Abstract
• PURPOSE:
To evaluate the relationship between the occurrence of optic disc hemorrhages (DH) and glaucoma progression as determined by multiple glaucoma testing modalities.
• DESIGN:
Prospective cohort study.
• METHODS:
A longitudinal study was undertaken of 124 open-angle glaucoma patients who had yearly disc photography, visual fields (VFs), spectral-domain optical coherence tomography (SD-OCT), retinal nerve fiber layer (RNFL) thickness scans, and optic nerve volume scans (Spectralis), all performed on the same day over a 5-year period. The minimum distance band (MDB) thickness, a 3-dimensional (3D) neuroretinal rim parameter, was calculated from optic nerve volume scans. Patients were classified as glaucoma progressors or glaucoma nonprogressors using event-based analysis.
• RESULTS:
Of 124 open-angle glaucoma patients, 19 (15.3%) had 1 or more DHs on yearly disc photographs. Presence of a DH was associated with localized 3D neuroretinal rim thickness progression (superior MDB progression; odds ratio: 3.96; P = .04) but not with global or inferior MDB progression (P = .14 and .81, respectively), DP progression (P = .08), VF progression (P = .45), or RNFL global, inferior, or superior progression (P = .17, .26, and .76, respectively). In the majority of patients with MDB progression (14/17 or 82%), the progression was noted before or concurrently with the first instance of DH.
• CONCLUSIONS:
Glaucoma progression detected by high-density 3D SD-OCT neuroretinal rim measurements preceded DH occurrence in the majority of patients. These findings support the hypothesis that DHs are indicators of ongoing glaucoma progression rather than discrete events that cause subsequent progression.
Glaucoma is a chronic blinding disease characterized by loss of retinal ganglion cells and characteristic morphological changes of the optic nerve. Optic disc hemorrhages (DH), splinter-shaped regions of bleeding straddling the optic disc rim, have been established as important risk factors for development and progression of glaucoma in several randomized prospective glaucoma trials.1-3 For example, in the OHTS (Ocular Hypertension Treatment Study), having a DH increased the likelihood of developing glaucoma 4-fold,1 whereas in the EMGT (Early Manifest Glaucoma Trial), DHs were associated with an increased likelihood of progression.3,4 However, the underlying etiology of DHs has remained elusive, and it is presently unclear whether DHs are the cause or the consequence of glaucoma progression.5,6
Prior studies investigating DHs and glaucoma progression have tended to focus on single glaucoma testing modalities (disc photographs,7,8 visual fields,9-11 or optical coherence tomography [OCT]12,13). A number of studies have demonstrated that the presence of a DH leads to a greater probability of visual field progression and more severe central damage.9-11 Akagi and associates12 demonstrated that rates of retinal nerve fiber layer (RNFL) thinning were significantly faster in DH quadrants than in non-DH quadrants and accelerated after DH occurred. Similarly, ganglion cell inner plexiform layer thinning rate was significantly more rapid in glaucomatous eyes with DH than in control eyes.13 However, to the best of our knowledge, no prior studies have investigated the relationship of DHs and progression using multiple glaucoma testing modalities simultaneously or have utilized new high-density 3D spectral domain-OCT (SD-OCT) protocols to detect progression.
Cross-sectional studies have shown that high-density 3D SD-OCT optic nerve measurements provide objective quantitative metrics that may be the same or better than the most commonly used 2D RNFL thickness parameters for diagnosing glaucoma.14-17 Specifically, the minimum distance band (MDB) thickness measures the neuroretinal rim in 3D space and is a high-density version of the low-density, commercially available Bruch membrane opening – minimum rim width.14-17 The MDB is calculated from an optic nerve volume scan, and it represents the neuroretinal rim as a band of tissue whose width is the shortest distance between the outer OCT-based disc border (ie, retinal pigment epithelium/Bruch membrane [RPE/BM]) and the inner cup surface (ie, internal limiting membrane [ILM]).14-16 In addition to providing objective quantitative neuroretinal rim thickness measurements,14,16 MDB thickness correlates well with structural assessments (ie, disc photography) and with functional assessments (ie, visual field mean deviation and pattern standard deviation).17 Furthermore, in a recent prospective study, we have demonstrated that MDB thickness measurements detected glaucoma progression 1 to 2 years earlier than disc photos and RNFL thickness measurements.18
In this study we evaluated open-angle glaucoma patients who were followed using 4 different glaucoma testing modalities (disc photography, Humphrey visual field testing, RNFL thickness measurements, and MDB thickness [ie, neuroretinal rim thickness in 3D space]) over a 5-year follow-up period. DHs were detected on disc photographs in a subset of patients, and the presence of a DH only led to escalation of glaucoma therapy if it was associated with structural and/or functional progression at a subsequent follow-up visit. We hypothesized that high-density MDB neuroretinal rim thinning would be associated with DHs and that our study may provide new insights into the pathophysiology of DHs.
PATIENTS AND METHODS
• PARTICIPANTS, CLINICAL EVALUATIONS, AND STUDY PROTOCOL:
The longitudinal SIG (SD-OCT in Glaucoma) study was prospectively approved by the Massachusetts Eye and Ear Institutional Review Board. All included patients provided informed consent in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. The SIG study included 2,000 study subjects who were recruited from the Massachusetts Eye and Ear Glaucoma Service since 2009. Complete eye examinations were performed by a glaucoma specialist (T.C.C.) and included ocular history, best-corrected visual acuity, refraction, Goldmann applanation tonometry, slitlamp biomicroscopy, gonioscopy, dilated fundus examination, ultrasound pachymetry, disc photography (Visucam Pro NM; Carl Zeiss Meditec), and visual field testing (Swedish Interactive Threshold Algorithm 24-2 test of the Humphrey visual field (HVF) analyzer 750i, Carl Zeiss Meditec).
Patients were eligible to participate in the SIG study if they consented to the research OCT imaging protocol on the same day as their complete eye examination, disc photography, and visual field testing. SD-OCT imaging was performed using the US Food and Drug Administration (FDA)-approved Spectralis SD-OCT machine (Heidelberg Engineering GmbH). Each eye was dilated and underwent peripapillary RNFL thickness circular scans and high-density optic nerve research volume scans. The RNFL scan circle was 12° in diameter (approximately 3.5-3.6 mm in diameter for a typical eye length). The research imaging protocol included an optic nerve volume scan which consisted of 193-raster B-scans with 20° × 20° field of view (approximately 6 mm × 6 mm). Automatic real-time function was enabled and set for 3 frames at each scan location. All study participants had at least 4 annual follow-up visits with repeat dilated clinical examinations, repeat routine clinical testing, and repeat high-density research optic nerve volume scans.
• DEFINITION OF OPEN-ANGLE GLAUCOMA AND GENERAL INCLUSION AND EXCLUSION CRITERIA:
The first 133 consecutive open-angle glaucoma patients were recruited for this study from the 2,000 subjects enrolled in the SIG study at Massachusetts Eye and Ear. Our study population consisted of patients with both primary and secondary open-angle glaucomas (ie, primary open angle, normal tension, pseudoexfoliation, and pigmentary glaucoma). All patients had characteristic glaucomatous optic nerve changes, corresponding visual field defects, and open angles on gonioscopic examination. In addition, primary open-angle glaucoma (POAG) patients had pretreatment intraocular pressures > 21 mm Hg; normal tension glaucoma patients had pre-treatment intraocular pressure ≤21 mm Hg; pseudoexfoliation glaucoma patients had pseudoexfoliative changes on slitlamp examination; and pigmentary glaucoma patients had pigment dispersion signs on the slitlamp examination.
Glaucomatous optic nerve changes were defined using the OHTS criteria, which included characteristic rim thinning in a generalized or localized pattern while demonstrating 1 or more of the following characteristics: change in the position of the vessels, development of a notch, development of an acquired pit, and development of localized or diffuse pallor. Disc hemorrhage, nerve fiber layer dropout, and a deep cup change was not a requirement for glaucoma.19 Visual fields were considered abnormal if 3 or more contiguous test locations on 1 side of the horizontal meridian was depressed by ≥5 dB with at least 1 point depressed ≥10 dB from normative values.20 Glaucoma staging was based on the Hodapp-Anderson-Parrish system, classifying subjects as having early stage glaucoma (mean deviation [MD] greater than −6 dB), moderate stage glaucoma (−12 dB less than MD less than or equal to −6 dB), or severe stage glaucoma (MD less than or equal to −12 dB).
General inclusion criteria included patients with spherical equivalent between −6.0 and +6.0 diopters, best-corrected visual acuity of 20/70 or better, good quality disc photographs, and reliable visual field test results, which were defined as ≤33% fixation losses, ≤33% false-positive results, and ≤33% false-negative results.21 SD-OCT scans included in the study needed to meet the following criteria: signal strength of at least 15 dB and completion of the 193-line raster scans for optic nerve volume scans. Exclusion criteria included congenital abnormalities of the anterior chamber, corneal scarring or opacities, severe nonproliferative or proliferative diabetic retinopathy, visual field loss due to a nonglaucomatous condition, or missing data. If both eyes of a patient were eligible for the study, 1 eye was selected using a random number generator in Excel 2013 software (Microsoft).
• SD-OCT DATA FROM RNFL THICKNESS SCANS AND OPTIC NERVE VOLUME SCANS:
The RNFL thickness was calculated by the Heidelberg SPECTRALIS SD-OCT software (Heidelberg Eye Explorer software version 5.4.8.0). The RNFL thickness values were reported as global (360°), quadrant (ie, inferior, superior, nasal, and temporal), and sector (ie, inferior-nasal, superior-nasal, inferior-temporal, and superior-temporal) values in an RNFL single examination report. From baseline RNFL thickness scans, artifacts were identified and classified according to the 12 artifact types as defined by Liu and associates.22
The 3D optic nerve volume scan raw data were exported, and the custom-designed program calculated MDB thickness with C++ software developed at Massachusetts Eye and Ear using the Open Source libraries Insight Segmentation and Registration Toolkit (ITK version 4.3, Insight Software Consortium, Kitware Inc.) and Open Source Computer Vision (OpenCV version 2.4.3). The MDB thickness was calculated by building a 3D model of the RPE/BM complex, which was reconstructed from the individual B-scans. Processing of the SD-OCT volume scans was described in detail previously.16 All 193 B-scan images of each patient were checked for segmentation artifacts by 1 of the authors (K.R.). Segmentation artifacts that consisted of either misidentification or improper segmentation of the ILM and RPE were manually deleted, and the software was then used to recalculate the measurements. The disc margin, defined by the RPE/BM termination, was identified by 100 points spaced by 3.6°. The central axis of the disc was determined by finding the centroid of the opening in the RPE/BM (ie, disc margin). For each angular interval of 3.6°, the shortest distance from the disc margin to the ILM was measured as the MDB thickness. MDB thickness values were determined for global (360°), for 90° quadrants (ie, inferior, superior, nasal, and temporal), and for 45 ° sectors (ie, inferior nasal, superior nasal, inferior temporal, and superior temporal).
• GLAUCOMA PROGRESSION ANALYSIS: DEFINING ONSET OF PROGRESSION:
Glaucoma progression analysis was determined separately for the disc photographs, visual fields, RNFL thickness measurements, and MDB thickness measurements using event-based analysis.18 The first instance each modality showed progression was defined as the date of progression for that modality.
• DISC PHOTOGRAPHY PROGRESSION AND PRESENCE OF DISC HEMORRHAGE:
All disc photographs were de-identified by 1 of the authors (K.R.). Glaucoma progression status was independently determined by 2 glaucoma specialists (M.A.M. and C.L.O.) using disc photographs that were placed in a random order by 1 of the authors (J.K.). The 2 graders were blinded to all clinical, visual field, and OCT data. The disc photography progression was determined using OHTS criteria described above. Each grader viewed all disc photographs and classified each eye as either progressed or stable. Any disagreement in the progression status between the 2 graders was resolved by a third glaucoma specialist (T.C.C.), until all 3 glaucoma specialists reached a unanimous determination of stable or progressed. All photographs were also evaluated for the presence of DHs. If a DH was detected in 1 or more disc photographs, the patient was classified as having had a DH over the course of the study. Patients without photographic evidence of DH were classified as non-DH subjects. In this study we focused on DHs that were detected using fundus photography obtained on visits during which the patient was dilated, and Humphrey visual field (HVF) testing and OCT imaging were performed. DHs that may have been detected during other clinic visits using undilated optic nerve examination were not assessed or included in this study.
• VISUAL FIELD PROGRESSION:
All visual fields were de-identified by 1 of the authors (K.R.). Glaucoma progression status was independently determined by 2 glaucoma specialists (M.A.M. and C.L.O.) using visual field analyses arranged by chronology. The 2 graders were blinded to all clinical and OCT data. The visual field progression was noted if at least 1 of the following were present: deepening of an existing scotoma, expanding of an existing scotoma, and/or new localized visual field defect observed in a previously normal part of the field.20,23,24 Each grader viewed all visual fields for each eye and classified each eye as either stable or progressed. Any disagreement of the progression status between the 2 graders was resolved by a third glaucoma specialist (T.C.C.), until all 3 glaucoma specialists reached a unanimous determination of stable or progressed.
• SD-OCT PROGRESSION: RNFL THINNING AND MDB NEURORETINAL RIM THINNING:
The RNFL thickness and MDB rim thickness were defined as showing glaucoma progression if serial measurements demonstrated a negative trend with values decreasing at a faster rate than expected for test-retest variability and for mean normal aging changes.25 The same changes also had to be confirmed in the last follow-up visit to avoid false positives. The cutoff values for test-retest variability used in this study were 5, 8, and 8 μm for RNFL thickness in the global, inferior, and superior quadrants, respectively; and 7, 5, and 5 μm for MDB thickness in the global, inferior, and superior quadrants, respectively.16,26-30 These cutoff values were based on past published reports as well as our group’s past publications.18,27,29,31 The normal rates of aging changes used in this study were −0.5 and −1.0 μ-m/year for RNFL thickness and MDB thickness, respectively.16,32
• STATISTICAL ANALYSIS:
Summary data were reported as means ± SD for continuous variables and as counts with percentages for categorical variables. Pre-enrollment power calculations revealed that 125 patients would be needed for initial enrollment in order to detect significant differences in OCT progression, that is, a change beyond expected intertest fluctuation, assuming 20% attrition over 5 years, for a final count of 100 patients at the last study visit. For comparisons between 2 groups, t-tests were used for continuous outcomes, and χ2 tests were used for categorical outcomes. ORs measuring the association between glaucoma progression status and DH were calculated using logistic regression. We also performed significance tests for the computed odds ratios using Fisher’s exact test to assess whether our results were sensitive to the relatively low number of progressors and DH events, but because there was high concordance between the results of the two approaches, we report results from only the logistic regression analyses here. All statistical analysis was performed using the statistical software R (version 3.3.3). The threshold for statistical significance was defined as P < .05.
RESULTS
The first 133 consecutive open-angle glaucoma patients were recruited for this study from the 2,000 subjects enrolled in the SIG study at Massachusetts Eye and Ear. Nine of 133 patients (6.8%) were excluded for the following reasons: 5 patients were missing at least one disc photograph or visual field test, 2 patients had incomplete volumetric scans, 1 patient had a possible non-glaucomatous visual field defect, and 1 patient exhibited algorithm failure. A total of 124 eyes from 124 included patients were analyzed in the final analysis. The average longitudinal follow-up period was about 5 years (66.9 ± 16.4 months). Out of 124 open angle patients, 19 patients (15.3%) had one or more DHs on routinely obtained disc photos during the follow-up period of approximately 5 years, while 105 patients did not (Table 1). Of note, the presence of a DH by itself was not an indication for escalation of glaucoma therapy.
TABLE 1.
Baseline Demographic and Clinical Characteristics of the 124 Open-Angle Glaucoma Study Patients
| Characteristics | Eyes Without DH | Eyes with DH | P Value |
|---|---|---|---|
| Number of eyes | 105 | 19 | |
| Follow-up period, mo | 6791 (16.81) | 61.32 (12.48) | .106 |
| Age at baseline, y | 68.57 (11.19) | 65.42 (13.66) | .278 |
| Female patients | 47 (44.8) | 15 (78.9) | .013 a |
| Race | .017 a | ||
| Caucasian | 76 (72.4) | 15 (78.9) | |
| Black | 19 (18.1) | 0 (0.0) | |
| Asian | 4 (3.8) | 4 (21.1) | |
| Hispanic | 3 (2.9) | 0 (0.0) | |
| Other | 3 (2.9) | 0 (0.0) | |
| Family history of glaucoma (1st-degree relative) | 31 (29.5) | 5 (26.3) | .993 |
| Type of glaucoma | .01 a | ||
| Primary open-angle glaucoma | 59 (56.2) | 8 (42.1) | |
| Normal tension glaucoma | 13 (12.4) | 8 (42.1) | |
| Pseudoexfoliation glaucoma | 23 (21.9) | 1 (5.3) | |
| Pigment-dispersion glaucoma | 10 (9.5) | 2 (10.5) | |
| Baseline severity of glaucoma | .065 | ||
| Mild (MD > −6 dB) | 63 (60.0) | 10 (52.6) | |
| Moderate (−12 dB < MD ≤ −6 dB) | 21 (20.0) | 8 (42.1) | |
| Severe (MD ≤ −12 dB) | 21 (20.0) | 1 (5.3) | |
| Baseline IOP, mm Hg | 15.33 (4.75) | 14.58 (4.11) | .518 |
| Baseline CCT, μm | 538.18 (38.49) | 546.32 (35.67) | .393 |
| Refractive error in spherical equivalent, D | −0.86 (2.08) | −1.34 (1.87) | .347 |
| Baseline vertical cup-to-disc ratio | 0.65 (0.20) | 0.60 (0.16) | .303 |
| Baseline visual field mean deviation, dB | −709 (6.59) | −5.76 (4.29) | .401 |
| Baseline visual field pattern, dB, mean ± SD | 5.53 ± 3.56 | 5.55 ± 3.73 | .983 |
The bolded values were the statistically significant values (with p < 0.05).
CCT = central corneal thickness; D = diopters; dB = decibels; DH = disc hemorrhage; IOP = intraocular pressure; MD = mean deviation; SD = standard deviation.
Data are mean ± SD for continuous variables and frequency count (%) for categorical variables. Severity of glaucoma was classified based on visual field MD.
Statistically significant P value < .05.
Table 1 shows baseline demographic and clinical characteristics of patients with and without DH. DH patients were more likely to be female (78.9% vs 44.8%; P = .013) and have normal tension glaucoma (42.1% vs 12.4%; P = .01). Furthermore, patients with DH were more likely to be Asian (42.1% vs 12.4%) and less likely to be African American (0% vs 18.1%; P = .017). There were no baseline differences in vertical cup-to-disc ratio or visual field parameters (mean deviation and pattern standard deviation) between the two groups (Table 1). Table 2 shows the average baseline values for the OCT parameters, 2D RNFL thickness and 3D MDB neuroretinal rim thickness (or MDB thickness). Interestingly, DH eyes had a greater global RNFL thickness at baseline (74.74 μm vs 66.05 μm; P = .03), as well as greater temporal quadrant RNFL thickness (65.26 μm vs 56.06 μm; P = .012) and inferonasal sector RNFL thickness (87.32 μm vs 72.03 μm; P = .022). There were no statistically significant differences between the two groups in terms of baseline global MDB thickness, quadrant MDB thickness, or sector MDB thickness (Table 2).
TABLE 2.
Baseline SD-OCT Diagnostic Parameters of the 124 Open-Angle Glaucoma Study Patients
| Region of Interest | Eyes Without DH | Eyes With DH | P Value |
|---|---|---|---|
| Global RNFL thickness, μm | 66.05 ± 16.26 | 74.74 ± 13.75 | .03 a |
| Quadrant RNFL thickness, μm | |||
| Inferior | 77.47 ± 25.87 | 84.32 ± 22.96 | .283 |
| Superior | 78.05 ± 22.86 | 86.68 ± 24.08 | .135 |
| Nasal | 53.70 ± 19.89 | 62.11 ± 16.48 | .085 |
| Temporal | 56.06 ± 14.81 | 65.26 ± 12.45 | .012 a |
| Sector RNFL thickness, μm | |||
| Inferonasal | 72.03 ± 27.73 | 87.32 ± 17.61 | .022 a |
| Superonasal | 72.08 ± 23.74 | 79.58 ± 24.88 | .211 |
| Inferotemporal | 82.93 ± 32.20 | 81.16 ± 37.27 | .829 |
| Superotemporal | 84.14 ± 28.52 | 94.00 ± 29.67 | .171 |
| Global MDB thickness, μm | 210.98 ± 56.40 | 226.04 ± 50.98 | .28 |
| Quadrant MDB thickness, μm | |||
| Inferior | 215.73 ± 74.09 | 210.23 ± 66.49 | .763 |
| Superior | 218.29 ± 74.35 | 250.12 ± 72.92 | .088 |
| Nasal | 224.50 ± 72.80 | 242.81 ± 73.25 | .315 |
| Temporal | 185.68 ± 67.35 | 201.07 ± 48.89 | .344 |
| Sector MDB thickness, μm | |||
| Inferonasal | 234.80 ± 80.10 | 222.77 ± 79.26 | .547 |
| Superonasal | 228.58 ± 82.08 | 264.21 ± 86.29 | .087 |
| Inferotemporal | 196.54 ± 80.13 | 197.64 ± 72.39 | .955 |
| Superotemporal | 212.82 ± 74.25 | 248.53 ± 67.33 | .053 |
DH = disc hemorrhage; MDB = minimum distance band; RNFL = retinal nerve fiber layer; SD-OCT = spectral-domain optical coherence tomography.
Data are mean ± SD.
Statistically significant P value < .05.
To determine if the presence of a DH is associated with an increased risk of progression of glaucoma, we compared the proportion of eyes exhibiting glaucoma progression using event-based criterion for all 4 modalities, ie, disc photography, visual fields, RNFL thickness, and MDB neuroretinal rim thickness (Table 3). Interestingly, presence of a DH was associated with localized 3D neuroretinal rim thickness progression (superior MDB progression; odds ratio [OR]: 3.96; P = .04), but not with global or inferior MDB progression (P = .14 and .81, respectively; Table 4). Presence of a disc hemorrhage was not associated with an increased risk for disc photo progression (P = .08), visual field progression (P = .45), or RNFL global, inferior or superior progression (P = .17, .26 and .76 respectively) in our dataset.
TABLE 3.
Proportion of Eyes With and Without Disc Hemorrhage Exhibiting Glaucoma Progression Over the Course of the Study
| Modality Detecting Progression |
Eyes Without DH n (%) | Eyes With DH n (%) |
|---|---|---|
| Disc photo | 14 (13.3) | 6 (31.6) |
| Visual field | 44 (41.9) | 10 (52.6) |
| Global RNFL | 14 (13.3) | 5 (26.3) |
| Inferior RNFL | 25 (23.8) | 7 (36.8) |
| Superior RNFL | 14 (13.3) | 3 (15.8) |
| Global MDB | 52 (49.5) | 13 (68.4) |
| Inferior MDB | 59 (56.2) | 10 (52.6) |
| Superior MDB | 60 (57.1) | 16 (84.2) |
| Inferotemporal MDB | 57 (54.3) | 10 (52.6) |
| Superotemporal MDB | 50 (47.6) | 13 (68.4) |
DH = disc hemorrhage; MDB = minimum distance band; RNFL = retinal nerve fiber layer.
Data are frequency count (%).
TABLE 4.
Presence of Disc Hemorrhage is a Risk Factor for Localized 3D Neuroretinal Rim Thickness Progression but not for Progression as Assessed by Other Modalities
| Modality Detecting Progression | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Disc photo | 2.97 | 0.79-10.21 | .08 |
| Visual field | 1.53 | 0.51-4.67 | .45 |
| RNFL | |||
| Global | 2.30 | 0.56-8.24 | .17 |
| Inferior | 1.86 | 0.56-5.80 | .26 |
| Superior | 1.22 | 0.20-5.11 | .72 |
| MDB | |||
| Global | 2.19 | 0.71-7.60 | .14 |
| Inferior | 0.87 | 0.29-2.63 | .81 |
| Superior | 3.96 | 1.04-22.49 | .04 a |
| Inferotemporal | 0.94 | 0.31-2.84 | 1.00 |
| Superotemporal | 2.37 | 0.77-8.20 | .13 |
MDB = minimum distance band; RNFL = retinal nerve fiber layer.
Statistically significant P value < .05.
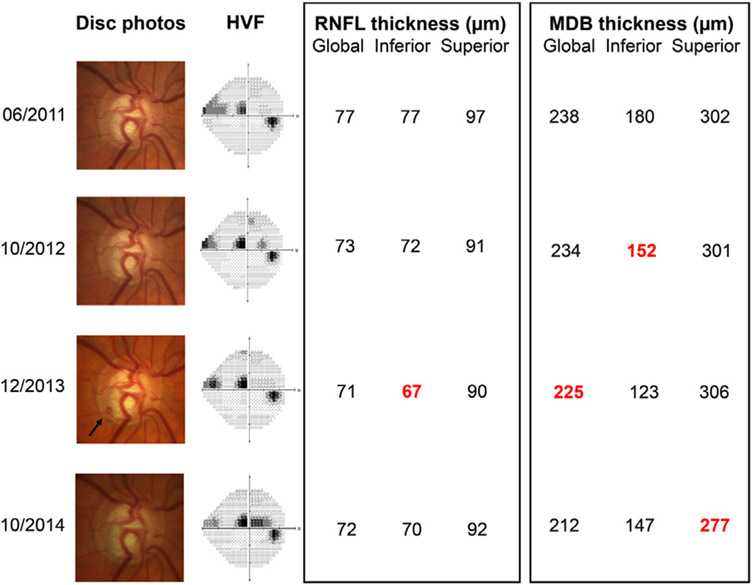
To further elucidate the relationship between DHs and glaucoma progression, we examined the timing of DHs and detection of progression across the four testing modalities (Table 5). Despite presence of a DH, many patients did not experience progression on disc photos (13/19, or 68%), visual field (9/19, or 47%), or RNFL (global, superior, and/or inferior; 11/19, or 58%) over the course of the study. Furthermore, only 2/19 (10.5%) of DH patients experienced an escalation in glaucoma therapy within a year after each DH occurrence (Table 5). On the other hand, most DH patients (17/19, or 89%) exhibited MDB progression (global, superior, and/or inferior), supporting the idea that three-dimensional neuroretinal rim thickness is a sensitive modality for determining progression in glaucoma. Notably, in the majority of patients with MDB progression (14/17, or 82%), the MDB progression was noted before or concurrently with the first instance of DH, supporting the notion that detectable optic nerve damage is present before a DH even occurs. A representative case illustrating the sensitivity of MDB thickness in detecting glaucoma progression prior to DH occurrence is shown in Figure 1.
TABLE 5.
Timing and Location of Disc Hemorrhage Occurrence and Glaucoma Progression in the Open-Angle Glaucoma Study Patients With Disc Hemorrhage
| Patient Number |
Glaucoma Severity |
Follow-up Duration (mo) |
DH Occurrence (mo) |
DH Location | DP Progression (mo) |
HVF Progression (mo) |
RNFL Progression (mo) |
MDB Progression (mo) |
MDB Progression Location |
Glaucoma Therapy Escalation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mild | 71 | 44 | IT | — | — | — | 10 | S | — |
| 2 | Mild | 64 | 41, 64 | IT, ST | — | — | — | 41 | G/S/I | — |
| 3 | Moderate | 47 | 31 | SN | — | — | — | 6, 31 | G, S | — |
| 4 | Moderate | 66 | 66 | ST | — | 54 | — | 54 | G/S | — |
| 5 | Mild | 68 | 46 | IT/I | — | — | — | 10 | S | — |
| 6 | Severe | 42 | 0, 42 | IT, IT | — | 14 | — | — | — | Yes (after 2nd DH) |
| 7 | Mild | 69 | 56 | I/ST | 26 | 13 | — | — | — | — |
| 8 | Moderate | 56 | 30, 56 | I, I | 56 | 56 | 14 | 14 | G/S/I | — |
| 9 | Mild | 76 | 30 | IT | — | 40 | 30 | 15, 30, 40 | I, G, S | — |
| 10 | Mild | 44 | 11 | N | — | — | — | 19, 44 | I, G/S | — |
| 11 | Moderate | 89 | 0 | IT | 55 | 55 | 25 | 25, 89 | G/I, S | — |
| 12 | Mild | 72 | 57 | I | — | — | — | 13 | G/S | — |
| 13 | Moderate | 62 | 0 | IT | — | 41 | 21 | 8 | G/S | — |
| 14 | Moderate | 56 | 24, 36, 48 | IT, IT, IT | 36 | — | — | 24 | I | — |
| 15 | Mild | 70 | 36 | IN | 36 | 53 | 18 | 18, 30 | G/S, I | — |
| 16 | Moderate | 65 | 12, 41, 53 | ST, ST, T | — | — | — | 12 | S | — |
| 17 | Mild | 49 | 49 | N | — | — | 21 | 21 | G/S | — |
| 18 | Mild | 46 | 34 | N | — | 34 | 18 | 18 | G/S/I | Yes |
| 19 | Moderate | 53 | 36, 53 | ST, ST | 36 | 11 | 36 | 11 | G/S/I | — |
DH = disc hemorrhage; DP = disc photo; G = global; HVF = Humphrey visual field; I = inferior; IN = inferonasal; IT = inferotemporal; MDB = minimum distance band; N = nasal; RNFL = retinal nerve fiber layer; S = superior; SN = superonasal; ST = superotemporal; T = temporal.
Time of DH occurrence and glaucoma progression is expressed as months from baseline visit. Events noted during the same visit are separated by a forward slash; events noted on subsequent visits are separated by a comma. Glaucoma therapy escalation refers to intensification of glaucoma therapy within 1 y after each documented instance of DH.
FIGURE 1.
A representative case demonstrating the sensitivity of MDB thickness in detecting glaucoma progression prior to DH occurrence. A 65-year-old Caucasian male with primary open-angle glaucoma and well-controlled intraocular pressure on latanoprost and timolol demonstrated an inferotemporal DH in 2013 (arrow) and Humphrey visual field progression in 2014. Significant progression on OCT RNFL thickness and MDB thickness is marked in red. Of note, whereas inferior RNFL progression was noted on the same visit as the DH, MDB progression was detected a year earlier, before the DH even occurred. DH = disc hemorrhage; MDB = minimum distance band; OCT = optical coherence tomography; RNFL = retinal nerve fiber layer.
We also examined the spatial relationship between the localized MDB progression and occurrence of DH. Interestingly, the quadrant in which MDB progression occurred did not necessarily correlate with the location of DH (Table 5). For example, superior MDB progression was the most common site of MDB change in our study, occurring in 16/19 (84%) of DH patients; however, out of those 16 patients, only 5 (31%) had a superior DH, while 8 (50%) had an inferior DH and 3 (19%) had a nasal DH (Table 5). Conversely, inferior DH were most common in our study (12/19, or 63% of patients); however, only 50% (6/12) of those patients exhibited inferior MDB progression. Therefore, in our dataset there was no clear spatial relationship between the location of MDB progression and DH occurrence.
DISCUSSION
In this study we investigated the relationship between DHs and glaucoma progression as determined by four different glaucoma testing modalities, including the novel high-density 3D spectral domain OCT (SD-OCT) optic nerve volume scans. The patients were followed prospectively for 5 years, and the presence of a DH only led to escalation of therapy if it was associated with subsequent structural and/or functional progression. Thus, our study can provide insight into the natural history of DHs and their relationship to glaucoma progression.
The key finding of our study is that DH patients had a significantly higher rate of localized 3D neuroretinal rim thickness progression (superior MDB progression; OR: 3.96; P = .04) (Table 4) than non-DH patients. The presence of a DH was not associated with a greater degree of disc photo progression, visual field progression, or RNFL progression in our dataset, in line with our previous finding that MDB thickness measurement is a very sensitive modality for detecting progression in glaucoma.18 Similarly, Cho and Kee33 demonstrated that, in patients with DHs, the rate of change of Bruch membrane opening-minimum rim width was significantly greater than that of RNFL, especially in the inferotemporal and superotemporal sectors. Notably, MDB progression in our dataset was detected in most patients (14/17 or 82%) before or concurrently with the first noted instance of DH (Table 5), suggesting that substantial and quantifiable optic nerve damage precedes DH occurrence.
This finding is in agreement with several prior studies, which also showed that optic nerve damage can precede DH occurrence. Two previous studies have found that DHs are more likely to occur in the areas of prior optic nerve focal rim notching7 and narrower neuroretinal rim at baseline,34 whereas Chung and associates8 showed that glaucomatous progression on disc photographs occurred both before and after a documented DH. De Moraes and associates11 showed that rapid and localized visual field progression detected using automated pointwise linear regression preceded development of a DH. Of note, if this methodological approach for detecting HVF progression is demonstrated to be more sensitive than the event-based expert assessment we used in our study, this may explain the lack of association between DH occurrence and HVF progression seen in our dataset. Although future studies are needed to directly compare trend-based versus event-based analysis, the event-based study design of the current study best affords extrapolation of our results to clinical practice, where the presence of progression needs to be determined at each clinic visit. In contrast, best pointwise linear regression models typically require 5 data points, which would not be available for this cohort until the end of the 5-year study period. In addition, the SD-OCT machine used in this study does not have FDA-approved RNFL software for trend analysis, which would also make a trend-based study design less clinically applicable. However, despite the nuances of various methods to detect glaucomatous disease progression, it is clear that significant structural and functional glaucoma progression can occur prior to DH detection.
How do these results compare to published large prospective trials? Several landmark studies have previously examined the relationship between DHs and glaucoma and found that DHs were a risk factor for glaucoma progression.1,2,4 However, in the CNTGS (Collaborative Normal Tension Glaucoma Study) and EMGT, the temporal relationship between DH occurrence and progression was not closely examined. In CNTGS, investigators looked solely at the association between glaucoma progression and DHs diagnosed at the time of enrollment,2 whereas in EMGT, DHs could have occurred at any time during the study period and the temporal relationship between the onset of DH, and progression was not investigated.4 In the OHTS trial, Budenz and associates1 found that having a DH was a strong risk factor for developing POAG, with the onset of glaucoma damage occurring on average 13 months after the DH was detected. However, the authors also found that the majority of patients with DH (86.7%) did not develop POAG during the study period.1 Furthermore, having the diagnosis of POAG increased the incidence of DH, with cumulative DH incidence increasing from 0.5% per year prior to development of POAG to 2.5% per year after the development of POAG.1 These findings indicate that, although DHs are clearly associated with glaucoma progression, the relationship between the two in terms of cause and effect is far more complex.
Another interesting finding from our study was that the degree of concordance between the quadrant in which DH occurred and where progression was detected was quite limited, with only 31% (5/16) patients with superior MDB progression also exhibiting a DH in the same (superior) quadrant (Table 5). Conversely, whereas the inferior DHs were most common in our study (12/19 or 63% of patients), only 50% of those patients (6/12) exhibited inferior MDB progression. This topographic noncorrespondence between DH location and area of progression is at first glance surprising but has been previously described. For example, 2 previous studies examining the degree of concordance of the location of the DH and the site of visual field progression found that the 2 were in agreement in only 44%35 and 65%9 of eyes. Similarly, a study by Gunvant and associates36 showed that RNFL thinning (as determined by GDx polarimetry measurements) was not restricted to regions corresponding to the location of the DH. This lack of topological agreement can be explained by 2 reasons: (1) many instances of glaucoma progression are not accompanied by a DH, and most DHs do not lead to glaucoma damage1; and (2) because DHs are transient phenomena,6 not all DHs will be captured with yearly fundus photography. Therefore, in this study, the observed DHs and disease progression may be 2 separate events. In agreement with the latter idea, a study by Nitta and associates,37 in which the patients were examined every 1 to 2 months, reported a much higher degree (88%) of topological correspondence between the site of DH and enlarging RNFL defects.
Taken all together, our data support the hypothesis that DHs represent indicators of ongoing glaucoma progression rather than being discrete events that cause subsequent progression.5 Current theories on the underlying pathophysiology of DHs fall into 2 camps: the vascular theory and the mechanical theory.5,6 According to the vascular theory, the primary cause of a DH is a microinfarction within the optic nerve head, whereby primary vascular dysregulation leads an increase in circulating levels of endothelin-1 and matrix metalloproteinase-9 and loss of the barrier function of the endothelial cells, resulting in extravasation of red blood cells and a DH.5,6 According to this theory, the primary cause of a DH is the underlying vascular insufficiency. The mechanical theory states that DHs result from mechanical disruption of the blood vessels in the optic nerve head from posterior bowing and glaucomatous remodeling of the lamina cribrosa.5,6 In addition, capillaries may also be mechanically disrupted through the process of proliferative reactive gliosis in response to glaucoma damage, in which the formation of a fibrous glial scar disrupts the capillary wall and leads to a DH.38 Our finding that significant optic nerve damage precedes DH occurrence is in agreement with the latter, mechanical theory of DH etiology, whereby ongoing glaucoma damage causes the DH and not the other way around.
In our study population, we also found that DHs were more likely to occur in women and patients with normal tension glaucoma (Table 1), a finding previously reported in several studies.39-41 For example, in a large population-based, cross-sectional study, Healey and associates39 found that DHs were present in 25% of patients with normal tension glaucoma, compared to 8% of patients with high-pressure glaucoma. DH patients in our study were also less likely to be Black and more likely to be Asian (Table 1). This finding is consistent with prior studies reporting a lower prevalence of DHs in patients of African descent than in those of European descent,42 and a high prevalence of DHs in Asian glaucoma patients, for example in Japan41 and South Korea.43 The patients with and without DH did not differ at baseline in terms of other clinical or demographic characteristics, including vertical cup-to-disc ratio, visual field parameters, or baseline global or quadrant MDB thickness. Of note, DH patients in our study did have a greater global RNFL thickness at baseline (P = .03) (Table 2) and a trend towards having less severe glaucoma than non-DH patients (as determined using Hodapp-Anderson-Parrish criteria; P = .065), consistent with prior studies that reported that DHs are detected more frequently in early to moderate than in advanced glaucoma.40,44
Our study had several advantages and disadvantages. To our knowledge, this is the first study comparing glaucoma progression in patients with and without DH using multiple commonly used clinical modalities (disc photography, visual fields, and 2D OCT RNFL thickness) and the novel high-density 3D OCT neuroretinal rim parameter, MDB thickness. Furthermore, our study was longitudinal and examined the natural history of DHs in an unbiased sample (ie, patients were not recruited based on presence of a DH, and their treatment was not modified solely because a DH was detected). A limitation of our study is that with yearly disc photographs we may not have captured all the DHs occurring in our patients. However, as DHs are transient phenomena that typically resolve after 6 to 12 weeks,6 this would remain a caveat even with more frequent testing and needs to be considered in most studies evaluating timing of DHs and glaucoma progression. Furthermore, our study design did not include a repeat test within a few weeks to confirm progression. Repeat confirmation testing may help to reduce the rate of false positives and false negatives,23,45,46 because false positives have been known to occur in the clinical setting and can lead to an initial overestimation of glaucoma progression.25
In summary, in this study we find that localized 3D neuroretinal rim thickness progression precedes DH occurrence in many glaucoma patients. These findings suggest that DHs should be seen as indicators of ongoing glaucoma damage, whose detection necessitates heightened clinical scrutiny but not necessarily immediate treatment intensification.
Supplementary Material
Funding/Support:
Teresa C. Chen is supported by a Harvard Catalyst National Institutes of Health award UL1 RR 025758, the Massachusetts Lions Eye Research Fund, an American Glaucoma Society Mid-Career Award, the Fidelity Charitable Fund, Department of Defense Small Business Innovation Research award DHP15-016. Milica A Margeta is supported by NIH/National Eye Instituted grants K12 EY016335 and K08 EY030160, American Glaucoma Society Young Clinician Scientist Award, and Research to Prevent Blindness Career Development Award.
Biography

Milica A. Margeta, MD PhD is an Assistant Professor of Ophthalmology at the Harvard Medical School and a full-time faculty member of the Massachusetts Eye and Ear Glaucoma Service. She is an NIH-funded clinician scientist whose primary research interest is in the role of neuroinflammation in the pathogenesis of glaucoma, with the ultimate goal of developing novel neuroprotective treatments for this blinding disease.

Teresa C. Chen, MD is an Associate Professor of Ophthalmology at the Harvard Medical School. She is a full-time faculty member of the Massachusetts Eye and Ear Glaucoma Service. Her research expertise is in imaging, the pediatric glaucomas, and keratoprosthesis surgery. She has published over 200 original articles, major reviews, and book chapters. Johannes de Boer, PhD and Dr. Chen were the first to image a human eye with video-rate spectral domain optical coherence tomography.
Footnotes
Financial Disclosure: Johannes F de Boer is the chair of the Scientific Advisory Board of the Center for Biomedical Optical Coherence Tomography Research and Translation (Harvard Medical School), and licenses to NIDEK Inc, Terumo Corp, Ninepoint Medical, and Heidelberg Engineering outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supplemental Material available at AJO.com.
Contributor Information
MILICA A. MARGETA, Department of Ophthalmology, Glaucoma Service, Massachusetts Eye and Ear, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA
KITIYA RATANAWONGPHAIBUL, Department of Ophthalmology, Glaucoma Service, Massachusetts Eye and Ear, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Glaucoma Research Unit, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Bangkok, Thailand.
EDEM TSIKATA, Department of Ophthalmology, Glaucoma Service, Massachusetts Eye and Ear, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
MICHELE ZEMPLENYI, Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
COURTNEY L. ONDECK, Department of Ophthalmology, Glaucoma Service, Massachusetts Eye and Ear, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Department of Ophthalmology, VA Boston Hospital, Boston, Massachusetts, USA
JANICE KIM, Harvard Medical School, Boston, Massachusetts, USA.
ANNE L. COLEMAN, Department of Ophthalmology, Stein Eye Institute, University of California Los Angeles, Los Angeles, California, USA
FEI YU, Department of Ophthalmology, Stein Eye Institute, University of California Los Angeles, Los Angeles, California, USA; Department of Biostatistics, University of California Los Angeles Fielding School of Public Health, Los Angeles, California, USA.
JOHANNES F. DE BOER, LaserLaB Amsterdam, Department of Physics and Astronomy, Vrijie Universiteit, Amsterdam, The Netherlands; Department of Ophthalmology, Vrijie Universiteit Medical Center, Amsterdam, The Netherlands
TERESA C. CHEN, Department of Ophthalmology, Glaucoma Service, Massachusetts Eye and Ear, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA
REFERENCES
- 1.Budenz DL, Anderson DR, Feuer WJ, et al. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113(12):2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drance S, Anderson DR, Schulzer M. Collaborative Normal-Tension Glaucoma Study G. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131(6):699–708. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–1972. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson B, Leske MC, Yang Z, Heijl A on behalf of Group E. Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology. 2008;115(11):2044–2048. [DOI] [PubMed] [Google Scholar]
- 5.Lee EJ, Kee HJ, Han JC, Kee C. Evidence-based understanding of disc hemorrhage in glaucoma. Surv Ophthalmol. May-Jun 2021;66(3):412–422 May-Jun 2021;66(3):412-422. [DOI] [PubMed] [Google Scholar]
- 6.Jasty U, Harris A, Siesky B, et al. Optic disc haemorrhage and primary open-angle glaucoma: a clinical review. Br J Ophthalmol. 2020;104(11):1488–1491. [DOI] [PubMed] [Google Scholar]
- 7.Law SK, Choe R, Caprioli J. Optic disk characteristics before the occurrence of disk hemorrhage in glaucoma patients. Am J Ophthalmol. 2001;132(3):411–413. [DOI] [PubMed] [Google Scholar]
- 8.Chung E, Demetriades AM, Christos PJ, Radcliffe NM. Structural glaucomatous progression before and after occurrence of an optic disc haemorrhage. Br J Ophthalmol. 2015;99(1):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000;129(6):707–714. [DOI] [PubMed] [Google Scholar]
- 10.Shukla AG, Sirinek PE, De Moraes CG, et al. Disc hemorrhages are associated with the presence and progression of glaucomatous central visual field defects. J Glaucoma. 2020;29(6):429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Moraes CG, Prata TS, Liebmann CA, Tello C, Ritch R, Liebmann JM. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest Ophthalmol Vis Sci. 2009;50(10):4727–4733. [DOI] [PubMed] [Google Scholar]
- 12.Akagi T, Zangwill LM, Saunders LJ, et al. Rates of local retinal nerve fiber layer thinning before and after disc hemorrhage in glaucoma. Ophthalmology. 2017;124(9):1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WJ, Kim YK, Park KH, Jeoung JW. Evaluation of ganglion cell-inner plexiform layer thinning in eyes with optic disc hemorrhage: a trend-based progression analysis. Invest Ophthalmol Vis Sci. 2017;58(14):6449–6456. [DOI] [PubMed] [Google Scholar]
- 14.Fan KC, Tsikata E, Khoueir Z, et al. Enhanced diagnostic capability for glaucoma of 3-dimensional versus 2-dimensional neuroretinal rim parameters using spectral domain optical coherence tomography. J Glaucoma. 2017;26(5):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shieh E, Lee R, Que C, et al. Diagnostic performance of a novel three-dimensional neuroretinal rim parameter for glaucoma using high-density volume scans. Am J Ophthalmol. 2016;169:168–178. [DOI] [PubMed] [Google Scholar]
- 16.Tsikata E, Lee R, Shieh E, et al. Comprehensive three-dimensional analysis of the neuroretinal rim in glaucoma using high-density spectral-domain optical coherence tomography volume scans. Invest Ophthalmol Vis Sci. 2016;57(13):5498–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen TC. Spectral domain optical coherence tomography in glaucoma: qualitative and quantitative analysis of the optic nerve head and retinal nerve fiber layer (an AOS thesis). Trans Am Ophthalmol Soc. 2009;107:254–281. [PMC free article] [PubMed] [Google Scholar]
- 18.Ratanawongphaibul K, Tsikata E, Zemplenyi M, et al. Earlier detection of glaucoma progression using high-density 3-dimensional spectral-domain OCT optic nerve volume scans. Ophthalmol Glaucoma. 2021. Mar 25 S2589-4196(21)00083–1. doi: 10.1016/j.ogla.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117(5):573–583. [DOI] [PubMed] [Google Scholar]
- 20.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126(4):487–497. [DOI] [PubMed] [Google Scholar]
- 21.Johnson CA, Keltner JL, Cello KE, et al. Baseline visual field characteristics in the ocular hypertension treatment study. Ophthalmology. 2002;109(3):432–437. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Simavli H, Que CJ, et al. Patient characteristics associated with artifacts in Spectralis optical coherence tomography imaging of the retinal nerve fiber layer in glaucoma. Am J Ophthalmol. 2015;159(3) 565–576.e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulzer M. Errors in the diagnosis of visual field progression in normal-tension glaucoma. Ophthalmology. 1994;101(9):1589–1594 discussion 1595. [DOI] [PubMed] [Google Scholar]
- 24.Drance S, Anderson DR, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131(6):699–708. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Saunders LJ, Zangwill LM, Daga FB, Crowston JG, Medeiros FA. Impact of normal aging and progression definitions on the specificity of detecting retinal nerve fiber layer thinning. Am J Ophthalmol. 2017;181:106–113. [DOI] [PubMed] [Google Scholar]
- 26.Reis ASC, Zangalli CES, Abe RY, et al. Intra- and interobserver reproducibility of Bruch membrane opening minimum rim width measurements with spectral domain optical coherence tomography. Acta Ophthalmol. 2017;95(7):e548–e555. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, de Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma. 2011;20(8):470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessel JM, Horn FK, Tornow RP, et al. Longitudinal analysis of progression in glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(5):3613–3620. [DOI] [PubMed] [Google Scholar]
- 29.Mwanza JC, Chang RT, Budenz DL. et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51(11):5724–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SH, Kim SH, Kim TW, Park KH, Kim DM. Reproducibility of retinal nerve fiber thickness measurements using the test-retest function of spectral OCT/SLO in normal and glaucomatous eyes. J Glaucoma. 2010;19(9):637–642. [DOI] [PubMed] [Google Scholar]
- 31.Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology. 2008;115(4):661–666. [DOI] [PubMed] [Google Scholar]
- 32.Bowd C, Zangwill LM, Weinreb RN, et al. Racial differences in rate of change of spectral-domain optical coherence tomography-measured minimum rim width and retinal nerve fiber layer thickness. Am J Ophthalmol. 2018;196:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho HK, Kee C. Comparison of rate of change between bruch’s membrane opening minimum rim width and retinal nerve fiber layer in eyes showing optic disc hemorrhage. Am J Ophthalmol. 2020;217:27–37. [DOI] [PubMed] [Google Scholar]
- 34.Furlanetto RL, De Moraes CG, Teng CC, et al. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2014;157(5):945–952. [DOI] [PubMed] [Google Scholar]
- 35.Rasker MT, van den Enden A, Bakker D, Hoyng PF. Deterioration of visual fields in patients with glaucoma with and without optic disc hemorrhages. Arch Ophthalmol. 1997;115(10):1257–1262. [DOI] [PubMed] [Google Scholar]
- 36.Gunvant P, Zheng Y, Essock EA, et al. Predicting subsequent visual field loss in glaucomatous subjects with disc hemorrhage using retinal nerve fiber layer polarimetry. J Glaucoma. 2005;14(1):20–25. [DOI] [PubMed] [Google Scholar]
- 37.Nitta K, Sugiyama K, Higashide T, Ohkubo S, Tanahashi T, Kitazawa Y. Does the enlargement of retinal nerve fiber layer defects relate to disc hemorrhage or progressive visual field loss in normal-tension glaucoma? J Glaucoma. 2011;20(3):189–195. [DOI] [PubMed] [Google Scholar]
- 38.Lee EJ, Han JC, Kee C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog Retin Eye Res. 2017;60:20–43. [DOI] [PubMed] [Google Scholar]
- 39.Healey PR, Mitchell P, Smith W, Wang JJ. Optic disc hemorrhages in a population with and without signs of glaucoma. Ophthalmology. 1998;105(2):216–223. [DOI] [PubMed] [Google Scholar]
- 40.Ozturker ZK, Munro K, Gupta N. Optic disc hemorrhages in glaucoma and common clinical features. Can J Ophthalmol. 2017;52(6):583–591. [DOI] [PubMed] [Google Scholar]
- 41.Tomidokoro A, Iwase A, Araie M, Yamamoto T, Kitazawa Y. Population-based prevalence of optic disc haemorrhages in elderly Japanese. Eye (Lond). 2009;23(5):1032–1037. [DOI] [PubMed] [Google Scholar]
- 42.Skaat A, De Moraes CG, Bowd C, et al. African Descent and Glaucoma Evaluation Study (ADAGES): racial differences in optic disc hemorrhage and beta-zone parapapillary atrophy. Ophthalmology. 2016;123(7):1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Dally LG, Ederer F, et al. The Advanced Glaucoma Intervention Study (AGIS): 14. Distinguishing progression of glaucoma from visual field fluctuations. Ophthalmology. 2004;111(11):2109–2116. [DOI] [PubMed] [Google Scholar]
- 44.Jonas JB, Xu L, Optic disk hemorrhages in glaucoma. Am J Ophthalmol. 1994;118(1):1–8. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Dally LG, Ederer F, et al. The Advanced Glaucoma Intervention Study (AGIS): 14. Distinguishing progression of glaucoma from visual field fluctuations. Ophthalmology. 2004;111(11):2109–2116. [DOI] [PubMed] [Google Scholar]
- 46.Keltner JL, Johnson CA, Quigg JM, Cello KE, Kass MA, Gordon MO. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118(9):1187–1194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.