Abstract
This study sought to compare the brachial and carotid hemodynamic response to hot water immersion (HWI) between healthy young men and women. Ten women (W) and 11 men (M) (24 ± 4 yr) completed a 60-min HWI session immersed to the level of the sternum in 40°C water. Brachial and carotid artery hemodynamics (Doppler ultrasound) were measured at baseline (seated rest) and every 15 min throughout HWI. Within the brachial artery, total shear rate was elevated to a greater extent in women [+479 (+364, +594) s−1] than in men [+292 (+222, +361) s−1] during HWI (P = 0.005). As shear rate is inversely proportional to blood vessel diameter and directly proportional to blood flow velocity, the sex difference in brachial shear response to HWI was the result of a smaller brachial diameter among women at baseline (P < 0.0001) and throughout HWI (main effect of sex, P < 0.0001) and a greater increase in brachial velocity seen in women [+48 (+36, +61) cm/s] compared with men [+35 (+27, +43) cm/s] with HWI (P = 0.047) which allowed for a similar increase in brachial blood flow between sexes [M: +369 (+287, +451) mL/min, W: +364 (+243, +486) mL/min, P = 0.943]. In contrast, no differences were seen between sexes in carotid total shear rate, flow, velocity, or diameter at baseline or throughout HWI. These data indicate the presence of an artery-specific sex difference in the hemodynamic response to a single bout of HWI.
Keywords: heat therapy, hyperthermia, sex differences, shear stress, thermal therapy
INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of death worldwide (1). In the United States, CVD currently accounts for approximately one of every three deaths (2), and it is predicted that over 40% of the US population will have some form of CVD by 2030 (3). Heat therapy, in the form of hot baths or sauna, has recently emerged as a promising means of improving cardiovascular health and reducing CVD burden. A 20+ year prospective cohort study in Finland revealed that regular sauna use was associated with a reduced risk of sudden cardiac death, coronary heart disease-, and CVD-related mortality, and all-cause mortality in men. Furthermore, within this study, an increased frequency and/or duration of sauna bathing was associated with progressively decreased risk of CVD mortality (4). Importantly, these findings were echoed in a subsequent study, which included both men and women (5). More recent research has begun to elucidate the mechanisms behind the aforementioned improvements in cardiovascular health (6–9). For example, Brunt et al. (10) demonstrated that 8 wk of hot water immersion (HWI) reduced carotid wall thickness and improved vascular endothelium-dependent dilation, arterial stiffness, and blood pressure (BP) in healthy young men and women.
Vascular shear stress, the frictional force exerted by blood against arterial walls, is thought to play a key role in mediating many of the vascular adaptations which accompany both acute heat exposure and chronic heat therapy (8). At rest, the pulsatile nature of blood flow results in both forward (antegrade) and backward (retrograde) vascular shear stress. During passive heat stress, the increase in cardiac output and peripheral vascular conductance and the redistribution of blood flow toward the cutaneous circulation promote large increases in both total and antegrade shear stress and reductions in retrograde shear stress (11–21). The magnitude and pattern of this altered shear profile acutely improves vascular endothelium-dependent dilation in a dose-dependent manner (22–24). Although studies investigating vascular function following heating have yielded inconsistent results (16–18), several studies have noted improved macrovascular endothelial function (brachial artery flow-mediated dilation), improved microvascular function (reactive hyperemia response; 15, 20, 25), and increased protection against impaired vascular function after ischemia reperfusion (26, 27) following acute heat exposure. Furthermore, repeated exposure to these episodic changes in vascular shear stress is thought to potentiate both the structural and functional vascular adaptations which accompany chronic heat therapy. The critical role that shear stress plays in eliciting vascular adaptation with heat therapy is best demonstrated by the work of Carter et al. (11). This study found that the strategic inflation of a cuff around one arm, which prevented the heat-induced episodic increases in blood flow and shear stress, abolished the improvements in endothelial function (measured as brachial flow-mediated dilation) accompanying an 8-wk lower limb heating intervention.
As heat therapy emerges as a promising cardiovascular therapeutic option, it is essential to consider how the shear stress response to a single bout of heat exposure may differ among different subject populations. Men and women both display systemic circulatory responses to an acute bout of heat stress, whereby increases in cardiac output and peripheral vascular conductance facilitate large increases in cutaneous blood flow (13, 14, 28, 29). These systemic adjustments not only allow for the dissipation of heat but also serve to alter conduit artery hemodynamics during passive heat stress. Indeed, previous studies conducted in both men and women have noted increases in total and antegrade shear and reductions in retrograde shear with passive heat stress (11–21). Importantly, however, the magnitude of the shear stress response to HWI may differ between men and women due to differences in conduit artery diameter. Women tend to have smaller diameter conduit arteries than men (e.g., 30–33) and, as shear stress is inversely proportional to blood vessel diameter, this may promote a greater increase in shear stress with passive heating in women than in men.
Although this collective evidence demonstrates the promise of heat therapy as a means of promoting vascular adaptation and improving cardiovascular health and the critical role of increased shear stress in potentiating beneficial vascular adaptation, no study to date has compared the hemodynamic response to a single bout of passive heat exposure between men and women. Therefore, the purpose of this study was to compare the brachial and carotid hemodynamic response to HWI between healthy, young men and women. We assessed rectal temperature, skin temperature (above the level of the water), brachial blood pressure, and heart rate (HR) as secondary measures. We hypothesized that men and women would both display increases in total and antegrade and decreases in retrograde shear rate with HWI, in both the brachial and carotid arteries, but that the magnitude of these hemodynamic response patterns would be greater in women than in men.
METHODS
Ethical Approval
This study was approved by the Institutional Review Board at the University of Oregon. Before participation, all the subjects provided oral and written informed consent as set forth by the Declaration of Helsinki.
Subjects
Twenty-one young (10 women and 11 men) volunteers participated in this study. All volunteers were healthy individuals with no history of CVD and not taking prescription medications other than hormonal contraceptives (3/10 women were taking oral hormonal birth control pills and 4/10 women tested had a levonorgestrel-releasing intrauterine device). Menstrual cycle phase was not controlled among female subjects. Based on reported menstrual cycle history, 4 out of 10 women were tested within 5 days of starting menstruation, 4 out of 10 women were tested in the luteal or high hormone phase, and the remaining 2 out of 10 women had irregular/absent menstrual cycles due to use of a levonorgestrel-releasing intrauterine device. Before the study day, the subjects were required to abstain from over-the-counter medications for >24 h, alcohol and caffeine for >12 h, heavy exercise for >24 h, and food for ≥4 h. Before the study day was started, euhydration was evaluated in all subjects by a first morning urine specific gravity of ≤1.024. If urine specific gravity was >1.024, subjects drank 5 mL·kg−1 of body weight before the HWI protocol. This urine sample was also used to conduct a pregnancy test among the female subjects.
Experimental Protocol
Experimental sessions were held in a 23 ± 1°C thermoneutral laboratory environment between the hours of 0900 and 1900. Dry nude body weight was measured before and after HWI to calculate body weight losses. Body surface area was estimated from measures of the subject height and weight (34). Subjects were instrumented with a sterile rectal thermistor probe (YSI series 400: Yellow Spring Instruments, Yellow Springs, OH), a telemetric chest strap heart rate (HR) monitor (Model RS400, Polar Electro, Lake Success, NY), and an automated blood pressure (BP) cuff on their upper right arm (Cardiocap 5, Datex-Ohmeda, St. Louis, MO). Subjects were additionally instrumented with copper-constantan thermocouples placed above the level of the water at standard locations on the upper back, forearm, and chest. Skin temperature (Tsk) is presented as the unweighted average of the temperature recorded at these three sites. After this initial instrumentation, the subjects were seated in a semi-reclined position for a 20-min period of rest. During this baseline period, a bolster was used to support the participants’ right arm at heart level. This baseline body and arm positioning mimicked the positioning maintained during HWI. After this seated rest and instrumentation period, baseline measurements of rectal temperature (Tre), Tsk, BP, and HR were collected. A 1-min baseline ultrasound scan of the brachial and carotid arteries was then completed.
After baseline measurements, the subjects were transferred into a 40°C HWI tub. Subjects remained immersed in the water to the level of mid to lower sternum for a period of 60 min as measurements were collected. Both arms and hands were supported at heart level and kept out of the water for the entirety of the immersion period. Tre, Tsk, BP, and HR were measured every 5 min. One-minute long brachial and carotid ultrasound assessments were completed every 15 min throughout the 60-min HWI protocol. Subjects were instructed to drink water ad libitum throughout the HWI protocol. Water consumption and fluid losses via sweating and urine production were recorded for each subject. When Tre reached 38.5°C, a nearby fan was turned on to promote subject comfort and slow the rate of further increases in Tre.
After the 60-min HWI bout, nude weight was measured a final time. If subjects lost >1% of their initial nude body weight, they were asked to drink additional fluids to make up the difference before leaving the laboratory.
Ultrasound
The brachial and carotid arteries were imaged using high-resolution Doppler ultrasonography (Terasont3000cv, Teratech, Burlington, MA) with a 10.0-MHz linear array ultrasound transducer probe and an insonation angle of 60°. Brachial measurements were made in the distal third of the upper arm, and carotid measurements were made 1–2 cm proximal to the carotid bulb. Ultrasound probe placement locations were marked following baseline measurements and the same area was imaged for repeated measurements during HWI. Ultrasound images were captured at 20 Hz using a video recording software (Camtasia, TechSmith, Okemos, MI) and analyzed using a custom-designed edge-detection and wall-tracking software (FMD/BloodFlow Software v1.1, Reed Electronics, Perth, WA, Australia; 35). With the use of this software, the Doppler flow velocity waveform envelopes were automatically traced, and mean blood flow velocity (cm/s) was subsequently calculated as half of this time-averaged peak envelope velocity. Blood vessel diameter (cm) was determined automatically along the highest quality portion of the B-mode image. Blood vessel diameter (cm) and blood flow velocity (cm/s) across each 1-min recording were used to calculate the blood flow and total, antegrade, and retrograde shear rates, and subsequently, oscillatory shear indices at each timepoint. Blood flow (mL/min) was calculated as the product of mean blood flow velocity and blood vessel cross-sectional area. Shear rate (s−1) was calculated as 4 · mean blood flow velocity/blood vessel diameter. Oscillatory shear index (arbitrary units, au) was calculated as |retrograde shear rate|/ (|antegrade shear rate| + |retrograde shear rate|) (36).
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 8.0.1. Unpaired, two-tailed t tests were used to compare subject characteristics, baseline values for each variable, the absolute change in each variable (calculated from baseline to the end of HWI), and the end of heating values for antegrade shear rate, retrograde shear rate, and oscillatory shear index between men and women. Paired, two-tailed t tests were additionally used to compare antegrade shear rate, retrograde shear rate, and oscillatory shear index values at baseline to the end of HWI values within men and women. One female subject was unable to complete the heating bout and exited the tub at 50 min of HWI, and we were unable to obtain quality carotid ultrasound scans in one female subject at 60 min of HWI. For these subjects, the end of heating measures and absolute change in each variable with heating were calculated using the last measure recorded during HWI. A two-way (sex × time) mixed-model analysis of variance was used to examine the effects of HWI on men and women with a priori contrasts of sex-time combinations. As such, we did not use a multiple comparisons adjustment when significant main or interaction effects of sex and/or time into heating were detected. Statistical significance was set at α = 0.05. Subject demographic data and experimental condition data (temperature, time of day, etc.) are presented as means and standard deviations (SDs). All other data are presented as means accompanied by 95% confidence intervals.
RESULTS
Subject Characteristics
Subject characteristics for the 11 men and 10 women who participated in the study are presented in Table 1.
Table 1.
Subject characteristics
| n | Age, yr | Height, cm | Weight, kg | Body Mass Index, kg·m−2 | Body Surface Area, m2 | Body Surface Area-To-Mass Ratio, cm2·kg-1 | |
|---|---|---|---|---|---|---|---|
| Men | 11 | 24 ± 4 | 179 ± 6 | 74 ± 5 | 23 ± 1 | 1.93 ± 0.10 | 260 ± 7 |
| Women | 10 | 24 ± 4 | 168 ± 8* | 63 ± 8* | 22 ± 2 | 1.71 ± 0.14* | 274 ± 13* |
Values are means ± SD. Data were compared between groups using an unpaired t test. *P < 0.01 women vs. men.
Temperature
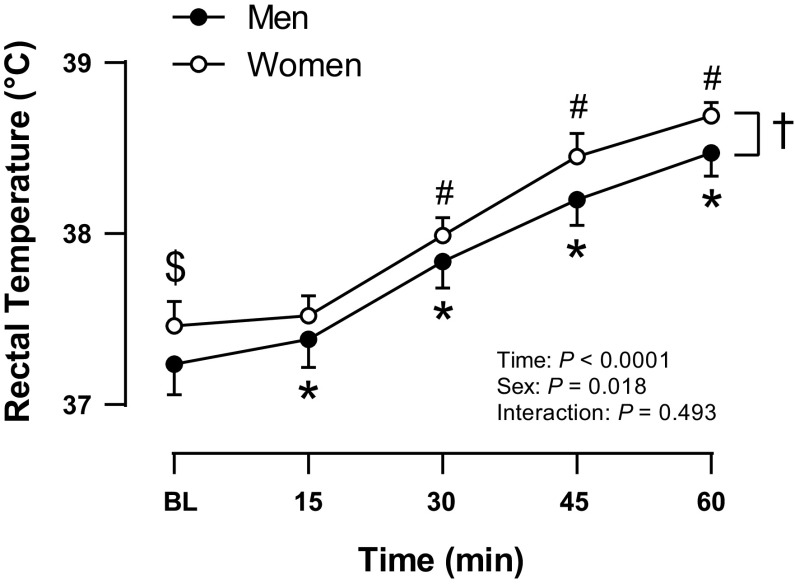
As shown in Fig. 1, Tre was ∼0.22°C higher in women (W) than in men (M) at baseline [W: 37.46 (37.32, 37.60)°C, M: 37.24 (37.06, 37.41)°C, P = 0.044]. Body core temperature increased similarly in men and women with HWI [M: +1.24 (1.06, 1.42)°C, W: +1.23 (1.10, 1.36)°C, P = 0.950], such that women had a higher Tre than men at all times. There were no sex differences in Tsk at baseline [M: 33.2 (32.5, 33.9)°C, W: 32.9 (32.3, 33.4)°C, P = 0.413] or throughout heating (P = 0.942). At baseline, the average Tsk was 33.0 (32.6, 33.5)°C. Tsk increased with HWI (P < 0.0001) and when Tre reached 38.5°C, skin temperature above the level of the water was 35.0 (34.5, 35.4)°C. At this time, a nearby fan was turned on, and by the end of HWI Tsk was 33.4 (33.0, 33.8)°C.
Figure 1.
Core temperature rise throughout the hot water immersion in men (filled circles, n = 11) and women (open circles, n = 10). Values are means and 95% confidence intervals. Baseline values of core temperature were compared between men and women using an unpaired t test, $P < 0.05 women vs. men at baseline. Data were analyzed using a two-way (sex × time) mixed-model analysis of variance, *P < 0.05 vs. baseline within men, #P < 0.05 vs. baseline within women, †P < 0.05 women vs. men (main effect of sex).
Central Hemodynamics
Central hemodynamic measures during HWI are presented in Table 2. There were no differences in mean arterial pressure between sexes at baseline (P = 0.211). Mean arterial pressure decreased with HWI and women displayed lower values than men, although there was no difference in the change in mean arterial pressure from baseline to the end of HWI between sexes [M: −6 (−10, −1) mmHg, W: −9 (−14, −4) mmHg, P = 0.280]. Men had a higher systolic blood pressure than women at baseline (P = 0.017) and this sex difference was maintained throughout HWI. Systolic blood pressure increased with HWI in both sexes and was above baseline values by 30 min HWI in men but was only elevated at 45 min HWI in women. Despite this differential response pattern, systolic blood pressure increased similarly between sexes by the end of HWI [M: +7 (−3, +17) mmHg, W: +6 (−4, +16) mmHg, P = 0.922]. There were no differences in diastolic blood pressure between sexes as diastolic blood pressure was similar between men and women at baseline (P = 0.636) and was reduced comparably by the end of HWI [M: −13 (−16, −9) mmHg, W: −16 (−21, −12) mmHg, P = 0.165]. As a result, women had a lower pulse pressure than men at baseline (P = 0.045), and pulse pressure increased comparably between sexes by the end of HWI [M: +19 (+9, +30) mmHg, W: +22 (+14, +31) mmHg, P = 0.627], such that men had a higher pulse pressure than women at all times. There were no differences in heart rate between sexes as heart rate was similar between men and women at baseline (P = 0.643) and increased comparably by the end of HWI [M: +35 (+27, +44) beats/min, W: +38 (+26, +50) beats/min, P = 0.698].
Table 2.
Central hemodynamic measures during hot water immersion in men and women
| Hot Water Immersion, min |
|||||
|---|---|---|---|---|---|
| Variable | Baseline | 15 | 30 | 45 | 60 |
| Mean arterial pressure, mmHg | |||||
| Men | 88 (84, 91) | 81 (76, 86)* | 83 (79, 87)* | 82 (76, 89)* | 82 (76, 87)* |
| Women† | 85 (81, 89) | 74 (69, 78)# | 76 (71, 80)# | 77 (72, 83)# | 75 (70, 81)# |
| Systolic blood pressure, mmHg | |||||
| Men | 122 (116, 128) | 123 (115, 131) | 130 (123, 138)* | 131 (121, 141)* | 129 (118, 140) |
| Women† | 112 (105, 118)$ | 108 (101, 116) | 113 (104, 122) | 120 (110, 130)# | 118 (108, 128) |
| Diastolic blood pressure, mmHg | |||||
| Men | 71 (68, 75) | 61 (57, 65)* | 59 (56, 63)* | 58 (52, 65)* | 59 (54, 63)* |
| Women | 70 (66, 74) | 56 (51, 60)# | 57 (53, 61)# | 56 (52, 60)# | 54 (49, 58)# |
| Pulse pressure, mmHg | |||||
| Men | 51 (43, 58) | 62 (54, 70)* | 71 (63, 78)* | 73 (64, 82)* | 70 (60, 80)* |
| Women† | 41 (35, 48)$ | 53 (45, 61)# | 57 (48, 65)# | 64 (55, 73)# | 64 (56, 72)# |
| Heart rate, beats/min | |||||
| Men | 67 (60, 74) | 88 (83, 94)* | 99 (93, 105)* | 104 (95, 113)* | 102 (94, 111)* |
| Women | 70 (60, 80] | 92 (85, 100)# | 107 (96, 119)# | 111 (100, 122)# | 107 (98, 117)# |
Mean arterial pressure, systolic blood pressure, diastolic blood pressure, pulse pressure, and heart rate at baseline and 15, 30, 45, and 60 min into hot water immersion in men (n = 11) and women (n = 10). Values are means and 95% confidence intervals. Baseline values for each variable were compared between men and women using an unpaired t test, $P < 0.05 women vs. men at baseline. Data were analyzed using a two-way (sex × time) mixed-model analysis of variance, *P < 0.05 vs. baseline within men, #P < 0.05 vs. baseline within women, †P < 0.05 women vs. men (main effect of sex).
Brachial Artery Hemodynamics in Men versus Women
Brachial hemodynamic measures during HWI are presented in Fig. 2 and Table 3. There were no differences in brachial artery blood flow between sexes as brachial artery blood flow was similar between men and women at baseline (P = 0.252) and increased comparably by the end of HWI [M: +369 (+287, +451) mL/min, W: +364 (+243, +486) mL/min, P = 0.943]. Brachial artery diameter was larger in men than in women at baseline (P < 0.0001) and this sex difference was maintained throughout HWI as brachial diameter increased similarly in men and women by the end of heating [M: +0.05 (+0.03, +0.07) cm, W: +0.06 (+0.05, +0.08) cm, P = 0.480]. Brachial blood flow velocity was similar between sexes at baseline (P = 0.351) and increased more with HWI in women than in men [M: +35 (+27, +43) cm/s, W: +48 (+36, +61) cm/s, P = 0.047]. Brachial total (P = 0.080) and antegrade (P = 0.096) shear rates were similar between sexes at baseline. Total brachial shear rate increased more with HWI in women than in men [M: +292 (+222, +361) s−1, W: +479 (+364, +594) s−1, P = 0.005]. This was due to a greater increase in antegrade shear rate among women [M: +272 (+202, +341) s−1, W: +467 (+356, +578) s−1, P = 0.003]. Retrograde shear rate was similar between men and women at baseline (P = 0.271). There was less retrograde shear with HWI and this response was comparable between sexes. Oscillatory shear index decreased comparably in men and women with HWI.
Figure 2.
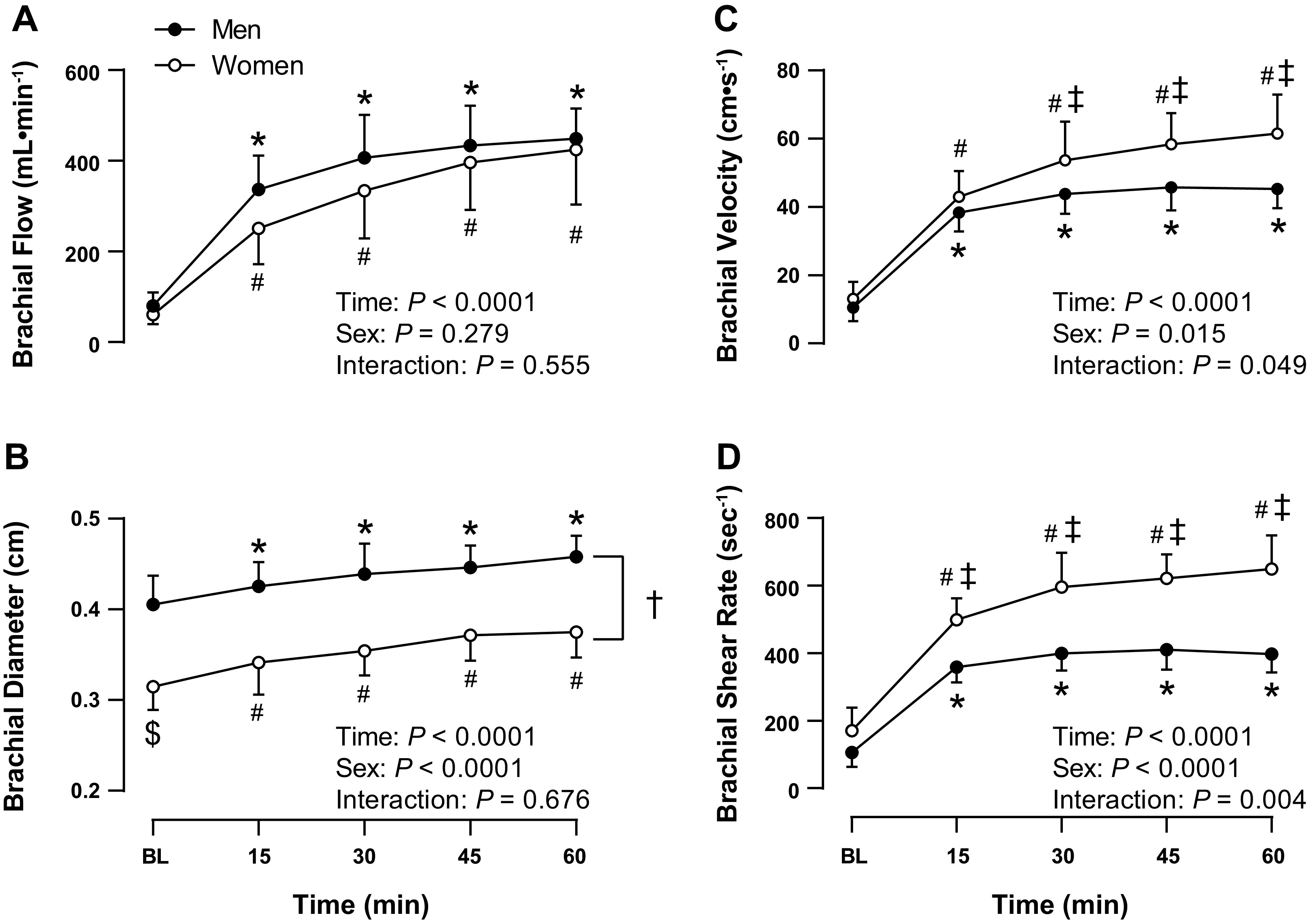
Brachial artery blood flow (A), diameter (B), velocity (C), and total shear rate (D) at baseline and 15, 30, 45, and 60 min into hot water immersion in men (closed circles, n = 11) and women (open circles, n = 10). Values are means and 95% confidence intervals. Baseline values for each variable were compared between men and women using an unpaired t test, $P < 0.05 women vs. men at baseline. Data were analyzed using a two-way (sex × time) mixed-model analysis of variance, *P < 0.05 vs. baseline within men, #P < 0.05 vs. baseline within women, †P < 0.05 women vs. men (main effect of sex), ‡P < 0.05 women vs. men at indicated time point.
Table 3.
Brachial and carotid hemodynamic measures in men and women
| Variable | Baseline | End of Heating |
|---|---|---|
| Brachial artery | ||
| Antegrade shear rate, s−1 | ||
| Men | 126 (91, 162) | 398 (344, 451)* |
| Women | 181 (118, 244) | 648 (548, 749)#‡ |
| Retrograde shear rate, s−1 | ||
| Men | −21 (−34, −8) | −1 (−5, 2)* |
| Women | −13 (−22, −4) | 0 (0, 0)# |
| Oscillatory shear index, au | ||
| Men | 0.16 (0.06, 0.25) | 0.00 (−0.01, 0.01)* |
| Women | 0.08 (0.02, 0.14) | 0.00 (0.00, 0.00)# |
| Carotid artery | ||
| Antegrade shear rate, s−1 | ||
| Men | 196 (162, 229) | 252 (215, 289)* |
| Women | 218 (193, 243) | 290 (254, 326)# |
| Retrograde shear rate, s−1 | ||
| Men | 0 (0, 0) | −2 (−3, 0)* |
| Women | 0 (0, 0) | −2 (−4, 0) |
| Oscillatory shear index, au | ||
| Men | 0.00 (0.00, 0.00) | 0.01 (0.00, 0.01)* |
| Women | 0.00 (0.00, 0.00) | 0.01 (0.00, 0.02) |
Brachial and carotid artery antegrade shear rate, retrograde shear rate, and oscillatory shear index at baseline and at the end of 60 min of hot water immersion. Values are means and 95% confidence intervals. Baseline values were compared with end of heating values within men and women using a paired t test, *P < 0.05 vs. baseline within men, #P < 0.05 vs. baseline within women. Values for each variable were compared between men and women using an unpaired t test; no differences were found at baseline and ‡P < 0.05 women vs. men at the end of heating.
Carotid Artery Hemodynamics in Men versus Women
As shown in Fig. 3, there were no sex differences in carotid artery blood flow (P = 0.526), diameter (P = 0.388), blood flow velocity (P = 0.245), or total (P = 0.259), antegrade (P = 0.253), or retrograde (P = 0.385) shear rate between sexes at baseline. Carotid artery blood flow increased comparably between sexes with HWI [M: +419 (+252, +586) mL/min, W: +383 (+248, +517) mL/min, P = 0.710]. This increase in carotid artery blood flow with HWI was supported by increases in carotid artery diameter [M: +0.06 (+0.03, +0.09) cm, W: +0.04 (+0.02, +0.06) cm, P = 0.311] and blood flow velocity [M: +13 (+7, +18) cm/s, W: +14 (+8, +21) cm/s, P = 0.690], which were displayed to a similar extent between sexes. As a result, total carotid shear rate increased comparably between sexes with HWI [M: +54 (+24, +85) s−1, W: +71 (+29, +112) s−1, P = 0.481]. This was due to an increased antegrade shear rate with HWI [M: +56 (+25, +87) s−1, W: +72 (+31, +113) s−1, P = 0.497], which was similar between sexes, as shown in Table 3. There was more retrograde shear with HWI and this response was comparable between sexes but only statistically significant among men. Similarly, oscillatory shear index increased in both men and women with HWI but this increase was only statistically significant among men.
Figure 3.
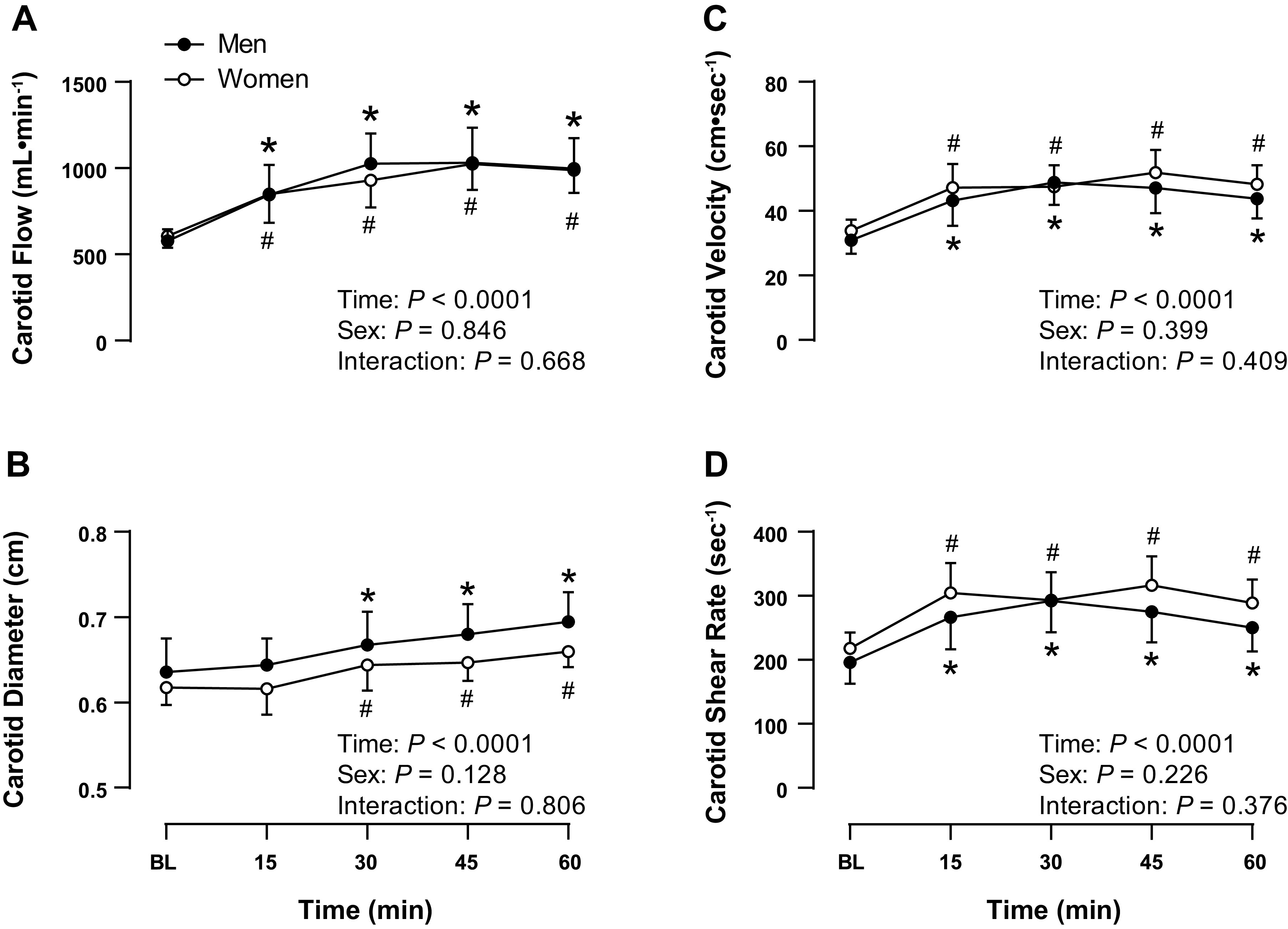
Common carotid artery blood flow (A), diameter (B), velocity (C), and total shear rate (D) at baseline and 15, 30, 45, and 60 min into hot water immersion in men (closed circles, n = 11) and women (open circles, n = 10). Values are means and 95% confidence intervals. Baseline values for each variable were compared between men and women using an unpaired t test; no differences were found. Data were analyzed using a two-way (sex × time) mixed-model analysis of variance, *P < 0.05 vs. baseline within men, #P < 0.05 vs. baseline within women.
DISCUSSION
Heat therapy is a promising means of improving cardiovascular health in both health and disease (6). As the increases in total and antegrade and reductions in retrograde shear stress patterns which accompany an acute bout of heat stress are thought to play a key role in mediating the beneficial vascular adaptations that accompany both acute and chronic heat exposure, it is important to consider how this shear stress stimulus may differ among different populations. Therefore, we aimed to compare the brachial and carotid hemodynamic response to 60 min of HWI between men and women. We found that HWI induced beneficial vascular shear stress patterns in the brachial and common carotid arteries of men and women. However, in the brachial artery, the magnitude of this shear stress response was greater in women than in men. These findings indicate the presence of an artery-specific sex difference in the hemodynamic response to HWI.
Core and Skin Temperature
At baseline, Tre was ∼0.22°C higher in women than in men. Although this difference in baseline Tre between men and women was statistically significant, the magnitude was small and fell within normal circadian and menstrual cycle variation. As there was no difference in the time of day that men and women were tested (average experiment start time M: 13:16 ± 01:37, W: 13:24 ± 02:32, P = 0.152), the increased core temperature observed among women may be attributable to the 0.3°C–0.5°C increase in core temperature, which accompanies the luteal phase of the menstrual cycle (37). Indeed, the 4 out of 10 women studied in the high hormone or luteal phase had a baseline Tre of 37.58 (37.37, 37.78)°C. Despite these initial differences, rectal temperature increased comparably between sexes and was elevated by an average of 1.23 (1.13, 1.33)°C by the end of HWI. This increase in Tre is well within the range reported by previous studies using HWI as a heating modality in men and women (10, 13, 14, 28). Furthermore, and consistent with the observations of Inoue et al. (28), there were no sex differences in Tsk above the level of the water at baseline or throughout HWI.
Central Hemodynamics
During the HWI intervention, mean arterial pressure decreased by an average of 7 (−11, −4) mmHg, as diastolic blood pressure was reduced and despite increases in systolic blood pressure, pulse pressure, and heart rate. Amin et al. (13) recently conducted a similar HWI protocol in which baseline measures were recorded during a semi-recumbent rest and HWI measures were recorded following 30 min of sternum-level immersion in a 42°C water bath. During this HWI intervention, mean arterial pressure did not change from baseline values, as diastolic blood pressure decreased by 13 mmHg and systolic blood pressure rose by 28 mmHg with HWI. The decrease in diastolic blood pressure observed in these studies is likely attributable to large increases in systemic vascular conductance, secondary to dramatic increases in skin blood flow, which accompany HWI. The increase in systolic blood pressure observed in these studies is likely the result of increases in cardiac output, facilitated primarily by increases in heart rate. Specifically, Amin et al. (13) observed a ∼3.7 L/min increase in cardiac output with HWI as heart rate increased by 38 beats/min and stroke volume did not change from baseline values. Similarly, we observed that heart rate increased by 36 beats/min by the end of HWI. We do not have an immediate explanation for the discrepancy in the magnitude of the increase in systolic blood pressure with HWI (7 mmHg in the present study vs. 28 mmHg observed by Amin et al.), which may be due to differences in the heating protocols used by the two studies. Although mean arterial pressure and diastolic blood pressure were similar between sexes at baseline, women displayed a lower baseline systolic blood pressure and pulse pressure than men. This difference in systolic blood pressure and pulse pressure was consistent with reference values showing that women of this age group have a lower blood pressure than men (39). Consistent with previous studies (28, 40), there were no differences in the change in mean arterial pressure, systolic blood pressure, diastolic blood pressure, pulse pressure, or heart rate from baseline to the end of heating between sexes.
Conduit Artery Hemodynamics
The brachial artery is a common site for the assessment of vascular health. Specifically, brachial flow-mediated dilation, an index of vascular endothelial function, is associated with coronary artery endothelial function (41, 42) and is a well-established predictor of CVD risk and events (43, 44). Several studies on the effect of acute heat exposure on vascular function in the brachial artery have noted improvements in brachial artery flow-mediated dilation and microvascular function (reactive hyperemia response) (15, 17, 20) following heating, whereas others have noted that prior heat exposure has a protective effect on brachial artery vascular function following ischemia reperfusion injury (26, 27). In addition, we and others have demonstrated that chronic heat therapy improves brachial artery flow-mediated dilation and provides protection from ischemia reperfusion injury (10, 11, 45–47). Although episodic changes in shear stress are thought to play a key role in mediating these beneficial vascular adaptations within the brachial artery, no study to date has compared the brachial shear stress response with HWI between men and women.
We observed that men and women displayed a similar brachial hemodynamic response pattern with HWI marked by increases in total and antegrade and reductions in retrograde shear rate. Other groups have previously demonstrated this shift in the vascular shear stress pattern with passive heat exposure in the radial (21), brachial (11, 13–17, 19, 20), common and superficial femoral (12–16, 18), and popliteal (19) arteries. The similar pattern of vascular shear stress with HWI among men and women is important as the simultaneous increase in antegrade shear and withdrawal of retrograde shear is thought to increase nitric oxide bioavailability and reduce vascular inflammation, oxidative stress, and circulating vasoconstrictors, and in doing so, is believed to be antiatherogenic and to improve endothelial function (8, 48). Importantly, despite this similar overall pattern of the brachial hemodynamics response to HWI between sexes, we found that the magnitude of the increase in total and antegrade shear rate was greater among women than among men. As shear rate is inversely proportional to blood vessel diameter and directly proportional to blood flow velocity, this was due to both a smaller brachial diameter and a greater increase in brachial velocity among women than men with HWI. The elevated brachial velocity among women allowed for a similar increase in brachial blood flow between sexes with HWI despite the smaller brachial diameter in women compared with men.
Carotid Artery
The carotid arteries, which are often subjected to low, oscillatory wall shear stress and flow separation at the carotid bifurcation, are particularly prone to the development of atherosclerotic lesions (49). Because of the critical role of the carotid arteries in supplying blood to the brain and their proclivity for vascular disease, the carotid arteries are a common site for the assessment of vascular health and disease. Specifically, common carotid wall thickness and stiffness are associated with atherosclerosis progression (50, 51) and CVD risk (52) and events (53). Our laboratory has demonstrated that 8–10 wk of heat therapy reduces carotid wall thickness in both young, healthy individuals and obese women with polycystic ovary syndrome (10, 45). Furthermore, Bikram or hot yoga regimens have been demonstrated to reduce carotid artery stiffness (β-stiffness and carotid artery compliance; 54). Although these vascular adaptations are believed to be mediated in part by the repeated, episodic change in vascular shear stress which accompanies acute heat exposure, it is unknown if the carotid shear stress response to HWI differs between sexes.
We observed that men and women displayed a similar carotid hemodynamic response pattern with HWI marked by modest increases in total and antegrade shear rate amid a small rise in retrograde shear rate. Unlike the brachial artery, there were no differences in the magnitude of this shear stress response between sexes. Although no study, to our knowledge, has reported common carotid shear stress patterns with HWI, these results are consistent with previous findings noting similar increases in common carotid artery blood flow when esophageal temperature was increased by 1.2°C via water-perfused suit heating (55). The limited increase in common carotid artery blood flow, and resulting shear stress patterns, likely stem in part from the low resistance and high blood flow of the resting cerebral circulation, which serves the high and nearly constant oxygen demand of the brain. Furthermore, cerebral autoregulation maintains tight control of brain blood flow and thus the common carotid artery displays comparably modest hemodynamic alterations during heat stress (56).
One important limitation of this experiment is that blood flow and shear rate were not measured in the internal or external carotid arteries. In the absence of these measures, we are unable to directly relate the observed alterations in common carotid artery hemodynamics to the blood flow and shear stress responses of the internal and the external carotid arteries with HWI. Previous studies have suggested that increases in common carotid blood flow during heat stress are primarily attributable to increases in extracranial cutaneous blood flow (external carotid artery). In contrast, internal carotid artery blood flow either does not change with moderate heating (<1°C increase in core temperature) or is decreased with severe heating (≥1°C increase in core temperature) due to hyperthermia-induced hypocapnia (55, 57–59). As the internal carotid artery is a significant location of atherosclerotic plaque buildup in the large cerebral vessels and may display a different hemodynamic response to heating than the common carotid artery, the assessment of internal carotid artery hemodynamics with HWI warrants future investigation.
Perspectives and Significance
Our findings offer important insight into the hemodynamic responses to acute passive heat exposure and suggest the presence of an artery-specific difference in the hemodynamic response to HWI between men and women. Although a “shear stress threshold” for eliciting vascular adaptation has not been identified our findings highlight the need for future studies to evaluate sex differences in the vascular response to both acute heat exposure and chronic heat therapy. Indeed, despite the growing interest in the changes in vascular function which follow a single bout of heat stress and chronic heat therapy, no study to date has examined sex differences which may exist within these responses. As heat therapy emerges as a promising cardiovascular therapeutic option, this remains an important area for future research.
GRANTS
This work was supported by an American Heart Association Grant 19TPA34890033, National Heart, Lung, and Blood Institute Grant HL144128, the William Townsend Porter Pre-doctoral Fellowship from the American Physiological Society (to E. A. Larson), the Kenneth and Kenda Singer Endowed Professorship in Human Physiology (to C. T. Minson), a University of Oregon Undergraduate Research Opportunity Program Mini Grant (to S. M. Harris), and a University of Oregon Robert D. Clark Honor’s College Thesis Research Grant (to S. M. Harris).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.R.E., V.E.B., S.M.H., and C.T.M. conceived and designed research; E.A.L., B.R.E., V.E.B., M.A.F., and S.M.H. performed experiments; E.A.L. analyzed data; E.A.L., J.R.H., and C.T.M. interpreted results of experiments; E.A.L., J.R.H., and C.T.M. prepared figures; E.A.L., J.R.H., and C.T.M. drafted manuscript; E.A.L., B.R.E., V.E.B., M.A.F., S.M.H., J.R.H., and C.T.M. edited and revised manuscript; E.A.L., B.R.E., V.E.B., M.A.F., S.M.H., J.R.H., and C.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the subjects for volunteering their time to participate in this study.
REFERENCES
- 1.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1736–1788, 2018. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 141: e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175: 542–548, 2015. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen T, Kunutsor SK, Khan H, Willeit P, Zaccardi F, Laukkanen JA. Sauna bathing is associated with reduced cardiovascular mortality and improves risk prediction in men and women: a prospective cohort study. BMC Med 16: 219, 2018. doi: 10.1186/s12916-018-1198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng JL, MacDonald MJ. Effect of heat stress on vascular outcomes in humans. J Appl Physiol (1985) 126: 771–781, 2019. doi: 10.1152/japplphysiol.00682.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullen T, Clarke ND, Hill M, Menzies C, Pugh CJA, Steward CJ, Thake CD. The health benefits of passive heating and aerobic exercise: to what extent do the mechanisms overlap? J Appl Physiol (1985) 129: 1304–1309, 2020. doi: 10.1152/JAPPLPHYSIOL.00608.2020. [DOI] [PubMed] [Google Scholar]
- 8.Brunt VE, Minson CT. Heat therapy: mechanistic underpinnings and applications to cardiovascular health. J Appl Physiol (1985) 130: 1684–1704, 2021. doi: 10.1152/japplphysiol.00141.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzey FK, Smith EC, Ruediger SL, Keating SE, Askew CD, Coombes JS, Bailey TG. The effect of heat therapy on blood pressure and peripheral vascular function: a systematic review and meta-analysis. Exp Physiol 106: 1317–1334, 2021. doi: 10.1113/EP089424. [DOI] [PubMed] [Google Scholar]
- 10.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter HH, Spence AL, Atkinson CL, Pugh CJA, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114: 859–865, 2014. doi: 10.1007/s00421-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 12.Chiesa ST, Trangmar SJ, González-Alonso J. Temperature and blood flow distribution in the human leg during passive heat stress. J Appl Physiol (1985) 120: 1047–1058, 2016. doi: 10.1152/japplphysiol.00965.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin SB, Hansen AB, Mugele H, Willmer F, Gross F, Reimeir B, Cornwell WK, Simpson LL, Moore JP, Romero SA, Lawley JS. Whole body passive heating versus dynamic lower body exercise: a comparison of peripheral hemodynamic profiles. J Appl Physiol (1985) 130: 160–171, 2021. doi: 10.1152/JAPPLPHYSIOL.00291.2020. [DOI] [PubMed] [Google Scholar]
- 14.Francisco MA, Colbert C, Larson EA, Sieck DC, Halliwill JR, Minson CT. Hemodynamics of postexercise versus post-hot water immersion recovery. J Appl Physiol (1985) 130: 1362–1372, 2021. doi: 10.1152/japplphysiol.00260.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng JL, Williams JS, Hoekstra SP, MacDonald MJ. Improvements in vascular function in response to acute lower limb heating in young healthy males and females. J Appl Physiol (1985) 131: 277–289., 2021. doi: 10.1152/japplphysiol.00630.2020. [DOI] [PubMed] [Google Scholar]
- 16.Coombs GB, Barak OF, Phillips AA, Mijacika T, Sarafis ZK, Lee AHX, Squair JW, Bammert TD, DeSouza NM, Gagnon D, Krassioukov AV, Dujic Z, DeSouza CA, Ainslie PN. Acute heat stress reduces biomarkers of endothelial activation but not macro- or microvascular dysfunction in cervical spinal cord injury. Am J Physiol Heart Circ Physiol 316: H722–H733, 2019. doi: 10.1152/ajpheart.00693.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coombs GB, Tremblay JC, Shkredova DA, Carr JMJR, Wakeham DJ, Patrician A, Ainslie PN. Distinct contributions of skin and core temperatures to flow-mediated dilation of the brachial artery following passive heating. J Appl Physiol (1985) 130: 149–159, 2021. doi: 10.1152/JAPPLPHYSIOL.00502.2020. [DOI] [PubMed] [Google Scholar]
- 18.Thomas KN, van Rij AM, Lucas SJE, Gray AR, Cotter JD. Substantive hemodynamic and thermal strain upon completing lower-limb hot-water immersion; comparisons with treadmill running. Temperature (Austin) 3: 286–297, 2016. doi: 10.1080/23328940.2016.1156215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas KN, van Rij AM, Lucas SJE, Cotter JD. Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol 312: R281–R291, 2017. doi: 10.1152/ajpregu.00404.2016. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JL, Au JS, MacDonald MJ. Peripheral artery endothelial function responses to altered shear stress patterns in humans. Exp Physiol 104: 1126–1135, 2019. doi: 10.1113/EP087597. [DOI] [PubMed] [Google Scholar]
- 21.Alali MH, Vianna LC, Lucas RAI, Junejo RT, Fisher JP. Impact of whole body passive heat stress and arterial shear rate modification on radial artery function in young men. J Appl Physiol (1985) 129: 1373–1382, 2020. doi: 10.1152/JAPPLPHYSIOL.00296.2020. [DOI] [PubMed] [Google Scholar]
- 22.Thijssen DHJ, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson CL, Carter HH, Dawson EA, Naylor LH, Thijssen DHJ, Green DJ. Impact of handgrip exercise intensity on brachial artery flow-mediated dilation. Eur J Appl Physiol 115: 1705–1713, 2015. doi: 10.1007/s00421-015-3157-1. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay JC, Grewal AS, Pyke KE. Examining the acute effects of retrograde versus low mean shear rate on flow-mediated dilation. J Appl Physiol (1985) 126: 1335–1342, 2019. doi: 10.1152/japplphysiol.01065.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero SA, Gagnon D, Adams AN, Cramer MN, Kouda K, Crandall CG. Acute limb heating improves macro- and microvascular dilator function in the leg of aged humans. Am J Physiol Heart Circ Physiol 312: H89–H97, 2017. doi: 10.1152/ajpheart.00519.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelland RE, Hemingway HW, Tomasco OG, Olivencia-Yurvati AH, Romero SA. Acute lower leg hot water immersion protects macrovascular dilator function following ischaemia–reperfusion injury in humans. Exp Physiol 105: 302–311, 2020. doi: 10.1113/EP088154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunt VE, Jeckell AT, Ely BR, Howard MJ, Thijssen DHJ, Minson CT. Acute hot water immersion is protective against impaired vascular function following forearm ischemia-reperfusion in young healthy humans. Am J Physiol Regul Integr Comp Physiol 311: R1060–R1067, 2016. doi: 10.1152/ajpregu.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue Y, Tanaka Y, Omori K, Kuwahara T, Ogura Y, Ueda H. Sex- and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure. Eur J Appl Physiol 94: 323–332, 2005. doi: 10.1007/s00421-004-1303-2. [DOI] [PubMed] [Google Scholar]
- 29.Gagnon D, Crandall CG, Kenny GP. Sex differences in postsynaptic sweating and cutaneous vasodilation. J Appl Physiol (1985) 114: 394–401, 2013. doi: 10.1152/japplphysiol.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashimoto M, Akishita M, Eto M, Ishikawa M, Kozaki K, Toba K, Sagara Y, Taketani Y, Orimo H, Ouchi Y. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation 92: 3431–3435, 1995. doi: 10.1161/01.CIR.92.12.3431. [DOI] [PubMed] [Google Scholar]
- 31.Krejza J, Arkuszewski M, Kasner SE, Weigele J, Ustymowicz A, Hurst RW, Cucchiara BL, Messe SR. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 37: 1103–1105, 2006. doi: 10.1161/01.STR.0000206440.48756.f7. [DOI] [PubMed] [Google Scholar]
- 32.Iwamoto Y, Maruhashi T, Fujii Y, Idei N, Fujimura N, Mikami S, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, Noma K, Nakashima A, Higashi Y. Intima-media thickness of brachial artery, vascular function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol 32: 2295–2303, 2012. doi: 10.1161/ATVBAHA.112.249680. [DOI] [PubMed] [Google Scholar]
- 33.Sandgren T, Sonesson B, Ahlgren AR, Länne T. The diameter of the common femoral artery in healthy human: influence of sex, age, and body size. J Vasc Surg 29: 503–510, 1999. doi: 10.1016/S0741-5214(99)70279-X. [DOI] [PubMed] [Google Scholar]
- 34.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303–313, 1989. [PubMed] [Google Scholar]
- 35.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol (1985) 91: 929–937, 2001. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- 36.Moore JE Jr, Xu C, Glagov S, Zarins CK, Ku DN. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 110: 225–240, 1994. doi: 10.1016/0021-9150(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 37.Hessemer V, Brück K. Influence of menstrual cycle on shivering, skin blood flow, and sweating responses measured at night. J Appl Physiol (1985) 59: 1902–1910, 1985. doi: 10.1152/jappl.1985.59.6.1902. [DOI] [PubMed] [Google Scholar]
- 39.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71: e13–e115, 2018. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 40.Meendering JR, Torgrimson BN, Houghton BL, Halliwill JR, Minson CT. Menstrual cycle and sex affect hemodynamic responses to combined orthostatic and heat stress. Am J Physiol Heart Circ Physiol 289: H631–H642, 2005. doi: 10.1152/ajpheart.00029.2005. [DOI] [PubMed] [Google Scholar]
- 41.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 42.Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M, Chayama K. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol 28: 460–466, 2005.doi: 10.1002/clc.4960281004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Ely BR, Francisco MA, Halliwill JR, Bryan SD, Comrada LN, Larson EA, Brunt VE, Minson CT. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 317: R630–R640, 2019. doi: 10.1152/ajpregu.00078.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. doi: 10.1016/s0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- 47.Bailey TG, Cable NT, Miller GD, Sprung VS, Low DA, Jones H. Repeated warm water immersion induces similar cerebrovascular adaptations to 8 weeks of moderate-intensity exercise training in females. Int J Sports Med 37: 757–765, 2016. doi: 10.1055/s-0042-106899. [DOI] [PubMed] [Google Scholar]
- 48.Tinken TM, Thijssen DHJ, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53: 502–514, 1983. doi: 10.1161/01.RES.53.4.502. [DOI] [PubMed] [Google Scholar]
- 50.Tu ST, Wang IW, Lin HF, Liao YC, Lin RT, Liu CS, Juo SHH. Carotid intima-media thickness and stiffness are independent risk factors for atherosclerotic diseases. J Investig Med 58: 786–790, 2010. doi: 10.2310/JIM.0b013e3181e8019d. [DOI] [PubMed] [Google Scholar]
- 51.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks APG, van der Kuip DAM, Hofman A, Witteman JCM. Association between arterial stiffness and atherosclerosis: the Rotterdam study. Stroke 32: 454–460, 2001. doi: 10.1161/01.STR.32.2.454. [DOI] [PubMed] [Google Scholar]
- 52.Mohammed M, Zito C, Cusmà-Piccione M, Di Bella G, Antonini-Canterin F, Taha NM, Di Bello V, Vriz O, Pugliatti P, Carerj S; Research Group of the Italian Society of Cardiovascular Echography (SIEC). Arterial stiffness changes in patients with cardiovascular risk factors but normal carotid intima-media thickness. J Cardiovasc Med (Hagerstown) 14: 622–628, 2013. doi: 10.2459/JCM.0B013E3283639721. [DOI] [PubMed] [Google Scholar]
- 53.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115: 459–467, 2007. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 54.Hunter SD, Dhindsa MS, Cunningham E, Tarumi T, Alkatan M, Nualnim N, Tanaka H. The effect of Bikram yoga on arterial stiffness in young and older adults. J Altern Complement Med 19: 930–934, 2013. doi: 10.1089/acm.2012.0709. [DOI] [PubMed] [Google Scholar]
- 55.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Morimoto K, Shibasaki M. Blood flow distribution during heat stress: cerebral and systemic blood flow. J Cereb Blood Flow Metab 33: 1915–1920, 2013. doi: 10.1038/jcbfm.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol 592: 841–859, 2014. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bain AR, Smith KJ, Lewis NC, Foster GE, Wildfong KW, Willie CK, Hartley GL, Cheung SS, Ainslie PN. Regional changes in brain blood flow during severe passive hyperthermia: effects of PaCO2 and extracranial blood flow. J Appl Physiol (1985) 115: 653–659, 2013. doi: 10.1152/japplphysiol.00394.2013. [DOI] [PubMed] [Google Scholar]
- 58.Brothers RM, Wingo JE, Hubing KA, Crandall CG. The effects of reduced end-tidal carbon dioxide tension on cerebral blood flow during heat stress. J Physiol 587: 3921–3927, 2009. doi: 10.1113/jphysiol.2009.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibbons TD, Tymko MM, Thomas KN, Wilson LC, Stembridge M, Caldwell HG, Howe CA, Hoiland RL, Akerman AP, Dawkins TG, Patrician A, Coombs GB, Gasho C, Stacey BS, Ainslie PN, Cotter JD. Global REACH 2018: the influence of acute and chronic hypoxia on cerebral haemodynamics and related functional outcomes during cold and heat stress. J Physiol 598: 265–284, 2020. doi: 10.1113/JP278917. [DOI] [PubMed] [Google Scholar]