lipid that accumulates in the absence of SifA, resulting in compartment destabilization. The nature of the lipid species is unclear, but they may be derived from membrane compartments targeted by the OSPB1-VapAB lipid exchange system, which directly associates with SseJ and is required for vacuole integrity in the presence of intact SifA function [12].
SifA clearly interfaces with the host endosomal system to prevent the SCV from degradation. The N-terminal region of SifA forms a complex with the host factor SifA-and-Kinesin-Interacting-Protein (SKIP) [17,18]. This interaction promotes kinesin-1-dependent movement along microtubules and regulates fission of host vesicle membranes to the SCV, thus maintaining vacuole integrity. Clues to how SifA maintains vacuole integrity can be gleaned from the studies on SKIP activity. Overexpression of SKIP induces kinesin-1-dependent anterograde movement of lysosomal compartments [19]. It was also shown recently that SifA-SKIP recruit HOPS (Homotypic fusion and Protein Sorting) complex to the SCV in order to access host late endosomal and lysosomal membrane [20]. These results presented the conundrum that fusion of lysosomal membrane maintains the vacuole but also delivers toxic lysosomal enzymes to the SCV. It was later demonstrated that SifA-SKIP interaction sequesters lysosomal Rab9, thereby inhibiting Rab9-dependent M6PR recruitment of the SCV [21]. Therefore, through the actions of SifA, Salmonella is able to hijack cargo movement for membrane acquisition while simultaneously evading fusion of toxic cargo with the SCV. One key to understanding how SifA coordinates these seemingly contradictory events was provided by real-time microscopy [19]. SifA appears to stimulate anterograde egress and fission of SCV membrane material, consistent with the protein driving removal of toxic membrane components from the SCV.
Based on these observations, we propose that SifA acts as a vacuole guard, preventing the vacuole from accumulating lipid components that destabilize the compartment. Note that in the case of S. flexneri, where no such guard exists, a bacterial effector drives alteration in the lipid content of the vacuole and association with membrane compartments that destabilize the vacuole. In both cases, the nature of the membrane damage that takes place is likely to be complex and may be generated by multiple sources, indicating that vacuole guards such as SifA prevent multiple types of membrane damage.
B. Legionella pneumophila SdhA protein.
Another intravacuolar pathogen, Legionella pneumophila encodes SdhA, a protein with properties strikingly similar to SifA[22]. L. pneumophila is an environmental pathogen of amoebae that causes human disease by growing within alveolar macrophages after inhalation of contaminated water supplies. Growth of the bacteria in a vacuolar compartment in all cell types requires the function of the bacterial Icm/Dot type IV secretion system (T4SS) that translocates approximately 300 proteins into the host cell [23,24]. The vast majority of these translocated effectors are devoted to construction of the Legionella-containing compartment (LCV) and interference with evolutionarily-conserved antimicrobial tactics such as xenophagy and the host unfolded protein response [25–27]. The SdhA protein is one of the few nonredundant L. pneumophila effectors, emphasizing its critical role in the disease process. Vacuoles harboring mutants lacking the SdhA protein become disrupted as the infection proceeds, releasing bacteria into the host cell cytosol. In primary macrophages, cytosolic bacteria are then degraded in an interferon-dependent fashion, resulting in Caspase 11- or 4- (mouse or human) dependent host cell death [28]. Therefore, the SdhA protein is a critical vacuole guard that acts to promote intracellular growth.
In another parallel to SifA, selection for second site mutants that stabilize the vacuole surrounding L. pneumophila identified the bacterial PlaA phospholipase as contributing to vacuole destabilization [29]. PlaA bears homology to Salmonella SseJ and, although the two proteina appear to have different substrate specificity, SdhA is able to cleave phospholipids and lysophospholipids. Similar to the situation with Salmonella, the ΔsdhAΔplaA strain restores vacuole integrity but the observed increase in intracellular growth is mild [29]. Therefore, it appears likely that a lipid accumulated on the ΔsdhA vacuole acts as a substrate for PlaA, with the action of the phospholipase destabilizing the LCV of the ΔsdhA strain. Destabilization appears to require interaction with host vesicle trafficking pathways. To identify host components involved in destabilizing the pathogen compartment, high throughput RNAi screens were performed, screening for disruptions that resulted in increased stability of the vacuole. Interestingly, RNAi species that increased stability of the Legionella-containing compartment were similar to those identified that support Shigella-containing compartment lysis [30]. These included regulators of endocytic vesicular traffic (Rab5 isoforms) and membrane recycling (Rab11b). This supports the conclusion that host-driven destabilization of pathogen-containing compartments may be exploited by the pathogen, as in the case of Shigella, or be blocked by vacuole guards, as observed with Salmonella and Legionella species.
C. Chlamydia trachomatis IncD/IncV proteins.
Chlamydia trachomatis hijacks the ER and Golgi to acquire lipids and maintain integrity of the vacuole surrounding replicating bacteria, called the inclusion membrane. The compartment integrity requires host-synthesized sphingomyelin [31]. In the mammalian host, ceramide, the precursor of sphingomyelin, is synthesized on the cytosolic surface of the endoplasmic reticulum. The host protein CERT binds to ER membrane proteins VAP-A and VAP-B and extracts newly synthesized ceramide. Ceramide is subsequently transported to the trans-Golgi by CERT and converted to sphingomyelin by sphingomyelin synthases. The Chlamydia protein IncD acquires sphingomyelin by directly recruiting host CERT-VAP complex to the inclusion [32,33]. Sphingomyelin synthases have shown to localize at the inclusion membrane by a vesicular pathway, thereby allowing the conversion of ceramide to sphingomyelin to occur directly on the membrane [34]. Without sphingomyelin, inclusion membrane disruption occurs.
The Chlamydia inclusion also establishes direct contacts with the ER by Chlamydia protein IncV, which inserts into the inclusion membrane and directly interacts with VAP by IncV FFAT motif [35]. This interaction is thought to tether the ER in close proximity to the inclusion. Therefore, in the case of Chlamydia the two vacuole guards act to ensure vacuolar membrane integrity by establishing transit of lipids into the membrane that stabilize the compartment as well as form docking sites to allow direct contact with the source of replication-supporting lipids. The importance of lipids transferred via the VAP-A/B system is emphasized by the fact that this transfer strategy is similarly critical for maintenance of an intact Salmonella-containing vacuole. In cells lacking both VAP-A and –B, the Salmonella vacuole loses integrity in fashion that phenocopies vacuole disruption observed after infection of cells with a sifA− strain [12]. Therefore, VAP-A/B transfer of lipids from the ER to pathogen-containing replication vacuoles may be a reoccurring motif in maintaining compartment integrity.
Regulation of Autophagy.
Autophagy is a conserved cellular pathway in eukaryotic cells that delivers cytoplasmic proteins and organelles to the lysosome for degradation. During bacterial uptake, carbohydrates that are exposed on the outside of cells face inward on vacuole membranes. These carbohydrates are signals for recruitment of autophagic machinery as a consequence of membrane damage. Galectins, such as galectin-8 recognize these carbohydrates. Galectin-8 in particular is known to recruit autophagy receptor NDP52 to the damaged membrane[36]. Autophagy protein LC3/Atg8 binds NDP52 and recruits the damaged vacuole to the autophagosome, where it ultimately fuses with the lysosome. Many pathogens inhibit recognition of disrupted vacuoles by the autophagy system. Legionella pneumophila interferes with the autophagy process of the host by the Icm/Dot translocated substrate RavZ, which is a cysteine protease that irreversibly cleaves ubiquitin-like protein Atg8 on membranes of nascent autophagosomes [25]. L. pneumophila spingosine-1 phosphate lyase (LpSpl) also inhibits autophagy by targeting host sphingolipid metabolism, which regulates the progression of autophagy [37].
In some cases, autophagy has shown to enhance the membrane integrity of the Salmonella-containing vacuole and the Mycobacterium-containing vacuole. It was demonstrated that autophagy proteins might repair SCV membrane that is damaged by the SPI-1 secretion system early on in infection. Investigators found that SCV dye retention was reduced in autophagic-deficient cells compared to WT cells [38]. Similarly, autophagy machinery was shown to repair the Mycobacterium-containing vacuole damage caused by the ESX-1 secretion system. Specifically, Atg8 was found localized around the damaged Mycobacterium-containing vacuole [39].
Recently, a complex connection between autophagy and integrity of the SCV has been described [40,41]. The Salmonella T3SS Spi-1, which is involved in the earliest steps of bacterial uptake and SCV establishment, drives translocation of SopF, which is required to maintain an intact vacuole shortly after uptake [40]. In some ways, the phenotype of a ΔsopF mutant appears quite similar to ΔsifA, with an approximately two-fold increase in disrupted SCVs at 1 hr after infection in the absence of SopF function. The authors noted, however, that 80% of the ΔsopF SCVs were encompassed by LC3 positive authophagous compartments at the same time point[40]. Although this could be an attempt to repair damaged vacuoles, the SopF mechanism of action argues that damage and LC3 recruitment are tightly connected. SopF has ADP ribosyltransferase activity that targets the v- ATPase inserted into the SCV [41]. This modification blocks the association of the v-ATPase with ATG16L1, an essential component of the LC3 transferase complex critical for authophagophore biosynthesis [41]. In vacuoles encompassing ΔsopF strains, the inability to modify the v-ATPase results in direct recruitment ATG16L1 and subsequent association of the LC3-driven autophagy machinery[41]. Yet to be determined is if the SCV damage is due to LC3 recruitment, or if damage and recruitment are two independent consequence of loss of SopF function.
Regulation of Retromer & Sorting Nexins
The retromer is a multi-subunit protein complex that mediates retrograde trafficking of cargo from endosomes to the trans Golgi network [42]. Cargo typically trafficked by the retromer are lysosome-related, such as MPRs and sortilin [43]. Modulation of retromer-dependent trafficking has emerged as a common used by intracellular pathogens to avoid vacuolar membrane damage and bacterial degradation by lysosomal components. Chlamydia substrate IncE disrupts retromer trafficking by directly binding to PX domains of retromer component SNX5/6 [44]. By binding SNX5, IncE inhibits the interaction between CI-MPR and SNX5, thus counteracting CI-MPR-mediated host restriction of Chlamydia [45]. Legionella translocated substrate RidL also inhibits retromer trafficking by binding to retromer subunit Vps29 [46]. RidL binds to a specific site of Vps29 that is also the competitive binding site TBC1d5, which regulates the interaction of the VPS complex with the targeted membrane [47–49]. In D. discoideum, deletion of host phosphoinositide 5-phosphatase OCRL reduces the amount of another retromer subunit, Vps5, recruited to the LCV [50].
In contrast, interaction with the retromer has been shown to maintain vacuole integrity in Coxiella and Salmonella. It is noteworthy that both Coxiella and Salmonella appear to replicate in acidic compartments, which may determine whether retromer components support or inhibit replication. The interaction seems to be direct, as Salmonella substrate SseC physically interacts with components of retromer [51]. Depletion of the retromer complex was found to disrupt the integrity of the SCV, indicating that SseC may be an important component in ensuring stable interaction. Additionally, the Salmonella phosphoinositide phosphatase, SopB, recruits SNX3, a retromer-associated protein, to the SCV. This recruitment induces formation of SNX3-containing tubules and promotes SCV maturation [52]. Similarly, retromer components have been implicated in regulating the formation of Coxiella-containing vacuole (CCV). RNAi treatment against retromer cargo-adapter genes VPS26, VPS29, and VPS35 or treatment against retromer-associated STX17 was shown to reduce C. burnetti intracellular replication [53]. As observed in the contrast between Salmonella and Shigella, events that can result in the loss replication vacuole integrity with one pathogen, could act to drive important steps in compartment biogenesis in another.
Conclusions.
It is clear from mutant studies that the host has numerous strategies to attack a replication vacuole. Not appreciated from previous work was the role of membrane trafficking events in disrupting membrane integrity. Traditionally, workers in the field of microbial pathogenesis had thought that the goal of constructing a microbe-containing vacuole was to synthesize a niche that avoids interacting with activities within the lysosome that are directly antimicrobial. Most surprising is that a membrane compartment, largely constructed of host components, could drive total destruction of a vacuole membrane. This is particularly unexpected, given that membrane trafficking events causing membrane dissolution are rarely observe. As a consequence, there are no molecular mechanisms for how this disruption can occur. Presumably, membrane destruction requires microbial products, and in several instances this has been observed. Almost certainly, the replication vacuole is established with a special lipid content that can tolerate the insertion of microbial proteins and the enzymatic activities associated with these products. Presumably, membrane docking events that destabilize the vacuole introduce components into the vacuolar membrane, resulting in loss of tolerance for these microbial factors. In several cases this has been demonstrated, as destabilization of the Salmonella- and Legionella-containing vacuoles results from the action of bacterial phospholipases that have no effect on membrane integrity, if traffic from poison compartments can be blocked by other microbial proteins.
The results from S. flexneri clearly show that microbial proteins can drive membrane trafficking events that destabilize the Shigella-containing compartment. For this pathogen, membrane dissolution is an essential step in the microbial replication cycle. When a similar event occurs with an intravacuolar pathogens, such as S. typhimurium and L. pneumophila, destabilization terminates microbial replication, making it necessary for the microorganism to carry the SifA and SdhA vacuole guards, respectively. Although the simplest model is that these vacuole guards act to prevent direct interaction with poison host compartments, work from Salmonella suggests that they can act to detoxify the replication vacuole membrane, funneling membranous material away from the vacuole after it has been deposited [19]. The nature of the destabilizing compartments is unclear, but both Chlamydia and Legionella translocate proteins that interfere with retromer-mediated traffic from the recycling compartment, making this compartment a particularly strong candidate for targeting by vacuole guards for some of these pathogens. That SifA also interferes with retrograde traffic of lysosomal mannose-6-phosphate receptor indicates that it could similarly prevent movement of material retrieved from the plasma membrane and directed to the lysosome via the recycling compartment.
This is a very exciting time for work focused on understanding the nature of events that maintain vacuole integrity. Most of the progress has been made at the level of genetics, and many key players have been identified. More difficult has been describing the mechanism of action of many of these proteins. Salmonella SifA is clearly one of the best characterized of this class, with binding partners identified and at least some activities described that appear to contribute to intracellular growth. Still a unified model for how membrane disruption occurs in the absence of SifA is elusive, preventing full understanding how SifA prevents this event from occurring. For other proteins that are less well-described, it is likely that further surprises will be encountered.
Acknowledgements
Work was supported by NIH grants R01-AI113211 and R21-AI11526 to RI. IA was supported by NIH Training Grant T32-GM00731. Work was supported by HHMI.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asrat S, Davis KM, Isberg RR: Modulation of the host innate immune and inflammatory response by translocated bacterial proteins. Cell Microbiol 2015, 17:785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Man SM, Place DE, Kuriakose T, Kanneganti TD: Interferon-inducible guanylate-binding proteins at the interface of cell-autonomous immunity and inflammasome activation. J Leukoc Biol 2017, 101:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackstadt T, Rockey DD, Heinzen RA, Scidmore MA: Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J 1996, 15:964–977. [PMC free article] [PubMed] [Google Scholar]
- 4.Omotade TO, Roy CR: Manipulation of Host Cell Organelles by Intracellular Pathogens. Microbiol Spectr 2019, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liss V, Hensel M: Take the tube: remodelling of the endosomal system by intracellular Salmonella enterica. Cell Microbiol 2015, 17:639–647. [DOI] [PubMed] [Google Scholar]
- 6.Beuzon CR, Meresse S, Unsworth KE, Ruiz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW: Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J 2000, 19:3235–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell G, Isberg RR: Innate Immunity to Intracellular Pathogens: Balancing Microbial Elimination and Inflammation. Cell Host Microbe 2017, 22:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellouk N, Weiner A, Aulner N, Schmitt C, Elbaum M, Shorte SL, Danckaert A, Enninga J: Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe 2014, 16:517–530. [DOI] [PubMed] [Google Scholar]
- 9.Weiner A, Mellouk N, Lopez-Montero N, Chang YY, Souque C, Schmitt C, Enninga J: Macropinosomes are Key Players in Early Shigella Invasion and Vacuolar Escape in Epithelial Cells. PLoS Pathog 2016, 12:e1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhle V, Abrahams GL, Hensel M: Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic 2006, 7:716–730. [DOI] [PubMed] [Google Scholar]
- 11.Rajashekar R, Liebl D, Chikkaballi D, Liss V, Hensel M: Live cell imaging reveals novel functions of Salmonella enterica SPI2-T3SS effector proteins in remodeling of the host cell endosomal system. PLoS One 2014, 9:e115423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolodziejek AM, Altura MA, Fan J, Petersen EM, Cook M, Brzovic PS, Miller SI: Salmonella Translocated Effectors Recruit OSBP1 to the Phagosome to Promote Vacuolar Membrane Integrity. Cell Rep 2019, 27:2147–2156 e2145. ** Shows that the SseJ phospholipase acts to support vacuolar integrity by recruiting members of the OPSBP1-VapA/B lipid exchange system, showing a parallel to work in Chlamydia.
- 13.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. : Caspase-11 cleaves gasdermin D for noncanonical inflammasome signalling. Nature 2015, 526:666–671. [DOI] [PubMed] [Google Scholar]
- 14.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J: Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 2015, 25:1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, Leffler H, Poirier F, Prevost MC, Lafont F, et al. : Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol 2010, 12:530–544. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Albert J, Yu XJ, Beuzon CR, Blakey AN, Galyov EE, Holden DW: Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol 2002, 44:645–661. [DOI] [PubMed] [Google Scholar]
- 17.Boucrot E, Henry T, Borg JP, Gorvel JP, Meresse S: The intracellular fate of Salmonella depends on the recruitment of kinesin. Science 2005, 308:1174–1178. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Wall DM, Castillo A, McCormick BA: Caspase-3 cleavage of Salmonella type III secreted effector protein SifA is required for localization of functional domains and bacterial dissemination. Gut Microbes 2019, 10:172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumont A, Boucrot E, Drevensek S, Daire V, Gorvel JP, Pous C, Holden DW, Meresse S: SKIP, the host target of the Salmonella virulence factor SifA, promotes kinesin-1-dependent vacuolar membrane exchanges. Traffic 2010, 11:899–911. [DOI] [PubMed] [Google Scholar]
- 20.Sindhwani A, Arya SB, Kaur H, Jagga D, Tuli A, Sharma M: Salmonella exploits the host endolysosomal tethering factor HOPS complex to promote its intravacuolar replication. PLoS Pathog 2017, 13:e1006700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGourty K, Thurston TL, Matthews SA, Pinaud L, Mota LJ, Holden DW: Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science 2012, 338:963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laguna RK, Creasey EA, Li Z, Valtz N, Isberg RR: A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc Natl Acad Sci U S A 2006, 103:18745–18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Valero L, Rusniok C, Carson D, Mondino S, Perez-Cobas AE, Rolando M, Pasricha S, Reuter S, Demirtas J, Crumbach J, et al. : More than 18,000 effectors in the Legionella genus genome provide multiple, independent combinations for replication in human cells. Proc Natl Acad Sci U S A 2019, 116:2265–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein D, Amaro F, Zusman T, Lifshitz Z, Cohen O, Gilbert JA, Pupko T, Shuman HA, Segal G: Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires. Nat Genet 2016, 48:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy A, Dancourt J, Mugo B, O’Connor TJ, Isberg RR, Melia TJ, Roy CR: The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 2012, 338:1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hempstead AD, Isberg RR: Inhibition of host cell translation elongation by Legionella pneumophila blocks the host cell unfolded protein response. Proc Natl Acad Sci U S A 2015, 112:E6790–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treacy-Abarca S, Mukherjee S: Legionella suppresses the host unfolded protein response via multiple mechanisms. Nat Commun 2015, 6:7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav, Copenhaver AM, Nguyen HT, Collman RG, Shin S: Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci U S A 2015, 112:6688–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creasey EA, Isberg RR: The protein SdhA maintains the integrity of the Legionella-containing vacuole. Proc Natl Acad Sci U S A 2012, 109:3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand IS, Choi W, Isberg RR: Components of the endocytic and recycling trafficking pathways interfere with the integrity of the Legionella-containing vacuole. BioRxiv 2019, 10.1101/781849v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson DK, Gu L, Rowe RK, Beatty WL: Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog 2009, 5:e1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derre I, Swiss R, Agaisse H: The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog 2011, 7:e1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumagai K, Elwell CA, Ando S, Engel JN, Hanada K: Both the N- and C- terminal regions of the Chlamydial inclusion protein D (IncD) are required for interaction with the pleckstrin homology domain of the ceramide transport protein CERT. Biochem Biophys Res Commun 2018, 505:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elwell CA, Jiang S, Kim JH, Lee A, Wittmann T, Hanada K, Melancon P, Engel JN: Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog 2011, 7:e1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stanhope R, Flora E, Bayne C, Derre I: IncV, a FFAT motif-containing Chlamydia protein, tethers the endoplasmic reticulum to the pathogen-containing vacuole. Proc Natl Acad Sci U S A 2017, 114:12039–12044. * Provides a molecular mechanism for maintenance of vacuolar membrane integrity by ensuring proper flow of membrane lipid components.
- 36.Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, Randow F: Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482:414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolando M, Escoll P, Buchrieser C: Legionella pneumophila restrains autophagy by modulating the host’s sphingolipid metabolism. Autophagy 2016, 12:1053–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreibich S, Emmenlauer M, Fredlund J, Ramo P, Munz C, Dehio C, Enninga J, Hardt WD: Autophagy Proteins Promote Repair of Endosomal Membranes Damaged by the Salmonella Type Three Secretion System 1. Cell Host Microbe 2015, 18:527–537. [DOI] [PubMed] [Google Scholar]
- 39. Lopez-Jimenez AT, Cardenal-Munoz E, Leuba F, Gerstenmaier L, Barisch C, Hagedorn M, King JS, Soldati T: The ESCRT and autophagy machineries cooperate to repair ESX-1-dependent damage at the Mycobacterium-containing vacuole but have opposite impact on containing the infection. PLoS Pathog 2018, 14:e1007501. *Demonstrates that host components that destabilize some pathogen vacuoles are important for biosynthesis of others
- 40. Lau N, Haeberle AL, O’Keeffe BJ, Latomanski EA, Celli J, Newton HJ, Knodler LA: SopF, a phosphoinositide binding effector, promotes the stability of the nascent Salmonella-containing vacuole. PLoS Pathog 2019, 15:e1007959. **First demonstration that the early steps of Salmonella vacuole biosynthesis dependent on the SPI-1 translocation system require guarding from host attack.
- 41. Xu Y, Zhou P, Cheng S, Lu Q, Nowak K, Hopp AK, Li L, Shi X, Zhou Z, Gao W, et al. : A Bacterial Effector Reveals the V-ATPase-ATG16L1 Axis that Initiates Xenophagy. Cell 2019, 178:552–566 e520. **First demonstration that the vacuolar ATPase differentiates xenophagy from the default autophagy network and that a bacterial effector targets this protein for protection from host attack.
- 42.Personnic N, Barlocher K, Finsel I, Hilbi H: Subversion of Retrograde Trafficking by Translocated Pathogen Effectors. Trends Microbiol 2016, 24:450–462. [DOI] [PubMed] [Google Scholar]
- 43.Mukadam AS, Seaman MN: Retromer-mediated endosomal protein sorting: The role of unstructured domains. FEBS Lett 2015, 589:2620–2626. [DOI] [PubMed] [Google Scholar]
- 44.Mirrashidi KM, Elwell CA, Verschueren E, Johnson JR, Frando A, Von Dollen J, Rosenberg O, Gulbahce N, Jang G, Johnson T, et al. : Global Mapping of the Inc-Human Interactome Reveals that Retromer Restricts Chlamydia Infection. Cell Host Microbe 2015, 18:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elwell CA, Czudnochowski N, von Dollen J, Johnson JR, Nakagawa R, Mirrashidi K, Krogan NJ, Engel JN, Rosenberg OS: Chlamydia interfere with an interaction between the mannose-6-phosphate receptor and sorting nexins to counteract host restriction. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finsel I, Ragaz C, Hoffmann C, Harrison CF, Weber S, van Rahden VA, Johannes L, Hilbi H: The Legionella effector RidL inhibits retrograde trafficking to promote intracellular replication. Cell Host Microbe 2013, 14:38–50. [DOI] [PubMed] [Google Scholar]
- 47.Yao J, Yang F, Sun X, Wang S, Gan N, Liu Q, Liu D, Zhang X, Niu D, Wei Y, et al. : Mechanism of inhibition of retromer transport by the bacterial effector RidL. Proc Natl Acad Sci U S A 2018, 115:E1446–E1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Romano-Moreno M, Rojas AL, Williamson CD, Gershlick DC, Lucas M, Isupov MN, Bonifacino JS, Machner MP, Hierro A: Molecular mechanism for the subversion of the retromer coat by the Legionella effector RidL. Proc Natl Acad Sci U S A 2017, 114:E11151–E11160. *Identifies likely mechanism of action of host protein that modulates vacuole biogenesis of a number of intravacuolar pathogens
- 49.Barlocher K, Welin A, Hilbi H: Formation of the Legionella Replicative Compartment at the Crossroads of Retrograde Trafficking. Front Cell Infect Microbiol 2017, 7:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Welin A, Weber S, Hilbi H: Quantitative Imaging Flow Cytometry of Legionella-Infected Dictyostelium Amoebae Reveals the Impact of Retrograde Trafficking on Pathogen Vacuole Composition. Appl Environ Microbiol 2018, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patrick KL, Wojcechowskyj JA, Bell SL, Riba MN, Jing T, Talmage S, Xu P, Cabello AL, Xu J, Shales M, et al. : Quantitative Yeast Genetic Interaction Profiling of Bacterial Effector Proteins Uncovers a Role for the Human Retromer in Salmonella Infection. Cell Syst 2018, 7:323–338 e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braun V, Wong A, Landekic M, Hong WJ, Grinstein S, Brumell JH: Sorting nexin 3 (SNX3) is a component of a tubular endosomal network induced by Salmonella and involved in maturation of the Salmonella-containing vacuole. Cell Microbiol 2010, 12:1352–1367. [DOI] [PubMed] [Google Scholar]
- 53.McDonough JA, Newton HJ, Klum S, Swiss R, Agaisse H, Roy CR: Host pathways important for Coxiella burnetii infection revealed by genome-wide RNA interference screening. MBio 2013, 4:e00606–00612. [DOI] [PMC free article] [PubMed] [Google Scholar]