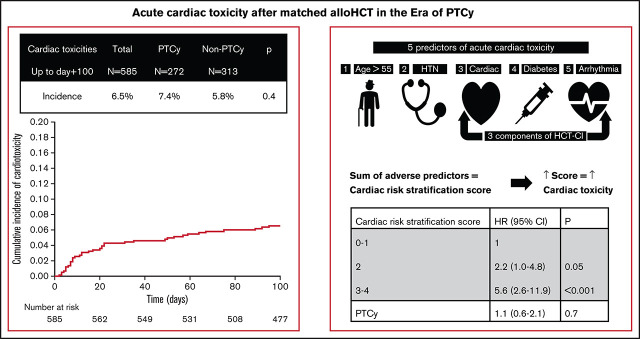
Key Points
The incidence of acute cardiac toxicity remains low and manageable after matched allo-HCT in the era of PTCy prophylaxis.
Older age, hypertension, arrhythmia, diabetes, and cardiac comorbidities increase this risk of cardiac toxicity but PTCy did not.
Visual Abstract
Abstract
Graft-versus-host disease (GVHD) is one of the leading causes of nonrelapse mortality (NRM) after allogeneic hematopoietic cell transplantation (allo-HCT). Posttransplant cyclophosphamide (PTCy) has shown promise in managing GVHD. However, cyclophosphamide has known cardiac toxicity, and few studies have evaluated the cardiac toxicities that arise after PTCy. We completed a retrospective analysis of patients who underwent matched-donor allo-HCT at our institution and who received PTCy- or non-PTCy–based GVHD prophylaxis, with the goal of determining the incidence of cardiac toxicities up to 100 days after allo-HCT. We included 585 patients in our analysis and found that 38 (6.5%) experienced cardiac toxicity after allo-HCT. The toxicities included arrhythmias (n = 21), heart failure (n = 14), pericardial effusion (n = 10), and myocardial infarction or ischemia (n = 7). Patients who received PTCy had a 7.4% incidence of cardiac toxicity, whereas non-PTCy recipients had an incidence of 5.8% (P = .4). We found that age >55 years (P = .02) and a history of hypertension (P = .01), arrhythmia (P = .003), diabetes (P = .04), and cardiac comorbidities (P < .001) were significant predictors of cardiac toxicity, whereas none of the preparative and GVHD prophylaxis regimens were predictive. From these findings, we proposed the use of a Cardiac Risk Stratification Score to quantify the risk of cardiac toxicity after allo-HCT. We found that a higher score correlated with an incidence of cardiac toxicity. Furthermore, the development of cardiac toxicity was associated with worse 1-year overall survival (OS) and NRM. The use of PTCy was associated with improvements in 1-year OS and NRM rates.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a lifesaving modality for many patients with hematologic malignancies.1 Over the past decade, several advances have been made to improve nonrelapse mortality (NRM) associated with conditioning regimens, including the replacement of cyclophosphamide and other alkylating agents with less toxic agents, such as fludarabine.2 Although improvement in NRM has been shown, NRM related to graft-versus-host disease (GVHD) remains one of the leading causes of death in allo-HCT survivors.
Currently, the standard GVHD prophylaxis regimen for allo-HCT involves the use of a calcineurin inhibitor and methotrexate (MTX), with or without anti-thymocyte globulin (ATG). The use of ATG produces lower rates of GVHD without improved survival.3 MTX is associated with increased rates of mucositis and renal dysfunction, which ultimately leads to increased rates of NRM.4 Because GVHD occurs in >50% of allo-HCT recipients, investigators have recently explored other GHVD prophylaxis options, including the use of posttransplant cyclophosphamide (PTCy).5
Cyclophosphamide is an alkylating agent of the nitrogen mustard class and has been used effectively in conditioning regimens for many years in allo-HCT. However, cyclophosphamide has historically been associated with increased rates of cardiac toxicity, including pericarditis and cardiomyopathy, as well as increased rates of cardiac compromise after allo-HCT.6-9 In recent years, PTCy has become the standard GVHD prophylaxis used in allo-HCT from haploidentical donors and has been used increasingly in allo-HCT from matched donors.5,10-13 The data detailing the incidence and the risk factors associated with developing cardiac toxicity in the era of PTCy-based GVHD prophylaxis are limited.
With the increasing use of PTCy-based GVHD prophylaxis at our institution, we performed this study to evaluate both the incidence and predictors of acute cardiac toxicity after matched allo-HCT. In particular, we sought to determine whether the use of PTCy was associated with increased cardiac toxicity.
Methods
Patients
We performed a retrospective review of all patients who underwent allo-HCT with an HLA-matched related donor (MRD) or matched unrelated donor (MUD) from 1 October 2016 to 30 April 2019. We excluded patients who received an allo-HCT from haploidentical or cord blood donors. MUDs were matched at HLA-A, -B, -C, and -DRB1. Most of the myeloablative preparative regimen were busulfan/fludarabine-based, whereas most of the reduced-intensity preparative regimens were fludarabine/melphalan-based. Both PTCy and non-PTCy GVHD prophylaxis were used in these preparative regimens. Cyclophosphamide and myeloablative doses of total-body irradiation were used in the preparative regimens of only 2.5% and 3% of patients, respectively. None of these patients received concurrent PTCy. PTCy-based GVHD prophylaxis consisted of cyclophosphamide, 50 mg/kg per day on days +3 and +4, and was administered with IV hydration and mesna, along with tacrolimus, with or without mycophenolate mofetil. Non-PTCy GVHD prophylaxis primarily consisted of tacrolimus and methotrexate 5 mg/m2 per day on days +1, +3, +6, and +11 with ATG (total dose of 4 mg/kg over 3 days) given to patients receiving a MUD transplant. This study was approved by the University of Texas MD Anderson Institutional Review Board and performed in accordance with the Declaration of Helsinki.
Definitions of cardiac toxicity
Cardiac toxicity was defined as any new episode of grade 2 or higher arrhythmia, heart failure, myocardial infarction or ischemia, pericarditis, or pericardial effusion that occurred up to day +100. These episodes and the type of toxicity were recorded contemporaneously in our departmental database and reviewed during the present study. Cardiac complications were defined and graded according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.14 Arrhythmias included atrial fibrillation, atrial flutter, supraventricular tachycardia, and ventricular arrhythmias. Heart failure and left ventricular systolic dysfunction were combined as 1 toxicity. Pericarditis and pericardial effusion (including pericardial tamponade) were defined according to the NCI CTCAE. Table 1 describes the cardiac toxicity grading in more detail.
Table 1.
Grading of cardiac toxicities after allo-HCT
| Cardiac toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Arrhythmia (including atrial fibrillation, atrial flutter, supraventricular tachycardia, ventricular arrhythmia) | Asymptomatic; intervention not indicated | Nonurgent medical intervention indicated | Symptomatic and incompletely controlled medically, or controlled with device, or ablation | Life-threatening consequences; urgent intervention indicated | Death |
| Heart failure (including left ventricular systolic dysfunction) |
Asymptomatic with laboratory or cardiac imaging abnormalities |
Symptoms with mild to moderate activity or exertion |
Severe with symptoms at rest or with minimal activity or exertion; intervention indicated |
Life-threatening consequences; urgent intervention indicated | Death |
| — | — | Symptomatic due to drop in ejection fraction (EF) responsive to intervention | Refractory or poorly controlled heart failure due to drop in EF; intervention such as ventricular assist device, IV vasopressor support, or heart transplant indicated | Death | |
| Myocardial infarction or ischemia (including acute coronary syndrome) |
— | Asymptomatic and cardiac enzymes minimally abnormal and no evidence of ischemic electrocardiogram (ECG) changes |
Severe symptoms; cardiac enzymes abnormal; hemodynamically stable; ECG changes consistent with infarction |
Life-threatening consequences; hemodynamically unstable | Death |
| — | Symptomatic, progressive angina; cardiac enzymes normal; hemodynamically stable | Symptomatic, unstable angina and/or acute myocardial infarction, cardiac enzymes abnormal, hemodynamically stable | Symptomatic, unstable angina and/or acute myocardial infarction, cardiac enzymes abnormal, hemodynamically unstable | Death | |
| Pericardial effusion (including pericardial tamponade) | — | Asymptomatic effusion size small to moderate | Effusion with physiologic consequences | Life-threatening consequences; urgent intervention indicated |
Death |
| Pericarditis | Asymptomatic, ECG or physical findings consistent with pericarditis | Symptomatic pericarditis | Pericarditis with physiologic consequences | Life-threatening consequences; urgent intervention indicated | Death |
Adapted from Common Terminology Criteria for Adverse Events, version 4.0.14
Data collection and analysis
Extracting data from the departmental database and using the electronic medical records, we obtained all patient demographics, allo-HCT–related characteristics, and pertinent comorbidities, including the Hematopoietic Cell Transplantation-specific Comorbidity Index (HCT-CI), a score commonly used to assess risk before allo-HCT.15 We also reviewed history of smoking, anthracycline exposure, hyperlipidemia, and hypertension (HTN), because these are risk factors that may affect cardiac toxicity, but are not included in the HCT-CI score.
Statistical analysis
The primary end points of the study were the incidence of cardiac toxicity at day +100 and the risk factors associated with development of cardiac toxicity after matched allo-HCT. The incidence of cardiac toxicity was estimated considering death before the development of toxicity as a competing risk. Predictors of toxicity were evaluated in univariate and multivariate analyses, by using Fine and Gray competing-risks regression analysis. In addition, we evaluated the impact of development of a cardiac toxicity on OS and NRM (using Cox’s proportional hazards and Fine and Gray regression analysis, respectively) considering the development of cardiac toxicity as a time-dependent variable. Significant predictors on univariate analysis were considered in multivariate analysis. The use of PTCy-based GVHD prophylaxis was considered in all multivariate models, irrespective of statistical significance in univariate analysis. The final regression model was determined by using backward elimination. First-degree interaction effects were tested for significant predictors and for the use of PTCy prophylaxis. Statistical analysis was defined at the P < .05 level. Analyses were primarily performed using STATA 14.0 (College Station, TX).
Results
General patient characteristics
A total of 585 adult patients met the study eligibility criteria and received an MRD transplant (n = 220) or MUD (n = 365) allo-HCT from October 2016 through April 2019. Patient, allo-HCT, and disease characteristics are presented in Table 2. The median age was 57 (range, 18-77) years, and most patients were male (58%) with acute myeloid leukemia/myelodysplastic syndrome (AML/MDS; 56%). This was a high-risk cohort, with 61% having active disease at the time of allo-HCT and 66% receiving a myeloablative preparative regimen. Notably, PTCy-based GVHD prophylaxis was given to a sizable proportion of patients (46%), allowing for a robust comparison with the patients who did not receive PTCy (54%). Patients who received PTCy were more often older (P < .001) with AML/MDS (P < .001), active disease (P = .02), and previous anthracycline exposure (P < .001) (Table 2).
Table 2.
Patient characteristics
| Characteristics | Total, N (%) N = 585 |
PTCy-based, n (%) n = 272 |
Non-PTCy, n (%) n = 313 |
P |
|---|---|---|---|---|
|
Sex Female Male |
243 (42) 342 (58) |
120 (44) 152 (56) |
123 (39) 190 (61) |
0.2 |
|
Age, median (interquartile range) years Age >55 Age ≤55 |
57 (18-77) 309 (53) 276 (47) |
61 (18-77) 180 (66) 92 (34) |
50 (18-74) 129 (41) 184 (59) |
<.001 <.001 |
|
Diagnosis AML/MDS Acute lymphocytic leukemia CML/MPD Chronic lymphocytic leukemia Lymphoma Other |
327 (56) 76 (13) 82 (14) 24 (4) 52 (9) 24 (4) |
189 (69) 9 (3) 50 (18) 4 (1) 12 (4) 8 (3) |
138 (44) 67 (21) 32 (10) 20 (6) 40 (13) 16 (5) |
<.001 |
|
Donor type MUD MRD |
365 (62) 220 (38) |
179 (66) 93 (34) |
186 (59) 127 (41) |
.1 |
|
Stem cell source Peripheral blood Bone marrow |
475 (81) 110 (19) |
229 (84) 43 (16) |
246 (79) 67 (21) |
.08 |
|
Disease status Active Remission |
356 (61) 229 (39) |
179 (66) 93 (34) |
177 (57) 136 (43) |
.02 |
|
Preparative regimen Reduced intensity Myeloablative |
201 (34) 384 (66) |
82 (30) 190 (70) |
119 (38) 194 (62) |
.05 |
|
History of anthracycline Yes No |
335 (57) 250 (43) |
122 (45) 150 (55) |
113 (36) 200 (64) |
<.001 |
|
History of HTN Yes No |
235 (40) 350 (60) |
81 (30) 191 (70) |
114 (36) 199 (64) |
.03 |
|
History of hyperlipidemia Yes No |
115 (20) 470 (80) |
60 (22) 212 (78) |
55 (18) 258 (82) |
.2 |
|
Smoker Yes No Missing |
227 (39) 356 (61) 2 (0) |
112 (41) 160 (59) 0 (0) |
115 (37) 196 (63) 2 (1) |
.4 |
|
Karnofsky Performance Scale <90 ≥90 Missing |
230 (39) 287 (49) 68 (12) |
117 (43) 133 (49) 22 (8) |
113 (36) 154 (49) 46 (15) |
.3 |
|
HCT-CI >3 ≤3 |
195 (33) 390 (67) |
81 (30) 191 (70) |
114 (36) 199 (64) |
.09 |
|
Cardiac* Yes No |
54 (8) 531 (92) |
22 (8) 250 (92) |
32 (10) 281 (90) |
.4 |
|
Arrhythmia† Yes No |
39 (7) 546 (93) |
17 (6) 255 (94) |
22 (7) 291 (93) |
.7 |
|
Heart valve‡ Yes No |
11 (2) 574 (98) |
5 (2) 267 (98) |
6 (2) 307(98) |
.6 |
|
Diabetes Yes No |
63 (11) 522 (89) |
30 (11) 242 (89) |
33 (11) 280 (89) |
.8 |
|
Pulmonary 0 2 3 |
335 (57) 190 (33) 60 (10) |
166 (61) 82 (30) 24(9) |
169 (54) 108 (34) 36 (11) |
.2 |
|
Obesity Yes No |
76 (13) 509 (87) |
38 (14) 234 (86) |
38 (12) 275 (88) |
.5 |
|
Infection Yes No |
78 (13) 507 (87) |
26 (10) 246(90) |
52 (17) 267 (83) |
.01 |
|
Hepatic 0 1 3 |
423 (72) 123 (21) 39 (7) |
197 (72) 59 (22) 16 (6) |
226 (72) 64 (20) 23 (7) |
.7 |
CML/MPD, chronic myeloid leukemia/myeloproliferative disease.
Cardiac includes coronary artery disease (≥1 vessel with coronary artery stenosis requiring medical treatment, stent, or bypass graft), congestive heart failure, myocardial infarction, or ejection fraction ≤50%.
Arrhythmia, atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias.
Heart valve, any heart valve disease except mitral valve prolapse.
Incidence of cardiac toxicity
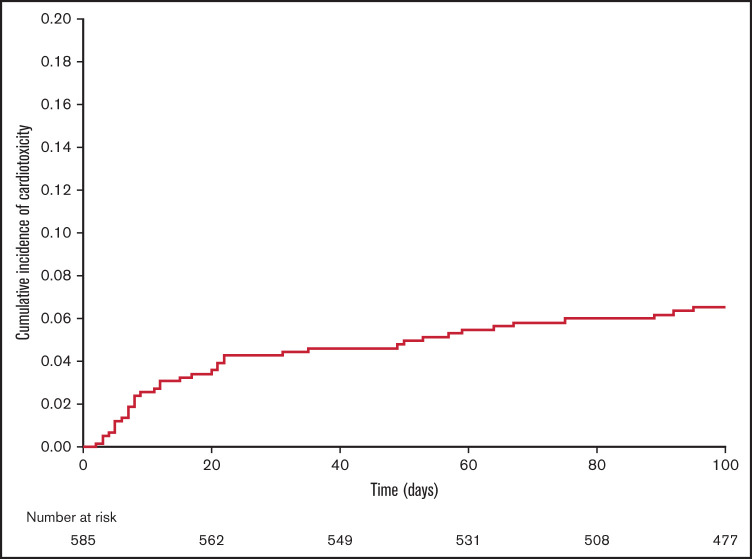
From day 0 to day +100 after matched allo-HCT, 38 patients developed a total of 52 episoces of cardiac toxicity, for an overall incidence of 6.5% (95% CI, 5-9; Figure 1). The cardiac toxicities included arrhythmias (n = 21), heart failure (n = 14), pericardial effusions (n = 10), and myocardial infarction or ischemia (n = 7). The median time to the development of cardiac toxicity was 20 days after allo-HCT (range, 3-95 days). Grade 2 toxicity was the maximum grade in 27 patients (52%), grade 3 in 11 patients (21%), grade 4 in 10 patients (19%), and grade 5 in 4 patients (8%). Patients who received PTCy-based GVHD prophylaxis had a 7.4% incidence of cardiac toxicity, whereas patients without PTCy had an incidence of 5.8% (P = .4). Nine (45%) patients with PTCy had multiple cardiac toxicities, compared with 5 (28%) of the non-PTCy–treated patients (P = .3). Details of the cardiac toxicities are presented in Table 3.
Figure 1.
Incidence of cardiac toxicity from day 0 to day +100 after allo-HCT.
Table 3.
Cardiac toxicity after allo-HCT
| Overall N = 585 |
PTCy n = 272 |
Non-PTCy n = 313 |
P | |
|---|---|---|---|---|
|
Incidence of cardiac toxicities (Day +100) (%) 95% CI |
6.5 (5-9) |
7.4 (4.7-10.9) |
5.8 (3.5-8.7) |
.4 |
| Patients with cardiac toxicity, n | 38 | 20 | 18 | — |
|
Episodes/patients, n (%) 1 ≥2 |
24 (63) 14 (37) |
11 (55) 9 (45) |
13 (72) 5 (28) |
.3 |
|
Type of cardiac toxicity, n (%) Arrhythmia Heart failure Myocardial infarction or ischemia Pericardial effusion |
n = 52 21 (40) 14 (27) 7 (13) 10 (19) |
n = 29 13 (45) 8 (28) 4 (14) 4 (14) |
n = 23 8 (35) 6 (26) 3 (13) 6 (26) |
.5 .9 .6 .2 |
Determining risk factors of cardiac toxicity
We performed a univariate analysis to determine patient, disease, and HCT characteristics (Table 4) that would predict the development of cardiac toxicity. Age >55 years (HR, 2.3; P = .02) and history of HTN (HR, 2.3; P = .01) were significant predictors. There were no associations observed regarding preparative regimen, GVHD prophylaxis (PTCy vs non-PTCy), or history of anthracycline exposure. However, we found that several individual comorbidities in the HCT-CI score were predictive of cardiac toxicity, such as cardiac disease (HR, 3.6; P < .001), arrhythmia (HR, 3.5; P = .003), and diabetes (HR, 2.3; P = .04).
Table 4.
Predictors of cardiac toxicity: univariate analysis
| Characteristics | Adults, n (%) N = 585 |
HR | 95% CI | P |
|---|---|---|---|---|
|
Age, y Age >55 Age ≤55 |
309 (53) 276 (47) |
2.3 1.0 |
1.1-4.5 |
.02 |
|
Disease status Active Remission |
356 61) 229 (39) |
2.1 1.0 |
1.0-4.5 |
.05 |
|
GVHD prophylaxis PTCy-based Non-PTCy |
272 (46) 313 (53) |
1.3 1.0 |
0.7-2.4 |
.4 |
|
Preparative regimen Reduced intensity Myeloablative |
201 (34) 384 (66) |
0.9 1.0 |
0.5-1.9 |
.9 |
|
Stem cell source Peripheral blood Bone marrow |
475 (81) 110 (19) |
0.9 1.0 |
0.4-1.9 |
.7 |
|
History of anthracycline Yes No |
335 (57) 250 (43) |
0.9 1.0 |
0.5-1.7 |
.8 |
|
History of HTN Yes No |
235 (40) 350 (60) |
2.3 1.0 |
1.2-4.5 |
.01 |
|
History of hyperlipidemia Yes No |
115 (20) 470 (80) |
1.5 1.0 |
0.7-2.9 |
.3 |
|
Smoker Yes No |
227 (39) 356 (61) |
1.6 |
0.8-3.0 |
.1 |
|
Karnofsky Performance Scale <90 ≥90 |
230 (39) 287 (49) |
0.7 1.0 |
0.4-1.4 |
.3 |
| Specific HCT-CI comorbidity | ||||
| Cardiac* Yes No |
54 (9) 531 (91) |
3.6 1.0 |
1.8-7.1 | <.001 |
| Arrhythmia† Yes No |
39 (7) 546 (93) |
3.5 1.0 |
1.5-7.9 |
.003 |
| Heart valve‡ Yes No |
11 (2) 574 (98) |
Not evaluable |
— |
.4 |
| Diabetes Yes No |
63 (11) 522 (89) |
2.3 — |
1.1-5 |
.04 |
| Pulmonary 0 2 3 |
335 (57) 190 (33) 60 (10) |
1.0 1.2 0.8 |
0.6-2.3 0.2-2.6 |
.6 .7 |
| Obesity Yes No |
76 (13) 509 (87) |
1.8 1.0 |
0.8-3.9 |
.1 |
Cardiac includes coronary artery disease (≥1 vessel coronary artery stenosis requiring medical treatment, stent, or bypass graft), congestive heart failure, myocardial infarction, and ejection fraction ≤50%.
Arrhythmia is defined as atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias.
Heart valve is any heart valve disease except mitral valve prolapse.
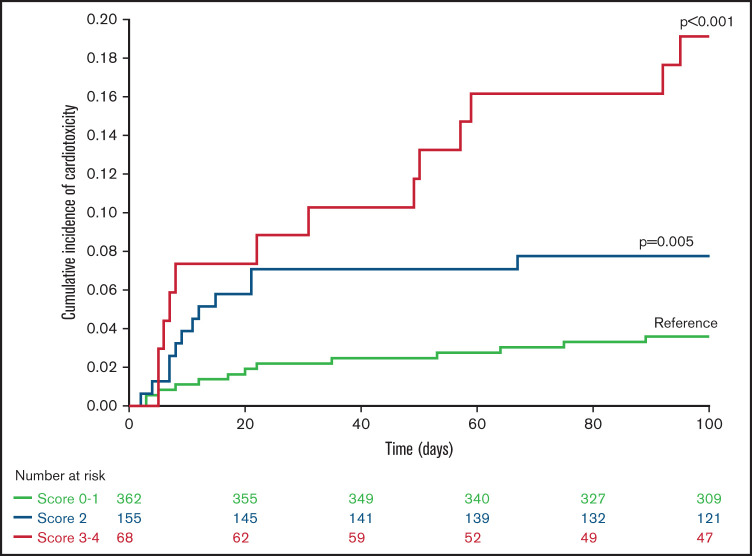
As expected, these cardiac comorbidities were strongly associated with age >55 years and with one another. Given these correlations and the comparable hazard ratios associated with each factor (Table 4), we devised a score that reflects the sum of the number of adverse predictors, including age >55 years, HTN, and cardiac comorbidities, arrhythmia, or diabetes, termed the Cardiac Risk Stratification Score, with a range of 0 to 5 points, although none of the patients in our cohort had all 5 risk factors. The day +100 cumulative incidence of cardiac toxicity was 4% (95% CI: 2-8), 4% (95% CI: 2-8), 8% (95% CI: 4-13), 13% (95% CI: 7-27), and 37% (95% CI: 20-71) in patients with a score of 0 (n = 192), 1 (n = 170), 2 (n = 155), 3 (n = 52), and 4 (n = 16), respectively. Using multivariate analysis, we found that higher scores were associated with cardiac toxicity (scores of 0 to 1 [reference], 2 [HR, 2.2; P = .05], and 3 and 4 [HR, 5.6; P < .001]). The cumulative incidence of cardiac toxicity increased in parallel with increasing score (Figure 2), reaching 19% (95% CI, 12-31) for patients with scores of 3 and 4, compared with 8%, (95% CI, 4-13) and 4% (95% CI: 2-6) for patients with scores of 2 and 0 and 1, respectively. Multivariate analysis adjusted for the proposed Cardiac Risk Stratification Score and the use of PTCy-based GVHD prophylaxis showed no significant effect (HR, 1.1; P = .7) of PTCy on cardiac toxicity (Table 5).
Figure 2.
Incidence of cardiac toxicity according to Cardiac Risk Stratification Score after allo-HCT.
Table 5.
Predictors of cardiac toxicity: multivariate analysis
| Covariable | HR (95% CI) | P |
|---|---|---|
|
Cardiac risk stratification score 0-1 2 3-4 |
1 2.2 (1.0-4.8) 5.6 (2.6-11.9) |
.05 <.001 |
| PTCy | 1.1 (0.6-2.1) | .7 |
Proposed Cardiac Risk Stratification Score includes 5 predictors of cardiac toxicity: age >55, HTN, cardiac, arrhythmia, and diabetes. Cardiac, includes coronary artery disease (≥1 vessel coronary artery stenosis requiring medical treatment, stent, or bypass graft), congestive heart failure, myocardial infarction, or ejection fraction ≤50%. Arrhythmia includes atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmia. Score 3-4 vs 2: HR, 2.5 (95% CI 1.2-5.5); P = 0.02.
Determining risk factors of OS and NRM
One-year OS was 71% (95% CI, 66-74) and 1-year NRM was 10% (95% CI, 8-13). The results of predictors of 1-year OS and NRM are described in Table 6 and 7, respectively. As expected, the development of cardiac toxicity was associated with worse 1-year OS (HR, 2.7; P < .001) and 1-year NRM (HR, 5.7; P < .001). In contrast, in both univariate and multivariate analyses, the use of PTCy-based GVHD prophylaxis was a predictor of improved 1-year OS (HR, 0.6; P = .001; HR, 0.5; P < .001) and improved 1-year NRM (HR, 0.4; P < .001; HR, 0.4; P < .001), respectively. Other factors for worse 1-year OS that remained significant in both the univariate and multivariate analyses included active disease (HR, 1.9; P < .001) and HCT-CI >3 (HR, 1.8; P < .001). Likewise, other factors for worse 1-year NRM included smoking (HR, 1.6, P = .004) and HCT-CI >3 (HR, 2.6; P < .001). Causes of death in the PTCy group included GVHD (n = 3), cardiac failure (n = 1), pneumonia/pulmonary failure (n = 2), and viral infection (n = 1). In the non-PTCy group, causes of death included GVHD (n = 3), pneumonia/pulmonary failure (n = 3), liver failure (n = 2), cardiac failure (n = 1), multiorgan failure (n = 1), viral infection (n = 2), and disease recurrence (n = 2).
Table 6.
Predictors of 1-y OS
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics |
Adults, n (%)
N = 585 |
HR | 95% CI | P | HR | 95% CI | P |
| Toxicity (time dependent) | 38 | 2.8 | 1.7-4.5 | <.001 | 2.7 | 1.7-4.4 | <.001 |
|
Age, y Age >55 Age ≤55 |
309 (53) 276 (47) |
1.1 1.0 |
0.8-1.5 |
.6 |
— |
— |
— |
|
Disease status Active Remission |
356 61) 229 (39) |
1.9 1.0 |
1.4-2.8 |
<.001 |
1.9 |
1.3-2.8 |
<.001 |
|
GVHD prophylaxis PTCy-based Non-PTCy |
272 (46) 313 (53) |
0.6 1.0 |
0.4-0.8 |
.001 |
0.5 |
0.4-0.7 |
<.001 |
|
Preparative regimen Reduced intensity Myeloablative |
201 (34) 384 (66) |
1.3 1.0 |
0.9-1.8 |
.08 |
— |
— |
— |
|
Stem cell source Peripheral blood Bone marrow |
475 (81) 110 (19) |
0.7 1.0 |
0.5-0.9 |
.04 |
— |
— |
— |
|
History of HTN Yes No |
235 (40) 350 (60) |
1.5 1.0 |
1.1-2.1 |
.008 |
— |
— |
— |
|
History of hyperlipidemia Yes No |
115 (20) 470 (80) |
1.05 1.0 |
0.7-1.5 |
.8 |
— |
— |
— |
|
Smoker Yes No |
227 (39) 356 (61) |
1.4 1.0 |
1.03-1.9 |
.03 |
— |
— |
— |
|
Karnovsky Performance Scale <90 ≥90 |
230 (39) 287 (49) |
1.4 1.0 |
1.03-2.1 | .03 | — | — | — |
| HCT-CI | |||||||
| >3 | 195 (33) | 1.9 | 1.4-2.6 | <.001 | 1.8 | 1.3-2.5 | <.001 |
| ≤3 | 390 (67) | 1.0 | — | — | — | ||
Table 7.
Predictors of 1-y NRM
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics |
Adults, n (%)
N = 585 |
HR | 95% CI | P | HR | 95% CI | P |
| Toxicity (time dependent) | 38 | 4.9 | 2.7-8.6 | <.001 | 5.7 | 3.1-10.5 | <.001 |
|
Age, y Age >55 Age ≤55 |
309 (53) 276 (47) |
1.3 1.0 |
0.9-2.1 |
.2 |
— |
— |
— |
|
Disease status Active Remission |
356 61) 229 (39) |
1.6 1.0 |
0.9-2.5 |
.05 |
— |
— |
— |
|
GVHD prophylaxis PTCy-based Non-PTCy |
272 (46) 313 (53) |
0.4 1.0 |
0.2-0.7 |
<.001 |
0.4 |
0.2-0.6 |
<.001 |
|
Preparative regimen Reduced intensity Myeloablative |
201 (34) 384 (66) |
1.5 1.0 |
0.9-2.3 |
.05 |
— |
— |
— |
|
Stem cell source Peripheral blood Bone marrow |
475 (81) 110 (19) |
0.6 1.0 |
0.4-1.0 |
.06 |
— |
— |
— |
|
History of HTN Yes No |
235 (40) 350 (60) |
1.6 1.0 |
1.1-2.5 |
.02 |
— |
— |
— |
|
History of hyperlipidemia Yes No |
115 (20) 470 (80) |
0.9 1.0 |
0.5-1.6 |
.7 |
— |
— |
— |
|
Smoker Yes No |
227 (39) 356 (61) |
1.6 1.0 |
1.05-2.4 |
.03 |
1.6 |
1.03-2.4 |
.04 |
|
KPS <90 ≥90 |
230 (39) 287 (49) |
1.5 1.0 |
0.9-2.3 |
.1 |
— |
— |
— |
| HCT-CI | |||||||
| >3 | 195 (33) | 2.8 | 1.8-4.3 | <.001 | 2.6 | 1.7-4.1 | <.001 |
| ≤3 | 390 (67) | 1.0 | — | — | — | — | — |
Discussion
In this study, we reviewed cardiac toxicity in patients at our institution who received a matched-donor allo-HCT and similar supportive care measures during the modern era of PTCy-based GVHD prophylaxis. We discovered a low incidence of acute cardiac toxicity, with a higher risk in older patients with cardiac comorbidities, arrhythmia, diabetes, and HTN. Importantly, we did not find a difference in cardiac toxicity between patients who received PTCy-based vs non-PTCy–based GVHD prophylaxis.
Cardiac toxicity after allo-HCT has been reported in 0.9% to 43% of patients.8 The incidence in our study fits within the lower limit of this range. Numerous patient risk factors have been associated with the development of cardiac toxicity after allo-HCT including age, smoking, HTN, hyperlipidemia, coronary artery disease, arrhythmia, prior cardiac event, and heart failure.8,9 Our findings confirm the link between many of these comorbidities and the development of cardiac toxicity after allo-HCT. In particular, our analysis determined that age >55, HTN, and 3 factors in the HCT-CI score (cardiac, arrhythmia, and diabetes) were significant predictors of development of cardiac toxicity by day +100 after allo-HCT. Lin et al performed a retrospective analysis evaluating cardiomyopathy after PTCy in 176 patients who underwent allo-HCT, including 141 from haploidentical donors. The researchers determined that age >60 years and HCT-CI ≥4 are predictors of post-HCT cardiomyopathy.7 Our study confirmed these results and suggests that age >55, HTN, and the cardiac, arrhythmia, and diabetes components of the HCT-CI score are driving forces in the development of cardiac toxicity after allo-HCT. In addition, our multivariate analysis demonstrated that a Cardiac Risk Stratification Score ≥2 is associated with a significant risk of developing cardiac toxicity compared with a score of ≤1. HCT physicians and providers should perform a thorough evaluation of these adverse predictors and discuss the risk of cardiac toxicity with patients before proceeding with allo-HCT. Future external studies are needed to analyze other cumulative risk factor combinations associated with cardiac toxicity and potentially validate the use of the proposed Cardiac Risk Stratification Score.
To our knowledge, this is the first study to review cardiac toxicity in only the matched allo-HCT setting during the current era of PTCy-based GVHD prophylaxis. Even though cyclophosphamide has been associated with cardiac toxicity, we found no significant difference in the incidence of cardiac toxicity between the patients receiving PTCy and non-PTCy GVHD prophylaxis. In addition, the patients who received PTCy in our study were older and more often had active disease, factors that are associated with increased toxicity and worse outcomes after allo-HCT. These results suggest that PTCy-based GVHD prophylaxis may not increase acute cardiac toxicity after matched allo-HCT. Lin et al also found no significant difference in the incidence of post-HCT cardiomyopathy between patients who received PTCy vs non-PTCy prophylaxis. Although 21.9% of the patients in their study developed cardiomyopathy after HCT with PTCy, they described the cardiomyopathy to be transient, reversible, and often sepsis induced.7 The patient populations reviewed in Lin et al and our present study are different, in that their study was primarily in haploidentical HCT recipients, whereas our study excluded those recipients. PTCy has established itself as the standard GVHD prophylaxis used after haploidentical HCT with acceptable rates of GVHD, relapse, and survival.16 Standard GVHD prophylaxis in the matched allo-HCT setting has historically included a calcineurin inhibitor with MTX, with or without ATG, with recent studies proposing that PTCy may be more beneficial in this setting as well.5 Our results are significant because cardiovascular risk is a current consideration in the decision of what GVHD prophylaxis platform to use in the matched donor allo-HCT setting. PTCy is often avoided in patients with a history of cardiac comorbidities. With similar rates of cardiac toxicity between the PTCy and non-PTCy groups, our results suggest that the preferred GVHD prophylaxis platform may not have to be altered because of cardiac comorbidities alone; however, this should be confirmed in prospective trials.
The improvement in outcomes for patients who received PTCy-based GVHD prophylaxis is an interesting finding that appears to be driven by a significant difference in NRM. Reviewing the causes of death among the 2 groups revealed a numerically higher number of deaths attributable to infectious causes, liver failure, and disease recurrence in the non-PTCy group. Although the numbers are small, we postulate that PTCy could provide an improvement in immune reconstitution of memory T cells that can protect against opportunistic infections.17 Another potential factor in the improvement of OS in the PTCy group could be related to the use of ATG in the non-PTCy group. Ten of the 14 patients with non-PTCy treatment who died were recipients of MUD allo-HCT and received ATG as part of their preparative regimen and GVHD prophylaxis. The use of ATG has been associated with delayed immune reconstitution and a higher incidence of infections.18,19 These factors may have contributed to the worse NRM and lower survival in the non-PTCy group.
The limitations of this study include its retrospective, single-center design and the lack of long-term follow-up. We focused on acute cardiac toxicities up to day +100 after allo-HCT and used objective definitions. Electrocardiograms or echocardiograms after allo-HCT are not routinely performed before day +100 at our institution, and the lack of these data are a major limitation of our retrospective comparison. Thus, the rates of cardiac toxicity may be underestimated because of potential asymptomatic cardiac toxicities that were not captured in our review. Longer follow-up is needed to evaluate for long-term cardiac toxicity, which has been shown to place a significant burden on survivors of allo-HCT.9,20 In addition, we were unable to consistently record other possible pre-HCT risk factors, such as cardiac biomarkers or analysis from cardiac imaging with details of diastolic function. Furthermore, the retrospective study design did not account for physician preference to avoid PTCy-based GVHD prophylaxis in patients with known cardiac comorbidities.
BMTCTN 1703 is a phase 3, prospective, randomized trial that compares tacrolimus/MTX to PTCy-based GVHD prophylaxis in allo-HCT.21 In addition to this study’s primary end point, it is also important to review the acute and long-term cardiac toxicities developed by patients in this trial. The results will add to the prospective data that are currently available concerning cardiac toxicity with PTCy-based GVHD prophylaxis in the matched allo-HCT setting.
In conclusion, the results of our study suggest that the incidence of cardiac toxicity is low in the current era of PTCy-based GVHD prophylaxis and that PTCy does not affect the development of acute cardiac toxicity after matched allo-HCT. The improved survival and toxicity outcomes in our study provide further support for the use of PTCy in patients with limited cardiac comorbidities.
Acknowledgment
The authors thank Mark Tanner for scientific editing and assistance with manuscript preparation.
Authorship
Contribution: J.Y. and L.W. designed the research, collected and analyzed the data, and wrote the manuscript; R.M.S. designed and performed the statistical analyses, interpreted the data, and wrote the manuscript; G.R. collected the data; J.B., E.S., and R.C. analyzed the data and edited the manuscript; and U.P. designed the research, analyzed the data, and wrote the manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Uday Popat, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: upopat@mdanderson.org.
References
- 1.Chow EJ, Wong K, Lee SJ, et al. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(6):794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhere V, Edelman S, Waller EK, et al. Myeloablative busulfan/cytoxan conditioning versus reduced-intensity fludarabine/melphalan conditioning for allogeneic hematopoietic stem cell transplant in patients with acute myelogenous leukemia. Leuk Lymphoma. 2018;59(4):837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kröger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43-53. [DOI] [PubMed] [Google Scholar]
- 4.Soltermann Y, Heim D, Medinger M, et al. Reduced dose of post-transplantation cyclophosphamide compared to ATG for graft-versus-host disease prophylaxis in recipients of mismatched unrelated donor hematopoietic cell transplantation: a single-center study. Ann Hematol. 2019;98(6):1485-1493. [DOI] [PubMed] [Google Scholar]
- 5.Bolaños-Meade J, Reshef R, Fraser R, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6(3):e132-e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Homsi AS, Roy TS, Cole K, Feng Y, Duffner U. Post-transplant high-dose cyclophosphamide for the prevention of graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(4):604-611. [DOI] [PubMed] [Google Scholar]
- 7.Lin CJ, Vader JM, Slade M, DiPersio JF, Westervelt P, Romee R. Cardiomyopathy in patients after posttransplant cyclophosphamide-based hematopoietic cell transplantation. Cancer. 2017;123(10):1800-1809. [DOI] [PubMed] [Google Scholar]
- 8.Qazilbash MH, Amjad AI, Qureshi S, et al. Outcome of allogeneic hematopoietic stem cell transplantation in patients with low left ventricular ejection fraction. Biol Blood Marrow Transplant. 2009;15(10):1265-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armenian SH, Bhatia S. Cardiovascular disease after hematopoietic cell transplantation--lessons learned. Haematologica. 2008;93(8):1132-1136. [DOI] [PubMed] [Google Scholar]
- 10.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014; 32(31):3497-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggeri A, Labopin M, Bacigalupo A, et al. Posttransplant cyclophosphamide for GVHD prophylaxis in HLA matched sibling or matched-unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events, Version 4.0 (version 4.03). 2009. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf/. Accessed on 28 November 2020
- 15.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs EJ. HLA-haploidentical blood or marrow transplantation with high-dose, post-transplantation cyclophosphamide. Bone Marrow Transplant. 2015;50(S2 Suppl 2):S31-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberto A, Castagna L, Zanon V, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood. 2015;125(18):2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storek J, Mohty M, Boelens JJ. Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015; 21(6):959-970. [DOI] [PubMed] [Google Scholar]
- 19.Figgins B, Hammerstrom A, Ariza-Heredia E, Oran B, Milton DR, Yeh J. Characterization of viral infections after antithymocyte globulin-based conditioning in adults undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(9):1837-1843. [DOI] [PubMed] [Google Scholar]
- 20.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155(1):21-32. [DOI] [PubMed] [Google Scholar]
- 21.DeFilipp Z, Burns LJ, Jaglowski SM, et al. A new standard in graft-versus-host disease prophylaxis? An introduction to Blood and Marrow Transplant Clinical Trials Network 1703. Biol Blood Marrow Transplant. 2020;26(12):e305-e308. [DOI] [PMC free article] [PubMed] [Google Scholar]