Abstract
Background and Aims
Persistent gastrogastric or jejunogastric fistula is theoretically a concerning sequela of EUS-directed transgastric ERCP/EUS (EDGE), as it may functionally reverse the malabsorptive mechanism of Roux-en-Y gastric bypass (RYGB). Prior EDGE studies, using predominantly 15-mm (diameter) lumen-apposing metal stents (LAMS) and fistula closure by primary intent, collectively report 9% persistent fistula rate, without a clear weight gain association. Our study determines the incidence of persistent fistula, and its association with unintentional weight gain, among recipients of EDGE via 20-mm LAMS followed by spontaneous fistula closure (secondary intent).
Methods
We conducted a dual-center prospective cohort study of 22 RYGB patients who underwent EDGE using 20-mm between 3/2018 and 10/2019. After LAMS extraction, all GGFs/JGFs were allowed to heal spontaneously. Objective testing for persistent fistula and total body weight (TBW) occurred a minimum of 8 weeks after LAMS extraction.
Results
Persistent fistula was identified in 9 patients (41%). Longer LAMS dwell time (median 77-days) was observed in the persistent fistula group, compared to those with durable spontaneous fistula closure (median 35-days) (p = 0.03). Weight gain of ≥ 5% TBW occurred in 56% (n = 5) of patients with persistent fistula, compared to 15% (n = 2) of patients with spontaneous fistula closure (p = 0.128). Four patients with symptomatic persistent fistulas underwent attempted endoscopic fistula closure a median 7.5 months after LAMS extraction. Durable fistula closure occurred in the single patient who received argon plasma coagulation plus endoscopic suturing, whereas fistula dehiscence occurred in 3/3 (100%) patients with endoscopic suturing monotherapy.
Conclusions
Larger LAMS diameter (20-mm), longer LAMS dwell time, and spontaneous fistula closure may be technical factors that increase the likelihood of post-EDGE persistent fistula. Post-EDGE persistent fistula has not been shown by ours or other studies to be significantly associated with unintentional weight gain; however, this may be due to small sample size. We question the utility of routine fistula closure by primary intent and suggest a personalized approach to post-EDGE fistula management.
Keywords: EUS-directed transgastric ERCP, EDGE, Lumen-apposing metal stent, Fistula, Rouxen-Y gastric bypass
Graphical Abstract
Background and Aims
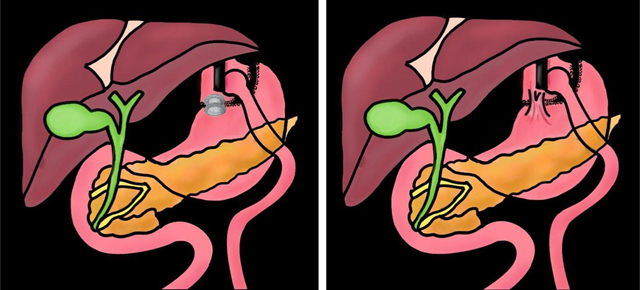
EUS-directed transgastric ERCP/EUS (EDGE) is an endoscopic method for treating pancreaticobiliary disorders in Roux-en-Y gastric bypass (RYGB) anatomy [1]. EDGE is a 2-in-1 procedure that consists of EUS-directed gastrogastrostomy or jejunogastrostomy (EUS-GG/JG) creation using a lumen-apposing metal stent (LAMS) (step 1), followed by, transgastric ERCP/EUS (step 2). The LAMS is removed after the completion of the transgastric intervention and maturation of the gastrogastric/jejunogastric fistula (GGF/JGF), to re-exclude the bypassed stomach. Closure of the GGF/JGF can occur via primary intent (i.e., endoscopic closure) or secondary intent (i.e., spontaneous closure) (Fig. 1). Persistent fistula refers to a GGF/JGF that fails to close by either primary or secondary intent (Fig. 2a, b). Theoretically, persistent fistula is a concerning sequela, as it may functionally reverse the bypass and/or lead to additional endoscopies for attempted fistula closure [2,3].
Fig. 1.
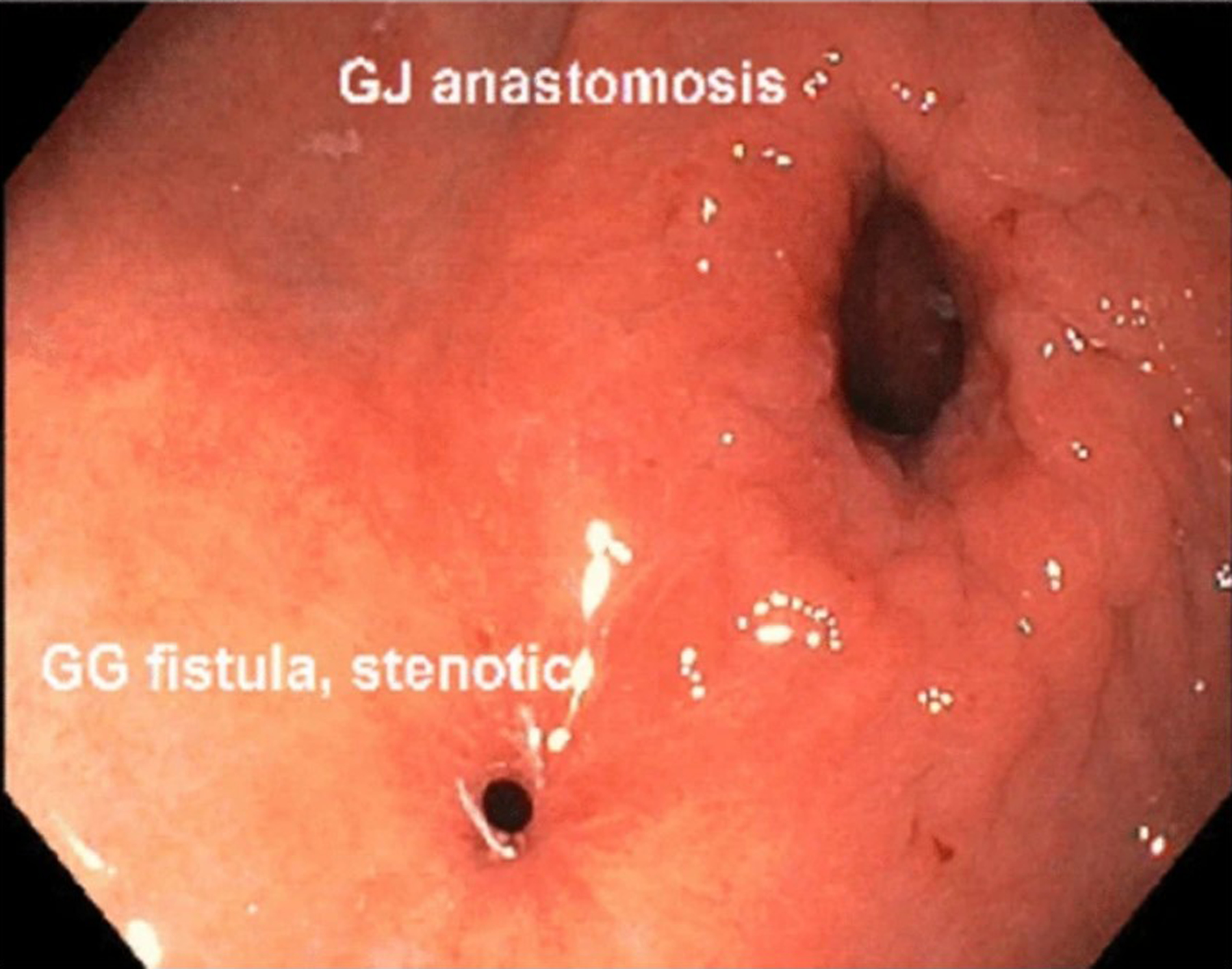
Endoscopic view of a gastrogastric fistula (GGF) that has almost spontaneously closed following LAMS extraction (i.e., fistula healing by secondary intent). The stenotic GGF is adjacent to the original surgical gastrojejunal anastomosis (GJA)
Figures 2.
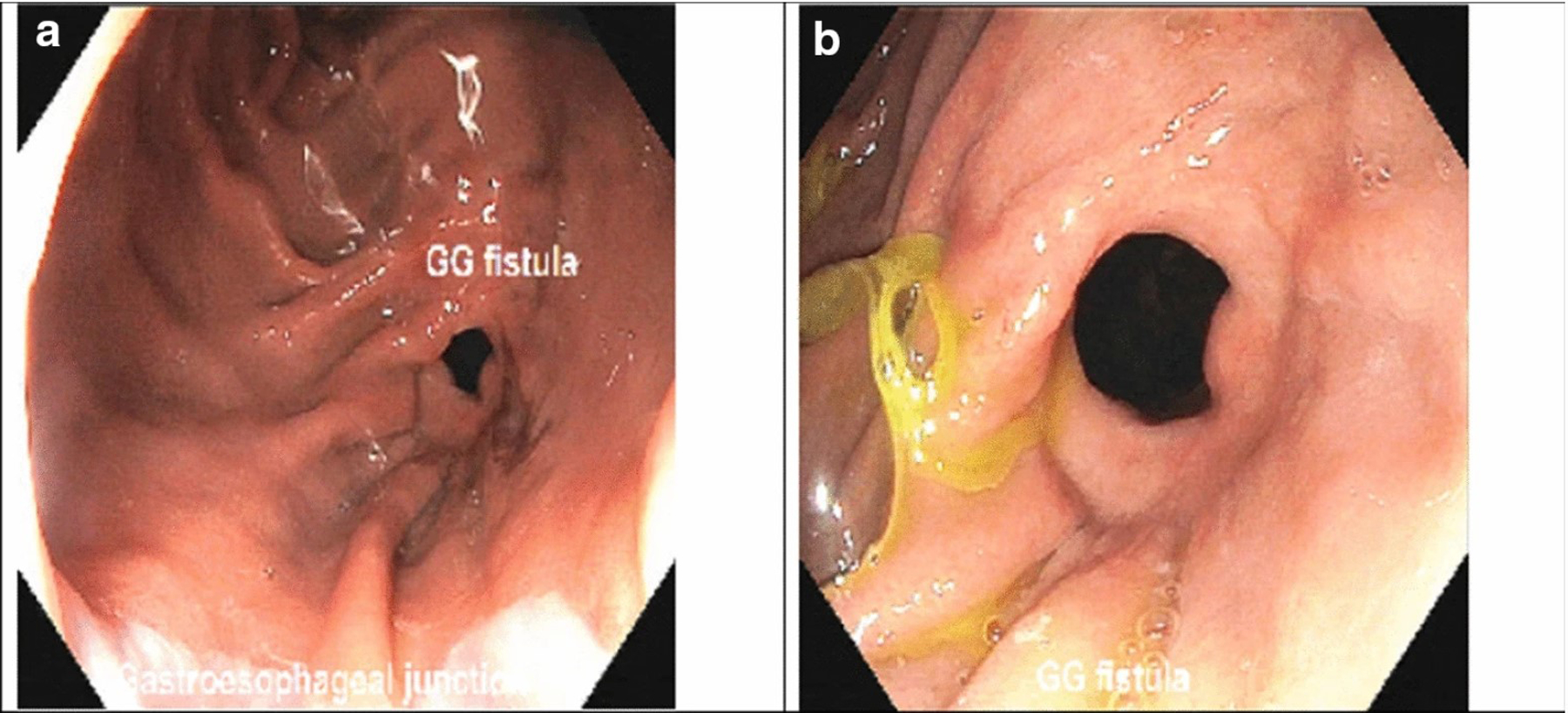
a, b Endoscopic views of a persistent GGF, which is seen along the surgical staple (division) line, in the gastric pouch
The natural history of an endoscopically created GGF/JGF in EDGE is unclear. Prior EDGE studies report persistent fistula rates of 0–12.5%, without a clear weight gain association [3–9]. These studies predominantly used 15-mm LAMS and varied forms of primary (endoscopic) fistula closure. Expert opinion now endorses use of 20-mm LAMS, followed by routine primary fistula closure [10]. Both recommendations are predicated on theoretical concepts that a wider LAMS conduit reduces duodenoscope friction (LAMS dislodgement) and that primary fistula closure decreases the risk of persistent fistula (weight gain). The problem with recommending routine primary fistula closure is the implication that persistent fistulas are detrimental, which is unknown. Along this line, if a persistent fistula is diagnosed after failed primary closure, up to 40% of patients will require more than one endoscopic reintervention to achieve successful fistula reclosure (i.e., more procedures, cost, and stress for the patient) [3]. The aim of our dual-center prospective cohort study is to determine the clinical significance of persistent fistulas occurring after EDGE using 20-mm LAMS, with spontaneous fistula closure (secondary intent). This knowledge, in turn, can allow us to prognose post-EDGE persistent fistulas and thereby determine if routine primary fistula closure is necessary.
Methods
We conducted a dual-center prospective cohort study of 22 RYGB patients who underwent EDGE using 20-mm × 10-mm electrocautery-enhanced lumen-apposing metal stents (ECE-LAMS; AXIOS stent and electrocautery-enhanced delivery system; Boston Scientific; Marlborough, MA, USA) at two tertiary care centers between March 2018 and October 2019. EDGE was performed by one endoscopist per institution (J.N., WVU; T.B., UNC). EDGE was defined as EUS-GG/JG creation via 20-mm ECE-LAMS, followed by transgastric (anterograde) ERCP or EUS. One advanced endoscopist per institution performed the procedures. Two patients were previously reported in a multicenter retrospective EDGE study [3]. Informed consent was obtained from each patient, including the theoretical benefits and drawbacks of spontaneous fistula closure. This study was approved by the Institutional Review Boards for Human Research and complied with Health Insurance Portability and Accountability Act regulations at each institution.
Inclusion criteria were patients with RYGB anatomy that underwent EDGE for any indication, except malignancy. We excluded malignancy because the underlying catabolic state would invariably confound our study question of whether a persistent fistula confers an increased risk of unintentional weight gain. After the completion of the transgastric intervention, the LAMS was extracted and the GGF or JGF was allowed to spontaneously heal (i.e., patients were disqualified if any form of primary fistula closure was attempted). LAMS dwell times varied among patients depending on the number of transgastric ERCPs required. The patients with a short LAMS dwell time generally only required a single ERCP before their pancreaticobiliary malady resolved (and EDGE could be declared completed). Patients were contacted by phone or office appointment and offered an objective test to confirm spontaneous fistula closure. Objective testing was performed at least 8 weeks after LAMS extraction and consisted of an upper gastrointestinal series (UGIS), computed tomography with oral contrast (CT), or esophagogastroduodenoscopy (EGD) (Fig. 3). Total body weight (kilograms) for each patient was recorded at the time of objective testing. A personalized plan was created for each patient who tested positive for persistent fistula (e.g., endoscopic closure, continued observation), which was left to the discretion of each advanced endoscopist.
Fig. 3.
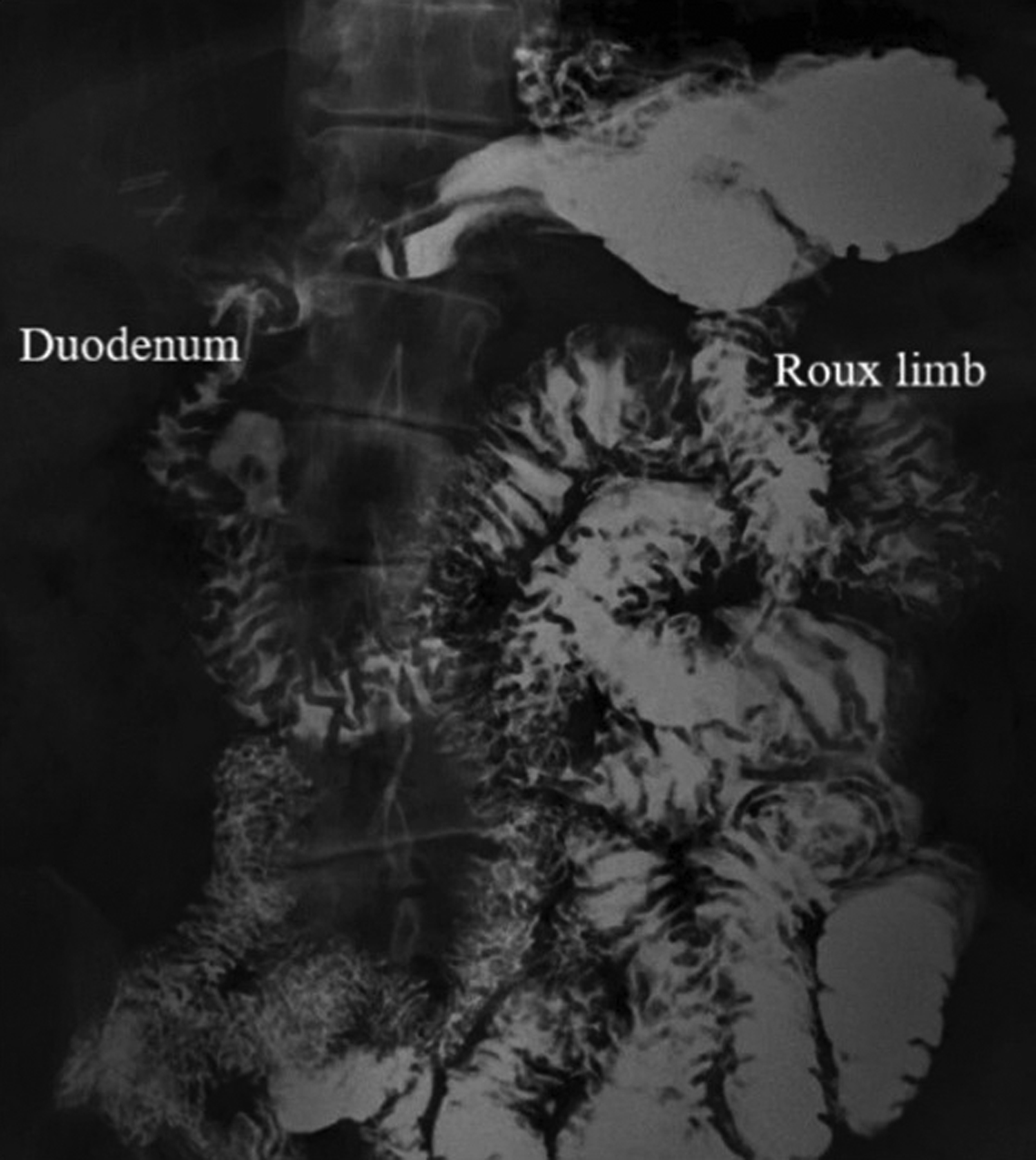
Upper gastrointestinal series (UGIS) with small bowel follow-through demonstrating a persistent jejunogastric fistula. The fistulous connection cannot be directly visualized; however, contrast opacification of both the jejunal Roux limb and gastric remnant/duodenum confirms the presence of a fistula
Primary and Secondary Study Outcomes
The primary outcome of this study was the incidence of persistent fistulas (GGF and JGF) and its association with unintentional weight gain. There is no universal definition of a “persistent fistula,” but for the purposes of this study, a fistula was considered “persistent” if detected at least 8 weeks after LAMS extraction. An 8-week interval was selected based on the study definition of persistent fistula put forth by Runge et al., the largest EDGE study to date [3]. Change in total body weight (TBW) was calculated for each patient by calculating the difference in pre-EDGE TBW (i.e., baseline TBW measured on the date of the index EDGE session) and post-EDGE TBW (i.e., measured at the time of objective testing for persistent fistula).
Secondary outcomes of this study included potential risk factors for persistent fistula, such as fistula type (GGF or JGF), LAMS dwell time (i.e., number of days from LAMS insertion to extraction), time interval from LAMS removal to objective testing for persistent fistula, and other demographic variables (e.g., diabetes mellitus, tobacco usage). Outcomes were also recorded for patients with persistent fistulas who elected to undergo secondary (fistula) closure (e.g., endoscopic suturing). Secondary (fistula) closure refers to attempted endoscopic closure of a persistent fistula that fails to heal spontaneously (i.e., failure to heal by secondary intention).
Procedural Description
EUS-GG/JG (Step 1)
All EDGE-related procedures were performed under general anesthesia with endotracheal intubation and under fluoroscopic-guidance. Prophylactic antimicrobials were not routinely administered. Baseline TBW was recorded the day of the index EDGE procedure (step 1). A linear echoendoscope (GFUCT180; Olympus, Central Valley, PA, USA) was used to intubate the esophagus and to reach the gastric pouch or jejunum (Roux limb). A puncture point was selected between the gastric pouch or jejunal Roux limb and the gastric remnant (bypassed stomach). Color Doppler imaging mode was used to avoid intervening blood vessels. The gastric remnant was accessed via transmural puncture with a 19-gauge aspiration needle. The gastric remnant was infused with at least 100 mL of contrast medium and sterile water, under endosonographic and fluoroscopic visualization. After sufficient distension, a 20-mm ECE-LAMS was deployed into the gastric remnant via freehand technique.
Transgastric ERCP/EUS (Step 2)
After creation of a GGF or JGF via 20-mm LAMS, transgastric ERCP or EUS was performed in either the same endoscopic session (i.e., single-session EDGE) or a later session (i.e., dual-session EDGE) [10]. LAMS anchoring was not performed. Transgastric interventions consisted of a variety of therapeutic pancreaticobiliary maneuvers (see results). In the event of intraprocedural LAMS dislodgement necessitating endoscopic salvage, 3–5 days of oral antimicrobials (e.g., fluoroquinolone) were prescribed.
LAMS Extraction (Step 3)
After completion of the transgastric intervention, the LAMS was extracted using a grasping forceps after a minimum 14-day dwell time (i.e., 14-days to allow fistula maturation). No form of primary (endoscopic) fistula closure was attempted after LAMS removal. No new medications were prescribed (e.g., proton pump inhibitor). Objective testing for persistent fistula was discussed with each patient and completed a minimum of 8 weeks after LAMS extraction. TBW was recorded at the time of objective testing for persistent fistula.
Fistula Closure (Step 4)
Patients with persistent fistulas were offered continued observation (i.e., serial weight measurements) or attempted endoscopic secondary fistula closure. Methods of fistula closure included endoscopic suturing monotherapy (Overstitch; Apollo Endosurgery, Austin, TX) or argon plasma coagulation [APC pulsed Effect 2,1 L/min, 30 W (ERBE USA, Marietta, GA)], to de-epithelialize the fistula margins, followed by endoscopic suturing. After attempted endoscopic fistula closure, an UGIS or EGD was obtained to confirm fistula closure.
Statistical Analysis
All statistical analysis was performed on R (Version 3.6.1). Categorical variables were reported as percentages. Quantitative variables were reported either as mean ± standard deviation or median with interquartile range (IQR). Fisher’s exact test and Wilcoxon rank-sum test were used to compare categorical and continuous variables, respectively. Because of our small sample size (n = 22), the permutation of regressor residuals (PRR) test was used to investigate the association between persistent fistula and LAMS dwell time, controlled for the effect of age and sex [11]. The PRR test is used for binary logistic regression with a small-sized data set. It was developed to overcome the limitation of statistical inference based on a large data set (e.g., exact conditional logistic regression), which can be inaccurate if applied to small data set logistic regression. The PRR test controls for type I error (i.e., rejecting a true null hypothesis).
Results
Study Cohort (n = 22)
Twenty-two patients (18 females; mean age 62.4 ± 9.5 years) underwent EDGE via 20-mm LAMS. Indications for EDGE included choledocholithiasis (n = 13), benign biliary stricture (n = 3), bile leak (n = 2), papillary stenosis (n = 1), intraductal papillary mucinous neoplasm (n = 1), chronic pancreatitis (n = 1), and benign gastric remnant wall thickening on CT (n = 1). Single-session EDGE was performed in 9 patients (41%) and dual-session EDGE in 13 patients (59%). A median 21-day interval elapsed between EUS-GG/JG creation and transgastric ERCP in the dual-session EDGE cohort. There was no association between type of EDGE (i.e., single-session EDGE or dual-session EDGE) and persistent fistula (p = 0.999). All patients underwent LAMS extraction and fistulas were allowed to spontaneously close. There were no access site infections or vascular injuries recorded. Eight patients (36%) were diabetic and 2 patients (9%) were active tobacco users. GGFs were endoscopically created in 12 patients (55%); the remaining 10 patients (45%) had JGFs. Median LAMS dwell time for the study cohort was 41.5 days (IQR1–3 29.7–74.5). Objective testing for persistent fistulas occurred a median 4.75 months (IQR1–3 2.7–9.75) after LAMS removal. Objective testing consisted of UGIS (n = 15), CT (n = 4), and EGD (n = 3). Persistent fistulas were diagnosed in 9/22 (41%) patients. Unintentional weight gain of ≥ 2.5% TBW occurred in 11 patients (50%), and 7 of these patients experienced weight gain of ≥ 5% TBW (32% of study population).
Persistent Fistula (n = 9) Versus Spontaneous Fistula Closure (n = 13)
No significant differences were found between patients with and without a persistent fistula in terms of age (p = 0.348), sex (p = 0.878), diabetes mellitus (p = 0.999), tobacco use (p = 0.999), fistula type (i.e., GGF vs JGF) (p = 0.999), or time interval from LAMS removal to objective testing for persistent fistula (p = 0.204) (Table 1). Patients with a persistent fistula had significantly longer median LAMS dwell time (77 days), compared to patients with fistula closure (35 days) (p = 0.03). The results from the PRR test revealed a significant association between LAMS dwell time and persistent fistula (p = 0.009), controlled for the effect of age and sex.
Table 1.
Comparison of patients with and without persistent fistula (GGF/JGF), after completion of EDGE using 20-mm LAMS with attempted spontaneous fistula closure (n = 22)
| Demographic, procedural characteristics, and unintentional weight gain | Fistula closure (n = 13) | Persistent fistula (n = 9) | p |
|---|---|---|---|
| Age (years), mean ± SD | 62.1 ± 9.5 | 64 ± 11.1 | 0.348* |
| Female, n (%) | 10 (77) | 8 (89) | 0.878† |
| Diabetes mellitus, n (%) | 5 (38) | 3 (33) | 0.999† |
| Tobacco use, n (%) | 1 (8) | 1 (11) | 0.999† |
| GGF (fistula type), n (%) | 7 (54) | 5 (56) | 0.999† |
| Single-session EDGE, n (%) | 5 (38) | 4 (44) | 0.999† |
| LAMS dwell time (days), median (Q1–Q3, IQR) | 35 (26–45) | 77 (42–124) | 0.03 * |
| Interval (months) from LAMS removal to objective testing for persistent fistula, median (Q1–Q3, IQR) | 3.6 (2.5–10.6, 8.1) | 5 (4.5–7.2, 2.7) | 0.204* |
| Weight gain ≥ 2.5% TBW, n (%) | 5 (38) | 6 (67) | 0.22† |
| Weight gain ≥ 5% TBW, n (%) | 2 (15) | 5 (56) | 0.128† |
Statistically significant value (p < 0.05) is given in bold
GGF Gastrogastric fistula, JGF Jejunogastric fistula, LAMS Lumen-apposing metal stent, Single-session EDGE EUS-GG/JG creation and transgastric ERCP within same procedure, Dual-session EDGE EUS-GG/JG creation and transgastric ERCP occurring in separate procedures, LAMS dwell time Time from LAMS deployment to extraction, Weight gain %TBW Percent increase of pre-EDGE total body weight, Interval from LAMS removal to objective testing for persistent fistula Months elapsed from the time of 20-mm LAMS removal to the time of objective testing for persistent fistula
Wilcoxon rank-sum test
Fisher’s exact test
A greater proportion of patients with persistent fistula had unintentional weight gain of at least 2.5% of their pre-EDGE TBW (n = 6, 67%), compared to patients with spontaneous fistula closure (n = 5, 38%) (Table 1). This difference was not statistically significant (p = 0.22). Likewise, patients with persistent fistulas were more likely to gain at least 5% of their pre-EDGE TBW (n = 5, 56%), compared to patients with fistula closure (n = 2, 15%) (p = 0.128). The average percent weight gain among the 5 patients with persistent fistulas was + 7.8%, compared to + 5.3% among the two patients with fistula closure (Table 1).
Attempted (Endoscopic) Secondary Closure of Persistent Fistula, After Failure of Spontaneous Fistula Closure (n = 4)
Four patients diagnosed with persistent fistula underwent attempted endoscopic secondary closure a median 7.5 months after LAMS extraction (Table 2). Indications included unintentional weight gain (n = 3) and failure to lose weight despite extensive lifestyle changes (n = 1). Endoscopic suturing monotherapy was employed in 3/4 (75%) patients, whereas APC followed by endoscopic suturing occurred in 1 (25%) patient. An UGIS or repeat EGD was ordered for each patient a median 3.5 months after attempted endoscopic secondary closure. All 3 patients who underwent endoscopic suturing monotherapy had persistent fistula recurrence, whereas the 1 patient who had APC + endoscopic suturing had durable fistula closure (Fig. 4a, b).
Table 2.
Attempted (endoscopic) secondary closure of persistent fistula, after failure of spontaneous fistula closure (n = 4)
| Patient | Interval (months) from LAMS extraction to secondary closure | Method of secondary fistula closure | Intraprocedural technical success of secondary fistula closure | Interval (months) from secondary closure to objective testing for persistent fistula | Persistent fistula recurrence after secondary closure |
|---|---|---|---|---|---|
| 1 | 17 | Endoscopic suturing | Yes | 4 | Yes (recurrence) |
| 2 | 7 | Endoscopic suturing | Yes | 2 | Yes (recurrence) |
| 3 | 6 | Endoscopic suturing | Yes | 12 | Yes (recurrence) |
| 4 | 8 | APC + Endoscopic suturing | Yes | 2 | No (fistula closure) |
Secondary (fistula) closure refers to attempted endoscopic closure of a persistent fistula that fails to heal spontaneously (i.e., failure to heal by secondary intention)
Fig. 4.
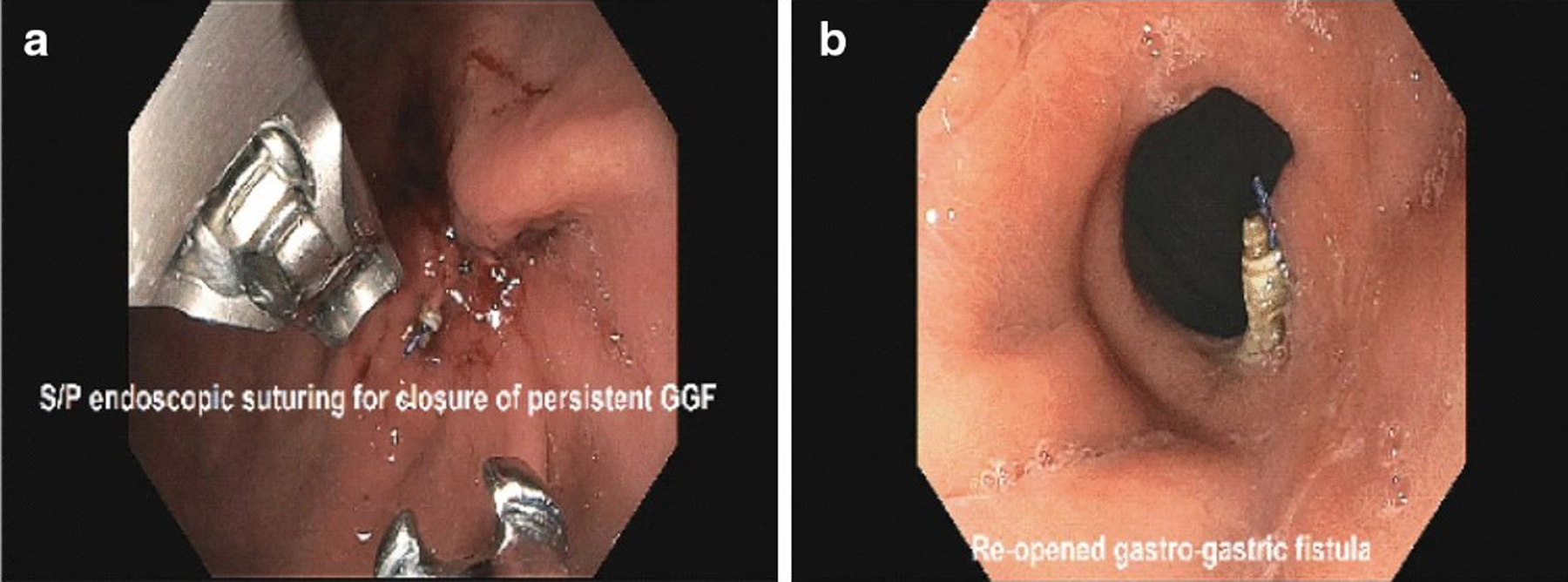
a Attempted secondary closure of a persistent GGF via endoscopic suturing monotherapy. b Persistent GGF recurrence after secondary closure via endoscopic suturing monotherapy [i.e., dehiscence after endoscopic suturing monotherapy, as shown in (a)]
Discussion
The concept of persistent fistula (GGF/JGF) as a risk factor for weight gain following EDGE intuitively makes sense. Reinclusion of the bypassed gut into the alimentary canal effectively cancels the malabsorptive mechanism of RYGB. However, the clinical concern of persistent fistula after EDGE is theoretical and extrapolated from the surgical literature. The surgical literature mostly describes experiences in the management of spontaneous GGFs after RYGB. There is virtually no data to support or refute the association between spontaneous GGF and unintentional weight gain [12–14]. Moreover, it is unknown whether the clinical behavior of a spontaneous GGF after RYGB can be reliably compared to a persistent GGF/JGF after EDGE, given etiological differences. Common causes of spontaneous fistula after RYGB include incomplete gastric transection by the surgeon, tissue ischemia near the GJA, and drainage of a contained leak (from the GJA or staple line) into the gastric remnant [13, 14]. Spontaneous fistulas caused by tissue ischemia and/or gastric leak are inflammatory in nature, and inflammation is a known anorexigenic factor [15, 16]. In contrast, the GGF/JFA in EDGE is created in a controlled manner via localized full-thickness thermal injury using electrocautery-enhanced LAMS.
The surgical literature reports 0.5–6% incidence of “symptomatic” GGF after RYGB, of which 27–55% of these patients subjectively report weight regain or poor weight loss [17–19]. A few case reports describe GGFs after RYGB associated with objective weight loss [20, 21]. Even though the clinical behavior of a post-RYGB spontaneous GGF is poorly understood, detection of a “symptomatic” GGF after RYGB is consequential as it often leads to revisional surgery. A study by Ribeiro-Parenti et al. found that all patients who reported weight regain in the setting of GGF underwent GGF/GJA resection with GJA revision (n = 5) [19]. Ironically, a study by Cucchi et al. found that RYGB revision itself may be a risk factor for GGF formation [i.e., RYGB revision preceded GGF formation in 5/6 (83%) patients] [17]. The suspected mechanism of GGF after RYGB revision is gastric pouch leak, which is more likely to occur due to increased dissection difficulty (adhesions, fibrosis) leading to gastric and/or vascular injury [17].
The association between post-EDGE persistent fistula and weight regain is difficult to determine given sparse existing data. Previous EDGE studies predominantly use 15-mm LAMS and report persistent fistula rates of 0–12.5% [3–9]. In these studies, a total of 151 EDGE patients were objectively tested for persistent fistula, of which 13/151 (9%) tests were positive (Table 3). Of the 13 patients with persistent fistulas, 15-mm LAMS was used in 10/13 (77%) and 20-mm LAMS in 3/13 (23%). Initial fistula closure techniques among these 13 patients varied from primary intent [i.e., APC (n = 7), endoscopic suturing (n = 2)] to secondary intent [i.e., spontaneous fistula closure (n = 3)]; the fistula closure method in one patient was unknown (n = 1). The rate of persistent fistula following primary intent fistula closure cannot be calculated in the largest known EDGE study by Runge et al., which accounts for 9/13 cases of persistent fistula reported in the literature (Table 3) [3]. TBW information is available for 12/13 patients with post-EDGE persistent fistulas. Weight loss occurred in 5/12 (41.67%), stable weight in 2/12 (16.67%), and weight gain ≥ 2.5% TBW in 5/12 (41.67%). 4/5 of weight regainers gained ≥ 5% TBW [i.e., 4/12 (33.3%)].
Table 3.
Persistent fistula rates and changes in total body weight (TBW); a review of existing EDGE studies
| Study (n) | LAMS Size, n(%) | Median LAMS dwell time, days (IQR1–3) | Primary fistula closure method, n (%) | No. pts. tested for persistent fistula | Persistent fistula, n (%) Fistula closure method | Persistent fistula and change in TBW, n (%) |
|---|---|---|---|---|---|---|
| Runge et al. [3] (178) | 20-mm, 66 (37) 15-mm, 112 (63) |
35 (22–54) | Suture, 57 (37) APC, 55 (36) Spont., 31 (20) Hclip, 7 (5) OTSC, 3 (2) |
90 | Total, 9 (10) APC, 7 (78) Spont., 2 (22) |
Weight loss, 4 (44) Stable weight, 1 (11) ≥2.5% TBW 4 (44) ≥5% TBW 3 (33) |
| James et al. [9] (19) | 15-mm, 19 (100) |
50 (36–73) | APC, 12 (63) Spont 6 (32) DPPS, 1 (5) |
11 | Total, 1 (9) Spont., 1 |
≥5% TBW, 1 |
| wang et al. [8] (10) | 15-mm, 10 (100) |
N/A | DPPS, 7 (100) | 7 | Total, 0 | |
| Bukhari et al. [7] (30) | 15-mm, 30 (100) |
7–91) | Suture, 15 (50) Spont or APC, 15 (50) |
23 | Total, 1 (4) Suture, 1 |
Stable weight, 1 |
| Ngamruengphong al. [6] (12) | 15-mm, 12 (100) |
N/A | Suture, 6 (50) APC, (25) OTSC, 3 (25) |
12 | Total, 1 (8) Suture, 1 |
Weight loss, 1 |
| Tyberg et al. [5] (10) | 15-mm, 10 (100) |
N/A | Suture, 7 (70) OTSC, 2 (20) Spont., 1 (10) |
8 | Total, 1 (12.5) N/A |
Unknown |
| Totals | 20-mm, 66 (25) 15-mm, 185 (74) |
Suture, 85 (38.1) APC, 64 (28.7) Spont, 37 (16.6) Spont. or APC, 15 (6.7) OTSC, 8 (3.6) Hclip, 7 (3.1) DPPS, 7 (3.1) |
151 | 13 (9) APC, 7 (54) Spont., 3 (23) Suture, 2 (15) N/A, 1 (8) |
Weight loss, 5/12 (41.67) Stable weight, 2/12 (16.67) ≥2.5% TBW, 5/12 (41.67) ≥5% TBW, 4/12 (33.3) |
|
| *Krafft et al. 2021 (22) | 20-mm, 22 (100) |
41.5 (29.7–74.5) | Spont., 22 (100) | 22 | Total, 9 (41) Spont., 9 (100) |
Weight loss, 1/9 (11) Stable weight, 2/9 (22) ≥2.5% TBW, 6/9 (67) ≥5 TBW, 5/9 (56) |
Values correspond to n (%), unless otherwise stated
LAMS Lumen-apposing metal stent, Spont. Spontaneous fistula closure (secondary intention), APC Argon plasma coagulation-mediated fistula closure, Suture Endoscopic suturing fistula closure, Hclip Hemoclipping fistula closure, OTSC Over-the-scope clip fistula closure, DPPS LAMS replaced with a double-pigtail plastic stent (DPPS) to promote fistula closure, Persistent fistula Gastrogastric/jejunogastric fistula persisting ≥ 8 weeks after LAMS extraction, Weight loss Defined as ≥ 2.5% loss of pre-EDGE (baseline) total body weight, Stable weight Defined as weight fluctuation between −2.5 and +2.5% of pre-EDGE TBW, Weight regain Defined as ≥ 2.5% or ≥ 5% pre-EDGE (baseline) total body weight
Current study
Our study has several unique methodological and results differences, compared to prior EDGE studies (Table 3). We followed patients who underwent EDGE using exclusively 20-mm LAMS, and all fistulas were allowed to heal spontaneously after LAMS extraction. By comparison, existing studies collectively utilize 20-mm LAMS (26%) and spontaneous fistula closure (16.6%) in a minority of the overall patient population (Table 3). The incidence of persistent fistula was much higher in our study (9/22, 41%), compared to prior EDGE studies (13/151, 9%). This suggests that larger LAMS diameter and the absence of fistula closure by primary intent may be technical factors that increase the likelihood of persistent fistula.
In our study, neither unintentional weight gain ≥ 2.5% TBW (p = 0.22) or ≥ 5% TBW (p = 0.128) was significantly associated with persistent fistula; however, there was a trend toward significance. Fistula type (GGF vs JGF) was not associated with persistent fistula (p = 0.999), which is in keeping with results from Runge et al. (p = 0.73) [3]. Longer LAMS dwell time was significantly associated with persistent fistula (p = 0.03); this held true on multivariate analysis after controlling for age and sex (p = 0.009). Similarly, Runge et al. found a trend toward significance for longer LAMS dwell time and persistent fistula (p = 0.09) [3].
Four of 9 patients (44%) with persistent fistulas elected to undergo attempted (endoscopic) secondary closure (Table 2). All 4 secondary closures of persistent fistulas were technically successful; however, persistent fistulas recurred (reopened) in 3/3 (100%) patients who received endoscopic suturing monotherapy. Fistula closure was durable in the 1 patient who had APC and endoscopic suturing for fistula closure. Although our sample size is small (n = 4), the failure of endoscopic suturing monotherapy for persistent fistula closure is important to report because this is the most commonly described method of primary fistula closure (38.1%) (Table 3).
At least 6 forms of post-EDGE endoscopic fistula closing techniques have been reported, most of which have been used for primary fistula closure at the time of LAMS extraction (Table 3). Less is known about techniques for closing persistent fistulas (i.e., secondary fistula closure). This begs the question of whether one technique is more effective than another? The primary literature on this topic is limited, but it appears that mucosal denudation prior to tissue approximation increases the likelihood of effective fistula closure. Flesher et al. performed an in vivo canine study to determine if gastric mucosal manipulation, prior to endoscopic suturing (tissue apposition), is necessary for tissue healing by primary intent [22], The results found that mucosal denudation (electrosurgical ablation or mucosal resection), prior to endoscopic suturing, achieved significantly better mucosal healing, compared to endoscopic suturing alone (p = 0.02). There was histologic evidence that mucosal ablation (prior to suturing) elicited a wound healing response consisting of organizing inflammation and fibrosis with giant cell reaction. In contrast, histologic sections of simple mucosal apposition (i.e., 2 weeks after endoscopic suturing) failed to show significant granulation tissue bridge formation. The findings of Flesher et al. appear to explain our 3 failed fistula closures using endoscopic suturing monotherapy, compared to our single success using APC and endoscopic suturing.
The primary limitations of our study are a relatively small sample size (n = 22) of patients that underwent EDGE (performed by two endoscopists), as well as the possibility of uncontrolled confounding variables that influence weight gain or loss after EDGE. Diet and lifestyle counseling was not monitored or controlled for. Also, it is not fully possible to control for unintentional weight gain that occurs because the patient’s baseline appetite is restored following resolution of the pancreaticobiliary malady. Another limitation of this study is that it only analyzes a patient cohort that underwent spontaneous fistula closure. Ideally, persistent fistula rates after attempted spontaneous closure (secondary intent) would have been compared to attempted fistula closure by primary intent. The primary strength of this study is that we are able to provide data on 9 patients with persistent fistula after EDGE. For perspective, the largest EDGE study to date detected 9 persistent fistulas from a sample size of 178 patients from 13 tertiary centers [3]. Therefore, we have almost doubled the amount of existing data on EDGE patients with persistent fistulas.
Conclusions
A 41% persistent fistula rate after EDGE with 20-mm LAMS (allowing for spontaneous fistula closure) is the highest reported incidence to date, compared to previous EDGE studies that collectively report 9% incidence. One possible explanation for this difference is that prior studies used 15-mm LAMS in 74% of patients (i.e., a smaller transmural defect), followed by primary (endoscopic) fistula closure in 83% of patients. We found that post-EDGE weight gains of ≥ 2.5% or ≥ 5% TBW were not significantly associated with persistent fistula; however, this may be due to small sample size, as there was a trend toward significance (i.e., p = 0.22 and p = 0.128, respectively). In light of our findings, we question the utility of routine fistula closure (primary intent) and suggest a personalized approach to clinical follow-up. Given that long LAMS dwell time (median 77-days) was significantly associated with persistent fistula (p = 0.03), select patients with long LAMS dwell time may be relatively good candidates for primary fistula closure. Regarding endoscopic management of symptomatic persistent fistulas, our sample size of secondary fistula closure patients (n = 4) is too small to recommend a best practice. However, it is notable that fistula margin apposition (via endoscopic suturing), without preceding mucosal denudation, resulted in 100% clinical failure (dehiscence).
Abbreviations
- AE
Adverse event
- DPPS
Double-pigtail plastic stent
- EGD
Esophagogastroduodenoscopy
- EDGE
EUS-directed transgastric ERCP/EUS
- ERCP
Endoscopic retrograde cholangiopancreatography
- EUS
Endoscopic ultrasound
- EUS-GG or JG
EUS-directed gastrogastrostomy or jejunogastrostomy creation
- GG
Gastrogastrostomy
- JG
Jejunogastrostomy
- GGF
Gastrogastric fistula
- JGF
Jejunogastric fistula
- LAMS
Lumen-apposing metal stent
- ECE-LAMS
Electrocautery-enhanced lumen-apposing metal stent
- Persistent fistula
A fistula (GGF/JGF) that persists at least 8 weeks after LAMS extraction
- Primary intent
Refers to fistula closure by endoscopic means (e.g., endoscopic suturing)
- Secondary intent
Refers to spontaneous fistula (GGF/JGF) closure
- Primary fistula closure
Refers to fistula closure by endoscopic means at the time of LAMS extraction
- Secondary fistula closure
Refers to endoscopic closure of a persistent fistula
- RYGB
Roux-en-Y gastric bypass
- UGIS
Upper gastrointestinal series
References
- 1.Prakash S, Elmunzer B, Forster EM, Cote G, Moran R. EUS-directed transgastric ERCP: systematic review to describe outcomes, adverse events and knowledge gaps. Endoscopy. 2021. 10.1055/a-1376-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster E, Elmunzer JB. Endoscopic retrograde cholangiopancreatography in patients with Roux-en-Y gastric bypass. Am J Gastroenterol. 2020;115:155–157. [DOI] [PubMed] [Google Scholar]
- 3.Runge TM, Chiang AL, Kowalski TE, James TW, Baron TH, Nieto J, Diehl D, Krafft MR, Nasr JY, Kumar V, Khara HS, Irani S, Patel A, Law R, Loren D, Schlachterman A, Hsueh W, Confer B, Stevens T, Chahal P, Al-Haddad M, Faisal MF, Pleskow D, Huggett M, Paranandi B, Trindade A, Brewer-Gutierrez OI, Ichkhanian Y, Dbouk M, Kumbhari V, Khashab MA. EUS-directed transgastric ERCP (EDGE): a retrospective, multicenter study. Endoscopy. 2020. 10.1055/a-1254-3942. [DOI] [PubMed] [Google Scholar]
- 4.Krafft MR, Fang W, Nasr JY. Shortened-interval dual-session EDGE reduces the risk of LAMS dislodgement while facilitating timely ERCP. Dig Dis Sci. 2020. 10.1007/s10620-020-06551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyberg A, Nieto J, Salgado D, Weaver K, Kedia P, Sharaiha RZ, Gaidhane M, Kahaleh M. Endoscopic ultrasound (EUS)-directed transgastric endoscopic retrograde cholangiopancreatography or EUS: mid-term analysis of an emerging procedure. Clin Endosc. 2017;50:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngamruengphong S, Nieto J, Kunda R, Kumbhari V, Chen YI, Bukhari M, El Zein MH, Bueno RP, Hajiyeva G, Ismail A, Chavez YH, Khashab M. Endoscopic ultrasound-guided creation of a transgastric fistula for the management of hepatobiliary disease in patients with Roux-en-Y gastric bypass. Endoscopy. 2017;49:549–552. [DOI] [PubMed] [Google Scholar]
- 7.Bukhari M, Kowalski T, Nieto J, Kunda R, Ahuja NK, Irani S, Shah A, Loren D, Brewer O, Sanaei O, Chen YI, Ngamruengphong S, Kumbhari V, Singh V, Aridi HD, Khashab MA. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc. 2018;88:486–494. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Thompson CC, Ryou M. Gastric access temporary for endoscopy (GATE): a proposed algorithm for EUS-directed transgastric ERCP in gastric bypass patients. Surg Endosc. 2019;33:2024–2033. [DOI] [PubMed] [Google Scholar]
- 9.James TW, Baron TH. Endoscopic ultrasound-directed transgastric ERCP (EDGE): a single-center US experience with follow-up data on fistula closure. Obes Surg. 2019;29:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran RA, Ngamruengphong S, Sanaei O, Fayad L, Singh VK, Kumbhari V, Khashab MA. EUS-directed transgastric access to the excluded stomach to facilitate pancreaticobiliary interventions in patients with Roux-en-Y gastric bypass anatomy. Endosc Ultrasound. 2019;8:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potter DM. A permutation test for inference in logistic regression with small- and moderate-sized data sets. Stat Med. 2005;24:693–708. [DOI] [PubMed] [Google Scholar]
- 12.Gumbs AA, Duffy AJ, Bell RL. Management of gastrogastric fistula after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006;2:117–121. 10.1016/j.soard.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Kane ED, Romanelli JR. Complications of Roux-en-Y gastric bypass. In: Reavis K, Barrett A, Kroh M, eds. Cham: Springer; 2018. [Google Scholar]
- 14.Palermo M, Acquafresca PA, Rogula T, Duza GE, Serra E. Late surgical complications after gastric by-pass: a literature review. Arq Bras Cir Dig. 2015;28:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bułdak Ł, Machnik G, Skudrzyk E et al. The impact of exenatide (a GLP1 agonist) on markers of inflammation and oxidative stress in normal human astrocytes subjected to various glycemic conditions. Exp Ther Med. 2019;17:2861–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunaldi VO, Farias GFA, de Rezende DT, Cairo-Nunes G, Riccioppo D, de Moura DTH, Santo MA, de Moura EGH. Argon plasma coagulation alone versus argon plasma coagulation plus full-thickness endoscopic suturing to treat weight regain after Roux-en-Y gastric bypass: a prospective randomized trial (with videos). Gastrointest Endosc. 2020;92:97–107. [DOI] [PubMed] [Google Scholar]
- 17.Cucchi SG, Pories WJ, MacDonald KG, Morgan EJ. Gastrogastric fistulas: a complication of divided gastric bypass surgery. Ann Surg. 1995;221:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrodeguas L, Szomstein S, Soto F et al. Management of gastrogastric fistulas after divided Roux-en-Y gastric bypass surgery for morbid obesity: analysis of 1,292 consecutive patients and review of literature. Surg Obes Relat Dis. 2005;1:467–474. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro-Parenti L, De Courville G, Daikha A, Arapis K, Chosidow D, Marmuse J-P. Classification, surgical management and outcomes of patients with gastrogastric fistula after Roux-En-Y gastric bypass. Surg Obes Relat Dis. 2017;13:243–249. [DOI] [PubMed] [Google Scholar]
- 20.Stanczyk M, Deveney CW, Traxler SA, McConnell DB, Jobe BA, O’Rourke RW. Gastro-gastric fistula in the era of divided Roux-en-Y gastric bypass: strategies for prevention, diagnosis, and management. Obes. Surg 2006;16:359–364. [DOI] [PubMed] [Google Scholar]
- 21.Gustavsson S, Sundbom M. Excellent weight result after Roux-en-Y gastric bypass in spite of gastro-gastric fistula. Obes Surg. 2003;13:457–459. [DOI] [PubMed] [Google Scholar]
- 22.Felsher J, Farres H, Chand B, Farver C, Ponsky J. Mucosal apposition in endoscopic suturing. Gastrointest Endosc. 2003;58:867–870. [DOI] [PubMed] [Google Scholar]


