Abstract
Purpose
Immune checkpoint blockade has led to a significant improvement of patient survival in metastatic colorectal cancer (CRC) with DNA mismatch repair-deficiency (dMMR)/microsatellite instability-high (MSI-H). However, not all these patients are sensitive to monoimmunotherapy. We firstly presented a case series of advanced dMMR/MSI-H CRCs treating with PD-1 inhibitor-based chemoradioimmunotherapy (CRIT).
Methods and Materials
We assessed the short-term efficacy and safety of CRIT in advanced dMMR/MSI-H CRCs, and also did next-generation sequencing (NGS) assays.
Results
Our analysis included five advanced dMMR/MSI-H CRCs who have received toripalimab-based CRIT. Toripalimab was given 240mg every three weeks, and the radiation dose was 45-50 gray in 25 fractions. Chemotherapy regimens consisted of CAPOX in three patients, capecitabine in one patient, and mFOLFOX6 in one patient. Initially, two patients displayed complete response (CR), and three patients achieved partial response (PR) on imaging findings. Afterwards, one PR patient was confirmed pathological complete response after surgery, leading to three CR cases in total. Hematological toxicity was the most common adverse effect, and only two patients developed mild immune-related adverse effects besides. All the treatment-related adverse events were under control. Based on the NGS results, the median intratumor heterogeneity was 0.19 (range 0-0.957), which was less in CR patients than PR patients (P = 0.019). Genetic mutations at DNA damage repair genes and the JAK1 gene were also observed.
Conclusions
For advanced dMMR/MSI-H CRC, anti-PD-1 based CRIT is effective and safe. Further studies are required to better clarify the potential role and mechanism of CRIT as a viable therapeutic strategy in this population.
Keywords: chemotherapy, radiotherapy, immunotherapy, programmed cell death protein 1 inhibitor, colorectal cancer
Introduction
The latest cancer statistics showed that the incidence and mortality of colorectal cancer (CRC) ranked third among all cancer in men and women (1). By the year 2030, there will be more than 2.2 million new cases and 1.1 million deaths of CRC (2). In the past ten years, immunotherapy especially immune checkpoint blockade (ICB) therapy, has become a major therapeutic strategy for multiple types of solid cancers. ICB, including programmed cell death protein-1 (PD-1) inhibitors and anti-cytotoxic T lymphocyte antigen-4 (CTLA-4) antibodies, have significantly improved patients’ survival in DNA mismatch repair-deficiency (dMMR)/microsatellite instability-high (MSI-H) metastatic colorectal cancer (mCRC) (3–8). As a result, the FDA has approved PD-1 inhibitors, pembrolizumab and nivolumab, as the second-line therapy for dMMR/MSI-H mCRC. Nivolumab ± ipilimumab or pembrolizumab alone were recommended for neoadjuvant treatments for resectable or unresectable synchronous liver and/or lung metastastic dMMR/MSI-H CRC by the NCCN guideline (9, 10), and for primary treatments for unresectable metachronous metastatic dMMR/MSI-H CRC who has received FOLFOX/CAPOX therapy within past 12 months (9, 10). However, there are still a large amount of dMMR/MSI-H CRCs who are resistant to ICB. To date, many ongoing clinical trials of combined ICB with chemotherapy, targeted therapy, or radiotherapy aim to improve the efficacy of ICB in these patients. For instance, chemoradiotherapy can upregulate PD-L1 expression in rectal cancer (11). PD-1 or PD-L1 antibodies did not only improve local control but also had a systemic efficacy in irradiated colon cancer mouse models (12). Besides, a preclinical study also showed that chemotherapy combined with ICB could enhance radiotherapy-induced abscopal effects (13). Therefore, it can be probably inferred that chemoradioimmunotherapy (CRIT) may improve the efficacy of ICB in dMMR/MSI-H CRC patients. Here, we firstly presented a retrospective case series of five advanced dMMR/MSI-H CRC patients who achieved good response to CRIT.
Materials and Methods
Study Design
From March 2019 to December 2019, advanced dMMR/MSI-H CRC patients receiving CRIT were retrospectively analyzed in this study.
This study was conducted following the Declaration of Helsinki and received the full approval of the Sun Yat-sen University Cancer Center Institutional Review Board on Medical Ethics (B2020-141-01). All patients provided written informed consent before therapy.
Next-Generation Sequencing (NGS)
Five formalin-fixed paraffin-embedded (FFPE) specimens of all patients were obtained before CRIT. All specimens were primary tumors except abdominal metastasis of patient 2.
Genomic DNAs were isolated from FFPE specimens and blood using the GeneRead DNA FFPE Kit (Qiagen) and Qiagen DNA blood mini kit (Qiagen), respectively. Then, extracted DNAs were amplified, purified, and analyzed using an NGS panel (YuceOneTM Plus X, Yucebio, China).
Sequencing reads with > 10% N rate and/or > 10% bases with quality score < 20 were filtered using SOAPnuke (Version 1.5.6). The clean reads were mapped to the UCSC reference human genome (version hg19) using the Burrows Wheeler Alignment tool (BWA, Version 0.7.12). SAMtools (Version 1.3) was used for alignment data conversion, sorting, and indexing. The duplicates were marked by SAMBLASTER (Version 0.1.22) to reduce biases in downstream analyses. The single nucleotide variants were detected using VarScan (Version 2.4) with parameters –min-coverage-normal 20 –min-coverage-tumor 20 –min-reads23. The mutations were filtered using a customized Perl script to eliminate false positives and annotated by SnpEff (Version 4.3).
Tumor mutational burden (TMB) was calculated using non-silent somatic mutations, including coding base substitution and indels. TMB > 20 muts/Mb was defined as TMB-High (TMB-H). Microsatellite instability (MSI) scores were analyzed by MSIsensor (Version 0.2). MSI scores > 20 were defined as MSI-High (MSI-H). Tumor neoantigen burden (TNB) was measured as the number of mutations that could generate neoantigens per megabase. TNB > 4.5 neos/Mb was defined as TNB-High (TNB-H). The ratio of subclone mutations to all mutations was interpreted as intratumor heterogeneity (ITH).
Frameshift, nonsense, and splice site alterations were classified as deleterious. Missense mutations reported as pathogenic by the Catalogue of Somatic Mutations in Cancer (COSMIC) (14) and/or ClinVar (15) databases, and/or with a SIFT score of <0.05 (16), were classified as deleterious.
Clinical Evaluation
Pretreatment tumors were staged according to the criteria of the American Joint Committee on Cancer 8th edition. Clinical response based imaging findings was assessed according to the RECIST 1.1 (17). For surgical specimens, no residual tumor cell was defined as pathological complete response (pCR). Follow-up data were collected from the follow-up platform of the hospital.
Statistical Analysis
Statistical analyses were performed using SPSS 26.0 statistical software (IBM, NY, USA). Comparisons between two groups were evaluated by Student t-test. p < 0.05 at two sides was considered statistically significant.
Results
Patient Demographic and Clinical Characteristics
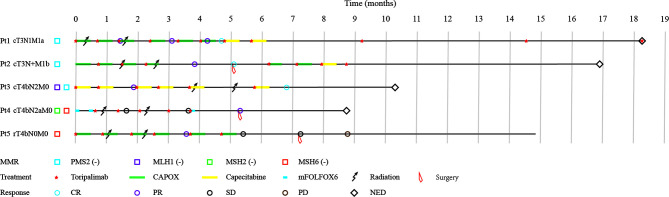
Overall, five dMMR/MSI-H CRC patients were identified and enrolled. The median age was 37 years old (range 27-64), and three (3/5) were male. Of all patients, primary tumors were located at right colon (n=3), left colon (n=1) and rectum (n=1). All patients were adenocarcinoma except one adenosquamous carcinoma. Two (2/5) patients had metastases: right kidney metastasis in patient 1, and liver and abdominal cavity metastases in patient 2. The other three patients had large tumors invaded adjacent organs and regions, including the gallbladder, duodenum, liver, and peritoneum of patient 3, peritoneum and abdominal wall of patient 4, and psoas major muscle and ureter of patient 5. Prior to this study, patient 4 had received three cycles of CAPOX, but no tumor regression. Besides, patient 5 had received surgery and adjuvant FOLFOX chemotherapy for the primary tumor but found the tumor regrowth a year later. The demographic, clinical, and therapeutic details of the patients were shown in Table 1 and Figure 1 .
Table 1.
Demographic features, clinical characteristics, and therapeutic regimens.
| Items | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Gender | Male | Male | Female | Female | Male |
| Age (years) | 37 | 27 | 62 | 35 | 64 |
| Tumor site | Rectum | Right colon | Right colon | Left colon | Right colon |
| Histology | Adenocarcinoma | Adenocarcinoma | Adenosquamous carcinoma | Adenocarcinoma | Adenocarcinoma |
| Stage | cT3N1M1a | cT3N+M1b | cT4bN2M0 | cT4bN2aM0 | rT4bN0M0 |
| Metastatic site | Right kidney | Liver and abdominal cavity | None | None | None |
| Invaded adjacent organ | NA | NA | Gallbladder, duodenum, liver, and peritoneum | Peritoneum and abdominal wall | Psoas major muscle, and ureter |
| MSH2 | + | + | + | – | + |
| MSH6 | + | + | + | – | – |
| PMS2 | – | – | – | + | + |
| MLH1 | + | + | – | + | + |
| Previous chemotherapy | No | No | No | CAPOX | FOLFOX |
| Previous surgery | No | No | No | No | Radical surgery |
| Combined chemotherapy | CAPOX | CAPOX | Capecitabine | mFOLFOX6 | CAPOX |
| Combined radiotherapy | 50Gy/25F | 50Gy/25F | 45Gy/25F | 48Gy/25F | 50Gy/25F |
| Surgery after ICB | No | Radical surgery | No | Radical surgery | Palliative surgery |
| Total course of ICB | 10 | 7 | 6 | 5 | 6 |
| Course of ICB for best response | 6 | 3 | 6 | 5 | 4 |
| Imaging response | CR | PR | CR | PR | PR |
| Pathology response | NA | pCR | NA | TRG(2ypT4bN0M0) | NA |
CR, complete response; ICB, immune checkpoint blockade; NA, not available; pCR, pathological complete response; PR, partial response; TRG, tumor regression response.
Figure 1.
Patient characteristics, treatments, and outcomes. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Treatment Strategies
All patients received PD-1 inhibitor-Toripalimab 240 mg intravenously once every three weeks with a median number of 6 cycles (range 5-11 cycles) and a median treatment duration of 5.8 months (range 3.1-18.3 months). Each individualized chemotherapy regimen was decided according to patients’ previous treatment and health conditions. Patient 3 received capecitabine as she was old and weak. The chemotherapy regime of patient 4 was switched to mFOLFOX6 because she was insensitive to CAPOX. Other patients all received CAPOX. All patients were also treated with intensity-modulated radiation therapy (IMRT) for the primary tumor at 45-50 gray in 25 fractions. After CRIT, patient 2 and patient 4 underwent radical surgery, whereas patient 5 underwent palliative surgery. The integral treatments for each patient were illustrated in Table 1 and Figure 1 .
Efficacy Results
After a median period of 4.4 months (range 3.1-6.8 weeks) from the first Toripalimab injection and 7.1 weeks (range 5.3-13.3 weeks) from the last IMRT, five patients all achieved objective responses, including two complete response (CR) and three partial responses (PR) according to RECIST 1.1. The two CR patients took a watch-and-wait strategy without surgery. Primary and metastases of patient 2 were confirmed pCR after surgery, resulting in three CR patients in total. Patient 4 underwent surgery and had a downstage tumor from T4bN2a to T4bN0, and the tumor regression grade was TRG2 based on NCCN guidelines. The primary tumor of patient 5 was smaller than the initial volume but found invaded the ureter, small intestine, and iliac blood vessels during surgery. As a result, patient 5 underwent palliative surgery for organ conservations. The images of all patients before and after CRIT were displayed in Supplementary Figures 1–5 . With a median follow-up time of 14.8 months (range 8.1-18.3), only patient 5 had progressive disease at 8.8 months after the first Toripalimab injection. All the other cases had no clinical signs of disease progression or recurrence.
Treatment-Related Adverse Events (TRAEs)
Till September 2020, all TRAEs happened for all patients were shown Table 2 . The most common adverse events were leukopenia and neutropenia. Most TRAEs in this study were at grade 1 or grade 2. Only one patient experienced grade 4 thrombocytopenia. One patient had grade 2 immune-related increased TSH, and therefore had levothyroxine therapy. All the adverse events were under control, and patients recovered during treatment.
Table 2.
Treatment-related adverse events.
| Adverse event | Grade 1 (n) | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) |
|---|---|---|---|---|
| Any | 4 | 5 | 3 | 1 |
| Leukopenia | 1 | 2 | 1 | 0 |
| Neutropenia | 3 | 0 | 1 | 0 |
| Anemia | 1 | 1 | 1 | 0 |
| Thrombocytopenia | 0 | 0 | 1 | 1 |
| ALT elevation | 0 | 2 | 0 | 0 |
| AST elevation | 3 | 0 | 0 | 0 |
| Increased creatinine | 1 | 0 | 0 | 0 |
| Diarrhea | 0 | 2 | 0 | 0 |
| Nausea | 0 | 1 | 0 | 0 |
| Vomit | 0 | 1 | 0 | 0 |
| Rash | 1 | 0 | 0 | 0 |
| TSH increased | 0 | 1 | 0 | 0 |
| fT3 decreased | 0 | 1 | 0 | 0 |
| Infection | 0 | 2 | 0 | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TSH, thyroid stimulating hormone; fT3, free triiodothyronine.
Genetic Analyses
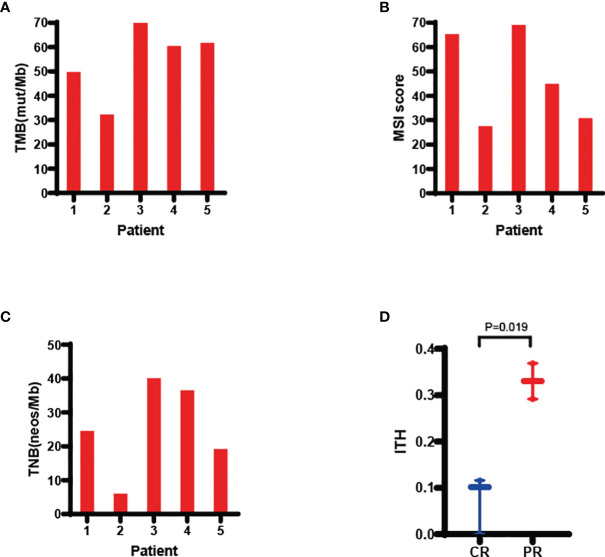
Based on NGS results shown in Figures 2A–D , the median value of TMB, MSI score, TNB and ITH were 60.48mut/Mb (range 32.31-77.18), 44.95 (range 27.45-69.06), 24.56 neos/Mb (range 5.98-40.09) and 0.116 (range 0-0.369), respectively. CR patients had a lower ITH than PR patients (p=0.019, Figure 2D ). TMB, MSI scores, and TNB did not correlate with treatment response ( Supplementary Figures 6A–C ).
Figure 2.
Molecular analyses of TMB (A), MSI score (B), TNB (C), and ITH (D). ITH was lower in CR than PR patients. Values were presented as median and range. Comparisons between CR and PR patients was tested using two-sided t-test. CR, complete response; CRIT, chemoradioimmunotherapy; ITH, intratumor heterogeneity; MSI, microsatellite instability; PR, partial response; TMB, tumor mutational burden; TNB, tumor neoantigen burden.
Additionally, 19 mutations in 12 DNA damage repair (DDR) genes were observed. Among them, most alternations were missense mutations (10/19, 52.6%), the remaining included frameshift mutations (6/19, 31.6%), splice site (2/19, 10.5%), and nonsense alterations (1/19, 5.3%). ATM (4/5, 80.0%) was the most commonly mutated DDR gene. All missense mutations were unique, of which only 7 were previously reported in COSMIC and/or ClinVar database and 5 of them were deleterious. In silico evaluation, another 2 potentially deleterious missense mutations were identified. The median number of deleterious DDR mutations patients harbored was 3 (range 1-6). All the DDR gene mutations were listed in Supplementary Table 1 . Genes involved in the mismatch repair pathway (6/10, 60%) were the most common ( Supplementary Table 2 ).
Furthermore, JAK1 deleterious mutation was observed in patients 1 (K924fs) and 3 (T533M), and JAK1 neutral mutation was found in patient 4 (R93H). The most frequently altered genes were APC (100%), FAT1 (80%), TRRAP (80%), KAT6B (80%), CIC (80%) and ATM (80%) ( Supplementary Figure 7 ).
Discussion
In this study, we firstly reported a retrospective case series of advanced dMMR/MSI-H CRC patients treated with CRIT. After treatment, all five patients achieved beneficial responses of pCR (1/5, 20%), cCR (2/5, 40%), or PR (2/5, 40%), leading to a 100% overall response rate. The good efficacy suggested that this strategy might be a promising treatment for advanced dMMR/MSI-H CRC.
It has been well studied that locally advanced rectal cancer can benefit from neoadjuvant chemoradiotherapy. Our previous work found that locally advanced unresectable colon cancer responded well to neoadjuvant chemoradiotherapy, with a 26.3%-38.1% pCR rate (18, 19). Consequently, neoadjuvant chemoradiotherapy has been recommended for T4b patients with local invasion of the sigmoid colon by the Chinese Society of Clinical Oncology (20). However, whether dMMR/MSI-H CRC patients could benefit from preoperative chemoradiotherapy as well as microsatellite instability stale or low CRC was still a controversial clinical question (21, 22). In our present cohort, a 100% objective response rate and 60% CR rate were achieved, indicating that they could benefit from CRIT.
For patients whose primary tumor did not invade adjacent organs, immunotherapy alone or combined with chemotherapy could result in CR (23, 24). So, radiotherapy and chemotherapy may not be essential for these patients. But, the two T3 patients with metastases were treated with CRIT to maximize the abscopal effect, and they finally achieved CR. Similarly, a preclinical study also showed that the abscopal tumor has the best response to triple therapy of cisplatin, radiation, and PD-1 inhibitor (13), implying that CRIT is effective for mCRC.
Though all patients in our study were effective, two patients with T4b colon cancer did not achieve CR. One of them was still unresectable after CRIT, and his disease progressed 8.8 months after the Toripalimab initiation. In the NICHE study (23), only one patient with T4b tumor and three of seven patients with T4a tumors did not achieve pCR. Also, some T4b tumors could not achieve CR after neoadjuvant PD-1 blockade in published case reports (24, 25). For dMMR/MSI-H CRC with large tumors invading multiple essential organs, the clinical response to single-agent immunotherapy or immunotherapy-based combination therapy is still unsatisfactory. Thus, it is necessary to explore optimized regimens of these therapeutic methods to achieve better clinical efficacy in these patients.
Up to now, DDR gene alterations, especially deleterious alternations, have been demonstrated to be associated with improved clinical outcomes in metastatic urothelial cancer (26), metastatic clear cell renal cell carcinoma (27), and non-small-cell lung cancer (28) treated with PD-(L)1 inhibitors. Likewise, a recent study also found that CRC patients with DDR mutations could obtain a better prognosis when using ICB (29). This was consistent with our patients that DDR genes alterations were frequent, which may contribute to their effective response to CRIT. Additionally, ATM was the most commonly mutated DDR gene in these CRC and patients with ATM mutations had a significantly better overall survival than those without when treated with ICB (30). In addition, ATM inhibitors potentiated anti-PD-1 therapy in the mouse model (30). It was also reported that ATM inhibition and radiation could enhance the efficacy of ICB by increasing tumoral immunogenicity (31). Therefore, ATM may be a potential biomarker of immunotherapy, and ATM inhibitors with ICB and radiation may be an efficacious therapeutic regimen. Interestingly, ATM, ATR, and LIG3 genes co-mutation was only found in two PR patients. This might imply that the molecular contributions of multiple DDR gene co-mutation may differ from single DDR gene mutation. Further studies are needed to disclose the effects and mechanisms of how DDR mutations impact sensitivity to CRIT.
Additionally, driver mutations of the JAK-STAT pathway may contribute to tumor progression during immunotherapy (32, 33). In our study, both of the two patients with pathogenic JAK1 mutation achieved CR, and the patient with neutral JAK1 mutation achieved PR. Perhaps, chemoradiotherapy can dismiss the negative impact of JAK1 mutations in some way.
There were some limitations to our study. Our study only enrolled five patients, and the follow-up period was short. Also, baseline characteristics and treatment strategies were not incoherent of all patients. Although we did genetic analysis, we could not illustrate the definite mechanism of CRIT. Consequently, our observations warrant further considerations and validations in a larger sample size. We are doing a phase II clinical study (NCT04301557) of Toripalimab combined with chemoradiotherapy for dMMR/MSI-H locally advanced CRC, which may provide more clinical evidence to clarify the role of CRIT for CRC.
In general, CRIT is effective and safe for advanced dMMR/MSI-H CRC. Further studies are required to investigate the potential role and mechanism of CRIT in this population.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The whole sequence data reported in this paper available in the Genome Warehouse in National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences, under accession number PRJCA007192 at https://ngdc.cncb.ac.cn/databases, upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Sun Yat-sen University Cancer Center Institutional Review Board on Medical Ethics. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: CJZ, TJ, and WWX. Methodology: CJZ, WWX, and YJX. Data curation: CJZ, QXW, and PQC. Investigation: YTZ, ZKZ, DFW, and HQL. Resources: ZFZ, RZ, and YHG. Software: CJZ and WWX. Writing - Original draft: CJZ, WWX, and YJX. Editing: CS. Writing-Review and Editing: RZ and YHG. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China under Grant 81672987 and 82073329; and the Natural Science Foundation of Guangdong Province under Grant 2020A1515011286.
Conflict of Interest
Authors YJX, YTZ, ZKZ, DFW, HQL and CS are employed by YuceBio Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MYC declared a shared affiliation with several of the authors (CJZ, TJ, QXW, ZFZ, PQC, RZ, WWX and YHG) to the handling editor at time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the medical staff and patients for their contribution to this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.784336/full#supplementary-material
Abbreviations
CRC, colorectal cancer; dMMR, mismatch repair-deficiency; MSI-H, microsatellite instability-high; CRIT, chemoradioimmunotherapy; PD-1, programmed cell death protein-1; CR, complete response; PR, partial response; pCR, pathological complete response; ICB, immune checkpoint blockade; CTLA-4, cytotoxic T lymphocyte antigen-4; mCRC, metastatic colorectal cancer; FFPE, formalin-fixed paraffin-embedded; TMB, tumor mutational burden; TNB, tumor neoantigen burden; ITH, intratumor heterogeneity; COSMIC, the Catalogue of Somatic Mutations in Cancer; TRAE, treatment-related adverse events; DDR, DNA damage repair.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 3. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors With Mismatch-Repair Deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez-Roca C, et al. Safety and Antitumor Activity of the Anti-PD-1 Antibody Pembrolizumab in Patients With Advanced Colorectal Carcinoma. PloS One (2017) 12(12):e0189848. doi: 10.1371/journal.pone.0189848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science (2017) 357(6349):409–13. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in Patients With Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol (2017) 18(9):1182–91. doi: 10.1016/s1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol (2018) 36(8):773–9. doi: 10.1200/jco.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 8. Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol (2020) 38(1):11–9. doi: 10.1200/jco.19.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network . (NCCN) Clinical Practice Guidelines in Oncology. Rectal Cancer. Version 2. (2021). Available at: https://www.nccn.org/patients/guidelines/cancers.aspx#rectal (Accessed 10 Sep 2021) [Google Scholar]
- 10. National Comprehensive Cancer Network . (NCCN) Clinical Practice Guidelines in Oncology. Colon Cancer. Version 3. (2021). Available at: https://www.nccn.org/patients/guidelines/cancers.aspx#colon (Accessed 10 Sep 2021) [Google Scholar]
- 11. Hecht M, Büttner-Herold M, Erlenbach-Wünsch K, Haderlein M, Croner R, Grützmann R, et al. PD-L1 Is Upregulated by Radiochemotherapy in Rectal Adenocarcinoma Patients and Associated With a Favourable Prognosis. Eur J Cancer (2016) 52–60. doi: 10.1016/j.ejca.2016.06.015 [DOI] [PubMed] [Google Scholar]
- 12. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res (2014) 74(19) 5458–68. doi: 10.1158/0008-5472.CAN-14-1258 [DOI] [PubMed] [Google Scholar]
- 13. Luo R, Firat E, Gaedicke S, Guffart E, Watanabe T, Niedermann G. Cisplatin Facilitates Radiation-Induced Abscopal Effects in Conjunction With PD-1 Checkpoint Blockade Through CXCR3/CXCL10-Mediated T-Cell Recruitment. Clin Cancer Res (2019) 25(23):7243–55. doi: 10.1158/1078-0432.Ccr-19-1344 [DOI] [PubMed] [Google Scholar]
- 14. Tate JG, Bamford S, Jubb HC, Sondka Z, Beare DM, Bindal N, et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res (2019) 47(D1):D941–d7. doi: 10.1093/nar/gky1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, et al. ClinVar: Public Archive of Interpretations of Clinically Relevant Variants. Nucleic Acids Res (2016) 44(D1):D862–8. doi: 10.1093/nar/gkv1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar P, Henikoff S, Ng PC. Predicting the Effects of Coding Non-Synonymous Variants on Protein Function Using the SIFT Algorithm. Nat Protoc (2009) 4(7):1073–81. doi: 10.1038/nprot.2009.86 [DOI] [PubMed] [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 18. Qiu B, Ding PR, Cai L, Xiao WW, Zeng ZF, Chen G, et al. Outcomes of Preoperative Chemoradiotherapy Followed by Surgery in Patients With Unresectable Locally Advanced Sigmoid Colon Cancer. Chin J Cancer (2016) 35(1):65. doi: 10.1186/s40880-016-0126-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang H, Yu X, Xiao WW, Wang QX, Zhou WH, Zeng ZF, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery in Patients With Unresectable Locally Advanced Colon Cancer: A Prospective Observational Study. Oncol Targets Ther (2018) 11:409–18. doi: 10.2147/ott.S150367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. CSOCOC DATGFCCWG . Chinese Society of Clinical Oncology (CSCO) Diagnosis and Treatment Guidelines for Colorectal Cancer 2018 (English Version). Chin J Cancer Res (2019) 31(1):117–34. doi: 10.21147/j.issn.1000-9604.2019.01.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, et al. Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis. Ann Surg (2020) 271(4):716–23. doi: 10.1097/sla.0000000000003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cercek A, Dos Santos Fernandes G, Roxburgh CS, Ganesh K, Ng S, Sanchez-Vega F, et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin Cancer Res (2020) 26(13):3271–9. doi: 10.1158/1078-0432.Ccr-19-3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat Med (2020) 26(4):566–76. doi: 10.1038/s41591-020-0805-8 [DOI] [PubMed] [Google Scholar]
- 24. Liu DX, Li DD, He W, Ke CF, Jiang W, Tang JH, et al. PD-1 Blockade in Neoadjuvant Setting of DNA Mismatch Repair-Deficient/Microsatellite Instability-High Colorectal Cancer. Oncoimmunology (2020) 9(1):1711650. doi: 10.1080/2162402x.2020.1711650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J, Cai J, Deng Y, Wang H. Complete Response in Patients With Locally Advanced Rectal Cancer After Neoadjuvant Treatment With Nivolumab. Oncoimmunology (2019) 8(12):e1663108. doi: 10.1080/2162402x.2019.1663108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MM, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J Clin Oncol (2018) 36(17):1685–94. doi: 10.1200/jco.2017.75.7740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ged Y, Chaim JL, DiNatale RG, Knezevic A, Kotecha RR, Carlo MI, et al. DNA Damage Repair Pathway Alterations in Metastatic Clear Cell Renal Cell Carcinoma and Implications on Systemic Therapy. J Immunother Cancer (2020) 8(1):e000230. doi: 10.1136/jitc-2019-000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricciuti B, Recondo G, Spurr LF, Li YY, Lamberti G, Venkatraman D, et al. Impact of DNA Damage Response and Repair (DDR) Gene Mutations on Efficacy of PD-(L)1 Immune Checkpoint Inhibition in Non-Small Cell Lung Cancer. Clin Cancer Res (2020) 26(15):4135–42. doi: 10.1158/1078-0432.Ccr-19-3529 [DOI] [PubMed] [Google Scholar]
- 29. Zhang W, Zhang L, Jiang H, Li Y, Wang S, Wang Q. Mutations in DNA Damage Response Pathways as a Potential Biomarker for Immune Checkpoint Blockade Efficacy: Evidence From a Seven-Cancer Immunotherapy Cohort. Aging (2021) 13(21):24136–54. doi: 10.18632/aging.203670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu M, Zhou M, Bao X, Pan D, Jiao M, Liu X, et al. ATM Inhibition Enhances Cancer Immunotherapy by Promoting mtDNA Leakage and cGAS/STING Activation. J Clin Invest (2021) 131(3):e139333. doi: 10.1172/JCI139333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Q, Green MA-O, Lang X, Lazarus J, Parsels JD, Wei S, et al. Inhibition of ATM Increases Interferon Signaling and Sensitizes Pancreatic Cancer to Immune Checkpoint Blockade Therapy. Cancer Res (2019) 79(15):3940–51. doi: 10.1158/0008-5472.CAN-19-0761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell (2016) 167(2):397–404.e9. doi: 10.1016/j.cell.2016.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov (2017) 7(2):188–201. doi: 10.1158/2159-8290.Cd-16-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The whole sequence data reported in this paper available in the Genome Warehouse in National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences, under accession number PRJCA007192 at https://ngdc.cncb.ac.cn/databases, upon reasonable request.