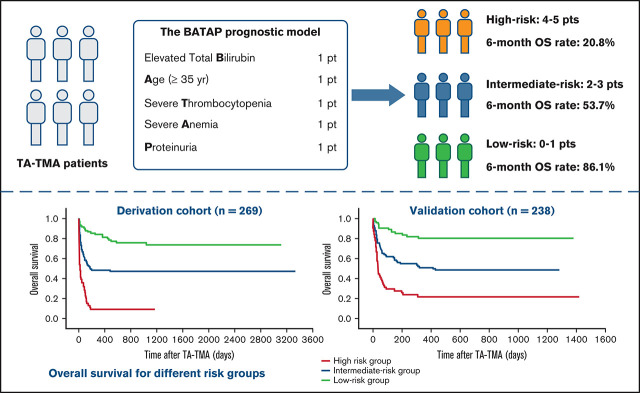
Key Points
A risk score model (BATAP) was established to facilitate the prognostic stratification of patients with TA-TMA following allo-HSCT.
The BATAP prognostic model showed robust predictive capacity through internal and external temporal validation.
Visual Abstract
Abstract
Transplant-associated thrombotic microangiopathy (TA-TMA) is a potentially life-threatening complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT). Information on markers for early prognostication remains limited, and no predictive tools for TA-TMA are available. We attempted to develop and validate a prognostic model for TA-TMA. A total of 507 patients who developed TA-TMA following allo-HSCT were retrospectively identified and separated into a derivation cohort and a validation cohort, according to the time of transplantation, to perform external temporal validation. Patient age (odds ratio [OR], 2.371; 95% confidence interval [CI], 1.264-4.445), anemia (OR, 2.836; 95% CI, 1.566-5.138), severe thrombocytopenia (OR, 3.871; 95% CI, 2.156-6.950), elevated total bilirubin (OR, 2.716; 95% CI, 1.489-4.955), and proteinuria (OR, 2.289; 95% CI, 1.257-4.168) were identified as independent prognostic factors for the 6-month outcome of TA-TMA. A risk score model termed BATAP (Bilirubin, Age, Thrombocytopenia, Anemia, Proteinuria) was constructed according to the regression coefficients. The validated c-statistic was 0.816 (95%, CI, 0.766-0.867) and 0.756 (95% CI, 0.696-0.817) for the internal and external validation, respectively. Calibration plots indicated that the model-predicted probabilities correlated well with the actual observed frequencies. This predictive model may facilitate the prognostication of TA-TMA and contribute to the early identification of high-risk patients.
Introduction
Transplant-associated thrombotic microangiopathy (TA-TMA) is a potentially life-threatening complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT) that can result in multiorgan injury and an increased risk for mortality.1-3 As part of the family of thrombotic microangiopathy (TMA) syndromes, TA-TMA is generated when endothelial injury (caused by multiple risk factors after transplantation) leads to intravascular platelet activation, generalized microthrombi formation, and microangiopathic hemolytic anemia (MAHA).3 The clinical manifestations of TA-TMA range from self-limited cytopenia to fulminant organ dysfunctions resulting in adverse survival.4 Such heterogeneity in disease severity may be ascribed to differences in recipient/donor characteristics, comorbidities, and/or concomitant disorders after transplantation.
The recognition of TA-TMA can be challenging because of the risk of tissue biopsy in allogeneic transplant recipients. Classic clinical features, including MAHA, thrombocytopenia, and organ involvement (especially renal and neurologic injury3,5), may also be initiated by drugs, infection, and graft-versus-host disease (GVHD), which further complicates the differential diagnosis.6 Several algorithms have been constructed to facilitate the diagnosis of TA-TMA based on clinical and laboratory criteria.7-10 As a consequence of the diversity in diagnostic criteria, the reported incidence (4-39% since 200411) and mortality of TA-TMA (40.2-91% since 20044,9,11-22) varied significantly among different case series.2 In a recent study, approximately half of TA-TMA patients suffered severe disease likely leading to adverse survival, which further indicated that heterogeneity in disease severity remained within the cohort using consistent diagnostic criteria.4,10 Therefore, renewed interest has emerged in the prognostic stratification of TA-TMA with the development of diagnostic algorithms.
Previously documented risk factors for poor outcome include advanced age, unrelated or haploidentical donors, elevated serum creatinine, proteinuria, and elevated serum C5b-C9 levels.3,4,13-15,23 In addition to these demographic and laboratory characteristics, clinical complications and comorbidities were also related to survival after TA-TMA. End-organ injuries, such as neurologic dysfunction,6 renal involvement,1,9,22 and significant gastrointestinal bleeding,20 were associated with an increased risk for mortality. Previous studies also reported adverse outcomes in TA-TMA patients with concomitant diffuse alveolar hemorrhage11 and acute GVHD.24 Considering the low response rate of current treatment strategies,3,6 severe cases that require immediate intensification of support need to be identified at an earlier stage, ideally as soon as the diagnosis of TMA. However, most of the clinical risk factors were not readily applicable at the initial presentation of disease. Nevertheless, statistical verification, such as external validation, might be vital for these candidate predictors. A prognostic model incorporating the patient risk factor profile may also be more precise and useful in individualized prognostic stratification, as has been demonstrated in the management of thrombotic thrombocytopenic purpura (TTP).25-29 To date, information on markers for early prognostication remains limited, and no validated predictive tool for TA-TMA is available in clinical practice.
Here, we report a prognostic model for TA-TMA following allo-HSCT derived from a large cohort study. The model performance is evaluated through internal and external temporal validation. By incorporating the patient characteristics and baseline laboratory data at the onset of TA-TMA, this predictive tool might help to identify high-risk patients and to assist with the administration of intensified therapy.
Methods
Study patients
Patients receiving allo-HSCT at Peking University People’s Hospital with a diagnosis of MAHA or evidence of microangiopathy (defined as the presence of schistocytes in a peripheral blood smear7-10 or histologic evidence of microangiopathy on tissue biopsy1,10) were retrospectively identified from 2010 to 2018. The histologic changes of microangiopathy in tissue specimens from kidney, lung, and gastrointestinal tract biopsy were reviewed according to previously published literature.1,10,30 Among these suspected cases, TA-TMA was diagnosed according to published criteria.9 Patients without fulfillment of the diagnostic criteria or complicated by other causes of MAHA were excluded from analysis. Patients with TA-TMA were separated into a derivation cohort and a validation cohort, according to the time of transplantation, to perform external temporal validation. The study protocol was approved by the central institutional review board at Peking University People’s Hospital, and informed consent was obtained from all patients.
Definition and management of TA-TMA
The diagnosis of TMA was based on published criteria9 that included (1) the presence of schistocytes in a peripheral blood smear or histologic evidence of microangiopathy on tissue biopsy, (2) de novo anemia defined as hemoglobin below the lower limit of normal or requirement of transfusion, (3) de novo, prolonged, or progressive thrombocytopenia with a platelet count < 50 000 per microliter or ≥50% decrease in the platelet count, (4) elevated lactate dehydrogenase above the institutional upper limit of normal (ULN), (5) decreased serum haptoglobin below the institutional lower limit of normal, and (6) a negative Coombs test, as well as the absence of concomitant coagulopathy (coagulation assays include prothrombin time and activated partial thromboplastin time).4,9 The time of diagnosis was determined as the date when all diagnostic criteria were fulfilled concomitantly. Management of TA-TMA included withdrawal or minimization of medication that may initiate endothelial injury, plasma infusion, and/or plasma exchange combined with corticosteroid administration, defibrotide, rituximab, and the treatment of concomitant conditions (eg, infections or GVHD).
Conditioning regimens and GVHD prophylaxis
Haploidentical stem cell transplantation is a suitable alternative for HLA-matched sibling donor transplantation.31,32 In this study, a majority of patients received allografts from HLA-partially matched related donors. These patients underwent antihuman thymocyte immunoglobulin (ATG)-based haploidentical stem cell transplantation through our previously established protocol,31-33 which features the combination of peripheral blood stem cell harvests and bone marrow harvests, ATG-based conditioning, and GVHD prophylaxis with cyclosporine A (CsA), methotrexate (MTX), and mycophenolate.34-38
In our study, most of the HLA-partially matched recipients received a myeloablative conditioning regimen consisting of busulfan (BU)/cyclophosphamide (CY) + ATG. Most of the HLA-matched recipients received the same regimen without ATG. A total body irradiation–based regimen was indicated for patients with specific status, such as subsequent hematopoietic stem cell transplantation (HSCT; with a history of prior transplantation). Most patients received CsA + mycophenolate + short-term MTX for GVHD prophylaxis, whereas a few patients were administered tacrolimus or sirolimus (mostly because of an intolerance to CsA).
Data collection
Complete blood count was monitored daily. Severe anemia was defined as hemoglobin < 70 g/L, and severe thrombocytopenia was defined as a platelet count < 15 000 per microliter (cutoff values were determined according to the optimized Youden index). Classification was based on nadir within ±3 days of TMA diagnosis to account for the potential effect of transfusions. The biochemistry panel was measured twice a week. Urinalysis was monitored once or twice per week from 1 week before transplantation to 60 days after transplantation and once every 2 weeks thereafter; it was repeated if abnormal values were observed. Proteinuria was defined as a urine protein/creatinine ratio ≥ 0.2 mg/mg or a protein concentration ≥ 30 mg/dL in random urinalysis on 2 consecutive tests.4,39,40 For prognostic analysis, baseline proteinuria was documented as positive when an abnormal test was present at least once within ±3 days of TMA diagnosis. The hematopoietic cell transplantation–specific comorbidity index (HCT-CI) was calculated, according to published criteria,41 prior to transplantation. White blood cell and platelet engraftment were defined as a neutrophil granulocyte count ≥ 500 per microliter and a platelet count ≥ 50 000 per microliter for 3 consecutive days without transfusions, respectively. Renal dysfunction was defined as the presence of acute kidney injury (AKI; elevated serum creatinine at least twofold from baseline) and/or the need for renal replacement therapy.7,22 To establish a model for early prognostication, data for the laboratory indicators were measured within ±3 days of TMA diagnosis (defined as the baseline), and the demographic or clinical variables were collected prior to or at the diagnosis of TA-TMA. Data about the immunosuppressive regimens prior to and after the diagnosis of TA-TMA were collected in the derivation cohort to explore the correlation between immunosuppressant modulation and clinical outcome.
Statistical analysis
Prognostic factors for TA-TMA were determined among patients in the derivation cohort. The outcome indicator was defined as the 6-month overall survival from the diagnosis of TA-TMA. Categorical variables were analyzed using the 2-tailed Fisher’s exact test. Continuous variables were analyzed using the nonparametric Mann-Whitney U test. Patient age, hemoglobin, platelet count, and total bilirubin (TBIL) were dichotomized for univariate and multivariate analyses; the cutoff values were determined as the thresholds with the optimal Youden index according to the receiver operating characteristic (ROC) curve. Candidate predictors (univariate P < .1) were included in the multivariate analysis using a backward stepwise logistic regression model. Variables remaining in the final model were identified as independent prognostic factors. A prognostic model was developed from the final logistic regression model according to established methods.42 A risk score was also constructed based on the regression coefficient of each variable. The performance of this predictive model was evaluated through internal validation using the bootstrap method with 1000 repetitions and external temporal validation using data from the validation cohort. Sensitivity analysis of the risk score was performed among patients undergoing different transplant protocols using the ROC curve. A multivariate logistic regression model was used to explore the correlation between immunosuppressant modulations and the 6-month overall survival of TA-TMA. Comparison of nonrelapse mortality among patients receiving different immunosuppressive regimens was conducted using the Fine-Gray competing risk model. A 2-sided P value < .05 was considered statistically significant. Data analysis was performed with SPSS 24.0 (SPSS Inc., Chicago, IL), R (R version 4.0.0), and SAS 9.4 (SAS Institute, Cary, NC) software.
Results
Study patients
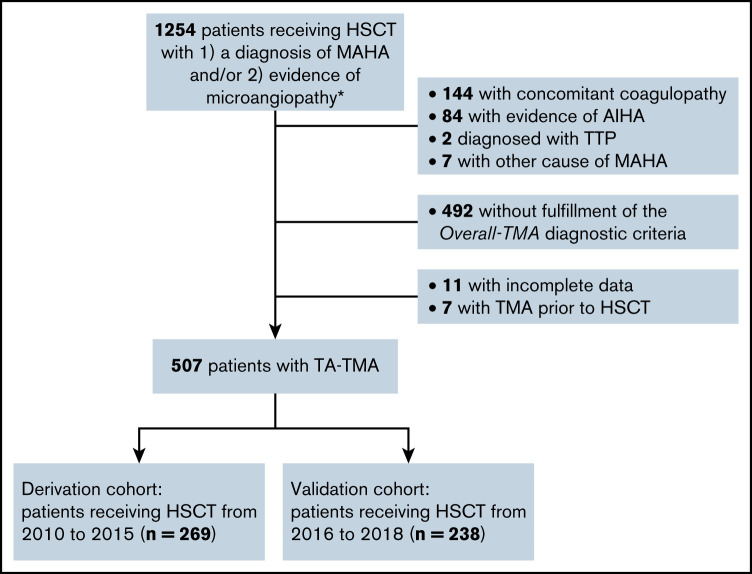
In total, 5337 patients underwent allo-HSCT at Peking University Institute of Hematology between 2010 and 2018. A total of 1254 patients with a diagnosis of MAHA and/or evidence of microangiopathy were retrospectively identified, among whom 507 patients met the inclusion criteria for this analysis (Figure 1). Patients with concomitant coagulopathy (n = 144), evidence of autoimmune hemolytic anemia (AIHA) (n = 84) or TTP (n = 2), or other specific causes of MAHA (n = 7) were excluded from the analysis. The patients receiving HSCT between 2010 and 2015 were included in the derivation cohort (n = 269), whereas those who received HSCT between 2016 and 2018 were included in the external validation cohort (n = 238). No significant difference in patient age, donor type, underlying disease, or 6-month overall survival was found between the derivation and validation cohorts (Table 1).
Figure 1.
Flowchart of study design. Patients receiving allo-HSCT with suspected TA-TMA were retrospectively identified from 2010 to 2018. The diagnosis was reviewed according to published criteria. *Evidence of microangiopathy was defined as the presence of schistocytes in a peripheral blood smear or histologic evidence of microangiopathy on tissue biopsy.
Table 1.
Clinical and laboratory characteristics among the patients with TA-TMA
| Characteristics | Derivation cohort (n = 269) | Validation cohort (n = 238) | P * |
|---|---|---|---|
| Clinical characteristics | |||
| Sex | .369 | ||
| Male | 161 (59.9) | 133 (55.9) | |
| Female | 108 (40.1) | 105 (44.1) | |
| Age at HSCT, median (range), y | 26 (3-62) | 29 (1-66) | .328 |
| Age at TA-TMA diagnosis, median (range), y | 26 (3-62) | 30 (1-67) | .334 |
| Time from HSCT to TA-TMA, median (IQR), d | 68 (42-143) | 59 (35-103) | .004 |
| Underlying disease | |||
| AML | 95 (35.3) | 91 (38.2) | .519 |
| ALL | 103 (38.3) | 74 (31.1) | .094 |
| CML | 18 (6.7) | 4 (0.2) | .007 |
| MDS/MPN | 32 (11.9) | 32 (13.4) | .688 |
| Other | 21 (7.8) | 37 (15.5) | .008 |
| HCT-CI | |||
| 0 | 196 (72.9) | 182 (76.5) | .360 |
| 1-2 | 66 (24.5) | 48 (20.2) | .244 |
| ≥3 | 7 (2.6) | 8 (3.4) | .794 |
| Donor type | |||
| Matched related | 28 (10.4) | 23 (9.7) | .883 |
| HLA-partially matched related | 239 (88.8) | 210 (88.2) | .889 |
| Matched unrelated | 2 (0.7) | 5 (2.1) | .261 |
| ABO compatibility | |||
| ABO matched | 141 (52.4) | 140 (58.8) | .153 |
| ABO mismatched | 128 (47.6) | 98 (41.2) | .153 |
| Conditioning regimen | |||
| BU/CY | 32 (11.9) | 29 (12.2) | 1.000 |
| BU/CY+ATG | 213 (79.2) | 191 (80.3) | .825 |
| TBI-based regimen | 12 (4.5) | 8 (3.4) | .649 |
| Other | 12 (4.5) | 10 (4.2) | 1.000 |
| Stem cell source | BM+PBSC | BM+PBSC | |
| GVHD prophylaxis | |||
| CsA+mycophenolate+MTX | 201 (74.7) | 202 (84.9) | .008 |
| Tacrolimus/sirolimus | 68 (25.3) | 36 (15.1) | .008 |
| Time to platelet engraftment, median (IQR), d† | 15 (12-22) | 15 (12-22) | .591 |
| Time to WBC engraftment, median (IQR), d† | 13 (11-16) | 13 (12-16) | .463 |
| Acute GVHD prior to TMA | |||
| None | 72 (26.7) | 74 (31.1) | .326 |
| I-II | 135 (50.2) | 105 (44.1) | .182 |
| III-IV | 62 (23.0) | 59 (24.8) | .677 |
| Donor lymphocyte infusion | 55 (20.4) | 38 (16.0) | .207 |
| Laboratory characteristics | |||
| Anemia (Hb < 70 g/L) | 128 (47.6) | 139 (58.4) | .016 |
| Thrombocytopenia (platelet count < 15 000/μL) | 131 (48.7) | 114 (47.9) | .859 |
| Elevated TBIL (>1.5 times ULN) | 162 (60.2) | 123 (51.7) | .060 |
| Hypoalbuminemia (<28 g/L) | 77 (28.6) | 75 (31.5) | .498 |
| Proteinuria (≥30 mg/dL) | 102 (37.9) | 101 (42.4) | .319 |
| Outcomes | |||
| 6-mo mortality rate | 125 (46.5) | 98 (41.2) | .245 |
| 1-y mortality rate | 129 (48.0) | 108 (45.4) | .593 |
Unless otherwise noted, data are n (%).
ALL, acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; BM, bone marrow; CML, chronic myeloid leukemia; Hb, hemoglobin; IQR, interquartile range; MDS, myelodysplastic syndrome; MPN, marrow proliferative neoplasm; PBSC, peripheral blood stem cell; TBI, total body irradiation; WBC, white blood cell.
Comparison between the derivation cohort and the validation cohort.
WBC and platelet engraftment: a neutrophil granulocyte count ≥500 per microliter and a platelet count ≥ 50 000 per microliter for 3 consecutive days without transfusions, respectively.
Clinical characteristics of patients with TA-TMA
The demographic and clinical characteristics of patients with TA-TMA are shown in Table 1. For the entire cohort (N = 507), the median age at the time of TA-TMA diagnosis was 28 years (interquartile range [IQR], 17-41). The median duration from the time of transplantation to the diagnosis of TA-TMA was 63 days (IQR, 38-121). Overall survival for patients with TA-TMA is shown in Figure 2. The 6-month survival rate was 56.0% (284/507), and the 1-year survival rate was 53.3% (270/507). Renal dysfunction was observed in 16.4% (83/507) of patients at the initial presentation of TA-TMA, 24.5% (124/507) of patients within 30 days of the diagnosis of TMA, and 29.6% (150/507) of patients within 3 months of TMA diagnosis. Patients with concomitant renal involvement at the diagnosis of TA-TMA exhibited significantly worse overall survival compared with those without renal dysfunction (P < .0001; supplemental Figure 1).
Figure 2.
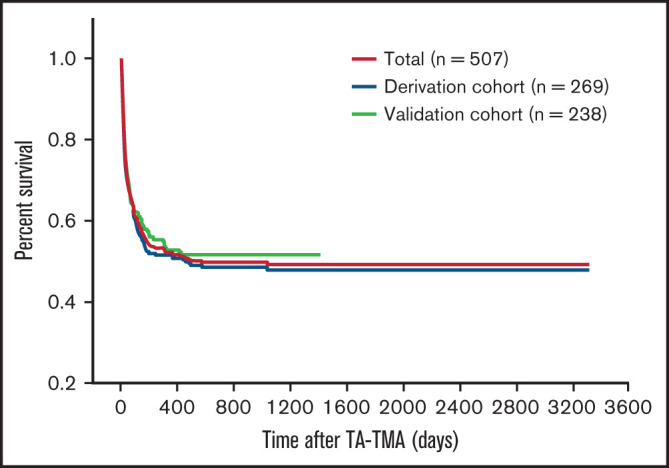
Survival analysis for patients with TA-TMA. No significant difference was observed in overall survival between the derivation cohort and the validation cohort.
Prognostic factors for patients with TA-TMA
In the derivation cohort, univariate analysis revealed that advanced age (≥35 years, 32%, P = .001), severe anemia (hemoglobin < 70 g/L, 47.6%, P < .0001), severe thrombocytopenia (platelet count < 15 000 per microliter, 48.7%, P < .0001), hypoalbuminemia (<28 g/L, 28.6%, P < .0001), elevated TBIL (>1.5 times ULN, 60.2%, P = .006), and proteinuria (37.5%, P < .0001) were associated with adverse prognosis (Table 1; supplemental Table 1). On multivariate analysis, advanced age (P = .007; odds ratio [OR], 2.371; 95% confidence interval [CI], 1.264-4.445), severe anemia (P = .001; OR, 2.836; 95% CI, 1.566-5.138), severe thrombocytopenia (P < .0001; OR, 3.871; 95% CI, 2.156-6.950), elevated TBIL (P = .001; OR, 2.716; 95% CI, 1.489-4.955), and proteinuria (P = .007; OR, 2.289; 95% CI, 1.257-4.168) were identified as independent prognostic factors for the 6-month outcome associated with TA-TMA (Table 2). Kaplan-Meier estimations of overall survival showed significant differences among patients with and without these indicators (supplemental Figure 2).
Table 2.
The BATAP risk score model according to multivariate analysis in the derivation cohort
| Variables | β | P | OR (95% CI) | Points |
|---|---|---|---|---|
| Age ≥35 y | 0.863 | .007 | 2.371 (1.264-4.445) | 1 |
| Hemoglobin <70 g/L | 1.043 | .001 | 2.836 (1.566-5.138) | 1 |
| Platelet count <15 000/μL | 1.353 | <.0001 | 3.871 (2.156-6.950) | 1 |
| TBIL >1.5 times ULN | 0.999 | .001 | 2.716 (1.489-4.955) | 1 |
| Proteinuria | 0.828 | .007 | 2.289 (1.257-4.168) | 1 |
The laboratory indicators were measured within ±3 d of the diagnosis of TA-TMA.
Prognostic model and risk stratification for patients with TA-TMA
A prognostic model termed BATAP (Bilirubin, Age, Thrombocytopenia, Anemia, Proteinuria) was developed from the multivariate logistic model. The equation for the BATAP prognostic model is as follows:
where
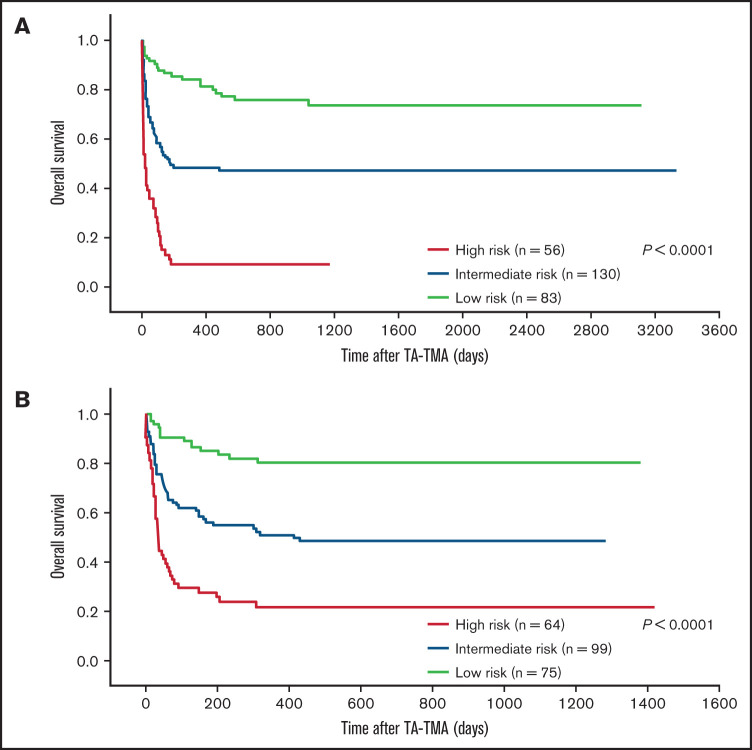
A risk heat map for the BATAP model is provided to visualize the risk estimates derived from the individual prognostic factor profile (Figure 3). To develop a simplified predictive tool for clinical practice, a BATAP risk score system was constructed according to the regression coefficients (1 point each for variable remaining in the final model) (Table 2). The distribution and prediction estimates for the risk score are shown in Figure 4 and supplemental Table 2. The lowest score (0 points) indicates a 7.4% risk, whereas the highest risk score (5 points) indicates an 83.3% risk for 6-month mortality. Patients were further separated into a low-risk group (BATAP risk score 0-1 points), an intermediate-risk group (2-3 points), and a high-risk group (4-5 points). Among these risk groups, significant differences were observed in the survival rates within 6 months of diagnosis (Table 3), as well as in the Kaplan-Meier estimations of overall survival (P < .001 for pairwise comparisons, log-rank test; Figure 5).
Figure 3.
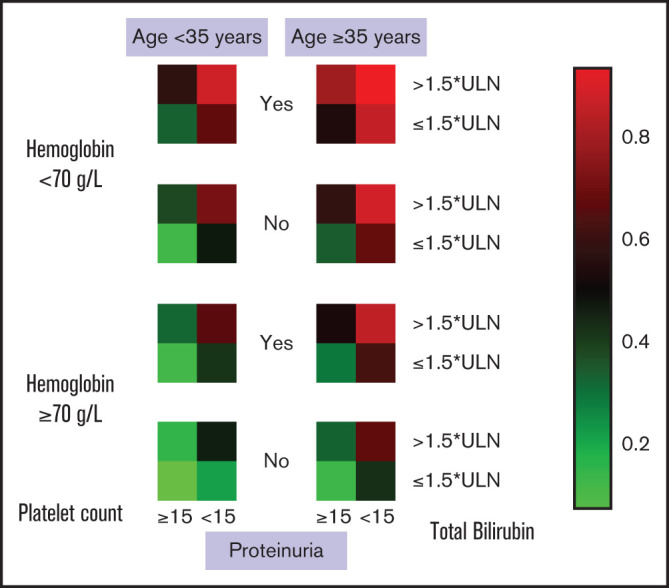
Individualized risk prediction for 6-month mortality in patients with TA-TMA. Heat map based on the BATAP prognostic model. Patient profile include 5 independent prognostic factors: age, hemoglobin, platelet count, TBIL, and proteinuria.
Figure 4.
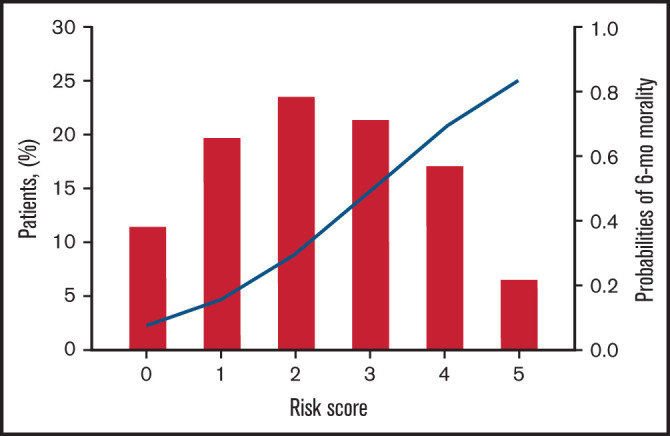
Distribution of BATAP risk score and corresponding risk estimates for 6-month mortality in patients with TA-TMA (N = 507). Blue line denotes the estimated 6-month mortality for each score.
Table 3.
Observed survival rates for patients with TA-TMA among different risk groups
| Risk group | Risk score | n (%) | 6-mo survival/death, n/n | 6-mo survival rate, % |
|---|---|---|---|---|
| Total cohort (N = 507) | ||||
| Low risk | 0-1 | 158 (31.1) | 136/22 | 86.1 |
| Intermediate risk | 2-3 | 229 (45.2) | 123/106 | 53.7 |
| High risk | 4-5 | 120 (23.7) | 25/95 | 20.8 |
| Derivation cohort (n = 269) | ||||
| Low risk | 0-1 | 83 (30.9) | 72/11 | 86.7 |
| Intermediate risk | 2-3 | 130 (48.3) | 66/64 | 50.8 |
| High risk | 4-5 | 56 (20.8) | 6/50 | 10.7 |
| Validation cohort (n = 238) | ||||
| Low risk | 0-1 | 75 (31.5) | 64/11 | 85.3 |
| Intermediate risk | 2-3 | 99 (41.6) | 57/42 | 57.6 |
| High risk | 4-5 | 64 (26.9) | 19/45 | 29.7 |
Figure 5.
Survival outcome according to the risk groups. Kaplan-Meier analysis among different risk groups in the derivation (A) and validation (B) cohorts. Significant differences in overall survival were observed between the risk groups (P < .0001, log-rank test, for each cohort).
Model performance
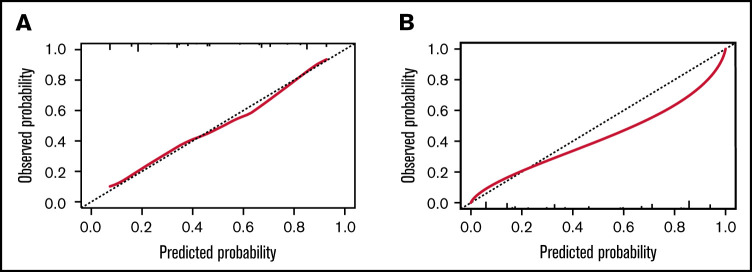
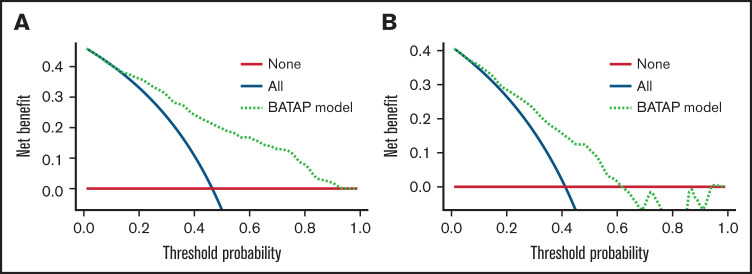
The BATAP prognostic model showed significant discriminatory capacity. The c-statistic was 0.816 (95% CI, 0.766-0.867) in the internal validation cohort using the bootstrap method, and it was 0.756 (95% CI, 0.696-0.817) in the external temporal validation cohort. ROC curves for the risk score are shown in supplemental Figure 3. The calibration plots also indicated an optimal agreement between the model-predicted and actual observed probabilities (Figure 6). To further evaluate the utility of the BATAP model in clinical practice, decision curve analysis was performed to assess the net benefit for patients with TA-TMA (Figure 7). A positive net benefit for a wide range of probability thresholds was observed in the internal validation and external validation cohorts.
Figure 6.
Calibration plots for the BATAP prognostic model with regard to the prediction of 6-month outcome. (A) The BATAP model in the derivation cohort. (B) The BATAP model in the validation cohort. The dashed diagonal line represents an ideal calibration plot.
Figure 7.
Decision curve analysis of the BATAP prognostic model. Decision curve analysis for the model in the derivation (A) and validation (B) cohorts. The black line assumes no patient died. The gray line assumes that all patients died. These 2 lines serve as references.
Discussion
A systematic review in 2004 summarized 35 published case series of TA-TMA using 20 diagnostic criteria; the reported incidence ranged from 0.5% to 63.6%, and the fatality ranged from 0% to 100%.43 Clinical studies since 2004 reported an incidence of 4% to 39%,4,11,13,16,18,19,21 with the overall survival rate ranging from 9% to 59.8%.4,6,9,11-22 In addition to the differences in study design (eg, retrospective or prospective nature), study population, and time of follow-up, the lack of uniformly accepted diagnostic criteria may also have contributed to this variability.2,3,9,44 In this retrospective cohort study, 9.5% (507/5337) of the allogeneic transplant recipients developed TA-TMA, which was consistent with previous studies using similar diagnostic algorithms.6,9,11,18,19 The 6-month overall survival rate for patients with TA-TMA was 56% (284/507), which was also consistent with previous reports (28.8-59.8% in cohort studies providing data for the 6-month outcome).16,18,21-23
A majority of our study patients underwent ATG-based unmanipulated stem cell transplantation using our established protocol,34 which has been reviewed and is increasingly used worldwide.31-33,35,36 Notably, the transplant protocols may be associated with the risk and outcome of TA-TMA. HLA disparity, conditioning regimens, and calcineurin inhibitor (CNI) exposure were well documented as potential risk factors,9,22,45 whereas recent studies report divergent evidence with regard to the prognostic role of donor type,9,21,45 conditioning, and immunosuppressive regimens3 in patients with TA-TMA. The consistency in observed incidence and outcomes suggests a likely similar TA-TMA profile among our study patients and previously reported cohorts. To further explore the stability and reliability of the BATAP risk score, a sensitivity analysis was performed; similar discriminative capacities were shown between patients undergoing different transplant protocols (supplemental Figure 4).
TA-TMA has been associated with significantly worse overall survival and nonrelapse mortality among allogeneic transplant recipients.10 Previous studies identified TA-TMA as an independent prognostic factor for 6-month overall survival among the posttransplant population.21,22 However, the high mortality rate may be multifactorial. In this regard, reported causes of death can include complications directly subsequent to TMA (eg, renal injury or neurologic dysfunction), as well as concomitant disorders (eg, severe GVHD or infections).11,17,18 Several studies have revealed the heterogeneity in disease severity within the TA-TMA population, which may be affected by organ involvement,6,9,23 concomitant morbidities,11 or other clinical factors. Considering the poor prognosis for severe cases and the low rate of response to current therapy, early-stage risk stratification at the time of diagnosis of TA-TMA may be of significant clinical relevance.
This study identified advanced age, severe anemia, severe thrombocytopenia, elevated TBIL, and proteinuria as independent prognostic factors for the 6-month outcome of TA-TMA. Among these predictors, patient age,15 anemia,4 and proteinuria4 were previously reported to be related to the overall survival of patients with TA-TMA, although different prognostic thresholds were proposed. Patients who died after TA-TMA presented with more severe anemia and more frequent proteinuria.4 In our study, the nadir hemoglobin concentrations within ±3 days of TMA diagnosis, rather than the value at the time of diagnosis, were collected for analysis to minimize the effect of transfusions; a nadir hemoglobin <7 g/dL was associated with adverse outcomes. Albuminuria was reported to be an indicator for renal dysfunction and a predictor for increased mortality following HSCT.1,46 A more recent study revealed that proteinuria in the TA-TMA population significantly affected overall survival and usually occurred prior to the diagnosis of TMA.4 Our findings verified the potential for proteinuria as an early-stage predictor of worse overall survival. The need for platelet transfusion was previously associated with worse prognosis in TA-TMA patients.17 Our findings suggest an optimal prognostic threshold for severe thrombocytopenia (platelet count <15 000 per microliter) to predict survival in TA-TMA. TBIL >1.5 times ULN, an indicator for moderate/severe hepatic morbidity in the HCT-CI risk score,41 was included in our model as an independent prognostic factor for TA-TMA. Elevated TBIL may imply the severity of hemolysis, as well as the degree of hepatic dysfunction, which may partially explain its prognostic role in patients with TMA.
Several prognostic models have been established to predict the clinical outcome, disease severity, or treatment response in patients with TTP.25-29 In this study, we developed and validated a prognostic model for TA-TMA based on patient risk factor profiles that showed good performance with regard to discrimination and calibration through internal and temporal external validation. According to the established risk score, patients with TA-TMA were divided into a low-risk group and a high-risk group. The observed survival rate correlated well within these risk groups, indicating its potential utility in prognostic stratification.
To the best of our knowledge, this is the first attempt to develop a prognostic model for TA-TMA in allo-HSCT recipients. The predictive model was derived from a large high-quality cohort with a consistent definition of TA-TMA, and the model performance was verified through internal validation and external temporal validation. Candidate predictors were clinically relevant and readily available by routine examination, which facilitates the application and generalizability of this model in clinical practice. Variables in the final model were all measured at baseline (within ±3 days of the TA-TMA diagnosis) to enable prognostic stratification at an early stage and assist with immediate intensive care for high-risk patients.
Our study has several potential limitations. First, the diversity in diagnosis algorithms for TA-TMA leads to limited transferability among different case series. In this study, TA-TMA is defined according to the criteria proposed by Cho et al,9 which have been well established and used widely in retrospective and prospective studies. Whether the present model is generalizable to populations using different diagnosis criteria requires further examination. Second, geographical validation was unavailable because of the single-center nature of our study. Although temporal validation indicated robust discriminative power, as well as good performance in the calibration plots, geographical validation of the model in other TA-TMA populations may be important to further assess its utility in clinical practice. Complement dysregulation was recently reported to have a significant role in the pathogenesis of TA-TMA. Elevated serum C5b-C9 levels at the diagnosis of TA-TMA were associated with adverse overall survival,4 and complement-targeting therapy, such as eculizumab, might be beneficial for patients with this risk factor.10,47-49 However, data for serum C5b-C9 levels or other indicators of complement activation were not available in this retrospective study.
Our findings indicate the feasibility of predicting the outcome of TA-TMA using routinely available clinical and laboratory characteristics. Significant heterogeneity was observed in clinical manifestations and overall survival among the patients fulfilling laboratory diagnostic criteria for TA-TMA, and the baseline risk factor profile may assist with early-stage prognostication. A formula was provided to estimate the risk of 6-month mortality through the BATAP prognostic model, and the risk estimate has been visualized as a heat map for convenient clinical use. We also developed the BATAP risk score as a simplified predictive tool and separated the study patients into 3 risk groups. Patients in the intermediate-risk group (2-3 points) showed similar 6-month overall survival (53.7%) compared with previously reported cohorts of TA-TMA,16,18,21-23 whereas the low-risk group (0-1 points) and high-risk group (4-5 points) showed significantly favorable and adverse outcomes, respectively. Therefore, patients in the high-risk group (those fulfilling ≥4 prognostic predictors) may require special concern and management.
Management of severe or refractory cases of TA-TMA has been challenging.1 Although exposure to CNIs is well documented as a risk factor for TA-TMA, whether CNI withdrawal can improve the clinical outcome remains controversial.11,13 GVHD flares subsequent to changes in immunosuppressive regimens may also lead to an increased risk for mortality.3 In our study, subanalyses were conducted among patients in the derivation cohort (n = 269) to explore the correlation between immunosuppressant modulation and clinical outcome. A total of 145 (53.9%) patients underwent withdrawal from (n = 86; 32.0%) or a reduction in (n = 59; 21.9%) CNIs after the diagnosis of TA-TMA (supplemental Table 3). Multivariate analysis and subgroup analysis stratified by the BATAP risk groups did not show significant differences in the 6-month overall survival (supplemental Figure 5) or nonrelapse mortality (supplemental Figure 6) between patients receiving different immunosuppressive regimens after TA-TMA. Other therapeutic options include the management of concomitant disorders (eg, infections or GVHD), plasma exchange, rituximab, and defibrotide. Therapeutic plasma exchange (TPE) in TA-TMA was not as beneficial as in TTP, with reported response rates ranging from 27% to 80% and mortality rates of 44% to 100%.1,43 Nevertheless, previous studies suggested that early initiation of therapeutic plasma exchange may be beneficial, especially for patients with documented factor H autoantibodies.50 Therefore, early intensification of supportive care and confining specific therapy to particular risk groups may improve their performance with regard to response rate and outcome. By facilitating early risk stratification among patients with TA-TMA, our prognostic model may assist with decision-making with regard to intensive management, as well as the timing of intervention. Notably, prompt and appropriate screening for TA-TMA, including the required clinical and laboratory markers among those with suspected manifestations, is of great clinical relevance for TMA diagnosis and, thereby, is fundamental for the early risk stratification and subsequent management.
In addition to overall survival, renal dysfunction (including AKI and chronic kidney disease) is a frequently used outcome indicator in TA-TMA.21,22 AKI is a well-documented prognostic risk factor among allogeneic transplant recipients.2,9,22 TA-TMA was also frequently associated with chronic kidney disease, another predictor for worse survival among patients receiving HSCT.1,18 Further investigation may focus on the frequency, risk factors, and outcomes of end-organ involvement, such as renal dysfunction, neurologic dysfunction, and gastrointestinal bleeding, because these complications may lead to worse survival and impaired quality of life in the long-term follow-up after transplantation.
Conclusions
In this study, patient age, severe anemia, severe thrombocytopenia, elevated TBIL, and proteinuria were identified as independent prognostic factors for the 6-month outcome of TA-TMA. A prognostic model (BATAP) for TA-TMA following allo-HSCT was developed and evaluated; it demonstrated robust predictive capacity. This predictive model might facilitate prognostication of TA-TMA and contribute to early identification of patients at higher risk for adverse outcomes. Further studies may focus on whether these high-risk patients could benefit from early administration of intensified care or other specific management.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and medical staff who participated in this study, and the department of medical record library for providing medical records.
This work was supported by the National Key Research and Development Program of China (2017YFA0105500 and 2017YFA0105503), the National Natural Science Foundation of China (81970113), the Key Program of the National Natural Science Foundation of China (81730004), the Beijing Natural Science Foundation (H2018206423), and the Beijing Municipal Science and Technology Commission (Z171100001017084).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: P.Z., Y.-j.W., and Y.H. analyzed data and wrote the manuscript; P.Z. and Y.-j.W. performed statistical analysis; X.-h.Z. designed the study, analyzed the data, and edited the manuscript; and the remaining authors collected data and helped to write and edit the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Hui Zhang, No. 11 Xizhimen South St, Xicheng District, Beijing 100044, China; e-mail: zhangxh@bjmu.edu.cn.
References
- 1.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118(6):1452-1462. [DOI] [PubMed] [Google Scholar]
- 2.Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA. Transplant-associated thrombotic microangiopathy: opening Pandora’s box. Bone Marrow Transplant. 2017;52(10):1355-1360. [DOI] [PubMed] [Google Scholar]
- 3.Khosla J, Yeh AC, Spitzer TR, Dey BR. Hematopoietic stem cell transplant-associated thrombotic microangiopathy: current paradigm and novel therapies. Bone Marrow Transplant. 2018;53(2):129-137. [DOI] [PubMed] [Google Scholar]
- 4.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(7):654-666. [DOI] [PubMed] [Google Scholar]
- 6.Gavriilaki E, Sakellari I, Batsis I, et al. Transplant-associated thrombotic microangiopathy: incidence, prognostic factors, morbidity, and mortality in allogeneic hematopoietic cell transplantation. Clin Transplant. 2018;32(9):e13371. [DOI] [PubMed] [Google Scholar]
- 7.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571-575. [DOI] [PubMed] [Google Scholar]
- 8.Ruutu T, Barosi G, Benjamin RJ, et al. ; European LeukemiaNet . Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92(1):95-100. [DOI] [PubMed] [Google Scholar]
- 9.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918-926. [DOI] [PubMed] [Google Scholar]
- 10.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li A, Wu Q, Davis C, et al. Transplant-associated thrombotic microangiopathy is a multifactorial disease unresponsive to immunosuppressant withdrawal. Biol Blood Marrow Transplant. 2019;25(3):570-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimoni A, Yeshurun M, Hardan I, Avigdor A, Ben-Bassat I, Nagler A. Thrombotic microangiopathy after allogeneic stem cell transplantation in the era of reduced-intensity conditioning: the incidence is not reduced. Biol Blood Marrow Transplant. 2004;10(7):484-493. [DOI] [PubMed] [Google Scholar]
- 13.Cutler C, Henry NL, Magee C, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):551-557. [DOI] [PubMed] [Google Scholar]
- 14.Martinez MT, Bucher C, Stussi G, et al. Transplant-associated microangiopathy (TAM) in recipients of allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. 2005;36(11):993-1000. [DOI] [PubMed] [Google Scholar]
- 15.Uderzo C, Bonanomi S, Busca A, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82(5):638-644. [DOI] [PubMed] [Google Scholar]
- 16.Oran B, Donato M, Aleman A, et al. Transplant-associated microangiopathy in patients receiving tacrolimus following allogeneic stem cell transplantation: risk factors and response to treatment. Biol Blood Marrow Transplant. 2007;13(4):469-477. [DOI] [PubMed] [Google Scholar]
- 17.Willems E, Baron F, Seidel L, Frère P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45(4):689-693. [DOI] [PubMed] [Google Scholar]
- 18.Labrador J, López-Corral L, López-Godino O, et al. Risk factors for thrombotic microangiopathy in allogeneic hematopoietic stem cell recipients receiving GVHD prophylaxis with tacrolimus plus MTX or sirolimus. Bone Marrow Transplant. 2014;49(5):684-690. [DOI] [PubMed] [Google Scholar]
- 19.Sakellari I, Gavriilaki E, Boussiou Z, et al. Transplant-associated thrombotic microangiopathy: an unresolved complication of unrelated allogeneic transplant for hematologic diseases. Hematol Oncol. 2017;35(4):932-934. [DOI] [PubMed] [Google Scholar]
- 20.Ye Y, Zheng W, Wang J, et al. Risk and prognostic factors of transplantation-associated thrombotic microangiopathy in allogeneic haematopoietic stem cell transplantation: a nested case control study. Hematol Oncol. 2017;35(4):821-827. [DOI] [PubMed] [Google Scholar]
- 21.Postalcioglu M, Kim HT, Obut F, et al. Impact of thrombotic microangiopathy on renal outcomes and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24(11):2344-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epperla N, Li A, Logan B, et al. Incidence, risk factors for and outcomes of transplant-associated thrombotic microangiopathy. Br J Haematol. 2020;189(6):1171-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruutu T, Hermans J, Niederwieser D, et al. ; EBMT Chronic Leukaemia Working Party . Thrombotic thrombocytopenic purpura after allogeneic stem cell transplantation: a survey of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2002;118(4):1112-1119. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XH, Liu X, Wang QM, et al. Thrombotic microangiopathy with concomitant GI aGVHD after allogeneic hematopoietic stem cell transplantation: risk factors and outcome. Eur J Haematol. 2018;100(2):171-181. [DOI] [PubMed] [Google Scholar]
- 25.Benhamou Y, Assié C, Boelle PY, et al. ; Thrombotic Microangiopathies Reference Center . Development and validation of a predictive model for death in acquired severe ADAMTS13 deficiency-associated idiopathic thrombotic thrombocytopenic purpura: the French TMA Reference Center experience. Haematologica. 2012;97(8):1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4(4):e157-e164. [DOI] [PubMed] [Google Scholar]
- 27.Falter T, Herold S, Weyer-Elberich V, et al. Relapse rate in survivors of acute autoimmune thrombotic thrombocytopenic purpura treated with or without rituximab. Thromb Haemost. 2018;118(10):1743-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li A, Khalighi PR, Wu Q, Garcia DA. External validation of the PLASMIC score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. J Thromb Haemost. 2018;16(1):164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gui RY, Huang QS, Cai X, et al. Development and validation of a prediction model (AHC) for early identification of refractory thrombotic thrombocytopenic purpura using nationally representative data. Br J Haematol. 2020;191(2):269-281. [DOI] [PubMed] [Google Scholar]
- 30.Siami K, Kojouri K, Swisher KK, Selby GB, George JN, Laszik ZG. Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: an autopsy study. Transplantation. 2008;85(1):22-28. [DOI] [PubMed] [Google Scholar]
- 31.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation [published correction appears in Nat Rev Clin Oncol. 2016;13(2):132]. Nat Rev Clin Oncol. 2016;13(1):10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun YQ, Chang YJ, Huang XJ. Update on current research into haploidentical hematopoietic stem cell transplantation. Expert Rev Hematol. 2018;11(4):273-284. [DOI] [PubMed] [Google Scholar]
- 33.Apperley J, Niederwieser D, Huang XJ, et al. Haploidentical hematopoietic stem cell transplantation: a global overview comparing Asia, the European Union, and the United States. Biol Blood Marrow Transplant. 2016;22(1):23-26. [DOI] [PubMed] [Google Scholar]
- 34.Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies [published correction appears in Bone Marrow Transplant. 2008;42(4):295]. Bone Marrow Transplant. 2006;38(4):291-297. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Chang YJ, Xu LP, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124(6):843-850. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956-3962. [DOI] [PubMed] [Google Scholar]
- 37.Lai YR, Chen YH, Hu DM, et al. Multicenter phase II study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG). J Hematol Oncol. 2014;7(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang YJ, Wang Y, Mo XD, et al. Optimal dose of rabbit thymoglobulin in conditioning regimens for unmanipulated, haploidentical, hematopoietic stem cell transplantation: long-term outcomes of a prospective randomized trial. Cancer. 2017;123(15):2881-2892. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Coresh J, Balk E, et al. ; National Kidney Foundation . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137-147. [DOI] [PubMed] [Google Scholar]
- 40.Kraft S, Bollinger N, Bodenmann B, et al. High mortality in hematopoietic stem cell transplant-associated thrombotic microangiopathy with and without concomitant acute graft-versus-host disease. Bone Marrow Transplant. 2019;54(4):540-548. [DOI] [PubMed] [Google Scholar]
- 41.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631-1660. [DOI] [PubMed] [Google Scholar]
- 43.George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion. 2004;44(2):294-304. [DOI] [PubMed] [Google Scholar]
- 44.Moiseev IS, Tsvetkova T, Aljurf M, et al. Clinical and morphological practices in the diagnosis of transplant-associated microangiopathy: a study on behalf of Transplant Complications Working Party of the EBMT. Bone Marrow Transplant. 2019;54(7):1022-1028. [DOI] [PubMed] [Google Scholar]
- 45.Batts ED, Lazarus HM. Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? Bone Marrow Transplant. 2007;40(8):709-719. [DOI] [PubMed] [Google Scholar]
- 46.Hingorani SR, Seidel K, Lindner A, Aneja T, Schoch G, McDonald G. Albuminuria in hematopoietic cell transplantation patients: prevalence, clinical associations, and impact on survival. Biol Blood Marrow Transplant. 2008;14(12):1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jodele S, Fukuda T, Vinks A, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasu S, Wu H, Satoskar A, et al. Eculizumab therapy in adults with allogeneic hematopoietic cell transplant-associated thrombotic microangiopathy. Bone Marrow Transplant. 2016;51(9):1241-1244. [DOI] [PubMed] [Google Scholar]
- 49.Masias C, Vasu S, Cataland SR. None of the above: thrombotic microangiopathy beyond TTP and HUS. Blood. 2017;129(21):2857-2863. [DOI] [PubMed] [Google Scholar]
- 50.Jodele S, Laskin BL, Goebel J, et al. Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy? Transfusion. 2013;53(3):661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.