Abstract
Objectives
There is limited literature on the prevalence and determinants of sarcopenia in the Indian predialysis chronic kidney disease (CKD) population. The current study attempts to characterize sarcopenia in CKD stages 3 & 4 using 3-compartment model dual-energy X-ray absorptiometry (DXA).
Methods
This is secondary data from a randomized trial on bicarbonate supplementation for preserving muscle mass. A 3-compartment DXA was done to assess body composition in 188 subjects aged 18 to 65, with stable kidney function. Sarcopenia was defined by Asian Working Group criteria - appendicular skeletal mass index < 5.4 kg/m2 in women and < 7 kg/m2 in men.
Results
Sarcopenia was present in 69.1% (n = 130). There was no difference in the prevalence of sarcopenia in CKD stage 3 (n = 62; 72.1%) vs CKD stage 4 (n = 68, 66.7%); P = 0.434. A lower body mass index (BMI) (OR 1.69; 95% CI 1.43, 2.01) and lower bicarbonate levels (OR 1.22; 95% CI 1.02, 1.47), and age (OR 0.95; 95% CI 0.91, 0.98) was independently associated with the muscle mass. A BMI cut-off of 18 failed to identify sarcopenia in 78.4% (n = 102) subjects (Kappa statistic 0.396). The receiver operating characteristic curve for mid-arm muscle circumference for identifying sarcopenia was 0.651 (95% CI 0.561, 0.740).
Conclusions
Sarcopenia is highly prevalent in CKD 3 and 4. Sarcopenic individuals are older, with a low BMI and lower bicarbonate levels. The anthropometric parameters and biochemical parameters did not help identify sarcopenia in the predialysis population.
Abbreviations: DXA, Dual energy X-ray absorptiometry
Keywords: Body composition, Muscle mass, Predialysis, Chronic kidney disease, DXA
1. Introduction
Chronic kidney disease (CKD) is associated with alterations in body composition, which appears ahead of the conventional markers of protein-energy wasting (PEW). CKD resembles a stage of accelerated ageing, with higher protein catabolic rates, resulting in relative changes of fat and muscle proportions without appreciable body weight reductions [1]. Sarcopenia is characterized by a loss of skeletal muscle mass, strength or function. In CKD, sarcopenia is not necessarily age-related and is highly prevalent in end-stage kidney disease patients on dialysis [2]. There is only limited literature on the prevalence and associations of sarcopenia in predialysis CKD. In patients with predialysis kidney disease, sarcopenia is associated with adverse long term outcomes [3,4].
The ideal method for the diagnosis of sarcopenia in patients with CKD is controversial. A decreased muscle mass is mandatory for diagnosing sarcopenia, whereas loss of physical function is considered a marker of its severity [5]. Accurate assessment of muscle mass in predialysis CKD needs dual-energy X-ray absorptiometry (DXA) or computed tomography [2]. However, previous studies on body composition in moderate to severe CKD were based on bioimpedance assays (BIA). BIA has limitations in predialysis CKD owing to the changes in the hydration status. Compared to DXA, the reliability of BIA is less reliable in states of over and under hydration [6,7]. The bias with increasing hydration tends to be less with DXA compared to BIA. There is no published literature on the prevalence of sarcopenia in the Indian predialysis population. The current study analysed sarcopenia prevalence and its clinical determinants in CKD stages 3 & 4 with a 3-compartment model DXA.
2. Methods
The data were collected between May 2015, and November 2016 from subjects enrolled in a clinical trial to test the efficacy of 6 months bicarbonate supplementation on preserving muscle mass and kidney function. The institute ethics committee approved the protocol (JIP/IEC/2014/10/475). Informed consent was taken from the participants. The study enrolled 188 patients of Indian ethnicity with CKD stages 3 & 4, aged > 18 and < 65 years, with stable renal function, with a minimum follow up of more than 3 months in the nephrology outpatient clinics. Patients with decompensated chronic liver disease, decompensated heart failure, morbid obesity (BMI ≥ 40 kg/m2), malignancy, chronic infections, bicarbonate therapy or immunosuppressants were excluded. The sample size was estimated to be 94 patients in each arm (total 188), with a mean difference of mid-arm circumference of 0.45 cm between the groups, with a standard deviation (SD) of 1.1, at 5% α error and 80% power, accommodating for a 10% loss of follow up. An intention to treat analysis showed that bicarbonate supplementation for 6 months leads to improvements in lean body mass (assessed by DXA) without changes in protein intake. The detailed protocol and results are published elsewhere.8 A thorough assessment of body composition was done at baseline, and the data is presented in this study. Demographic, clinical, and anthropometric parameters were collected at the time of enrollment. Biochemical parameters included the serum proteins, calcium, phosphorus, lipid profile, urea, creatinine and uric acid, analysed using a AU 5800 autoanalyser (Beckmann Coulter Inc, Brea, CA, USA). Venous bicarbonate levels were done with an ABL FLEX blood gas analyser (Radiometer Medical, Copenhagen, Denmark). A 3-day diet diary was used to document the nutrition intake. Subjects were instructed to record all foods and beverages details in the 3 days preceding the hospital visit. The nitrogen content of each food taken was converted to protein using the Jones Factor, specific for each food. When a specific correction factor was not available, a conversion factor of 6.25 was used.
2.1. Anthropometric data
The anthropometric measurements included body weight, height, mid-arm circumference, and triceps skinfold thickness (SFT). Bodyweight was measured to the nearest 0.1 kg on a balance beam scale with a margin of error of 0.01 kg. Height was measured to the nearest 0.5 cm, with patients standing erect with the head in the Frankfort plane. According to standard techniques, the triceps SFT was measured in the non-dominant arm using Slim Guide Skin Fold Calliper (Creative Health Products, Ann Arbour, MI, USA). The mid-arm circumference (MAMC) was measured using a flexible plastic tape with a graduated scale, at the point halfway between the acromion of the scapula and the olecranon of the ulna, in a sitting position with the arm relaxed and flexed at 90 degrees, with tape adjusted to surround the arm and avoiding skin compression. The average value of 3 consecutive measurements was taken. MAMC was calculated using Bishop's formula [MAMC = 1⁄4 mid-arm circumference [0.314 x triceps SFT (mm)]. A single trained observer performed all measurements.
2.2. Body composition
A DXA scan (Discovery Wi System, Hologic Inc, Bedford, MA, USA) was used to assess the body composition. A 3-compartment model was used for estimating whole body fat, LBM, and mineral density using the software provided by the manufacturer (QDR software version 13.4.1; Hologic Inc, Bedford, MA, USA). Patients were clinically euvolemic at the time of DXA scans. Diuretics were prescribed for patients who presented with edema, and measurements were done when they were euvolemic. The TNF-α level was measured by a commercially available ELISA (Quantikine HS human TNF-α Immunoassay kit, R & D Systems, Minneapolis, MN, USA).
2.3. Definitions
The revised consensus criteria for obesity in Indians were used for defining obesity and abdominal obesity [9]. According to Indian guidelines, a BMI of 18–22.9 kg/m2 is normal, 23–24.9 kg/m2 is considered overweight, and > 25 kg/m 2 is considered obese. An abdominal circumference higher than 80 cm in females and 90 cm in males is deemed abdominal obesity. Sarcopenia was defined as per the Asian Working group criteria - appendicular skeletal mass index (ASMI) < 5.4 kg/m2 in women and < 7 kg/m2 in men [10].
2.4. Statistical methods
All categorical variables were expressed as percentages; continuous variables were expressed as the mean with 95% confidence intervals (CI) or median with interquartile range (IQR), wherever appropriate. Chi-square test and student's t-test were used to compare categorical and continuous data across the group. Kappa statistic was used to assess the agreement between different definitions of obesity. A logistic regression analysis was done to determine the effect of preselected variables on sarcopenia. P-values < 0.05 were considered statistically significant. The data were analysed using the statistical software SPSS, version 27.0 (IBM, Armonk, NY, USA).
3. Results
The mean age was 50.2 years (95% CI 48.5–51.9). A total of 86 participants (45.7%) had CKD stage 3, and 102 (54.3%) had CKD stage 4. The predominant aetiology was CKD of unidentified aetiology (52.1%). A total of 22.3% had diabetes mellitus. The clinical parameters, anthropometric assessment and body composition across the various CKD stages are given in Table 1.
Table 1.
Characteristics of the population according to CKD stage.
| Parameter | CKD stage 3 (n = 86) | CKD 4 (n = 102) | P-value |
|---|---|---|---|
| Agea | 49.9 (47.5, 52.4) | 50.4 (48.2,52.7) | 0.776 |
| Gender (male), n % | 67 (77.9) | 67 (65.7) | 0.076 |
| Albumina | 4.0 (3.9,4.1) | 4.2 (3.7,4.7) | 0.443 |
| Cholesterola | 176 (167,185) | 174.1 (166,184) | 0.700 |
| Calciuma | 9.1 (8.9,9.2) | 9.1 (9.0,9.3) | 0.370 |
| Phosphorousa | 3.4 (3.2,35) | 3.6 (3.4, 3.8) | 0.083 |
| Uric acida | 5.5 (5.2, 5.9) | 6.0 (5.6,6.4) | 0.113 |
| Midarm muscle circumferencea | 23 (22.5,23.5) | 22.7 (22.3,23.1) | 0.663 |
| Body mass indexa | 21.5 (20.8,22.1) | 21.1 (20.5, 21.7) | 0.368 |
| Lean Tissue indexa | 6.2 (5.6,6.9) | 6.3 (5.9,6.8) | 0.611 |
| Fat tissue indexa | 14.6 (14.1, 14.9) | 14.1 (13.7, 14.4) | 0.666 |
| Appendicular skeletal mass Index (ASMI)a | 6.3 (6.1, 6.5) | 6.1 (5.9, 6.3) | 0.201 |
CKD, chronic kidney disease; Lean Tissue Index, lean mass (kg)/height, (m2); fat tissue index, fat mass (kg)/height, (m2); ASMI, appendicular skeletal mass index; appendicular skeletal mass (kg)/height (m2).
mean with 95% confidence intervals.
Sarcopenia was present in 69.1% (n = 130); there was no difference in the proportion of sarcopenia in CKD 3 (n = 62; 72.1%), and CKD 4 (n = 68, 66.7%); P = 0.434. The characteristics of those with and without sarcopenia are compared in Table 2. Those with sarcopenia had lower MAMC, serum phosphorus levels, more acidosis, lower lean and fat tissue indices and lower bone mass. The lean body mass positively correlated with the bone mass (r = 0.736; P < 0.001).
Table 2.
Clinical, biochemical, anthropometric parameters and body composition in patients with and without sarcopenia
| Parameter | Sarcopenia (n = 130) | No Sarcopenia (n = 58) | P-value |
|---|---|---|---|
| Age > 40, n (%) | 111 (85.4) | 41 (70.7%) | 0.018 |
| Gender (male), n (%) | 93 (71.5) | 41 (70.7) | 1.00 |
| Diabetes mellitus, n (%) | 26 (20) | 16 (27.6) | 0.260 |
| Urea, mg/dLa | 54 (51.4, 56.6) | 55 (51.2 58.8) | 0.627 |
| Glomerular filtration rate, ml/1.73m2/mina | 30.3 (28.4, 32.2) | 30.5 (27.8, 33.3) | 0.901 |
| Albumin, g/dLa | 4.0 (3.8, 4.2) | 4.0 (3.9, 4.2) | 0.594 |
| Cholesterol, mg/dLa | 173 (166,180) | 180 (169,190) | 0.250 |
| Calcium, mg/dLa | 9.1 (9.0,9.2) | 9.1 (8.9,9.3) | 0.965 |
| Phosphorous, mg/dLa | 3.4 (3.3,3.5) | 3.7 (3.4,4.1) | 0.029 |
| Uric acid, mg/dLa | 5.8 (5.4,6.1) | 5.8 (5.4,6.2) | 0.992 |
| Protein intake, g/daya | 34.2 (28.8,39.5) | 36.6 (28.3,41.8) | 0.647 |
| Venous bicarbonate, mEq/La | 17.9 (17.5,18.3) | 18.6 (18.1,19.1) | 0.042 |
| TNF α, pg/mLb, c | 22.9 (19.3,32.5) | 20.7 (18.5,25.9) | 0.221 |
| Anthropometry | |||
| Midarm muscle circumference, cma | 22.5 (22.1,22.9) | 23.6 (23,24.1) | 0.001 |
| Abdominal circumference, cma | 79 (77.8, 80.2) | 80.7 (79.1, 81.3) | 0.114 |
| Body weight, kga | 51.8 (50.4,53.3) | 58.6 (56.7,60.5) | < 0.001 |
| Body mass indexa | 20.3 (19.8, 20.7) | 23.5 (22.8, 24.2) | < 0.001 |
| Body mass index > 23, n (%) | 49 (37.6) | 26 (44.8) | 0.223 |
| Body composition | |||
| Lean tissue indexa | 13.6 (13.4,13.8) | 15.8 (15.4,16.2) | < 0.001 |
| Fat tissue indexa | 5.9 (5.5, 6.3) | 7.1 (6.4,7.8) | 0.002 |
| Appendicular skeletal mass indexa | 5.8 (5.7, 5.9) | 7.2 (6.9, 7.4) | < 0.001 |
| Bone mineral mass, kgb | 1.85 (1.60,2.09) | 2.03 (1.62,2.22) | 0.044 |
| Visceral adipose tissue mass, gb | 422 (231,615) | 519 (360,683) | 0.029 |
TNF, tumor necrosis factor.
mean with 95% confidence intervals.
median with interquartile range.
51 subjects had undetectable TNF α levels; the lowest limit of detection was 15.5 pg/mL.
A logistic regression model was created to assess the effect of clinical variables like age, gender, glomerular filtration rate, venous bicarbonate and BMI on the presence of sarcopenia. Age, BMI, eGFR, and bicarbonate levels were analysed as continuous variables. BMI, bicarbonate levels and age were independent predictors of sarcopenia on a multivariate regression (Table 3).
Table 3.
Logistic regression for predictors of muscle mass
| Parameter | Univariate OR (95% CI) | Multivariate OR (95% CI) |
|---|---|---|
| Age, yr | 0.98 (0.95,1.01) | 0.95 (0.91, 0.98) |
| Gender | 0.96 (0.48,1.89) | 1.88 (0.76, 4.66) |
| Glomerular filtration rate, ml/1.73m2/min | 1.00 (0.98,1.03) | 0.98 (0.95,1.01) |
| Bicarbonate, mEq/L | 1.16 (1.00, 1.37) | 1.22 (1.02, 1.47) |
| Body mass index | 1.53 (1.32, 1.77) | 1.69 (1.43, 2.01) |
OR, odds ratio.
3.1. Utility of anthropometric parameters in identifying sarcopenia
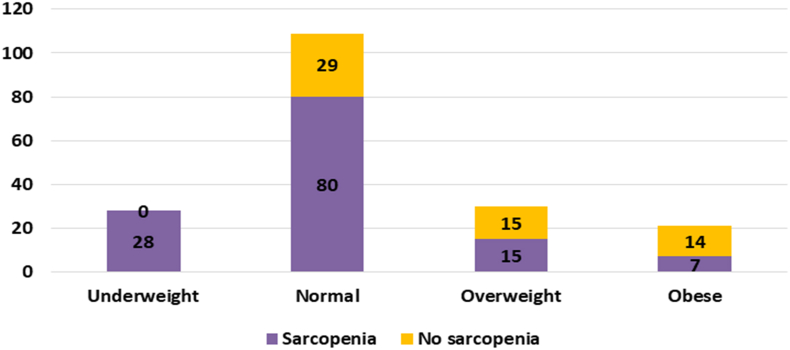
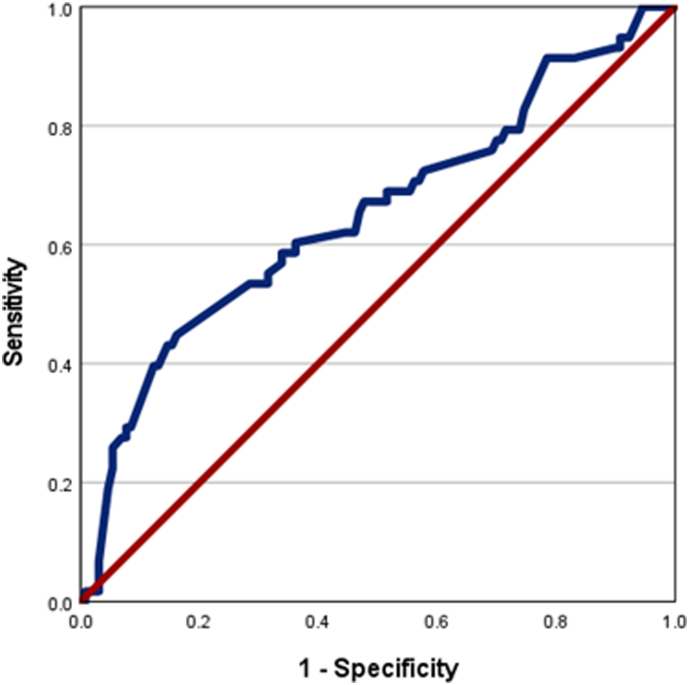
The prevalence of sarcopenia among the various BMI categories are shown in Fig. 1; (underweight 100%, normal 73.3%, overweight 50% and obese 33.3%). The proportion of overweight and obese subjects (BMI > 23) were similar between the sarcopenic and non-sarcopenic groups (Table 2). A BMI cut-off of 18 would fail to identify sarcopenia in 78.4% (n = 102) subjects with sarcopenia (Kappa statistic 0.396). Abdominal circumference did not help identify sarcopenia. The receiver operating characteristic curve for mid-arm muscle circumference for identifying sarcopenia was 0.651 (95% CI, 0.561, 0.740); Fig. 2. None of the biochemical or nutritional parameters differed between sarcopenic and non-sarcopenic individuals.
Fig. 1.
Prevalence of sarcopenia among the various BMI categories.
Fig. 2.
Receiver operating characteristic curve for midarm muscle circumference and sarcopenia.
4. Discussion
Muscle weakness and loss of muscle strength are common in CKD. Sarcopenia induces fatigue and physical inactivity, accelerating the functional loss of skeletal muscle, creating a vicious cycle [11]. Sarcopenia is contributed by multiple factors, including accumulation of uremic toxins, inflammation, insulin resistance, malnutrition, divalent ion metabolism disorders, vitamin D deficiency, anemia, metabolic acidosis, hormonal imbalances, and overzealous protein restriction. Sarcopenia is associated with increased mortality, a higher prevalence of cardiovascular disease, and faster CKD progression [4,12,13].
Most of the published literature on sarcopenia is limited to the elderly population on dialysis. There is only limited literature on sarcopenia in predialysis CKD. There are considerable variations in the reported sarcopenia prevalence; the rates vary from 2.6% in CKD stage 1, progressively increasing with later stages of CKD [14,15]. A study from Brazil reported sarcopenia in 65.5% of patients with CKD 3b-5; however, the cohort was 2 decades older than the present study [15]. A sarcopenia prevalence of 28.1% is reported from Korea, in a cohort comprising of predominantly elderly individuals with late CKD stage 3 [16]. Another Brazilian study reported a sarcopenia prevalence of only 5.9% in non-dialysis CKD [4]. The proportion of subjects with sarcopenia in the present study is considerably higher compared to the existing literature. The possible reasons may be ethnic as well as dietary practices. The staple diet is primarily rice-based with a low intake of high biological value proteins. The long-term intake of suboptimal diets may be a contributory factor. Indian ethnicity appears to be associated with low muscle mass. There is no published literature on sarcopenia prevalence in the Indian predialysis population. In a multicontinental study involving a healthy elderly population, 17.5% of Indians had sarcopenia, a considerably higher figure than other Asian countries and Europe [17]. Similarly, a hospital-based study from South India reported a sarcopenia prevalence of 54% among older adults who underwent imaging for non-malignant indications [18].
We observed that those with sarcopenia were older, with lower BMI, with a higher degree of metabolic acidosis. Advanced age is an established risk factor for sarcopenia [19]. Pro-inflammatory cytokines such as interleukin-1, interleukin-6, and TNFα levels are elevated in ESRD and contribute to insulin resistance and enhanced muscle catabolism [20,21]. However, there is a lack of literature on the direct causative role of these cytokines in predialysis patients. The current study did not show a relationship between the pro-inflammatory marker TNF-α and sarcopenia. Also, we found that the dietary intake of protein was similar between the groups. A recent study failed to show an association between dietary protein intake, inflammatory markers and sarcopenia in subjects with CKD not on dialysis [22]. Mechanisms other than the nutritional intake may be significant players in the causation of sarcopenia.
Those with sarcopenia were found to have a greater degree of metabolic acidosis. Metabolic acidosis induces insulin resistance, enhances mitochondrial stress, and activates multiple downstream cytokine mediators, leading to enhanced muscle catabolism [23,24]. Correction of metabolic acidosis was associated with better preservation of total muscle mass [8]. Also, we observed that those with sarcopenia had lower bone mineral content. There is only limited literature on the association between sarcopenia and osteoporosis in CKD [25,26]. In addition to the mechanical interactions, paracrine interactions also occur between muscle and bone. Essentially, bone and muscle function as a single functional unit; changes in 1 compartment may be transmitted to the other [27].
There was no agreement between BMI and sarcopenia, whereas a modest relationship was observed with MAMC. The reported prevalence of sarcopenia in CKD varies based on the operational definitions. Compared with BIA, a higher prevalence of sarcopenia is documented with anthropometric methods such as MAMC and subjective global assessment among older adults with CKD [4,22]. The agreement between anthropometry and sarcopenia identified by DXA is often lacking. Similarly, muscle strength based definitions tend to over diagnose sarcopenia compared to muscle mass based criteria assessed by DXA and BIA [28]. Application of diagnostic criteria like the European Working Group on Sarcopenia in Older People (EWGSOP2) criteria leads to a higher identification rate of sarcopenia. However, subjective measurements based on muscle strength were not helpful in the risk stratification of predialysis CKD patients. On the other hand, muscle mass-based definition correlated well with long term morbidity and mortality [28,29]. It appears that functional deficits may precede the changes in muscle mass, and often the association is nonlinear [19].
This study's strengths include recruiting a stable predialysis population and standardized measurement techniques for anthropometry and body composition. However, the protein intake was based on a diet diary, which is subject to recall bias. We do not have data on net nitrogen balance. We do not have data on muscle strength to quantify the magnitude of sarcopenia, which is a major limitation. The primary endpoint drove the sample size calculations, ie, the changes in the midarm circumference. A sample size of 173 would be sufficient to identify a sarcopenia prevalence of 69% (the prevalence rate in the present study) at an alpha error of 0.05 and relative precision of 10%. However, the study may be underpowered to assess the determinants of sarcopenia.
This is the first report on the prevalence of sarcopenia in predialysis CKD from India to the best of our knowledge. None of the biochemical or conventional anthropometric parameters helped diagnose sarcopenia. The study underscores the need to look into the prevalence of sarcopenia actively. An early diagnosis of sarcopenia would aid in the early institution of remedial measures, thus improving the long-term morbidity and mortality. It appears that the Indian ethnicity is associated with higher sarcopenia chances, which may be augmented by concomitant chronic illnesses. Appropriate dietary counselling should be emphasised to ensure that the dietary restrictions associated with CKD do not compromise protein intake. We found that acidosis correction improved the muscle mass, without increments in protein intake [8]. Physical activity needs to be encouraged, and acidosis needs to be corrected to prevent loss of muscle mass. Further longitudinal studies are required to assess the impact of sarcopenia on morbidity and mortality in Indian patients with CKD.
5. Conclusions
A high proportion of individuals with predialysis CKD have sarcopenia. A normal BMI does not exclude sarcopenia. Only the MAMC showed a modest association with sarcopenia. The biochemical markers do not help identify sarcopenia in moderate to severe CKD. Acidosis, advanced age and low BMI are predictors of sarcopenia.
CRediT author statement
Avinash Kumar Dubey: Conceptualization, Methodology, Formal analysis, Data curation, Writing - Original draft.
Jayaprakash Sahoo: Conceptualization, Methodology, Data curation, Writing – Review & editing.
Balasubramaniyan Vairappan: Conceptualization, Methodology, Data curation, Writing – Review & editing.
Sreejith Parameswaran: Conceptualization, Methodology, Data curation, Writing – Review & editing.
Priyamvada PS: Conceptualization, Methodology, Data curation, Writing – Original draft, Writing – Review & editing.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
Funding- Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India 605006. ORCID Avinash Kumar Dubey: 0000-0002-6108-3108. Jayaprakash Sahoo: 0000-0002-8805-143X. Balasubramaniyan Vairappan: 0000-0003-1708-4864. Sreejith Parameswaran: 0000-0001-5939-6300. Priyamvada PS: 0000-0003-0731-657X.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Bellizzi V., Scalfi L., Terracciano V., De Nicola L., Minutolo R., Marra M., et al. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J Am Soc Nephrol. 2006;17:1481–1487. doi: 10.1681/ASN.2005070756. [DOI] [PubMed] [Google Scholar]
- 2.Sabatino A., Cuppari L., Stenvinkel P., Lindholm B., Avesani C.M. Sarcopenia in chronic kidney disease: what have we learned so far? J Nephrol. 2021;34:1347–1372. doi: 10.1007/s40620-020-00840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang Y.T., Wu H.L., Guo H.R., Cheng Y.Y., Tseng C.C., Wang M.C., et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant. 2011;26:3588–3595. doi: 10.1093/ndt/gfr013. [DOI] [PubMed] [Google Scholar]
- 4.Pereira R.A., Cordeiro A.C., Avesani C.M., Carrero J.J., Lindholm B., Amparo F.C., et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015;30:1718–1725. doi: 10.1093/ndt/gfv133. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrero J.J., Johansen K.L., Lindholm B., Stenvinkel P., Cuppari L., Avesani C.M. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90:53–66. doi: 10.1016/j.kint.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Kyle U.G., Bosaeus I., De Lorenzo A.D., Deurenberg P., Elia M., Manuel Gómez J., et al. Bioelectrical impedance analysis - Part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Dubey A.K., Sahoo J., Vairappan B., Haridasan S., Parameswaran S., Priyamvada P.S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: a randomized controlled trial. Nephrol Dial Transplant. 2020;35:121–129. doi: 10.1093/ndt/gfy214. [DOI] [PubMed] [Google Scholar]
- 9.Misra A., Chowbey P., Makkar B.M., Vikram N.K., Wasir J.S., Chadha D., et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Phys India. 2009;57:163–170. [PubMed] [Google Scholar]
- 10.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S., et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Hirai K., Ookawara S., Morishita Y. Sarcopenia and physical inactivity in patients with chronic kidney disease. Nephro-Urol Mon. 2016;8:37443. doi: 10.5812/numonthly.37443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai S., Muscaritoli M., Andreozzi P., Sgreccia A., De Leo S., Mazzaferro S., et al. Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition. 2019;62:108–114. doi: 10.1016/j.nut.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Navaneethan S.D., Kirwan J.P., Arrigain S., Schold J.D. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999-2004. BMC Nephrol. 2014;15 doi: 10.1186/1471-2369-15-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon S.J., Kim T.H., Yoon S.Y., Chung J.H., Hwang H.J. Relationship between stage of chronic kidney disease and sarcopenia in Korean aged 40 years and older using the Korea National Health and Nutrition Examination Surveys (KNHANES IV-2, 3, and V-1, 2), 2008-2011. PLoS One. 2015:10. doi: 10.1371/journal.pone.0130740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souza V.A., Oliveira D., Barbosa S.R., Corrêa J.O.D.A., Colugnati F.A.B., Mansur H.N., et al. In: Shimosawa T., editor. vol. 12. 2017. (Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors). PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An J.N., Kim J.-K., Lee H.-S., Kim S.G., Kim H.J., Song Y.R. Late stage 3 chronic kidney disease is an independent risk factor for sarcopenia, but not proteinuria. Sci Rep. 2021;11:18472. doi: 10.1038/s41598-021-97952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tyrovolas S., Koyanagi A., Olaya B., Ayuso-Mateos J.L., Miret M., Chatterji S., et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7:312–321. doi: 10.1002/jcsm.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sreepriya P.R., Pillai S.S., Nair njana N.K.K., Rahul A., Pillai S., Nair A.T.S. Prevalence and associated factors of sarcopenia among patients underwent abdominal CT scan in tertiary care hospital of South India. J Frailty Sarcopenia Falls. 2020:79–85. doi: 10.22540/JFSF-05-079. 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenvinkel P., Carrero J.J., Von Walden F., Ikizler T.A., Nader G.A. Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant. 2016;31:1070–1077. doi: 10.1093/ndt/gfv122. [DOI] [PubMed] [Google Scholar]
- 20.Wang X.H., Mitch W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10:504–516. doi: 10.1038/nrneph.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung W.W., Paik K.H., Mak R.H. vol. 25. Springer; 2010. Inflammation and cachexia in chronic kidney disease; pp. 711–724. (Pediatric nephrology). [DOI] [PubMed] [Google Scholar]
- 22.Vettoretti S., Caldiroli L., Armelloni S., Ferrari C., Cesari M., Messa P. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced CKD. Nutrients. 2019;11 doi: 10.3390/nu11061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X.H., Mitch W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014;10:504–516. doi: 10.1038/nrneph.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalupsky M., Goodson D.A., Gamboa J.L., Roshanravan B. New insights into muscle function in chronic kidney disease and metabolic acidosis. Curr Opin Nephrol Hypertens. 2021;30:369–376. doi: 10.1097/MNH.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 25.Chiu T.H., Chen S.C., Yu H.C., Hsu J.S., Shih M.C., Jiang H.J., et al. Association between geriatric nutrition risk index and skeletal muscle mass index with bone mineral density in post-menopausal women who have undergone total thyroidectomy. Nutrients. 2020;12:1–12. doi: 10.3390/nu12061683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S.C., Chung W.S., Wu P.Y., Huang J.C., Chiu Y.W., Chang J.M., et al. Associations among geriatric nutrition risk index, bone mineral density, body composition and handgrip strength in patients receiving hemodialysis. Nutrition. 2019;65:6–12. doi: 10.1016/j.nut.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Hirschfeld H.P., Kinsella R., Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017;28:2781–2790. doi: 10.1007/s00198-017-4151-8. [DOI] [PubMed] [Google Scholar]
- 28.Lamarca F., Carrero J.J., Rodrigues J.C.D., Bigogno F.G., Fetter R.L., Avesani C.M. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging. 2014;18:710–717. doi: 10.1007/s12603-014-0505-5. [DOI] [PubMed] [Google Scholar]
- 29.Guida B., Maro M.D., Lauro M.D., Lauro T.D., Trio R., Santillo M., et al. Identification of sarcopenia and dynapenia in CKD predialysis patients with EGWSOP2 criteria: an observational, cross-sectional study. Nutrition. 2020;78:110815. doi: 10.1016/j.nut.2020.110815. [DOI] [PubMed] [Google Scholar]