Abstract
Background
Previous population‐based studies on second primary cancers (SPCs) in urothelial cancers have focused on known risk factors in bladder cancer patients without data on other urothelial sites of the renal pelvis or ureter.
Aims
To estimate sex‐specific risks for any SPCs after urothelial cancers, and in reverse order, for urothelial cancers as SPCs after any cancer. Such two‐way analysis may help interpret the results.
Methods
We employed standardized incidence ratios (SIRs) to estimate bidirectional relative risks of subsequent cancer associated with urothelial cancers. Patient data were obtained from the Swedish Cancer Registry from years 1990 through 2015.
Results
We identified 46 234 urinary bladder cancers (75% male), 940 ureteral cancers (60% male), and 2410 renal pelvic cancers (57% male). After male bladder cancer, SIRs significantly increased for 9 SPCs, most for ureteral (SIR 41.9) and renal pelvic (17.2) cancers. In the reversed order (bladder cancer as SPC), 10 individual FPCs were associated with an increased risk; highest associations were noted after renal pelvic (21.0) and ureteral (20.9) cancers. After female bladder cancer, SIRs of four SPCs were significantly increased, most for ureteral (87.8) and pelvic (35.7) cancers. Female bladder, ureteral, and pelvic cancers associated are with endometrial cancer.
Conclusions
The risks of recurrent urothelial cancers were very high, and, at most sites, female risks were twice over the male risks. Risks persisted often to follow‐up periods of >5 years, motivating an extended patient follow‐up. Lynch syndrome‐related cancers were associated with particularly female urothelial cancers, calling for clinical vigilance.
Keywords: cancer etiology, relative risk, renal pelvic cancer, second primary cancer, ureter cancer, urothelial cancer
1. INTRODUCTION
Urothelial carcinomas include bladder cancer (90‐95% of all) and cancer of the upper urinary tract (UUT), of which two‐thirds are located in the renal pelvis and the remaining in the ureter. 1 Bladder cancer is characterized by male excess, ranging from three‐ to sixfold. 2 , 3 International incidence trends have found correlation with the regional smoking prevalence. 2 , 4 Bladder cancer incidence in Swedish men has been low and relatively stable at about 20/100 000, while for women, the rate has increased, reaching an incidence of about 6/100 000. 5 , 6 Smoking prevalence is not the only explanation to the sex difference in bladder cancer incidence in Sweden, because smoking prevalence in men and women has been approximately equal since 1980 and dropping from 30% to below 10%; around 1950, half of men smoked compared to 10% of women 6 (www.pnlee.co.uk/downloads/iss/iss-sweden_111024.pdf). UUT cancers show also male excess. 1 All urothelial cancers share a number of risk factors, including smoking, occupational exposures, family history, and association with Lynch syndrome. 1 , 7 , 8 , 9 However, while smoking appears to be the main risk factor for bladder cancer compared to Lynch syndrome, the opposite may be the case for UUT cancers. 10 , 11 Colorectal cancer and endometrial cancers are traditional hallmarks of Lynch syndrome (www.lscarisk.org). 12 , 13 Few guidelines to date suggest urological follow‐up with Lynch patients. 1
In Sweden, survival has slightly improved for bladder cancer since about 1980. 14 , 15 , 16 In 2012‐2016, the relative 5‐year survival for female bladder cancer was 72% but it was higher, 77% for men (http://www-dep.iarc.fr/NORDCAN/english/frame.asp). Early detection, novel imaging technologies, and improvements in treatment have contributed to positive trends in bladder cancer survival. 5 At bladder cancer diagnosis, some 20‐25% of patients present with muscle invasive tumors, and the remaining patients have superficial tumors, which can later progress to invasive cancer. 17 Surgery is the main treatment mode for urothelial cancers. For bladder cancer, non‐muscle‐invasive tumors are transuretherally resected while muscle‐invasive tumors are typically treated with cystectomy; both of which can be supplemented with chemotherapy or immunotherapy. 5 , 17 Radiotherapy may be used for bladder preservation. 18 For UUT cancers, treatment may involve removal of the ipsilateral ureter and kidney. 1
Improved survival implies that the likelihood of second primary cancers (SPCs) increases. SPCs after bladder cancer show typically high risks of tobacco‐related cancers of the lung and head and neck. 19 , 20 , 21 , 22 Studies on SPCs in UUT cancer patients are limited, and one of the problems is to distinguish independent SPCs from recurrences. 21 Recurrence of UUT urothelial cancer into the bladder is relatively common, while seeding from the bladder into UUT is rarer. 1 , 23 , 24 , 25 Plausible etiologies for SPCs are many, but probably the most important ones are intensive medical surveillance after the diagnosis of first primary cancer (FPC), therapy for FPC, shared genetic or nongenetic risk factors between FPC and SPC and immune dysfunction, or interactions between these. 26 , 27 , 28 As data on the possible risk factors for SPC are usually limited, we have devised a bidirectional analysis as a tool to help etiological search. 26 , 27 , 28 , 29
In this analysis, we want to define risks for specific subsequent cancers related to bladder and UUT cancers using Swedish nation‐wide data. Risks for SPCs are assessed pair‐wise as FPC and SPC, that is, the standardized incidence ratio (SIR) of lung cancer was assessed after bladder cancer, and, alternatively, SIR of bladder cancer was assessed after lung cancer. The bidirectional analysis will help to distinguish, at least to some extent, the influence of treatment and medical surveillance on SIR because two different cancers are usually treated and diagnosed in different ways. As the previous literature has focused on SPCs after bladder cancer, we hypothesized that the novel type of bidirectional analysis will be able to produce novel qualified data on risks for bladder and UUT cancers.
2. MATERIALS AND METHODS
2.1. Diagnostic codes and nomenclature
We considered bladder and UUT cancers diagnosed from 1990 to 2015 in the Swedish Cancer Registry using International Classification of Diseases (ICD) version 7 and later codes. The project database is located at the Center for Primary Health Care Research in Malmö, Sweden.
Code 1810 was used for bladder cancers and, of these, 98% are transitional cell carcinomas. 30 The code for ureter cancer was 1811. 31 Urethral cancer was not considered because of its rarity and late introduction of a specific diagnostic code. Kidney cancer (ie, renal + pelvis/calyx) was identified with code 180, renal cell cancer (RCC) with code 1800, and pelvic/calyx cancer with 1801. The correctness of classification of UUT cancer in the Cancer Registry has been evaluated, and 93% were found to be correct; the misclassification frequently involved other urinary tract tumors. 32 In this article, we consider a possible “recurrence” when a second urothelial cancer occurred at the same anatomic site or at another urothelial site, following the practice of the European Association of Urology. 1
2.2. Patient follow‐up periods and methods
Bladder and UUT cancer patients were followed from year 1990 through year 2015 for diagnosis of any common SPC, and, conversely in a reverse order, any common cancer was FPC and bladder, and UUT cancers were SPCs. The other cancers include any of 21 common male and 22 female primary cancers. For a proper bidirectional analysis, we excluded cancers for which 1‐year survival was less than 50% (esophagus, pancreas). Patients were followed for SPCs from the diagnosis of FPC until the end of 2015 or immigration or death, whichever came earliest. Only discordant (different) FPC‐SPC pairs were included without applying any lag time between the two diagnoses. The upper aerodigestive tract (UAT) included the lip, oral cavity, pharynx, and larynx. For skin cancer, only squamous cell carcinoma (SCC) was included. For the risk of “all” cancers, bladder and UUT cancers were excluded. The SIR for “all” cancers was weighed according to person‐years at risk. No latency time was apply between diagnoses of FPC and SPC because in the Swedish Cancer Registry, practically all cancers are histologically verified and thus true cancers. 31 The application of a latency time would have caused bias because a large number of true SPCs would have been missed.
2.3. Calculation of relative risk
Sex‐specific SIRs were calculated to measure the risk of SPCs as the ratio of observed to expected number of cases. For risk of a certain SPC, the expected number of cases was calculated by strata‐specific person years in patients with diagnosis of first primary bladder or UUT cancers, multiplied by strata‐specific incidence rates of the same SPC as FPC in the general population. The strata were specified by sex, 5‐year age group, 5‐year‐calendar period, socioeconomic status, and place of residence. In the reverse analysis, SIRs for second bladder and UUT cancers were calculated in the same way. The two‐tailed 95% confidence intervals (95% CIs) of SIRs were calculated by assuming a Poisson distribution. The expected numbers can be obtained by dividing observed numbers with SIR. The method of SIR calculation is based on indirect standardization, and it is particularly suitable for datasets with small case numbers because the expected numbers are calculated from the large background population, all Sweden in this case. 33
Bidirectional SIRs for bladder cancer were summarized in forest plots. Pearson correlation coefficient was used to estimate the relation between the bidirectional SIRs for bladder cancer (FPC vs SPC) as well as the SIRs of the common cancers in men and women (men vs women). In addition, we carried out period‐specific analysis by calculating SIRs during 1, 2‐5, and >5 years after first primary cancer diagnosis. All the statistical analyses were performed in SAS 9.4, and forest plot was generated in R 3.3.6. In order to simplify the tables, we did not show data for cancers with less than five cases in any comparison, unless the SIR was significant. The difference between two SIRs was considered significant when their 95% CIs did not overlap. Only significant SIRs were commented on. In the tables, some lines for the urothelial cancers were repeated in the reverse analyses, but they were kept to help comparisons.
3. RESULTS
During the follow‐up (inclusion) period of 1990 to 2015, we identified 46 234 bladder cancers, 940 ureter cancers, and 2410 renal pelvic cancers (Table 1). The total number of other cancers considered as SPCs or FPCs was 513 693 for men and 496 600 for women in the concurrent Swedish population of 6.2 million men and 6.2 million women.
TABLE 1.
Number and median age at diagnosis of the bladder and upper urinary tract cancers identified from 1990 to 2015
| Cancer | N (percentage) | Median (lower and upper quartiles) age at diagnosis | ||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Bladder | 34 676 (75%) | 11 558 (25%) | 73 (65‐80) | 74 (65‐82) |
| Ureter | 564 (60%) | 376 (40%) | 72 (65‐79) | 74 (67‐80) |
| Renal pelvic | 1374 (57%) | 1036 (43%) | 77 (63‐77) | 73 (65‐80) |
Bidirectional SIRs for male SPCs for bladder and UUT cancers are shown in Table 2. After bladder cancer, SIRs were significantly increased for 10 SPCs (counting RCC and renal pelvis but not kidney), most for ureteral (SIR 41.9), renal pelvic (17.20), and small intestinal (2.38) cancers. SIR for RCC was 2.20. The overall SIR for any SPC was 1.56. In the reversed order (bladder cancer as SPC), 10 individual FPCs were associated with an increased risk; highest associations were noted after renal pelvic (21.0), ureteral (20.9), and testicular (2.01) cancers. Second bladder cancer risk was 1.45 after RCC. The SIR for all cancers was 1.28. Only five cancers (ureteral, pelvic, RCC, lung and prostate cancers) were bidirectionally associated. For six cancer pairs, the bidirectional SIRs differed significantly (ie, the 95% CIs did not overlap); for ureteral, stomach, lung, RCC, and prostate cancers, the SIRs were higher when these cancers were SPCs than in the reverse order; for skin SCC, the opposite was the case. The data are summarized in Figure 1A.
TABLE 2.
Male risks of SPCs after bladder, ureteral, and renal pelvic cancers and these cancers as SPCs
| Cancer A | Cancer B | Cancer A followed by cancer B | Cancer B followed by cancer A | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | SIR | 95% CI | N | SIR | 95% CI | ||||
| Bladder | UAT | 110 | 1.18 | 0.97 | 1.43 | 143 | 1.51 | 1.28 | 1.78 |
| Stomach | 141 | 1.28 | 1.08 | 1.51 | 46 | 0.75 | 0.55 | 1.01 | |
| Small intestine | 41 | 2.38 | 1.71 | 3.23 | 19 | 1.37 | 0.82 | 2.15 | |
| CRC | 564 | 1.08 | 0.99 | 1.17 | 521 | 1.11 | 1.02 | 1.21 | |
| Liver | 117 | 1.41 | 1.17 | 1.69 | 23 | 0.78 | 0.50 | 1.17 | |
| Lung | 683 | 2.08 | 1.93 | 2.25 | 174 | 1.31 | 1.12 | 1.52 | |
| Breast | 10 | 1.52 | 0.72 | 2.81 | 9 | 1.34 | 0.61 | 2.56 | |
| Prostate | 2832 | 1.73 | 1.67 | 1.8 | 2518 | 1.25 | 1.20 | 1.30 | |
| Testis | 5 | 1.41 | 0.45 | 3.32 | 23 | 2.01 | 1.27 | 3.01 | |
| Male genital | 5 | 0.35 | 0.11 | 0.83 | 19 | 1.31 | 0.79 | 2.05 | |
| Kidney | 369 | 3.98 | 3.58 | 4.4 | 309 | 3.54 | 3.16 | 3.96 | |
| RCC | 140 | 2.20 | 1.85 | 2.60 | 97 | 1.45 | 1.17 | 1.77 | |
| Renal pelvis | 195 | 17.2 | 14.9 | 19.8 | 199 | 21.0 | 18.2 | 24.1 | |
| Ureter | 211 | 41.9 | 36.4 | 48.0 | 83 | 20.9 | 16.6 | 25.9 | |
| Melanoma | 135 | 0.93 | 0.78 | 1.1 | 174 | 1.08 | 0.92 | 1.25 | |
| Skin SCC | 364 | 0.95 | 0.86 | 1.05 | 337 | 1.35 | 1.21 | 1.51 | |
| Nervous system | 61 | 1.17 | 0.89 | 1.5 | 49 | 0.96 | 0.71 | 1.26 | |
| Thyroid | 15 | 1.54 | 0.86 | 2.54 | 13 | 1.18 | 0.62 | 2.02 | |
| Endocrine | 41 | 1.68 | 1.21 | 2.28 | 48 | 1.14 | 0.84 | 1.51 | |
| Connective tissue | 29 | 1.33 | 0.89 | 1.91 | 27 | 1.34 | 0.88 | 1.95 | |
| NHL | 7 | 1.21 | 0.48 | 2.51 | 12 | 1.80 | 0.93 | 3.15 | |
| Hodgkin lymphoma | 140 | 1.06 | 0.89 | 1.25 | 141 | 1.29 | 1.08 | 1.52 | |
| Myeloma | 56 | 0.95 | 0.72 | 1.23 | 24 | 0.58 | 0.37 | 0.87 | |
| Leukemia | 151 | 1.23 | 1.04 | 1.44 | 106 | 1.11 | 0.91 | 1.35 | |
| All | 6510 | 1.56 | 1.53 | 1.60 | 4919 | 1.28 | 1.25 | 1.32 | |
| Ureter | CRC | 5 | 0.85 | 0.27 | 2.00 | 9 | 1.22 | 0.55 | 2.32 |
| Lung | 8 | 2.12 | 0.91 | 4.20 | 2 | 0.90 | 0.09 | 3.32 | |
| Prostate | 20 | 1.07 | 0.65 | 1.66 | 32 | 1.00 | 0.69 | 1.42 | |
| Kidney | 20 | 17.9 | 10.93 | 27.74 | 20 | 14.2 | 8.67 | 22.0 | |
| RCC | 5 | 6.46 | 2.04 | 15.2 | 2 | 1.84 | 0.17 | 6.76 | |
| Renal pelvis | 14 | 108.6 | 59.2 | 182.8 | 18 | 129.0 | 76.3 | 204.3 | |
| Bladder | 83 | 20.9 | 16.6 | 25.9 | 211 | 41.9 | 36.4 | 48.0 | |
| All | 167 | 2.99 | 2.56 | 3.48 | 293 | 4.46 | 3.96 | 5.00 | |
| Renal pelvis | CRC | 12 | 0.85 | 0.43 | 1.48 | 22 | 1.32 | 0.83 | 2.00 |
| Lung | 13 | 1.39 | 0.74 | 2.39 | 13 | 1.12 | 0.40 | 2.46 | |
| Prostate | 54 | 1.17 | 0.88 | 1.53 | 86 | 1.19 | 0.95 | 1.46 | |
| RCC | 5 | 2.64 | 0.83 | 6.22 | 10 | 3.94 | 2.70 | 12.5 | |
| Bladder | 199 | 21.0 | 18.2 | 24.1 | 195 | 17.2 | 14.9 | 19.8 | |
| Ureter | 18 | 129.0 | 76.3 | 204.3 | 14 | 108.6 | 59.2 | 182.8 | |
| All | 357 | 2.73 | 2.40 | 3.03 | 380 | 2.53 | 2.28 | 2.80 | |
Note: Bold values show that the 95% CI does not overlap with 1.00.
Abbreviations: CRC, colorectal cancer; N, patient number; NHL, non‐Hodgkin lymphoma; RCC, renal cell carcinoma; SCC, squamous cell carcinoma; SIR, standardized incidence ratio; SPC, second primary cancer; UAT, upper aerodigestive tract; 95% CI, 95% confidence interval.
FIGURE 1.
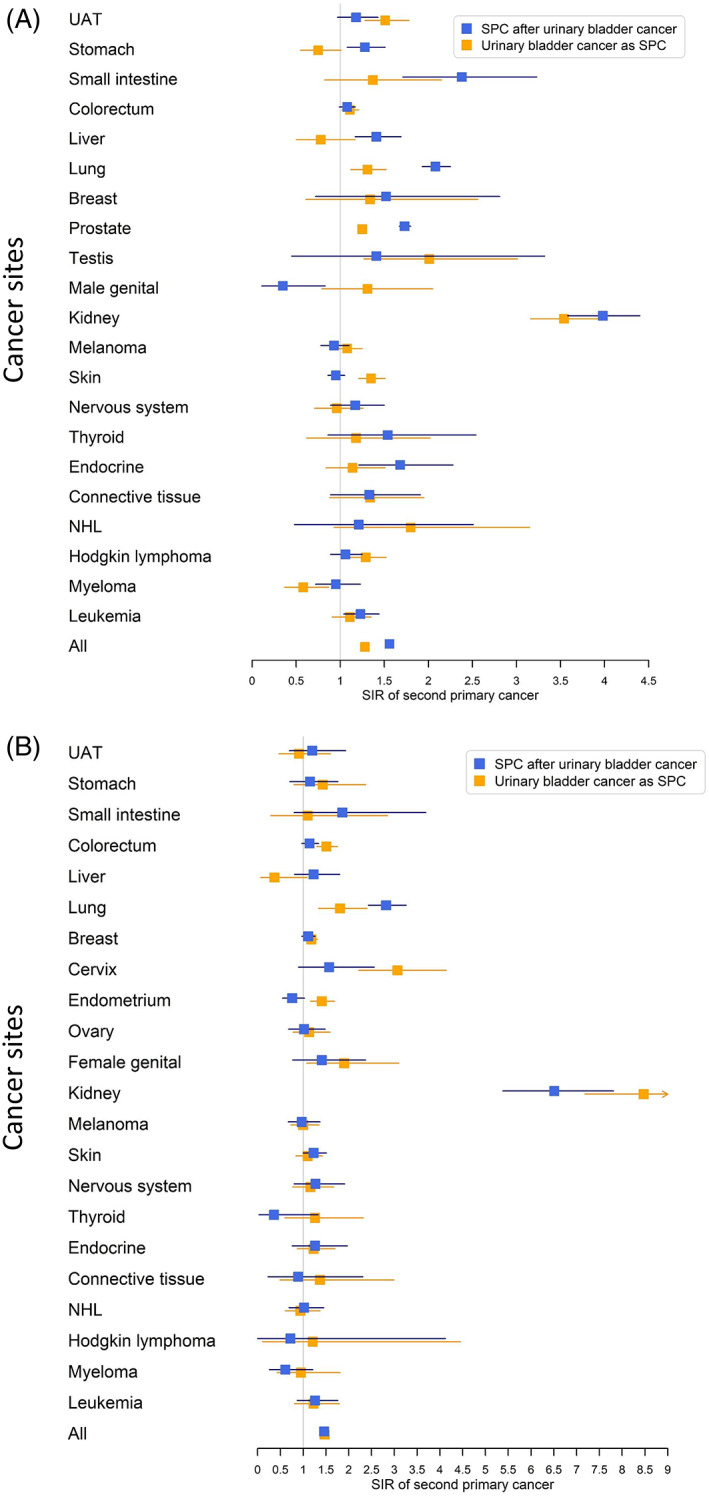
Forest plot on standardized incidence ratios (SIRs) of second primary cancers (SPCs) after urinary bladder cancer and that of urinary bladder cancer after other cancers in men (A) and women (B). SIR for all cancers excluded bladder cancer. SPC, second primary cancer; SIR, standardized incidence ratio; UAT, upper aerodigestive tract; NHL, non‐Hodgkin lymphoma
For ureteral cancer, second RCC (6.46), pelvic (108.6), and bladder (20.9) cancers were the only significantly increased SPCs (Table 2). Bladder cancer accounted for half of all SPCs. Ureteral cancer as SPC was associated with renal pelvic (129.0, the highest risk recoded in this study) and bladder (41.9) cancers. After renal pelvic cancer, bladder (21.0) and ureteral (129.0) cancers were increased. Bladder cancer accounted for more than half of all SPCs. In the reverse order, the same cancers and RCC (3.94) showed associations.
Similar analysis is shown for female cancer in Table 3. After bladder cancer, SIRs of five SPCs were significantly increased, including ureteral (87.8), pelvic (35.7), RCC (2.26), lung (2.82), and skin (1.23) cancers. The overall risk was 1.46. In the reverse order (bladder cancer as SPC), nine individual FPCs were associated with an increased risk; SIR of bladder cancer was highest after ureteral (69.9), renal pelvic (60.5), and cervical (3.07) cancers. Notably, SIRs for bladder cancer were increased after all female cancers (except ovarian cancer), including genital (1.90) and endometrial (1.41) cancers. A bidirectional increase in SIRs was found only for pelvic, ureteral, RCC, and lung cancers. The difference between bidirectional SIRs was significant for lung, endometrial, and pelvic cancers; for the latter two cancers, bladder cancer risk was higher when diagnosed as SPC. The overall SIR was 1.47. The data are summarized in Figure 1B.
TABLE 3.
Female risks of SPCs after bladder, ureteral, and renal pelvic cancers and these cancer as SPCs
| Cancer A | Cancer B | Cancer A followed by cancer B | Cancer B followed by cancer A | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | SIR | 95% CI | N | SIR | 95% CI | ||||
| Bladder | UAT | 17 | 1.20 | 0.70 | 1.93 | 12 | 0.91 | 0.47 | 1.6 |
| Stomach | 21 | 1.15 | 0.71 | 1.76 | 15 | 1.43 | 0.80 | 2.37 | |
| Small intestine | 8 | 1.86 | 0.80 | 3.69 | 4 | 1.10 | 0.29 | 2.85 | |
| CRC | 148 | 1.14 | 0.97 | 1.34 | 183 | 1.51 | 1.30 | 1.75 | |
| Liver | 27 | 1.23 | 0.81 | 1.8 | 3 | 0.37 | 0.07 | 1.09 | |
| Lung | 181 | 2.82 | 2.43 | 3.26 | 49 | 1.81 | 1.34 | 2.4 | |
| Breast | 240 | 1.11 | 0.97 | 1.26 | 382 | 1.18 | 1.07 | 1.31 | |
| Cervix | 16 | 1.57 | 0.90 | 2.56 | 43 | 3.07 | 2.22 | 4.14 | |
| Endometrium | 43 | 0.76 | 0.55 | 1.03 | 118 | 1.41 | 1.16 | 1.69 | |
| Ovary | 28 | 1.02 | 0.68 | 1.48 | 33 | 1.13 | 0.78 | 1.59 | |
| Female genital | 14 | 1.41 | 0.77 | 2.37 | 16 | 1.90 | 1.08 | 3.1 | |
| Kidney | 116 | 6.51 | 5.38 | 7.81 | 153 | 8.47 | 7.18 | 9.92 | |
| RCC | 27 | 2.26 | 1.49 | 3.29 | 30 | 2.14 | 1.44 | 3.06 | |
| Renal pelvis | 82 | 35.7 | 28.4 | 44.3 | 117 | 60.5 | 50.1 | 72.6 | |
| Ureter | 83 | 87.8 | 70.0 | 108.9 | 49 | 69.9 | 51.5 | 92.1 | |
| Melanoma | 33 | 0.97 | 0.67 | 1.37 | 44 | 1.00 | 0.73 | 1.35 | |
| Skin SCC | 95 | 1.23 | 1.00 | 1.51 | 61 | 1.10 | 0.84 | 1.42 | |
| Nervous system | 23 | 1.27 | 0.80 | 1.91 | 28 | 1.16 | 0.77 | 1.67 | |
| Thyroid | 2 | 0.36 | 0.03 | 1.33 | 10 | 1.26 | 0.60 | 2.32 | |
| Endocrine | 19 | 1.26 | 0.76 | 1.97 | 38 | 1.23 | 0.87 | 1.7 | |
| Connective tissue | 4 | 0.89 | 0.23 | 2.31 | 6 | 1.37 | 0.49 | 2.99 | |
| NHL | 31 | 1.02 | 0.69 | 1.45 | 26 | 0.94 | 0.61 | 1.37 | |
| Hodgkin lymphoma | 1 | 0.72 | 0.00 | 4.12 | 2 | 1.21 | 0.11 | 4.45 | |
| Myeloma | 8 | 0.61 | 0.26 | 1.21 | 9 | 0.95 | 0.43 | 1.81 | |
| Leukemia | 34 | 1.26 | 0.87 | 1.76 | 27 | 1.23 | 0.81 | 1.79 | |
| All | 1329 | 1.46 | 1.38 | 1.54 | 1359 | 1.47 | 1.39 | 1.55 | |
| Ureter | CRC | 7 | 2.22 | 0.88 | 4.60 | 9 | 2.13 | 0.97 | 4.06 |
| Lung | 6 | 4.10 | 1.48 | 8.99 | 2 | 1.98 | 0.19 | 7.29 | |
| Breast | 12 | 2.33 | 1.20 | 4.09 | 17 | 1.50 | 0.87 | 2.4 | |
| Cervix | 0 | — | — | — | 3 | 6.46 | 1.22 | 19.1 | |
| Endometrium | 3 | 2.23 | 0.42 | 6.61 | 15 | 4.85 | 2.71 | 8.03 | |
| Female genital | 1 | 4.04 | 0 | 23.17 | 2 | 6.95 | 0.66 | 25.6 | |
| Kidney | 8 | 18.5 | 7.89 | 36.57 | 15 | 22.5 | 12.6 | 37.2 | |
| RCC | 1 | 3.48 | 0.00 | 20.0 | 1 | 1.94 | 0 | 11.1 | |
| Renal pelvis | 6 | 197.8 | 38.8 | 236.2 | 13 | 191.5 | 101.5 | 328.3 | |
| Bladder | 49 | 69.9 | 51.5 | 92.1 | 83 | 87.8 | 70.0 | 108.9 | |
| All | 94 | 3.88 | 3.13 | 4.75 | 159 | 4.74 | 4.03 | 5.54 | |
| Renal pelvis | CRC | 20 | 2.26 | 1.38 | 3.49 | 9 | 0.88 | 0.4 | 1.68 |
| Lung | 5 | 1.14 | 0.36 | 2.68 | 7 | 2.72 | 1.08 | 5.64 | |
| Breast | 13 | 0.89 | 0.47 | 1.52 | 30 | 1.06 | 0.72 | 1.52 | |
| Cervix | 0 | — | — | — | 2 | 1.66 | 0.16 | 6.10 | |
| Endometrium | 4 | 1.02 | 0.27 | 2.64 | 14 | 1.88 | 1.02 | 3.16 | |
| Female genital | 2 | 0.52 | 0 | 2.95 | 0 | — | — | — | |
| RCC | 1 | 1.17 | 0 | 6.72 | 8 | 6.32 | 2.70 | 12.5 | |
| Bladder | 117 | 60.5 | 50.1 | 72.6 | 82 | 35.7 | 28.4 | 44.3 | |
| Ureter | 13 | 191.5 | 101.5 | 328.3 | 6 | 197.8 | 38.8 | 236.2 | |
| All | 205 | 3.08 | 2.68 | 3.53 | 194 | 2.35 | 2.03 | 2.70 | |
Note: Bold values show that the 95% CI does not overlap with 1.00.
Abbreviations: CRC, colorectal cancer; N, patient number; NHL, non‐Hodgkin lymphoma; SCC squamous cell carcinoma; SIR, standardized incidence ratio; SPC, second primary cancer; UAT, upper aerodigestive tract; 95% CI, 95% confidence interval.
After ureteral cancer, increased risks of SPCs were noted for renal pelvic (197.8), bladder (69.9), lung (4.10), and breast (2.33) cancers (Table 2). In the reverse order, SIRs of ureteral cancer were increased after pelvic (191.5), bladder (87.8), cervical (6.46), and endometrial cancers (4.85). After pelvic cancer, second ureteral, bladder, and colorectal (2.26) cancers were increased. In the reverse order, second pelvic cancer was increased after ureteral, bladder, lung (2.72), endometrial (1.88), and RCC (6.32) cancers. Bladder cancer was by far the most common SPC after urethral and pelvic cancers.
We carried out analysis of risk of SPCs depending on the follow‐up time (1 year, 2‐5 years, and >5 years) for male urothelial carcinomas (Table S1). While the SIR was elevated for many SPCs in the first year of follow‐up, that of second lung, RCC, renal pelvic, and ureteral cancers remained elevated throughout the follow‐up time. Of note, second RCC was significant only in the first follow‐up period. Risk of second liver cancer was increased in periods 1 and 2; UAT and stomach cancers and leukemia only in period 2; and NHL only in period 3. Colorectal cancer showed a borderline increase in periods 2 and 3. In the reverse order, second bladder cancer risk was increased after ureteral, prostate, and skin cancers through all periods; association with male genital cancers was significant in periods 1; associations with UAT, colorectal, and lung cancers were significant in periods 2 and 3; and associations with small intestinal, testicular, connective tissue cancers, NHL, and Hodgkin lymphoma were significant in the last period. For ureter and renal pelvic cancers, only bladder and other UUT cancers were significant.
Similar analysis of risk of SPCs for female urothelial carcinomas is shown in Table S2, and association between UUT sites was high throughout the follow‐up time. Risk of second lung cancers remained elevated throughout the follow‐up time. Risk of skin cancer was elevated in period 2 and nervous system cancer in period 3. Risk of breast cancer was elevated after ureteral cancer in period 3. Risk of colorectal cancer was increased after renal pelvic cancer in periods 1 and 2. In the reverse order, bladder cancer as SPC, colorectal and cervical cancers were associated with increased risk through all periods, and renal cell cancer in periods 1 and 2. Associations with breast and endometrial cancers were found in periods 2 and 3, while those with ovarian and lung cancers included period 3. SIR of second ureter cancer was increased after endometrial cancer (6.26) in the last period. Second renal pelvic cancer was increased after colorectal cancer in periods 1 and 2.
Correlation coefficients for the pairwise SIRs were calculated for bladder cancer based on the main sites from Tables 1 and 2 (Table 4). Male vs female Pearson correlation coefficient was 0.92 for SPC after bladder cancer and 0.88 in the reverse order (both P < .0001). For SPC vs FPC, the correlation in men was 0.72 (P < .0002) and in women it was 0.92 (P < .0001).
TABLE 4.
Correlation analysis between first and second primary cancers in men and women
| Type of correlation | No of pairs | Pearson correlation coefficient | P value | |
|---|---|---|---|---|
| Men vs Women | Risk of SPC after bladder cancer | 18 | 0.92 | <.0001 |
| Risk of bladder cancer as SPC | 18 | 0.88 | <.0001 | |
| Risk of SPC vs risk as FPC | Bladder in men | 21 | 0.72 | .0002 |
| Bladder in women | 20 | 0.92 | <.0001 | |
Note: Bold values show that the 95% CI does not overlap with 1.00.
Abbreviations: FPC, first primary cancer; SPC, second primary cancer.
4. DISCUSSION
4.1. Novel approach
The observation with direct clinical implications was the very high mutual associations of bladder, ureteral, and renal pelvic cancers with each other as FPC and SPCs. Although the recurrence risk is well known in urology, it has never been defined before with the present precision. 1 , 34 , 35 Among all cancer considered, relative risks of 10 cancers were significant as SPCs after bladder cancer, and second bladder cancer was significant after 10 FPCs, only five male cancers showed a bidirectional association. For women, four cancers were bidirectionally increased. Bidirectional associations of bladder cancer were shared for men and women for ureteral, renal pelvic, RCC, and lung cancers. The correlation analysis showed high concordance (P < .00021) between men and women when SPCs were compared after bladder cancer or in the reverse order, bladder cancer as SPC. Although previous studies have identified risks of smoking‐related SPCs after bladder cancer, the novel results in this study define bidirectional risks between many sites not related to smoking. 19 , 20 , 21 , 22
In the below discussion, we compare the consistency of the results internally and externally (with published literature) and speculate about the putative mechanisms, keeping in mind the limitations of observational epidemiology.
4.2. Urological sites
Urothelial carcinomas have been described as a pan‐urothelial disease with a propensity to recur throughout these sites. 24 , 36 As with many other cancers, even intratumoral heterogenicity has been described. 37 However, a recent literature review concluded that most recurrent urothelial tumors are monoclonal which would imply that the mechanism of spread would be intraluminal seeding or intraepithelial migration. 24 These in turn would imply that the direction of urine flow and anatomic vicinity would play a role. In a Spanish study, concomitant primary urothelial tumors were found in 17% of the patients. 36 The likelihood of finding a concomitant bladder cancer increased by anatomic location of the primary tumor, being 10, 18, and 33% in patients with primary caliceal/renal pelvic, upper ureteral, and lower ureteral cancers, in line with the above predictions. The pan‐urothelial disease is the likely explanation why the risks among urothelial sites far exceeded those between urothelial and nonurothelial sites.
In the present study, the male risk of second bladder cancer was equally high (SIR 21) when renal pelvic or ureteral cancers were FPCs, but, for women, the risks were slightly higher after ureteral (69.9) than after pelvic (60.5) cancers. Most urothelial cancer associations were higher in women than in men. The female SIRs were more than doubled and significantly higher compared to male rates for FCP‐SPC pairs of bladder‐pelvis (SIR in women 35.7), pelvis‐bladder (60.5), bladder‐ureter (87.8), and ureter‐bladder (69.9). Although the risks were highest during the year of diagnosis, they persisted often to follow‐up period of >5 years (conditional on patient survival). These risks were exquisitely high and clinically relevant, motivating an extended patient follow‐up. Bladder cancer was by far the most common SPC after first ureteral and renal pelvic cancers, accounting for at least half of all SPCs.
Of urological interest is also the possible SPC risks between bladder, RCC, and prostate cancers. The SIRs for second male RCC and prostate cancer were 2.20 and 1.73, respectively, and prostate cancer accounted for 44% of all SPCs after bladder cancer. Male SIRs for bladder cancer as SPC after these cancers were more modest, 1.45 and 1.25, respectively, but second bladder cancer following prostate cancer accounted for 51% of all bladder cancers as SPC. SIR for second RCC in women was 2.26 and in the reverse order 2.14. However, when analyzed by follow‐up time, the risk for second prostate cancer was increased only in the year of bladder cancer diagnosis, which suggests that the excess risk was due to surveillance bias. The conclusion about second RCC was similar for men but for women remained unclear. Thus, the study provided no evidence for association of urothelial cancers and RCC or prostate cancer.
4.3. Urological with other sites
For the other cancers, we considered the multiple independent results for consistency in order to conclude about true associations. These included results between both sexes in bidirectional analysis and, in order to exclude surveillance bias, only considering the follow‐up times 2‐5 years and >5 years after diagnosis of FPC (thus yielding eight comparisons for cancers diagnosed in both sexes). Lung cancer was significant in seven associations, colorectal cancer in four, UAT, prostate, and skin cancers in three, and breast, cervical, and endometrial cancers and non‐Hodgkin lymphoma in two. The associations with lung and upper aerodigestive tract cancers were most likely related to smoking and that of prostate cancer to residual surveillance bias. The risk for cervical cancer (and, for female, genital cancer) was most likely an example of long‐term surveillance bias, considering risk reduction along the follow‐up time, anatomic vicinity, and known viral etiology. We have previously observed such long‐term surveillance bias for urolithiasis with various cancers, particularly for cancers in anatomic vicinity. 38 In another study on female vaginal and vulvar cancer, which share the viral etiology with cervical cancer, we have shown that the risk for second bladder cancer was 1.88 (N = 45, 95% CI 1.35‐2.44). 39
The associations of bladder, ureteral, and renal pelvic cancers with colorectal, small intestinal, endometrial, and ovarian cancers may signal the contribution of genetic factors. Bladder cancer is considered a minor component in Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome), caused by germline mutations in mismatch repair genes. 7 It is plausible that the increased risks of second ureteral (4.85) and pelvic (1.88) cancers after endometrial cancer were contributed by Lynch syndrome. 10 , 11 The reason for the higher associations of the Lynch syndrome‐related cancers in women compared to men may be due to the lower female population incidence of urothelial cancers, particularly of bladder cancer. The difference in population incidence was probably in part related to historic higher smoking level in men compared to women, as pointed out in Introduction. However, as in all population level studies, we lacked the definitive genetic evidence, but want to remind that the diagnostic guidelines based on the Amsterdam or Bethesda criteria emphasize family history. 40
Among the remaining associations with bladder cancer, using the above criteria for consistency, skin SCC had three and non‐Hodgkin lymphoma two associations. The common denominator for these two cancer is that they are a hallmark of immune disturbance and are vastly elevated in immune suppressed patients. 41 , 42 It is thus plausible that immune dysfunctions may contribute to the associations of bladder cancers with skin cancer and NHL. 41 Finally, there were solitary associations for male bladder cancers as SPC, which emerged >5 years after diagnosis of testicular cancer and Hodgkin lymphoma. These are early‐onset cancers treated with intense chemotherapy and/or radiotherapy with high risk of SPCs, including bladder cancer. 43 , 44 , 45
4.4. Strengths and limitations
This study has a number of strengths, the foremost being a nation‐wide coverage and access to a high‐level cancer registry data. 31 , 46 Because of linkage to the censuses, we had information on socio‐economic and residential background data covering the whole population, which is unique in nation‐wide studies. Socio‐economic data are highly correlated with lung cancer incidence in Sweden and thus provide a proxy of smoking level. 47 , 48 , 49 This kind of studies with numerous comparisons will produce some significant associations by chance. Assessing both sexes separately and comparing the consistency was helpful in avoiding chance findings. The bidirectional design is another strength in helping to interpret the associations. Ureteral and renal pelvic cancers are rare, and all related case numbers were low, affording low statistical power. The major limitation was that data on treatment were lacking, but we assume that surgery has been the primary treatment for bladder and UUT cancers.
5. CONCLUSIONS
We showed that many cancers were associated with bladder cancer as SPCs or, in the reverse order, bladder cancer as SPC. The risks of recurrence of urothelial cancers were very high, and, at most sites, female risks were twice over the male risks. Risks persisted often through follow‐up periods of >5 years, motivating an extended patient follow‐up. Apart from smoking‐related cancers, immune dysfunction was suggested to contribute to associations with skin cancer and non‐Hodgkin lymphoma. Lynch syndrome‐related cancers were associated with urothelial cancers, providing population‐level justification for diagnostic and management recommendations. 1
AUTHOR CONTRIBUTIONS
Kristina Sundquist: Funding acquisition; investigation; project administration; resources; software; visualization; writing‐review & editing; Funding and administration. Jan Sundquist: Funding acquisition; investigation; project administration; resources; software; visualization; writing‐review & editing; Acquisition of data; Funding and administration. Asta Försti: Formal analysis; investigation; validation; visualization; writing‐review & editing; Statistical analysis and interpretation. Otto Hemminki: Formal analysis; investigation; validation; visualization; writing‐review & editing; Statistical analysis and interpretation. Guoqiao Zheng: Design; Statistical analysis and interpretation. Kari Hemminki: Design; Funding and administration. All authors: Manuscript writing; Approval of the final text.
CONFLICT OF INTEREST
None.
ETHICAL STATEMENT
The study was approved on 25 January 2013, by the Regional Ethical Review Board in Lund without requirement for informed consent (registration number 2012/795). Instead, the Ethical Review Board obliged us to advertise in the newspapers in order to inform the public that their data would be used for secondary purposes. After that, around 40 individuals were excluded (≈40 excluded individuals/≈10 million individuals in the Swedish population).
Supporting information
TABLE S1 Male risks of SPCs after bladder, ureteral or renal pelvic cancers and these cancer as SPCs stratified by follow‐up time after first primary cancer diagnosis
TABLE S2 Female risks of SPCs after bladder, ureteral or renal pelvic cancers and these cancer as SPCs stratified by follow‐up time after first primary cancer diagnosis
ACKNOWLEDGMENTS
O.H. was supported by Biomedicum Helsinki‐säätiö and Finska läkaresällskapet grants. G.Z was a doctoral student supported by the China Scholarship Council (201606100057). The study was supported by the European Union's Horizon 2020 research and innovation program, Grant No. 856620 (Chaperon) and The Swedish Research Council.
Zheng G, Sundquist K, Sundquist J, Försti A, Hemminki O, Hemminki K. Bladder and upper urinary tract cancers as first and second primary cancers. Cancer Reports. 2021;4:e1406. 10.1002/cnr2.1406
Funding information Biomedicum Helsinki‐säätiö; China Scholarship Council, Grant/Award Number: 201606100057; European Union's Horizon 2020 research and innovation program, grant, Grant/Award Number: No 856620 (Chaperon); Finska läkaresällskapet grants; The Swedish Research Council
DATA AVAILABILITY STATEMENT
DATA AVAILABILITY The data that support the findings of this study are available from Lund University but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available.
REFERENCES
- 1. Roupret M, Babjuk M, Comperat E, et al. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111‐122. [DOI] [PubMed] [Google Scholar]
- 2. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96‐108. [DOI] [PubMed] [Google Scholar]
- 3. Cai Q, Chen Y, Xin S, et al. Temporal trends of bladder cancer incidence and mortality from 1990 to 2016 and projections to 2030. Transl Androl Urol. 2020;9(2):153‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teoh JY, Huang J, Ko WY, et al. Global trends of bladder cancer incidence and mortality, and their associations with tobacco use and gross domestic product per capita. Eur Urol. 2020;78(6):893‐906. [DOI] [PubMed] [Google Scholar]
- 5. Malmstrom PU, Gardmark T, Sherif A, et al. Incidence, survival and mortality trends of bladder cancer in Sweden 1997‐2016. Scand J Urol. 2019;53(4):193‐199. [DOI] [PubMed] [Google Scholar]
- 6. Patja K, Hakala SM, Boström G, Nordgren P, Haglund M. Trends of tobacco use in Sweden and Finland: do differences in tobacco policy relate to tobacco use? Scand J Public Health. 2009;37(2):153‐160. [DOI] [PubMed] [Google Scholar]
- 7. Kohlmann W, Gruber SB. Lynch syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews(R). Seattle, WA: University of Washington, Seattle University of Washington; 1993. [Google Scholar]
- 8. Yu H, Hemminki O, Forsti A, Sundquist K, Hemminki K. Familial urinary bladder cancer with other cancers. Eur Urol Oncol. 2018;1(6):461‐466. [DOI] [PubMed] [Google Scholar]
- 9. Aragon‐Ching JB, Nizam A, Henson DE. Carcinomas of the renal pelvis, ureters, and urinary bladder share a carcinogenic field as revealed in epidemiological analysis of tumor registry data. Clin Genitourin Cancer. 2019;17(6):436‐442. [DOI] [PubMed] [Google Scholar]
- 10. Moller P, Seppala TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75years of age: a report from the prospective Lynch syndrome database. Gut. 2018;67(7):1306‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wischhusen JW, Ukaegbu C, Dhingra TG, et al. Clinical factors associated with urinary tract cancer in individuals with Lynch syndrome. Cancer Epidemiol Biomark Prev. 2020;29(1):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dominguez‐Valentin M, Sampson JR, Seppälä TT, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the prospective Lynch syndrome database. Genet Med. 2020;22(1):15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high‐risk assessment: colorectal, version 2.2019. J Natl Compr Cancer Network. 2019;17(9):1032‐1041. [DOI] [PubMed] [Google Scholar]
- 14. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999‐2007 by country and age: results of EUROCARE‐5—a population‐based study. Lancet Oncol. 2014;15(1):23‐34. [DOI] [PubMed] [Google Scholar]
- 15. Engholm G, Hakulinen T, Gislum M, et al. Trends in the survival of patients diagnosed with kidney or urinary bladder cancer in the Nordic countries 1964‐2003 followed up to the end of 2006. Acta Oncol. 2010;49(5):655‐664. [DOI] [PubMed] [Google Scholar]
- 16. Lundberg FE, Andersson TM, Lambe M, et al. Trends in cancer survival in the Nordic countries 1990‐2016: the NORDCAN survival studies. Acta Oncol. 2020;59(11):1266‐1274. [DOI] [PubMed] [Google Scholar]
- 17. Nilsson S, Ragnhammar P, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in urothelial bladder cancer. Acta Oncol. 2001;40(2–3):371‐390. [DOI] [PubMed] [Google Scholar]
- 18. Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology guidelines on muscle‐invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82‐104. [DOI] [PubMed] [Google Scholar]
- 19. Bermejo JL, Sundquist J, Hemminki K. Bladder cancer in cancer patients: population‐based estimates from a large Swedish study. Br J Cancer. 2009;101(7):1091‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muller J, Grosclaude P, Lapotre‐Ledoux B, et al. Trends in the risk of second primary cancer among bladder cancer survivors: a population‐based cohort of 10 047 patients. BJU Int. 2016;118(1):53‐59. [DOI] [PubMed] [Google Scholar]
- 21. Kwon WA, Joung JY, Lim J, et al. Risk of second primary cancer among bladder cancer patients: a population‐based cohort study in Korea. BMC Cancer. 2018;18(1):617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adjei Boakye E, Buchanan P, Hinyard L, et al. Trends in the risk and burden of second primary malignancy among survivors of smoking‐related cancers in the United States. Int J Cancer. 2019;145(1):143‐153. [DOI] [PubMed] [Google Scholar]
- 23. Naser‐Tavakolian A, Ghodoussipour S, Djaladat H. Upper urinary tract recurrence following bladder cancer therapy: a review of surveillance and management. Curr Opin Urol. 2019;29(3):189‐197. [DOI] [PubMed] [Google Scholar]
- 24. van Doeveren T, van de Werken HJG, van Riet J, et al. Synchronous and metachronous urothelial carcinoma of the upper urinary tract and the bladder: are they clonally related? A systematic review. Urol Oncol. 2020;38:590‐598. [DOI] [PubMed] [Google Scholar]
- 25. Nuhn P, Novara G, Seitz C, et al. Prognostic value of prior history of urothelial carcinoma of the bladder in patients with upper urinary tract urothelial carcinoma: results from a retrospective multicenter study. World J Urol. 2015;33(7):1005‐1013. [DOI] [PubMed] [Google Scholar]
- 26. Chattopadhyay S, Hemminki A, Forsti A, Sundquist K, Sundquist J, Hemminki K. Second primary cancers in patients with invasive and in situ squamous cell skin carcinoma, Kaposi sarcoma and Merkel cell carcinoma: role for immune mechanisms? J Invest Dermatol. 2019;140(1). 10.1016/j.jid.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 27. Chattopadhyay S, Sud A, Zheng G, et al. Second primary cancers in non‐Hodgkin lymphoma: bi‐directional analyses suggesting role for immune dysfunction. Int J Cancer. 2018;143:2449‐2457. [DOI] [PubMed] [Google Scholar]
- 28. Travis LB, Demark Wahnefried W, Allan JM, Wood ME, Ng AK. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10(5):289‐301. [DOI] [PubMed] [Google Scholar]
- 29. Liu H, Hemminki K, Sundquist J. Renal cell carcinoma as first and second primary cancer: etiological clues from the Swedish family—cancer database. J Urol. 2011;185(6):2045‐2049. [DOI] [PubMed] [Google Scholar]
- 30. Hemminki K, Yu H, Hemminki O, Sundquist J. RE: familial cancer clustering of urothelial cancer: a population‐based case‐control study. J Natl Cancer Inst. 2018;110(11):1277‐1278. [DOI] [PubMed] [Google Scholar]
- 31. Centre for Epidemiology . Cancer Incidence in Sweden 2012. Stockholm, Sweden: The National Board of Health and Welfare; 2013. [Google Scholar]
- 32. Holmang S, Amsler‐Nordin S, Carlson K, Holmberg E, Johansson SL. Completeness and correctness of registration of renal pelvic and ureteral cancer in the Swedish Cancer Registry. Scand J Urol Nephrol. 2008;42(1):12‐17. [DOI] [PubMed] [Google Scholar]
- 33. dos Santos Silva I. Cancer Epidemiology: Principles and Methods. Lyon, France: IARC; 1999. 442 p. [Google Scholar]
- 34. Seisen T, Granger B, Colin P, et al. A systematic review and meta‐analysis of clinicopathologic factors linked to Intravesical recurrence after radical nephroureterectomy to treat upper tract urothelial carcinoma. Eur Urol. 2015;67(6):1122‐1133. [DOI] [PubMed] [Google Scholar]
- 35. Lonergan PE, Porten SP. Bladder tumor recurrence after urothelial carcinoma of the upper urinary tract. Transl Androl Urol. 2020;9(4):1891‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cosentino M, Palou J, Gaya JM, Breda A, Rodriguez‐Faba O, Villavicencio‐Mavrich H. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol. 2013;31(1):141‐145. [DOI] [PubMed] [Google Scholar]
- 37. Li H, Zhang Q, Shuman L, et al. Evaluation of PD‐L1 and other immune markers in bladder urothelial carcinoma stratified by histologic variants and molecular subtypes. Sci Rep. 2020;10(1):1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hemminki K, Hemminki O, Försti A, Sundquist K, Sundquist J, Li X. Surveillance bias in cancer risk after unrelated medical conditions: example urolithiasis. Sci Rep. 2017;7:8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang L, Hemminki O, Chen T, et al. Familial clustering, second primary cancers and causes of death in penile. Vulvar Vaginal Cancers Sci Rep. 2019;9(1):11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hortlund M, Arroyo Muhr LS, Storm H, Engholm G, Dillner J, Bzhalava D. Cancer risks after solid organ transplantation and after long‐term dialysis. Int J Cancer. 2017;140(5):1091‐1101. [DOI] [PubMed] [Google Scholar]
- 42. Rama I, Grinyo JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. 2010;6(9):511‐519. [DOI] [PubMed] [Google Scholar]
- 43. Hemminki K, Liu H, Sundquist J. Second cancers after testicular cancer diagnosed after 1980 in Sweden. Ann Oncol. 2010;21:1546‐1551. [DOI] [PubMed] [Google Scholar]
- 44. Zhang L, Hemminki O, Chen T, et al. Second cancers and causes of death in patients with testicular cancer in Sweden. PLoS One. 2019;14(3):e0214410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N Engl J Med. 2015;373(26):2499‐2511. [DOI] [PubMed] [Google Scholar]
- 46. Ji J, Sundquist K, Sundquist J, Hemminki K. Comparability of cancer identification among death registry, cancer registry and hospital discharge registry. Int J Cancer. 2012;131:2085‐2093. [DOI] [PubMed] [Google Scholar]
- 47. Hemminki K, Li X. Level of education and the risk of cancer in Sweden. Cancer Epidemiol Biomark Prev. 2003;12:796‐802. [PubMed] [Google Scholar]
- 48. Hemminki K, Zhang H, Czene K. Socioeconomic factors in cancer in Sweden. Int J Cancer. 2003;105:692‐700. [DOI] [PubMed] [Google Scholar]
- 49. Lipfert FW, Wyzga RE. Longitudinal relationships between lung cancer mortality rates, smoking, and ambient air quality: a comprehensive review and analysis. Crit Rev Toxicol. 2019;49(9):790‐818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Male risks of SPCs after bladder, ureteral or renal pelvic cancers and these cancer as SPCs stratified by follow‐up time after first primary cancer diagnosis
TABLE S2 Female risks of SPCs after bladder, ureteral or renal pelvic cancers and these cancer as SPCs stratified by follow‐up time after first primary cancer diagnosis
Data Availability Statement
DATA AVAILABILITY The data that support the findings of this study are available from Lund University but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available.


