Abstract
Enzyme-based therapeutics (EBTs) have the potential to tap into an almost unmeasurable amount of enzyme biodiversity and treat myriad conditions. Although EBTs were some of the first biologics used clinically, the rate of development of newer EBTs has lagged behind that of other biologics. Here, we review the history of EBTs, and discuss the state of each class of EBT, their potential clinical advantages, and the unique challenges to their development. Additionally, we discuss key remaining technical barriers that, if addressed, could increase the diversity and rate of the development of EBTs.
Keywords: enzyme based therapy, enzyme, therapy, biologic, therapeutic enzymes, clinical, pre-clinical, early investigational, biotechnology, history
Introduction
Enzymes have a unique therapeutic potential rooted in their ability to not only catalyze chemical reactions, but also perform reactions with environmental sensitivity (i.e., catalytic rates that are dependent on environmental variables, temperature, pH, cofactor levels, activators, inhibitors, etc). Both of these abilities allow for diverse therapeutic functions (Figure 1; Table S1 in the supplemental information online), ranging from supplementing essential digestion and cellular metabolism to targeting infectious diseases. Their therapeutic potential is expansive given the biodiversity of heterologous enzymes that have the ability to perform either unique chemistry in the human body or chemistry with different environmental sensitivity.1–3
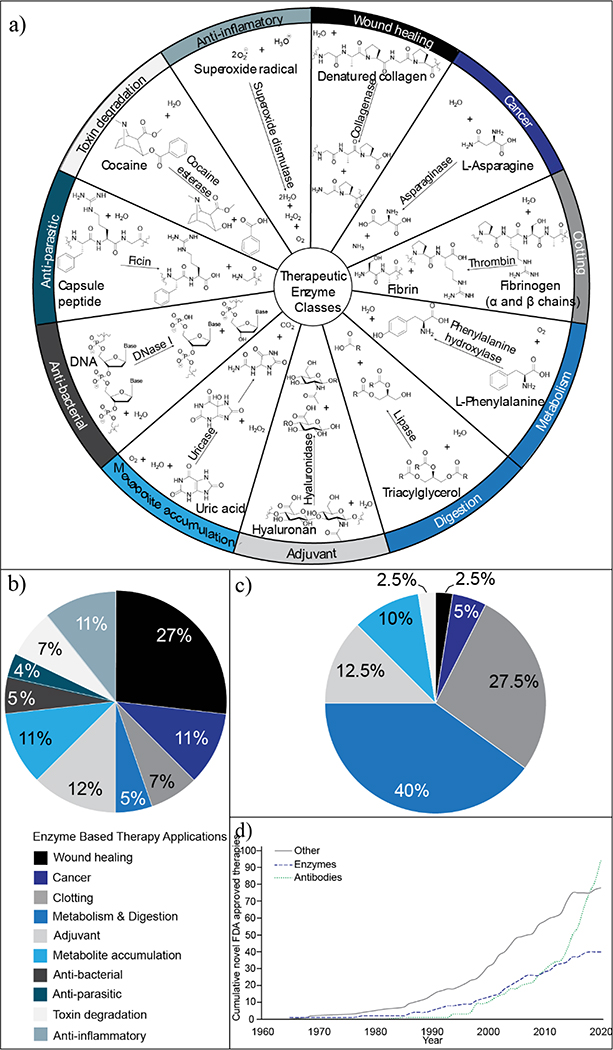
Figure 1.
Overview of enzyme-based therapies (EBTs). (a) Classes of therapeutic enzyme application and an example from each class. (b) Distribution of therapeutic enzyme applications before 1962, when the US Food and Drug Administration (FDA) approval process was established (N=32). EBT applications are listed with their corresponding colors and begin at the 12 o’clock position on the pie chart. Labeling continues in the order listed moving in the clockwise direction. For more information, see Table S2 in the supplemental information online. (c) Distribution of therapeutic enzyme applications with FDA approval in 2020 (N=39). Color and labeling scheme is consistent that in (b). For more information, see Table S3 in the supplemental information online. (d) Growth of novel biologics with FDA approval over time stratified as antibodies, enzymes, or other (such as hormones, growth factors, and cytokines). For more information, see Table S4 in the supplemental information online.
Early EBTs took advantage of this biodiversity (Figure 1; Table S2 in the supplemental information online). These therapies had promising clinical success in a range of applications, including wound debridement, and as antiparasitics and antitoxins (via toxin degradation). Despite this historical success, few early EBTs gained or maintained US Food and Drug Administration (FDA) approval.4,5 The lack of FDA approval can be explained primarily by safety concerns and, over the years, the diversity of therapeutic enzyme applications and their origins have dwindled6,7 (Figure 1b,c; Tables S1–S3 in the supplemental information online). These early enzymes often had unwanted promiscuous activity at sites other than those targeted, resulting in adverse events.6,8,9 Additionally, enzymes from nonhuman sources posed a greater safety and tolerability risk because of their inherent immunogenicity.7,9,10 Given these safety concerns, with the advent of recombinant DNA technology, human enzymes replaced their early isozyme predecessors whenever possible.5,7 Several heterologous enzymes remained therapeutically viable because of their oral or topical routes of administration (which pose a lower risk for immunogenicity) or via development of immune-tolerant enzyme conjugates, such as PEGylated variants5,11,12 (although anti-PEG antibodies are now becoming a problem in the clinic13). Today, enzymes are primarily used in replacement therapy. Most of the 39 unique FDA-approved enzymes are recombinant human enzymes (Figure 1c; Table S1 in the supplemental information online).5 In this review, we share a historical perspective of these therapies, discuss the current state and potential of enzymes as therapeutics, and highlight key remaining challenges and opportunities in advancing this class of therapeutics.
A historical perspective of enzyme therapeutics
Historically, the development of enzymatic therapeutics has paralleled biotechnological advances, although the rate of EBT development has declined over the past decade (Figure 1d; Table S4 in the supplemental information online). Here, we briefly review the history of EBTs to understand the diverse applications of early EBTs, and the recent decline in new drugs.
One of the earliest protein therapeutics was trypsin, which was used clinically to treat tumors during the early 1900s.14 Although localized injections decreased tumor size, trypsin degrades proteins nonspecifically and also digested healthy tissue.14 When studies in mice confirmed that trypsin was not specific against cancer, it was no longer considered a safe treatment.15 Over the following decades, another protease, pepsin, began to have clinical use in treating gastritis (taken orally); however, injected EBTs required further development.16
The field of biologics was revolutionized in 1922 with insulin developed as the first injectable protein-based therapeutic. The efficacy of insulin and need for large-scale production catalyzed a wave of interest in protein therapeutics, protein purification, and modern biotechnology, and the clinical potential of EBTs.17 Equipped with these new protein purification techniques, there was a wave of enzymes under clinical investigation during the 1930s. These included the plant proteases ficin and papain, which had a history of use in traditional medicine as anthelmintics.18,19 Ficin, isolated from Ficus laurifolia latex, degrades the mucosa of both hookworm and Ascaris, killing these worms within an hour in vitro.18 The therapeutic potential of ficin was short-lived once it was found to have unwanted proteolytic activity against lesions in the digestive tract, causing erosion and hemorrhaging.6,19 Papain was evaluated, both in animals and humans, for its ability to clear peritoneal adhesions.20 It was hypothesized to degrade fibrin deposits, but studies reported conflicting results, attributed to variations in enzyme purity.21 Once enzyme purification procedures had been improved, pure formulations caused severe hemorrhaging and lethality in rats. Additionally, lab personnel who inhaled papain had severe and even lethal anaphylactic reactions, which halted attempts to develop therapeutic applications that required papain injection or pulmonary administration.21
Protein therapeutics and enzymes saw another wave of development during the 1940s with the advancement of the Cohn process to fractionate human blood.22 Isolation of human blood products was driven by wartime necessity to treat shock (treated with albumin), severe bleeding (treated with clotting proteins, zymogens, and thrombin), and burns.23 Fibrinogen, prothrombin, and thrombin were also incorporated into medical materials, such as surgical instruments, films, and foams, which improved outcomes for skin grafts, wound healing, and burns.24 Additionally, the need for debridement expanded the utility of topically applied enzymes. Streptokinase–streptodornase mixtures, derived from hemolytic streptococci, were used to degrade necrotic tissue and hydrolyze DNA released from injured cells. The use of these enzymes decreased viscosity and improved permeability, enhancing the local delivery of antibiotics, which led to improved clinical outcomes.25
Research during the 1950s focused on expanding these applications with heterologous enzymes. New enzymes were evaluated for efficacy in wound treatment, including a Clostridium collagenase that selectively degrades denatured collagen.6 Additionally, enzymes that were successful for debridement were used to treat bacterial infections in the lungs and sinuses, both as an adjuvant to improve the delivery of antibiotics, and by decreasing sputum viscosity to improve clearance.26 However, administering these enzymes occasionally resulted in anaphylactic reactions.27
Despite these adverse effects, continued research efforts took advantage of enzymes with novel activities. For example, β-lactamases obtained from penicillin-resistant bacteria were exploited to treat penicillin hypersensitivity reactions that were unresponsive to antihistamines.28 β-lactamase improved symptoms (urticaria and angioedema) in most patients initially studied, and removed penicillin from circulation within an hour.28,29 Unfortunately, cases of anaphylactic reactions halted further investigation.30 Additional examples of toxin degradation applications can be found in Table S1 in the supplemental information online.5
In 1962 the Kefauver–Harris amendment improved regulation and required proven safety and efficacy of all therapeutics. This influenced ongoing and future research and development of enzymes.31 This safeguard was necessary, but limited approved enzymes to human blood products. Early debridement agents quickly gained approval, but the only injectable bacterial enzyme approved (until the early 2000s) was L-asparaginase.5 In 1978, insulin became the first protein therapeutic produced with recombinant DNA17 and, equipped with the technology to produce additional recombinant human enzymes coupled with the Orphan drug act of 1983, human-derived enzyme replacement therapies and clotting factors experienced the largest growth in approvals.
The therapeutic potential of enzymes
EBT applications
EBTs can address a range of diseases (Figure 1a) and are used as enzyme replacement therapies, in wound healing, in clotting (both coagulants and anticoagulants), as antineoplastic agents, as adjuvants to improve permeability, in degrading toxins and toxic metabolites, and as antibacterials, antiparasitics, and anti-inflammatories. Here, we discuss the current state of enzymes in each of these key application areas in turn. A complete summary of therapeutic enzymes, their clinical uses and adverse effects can be found in Table S1 in the supplemental information online.5
Metabolism and digestion
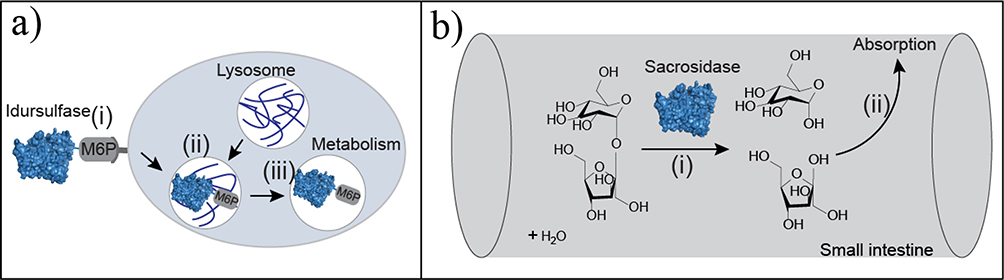
EBTs that supplement deficiencies in metabolism and/or digestion are the largest class of FDA-approved enzyme therapies, comprising 40% of EBTs. These enzymes have had the shortest clinical development time of any biologics, with an average of only 5.9 years compared with 7.8 years for monoclonal antibodies and 8.3 years for receptor modulators.32 This shorter barrier to approval and market entry can be attributed to four factors. First, metabolic enzyme replacement therapies are primarily recombinant human enzymes, where there is no need to evolve or engineer a novel activity (Figure 2a). Second, these therapies tend to have a lower risk for hypersensitivity reactions. However, immune tolerance varies within these patient populations. If a patient is missing an enzyme altogether, they have a higher likelihood of an immune response. Those with less severe mutations or partial activity tend to tolerate these therapies better than do those with more irregular phenotypes resulting from insertions, deletions, and complex rearrangements of the natural genes.33,34 Third, many of these enzyme therapies are treatments for rare genetic disorders, and are often granted orphan drug status.35,36 Orphan drug status offers tax incentives, grant availability, seven years of marketing exclusivity in the USA, and, on average, shorter FDA review timelines.35,36 Lastly, enzymes that supplement digestion, such as pancrelipase and sacrosidase, do so in the digestive tract (Figure 2b), where there is smaller risk for immunogenicity compared with systemically administered drugs.
Figure 2.
Metabolic and digestive enzyme therapeutics. (a) Metabolic enzyme replacement therapies, such as idursulfatase, which treats lysosomal storage disorders, enter cells via mannose-6-phosphate (M6P)-mediated endocytosis (i). The endosome fuses with the lysosome (ii) and idursulfatase metabolizes the accumulated substrate(iii). (b) The digestive enzyme therapy sacrosidase improves absorption in the small intestine. Sacrosidase hydrolyzes sucrose (i) into its monomers (glucose and fructose) to improve absorption (ii) and limit fermentation by bacteria in the gut.
Although there are incentives to decrease the risks associated with the development of enzyme therapies for genetic diseases, by their very nature, orphan diseases have a limited patient population, offering smaller returns on the investment of development efforts. As a result, commercial research and development (R&D) efforts have focused on more prevalent rare diseases. For example, 45 drugs (including biologics and small molecules) have been developed to treat lysosomal storage disorders, which have a prevalence of ≥5 per 1 000 000, but only 25 drugs have been developed for lysosomal storage diseases, for which there is a prevalence of <5 per 1 000 000, and there have been no drugs developed for 17 lysosomal storage disorders classified as very rare.35 Therefore, although enzyme replacement therapies work, there is limited incentive for growth in treating other very rare metabolic disorders. The development of new drugs for these smaller patient populations will likely require additional incentivization, such as further extensions of marketing exclusivity.
Clotting
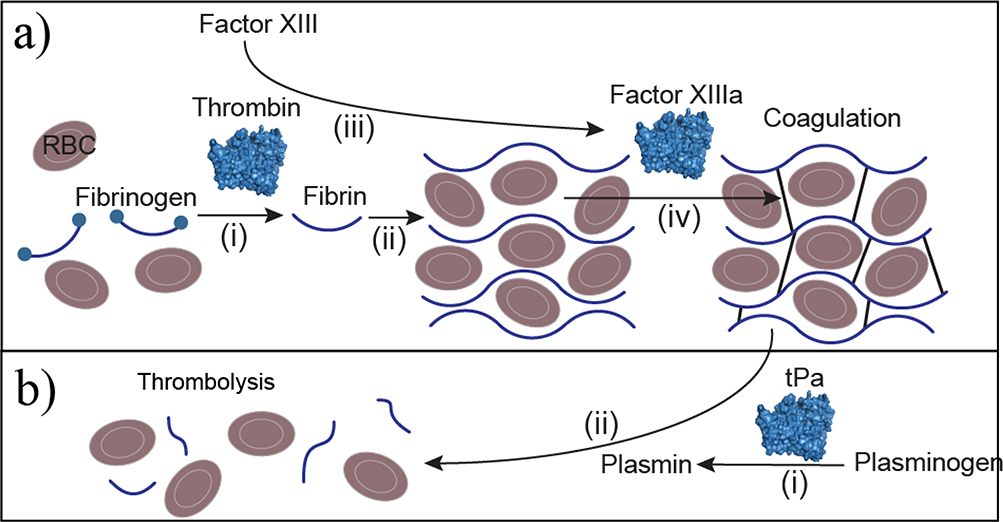
EBTs used to manage clotting can be divided into coagulation and thrombolytic treatments. Enzyme-based coagulation is either administered systemically to supplement missing clotting factors to treat hemophilia (similar to ERTs) or applied locally to stop surgical bleeding. For surgical bleeding, thrombin is applied at the site of the bleed to activate the clotting cascade (Figure 3a). Thrombin formulations are derived from human plasma, recombinantly produced, or extracted from bovine plasma. Recombinantly produced thrombin is often preferred because human blood products carry some risk of viral infection.37 Table S1 in the supplemental information online provides a classification of EBTs by manufacturing method (either extraction from a native source or recombinant production). Additionally, bovine thrombin has a higher incidence of antidrug antibody formation compared with recombinant human thrombin (21.5% versus 1.5%).38 Bovine antidrug antibodies are cross-reactive against human thrombin and Factor V, which in combination can have lasting adverse effects, such as inhibiting thrombin activity and prolonging clotting time (thrombin time) compared with treatment with human thrombin (by eightfold).39
Figure 3.
Clotting enzyme therapies. (a) Coagulation enzymes can catalyze clot formation directly, such as in the case of thrombin, which converts soluble fibrinogen to insoluble fibrin (i), which polymerizes to form the primary scaffold around red blood cells (RBCs) needed for clotting. (ii). Thrombin also activates clotting factors that are zymogen proteases, such as factor XIII (iii). Activated factor XIII (factor XIIIa) crosslinks fibrin (iv) during coagulation to secure the fibrin network leading to coagulation. (b) Anticoagulation is performed with the protease tissue plasminogen activator (tPa), which converts plasminogen to plasmin (i). Plasmin proteolyzes fibrin to fibrin degradation products (ii) to disassemble the clot, resulting in thrombolysis.
Thrombolytic EBTs dissolve clots to treat acute myocardial infarction, thromboembolism, and ischemic stroke. Enzyme therapies that have been used clinically for thrombolysis are streptokinase, staphylokinase, tissue plasminogen activator (tPA), and urokinase.5 However, only tPA and its variants are FDA approved. Both tPA and urokinase are serine proteases that directly cleave/activate plasminogen to produce plasmin to degrade fibrin clots (Figure 3b). By contrast, streptokinase and staphylokinase are microbial enzymes that must first complex with plasminogen before they have catalytic activity against plasminogen.40 Given that streptokinase and staphylokinase are microbial enzymes, they have higher rates of immunogenicity, and efforts have been made to reduce antigenicity with epitope engineering to disrupt antidrug antibody binding.41,42 Both tPA and urokinase are human proteases but tPA has the advantage of a fibrin-binding domain. The tPA variant tenecteplase has six amino acid substitutions that improve fibrin-binding affinity, prolong half-life, and improve resistance to plasminogen activator inhibitor-1. Combined, these improved functions allow for bolus administration rather than infusion as well as fewer adverse bleeding events relative to recombinant human tPA (alteplase).43 Additional attempts to improve tPA and urokinase specificity with fusions to antibodies directed against fibrin, platelets, and damaged endothelial cells are reviewed elsewhere.8
Cancer treatment
Antineoplastic enzymes are used in four ways. First, enzymes can be used to deplete specific amino acids essential for the growth of auxotrophic carcinomas.10 Second, enzymatic depletion of metabolites involved in cell signaling pathways that promote tumorigenesis can have antineoplastic effects. Third, antibody-directed enzyme prodrug therapy (ADEPT) uses enzymes conjugated to antibodies along with prodrugs to target drug activity to tumors. Last, enzymes such as hyaluronidase can be used to degrade the diseased extracellular matrix (ECM) of tumors, which can act as a barrier to the delivery of chemotherapy.
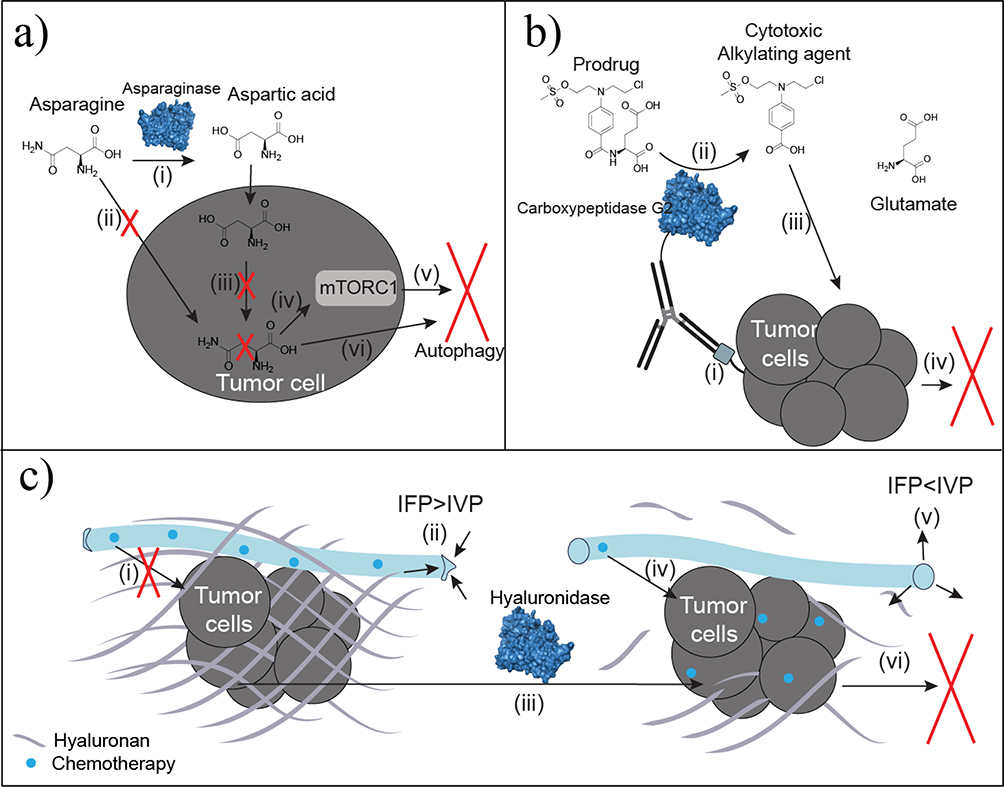
The most notable depletion therapy, L-asparaginase, which eliminates asparagine, has been used for decades to treat lymphoma and leukemia. These cancers often lack or have low expression of L-asparagine synthetase (Figure 4a).44 Treatment with PEGylated L-asparaginase alone can lead to remission rates of 40–60% in acute lymphoblastic leukemia.45 By contrast, treatment with the chemotherapeutics vincristine and prednisone alone has a remission rate of 86%.46 However, when PEGylated L-asparaginase is combined with vincristine and prednisone, remission rates improved to 93%.46 Approved formulations are derived from two microbial sources (Escherichia coli or Erwinia chrysanthemi). As a result, asparaginase has been the focus of several deimmunization efforts, but none have progressed beyond mouse models.47 Additional cancers have auxotrophy for other nonessential amino acids, such as arginine, methionine, glutamine, leucine, lysine, tyrosine, and phenylalanine.10,48 Similar systemic depletion efforts are under investigation with these amino acids.5 These ongoing studies can be grouped into two approaches.
Figure 4.
Antineoplastic enzyme therapies. (a) Asparaginase converts circulating asparagine to aspartic acid (i). Asparagine depletion limits cellular uptake of asparagine (ii). Auxotrophic tumors lack asparagine synthetase needed to convert intracellular aspartic acid into asparagine (iii). Decreased intracellular asparagine regulates mammalian target of rapamycin complex 1 (mTORC1) (iv) to promote autophagy based on starvation signaling (v). Decreased asparagine also promotes autophagy by inhibiting protein synthesis because of limited precursor availability (vi). (b) Antibody-directed enzyme prodrug therapy (ADEPT) utilizes antibody specificity for irregular or overexpressed tumor antigens (i) to localize the conjugated enzyme, such as carboxypeptidase G2, at the tumor site. Carboxypeptidase G2 converts the nontoxic prodrug to the toxic benzoic acid mustard drug (ii) shown to increase potency and localization of the drug over traditional chemotherapeutic infusion. Uptake of the cytotoxic agent (iii) results in DNA alkylation and subsequent cell death (iv). (c) Thickened hyaluronan surrounding tumors is a physical barrier to chemotherapeutic diffusion (i). Increased hyaluronan also elevates interstitial fluid pressure (IFP) to be greater than intravascular pressure (IVP), which collapses the vasculature to limit diffusion and delivery (ii). Hyaluronidase degrades hyaluronan surrounding the tumor (iii). By eliminating the physical barrier, chemotherapeutics readily diffuse to improve tumor uptake (iv). Interstitial fluid pressure decreases below IVP for reperfusion and reversal of vascular collapse (v). The improved chemotherapeutic delivery and permeability improves chemotherapeutic uptake at the tumor to induce apoptosis (vi).
The first approach parallels L-asparaginase by utilizing the microbial enzymes arginine deaminase, methionine-γ-lyase, phenylalanine ammonia lyase, and lysine oxidase for depletion.5 Predictably, these microbial enzymes are all limited by immunogenicity and short half-lives in vivo, which range from 1.5 to 7 h. Improvements have been made via PEGylation and encapsulation.49,50 Given that PEGylated phenylalanine ammonia lyase (Palynziq) was recently approved for phenylketonuria, and has demonstrated efficacy against colorectal cancer, it is a likely candidate for further clinical evaluation.5,51
The second approach utilizes human enzymes, which have a reduced risk of immunogenicity, but often require engineering to either improve substrate affinity (by decreasing Km) or activity for a new substrate. For example, human arginase-I has a Km of 1–5 mM, whereas serum concentration ranges from 80 to 120 μM,10 substitution of the cofactor Mn2+ with Co2+ decreases the Km to ~0.2 mM, which improves in vitro cytotoxicity based on an ~15-fold decrease in IC50 values.52 As another example, the closest human homolog to microbial methionine-γ-lyase, cystathionine-γ-lyase, has been successfully engineered to enable methionine degradation, decreasing serum methionine concentrations in mouse models.53
Circulating amino acid concentrations are also known regulators of the mammalian target of rapamycin complex 1 (mTORC1), which regulates cell growth and is essential for cancer progression.54 The metabolite kynurenine, a product of upregulated tryptophan catabolism, is elevated in the tumor microenvironment. Kynurenine suppresses effector T cells through transcriptional regulation, which promotes tumor immune escape.55 Degrading extracellular kynurenine with kynureninase is an attractive option to reprogram effector T cells; however, the human kynureninase has a low catalytic efficiency relative to microbial enzymes, which have a 500–700-fold increase in Kcat/Km. As a result, the human enzyme had no therapeutic effect on tumor growth in mice, whereas Pseudomonas fluorescens kynureninase reduced serum concentration 100-fold, improved median survival by 45%, and significantly increased the concentration of CD8+ effector T cells.56 Given the likelihood of an immune response to microbial enzymes, improving the turnover of human kynureninase is a promising next step.
The third subclass of enzymes used in cancer therapy are applied in ADEPT, which is a two-step procedure in which an enzyme conjugated to a tumor-specific antibody is administered. In the second step, a prodrug is administered and converted into a cytotoxic drug with a short half-life specifically at the tumor site (Figure 4b). Given the heterologous activity required to convert a prodrug to a drug, microbial enzymes are primarily used.57 Although bacterial enzyme carboxypeptidase G2 is the most common enzyme used in clinical trials, antidrug antibodies have been reported in 97% of patients.58 Options for improving immune tolerance include co-administration with an immunosuppressant or epitope engineering.59 Mapping B cell epitopes of carboxypeptidase G2 and subsequent mutagenesis reduced immunogenicity by 38.5–99% in vitro, and steric hindrance of epitopes with a C-terminal tag had the best results.60 This improvement also translated to humans in the clinic, with only 23% of patients developing antidrug antibodies after a single administration. However, this modification reduced specific enzyme activity by 45%, which reduced rates of prodrug activation.60
ADEPT has been also limited by low enzyme affinities for the prodrug relative to serum concentrations, or by prodrugs that are cytotoxic without enzyme activation.61 Both of these limitations have been addressed either by improving the Km of the enzyme for the substrate, or developing less-toxic prodrugs.62,63 Improved prodrug activation has been achieved by engineering enzymes (nitroreductases) to have Km s decreased by 1.2–14 fold while maintaining catalytic efficiency.61,64 This reduction in Km is necessary when peak prodrug plasma concentrations are orders of magnitude lower than the Km of the wild-type enzyme. Given the advances in ADEPT and antineoplastic enzymes, and the tools available to address the challenges, there is room for improvement over the two asparaginases approved in the class. A complete list of antineoplastic enzymes and their challenges is provide in Table S1 in the supplemental information online.5
Adjuvants
EBTs are also commonly used as adjuvants to improve the delivery of antibiotics, chemotherapeutics, and even antibodies. As noted previously, deoxyribonuclease has been used as an adjuvant for wound treatment to improve antibiotic penetration.6 This technique has also been applied in the treatment of respiratory infections that are common with cystic fibrosis, and hyaluronidase has long been used to enhance the permeability of injected contrast agents and therapies.5,65
Enzyme adjuvants are also under investigation in cancer to degrade diseased irregular ECM components for improved chemotherapeutic permeability (Figure 4c). Specifically, hyaluronan and collagen-I levels are elevated in many tumors. This fibrous tissue imposes limitations on the perfusion and delivery of therapeutics and negatively correlates with survival.66 Infusions of PEGylated recombinant human hyaluronidase, which degrades the thickened hyaluronan, increased tumor perfusion by 16–547% in clinical trials.67 In tumors with high concentrations of hyaluronan, co-administration of hyaluronidase with gemcitabine improved progression-free survival by 2.9 months for patients with stage IV metastatic pancreatic ductal adenocarcinoma.67,68 Additional clinical trials of hyaluronidase in combination with trastuzumab have shown improved subcutaneous administration leading to comparable efficacy with infused therapies at decreased treatment costs.69 In addition to hyaluronan degradation, excess collagen has been targeted with collagenase in mouse models.70 Collagenase administration also improved monoclonal antibody uptake 1.1–4-fold in xenografts.70
Despite these promising results, antineoplastic adjuvants are contentious. Constitutive hyaluronan and collagen degradation can promote metastasis because the ECM barrier also impedes tumor migration.71 Additionally, systemic administration can cause off-target hyaluronan and collagen degradation, increasing toxicity.70 Improvements in biomarker characterization are being investigated to determine whether a tumor has low or high levels of ECM components because only tumors with thickened ECM benefit from the adjuvants.72 Additionally, adjuvant delivery can be localized. Nanoparticles and hydrogels have been used to improve tumor-specific delivery.73–75 An interesting next step would be using enzyme engineering to improve activity in the presence of irregular stimuli in the tumor microenvironment, such as lower tumor pH or irregular tumor metabolite profiles.
Wound healing
EBTs that are used to promote wound healing focus on enzymatic debridement (Figure 5a), and include proteases and deoxyribonucleases. Proteases include bacterial collagenases, serine proteases, and cysteine proteases.5 These proteases remove necrotic tissue from the wound bed to leave a healthy scaffold for re-epithelialization. This debridement also increases the production of anti-inflammatory cytokines (IL-10 and TGF-β) and decreases production of proinflammatory cytokines (TNF-α and IL-1β) to promote healing and decrease inflammation.76 Deoxyribonuclease also helps to minimize infection and inflammation by degrading DNA released from degenerating cells that can contribute to increased viscosity in wounds.77 In combination, these therapies allow for better penetration of antibiotics and decreases bacterial loads, decreasing infection and leading to better clinical outcomes.
Figure 5.
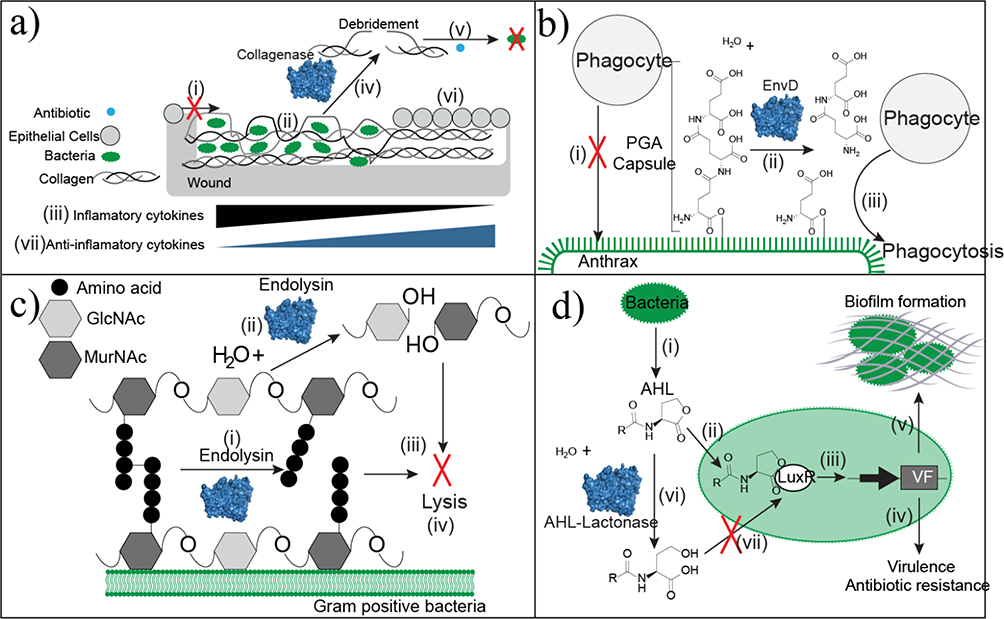
Approaches of antibacterial enzyme therapeutics. (a) Wound beds that have excessive denatured collagen prevent epithelial cell migration (i) because of the unstable scaffold. Bacteria colonize within the denatured collagen (ii). Infected tissue accumulates the inflammatory cytokines TNF-α and IL-1β. (iii). Debridement with collagenase specifically cleaves collagen at the sites of denaturation to remove necrotic and unsuitable tissue (iv). Debridement decreases bacterial loads and improves antibiotic penetration as an adjuvant (v). Collagen in its native conformation serves as a healthy scaffold for re-epithelialization. This tissue remodeling is associated with an increase in anti-inflammatory cytokines. (b) Anthrax is protected from phagocytosis by the poly-glutamic acid (PGA) capsule (i). EnvD depolymerizes the protective PGA capsule (ii) leaving anthrax susceptible to phagocytosis (iii). (c) Endolysin strips the peptidoglycan cell wall of Gram-positive bacteria through two mechanisms of action. It hydrolyzes either the peptide cross-linkages within the cell wall (i) or the glycosidic linkage between N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc). Continued depolymerization strips the capsule (iii), which causes bacterial lysis (iv). (d) Bacteria produce the quorum-sensing molecule N-acyl-L homoserine lactone (AHL) (i). AHL binds to the transcription factor LuxR (ii) to activate transcription of genes controlled by the Lux operon (iii). These genes are linked to virulence factors, antibiotic resistance genes (iv), and biofilm formation (v), which limits antibiotic penetration. AHL lactonase hydrolyzes the ester bond of the lactone ring (vi). This structural change disrupts LuxR binding (vii) to block transcription of genes linked to pathogenicity.
Antibacterials and antiparasitics
EBTs have efficacy in both antibacterial and antiparasitic applications, but have not yet been FDA approved (except for deoxyribonuclease as an antibacterial adjuvant). However, antibiotic resistance is on the rise,78 and EBTs could be used as novel solutions to this growing problem. Current enzymatic antiparasitics and antibacterials depolymerize capsular components or disrupt quorum-sensing signals required for bacterial virulence. These EBTs have shown promise in recent in vivo studies.79 As early as the 1930s, a depolymerase isolated from Bacillus was found to degrade the capsular polysaccharide of type III pneumococci and was used as a prophylactic and curative treatment for pneumonia in mice and nonhuman primates.80,81 Although this enzyme did not undergo further investigation, capsule depolymerases were recently demonstrated to be effective in vivo against Bacillus anthraci and E. coli K1, which cause anthrax and neonatal bacterial meningitis, respectively. The pathogenesis of these bacteria is enhanced by the protective poly-γ-D-glutamic acid capsule, which suppresses the humoral immune response (Figure 5b). The enzyme EnvD cleaves the γ‐glutamyl bonds between D‐glutamate residues in the capsule to promote phagocytosis of anthrax. EnvD treatment significantly improved survival after exposure to 70% and decreased symptoms relative to the control group.82,83 Similarly, enzyme-mediated degradation of the E. coli K1 polysialic acid capsule with endosialidase E effectively treated neonatal bacterial meningitis in rats. Treatment with endosialidase E prevented death, and significantly decreased the inflammatory response, in nearly all neonatal rats, whereas the control group did not survive.84
Bacterial lysis, specifically via hydrolysis of the peptidoglycan cell wall, has been promising in preclinical trials with bacteriophage-encoded endolysins that have evolved to lyse bacteria at the end of bacteriophage lytic cycles (Figure 5c).79,85 Endolysins are fast-acting (within minutes) and specific for their target because they comprise both a catalytic domain (specific to the peptidoglycan bond cleavage) and a specific cell wall-binding domain.79,85 Clinical trials are ongoing in the treatment of staphylococcal infections.79,86 Limitations to endolysin therapies have included: narrow specificity, slow permeability through the outer membrane of Gram-negative bacteria, and short-half life. Engineering efforts to address these challenges were recently reviewed 86. Briefly, therapeutic activity has been altered by: (i) mutating the catalytic or cell-binding domain to alter specificity; (ii) fusions of multiple catalytic domains and/or multiple cell binding domains; (iii) fusions with membrane-permeabilizing peptides; and (iv) Fc fusions to improve half-life. Although some patients in Phase I clinical trials developed anti-endolysin antibodies, the impact of antidrug antibodies on efficacy has not yet been studied.87
Enzyme degradation of quorum-signaling molecules can disrupt communication and decrease production of virulence factors (Figure 5d). Enzymatic quorum quenching has focused primarily on the hydrolysis of N-acyl-L homoserine lactones (AHLs), signals that enhance the production of virulence factors and pathogenicity.88 AHLs are quenched, in vitro, with either AHL acylases, which hydrolyze the amide bond between the lactone ring and acyl side chain, or AHL lactonase, which hydrolyzes the ester bond of the lactone ring.88,89 A common challenge for AHL lactonases and AHL acylases is low catalytic efficiency, and enzyme engineering efforts to improve efficiency were recently reviewed.89 However, further characterization is needed to evaluate their biocompatibility and potential efficacy in vivo.
Not only can antibacterial therapies take inspiration from naturally occurring enzymes, but several antiparasitic enzymes are also present in the latex of many plants. As noted previously, papain and ficin have historical antiparasitic activity in the gut, but off-target effects in the presence of digestive tract lesions raised safety concerns.6,18 Antiparasitic activity has also been demonstrated with other plant-based proteases, including bromelain, chymopapain, and milkweed latex5. In vitro incubations with the Heligmosomoides bakeri nematode have EC50 values ranging from 3 to 150 μM, with most of these enzymes tolerating gastric acidity.90 Better characterization of these enzymes and their consensus sequences, in combination with mutagenesis, could lead to novel and more specific vermifuges to combat evolving resistance.
Toxin degradation
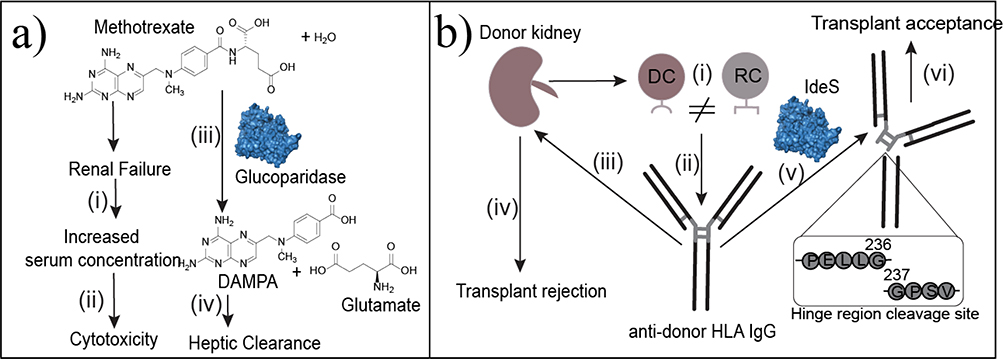
Drugs and toxins accumulate in the body because of ingestion or impaired renal clearance. Historically, EBTs have been used to treat poisoning by poison ivy, atropine, cyanide, and, more recently, cocaine.5 Additionally, enzymatic digestion of ingested allergens has gained attention as a therapy for celiac disease.91,92 Unfortunately, there is only one FDA-approved therapy in this class, glucarpidase, which converts methotrexate into less-toxic products (Figure 6a).
Figure 6.
Enzyme-based therapies for toxin clearance or accumulated products of metabolism (metabolites and proteins). (a) Methotrexate is well tolerated in cancer therapy until renal failure increases serum concentration (i) to toxic levels (ii). Glucoparidase hydrolyzes the amide bond in methotrexate to produce DAMPA and glutamate. These products can be metabolized in the liver (iv) for clearance. (b) Organ transplants are HLA incompatible when donor cells (DCs) have MHCs encoded by a different HLA group than the recipient’s cells (RCs) (i). In response, the recipient produces IgG antibodies against the donor’s HLA type (ii). These antibodies bind to the donor cells (iii) to facilitate donor cell opsonization (iv), which causes transplant rejection. Imlifidase (IdeS) cleaves the antidonor IgG at the hinge region (v) separating the Fc region from the Fab region to disrupt the humoral immune response. This cleavage leads to transplant acceptance (vi).
Therapies to treat poisoning used to be more common. Cocaine esterase and atropine esterase were originally isolated from rabbit serum and used to treat poisoning.93 Similarly, purified rhodanese, a sulfur transferase, was previously used in combination with sodium thiosulfate to expedite cyanide detoxification.94 These enzymes are not currently used in the clinic and are limited by short serum-half lives. Cocaine esterase could have broader utility, but it is a multidomain protein limited by a 15-min half-life and thermal instability at 37°C. Engineering efforts have focused on increasing thermal stability by introducing stronger interdomain interactions.95,96 Another limitation of cocaine esterase and toxin degradation in general is substrate affinity. Cocaine esterase has a Km ranging from 7.3 to 21 μM, whereas cocaine overdose fatalities occur when blood cocaine concentrations reach only 3–9 mg/l (2–10 μM).97 Cocaine esterase variants have been engineered with improved affinity, making it a good candidate for further investigation.95,96
As noted previously, an early EBT (bacterially derived β-lactamase) was used to treat penicillin anaphylaxis.29,30 Orally delivered therapies have a lower likelihood of eliciting an immune response, and current approaches to treat allergic reactions rely on enzymes that can degrade ingested allergens. Specifically, recent work has been focused on EBTs to treat celiac disease.98 In celiac disease, proline and glutamine-rich residues found in α-gliadin trigger a T cell-based immune response. There are more than 30 known proline and glutamine-rich T cell epitopes that contribute to celiac disease.92,98 To date, prolyl endopeptidase isolated from the acidophile Sphingomonas capsulata, and kumamolisin-As from Alicyclobacillus sendaiensis have been engineered to digest the immunogenic proline and glutamine-rich sequences. Prolyl endopeptidase engineering improved specific activity by 20% for proline and glutamine-rich sequences.91 Kumamolisin-As has been engineered for improved α-gliadin selectivity over other peptide sequences.92,98 The improved Kumamolisin-As variant performed well in simulated gastric digests of bread and wheat beer, where peptides degraded to under 20 ppm, which meets the FDA requirement for gluten-free labeling.92 Based on these results, acidophilic bacterial proteases selective for immunogenic gluten peptides are promising prophylactics or emergency therapies and a combination of engineered proteases could ensure that all α-gliadin epitopes are degraded.
Limited clearance can also cause molecules to accumulate to toxic levels. For example, impaired renal clearance causes methotrexate toxicity in patients with cancer. Methotrexate toxicity is treated with carboxypeptidase G2 (glucarpidase), also discussed above, which hydrolyzes methotrexate into glutamate and 2,4-diamino-N10-methylpteroic acid. which are less toxic.99 Carboxypeptidase G2 elicits an immune response, with 17% of patients developing antidrug antibodies, with <1% having a hypersensitivity reaction.100 Although carboxypeptidase G2 has been deimmunized for ADEPT therapy, this variant has not yet been broadly used in the clinic.101
Degradation of accumulated metabolites
EBTs can also be used to degrade accumulated products of metabolism, which can either be specific proteins or metabolites. Analogous to toxin accumulation, proteins and metabolites accumulate because of increased production or impaired clearance. For example, protein accumulation causes Dupuytren’s contracture and Peyronie’s disease, vitreomacular adhesions, Alzheimer’s disease (AD), and transplant rejection. Metabolite accumulation is responsible for uric acid crystallization, which causes gout. Currently, EBTs are either used clinically or under investigation as treatments to target protein and metabolite accumulation.
Dupuytren’s contracture and Peyronie’s disease are caused by collagen deposits in the hands and penis, respectively. Both of these disorders are treated with Clostridium histolyticum collagenase, which is FDA approved for local injection. Collagenase injections have improved joint range of motion by up to 35° and reduced penile curvature by 34–37%.102,103 Nearly all patients develop anticollagenase antibodies, but there have been no reports of anaphylaxis.102,103 The impact of antibody formation on efficacy has not yet been studied. To date, because collagenase treatment is only used as a non-invasive alternative to surgery, there have not been any attempts at deimmunization.
Similarly, build-up of collagen, fibronectin, and laminin can cause vitreomacular adhesions, which can lead to a macular hole and disrupted vision.104 Ocriplasmin is a recombinant truncated human plasmin that is also administered as a local injection to facilitate vitreous liquefaction as an alternative to a vitrectomy.105 Since the eye is immune privileged and ocriplasmin is human, it has been well tolerated, with mostly transient adverse events that are not significantly different from a placebo. Ocriplasmin is primarily limited by efficacy, with a success rate in macular hole closure of only ~40%.105
In addition to FDA-approved proteases, an active area of protease research involves selectively targeting amyloid beta that aggregates into oligomers that form plaques in AD.106 Engineering efforts have focused on three priorities in an ideal therapeutic candidate: high specific activity for amyloid beta, low activity for off-target sequences, and immune tolerance. Therefore, human proteases with a low level of pre-existing activity for amyloid beta have been templates for most engineering efforts.107,108 The two human proteases engineered for amyloid beta selectivity are human kallikrein 7 and neprilysin.107,108 Human kallikrein 7 was engineered to increase selectivity by decreasing off-target activity, with the most-selective variant having 80% of the wild-type activity for amyloid beta, and at least a twofold reduction in activity toward other physiological substrates (cell adhesion proteins).109 Although this selectivity improves the therapeutic potential, neprilysin engineering had success at both improving amyloid beta degradation (~18 fold) and decreasing activity toward off-target neuropeptide and natriuretic substrates ( from 3 to >1000-fold).108 Future work is needed to evaluate these potential therapies in vivo.
The last EBT for protein accumulation directly targets the longstanding challenge of immunogenicity. The accumulation of antidrug antibodies is often a limitation for many protein therapeutics as well as organ transplants and gene therapies. Specifically, transplants can be rejected because of the antidonor HLA IgG-mediated immune response, and pre-existing anti-adeno-associated virus (AAV) vector antibodies decrease transduction efficiency during gene therapy. Higher titers of unwanted antibodies are associated with worse outcomes for each.110,111 To mitigate these unwanted immune responses, microbial endopeptidase, imlifidase (IdeS), is infused to cleave IgG at the lower hinge between G236 and G237112 (Figure 6b). This cleavage separates the Fc region from the Fab region, disrupting the humoral immune response to allow for immunogenic therapies to evade the immune system. IdeS circulates for 24–48 h. This approach has depleted donor-specific antibodies in Phase I–II clinical trials, and 96% of patients who were HLA incompatible had successful initial transplants.112 Additionally, recent in vitro and in vivo studies used IdeS to deplete anti-AAV IgG to improve transduction in gene therapy. In vitro incubation of IdeS with patient blood significantly decreased anti-AAV antibodies after 24 h of incubation. This approach has been tested in nonhuman primates in in vivo experiments, which resulted in enhanced transduction efficiency and transgene expression in the pretreated primate relative to the control.113 Based on these results, IdeS is a promising EBT for applications in which improving immune tolerance is needed, such as the administration of other biologics, and could have a broader impact in treatment of autoimmune diseases.114
Lastly, there are also two FDA-approved enzymes to treat metabolite accumulation. Uric acid can accumulate beyond solubility limits and crystallize in joints, causing gout. Uricase converts uric acid into a less toxic product, allantoin, which has improved solubility and clearance, decreasing crystal formation. The two FDA-approved uricases are a non-PEGylated uricase from Aspergillus flavus and a PEGylated chimeric porcine/baboon uricase.5 Predictably, both of these enzymes have unwanted immune responses. At least 25% of patients who take the A. flavus uricase develop antidrug antibodies that decrease efficacy, and the anaphylaxis rate ranges from 3.9 to 7.3%.115,116 Similarly, repeated administration of PEGylated chimeric porcine/baboon uricase results in 41–89% of patients developing antidrug antibodies and 6.5% anaphylactic reactions.117–119 Higher antibody titers are also associated with infusion reactions and decreased efficacy, as measured by uric acid levels.119,120 Preliminary attempts to map antigenic regions and deimmunize uricase have been performed in silico, but have not been experimentally validated.121 A combination of in silico and in vitro approaches has been used to engineer a variant that is less immuno reactive, although this variant also has a 60% decrease in catalytic efficiency.117 Therefore, although these uricase therapies can be effective, better deimmunization algorithms and preclinical models are needed to design variants for improved clinical translation.
Anti-inflammatories
Anti-inflammatory enzymes primarily target inflammatory cytokines in three ways. First, enzymes that scavenge reactive oxygen species (ROS) interfere with cytokine transcription. Second, enzymes can degrade circulating inflammatory cytokines directly. Third, enzymes can prevent cytokine release by degrading receptors that are required for exocytosis.
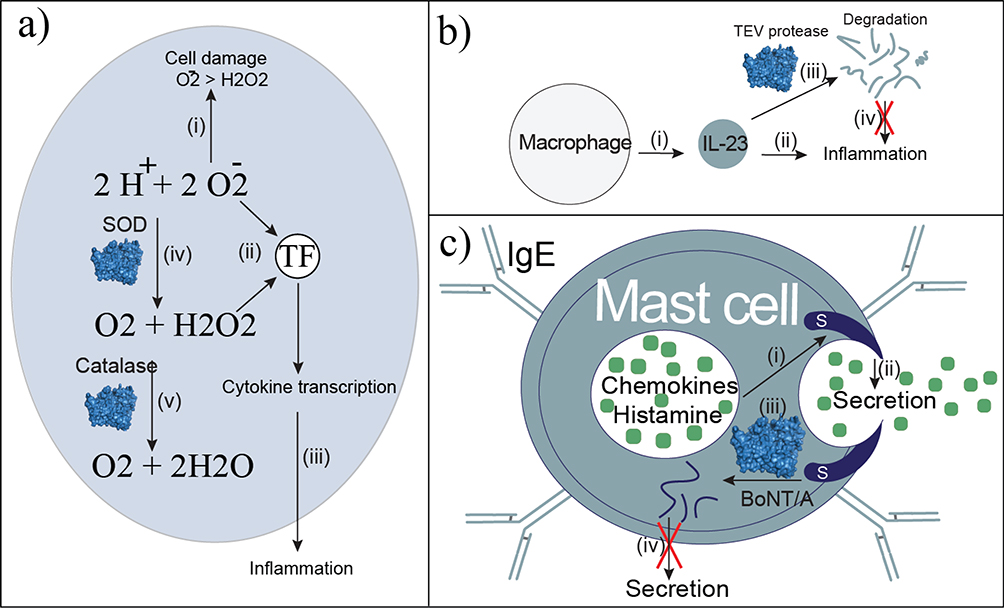
The first approach targeting ROS is a broadly applicable therapeutic strategy because ROS contributes to the pathogenesis of several diseases, such as bacterial infections, cancer, ischemic injury, and drug-induced liver toxicity.122 Specifically, the superoxide anion and hydrogen peroxide accumulate, causing cell damage and death.122 EBTs treat inflammation by supplementing innate antioxidant-scavenging enzymes, converting ROS into less toxic or harmless products (Figure 7a). Given that ROS activate the transcription factor NF-κb, which mediates proinflammatory cytokine expression, these scavenging enzymes limit inflammation.123,124
Figure 7.
Anti-inflammatory enzyme therapies. (a) Reactive oxygen species (ROS) cause cell damage and death (i) with the superoxide anion being more reactive than hydrogen peroxide. Both ROS activate the transcription factor (TF) NF-κb (ii), which activates proinflammatory cytokine production. Cytokine release (iii) signals the inflammatory response. The enzyme therapy superoxide dismutase (SOD) converts the superoxide anion into oxygen and hydrogen peroxide (iv), which is less harmful, and limits cytokine transcription. Catalase converts hydrogen peroxide into oxygen and water, which is nontoxic and does not activate inflammatory cytokine transcription. (b) Macrophages produce IL-23 (i), which contributes to inflammation in rheumatoid arthritis (ii). Engineered Tobacco Etch Virus (TEV) protease degrades IL-23 (iii) to disrupt inflammation. (c) Cytokine release is dependent on SNAP-23 located within mast cells (i). Secretion of chemokines and histamine (ii) leads to inflammation and allergic reactions. Engineered botulinum neurotoxin type A (BoNT/A) degrades SNAP-23 (iii). Without SNAP-23, secretion is blocked (iv).
Superoxide dismutase (SOD), which converts the superoxide anion into hydrogen peroxide, has been used therapeutically.125 Subsequently hydrogen peroxide is converted into water by catalase, which has also been used as an anti-inflammatory.126 Although humans have superoxide dismutases, from the late 1970s through the 1990s, bovine SOD was used clinically as an anti-inflammatory agent. Bovine SOD was effective as both a systemic therapy to decrease ROS-mediated adverse effects of radiation after chemotherapy, and as a local injection to decrease osteoarthritis pain resulting from inflammation.127–130 However, in 1994, it was withdrawn because of severe anaphylactic reactions and some deaths.4 Intuitively, recombinant human SOD is likely an immune-tolerant solution. Recombinant human SOD has demonstrated anti-inflammatory efficacy in vivo by significantly reducing inflammatory cytokine concentrations, along with inflammation-related symptoms, in animal models.131–133 Efficacy has also been tested in clinical trials with recombinant human SOD encapsulated in liposomes for the topical treatment of Peyronie’s disease. Pain significantly decreased in 89% of patients, along with improvements in other symptoms, with no adverse events.134
Despite this success, two limitations remain for the broader use of human SOD. First, recombinant human SOD has a short serum half-life of only ~7–25 min.135,136 Second, anti-inflammatory activity is more effective when SOD is concentrated at the site of ROS production. Research efforts have focused on SOD conjugation or encapsulation to address these two challenges, with both approaches leading to an improved half-life.135,136 Given that SOD needs to cross cell membranes for efficacy, alternatives to PEG, which enable improved cellular uptake, have been investigated, with ~eightfold improvement in the half-life.8,135 Targeting moieties (such as antibodies and ligands) have also been used to improve site-specific delivery, leading to improvements in cell viability, and inhibition of inflammation and cytokine concentration compared with nontargeted controls.8,137 Lastly, targeting digestive tract inflammation with oral SOD has proven challenging because of proteolytic degradation, yet recent enzyme engineering has been used to improve resistance to pepsin and trypsin for more effective management of oxidative stress in the gastrointestinal tract.138
Recombinant human catalase has also been effective in vitro and in vivo, but has not moved to clinical trials.139,140 Similar to SOD, catalase is limited by a short half-life (~23 min) and the need for site-specific targeting.141 Similar approaches to those used for SOD (conjugation and encapsulation) have been used to enhance ROS inhibition via catalase, with in vivo success in treating cancer, Parkinson’s disease, and traumatic brain injury.126,140,142 Given that human SOD and catalase have high catalytic efficiencies (8×108 s−1 M−1 and 7.3×106 s−1 M−1), protein engineering efforts that improve protease resistance in the serum, while maintaining catalytic efficiency, can significantly improve systemic administration.143,144
The second type of anti-inflammatory EBTs lead to direct cytokine degradation (Figure 7b). This approach is in the early investigational stages with the Tobacco Etch Virus (TEV) protease. Specifically, TEV protease was engineered to cleave solvent-exposed residues on the proinflammatory cytokine IL-23, as a potential therapeutic for rheumatoid arthritis and psoriasis.145 Wild-type TEV protease has no pre-existing activity for this substrate. More than ~2500 generations of phage-assisted continuous evolution led to a variant with more than 20 mutations, and ~15% of the catalytic efficiency for the IL-23 substrate, compared with the native substrate, and inactivated IL-23 in vitro. Although this directed evolution approach did not include counter-selection for off-target activity, a second generation of this method was recently developed with counter-selection to overcome this limitation.146
The last group of anti-inflammatory EBTs prevents vasoactive amine release from mast cells involved in inflammation. Specifically, vasoactive amine release is dependent on the SNARE protein, SNAP-23, which is an attractive therapeutic target (Figure 7c).147,148 Interestingly, SNAP-23 is an isoform of SNAP-25, which is the target of the enzymatic light chain of botulinum neurotoxin type A (BoNT/A), also known as Botox, which is used therapeutically to inhibit neurotransmitter release at cholinergic nerve terminals. SNAP-25 has >75% sequence similarity to SNAP-23, but BoNT/A has little activity on SNAP-23. Therefore, the enzymatic domain of BoNT/A has been engineered for SNAP-23 degradation. A variant was identified with improved selectivity for SNAP-23, which has a twofold increase in SNAP-23 catalytic efficiency and a decrease in activity on SNAP-25 (> 95% relative to the wild type).148
Challenges to the development of new EBTs
As highlighted in the discussion above and as can be seen in Table S1 in the supplemental information online,5 one of the most crucial challenges in the development of EBTs is unwanted immune responses, whether these are antidrug antibodies that facilitate clearance or activity neutralization, or hypersensitivity responses. As a result of these unwanted immune responses, development of microbial EBTs has lagged in favor of human enzymes. As discussed, human enzymes are primarily used in replacement therapies, and two more are in clinical development. One new ERT, olipudase alfa, is the only therapy in development to treat sphingomyelinase deficiency.149 The second, avalglucosidase alfa, is the next generation of alglucosidase alfa, with a 15-fold increase in mannose-6-phosphate concentration, aimed to improve cellular uptake through the mannose-6-phosphate receptor.150 Given that many ERTs rely on this method of uptake, it is a likely approach to improve the efficacy of other approved ERTs for lysosomal storage disorders.
Although recombinant production of enzymes led to rapid growth in EBT approvals from the 1980s through 2010, since 2010, new enzyme therapy approvals have plateaued relative to antibodies and protein therapeutics as a whole (Figure 1c; Table S4 in the supplemental information online), which can be attributed to new production technologies, such as CHO and HEK cell culture, making the recombinant production of ‘human-like’ proteins more feasible.151,152 Therefore, although enzyme engineering over the past decade has also made leaps in progress, these capabilities have not translated well to new clinical capabilities and approvals for new EBTs.153–155 This plateau is surprising because EBTs have shorter average development timelines compared with other biologics and small molecules.32 However, by combining recombinant DNA, new production technologies, and enzyme engineering, human enzymes have been engineered with either new activity (such as cystathionine-γ-lyase for L-methionine depletion) or improved kinetics for therapeutic activity (such as neprilysin for amyloid beta proteolysis). Applying these approaches to other human enzymes can address the need for both novel, effective, and safe EBTs with low immunogenicity and rapid development.
New improved technologies for deimmunization or immunoevasion are a crucial challenge in the development of new EBTs and are of particular importance when considering nonhuman enzymes.156–158 In fact, as this review discusses, there are existing numerous examples of enzymes with therapeutic activity that might be of clinical use if deimmunized. Toward this goal, deimmunization has been used on lysostaphin to remove T cell epitopes, which limited antidrug antibody responses and remained effective after repeated doses (even with pre-existing wild-type immunity) in human HLA transgenic mice.159 Although it is recognized that in vitro assays and mouse models are not perfect predictors of clinical behavior, the engineered lysostaphin variant has performed well with preclinical metrics that support the need for clinical evaluation.
Beyond epitope-engineering approaches, there are additional approaches to prolong the half-life.11,13,160 PEGylation is routinely deployed to reduce immunogenicity, and decrease clearance to prolong the half-life, although anti-PEG immunity can develop. Therefore, the development of alternatives to PEGylation is an active area of research that has been reviewed recently.13 Beyond synthetic conjugates, to address immunogenicity specifically, half-life can be prolonged with Fc-fusions. This approach prolongs circulation both by decreasing renal clearance because of the increased molecular weight, and limiting degradation with Fc recycling.160 Recently, Palleon pharmaceuticals developed the Fc fusion enzyme E −602 (bi-sialidase), which is intended to digest dense sialic acid-containing glycans on the surface of T cells that block the CD28 receptor needed for activation.161 An investigation new drug application has been filed for E −602 (Bi-sialidase) and it is expected to begin Phase I clinical trials this year.
Concluding remarks
Assuming challenges in deimmunization can be overcome, the potential of EBTs is almost limitless, far beyond the current 39 FDA-approved enzymes.5 Out of these approved enzymes, 66% are produced recombinantly, but, as a whole, <20% of the FDA-approved enzymes have engineered sequences (Table S1 in the supplemental information online). There are millions of nonhuman enzymes in nature, with an even greater number considering advancements in protein engineering and directed evolution, which have the potential to generate novel enzymes with new or altered functions.162 Therefore, by coupling recombinant protein expression with advancements in enzyme engineering, there are known approaches to address the challenges associated with EBTs with directed evolution for improved safety and efficacy, enabling a huge potential growth in novel therapeutics.
Lastly, with topical or oral administration, several therapeutic enzymes are viable without deimmunization. In fact, there is already a therapy in Phase III clinical trials, NexoBrid, for topical debridement.163,164 Specifically, anti-inflammatory and antibacterial enzymes have great potential for growth because they could be combined with debridement agents for topical administration to promote wound healing.
Supplementary Material
Highlights:
An overview of applications of enzyme based therapies (EBTs)
Provides historical, clinical, and regulatory context for EBT market growth and decline
A review of both approved and investigational therapies.
Discusses challenges facing EBTs that could be addressed with enzyme engineering.
Specific, relevant examples of targets for enzyme engineering for improved EBTs
Acknowledgments
We would like to acknowledge the following support: the North Carolina Biotechnology Center 2018-BIG-6503, NIH R61 AI140485–01. J.N.H. was supported, in part, by the NIH Biotechnology Training Grant (T32GM008555).
Author biographies

Jennifer N. Hennigan received her BS in Chemistry from Stetson University in 2015. She is currently a PhD candidate in the Chemistry Department at Duke University. Her research interests focus on protein therapeutics. This includes analyzing current biologics and their development trends, recognizing gaps in therapeutic or production capabilities, and identifying potential solutions to overarching challenges in the field from a research and development perspective.

Michael D. Lynch is an assistant professor in the Department of Biomedical Engineering at Duke University. His team’s work is focused on standardizing bioengineering and synthetic biology tools. Engineered proteins, enzymes, and cells have utility in numerous applications from fuels and chemicals production to the manufacture of foods, agricultural products, proteins and enzymes, and in the development of pharmaceuticals and other therapeutics.
Footnotes
Declaration of Interest
M.D.L. has a financial interest in DMC Biotechnologies, Inc. M.D.L. and J.N.H. have financial interests in Roke Biotechnologies, Inc.
Teaser: Enzyme-based therapies have a rich history and significant clinical potential. We review the history and classes of enzyme based therapies, key successes, challenges, areas for growth, and factors that have guided clinical development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parte S, Sirisha VL, D’Souza JS. Biotechnological applications of marine enzymes from algae, bacteria, fungi, and sponges. Adv Food Nutr Res 2017; 80: 75–106. [DOI] [PubMed] [Google Scholar]
- 2.Fornbacke M, Clarsund M. Cold-adapted proteases as an emerging class of therapeutics. Infect Dis Ther 2013; 2(1): 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taipa MÂ, Fernandes P, de Carvalho CCCR. Production and purification of therapeutic enzymes. In: Labrou N, ed. Therapeutic Enzymes: Function and Clinical Implications. Singapore: Springer; 2019: 1–24. [DOI] [PubMed] [Google Scholar]
- 4.Stephens M Appendix I: Drug products withdrawn from the market for safety reasons. In: Talbot J, Waller P, eds. Stephens’ Detection of New Adverse Drug Reactions. Hoboken; John Wiley & Sons; 2005: 667–702. [Google Scholar]
- 5.Lynch M A compilation of investigational, preclinical and clinical enzyme based therapeutics. www.narcis.nl/dataset/RecordID/oai%3Aeasy.dans.knaw.nl%3Aeasy-dataset%3A211738 [Accessed September 9, 2021].
- 6.Sherry S, Fleteher AP. Proteolytic enzymes: a therapeutic evaluation. Clinical Pharmacology & Therapeutics 1960; 1(2): 202–226. [Google Scholar]
- 7.Labrou N, ed. Therapeutic Enzymes: Function and Clinical Implications. Singapore; Springer; 2019. [Google Scholar]
- 8.Dean SN, Turner KB, Medintz IL, Walper SA. Targeting and delivery of therapeutic enzymes. Ther Deliv 2017; 8(7): 577–595. [DOI] [PubMed] [Google Scholar]
- 9.Baldo BA. Enzymes approved for human therapy: indications, mechanisms and adverse effects. BioDrugs 2015; 29(1): 31–55. [DOI] [PubMed] [Google Scholar]
- 10.Cantor JR, Panayiotou V, Agnello G, Georgiou G, Stone EM. Engineering reduced-immunogenicity enzymes for amino acid depletion therapy in cancer. Methods Enzymol 2012; 502: 291–319. [DOI] [PubMed] [Google Scholar]
- 11.Menacho-Melgar R, Decker JS, Hennigan JN, Lynch MD. A review of lipidation in the development of advanced protein and peptide therapeutics. J Control Release 2019; 295: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milton Harris J, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003; 2(3): 214–221. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Sun F, Liu S, Jiang S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release 2016; 244(Pt B): 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graves WP. Report on the trypsin treatment of cancer. Boston Medical and Surgical Journal 1907; 156: 129–131. [Google Scholar]
- 15.Rushmore S The effect of trypsin on cancer and on the germ cells in mice. J Med Res 1909; 21(3): 591–596. [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein JW. Drugs in gastric therapeutics. Med Rec 1911; 79(17): 767. [Google Scholar]
- 17.Rosenfeld L Insulin: discovery and controversy. Clin Chem 2002; 48(12): 2270–2288. [PubMed] [Google Scholar]
- 18.Robbins BH. A proteolytic enzyme in ficin, the anthelmintic principle of leche de higueron. J Biol Chem 1930; 87(2): 251–257. [Google Scholar]
- 19.Lamson PD, Brown HW, Ward CB. Anthelmintics: some therapeutic and practical considerations on their use. JAMA 1932; 99(4): 292–295. [Google Scholar]
- 20.Elliot RHE, Mellney FL. Papain and peritoneal adhesions. Surgery 1937; 1: 785–791. [Google Scholar]
- 21.Stevens LE. A reassessment of papain in preventing peritoneal adhesions. Am J Surg 1968; 115(4): 535–539. [DOI] [PubMed] [Google Scholar]
- 22.Cohn EJ. The separation of blood into fractions of therapeutic value. Ann Intern Med 1947; 26(3): 341–352. [DOI] [PubMed] [Google Scholar]
- 23.Warren JV, Stead EA, Merrill AJ, Brannon ES. Chemical, clinical, and immunological studies on the products of human plasma fractionation. IX. The treatment of shock with concentrated human serum albumin: a preliminary report. J Clin Invest 1944; 23(4): 506–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferry JD, Morrison PR. Chemical, clinical, and immunological studies on the products of human plasma fractionation. XVI. Fibrin clots, fibrin films, and fibrinogen plastics. J Clin Invest 1944; 23(4): 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherry S, Goeller JP. The extent of the enzymatic degradation of desoxyribonucleic acid (DNA) in purulent exudates by streptodornase. J Clin Invest 1950; 29(12): 1588–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farber LA. Sputum cytology in patients following enzyme aerosol therapy. Dis Chest 1957; 31(2): 169–179. [DOI] [PubMed] [Google Scholar]
- 27.Goehring WO, Grant JJ. Allergic reaction to streptokinase-streptodornase solution given intrapleurally. JAMA 1953; 152(15): 1429–1430. [DOI] [PubMed] [Google Scholar]
- 28.Becker RM. A new concept in the treatment of penicillin reactions: use of penicillinase. Ann Intern Med 1958; 48(6): 1228–1242. [DOI] [PubMed] [Google Scholar]
- 29.Becker RM. Effect of penicillinase on circulating penicillin. N Engl J Med 1956; 254(20): 952–953. [DOI] [PubMed] [Google Scholar]
- 30.Hyman AL. Anaphylactic shock after therapy with penicillinase. J Am Med Assoc 1959; 169(6): 593–594. [DOI] [PubMed] [Google Scholar]
- 31.Gad SC. Drug Safety Evaluation. Hoboken; John Wiley & Sons; 2016. [Google Scholar]
- 32.Kinch MS. An overview of FDA-approved biologics medicines. Drug Discov Today 2015; 20(4): 393–398. [DOI] [PubMed] [Google Scholar]
- 33.Kuriakose A, Chirmule N, Nair P. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. J Immunol Res 2016; 2016: 1298473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Lozier J, Johnson G, Kirshner S, Verthelyi D, Pariser A, et al. Neutralizing antibodies to therapeutic enzymes: considerations for testing, prevention and treatment. Nat Biotechnol 2008; 26(8): 901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mechler K, Mountford WK, Hoffmann GF, Ries M. Pressure for drug development in lysosomal storage disorders – a quantitative analysis thirty years beyond the US orphan drug act. Orphanet J Rare Dis 2015; 10: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melnikova I Rare diseases and orphan drugs. Nat Rev Drug Discov 2012; 11(4): 267–268. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz B, Busch M. Estimating the pathogen safety of manufactured human plasma products: application to fibrin sealants and to thrombin. Transfusion 2008; 48(8): 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng CM, Meyer-Massetti C, Kayser SR. A review of three stand-alone topical thrombins for surgical hemostasis. Clin Ther 2009; 31(1): 32–41. [DOI] [PubMed] [Google Scholar]
- 39.Rapaport SI, Zivelin A, Minow RA, Hunter CS, Donnelly K. Clinical significance of antibodies to bovine and human thrombin and factor V after surgical use of bovine thrombin. Am J Clin Pathol 1992; 97(1): 84–91. [DOI] [PubMed] [Google Scholar]
- 40.Ali MR, Salim Hossain M, Islam MA, Saiful Islam Arman M, Sarwar Raju G, Dasgupta P, et al. Aspect of thrombolytic therapy: a review. ScientificWorldJournal 2014; 2014: 586510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y, Shi Y, Zhou J, Yang W, Bai L, Wang S, et al. Structure-based antigenic epitope and PEGylation improve the efficacy of staphylokinase. Microb Cell Fact 2017; 16(1): 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang M, Chen Y, Fu W, Zou M, Wang Y, Xing W, et al. Construction of a novel staphylokinase (SAK) mutant with low immunogenicity and its evaluation in rhesus monkey. Int J Biol Macromol 2020; 146: 781–789. [DOI] [PubMed] [Google Scholar]
- 43.Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke. Stroke 2019; 50(8): 2156–2162. [DOI] [PubMed] [Google Scholar]
- 44.Haskell CM, Canellos GP, Leventhal BG, Carbone PP, Block JB, Serpick AA, et al. L-asparaginase: therapeutic and toxic effects in patients with neoplastic disease. N Engl J Med 1969; 281(19): 1028–1034. [DOI] [PubMed] [Google Scholar]
- 45.Richards NGJ, Kilberg MS. Asparagine synthetase chemotherapy. Annu Rev Biochem 2006; 75: 629–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortega JA, Nesbit ME Jr, Donaldson MH, Hittle RE, Weiner J, Karon M, et al. L-Asparaginase, vincristine, and prednisone for induction of first remission in acute lymphocytic leukemia. Cancer Res 1977; 37(2): 535–540. [PubMed] [Google Scholar]
- 47.Fonseca MHG, Fiúza T da S, de Morais SB, Souza T de ACB de, Trevizani R. Circumventing the side effects of L-asparaginase. Biomed Pharmacother 2021; 139: 111616. [DOI] [PubMed] [Google Scholar]
- 48.Dhankhar R, Gupta V, Kumar S, Kapoor RK, Gulati P. Microbial enzymes for deprivation of amino acid metabolism in malignant cells: biological strategy for cancer treatment. Appl Microbiol Biotechnol 2020; 104(7): 2857–2869. [DOI] [PubMed] [Google Scholar]
- 49.Yang J, Tao R, Wang L, Song L, Wang Y, Gong C, et al. Thermosensitive micelles encapsulating phenylalanine ammonia lyase act as a sustained and efficacious therapy against colorectal cancer. J Biomed Nanotechnol 2019; 15(4): 717–727. [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Wang J, Lu Q, Xu J, Kobayashi Y, Takakura T, et al. PEGylation confers greatly extended half-life and attenuated immunogenicity to recombinant methioninase in primates. Cancer Res 2004; 64(18): 6673–6678. [DOI] [PubMed] [Google Scholar]
- 51.Stith WJ, Hodgins DS, Abell CW. Effects of phenylalanine amonia-lyase and phenylalanine deprivation on murine leukemic lymphoblasts in vitro. Cancer Res 1973; 33(5): 966–971. [PubMed] [Google Scholar]
- 52.Stone EM, Glazer ES, Chantranupong L, Cherukuri P, Breece RM, Tierney DL, et al. Replacing Mn(2+) with Co(2+) in human arginase i enhances cytotoxicity toward l-arginine auxotrophic cancer cell lines. ACS Chem Biol 2010; 5(3): 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu WC, Saha A, Yan W, Garrison K, Lamb C, Pandey R, et al. Enzyme-mediated depletion of serum l-Met abrogates prostate cancer growth via multiple mechanisms without evidence of systemic toxicity. Proc Natl Acad Sci U S A 2020; 117(23): 13000–13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimobayashi M, Hall MN. Multiple amino acid sensing inputs to mTORC1. Cell Res 2016; 26(1): 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campesato LF, Budhu S, Tchaicha J, Weng CH, Gigoux M, Cohen IJ, et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat Commun 2020; 11(1): 4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triplett TA, Garrison KC, Marshall N, Donkor M, Blazeck J, Lamb C, et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat Biotechnol 2018; 36(8): 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma SK, Bagshawe KD. Antibody directed enzyme prodrug therapy (ADEPT): trials and tribulations. Adv Drug Deliv Rev 2017; 118: 2–7. [DOI] [PubMed] [Google Scholar]
- 58.Francis RJ, Sharma SK, Springer C, Green AJ, Hope-Stone LD, Sena L,et al. A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. Br J Cancer 2002; 87(6): 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bagshawe KD, Sharma SK. Cyclosporine delays host immune response to antibody enzyme conjugate in ADEPT. Transplant Proc 1996; 28(6): 3156–3158. [PubMed] [Google Scholar]
- 60.Mayer A, Sharma SK, Tolner B, Minton NP, Purdy D, Amlot P, et al. Modifying an immunogenic epitope on a therapeutic protein: a step towards an improved system for antibody-directed enzyme prodrug therapy (ADEPT). Br J Cancer 2004; 90(12): 2402–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Copp JN, Mowday AM, Williams EM, Guise CP, Ashoorzadeh A, Sharrock AV, et al. Engineering a multifunctional nitroreductase for improved activation of prodrugs and PET probes for cancer gene therapy. Cell Chem Biol 2017; 24(3): 391–403. [DOI] [PubMed] [Google Scholar]
- 62.Adiyala PR, Tekumalla V, Sayeed IB, Nayak VL, Nagarajan A, Shareef MA, et al. Development of pyrrolo[2,1-c][1,4]benzodiazepine β-glucoside prodrugs for selective therapy of cancer. Bioorg Chem 2018; 76: 288–293. [DOI] [PubMed] [Google Scholar]
- 63.Di Y, Ji S, Wolf P, Krol ES, Alcorn J. Enterolactone glucuronide and β-glucuronidase in antibody directed enzyme prodrug therapy for targeted prostate cancer cell treatment. AAPS PharmSciTech 2017; 18(6): 2336–2345. [DOI] [PubMed] [Google Scholar]
- 64.Grove JI, Lovering AL, Guise C, Race PR, Wrighton CJ, White SA,et al. Generation of Escherichia coli nitroreductase mutants conferring improved cell sensitization to the prodrug CB1954. Cancer Res 2003; 63(17): 5532–5537. [PubMed] [Google Scholar]
- 65.Locke KW, Maneval DC, LaBarre MJ. ENHANZE® drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv 2019; 26(1): 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C,, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res 2015; 21(15): 3561–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 2016; 22(12): 2848–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Mackelenbergh MG, Stroes CI, Spijker R, van Eijck CHJ, Wilmink JW, Bijlsma MF, et al. Clinical trials targeting the stroma in pancreatic cancer: a systematic review and meta-analysis. Cancers 2019; 11(5). doi: 10.3390/cancers11050588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duco MR, Murdock JL, Reeves DJ. Trastuzumab/Hyaluronidase-oysk: a new option for patients with HER2-positive breast cancer. Ann Pharmacother 2020; 54(3): 254–261. [DOI] [PubMed] [Google Scholar]
- 70.Dolor A, Szoka FC Jr. Digesting a path forward: the utility of collagenase tumor treatment for improved drug delivery. Mol Pharm 2018; 15(6): 2069–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu M, Tolg C, Turley E. Dissecting the dual nature of hyaluronan in the tumor microenvironment. Front Immunol 2019; 10: 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taverna D, Printz M, LaBarre MJ, Sekulovich RE. Plasma hyaluronan is a predictive marker for pegvorhyaluronidase alfa (PEGPH20; PVHA) response in a phase II study of pancreatic ductal adenocarcinoma (PDAC). J Clin Orthod 2019; 37(4_suppl): 404–404. [Google Scholar]
- 73.Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB, et al. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett 2016; 16(5): 3268–3277. [DOI] [PubMed] [Google Scholar]
- 74.Zinger A, Koren L, Adir O, Poley M, Alyan M, Yaari Z, et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano 2019; 13(10): 11008–11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan A, Wang Z, Chen B, Dai W, Zhang H, He B, et al. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv 2018; 25(1): 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Das A, Datta S, Roche E, Chaffee S, Jose E, Shi L, et al. Novel mechanisms of Collagenase Santyl Ointment (CSO) in wound macrophage polarization and resolution of wound inflammation. Sci Rep 2018; 8(1): 1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin SJ, Corrado OJ, Kay EA. Enzymatic debridement for necrotic wounds. J Wound Care 1996; 5(7): 310–311. [DOI] [PubMed] [Google Scholar]
- 78.Pärnänen KMM, Narciso-da-Rocha C, Kneis D, Berendonk TU, Cacace D, Do TT, et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci Adv 2019; 5(3): eaau9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dams D, Briers Y. Enzybiotics: Enzyme-based antibacterials as therapeutics. In: Labrou N, ed. Therapeutic Enzymes: Function and Clinical Implications. Singapore; Springer; 2019: 233–253. [DOI] [PubMed] [Google Scholar]
- 80.Avery OT, Dubos R. The protective action of a specific enzyme against type III Pneumococcus infection in mice. J Exp Med 1931; 54(1): 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Francis T, Terrell EE, Dubos R, Avery OT. Experimental type III Pneumococcus pneumonia in monkeys: II. treatment with an enzyme which decomposes the specific capsular polysaccharide of Pneumococcus type III. J Exp Med 1934; 59(5): 641–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Negus D, Taylor PW. A poly-γ-(D)-glutamic acid depolymerase that degrades the protective capsule of Bacillus anthracis. Mol Microbiol 2014; 91(6): 1136–1147. [DOI] [PubMed] [Google Scholar]
- 83.Negus D, Vipond J, Hatch GJ, Rayner EL, Taylor PW. Parenteral administration of capsule depolymerase EnvD prevents lethal inhalation anthrax infection. Antimicrob Agents Chemother 2015; 59(12): 7687–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zelmer A, Martin MJ, Gundogdu O, Birchenough G, Lever R, Wren BW,et al. Administration of capsule-selective endosialidase E minimizes upregulation of organ gene expression induced by experimental systemic infection with Escherichia coli K1. Microbiology 2010; 156(Pt 7): 2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haddad Kashani H, Schmelcher M, Sabzalipoor H, Seyed Hosseini E, Moniri R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev 2018; 31(1). doi: 10.1128/CMR.00071-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gerstmans H, Criel B, Briers Y. Synthetic biology of modular endolysins. Biotechnol Adv 2018; 36(3): 624–640. [DOI] [PubMed] [Google Scholar]
- 87.Jun SY, Jang IJ, Yoon S, Jang K, Yu KS, Cho JY, et al. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob Agents Chemother 2017; 61(6). doi: 10.1128/AAC.02629-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan K-G, Liu Y-C, Chang C-Y. Inhibiting N-acyl-homoserine lactone synthesis and quenching Pseudomonas quinolone quorum sensing to attenuate virulence. Front Microbiol 2015; 6: 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murugayah SA, Gerth ML. Engineering quorum quenching enzymes: progress and perspectives. Biochem Soc Trans 2019; 47(3): 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Behnke JM, Buttle DJ, Stepek G, Lowe A, Duce IR. Developing novel anthelmintics from plant cysteine proteinases. Parasit Vectors 2008; 1(1): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ehren J, Govindarajan S, Morón B, Minshull J, Khosla C. Protein engineering of improved prolyl endopeptidases for celiac sprue therapy. Protein Eng Des Sel 2008; 21(12): 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolf C, Siegel JB, Tinberg C, Camarca A, Gianfrani C, Paski S, et al. Engineering of Kuma030: a gliadin peptidase that rapidly degrades immunogenic gliadin peptides in gastric conditions. J Am Chem Soc 2015; 137(40): 13106–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shirkey HC, Schmidt GC, Miller RG, Honkomp L, Flamm G. Animal sera and specific enzymes inthe treatment of poisoning. J Pediatr 1962; 60(5): 711–715. [DOI] [PubMed] [Google Scholar]
- 94.Frankenberg L Enzyme therapy in cyanide poisoning: effect of rhodanese and sulfur compounds. Arch Toxicol 1980; 45(4): 315–323. [DOI] [PubMed] [Google Scholar]
- 95.Gao D, Narasimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, et al. Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol 2009; 75(2): 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brim RL, Nance MR, Youngstrom DW, Narasimhan D, Zhan CG, Tesmer JJ, et al. A thermally stable form of bacterial cocaine esterase: a potential therapeutic agent for treatment of cocaine abuse. Mol Pharmacol 2010; 77(4): 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kontak A, Vongpatanasin W, Victor RG. Cocaine overdose. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, eds. Primer on the Autonomic Nervous System (Third Edition). London; Academic Press; 2012: 577–581. [Google Scholar]
- 98.Sollid LM, Qiao S-W, Anderson RP, Gianfrani C, Koning F. Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 2012; 64(6): 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Green JM. Glucarpidase to combat toxic levels of methotrexate in patients. Ther Clin Risk Manag 2012; 8: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramsey LB, Balis FM, O’Brien MM, Schmiegelow K, Pauley JL, Bleyer A, et al. Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 2018; 23(1): 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.AlQahtani AD, Al-Mansoori L, Bashraheel SS, Rashidi FB, Al-Yafei A, Elsinga P, et al. Production of ‘biobetter’ glucarpidase variants to improve drug detoxification and antibody directed enzyme prodrug therapy for cancer treatment. Eur J Pharm Sci 2019; 127: 79–91. [DOI] [PubMed] [Google Scholar]
- 102.Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010; 35(12): 2027–2038.e1. [DOI] [PubMed] [Google Scholar]
- 103.Gelbard M, Goldstein I, Hellstrom WJ, McMahon CG, Smith T, Tursi J, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol 2013; 190(1): 199–207. [DOI] [PubMed] [Google Scholar]
- 104.Grinton M, Steel DH. Cochrane Corner: Ocriplasmin—why isn’t it being used more? Eye 2019; 33(8): 1195–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med 2012; 367(7): 606–615. [DOI] [PubMed] [Google Scholar]
- 106.Sikanyika NL, Parkington HC, Smith AI, Kuruppu S. Powering amyloid beta degrading enzymes: a possible therapy for Alzheimer’s disease. Neurochem Res 2019; 44(6): 1289–1296. [DOI] [PubMed] [Google Scholar]
- 107.Guerrero JL, O’Malley MA, Daugherty PS. Intracellular FRET-based screen for redesigning the specificity of secreted proteases. ACS Chem Biol 2016; 11(4): 961–970. [DOI] [PubMed] [Google Scholar]
- 108.Webster CI, Burrell M, Olsson LL, Fowler SB, Digby S, Sandercock A, et al. Engineering neprilysin activity and specificity to create a novel therapeutic for Alzheimer’s disease. PLoS ONE 2014; 9(8): e104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu Y, Prassas I, Dimitromanolakis A, Diamandis EP. Novel biological substrates of human kallikrein 7 identified through degradomics. J Biol Chem 2015; 290(29): 17762–17775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meadows AS, Pineda RJ, Goodchild L, Bobo TA, Fu H. Threshold for pre-existing antibody levels limiting transduction efficiency of systemic rAAV9 gene delivery: relevance for translation. Mol Ther Methods Clin Dev 2019; 13: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang R Donor-specific antibodies in kidney transplant recipients. Clin J Am Soc Nephrol 2018; 13(1): 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jordan SC, Lorant T, Choi J, Kjellman C, Winstedt L, Bengtsson M, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 2017; 377(5): 442–453. [DOI] [PubMed] [Google Scholar]
- 113.Leborgne C, Barbon E, Alexander JM, Hanby H, Delignat S, Cohen DM, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med 2020; 26(7): 1096–1101. [DOI] [PubMed] [Google Scholar]
- 114.Collin M, Björck L. Toward clinical use of the IgG specific enzymes IdeS and EndoS against antibody-mediated diseases. Methods Mol Biol 2017; 1535: 339–351. [DOI] [PubMed] [Google Scholar]
- 115.Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet 2011; 377(9760): 165–177. [DOI] [PubMed] [Google Scholar]
- 116.Ueng S Rasburicase (Elitek): a novel agent for tumor lysis syndrome. Proc 2005; 18(3): 275–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nyborg AC, Ward C, Zacco A, Chacko B, Grinberg L, Geoghegan JC, et al. A therapeutic uricase with reduced immunogenicity risk and improved development properties. PLoS ONE 2016; 11(12): e0167935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, et al. Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther 2014; 16(2): R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 2011; 306(7): 711–720. [DOI] [PubMed] [Google Scholar]
- 120.Guttmann A, Krasnokutsky S, Pillinger MH, Berhanu A. Pegloticase in gout treatment - safety issues, latest evidence and clinical considerations. Ther Adv Drug Saf 2017; 8(12): 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nelapati AK, Das BK, Ponnan Ettiyappan JB, Chakraborty D. In-silico epitope identification and design of Uricase mutein with reduced immunogenicity. Process Biochem 2020; 92: 288–302. [Google Scholar]
- 122.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol 2012; 2012: 936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Chem Biol 1995; 2(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 124.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24(10): R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Younus H Therapeutic potentials of superoxide dismutase. Int J Health Sci 2018; 12(3): 88. [PMC free article] [PubMed] [Google Scholar]
- 126.Lutton EM, Razmpour R, Andrews AM, Cannella LA, Son YJ, Shuvaev VV, et al. Acute administration of catalase targeted to ICAM-1 attenuates neuropathology in experimental traumatic brain injury. Sci Rep 2017; 7(1): 3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sanchiz F, Millá A, Artola N, Julià JC, Moya LM, Pedro A, et al. Prevention of radioinduced cystitis by orgotein: a randomized study. Anticancer Res 1996; 16(4A): 2025–2028. [PubMed] [Google Scholar]
- 128.Gammer W, Brobäck LG. Clinical comparison of orgotein and methylprednisolone acetate in the treatment of osteoarthrosis of the knee joint. Scand J Rheumatol 1984; 13(2): 108–112. [DOI] [PubMed] [Google Scholar]
- 129.McIlwain H, Silverfield JC, Cheatum DE, Poiley J, Taborn J, Ignaczak T, et al. Intra-articular orgotein in osteoarthritis of the knee: a placebo-controlled efficacy, safety, and dosage comparison. Am J Med 1989; 87(3): 295–300. [DOI] [PubMed] [Google Scholar]
- 130.Menander-Huber KB, Edsmyr F, Huber W. Orgotein (superoxide dismutase): a drug for the amelioration of radiation-induced side effects. A double-blind, placebo-controlled study in patients with bladder tumours. Urol Res 1978; 6(4): 255–257. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, Wang J-Z, Wu Y-J, Li W-G. Anti-inflammatory effect of recombinant human superoxide dismutase in rats and mice and its mechanism. Acta Pharmacol Sin 2002; 23(5): 439–444. [PubMed] [Google Scholar]
- 132.Guillaume M, Rodriguez-Vilarrupla A, Gracia-Sancho J, et al. Recombinant human manganese superoxide dismutase reduces liver fibrosis and portal pressure in CCl4-cirrhotic rats. J Hepatol 2013; 58(2): 240–246. [DOI] [PubMed] [Google Scholar]
- 133.Kopincova J, Kolomaznik M, Mikolka P, Kosutova P, Topercerova J, Matasova K Jr, et al. Recombinant human superoxide dismutase and N-acetylcysteine addition to exogenous surfactant in the treatment of meconium aspiration syndrome. Molecules 2019; 24(5): 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riedl CR, Sternig P, Gallé G, Langmann F, Vcelar B, Vorauer K, et al. Liposomal recombinant human superoxide dismutase for the treatment of Peyronie’s disease: a randomized placebo-controlled double-blind prospective clinical study. Eur Urol 2005; 48(4): 656–661. [DOI] [PubMed] [Google Scholar]
- 135.Hallewell RA, Laria I, Tabrizi A, Carlin G, Getzoff ED, Tainer JA, et al. Genetically engineered polymers of human CuZn superoxide dismutase. Biochemistry and serum half-lives. J Biol Chem 1989; 264(9): 5260–5268. [PubMed] [Google Scholar]
- 136.Xu L, Ji X, Zhao N, Song C, Wang F, Liu C. The conjugation of Cu/Zn superoxide dismutase (SOD) to O-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride (O-HTCC) enhances its therapeutic potential against radiation-induced oxidative damage. Polym Chem 2016; 7(9): 1826–1835. [Google Scholar]
- 137.Shuvaev VV, Kiseleva RY, Arguiri E, Villa CH, Muro S, Christofidou-Solomidou M, et al. Targeting superoxide dismutase to endothelial caveolae profoundly alleviates inflammation caused by endotoxin. J Control Release 2018; 272: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yan XX, Li HL, Zhang YT, Wu SY, Lu HL, Yu XL, et al. A new recombinant MS-superoxide dismutase alleviates 5-fluorouracil-induced intestinal mucositis in mice. Acta Pharmacol Sin 2020; 41(3): 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shi X-L, Shi Z-H, Huang H, Zhu H-G, Zhou P, Ju D. Therapeutic effect of recombinant human catalase on H1N1 influenza-induced pneumonia in mice. Inflammation 2010; 33(3): 166–172. [DOI] [PubMed] [Google Scholar]
- 140.Zhang R, Song X, Liang C, Yi X, Song G, Chao Y, et al. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemoradiotherapy of cancer. Biomaterials 2017; 138: 13–21. [DOI] [PubMed] [Google Scholar]
- 141.Turrens JF, Crapo JD, Freeman BA. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest 1984; 73(1): 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eom SA, Kim DW, Shin MJ, Ahn EH, Chung SY, Sohn EJ, et al. Protective effects of PEP-1-catalase on stress-induced cellular toxicity and MPTP-induced Parkinson’s disease. BMB Rep 2015; 48(7): 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Switala J, Loewen PC. Diversity of properties among catalases. Arch Biochem Biophys 2002; 401(2): 145–154. [DOI] [PubMed] [Google Scholar]
- 144.Hsu JL, Hsieh Y, Tu C, O’Connor D, Nick HS, Silverman DN. Catalytic properties of human manganese superoxide dismutase. J Biol Chem 1996; 271(30): 17687–17691. [DOI] [PubMed] [Google Scholar]
- 145.Packer MS, Rees HA, Liu DR. Phage-assisted continuous evolution of proteases with altered substrate specificity. Nat Commun 2017; 8(1): 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blum TR, Liu H, Packer MS, Xiong X, Lee PG, Zhang S, et al. Phage-assisted evolution of botulinum neurotoxin proteases with reprogrammed specificity. Science 2021; 371(6531): 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Frank SPC, Thon K-P, Bischoff SC, Lorentz A. SNAP-23 and syntaxin-3 are required for chemokine release by mature human mast cells. Mol Immunol 2011; 49(1–2): 353–358. [DOI] [PubMed] [Google Scholar]
- 148.Sikorra S, Litschko C, Müller C, Thiel N, Galli T, Eichner T, et al. Identification and characterization of botulinum neurotoxin a substrate binding pockets and their re-engineering for human SNAP-23. J Mol Biol 2016; 428(2 Pt A): 372–384. [DOI] [PubMed] [Google Scholar]
- 149.Diaz GA, Jones SA, Scarpa M, Mengel KE, Giugliani R, Guffon N, et al. One-year results of a clinical trial of olipudase alfa enzyme replacement therapy in pediatric patients with acid sphingomyelinase deficiency. Genet Med. 2021; 23: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kushlaf H, Attarian S, Borges JL, Bouhour F, Chien Y-H, Choi Y-C, et al. Efficacy and safety results of the avalglucosidase alfa Phase 3 COMET trial in late-onset Pompe disease patients. Neurology 2021; 96(15 Suppl.): 4195. [Google Scholar]
- 151.Almo SC, Love JD. Better and faster: improvements and optimization for mammalian recombinant protein production. Curr Opin Struct Biol 2014; 26: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Böhm E, Seyfried BK, Dockal M, Graninger M, Hasslacher M, Neurath M, et al. Differences in N-glycosylation of recombinant human coagulation factor VII derived from BHK, CHO, and HEK293 cells. BMC Biotechnol 2015; 15: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Renata H, Wang ZJ, Arnold FH. Expanding the enzyme universe: accessing non-natural reactions by mechanism-guided directed evolution. Angew Chem Int Ed Engl 2015; 54(11): 3351–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen K, Arnold FH. Engineering new catalytic activities in enzymes. Nature Catalysis 2020; 3(3): 203–213. [Google Scholar]
- 155.Yang T, Ye Z, Lynch MD. ‘Multi-agent’ screening improves the efficiency of directed enzyme evolution. bioRxiv Published online April 6, 2021. 10.1101/2021.04.06.438652 [DOI] [Google Scholar]
- 156.Parker AS, Zheng W, Griswold KE, Bailey-Kellogg C. Optimization algorithms for functional deimmunization of therapeutic proteins. BMC Bioinformatics 2010; 11: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zinsli LV, Stierlin N, Loessner MJ, Schmelcher M. Deimmunization of protein therapeutics - recent advances in experimental and computational epitope prediction and deletion. Comput Struct Biotechnol J 2021; 19: 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sauna ZE, Lagassé D, Pedras-Vasconcelos J, Golding B, Rosenberg AS. Evaluating and mitigating the immunogenicity of therapeutic proteins. Trends Biotechnol 2018; 36(10): 1068–1084. [DOI] [PubMed] [Google Scholar]
- 159.Zhao H, Brooks SA, Eszterhas S, Heim S, Li L, Xiong YQ, et al. Globally deimmunized lysostaphin evades human immune surveillance and enables highly efficacious repeat dosing. Science Advances 2020; 6(36): eabb9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zaman R, Islam RA, Ibnat N, Othman I, Zaini A, Lee CY, et al. Current strategies in extending half-lives of therapeutic proteins. J Control Release 2019; 301: 176–189. [DOI] [PubMed] [Google Scholar]
- 161.Palleon Pharmaceuticals Pipeline. https://palleonpharma.com/pipeline/ [Accessed July 9, 2021].
- 162.Li Y, Cirino PC. Recent advances in engineering proteins for biocatalysis. Biotechnol Bioeng 2014; 111(7): 1273–1287. [DOI] [PubMed] [Google Scholar]
- 163.Rosenberg L, Shoham Y, Krieger Y, Rubin G, Sander F, Koller J, et al. Minimally invasive burn care: a review of seven clinical studies of rapid and selective debridement using a bromelain-based debriding enzyme (Nexobrid®). Ann Burns Fire Disasters 2015; 28(4): 264–274. [PMC free article] [PubMed] [Google Scholar]
- 164.A Study to Evaluate the Efficacy and Safety of NexoBrid in Subjects With Thermal Burns. https://clinicaltrials.gov/ct2/show/NCT02148705 [Accessed July 9, 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.