Abstract
Background
The use of endocrine therapy for early-stage breast cancer, particularly aromatase inhibitor therapy has been associated with an increased risk of osteoporosis and fracture in clinical trials. We sought to validate this observation in real-world practice.
Methods
We used health administrative data collected from post-menopausal women (aged ≥66 years) who were diagnosed with breast cancer and started on adjuvant endocrine therapy from 2005 to 2012. Patients were classified by use of either an aromatase inhibitor or tamoxifen and followed until 2017 for a new diagnosis of an osteoporotic fracture. A multivariable analysis using a Cox proportional hazards model was adjusting for age, medical co-morbidities, medication use and duration of endocrine therapy.
Results
We identified 12,077 patients of whom 73% were treated with an aromatase inhibitor as compared to 27% with tamoxifen. Our multivariable analysis did not demonstrate any significant difference in the rate of osteoporotic fracture between patients treated with an aromatase inhibitor when compared with tamoxifen [Hazard ratio (HR) = 1.09; 95% confidence interval (CI) = 0.96–1.23, p-value = 0.18]. The 5-year rate of osteoporotic fracture for patients treated with either an aromatase inhibitor or tamoxifen was 7.5% and 6.9%, respectively. A completed sensitivity analysis did observe a decreased risk of fracture associated with tamoxifen usage over time.
Conclusion
We could not detect a significant difference in the rate of osteoporotic fracture among patients treated with an aromatase inhibitor versus tamoxifen. Nonetheless, the risk with tamoxifen was numerically lower and significantly decreased when accounting for total duration of endocrine therapy.
Keywords: Breast cancer, Endocrine therapy, Osteoporosis, Fracture
Highlights
-
•
Our real-world study investigated the osteoporotic fracture risk among early-stage post-menopausal breast cancer patients.
-
•
No significant difference in fracture rates was observed among patients treated with aromatase inhibitors versus tamoxifen.
-
•
The risk of osteoporotic fracture decreased with tamoxifen usage over time.
-
•
Bone health should be carefully monitored and optimized among breast cancer patients recieving endocrine therapy.
1. Introduction
Endocrine therapy, a standard treatment for early-stage breast cancer, is associated with a significant reduction in disease recurrence and improvement in overall survival following a 5–10 year course of treatment [1]. Aromatase inhibitors are the preferred endocrine therapy agents among post-menopausal women diagnosed with breast cancer, supported by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis showing a decreased risk of recurrence among patients treated with aromatase inhibitors as compared to tamoxifen [2].
Endocrine therapy is associated with an increased risk of osteoporosis and osteoporotic fracture, particularly among patients receiving treatment with an aromatase inhibitor [3]. Aromatase inhibitors function as pure antiestrogens, inhibiting the function of the aromatase enzyme and peripheral conversion to estrogen as compared to tamoxifen which acts as a selective estrogen receptor modulator. The antiestrogen effects of aromatase inhibitors are associated with increased bone turnover and bone loss as compared to tamoxifen which has been postulated to have bone protective effects [4,5]. Researchers have observed higher rates of fracture among patients using aromatase inhibitors when compared to tamoxifen in most major clinical trials with fracture rates ranging from 5 to 11% [[6], [7], [8], [9]].
The risk of fracture was not the primary outcome of most clinical trials and there is a concern that rates of osteoporotic fracture may be significantly higher in the general population [3]. The real-world incidence of fracture following adjuvant endocrine therapy is not well established. The purpose of this study is to compare the population-based rate of osteoporotic fracture among post-menopausal women treated with either aromatase inhibitor or tamoxifen for early-stage breast cancer in Ontario, Canada.
2. Materials and methods
2.1. Design
We conducted a retrospective cohort study among post-menopausal women (aged ≥66 years) with early-stage breast cancer who initiated treatment with adjuvant endocrine therapy between April 1st, 2005 and March 31st, 2012. Early-stage breast cancer patients, identified through the Ontario Cancer Registry, were required to have undergone definitive breast cancer surgery and demonstrate early-stage disease (stage I-III) where pathology was available. The study was conducted in Ontario, Canada using linked, population-based, health care administrative databases at ICES, an independent, non-profit research institute whose legal status under Ontario's health information privacy law allows it to collect and analyze health care and demographic data for health system evaluation and improvement [10]. ICES is a prescribed entity under section 45 of Ontario's Personal Health Information Protection Act (PHIPA). Section 45 is the provision that enables analysis and compilation of statistical information related to the management, evaluation and monitoring of, allocation of resources to, and planning for the health system. Section 45 authorizes health information custodians to disclose personal health information to a prescribed entity, like ICES, without consent for such purposes. Projects conducted wholly under section 45, by definition, do not require review by a Research Ethics Board.
2.1.1. Data sources
Administrative health care databases capturing physician billings, hospitalizations and medications were linked using unique, encoded identifiers and analyzed at ICES. Codes from the International Classification of Diseases 10th revision, Canadian Classification of Health Interventions and Drug Identification Numbers were used to identify all diagnoses, procedures and medications. Descriptions of databases and definitions of variables and administrative codes are listed in the Data Supplement (Tables 1–5).
2.1.2. Study population
We included patients aged 66 and older with recently diagnosed breast cancer who began adjuvant endocrine therapy (tamoxifen or an aromatase inhibitor: letrozole, anastrozole or exemestane) between 2005 and 2012. The majority of Ontario residents have universal coverage for physician services and hospitalizations. Starting at age 65 years, prescription medications are also covered allowing for a 1-year look-back of previous medication usage for our cohort. Patients were excluded from the cohort if they had a previous history of dementia, lived in long-term care, did not have a record in the Ontario Cancer Registry (OCR), did not have breast cancer surgery within the previous year, or were diagnosed with pre-malignant or metastatic breast cancer within 1-year of starting endocrine therapy. They were also excluded if they had previous endocrine therapy within 1-year or had a diagnosis of a second malignancy within the previous 10-years.
2.1.3. Patient characteristics
Patients were classified according to their first endocrine therapy prescription of either an aromatase inhibitor or tamoxifen and we collected the following patient characteristics: age, neighborhood-level income quintile, rural residence (community of <10,000 persons), type of breast surgery, previous receipt of chemotherapy and/or radiation, cancer stage (AJCC 7th edition), Charlson comorbidity index, duration of endocrine therapy (years) and any switch of prescribed endocrine therapy [11,12]. We also recorded baseline co-morbidities and healthcare history including: history of osteoporosis or osteoporotic fracture, previous use of corticosteroids, previous use of a bisphosphonate, number of hospitalizations (previous 3 years), number of hospital visits (previous 1 year), number of prescription medications (previous 1 year) and bisphosphonate or denosumab use while receiving endocrine therapy [13].
2.1.4. Outcomes
Our primary outcome was the incidence of osteoporotic fracture among patients treated with an aromatase inhibitor as compared to tamoxifen (enrollment from 2005 to 2012) with patient follow-up until March 31, 2017. We identified osteoporotic fracture using an algorithm adapted from the Canadian Chronic Disease Surveillance Osteoporosis Working Group definition that is based on previous case validations and studies investigating population-based osteoporosis prevalence and fracture incidence [13]. The algorithm identifies an osteoporotic fracture if a patient meets one of three conditions: ≥1 hospitalization associated with a fracture of the forearm, humerus, vertebrae, hip, pelvis or femur, 2) ≥1 physician visit for an vertebral or unspecified fracture or 3) ≥2 physician visits within a 3 month period for a fracture of the forearm, humerus or pelvis [13]. The date of first fracture was recorded as the date of either first hospitalization or physician visit associated with the fracture diagnosis. If greater than two physician visits were required within a 3-month period, the date of fracture was recorded as the last physician visits which fulfilled the criteria. The date of first fracture was retained for the analysis.
2.1.5. Statistical analysis
Characteristics among patients receiving an aromatase inhibitor versus tamoxifen were compared using students t-test, chi-square, and Kruskal-Wallis tests as applicable. A standardized difference of ≥0.10 were considered statistically significant.
A univariable time-to-event analysis was performed to evaluate the potential association between type of endocrine therapy and risk of developing of osteoporotic fracture up to the maximum follow-up date of March 31, 2017, allowing for a potential 5–12 year period of follow-up. Subjects were censored if they switched endocrine therapy (aromatase inhibitor to tamoxifen or vice versa), reached the end of the follow-up period or died. We also calculated a separate 5-year event rate to compare the cumulative incidence of osteoporotic fracture among patients started either on an aromatase inhibitor or tamoxifen.
A cause-specific multivariable Cox proportional hazards model was estimated to evaluate the possible association between type of endocrine therapy (aromatase inhibitor versus tamoxifen) and the risk of developing an osteoporotic fracture. We chose a Cox proportional hazards model due to the presence of incomplete follow-up time. The multivariable model adjusted for other important confounding factors including age, Charlson comorbidity index, previous medical co-morbidities (osteoporosis of history of osteoporotic fracture) previous medication history (use of corticosteroids or a bisphosphonate), previous use of chemotherapy and duration of endocrine therapy. The cancer stage variable was not included in the multivariable model due to large amounts of missing data. Patients with missing neighborhood income quintile were also excluded. The analysis was an intention to treat analysis and the duration of endocrine therapy variable was recorded as a time-dependent covariate measured on an annual basis up to a maximum of 5 years. The proportional hazards assumption was assessed by plotting Schoenfeld residuals against rank-transformed time.
A univariable and multivariable sensitivity analysis was also conducted using a more specific definition of osteoporotic fracture which excluded any fracture associated with radiation therapy within 60 days, as these fractures may have represented pathologic fractures associated with the patients underlying breast cancer diagnosis. We also measured the rate of osteoporotic fracture in a select group of high-risk patients for osteoporotic fracture defined as patients ≥75 years of age who had a history of osteoporosis or osteoporotic fracture or had a previous history of bisphosphonate at initiation of endocrine therapy. An additional sensitivity analysis was also conducted modelling the effect of duration of endocrine therapy for both an aromatase inhibitor and tamoxifen using two separate time-dependent covariates and controlling for bisphosphonate or denosumab use while receiving endocrine therapy. A Fine and Gray competing risk model was also performed using death as a competing risk for developing an osteoporotic fracture.
All tests were two sided and a p-value of 0.05 or less was considered statistically significant. No statistical corrections for multiple testing were conducted. Statistical analyses were performed using the SAS software program version 9.4 (SAS Institute, Cary, NC).
3. Results
A total of 12,077 patients were identified as receiving endocrine therapy from 2005 to 2012 after applying exclusions (Fig. 1). The median age of the cohort was 73 years (IQR 69–78). Seventy-three percent of patients started therapy with an aromatase inhibitor as compared to 27% originally treated with tamoxifen. Sixty-one percent of patients completed ≥5 years of therapy and 21% of patients switched therapy during their course of treatment. In the observed cohort, 64% percent of patients had a lumpectomy, 19% and 27% percent were treated with adjuvant chemotherapy and radiation respectively. Staging information was missing in 34% percent of patients. Among those with recorded staging information, 46% were Stage I, 42% Stage II and 12% Stage III disease at presentation.
Fig. 1.
Consort diagram.
Table 1 demonstrates the baseline characteristics among patients receiving an initial prescription for either an aromatase inhibitor or tamoxifen. There were significant differences noted between the two groups regarding age, stage, adjuvant chemotherapy, baseline history of osteoporosis and/or osteoporotic fracture and previous use of corticosteroids and bisphosphonate therapy based on a standardized difference of ≥0.10.
Table 1.
Baseline characteristics of post-menopausal breast cancer patients according to type of endocrine therapy treated in Ontario, Canada from 2005 to 2012 (n = 12,077).
| Baseline Characteristics | Endocrine therapy |
Standardized Differences | |
|---|---|---|---|
| Aromatase Inhibitor n = 8770 | Tamoxifen n = 3307 | ||
| Age (years) | |||
| Median (IQR) |
72 (69–78) |
74 (69–80) |
0.23 |
| Income quintile, n (%) | |||
| 1 (low) | 1688 (19%) | 627 (19%) | 0.01 |
| 2 | 1827 (21%) | 669 (20%) | 0.01 |
| 3 | 1708 (19%) | 656 (20%) | 0.01 |
| 4 | 1707 (19%) | 646 (20%) | 0 |
| 5 (high) | 1818 (21%) | 697 (21%) | 0.01 |
| Missing |
22 (<1%) |
12 (<1%) |
0.02 |
| Rural (<10,000 residents) | 1169 (13%) | 531 (16%) | 0.08 |
| Type of surgery, n (%) | |||
| Mastectomy | 3128 (36%) | 1142 (35%) | 0.02 |
| Lumpectomy | 5589 (64%) | 2149 (65%) | 0.03 |
| Missing |
53 (<1%) |
16 (<1%) |
0.02 |
| Radiation therapy, n (%) | 2277 (26%) | 948 (29%) | 0.06 |
| Chemotherapy, n (%) | 1949 (22%) | 378 (11%) | 0.29 |
| Cancer stage, n (%) | |||
| I | 2672 (31%) | 1054 (32%) | 0.03 |
| II | 2605 (30%) | 739 (22%) | 0.17 |
| III | 802 (9%) | 132 (4%) | 0.21 |
| Missing |
2691 (31%) |
1382 (42%) |
0.23 |
| Charlson comorbidity index, n (%) | |||
| ≤1 (low) | 678 (8%) | 280 (9%) | 0.03 |
| 2 | 2285 (26%) | 999 (30%) | 0.09 |
| ≥3 (high) | 1915 (22%) | 596 (18%) | 0.1 |
| No hospitalization |
3892 (44%) |
1432 (43%) |
0.02 |
| Medical co-morbidities, n (%) | |||
| Osteoporosis and/or history of fracture |
1471 (17%) |
834 (25%) |
0.21 |
| Previous medications, n (%) | |||
| Corticosteroids | 1788 (20%) | 390 (12%) | 0.24 |
| Bisphosphonates |
1983 (23%) |
1188 (36%) |
0.30 |
| No. of hospitalizations (previous 3-years) | |||
| Median (IQR) |
0 (0–0) |
0 (0–0) |
0.02 |
| No. of hospital visits (previous year) | |||
| Median (IQR) |
8 (5–13) |
8 (5–13) |
0.03 |
| No. of prescriptions (previous year) | |||
| Median (IQR) | 7 (4-11) | 7 (4-11) | 0.03 |
| Duration of endocrine therapy, n (%) | |||
| 1 year | 876 (10%) | 498 (15%) | 0.15 |
| 2 years | 837 (10%) | 446 (14%) | 0.12 |
| 3 years | 641 (7%) | 479 (15%) | 0.23 |
| 4 years | 575 (7%) | 347 (11%) | 0.14 |
| ≥5 years |
5841 (67%) |
1537 (47%) |
0.41 |
| Switch of endocrine therapy, n (%) | 1355 (16%) | 1214 (37%) | 0.5 |
| Medication use during endocrine therapy, n (%) | |||
| Bisphosphonates | 4230 (48%) | 1546 (47%) | 0.03 |
| Bisphosphonates or denosumab | 4307 (49%) | 1579 (48%) | 0.03 |
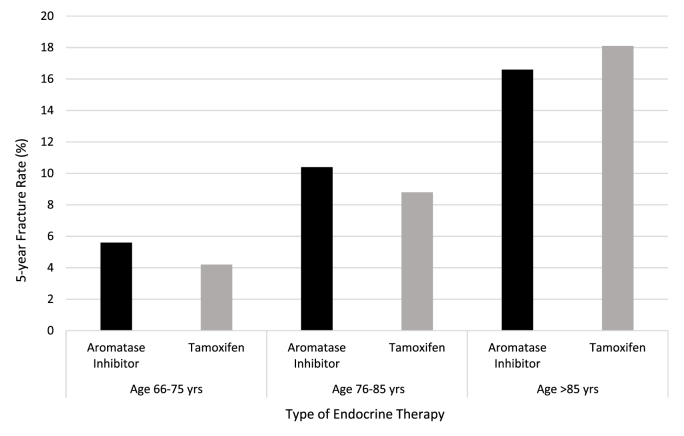
In a univariable analysis, the cumulative incidence of osteoporotic fracture was 12.0% (n = 1049) among patients treated with an aromatase inhibitor and 11.0% (n = 364) among patients treated with tamoxifen (Table 2). The unadjusted risk of osteoporotic fracture was not significantly different among patients treated with an aromatase inhibitor as compared to tamoxifen [Hazard Ratio (HR) = 0.89, 95% confidence internal (CI) 0.79–1.00, p-value = 0.06]. The 5-year fracture rate was 7.5% and 6.9% among patients treated with aromatase inhibitors and tamoxifen respectively. The 5-year rate of fracture at age of study entry is also presented in Fig. 2 and the cumulative incidence fracture curve in the Data Supplement (Fig. 1). Fracture sites included forearm (45%), hip (27%), vertebrae (16%), pelvis (10%), and unspecified (2%).
Table 2.
Univariable incidence of developing an osteoporotic fracture according to endocrine therapy among post-menopausal women diagnosed with early-stage breast cancer treated in Ontario, Canada from 2005 to 2012 (N = 12,077) and among patients classified as high-risk for fracture (n = 1913).
| Characteristics | Endocrine Therapy | Event rate n (%) |
HR | 95% CI | P-value |
|---|---|---|---|---|---|
| Osteoporotic fracture | Aromatase Inhibitor | 1049 (12.0%) | 0.89 | 0.79–1.00 | 0.06 |
| Tamoxifen | 364 (11.0%) | Reference | |||
| Osteoporotic facture (among high-risk patientsa) | Aromatase Inhibitor | 240 (20.5%) | 1.13 | 0.91–1.40 | 0.27 |
| Tamoxifen | 123 (16.6%) | Reference | |||
HR: hazard ratio, CI: confidence interval.
Patients censored at death, end of follow-up (March 2017) and upon a switch of endocrine therapy (aromatase inhibitor to tamoxifen or vice versa).
High-risk patients were defined as age ≥75 years and history of osteoporosis or osteoporotic fracture or previous use of a bisphosphonate.
Fig. 2.
5-year osteoporotic fracture rate by patients age and type of endocrine therapy among post menopausal women treated for early-stage breast cancer in Ontario, Canada from 2005 to 2012 (n = 12,077).
In our adjusted analysis presented in Table 3, the risk of developing an osteoporotic fracture was also not significantly higher in patients treated with an aromatase inhibitor as compared to tamoxifen (HR = 1.09, 95% CI: 0.96–1.23, p-value = 0.18). In the multivariable model, there were 1413 (11.7%) fracture events, 2261 (18.7%) deaths, 2487 (20.6%) patients censored for switching endocrine therapy and 5916 (49.0%) patients who reached the end of follow-up. There were 1709 (19.5%) deaths among patients treated with aromatase inhibitors as compared to 552 (16.7%) deaths among patients treated with tamoxifen. The median follow-up time was 5.9 years. The following variables were also significant in the adjusted model: age (HR = 1.07, 95% CI: 1.06–1.08, p-value=<0.001), Charlson comorbidity index ≥2 (HR = 1.18, 95%CI: 1.06–1.31, p-value = 0.002), history of osteoporosis or osteoporotic fracture (HR = 1.42, 95% CI: 1.24–1.62, p-value<0.001), history of corticosteroid use (HR = 1.30, 95%CI: 1.06–1.60, p-value = 0.012), history of bisphosphonate use (HR = 1.19; 95%CI: 1.05–1.35, p-value = 0.006). Previous use of chemotherapy, type of endocrine therapy and duration of endocrine therapy measured as a time-dependent covariate were not significant.
Table 3.
Multivariable Cox proportional hazards model for factors influencing the risk of osteoporotic fracture among post-menopausal women treated for breast cancer in Ontario, Canada from 2005 to 2012 (N = 12,077).
| Characteristics | Units | HR | 95% CI | P-value |
|---|---|---|---|---|
| Endocrine therapy | AI vs. Tamoxifen | 1.09 | 0.96–1.23 | 0.18 |
| Age | Per Year | 1.07 | 1.06–1.08 | <.0001 |
| Charlson comorbidity index | High vs. Low | 1.18 | 1.06–1.31 | 0.002 |
| History of osteoporosis or osteoporotic fracture | Yes vs. No | 1.42 | 1.24–1.62 | <0.001 |
| Previous use of steroids | Yes vs. No | 1.30 | 1.06–1.60 | 0.012 |
| Previous use of bisphosphonate | Yes vs. No | 1.19 | 1.05–1.35 | 0.006 |
| Previous use of chemotherapy | Yes vs. No | 0.89 | 0.72–1.10 | 0.27 |
| Duration of endocrine therapya | Per Year | 0.97 | 0.91–1.02 | 0.20 |
AI: Aromatase inhibitor, HR: hazard ratio, CI: confidence interval.
Charlson comorbidity index: High (≥2) vs. Low (0–1 or no hospitalization).
Patients censored at death, end of follow-up (March 2017) and upon a switch of endocrine therapy (aromatase inhibitor to tamoxifen or vice versa).
Time-dependent co-variate.
A sensitivity analysis was also performed excluding fractures treated with radiation within 60 days. This only excluded 28 (<1%) fracture events and the results of the multivariable analysis comparing the risk of developing an osteoporotic fracture in patients treated with an aromatase inhibitor as compared to tamoxifen did not significantly change [Data Supplement Table 6: (HR = 1.09, 95% CI: 0.96–1.23, p-value = 0.19)]. The cumulative incidence of osteoporotic fracture was also calculated among patients at high risk of osteoporotic fracture (defined as age ≥75 year of age and previous baseline history of osteoporosis or osteoporotic fracture or previous use of a bisphosphonate). The high-risk fracture rate was 20.5% (n = 240) among patient treated with an aromatase inhibitor and 16.6% (n = 123) among patients treated with tamoxifen (Table 2). The unadjusted risk of osteoporotic fracture was not significantly different among high-risk patients treated with an aromatase inhibitor as compared to tamoxifen [HR = 1.13, 95% CI: 0.91–1.40, p-value = 0.27]. The 5-year fracture rate was 13.5% and 12.2% for patients receiving aromatase inhibitors and tamoxifen, respectively.
The sensitivity analysis using two time-dependent covariates modelling duration of aromatase inhibitor and tamoxifen treatment observe a decreased risk of fracture associated with tamoxifen use over time [Data Supplement: Table 7: (HR = 0.90; 95%CI: 0.83–0.97; p-value = 0.007)]. Conversely, fracture risk was unchanged when investigating duration of aromatase inhibitor therapy over time. No significant difference in fracture risk according to first prescription of an aromatase inhibitor or tamoxifen was observed even when controlling for bisphosphonate or denosumab use while on endocrine therapy [HR = 0.87; 95% CI: 0.64–1.17; p-value = 0.35]. The Fine and Gray competing risk model did not demonstrate any significant difference in osteoporotic fracture risk among patients treated with an aromatase inhibitor as compared to tamoxifen when adjusting for death as a competing risk to developing a fracture event [Data Supplement: Table 8: HR = 1.07, 95% CI: 0.94–1.21, p-value = 0.30]
4. Discussion
Our study did not detect a significant difference in osteoporotic fracture risk among post-menopausal women treated either with an aromatase inhibitor or tamoxifen for early breast cancer in Ontario, Canada. The osteoporotic fracture risk was 7.5% among patients treated with an aromatase inhibitor as compared to 6.9% in patients treated with tamoxifen with 5-years follow-up. After adjustment in the multivariable analysis, the risk of fracture was not significantly different (HR = 1.09, 95% CI: 0.96–1.23; p-value = 0.18).
Large clinical trials have demonstrated an increased risk of fracture among patients receiving an aromatase inhibitor as compared to tamoxifen. The EBCTCG meta-analysis noted a higher 5-year fracture risk of 8.2% versus 5.5% among patients treated with aromatase inhibitor as compared to tamoxifen [14]. While we expected to observe a higher rate of fracture among patients treated with an aromatase inhibitor, we did not observe any significant difference in comparison with patients treated with tamoxifen. This may reflect unmeasured confounding as oncologists may have preferentially offered tamoxifen to patients viewed at higher risk of osteoporotic fracture. A large study by Schimdt et al. from the United Kingdom using health administrative data was able to demonstrate a significant increased risk of fracture among patients treated with aromatase inhibitors [15]. We were not able to control for height, weight, smoking status, alcohol consumption and baseline bone mineral density which are important elements of the Fracture Risk Assessment Tool (FRAX), commonly used in clinical practice to estimate a patient's future risk of fracture, as this information was not available in our health administrative databases [16]. Our study also reflects an older post-menopausal population where fracture risk may be more influenced by age, medical co-morbidities and pre-existing osteoporosis rather than medication associated effects.
Two of our study's greatest strengths are the use of comprehensive real-world population of breast cancer patients from Ontario, Canada and use of a strict clinical fracture definition adapted from the Canadian Chronic Disease Surveillance Osteoporosis Working Group [13]. Our results are consistent with a previous Canadian study using health administrative data, which also did not demonstrate a higher risk of fracture among patients treated with aromatase inhibitors as compared to non-aromatase inhibitor users or the general population [17]. Additionally, our sensitivity analysis did demonstrate an association between longer durations of tamoxifen therapy and a decreased risk of osteoporotic fracture, consistent with previously published reports demonstrating tamoxifen's more favorable effect on bone re-modelling, formation and density [5].
Nonetheless, our analysis does provide valuable estimates of osteoporotic fracture risk among early-stage breast cancer patients treated with endocrine therapy. The incidence of osteoporotic fracture observed in our study is consistent with the EBCTCG meta-analysis and large-scale clinical trials [2]. Other clinical trials have suggested higher rates of osteoporotic fracture including the ABCSG-18 clinical trial investigating adjuvant denosumab [18]. In this study the fracture rate was 26.2% at 7-years, significantly higher than the rates observed in our current study. We acknowledge the possibility that our study using health administrative data may underestimate osteoporotic fracture incidence, especially in regard to vertebral or milder fracture events. Our fracture definition cannot truly differentiate the cause of fracture (osteoporotic, traumatic, or pathologic), however, our sensitivity analysis results did not significantly change after restricting potential cases of pathologic fracture who received radiation treatment within 60 days of their fracture event. Additionally, our real-world data did not show a reduction in osteoporotic fracture with the concomitant use of a bisphosphonate. Denosumab does significantly lower the risk of osteoporotic fracture as demonstrated in the ABCSG-18 and D-CARE clinical trials, however, we were not able to confirm this observation as denosumab utilization was relatively uncommon during our study time period [18,19].
Our analysis is also focused on older post-menopausal women and the finding should not represent fracture risk in pre- or peri-menopausal breast cancer patients. Our study also pre-dates the use of adjuvant bisphosphonate therapy in breast cancer which may also potentially reduce risk of osteoporotic fracture among post-menopausal women [20]. Further studies among patients treated with adjuvant bisphosphonates are warranted.
Our study is one of a few population-based studies of fracture in early-stage breast cancer patients among the general population. While our study did not observe a significant difference in fracture among postmenopausal breast cancer patients using either aromatase inhibitors or tamoxifen, the study does give reasonable estimates for fracture in a real-world population-based setting in a country with publicly funded health care. Of note, the fracture risk among high-risk patients (age ≥75 years and previous baseline history of osteoporosis or osteoporotic fracture or previous use of a bisphosphonate) was 20.5% after starting an aromatase inhibitor. Patients and clinicians need to be aware of a significant fracture risk with endocrine therapy and appropriate surveillance and optimized treatment of osteoporosis is required irrespective of choice of endocrine therapy.
Declaration of competing interest
KIP has received honoraria from Pfizer, Roche, Amgen, Novartis, Eisai, Genomic Health, and Myriad Genetics Laboratories. She has also consulted for Pfizer and Gilead Sciences and received royalties from UpToDate. TV has consulted for Novartis and Roche. RF has consulted for Novartis, Janssen, Pfizer and Bayer. JR has received honoraria from Roche and consulted for Lilly, Merck and Novartis. DND has received honoraria from Amgen and consulted for Novartis. All other authors report no conflicts.
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and Ministry of Long-Term Care (MLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). This research was also funded by a Medical Oncology Research Fund (MORF). Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI) and Cancer Care Ontario (CCO). The opinions, results, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of the funding or data sources. We thank IQVIA Solutions Canada Inc. for use of their Drug Information Database. No endorsement by ICES, MOHLTC, AMOSO, SSMD, LHRI, CIHI, or CCO is intended or should be inferred.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.09.010.
Author contributions
PSB, ML, BL, LR, SZS, KIP, JR, TV, RF, DND, KKWC and CCE created the study design and conception. Data analysis and interpretation was performed by PSB, ML, BL and AO. PSB, ML, BL, LR, SZS, KIP, JR, TV, RF, DND, KKWC and CCE were responsible for writing the original drafted manuscript and all authors approved the final manuscript.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Burstein H.J., et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 3.Jerzak K.J., et al. Bone-targeted therapy in early breast cancer. Oncology (Williston Park) 2018;32(11):562–569. [PubMed] [Google Scholar]
- 4.Perez E.A., Weilbaecher K. Aromatase inhibitors and bone loss. Oncology (Williston Park) 2006;20(9):1029–1039. discussion 1039-40, 1042, 1048. [PMC free article] [PubMed] [Google Scholar]
- 5.Love R.R., et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326(13):852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 6.Howell A., et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 7.Coates A.S., et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25(5):486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 8.Coleman R.E., et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8(2):119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 9.Goss P.E., et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Canc Inst. 2005;97(17):1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 10.ICES www.ices.on.ca
- 11.Charlson M.E., et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell S., Canadian G. Chronic Disease Surveillance System Osteoporosis Working, Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: results from a feasibility study. Arch Osteoporos. 2013;8:143. doi: 10.1007/s11657-013-0143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists' Collaborative G., et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt N., et al. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res Treat. 2016;155(1):151–157. doi: 10.1007/s10549-015-3661-3. [DOI] [PubMed] [Google Scholar]
- 16.Leslie W.D., et al. Construction of a FRAX(R) model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int. 2011;22(3):817–827. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- 17.Leslie W.D., et al. Fracture risk in women with breast cancer initiating aromatase inhibitor therapy: a registry-based cohort study. Oncologist. 2019;24(11):1432–1438. doi: 10.1634/theoncologist.2019-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnant M., et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 19.Coleman R., et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(1):60–72. doi: 10.1016/S1470-2045(19)30687-4. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.