Clinical problem
With the expanding use of botulinum neurotoxin injections for the management of masseter hypertrophy, bruxism, and facial slimming and contouring, it is important to recognize the related and at times overlooked complications of these procedures, including paradoxical masseteric bulging (PMB). According to the recent literature, PMB has an incidence rate between 0.5% and 18.8% (Yeh et al., 2018). Herein, we propose recommendations for the prevention and correction of PMB.
Solution
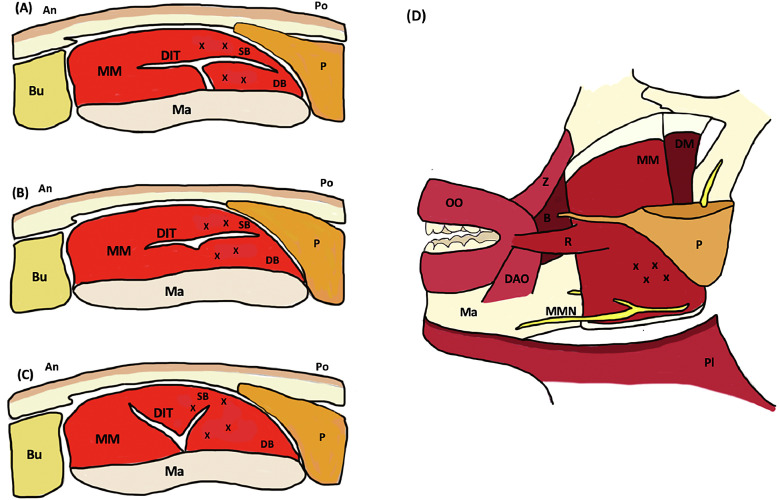
PMB is thought to arise from the presence of a broad tendon structure, known as the deep inferior tendon (DIT), located within the superficial layer of the masseter muscle (Lee et al., 2016). When injected underneath the DIT, toxin is blocked from diffusing into the more superficial muscle fibers, creating a discrepancy between the contractile capabilities of the deep and superficial layers. In some cases, overcompensation from nonparalyzed superficial muscle fibers creates prominent bulging (Suppl. Video). The reverse scenario may also occur if a more superficial injection fails to penetrate through the DIT and deeper fibers remain unaffected by toxin. To help prevent the development of PMB, care must be taken to evenly space injections both above and below the DIT and to accurately demarcate and treat the entire muscle. Prior to injection, the full extent of the masseter and anatomical landmarks (Fig. 1) should be identified and marked using visualization and palpation while the patient is clenching. Layer-by-layer injections may then be administered into the superficial and deep bellies of the masseter to ensure equal distribution of toxin, noting that the division pattern of the DIT may vary slightly among individuals (Fig. 1; Lee et al., 2016; 2019). For patients in whom PMB has already developed, we demonstrate correction with the administration of additional units of botulinum neurotoxin carefully injected into the paradoxically contracted muscle fibers. This correction yielded improvement within 1 week and complete correction within 2 weeks (Fig. 2).
Fig. 1.
Anatomical view of the masseter muscle with suggested placement of toxin injections (x) and various division patterns of deep inferior tendon. (A) Compartmentalized pattern. (B) Transversely divided pattern. (C) Longitudinally divided pattern. (D) Profile view. An, anterior; B, buccinator; Bu, buccal fat; DAO, depressor angularis oris; DB, deep belly of superficial masseter; DIT, deep inferior tendon; DM, deep and middle layers of masseter; Ma, mandible; MM, superficial masseter muscle; MMN, marginal mandibular branch of facial nerve; OO, orbicularis oris; P, parotid gland; Pl, platysma (cut); Po, posterior; R, risorius; SB, superficial belly of superficial masseter; Z, zygomaticus. Figure adapted from Lee et al. (2019) and Peng & Peng (2018).
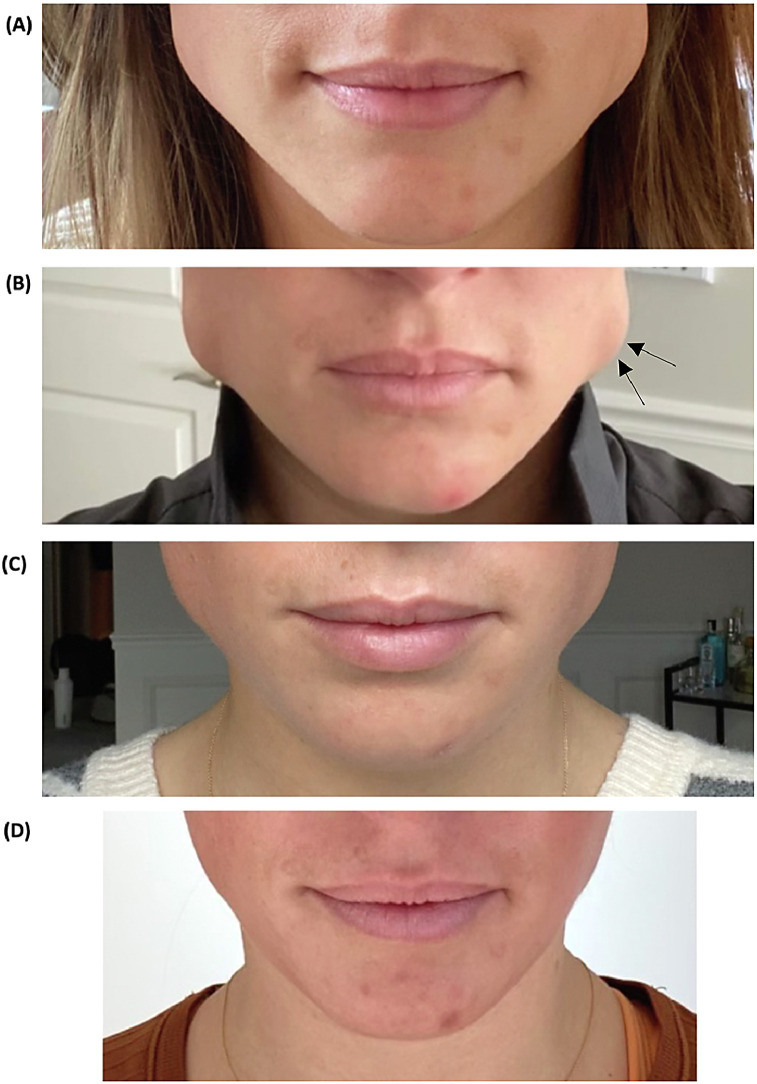
Fig. 2.
In all components of the image, the patient is clenching the jaw. Arrows indicate placement of corrective injections. (A) Masseter hypertrophy before treatment. (B) Paradoxical masseteric bulging after initial botulinum neurotoxin (BoNT) injections (20 units per side). (C) Two weeks after corrective BoNT injections (16 additional corrective units per side). (D) Two months after corrective BoNT injections.
Conflicts of interest
None.
Acknowledgments
Funding
None.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijwd.2021.03.002.
Appendix. Supplementary materials
References
- Lee H.J., Choi Y.J., Lee K.W., Hu K.S., Kim S.T., Kim H.J. Ultrasonography of the internal architecture of the superficial part of the masseter muscle in vivo. Clin Anat. 2019;32(3):446–452. doi: 10.1002/ca.23337. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Kang I.W., Seo K.K., Choi Y.J., Kim S.T., Hu K.S., et al. The anatomical basis of paradoxical masseteric bulging after botulinum neurotoxin type A injection. Toxins (Basel) 2016;9(1):14. doi: 10.3390/toxins9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H.P., Peng J.H. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J Cosmet Dermatol. 2018;17(1):33–38. doi: 10.1111/jocd.12473. [DOI] [PubMed] [Google Scholar]
- Yeh Y.T., Peng J.H., Peng H.P. Literature review of the adverse events associated with botulinum toxin injection for the masseter muscle hypertrophy. J Cosmet Dermatol. 2018;17(5):675–687. doi: 10.1111/jocd.12721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.