Abstract
Background:
Over 80% of the global population consider themselves religious with even more identifying as spiritual, but the neural substrates of spirituality and religiosity remain unresolved.
Methods:
In two independent brain lesion datasets (N1=88; N2=105), we apply lesion network mapping to test whether lesion locations associated with spiritual and religious belief map to a specific human brain circuit.
Results:
We found that brain lesions associated with self-reported spirituality map to a brain circuit centered on the periaqueductal grey. Intersection of lesion locations with this same circuit aligned with self-reported religiosity in an independent dataset, as well as prior reports of lesions associated with hyper-religiosity. Lesion locations causing delusions and alien limb syndrome also intersected this circuit.
Conclusions:
These findings suggest that spirituality and religiosity map to a common brain circuit centered on the periaqueductal grey, a brainstem region previously implicated in fear conditioning, pain modulation, and altruistic behavior.
Introduction
Spiritual and religious behaviors have been present since early stages of human evolution(1) and played a significant role in shaping most human societies(1–5). Today, over 80% of the global population identify as religious, and even more as spiritual(2,6). Defining and measuring these behaviors scientifically is possible. Spirituality, or more precisely spiritual acceptance, has been defined as “a stable shift in worldview towards belief in forces that cannot be rationally comprehended or objectively proven.”(7,8) It has been measured using the Temperament and Character Inventory (TCI), which includes questions about “being directed by a spiritual force”, “miracles”, “religious experiences” and “purpose”. Religiosity has been defined as participation in a “unified system of beliefs and practices relative to sacred things.”(3) There is no accepted standard for measuring religiosity, but it can be assessed via simple self-report to the question “Do you consider yourself to be a religious person?”(9)
The biological basis for spirituality and religiosity has been investigated using genetics, neurotransmitter levels, and functional neuroimaging(10–16). Functional neuroimaging has identified many different brain regions whose activity is correlated with spirituality or religiosity, but whether these regions are causally involved in these behaviors is unknown.
Patients with brain disorders can provide unique insight into the neural substrate of spirituality and religiosity that can complement data from functional neuroimaging(17–23). Patients with temporal lobe epilepsy can present with hyper-religious symptoms(18–21), which has been linked to hippocampal as opposed to amygdala pathology(24). Patients with parietal lobe damage can experience increased spirituality(22), and patients with frontal lobe damage can show increased religious fundamentalism(23,25). Such patients can allow for causal inferences between neuroanatomy and spiritual or religious behaviors, but multiple different brain regions have been implicated.
Recently, it has become possible to map complex behavior to human brain circuits based on locations of brain damage that modulate the behavior and a wiring diagram of the human brain termed the human connectome(26). This technique, termed “lesion network mapping”, is particularly helpful when lesions causing similar symptoms occur in multiple different brain locations. Lesion network mapping has identified human brain circuits associated with amnesia, delusions, hallucinations, and even disorders of free will(27–30). Here, we use this technique to test whether lesion locations associated with spiritual and religious belief map to a specific human brain circuit.
Methods
Lesion dataset for spiritual acceptance
We analyzed a previously published dataset(22) in which neurosurgical patients were recruited for the purpose of studying temperament and character changes following brain tumor resection (N = 88, Figure 1; Supplementary Methods). For the current study, we focused on the “spiritual acceptance” subscale of the “self-transcendence” domain because it has previously been validated as a metric of spirituality and religiosity(8,10,13). Note this differs from a prior report on this dataset(22), which focused on the broader self-transcendence category.
Figure 1 -. Lesion locations associated with changes in spirituality occur in many different brain locations.
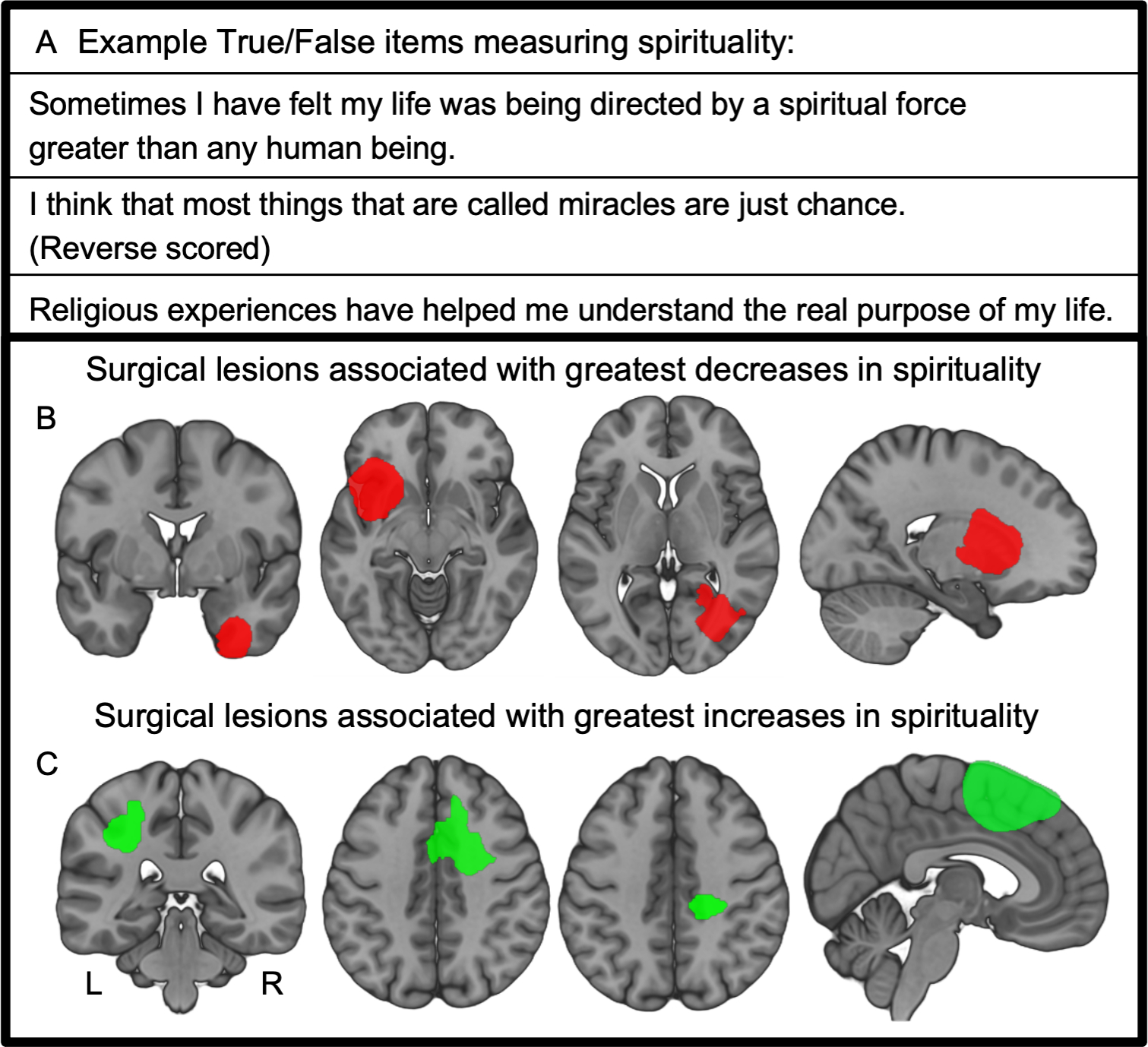
(A) Spirituality (or, more precisely “spiritual acceptance”) is measured by the Temperament and Character Inventory using a series of True or False self-report items. Spirituality is calculated as a single-value score based on participant responses across the items. Spirituality scores were obtained before and after neurosurgical resection of brain tumors, and changes in spirituality calculated from these longitudinal time points. (B) Lesion locations from the four patients with the greatest decrease in spiritual acceptance following neurosurgery; (C) lesion locations from the four patients with the greatest increase in spiritual acceptance.
Lesion network mapping of spiritual acceptance
We used lesion network mapping and previously validated methods to derive a brain network for spiritual acceptance in a data-driven fashion(26,27) (Figure 2). First, resting state functional connectivity between each lesion location and the rest of the brain was computed using a publicly available normative connectome dataset from 1000 healthy right-handed subjects (42.7% male subjects, ages 18–35 years, mean age 21.3 years)(31,32). This connectome dataset was processed in accordance with the strategy of Fox et al., 2005(33), which results in a map of brain regions functionally connected to each lesion location referred to as a lesion network(26,27). Second, we identified the peak connection most associated with changes in spiritual acceptance using voxelwise permutation analysis of linear models (PALM) with changes in spiritual acceptance as a behavioral covariable (Figure 2). The peak voxelwise association was identified and the coordinates recorded in MNI space. By definition, functional connectivity with this peak coordinate (using the same normative connectome described above) defines a brain network that best aligns with lesion locations decreasing or increasing spirituality. We previously used this same approach to define brain networks for memory(27) and depression(34). Because we were searching for the peak voxelwise association to define a spirituality network, this analysis was not corrected for multiple comparisons across all brain voxels. This peak should therefore be considered descriptive until validated in an independent dataset.
Figure 2 -. Data-driven method for identifying a lesion network for spiritual acceptance.
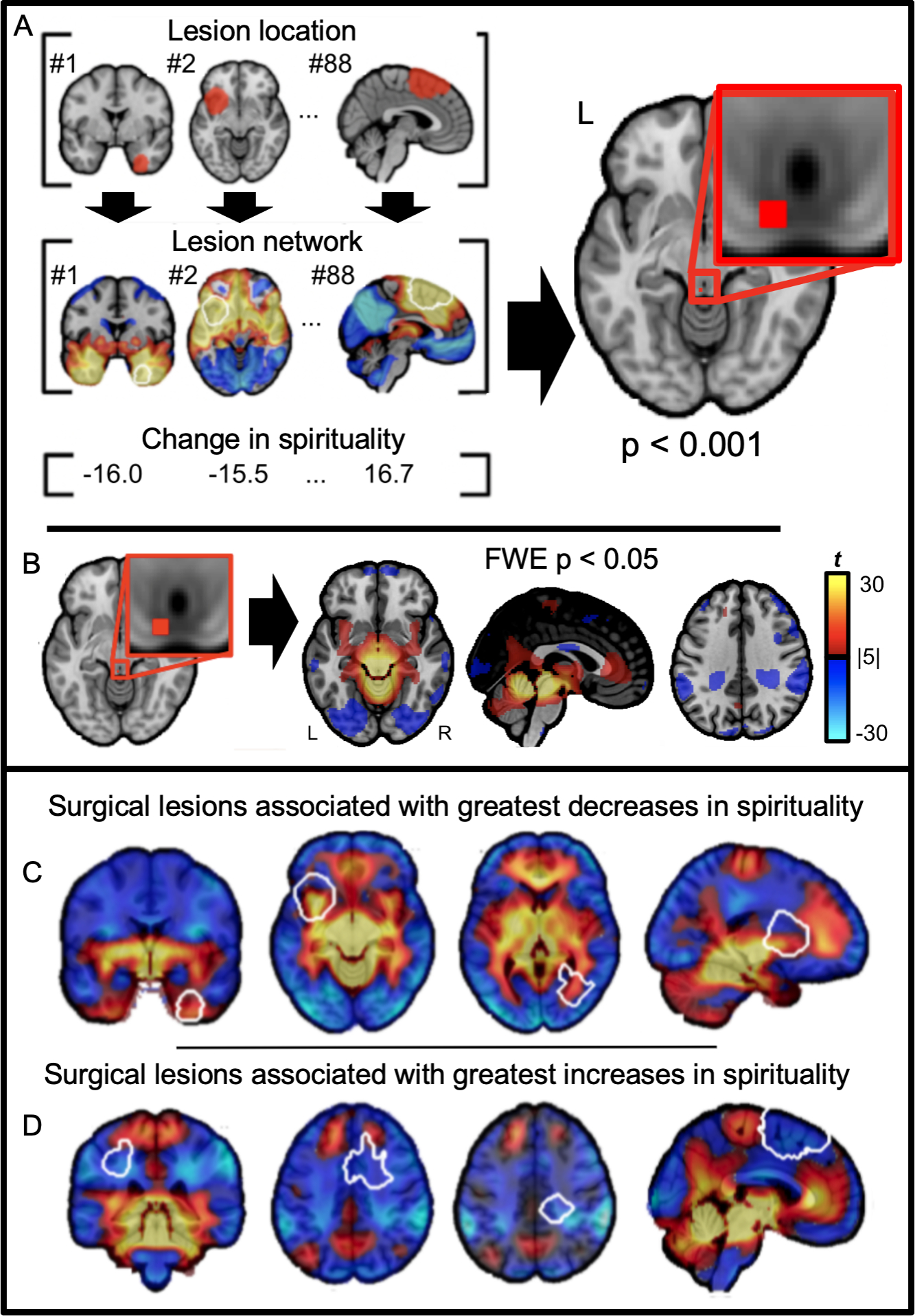
(A) The network of brain regions functionally connected to each lesion location was computed using resting state functional connectivity data from a large database of healthy volunteers (N = 1000). Lesion locations and lesion networks are shown for 3 of the 88 neurosurgical cases. Positively connected voxels are shown in warm colors, while negatively connected voxels are shown in cool colors. The peak voxelwise association between lesion connectivity and changes in spiritual acceptance was identified (image shown at uncorrected p < 0.001). (B) Functional connectivity with this peak was computed using the same resting state functional connectivity database from healthy volunteers (N = 1000) to derive a brain circuit for spirituality (image shown after voxelwise correction for multiple comparisons, FWE p < 0.05). (C & D) Circular demonstration that our brain circuit for spirituality aligns with lesion locations associated with decreased spirituality (C) or increased spirituality (D). Lesions locations associated with increased spirituality intersect negatively connected regions (cool colors) while legions associated with decreased spirituality intersect positively connected regions (warm colors).
To test for robustness, we repeated this PALM analysis including lesion size as a covariate. To test for specificity to spiritual acceptance, we repeated this PALM analysis using all seven TCI measures available in this dataset as covariates while also controlling for lesion size.
Validation in an independent dataset
To validate these data-driven findings, we analyzed a second independent dataset from patients with lesions caused by penetrating head trauma from combat during the Vietnam War (N = 105). Religiosity was assessed via questionnaire (“Do you consider yourself to be a religious person?”, “Yes” or “No”) administered several decades after brain injury, during Phase 4 of the Vietnam Head Injury Study(35). Lesion locations were outlined on CT scans and transformed to MNI space as described previously(35).
We calculated functional connectivity between each head trauma lesion location (N = 105) with the peak coordinate identified from our neurosurgical dataset. In other words, the data-driven result from our neurosurgical dataset (discovery) was used as an a priori region of interest (ROI) in the analysis of our independent head trauma dataset (validation). Using our normative connectome and previously reported methods(36), we computed the Pearson correlation between fMRI timeseries extracted from each lesion location with the timeseries extracted from our a priori ROI. The resulting r values were converted to a normal distribution using Fischer’s r to z transform, then averaged across the 1000 subjects, resulting in a single value that reflects the functional connectivity between each lesion location and our a priori ROI (spirituality peak). We then used a two-sample two-tailed t-test to compare connectivity values between non-religiously self-identified individuals (n = 25) versus religiously self-identified individuals (n = 80).
In a related analysis, we tested whether intersection of lesion locations from our head trauma dataset (N = 105) with the spirituality circuit derived from our neurosurgical dataset was associated with religiosity. A circuit damage score was computed by overlapping each head trauma lesion (N = 105) with the circuit map defined by functional connectivity to the peak coordinate from the neurosurgical dataset (N = 88). We then calculated the sum of functional connectivity values for all voxels within the lesion trace(34,37). We again used a two-sample two-tailed t-test to compare circuit damage score values between non-religiously self-identified (n = 25) versus religiously self-identified individuals (n = 80). For visualization purposes, we overlaid lesion locations from non-religious versus religious individuals on the brain circuit derived from our spirituality dataset (Figure 3).
Figure 3 -. Cross-validation of lesion network mapping results across two independent datasets.
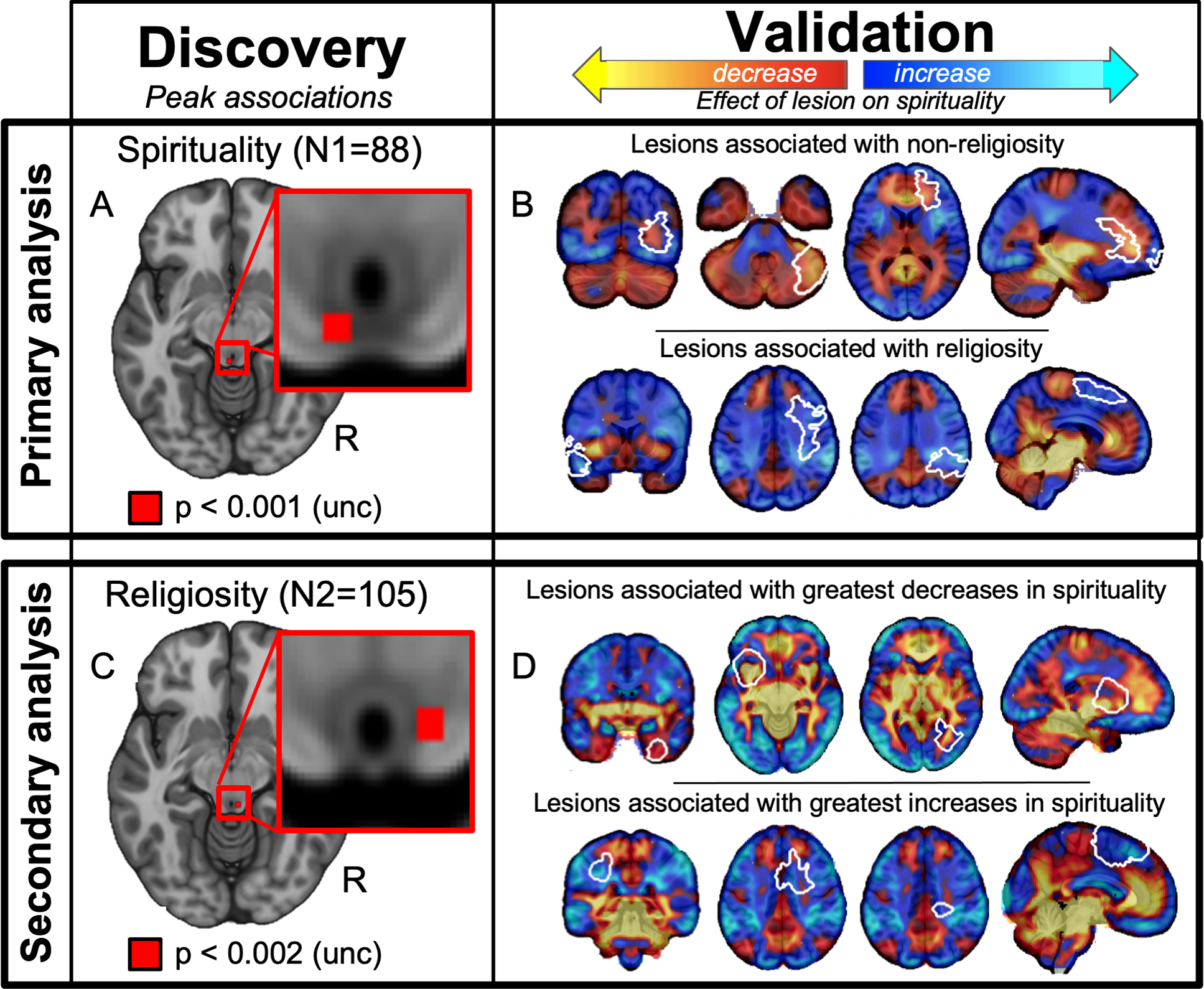
(A) Discovery: Lesion network mapping of spiritual acceptance in a neurosurgical dataset (N = 88) identified a peak association in the periaqueductal grey (PAG, uncorrected p < 0.001; z = −10). (B) Cross-validation: Functional connectivity between this PAG location and lesion locations from an independent dataset of head trauma lesions (N = 105) was associated with religiosity (white outlines showing 8 of 105 lesions). Positive functional connectivity with the PAG is shown in warm colors (intersecting lesion locations associated with non-religiosity) while negative functional connectivity with the PAG is shown in cool colors (intersecting lesion locations associated with religiosity). (C) Discovery: Lesion network mapping of religiosity in a head trauma dataset (N = 105) also identified a peak association in the PAG (uncorrected p < 0.002; z = −11). (D) Cross-validation: Functional connectivity between this PAG location and lesion locations from an independent dataset of neurosurgical lesions (N = 88) was associated with changes in spirituality (white outlines showing 8 of 105 lesions). Positive functional connectivity with the PAG is shown in warm colors (intersecting lesion locations associated with decreased spirituality) while negative functional connectivity with the PAG is shown in cool colors (intersecting lesion locations associated with increased spirituality).
Swapping discovery and validation datasets
To ensure that results were not dependent on which dataset we used for discovery versus validation, we repeated analyses using the head trauma dataset to define a data-driven network for religiosity (discovery) and the independent neurosurgical dataset to test whether this network was related to lesion induced changes in spirituality (validation). For a more detailed description please see Supplementary Methods.
Voxel-based Lesion Symptom Mapping (VLSM)
To test whether or results depended on connectivity or could be obtained based on lesion location alone, we repeated all analyses using voxel-based lesion symptom mapping (see Supplementary Methods).
Robustness to methodological changes
To ensure our lesion network mapping results were not dependent on methods used for processing resting state functional connectivity, we repeated our analyses using a human connectome processed without global signal regression (Supplementary Methods)(36). We repeated lesion network mapping analysis for spirituality (in the neurosurgical dataset) and religiosity (in the head trauma dataset) using this alternative connectome.
To ensure our lesion network mapping results were not dependent on the peak voxel, we repeated our lesion network analyses using the top 1% and 5% of voxels rather than only the peak voxel. This analysis was performed separately on our spirituality dataset and our religiosity dataset, each processed using two different connectome processing strategies described above. This resulted in 8 total maps (4 maps with the 1% cutoff and 4 maps at the 5% cutoff). Of these four maps for each voxel cutoff, two maps were voxelwise associations with spirituality (with and without global signal regression), and two maps were voxelwise associations with religiosity (with and without global signal regression). We performed a conjunction analysis by binarizing each map and overlapping them, showing results that are independent of dataset and these methodological changes.
Characterization of the spirituality network
We identified local maxima in our spirituality network using a clustering analysis (FSL version 6.0, 2018 release). No a priori threshold was applied for clustering or local maxima searching. The top ten positive and negative peaks were identified and recorded.
Literature-based case reports of hyper-religiosity
Case reports of patients with lesion-induced hyper-religiosity were identified using a systematic literature search (see Supplementary Materials). Lesion location was traced by hand from the published image onto the MNI template brain using 3D slicer (available at https://www.slicer.org) (Figure 5). Although prior work has shown high test-retest reproducibility of these tracings (37), the tracings were repeated by a second person blind to the lesion network mapping results of this study (Supplementary Figure 3). Intersections of each lesion location with our spirituality circuit were quantified by summing the t-values of each voxel in our spirituality circuit that fell within each lesion trace(34).
Figure 5 -. Our brain circuit for spiritual acceptance aligns with prior literature on hyper-religiosity.
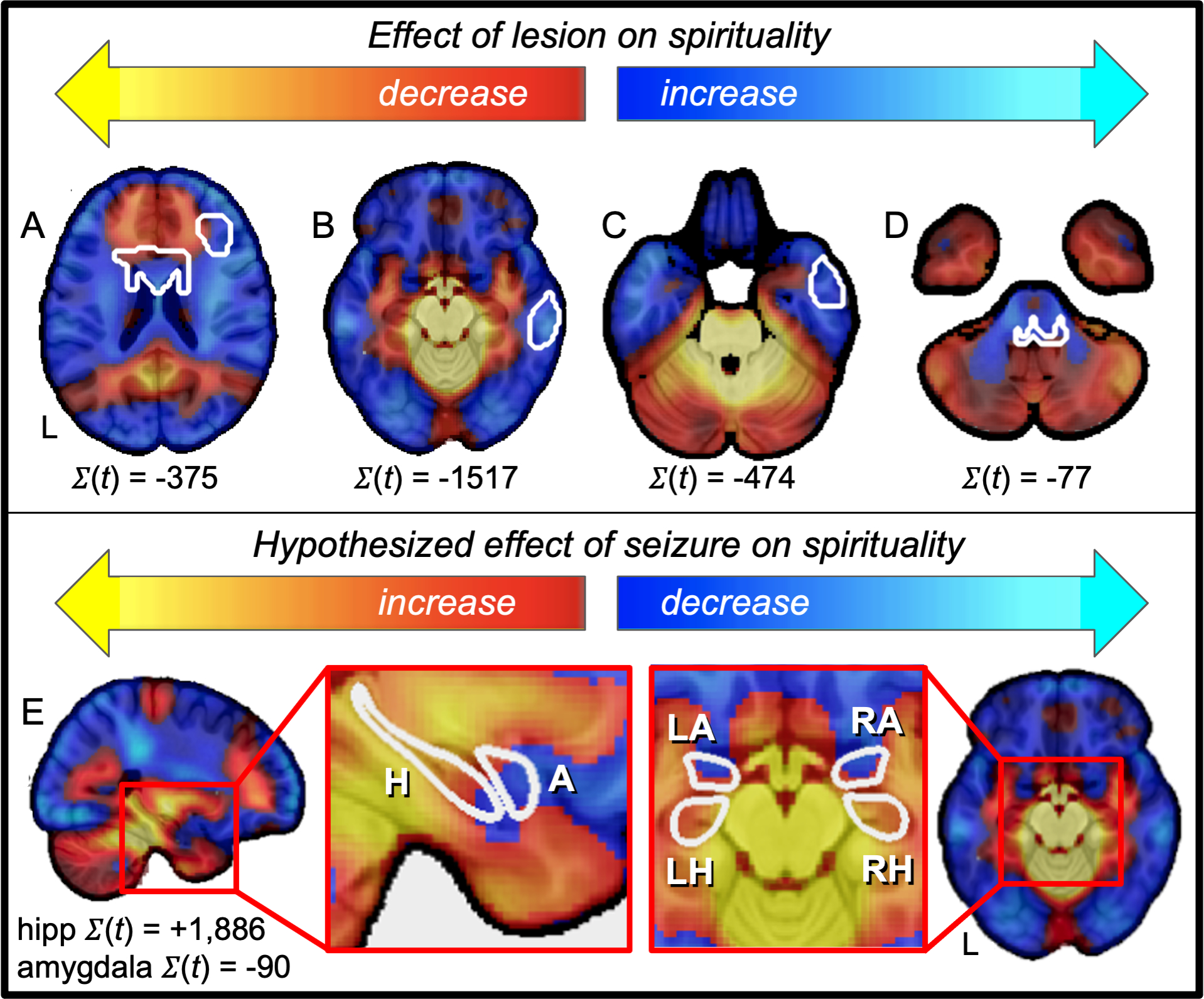
(top row, A – D) Case reports of lesion locations associated with hyper-religiosity (white outlines) intersect negative nodes of our spirituality circuit. (E) Brain regions previously associated with seizure-induced hyper-religiosity (hippocampus) intersects positive nodes of our spirituality circuit, but not adjacent brain regions not associated with hyper-religiosity (amygdala). Positive PAG connectivity is shown in warm colors while negative PAG connectivity is shown in cool colors.
Intersection of hyper-religiosity seizure zones with brain circuit findings
To explore whether spirituality circuit topography aligns with previously-published descriptions of hyper-religiosity in the context of mesial temporal lobe epilepsy(24) we leveraged a prior study linking hyper-religiosity to neuroanatomy(24). Specifically, hyper-religiosity was associated with hippocampal but not amygdala atrophy. We therefore computed the intersection of our circuit with anatomical masks of the hippocampus and the amygdala from the Harvard-Oxford neuroanatomical atlas. Intersection was quantified by summing the t-values of each voxel in our spirituality circuit that fell within the hippocampus and amygdala masks(34).
Relationship to lesions associated with other neurological or psychiatric symptoms
Spirituality circuit damage scores were calculated as described above for 356 symptom-causing lesions spanning twelve unique symptoms (Figure 6)(34,37). These twelve symptoms represent all lesion network mapping studies previously published by our laboratory at the time of manuscript preparation. In other words, these symptoms were not selected on the basis of any a priori hypothesis for which symptoms should align with our spirituality and religiosity circuit. These twelve queried symptoms included akinetic mutism (n = 28)(30), alien limb (n = 50)(30), amnesia (n = 53)(27), asterixis (n = 30)67, criminality (n = 17)19, delusions (n = 15)68, expressive aphasia (n = 12)(29), freezing of gait (n = 14)(38), hallucinations (n = 15)(39), hemichorea (n = 29)(38), pain (n = 24)(29), and parkinsonism (n = 29)(40). A one-way analysis of variance (ANOVA) was performed across symptom categories to test for preferential relationships between specific categories of symptom-causing lesions and the spirituality circuit. Post-hoc one-sample t-tests were performed on spirituality circuit damage for each individual symptom to quantitatively characterize the relationships between symptom-causing lesions and the spirituality circuit.
Figure 6 -. Lesion locations associated with other neurological and psychiatric symptoms intersect our spirituality circuit.
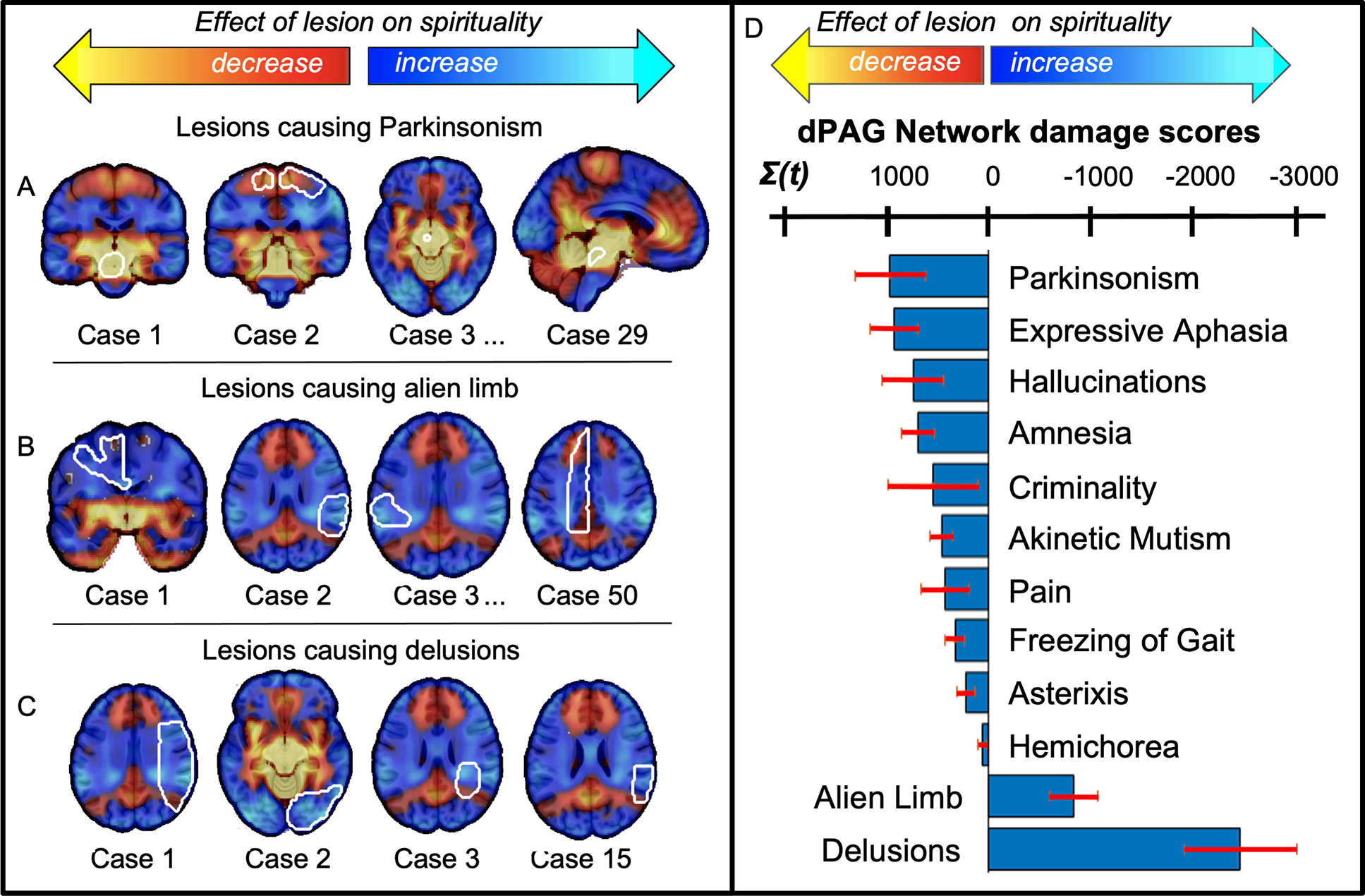
Lesion locations (white outlines) associated with parkinsonism (A, showing 4 of 29 cases) intersected positive nodes of our spirituality circuit, similar to lesions associated with non-religiosity. Lesion locations associated with alien limb syndrome (B, showing 4 of 50 cases) delusions (c, showing 4 of 15 cases) and delusions (C, showing 4 of 15 cases) showed the strongest intersection with negative nodes of our spirituality circuit, similar to lesion locations associated with religiosity. The sum of voxel intensities within lesion locations associated with twelve different neurological and psychiatric symptoms (N = 356) are shown in a bar graph (D). Error bars reflect standard error across different lesion locations within each lesion syndrome.
Results
Lesion network mapping of spiritual acceptance
Of the eighty-eight neurosurgical patients, thirty patients showed a decrease, twenty-nine of these patients showed an increase, and twenty-nine showed no change in self-reported spiritual belief before and after neurosurgical brain tumor resection (Supplementary Tables 1 & 2)(7,22). Lesion locations were heterogeneously distributed throughout the brain (Figure 1B, C).
Using lesion network mapping (Figure 2), the peak association with changes in spiritual acceptance was connectivity between lesion locations and the periaqueductal grey (PAG) (MNI: x = −2, y = −36, z = −10, uncorrected p < 0.001, Figure 2A). Functional connectivity with this PAG location thus defines a brain circuit that best aligns with lesion locations that modulate spirituality (Figure 2B), such that lesion locations associated with decreased spirituality intersect positive nodes in this map while lesion locations associated with increased spirituality intersect negative nodes (Figure 2C, D).
Connectivity between lesion locations and the PAG was still associated with changes in spirituality after controlling for lesion size (p = 0.002). Lesion connectivity to PAG was also specific for spiritual acceptance when controlling for all seven TCI measures of temperament and character (p = 0.02).
Validation in an independent dataset
Of the 105 patients who completed a questionnaire about religiosity after penetrating head trauma (Supplementary Tables 1 & 2), 24% identified as non-religious and 76% self-identified as religious. Functional connectivity between lesion locations in this independent dataset (N = 105) and the PAG hub of our spirituality circuit (defined using our neurosurgical dataset) was significantly associated with whether subjects self-identified as non-religious or religious (p < 0.01). Circuit damage scores for damage caused by lesions in this independent dataset (N = 105) to the spirituality circuit (defined using our neurosurgical dataset) were also significantly associated with self-identification as non-religious or religious (p < 0.03) To illustrate this cross-dataset convergence, we show lesion locations from the head trauma dataset overlaid on the spirituality circuit derived from our neurosurgical dataset (Figure 3B).
Swapping discovery and validation datasets
Using our head trauma lesion dataset (N = 105) to derive a data-driven lesion network for religiosity, we again found a peak association in the PAG (MNI: x = 3, y = −35, z = −11, uncorrected p < 0.002, Figure 3B). The peak association for religiosity in this independent dataset was within 4 mm of the peak association for spirituality (Figure 3A, C). As before, this relationship persisted after controlling for lesion size (p = 0.003) and was specific to religiosity when controlling for other behavioral measures (p = 0.003). Functional connectivity between neurosurgical lesion locations (N = 88) and the PAG hub of our religiosity circuit (defined in the independent head trauma dataset) was significantly associated with changes in spiritual acceptance (p < 0.02). Circuit damage scores for neurosurgical lesion damage to the religiosity circuit was also significantly associated with changes in spiritual acceptance (p < 0.05) (Figure 3D).
Voxel-based lesion symptom mapping (VLSM)
Using voxel lesion symptom mapping (VLSM), no voxels were associated with changes in spiritual acceptance at the uncorrected threshold of p < 0.001 (matching the peak voxelwise association discovered from lesion network mapping) and no voxels were associated with self-identified religiosity at the uncorrected threshold of p < 0.002 (matching the peak voxelwise association discovered from lesion network mapping). Using the unthresholded VLSM maps and testing for cross-dataset validation, there was no association between our VLSM map for spirituality and lesion locations associated with religiosity (p = 0.98) and no association between our VLSM map for religiosity and lesion locations associated with changes in spiritual acceptance (p = 0.76).
Robustness to methodological changes
To ensure that our data-driven localization to the PAG was independent of our specific methods, we repeated our lesion network mapping analysis using a connectome processed without global signal regression, in each case looking at the top 1% and 5% of voxels rather than just the peak association. Results were robust to these processing changes, again identifying a brain circuit for spirituality and religiosity centered on the PAG (Figure 4).
Figure 4 -. Conjunction of lesion network mapping results using different analysis approaches shows consistent localization to the PAG:
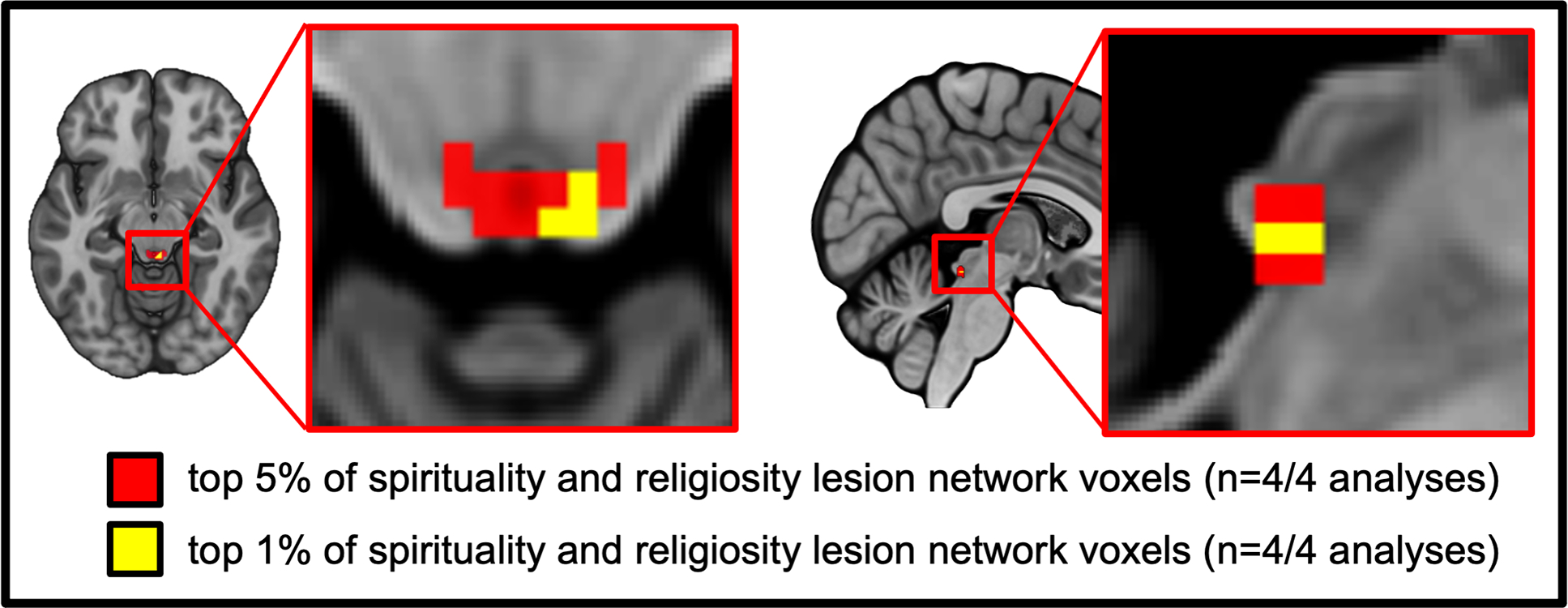
the top 5% of voxels (red) and top 1% of voxels (yellow) identified across our two independent datasets analyzed using two different connectome processing strategies (i.e., connectomes processed either with or without global signal regression).
Characterization of PAG functional connectivity network
Our spirituality circuit (defined by functional connectivity to the PAG) includes positive connectivity to subcortical and limbic regions and negative connectivity to frontoparietal networks and cortical regions previously implicated in “reasoning” (for peak coordinates see Supplementary Table 3, for overlap images see Supplementary Figure 1.
Alignment with prior literature on hyper-religiosity
Our systematic literature search identified four case reports of lesions associated with hyper-religiosity (Supplementary Figure 2, Supplementary Table 4). Each lesion location intersected negative nodes of our brain circuit, similar to lesions from our initial datasets associated with increased spirituality or religiosity (Figure 5A–D). Exploratory analyses of brain regions linked to seizure-induced hyper-religiosity also align well with our circuit (Figure 5E).
Relationship to lesions associated with other neurological or psychiatric symptoms
Finally, in our examination of 356 lesion locations associated with a range of other neurological and psychiatric symptoms, we found that lesion locations associated with certain symptoms intersected our spirituality circuit more so than others (one-way ANOVA, F (11) = 6.1, p = 10−8) (Figure 6). Specifically, lesions causing parkinsonism (t (28) = 2.7, p = 0.01, 95% CI [243, 1,668]) intersected positive areas of our circuit, similar to lesions associated with decreased spirituality (Figure 6). Lesions causing delusions (t (14) = −4.4, p = 0.001, [−3,667, −1,253] = 95% CI) and alien limb syndrome (t (49) = −3.5, p = 0.001, [−1,320, −352] = 95% CI), intersected negative regions on our map similar to lesion locations associated with increased spirituality and religiosity (Figure 6).
Discussion
Brain lesions associated with changes in spiritual acceptance map to a functionally connected brain circuit centered on the periaqueductal grey (PAG). Intersection of lesion locations with this spirituality circuit was associated with self-reported religiosity in an independent dataset, intersected prior case reports of hyper-religiosity, and intersected lesion locations associated with delusions and alien limb syndrome.
Our finding that spirituality and religiosity map better to a functionally connected brain circuit than an individual brain region is consistent with recent results across a range of complex human behaviors(27–30) and may help explain why prior studies have implicated multiple different brain regions(6,14,15,17,41). Our spirituality circuit is defined by connectivity to one focal brain region (the PAG), similar to prior work identifying a memory circuit defined by connectivity to the subiculum or a depression circuit defined by connectivity to the left dorsal lateral prefrontal cortex(27,34). In each case, lesion locations disrupting the behavior map to a brain circuit, but the circuit is defined by connectivity to one specific brain region that may play a critical role in mediating the behavior.
The PAG has been implicated in numerous functions including fear conditioning(42), pain modulation(43), altruistic behaviors(44) and unconditional love(45). It is anatomically connected to both the limbic system and prefrontal cortex(44) and enriched in receptors implicated in pain regulation (e.g. mu-opiate), and pair bonding (e.g. oxytocin)(44,46,47). Although speculative, these classic PAG functions may align with aspects of spirituality and religiosity. For example, religiosity increases under threat or after natural disasters(48) consistent with the role of the PAG in fear conditioning(42). Spirituality can alleviate pain and augment placebo(49), consistent with the role of the PAG in opiate and non-opiate analgesia(43,50). Finally, spirituality and religiosity have been linked to, if not equated with, unconditional love(51,52), consistent with the role of the PAG in maternal and pair-bonding(45,53–58), unconditional love(45), maternal love(59), non-sexual love(57), compassion(60), and the duration of long-term relationships(53). These findings of shared brain circuitry for spiritual acceptance and altruism are also convergent with the hypothesis that spiritual beliefs facilitated the expansion of prosociality over the course of human evolution(61). As such, although the PAG was not an a priori region of interest prior to our study, it has been implicated in many functions that could be relevant for spirituality and religiosity.
Notably, the negative functional topography in our PAG-defined circuit for spirituality and religiosity aligns with the frontoparietal control network(31), previously implicated in executive control, as well as brain regions previously implicated in neuroimaging studies of “Reasoning” (Supplementary Figure 1). This result is consistent with prior work suggesting that spiritual acceptance is “the opposite of rational materialism”(7,8) and priori work suggesting that negatively corelated brain networks represent “opposing functions”(33).
Medically, hyper-religiosity has been noted following focal brain lesions and in patients with mesial temporal lobe epilepsy for many decades (18–21). Lesion locations in these case reports align well with our spirituality circuit. Whether seizure onset zones associated with hyper-religiosity align with our circuit and whether hyper-religiosity is driven by regional hyperactivity during seizures or hypoactivity between seizures remains unclear (21). Our exploratory results support the latter, as atrophy locations associated with hyper-religiosity intersect positive nodes of our spirituality circuit while lesions associated with hyper-religiosity intersect negative nodes(24) (Figure 5). The fact that hyper-religiosity can resolve after resection of the medial temporal further supports this finding(21). Whether seizure propagation to the PAG is related to hyper-religiosity is a testable hypothesis for future work but is potentially consistent with brainstem propagation of mesial temporal seizures(62,63) and atrophy of the PAG in patients with mesial temporal epilepsy(64).
We also examined our database of lesions associated with neurological and psychiatric symptoms to see which, if any, of these symptoms share neuroanatomy with spirituality. Similarities between lesions associated with delusions and increased spirituality suggest a shared neural substrate, potentially consistent with shared features such as strongly-held fixed beliefs or the occurrence of religious content in patients with delusions(65–68). Our data also suggest a shared neural substrate between spirituality and “alien limb” phenomenon, both of which can be associated with feelings of control by an external agent(30,69). This relationship may have clinical value, such as surrendering to a “higher power” in the context of addiction treatment(70,71). Finally, our results suggest an inverse association between spirituality and lesions associated with parkinsonism, potentially consistent with decreased religiosity in patients with Parkinson’s disease(72,73).
It is important to note that a shared neural substrate between two phenomena may be helpful for understanding shared features and associations, but these results should not be over-interpreted. For example, our results do not imply that religion is a delusion, that historical religious figures suffered from alien limb syndrome, or that Parkinson’s disease arises due to a lack of religious faith. Similarly, our results have no bearing on the truth of any particular religious or spiritual belief.
There are several limitations in the current study. First, participants in both our spirituality and religiosity datasets came from predominantly Christian cultures, which may limit generalizability to other cultures and religious traditions, and our assessment of religiosity in our head trauma dataset was limited to a single yes/no question, which does not capture the wide variety of religious beliefs, behaviors, or contributing factors such as exposure to religiosity during their youth. Second, our religiosity dataset was mostly Caucasian, older males, which may not generalize to other ethnicities, ages or genders. Third, we investigated spirituality and religiosity as single behaviors, but different aspects of spirituality and religiosity may map to different brain circuits, an important topic for future work(17,41). Fourth, our localization of spirituality and religiosity to a brain circuit centered on the PAG was a post-hoc discovery in the neurosurgical dataset (N = 88) which did not survive correction for multiple comparisons across all brain voxels; however, this limitation is largely mitigated by validation and replication of this finding in a second independent dataset (head trauma dataset, N = 105), in which the PAG circuit from the neurosurgical dataset was used as an a priori hypothesis. Relatedly, the lesions that we studied do not directly intersect with the PAG, and PAG involvement is inferred from connectivity to lesion rather than from direct lesion location. Additionally, our lesion networks explain only a small amount of behavioral variance, and there are undoubtedly many other factors contributing to these complex behaviors. Finally, the function of the PAG is based largely on animal studies, and any relationship between these functions and features of religiosity and spirituality should be considered speculative.
Our data provide several testable hypotheses for future work. First, we hypothesize that intersection of neurosurgical lesions with our PAG circuit will explain variance in spirituality or religiosity measured pre and post intervention (as in the neurosurgical dataset). Second, we hypothesize that intersection of stroke lesions with our PAG circuit will be associated with measures of spirituality and religiosity assessed after the lesion (as in the head trauma dataset). Finally, we hypothesize that intersection of seizure onset zones with our PAG circuit will be associated with the presence or absence of seizure-induced hyper-religiosity.
In conclusion, our study demonstrates that lesions associated with spirituality and religiosity map to a human brain circuit defined by connectivity to the periaqueductal grey. This brain circuit aligns with lesion locations from prior case reports of hyper-religiosity and with lesion locations previously associated with strongly held fixed beliefs and feelings of control by an external agent.
Supplementary Material
Acknowledgments
Funding: ALC was supported by an NIH Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant (T32MH112510) and the Shields Research Grant from the Child Neurology Foundation. MDF was supported by the Sidney R. Baer, Jr. Foundation, the Nancy Lurie Marks Foundation, the Mather’s Foundation, the Kaye Family Research Endowment, and the NIH (grants R01 MH113929, R01 MH115949, and R01 AG060987). None of the funding agencies had a role in the design and conduct of the study, in the collection, management, analysis and interpretation of the data, in the preparation, review or approval of the manuscript, nor in the decision to submit the manuscript for publication.
Footnotes
Competing interests:
All authors report no biomedical financial interests or potential conflicts of interest.
Data and materials availability:
Data, code, and materials used in the analysis are available upon reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atran S In gods we trust: The evolutionary landscape of religion. Oxford University Press; 2002. [Google Scholar]
- 2.Dawkins R, Ward L. The god delusion. Houghton Mifflin Company Boston; 2006. [Google Scholar]
- 3.Durkheim E, Swain JW. The elementary forms of the religious life. Courier Corporation; 2008. [Google Scholar]
- 4.Freud S Civilization and its discontents. Broadview Press; 2015. [Google Scholar]
- 5.Laine JW. Meta-religion: religion and power in world history. Univ of California Press; 2014. [Google Scholar]
- 6.Hackett C, Stonawski M, McClendon D. The changing global religious landscape. Pew Res Cent. 2017; [Google Scholar]
- 7.Cloninger CR, Svrakic DM, Przybeck TR. A Psychobiological Model of Temperament and Character. Arch Gen Psychiatry. 1993. Dec 1;50(12):975–90. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Romeu A Self-transcendence as a measurable transpersonal construct. J Transpers Psychol. 2010;42(1):26. [Google Scholar]
- 9.Cohen-Zimerman S, Cristofori I, Zhong W, Bulbulia J, Krueger F, Gordon B, et al. Neural underpinning of a personal relationship with God and sense of control: A lesion-mapping study. Cogn Affect Behav Neurosci. 2020. Jun;20(3):575–87. [DOI] [PubMed] [Google Scholar]
- 10.Borg J, Andrée B, Soderstrom H, Farde L. The serotonin system and spiritual experiences. Am J Psychiatry. 2003. Nov;160(11):1965–9. [DOI] [PubMed] [Google Scholar]
- 11.Comings DE, Gonzales N, Saucier G, Johnson PJ, MacMurray JP. The DRD4 gene and the spiritual transcendence scale of the character temperament index. Psychiatr Genet. 2000. Dec;10(4):185–9. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzi C, Serretti A, Mandelli L, Tubazio V, Ploia C, Smeraldi E. 5-HT1A polymorphism and self-transcendence in mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;137B(1):33–5. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson KW, Damberg M, Ohrvik J, Leppert J, Lindström L, Anckarsäter H, et al. Genes encoding for AP-2beta and the Serotonin Transporter are associated with the Personality Character Spiritual Acceptance. Neurosci Lett. 2007. Jan 16;411(3):233–7. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson MA, Nielsen JA, King JB, Dai L, Giangrasso DM, Holman R, et al. Reward, salience, and attentional networks are activated by religious experience in devout Mormons. Soc Neurosci. 2018;13(1):104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beauregard M, Paquette V. Neural correlates of a mystical experience in Carmelite nuns. Neurosci Lett. 2006;405(3):186–90. [DOI] [PubMed] [Google Scholar]
- 16.Rim JI, Ojeda JC, Svob C, Kayser J, Drews E, Kim Y, et al. Current Understanding of Religion, Spirituality, and Their Neurobiological Correlates. Harv Rev Psychiatry. 2019. Oct;27(5):303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grafman J, Cristofori I, Zhong W, Bulbulia J. The neural basis of religious cognition. Curr Dir Psychol Sci. 2020;29(2):126–33. [Google Scholar]
- 18.Waxman SG, Geschwind N. The interictal behavior syndrome of temporal lobe epilepsy. Arch Gen Psychiatry. 1975;32(12):1580–6. [DOI] [PubMed] [Google Scholar]
- 19.Geschwind N Behavioural changes in temporal lobe epilepsy1. Psychol Med. 1979;9(2):217–9. [DOI] [PubMed] [Google Scholar]
- 20.Ogata A, Miyakawa T. Religious experiences in epileptic patients with a focus on ictus-related episodes. Psychiatry Clin Neurosci. 1998;52(3):321–5. [DOI] [PubMed] [Google Scholar]
- 21.Devinsky O, Lai G. Spirituality and religion in epilepsy. Epilepsy Behav. 2008;12(4):636–43. [DOI] [PubMed] [Google Scholar]
- 22.Urgesi C, Aglioti SM, Skrap M, Fabbro F. The spiritual brain: selective cortical lesions modulate human self-transcendence. Neuron. 2010;65(3):309–19. [DOI] [PubMed] [Google Scholar]
- 23.Zhong W, Cristofori I, Bulbulia J, Krueger F, Grafman J. Biological and cognitive underpinnings of religious fundamentalism. Neuropsychologia. 2017;100:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuerfel J, Krishnamoorthy ES, Brown RJ, Lemieux L, Koepp M, Tebartz van Elst L, et al. Religiosity is associated with hippocampal but not amygdala volumes in patients with refractory epilepsy. J Neurol Neurosurg Psychiatry. 2004. Apr;75(4):640–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asp E, Ramchandran K, Tranel D. Authoritarianism, religious fundamentalism, and the human prefrontal cortex. Neuropsychology. 2012. Jul;26(4):414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379(23):2237–45. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson MA, Lim C, Cooke D, Darby RR, Wu O, Rost NS, et al. A human memory circuit derived from brain lesions causing amnesia. Nat Commun. 2019;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darby RR, Horn A, Cushman F, Fox MD. Lesion network localization of criminal behavior. Proc Natl Acad Sci. 2018;115(3):601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS Jr, et al. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138(10):3061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darby RR, Joutsa J, Burke MJ, Fox MD. Lesion network localization of free will. Proc Natl Acad Sci. 2018;115(42):10792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011. Sep;106(3):1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes AJ, Hollinshead MO, O’Keefe TM, Petrov VI, Fariello GR, Wald LL, et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Sci Data. 2015;2:150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005. Jul 5;102(27):9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padmanabhan JL, Cooke D, Joutsa J, Siddiqi SH, Ferguson MA, Darby RR, et al. A human depression circuit derived from focal brain lesions. Biol Psychiatry. 2019. Nov 15;86(10):749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raymont V, Salazar AM, Krueger F, Grafman J. “Studying injured minds” - the Vietnam head injury study and 40 years of brain injury research. Front Neurol. 2011;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snider SB, Hsu J, Darby RR, Cooke D, Fischer D, Cohen AL, et al. Cortical lesions causing loss of consciousness are anticorrelated with the dorsal brainstem. Hum Brain Mapp. 2020. Apr 15;41(6):1520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotovio G, Talmasov D, Barahona-Corrêa JB, Hsu J, Senova S, Ribeiro R, et al. Mapping mania symptoms based on focal brain damage. J Clin Invest. 2020. Oct 1;130(10):5209–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol. 2017. Jan;81(1):129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim NY, Hsu J, Talmasov D, Joutsa J, Soussand L, Wu O, et al. Lesions causing hallucinations localize to one common brain network. Mol Psychiatry. 2021. Apr;26(4):1299–309. [DOI] [PubMed] [Google Scholar]
- 40.Joutsa J, Horn A, Hsu J, Fox MD. Localizing parkinsonism based on focal brain lesions. Brain J Neurol. 2018. Aug 1;141(8):2445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapogiannis D, Barbey AK, Su M, Zamboni G, Krueger F, Grafman J. Cognitive and neural foundations of religious belief. Proc Natl Acad Sci. 2009;106(12):4876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993. Dec;107(6):1093–8. [DOI] [PubMed] [Google Scholar]
- 43.Hosobuchi Y Dorsal periaqueductal gray-matter stimulation in humans. Pacing Clin Electrophysiol PACE. 1987. Jan;10(1 Pt 2):213–6. [DOI] [PubMed] [Google Scholar]
- 44.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage. 2012. Mar;60(1):505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beauregard M, Courtemanche J, Paquette V, St-Pierre EL. The neural basis of unconditional love. Psychiatry Res. 2009. May 15;172(2):93–8. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins JS, Ang VT, Hawthorn J, Rossor MN, Iversen LL. Vasopressin, oxytocin and neurophysins in the human brain and spinal cord. Brain Res. 1984. Jan 16;291(1):111–7. [DOI] [PubMed] [Google Scholar]
- 47.Back FP, Carobrez AP. Periaqueductal gray glutamatergic, cannabinoid and vanilloid receptor interplay in defensive behavior and aversive memory formation. Neuropharmacology. 2018. Jun;135:399–411. [DOI] [PubMed] [Google Scholar]
- 48.Koenig HG. In the wake of disaster: Religious responses to terrorism and catastrophe. Templeton Foundation Press; 2006. [Google Scholar]
- 49.Kohls N, Sauer S, Offenbächer M, Giordano J. Spirituality: an overlooked predictor of placebo effects? Philos Trans R Soc Lond B Biol Sci. 2011. Jun 27;366(1572):1838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva C, McNaughton N. Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? A comprehensive review. Prog Neurobiol. 2019. Jun;177:33–72. [DOI] [PubMed] [Google Scholar]
- 51.Goleman D Healing emotions: Conversations with the Dalai Lama on mindfulness, emotions, and health. Shambhala Publications; 2003. [Google Scholar]
- 52.Pope Benedict XVI. God Is Love--Deus Caritas Est: Encyclical Letter. USCCB Publishing; 2006. [Google Scholar]
- 53.Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci. 2012. Feb;7(2):145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000. Nov 27;11(17):3829–34. [DOI] [PubMed] [Google Scholar]
- 55.Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004. Mar;21(3):1155–66. [DOI] [PubMed] [Google Scholar]
- 56.Cacioppo S, Bianchi-Demicheli F, Frum C, Pfaus JG, Lewis JW. The common neural bases between sexual desire and love: a multilevel kernel density fMRI analysis. J Sex Med. 2012. Apr;9(4):1048–54. [DOI] [PubMed] [Google Scholar]
- 57.Diamond LM, Dickenson JA. The neuroimaging of love and desire: review and future directions. Clin Neuropsychiatry. 2012. Feb 1;9(1). [Google Scholar]
- 58.Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev. 2012. Jul;36(6):1481–509. [DOI] [PubMed] [Google Scholar]
- 59.Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry. 2008. Feb 15;63(4):415–23. [DOI] [PubMed] [Google Scholar]
- 60.Kim JJ, Cunnington R, Kirby JN. The neurophysiological basis of compassion: An fMRI meta-analysis of compassion and its related neural processes. Neurosci Biobehav Rev. 2020. Jan;108:112–23. [DOI] [PubMed] [Google Scholar]
- 61.Purzycki BG, Apicella C, Atkinson QD, Cohen E, McNamara RA, Willard AK, et al. Moralistic gods, supernatural punishment and the expansion of human sociality. Nature. 2016. Feb 18;530(7590):327–30. [DOI] [PubMed] [Google Scholar]
- 62.Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex N Y N 1991. 2004. Aug;14(8):892–902. [DOI] [PubMed] [Google Scholar]
- 63.Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain J Neurol. 2009. Apr;132(Pt 4):999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mueller SG, Bateman LM, Laxer KD. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. NeuroImage Clin. 2014;5:208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraepelin E Dementia praecox and paraphrenia. Livingstone. 1919; [Google Scholar]
- 66.Kraepelin E Manic depressive insanity and paranoia. J Nerv Ment Dis. 1921;53(4):350. [Google Scholar]
- 67.Bell V, Raihani N, Wilkinson S. De-rationalising delusions. PsyArXiv. [Google Scholar]
- 68.Bronstein MV, Pennycook G, Joormann J, Corlett PR, Cannon TD. Dual-process theory, conflict processing, and delusional belief. Clin Psychol Rev. 2019. Aug;72:101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taves A Revelatory events: Three case studies of the emergence of new spiritual paths. Princeton University Press; 2016. [Google Scholar]
- 70.Cole B, Pargament K. Spiritual surrender: A paradoxical path to control. 1999; [Google Scholar]
- 71.Miller W, Thoresen C. Spirituality, religion, and health: An emerging research field. Am Psychol. 2003;58(1):24. [DOI] [PubMed] [Google Scholar]
- 72.McNamara P, Durso R, Brown A. Religiosity in patients with Parkinson’s disease. Neuropsychiatr Dis Treat. 2006. Sep;2(3):341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butler PM, McNamara P, Durso R. Deficits in the automatic activation of religious concepts in patients with Parkinson’s disease. J Int Neuropsychol Soc JINS. 2010. Mar;16(2):252–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


