Abstract
Sex-related differences in respiratory modulation of sympathetic activity have been observed in rodent models of sleep apnea [intermittent hypoxia (IH)]. In light of sex disparities in the respiratory response to acute IH in humans as well as changes in respiratory modulation of muscle sympathetic nerve activity (MSNA) in clinical sleep apnea, we examined sex-related differences in respiratory modulation of MSNA following acute IH. We hypothesized that respiratory modulation of MSNA would be altered in both male and female participants after IH; however, the respiratory patterning of MSNA following IH would be sex specific. Heart rate, MSNA, and respiration were evaluated in healthy male (n = 21, 30 ± 5 yr) and female (n = 10, 28 ± 5 yr) participants during normoxic rest before and after 30 min of IH. Respiratory modulation of MSNA was assessed by fitting polynomials to cross-correlation histograms constructed between sympathetic spikes and respiration. MSNA was elevated after IH in male (20 ± 6 to 24 ± 8 bursts/min) and female (19 ± 8 to 22 ± 10 bursts/min) participants (P < 0.01). Both male and female participants exhibited respiratory modulation of MSNA (P < 0.01); however, the pattern differed by sex. After IH, modulation of MSNA within the breath was reduced in male participants (P = 0.03) but increased in female participants (P = 0.02). Both male and female adults exhibit changes in respiratory patterning of MSNA after acute IH; however, this pattern differs by sex. These data support sex disparities in respiratory modulation of MSNA and may have implications for conditions such as sleep apnea.
Keywords: intermittent hypoxia, muscle sympathetic nerve activity, women
BACKGROUND
Efferent sympathetic nervous system activity fluctuates throughout the respiratory cycle [i.e., respiratory modulation of muscle sympathetic nerve activity (MSNA)] (1). In humans, MSNA is inhibited when lung volume is highest (peak inspiration) and MSNA is maximal when lung volume is lowest (end expiration) (2–5). In patients with sleep apnea, who experience intermittent bouts of low oxygen during sleep (intermittent hypoxia), changes in respiratory modulation of sympathetic activity are observed (6). Specifically, individuals with sleep apnea exhibit greater sympathetic activity after peak inspiration and less activity during expiration compared with healthy control subjects (6). Consistent with data from patients, preclinical rodent models display changes in respiratory modulation of sympathetic activity after exposure to intermittent hypoxia (7–10). Notably, the effect of intermittent hypoxia on respiratory modulation of sympathetic activity in rodents differs by sex (reviewed in Ref. 7). In male animals, thoracic sympathetic activity is highest in late expiration after intermittent hypoxia (9, 10). In contrast, after intermittent hypoxia in female rats, sympathetic activity is greater during inspiration (7, 8). Whether these sex-related differences in respiratory modulation of sympathetic activity after intermittent hypoxia translate to humans is unknown. However, sex-related differences in factors that influence within-breath modulation of MSNA (e.g., changes in lung volume, breathing rate, baroreceptor feedback, and central/peripheral chemoreceptor activation) have been identified (11, 12) and may thus support the presence of sex-related differences in respiratory modulation of sympathetic activity after intermittent hypoxia.
Differences in respiratory-linked rhythmic increases in sympathetic activity may have differential effects on norepinephrine release from sympathetic nerve terminals, peripheral vascular tone, and ultimately blood pressure (13). As such, changes in respiratory modulation of sympathetic activity have been implicated in the development of neurogenic hypertension (13–15). We have shown previously that both male and female study participants exhibit higher MSNA after acute intermittent hypoxia; however, only male participants exhibit an increase in blood pressure (16). In a secondary analysis of previously collected data, we explored sex-related changes in respiratory modulation of MSNA after acute intermittent hypoxia in humans. We hypothesized that respiratory modulation of MSNA would be altered in both male and female participants after acute intermittent hypoxia. We further hypothesized, on the basis of rodent models (7), that changes in respiratory patterning of MSNA after intermittent hypoxia would be sex specific.
METHODS
Participants
All participants (21 males, 10 females) were young (<45 yr of age), healthy, nonobese [body mass index (BMI) < 30 kg/m2] nonsmokers without chronic diseases and taking no medications known to affect cardiovascular or autonomic function. Female participants (n = 10) were premenopausal, not pregnant (confirmed by negative pregnancy test ≤24 h before participation), and determined by self-report to be in the early follicular phase of the menstrual cycle (n = 3) or placebo phase of oral contraceptive use (n = 7). Experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic (16-004563) and University of Missouri (2007973), were in accordance with institutional guidelines, and conformed to the Declaration of Helsinki. Written informed consent was obtained from all participants on a screen visit. Data presented here were retrospectively analyzed to address the proposed novel hypotheses, and data unrelated to respiratory modulation of MSNA were published previously from a subset of participants (16, 17).
Instrumentation
Participants were asked to refrain from alcohol, caffeine, nonsteroidal anti-inflammatory drugs, and exercise for 24 h before the study visit and were admitted to the laboratory in the morning after an overnight fast. Participants rested supine during instrumentation, which included electrocardiography (lead II) and finger pulse oximetry. In n = 21 (15 males, 6 females), a 20-gauge, 5-cm catheter was placed in the brachial artery under aseptic conditions after local anesthesia for beat-by-beat arterial blood pressure measurement. In n = 10 (6 males, 4 females), arterial blood pressure was assessed noninvasively by finger photoplethysmography calibrated to automated upper arm sphygmomanometry measurements. Importantly, beat-to-beat changes in noninvasive blood pressure measurements closely follow changes in intra-arterial pressure (18, 19).
MSNA was recorded with the technique of microneurography. Multiunit MSNA was recorded with a tungsten microelectrode. The microelectrode was placed percutaneously into the peroneal nerve, posterior to the fibular head, under direct two-dimensional (2-D) ultrasound guidance (20). A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretch evoked mechanoreceptive impulses, and no afferent neural response was evoked by skin stimuli. A reference electrode was positioned subcutaneously ∼4 cm from the recording electrode. The recorded signal was amplified and band-pass filtered (700–2,000 Hz) (n = 21: Nerve Traffic Analyzer, model 662c-3; University of Iowa Bioengineering, Iowa City, IA; n = 10: NeuroAmpEx, ADInstruments, Dunedin, New Zealand).
Acute Intermittent Hypoxia
Acute intermittent hypoxia was achieved for 30 min with methods published previously (16, 17). Briefly, participants were instrumented with a mask connected to a nonrebreathing valve, and breathing was spontaneous. Respiration was monitored by use of a piezo respiratory belt transducer. Breath-by-breath tidal volume (n = 21: Universal Ventilation meter, VacuMed, Ventura, CA; n = 10: Pneumotachograph with differential pressure amplifier PA-1, series 1110, Hans Rudolph, Shawnee, KS), respiratory rate, and inspired/expired gases (n = 21: GE Datex-Ohmeda Cardiocap/5, GE Healthcare; n = 10: Gemini 14-10000 Respiratory Monitor, CWE Inc.) were monitored continuously. Intermittent hypoxia was achieved by alternating between a hypercapnic hypoxic gas cylinder (3% carbon dioxide, 5% oxygen) for 30 s and room air (21% oxygen) to target 15 hypoxic events over 30 min. A 50-L meteorological balloon served as a volume reservoir. This technique results in arterial desaturation events similar to those observed in mild to moderate sleep apnea (5–10% desaturations; 15–30 events per hour), and duration was chosen based on prior work showing that a consistent rise in MSNA is achieved within this time frame (21–23). A hypoxic ventilatory response test was conducted immediately before and after acute intermittent hypoxia. The test took ∼15 min to complete and resulted in an additional 6–10 desaturation events. Results of the hypoxic ventilatory response test were published previously (16, 17).
Respiratory Modulation of MSNA
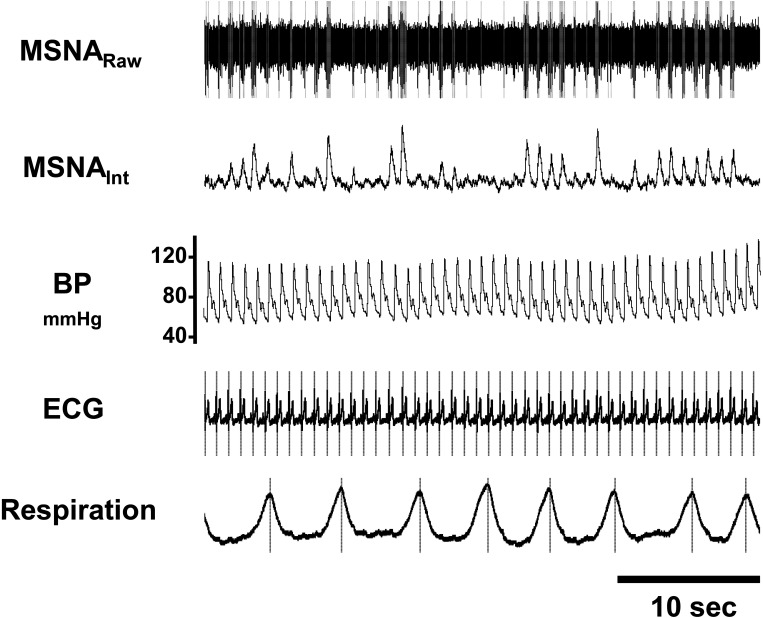
Respiratory patterning of MSNA was assessed during ∼5 min of quiet normoxic rest before and after the hypoxic ventilatory response test accompanying acute intermittent hypoxia. The raw filtered MSNA signal was integrated (time constant 0.1) to display multiunit bursts of sympathetic activity; however, the primary analysis was conducted on the raw, negative-going sympathetic spikes, similar to work conducted previously (6, 24, 25). To identify only raw, negative-going spikes of sympathetic origin (half-width 0.2–0.6 ms), discriminator levels were adjusted with window discriminator software (Spike Histogram, LabChart, ADInstruments) (6, 24,25). The times of occurrence of R waves (ECG) and inspiratory peaks (respiratory signal) were computed with Peak Analysis software (LabChart, ADInstruments). To account for conduction delays between the R wave (ECG) and MSNA in the peroneal nerve (26), the neurogram was shifted back in time (∼1.2 s) relative to the R wave (see Fig. 1).
Figure 1.
Multiunit recording of muscle sympathetic nerve activity (MSNA) from a male research participant. Discriminated spikes were extracted from the nerve recording (dashed lines). The times of occurrence of each heartbeat (R waves) and end of each inhale (inspiratory peaks) are also shown (dashed lines). These timing events were used to generate the cross-correlation and autocorrelation histograms. BP, blood pressure.
Autocorrelation histograms for cardiac (R wave, ECG) signals were generated in 50-ms bins with Spike Histogram software. Cross-correlation histograms between negative sympathetic spikes and R-R intervals were similarly generated with Spike Histogram software. Discriminator levels were adjusted to ensure that negative-going (C fiber) spikes exhibited cardiac modulation. The same discriminator settings were applied to cross correlograms between the MSNA and R waves of the ECG and MSNA and peaks of the respiratory signal (24, 25) (see representative data in Fig. 2).
Figure 2.
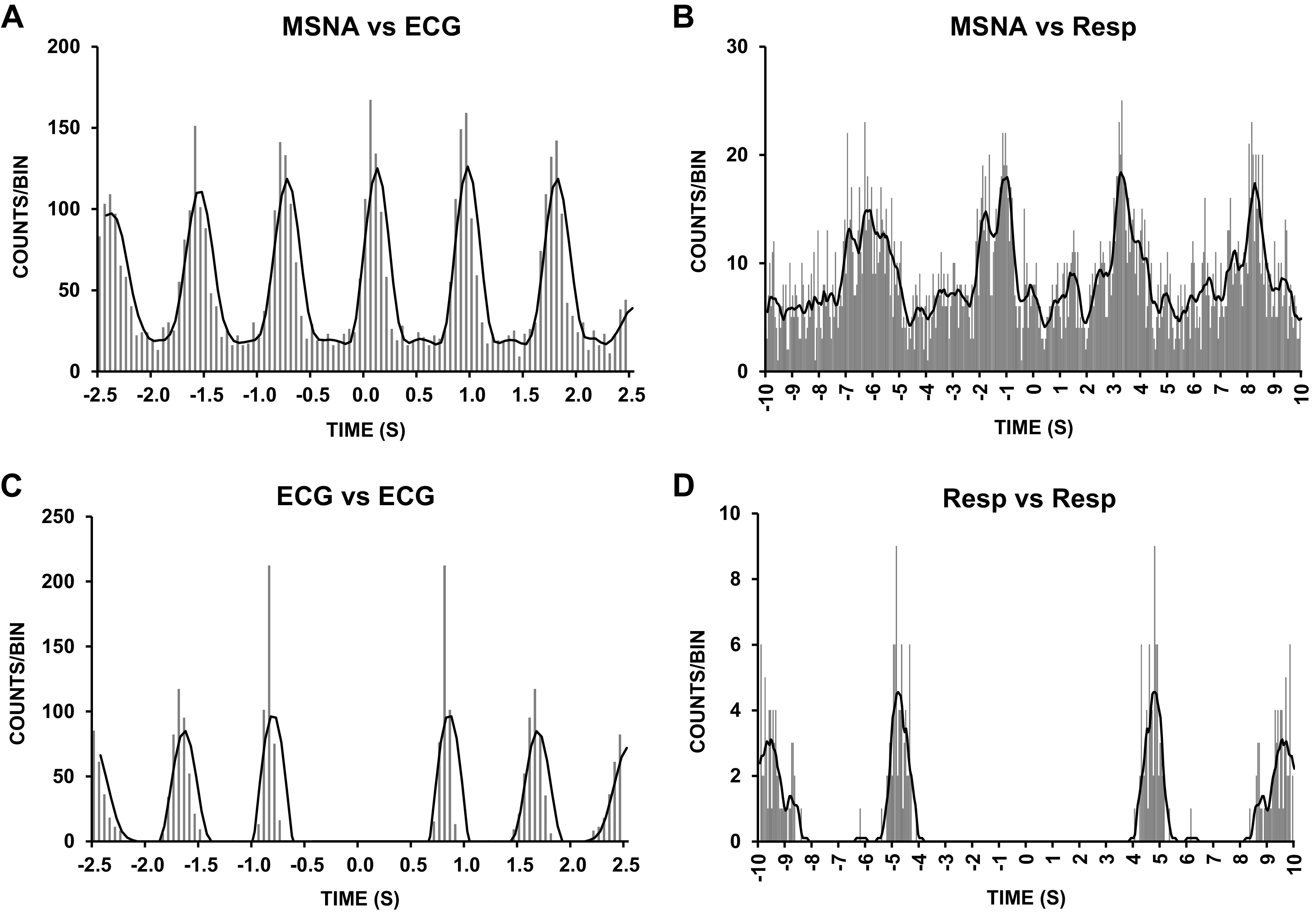
Correlations between muscle sympathetic nerve activity (MSNA) and ECG and respiration (Resp): cross-correlation histograms (top) and autocorrelation histograms (bottom) between sympathetic spikes and ECG and respiration. Data were obtained from the same participant illustrated in Fig. 1. Smoothed polynomials (black lines) have been fitted to the histograms. The numbers on the y-axes refer to the numbers of spikes per 50-ms bin. Time 0 corresponds to the triggering event (A and C: R waves; B and D: inspiratory peaks) in the cross or autocorrelograms.
Smoothed polynomial curves were fitted to histogram data (GraphPad Prism 8.4.3). Second-order polynomials were used to fit curves to cardiac cross correlograms, and zero-order polynomials were used to fit curves to respiratory cross correlograms (24, 25). Smoothed polynomial data were normalized by setting peak event counts in cross correlograms from each participant to 100%. In each study participant, the relative numbers of sympathetic spikes in different phases of the respiratory cycle were calculated. Because within-breath sympathetic modulation is influenced by the phase of respiration as well as the volume of lung inflation, data are presented relative (%) to each individual’s average inspiratory and expiratory time (Fig. 3) and binned by low lung volumes (inspiration, expiration) or high lung volumes (peak inspiration) (25). Modulation of MSNA within the breath was quantified as the difference in the relative number of MSNA spikes at the peak and the number at the trough for each participant [modulation index = (peak − trough)/peak × 100] (24, 25).
Figure 3.
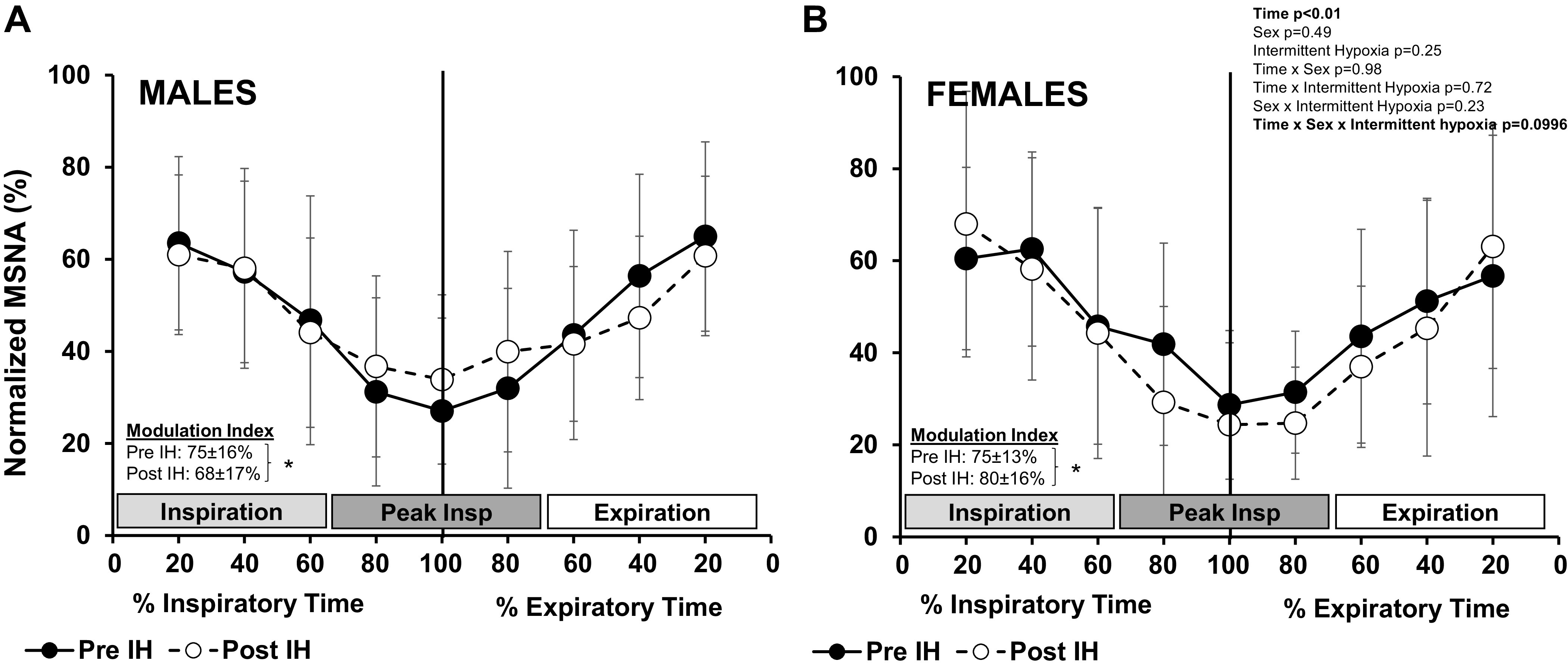
Cross-correlation histograms between sympathetic spikes and respiration: normalized data (means ± SD) of male (n = 21) (A) and female (n = 10) (B) participants before and after acute intermittent hypoxia (IH). Muscle sympathetic nerve activity (MSNA) spikes were measured in 50-ms bins around the inspiratory peak (time 0, vertical line) and normalized by setting peak event counts in cross correlograms in each participant to 100%. Data are reported relative to respiratory phase (% inspiratory/expiratory time) and were analyzed with a 3-way mixed-effect model with repeated measures. Modulation index was calculated [(peak − trough)/peak × 100] for each individual and analyzed with a paired t test. *P < 0.05, pre- vs. post-IH.
Data Analysis
Hemodynamic and respiratory variables were recorded at 1,000 Hz and MSNA signals were recorded at 10,000 Hz with a computer data acquisition system (PowerLab, ADInstruments) and stored for off-line analysis (LabChart, ADInstruments). Measurements during normoxic rest were analyzed over an ∼5-min baseline period before and immediately after the last acute hypoxic exposure once oxygen saturation levels returned to baseline. This timing was identical across all participants.
Phase of respiration was determined based on a change in tidal volume above (expiration) or below (inspiration) zero (Peak analysis, LabChart). Total breath duration (TTot) was automatically derived, and inspiratory duration (Ti, time below zero) was analyzed by hand for each participant by a single investigator (J. K. Limberg). To evaluate the effect of intermittent hypoxia on the temporal profile of the respiratory modulation of blood pressure, cycle-triggered averages of mean arterial blood pressure were measured in 100-ms bins (Scope, LabChart, ADInstruments) and a change (Δ) in blood pressure from the inspiratory peak was calculated. Blood pressure data are similarly presented relative (%) to each individual’s average inspiratory and expiratory time (Fig. 4).
Figure 4.
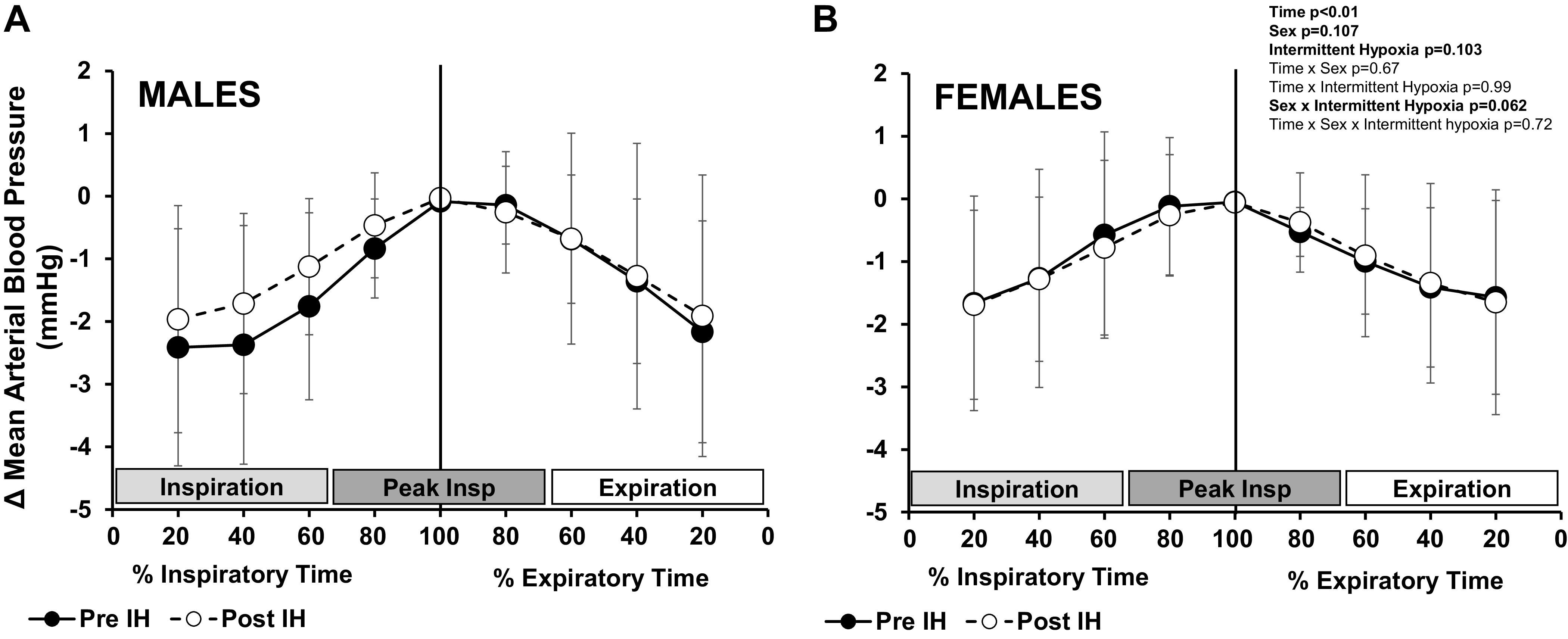
Cycle-triggered averages of mean blood pressure. Cycle-triggered averages (means ± SD) of mean arterial blood pressure were measured in male (n = 21) (A) and female (n = 10) (B) participants in 100-ms bins around the inspiratory peak (time 0, vertical line) before and after acute intermittent hypoxia (IH). A change (Δ) in mean arterial blood pressure from the inspiratory peak was calculated. Data are reported relative to respiratory phase (%inspiratory/expiratory time) and were analyzed with a 3-way mixed-effect model with repeated measures.
Statistical analysis was completed with GraphPad Prism version 8 (GraphPad Software, LLC). Participant demographics were compared with Student’s unpaired t test. The effects of sex (male, female), time (% inspiratory/expiratory time), and/or intermittent hypoxia (pre-intermittent hypoxia, post-intermittent hypoxia) on main outcome variables were assessed with a two (Tables 1 and 2)- or three (Figs. 3 and 4)-way mixed-effect model with repeated measures. Data are reported as means ± standard deviation (SD).
Table 1.
Respiratory variables before and after acute intermittent hypoxia
| Males |
Females |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Pre-IH | Post-IH | Pre-IH | Post-IH | IH | Sex | Interaction | |
| Minute ventilation, L/min | 6.1 ± 1.5 | 7.3 ± 2.2 | 5.7 ± 1.9 | 6.1 ± 1.6 | 0.04 | 0.18 | 0.32 |
| Tidal volume, mL/breath | 584 ± 230 | 617 ± 243 | 493 ± 188 | 508 ± 240 | 0.49 | 0.23 | 0.79 |
| TTot, s | 6.1 ± 2.0 | 5.8 ± 1.7 | 5.2 ± 1.4 | 5.4 ± 2.6 | 0.75 | 0.34 | 0.37 |
| TI, s | 2.6 ± 1.0 | 2.5 ± 0.7 | 2.5 ± 0.5 | 2.4 ± 0.6 | 0.48 | 0.82 | 0.62 |
| TI/TTot | 0.42 ± 0.08 | 0.43 ± 0.08 | 0.49 ± 0.08 | 0.49 ± 0.09 | 0.57 | 0.03 | 0.79 |
Values are presented as means ± SD from n = 21 male and n = 10 female participants. Normoxic resting data were analyzed over an ∼5-min baseline before and after acute intermittent hypoxia (IH). Data were analyzed with a 2-way mixed-effect model with repeated measures to assess the effect of sex (male, female) and condition (pre-IH, post-IH) and the interaction of sex and condition. Significant P values are in bold. TI/TTot, inspiratory time as a fraction of total breath time.
Table 2.
Normalized data computed from averaged cross-correlation histograms for MSNA and respiration
| Males |
Females |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Pre-IH | Post-IH | Pre-IH | Post-IH | IH | Sex | Interaction | |
| Inspiration | 56 ± 18 | 54 ± 17 | 56 ± 19 | 57 ± 22 | 0.89 | 0.82 | 0.76 |
| Peak Inspiration | 30 ± 19 | 37 ± 18 | 34 ± 14 | 26 ± 14 | 0.86 | 0.56 | 0.03 |
| Expiration | 55 ± 18 | 50 ± 13 | 51 ± 19 | 48 ± 20 | 0.18 | 0.62 | 0.56 |
Values are presented as means ± SD from male (n = 21) and female (n = 10) participants. Smoothed polynomial data were normalized by setting peak event counts in cross correlograms between muscle sympathetic nerve activity (MSNA) and peaks of the respiratory signal in each participant to 100%. In each study participant, the relative numbers of sympathetic spikes in different phases of the respiratory cycle were calculated. Data were analyzed with a 2-way mixed-effect model with repeated measures to assess the effect of sex (male, female) and condition [pre-intermittent hypoxia (IH), post-IH] and the interaction of sex and condition. Significant P value is in bold.
RESULTS
Data from 31 young healthy individuals (21 males, 10 females) are included in the present investigation. Groups did not differ by age (males: 30 ± 5 yr, females: 28 ± 5 yr, P = 0.17) or body mass index (males: 26 ± 2 kg/m2, females: 25 ± 3 kg/m2, P = 0.37). Acute intermittent hypoxia consisted of repeated reductions in inspired oxygen (males: 98 ± 16 to 49 ± 16 mmHg, females: 111 ± 17 to 46 ± 14 mmHg; main effect of intermittent hypoxia, P < 0.01; interaction of intermittent hypoxia and sex, P = 0.09) resulting in falls in arterial oxygen saturation () that did not differ between male (baseline : 97.8 ± 1.1%, average nadir : 91.5 ± 3.1%) and female (baseline : 98.4 ± 1.8%, average nadir : 91.6 ± 2.7%) participants (effect of intermittent hypoxia, P < 0.01; interaction of intermittent hypoxia and sex, P = 0.68). During intermittent exposures to hypoxia, end-tidal carbon dioxide was acutely increased (males: 42.5 ± 4.3 to 44.7 ± 3.9 mmHg; females: 40.5 ± 2.8 to 42.6 ± 2.6 mmHg; main effect of intermittent hypoxia, P < 0.01; interaction of intermittent hypoxia and sex, P = 0.99).
Effect of Intermittent Hypoxia on Resting Variables
Data were analyzed over an ∼5-min period of normoxic rest before and immediately after the hypoxic ventilatory response test that accompanied acute intermittent hypoxia, once oxygen saturation returned to baseline. As a result, end-tidal pressure of oxygen () did not differ from baseline after intermittent hypoxia in male (100 ± 16 to 102 ± 14 mmHg) or female (111 ± 16 to 110 ± 13 mmHg) participants (main effect of intermittent hypoxia, P = 0.88; interaction of intermittent hypoxia and sex, P = 0.66).
Minute ventilation increased after intermittent hypoxia (main effect of intermittent hypoxia, P = 0.04), and the response did not differ between the sexes (interaction of intermittent hypoxia and sex, P = 0.32) (Table 1). This mild hyperventilation resulted in a reduction in end-tidal pressure of carbon dioxide () during the post-intermittent hypoxia measurement period in both male (43.7 ± 4.1 to 41.4 ± 4.3 mmHg) and female (40.3 ± 1.7 to 38.5 ± 2.4 mmHg) participants (main effect of intermittent hypoxia, P < 0.01; interaction of intermittent hypoxia and sex, P = 0.66). There were no differences in respiratory period (TTot) or tidal volume between groups (main effect of sex, P = 0.34 and P = 0.23, respectively) before or after intermittent hypoxia (interaction of intermittent hypoxia and sex, P = 0.37 and P = 0.79, respectively). Although inspiratory time as a fraction of total breath time (TI/TTot) was greater in female than male participants (main effect of sex, P = 0.03), there was no effect of intermittent hypoxia (main effect of intermittent hypoxia, P = 0.57; interaction of intermittent hypoxia and sex, P = 0.79).
Heart rate increased after intermittent hypoxia in both male (59 ± 7 to 60 ± 9 beats/min) and female (64 ± 9 to 65 ± 9 beats/min) participants (main effect of intermittent hypoxia, P = 0.03), and this did not differ by sex (interaction of intermittent hypoxia and sex, P = 0.97). Any increase in mean arterial blood pressure after intermittent hypoxia tended to be greater in male (95 ± 7 to 97 ± 8 mmHg) than female (95 ± 11 to 94 ± 10 mmHg) participants (interaction of intermittent hypoxia and sex, P = 0.09).
Compared with baseline, multiunit MSNA burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats) increased after intermittent hypoxia in both male (20 ± 6 to 24 ± 8 bursts/min; 34 ± 11 to 41 ± 13 bursts/100 heartbeats) and female (19 ± 8 to 22 ± 10 bursts/min; 30 ± 13 to 35 ± 15 bursts/100 heartbeats) participants (main effect of intermittent hypoxia, P < 0.01 for both). The increase in MSNA with intermittent hypoxia did not differ between the sexes (interaction of intermittent hypoxia and sex: burst frequency P = 0.65, burst incidence P = 0.59).
Respiratory Modulation
Normalized cross-correlation histograms were constructed for all participants. MSNA was modulated by respiration such that MSNA counts were lowest at inspiratory peak and highest during early inspiration/late expiration (main effect of time, P < 0.01; Fig. 3). This modulation of MSNA within the breath was observed in both male (Fig. 3A) and female (Fig. 3B) participants (main effect of time, P < 0.01; main effect of sex, P = 0.49; interaction of time and sex, P = 0.98). There was a trend for sex-related differences in the effect of intermittent hypoxia on respiratory modulation of MSNA (interaction of time, sex, and intermittent hypoxia, P = 0.099; Fig. 3). As a result, the modulation index decreased in male participants after intermittent hypoxia (P = 0.03), whereas the modulation index increased after intermittent hypoxia in female participants (P = 0.02). This was observed both within groups (Fig. 3) and when the effect of intermittent hypoxia on the modulation index was compared between groups (interaction of intermittent hypoxia and sex, P = 0.047). When respiratory time (Fig. 3) was divided into thirds [inspiration, inspiratory peak, and expiration (25)], sex differences in the effect of intermittent hypoxia on sympathetic activity around the inspiratory peak were observed (interaction of sex and intermittent hypoxia, P = 0.03; Table 2). No group differences were observed in the early inspiratory or late expiratory phases (P = 0.76 and P = 0.56, respectively; Table 2).
To illustrate the temporal profile of respiratory modulation of mean arterial blood pressure, cycle-triggered averages of mean arterial blood pressure were measured around the inspiratory peak (time 0) and a change (Δ) from inspiratory peak was calculated (Fig. 4). Blood pressure was modulated by respiration, such that blood pressure increased during inspiration and decreased during expiration (main effect of time, P < 0.01; Fig. 4). This modulation of blood pressure within the breath was observed in both male (Fig. 4A) and female (Fig. 4B) participants (main effect of time, P < 0.01; interaction of time and sex, P = 0.67). The temporal profile of mean arterial blood pressure throughout the respiratory cycle was maintained after intermittent hypoxia (interaction of intermittent hypoxia and time, P = 0.99). However, after intermittent hypoxia sex-related differences in the change in blood pressure during the respiratory cycle approached significance (main effect of sex, P = 0.11; main effect of intermittent hypoxia, P = 0.10; interaction of sex and intermittent hypoxia, P = 0.06).
DISCUSSION
The main findings from the present investigation are threefold. First, respiratory modulation of MSNA is altered after acute intermittent hypoxia exposure in healthy young male and female participants. Second, intermittent hypoxia-mediated changes in within-breath modulation of MSNA differ by sex. In female participants, modulation of MSNA within the breath was greater after acute intermittent hypoxia, whereas male participants exhibited less respiratory modulation of MSNA after intermittent hypoxia. Third, these sex-related differences in respiratory modulation of MSNA after intermittent hypoxia were accompanied by potential sex differences in the modulation of blood pressure during the respiratory cycle.
Sympathetic vasoconstrictor activity exhibits profound within-breath modulation (2–5). Intermittent hypoxia exposure increases MSNA (21–23, 27), and this effect persists beyond the duration of the hypoxic exposure. Sympathetic activity increases similarly in both male (10) and female (8) rodents after long-term exposure to intermittent hypoxia. As shown previously by our group (16), MSNA is also increased from baseline levels after acute intermittent hypoxia in this cohort of healthy young adults, and this increase does not differ between male and female participants. In the absence of sex differences in the increase in MSNA after intermittent hypoxia, sex-related differences in respiratory modulation of MSNA were observed (Fig. 3, Table 2). Compared with baseline, male participants exhibited less modulation of MSNA after intermittent hypoxia, most likely due to an attenuation of peak inspiratory inhibition of MSNA. In contrast, peak inspiratory inhibition of MSNA was preserved or even augmented in female participants, resulting in a greater modulation index after intermittent hypoxia. In humans, MSNA is lowest during inspiration (when lung volume is high) and is highest during expiration (when lung volume is low) (2–5). This pattern differs from what is observed in preclinical rodent models (sympathetic activity is highest during inspiration and lowest during expiration) (8, 10). Despite species differences, sex-related differences in the effect of intermittent hypoxia on respiratory-sympathetic coupling are similarly observed in rodents (7–10). These sex-related differences are likely due to intrinsic sex differences rather than differences in sex hormones (e.g., estradiol) given that animal studies were conducted in sexually immature rats (7–10). Furthermore, human studies were conducted during the early follicular phase of the menstrual cycle or placebo phase of oral contraceptive use, when estradiol levels are relatively matched between sexes. With this, it may be reasonable to propose that sex-related differences observed in the present study would be amplified when estradiol and/or progesterone levels are elevated, and future studies in this area are warranted.
In line with the present findings, patients with sleep apnea exhibit changes in the temporal profile of the respiratory modulation of MSNA measured from normalized MSNA. Fatouleh et al. (6) found that temporal coupling of MSNA was higher in the postinspiratory phase than during inspiration or expiration in patients with sleep apnea (18 males, 3 females) and these changes were reversed after continuous positive airway pressure (CPAP) treatment, supporting a direct role for intermittent hypoxic exposures in changes in respiratory modulation of MSNA. Consistent with data from Fatouleh and colleagues (6), and using similar methodologies, we observed changes in the temporal profile of the respiratory modulation of MSNA after acute intermittent hypoxia exposure, including a lower modulation index (Fig. 3) and greater relative sympathetic activity in the peak inspiratory phase (Table 2) in the male participants studied. In contrast, Nardone and colleagues (28) recently showed that acute intermittent hypoxia increased resting MSNA and blood pressure without altering within-breath respiratory modulation of MSNA in nine male participants. In addition to a relatively small sample size and a lack of female participants, Nardone and colleagues assessed respiratory modulation of MSNA in terms of sympathetic burst occurrence (bursts/min) (28) rather than the coherence between respiration and MSNA, as in the present study. Recent data from Badrov and colleagues (29) show that conclusions from multiunit integrated MSNA burst analysis alone (i.e., bursts/min) may miss subtle but important sex-related differences in neuronal firing between groups of individuals at increased cardiovascular disease risk (e.g., hypertension). Combining the aforementioned results with both human and preclinical data, we conclude that relationships between respiratory and sympathetic nervous system activation are altered after intermittent hypoxia and these changes differ between male and female participants.
Contributing Mechanisms
Present data were collected prospectively by two centers, and the collected data were merged to assess the hypothesis presented here. Because this was a secondary analysis of prospectively collected data, the present experimental design does not allow for direct assessment of contributing mechanisms. That being said, there are several factors that influence how breathing modulates MSNA and its timing within the breath, including changes in lung volume, breathing frequency, baroreceptor feedback, and central/peripheral chemoreceptor activation (reviewed in Ref. 30). Vermeulen and colleagues (12) recently reported ventilatory long-term facilitation after acute intermittent hypoxia exposure in healthy humans. The authors further showed that male (n = 10) and female (n = 9) participants achieved intermittent hypoxia-mediated ventilatory long-term facilitation through different respiratory recruitment patterns (i.e., differences in the reliance on tidal volume vs. breathing frequency) (12). Importantly, the respiratory pattern was not constrained with the use of a metronome, thus allowing participants to breathe spontaneously throughout the protocol. In contrast to data from Vermeulen and colleagues (12), we did not observe sex-specific changes in respiratory recruitment patterns with intermittent hypoxia (Table 1). Differences in intermittent hypoxia protocol severity and duration (longer hypoxic stimulus, less recovery time, greater number of cycles), and the degree to which carbon dioxide was controlled, likely contribute to any discrepancies. Although the hypercapnic hypoxic gas mixture used in the present study was effective at preventing hypocapnia typical of hypoxia-induced hyperventilation, the level of hypercapnia achieved is less than that used by Vermeulen et al. (12) (+4 mmHg above baseline), which may be more similar to events that occur during sleep apnea where hypercapnia is present.
Given that we did not observe any sex-related differences in the effect of intermittent hypoxia on lung volume and/or breathing frequencies, it is unlikely that such changes explain observed sex differences in respiratory modulation of MSNA. Notably, we observed female participants spending more time during inspiration compared with male participants both before and after acute intermittent hypoxia. Other groups have also observed sex differences in resting respiratory function, as well as changes across different phases of the menstrual cycle (31). Within-breath modulation of MSNA is more pronounced when inspiratory time is prolonged (5, 32). Thus, it is reasonable to speculate that group differences in inspiratory time may contribute to sex differences in the magnitude of inhibition of MSNA during peak inspiration. Importantly, any differences in inspiratory time between sexes were observed both before and after the intermittent hypoxia exposure and are unlikely to contribute directly to observed sex differences in the response to the intermittent hypoxia protocol. Furthermore, differences in respiratory modulation of MSNA are unlikely to be due to any sex-related difference in work of breathing, given prior work suggesting that resistive work of breathing does not differ between sexes until minute ventilation reaches ∼45 L/min (33).
Finally, the sensitivity of the sympathetic nervous system to baroreceptor influence fluctuates with breathing. Specifically, the slope of the relationship between changes in MSNA and blood pressure is greatest during low lung volumes and falls at higher lung volumes (28, 34, 35). Sex-related differences in baroreflex sensitivity after acute intermittent hypoxia were not observed in prior work from our group (16), and others have similarly reported no effect of intermittent hypoxia on resting (36) or within-breath (28) baroreflex gain, albeit the latter study was conducted in male participants only. As such, additional studies are required to further elucidate the mechanism by which these neural networks may influence respiratory-sympathetic coupling differently in male and female participants.
Perspectives and Significance
In preclinical rodent models, changes in respiratory modulation of MSNA have been implicated in the development of hypertension after intermittent hypoxia exposure (7). Consistent with this, an increase in sympathetic activity during specific periods of the respiratory cycle may lead to increased neurotransmitter release, peripheral vasoconstriction, and ultimately blood pressure in rat models of hypertension (14, 15). Reported differences in respiratory modulation of MSNA are likely clinically significant and may contribute to heightened MSNA and blood pressure in individuals with sleep apnea (6, 37). Given the potential sex differences in respiratory modulation of MSNA in the present study, it is interesting to note that the prevalence of hypertension in patients with sleep apnea may also differ by sex (38–40). Consistent with this, we have previously shown that the blood pressure response to acute intermittent hypoxia is attenuated in female participants compared with male participants, despite similar increases in MSNA (16). In the present post hoc analysis, we include data from a subset of the original cohort. With this smaller number of participants, we were underpowered to detect sex differences in the effect of intermittent hypoxia on blood pressure (P = 0.09; Cohen’s d = 0.696).
Although we clearly observed modulation of mean arterial blood pressure (±3 mmHg) by respiration of magnitude similar to prior work (41), there was no effect of intermittent hypoxia on respiratory patterning of blood pressure in the female participants studied (Fig. 4B). In contrast, male participants appear to exhibit less within-breath change in mean arterial blood pressure after intermittent hypoxia (Fig. 4A). We speculate that the observed differences in respiratory modulation of MSNA might contribute, at least in part, to sex differences in overall blood pressure changes after intermittent hypoxia. Neurovascular transduction (the transmission of MSNA to vascular tone) increases after acute intermittent hypoxia exposure in male participants (22). When combined with diminished peak inspiratory inhibition of MSNA, these changes may contribute to enhanced sympathetically mediated vasoconstriction after intermittent hypoxia and ultimately higher blood pressures. However, it is important to acknowledge that any sex-related differences in the blood pressure response to acute intermittent hypoxia were modest [2–4 mmHg (16)] and any changes in within-breath modulation of blood pressure (P = 0.06) are unlikely to be the sole contributor to increases in blood pressure after intermittent hypoxia exposure. Future work will be necessary to determine whether the sex-related differences in respiratory-sympathetic coupling observed contribute to sex disparities in high blood pressure for patients with sleep apnea.
Conclusions
The present findings highlight sex-related differences in respiratory modulation of MSNA. We show that both male and female adults exhibit alternations in respiratory patterning of MSNA after exposure to acute intermittent hypoxia; however, the patterning of sympathetic activity within the breath is sex specific. The results advance our understanding of sex-specific changes in modulation of sympathetic activity after intermittent hypoxia in humans. How sex differences in respiratory modulation of MSNA influence blood pressure regulation long term and the possible clinical implications (i.e., sleep apnea) remain to be elucidated.
GRANTS
This study was supported by National Institutes of Health Grants AHA15SDG25080095 (to J.K.L.), HL130339 (to J.K.L.), HL153523 (to J.K.L.), and U54 AG044170 (to S.E.B.) and by the Mayo Clinic Center for Biomedical Discovery (to J.K.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.B. and J.K.L. conceived and designed research; C.L.I., E.P.O., D.W.J., S.E.B., J.L.H., C.M.M., and J.K.L. performed experiments; J.S.E., C.L.I., E.P.O., D.W.J., and J.K.L. analyzed data; J.S.E., C.L.I., D.W.J., S.E.B., C.M.M., and J.K.L. interpreted results of experiments; J.S.E. and J.K.L. prepared figures; J.S.E. and J.K.L. drafted manuscript; J.S.E., C.L.I., E.P.O., D.W.J., S.E.B., J.L.H., C.M.M., and J.K.L. edited and revised manuscript; J.S.E., C.L.I., E.P.O., D.W.J., S.E.B., J.L.H., C.M.M., and J.K.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our research participants as well as Drs. Vaughan Macefield and Chloe Taylor for assistance with data analysis and Drs. Jerome Dempsey and Barbara Morgan for helpful feedback during the writing process.
REFERENCES
- 1.Malpas SC. The rhythmicity of sympathetic nerve activity. Prog Neurobiol 56: 65–96, 1998. doi: 10.1016/S0301-0082(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 2.Eckberg DL, Wallin BG, Fagius J, Lundberg L, Torebjörk HE. Prospective study of symptoms after human microneurography. Acta Physiol Scand 137: 567–569, 1989. doi: 10.1111/j.1748-1716.1989.tb08804.x. [DOI] [PubMed] [Google Scholar]
- 3.Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand 74: 96–108, 1968. doi: 10.1111/j.1365-201X.1968.tb10904.x. [DOI] [PubMed] [Google Scholar]
- 4.Macefield VG, Wallin BG. Modulation of muscle sympathetic activity during spontaneous and artificial ventilation and apnoea in humans. J Auton Nerv Syst 53: 137–147, 1995. doi: 10.1016/0165-1838(94)00173-H. [DOI] [PubMed] [Google Scholar]
- 5.Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ Res 72: 440–454, 1993. doi: 10.1161/01.RES.72.2.440. [DOI] [PubMed] [Google Scholar]
- 6.Fatouleh R, McKenzie DK, Macefield VG. Respiratory modulation of muscle sympathetic nerve activity in obstructive sleep apnoea. Exp Physiol 99: 1288–1298, 2014. doi: 10.1113/expphysiol.2013.077511. [DOI] [PubMed] [Google Scholar]
- 7.Souza GM, Amorim MR, Moraes DJ, Machado BH. Sex differences in the respiratory-sympathetic coupling in rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol 256: 109–118, 2018. doi: 10.1016/j.resp.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Souza GM, Bonagamba LG, Amorim MR, Moraes DJ, Machado BH. Inspiratory modulation of sympathetic activity is increased in female rats exposed to chronic intermittent hypoxia. Exp Physiol 101: 1345–1358, 2016. doi: 10.1113/EP085850. [DOI] [PubMed] [Google Scholar]
- 9.Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol 94: 972–983, 2009. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]
- 10.Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586: 3253–3265, 2008. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usselman CW, Steinback CD, Shoemaker JK. Effects of one’s sex and sex hormones on sympathetic responses to chemoreflex activation. Exp Physiol 101: 362–367, 2016. doi: 10.1113/EP085147. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen TD, Benbaruj J, Brown CV, Shafer BM, Floras JS, Foster GE. Peripheral chemoreflex contribution to ventilatory long-term facilitation induced by acute intermittent hypercapnic hypoxia in males and females. J Physiol 598: 4713–4730, 2020. doi: 10.1113/JP280458. [DOI] [PubMed] [Google Scholar]
- 13.Briant LJ, O’Callaghan EL, Champneys AR, Paton JF. Respiratory modulated sympathetic activity: a putative mechanism for developing vascular resistance? J Physiol 593: 5341–5360, 2015. doi: 10.1113/JP271253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol 587: 597–610, 2009. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toney GM, Pedrino GR, Fink GD, Osborn JW. Does enhanced respiratory-sympathetic coupling contribute to peripheral neural mechanisms of angiotensin II-salt hypertension? Exp Physiol 95: 587–594, 2010. doi: 10.1113/expphysiol.2009.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob DW, Ott EP, Baker SE, Scruggs ZM, Ivie CL, Harper JL, Manrique-Acevedo CM, Limberg JK. Sex differences in integrated neurocardiovascular control of blood pressure following acute intermittent hypercapnic hypoxia. Am J Physiol Regul Integr Comp Physiol 319: R626–R636, 2020. doi: 10.1152/ajpregu.00191.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott EP, Jacob DW, Baker SE, Holbein WW, Scruggs ZM, Shoemaker JK, Limberg JK. Sympathetic neural recruitment strategies following acute intermittent hypoxia in humans. Am J Physiol Regul Integr Comp Physiol 318: R961–R971, 2020. doi: 10.1152/ajpregu.00004.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989. doi: 10.1161/01.HYP.13.6.647. [DOI] [PubMed] [Google Scholar]
- 19.Petersen ME, Williams TR, Sutton R. A comparison of non-invasive continuous finger blood pressure measurement (Finapres) with intra-arterial pressure during prolonged head-up tilt. Eur Heart J 16: 1641–1654, 1995. doi: 10.1093/oxfordjournals.eurheartj.a060790. [DOI] [PubMed] [Google Scholar]
- 20.Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci 162: 89–93, 2011. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 121: 87–93, 2005. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Stuckless TJ, Vermeulen TD, Brown CV, Boulet LM, Shafer BM, Wakeham DJ, Steinback CD, Ayas NT, Floras JS, Foster GE. Acute intermittent hypercapnic hypoxia and sympathetic neurovascular transduction in men. J Physiol 598: 473–487, 2020. doi: 10.1113/JP278941. [DOI] [PubMed] [Google Scholar]
- 23.Xie A, Skatrud JB, Crabtree DC, Puleo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol (1985) 89: 1333–1339, 2000. doi: 10.1152/jappl.2000.89.4.1333. [DOI] [PubMed] [Google Scholar]
- 24.Fatouleh R, Macefield VG. Cardiorespiratory coupling of sympathetic outflow in humans: a comparison of respiratory and cardiac modulation of sympathetic nerve activity to skin and muscle. Exp Physiol 98: 1327–1336, 2013. doi: 10.1113/expphysiol.2013.072421. [DOI] [PubMed] [Google Scholar]
- 25.Fatouleh R, Macefield VG. Respiratory modulation of muscle sympathetic nerve activity is not increased in essential hypertension or chronic obstructive pulmonary disease. J Physiol 589: 4997–5006, 2011. doi: 10.1113/jphysiol.2011.210534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci 47: 433–448, 1980. doi: 10.1016/0022-510X(80)90098-2. [DOI] [PubMed] [Google Scholar]
- 27.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 96: 754–761, 2004. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- 28.Nardone M, Teixeira AL, Incognito AV, Vermeulen TD, Shafer BM, Millar PJ, Foster GE. Within-breath sympathetic baroreflex sensitivity is modulated by lung volume but unaffected by acute intermittent hypercapnic hypoxia in men. Am J Physiol Heart Circ Physiol 319: H213–H221, 2020. doi: 10.1152/ajpheart.00296.2020. [DOI] [PubMed] [Google Scholar]
- 29.Badrov MB, Okada Y, Yoo JK, Vongpatanasin W, Shoemaker JK, Levine BD, Fu Q. Sex Differences in the sympathetic neural recruitment and hemodynamic response to head-up tilt in older hypertensives. Hypertension 75: 458–467, 2020. doi: 10.1161/HYPERTENSIONAHA.119.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol 130: 3–20, 2002. doi: 10.1016/S0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 31.Macnutt MJ, De Souza MJ, Tomczak SE, Homer JL, Sheel AW. Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J Appl Physiol (1985) 112: 737–747, 2012. [ doi: 10.1152/japplphysiol.00727.2011. [DOI] [PubMed] [Google Scholar]
- 32.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol 537: 277–289, 2001. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominelli PB, Molgat-Seon Y, Bingham D, Swartz PM, Road JD, Foster GE, Sheel AW. Dysanapsis and the resistive work of breathing during exercise in healthy men and women. J Appl Physiol (1985) 119: 1105–1113, 2015. [ doi: 10.1152/japplphysiol.00409.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol 304: 489–502, 1980. doi: 10.1113/jphysiol.1980.sp013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol 365: 181–196, 1985. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol 574: 605–613, 2006. doi: 10.1113/jphysiol.2006.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283: 1829–1836, 2000. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 39.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 40.Yu Q, Yin G, Zhang P, Song Z, Chen Y, Zhang D, Hu W. Distinct associations between hypertension and obstructive sleep apnea in male and female patients. PLoS One 9: e113076, 2014. doi: 10.1371/journal.pone.0113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res 85: 457–469, 1999. doi: 10.1161/01.RES.85.5.457. [DOI] [PubMed] [Google Scholar]