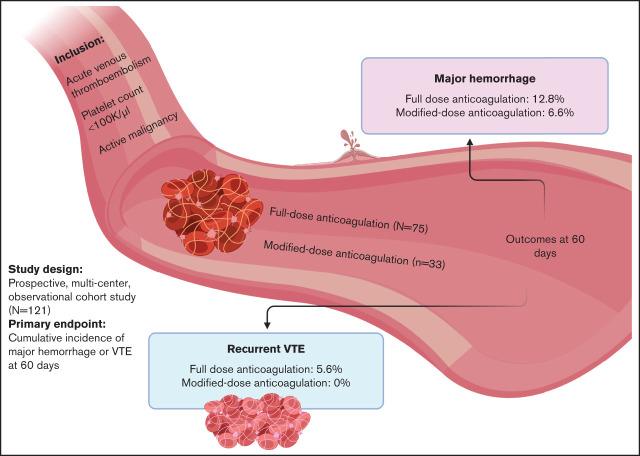
Key Points
In patients with cancer with VTE and thrombocytopenia, modified-dose anticoagulation was associated with a lower rate of major hemorrhage.
In this cohort, recurrent VTE was not observed after initiation of modified-dose anticoagulation.
Visual Abstract
Abstract
Venous thromboembolism (VTE) with concurrent thrombocytopenia is frequently encountered in patients with cancer. Therapeutic anticoagulation in the setting of thrombocytopenia is associated with a high risk of hemorrhage. Retrospective analyses suggest the utility of modified-dose anticoagulation in this population. To assess the incidence of hemorrhage or thrombosis according to anticoagulation strategy, we performed a prospective, multicenter, observational study. Patients with active malignancy, acute VTE, and concurrent thrombocytopenia (platelet count <100 000/µL) were enrolled. The cumulative incidences of hemorrhage or recurrent VTE were determined considering death as a competing risk. Primary outcomes were centrally adjudicated and comparisons made according to initial treatment with full-dose or modified-dose anticoagulation. A total of 121 patients were enrolled at 6 hospitals. Seventy-five patients were initially treated with full-dose anticoagulation (62%) and 33 (27%) with modified-dose anticoagulation; 13 (11%) patients received no anticoagulation. Most patients who received modified-dose anticoagulation had a hematologic malignancy (31 of 33 [94%]) and an acute deep vein thrombosis (28 of 33 [85%]). In patients who initially received full-dose anticoagulation, the cumulative incidence of major hemorrhage at 60 days was 12.8% (95% confidence interval [CI], 4.9-20.8) and 6.6% (95% CI, 2.4-15.7) in those who received modified-dose anticoagulation (Fine-Gray hazard ratio, 2.18; 95% CI, 1.21-3.93). The cumulative incidence of recurrent VTE at 60 days in patients who initially received full-dose anticoagulation was 5.6% (95% CI, 0.2-11) and 0% in patients who received modified-dose anticoagulation. In conclusion, modified-dose anticoagulation appears to be a safe alternative to therapeutic anticoagulation in patients with cancer who develop deep vein thrombosis in the setting of thrombocytopenia.
Introduction
Thromboembolism is a frequent complication of cancer. It is the second-leading cause of death in patients with cancer and is associated with worsened prognosis and quality of life.1,2 Patients with cancer diagnosed with venous thromboembolism (VTE) are more likely to develop recurrent thrombosis and bleeding complications while on anticoagulation compared with patients with VTE who do not have an active malignancy.1-4
Thrombocytopenia is common in cancer, either due to the underlying disease or as a side effect of antineoplastic therapy. In hematologic malignancies, which are frequently associated with VTE, platelet counts often decline to <50 000/µL.5 A retrospective cohort study of 2537 patients with hematologic malignancies reported a 28% incidence of thrombocytopenia to <100 000/µL, with 16% of patients experiencing platelet counts <50 000/µL.6 Certain chemotherapeutics used in the treatment of solid tumors such as gemcitabine and platinum-based agents are frequently associated with thrombocytopenia (30%-50% incidence), with platelet counts <50 000/µL occurring in 5% to 10% of patients.7,8 The appropriate management of acute VTE and thrombocytopenia is uncertain. Treatment guidelines include recommendations regarding the administration of full therapeutic or modified-dose anticoagulation, with or without target platelet transfusion goals.9,10 The relative safety or efficacy of these approaches has not been established. In a retrospective cohort study of 1514 patients undergoing allogeneic stem cell transplantation at John Hopkins Hospital, approximately one-third of patients experienced a hemorrhage when full-dose anticoagulation was administered along with platelet transfusion support.11 Other studies have similarly suggested a high rate of hemorrhage as well as recurrent VTE in this patient population when treated with full-dose anticoagulation.4 A retrospective quality assurance project conducted at Memorial Sloan Kettering Cancer Center identified modified-dose anticoagulation as a potentially safer alternative to full-dose anticoagulation in the setting of severe thrombocytopenia.12
Based on the available data, it is evident that patients with cancer who develop acute VTE in the setting of thrombocytopenia are at increased risk of both hemorrhage and recurrent VTE. However, prospective, multi-institutional data to inform management in this setting are lacking. We initiated a prospective, observational, multicenter cohort study to assess rates of hemorrhage and recurrent VTE among patients with cancer, acute VTE, and thrombocytopenia treated with modified or therapeutic doses of anticoagulation.
Methods
Study design
A prospective cohort study was conducted at 6 academic hospitals in the United States through VENUS (the Venous thromboEmbolism Network US). The institutional review board at each participating hospital approved the protocol, and all patients provided written informed consent before study procedures. Eligible patients were ≥18 years of age with histologically confirmed active malignancy, defined as a cancer diagnosis or having received cancer-directed therapy within 6 months of study enrollment. Patients were required to have radiologically confirmed VTE and concurrent thrombocytopenia with a platelet count <100 000/µL within 72 hours of VTE diagnosis (either before or after VTE diagnosis). Patients were required to enroll within 7 days of VTE diagnosis. The initial anticoagulation strategy was obtained from the medical chart and classified as either full dose with platelet transfusion support, modified dose (eg, unfractionated heparin with decreased activated partial thromboplastin time or anti–factor Xa targets, enoxaparin dose <1.5 mg/kg per 24 hours, 2.5 mg apixaban twice daily, or 10 mg rivaroxaban daily), or not started.
Study assessments
Study assessments were performed at 30 and 60 days after enrollment and included laboratory studies, review of anticoagulation status and compliance, and assessment for hemorrhage and recurrent VTE events. Recurrent VTE was objectively confirmed by imaging studies or documentation. Hemorrhage events (major hemorrhage and clinically relevant nonmajor bleeding) were classified according to International Society on Thrombosis and Haemostasis criteria.13,14 Hemorrhage and recurrent VTE outcomes were centrally adjudicated by 2 physicians. At each study visit, adherence to the documented anticoagulation and/or platelet transfusion plans was assessed and classified as “excellent” (ie, frequent dose adjustment based on platelet count or full-dose anticoagulation maintained with platelet transfusion support), “poor” (ie, anticoagulation not adjusted based on platelet count or treatment plan not followed more often than not), or “mixed” by the site investigator.
Statistical analysis
The primary end points of the study were hemorrhage and recurrent VTE events within 60 days of the initial VTE event (time zero representing the date of the initial VTE). Primary analyses were according to intention-to-treat based on the initial anticoagulation regimen at the time of enrollment. No formal sample size was determined, but there was a target enrollment of 200 patients to provide point estimates of event rates. The study stopped early due to the COVID-19 pandemic resulting in an extended cessation of patient enrollment across all study sites. Variables were compared between the full-dose, modified-dose, and no anticoagulation cohorts by using Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. The method of Kaplan and Meier was used to estimate survival, and the log-rank test was used to compare survival between the groups. The cumulative incidences of hemorrhage and recurrent VTE were determined with death considered as a competing risk and sub-hazard ratio calculated by using the Fine-Gray regression.15 Two-sided P values <.05 were considered statistically significant. Patients who were still alive were censored at the date of last contact. Patients who were discharged to hospice were considered deceased at the date of last contact.
Results
A total of 121 patients were enrolled (Table 1) from March 2018 to August 2020. The majority of patients (n = 75 [62%]) were treated with full-dose anticoagulation and 33 (27%) patients with modified-dose anticoagulation; 13 (11%) patients received no anticoagulation. Baseline characteristics differed between the treatment cohorts in some aspects. A greater proportion of patients with hematologic malignancies initially received modified-dose anticoagulation, whereas patients with solid tumors predominantly received full-dose anticoagulation. Of the 45 patients enrolled with qualifying pulmonary emboli, 80% received full-dose anticoagulation. Among the 101 patients enrolled with deep vein thrombosis (DVT), 59% received full-dose anticoagulation, 38% received modified-dose anticoagulation, and 12% were not anticoagulated. A total of 48 patients had index upper extremity DVT, 38 (79%) of which were catheter associated. Low-molecular-weight heparin (LMWH) was the most common anticoagulant used for both full-dose and modified-dose anticoagulation. A total of 16 patients were initially started on a direct oral anticoagulant (DOAC), with 13 receiving apixaban and 3 rivaroxaban. In patients started on full-dose anticoagulation with platelet transfusion support, the platelet transfusion threshold ranged from 10 000 to 50 000/µL. The most common transfusion threshold was a platelet count <50 000/µL (74%). In patients started on modified-dose anticoagulation, the most common dose modification strategy was half-dose enoxaparin (0.5 mg/kg every 12 hours).
Table 1.
Baseline characteristics
| Characteristic | Full-dose (n = 75) | Modified-dose (n = 33) | None (n = 13) |
|---|---|---|---|
| Male sex, n (%) | 39 (52) | 16 (48) | 10 (77) |
| Mean age at enrollment (range), y | 59 (23-88) | 60 (26-78) | 65 (42-78) |
| Inpatient, n (%) | 70 (93) | 30 (91) | 11 (85) |
| Cancer diagnosis, n (%) | |||
| Hematologic malignancy | 43 (57) | 31 (94) | 11 (85) |
| Solid tumor | 32 (43) | 2 (6) | 2 (15) |
| Comorbidities, n (%) | |||
| Hypertension | 38 (51) | 15 (45) | 7 (54) |
| Chronic kidney disease | 0 | 2 (6) | 1 (8) |
| Cirrhosis | 2 (3) | 0 | 0 |
| History of major hemorrhage | 2 (3) | 0 | 0 |
| Brain metastases | 7 (9) | 0 | 1 (8) |
| Median baseline laboratory values (IQR) | |||
| Hemoglobin, g/dL | 8.7 (7.4-10.7) | 7.9 (7.5-9.7) | 7.4 (7.0-8.7) |
| Platelet count, K/µL | 65 (47-88) | 37 (24-48) | 16 (14-32) |
| Prothrombin time, s | 14.1 (13-15.3) | 13 (12.1-13.8) | 13.8 (13.5-14.6) |
| Creatinine, mg/dL | 0.8 (0.6-1.0) | 0.8 (0.5-1.1) | 0.8 (0.7-1.8) |
| Total bilirubin, mg/dL | 0.6 (0.4-1.1) | 0.5 (0.4-0.7) | 0.8 (0.5-0.9) |
| Index VTE event | |||
| Proximal lower extremity DVT | 19 (25) | 6 (18) | 2 (15) |
| Distal lower extremity DVT | 14 (19) | 6 (18) | 2 (15) |
| Upper extremity DVT | 23 (31) | 17 (81) | 8 (62) |
| Pulmonary embolism | 36 (48) | 7 (21) | 2 (15) |
| Mesenteric thrombosis | 4 (5) | 0 | 0 |
| Anticoagulant, n (%) | |||
| LMWH | 40 (53) | 26 (79) | 0 |
| Unfractionated heparin | 23 (31) | 3 (9) | 0 |
| Direct oral anticoagulant | 12 (16) | 4 (12) | 0 |
IQR, interquartile range.
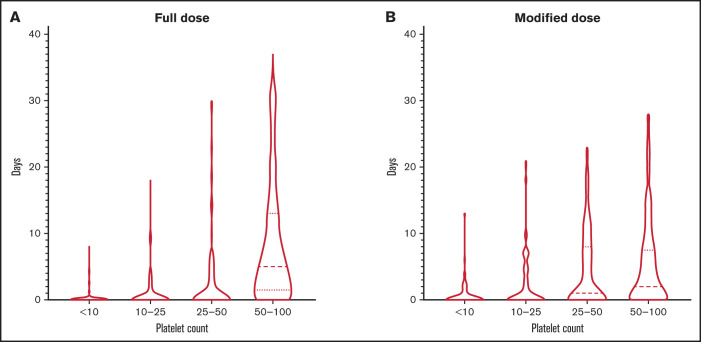
The median platelet count was higher in patients who were initially started on full-dose anticoagulation, with a median of 65 000/µL compared with 37 000/µL in the modified-dose anticoagulation cohort (P < .001). The median platelet count was 16 000/µL in the group that received no anticoagulation. During follow-up, platelet count nadir at 30 days remained significantly lower in patients treated with modified-dose anticoagulation (26 000/µL; P = .02) and no anticoagulation (8000/µL; P < .001) than those receiving full-dose anticoagulation (42 000/µL). The distribution of duration of thrombocytopenia over 60-day follow-up in the full-dose and modified-dose cohorts is shown in Figure 1. The mean duration of severe thrombocytopenia (platelet count ≤50 000/µL) was 9.73 days in the full-dose cohort and 13.76 days in the modified-dose cohort (P = .07).
Figure 1.
Duration and severity of thrombocytopenia. Duration of thrombocytopenia in patients receiving full-dose (A) or modified-dose (B) anticoagulation.
During the study follow-up period, changes in anticoagulant prescribed were frequent. In 26 patients who initially received unfractionated heparin, 9 patients switched to LMWH and 8 switched to a DOAC. An additional 15 patients were switched from LMWH to a DOAC during follow-up. Changes in anticoagulation intensity occurred much less frequently, with 3 patients switching from modified-dose to full-dose anticoagulation and 4 patients switching from full-dose to modified-dose anticoagulation during follow-up. Among 134 follow-up visits in patients initiated on full-dose anticoagulation, 127 (94.8%) were characterized as having excellent adherence to the documented anticoagulation strategy (ie, appropriate dose adjustments and/or administration of platelet transfusion support based on threshold platelet counts). Similarly, in the modified-dose anticoagulation cohort, 49 (89.1%) of 55 visits were characterized as excellent adherence. There was no difference in quality of adherence between the full-dose and modified-dose anticoagulation cohorts (P = .21).
Hemorrhage
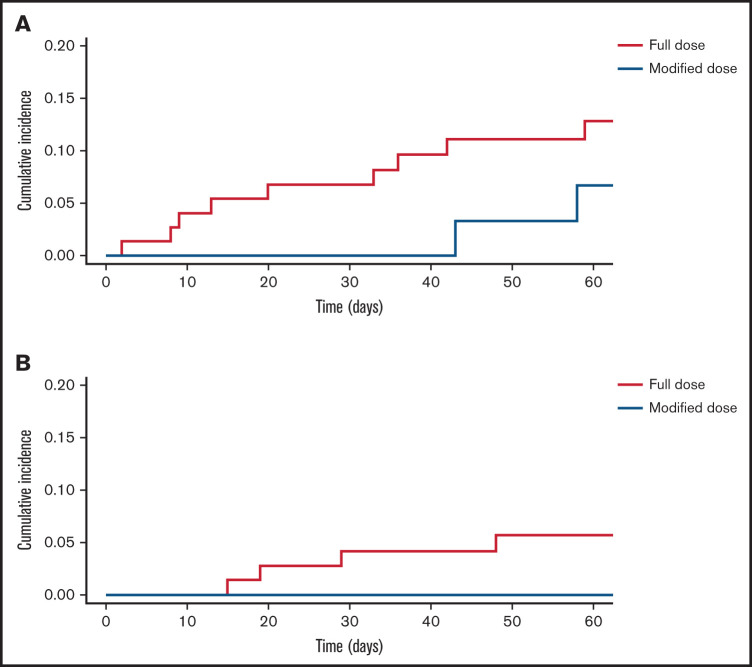
There were 23 hemorrhagic events during the 60-day study period (Table 2). Eighteen of the hemorrhagic events occurred in patients who initially received full-dose anticoagulation, and the remaining 5 occurred in patients started on modified-dose anticoagulation. The cumulative incidence of total hemorrhage at 60 days was 24% (95% confidence interval [CI], 13.9-34.1) in patients who initially received full-dose anticoagulation compared with 15.9% (95% CI, 2.8-28.9) in the modified-dose anticoagulation group. In patients who initially received full-dose anticoagulation, the cumulative incidence of major hemorrhage at 60 days was 12.8% (95% CI, 4.9-20.8) compared with 6.6% (95% CI, 2.4-15.7) in the modified-dose anticoagulation group (Fine-Gray hazard ratio, 2.18; 95% CI, 1.21-3.93) (Figure 2). Four of the five patients initially started on modified-dose anticoagulation who subsequently experienced a hemorrhage were receiving full-dose anticoagulation at the time of the event. Of the 11 total major hemorrhagic events, only 1 patient was receiving modified-dose anticoagulation at the time of the bleed. One fatal hemorrhage (an upper gastrointestinal hemorrhage) occurred in a patient on full-dose anticoagulation. There were no hemorrhagic events in the cohort receiving no anticoagulation. Among the 11 patients who experienced a major hemorrhage, the median time from index VTE to major hemorrhage was 20 days in patients started on full-dose anticoagulation and 50.5 days in patients started on modified-dose anticoagulation.
Table 2.
Hemorrhagic events during the 60-day study period according to initial anticoagulation strategy
| Hemorrhage characteristics | Full-dose (n = 75) | Modified-dose (n = 33) | None (n = 13) |
|---|---|---|---|
| Total hemorrhage, n (%) | 18 (24) | 5 (15) | 0 |
| Severity of hemorrhage, n (%) | |||
| Fatal hemorrhage | 1 (1) | 0 | 0 |
| Major hemorrhage | 8 (11) | 2 (6) | 0 |
| Clinically relevant nonmajor hemorrhage | 9 (12) | 2 (6) | 0 |
| Minor hemorrhage | 0 | 1 (3) | 0 |
| Type of hemorrhage | |||
| Intracranial hemorrhage | 2 (3) | 1 (3) | 0 |
| Ocular | 0 | 1 (3) | 0 |
| Upper gastrointestinal bleed | 1 (1) | 0 | 0 |
| Lower gastrointestinal bleed | 7 (9) | 1 (3) | 0 |
| Retroperitoneal | 1 (1) | 0 | 0 |
| Hematuria | 4 (5) | 0 | 0 |
| Mucocutaneous | 4 (5) | 2 (6) | 0 |
| Intramuscular | 1 (1) | 0 | 0 |
| Anticoagulant dose at time of hemorrhage | |||
| Full-dose | 15 (20) | 4 (12) | — |
| Modified-dose | 3 (4) | 1 (3) | — |
| None | 1 (1) | 0 | — |
| Anticoagulant at time of hemorrhage | |||
| LMWH | 10 (13) | 2 (6) | — |
| Unfractionated heparin | 4 (5) | 1 (3) | — |
| Direct oral anticoagulant | 4 (5) | 2 (6) | — |
| Median days with platelet count <100 000/µL | 20 | 16 | 20 |
Figure 2.
Major hemorrhage and recurrent VTE. Cumulative incidence of major hemorrhage (A) and recurrent VTE (B) according to initial anticoagulation regimen. Full-dose (blue); modified dose (red).
Recurrent VTE.
There were 5 recurrent VTE events (Table 3). Four occurred in patients who initially received full-dose anticoagulation and 1 occurred in a patient who did not receive anticoagulation. No patient who initially received modified-dose anticoagulation experienced a recurrent VTE. The cumulative incidence of recurrent VTE at 60 days in patients who initially received full-dose anticoagulation was 5.6% (95% CI, 0.2-11) and 0% in patients who initially received modified-dose anticoagulation (Figure 2).
Table 3.
Recurrent VTE events during the 60-day study period according to initial anticoagulation strategy
| VTE characteristics | Full-dose (n = 75) | Modified-dose (n = 33) | None (n = 13) |
|---|---|---|---|
| Total recurrent VTE events | 4 (5) | 0 | 1 (8) |
| Recurrent VTE event | |||
| Distal lower extremity DVT | 1 (1) | 0 | 0 |
| Proximal lower extremity DVT | 2 (3) | 0 | 0 |
| Upper extremity DVT | 0 | 0 | 1 (8) |
| Pulmonary embolism | 1 (1) | 0 | 0 |
| Anticoagulant dose at time of recurrent VTE | |||
| Full-dose | 2 (3) | 0 | 0 |
| Modified-dose | 0 | 0 | 0 |
| None | 2 (3) | 0 | 1 (8) |
| Anticoagulant at time of recurrent VTE | |||
| LMWH | 1 (1) | 0 | 0 |
| Unfractionated heparin | 1 (1) | 0 | 0 |
| Direct oral anticoagulant | 0 | 0 | 0 |
Among the 5 patients who experienced recurrent VTE, the median time from index VTE to recurrence was 19 days. Recurrent VTE events included a segmental pulmonary embolism in a patient who initially had only an upper extremity DVT, an upper extremity DVT, 2 proximal lower extremity DVTs, and a distal lower extremity DVT. Three of five patients were receiving full-dose heparin anticoagulation at the time of recurrent VTE. The other 2 patients were not receiving anticoagulation at the time of recurrent VTE due to prolonged thrombocytopenia in one case and a recent retroperitoneal hemorrhage in another. A single patient who was initially not started on anticoagulation developed a recurrent VTE. The index event was an upper extremity DVT. Propagation of the upper extremity DVT was noted 11 days after initial recognition, prompting the initiation of full-dose anticoagulation.
Hemorrhage and thrombosis during exposure to DOACs.
A total of 16 patients were initially started on a DOAC (15 patients starting full-dose anticoagulation and 1 modified-dose anticoagulation) and 27 were switched to a DOAC during follow-up. Of the patients started on a DOAC, 2 had luminal gastrointestinal malignancies, and 1 had bladder cancer. The median platelet count at the time of DOAC initiation was 51 000/µL (range, 12 000-98 000/µL). Among patients initially started on a DOAC, median exposure to the DOAC was 62 days, with only 1 patient switching to LMWH during follow-up. Three hemorrhagic events occurred in patients initially started on a DOAC during the 60-day follow-up period, correlating to a cumulative incidence of hemorrhage of 20% (95% CI, 0-40). Two of these events were lower gastrointestinal hemorrhage adjudicated as major hemorrhages. All 3 events occurred while on a full-dose DOAC. An additional 3 hemorrhagic events occurred in patients switched to a DOAC during follow-up. The events included a lower gastrointestinal hemorrhage and 2 patients with hematuria. All were adjudicated as clinically relevant nonmajor bleeding. There were no recurrent VTE events in patients who were initially started on and/or were actively receiving a DOAC.
Survival
A total of 22 patients died during the 60-day follow-up period. Median survival was not reached in either cohort. Survival at 60 days was 81.5% in the full-dose anticoagulation cohort and 80.1% in the modified-dose anticoagulation cohort (log-rank test, P = .90).
Discussion
Anticoagulation management in patients with cancer who develop VTE in the setting of thrombocytopenia is challenging given the high risk of hemorrhage balanced against the risk of recurrent thrombosis. Retrospective studies suggested that modified-dose anticoagulation is a viable alternative to full-dose anticoagulation with platelet transfusion support.11,12 In the TROVE (Thrombocytopenia Related Outcomes with Venous thromboEmbolism) study, we observed a lower rate of major hemorrhage in patients with thrombocytopenia and VTE initially treated with modified-dose anticoagulation relative to those initially managed with full-dose anticoagulation. The rates of recurrent VTE were low in both cohorts.
To the best of our knowledge, this analysis represents the first prospective study assessing the rates of hemorrhage and recurrent thrombosis according to anticoagulation strategy in patients with thrombocytopenia at the time of VTE. The reported rates of hemorrhage with modified-dose anticoagulation in smaller single-institution cohorts are highly variable, ranging from 0% to 33%.4,12,16,17 Retrospective comparisons of hemorrhagic outcomes between the 2 anticoagulation strategies generally show favorable hemorrhagic outcomes with modified-dose anticoagulation.4,16,18 In this study, the absence of major hemorrhage at 30 days with modified-dose anticoagulation provides prospective evidence that this approach seems safe in the management of DVT in the setting of thrombocytopenia. The substantial rates of major hemorrhage observed in both cohorts by 60 days’ follow-up highlight the high-risk nature of this patient population and the need for prospective data to inform clinical decision-making.
There were very few recurrent VTE events documented in this study. This stands in contrast to some retrospective studies that have reported rates of recurrent thrombosis in excess of 40%.4,18 The observation period in the current study was 60 days, which may account for the lower rates of recurrent VTE observed. All thrombotic events were centrally adjudicated with clear criteria for progression or recurrence. Although this study was underpowered to conclude that the 2 anticoagulation approaches were similarly effective, there were no cases of recurrent or progressive VTE among patients initially receiving modified-dose anticoagulation.
Because anticoagulation management was not randomized in this study, not unexpectedly, there were baseline imbalances, including more patients with pulmonary emboli receiving full-dose anticoagulation. There are limited prospective data available showing the efficacy of modified-dose anticoagulation in higher risk VTE, and guidance from the International Society on Thrombosis and Haemostasis is full-dose anticoagulation with platelet transfusion support in patients with severe thrombocytopenia and a high risk of thrombus progression such as pulmonary embolism and proximal lower extremity DVT.10 With only 7 patients with pulmonary embolism receiving modified-dose anticoagulation, we were unable to conclude that modified-dose anticoagulation is an effective strategy for this indication, although none of the 7 patients developed recurrent VTE during the 60-day follow-up. A prior study included 26 patients with pulmonary emboli treated with modified-dose anticoagulation, and none developed recurrent or progressive VTE.12
The 2 cohorts were also imbalanced according to underlying cancer diagnosis. Almost all patients in the modified-dose arm carried a hematologic malignancy diagnosis compared with 57% in the full-dose anticoagulation arm. Accordingly, the severity of thrombocytopenia, both in terms of depth and duration of thrombocytopenia, was more pronounced in the modified-dose anticoagulation arm compared with the full-dose anticoagulation arm. As such, the modified-dose anticoagulation arm represented a higher hemorrhagic risk population relative to the full-dose anticoagulation arm. Despite this imbalance, there were no major hemorrhages observed in those patients who received modified-dose anticoagulation, providing additional reassurance regarding the safety of this approach during periods of thrombocytopenia.
In the TROVE study, patients received anticoagulation at the discretion of the treating clinician. Although patients were prospectively followed up, a treatment regimen was not prescribed per a defined protocol. Qualitative treatment adherence assessments were performed at study visits, and overall the adherence to the prescribed anticoagulation strategy was classified as excellent. However, this study was not designed to validate a specific anticoagulant or dosing regimen. Criteria for modified-dose anticoagulation was below therapeutic dosing (ie, LMWH <1.5 mg/kg daily), with the most common regimen being LMWH according to the treatment plan described by Mantha et al.12 For initial treatment of VTE, the most common therapeutic and modified-dose anticoagulation regimen was LMWH. Although transition from full-dose to modified-dose anticoagulation or vice versa was uncommon, several patients switched to alternative therapeutic anticoagulants (eg, unfractionated heparin to full-dose LMWH).
DOACs have established efficacy relative to LMWH for the treatment of cancer-associated thrombosis, although there is concern for increased rates of bleeding in luminal gastrointestinal and possible genitourinary and gynecologic malignancies.19-21 The phase 3 randomized trials had different eligibility platelet cutoffs ranging between 50 000/µL and 100 000/µL. In these studies, very few patients were enrolled with platelet counts <100 000/µL, and outcomes during periods of thrombocytopenia have not been described. Our study represents the largest experience to date with DOACs in patients with cancer-associated thrombosis and thrombocytopenia. In our study, 6 of the 23 hemorrhagic events occurred in patients receiving a DOAC at the time of the event. As such, the safety of DOACs during periods of thrombocytopenia remains unclear, and additional data are required.
Although full-dose anticoagulation with platelet transfusion support seems to be the most common treatment strategy for the management of VTE associated with thrombocytopenia in our study, it poses challenges both conceptually and practically. The justification of supplementing platelets on the backdrop of an existing thrombus to permit full-dose anticoagulation is largely theoretical. Transfusion of platelets in this setting has been associated with an increased risk of thrombosis.22 Aggressive platelet transfusion poses additional challenges and potential complications (including transfusion reactions and allo-immunization in a heavily transfused population),23,24 somewhat arbitrary transfusion thresholds, and intense resource utilization. A modified-dose anticoagulation strategy without platelet transfusion targets addresses some of these issues and permits a degree of anticoagulation fine-tuning based on severity of thrombocytopenia.
A key limitation of this study was its lack of randomization for treatment allocation and the imbalanced baseline characteristics in both arms, which could influence outcomes. The adherence to the documented anticoagulation strategy (including platelet transfusion management) was determined by local investigators and not centrally adjudicated. Strengths of this study include enrollment of patients at multiple centers nationally, prospective data collection, and central adjudication of all qualifying hemorrhagic or thrombotic events.
In conclusion, the TROVE study showed that in select patients with cancer who develop VTE in the setting of thrombocytopenia, modified-dose anticoagulation was well tolerated with a low rate of recurrent VTE. In the setting of DVT, this study adds to the accumulating data that modified-dose anticoagulation seems to be both efficacious and safe during periods of thrombocytopenia.
Acknowledgments
The TROVE study was conducted without formal funding support. Logistical support was provided by the VENUS Network as well as by Beth Israel Deaconess Medical Center. The graphical abstract was generated with BioRender.
Authorship
Contribution: B.J.C. and J.I.Z. designed the study; B.J.C., T.-F.W., G.G., A.A.H., M.G., G.C.C., V.I.S., T.B., A.B., L.B.K., and J.I.Z. enrolled patients and collected data; B.J.C. and T.-F.W. adjudicated study outcomes; B.J.C., S.R., D.N., and J.I.Z. performed statistical analysis; and B.J.C. and J.I.Z. drafted the article, which all authors reviewed and approved before submission.
Correspondence: Jeffrey I. Zwicker, Division of Hematology and Hematologic Malignancies, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; e-mail: jzwicker@bidmc.harvard.edu.
References
- 1.Khorana AA. Cancer and thrombosis: implications of published guidelines for clinical practice. Ann Oncol. 2009;20(10):1619-1630. [DOI] [PubMed] [Google Scholar]
- 2.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH. Thromboprophylaxis with low-molecular-weight heparin in medical patients with cancer. Cancer. 2009;115(24):5637-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopolovic I, Lee AY, Wu C. Management and outcomes of cancer-associated venous thromboembolism in patients with concomitant thrombocytopenia: a retrospective cohort study. Ann Hematol. 2015;94(2):329-336. [DOI] [PubMed] [Google Scholar]
- 5.Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133(suppl 2):S63-S69. [DOI] [PubMed] [Google Scholar]
- 6.Shaw JL, Nielson CM, Park JK, Marongiu A, Soff GA. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106(5):662-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barni S, Labianca R, Agnelli G, et al. Chemotherapy-associated thromboembolic risk in cancer outpatients and effect of nadroparin thromboprophylaxis: results of a retrospective analysis of the PROTECHT study. J Transl Med. 2011;9(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moik F, Makatsariya A, Ay C. Challenging anticoagulation cases: cancer-associated venous thromboembolism and chemotherapy-induced thrombocytopenia – a case-based review of clinical management. Thromb Res. 2021;199:38-42. [DOI] [PubMed] [Google Scholar]
- 9.Khorana AA. The NCCN Clinical Practice Guidelines on Venous Thromboembolic Disease: strategies for improving VTE prophylaxis in hospitalized cancer patients. Oncologist. 2007;12(11):1361-1370. [DOI] [PubMed] [Google Scholar]
- 10.Samuelson Bannow BT, Lee A, Khorana AA, et al. Management of cancer-associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(6):1246-1249. [DOI] [PubMed] [Google Scholar]
- 11.Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood. 2008;112(3):504-510. [DOI] [PubMed] [Google Scholar]
- 12.Mantha S, Miao Y, Wills J, Parameswaran R, Soff GA. Enoxaparin dose reduction for thrombocytopenia in patients with cancer: a quality assessment study. J Thromb Thrombolysis. 2017;43(4):514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694. [DOI] [PubMed] [Google Scholar]
- 14.Barnaby DP, Wollowitz A, White D, et al. Generalizability and effectiveness of butterfly phlebotomy in reducing hemolysis. Acad Emerg Med. 2016;23(2):204-207. [DOI] [PubMed] [Google Scholar]
- 15.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 16.Samuelson Bannow BR, Lee AYY, Khorana AA, et al. Management of anticoagulation for cancer-associated thrombosis in patients with thrombocytopenia: a systematic review. Res Pract Thromb Haemost. 2018;2(4):664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houghton DE, Key NS, Zakai NA, Laux JP, Shea TC, Moll S. Analysis of anticoagulation strategies for venous thromboembolism during severe thrombocytopenia in patients with hematologic malignancies: a retrospective cohort. Leuk Lymphoma. 2017;58(11):2573-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanal N, Bociek RG, Chen B, et al. Venous thromboembolism in patients with hematologic malignancy and thrombocytopenia. Am J Hematol. 2016;91(11):E468-E472. [DOI] [PubMed] [Google Scholar]
- 19.Raskob GE, van Es N, Verhamme P, et al. ; Hokusai VTE Cancer Investigators . Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615-624. [DOI] [PubMed] [Google Scholar]
- 20.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017-2023. [DOI] [PubMed] [Google Scholar]
- 21.Agnelli G, Becattini C, Meyer G, et al. ; Caravaggio Investigators . Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599-1607. [DOI] [PubMed] [Google Scholar]
- 22.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168(21):2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goswamy RV, Wilson NR, Tannenbaum DJ, Aung FM, Hernandez CMR. Practice patterns and clinical outcomes of platelet alloimmunization in a comprehensive cancer center. Transfus Apher Sci. 2021;60(3):103096. [DOI] [PubMed] [Google Scholar]
- 24.Samuelson Bannow BT, Walter RB, Gernsheimer TB, Garcia DA. Patients treated for acute VTE during periods of treatment-related thrombocytopenia have high rates of recurrent thrombosis and transfusion-related adverse outcomes. J Thromb Thrombolysis. 2017;44(4):442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]