Key Points
Adult ES is a rare, often severe, and potentially fatal condition.
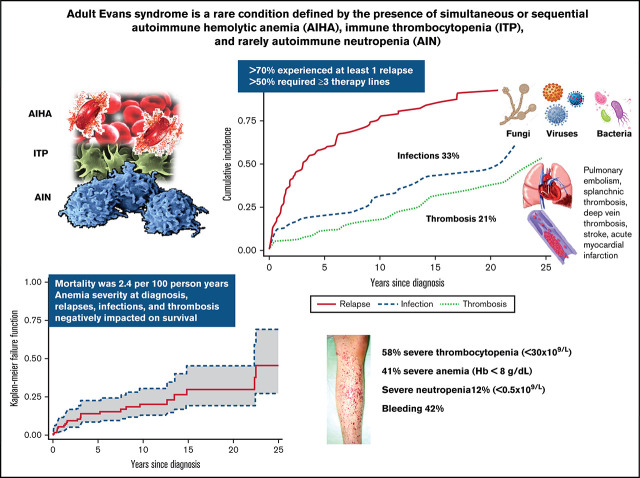
ES is marked by frequent relapses, high therapy burden, and increased risk of infection/thrombosis, significantly affecting survival.
Visual Abstract
Abstract
Evans syndrome (ES) is a rare condition, defined as the presence of 2 autoimmune cytopenias, most frequently autoimmune hemolytic anemia and immune thrombocytopenia (ITP) and rarely autoimmune neutropenia. ES can be classified as primary or secondary to various conditions, including lymphoproliferative disorders, other systemic autoimmune diseases, and primary immunodeficiencies, particularly in children. In adult ES, little is known about clinical features, disease associations, and outcomes. In this retrospective international study, we analyzed 116 adult patients followed at 13 European tertiary centers, focusing on treatment requirements, occurrence of complications, and death. ES was secondary to or associated with underlying conditions in 24 cases (21%), mainly other autoimmune diseases and hematologic neoplasms. Bleeding occurred in 42% of patients, mainly low grade and at ITP onset. Almost all patients received first-line treatment (steroids with or without intravenous immunoglobulin), and 23% needed early additional therapy for primary refractoriness. Additional therapy lines included rituximab, splenectomy, immunosuppressants, thrombopoietin receptor agonists, and others, with response rates >80%. However, a remarkable number of relapses occurred, requiring ≥3 therapy lines in 54% of cases. Infections and thrombotic complications occurred in 33% and 21% of patients, respectively, mainly grade ≥3, and correlated with the number of therapy lines. In addition to age, other factors negatively affecting survival were severe anemia at onset and occurrence of relapse, infection, and thrombosis. These data show that adult ES is often severe and marked by a relapsing clinical course and potentially fatal complications, pinpointing the need for high clinical awareness, prompt therapy, and anti-infectious/anti-thrombotic prophylaxis.
Introduction
Evans syndrome (ES) is a rare condition characterized by the association of multiple autoimmune cytopenias (AICs), usually autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP) and rarely autoimmune neutropenia (AIN). In 1951, Evans et al1 described the presence of thrombocytopenia in patients with AIHA as well as the occurrence of red blood cell sensitization with or without overt hemolysis in patients with ITP. They proposed a spectrum-like relationship between the 2 diseases, likely resulting from autoantibodies, the production of which could be decreased by splenectomy. Since then, the syndrome has been further characterized, with an estimated incidence of 1 to 9 cases per million people per year, with up to 50% of ES cases being secondary to a variety of conditions, including infections, primary immunodeficiencies (PIDs; particularly in children), systemic autoimmune diseases (eg, systemic lupus erythematosus and rheumatoid arthritis), lymphoproliferative syndromes, and hematopoietic stem cell transplantation.2,3 Recent prospective studies highlighted the clinical features, including disease associations and outcomes, in pediatrics4-6; however, less is known about adult patients, with the largest series of 68 cases dating back to 2008.7 In this collaborative international study, we retrospectively analyzed 116 patients with ES focusing on baseline characteristics, treatment requirements, occurrence of complications, and death. We show that ES in adults is marked by many relapses, severe complications, and high mortality, related to the severity of anemia at presentation and thrombotic and infectious complications.
Patients and methods
Patients and diagnosis
In this observational and retrospective multicenter study, we investigated 116 adult patients diagnosed with ES followed at 13 European tertiary centers. ITP, AIHA, and, to a lesser extent, AIN were diagnosed according to current guidelines.8-10 If ITP, AIHA, and AIN were not diagnosed concurrently, the diagnosis of the second AIC also required the exclusion of the effects of recent myelosuppressive therapies used for the initial cytopenia. AIHA was classified according to the positivity of the direct antiglobulin test (DAT) as warm (either immunoglobulin G positive [IgG+] or IgG+ plus complement C at low titer), cold (C+), mixed (IgG plus complement+ autoagglutination at room temperature and high-titer cold agglutinins), or yet atypical (DAT−, IgA+). Immunohematologic assessment was performed at ES diagnosis. Anemia severity was categorized according to previous scores for AIHA11 as moderate (hemoglobin [Hb] 8-10 g/dL), severe (Hb 6-8 g/dL), or very severe (Hb <6 g/dL). ITP was categorized as severe (platelets [PLTs] <30 × 109/L) or moderate (PLTs 30-100 × 109/L). AIN was classified as severe (absolute neutrophil count [ANC] <0.5 × 109/L), moderate (ANC 0.5-1 × 109/L), or mild (ANC >1 × 109/L). The positivity of anti-PLT autoantibodies and anti-neutrophil autoantibodies was registered when available. The study was conducted according to the Declaration of Helsinki and approved by the local ethics committee (Comitato Etico di Milano Area 2).
Clinical and hematologic parameters and outcomes
Hematologic data, associated conditions at diagnosis, and relative investigations were retrospectively collected. Where available, bone marrow features, imaging data (ultrasounds and computed tomography [CT] scans), levels of total IgG, IgA, and IgM, lymphocyte subpopulations, data on positivity of antinuclear antibodies, extractable nuclear antigens, anti-DNA autoantibodies, antiphospholipid autoantibodies (lupus anticoagulant [LAC], anticardiolipin autoantibodies, and anti–β2 glycoprotein 1) were registered. Collected data were tested at the time of ES diagnosis. Genetic testing results were collected when available. A subgroup of patients was studied with a next-generation sequencing (NGS) panel specifically designed to assess somatic mutations in genes commonly associated with myeloid neoplasms. NGS study was performed by next-generation sequence technology (Ion Torrent S5; Ion Reporters software [version 5.2]) that evaluates mutational status of 69 potentially oncogenic genes present in the Oncomine Myeloid Research Assay diagnostic panel, including hotspot genes (ABL1, BRAF, CBL, CSFR3, DNMT3A, FLT3, GATA2, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MOL, MYD88, NPM1, NRAS, PTPN11, SETPB1, SF3B1, SRSF2, U2AF1, and WT1) full genes (ASXL1, BCOR, CALR, CEBPA, ETV6, EZH2, IKZF1, NF1, PHF6, PRPF8, RB1, RUNX1, SH2B3, STAG2, TET2, TP53, and ZRSR2), and fusion transcripts (ABL1, ALK, BCL2, BRAF, CCDN1, CREBBP, EGFR, ETV6, FGFR1, FGFR2, FUS, HMGA2, JAK2, KMT2A, MECOM, MET, MLLT10, MLLT3, MYBL1, MYH11, NTRK3, NUP214, PDGFRA, PDGFRB, RARA, RBM15, RUNX1, TCF3, and TFE3). Only variants with an allelic frequency >5% were reported. A further subgroup of patients was studied with an NGS panel specifically designed to reveal mutations in genes commonly associated with PIDs, including TNFRSF6, CTLA4, STAT3, PIK3CD, CBL, ADAR1, LRBA, RAG1, and KRAS.4
The treatments administered were collected, including steroids, intravenous immunoglobulins (IVIGs), rituximab, splenectomy, cytotoxic immunosuppression (immunosuppressive therapy, including azathioprine, cyclosporin A, mycophenolate mofetil, and cyclophosphamide), thrombopoietin receptor agonists (TPO-RAs) eltrombopag and romiplostim, recombinant erythropoietin, and plasma exchange (PEX). Treatment outcome was evaluated as overall response, described as PLTs >30 × 109/L with at least a doubling of the baseline count in ITP and Hb >10 g/dL without transfusion and with reduction of markers of hemolysis in AIHA.11 Relapses of any cytopenia (ITP, AIHA, and/or AIN), either alone or combined, were registered and considered in the analysis; relapse-free survival after first ES therapy calculated. Infectious, thrombotic, and bleeding complications were collected and graded according to the Common Terminology Criteria for Adverse Events (version 5).12 Occurrence of death and relative causes were registered for all patients, and overall survival (OS) from ES diagnosis was estimated.
Statistical analysis
Kruskal-Wallis and χ2 tests were used for comparison of quantitative and categorical variables, respectively. We performed 3 types of survival/incidence analyses after truncating follow-up time (time since diagnosis) at 25 years. Firstly, we analyzed cumulative incidence of selected intermediate events (first occurrence of relapse, infection, or thrombosis; a patient may have experienced >1 intermediate event). In these analyses, we calculated and plotted cumulative incidence for each type of event. Death resulting from any cause was treated as a competing event.13 Secondly, we analyzed OS and mortality using the Kaplan-Meier estimator. Thirdly, we analyzed OS and death rates after each type of intermediate event. To avoid immortal-time bias,14 each event was considered as a time-dependent variable (ie, a patient who experienced an intermediate event was classified as not having that intermediate event until its occurrence and as having the event after that event occurred). In these analyses, we calculated death rates (per 100 person-years). For all outcomes (intermediate events and death), hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for selected variables using univariate and multivariable proportional Cox hazards regression models. When analyzing mortality, we included in the model the covariates age, relapse, infection, and thrombosis (all treated as time-varying variables). Statistical analyses were performed with Stata software (StataCorp, College Station, TX).
Results
Baseline features
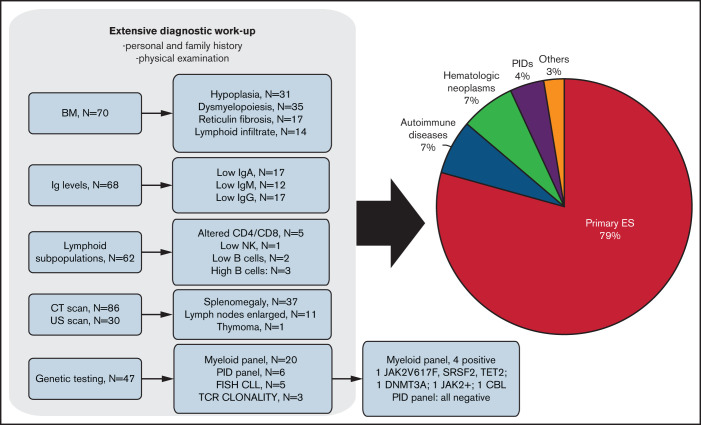
Overall, 116 patients (51% female) with a median age at ES diagnosis of 51 years (range, 1.9-94.8 years) and followed for a median of 7.5 years (range, 2-31 years) were included in the study. Three patients experienced their first cytopenia in infancy (Figure 1) but were referred to the participating center for the first time in adulthood when the second cytopenia occurred, leading to ES diagnosis; their hematologic parameters at the first episode were not included in the analysis. Of note, a comprehensive workup (lymphoid subpopulation, Ig level, CT scan, and PID gene panel) excluded a PID in these cases. Table 1 shows clinical and laboratory baseline characteristics of the patients. Regarding ES type, a majority of cases had combined ITP and AIHA (81%), followed by the triple combination of ITP, AIHA, and AIN in 10% of patients. Isolated thrombocytopenia, anemia, and their simultaneous occurrence had the same frequency at ES onset (1 of 3 each), whereas neutropenia was rarer (4%). Hematologic parameters at disease onset were highly variable, depending on the prevailing AIC: 41% had severe anemia (Hb <8 g/dL), 47% had increased LDH (>1.5× upper limit of normal [ULN]), 58% had severe thrombocytopenia (<30 × 109/L), and 12% had severe neutropenia (<0.5 × 109/L). Regarding autoimmunity tests (Table 1), 89% of tested patients had a positive DAT, mainly for warm IgG with or without complement (86%), 6 patients had DAT− AIHA (diagnosed after the exclusion of all other causes of hemolysis and given response to steroids), and 84% displayed positive anti-PLT autoantibodies. Anti-neutrophil antibodies were tested in only 19 cases and were positive in 45%. Finally, non–organ-specific autoantibodies were positive in >50% of cases (including antinuclear antibodies, extractable nuclear antigens, and anti-DNA), and antiphospholipids (including anticardiolipin, anti–β2 glycoprotein 1, and LAC) were positive in up to 18% of tested cases.
Figure 1.
Extensive workup leading to the diagnosis of secondary ES forms. Lower limit of normal of IgG <800 mg/dL, IgA <90 mg/dL, and IgM <60 mg/dL. BM, bone marrow; CLL, chronic lymphocytic leukemia; FISH, fluorescence in situ hybridization; NK, natural killer; TCR, T-cell receptor; US, ultrasound.
Table 1.
Clinical and hematologic features of patients with ES overall and by primary and secondary cases
| All patients (N = 116) |
Primary (n = 92) |
Secondary (n = 24) |
|
|---|---|---|---|
| Demographics | |||
| Age, y | 50.2 (1.9-94.8) | 50.7 (1.9-94.8) | 52.6 (7.6-79.5) |
| Sex | |||
| Male | 57 (49) | 42 (46) | 15 (63) |
| Female | 59 (51) | 50 (54) | 9 (37) |
| Laboratory values | |||
| Hb, g/dL | 8.5 (2.8-17) | 8.9 (2.8-17.0) | 7.6 (3.7-13.5) |
| PLTs, × 109/L | 20 (1-727) | 20 (1-727) | 21 (1-324) |
| ANC, × 109/L | 3.0 (0.2-15.2) | 3.0 (0.2-14.3) | 2.8 (0.2-15.2) |
| LDH, ×ULN | 1.7 (0.5-16) | 1.7 (0.6-16) | 1.5 (0.5-11) |
| IgG, g/dL | 1002 (52-5660) | 1030 (52-5660) | 862 (113-1440) |
| IgA, g/dL | 155 (24-1257) | 145 (24-1257) | 143 (40-375) |
| IgM, g/dL | 88 (10-1800) | 83 (10-1800) | 88 (27-475) |
| DAT+ | 103 (89) | 80 (87) | 23 (96) |
| IgG | 64 (62) | 48 (60) | 16 (70) |
| IgG + C | 25 (24) | 20 (25) | 5 (22) |
| C | 5 (5) | 5 (6.25) | 0 (0) |
| Mixed | 7 (7) | 6 (7.5) | 1 (4) |
| Atypical | 2 (2) | 1 (1.25) | 1 (4) |
| DAT− AIHA* | 6 (5) | 5 (5) | 1 (4) |
| Positive anti-PLTs (n = 49) | 41 (84) | 37(82)† | 4 (100) |
| Positive antineutrophils (n = 42) | 10 (45) | 8 (42)‡ | 2 (67) |
| ANA (n = 94) | 34 (36) | 29/74 | 5/20 |
| ENA (n = 69) | 6 (7) | 4/57 | 2/12 |
| Anti-DNA (n = 72) | 4 (56) | 2/58 | 2/14 |
| Anticardiolipin antibodies (n = 70) | 12 (17) | 11/56 | 1/14 |
| Anti–β2 glycoprotein 1 antibodies (n = 51) | 9 (18) | 7/46 | 2/5 |
| LAC (n = 60) | 9 (15) | 4/49 | 5/11 |
| Disease categories | |||
| Cytopenia at onset | |||
| ITP | 39 (34) | 34 (37) | 5 (21) |
| AIHA | 36 (31) | 30 (33) | 6 (25) |
| AIN | 5 (4) | 4 (4) | 1 (4) |
| Concomitant | 36 (31) | 24 (26) | 12 (50) |
| ES type | |||
| ITP and AIHA | 94 (81) | 75 (82) | 19 (79) |
| ITP and AIN | 7 (6) | 6 (6) | 1 (4) |
| AIHA and AIN | 3 (3) | 3 (3) | 0 (0) |
| ITP, AIHA, and AIN | 12 (10) | 8 (9) | 4 (17) |
| Splenomegaly | 37 (32) | 26 (28) | 11 (46) |
| Complications and outcomes | |||
| Infection | 39 (34) | 26 (28) | 13 (54) |
| Thrombosis | 25 (22) | 17 (18) | 8 (33) |
| Death§ | 23 (21) | 19 (21) | 4 (17) |
Demographics and laboratory data reflect those registered at first ES diagnosis. Values are given as median (range) or n (%).
ANA, antinuclear antibody; ENA, extractable nuclear antigen; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Patients with DAT− AIHA were diagnosed after exclusion of all other causes of hemolytic anemia and response to steroids; percentages calculated based on 109 total AIHA cases.
n = 45 tested.
n = 19.
6 patients lost to follow-up.
Extensive workup aimed at excluding secondary forms
Figure 1 shows the extensive diagnostic workup performed to evaluate underlying disease in adult patients with ES. On the whole, 59% of patients were tested for Ig levels and 53% for lymphoid subpopulations, 74% underwent a CT scan (the remaining had abdomen ultrasound), and 61% underwent bone marrow evaluation. Ig levels were below the normal range in 25% of patients, and lymphoid subpopulations showed an altered CD4/CD8 ratio in 8%, low B cells in 3%, increased B cells in 5%, and low NK in 1% of cases. At CT/ultrasound scan, one-third of patients showed splenomegaly, and 13% had enlarged lymph nodes. At bone marrow evaluation, most patients displayed erythroid and megakaryocytic hyperplasia (n = 27 and 18, respectively), 39% had dysplastic features of either erythroid or megakaryocytic lineage, and 24% had an increase of reticulin fibrosis (World Health Organization grade MF1). Notably, a lymphoid infiltrate was present in 20% of cases, mainly polyclonal, except for 2 patients with underlying lymphoma. Interestingly, 31% of patients underwent genetic testing: 5 were studied with a fluorescence in situ hybridization panel for chronic lymphocytic leukemia, 3 were tested for T-cell receptor clonality by polymerase chain reaction, and 34% were tested with an NGS panel. The latter included 1 panel for the assessment of mutations in genes commonly mutated in myeloid neoplasms, performed in 20 patients with some features of bone marrow failure (hypoplasia, dyserythropoiesis, and reticulin fibrosis). The test produced positive results in 4 patients: 1 patient had 3 mutations (JAK2, SRSF2, and TET2) and the other 3 patients had 1 mutation each (DNMT3A, JAK2, and CBL). Moreover, 6 patients (mainly young adults) were tested with an NGS panel for genes commonly mutated in PIDs; all were negative.
Secondary ES
ES was secondary to or associated with an underlying condition in 24 cases (21%), mainly other autoimmune conditions (n = 8, including Hashimoto thyroiditis, n = 1; antiphospholipid syndrome, n = 2; systemic lupus erythematosus, n = 1; undifferentiated connective tissue disease, n = 1; polymyalgia rheumatica, n = 1; posterior uveitis, n = 1; and autoimmune hepatitis, n = 1), hematologic neoplasms (n = 8, including chronic lymphocytic leukemia, n = 5; Castleman disease, n = 1; multiple myeloma, n = 1; and chronic myelomonocytic leukemia, n = 1), and primary immunodeficiencies (n = 5, including common variable immunodeficiency, n = 2; autoimmune lymphoproliferative syndrome, n = 1; Kabuki syndrome, n = 1; and nonfamilial hypogammaglobulinemia, n = 1); rarer associations were solid cancers (thymoma, n = 1), paroxysmal nocturnal hemoglobinuria (n = 1), and heart transplantation (n = 1). Secondary ES displayed lower Hb values (P = .04) and lower total IgG levels (P = .04) compared with primary cases. Moreover, patients with secondary ES presented more frequently with multiple cytopenias at onset (P = .02).
Bleeding manifestations
Forty-nine patients experienced a bleeding episode, mainly grade 1 or 2 (skin or oral bleeding; 82%) and mainly occurring at disease onset, concomitantly with severe thrombocytopenia. Grade ≥3 episodes included 4 grade 3 (nasal, n = 1; metrorrhagias, n = 3; and gastrointestinal bleeding, n = 1), 3 grade 4 (intraalveolar hemorrhage, n = 1; and gastrointestinal bleeding, n = 2), and 2 fatalities (pulmonary bleeding, n = 1; and intracranial hemorrhage, n = 1).
Treatments and relapses
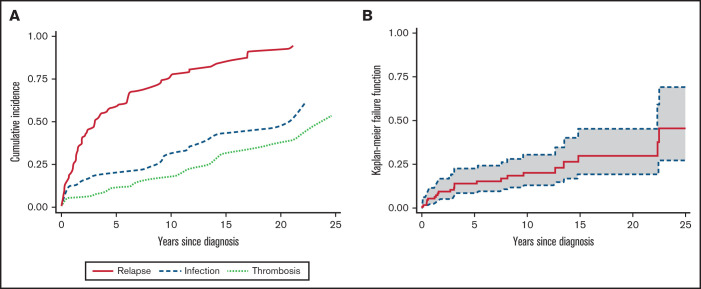
All patients but 1 received at least 1 line of therapy; 76% of cases required at least 2 lines, and 54% required ≥3 lines. First-line therapies included steroids alone (57%) or treatment associated with IVIGs (20% of cases for both ITP and AIHA). Additional frontline treatments in primary refractory cases (23%) comprised rituximab (n = 10), immunosuppressants (n = 8), splenectomy (n = 3), danazol (n = 3), TPO-RAs (n = 2), and PEX (n = 1). Table 2 displays the total number of the various treatments administered during follow-up, along with the response rates in all patients and in ITP and AIHA separately (of note, 37 steroid and 13 rituximab courses were administered for concomitant AIHA and ITP). Supplemental Table 1 details the distribution of specific therapies across the various lines of treatment, according to the relapsing cytopenia; numbers of responders are also provided. Regarding steroids, most patients were restarted on prednisone or methylprednisolone at each relapse, either alone or in combination with an additional line of treatment (supplemental Table 1). The median duration of the first steroid course was of 9.7 months (range, 0.3-36 months; comprising the initial full-dose course of 1 mg/kg per day and a tapering/low-dose maintenance period). Overall, more than half of the patients received rituximab, mainly for AIHA, one-third received an immunosuppressor (azathioprine, n = 10; cyclosporine, n = 6; cyclophosphamide, n = 8; and mycophenolate mofetil, n = 8), one-third received a TPO-RA (n = 27), and 10% underwent splenectomy. Interestingly, overall response rates were invariably >80%. The median duration of response (DOR; ie, the time from treatment to the next relapse) to rituximab was 16 months (range, 1.9-197 months) for AIHA, 16 months (range, 10-56 months) for ITP, and 31 months (range, 10-161 months) for concomitant ITP and AIHA, whereas 30% of patients never relapsed. DOR for splenectomy was 75 months (range, 19-171 months) for ITP and 16 months (range, 2-109 months) for AIHA; 31% never relapsed. Finally, a minority of patients (8%) received other treatments, including danazol (n = 5), PEX (n = 2), recombinant erythropoietin (n = 1), and the oral phosphatidylinositol 3-kinase inhibitor parsaclisib in a clinical trial (n = 1). In patients with AIN, no specific treatment was administered aside from anti-infective therapy or granulocyte colony-stimulating factor (n = 3) in case of infection. During follow-up, 165 cases of ITP, 103 cases of AIHA, and 16 concomitant relapses occurred. Figure 2A shows cumulative incidence curves for relapses and infectious and thrombotic events. Factors associated with a lower risk of relapse after first treatment were the frontline use of IVIGs in association with steroids (HR, 0.48; 95% CI, 0.27-0.92; P = .03), along with female sex (HR, 0.56; 95% CI, 0.36-0.86; P < .01) and presence of normal IgM level (HR, 0.47; 95% CI, 0.23-0.97; P = .04). No associations have been found between relapse occurrence and autoantibody positivity, nor with presence of molecular abnormalities by NGS. Notably, during the study period, patients with secondary ES did not receive treatment for the underlying condition, except for substitutive IVIGs in those with common variable immunodeficiency.
Table 2.
Total therapies administered in patients with ES and relative responses
| Treatment administered | ||||
|---|---|---|---|---|
| Any cytopenia (n = 115) |
AIHA (n = 38) |
ITP (n = 40) |
ITP + AIHA (n = 37) |
|
| Steroids alone | ||||
| N of patients | 84 | 18 | 29 | 37 |
| ORR | 81/84 (96) | 17/18 (94) | 27/29 (93) | 37/37 (100) |
| Steroids + IVIGs | ||||
| N of patients | 42 | 3 | 29 | 10 |
| ORR | 39/42 (93) | 2/3 (67) | 27/29 (93) | 8/10 (80) |
| Rituximab | ||||
| N of patients | 46 | 23 | 10 | 13 |
| ORR | 44/46 (96) | 23/23 (100) | 8/10 (80) | 13/13 (100) |
| IST | ||||
| N of patients | 26 | 15 | 11 | — |
| ORR | 25/26 (96) | 15/15 (100) | 10/11 (91) | — |
| Eltrombopag | ||||
| N of patients | 19 | — | 19 | — |
| ORR | 18/19 (95) | — | 18/19 (95) | — |
| Romiplostim | ||||
| N of patients | 8 | — | 8 | — |
| ORR | 7/8 (88) | — | 7/8 (88) | — |
| Splenectomy | ||||
| N of patients | 12 | 7 | 5 | — |
| ORR | 12/12 (100) | 7/7 (100) | 5/5 (100) | — |
| Danazol | ||||
| N of patients | 5 | 3 | 2 | — |
| ORR | 5/5 (100) | 3/3 (100) | 2/2 (100) | — |
Values are given as n (%). N of patients requiring 1 line of therapy only, 26 (23%); 2 lines, 26 (23%); 3 lines, 30 (26%); >4 lines, 33 (28%). A 79-y-old man with chronic lymphocytic leukemia was not treated; his Hb level stabilized at ∼10 g/dL, and PLTs never dropped below 30 × 109/L.
ORR, overall response rate.
Figure 2.
Cumulative incidence of relapse, infection, and thrombosis and overall mortality in adult patients with ES. (A) Cumulative incidence of relapses and infectious and thrombotic events, with death treated as a competing event. (B) Overall mortality; dotted line represents 95% CI.
Infectious complications
Thirty-nine patients (33%) experienced at least 1 infectious episode after ES diagnosis, mainly grade 3 or 4 (77%), with 3 fatal events. Infections included bacterial pneumonia (n = 13), sepsis with bacteremia (n = 8, including Salmonella, n = 1; Pseudomonas aeruginosa, n = 1; Staphylococcus aureus, n = 3; and Streptococcus pneumoniae, n = 1), urinary tract infection (n = 4), abscess (n = 3, including cutaneous, n = 1; and perianal, n = 2), Pneumocystis jirovecii pneumonia (n = 2), meningitis (n = 2), herpes virus reactivation (simplex, n = 1; and zoster, n = 3), tuberculosis, hepatitis C virus [HCV] infection, ileitis, and ocular infection. Infections were significantly more frequent in patients with secondary ES (54% vs 28% in primary cases; P = .01), with positive antineutrophil autoantibodies (75% vs 25%; P = .03), and with splenomegaly (46% vs 23% in those with normal spleen; P = .01). Moreover, patients with grade ≥4 infections had low ANCs compared with those with grade <4 episodes (P = .04). Importantly, infections were significantly associated with the number of therapy lines (69% in those receiving ≥2 therapy lines vs 35%; P = .01), but not with a specific treatment. Cox regression models confirmed that an underlying lymphoproliferative disorder (HR, 2.7; 95% CI, 1.1-6.8; P = .02) and splenomegaly (HR, 2.04; 95% CI, 1.03-4.02; P = .03) were associated with infectious risk. Notably, patients with concomitant AIHA and ITP (HR, 2.48; 95% CI, 1.05-5.8; P = .03) and very severe anemia (Hb <6 g/dL; HR, 3.4; 95% CI, 1.3-8.9; P = .01) at onset displayed a higher cumulative incidence of infections.
Thrombotic complications
Thrombotic episodes occurred in 21% of cases; 72% were grade ≥3, and 1 event was fatal. Thromboses included 10 pulmonary embolisms (fatal event, n = 1), 7 deep venous thromboses of the lower limbs, 2 strokes, 3 splanchnic thromboses, 1 acute myocardial infarction, 1 atrial thrombus, and 1 superficial thrombophlebitis. Thromboses were significantly more frequent in patients with antiplatelet antibodies (64% vs 35%; P = .02), antineutrophil antibodies (75% vs 25%; P = .03), and LAC (78% vs 25%; P = .002). In cause-specific Cox regression models, the risk of thrombosis was higher in patients with Hb <6 g/dL (HR, 4.38; 95% CI, 1.48-12.9; P < .01), LDH >1.5× ULN (HR, 2.7; 95% CI, 0.98-7.4; P = .05), LAC (HR, 4.6; 95% CI, 1.52-10.6; P = .005), and primary refractory disease (ie, those who required drugs different from steroids with or without IVIGs as first-line treatment; HR, 3.4; 95% CI, 1.4-8.3; P < .01). Finally, thrombotic events were more frequent in patients with grade ≥4 infections (40% vs 16%; P = .04).
Survival
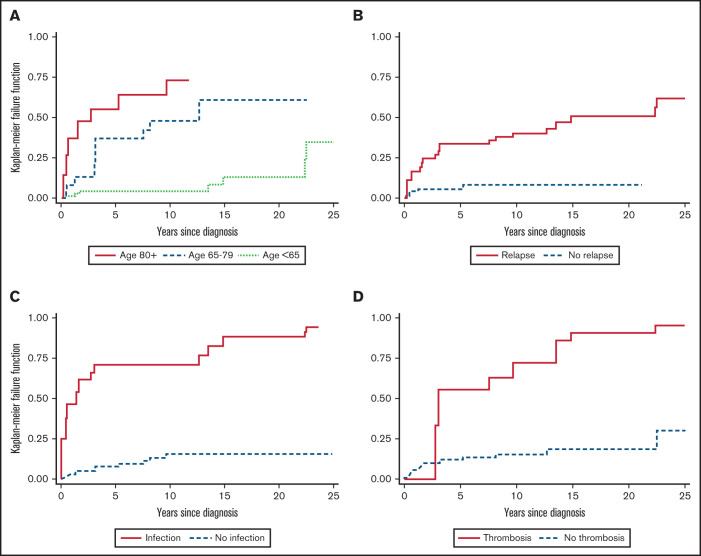
During the study period, 23 patients (19.8%) died, 10 as a result of ES relapse and/or complications (infections, n = 3; pulmonary embolism, n = 1; sepsis and multiorgan failure, n = 4; and bleeding, n = 2) and 13 as a result of other and unrelated causes (renal cancer, n = 2; advanced age, n = 8; and unknown, n = 3). Overall, the mortality rate was 2.4 per 100 person-years (23 deaths and 963 person-years of observation; Figure 2B), resulting in an OS rate of 54% (95% CI, 31%-73%). The median OS was not reached after a median follow-up of 7.3 years (mean, 8.3 year; 25th-75th percentiles, 1.9-12.6 years) from the initial diagnosis. As shown in Table 3, death was significantly associated with lower Hb level at onset (P = .04), mixed or atypical AIHA (55% vs 17%; P = .01), and age (P = .001). Cox regression analysis confirmed a higher risk of death in patients with Hb <8 g/dL (HR, 3; 95% CI, 0.97-9.36; P = .05) and Hb <6 g/dL (HR, 3.94; 95% CI, 0.99-11.3; P = .05) and in elderly patients (age >65 years; HR, 5.46; 95% CI, 1.71-17.42; P < .001). Importantly, cumulative incidence of mortality was higher in patients experiencing at least 1 relapse (HR, 6.16; 95% CI, 2.1-18.09; P = .001), thrombosis (HR, 6.76; 95% CI, 2.66-17.2; P < .001), and infections (HR, 10.16; 95% CI, 4.2-24.4; P < .001; Figure 3). Multivariate analysis (supplemental Table 2) confirmed the independent value of relapse (P = .03), thrombosis (P = .002), and infections (P < .001) in predicting OS. The latter 2 maintained their detrimental impact on OS when considering ES-related causes only (supplemental Table 3). Finally, experiencing at least 1 event (relapse, thrombosis, or infection) increased the risk of death by sixfold and 2 events by 20-fold.
Table 3.
Clinical and hematologic characteristics of patients with ES according to death occurrence
| Alive (n = 93) |
Dead (n = 23) |
|
|---|---|---|
| Demographics | ||
| Age, y | 46.7 (1.9-84.6) | 71.4 (18.3-94.8)* |
| Sex | ||
| Male | 45 (48) | 11 (48) |
| Female | 48 (52) | 12 (52) |
| Laboratory values | ||
| Hb, g/dL | 8.9 (2.8-17.0) | 6.6 (3.0-13.3)** |
| PLTs, × 109/L | 19 (1-564) | 39 (2-727) |
| ANC, × 109/L | 2.50 (0.2-15.2) | 3.1 (0.25-9.0) |
| LDH, ×ULN | 1.6 (0.52-11.11) | 2 (1-16) |
| IgG, g/dL | 955 (52-5660) | 1052 (340-1618) |
| IgA, g/dL | 143 (24-1257) | 165 (65-260) |
| IgM, g/dL | 95 (10-1800) | 82 (39-218) |
| DAT+ | 82 (88) | 21 (91) |
| IgG | 53 (65) | 11 (52) |
| IgG + C | 20 (24) | 5 (24) |
| C | 5 (6) | 0 (0) |
| Mixed | 4 (5) | 3 (14)*** |
| Atypical | // | 2 (10)*** |
| Positive antiplatelets (n = 38) | 32 (84) | 9 (82) |
| Positive antineutrophils (n = 22) | 10 (45) | 1 (20) |
| Complications | ||
| Infection | 27 (29) | 12 (52) |
| Thrombosis | 18 (19) | 7 (30) |
Demographics and laboratory data reflect those collected at disease onset. Values are given as median (range) or n (%).
LDH, lactate dehydrogenase; ULN, upper limit of normal.
P = .001, **P < .05, ***P = .01.
Figure 3.
Cumulative mortality in adult ES patients according to time-dependent variables. Cumulative mortality according to age (A) and occurrence of relapses (B), infections (C), and thromboses (D).
Discussion
Here we describe the largest series of adult patients with ES with granular clinical information ever reported and show that the disease may be very severe, highly relapsing, and marked by several sometimes fatal complications, particularly bleeding, infection, and thrombosis.
Although patients with ES displayed initial high response rates (>80%) to various therapies, including rituximab, splenectomy, TPO-RAs, and immunosuppressive treatments, comparable if not higher than those in patients with AIHA and ITP separately,8,9,11 one-fourth of patients can be considered primary refractory. In fact, they required additional frontline treatments beyond steroids with or without IVIGs and showed increased risk of complications and worse outcomes, thus representing a true unmet need. Furthermore, a remarkably high relapse rate was observed in the whole cohort, with 70% of cases requiring ≥2 lines of therapy. In fact, response duration to main second-line treatments (rituximab and splenectomy) was inferior to DORs in AIHA and ITP separately.8,11,15 Interestingly, the early use of IVIGs in combination with steroids was associated with a lower risk of early relapse, although this potentially protective effect will require prospective confirmation. The several courses of steroids at each relapse underline that we still rely on this drug to induce a quick response in the acute phase, because other treatments (ie, immunosuppressive therapy, rituximab, and novel molecules) take time to work. This happens at the price of several adverse effects that warrant appropriate monitoring. Finally, the association between low IgM levels and higher risk of relapse after first treatment may be linked to a deeper underlying immune dysregulation in chronic/relapsing disease, as observed in ES secondary to PIDs or lymphoproliferative syndromes (often marked by hypogammaglobulinemia).
Infections and thromboses complicate approximately one-third of cases and mostly occur in the first 2 to 3 years within diagnosis, and their frequency increases along with the number of therapy lines, suggesting an additional iatrogenic effect. However, treatments such as cytotoxic immunosuppressants, TPO-RAs, and splenectomy were not statistically related to the occurrence of complications, possibly indicating that repeated steroid therapy at each relapse may have a prominent role. Additionally, some disease-related risk factors emerged for infections, including presence of secondary ES and splenomegaly, pinpointing the need for anti-infectious prophylaxis in this subset. Moreover, the concomitant onset of ITP and AIHA, the severity of anemia, and the presence of neutropenia with positive antineutrophil autoantibodies emerged as additional risk factors for infectious complications. Altogether, these findings suggest that the extent of the immune dysregulation may be associated with an impairment of immune competence. It is difficult to give recommendations for prophylaxis, except for testing for HBV/HCV status and quantiferon testing for tuberculosis in patients who are candidates for immunosuppression with high-dose steroids, rituximab, or cytotoxic drugs. Vaccinations against capsulated bacteria (meningococcus, Haemophilus, and pneumococcus) are mandatory before splenectomy. Prophylaxis with cotrimoxazole (ie, 800 mg orally every other day) is advisable in patients receiving >25 mg per day of prednisone (or equivalent) for >4 weeks, antiviral agents (eg, lamivudine) should be administered to patients at risk for HBV reactivation, along with close surveillance. Chronic HCV infections should be referred for possible eradication before immunosuppression. If tuberculosis is suspected, the infectious disease specialist may indicate specific prophylaxis. Finally, patient education is fundamental, along with prompt referral to medical attention/emergency room in case of severe infection.16
Thrombotic complications, observed in 20% of cases, were fairly more frequent than in AIHA alone, where they have been reported in ∼10% to 15% of cases.11,15,17-19 Thrombosis was associated with severe hyperhemolytic disease (ie, Hb <6 g/dL and LDH >1.5× ULN), but not with previous splenectomy as described for primary AIHA.11,15,19 Thrombotic risk in ES also seems higher than that observed in isolated ITP, where it is only slightly augmented as compared with the general population but increases by threefold if TPO-RAs are administered.20 In contrast, TPO-RAs were not associated with thrombotic complications in ES, although the limited number of episodes does not allow definite conclusions. The physiopathology of thrombosis in ES likely encompasses the contribution of hemolysis (nitric oxide depletion) and that of procoagulant young platelets released in ITP.20,21 Moreover, the risk seems to increase in primary refractory patients, consistent with a highly active and severe disease positive for multiple autoantibodies, particularly LAC. Not surprisingly, thrombotic complications are more frequent during infections, which may fuel the vicious circle of thromboinflammation.22 These findings suggest the use of anticoagulant prophylaxis may be beneficial in hyperhemolytic patients with ES and infections, provided PLT counts are safe. A comprehensive evaluation of all risk factors is advised, including thrombophilia screening at diagnosis (particularly LAC), because thrombotic episodes have also been observed in thrombocytopenic patients.8,23-25 As expected, bleeding complications are frequent (approximately half of cases), concomitant with ITP, and mainly low grade. However, bleeding may account for early mortality when occurring at disease onset, whereas at relapse, it is generally milder as a result of patient education and medical surveillance.24
We found a prevalence of underlying disease of ∼20%, lower than that reported in a previous French study7 and in adult warm AIHA and much lower than that observed in recent prospective pediatric experiences.4-6 This difference may be related to the greater frequency of PIDs in children (>60% of ES cases)4,6 and possible underdiagnosis of milder adult forms, where extensive workups and immunologic/molecular studies are not routinely performed. It is worth mentioning that in adults it may be more difficult to select the appropriate genetic tests a priori. In fact, whereas in young adults the exclusion of an associated PID may be pivotal, in the elderly the assessment of underlying bone marrow failure through myeloid NGS panels may be preferred. As a matter of fact, in the subgroup analyzed, no patients displayed a typical PID mutation, whereas 20% of patients had a mutation indicative of clonal hematopoiesis of indetermined potential, which in cytopenic patients may be termed clonal cytopenia of unknown significance. These forms are more prone to evolve into myeloid neoplasms and deserve appropriate follow-up with repeated bone marrow evaluation in case of clinical changes. Finally, an underlying lymphoproliferative disorder might have been underestimated in our series because of a paucisymptomatic or indolent course, notwithstanding extensive investigations (bone marrow evaluation and imaging studies). We suggest that in adult ES, the workup should exclude lymphoproliferative syndromes that are most commonly associated in this setting. Even rarer associations, such as PIDs, should be extensively investigated and excluded (by history of recurrent infections, immunoglobulin level evaluation, lymphocyte subpopulations, and genetic testing).
Concerning survival, the severity of anemia at diagnosis was the main predictor of mortality, similar to what has been reported in primary AIHA, where each gram of Hb reduction yielded a 7% greater risk of relapse.15 Regarding time-dependent variables, complications were the main risk factors for a fatal outcome in our series, in line with a recent Danish epidemiologic study.3 The latter reported that bleeding and infections were the main causes of death, resulting in a median OS of only 7.2 years. Moreover, all-cause mortality was higher in patients with ES than in the general population, and the proportion of deaths resulting from bleeding was greater in ES than in isolated ITP.3 Our results strengthen the evidence of the dismal impact of complications on survival. In fact, the occurrence of at least 1 of either relapse, infection, or thrombosis increased the risk of death by sixfold, and the presence of 2 increased the risk by >20-fold, regardless of age. On the whole, mortality was comparable to that of low-grade hematologic malignancies, such as low-risk myelodysplastic syndromes and indolent lymphomas,26,27 despite the markedly younger median age of patients with ES.
Our study has several limitations, particularly regarding the retrospective nature of the analysis, the heterogeneous workup (genetic testing included various methods and panels), and the lack of uniformity in multi-institutional management. Other possible confounders include difficulties in analyzing each ES relapse and evaluating the treatments used for each cytopenia. However, the impact of relapses and ES complications on outcomes clearly emerged from the analysis and supports some important clinical warnings. An international prospective registry for adult ES would allow the collection of clearer data on disease course, treatment, and complications, because forms of adult ES are often excluded from clinical trials for ITP and AIHA, and therefore, a specific approach is lacking. In conclusion, adult ES is a rare and often severe disease requiring high clinical awareness, accurate diagnosis to assess secondary forms, and prompt treatment. Therapeutic strategies should take into account the risk of infection, thrombosis, and relapse. Supportive care including prophylactic measures for infectious and thrombotic complications is warranted.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Contribution: B.F., J.A.G., T.J.G.-L., and W.B. conceived the study; B.F., J.A.G., and W.B. wrote the manuscript; D.C. performed the statistical analysis. All authors followed the patients and/or collected data and revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: B.F. has received consultancy honoraria from Momenta, Novartis, and Amgen. M.M. has received consultancy honoraria from Amgen and Novartis. D.L.H. has received honoraria from Alexion and Novartis. A.P. has received advisor honoraria from Sanofi, Novartis, Pfizer, and Takeda. M.E.M.-C. has received research grants and speaker honoraria from Amgen, Novartis, Sobi, and Takeda and acted as a consultant for Amgen, Novartis, Grifols, Takeda, Sanofi, and Sobi. V.M. has received advisor honoraria from Amgen, Novartis, and Sobi and research funding from Grifols. M.C. reports nonfinancial support from Roche, Novo Nordisk, and Sobi and honoraria from Daiichi Sankyo. J.M.V. has received travel/conference support from Celgene. A.G. has received honoraria from Novo Nordisk, consultancy honoraria from Agios, Novartis, and Bristol-Myers Squibb, and research funding from Saniona. H.F. has received research funding from Novartis, Gilead, AbbVie, and Alexion. T.J.G.-L. has received research grants and speaker honoraria from Amgen and Novartis and consultancy honoraria from Amgen, Novartis, Grifols, Momenta, Sobi, and Argenx. W.B. has received consultancy honoraria from Alexion, Novartis, Agios, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Bruno Fattizzo, Department of Oncology and Onco-hematology, University of Milan, via Festa del Perdono 7, 20122, Milan, Italy; e-mail bruno.fattizzo@unimi.it.
References
- 1.Evans RS, Takahashi K, Duane RT, Payne R, Liu C. Primary thrombocytopenic purpura and acquired hemolytic anemia; evidence for a common etiology. AMA Arch Intern Med. 1951;87(1):48-65. [DOI] [PubMed] [Google Scholar]
- 2.Audia S, Grienay N, Mounier M, Michel M, Bonnotte B. Evans’ syndrome: from diagnosis to treatment. J Clin Med. 2020;9(12):3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen DL, Möller S, Andersen K, Gaist D, Frederiksen H. Evans syndrome in adults - incidence, prevalence, and survival in a nationwide cohort. Am J Hematol. 2019;94(10):1081-1090. [DOI] [PubMed] [Google Scholar]
- 4.Hadjadj J, Aladjidi N, Fernandes H, et al. ; members of the French Reference Center for Pediatric Autoimmune Cytopenia (CEREVANCE) . Pediatric Evans syndrome is associated with a high frequency of potentially damaging variants in immune genes. Blood. 2019;134(1):9-21. [DOI] [PubMed] [Google Scholar]
- 5.Mannering N, Hansen DL, Frederiksen H. Evans syndrome in children below 13 years of age - a nationwide population-based cohort study. PLoS One. 2020;15(4):e0231284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pincez T, Fernandes H, Leblanc T, et al. Long term follow-up of pediatric-onset Evans syndrome: broad immunopathological manifestations and high treatment burden [published online ahead of print 14 January 2021]. Haematologica. doi: 10.3324/haematol.2020.271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114(15):3167-3172. [DOI] [PubMed] [Google Scholar]
- 8.Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia [published correction appears in Blood Adv. 2020;4(2):252]. Blood Adv. 2019;3(23):3829-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the first international consensus meeting. Blood Rev. 2020;41:100648. [DOI] [PubMed] [Google Scholar]
- 10.Fattizzo B, Zaninoni A, Consonni D, et al. Is chronic neutropenia always a benign disease? Evidences from a 5-year prospective study. Eur J Intern Med. 2015;26(8):611-615. [DOI] [PubMed] [Google Scholar]
- 11.Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930-2936. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 27 February 2021.
- 13.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41(3):861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renoux C, Azoulay L, Suissa S. Biases in evaluating the safety and effectiveness of drugs for covid-19: designing real-world evidence studies. Am J Epidemiol. 2021;190(8):1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcellini W, Zaninoni A, Fattizzo B, et al. Predictors of refractoriness to therapy and healthcare resource utilization in 378 patients with primary autoimmune hemolytic anemia from eight Italian reference centers. Am J Hematol. 2018;93(9):E243-E246. [DOI] [PubMed] [Google Scholar]
- 16.Giannotta JA, Fattizzo B, Cavallaro F, Barcellini W. Infectious complications in autoimmune hemolytic anemia. J Clin Med. 2021;10(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broome CM, Cunningham JM, Mullins M, et al. Increased risk of thrombotic events in cold agglutinin disease: A 10-year retrospective analysis. Res Pract Thromb Haemost. 2020;4(4):628-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berentsen S, Barcellini W, D’Sa S, et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood. 2020; 136(4):480-488. [DOI] [PubMed] [Google Scholar]
- 19.Ho G, Brunson A, Keegan THM, Wun T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with autoimmune hemolytic anemia. Blood Cells Mol Dis. 2020;81:102388. [DOI] [PubMed] [Google Scholar]
- 20.Rodeghiero F. Is ITP a thrombophilic disorder? Am J Hematol. 2016;91(1):39-45. [DOI] [PubMed] [Google Scholar]
- 21.Audia S, Bach B, Samson M, et al. Venous thromboembolic events during warm autoimmune hemolytic anemia. PLoS One. 2018;13(11):e0207218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fattizzo B. Evans syndrome and infections: a dangerous cocktail to manage with caution. Blood Transfus. 2021;19(1):5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuelson Bannow BT, Lee A, Khorana AA, et al. Management of cancer-associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(6):1246-1249. [DOI] [PubMed] [Google Scholar]
- 24.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. [DOI] [PubMed] [Google Scholar]
- 25.Boulware R, Refaai MA. Why do patients with immune thrombocytopenia (ITP) experience lower bleeding events despite thrombocytopenia? Thromb Res. 2020;187:154-158. [DOI] [PubMed] [Google Scholar]
- 26.Dada R. Diagnosis and management of follicular lymphoma: a comprehensive review. Eur J Haematol. 2019;103(3):152-163. [DOI] [PubMed] [Google Scholar]
- 27.de Swart L, Smith A, Johnston TW, et al. Validation of the revised international prognostic scoring system (IPSS-R) in patients with lower-risk myelodysplastic syndromes: a report from the prospective European LeukaemiaNet MDS (EUMDS) registry. Br J Haematol. 2015;170(3):372-383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.