Abstract
Osteoarthritis (OA) is a joint disease that is pervasive in life, and the incidence and mortality of OA are increasing, causing many adverse effects on people’s life. Therefore, it is very vital to identify new biomarkers and therapeutic targets in the clinical diagnosis and treatment of OA. ncRNA is a nonprotein-coding RNA that does not translate into proteins but participates in protein translation. At the RNA level, it can perform biological functions. Many studies have found that miRNA, lncRNA, and circRNA are closely related to the course of OA and play important regulatory roles in transcription, post-transcription, and post-translation, which can be used as biological targets for the prevention, diagnosis, and treatment of OA. In this review, we summarized and described the various roles of different types of miRNA, lncRNA, and circRNA in OA, the roles of different lncRNA/circRNA-miRNA-mRNA axis in OA, and the possible prospects of these ncRNAs in clinical application.
Keywords: osteoarthritis, miRNA, lncRNA, circRNA, lncRNA/circRNA-miRNA-mRNA axis
Introduction
Osteoarthritis (OA) is a joint disease that is pervasive in life. It is largely caused by cartilaginous injury and affects the whole joint tissue (Pereira et al., 2015). Nearly half of people over 65 suffer from OA.(Sakalauskienė and Jauniškienė, 2010; Glyn-Jones et al., 2015). Globally, the incidence and mortality of OA are increasing (Bijlsma et al., 2011). Arthrodynia, swelling, and inability to move freely are the main symptoms of OA and cause many adverse effects on people’s lives. Several risk factors (Prieto-Alhambra et al., 2014), including age, sex, obesity, genetics, and joint damage, have been linked to OA progression (Felson et al., 2000; Vincent, 2019; Abramoff and Caldera, 2020). Articular cartilage degeneration and secondary osteogenesis are the main pathological manifestations of OA (Burr and Gallant, 2012). The long-term development of OA will not only affect people’s behaviors and activities but also cause depression, anxiety, and other negative emotions (Litwic et al., 2013). To provide more perfect, targeted treatment for patients with OA, the progression of OA needs to be studied. The specific pathogenesis of OA may be related to metalloproteinases (Mehana et al., 2019), cytokines (Boehme and Rolauffs, 2018), signaling pathways (Rigoglou and Papavassiliou, 2013), and noncoding RNA (ncRNA) (Sondag and Haqqi, 2016).
ncRNA is a nonprotein-coding RNA that does not translate into proteins but participates in protein translation. At the RNA level, it can perform biological functions (Wu et al., 2019). microRNA (miRNA), long ncRNA (lncRNA), circular RNA (circRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), small interfering RNA (siRNA), short hairpin RNA (shRNA) and Piwi-interactingRNA (piRNA) are the main ncRNAs(Chen et al., 2021). Studies have found that ncRNA is closely related to the occurrence of several diseases for the past few years (Esteller, 2011; Wang et al., 2019b). For example, promoter CpG methylation of two genes encoding members of the miR-200 family can easily lead to the occurrence and development of breast and colorectal cancer (Lim et al., 2013); miR-34b/c is a critical tumor suppressor. The methylation of miR-34b/c CpG island leads to the silence of miR-34b/c, thus increasing the incidence of tumors (Toyota et al., 2008); the decreased expression of miR-133 may induce myocardial hypertrophy by targeting the beta-1 adrenergic receptor pathway (Castaldi et al., 2014). Many studies have also found that miRNA, lncRNA, and circRNA are closely related to the course of OA, and play important regulatory roles in transcription, post-transcription, and post-translation (Li et al., 2019b; Zhang et al., 2021e). The interaction between lncRNA/circRNA, miRNA, and mRNA has attracted increasing attention. For example, lncRNA/circRNA can bind to miRNA, reduce the inhibitory effect of miRNA on mRNA, participate in regulating the progress of chondrocyte proliferation and apoptosis, extracellular matrix (ECM) degradation and inflammatory response in the progress of OA. Furthermore, lncRNA-p21 could induce chondrocyte apoptosis and slow the process of OA by binding to miR-451 and promoting the expression of downstream target gene mRNA (Tang et al., 2018a). This review describes the roles of miRNA, lncRNA, and circRNA in OA and the role of the lncRNA/circRNA–miRNA–mRNA axis in OA.
miRNAs and OA
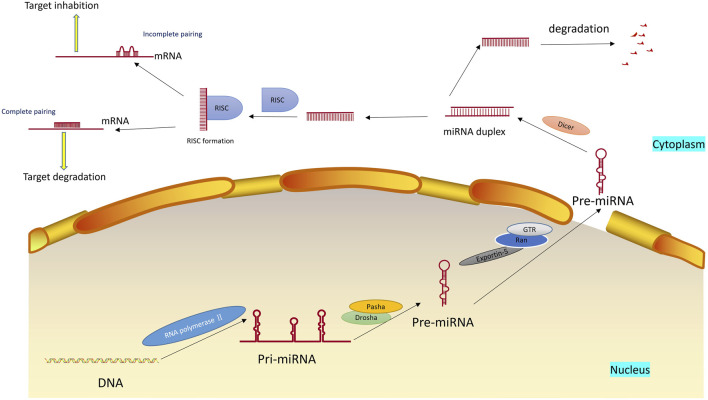
miRNA is a single-stranded RNA molecule with a length of about 20–24 nucleotides (Correia de Sousa et al., 2019). It belongs to one type of ncRNA and widely exists in eukaryotes to regulate the expression of other genes. miRNA regulates gene expression based on complete or incomplete pairing with mRNA. In most cases, the single-stranded miRNA in the complex is paired with the 3′UTR of the target mRNA in an incomplete complementary manner, blocking the translation of the gene and regulating gene expression. This process, called translation inhibition, is mainly found in animal cells. When the miRNA is completely complementary to the 3′UTR of the target mRNA, the mRNA in the complementary region would be specifically broken, eventually leading to gene silencing, and the process called post-transcriptional gene silencing, which will eventually lead to the degradation of target mRNA, mainly exists in plant cells (Liu et al., 2014a). The same gene can be regulated by multiple miRNAs, and multiple target genes can be regulated by the same miRNA (Iacona and Lutz, 2019). The formation and mechanism of miRNA are shown in Figure 1.
FIGURE 1.
Formation and mechanism of miRNA. miRNAs are first transcripted into longer primary miRNAs in the nucleus and then processed into hairpin RNAs of 60–70 nucleotides in the nucleus by Drosha, Pasha et al. The precursor miRNAs are transported out of the cell nucleus with the help of the Ran-GTP-dependent nucleoplasmic/cell transporter Exprotin-5 and split into 21–25 nucleotide length double-stranded miRNAs in the cytoplasm by Dicer. Subsequently, the double helix is derotated by the action of the derotation enzyme, and one of the strands is integrated into the RNA-induced silencing complex (RISC), an asymmetric RISC assembly is formed, and the other chain is immediately degraded.
With the deepening of research, miRNAs have been discovered and studied increasingly, and they have become a potential target in disease prevention and treatment. miRNA has many functions roles in human diseases, such as regulating cell autophagy (Li et al., 2019h), epigenesis (Yao et al., 2019), glucose metabolism (Fu et al., 2015). Chen et al. (2018c) developed a computational model for disease association prediction to detect potential miRNA-disease associations accurately and efficiently. By studying three common human cancers (Zhang et al., 2021b), namely, colon cancer, esophagus cancer, and kidney cancer, many miRNAs were confirmed to be connected with the three kinds of cancer. In addition, many studies have proven that miRNA is related to the pathological processes of intervertebral disc degeneration (Shi et al., 2021b), muscle atrophy (Zhang et al., 2021a), and cardiovascular diseases (Liu et al., 2021a).
Currently, growing findings reveal that miRNA expression level changes exist in various tissues of patients with OA, leading to abnormal target gene expression. miRNA has many functions in OA, such as regulating cell autophagy and apoptosis (Yu et al., 2019b), inflammatory reaction (Sui et al., 2019), and cartilage degradation (Guo et al., 2020). Changes in miRNA expression levels in different tissues can be experimented with by gene sequencing. Gene sequencing is a new type of gene detection technology, which can analyze and determine the whole sequence of genes from blood or saliva to predict the possibility of suffering from various diseases and lock in individual diseased genes for early prevention and treatment. Zhou et al. (2020b) revealed 21 differentially expressed miRNAs in synovial tissues from OA patients compared with normal controls by gene sequencing technology. The expression levels of the first two DEmiRNAs(hsa-miR-17-5p and hsa-miR-20b-5p), which cover most of the DEmRNAs, were analyzed and found to be down-regulated in OA, which was also confirmed by qRT-PCR verification. Ntoumou et al. (2017) assessed differential miRNA expression by microarray analysis in the serum of patients with OA. Compared with the control group, 279 miRNAs were differentially expressed in OA. This study focused on analyzing and studying three differentially expressed miRNAs: hsa-miR-140-3p, hsa-miR-671-3p, and hsa-miR-33b-3p. We found that the expression of these three miRNAs was down-regulated in the serum of OA patients. Through serum microRNA array analysis and bioinformatics analysis, they determined that these three miRNAs were potential OA biomarkers involved in the metabolic processes of insulin and cholesterol. OA is a metabolic disease, and insulin resistance plays a vital role in metabolic syndrome. Therefore, the metabolic processes of insulin and cholesterol in the body are closely related to OA. In addition, based on RNA sequencing and miRNA analysis, Wu et al. (2021a) identified that miR-210-5p is highly enriched in the exosomes of OA sclerotic subchondral osteoblasts, triggering the expression of genes associated with catabolism in articular chondrocytes. Therefore, the abnormal up-regulation of miR-210-5p in exosomes could serve as a marker for OA. Notably, miRNA show obvious tissue specificity in different OA tissues. For example, the expression of miR-125b-5p in synovial fluid and chondrocytes is different in OA patients. Ge et al. (2017) found by PCR that miR-125b-5p in synovial fluid was significantly up-regulated in OA patients compared with normal subjects, promoting synovial cell apoptosis by targeting syvn1. Rasheed et al. (2019) treated chondrocytes with IL-1β to construct OA cell models and determined the expression of miR-125b-5p using Taqman analysis. They found that miR-125b-5p in chondrocytes was significantly down-regulated compared to healthy individuals and regulated inflammatory genes in OA chondrocytes by targeting TRAF6. Our appeal study found that expression levels of multiple miRNAs in the synovial membrane, cartilage, and subchondral bone were altered in OA patients compared to healthy individuals. In addition, even in the same tissue, if in different stages of development, the expression of miRNAs may be different. For example, in different stages of the knee joint cartilage of rats, Sun et al. (2011) used Solexa sequencing and RT-qPCR detection for the expression of miRNAs. They tested the miRNAs in the rat knee joint cartilage at the starting point, on Day 21 and Day 42, and found that the expression of miRNAs was different at each stage. Among them, 4 representative miRNAs were selected for further analysis. Compared with the initial stage, the expressions of aggrecan, colia1, and ColXa1 were up-regulated on day 21. The expression of ColXa1 was up-regulated on day 42, whereas those of aggrecan and colia1 were down-regulated. The expression of Sox9 showed minimal change during the three stages. Gabler et al. (2015) found that miRNA could control the differentiation of chondrocytes and regulate the occurrence of OA. During the development of human bone marrow mesenchymal stem cells (HMSCs), the expression of miRNA in different development stages is also different. By microarray analysis, the miR spectra of HMSCs in patients with OA at different development time points were measured. Among the 1,349 detected miRNAs, 553 were expressed in cartilage formation, they further performed miRNAs detection at 7, 14, 21, and 42 days after cartilage formation and found that their expression of miRNAs was also different. In summary, the expression of miRNAs in OA patients is different in different tissues and between different stages of development of the same tissue.
It is well known that many intracellular signaling pathways, such as nuclear factor-kappaB(NF-κB) and transforming growth factor β (TGF-β) played an vital roles in the pathogenesis of OA (Nishimura et al., 2020). In recent years, more studies discovered that miRNA can delay the pathological process of OA by promoting or inhibiting these pathways (Xu et al., 2016). NF-κB is an essential nuclear transcription factor in cells participating in the inflammatory and immune response of the body and apoptosis regulation (Lawrence, 2009). For example, as the 3′UTR of NF-κB contains the binding site of miR-143 and miR-124, when the DNA methylation degree of miR-143 and miR-124 promoters is reduced, the expression of miR-143 and miR-124 is up-regulated, and the transcription process is activated, thereby inhibiting the NF-κB signaling pathway, inhibiting apoptosis and delaying the progression of OA (Qiu et al., 2020). Similarly, When the expression levels of miR-34a and miR-181a were decreased, the expression of the BCL2 gene was increased, thereby limiting the term of NF-κ B translocation into the nucleus in OA Chondrocytes cultures and eventually reducing apoptosis and oxidative stressl (Cheleschi et al., 2019). The TGF-β signaling pathway is involved in many cellular processes in mature organisms and developing embryos, including cell growth, differentiation, apoptosis, dynamic cell balance, and other cellular functions. By promoting or inhibiting the TGF-β signaling pathway, we can regulate the cellular processes, thereby inducing or delaying the progression of OA (Shen et al., 2014). Hu et al. (2019b) established OA mouse models. QPCR and Western blot were used to compare the expression of miR-455-3p and PAK2 in the cartilage of healthy individuals and patients with OA, and the luciferase reporter gene was used to analyze the interaction between them. The results showed that miR-455-3p could inhibit the expression of pak 2, promote the TGF-β signaling pathway, and ultimately inhibit OA by directly targeting PAK2 3′UTR. In summary, various miRNAs are involved in regulating OA progression by handling a variety of intracellular signaling pathways. In addition, increasing evidence also emphasizes that changes in the expression of many miRNAs can also directly regulate the development of OA. The specific information of these miRNAs is listed in Table 1.
TABLE 1.
Functional characterization of the miRNAs in OA.
| miRNA | Expression | Target gene(s) | Tissue/cell source | Region | Model | Functions | Reference |
|---|---|---|---|---|---|---|---|
| miR-103 | Up | SPHK1 | Cartilage tissue | knee joint, hip joint | OA rat model | Apoptosis | Li et al. (2019a) |
| Up | Sox6 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Chen and Wu, (2019) | |
| miR-34a | Up | TGIF2 | Synovial fluid | knee joint | OA cell model | Apoptosis | Luo et al. (2019a) |
| Up | DLL1 | Cartilage tissue | knee joint, hip joint | OA rat model | Apoptosis | Zhang et al. (2018d) | |
| Up | SIRT1/p53 | Cartilage tissue | knee joint | OA rat model | Apoptosis | Yan et al. (2016) | |
| Up | Cyr61 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Yang et al. (2018a) | |
| Up | —— | Cartilage tissue | knee joint | OA rat model | Apoptosis | Tao et al. (2020) | |
| Up | —— | Cartilage tissue | knee joint | OA rat model | Apoptosis | Abouheif et al. (2010) | |
| miR-486-5p | Up | SMAD2 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Shi et al. (2018) |
| miR-375 | Up | JAK2 | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Zou et al. (2019) |
| Up | ATG2B | Cartilage tissue | knee joint | OA mouse model | Autophagy | Li et al. (2020c) | |
| miR-29b | Up | PTHLH | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Dou et al. (2020) |
| Up | Wnt5a | —— | —— | OA mouse model | cartilage degradation | Sun et al. (2020) | |
| Up | COL2A1, COL1A2 | Cartilage tissue | knee joint, hip joint | OA mouse model | Apoptosis | Moulin et al. (2017) | |
| Up | COL1A1, COL3A1 | Cartilage tissue | knee joint, hip joint | OA cell model | Apoptosis | Mayer et al. (2017) | |
| miR-29b-3p | Up | PGRN | Cartilage tissue | knee joint | OA rat model | Apoptosis | Chen et al. (2017) |
| miR-124A | Up | QKI, MAP 1B | Cartilage tissue | knee joint | OA rat model | cartilage degradation | Jiang et al. (2020b) |
| miR-455-3p | Up | PAK2 | Cartilage tissue | knee joint | OA mouse model | cartilage degradation | Hu et al. (2019b) |
| Up | COL2A1 | Cartilage tissue | —— | OA cell model | Apoptosis, Inflammation | Cheng et al. (2020) | |
| Up | PTEN | Bone marrow, Cartilage tissue | —— | OA mouse model | Apoptosis, Inflammation | Wen et al. (2020) | |
| miR-30b | Up | ERG | Cartilage tissue | knee joint | OA cell model | cartilage degradation | Li et al. (2015) |
| miR-181 | Up | PTEN | Cartilage tissue | knee joint | OA cell model | Apoptosis | Wu et al. (2017b) |
| miR-324-5p | Up | Gpc1 | Cartilage tissue | —— | OA cell model | —— | Woods et al. (2019) |
| miR-146a | Up | TRAF6 | Cartilage tissue | knee joint, hip joint | OA cell model | Apoptosis | Zhong et al. (2017) |
| Up | Camk2d, Ppp3r2 | Cartilage tissue | knee joint | OA mouse model | cartilage degradation | Zhang et al. (2017) | |
| Up | Smad4 | Cartilage tissue | knee joint | OA rat model | Apoptosis | Li et al. (2012) | |
| Up | CXCR4 | Cartilage tissue | —— | OA mouse model | nflammation | Sun et al. (2017) | |
| miR-146a-5p | Up | TRAF6 | Cartilage tissue | hip joint | OA cell model | Apoptosis | Shao et al. (2020) |
| Up | TXNIP | SW1353 and C28/I2 cells | —— | —— | Apoptosis, Inflammation | Zhao and Gu, (2020) | |
| Up | —— | Cartilage tissue, Blood | —— | OA cell model | cartilage degradation, Inflammation | Skrzypa et al. (2019) | |
| miR-146b | Up | A2M | Cartilage tissue | knee joint | OA mouse model | Apoptosis, cartilage degradation | Liu et al. (2019d) |
| Up | —— | Bone marrow, Cartilage tissue | —— | OA cell model | Apoptosis | Budd et al. (2017) | |
| miR-1236 | Up | rs4246215 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Wang et al. (2020b) |
| miR-10a-5p | Up | HOXA3 | Cartilage tissue, Blood | —— | OA mouse model | Apoptosis, cartilage degradation | Li et al. (2020b) |
| Up | HOXA1 | Cartilage tissue | hip joint | OA mouse model | Apoptosis | Ma et al. (2019b) | |
| miR-27b-3p | Up | KDM4B | Cartilage tissue | knee joint | OA rat model | Inflammation | Zhang et al. (2020c) |
| miR-483-5p | Up | Matn3, Timp2 | Cartilage tissue | knee joint | OA mouse model | cartilage degradation | Wang et al. (2017b) |
| miR-340-5p | Up | FMOD | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Zhang et al. (2018c) |
| miR-195 | Up | PTHrP | Cartilage tissue | knee joint | OA rat model | Apoptosis | Cao et al. (2019b) |
| miR-195-5p | Up | REGγ | Cartilage tissue | —— | OA mouse model | Apoptosis | Shu et al. (2019) |
| miR-23b-3p | Up | COL11A2 | Cartilage tissue | knee joint | OA mouse model | inflammation | Yang et al. (2019b) |
| miR-448 | Up | matrilin-3 | Cartilage tissue | knee joint | OA cell model | Apoptosis, cartilage degradation | Yang et al. (2018b) |
| miR-203 | Up | ERα | Blood, Cartilage tissue | —— | OA rat model | cartilage degradation | Tian et al. (2019) |
| Up | MCL-1 | Cartilage tissue | —— | OA cell model | Apoptosis, cartilage degradation, Inflammation | Zhao et al. (2017) | |
| miR-203a | Up | Smad3 | Cartilage tissue | knee joint | OA cell model | cartilage degradation, Inflammation | An et al. (2020) |
| miR-21 | Up | GDF-5 | Cartilage tissue | —— | OA cell model | Apoptosis | Zhang et al. (2014) |
| miR-21-5p | Up | FGF18 | Cartilage tissue | knee joint | OA mouse model | Apoptosis, cartilage degradation | Wang et al. (2019e) |
| miR-218-5p | Up | PIK3C2A | Cartilage tissue | knee joint | OA mouse model | cartilage degradation, Apoptosis | Lu et al. (2017) |
| miR-449a | Up | GDF5 | Cartilage tissue | —— | OA cell model | cartilage degradation | Wu et al. (2018a) |
| miR-125b-5p | Up | SYVN1 | Synovial fluid | —— | OA cell model | Apoptosis | Ge et al. (2017) |
| miR-384-5p | Up | SOX9 | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Zhang et al. (2020i) |
| miR-23a-3p | Up | SMAD3 | Cartilage tissue | —— | OA cell model | cartilage degradation | Kang et al. (2016a) |
| miR-139 | Up | MCPIP1 | Cartilage tissue | —— | OA cell model | Apoptosis | Makki and Haqqi, (2015) |
| miR-206 | Up | —— | Cartilage tissue | knee joint | OA cell model | Apoptosis | Ni et al. (2018) |
| miR-382-3p | Up | CX43 | Cartilage tissue | knee joint | OA cell model | Inflammation | Lei et al. (2019) |
| miR-101 | Up | Sox9 | Synovial fluid | knee joint | OA rat model | cartilage degradation | Dai et al. (2015) |
| miR-30a | Up | Sox9 | Cartilage tissue | knee joint | OA cell model | cartilage degradation, Inflammation | Chang et al. (2016) |
| Up | DLL4 | bone marrow | —— | OA rat model | Cell differentiation | Tian et al. (2016) | |
| miR-216b | Up | Smad3 | Cartilage tissue | knee joint | OA cell model | cartilage degradation | He et al. (2017) |
| miR-128a | Up | Atg12 | Cartilage tissue | knee joint | OA rat model | Autophagy | Lian et al. (2018) |
| miR-20a | Up | IkBβ | Cartilage tissue, blood | —— | OA rat model | Inflammation | Zhao and Gong, (2019) |
| miR-136 | Up | Mcl-1 | Cartilage tissue | —— | OA cell model | Apoptosis, cartilage degradation, Inflammation | Wang and Kong, (2018) |
| miR-130b | Up | SOX9 | Bone marrow, Cartilage tissue | —— | OA rat model | Cell differentiation | Zhang et al. (2021c) |
| miR-132-3p | Up | ADAMTS-5 | Bone marrow, Cartilage tissue | —— | OA rat model | Cell differentiation | Zhou et al. (2018c) |
| miR-1246 | Up | HNF4γ | Cartilage tissue | —— | OA mouse model | Inflammation | Wu et al. (2017a) |
| miR-9 | Up | —— | Cartilage tissue | —— | OA mouse model | Apoptosis, cartilage degradation, Inflammation | Zhang et al. (2019e) |
| miR-222 | Up | HDAC-4 | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Song et al. (2015) |
| miR-155 | Up | PIK3R1 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Fan et al. (2020) |
| miR-33a | Up | Smad7 | Cartilage tissue | knee joint | OA cell model | Cell differentiation | Kostopoulou et al. (2015) |
| miR-93 | Down | TLR4 | Cartilage tissue, Synovial fluid | knee joint | OA mouse model | Apoptosis, inflammation | Ding et al. (2019) |
| miR-93-5p | Down | TCF4 | Cartilage tissue | knee joint | OA rat model | Apoptosis | Xue et al. (2019) |
| miR-92a-3p | Down | WNT5A | Bone marrow, Cartilage tissue | —— | OA mouse model | cartilage degradation | Mao et al. (2018b) |
| miR-92a-3p | Down | HDAC2 | Bone marrow, Cartilage tissue | —— | OA cell model | cartilage degradation | Mao et al. (2017b) |
| miR-92a-3p | Down | ADAMTS-4, ADAMTS-5 | Cartilage tissue | knee joint | OA cell model | cartilage degradation, Inflammation | Mao et al. (2017a) |
| miR-107 | Down | TRAF3 | Cartilage tissue | knee joint | OA rat model | Autophagy and apoptosis | Zhao et al. (2019b) |
| miR-101a-3p | Down | UBE2D1, FZD4 | Cartilage tissue | —— | OA rat model | Apoptosis | Mao et al. (2021a) |
| miR-671 | Down | —— | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Zhang et al. (2019a) |
| miR-671-3p | Down | TRAF3 | Cartilage tissue | knee joint | OA cell model | cartilage degradation, Inflammation, Apoptosis | Liu et al. (2019e) |
| miR-140 | Down | —— | Synovial fluid, Cartilage tissue | knee joint | OA cell model | cartilage degradation | Si et al. (2016) |
| Down | RALA | Cartilage tissue | knee joint | OA cell model | Cell differentiation | Karlsen et al. (2014) | |
| Down | IGFBP-5 | Cartilage tissue | knee joint | OA cell model | cartilage degradation | Tardif et al. (2009) | |
| Down | IGFBP5 | Cartilage tissue | knee joint | OA cell model | inflammation | Karlsen et al. (2016) | |
| Down | ADAMTS5 | Cartilage tissue | —— | OA mouse model | cartilage degradation | Miyaki et al. (2010) | |
| Down | MMP-13 | Cartilage tissue | —— | OA cell model | cartilage degradation | (Liang et al., 2012; Liang et al., 2016) | |
| Down | SMAD1 | Cartilage tissue | —— | OA cell model | Apoptosis | Li et al. (2018a) | |
| Down | NFAT3, SMAD3 | Cartilage tissue | knee joint | OA cell model | inflammation | Tardif et al. (2013) | |
| miR-140-3p | Down | CXCR4 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Ren et al. (2020) |
| miR-140-5p | Down | SMAD3 | —— | —— | OA mouse model | inflammation | Li et al. (2019d) |
| Down | HMGB1 | Cartilage tissue | knee joint | OA cell model | inflammation | Wang et al. (2020d) | |
| Down | FUT1 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Wang et al. (2018b) | |
| miR-33b-3p | Down | DNMT3A | Cartilage tissue | knee joint | OA cell model | Apoptosis | Ma et al. (2019a) |
| miR-766-3p | Down | AIFM1 | Cartilage tissue | —— | OA cell model | cartilage degradation | Li et al. (2020g) |
| miR-26a | Down | —— | Cartilage tissue | knee joint | OA rat model | inflammation | Zhao et al. (2019c) |
| miR-26a/miR-26b | Down | FUT4 | Cartilage tissue | knee joint | OA rat model | Apoptosis | Hu et al. (2018a) |
| miR-26a-5p | Down | PTGS2 | Bone marrow, Synovial fluid | —— | OA rat model | Apoptosis, Inflammation | Jin et al. (2020) |
| miR-377-3p | Down | ITGA6 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Tu et al. (2020) |
| miR-410-3p | Down | HMGB1 | Synovial fluid, Cartilage tissue | knee joint | OA mouse model | Apoptosis, Inflammation | Pan et al. (2020) |
| miR-142-3p | Down | HMGB1 | Cartilage tissue | knee joint | OA mouse model | Apoptosis, Inflammation | Wang et al. (2016c) |
| miR-210 | Down | HIF-3α | Cartilage tissue | knee joint | OA cell model | Apoptosis, cartilage degradation | Li et al. (2016) |
| Down | DR6 | Cartilage tissue | knee joint | OA rat model | Apoptosis, Inflammation | Zhang et al. (2015) | |
| miR-122 | Down | SIRT1 | Cartilage tissue | knee joint | OA cell model | cartilage degradation | Bai et al. (2020a) |
| miR-337-3p | Down | PTEN | Cartilage tissue | knee joint | OA cell model | Apoptosis | Huang et al. (2017) |
| miR-129-3p | Down | CPEB1 | Cartilage tissue | knee joint | OA rat model | Apoptosis | Chen et al. (2020d) |
| miR-675-3p | Down | GNG5 | Cartilage tissue | knee joint | OA cell model | Apoptosis, cartilage degradation | Shen et al. (2020b) |
| miR-132 | Down | PTEN | Cartilage tissue, Blood | —— | OA rat model | Apoptosis | Zhang et al. (2021d) |
| miR-137 | Down | TCF4 | Cartilage tissue | knee joint | OA rat model | Apoptosis, inflammation | Wang et al. (2020a) |
| miR-320c | Down | β-catenin | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Hu et al. (2019a) |
| miR-29a | Down | Bax | Cartilage tissue | —— | OA cell model | Apoptosis | Miao et al. (2019) |
| Down | VEGF | Synovial fluid | knee joint | OA cell model | cartilage degradation, Inflammation | Ko et al. (2017) | |
| miR-193b-3p | Down | MMP-19 | Cartilage tissue | knee joint | OA cell model | Inflammation | Chang et al. (2018) |
| miR-193b-3p | Down | HDAC3 | Cartilage tissue | knee joint | OA mouse model | cartilage degradation | Meng et al. (2018) |
| miR-193b-5p | Down | HDAC7 | Cartilage tissue | knee joint, hip joint | OA cell model | Inflammation | Zhang et al. (2019b) |
| miR-136-5p | Down | ELF3 | Bone marrow, Cartilage tissue | —— | OA mouse model | Apoptosis, cartilage degradation | Chen et al. (2020e) |
| miR-374a-3p | Down | WNT5B | —— | —— | OA cell model | Apoptosis | Shi and Ren, (2020) |
| miR-19b-3p | Down | GRK6 | Cartilage tissue | knee joint, hip joint | OA cell model | cartilage degradation, Inflammation | Duan et al. (2019a) |
| miR-221-3p | Down | SDF1/CXCR4 | Cartilage tissue | knee joint | OA cell model | cartilage degradation | Zheng et al. (2017) |
| miR-502-5p | Down | TRAF2 | Cartilage tissue | knee joint, hip joint | OA cell model | cartilage degradation, Inflammation | Zhang et al. (2016b) |
| miR-31 | Down | CXCL12 | Cartilage tissue | —— | OA cell model | Apoptosis | Dai et al. (2019) |
| miR-488 | Down | ZIP-8 | Cartilage tissue | knee joint | OA mouse model | cartilage degradation | Song et al. (2013) |
| miR-125b | Down | ADAMTS-4 | Cartilage tissue | knee joint | OA cell model | —— | Matsukawa et al. (2013) |
| miR-181c | Down | NEAT1 | Synovial fluid | —— | OA cell model | Apoptosis, Inflammation | Wang et al. (2017d) |
| miR-615-3p | Down | —— | bone marrow | —— | OA rat model | Inflammation | Zhou et al. (2018a) |
| miR-211-5p | Down | Fibulin-4 | Cartilage tissue | —— | OA rat model | cartilage degradation, Inflammation | Liu and Luo, (2019) |
| miR-19a | Down | SOX9 | Cartilage tissue | knee joint | OA cell model | Apoptosis | Yu and Wang, (2018) |
| miR-503-5p | Down | SGK1 | Cartilage tissue | knee joint | OA rat model | Apoptosis, Inflammation | Wang et al. (2021b) |
| miR-33 | Down | CCL2 | Cartilage tissue | —— | OA mouse model | Inflammation | Wei et al. (2016) |
| miR-27 | Down | Leptin | Cartilage tissue | —— | OA rat model | Inflammation | Zhou et al. (2017) |
| miR-186 | Down | SPP1 | Cartilage tissue | —— | OA mouse model | Apoptosis | Lin et al. (2019) |
| miR-149 | Down | TAK1 | Cartilage tissue | —— | OA cell model | Inflammation | Chen et al. (2018a) |
| miR-204-5p | Down | Runx2 | Cartilage tissue | knee joint | OA rat model | Apoptosis | Cao et al. (2018a) |
| miR-128-3p | Down | WISP1 | Cartilage tissue | knee joint | OA cell model | Apoptosis, cartilage degradation, Inflammation | Chen and Li, (2020) |
| miR-320 | Down | MMP-13 | Cartilage tissue | —— | OA mouse model | Inflammation | Meng et al. (2016) |
| miR-558 | Down | COX-2 | Cartilage tissue | knee joint | OA cell model | Inflammation | Park et al. (2013) |
| miR-634 | Down | PIK3R1 | Cartilage tissue | —— | OA cell model | cartilage degradation | Cui et al. (2016) |
| miR-24 | Down | C-myc | Cartilage tissue | knee joint | OA rat model | Apoptosis | Wu et al. (2018b) |
| miR-365 | Down | HIF-2α | Cartilage tissue | knee joint | OA cell model | Apoptosis | Hwang et al. (2017) |
| miR-126-3p | Down | —— | Synovial fluid | knee joint | OA rat model | cartilage degradation, Inflammation | Zhou et al. (2021d) |
| miR-520c-3p | Down | GAS2 | Cartilage tissue | hip joint | OA cell model | Apoptosis, cartilage degradation | Peng et al. (2021) |
| miR-1207-5p | Down | CX3CR1 | Cartilage tissue | —— | OA cell model | Apoptosis, cartilage degradation | Liu et al. (2020b) |
| miR-152 | Down | TCF-4 | Cartilage tissue | knee joint, hip joint | OA rat model | Apoptosis | Wan et al. (2020) |
| miR-296-5p | Down | TGF-β | Cartilage tissue | knee joint | OA cell model | Apoptosis, cartilage degradation | Cao et al. (2020) |
| miR-373 | Down | P2X7R | Cartilage tissue, Blood | —— | OA cell model | cartilage degradation, Inflammation | Zhang et al. (2018e) |
| miR-25-3p | Down | IGFBP7 | Cartilage tissue | —— | OA rat model | Apoptosis | He and Deng, (2021) |
| miR-95-5p | Down | HDAC2, HDAC8 | Bone marrow, Cartilage tissue | —— | OA cell model | cartilage degradation | Mao et al. (2018a) |
| miR-181a | Down | GPD1L | Cartilage tissue | knee joint | OA cell model | Apoptosis | Zhai et al. (2017) |
| miR-411 | Down | HIF-1α | Cartilage tissue | —— | OA cell model | autophagy | Yang et al. (2020b) |
| miR-98 | Down | Bcl-2 | Cartilage tissue | —— | OA mouse model | Apoptosis | Wang et al. (2017c) |
| Down | Bcl-2 | Cartilage tissue | —— | OA rat model | cartilage degradation、Apoptosis | Wang et al. (2016b) | |
| Down | —— | Cartilage tissue | knee joint | OA rat model | Apoptosis | Wang et al. (2016a) | |
| miR-125b-5p | Down | TRAF6 | Cartilage tissue | knee joint, hip joint | OA cell model | Inflammation | Rasheed et al. (2019) |
| miR-27a | Down | TLR4 | Cartilage tissue | knee joint, hip joint | OA rat model | cartilage degradation、Inflammation | Qiu et al. (2019) |
| Down | NF-κB | Cartilage tissue | knee joint | OA rabbit model | Apoptosis, Inflammation | Zhang et al. (2019c) | |
| Down | PLK2 | Cartilage tissue | knee joint | OA rat model | Apoptosis | Liu et al. (2019c) | |
| miR-15a-5p | Down | PTHrP | Cartilage tissue | knee joint | OA cell model | Apoptosis | Duan et al. (2019b) |
| miR-9-5p | Down | Tnc | Cartilage tissue | knee joint, hip joint | OA mouse model | Apoptosis | Chen et al. (2019a) |
| miR-145 | Down | BNIP3 | Cartilage tissue | knee joint | OA mouse model | Apoptosis | Wang et al. (2020c) |
| miR-145 | Down | MKK4 | Cartilage tissue | —— | OA rat model | cartilage degradation | Hu et al. (2017) |
| miR-145 | Down | TNFRSF11B | Cartilage tissue | knee joint | OA cell model | Apoptosis | Wang et al. (2017a) |
Abbreviations: SPHK1, sphingosine kinase-1; SOX9, SRY-Box 9; DLL1, delta-like protein 1; SIRT1, silent information regulator 1; SMAD2, SMAD, family member 2; PTHLH, parathyroid hormone-like hormone; Wnt5a, wnt family member 5A; PGRN, progranulin; MAP, 1B, microtubule associated protein 1B; Gpc1, glypican 1; Ppp3r2, calcineurin B, type II, protein phosphatase 3; TXNIP, thioredoxin-interacting protein; A2M, alpha-2-macroglobulin; KDM4B, lysine demethylase 4B; Matn3, cartilage matrix protein matrilin 3; Timp2, tissue inhibitor of metalloproteinase 2; PTHrP, parathyroid hormone-related protein; MCL-1, myeloid cell leukemia-1; GDF-5, growth differentiation factor 5; FGF18, fibroblast growth factor 18; GDF5, growth differentiation factor 5; SYVN1, synoviolin 1; CX43, connexin 43; TLR4, toll-like receptor 4; TCF4, transcription factor 4; HDAC2, histone deacetylase 2; ADAMTS-4, aggrecanase-1; TRAF3, TNF, receptorassociated factor 3; UBE2D1, ubiquitin-conjugating enzyme 2D1; FZD4, frizzled class receptor 4; MMP-13, matrix metalloproteinase-13; FUT1, fucosyltransferase 1; DNMT3A, DNA, methyltransferase 3A; DR6, death receptor 6; GNG5, G-protein subunit g 5; HDAC7, histone deacetylase 7; HDAC3, histone deacetylase 3; ELF3, E74-like factor 3; TRAF2, TNF, receptorassociated factor 2; SPP1, phosphoprotein 1; COX-2, cyclooxygenase-2; HIF-2α, hypoxia-inducible factor-2α; GAS2, Growth arrest-specific 2; P2X7R, P2X7 receptor; IGFBP7, insulin-like growth factor-binding protein 7; HIF-1α, hypoxia-inducible factor 1 alpha; Bcl-2, B-cell lymphoma 2; PLK2, polo-like kinase 2.
lncRNAs and OA
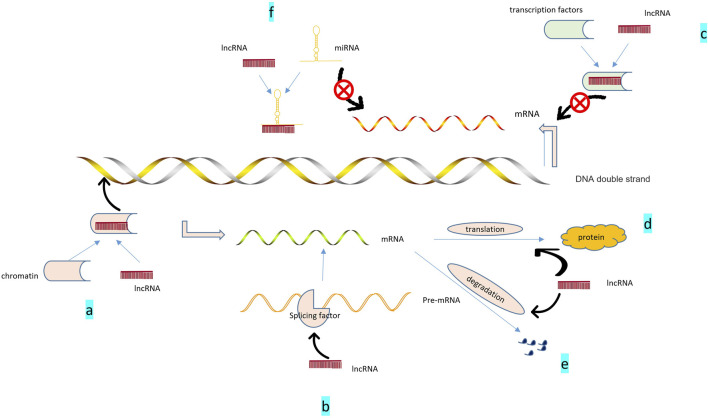
lncRNAs are ncRNAs with a length of more than 200 nucleotides that have little or no protein-coding potential, and account for more than 80% of total lncRNAs(Ponting et al., 2009). At first, lncRNA was considered the “noise” of genome transcription, with no biological function, and its mechanism of action was only in situ regulation, through recruitment and formation of chromatin modification complexes [such as IGF2RRNA antisense (AIR), XIST] to silence the transcription of neighboring genes. As more detection techniques were applied to RNA studies, such as microarray, RNA sequencing (RNA-seq), Northern blot, and real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) (Zhu et al., 2013), more biological functions of lncRNAs gradually being discovered. Recent studies have discovered several mechanisms of action of lncRNA, which can interact with proteins, DNA, and RNA to regulate many biological processes (Zhu et al., 2013). For example, lncRNA MALAT1 acts on miR-150-5P and AKT3 to regulate cell proliferation and apoptosis (Zhang et al., 2019g), thus participating in the growth and development of the body and the pathological process of diseases (Kopp and Mendell, 2018) (Figure 2).
FIGURE 2.
Role of lncRNAs: 1. Epigenetic regulation: (A). lncRNA recruits chromatin remodeling and modification complexes to specific sites; regulates DNA or RNA methylation status, chromosome structure; and promotes the expression of related genes.2. Transcriptional regulation: (B). lncRNA can help generate mature mRNA by promoting the binding of pre-mRNA to alternative splicing factors; (C). ncRNA binding with transcription factors can inhibit the activity of target genes and inhibit their gene expression. 3. Post-transcriptional regulation: (D). Participation in mRNA translation; (E). Involvement in mRNA degradation. 4. Regulation of miRNA: (F). ncRNA can act as sponges of miR-compete for miR and alleviate the inhibition of target genes.
lncRNA is closely related to cell growth, differentiation, and senescence. In addition, lncRNA has a special relationship with some human diseases, such as cardiovascular diseases (Huang, 2018), nervous system diseases (Zhang et al., 2019f), and immune-mediated diseases (Zhou et al., 2018b). In the recently updated database of lncRNA-related diseases, more than 200,000 lncRNAs have been recorded in their association with diseases (Bao et al., 2019).
lncRNA can regulate chondrocyte proliferation and apoptosis, inflammatory response, and extracellular matrix degradation, and promote the repair and stability of articular cartilage. Recent studies have shown an essential relationship between some changes or disorders of lncRNAs and the occurrence and development of OA. There are many studies to detect the expression of lncRNA in OA patients. Yang et al. (2021a) examined the lncRNA profiles of patients with OA and healthy individuals by RNA sequencing. They found that 25 lncRNAs are differentially expressed in patients with OA compared with the control group. Through microarray analysis, Xing et al. (2014) detected the expression of lncRNA in KOA cartilage and normal cartilage and further verified it by real-time polymerase chain reaction (RT-PCR). They found that the expression of 121 lncRNAs in KOA is different from normal cartilage: 73 up-regulated lncRNAs and 48 down-regulated lncRNAs. Among the up-regulated lncRNAs, HOTAIR is the most up-regulated. Pearson et al. (2016) separated OA chondrocytes through collagenase digestion and analyzed lncRNA expression through RNA sequencing (RNAseq) and qPCR. Finally, 983 lncRNAs were identified in OA chondrocytes. A total of 125 differentially expressed lncRNAs were identified after interleukin-1B (IL-1B) stimulation. Through microarray and qPCR analysis, Liu et al. (2014b) compared the expression of lncRNA in OA cartilage and normal cartilage, and found 152 differentially expressed lncRNAs in OA cartilage. Compared with normal cartilage, 82 increased lncRNAs and 70 decreased lncRNAs were in OA cartilage. Using mRNA and lncRNA microarray analysis, Zhang et al. (2020a) found that 990 lncRNAs were different in OA chondrocytes compared with the control group: 666 up-regulated, 324 down-regulated. In addition, 546 mRNAs had a different expression: 419 up-regulated, 127 down-regulated. Six lncRNAs (ENST00000606283.1, ENST00000436872.1, ENST00000488584.1, ENST00000603682.1, XR-245446.2, and ENST00000605586.1) were tested by qPCR. The results were consistent with the test results. In summary, through the detection of lncRNA expression levels in the chondrocytes of OA patients and healthy individuals, we can finally find that there are differences in the expression of a variety of lncRNAs. In addition to the lncRNAs of appeal, several lncRNAs are closely related to the progress of OA, as shown in Table 2.
TABLE 2.
Functional characterization of the lncRNAs in OA.
| lncRNA | Expression | Target genes | Related genes | Tissue/cell source | Region | Model | Functions | Reference |
|---|---|---|---|---|---|---|---|---|
| ANRIL | Up | miRNA-122-5p | DUSP4 | Cartilage tissue, synoviocytes | knee joint | OA cell model | Cell proliferation and apoptosis | Li et al. (2019e) |
| CASC2 | Up | —— | IL-17 | Blood, Synovial fluid, chondrocyte | —— | OA cell model | Cell proliferation and apoptosis | Huang et al. (2019d) |
| CIR | Up | —— | —— | Cartilage tissue | hip joint | OA rat model | Cell autophagy | Wang et al. (2018a) |
| Up | miR-130a | Bim | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Lu et al. (2018) | |
| Up | miR-27b | —— | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Li et al. (2017b) | |
| HOTAIR | Up | —— | MMP | Cartilage tissue, Synovial fluid | temporomandibular | OA rabbit model | Cell proliferation and apoptosis | Zhang et al. (2016a) |
| Up | —— | WIF-1 | SW1353 cells | knee joint | OA cell model | Degradation of extracellular matrix | Yang et al. (2020c) | |
| Up | —— | —— | Synovial fluid, Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Liang et al. (2021) | |
| Up | —— | —— | Synovial tissue | knee joint | 0A rat model | Cell proliferation and apoptosis, inflammation | Mao et al. (2019b) | |
| Up | miR-20b | PTEN | Cartilage tissue | knee joint | OA mouse model | Cell proliferation and apoptosis, Degradation of extracellular matrix | Chen et al. (2020f) | |
| Up | miR-130a-3p | —— | Cartilage tissue | —— | OA human model | Cell proliferation and apoptosis, Cell autophagy | He and Jiang, (2020) | |
| Up | miR-17-5p | FUT2 | Cartilage tissue | knee joint | 0A rat model | Cell proliferation and apoptosis, Degradation of extracellular matrix | Hu et al. (2018b) | |
| LOC101928134 | Up | —— | IFNA1 | Synovial fluid, Cartilage tissue | knee joint | 0A rat model | Cell proliferation and apoptosis, Degradation of extracellular matrix | Yang et al. (2019a) |
| LINC00671 | Up | —— | Smurf2 | Cartilage tissue | knee joint | OA mouse model | Degradation of extracellular matrix | Chen and Xu, (2021) |
| TM1P3 | Up | —— | —— | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Li et al. (2019g) |
| GAS5 | Up | miR-144 | mTOR | Cartilage tissue | knee joint | 0A rat model | Cell proliferation and apoptosis | Ji et al. (2021) |
| Up | miR-137 | —— | Blood, cartilage tissues | —— | OA cell model | Cell proliferation and apoptosis | Gao et al. (2020) | |
| Up | miR-21 | —— | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Song et al. (2014) | |
| SAMD14-4 | Up | —— | COL1A1, COL1A2 | Cartilage tissue | knee joint | OA cell model | inflammation | Zhang et al. (2019d) |
| KLF3-AS1 | Up | miR-206 | GIT1 | Cartilage tissue | knee joint | OA mouse model | Cell proliferation and apoptosis | Liu et al. (2018) |
| CTBP1-AS2 | Up | miR-130a | —— | Cartilage tissue, Synovial fluid | knee join、hip join | OA cell model | Cell proliferation and apoptosis | Zhang et al. (2020d) |
| H19 | Up | miR-140-5p | —— | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Yang et al. (2020a) |
| Up | miR-675 | —— | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Steck et al. (2012) | |
| Up | miR-106b-5p | TIMP2 | Cartilage tissue, Synovial fluid | knee joint | OA cell model | Degradation of extracellular matrix | Tan et al. (2020) | |
| Up | miR-29a-3p | FOS | Astrocytes | —— | OA rat model | inflammation | Yang et al. (2021b) | |
| PART1 | Up | miR-373-3p | SOX4 | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis, Degradation of extracellular matrix | Zhu and Jiang, (2019) |
| LOXL1-AS1 | Up | miR-423-5p | KDM5C | Cartilage tissue | knee join, hip join | OA cell model | Cell proliferation and apoptosis | Chen et al. (2020c) |
| MALAT1 | Up | miR-145 | ADAMTS5 | Cartilage tissue | —— | OA cell model | Degradation of extracellular matrix | Liu et al. (2019a) |
| Up | miR-146a-PI3K | —— | Cartilage tissue | —— | OA rat model | Degradation of extracellular matrix | Li et al. (2020d) | |
| TUG1 | Up | miR-195 | MMP-13 | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Tang et al. (2018b) |
| Up | miR-320c | MMP-13 | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix, Cell proliferation and apoptosis | Han and Liu, (2021) | |
| XIST | Up | miR-211 | CXCR4 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Li et al. (2018b) |
| Up | miR-149-5p | DNMT3A | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Liu et al. (2020c) | |
| Up | miR-1277-5p | —— | Cartilage tissue | knee join, hip join | OA rat model | Degradation of extracellular matrix | Wang et al. (2019d) | |
| FOXD2-AS1 | Up | miR-27a-3p | TLR4 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Wang et al. (2019f) |
| Up | miR-206 | CCND1 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Cao et al. (2018b) | |
| NEAT1 | Up | miR-543 | PLA2G4A | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Xiao et al. (2021) |
| Up | miR-16-5p | —— | ATDC5 | knee joint | OA cell model | Cell proliferation and apoptosis | Li et al. (2020a) | |
| Up | miR-193a-3p | SOX5 | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Liu et al. (2020a) | |
| IGHCγ1 | Up | miR-6891-3p | TLR4 | PBMCs | —— | OA cell model | inflammation | Zhang et al. (2020g) |
| LINC00511 | Up | miR-150-5p | SP1 | ATDC5 | —— | OA cell model | Cell proliferation and apoptosis | Zhang et al. (2020m) |
| PVT1 | Up | miR-488-3p | —— | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Li et al. (2017c) |
| Up | miR-27b-3p | TRAF3 | Cartilage tissue | —— | OA cell model | inflammation | Lu et al. (2020) | |
| Up | miR-26b | —— | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Ding et al. (2020) | |
| Up | miR-149 | —— | Cartilage tissue | knee joint | OA cell model | inflammation | Zhao et al. (2018) | |
| Up | miR-211-3p | —— | SW982 cells, Chondrocytes | —— | OA rat model | Cell proliferation and apoptosis | Xu et al. (2020) | |
| CASC19 | Up | miR-152-3p | DDX6 | Cartilage tissue | —— | OA cell model | inflammation | Zhou et al. (2021a) |
| CHRF | Up | miR-146a | —— | ATDC5 | —— | OA cell model | inflammation | Yu et al. (2019a) |
| HOTTIP | Up | miR-663a | —— | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | He et al. (2021b) |
| Up | miR-455-3p | CCL3 | Chondrocytes, Bone marrow | knee join, hip join | OA cell model | Degradation of extracellular matrix | Mao et al. (2019a) | |
| DANCR | Up | miR-216a-5p | JAK2, STAT3 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Zhang et al. (2018b) |
| Up | miR-1275 | MMP-13 | SFMSCs, Synovial fluid | —— | OA cell model | Cell proliferation and apoptosis | Fang et al. (2019) | |
| Up | miR-577 | —— | Cartilage tissue | knee join, hip join | OA cell model | Cell proliferation and apoptosis | Fan et al. (2018) | |
| TNFSF10 | Up | miR-376-3p | FGFR1 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Huang et al. (2019a) |
| ARFRP1 | Up | miR-15a-5p | TLR4 | Cartilage tissue | knee joint | OA cell model | inflammation | Zhang et al. (2020b) |
| LINC00461 | Up | miR-30a-5p | —— | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Zhang et al. (2020n) |
| BLACAT1 | Up | miR-142-5p | —— | BMSCs, Bone marrow | —— | OA rat model | Cell proliferation and apoptosis | Ji et al. (2020) |
| MCM3AP-AS1 | Up | miR-1423p | HMGB1 | Synovial fluid, chondrocyte | knee join, hip join | OA cell model | Cell proliferation and apoptosis | Gao et al. (2019b) |
| MCM3AP-AS1 | Down | miR-138-5p | SIRT1 | Cartilage tissue | knee joint | OA cell model | inflammation | Shi et al. (2021a) |
| PCAT-1 | Up | miR-27b-3p | —— | Cartilage tissue | knee join, hip join | OA cell model | Cell proliferation and apoptosis | Zhou et al. (2021c) |
| PMS2L2 | Up | miR-203 | —— | ATDC5 | —— | OA cell model | inflammation | Li et al. (2019f) |
| LINC01534 | Up | miR-140-5p | —— | Cartilage tissue | knee joint | OA cell model | inflammation | Wei et al. (2019) |
| MIR22HG | Up | miR-9-3p | ADAMTS5 | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Long et al. (2021) |
| PCGEM1 | Up | miR-770 | —— | Synovial fluid | —— | OA mouse model | Cell proliferation and apoptosis | Kang et al. (2016b) |
| DILC | Down | —— | IL-6 | Blood, Synovial fluid | —— | OA cell model | inflammation | Huang et al. (2019b) |
| PACER | Down | —— | HOTAIR | Blood | —— | OA cell model | Cell proliferation and apoptosis | Jiang et al. (2019) |
| MIR4435-2HG | Down | —— | —— | Blood, Synovial fluid | knee joint | OA cell model | Cell proliferation and apoptosis | Xiao et al. (2019b) |
| HAND2-AS1 | Down | —— | IL-6 | Blood, Synovial fluid | knee joint | OA cell model | inflammation | Si et al. (2021) |
| ANCR | Down | —— | TGF-β1 | Blood | —— | OA cell model | Cell proliferation and apoptosis | Li et al. (2019c) |
| ROR | Down | —— | —— | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Yang et al. (2018c) |
| FAS-AS1 | Down | —— | —— | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Zhu et al. (2018) |
| lncRNA-NR024118 | Down | —— | —— | ATDC5 | —— | OA mouse model | inflammation | Mei et al. (2019) |
| FER1L4 | Down | —— | IL-6 | Blood, Synovial fluid | —— | OA cell model | inflammation | He et al. (2021a) |
| ZFAS1 | Down | —— | —— | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Ye et al. (2018) |
| MEG3 | Down | —— | VEGF | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Su et al. (2015) |
| Down | —— | TRIB2 | Synovial fluid | knee joint | OA cell model | Cell proliferation and apoptosis | You et al. (2019) | |
| Down | miR-361-5p | FOXO1 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Wang et al. (2019a) | |
| Down | miR-16 | SMAD7 | Cartilage tissue | —— | OA rat model | Cell proliferation and apoptosis | Xu and Xu, (2017) | |
| Down | miR-93 | TGFBR2 | Cartilage tissue | knee joint | OA rat model | Degradation of extracellular matrix | Chen et al. (2019b) | |
| MALAT-1 | Down | —— | —— | Cartilage tissue | knee joint | OA rat model | Cell proliferation and apoptosis | Gao et al. (2019a) |
| SNHG7 | Down | miR-34a-5p | SYVN1 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Tian et al. (2020) |
| Down | miR-214-5p | PPARGC1B | Cartilage tissue | knee joint | OA cell model | inflammation | Xu et al. (2021) | |
| SNHG9 | Down | miR-34a | —— | Cartilage tissue, Synovial fluid | knee joint | OA cell model | Cell proliferation and apoptosis | Zhang et al. (2020e) |
| NKILA | Down | miR-145 | SP1 | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Xue et al. (2020) |
| SNHG5 | Down | miR-10a-5p | H3F3B | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Jiang et al. (2020a) |
| Down | miR-26a | SOX2 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Shen et al. (2018) | |
| PART-1 | Down | miR-590-3p | TGFBR2/Smad3 | Cartilage tissue | knee join, hip join | OA cell model | Cell proliferation and apoptosis | Lu et al. (2019) |
| OIP5-AS1 | Down | miR-29b-3p | PGRN | Cartilage tissue | knee joint | OA cell model | inflammation | Zhi et al. (2020) |
| Down | miR-30a-5p | —— | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Qin et al. (2021) | |
| DNM3OS | Down | miR-126 | CHON-001 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | |
| LINC00623 | Down | miR-101 | HRAS | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Lü et al. (2020) |
| ATB | Down | miR-223 | —— | ATDC5 | —— | OA mouse model | inflammation | Ying et al. (2019) |
| HOTAIRM1-1 | Down | miR-125b | BMPR2 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Xiao et al. (2019c) |
| HULC | Down | miR-101 | —— | Cartilage tissue | knee joint | OA cell model | inflammation | Chu et al. (2019) |
| SNHG15 | Down | miR-141-3p | BCL2L13 | Cartilage tissue | knee joint | OA rat model | Cell proliferation and apoptosis | Zhang et al. (2020k) |
| LINC00662 | Down | miR-15b-5p | GPR120 | Cartilage tissue | knee joint | OA rat model | inflammation | Lu and Zhou, (2020) |
| LUADT1 | Down | miR-34a | SIRT1 | Synovial fluid, chondrocytes | knee join, hip join | OA cell model | Cell proliferation and apoptosis | Ni et al. (2020b) |
| UFC1 | Down | miR-34a | —— | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Zhang et al. (2016c) |
Abbreviations: PBMCs, peripheral blood mononuclear cells; MMP, metalloproteinases; WIF-1, Wnt inhibitory factor 1; FUT2, fucosyltransferase 2; KDM5C, lysine demethylase 5C; DNMT3A, DNA, methyltransferase 3A; SIRT1, silent information regulator-1; TRIB2, Tribbles homolog 2; TGFBR2, transforming growth factor β receptor type II; KLF4, Krüppel-like factor 4; PPARGC1B, PPARG, coactivator 1 beta; H3F3B, H3 histone family 3B; Smad3, SMAD, family member 3; PGRN=progranulin; DNM3OS, dynamin 3 opposite strand; HRAS, Harvey rat sarcoma viral oncogene homolog; BMPR2, bone morphogenetic protein receptor 2.
circRNA and OA
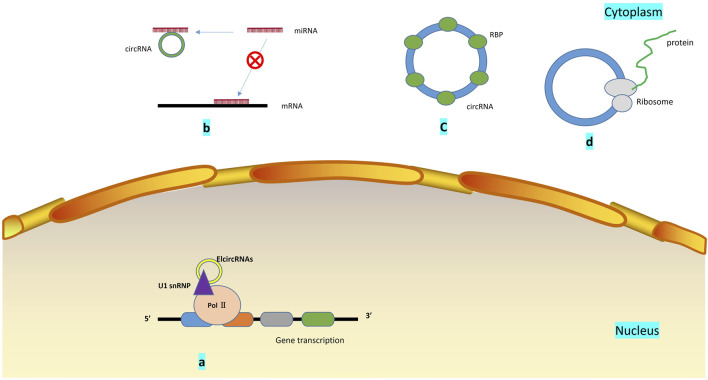
The circRNA molecule is in a closed-loop structure and is not affected by RNA exonuclease. They are mainly in the cytoplasm or stored in exosomes. They are stable and not easily degradable, and widely exist in many eukaryotes. circRNAs are formed by reverse splicing through nonclassical splicing. One model believes that in the transcription of pre-RNA, due to the partial folding of RNA, the originally nonadjacent exons are pulled closer, and exon jumping occurs, resulting in the formation of circular RNA intermediates in the region to be crossed. Moreover, ring RNA molecules composed of exons are formed by lasso splicing. Another model suggests that the reverse complementary sequence located in the intron region leads to intron region pairing mediated reverse splicing, resulting in the formation of circular RNA molecules (Chen and Yang, 2015). To date, the biological functions of circRNAs that have been discovered mainly include interactions with miRNAs(Cao et al., 2019a), binding of regulatory proteins (Zang et al., 2020), transcription of regulatory genes (Zhang, 2020), and coding functions (Lei et al., 2020) (Figure 3). For example, circRNA.33186 increased MMP-13 expression by interacting with miR-127-5p to regulate cell proliferation and apoptosis (Zhou et al., 2019b).
FIGURE 3.
Biological functions of circRNA: (A). Regulation of gene transcription: Elcircrna can interact with small nuclear ribonucleoproteins and bind to RNA polymerase II; (B). miRNA sponge: circRNA contains miRNA binding sites, which can block miRNA binding to mRNA and promote or inhibit the expression of related genes by sponging miRNA; (C). circRNAs bind to mRNA regulatory binding protein, which influences the stability of mRNA, and may change the splicing pattern of circRNA; (D). By being translated by ribosomes and encoding polypeptides, several circRNAs can play a role in regulating and controlling human physiological processes.
Bipartite Network Projection allocates resources according to the known associations between different miRNAs and diseases, entirely using the similarity information of miRNA and diseases to predict various conditions accurately (Chen et al., 2018b). KATZ Measure is a graph-based calculation method, which converts the calculation of the similarity between lncRNA and diseases into the problem of similarity calculation between nodes in heterogeneous networks to predict the correlation between lncRNA and conditions. The integration of the two can recognize the association of circRNA with the disease (Chen, 2015). Through Bipartite Network Projection and KATZ Measure (Zhao et al., 2019a), many circRNAs related to diseases have been discovered, and circRNAs are involved in the diagnosis and treatment of atherosclerosis (Zhang et al., 2018a), cancer (Li et al., 2020f), cardiac hyperplasia (Li et al., 2020e), and other diseases. There are many experimental studies related to circRNA and diseases, and the main research types are cell experiments or animal experiments. Through these experiments, we have found multiple action mechanisms of circRNA on various conditions. For example, circRNA_100367 acts as a signaling molecule that regulates esophageal squamous cell carcinoma through the Wnt3 signaling pathway (Liu et al., 2019b); circRNA_0016624 regulates gene-based expression of interest in osteoporosis patients via sponge miR-98 (Yang et al., 2020d); circRNA_100395 mitigates the progression of breast cancer by directly targeting MAPK6 (Yu et al., 2020).
In addition, several circRNAs participate in the development of OA and the OA of the abnormal expression in various tissues. For example, Xiao et al. (2019a) used illumina sequencing platform to detect circRNA expression in patients with mild and severe KOA. In this paper, 197 differentially expressed circRNAs were identified. Among them, the up-regulation amplitude of Hg38_circ_0007474 is the largest, and the down-regulation amplitude of hg38_circ_0000118 is the largest. Further analysis of the three circRNAs selected from hsa_circ_0045714, hsa_circ_0005567, and hsa_circ_0002485 found that all three circRNAs can inhibit the function of the corresponding miRNA by serving as a sponge for miRNAs and indirectly promote its downstream process, thereby participating in the development of OA. Wang et al. (2019h) used microarray analysis to screen for circRNA expression in healthy and KOA articular cartilage. They found 1,380 circRNAs differentially expressed in the articular cartilage of knee joints of healthy individuals and patients with OA. Meanwhile, constructing a circRNA-miRNA network verified the ten most likely target genes related to circRNA. It was finally discovered that hsa_circ RNA_003231 might be involved in the occurrence and progression of OA. Zhou et al. (2018e) established OA models in interleukin-1β (IL1β)-treated mouse articular chondrocytes (MACs) to study the expression and function of circRNAs in OA using new sequencing methods and bioinformatic analysis. Compared with the control group, 255 circRNAs were differentially expressed in MACs treated with IL-1 β: 119 up-regulated, 136 down-regulated. Mmu-circRNA-30365 and Mmu-circRNA36866 were two substantially different circRNAs, and their specific expression changes in patients with OA and normal individuals were verified by QRT-PCR. Liu et al. (2016) analyzed circRNA expression between OA and normal cartilage samples by hierarchical clustering analysis and found that compared with normal cartilage, 71 circRNAs were differentially expressed (16 were increased, and 55 were decreased) in OA cartilage. In this study, we focused on the research of circRNA-CER. We found that this circRNA could compete with MMP13 for miR-136 and participate in the degradation of the extracellular matrix of chondrocytes. The above examples fully prove that the expression levels of circRNA in OA patients and healthy individuals are different, and these differentially expressed circRNA has a special relationship with the progression of OA.
Several studies have reported the functions and mechanisms of several circRNAs in OA, but relevant studies are few. Zhou et al. (2018d) established rat OA models, predicted the function of circRNA_ATP9b in rat knee chondrocytes through bioinformatic analysis, and finally found that circRNA_ATP9b regulated the degradation of extracellular matrix through sponge miR-138-5p, thereby controlling the progression of OA. Moreover, circRNA_ATP9b expression was increased, and miR-138-5p expression was down-regulated in IL-1β-induced chondrocytes. circRNA_ATP9b regulated the expression of related genes by targeting miR-138-5p. Li et al. (2017a) analyzed the dual-luciferase reporter genes and found that the transcriptional activity of miR-193b can be inhibited by overexpression of hsa_circ_0045714. Overexpression of hsa_circ_0045714 can also up-regulate the expression of insulin-like growth factor 1 receptor (IGF1R) because IGF1R is a crucial target gene of miR-193b. It is associated with cell proliferation and apoptosis. Further studies on the progression of circRNA in OA are presented in Table 3.
TABLE 3.
Functional characterization of the circRNAs in OA.
| CircRNA | Expression | Target genes | Related genes | Tissue/cell source | Region | Model | Functions | Reference |
|---|---|---|---|---|---|---|---|---|
| CircVCAN | Up | —— | —— | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Ma et al. (2020) |
| hsa_circ_0000448 | Up | —— | —— | Synovial tissues | Temporomandibular joint | OA cell model | Degradation of extracellular matrix | Hu et al. (2019c) |
| hsa_circ_0037658 | Up | —— | —— | Cartilage tissue | —— | OA cell model | Cell autophagy | Sui et al. (2021) |
| hsa_circ_0032131 | Up | —— | —— | Blood | —— | OA cell model | Cell proliferation and apoptosis | Wang et al. (2019g) |
| Up | miR-502-5p | PRDX3 | Cartilage tissue | —— | OA rat model | Cell proliferation and apoptosis | Xu and Ma, (2021) | |
| CircRNA.33186 | Up | miR-127-5p | —— | Cartilage tissue | knee joint | OA mouse model | Cell proliferation and apoptosis | Zhou et al. (2019b) |
| CircRNA_0092516 | Up | miR-337-3p | PTEN | Cartilage tissue | knee joint | OA mouse model | Cell proliferation and apoptosis | Huang et al. (2021) |
| CircGCN1L1 | Up | miR-330-3p | TNF-α | Synovial fluid | Temporomandibular joint | OA rat model | Cell proliferation and apoptosis | Zhu et al. (2020) |
| CircRNA-UBE2G1 | Up | miR-373 | HIF-1a | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Chen et al. (2020b) |
| CircRNA HIPK3 | Up | miR-124 | SOX8 | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Wu et al. (2020) |
| CircTMBIM6 | Up | miR-27a | MMP13 | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Bai et al. (2020b) |
| CircPSM3 | Up | miRNA-296-5p | —— | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Ni et al. (2020a) |
| hsa_circ_0005105 | Up | miR-26a | NAMPT | Cartilage tissue | —— | OA cell model | Degradation of extracellular matrix | Wu et al. (2017c) |
| CircRNA-CDR1as | Up | miRNA-641 | —— | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Zhang et al. (2020j) |
| CircRNA Atp9b | Up | miR-138-5p | —— | Cartilage tissue | knee joint | OA mouse model | Degradation of extracellular matrix | Zhou et al. (2018d) |
| Circ_0116061 | Up | miR-200b-3p | SMURF2 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis, inflammation | Zheng et al. (2021) |
| Circ-BRWD1 | Up | miR-1277 | TRAF6 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis, Degradation of extracellular matrix, inflammation | Guo et al. (2021) |
| Circ-SPG11 | Up | miR-337-3p | ADAMTS5 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis, Degradation of extracellular matrix, inflammation | Liu et al. (2021b) |
| Circ_SLC39A8 | Up | miR-591 | IRAK3 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis, Degradation of extracellular matrix, inflammation | Yu et al. (2021) |
| Circ-PRKCH | Up | miR-140-3p | ADAM10 | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Zhao et al. (2021) |
| CircCDH13 | Up | miR-296-3p | PTEN | Cartilage tissue | hip joint | OA mouse model | Cell proliferation and apoptosis, Degradation of extracellular matrix | Zhou et al. (2021e) |
| Circ-IQGAP1 | Up | miR-671-5p | TCF4 | Cartilage tissue | knee joint, hip joint | OA cell model | Cell proliferation and apoptosis, Degradation of extracellular matrix, inflammation | Xi et al. (2021) |
| Circ_0136,474 | Up | miR-127-5p | MMP-13 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Li et al. (2019i) |
| Circ_RUNX2 | Up | —— | RUNX2 | Blood | —— | OA cell model | Degradation of extracellular matrix | Wang et al. (2021a) |
| CircRSU1 | Up | miR-93-5p | MAP3K8 | Cartilage tissue | knee joint | OA mouse model | Degradation of extracellular matrix | Yang et al. (2021c) |
| CircRNA3503 | Down | —— | —— | Synovial fluid | —— | OA cell model | Degradation of extracellular matrix | Tao et al. (2021) |
| CircPDE4B | Down | —— | RIC8A, MID1 | Cartilage tissue | —— | OA mouse model | Degradation of extracellular matrix | Shen et al. (2021) |
| CircSERPINE2 | Down | miR-1271 | —— | Cartilage tissue | —— | OA cell model | Degradation of extracellular matrix, Cell proliferation and apoptosis | Shen et al. (2019) |
| Down | miR-495 | TGFBR2 | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Zhang et al. (2020h) | |
| CiRS-7 | Down | miR-7 | —— | Cartilage tissue | —— | OA rat model | Inflammation | Zhou et al. (2020a) |
| Down | miR-7 | —— | Blood | —— | OA cell model | Cell proliferation and apoptosis | Zhou et al. (2019a) | |
| CircCDK14 | Down | miR-125a-5p | Smad2 | Cartilage tissue | knee joint | 0A rabbit model | Cell proliferation and apoptosis | Shen et al. (2020a) |
| CircPDE4D | Down | miR-103a-3p | FGF18 | Cartilage tissue | knee joint | OA mouse model | Degradation of extracellular matrix | Wu et al. (2021b) |
| CircRNA_0001236 | Down | miR-3677-3p | Sox9 | Bone marrow, Cartilage tissue | —— | OA mouse model | Degradation of extracellular matrix | Mao et al. (2021b) |
| CircRNA-9119 | Down | miRNA-26a | PTEN | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Chen et al. (2020a) |
| Hsa_circ_0005567 | Down | miR-495 | ATG14 | Cartilage tissue | —— | OA cell model | Cell autophagy and apoptosis | Zhang et al. (2020f) |
| CircRNA-CER | Up | MiR-136 | MMP13 | —— | knee joint | OA cell model | Degradation of extracellular matrix | Liu et al. (2016) |
| CircHYBID | Down | hsa-miR-29b-3p | TGF-β1 | Cartilage tissue | knee joint | OA cell model | Degradation of extracellular matrix | Liao et al. (2021) |
| CircADAMTS6 | Down | miR-431-5p | —— | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Fu et al. (2021) |
| Hsa_circ_0045714 | Down | miR-193b | IGF1R | Cartilage tissue | knee joint | OA cell model | Cell proliferation and apoptosis | Li et al. (2017a) |
| Circ_0020093 | Down | miR-23b | SPRY1 | Cartilage tissue | —— | OA cell model | Degradation of extracellular matrix | Feng et al. (2021) |
| CircANKRD36 | Down | miR-599 | Casz1 | Cartilage tissue | —— | OA cell model | Cell proliferation and apoptosis | Zhou et al. (2021b) |
| CircSLC7A2 | Down | miR-4498 | TIMP3 | Cartilage tissue | —— | OA mouse model | Degradation of extracellular matrix, Cell proliferation and apoptosis, inflammation | Ni et al. (2021) |
Abbreviations: TNF-α, tumor necrosis factor-α; LEF1, lymphoid enhancer-binding factor 1; NAMPT, nicotinamide phosphoribosyltransferase; SMURF2, Smad ubiquitin regulatory factor 2; TRAF6, TNF, receptorassociated factor 6; ADAM10, a-disintegrin and metallopeptidase domain 10; PTEN, phosphatase and tensin homolog; MID1, midline 1; TGFBR2, transforming growth factor-β receptor 2; SPRY1, sprouty 1.
Interactions Between lncRNAs, miRNAs and mRNAs in OA
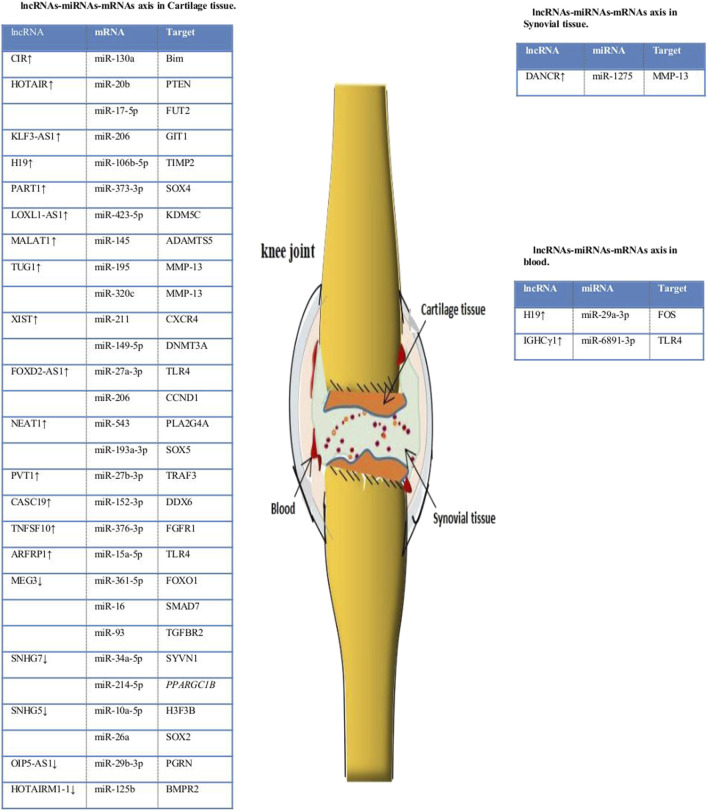
Studies have shown that lncRNA–miRNA–mRNA axis plays a vital control effect in the progression of several diseases, such as cardiovascular disease and cancer (He et al., 2018; Wang et al., 2019c). The mechanisms of interaction of lncRNAs, miRNAs, and mRNAs in various diseases are as follows: 1) The structure of most lncRNAs is similar to mRNAs, and miRNAs binding to mRNAs can reduce the expression of lncRNAs. lncRNA and miRNA compete to bind the 3′-UTR of target gene mRNA, thereby indirectly inhibiting the interaction between miRNA and mRNA. For example, in Alzheimer’s disease, the post-transcriptional regulation of BACE1 involves miR-485-5p, and the specific antisense transcription of BACE1 forms lncRNA-BacE1-As, which compete with lncRNA-Bace1-As to bind to the binding sites of related mRNAs (Faghihi et al., 2010). 2) lncRNAs sponge miRNAs as competitive endogenous RNAs (ceRNAs). lncRNA molecules contain miRNA binding sites, which can bind to miRNA, inhibit the interaction between miRNA and mRNA, improve the expression level of related mRNA, and regulate the expression of target genes. For example, Zhang et al. (2020l) constructed a complete mRNA-LncRNA-miRNA ceRNA regulatory network; lncRNAs ENST00000326237.3, ENST00000399702.5, and ENST00000463727.1 were found to regulate related genes through competitive binding of the same miRNA has-miR-1260a. Kong et al. (2019) demonstrated that lncRNA—CDC6 can further regulate CDC6 expression through direct uptake of miR-215 as a ceRNA. Luan et al.(Luan and Wang, 2018) found that in cervical cancer, XLOC_006390 may act as ceRNA and bind with miR-331-3p and miR-338-3p, thus regulating the expression of genes related to cervical cancer. 3) miRNAs mediate the degradation of lncRNAs. For example, miRNA-150 is the target gene for lncRNA CASC11 in human plasma, and increased concentrations of miRNA-150 decrease the activity of lncRNA CASC11(Luo et al., 2019b). 4) lncRNAs act as miRNAs precursors. For example, Tao et al. (2017) found that miR-869a and miR-160c could be clipped from lncRNAs npc83 and npc521. However, in OA, lncRNA mainly binds to miRNA as a competitive endogenous RNA (ceRNA), inhibiting its target genes’ expression and regulating OA’s progression by regulating cell proliferation, apoptosis, autophagy and extracellular matrix (ECM) degradation (Figure 4).
FIGURE 4.
lncRNA–miRNA–mRNA axis in OA. lncRNA can combine with miRNA to promote the expression of related target genes. PTEN = phosphatase and tensin homolog; FUT2 = fucosyltransferase 2; Timp2 = tissue inhibitor of metalloproteinase 2; KDM5C = lysine demethylase 5C; DNMT3A = DNA methyltransferase 3A; TLR4 = toll-like receptor 4; CCND1 = cyclin D1; KLF4 = Krüppel-like factor 4; SYVN1 = synoviolin 1; PPARGC1B = PPARG coactivator 1 beta; H3F3B = H3 histone family 3B; PGRN = progranulin; DNM3OS = dynamin 3 opposite strand; BMPR2 = bone morphogenetic protein receptor 2.
There are many examples where lncRNA functions as a binding of ceRNA to miRNA in OA. For example, Zhang et al. (2020m) took IL -1β-induced OA chondrocytes as the research object to study the molecular mechanism of LINC00511 in regulating OA. The study found that the expression of LINC00511 was up-regulated, and the lncRNA could be used as a sponge of miR-150-5p and combined with 3′-UTR of transcription factor inhibit the proliferation of chondrocytes, promote apoptosis and degradation of ECM, and finally regulate OA. Liu et al. (2018) established an OA chondrocyte model induced by IL -1β and an OA mouse model caused by collagenase. The experiments were performed in vivo and in vitro at two levels, and the cell state was examined by the CCK-8 method and flow cytometry. Studies have found that KLF3-AS1, as a ceRNA interacting with miR-206, promotes the expression of GIT1 and then promotes the proliferation of chondrocytes and inhibits apoptosis, ultimately alleviating the progression of OA. Likewise, Tian et al. (2020) studied the relationship between SNHG7, miR-34a-5p, and SYVN1 in human chondrocytes. It has been found that in OA tissues, SNHG7 is down-regulated, and SNHG7 can regulate SYVN1 by sponging miR-34a-5p, thereby promoting cell proliferation and inhibiting apoptosis and autophagy. In addition, studies have found that lncRNA XIST is up-regulated in OA articular cartilage. Like a sponge, XIST regulates the target proteins miR-211, miR-17-5p, miR-149-5p, and miR-27b-3p, thereby promoting the proliferation and apoptosis of chondrocytes and finally inducing OA (Li et al., 2018b; Zhu et al., 2021). These results suggest that lncRNAs can act as miRNA sponges in the interaction of lncRNAs, miRNAs, and mRNA in OA.
Interactions Between circRNAs, miRNAs and mRNAs in OA
Currently, research on the mechanism of interactions between circRNAs, miRNAs, and mRNAs is growing (Peng et al., 2020). circRNAs and miRNAs are closely related to the expression of disease-related mRNAs, and interactions between circRNAs, miRNAs, and mRNAs may be involved in the pathological mechanism of OA (Figure 5). At present, research on the interaction mechanism of circRNAs, miRNAs, and mRNAs is not comprehensive. Relevant research has three main types: 1) circRNAs interact with miRNAs. miRNA interacts with mRNA to inhibit mRNA expression. circRNA molecules contain miRNA binding sites, which can sponge miRNA and release miRNA’s inhibitory effect on target genes. For example, Hansen et al. (2013) found that CiRS-7 could sponge miR-7, inhibit the binding of miR-7 and its target genes, and indirectly promote the expression of related mRNA. Other research suggests that hsa_circ_101237, like a sponge for miRNA490-3p, promotes the expression of its target gene MAPK1. In patients with lung cancer, hsa_circ_101237 expression is up-regulated, thereby promoting the proliferation, differentiation, and migration of lung cancer cells (Zhang et al., 2020o). 2) circRNA can regulate the splicing of pre-mRNA, thus affecting the production of protein. 3) circRNA can pair with targeted mRNA directly through local bases. As the circRNA molecule is rich in miRNA binding sites, the circ RNA molecule functions as a miRNA sponge in cells so that the inhibition effect of the miRNA on target genes can be released, and the expression level of the target genes is increased. Therefore, in OA, the interaction mechanism of circRNA, miRNA, and mRNA is mainly circRNA sponging miRNA (Kulcheski et al., 2016). Many circRNA expressions in OA have been changed, and OA is regulated by adsorbing a specific miRNA. For example, hsa_circ_0005567 is down-regulated in OA patients and, by competitively binding to miR-495, terminates Atg14 expression and eventually induces human chondrocyte apoptosis (Zhang et al., 2020f); hsa_circ_0032131 is up-regulated in the human body, and knocking out hsa_circ_0032131 inactivates the STAT3 signaling pathway by sponging miR-502-5p, thereby relieving symptoms of OA in the body (Xu and Ma, 2021); circPSM3 is up-regulated in OA chondrocytes, and its low expression promotes chondrogenesis and OA development. circPSM3 can inhibit OA chondrogenesis by sponging miRNA-296-5p (Ni et al., 2020a). All these results prove the mechanism of circRNA sponge miRNA in osteoarthritis.
FIGURE 5.
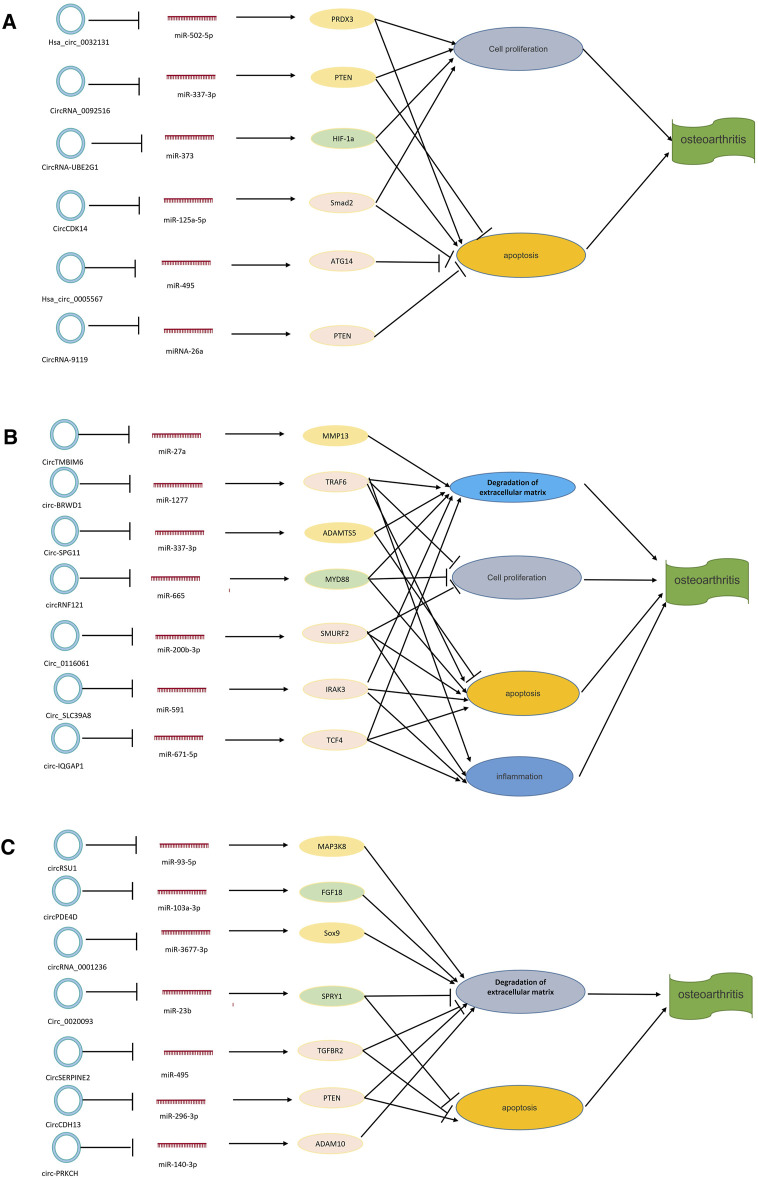
circRNA–miRNA–mRNA axis in OA. circRNAs can combine with miRNAs to promote the expression of related target genes. (A) circRNAs that play a role in cell proliferation and apoptosis. (B) circRNAs that play a role in degradation of the extracellular matrix and apoptosis. (C) circRNAs that play a role in degradation of the extracellular matrix, cell proliferation, apoptosis, and inflammation. NAMPT = nicotinamide phosphoribosyltransferase; MMP13 = matrix metalloproteinase.
Other studies have found interactions between circRNA, miRNA, and mRNA. Shen et al. (2020a) established a rabbit model of OA and studied the role and mechanism of circCDK14 in OA by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and other methods. miR-125a-5p is a downstream target protein of circCDK14, while Smad2 is an mRNA target protein of circCDK14. The mechanism of action of circCDK14 in OA is to down-regulate the expression of Smad2 through the sponge action of miR-125a-5p, resulting in dysfunction of the TGF-β signaling pathway. Chen et al. (2020a) studied the expression and action mechanism of circRNA-9119 in OA patients using bioinformatics prediction and double luciferase reporter gene detection. They found that the expression of circRNA-9119 was down-regulated to provide a sponge effect on miR-26a. At the same time, miR-26a targeted the 3' -UTR of PTEN to promote cell proliferation and inhibit apoptosis. Their results all demonstrated the mechanism of the interaction between circRNAs, miRNAs, and mRNAs in OA.
Clinical Implications
At present, the incidence of OA is very high, and its pathogenesis is still unclear. Studying the specific pathological process and molecular pathway of OA is of great clinical significance (Duan et al., 2020). First, ncRNA can be used to diagnose OA. The expression of many ncRNAs between patients with OA and normal individuals have remarkable differences, which can be seen in humans and animals. For example, Huang et al. (2019c) showed that miRNA-204 and miRNA-211 are decreased in OA, resulting in Runx2 accumulation in multiple types of joint cells and elevated OA markers, and leading to total joint degeneration. Second, several ncRNAs are associated with the prognosis of OA. Rousseau et al. (2020) took the miRNAs in the serum of female patients with KOA as the research objects. He first made a preliminary screening of the research objects through next-generation sequencing and then further analyzed the research objects through RT-QPCR. He found that miR-146A-5p is up-regulated in patients with mild OA, and the prognosis of OA caused by the up-regulation of miRNA is relatively good. In addition, the increase of miR-186-5p in an individual means that the individual might have the imaging changes of OA in the past 4 years, which could be prevented in advance to avoid the occurrence of OA as much as possible. Finally, several ncRNAs can be used for the treatment of OA. Several new drugs can be developed to promote or inhibit several ncRNAs, or change the pathway of action of ncRNA to treat OA. For example, miR-93 is down-regulated in mice with OA and lipopolysaccharide-treated chondrocytes, and acts directly on TLR4 to exert biological effects. miR-93 regulates OA by inhibiting the TLR4/NF-κB pathway, lipopolysaccharide-induced inflammation, and apoptosis. In patients with OA and down-regulation of miR-93, corresponding drugs can be developed to promote its up-regulation and inhibit the aggravation of OA (Ding et al., 2019). These studies indicate that ncRNA has great potential for clinical use in OA. At present, most of the tissue comes from cartilage and is found in the knee joint, and the chondrocytes are cultured to construct the OA cell model. Further research is needed, and more clinical trials must be explored to find biomarkers associated with OA while developing the immense potential of ncRNA.
Conclusion
In recent years, ncRNAs have become one of the most widely studied fields in the development of OA. However, the studies on the regulation of miRNA, lncRNA, and circRNA in diseases and their use as indicators for diagnosis or treatment of OA are still in the early stages, and the mechanism of action ofOA, which may involve multiple signaling pathways, is still unclear. This study reviews theinteractions between lncRNA/circRNA and miRNA in OA. Through high-throughput sequencingtechnologies such as microarray analysis and RNA sequencing, the findings reveal that a large number of miRNA, lncRNA, and circRNA are dysregulated in patients with OA, and the clinical trials related to ncRNA and OA are summarized. The present research progress of ncRNA in the prevention, diagnosis, and treatment of OA is illustrated, which provides a basis for the treatment of OA by ncRNA in the future.
Author Contributions
X-AZ and X-QW: conceptualization, project administration, and funding acquisition. HK, X-AZ, and X-QW: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Innovative Talents Support Program for Universities of Liaoning Province, No. WR2019024; Shanghai Frontiers Science Research Base of Exercise and Metabolic Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abouheif M. M., Nakasa T., Shibuya H., Niimoto T., Kongcharoensombat W., Ochi M. (2010). Silencing microRNA-34a Inhibits Chondrocyte Apoptosis in a Rat Osteoarthritis Model In Vitro . Rheumatology 49 (11), 2054–2060. 10.1093/rheumatology/keq247 [DOI] [PubMed] [Google Scholar]
- Abramoff B., Caldera F. E. (2020). Osteoarthritis. Med. Clin. North America 104 (2), 293–311. 10.1016/j.mcna.2019.10.007 [DOI] [PubMed] [Google Scholar]
- An Y., Wan G., Tao J., Cui M., Zhou Q., Hou W. (2020). Down-regulation of microRNA-203a Suppresses IL-1β-induced Inflammation and Cartilage Degradation in Human Chondrocytes through Smad3 Signaling. Biosci. Rep. 40 (3), BSR20192723. 10.1042/BSR20192723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Chen K., Zhan J., Wu M. (2020a). miR-122/SIRT1 axis Regulates Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Biosci. Rep. 40 (6), BSR20191908. 10.1042/BSR20191908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z. M., Kang M. M., Zhou X. F., Wang D. (2020b). CircTMBIM6 Promotes Osteoarthritis-Induced Chondrocyte Extracellular Matrix Degradation via miR-27a/MMP13 axis. Eur. Rev. Med. Pharmacol. Sci. 24 (15), 7927–7936. 10.26355/eurrev_202008_22475 [DOI] [PubMed] [Google Scholar]
- Bao Z., Yang Z., Huang Z., Zhou Y., Cui Q., Dong D. (2019). LncRNADisease 2.0: an Updated Database of Long Non-coding RNA-Associated Diseases. Nucleic Acids Res. 47 (D1), D1034–D1037. 10.1093/nar/gky905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma J. W., Berenbaum F., Lafeber F. P. (2011). Osteoarthritis: an Update with Relevance for Clinical Practice. The Lancet 377 (9783), 2115–2126. 10.1016/S0140-6736(11)60243-2 [DOI] [PubMed] [Google Scholar]
- Boehme K. A., Rolauffs B. (2018). Onset and Progression of Human Osteoarthritis-Can Growth Factors, Inflammatory Cytokines, or Differential miRNA Expression Concomitantly Induce Proliferation, ECM Degradation, and Inflammation in Articular Cartilage? Int. J. Mol. Sci. 19 (8), 2282. 10.3390/ijms19082282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd E., de Andrés M. C., Sanchez-Elsner T., Oreffo R. O. C. (2017). MiR-146b Is Down-Regulated during the Chondrogenic Differentiation of Human Bone Marrow Derived Skeletal Stem Cells and Up-Regulated in Osteoarthritis. Sci. Rep. 7, 46704. 10.1038/srep46704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D. B., Gallant M. A. (2012). Bone Remodelling in Osteoarthritis. Nat. Rev. Rheumatol. 8 (11), 665–673. 10.1038/nrrheum.2012.130 [DOI] [PubMed] [Google Scholar]
- Cao J., Han X., Qi X., Jin X., Li X. (2018a). miR-204-5p I-nhibits the O-ccurrence and D-evelopment of O-steoarthritis by T-argeting Runx2. Int. J. Mol. Med. 42 (5), 2560–2568. 10.3892/ijmm.2018.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Wang Y., Wang Q., Huang J. (2018b). LncRNA FOXD2-AS1 Regulates Chondrocyte Proliferation in Osteoarthritis by Acting as a Sponge of miR-206 to Modulate CCND1 Expression. Biomed. Pharmacother. 106, 1220–1226. 10.1016/j.biopha.2018.07.048 [DOI] [PubMed] [Google Scholar]
- Cao M., Zhang L., Wang J.-H., Zeng H., Peng Y., Zou J., et al. (2019a). Identifying circRNA-Associated-ceRNA Networks in Retinal Neovascularization in Mice. Int. J. Med. Sci. 16 (10), 1356–1365. 10.7150/ijms.35149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Duan Z., Yan Z., Li Y., Li L., Sun J., et al. (2019b). miR-195 Contributes to Human Osteoarthritis via Targeting PTHrP. J. Bone Miner Metab. 37 (4), 711–721. 10.1007/s00774-018-0973-5 [DOI] [PubMed] [Google Scholar]
- Cao Z., Liu W., Qu X., Bi H., Sun X., Yu Q., et al. (2020). miR-296-5p Inhibits IL-1β-induced Apoptosis and Cartilage Degradation in Human Chondrocytes by Directly Targeting TGF-β1/CTGF/p38MAPK Pathway. Cell Cycle 19 (12), 1443–1453. 10.1080/15384101.2020.1750813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldi A., Zaglia T., Di Mauro V., Carullo P., Viggiani G., Borile G., et al. (2014). MicroRNA-133 Modulates the β 1 -Adrenergic Receptor Transduction Cascade. Circ. Res. 115 (2), 273–283. 10.1161/CIRCRESAHA.115.303252 [DOI] [PubMed] [Google Scholar]
- Chang T., Xie J., Li H., Li D., Liu P., Hu Y. (2016). MicroRNA-30a Promotes Extracellular Matrix Degradation in Articular Cartilageviadownregulation of Sox9. Cell Prolif. 49 (2), 207–218. 10.1111/cpr.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z. k., Meng F. g., Zhang Z. q., Mao G. p., Huang Z. y., Liao W. m., et al. (2018). MicroRNA‐193b‐3p Regulates Matrix Metalloproteinase 19 Expression in Interleukin‐1β‐induced Human Chondrocytes. J. Cel. Biochem. 119 (6), 4775–4782. 10.1002/jcb.26669 [DOI] [PubMed] [Google Scholar]
- Cheleschi S., Tenti S., Mondanelli N., Corallo C., Barbarino M., Giannotti S., et al. (2019). MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-Κb Pathway in Human Osteoarthritic Chondrocytes. Cells 8 (8), 874. 10.3390/cells8080874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Xu Y. (2021). Long Noncoding RNA LINC00671 Exacerbates Osteoarthritis by Promoting ONECUT2-Mediated Smurf2 Expression and Extracellular Matrix Degradation. Int. immunopharmacology 90, 106846. 10.1016/j.intimp.2020.106846 [DOI] [PubMed] [Google Scholar]
- Chen C., Yin P., Hu S., Sun X., Li B. (2020a). Circular RNA-9119 Protects IL-1β-treated Chondrocytes from Apoptosis in an Osteoarthritis Cell Model by Intercepting the microRNA-26a/PTEN axis. Life Sci. 256, 117924. 10.1016/j.lfs.2020.117924 [DOI] [PubMed] [Google Scholar]
- Chen G., Liu T., Yu B., Wang B., Peng Q. (2020b). CircRNA-UBE2G1 Regulates LPS-Induced Osteoarthritis through miR-373/HIF-1a axis. Cell Cycle 19 (13), 1696–1705. 10.1080/15384101.2020.1772545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yang J., Tan Z. (2019a). Upregulation of microRNA‐9‐5p Inhibits Apoptosis of Chondrocytes through Downregulating Tnc in Mice with Osteoarthritis Following Tibial Plateau Fracture. J. Cel Physiol 234 (12), 23326–23336. 10.1002/jcp.28900 [DOI] [PubMed] [Google Scholar]
- Chen J., Wu X. (2019). MicroRNA-103 Contributes to Osteoarthritis Development by Targeting Sox6. Biomed. Pharmacother. 118, 109186. 10.1016/j.biopha.2019.109186 [DOI] [PubMed] [Google Scholar]
- Chen K., Fang H., Xu N. (2020c). LncRNA LOXL1-AS1 Is Transcriptionally Activated by JUND and Contributes to Osteoarthritis Progression via Targeting the miR-423-5p/KDM5C axis. Life Sci. 258, 118095. 10.1016/j.lfs.2020.118095 [DOI] [PubMed] [Google Scholar]
- Chen K., Zhu H., Zheng M.-Q., Dong Q.-R. (2019b). LncRNA MEG3 Inhibits the Degradation of the Extracellular Matrix of Chondrocytes in Osteoarthritis via Targeting miR-93/TGFBR2 Axis. Cartilage 2019, 194760351985575. 10.1177/1947603519855759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-L., Yang L. (2015). Regulation of circRNA Biogenesis. RNA Biol. 12 (4), 381–388. 10.1080/15476286.2015.1020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li Q., Wang J., Jin S., Zheng H., Lin J., et al. (2017). MiR-29b-3p Promotes Chondrocyte Apoptosis and Facilitates the Occurrence and Development of Osteoarthritis by Targeting PGRN. J. Cel. Mol. Med. 21 (12), 3347–3359. 10.1111/jcmm.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Wu S., Wu Y., Chen L., Pang Q. (2018a). MiR-149 Suppresses the Inflammatory Response of Chondrocytes in Osteoarthritis by Down-Regulating the Activation of TAK1/NF-Κb. Biomed. Pharmacother. 101, 763–768. 10.1016/j.biopha.2018.02.133 [DOI] [PubMed] [Google Scholar]
- Chen R., Ye B., Xie H., Huang Y., Wu Z., Wu H., et al. (2020d). miR-129-3p Alleviates Chondrocyte Apoptosis in Knee Joint Fracture-Induced Osteoarthritis through CPEB1. J. Orthop. Surg. Res. 15 (1), 552. 10.1186/s13018-020-02070-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Li B. (2020). MiR-128-3p Post-Transcriptionally Inhibits WISP1 to Suppress Apoptosis and Inflammation in Human Articular Chondrocytes via the PI3K/AKT/NF-κB Signaling Pathway. Cel Transpl. 29, 096368972093913. 10.1177/0963689720939131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Guo D.-Y., Yin T.-L., Yang J. (2021). Non-Coding RNAs Regulate Placental Trophoblast Function and Participate in Recurrent Abortion. Front. Pharmacol. 12, 646521. 10.3389/fphar.2021.646521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. (2015). KATZLDA: KATZ Measure for the lncRNA-Disease Association Prediction. Sci. Rep. 5, 16840. 10.1038/srep16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Shi Y., Xue P., Ma X., Li J., Zhang J. (2020e). Mesenchymal Stem Cell-Derived Exosomal microRNA-136-5p Inhibits Chondrocyte Degeneration in Traumatic Osteoarthritis by Targeting ELF3. Arthritis Res. Ther. 22 (1), 256. 10.1186/s13075-020-02325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Xie D., Wang L., Zhao Q., You Z.-H., Liu H. (2018b). BNPMDA: Bipartite Network Projection for MiRNA-Disease Association Prediction. Bioinformatics (Oxford, England) 34 (18), 3178–3186. 10.1093/bioinformatics/bty333 [DOI] [PubMed] [Google Scholar]
- Chen X., Zhou Z., Zhao Y. (2018c). ELLPMDA: Ensemble Learning and Link Prediction for miRNA-Disease Association Prediction. RNA Biol. 15 (6), 1–12. 10.1080/15476286.2018.1460016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang L., Li E., Zhang G., Hou Y., Yuan W., et al. (2020f). Long-chain Non-coding RNA HOTAIR Promotes the Progression of Osteoarthritis via Sponging miR-20b/PTEN axis. Life Sci. 253, 117685. 10.1016/j.lfs.2020.117685 [DOI] [PubMed] [Google Scholar]
- Cheng F., Hu H., Sun K., Yan F., Geng Y. (2020). miR-455-3p Enhances Chondrocytes Apoptosis and Inflammation by Targeting COL2A1 in the In Vitro Osteoarthritis Model. Biosci. Biotechnol. Biochem. 84 (4), 695–702. 10.1080/09168451.2019.1690974 [DOI] [PubMed] [Google Scholar]
- Chu P., Wang Q., Wang Z., Gao C. (2019). Long Non-coding RNA Highly Up-Regulated in Liver Cancer Protects Tumor Necrosis Factor-Alpha-Induced Inflammatory Injury by Down-Regulation of microRNA-101 in ATDC5 Cells. Int. immunopharmacology 72, 148–158. 10.1016/j.intimp.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Correia de Sousa M., Gjorgjieva M., Dolicka D., Sobolewski C., Foti M. (2019). Deciphering miRNAs' Action through miRNA Editing. Int. J. Mol. Sci. 20 (24), 6249. 10.3390/ijms20246249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Wang S., Cai H., Lin Y., Zheng X., Zhang B., et al. (2016). Overexpression of microRNA-634 Suppresses Survival and Matrix Synthesis of Human Osteoarthritis Chondrocytes by Targeting PIK3R1. Sci. Rep. 6, 23117. 10.1038/srep23117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Zhang X., Hu X., Liu Q., Man Z., Huang H., et al. (2015). Silencing of miR-101 Prevents Cartilage Degradation by Regulating Extracellular Matrix-Related Genes in a Rat Model of Osteoarthritis. Mol. Ther. 23 (8), 1331–1340. 10.1038/mt.2015.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Liu S., Xie X., Ding M., Zhou Q., Zhou X. (2019). MicroRNA-31 P-romotes C-hondrocyte P-roliferation by T-argeting C-X-C M-otif C-hemokine L-igand 12. Mol. Med. Rep. 19 (3), 2231–2237. 10.3892/mmr.2019.9859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L.-B., Li Y., Liu G.-Y., Li T.-H., Li F., Guan J., et al. (2020). Long Non-coding RNA PVT1, a Molecular Sponge of miR-26b, Is Involved in the Progression of Hyperglycemia-Induced Collagen Degradation in Human Chondrocytes by Targeting CTGF/TGF-β Signal Ways. Innate Immun. 26 (3), 204–214. 10.1177/1753425919881778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Wang L., Zhao Q., Wu Z., Kong L. (2019). MicroRNA-93 I-nhibits C-hondrocyte A-poptosis and I-nflammation in O-steoarthritis by T-argeting the TLR4/NF-κB S-ignaling P-athway. Int. J. Mol. Med. 43 (2), 779–790. 10.3892/ijmm.2018.4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou P., He Y., Yu B., Duan J. (2020). Downregulation of microRNA-29b by DNMT3B Decelerates Chondrocyte Apoptosis and the Progression of Osteoarthritis via PTHLH/CDK4/RUNX2 axis. Aging 13 (5), 7676–7690. 10.18632/aging.103778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L., Duan D., Wei W., Sun Z., Xu H., Guo L., et al. (2019a). MiR-19b-3p Attenuates IL-1β Induced Extracellular Matrix Degradation and Inflammatory Injury in Chondrocytes by Targeting GRK6. Mol. Cel Biochem 459 (1-2), 205–214. 10.1007/s11010-019-03563-2 [DOI] [PubMed] [Google Scholar]
- Duan L., Liang Y., Xu X., Wang J., Li X., Sun D., et al. (2020). Noncoding RNAs in Subchondral Bone Osteoclast Function and Their Therapeutic Potential for Osteoarthritis. Arthritis Res. Ther. 22 (1), 279. 10.1186/s13075-020-02374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z.-X., Huang P., Tu C., Liu Q., Li S.-Q., Long Z.-L., et al. (2019b). MicroRNA-15a-5p Regulates the Development of Osteoarthritis by Targeting PTHrP in Chondrocytes. Biomed. Research International 2019, 1–11. 10.1155/2019/3904923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. (2011). Non-coding RNAs in Human Disease. Nat. Rev. Genet. 12 (12), 861–874. 10.1038/nrg3074 [DOI] [PubMed] [Google Scholar]
- Faghihi M. A., Zhang M., Huang J., Modarresi F., Van der Brug M. P., Nalls M. A., et al. (2010). Evidence for Natural Antisense Transcript-Mediated Inhibition of microRNA Function. Genome Biol. 11 (5), R56. 10.1186/gb-2010-11-5-r56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Yuan J., Xie J., Pan Z., Yao X., Sun X., et al. (2018). Long Non-protein Coding RNA DANCR Functions as a Competing Endogenous RNA to Regulate Osteoarthritis Progression via miR-577/SphK2 axis. Biochem. biophysical Res. Commun. 500 (3), 658–664. 10.1016/j.bbrc.2018.04.130 [DOI] [PubMed] [Google Scholar]
- Fan Z., Liu Y., Shi Z., Deng K., Zhang H., Li Q., et al. (2020). MiR‐155 Promotes Interleukin‐1β‐induced Chondrocyte Apoptosis and Catabolic Activity by Targeting PIK3R1‐mediated PI3K/Akt Pathway. J. Cel Mol Med 24 (15), 8441–8451. 10.1111/jcmm.15388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P., Zhang L. X., Hu Y., Zhang L., Zhou L. W. (2019). Long Non-coding RNA DANCR Induces Chondrogenesis by Regulating the miR-1275/MMP-13 axis in Synovial Fluid-Derived Mesenchymal Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 23 (23), 10459–10469. 10.26355/eurrev_201912_19685 [DOI] [PubMed] [Google Scholar]
- Felson D. T., Lawrence R. C., Dieppe P. A., Hirsch R., Helmick C. G., Jordan J. M., et al. (2000). Osteoarthritis: New Insights. Part 1: the Disease and its Risk Factors. Ann. Intern. Med. 133 (8), 635–646. 10.7326/0003-4819-133-8-200010170-00016 [DOI] [PubMed] [Google Scholar]
- Feng M., Jing L., Cheng J., An S., Huang J., Yan Q. (2021). Circ_0020093 Ameliorates IL-1β-induced Apoptosis and Extracellular Matrix Degradation of Human Chondrocytes by Upregulating SPRY1 via Targeting miR-23b. Mol. Cel Biochem 476 (10), 3623–3633. 10.1007/s11010-021-04186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Li L., Wang B., Wu J., Li H., Han Y., et al. (2021). CircADAMTS6/miR‐431‐5p axis Regulate Interleukin‐1β Induced Chondrocyte Apoptosis. J. Gene Med. 23 (2), e3304. 10.1002/jgm.3304 [DOI] [PubMed] [Google Scholar]
- Fu X., Dong B., Tian Y., Lefebvre P., Meng Z., Wang X., et al. (2015). MicroRNA-26a Regulates Insulin Sensitivity and Metabolism of Glucose and Lipids. J. Clin. Invest. 125 (6), 2497–2509. 10.1172/JCI75438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabler J., Ruetze M., Kynast K. L., Grossner T., Diederichs S., Richter W. (2015). Stage-Specific miRs in Chondrocyte Maturation: Differentiation-dependent and Hypertrophy-Related miR Clusters and the miR-181 Family. Tissue Eng. A 21 (23-24), 2840–2851. 10.1089/ten.TEA.2015.0352 [DOI] [PubMed] [Google Scholar]
- Gao G. C., Cheng X. G., Wei Q. Q., Chen W. C., Huang W. Z. (2019a). Long Noncoding RNA MALAT‐1 Inhibits Apoptosis and Matrix Metabolism Disorder in Interleukin‐1β‐induced Inflammation in Articular Chondrocytes via the JNK Signaling Pathway. J. Cel Biochem 120 (10), 17167–17179. 10.1002/jcb.28977 [DOI] [PubMed] [Google Scholar]
- Gao S. T., Yu Y. M., Wan L. P., Liu Z. M., Lin J. X. (2020). LncRNA GAS5 Induces Chondrocyte Apoptosis by Down-Regulating miR-137. Eur. Rev. Med. Pharmacol. Sci. 24 (21), 10984–10991. 10.26355/eurrev_202011_23582 [DOI] [PubMed] [Google Scholar]
- Gao Y., Zhao H., Li Y. (2019b). LncRNA MCM3AP-AS1 Regulates miR-142-3p/HMGB1 to Promote LPS-Induced Chondrocyte Apoptosis. BMC Musculoskelet. Disord. 20 (1), 605. 10.1186/s12891-019-2967-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F.-X., Li H., Yin X. (2017). Upregulation of microRNA-125b-5p Is Involved in the Pathogenesis of Osteoarthritis by Downregulating SYVN1. Oncol. Rep. 37 (4), 2490–2496. 10.3892/or.2017.5475 [DOI] [PubMed] [Google Scholar]
- Glyn-Jones S., Palmer A. J. R., Agricola R., Price A. J., Vincent T. L., Weinans H., et al. (2015). Osteoarthritis. The Lancet 386 (9991), 376–387. 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- Guo Y., Tian L., Du X., Deng Z. (2020). MiR-203 Regulates Estrogen Receptor α and Cartilage Degradation in IL-1β-stimulated Chondrocytes. J. Bone Miner Metab. 38 (3), 346–356. 10.1007/s00774-019-01062-4 [DOI] [PubMed] [Google Scholar]
- Guo Z., Wang H., Zhao F., Liu M., Wang F., Kang M., et al. (2021). Exosomal Circ-BRWD1 Contributes to Osteoarthritis Development through the Modulation of miR-1277/TRAF6 axis. Arthritis Res. Ther. 23 (1), 159. 10.1186/s13075-021-02541-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Liu L. (2021). Long Noncoding RNA TUG1 Regulates Degradation of Chondrocyte Extracellular Matrix via miR-320c/MMP-13 axis in Osteoarthritis. Open Life Sci. 16 (1), 384–394. 10.1515/biol-2021-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. B., Jensen T. I., Clausen B. H., Bramsen J. B., Finsen B., Damgaard C. K., et al. (2013). Natural RNA Circles Function as Efficient microRNA Sponges. Nature 495 (7441), 384–388. 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- He B., Jiang D. (2020). HOTAIR‐induced Apoptosis Is Mediated by Sponging miR‐130a‐3p to Repress Chondrocyte Autophagy in Knee Osteoarthritis. Cell Biol Int 44 (2), 524–535. 10.1002/cbin.11253 [DOI] [PubMed] [Google Scholar]
- He J.-H., Han Z.-P., Zou M.-X., Wang L., Lv Y. B., Zhou J. B., et al. (2018). Analyzing the LncRNA, miRNA, and mRNA Regulatory Network in Prostate Cancer with Bioinformatics Software. J. Comput. Biol. 25 (2), 146–157. 10.1089/cmb.2016.0093 [DOI] [PubMed] [Google Scholar]
- He J., Wang L., Ding Y., Liu H., Zou G. (2021a). lncRNA FER1L4 Is Dysregulated in Osteoarthritis and Regulates IL-6 Expression in Human Chondrocyte Cells. Sci. Rep. 11 (1), 13032. 10.1038/s41598-021-92474-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Zhang J., Wang D. (2017). Down-regulation of microRNA-216b Inhibits IL-1β-induced Chondrocyte Injury by Up-Regulation of Smad3. Biosci. Rep. 37 (2), BSR20160588. 10.1042/BSR20160588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Deng L. (2021). Potential of miR-25-3p in protection of Chondrocytes: Emphasis on Osteoarthritis. Folia Histochem. Cytobiol. 59 (1), 30–39. 10.5603/FHC.a2021.0004 [DOI] [PubMed] [Google Scholar]
- He X., Gao K., Lu S., Wu R. (2021b). LncRNA HOTTIP Leads to Osteoarthritis Progression via Regulating miR-663a/Fyn-Related Kinase axis. BMC Musculoskelet. Disord. 22 (1), 67. 10.1186/s12891-020-03861-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Zhao X., Wang C., Geng Y., Zhao J., Xu J., et al. (2017). MicroRNA-145 Attenuates TNF-α-Driven Cartilage Matrix Degradation in Osteoarthritis via Direct Suppression of MKK4. Cell Death Dis 8 (10), e3140. 10.1038/cddis.2017.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Wang Z., Pan Y., Ma J., Miao X., Qi X., et al. (2018a). MiR-26a and miR-26b Mediate Osteoarthritis Progression by Targeting FUT4 via NF-Κb Signaling Pathway. Int. J. Biochem. Cel Biol. 94, 79–88. 10.1016/j.biocel.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Hu J., Wang Z., Shan Y., Pan Y., Ma J., Jia L. (2018b). Long Non-coding RNA HOTAIR Promotes Osteoarthritis Progression via miR-17-5p/FUT2/β-Catenin axis. Cel Death Dis 9 (7), 711. 10.1038/s41419-018-0746-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Mao G., Zhang Z., Wu P., Wen X., Liao W., et al. (2019a). MicroRNA-320c Inhibits Development of Osteoarthritis through Downregulation of Canonical Wnt Signaling Pathway. Life Sci. 228, 242–250. 10.1016/j.lfs.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Hu S., Zhao X., Mao G., Zhang Z., Wen X., Zhang C., et al. (2019b). MicroRNA-455-3p Promotes TGF-β Signaling and Inhibits Osteoarthritis Development by Directly Targeting PAK2. Exp. Mol. Med. 51 (10), 1–13. 10.1038/s12276-019-0322-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhu H., Bu L., He D. (2019c). Expression Profile of Circular RNA S in TMJ Osteoarthritis Synovial Tissues and Potential Functions of Hsa_circ_0000448 with Specific Back-Spliced junction. Am. J. Transl Res. 11 (9), 5357–5374. [PMC free article] [PubMed] [Google Scholar]
- Huang B., Yu H., Li Y., Zhang W., Liu X. (2019a). Upregulation of Long Noncoding TNFSF10 Contributes to Osteoarthritis Progression through the miR‐376‐3p/FGFR1 axis. J. Cel Biochem 120 (12), 19610–19620. 10.1002/jcb.29267 [DOI] [PubMed] [Google Scholar]
- Huang J., Liu L., Yang J., Ding J., Xu X. (2019b). lncRNA DILC Is Downregulated in Osteoarthritis and Regulates IL‐6 Expression in Chondrocytes. J. Cel Biochem 120 (9), 16019–16024. 10.1002/jcb.28880 [DOI] [PubMed] [Google Scholar]
- Huang J., Zhao L., Fan Y., Liao L., Ma P. X., Xiao G., et al. (2019c). The microRNAs miR-204 and miR-211 Maintain Joint Homeostasis and Protect against Osteoarthritis Progression. Nat. Commun. 10 (1), 2876. 10.1038/s41467-019-10753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Wang J., Zhou Y., Zhao Y., Hang D., Cao Y. (2019d). LncRNA CASC2 Is Up-Regulated in Osteoarthritis and Participates in the Regulation of IL-17 Expression and Chondrocyte Proliferation and Apoptosis. Biosci. Rep. 39 (5), BSR20182454. 10.1042/BSR20182454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. (2018). The Novel Regulatory Role of lncRNA-miRNA-mRNA axis in Cardiovascular Diseases. J. Cel Mol Med 22 (12), 5768–5775. 10.1111/jcmm.13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Ma W., Xiao J., Dai X., Ling W. (2021). CircRNA_0092516 Regulates Chondrocyte Proliferation and Apoptosis in Osteoarthritis through the miR-337-3p/PTEN axis. J. Biochem. 169 (4), 467–475. 10.1093/jb/mvaa119 [DOI] [PubMed] [Google Scholar]
- Huang Z., Zhang N., Ma W., Dai X., Liu J. (2017). MiR-337-3p Promotes Chondrocytes Proliferation and Inhibits Apoptosis by Regulating PTEN/AKT axis in Osteoarthritis. Biomed. Pharmacother. 95, 1194–1200. 10.1016/j.biopha.2017.09.016 [DOI] [PubMed] [Google Scholar]
- Hwang H. S., Park S. J., Lee M. H., Kim H. A. (2017). MicroRNA-365 Regulates IL-1β-induced Catabolic Factor Expression by Targeting HIF-2α in Primary Chondrocytes. Sci. Rep. 7 (1), 17889. 10.1038/s41598-017-18059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacona J. R., Lutz C. S. (2019). miR‐146a‐5p: Expression, Regulation, and Functions in Cancer. WIREs RNA 10 (4), e1533. 10.1002/wrna.1533 [DOI] [PubMed] [Google Scholar]
- Ji Q., Qiao X., Liu Y., Wang D. (2021). Expression of Long-Chain Noncoding RNA GAS5 in Osteoarthritis and its Effect on Apoptosis and Autophagy of Osteoarthritis Chondrocytes. Histol. Histopathol 36 (4), 475–484. 10.14670/HH-18-312 [DOI] [PubMed] [Google Scholar]
- Ji Y., Fang Q. Y., Wang S. N., Zhang Z. W., Hou Z. J., Li J. N., et al. (2020). Lnc-RNA BLACAT1 Regulates Differentiation of Bone Marrow Stromal Stem Cells by Targeting miR-142-5p in Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 24 (6), 2893–2901. 10.26355/eurrev_202003_20653 [DOI] [PubMed] [Google Scholar]
- Jiang H., Pang H., Wu P., Cao Z., Li Z., Yang X. (2020a). LncRNA SNHG5 Promotes Chondrocyte Proliferation and Inhibits Apoptosis in Osteoarthritis by Regulating miR-10a-5p/H3F3B axis. Connect. Tissue Res. 2020, 1–10. 10.1080/03008207.2020.1825701 [DOI] [PubMed] [Google Scholar]
- Jiang L., Sun X., Kong H. (2020b). microRNA-9 Might Be a Novel Protective Factor for Osteoarthritis Patients. Hereditas 157 (1), 15. 10.1186/s41065-020-00128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Liu J., Luo T., Chen Q., Lu M., Meng D. (2019). LncRNA PACER Is Down-Regulated in Osteoarthritis and Regulates Chondrocyte Apoptosis and lncRNA HOTAIR Expression. Biosci. Rep. 39 (6), BSR20190404. 10.1042/BSR20190404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Ren J., Qi S. (2020). Human Bone Mesenchymal Stem Cells-Derived Exosomes Overexpressing microRNA-26a-5p Alleviate Osteoarthritis via Down-Regulation of PTGS2. Int. immunopharmacology 78, 105946. 10.1016/j.intimp.2019.105946 [DOI] [PubMed] [Google Scholar]
- Kang L., Yang C., Song Y., Liu W., Wang K., Li S., et al. (2016a). MicroRNA-23a-3p Promotes the Development of Osteoarthritis by Directly Targeting SMAD3 in Chondrocytes. Biochem. biophysical Res. Commun. 478 (1), 467–473. 10.1016/j.bbrc.2016.06.071 [DOI] [PubMed] [Google Scholar]
- Kang Y., Song J., Kim D., Ahn C., Park S., Chun C.-H., et al. (2016b). PCGEM1 Stimulates Proliferation of Osteoarthritic Synoviocytes by Acting as a Sponge for miR-770. J. Orthop. Res. 34 (3), 412–418. 10.1002/jor.23046 [DOI] [PubMed] [Google Scholar]
- Karlsen T. A., de Souza G. A., Ødegaard B., Engebretsen L., Brinchmann J. E. (2016). microRNA-140 Inhibits Inflammation and Stimulates Chondrogenesis in a Model of Interleukin 1β-Induced Osteoarthritis. Mol. Ther. - Nucleic Acids 5 (10), e373. 10.1038/mtna.2016.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen T. A., Jakobsen R. B., Mikkelsen T. S., Brinchmann J. E. (2014). microRNA-140 Targets RALA and Regulates Chondrogenic Differentiation of Human Mesenchymal Stem Cells by Translational Enhancement of SOX9 and ACAN. Stem Cell Dev. 23 (3), 290–304. 10.1089/scd.2013.0209 [DOI] [PubMed] [Google Scholar]
- Ko J.-Y., Lee M. S., Lian W.-S., Weng W.-T., Sun Y.-C., Chen Y.-S., et al. (2017). MicroRNA-29a Counteracts Synovitis in Knee Osteoarthritis Pathogenesis by Targeting VEGF. Sci. Rep. 7 (1), 3584. 10.1038/s41598-017-03616-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Duan Y., Sang Y., Li Y., Zhang H., Liang Y., et al. (2019). LncRNA-CDC6 Promotes Breast Cancer Progression and Function as ceRNA to Target CDC6 by Sponging microRNA‐215. J. Cel Physiol 234 (6), 9105–9117. 10.1002/jcp.27587 [DOI] [PubMed] [Google Scholar]
- Kopp F., Mendell J. T. (2018). Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 172 (3), 393–407. 10.1016/j.cell.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulou F., Malizos K. N., Papathanasiou I., Tsezou A. (2015). MicroRNA-33a Regulates Cholesterol Synthesis and Cholesterol Efflux-Related Genes in Osteoarthritic Chondrocytes. Arthritis Res. Ther. 17, 42. 10.1186/s13075-015-0556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcheski F. R., Christoff A. P., Margis R. (2016). Circular RNAs Are miRNA Sponges and Can Be Used as a New Class of Biomarker. J. Biotechnol. 238, 42–51. 10.1016/j.jbiotec.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Lawrence T. (2009). The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harbor Perspect. Biol. 1 (6), a001651. 10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J., Fu Y., Zhuang Y., Zhang K., Lu D. (2019). miR‐382‐3p Suppressed IL‐1β Induced Inflammatory Response of Chondrocytes via the TLR4/MyD88/NF‐κB Signaling Pathway by Directly Targeting CX43. J. Cel Physiol 234 (12), 23160–23168. 10.1002/jcp.28882 [DOI] [PubMed] [Google Scholar]
- Lei M., Zheng G., Ning Q., Zheng J., Dong D. (2020). Translation and Functional Roles of Circular RNAs in Human Cancer. Mol. Cancer 19 (1), 30. 10.1186/s12943-020-1135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.-F., Zhang Y., Xiao J., Wang F., Li M., Guo X.-Z., et al. (2017a). Hsa_circ_0045714 Regulates Chondrocyte Proliferation, Apoptosis and Extracellular Matrix Synthesis by Promoting the Expression of miR-193b Target Gene IGF1R. Hum. Cel. 30 (4), 311–318. 10.1007/s13577-017-0177-7 [DOI] [PubMed] [Google Scholar]
- Li C., Hu Q., Chen Z., Shen B., Yang J., Kang P., et al. (2018a). MicroRNA-140 Suppresses Human Chondrocytes Hypertrophy by Targeting SMAD1 and Controlling the Bone Morphogenetic Protein Pathway in Osteoarthritis. Am. J. Med. Sci. 355 (5), 477–487. 10.1016/j.amjms.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Li D., Sun Y., Wan Y., Wu X., Yang W. (2020a). LncRNA NEAT1 Promotes Proliferation of Chondrocytes via Down‐regulation of miR‐16‐5p in Osteoarthritis. J. Gene Med. 22 (9), e3203. 10.1002/jgm.3203 [DOI] [PubMed] [Google Scholar]
- Li F., Yao J., Hao Q., Duan Z. (2019a). miRNA-103 Promotes Chondrocyte Apoptosis by Down-Regulation of Sphingosine Kinase-1 and Ameliorates PI3K/AKT Pathway in Osteoarthritis. Biosci. Rep. 39 (10), BSR20191255. 10.1042/BSR20191255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.-Z., Xu X.-H., Lin N., Wang D.-W., Lin Y.-M., Su Z.-Z., et al. (2020b). Overexpression of miR-10a-5p Facilitates the Progression of Osteoarthritis. Aging 12 (7), 5948–5976. 10.18632/aging.102989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li Z., Pi Y., Chen Y., Mei L., Luo Y., et al. (2020c). MicroRNA-375 Exacerbates Knee Osteoarthritis through Repressing Chondrocyte Autophagy by Targeting ATG2B. Aging 12 (8), 7248–7261. 10.18632/aging.103073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xie S., Li H., Zhang R., Zhang H. (2020d). LncRNA MALAT1 Mediates Proliferation of LPS Treated-Articular Chondrocytes by Targeting the miR-146a-PI3K/Akt/mTOR axis. Life Sci. 254, 116801. 10.1016/j.lfs.2019.116801 [DOI] [PubMed] [Google Scholar]
- Li H., Xu J.-D., Fang X.-H., Zhu J.-N., Yang J., Pan R., et al. (2020e). Circular RNA circRNA_000203 Aggravates Cardiac Hypertrophy via Suppressing miR-26b-5p and miR-140-3p Binding to Gata4. Cardiovasc. Res. 116 (7), 1323–1334. 10.1093/cvr/cvz215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Yang H. H., Sun Z. G., Tang H. B., Min J. K. (2019b). Whole-transcriptome Sequencing of Knee Joint Cartilage from Osteoarthritis Patients. Bone Jt. Res. 8 (7), 290–303. 10.1302/2046-3758.87.BJR-2018-0297.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Huang J., Dai L., Yu D., Chen Q., Zhang X., et al. (2012). miR-146a, an IL-1β Responsive miRNA, Induces Vascular Endothelial Growth Factor and Chondrocyte Apoptosis by Targeting Smad4. Arthritis Res. Ther. 14 (2), R75. 10.1186/ar3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lv G., Wang B., Kuang L. (2018b). The Role of lncRNA XIST/miR-211 axis in Modulating the Proliferation and Apoptosis of Osteoarthritis Chondrocytes through CXCR4 and MAPK Signaling. Biochem. biophysical Res. Commun. 503 (4), 2555–2562. 10.1016/j.bbrc.2018.07.015 [DOI] [PubMed] [Google Scholar]
- Li L., Yang C., Liu X., Yang S., Ye S., Jia J., et al. (2015). Elevated Expression of microRNA-30b in Osteoarthritis and its Role in ERG Regulation of Chondrocyte. Biomed. Pharmacother. 76, 94–99. 10.1016/j.biopha.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Li Q., Zhang Z., Guo S., Tang G., Lu W., Qi X. (2019c). LncRNA ANCR Is Positively Correlated with Transforming Growth Factor‐β1 in Patients with Osteoarthritis. J. Cel Biochem 120 (9), 14226–14232. 10.1002/jcb.28881 [DOI] [PubMed] [Google Scholar]
- Li W., Zhao S., Yang H., Zhang C., Kang Q., Deng J., et al. (2019d). Potential Novel Prediction of TMJ-OA: MiR-140-5p Regulates Inflammation through Smad/TGF-β Signaling. Front. Pharmacol. 10, 15. 10.3389/fphar.2019.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Huang T. L., Zhang G. D., Jiang J. T., Guo P. Y. (2019e). LncRNA ANRIL Impacts the Progress of Osteoarthritis via Regulating Proliferation and Apoptosis of Osteoarthritis Synoviocytes. Eur. Rev. Med. Pharmacol. Sci. 23 (22), 9729–9737. 10.26355/eurrev_201911_19535 [DOI] [PubMed] [Google Scholar]
- Li X., Ding J., Wang X., Cheng Z., Zhu Q. (2020f). NUDT21 Regulates circRNA Cyclization and ceRNA Crosstalk in Hepatocellular Carcinoma. Oncogene 39 (4), 891–904. 10.1038/s41388-019-1030-0 [DOI] [PubMed] [Google Scholar]
- Li X., Yu M., Chen L., Sun T., Wang H., Zhao L., et al. (2019f). RETRACTED: LncRNA PMS2L2 Protects ATDC5 Chondrocytes against Lipopolysaccharide-Induced Inflammatory Injury by Sponging miR-203. Life Sci. 217, 283–292. 10.1016/j.lfs.2018.12.020 [DOI] [PubMed] [Google Scholar]
- Li Y.-F., Li S.-H., Liu Y., Luo Y.-T. (2017b). Long Noncoding RNA CIR Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis by Acting as a Sponge for Mir-27b. Cell Physiol Biochem 43 (2), 602–610. 10.1159/000480532 [DOI] [PubMed] [Google Scholar]
- Li Y., Li S., Luo Y., Liu Y., Yu N. (2017c). LncRNA PVT1 Regulates Chondrocyte Apoptosis in Osteoarthritis by Acting as a Sponge for miR-488-3p. DNA Cel. Biol. 36 (7), 571–580. 10.1089/dna.2017.3678 [DOI] [PubMed] [Google Scholar]
- Li Y., Li Z., Li C., Zeng Y., Liu Y. (2019g). Long Noncoding RNA TM1P3 Is Involved in Osteoarthritis by Mediating Chondrocyte Extracellular Matrix Degradation. J. Cel Biochem 120 (8), 12702–12712. 10.1002/jcb.28539 [DOI] [PubMed] [Google Scholar]
- Li Y., Zhou D., Ren Y., Zhang Z., Guo X., Ma M., et al. (2019h). Mir223 Restrains Autophagy and Promotes CNS Inflammation by Targeting ATG16L1. Autophagy 15 (3), 478–492. 10.1080/15548627.2018.1522467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Cheng J., Liu J. (2020g). Baicalin Protects Human OA Chondrocytes against IL-1β-Induced Apoptosis and ECM Degradation by Activating Autophagy via MiR-766-3p/AIFM1 Axis. Drug Des. Devel Ther. 14, 2645–2655. 10.2147/DDDT.S255823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Meng D., Li G., Xu J., Tian K., Li Y. (2016). Overexpression of microRNA-210 Promotes Chondrocyte Proliferation and Extracellular Matrix Deposition by Targeting HIF-3α in Osteoarthritis. Mol. Med. Rep. 13 (3), 2769–2776. 10.3892/mmr.2016.4878 [DOI] [PubMed] [Google Scholar]
- Li Z., Yuan B., Pei Z., Zhang K., Ding Z., Zhu S., et al. (2019i). Circ_0136474 and MMP‐13 Suppressed Cell Proliferation by Competitive Binding to miR‐127‐5p in Osteoarthritis. J. Cel Mol Med 23 (10), 6554–6564. 10.1111/jcmm.14400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian W.-S., Ko J.-Y., Wu R.-W., Sun Y.-C., Chen Y.-S., Wu S.-L., et al. (2018). MicroRNA-128a Represses Chondrocyte Autophagy and Exacerbates Knee Osteoarthritis by Disrupting Atg12. Cel Death Dis 9 (9), 919. 10.1038/s41419-018-0994-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q., Asila A., Deng Y., Liao J., Liu Z., Fang R. (2021). Osteopontin‐induced lncRNA HOTAIR Expression Is Involved in Osteoarthritis by Regulating Cell Proliferation. BMC Geriatr. 21 (1), 57. 10.1186/s12877-020-01993-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Duan L., Xiong J., Zhu W., Liu Q., Wang D., et al. (2016). E2 Regulates MMP-13 via Targeting miR-140 in IL-1β-induced Extracellular Matrix Degradation in Human Chondrocytes. Arthritis Res. Ther. 18 (1), 105. 10.1186/s13075-016-0997-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z.-J., Zhuang H., Wang G.-X., Li Z., Zhang H.-T., Yu T.-Q., et al. (2012). MiRNA-140 Is a Negative Feedback Regulator of MMP-13 in IL-1β-stimulated Human Articular Chondrocyte C28/I2 Cells. Inflamm. Res. 61 (5), 503–509. 10.1007/s00011-012-0438-6 [DOI] [PubMed] [Google Scholar]
- Liao H.-X., Zhang Z.-H., Chen H.-L., Huang Y.-M., Liu Z.-L., Huang J. (2021). CircHYBID Regulates Hyaluronan Metabolism in Chondrocytes via Hsa-miR-29b-3p/TGF-Β1 axis. Mol. Med. 27 (1), 56. 10.1186/s10020-021-00319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y., Wright J. A., Attema J. L., Gregory P. A., Bert A. G., Smith E., et al. (2013). Epigenetic Modulation of the miR-200 Family Is Associated with Transition to a Breast Cancer Stem Cell-like State. J. Cel. Sci. 126 (Pt 10), 2256–2266. 10.1242/jcs.122275 [DOI] [PubMed] [Google Scholar]
- Lin Z., Tian X. Y., Huang X. X., He L. L., Xu F. (2019). microRNA‐186 Inhibition of PI3K-AKT Pathway via SPP1 Inhibits Chondrocyte Apoptosis in Mice with Osteoarthritis. J. Cel Physiol 234 (5), 6042–6053. 10.1002/jcp.27225 [DOI] [PubMed] [Google Scholar]
- Litwic A., Edwards M. H., Dennison E. M., Cooper C. (2013). Epidemiology and burden of Osteoarthritis. Br. Med. Bull. 105, 185–199. 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li J., Cairns M. J. (2014a). Identifying miRNAs, Targets and Functions. Brief. Bioinformatics 15 (1), 1–19. 10.1093/bib/bbs075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Ren S., Zhao S., Wang Y. (2019a). LncRNA MALAT1/MiR-145 Adjusts IL-1β-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med. J. 60 (11), 1081–1092. 10.3349/ymj.2019.60.11.1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Liu X., Yang Y., Sun Z., Deng S., Jiang Z., et al. (2020a). NEAT1/miR‐193a‐3p/SOX5 axis Regulates Cartilage Matrix Degradation in Human Osteoarthritis. Cel Biol Int 44 (4), 947–957. 10.1002/cbin.11291 [DOI] [PubMed] [Google Scholar]
- Liu H., Luo J. (2019). miR-211-5p Contributes to Chondrocyte Differentiation by Suppressing Fibulin-4 Expression to Play a Role in Osteoarthritis. J. Biochem. 166 (6), 495–502. 10.1093/jb/mvz065 [DOI] [PubMed] [Google Scholar]
- Liu J., Liu Y., Wang F., Liang M. (2021a). miR-204: Molecular Regulation and Role in Cardiovascular and Renal Diseases. Hypertension 78 (2), 270–281. 10.1161/HYPERTENSIONAHA.121.14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xue N., Guo Y., Niu K., Gao L., Zhang S., et al. (2019b). CircRNA_100367 Regulated the Radiation Sensitivity of Esophageal Squamous Cell Carcinomas through miR-217/Wnt3 Pathway. Aging 11 (24), 12412–12427. 10.18632/aging.102580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang X., Dai L., Hu X., Zhu J., Li L., et al. (2014b). Long Noncoding RNA Related to Cartilage Injury Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Arthritis Rheumatol. 66 (4), 969–978. 10.1002/art.38309 [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhang X., Hu X., Dai L., Fu X., Zhang J., et al. (2016). Circular RNA Related to the Chondrocyte ECM Regulates MMP13 Expression by Functioning as a MiR-136 'Sponge' in Human Cartilage Degradation. Sci. Rep. 6, 22572. 10.1038/srep22572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Zha Z., Wang H. (2019c). Upregulation of microRNA‐27a Inhibits Synovial Angiogenesis and Chondrocyte Apoptosis in Knee Osteoarthritis Rats through the Inhibition of PLK2. J. Cel Physiol 234 (12), 22972–22984. 10.1002/jcp.28858 [DOI] [PubMed] [Google Scholar]
- Liu X. C., Xu L., Cai Y. L., Zheng Z. Y., Dai E. N., Sun S. (2020b). MiR‐1207‐5p/CX3CR1 axis Regulates the Progression of Osteoarthritis via the Modulation of the Activity of NF‐κB Pathway. Int. J. Rheum. Dis. 23 (8), 1057–1065. 10.1111/1756-185X.13898 [DOI] [PubMed] [Google Scholar]
- Liu X., Liu L., Zhang H., Shao Y., Chen Z., Feng X., et al. (2019d). MiR-146b Accelerates Osteoarthritis Progression by Targeting Alpha-2-Macroglobulin. Aging 11 (16), 6014–6028. 10.18632/aging.102160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li Q., Gao Z., Lei F., Gao X. (2021b). Circ-SPG11 Knockdown Hampers IL-1β-induced Osteoarthritis Progression via Targeting miR-337-3p/ADAMTS5. J. Orthop. Surg. Res. 16 (1), 392. 10.1186/s13018-021-02526-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lin L., Zou R., Wen C., Wang Z., Lin F. (2018). MSC-derived Exosomes Promote Proliferation and Inhibit Apoptosis of Chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in Osteoarthritis. Cell Cycle 17 (21-22), 2411–2422. 10.1080/15384101.2018.1526603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu K., Tang C., Shi Z., Jing K., Zheng J. (2020c). Long Non-coding RNA XIST Contributes to Osteoarthritis Progression via miR-149-5p/DNMT3A axis. Biomed. Pharmacother. 128, 110349. 10.1016/j.biopha.2020.110349 [DOI] [PubMed] [Google Scholar]
- Liu Z., Chen S., Yang Y., Lu S., Zhao X., Hu B., et al. (2019e). MicroRNA-671-3p R-egulates the D-evelopment of K-nee O-steoarthritis by T-argeting TRAF3 in C-hondrocytes. Mol. Med. Rep. 20 (3), 2843–2850. 10.3892/mmr.2019.10488 [DOI] [PubMed] [Google Scholar]
- Long H., Li Q., Xiao Z., Yang B. (2021). LncRNA MIR22HG Promotes Osteoarthritis Progression via Regulating miR-9-3p/ADAMTS5 Pathway. Bioengineered 12 (1), 3148–3158. 10.1080/21655979.2021.1945362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Li Z., Hu S., Cai Y., Peng K. (2019). LncRNA PART‐1 Targets TGFBR2/Smad3 to Regulate Cell Viability and Apoptosis of Chondrocytes via Acting as miR‐590‐3p Sponge in Osteoarthritis. J. Cel Mol Med 23 (12), 8196–8205. 10.1111/jcmm.14690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü G., Li L., Wang B., Kuang L. (2020). LINC00623/miR-101/HRAS axis Modulates IL-1β-mediated ECM Degradation, Apoptosis and Senescence of Osteoarthritis Chondrocytes. Aging 12 (4), 3218–3237. 10.18632/aging.102801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Ji M.-L., Zhang X.-J., Shi P.-L., Wu H., Wang C., et al. (2017). MicroRNA-218-5p as a Potential Target for the Treatment of Human Osteoarthritis. Mol. Ther. 25 (12), 2676–2688. 10.1016/j.ymthe.2017.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Zhou E. (2020). Long Noncoding RNA LINC00662‐miR‐15b‐5p Mediated GPR120 Dysregulation Contributes to Osteoarthritis. Pathol. Int. 70 (3), 155–165. 10.1111/pin.12875 [DOI] [PubMed] [Google Scholar]
- Lu X., Yu Y., Yin F., Yang C., Li B., Lin J., et al. (2020). Knockdown of PVT1 Inhibits IL-1β-induced Injury in Chondrocytes by Regulating miR-27b-3p/TRAF3 axis. Int. immunopharmacology 79, 106052. 10.1016/j.intimp.2019.106052 [DOI] [PubMed] [Google Scholar]
- Lu Z., Luo M., Huang Y. (2018). lncRNA‐CIR Regulates Cell Apoptosis of Chondrocytes in Osteoarthritis. J. Cel Biochem 120, 7229–7237. 10.1002/jcb.27997 [DOI] [PubMed] [Google Scholar]
- Luan X., Wang Y. (2018). LncRNA XLOC_006390 Facilitates Cervical Cancer Tumorigenesis and Metastasis as a ceRNA Against miR-331-3p and miR-338-3p. J. Gynecol. Oncol. 29 (6), e95. 10.3802/jgo.2018.29.e95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Liang J. S., Gong J., Zhang H. L., Feng Z. J., Yang H. T., et al. (2019a). The Function of microRNA-34a in Osteoarthritis. Bratisl Lek Listy 120 (5), 386–391. 10.4149/BLL_2019_063 [DOI] [PubMed] [Google Scholar]
- Luo H., Xu C., Le W., Ge B., Wang T. (2019b). lncRNA CASC11 Promotes Cancer Cell Proliferation in Bladder Cancer through miRNA‐150. J. Cel Biochem 120 (8), 13487–13493. 10.1002/jcb.28622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F., Li G., Yu Y., Xu J., Wu X. (2019a). MiR-33b-3p Promotes Chondrocyte Proliferation and Inhibits Chondrocyte Apoptosis and Cartilage ECM Degradation by Targeting DNMT3A in Osteoarthritis. Biochem. biophysical Res. Commun. 519 (2), 430–437. 10.1016/j.bbrc.2019.09.022 [DOI] [PubMed] [Google Scholar]
- Ma H. R., Mu W. B., Zhang K. Y., Zhou H. K., Jiang R. D., Cao L. (2020). CircVCAN Regulates the Proliferation and Apoptosis of Osteoarthritis Chondrocyte through NF-Κb Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 24 (12), 6517–6525. 10.26355/eurrev_202006_21635 [DOI] [PubMed] [Google Scholar]
- Ma Y., Wu Y., Chen J., Huang K., Ji B., Chen Z., et al. (2019b). miR-10a-5p Promotes Chondrocyte Apoptosis in Osteoarthritis by Targeting HOXA1. Mol. Ther. - Nucleic Acids 14, 398–409. 10.1016/j.omtn.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki M. S., Haqqi T. M. (2015). miR-139 Modulates MCPIP1/IL-6 Expression and Induces Apoptosis in Human OA Chondrocytes. Exp. Mol. Med. 47, e189. 10.1038/emm.2015.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D., Wu M., Wei J., Zhou X., Yang L., Chen F. (2021a). MicroRNA‐101a‐3p Could Be Involved in the Pathogenesis of Temporomandibular Joint Osteoarthritis by Mediating UBE2D1 and FZD4. J. Oral Pathol. Med. 50 (2), 236–243. 10.1111/jop.13131 [DOI] [PubMed] [Google Scholar]
- Mao G., Hu S., Zhang Z., Wu P., Zhao X., Lin R., et al. (2018a). Exosomal miR-95-5p Regulates Chondrogenesis and Cartilage Degradation via Histone Deacetylase 2/8. J. Cel Mol Med 22 (11), 5354–5366. 10.1111/jcmm.13808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Kang Y., Lin R., Hu S., Zhang Z., Li H., et al. (2019a). Long Non-coding RNA HOTTIP Promotes CCL3 Expression and Induces Cartilage Degradation by Sponging miR-455-3p. Front. Cel Dev. Biol. 7, 161. 10.3389/fcell.2019.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Wu P., Zhang Z., Zhang Z., Liao W., Li Y., et al. (2017a). MicroRNA-92a-3p Regulates Aggrecanase-1 and Aggrecanase-2 Expression in Chondrogenesis and IL-1β-Induced Catabolism in Human Articular Chondrocytes. Cel Physiol Biochem 44 (1), 38–52. 10.1159/000484579 [DOI] [PubMed] [Google Scholar]
- Mao G., Xu Y., Long D., Sun H., Li H., Xin R., et al. (2021b). Exosome-transported circRNA_0001236 Enhances Chondrogenesis and Suppress Cartilage Degradation via the miR-3677-3p/Sox9 axis. Stem Cel Res Ther 12 (1), 389. 10.1186/s13287-021-02431-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Zhang Z., Hu S., Zhang Z., Chang Z., Huang Z., et al. (2018b). Exosomes Derived from miR-92a-3p-Overexpressing Human Mesenchymal Stem Cells Enhance Chondrogenesis and Suppress Cartilage Degradation via Targeting WNT5A. Stem Cel Res Ther 9 (1), 247. 10.1186/s13287-018-1004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Zhang Z., Huang Z., Chen W., Huang G., Meng F., et al. (2017b). MicroRNA-92a-3p Regulates the Expression of Cartilage-specific Genes by Directly Targeting Histone Deacetylase 2 in Chondrogenesis and Degradation. Osteoarthritis and cartilage 25 (4), 521–532. 10.1016/j.joca.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Mao T., He C., Wu H., Yang B., Li X. (2019b). Silencing lncRNA HOTAIR Declines Synovial Inflammation and Synoviocyte Proliferation and Promotes Synoviocyte Apoptosis in Osteoarthritis Rats by Inhibiting Wnt/β-Catenin Signaling Pathway. Cell Cycle 18 (22), 3189–3205. 10.1080/15384101.2019.1671716 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matsukawa T., Sakai T., Yonezawa T., Hiraiwa H., Hamada T., Nakashima M., et al. (2013). MicroRNA-125b Regulates the Expression of Aggrecanase-1 (ADAMTS-4) in Human Osteoarthritic Chondrocytes. Arthritis Res. Ther. 15 (1), R28. 10.1186/ar4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U., Benditz A., Grässel S. (2017). miR-29b Regulates Expression of Collagens I and III in Chondrogenically Differentiating BMSC in an Osteoarthritic Environment. Sci. Rep. 7 (1), 13297. 10.1038/s41598-017-13567-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehana E.-S. E., Khafaga A. F., El-Blehi S. S. (2019). The Role of Matrix Metalloproteinases in Osteoarthritis Pathogenesis: An Updated Review. Life Sci. 234, 116786. 10.1016/j.lfs.2019.116786 [DOI] [PubMed] [Google Scholar]
- Mei X., Tong J., Zhu W., Zhu Y. (2019). lncRNA-NR024118 O-verexpression R-everses LPS-induced I-nflammatory I-njury and A-poptosis via NF-κB/Nrf2 S-ignaling in ATDC5 C-hondrocytes. Mol. Med. Rep. 20 (4), 3867–3873. 10.3892/mmr.2019.10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Li Z., Zhang Z., Yang Z., Kang Y., Zhao X., et al. (2018). MicroRNA-193b-3p Regulates Chondrogenesis and Chondrocyte Metabolism by Targeting HDAC3. Theranostics 8 (10), 2862–2883. 10.7150/thno.23547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Zhang Z., Chen W., Huang G., He A., Hou C., et al. (2016). MicroRNA-320 Regulates Matrix Metalloproteinase-13 Expression in Chondrogenesis and Interleukin-1β-Induced Chondrocyte Responses. Osteoarthritis and cartilage 24 (5), 932–941. 10.1016/j.joca.2015.12.012 [DOI] [PubMed] [Google Scholar]
- Miao G., Zang X., Hou H., Sun H., Wang L., Zhang T., et al. (2019). Bax Targeted by miR-29a Regulates Chondrocyte Apoptosis in Osteoarthritis. Biomed. Research International 2019, 1–9. 10.1155/2019/1434538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki S., Sato T., Inoue A., Otsuki S., Ito Y., Yokoyama S., et al. (2010). MicroRNA-140 Plays Dual Roles in Both Cartilage Development and Homeostasis. Genes Dev. 24 (11), 1173–1185. 10.1101/gad.1915510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin D., Salone V., Koufany M., Clément T., Behm-Ansmant I., Branlant C., et al. (2017). MicroRNA-29b Contributes to Collagens Imbalance in Human Osteoarthritic and Dedifferentiated Articular Chondrocytes. Biomed. Research International 2017, 1–12. 10.1155/2017/9792512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. L., Dang X. Q., Shi Z. B. (2020a). CircPSM3 Inhibits the Proliferation and Differentiation of OA Chondrocytes by Targeting miRNA-296-5p. Eur. Rev. Med. Pharmacol. Sci. 24 (7), 3467–3475. 10.26355/eurrev_202004_20805 [DOI] [PubMed] [Google Scholar]
- Ni S., Xu C., Zhuang C., Zhao G., Li C., Wang Y., et al. (2020b). LncRNA LUADT1 Regulates miR‐34a/SIRT1 to Participate in Chondrocyte Apoptosis. J. Cel Biochem 122, 1003–1008. 10.1002/jcb.29637 [DOI] [PubMed] [Google Scholar]
- Ni W., Jiang C., Wu Y., Zhang H., Wang L., Yik J. H. N., et al. (2021). CircSLC7A2 Protects against Osteoarthritis through Inhibition of the miR‐4498/TIMP3 axis. Cell Prolif 54 (6), e13047. 10.1111/cpr.13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z., Shang X., Tang G., Niu L. (2018). Expression of miR-206 in Human Knee Articular Chondrocytes and Effects of miR-206 on Proliferation and Apoptosis of Articular Chondrocytes. Am. J. Med. Sci. 355 (3), 240–246. 10.1016/j.amjms.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Nishimura R., Hata K., Takahata Y., Murakami T., Nakamura E., Ohkawa M., et al. (2020). Role of Signal Transduction Pathways and Transcription Factors in Cartilage and Joint Diseases. Int. J. Mol. Sci. 21 (4), 1340. 10.3390/ijms21041340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntoumou E., Tzetis M., Braoudaki M., Lambrou G., Poulou M., Malizos K., et al. (2017). Serum microRNA Array Analysis Identifies miR-140-3p, miR-33b-3p and miR-671-3p as Potential Osteoarthritis Biomarkers Involved in Metabolic Processes. Clin. Epigenet 9, 127. 10.1186/s13148-017-0428-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Dai H., Wang L., Lin S., Tao Y., Zheng Y., et al. (2020). MicroRNA-410-3p Modulates Chondrocyte Apoptosis and Inflammation by Targeting High Mobility Group Box 1 (HMGB1) in an Osteoarthritis Mouse Model. BMC Musculoskelet. Disord. 21 (1), 486. 10.1186/s12891-020-03489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J., Cheon E. J., Kim H. A. (2013). MicroRNA-558 Regulates the Expression of Cyclooxygenase-2 and IL-1β-induced Catabolic Effects in Human Articular Chondrocytes. Osteoarthritis and cartilage 21 (7), 981–989. 10.1016/j.joca.2013.04.012 [DOI] [PubMed] [Google Scholar]
- Pearson M. J., Philp A. M., Heward J. A., Roux B. T., Walsh D. A., Davis E. T., et al. (2016). Long Intergenic Noncoding RNAs Mediate the Human Chondrocyte Inflammatory Response and Are Differentially Expressed in Osteoarthritis Cartilage. Arthritis Rheumatol. 68 (4), 845–856. 10.1002/art.39520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Deng M., Ma Y., Hu W., Liang F. (2021). miR-520c-3p Regulates IL-1β-stimulated Human Chondrocyte Apoptosis and Cartilage Degradation by Targeting GAS2. J. Orthop. Surg. Res. 16 (1), 347. 10.1186/s13018-021-02466-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P., Zhang B., Huang J., Xing C., Liu W., Sun C., et al. (2020). Identification of a circRNA-miRNA-mRNA Network to Explore the Effects of circRNAs on Pathogenesis and Treatment of Spinal Cord Injury. Life Sci. 257, 118039. 10.1016/j.lfs.2020.118039 [DOI] [PubMed] [Google Scholar]
- Ponting C. P., Oliver P. L., Reik W. (2009). Evolution and Functions of Long Noncoding RNAs. Cell 136 (14), 629–641. 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Prieto-Alhambra D., Judge A., Javaid M. K., Cooper C., Diez-Perez A., Arden N. K. (2014). Incidence and Risk Factors for Clinically Diagnosed Knee, Hip and Hand Osteoarthritis: Influences of Age, Gender and Osteoarthritis Affecting Other Joints. Ann. Rheum. Dis. 73 (9), 1659–1664. 10.1136/annrheumdis-2013-203355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G. H., Yang W. C., Yao J. N., Zhao Y., Wu X. J. (2021). LncRNA OIP5-AS1 Affects the Biological Behaviors of Chondrocytes of Patients with Osteoarthritis by Regulating Micro-30a-5p. Eur. Rev. Med. Pharmacol. Sci. 25 (3), 1215–1224. 10.26355/eurrev_202102_24825 [DOI] [PubMed] [Google Scholar]
- Qiu B., Xu X., Yi P., Hao Y. (2020). Curcumin Reinforces MSC‐derived Exosomes in Attenuating Osteoarthritis via Modulating the miR‐124/NF‐kB and miR‐143/ROCK1/TLR9 Signalling Pathways. J. Cel Mol Med 24 (18), 10855–10865. 10.1111/jcmm.15714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu W.-J., Xu M.-Z., Zhu X.-D., Ji Y.-H. (2019). MicroRNA-27a Alleviates IL-1β-induced Inflammatory Response and Articular Cartilage Degradation via TLR4/NF-Κb Signaling Pathway in Articular Chondrocytes. Int. immunopharmacology 76, 105839. 10.1016/j.intimp.2019.105839 [DOI] [PubMed] [Google Scholar]
- Rasheed Z., Rasheed N., Abdulmonem W. A., Khan M. I. (2019). Author Correction: MicroRNA-125b-5p Regulates IL-1β Induced Inflammatory Genes via Targeting TRAF6-Mediated MAPKs and NF-Κb Signaling in Human Osteoarthritic Chondrocytes. Sci. Rep. 9 (1), 14729. 10.1038/s41598-019-50844-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T., Wei P., Song Q., Ye Z., Wang Y., Huang L. (2020). MiR-140-3p Ameliorates the Progression of Osteoarthritis via Targeting CXCR4. Biol. Pharm. Bull. 43 (5), 810–816. 10.1248/bpb.b19-00959 [DOI] [PubMed] [Google Scholar]
- Rigoglou S., Papavassiliou A. G. (2013). The NF-Κb Signalling Pathway in Osteoarthritis. Int. J. Biochem. Cel Biol. 45 (11), 2580–2584. 10.1016/j.biocel.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Rousseau J.-C., Millet M., Croset M., Sornay-Rendu E., Borel O., Chapurlat R. (2020). Association of Circulating microRNAs with Prevalent and Incident Knee Osteoarthritis in Women: the OFELY Study. Arthritis Res. Ther. 22 (1), 2. 10.1186/s13075-019-2086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakalauskienė G., Jauniškienė D. (2010). Osteoarthritis: Etiology, Epidemiology, Impact on the Individual and Society and the Main Principles of Management. Medicina (Kaunas) 46 (11), 790–797. [PubMed] [Google Scholar]
- Shao J., Ding Z., Peng J., Zhou R., Li L., Qian Q., et al. (2020). MiR-146a-5p Promotes IL-1β-induced Chondrocyte Apoptosis through the TRAF6-Mediated NF-kB Pathway. Inflamm. Res. 69 (6), 619–630. 10.1007/s00011-020-01346-w [DOI] [PubMed] [Google Scholar]
- Shen H., Wang Y., Shi W., Sun G., Hong L., Zhang Y. (2018). LncRNA SNHG5/miR-26a/SOX2 Signal axis Enhances Proliferation of Chondrocyte in Osteoarthritis. Acta Biochim. Biophys. Sinica 50 (2), 191–198. 10.1093/abbs/gmx141 [DOI] [PubMed] [Google Scholar]
- Shen J., Li S., Chen D. (2014). TGF-β Signaling and the Development of Osteoarthritis. Bone Res. 2, 14002. 10.1038/boneres.2014.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Yang Y., Liu G., Chen W., Chen J., Wang Q., et al. (2020a). CircCDK14 Protects against Osteoarthritis by Sponging miR-125a-5p and Promoting the Expression of Smad2. Theranostics 10 (20), 9113–9131. 10.7150/thno.45993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Wu Y., Chen J., Xie Z., Huang K., Wang G., et al. (2019). CircSERPINE2 Protects against Osteoarthritis by Targeting miR-1271 and ETS-Related Gene. Ann. Rheum. Dis. 78 (6), 826–836. 10.1136/annrheumdis-2018-214786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Yang Y., Shen P., Ma J., Fang B., Wang Q., et al. (2021). circPDE4B Prevents Articular Cartilage Degeneration and Promotes Repair by Acting as a Scaffold for RIC8A and MID1. Ann. Rheum. Dis. 80 (9), 1209–1219. 10.1136/annrheumdis-2021-219969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.-F., Cheng Y., Dong Q.-R., Zheng M.-Q. (2020b). MicroRNA-675-3p Regulates IL-1β-stimulated Human Chondrocyte Apoptosis and Cartilage Degradation by Targeting GNG5. Biochem. biophysical Res. Commun. 527 (2), 458–465. 10.1016/j.bbrc.2020.04.044 [DOI] [PubMed] [Google Scholar]
- Shi F.-L., Ren L.-X. (2020). Up-regulated miR-374a-3p Relieves Lipopolysaccharides Induced Injury in CHON-001 Cells via Regulating Wingless-type MMTV Integration Site Family Member 5B. Mol. Cell. probes 51, 101541. 10.1016/j.mcp.2020.101541 [DOI] [PubMed] [Google Scholar]
- Shi J., Cao F., Chang Y., Xin C., Jiang X., Xu J., et al. (2021a). Long Non-coding RNA MCM3AP-AS1 Protects Chondrocytes ATDC5 and CHON-001 from IL-1β-induced Inflammation via Regulating miR-138-5p/SIRT1. Bioengineered 12 (1), 1445–1456. 10.1080/21655979.2021.1905247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Guo K., Su S., Li J., Li C. (2018). miR-486-5p I-s U-pregulated in O-steoarthritis and I-nhibits C-hondrocyte P-roliferation and M-igration by S-uppressing SMAD2. Mol. Med. Rep. 18 (1), 502–508. 10.3892/mmr.2018.8931 [DOI] [PubMed] [Google Scholar]
- Shi J., Wang S., He Q., Liu K., Zhao W., Xie Q., et al. (2021b). TNF‐α Induces Up‐regulation of MicroRNA‐27a via the P38 Signalling Pathway, Which Inhibits Intervertebral Disc Degeneration by Targeting FSTL1. J. Cel Mol Med 25 (15), 7146–7156. 10.1111/jcmm.16745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., Long J., Guo W., Ye W. (2019). MicroRNA-195-5p I-nhibitor P-revents the D-evelopment of O-steoarthritis by T-argeting REGγ. Mol. Med. Rep. 19 (6), 4561–4568. 10.3892/mmr.2019.10124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H.-b., Zeng Y., Zhou Z.-k., Pei F.-x., Lu Y.-r., Cheng J.-q., et al. (2016). Expression of miRNA-140 in Chondrocytes and Synovial Fluid of Knee Joints in Patients with Osteoarthritis. Chin. Med. Sci. J. 31 (4), 207–212. 10.1016/s1001-9294(17)30002-0 [DOI] [PubMed] [Google Scholar]
- Si Z., Zhou S., Shen Z., Luan F., Yan J. (2021). lncRNA HAND2-AS1 Is Downregulated in Osteoarthritis and Regulates IL-6 Expression in Chondrocytes. J. Orthop. Surg. Res. 16 (1), 68. 10.1186/s13018-021-02216-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Skrzypa M., Szala D., Gablo N., Czech J., Pajak J., Kopanska M., et al. (2019). miRNA-146a-5p Is Upregulated in Serum and Cartilage Samples of Patients with Osteoarthritis. Pol. Przegl Chir 91 (3), 1–5. 10.5604/01.3001.0013.0135 [DOI] [PubMed] [Google Scholar]
- Sondag G. R., Haqqi T. M. (2016). The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 18 (8), 56. 10.1007/s11926-016-0604-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Ahn C., Chun C.-H., Jin E.-J. (2014). A Long Non-coding RNA, GAS5, Plays a Critical Role in the Regulation of miR-21 during Osteoarthritis. J. Orthop. Res. 32 (12), 1628–1635. 10.1002/jor.22718 [DOI] [PubMed] [Google Scholar]
- Song J., Jin E.-H., Kim D., Kim K. Y., Chun C.-H., Jin E.-J. (2015). MicroRNA-222 Regulates MMP-13 via Targeting HDAC-4 during Osteoarthritis Pathogenesis. BBA Clin. 3, 79–89. 10.1016/j.bbacli.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Kim D., Lee C. H., Lee M. S., Chun C.-H., Jin E.-J. (2013). MicroRNA-488 Regulates Zinc Transporter SLC39A8/ZIP8 during Pathogenesis of Osteoarthritis. J. Biomed. Sci. 20, 31. 10.1186/1423-0127-20-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck E., Boeuf S., Gabler J., Werth N., Schnatzer P., Diederichs S., et al. (2012). Regulation of H19 and its Encoded microRNA-675 in Osteoarthritis and under Anabolic and Catabolic In Vitro Conditions. J. Mol. Med. 90 (10), 1185–1195. 10.1007/s00109-012-0895-y [DOI] [PubMed] [Google Scholar]
- Su W., Xie W., Shang Q., Su B. (2015). The Long Noncoding RNA MEG3 Is Downregulated and Inversely Associated with VEGF Levels in Osteoarthritis. Biomed. Research International 2015, 1–5. 10.1155/2015/356893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui C., Liu D., Que Y., Xu S., Hu Y. (2021). Knockdown of Hsa_circ_0037658 Inhibits the Progression of Osteoarthritis via Inducing Autophagy. Hum. Cel. 34 (1), 76–85. 10.1007/s13577-020-00440-9 [DOI] [PubMed] [Google Scholar]
- Sui C., Zhang L., Hu Y. (2019). MicroRNA-let-7a I-nhibition I-nhibits LPS-induced I-nflammatory I-njury of C-hondrocytes by T-argeting IL6R. Mol. Med. Rep. 20 (3), 2633–2640. 10.3892/mmr.2019.10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. L., Yan J. F., Yu S. B., Zhao J., Lin Q. Q., Jiao K. (2020). MicroRNA-29b Promotes Subchondral Bone Loss in TMJ Osteoarthritis. J. Dent Res. 99 (13), 1469–1477. 10.1177/0022034520937617 [DOI] [PubMed] [Google Scholar]
- Sun J., Zhong N., Li Q., Min Z., Zhao W., Sun Q., et al. (2011). MicroRNAs of Rat Articular Cartilage at Different Developmental Stages Identified by Solexa Sequencing. Osteoarthritis and cartilage 19 (10), 1237–1245. 10.1016/j.joca.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Sun T., Li X., Song H., Gao F., Zhou G., Li X., et al. (2017). MiR-146a Aggravates LPS-Induced Inflammatory Injury by Targeting CXCR4 in the Articular Chondrocytes. Cel Physiol Biochem 44 (4), 1282–1294. 10.1159/000485488 [DOI] [PubMed] [Google Scholar]
- Tan F., Wang D., Yuan Z. (2020). The Fibroblast-like Synoviocyte Derived Exosomal Long Non-coding RNA H19 Alleviates Osteoarthritis Progression through the miR-106b-5p/TIMP2 Axis. Inflammation 43 (4), 1498–1509. 10.1007/s10753-020-01227-8 [DOI] [PubMed] [Google Scholar]
- Tang L., Ding J., Zhou G., Liu Z. (2018a). LncRNA-p21 P-romotes C-hondrocyte A-poptosis in O-steoarthritis by A-cting as a S-ponge for miR-451. Mol. Med. Rep. 18 (6), 5295–5301. 10.3892/mmr.2018.9506 [DOI] [PubMed] [Google Scholar]
- Tang L. P., Ding J. B., Liu Z. H., Zhou G. J. (2018b). LncRNA TUG1 Promotes Osteoarthritis-Induced Degradation of Chondrocyte Extracellular Matrix via miR-195/MMP-13 axis. Eur. Rev. Med. Pharmacol. Sci. 22 (24), 8574–8581. 10.26355/eurrev_201812_16620 [DOI] [PubMed] [Google Scholar]
- Tao H., Cheng L., Yang R. (2020). Downregulation of miR-34a Promotes Proliferation and Inhibits Apoptosis of Rat Osteoarthritic Cartilage Cells by Activating PI3K/Akt Pathway. Clin. Interv. Aging 15, 373–385. 10.2147/CIA.S241855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S.-C., Huang J.-Y., Gao Y., Li Z.-X., Wei Z.-Y., Dawes H., et al. (2021). Small Extracellular Vesicles in Combination with Sleep-Related circRNA3503: A Targeted Therapeutic Agent with Injectable Thermosensitive Hydrogel to Prevent Osteoarthritis. Bioactive Mater. 6 (12), 4455–4469. 10.1016/j.bioactmat.2021.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S.-C., Yuan T., Zhang Y.-L., Yin W.-J., Guo S.-C., Zhang C.-Q. (2017). Exosomes Derived from miR-140-5p-Overexpressing Human Synovial Mesenchymal Stem Cells Enhance Cartilage Tissue Regeneration and Prevent Osteoarthritis of the Knee in a Rat Model. Theranostics 7 (1), 180–195. 10.7150/thno.17133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif G., Hum D., Pelletier J.-P., Duval N., Martel-Pelletier J. (2009). Regulation of the IGFBP-5 and MMP-13 Genes by the microRNAs miR-140 and miR-27a in Human Osteoarthritic Chondrocytes. BMC Musculoskelet. Disord. 10, 148. 10.1186/1471-2474-10-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif G., Pelletier J.-P., Fahmi H., Hum D., Zhang Y., Kapoor M., et al. (2013). NFAT3 and TGF-Β/smad3 Regulate the Expression of miR-140 in Osteoarthritis. Arthritis Res. Ther. 15 (6), R197. 10.1186/ar4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F., Wang J., Zhang Z., Yang J. (2020). LncRNA SNHG7/miR-34a-5p/SYVN1 axis Plays a Vital Role in Proliferation, Apoptosis and Autophagy in Osteoarthritis. Biol. Res. 53 (1), 9. 10.1186/s40659-020-00275-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Su Z., Ma X., Wang F., Guo Y. (2019). Inhibition of miR-203 Ameliorates Osteoarthritis Cartilage Degradation in the Postmenopausal Rat Model: Involvement of Estrogen Receptor α. Hum. Gene Ther. Clin. Develop. 30 (4), 160–168. 10.1089/humc.2019.101 [DOI] [PubMed] [Google Scholar]
- Tian Y., Guo R., Shi B., Chen L., Yang L., Fu Q. (2016). MicroRNA-30a Promotes Chondrogenic Differentiation of Mesenchymal Stem Cells through Inhibiting Delta-like 4 Expression. Life Sci. 148, 220–228. 10.1016/j.lfs.2016.02.031 [DOI] [PubMed] [Google Scholar]
- Toyota M., Suzuki H., Sasaki Y., Maruyama R., Imai K., Shinomura Y., et al. (2008). Epigenetic Silencing of microRNA-34b/c and B-Cell Translocation Gene 4 Is Associated with CpG Island Methylation in Colorectal Cancer. Cancer Res. 68 (11), 4123–4132. 10.1158/0008-5472.CAN-08-0325 [DOI] [PubMed] [Google Scholar]
- Tu Y., Ma T., Wen T., Yang T., Xue L., Cai M., et al. (2020). MicroRNA-377-3p Alleviates IL-1β-caused Chondrocyte Apoptosis and Cartilage Degradation in Osteoarthritis in Part by Downregulating ITGA6. Biochem. biophysical Res. Commun. 523 (1), 46–53. 10.1016/j.bbrc.2019.11.186 [DOI] [PubMed] [Google Scholar]
- Vincent T. L. (2019). Mechanoflammation in Osteoarthritis Pathogenesis. Semin. Arthritis Rheum. 49 (3S), S36–S38. 10.1016/j.semarthrit.2019.09.018 [DOI] [PubMed] [Google Scholar]
- Wan D., Qu Y., Ai S., Cheng L. (2020). miR-152 Attenuates Apoptosis in Chondrocytes and Degeneration of Cartilages in Osteoarthritis Rats via TCF-4 Pathway. Dose-Response 18 (4), 155932582094691. 10.1177/1559325820946918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Hu N., Zhang Y., Chen Y., Su C., Lv Y., et al. (2019a). MEG3 Promotes Proliferation and Inhibits Apoptosis in Osteoarthritis Chondrocytes by miR-361-5p/FOXO1 axis. BMC Med. Genomics 12 (1), 201. 10.1186/s12920-019-0649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-L., Peng J.-P., Chen X.-D. (2018a). LncRNA-CIR Promotes Articular Cartilage Degeneration in Osteoarthritis by Regulating Autophagy. Biochem. biophysical Res. Commun. 505 (3), 692–698. 10.1016/j.bbrc.2018.09.163 [DOI] [PubMed] [Google Scholar]
- Wang C., Li N., Liu Q., Su L., Wang S., Chen Y., et al. (2021a). The Role of circRNA Derived from RUNX2 in the Serum of Osteoarthritis and its Clinical Value. J. Clin. Lab. Anal. 35 (7), e23858. 10.1002/jcla.23858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-D., Zhao X.-W., Zhang Y.-G., Kong Y., Niu S.-S., Ma L.-F., et al. (2017a). Effects of miR-145 on the Inhibition of Chondrocyte Proliferation and Fibrosis by Targeting TNFRSF11B in Human Osteoarthritis. Mol. Med. Rep. 15 (1), 75–80. 10.3892/mmr.2016.5981 [DOI] [PubMed] [Google Scholar]
- Wang G.-L., Wu Y.-B., Liu J.-T., Li C.-Y. (2016a). Upregulation of miR-98 Inhibits Apoptosis in Cartilage Cells in Osteoarthritis. Genet. Test. Mol. biomarkers 20 (11), 645–653. 10.1089/gtmb.2016.0011 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang H., Sun Q., Wang Y., Yang J., Yang J., et al. (2017b). Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol. Ther. 25 (3), 715–727. 10.1016/j.ymthe.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang J., Chen L., Jin S., Lin J., Zheng H., Zhang H., et al. (2017c). Altered Expression of microRNA-98 in IL-1β-induced Cartilage Degradation and its Role in Chondrocyte Apoptosis. Mol. Med. Rep. 16 (3), 3208–3216. 10.3892/mmr.2017.7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen L., Jin S., Lin J., Zheng H., Zhang H., et al. (2016b). MiR-98 Promotes Chondrocyte Apoptosis by Decreasing Bcl-2 Expression in a Rat Model of Osteoarthritis. Acta Biochim. Biophys. Sin 48 (10), 923–929. 10.1093/abbs/gmw084 [DOI] [PubMed] [Google Scholar]
- Wang J., Fang L., Ye L., Ma S., Huang H., Lan X., et al. (2020a). miR-137 Targets the Inhibition of TCF4 to Reverse the Progression of Osteoarthritis through the AMPK/NF-κB Signaling Pathway. Biosci. Rep. 40 (6), BSR20200466. 10.1042/BSR20200466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhu S., Meng N., He Y., Lu R., Yan G.-R. (2019b). ncRNA-Encoded Peptides or Proteins and Cancer. Mol. Ther. 27 (10), 1718–1725. 10.1016/j.ymthe.2019.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Cho K. B., Li Y., Tao G., Xie Z., Guo B. (2019c). Long Noncoding RNA (lncRNA)-Mediated Competing Endogenous RNA Networks Provide Novel Potential Biomarkers and Therapeutic Targets for Colorectal Cancer. Int. J. Mol. Sci. 20 (22), 5758. 10.3390/ijms20225758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang W., Zhang F., Deng Y., Long Z. (2017d). NEAT1/miR‐181c Regulates Osteopontin (OPN)‐Mediated Synoviocyte Proliferation in Osteoarthritis. J. Cel. Biochem. 118 (11), 3775–3784. 10.1002/jcb.26025 [DOI] [PubMed] [Google Scholar]
- Wang T., Liu Y., Wang Y., Huang X., Zhao W., Zhao Z. (2019d). Long Non-coding RNA XIST Promotes Extracellular Matrix Degradation by Functioning as a Competing Endogenous RNA of miR-1277-5p in Osteoarthritis. Int. J. Mol. Med. 44 (2), 630–642. 10.3892/ijmm.2019.4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.-T., Huang Z.-P., Sui S., Liu J.-H., Yu D.-M., Wang W.-B. (2020b). microRNA-1236 Promotes Chondrocyte Apoptosis in Osteoarthritis via Direct Suppression of PIK3R3. Life Sci. 253, 117694. 10.1016/j.lfs.2020.117694 [DOI] [PubMed] [Google Scholar]
- Wang W. F., Liu S. Y., Qi Z. F., Lv Z. H., Ding H. R., Zhou W. J. (2020c). MiR-145 Targeting BNIP3 Reduces Apoptosis of Chondrocytes in Osteoarthritis through Notch Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 24 (16), 8263–8272. 10.26355/eurrev_202008_22622 [DOI] [PubMed] [Google Scholar]
- Wang X.-B., Zhao F.-C., Yi L.-H., Tang J.-L., Zhu Z.-Y., Pang Y., et al. (2019e). MicroRNA-21-5p as a Novel Therapeutic Target for Osteoarthritis. Rheumatology (Oxford) 58, 1485–1497. 10.1093/rheumatology/kez102 [DOI] [PubMed] [Google Scholar]
- Wang X., Guo Y., Wang C., Yu H., Yu X., Yu H. (2016c). MicroRNA-142-3p Inhibits Chondrocyte Apoptosis and Inflammation in Osteoarthritis by Targeting HMGB1. Inflammation 39 (5), 1718–1728. 10.1007/s10753-016-0406-3 [DOI] [PubMed] [Google Scholar]
- Wang Y., Cao L., Wang Q., Huang J., Xu S. (2019f). LncRNA FOXD2-AS1 Induces Chondrocyte Proliferation through Sponging miR-27a-3p in Osteoarthritis. Artif. Cell nanomedicine, Biotechnol. 47 (1), 1241–1247. 10.1080/21691401.2019.1596940 [DOI] [PubMed] [Google Scholar]
- Wang Y., Kong D. (2018). Retracted : MicroRNA‐136 Promotes Lipopolysaccharide‐induced ATDC5 Cell Injury and Inflammatory Cytokine Expression by Targeting Myeloid Cell Leukemia 1. J. Cel Biochem 119 (11), 9316–9326. 10.1002/jcb.27208 [DOI] [PubMed] [Google Scholar]
- Wang Y., Shen S., Li Z., Li W., Weng X. (2020d). MIR-140-5p Affects Chondrocyte Proliferation, Apoptosis, and Inflammation by Targeting HMGB1 in Osteoarthritis. Inflamm. Res. 69 (1), 63–73. 10.1007/s00011-019-01294-0 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu C., Yang Y., Ren Z., Lammi M. J., Guo X. (2019g). Preliminary Exploration of Hsa_circ_0032131 Levels in Peripheral Blood as a Potential Diagnostic Biomarker of Osteoarthritis. Genet. Test. Mol. biomarkers 23 (10), 717–721. 10.1089/gtmb.2019.0036 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu C., Zhang F., Zhang Y., Ren Z., Lammi M. J., et al. (2019h). Screening for Differentially Expressed Circular RNAs in the Cartilage of Osteoarthritis Patients for Their Diagnostic Value. Genet. Test. Mol. biomarkers 23 (10), 706–716. 10.1089/gtmb.2019.0108 [DOI] [PubMed] [Google Scholar]
- Wang Z., Hu J., Pan Y., Shan Y., Jiang L., Qi X., et al. (2018b). miR-140-5p/miR-149 Affects Chondrocyte Proliferation, Apoptosis, and Autophagy by Targeting FUT1 in Osteoarthritis. Inflammation 41 (3), 959–971. 10.1007/s10753-018-0750-6 [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhou N., Wang W., Yu Y., Xia L., Li N. (2021b). HDAC2 Interacts with microRNA-503-5p to Regulate SGK1 in Osteoarthritis. Arthritis Res. Ther. 23 (1), 78. 10.1186/s13075-020-02373-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M., Xie Q., Zhu J., Wang T., Zhang F., Cheng Y., et al. (2016). MicroRNA-33 Suppresses CCL2 Expression in Chondrocytes. Biosci. Rep. 36 (3), BSR20160068. 10.1042/BSR20160068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., He S., Wang Z., Dong J., Xiang D., Li Y., et al. (2019). LINC01534 Promotes the Aberrant Metabolic Dysfunction and Inflammation in IL-1β-Simulated Osteoarthritic Chondrocytes by Targeting miR-140-5p. Cartilage 2019, 194760351988878. 10.1177/1947603519888787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Li H., Sun H., Zeng A., Lin R., Zhao J., et al. (2020). MiR-455-3p Reduces Apoptosis and Alleviates Degeneration of Chondrocyte through Regulating PI3K/AKT Pathway. Life Sci. 253, 117718. 10.1016/j.lfs.2020.117718 [DOI] [PubMed] [Google Scholar]
- Woods S., Barter M. J., Elliott H. R., McGillivray C. M., Birch M. A., Clark I. M., et al. (2019). miR-324-5p Is up Regulated in End-Stage Osteoarthritis and Regulates Indian Hedgehog Signalling by Differing Mechanisms in Human and Mouse. Matrix Biol. 77, 87–100. 10.1016/j.matbio.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.-P., Zhang J.-L., Wang J.-Y., Cui M.-X., Jia J.-L., Liu X.-H., et al. (2017a). MiR-1246 Promotes LPS-Induced Inflammatory Injury in Chondrogenic Cells ATDC5 by Targeting HNF4γ. Cel Physiol Biochem 43 (5), 2010–2021. 10.1159/000484162 [DOI] [PubMed] [Google Scholar]
- Wu J., Zou M., Ping A., Deng Z., Cai L. (2018a). MicroRNA-449a Upregulation Promotes Chondrocyte Extracellular Matrix Degradation in Osteoarthritis. Biomed. Pharmacother. 105, 940–946. 10.1016/j.biopha.2018.06.074 [DOI] [PubMed] [Google Scholar]
- Wu Q., Yuan Z. H., Ma X. B., Tang X. H. (2020). Low Expression of CircRNA HIPK3 Promotes Osteoarthritis Chondrocyte Apoptosis by Serving as a Sponge of miR-124 to Regulate SOX8. Eur. Rev. Med. Pharmacol. Sci. 24 (15), 7937–7945. 10.26355/eurrev_202008_22476 [DOI] [PubMed] [Google Scholar]
- Wu X.-F., Zhou Z.-H., Zou J. (2017b). MicroRNA-181 Inhibits Proliferation and Promotes Apoptosis of Chondrocytes in Osteoarthritis by Targeting PTEN. Biochem. Cel Biol. 95 (3), 437–444. 10.1139/bcb-2016-0078 [DOI] [PubMed] [Google Scholar]
- Wu X., Crawford R., Xiao Y., Mao X., Prasadam I. (2021a). Osteoarthritic Subchondral Bone Release Exosomes that Promote Cartilage Degeneration. Cells 10 (2), 251. 10.3390/cells10020251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. H., Liu W., Zhang L., Liu X. Y., Wang Y., Xue B., et al. (2018b). Retracted : Effects of microRNA‐24 Targeting C‐myc on Apoptosis, Proliferation, and Cytokine Expressions in Chondrocytes of Rats with Osteoarthritis via MAPK Signaling Pathway. J. Cel. Biochem. 119 (10), 7944–7958. 10.1002/jcb.26514 [DOI] [PubMed] [Google Scholar]
- Wu Y., Hong Z., Xu W., Chen J., Wang Q., Chen J., et al. (2021b). Circular RNA circPDE4D Protects against Osteoarthritis by Binding to miR-103a-3p and Regulating FGF18. Mol. Ther. 29 (1), 308–323. 10.1016/j.ymthe.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Lu X., Shen B., Zeng Y. (2019). The Therapeutic Potential and Role of miRNA, lncRNA, and circRNA in Osteoarthritis. Curr. Gene Ther. 19 (4), 255–263. 10.2174/1566523219666190716092203 [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang Y., Zhang Y., Wang J.-J. (2017c). CircRNA Hsa_circ_0005105 Upregulates NAMPT Expression and Promotes Chondrocyte Extracellular Matrix Degradation by Sponging miR-26a. Cel Biol Int 41 (12), 1283–1289. 10.1002/cbin.10761 [DOI] [PubMed] [Google Scholar]
- Xi P., Zhang C. l., Wu S. y., Liu L., Li W. j., Li Y. m. (2021). CircRNA circ‐IQGAP1 Knockdown Alleviates Interleukin‐1β‐Induced Osteoarthritis Progression via Targeting miR‐671‐5p/TCF4. Orthop. Surg. 13 (3), 1036–1046. 10.1111/os.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Xia Z., Feng B., Bian Y., Fan Y., Li Z., et al. (2019a). Circular RNA Expression Profile of Knee Condyle in Osteoarthritis by Illumina HiSeq Platform. J. Cel Biochem 120 (10), 17500–17511. 10.1002/jcb.29014 [DOI] [PubMed] [Google Scholar]
- Xiao P., Zhu X., Sun J., Zhang Y., Qiu W., Li J., et al. (2021). LncRNA NEAT1 Regulates Chondrocyte Proliferation and Apoptosis via Targeting miR-543/PLA2G4A axis. Hum. Cel. 34 (1), 60–75. 10.1007/s13577-020-00433-8 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Bao Y., Tang L., Wang L. (2019b). LncRNA MIR4435-2HG Is Downregulated in Osteoarthritis and Regulates Chondrocyte Cell Proliferation and Apoptosis. J. Orthop. Surg. Res. 14 (1), 247. 10.1186/s13018-019-1278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Yan X., Yang Y., Ma X. (2019c). Downregulation of Long Noncoding RNA HOTAIRM1 Variant 1 Contributes to Osteoarthritis via Regulating miR-125b/BMPR2 axis and Activating JNK/MAPK/ERK Pathway. Biomed. Pharmacother. 109, 1569–1577. 10.1016/j.biopha.2018.10.181 [DOI] [PubMed] [Google Scholar]
- Xing D., Liang J.-q., Li Y., Lu J., Jia H.-b., Xu L.-y., et al. (2014). Identification of Long Noncoding RNA Associated with Osteoarthritis in Humans. Orthopaedic Surg. 6 (4), 288–293. 10.1111/os.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Li Y.-y., Ma J., Pei F.-x. (2016). Roles of microRNA and Signaling Pathway in Osteoarthritis Pathogenesis. J. Zhejiang Univ. Sci. B 17 (3), 200–208. 10.1631/jzus.B1500267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Ma X. (2021). Hsa_circ_0032131 Knockdown Inhibits Osteoarthritis Progression via the miR-502-5p/PRDX3 axis. Aging 13 (11), 15100–15113. 10.18632/aging.203073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Pei Y., Lu J., Liang X., Li Y., Wang J., et al. (2021). LncRNA SNHG7 Alleviates IL-1β-induced Osteoarthritis by Inhibiting miR-214-5p-Mediated PPARGC1B Signaling Pathways. Int. immunopharmacology 90, 107150. 10.1016/j.intimp.2020.107150 [DOI] [PubMed] [Google Scholar]
- Xu J., Xu Y. (2017). The lncRNA MEG3 Downregulation Leads to Osteoarthritis Progression via miR-16/SMAD7 axis. Cell Biosci 7, 69. 10.1186/s13578-017-0195-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Meng Z., Xian X.-M., Deng M.-H., Meng Q.-G., Fang W., et al. (2020). LncRNA PVT1 Induces Chondrocyte Apoptosis through Upregulation of TNF-α in Synoviocytes by Sponging miR-211-3p. Mol. Cell. probes 52, 101560. 10.1016/j.mcp.2020.101560 [DOI] [PubMed] [Google Scholar]
- Xue H., Yu P., Wang W. Z., Niu Y. Y., Li X. (2020). The Reduced lncRNA NKILA Inhibited Proliferation and Promoted Apoptosis of Chondrocytes via miR-145/sp1/nf-Κb Signaling in Human Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 24 (2), 535–548. 10.26355/eurrev_202001_20030 [DOI] [PubMed] [Google Scholar]
- Xue H., Tu Y., Ma T., Wen T., Yang T., Xue L., et al. (2019). miR-93-5p Attenuates IL-1β-induced Chondrocyte Apoptosis and Cartilage Degradation in Osteoarthritis Partially by Targeting TCF4. Bone 123, 129–136. 10.1016/j.bone.2019.03.035 [DOI] [PubMed] [Google Scholar]
- Yan S., Wang M., Zhao J., Zhang H., Zhou C., Jin L., et al. (2016). MicroRNA-34a Affects Chondrocyte Apoptosis and Proliferation by Targeting the SIRT1/p53 Signaling Pathway during the Pathogenesis of Osteoarthritis. Int. J. Mol. Med. 38 (1), 201–209. 10.3892/ijmm.2016.2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Ni J., Long H., Huang J., Yang C., Huang X. (2018a). IL‐1β‐induced miR‐34a Up‐regulation Inhibits Cyr61 to Modulate Osteoarthritis Chondrocyte Proliferation through ADAMTS‐4. J. Cel. Biochem. 119 (10), 7959–7970. 10.1002/jcb.26600 [DOI] [PubMed] [Google Scholar]
- Yang B., Xu L., Wang S. (2020a). Regulation of lncRNA-H19/miR-140-5p in Cartilage Matrix Degradation and Calcification in Osteoarthritis. Ann. Palliat. Med. 9 (4), 1896–1904. 10.21037/apm-20-929 [DOI] [PubMed] [Google Scholar]
- Yang D. W., Zhang X., Qian G. B., Jiang M. J., Wang P., Wang K. Z. (2019a). Downregulation of Long Noncoding RNA LOC101928134 Inhibits the Synovial Hyperplasia and Cartilage Destruction of Osteoarthritis Rats through the Activation of the Janus Kinase/signal Transducers and Activators of Transcription Signaling Pathway by Upregulating IFNA1. J. Cel Physiol 234 (7), 10523–10534. 10.1002/jcp.27730 [DOI] [PubMed] [Google Scholar]
- Yang F., Huang R., Ma H., Zhao X., Wang G. (2020b). miRNA-411 Regulates Chondrocyte Autophagy in Osteoarthritis by Targeting Hypoxia-Inducible Factor 1 Alpha (HIF-1α). Med. Sci. Monit. 26, e921155. 10.12659/MSM.921155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Tang K., Qiao L., Li Y., Sun S. (2021a). Identification of Critical Genes and lncRNAs in Osteolysis after Total Hip Arthroplasty and Osteoarthritis by RNA Sequencing. Biomed. Research International 2021, 1–13. 10.1155/2021/6681925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wu D., Li H., Chen N., Shang Y. (2018b). Downregulation of microRNA-448 Inhibits IL-1β-induced Cartilage Degradation in Human Chondrocytes via Upregulation of Matrilin-3. Cell Mol Biol Lett 23, 7. 10.1186/s11658-018-0072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Yao Y., Zhao D., Zou H., Lai C., Xiang G., et al. (2021b). LncRNA H19 Secreted by Umbilical Cord Blood Mesenchymal Stem Cells through microRNA-29a-3p/FOS axis for central Sensitization of Pain in Advanced Osteoarthritis. Am. J. Transl Res. 13 (3), 1245–1256. [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Zhou Y., Cai P., Fu W., Wang J., Wei Q., et al. (2019b). Downregulation of microRNA-23b-3p Alleviates IL-1β-induced Injury in Chondrogenic CHON-001 Cells. Drug Des. Devel Ther. 13, 2503–2512. 10.2147/DDDT.S211051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Shen P., Yao T., Ma J., Chen Z., Zhu J., et al. (2021c). Novel Role of circRSU1 in the Progression of Osteoarthritis by Adjusting Oxidative Stress. Theranostics 11 (4), 1877–1900. 10.7150/thno.53307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xing D., Wang Y., Jia H., Li B., Li J. J. (2020c). A Long Non-coding RNA, HOTAIR, Promotes Cartilage Degradation in Osteoarthritis by Inhibiting WIF-1 Expression and Activating Wnt Pathway. BMC Mol. Cel Biol 21 (1), 53. 10.1186/s12860-020-00299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yujiao W., Fang W., Linhui Y., Ziqi G., Zhichen W., et al. (2020d). The Roles of miRNA, lncRNA and circRNA in the Development of Osteoporosis. Biol. Res. 53 (1), 40. 10.1186/s40659-020-00309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Tang Y., Lu H., Shi B., Ye Y., Xu G., et al. (2018c). Retracted : Long Non‐coding RNA Reprogramming (lncRNA‐ROR) Regulates Cell Apoptosis and Autophagy in Chondrocytes. J. Cel. Biochem. 119 (10), 8432–8440. 10.1002/jcb.27057 [DOI] [PubMed] [Google Scholar]
- Yao Q., Chen Y., Zhou X. (2019). The Roles of microRNAs in Epigenetic Regulation. Curr. Opin. Chem. Biol. 51, 11–17. 10.1016/j.cbpa.2019.01.024 [DOI] [PubMed] [Google Scholar]
- Ye D., Jian W., Feng J., Liao X. (2018). Role of Long Noncoding RNA ZFAS1 in Proliferation, Apoptosis and Migration of Chondrocytes in Osteoarthritis. Biomed. Pharmacother. 104, 825–831. 10.1016/j.biopha.2018.04.124 [DOI] [PubMed] [Google Scholar]
- Ying H., Wang Y., Gao Z., Zhang Q. (2019). Long Non-coding RNA Activated by Transforming Growth Factor Beta Alleviates Lipopolysaccharide-Induced Inflammatory Injury via Regulating microRNA-223 in ATDC5 Cells. Int. immunopharmacology 69, 313–320. 10.1016/j.intimp.2019.01.056 [DOI] [PubMed] [Google Scholar]
- You D., Yang C., Huang J., Gong H., Yan M., Ni J. (2019). Long Non-coding RNA MEG3 Inhibits Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells by Epigenetically Inhibiting TRIB2 via Methyltransferase EZH2. Cell Signal. 63, 109379. 10.1016/j.cellsig.2019.109379 [DOI] [PubMed] [Google Scholar]
- Yu C., Shi D., Li Z., Wan G., Shi X. (2019a). Retracted : Long Noncoding RNA CHRF Exacerbates IL‐6‐induced Inflammatory Damages by Downregulating microRNA‐146a in ATDC5 Cells. J. Cel Physiol 234 (12), 21851–21859. 10.1002/jcp.28749 [DOI] [PubMed] [Google Scholar]
- Yu C., Wang Y. (2018). RETRACTED: MicroRNA-19a Promotes Cell Viability and Migration of Chondrocytes via Up-Regulating SOX9 through NF-Κb Pathway. Biomed. Pharmacother. 98, 746–753. 10.1016/j.biopha.2017.11.132 [DOI] [PubMed] [Google Scholar]
- Yu J., Qin Y., Zhou N. (2021). Knockdown of Circ_SLC39A8 Protects against the Progression of Osteoarthritis by Regulating miR-591/IRAK3 axis. J. Orthop. Surg. Res. 16 (1), 170. 10.1186/s13018-021-02323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Zhao B., He Q., Zhang Y., Peng X. B. (2019b). microRNA‐206 Is Required for Osteoarthritis Development through its Effect on Apoptosis and Autophagy of Articular Chondrocytes via Modulating the Phosphoinositide 3‐kinase/protein Kinase B‐mTOR Pathway by Targeting Insulin‐like Growth Factor‐1. J. Cel Biochem 120 (4), 5287–5303. 10.1002/jcb.27803 [DOI] [PubMed] [Google Scholar]
- Yu X. P., Liu C. G., Qiu F., Xu Y. Q., Xing F., Yin J. Q., et al. (2020). CircRNA_100395 Protects Breast Carcinoma Deterioration by Targeting MAPK6. Eur. Rev. Med. Pharmacol. Sci. 24 (23), 12216–12223. 10.26355/eurrev_202012_24012 [DOI] [PubMed] [Google Scholar]
- Zang J., Lu D., Xu A. (2020). The Interaction of circRNAs and RNA Binding Proteins: An Important Part of circRNA Maintenance and Function. J. Neurosci. Res. 98 (1), 87–97. 10.1002/jnr.24356 [DOI] [PubMed] [Google Scholar]
- Zhai X., Meng R., Li H., Li J., Jing L., Qin L., et al. (2017). miR-181a Modulates Chondrocyte Apoptosis by Targeting Glycerol-3-Phosphate Dehydrogenase 1-Like Protein (GPD1L) in Osteoarthritis. Med. Sci. Monit. 23, 1224–1231. 10.12659/msm.899228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Sun M., Wang J., Ma C., Hao T., Liu G., et al. (2019a). MiR-671 Ameliorates the Progression of Osteoarthritis In Vitro and In Vivo . Pathol. - Res. Pract. 215 (7), 152423. 10.1016/j.prp.2019.04.015 [DOI] [PubMed] [Google Scholar]
- Zhang C.-Y., Yang C.-Q., Chen Q., Liu J., Zhang G., Dong C., et al. (2021a). miR-194-Loaded Gelatin Nanospheres Target MEF2C to Suppress Muscle Atrophy in a Mechanical Unloading Model. Mol. Pharmaceutics 18 (8), 2959–2973. 10.1021/acs.molpharmaceut.1c00121 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang P., Jiang P., Lv Y., Dong C., Dai X., et al. (2016a). Upregulation of lncRNA HOTAIR Contributes to IL-1β-induced MMP Overexpression and Chondrocytes Apoptosis in Temporomandibular Joint Osteoarthritis. Gene 586 (2), 248–253. 10.1016/j.gene.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang Z., Chang Z., Mao G., Hu S., Zeng A., et al. (2019b). miR‐193b‐5p Regulates Chondrocytes Metabolism by Directly Targeting Histone Deacetylase 7 in Interleukin‐1β‐induced Osteoarthritis. J. Cel Biochem 120 (8), 12775–12784. 10.1002/jcb.28545 [DOI] [PubMed] [Google Scholar]
- Zhang D., Cao X., Li J., Zhao G. (2015). MiR-210 Inhibits NF-Κb Signaling Pathway by Targeting DR6 in Osteoarthritis. Sci. Rep. 5, 12775. 10.1038/srep12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Wang K., Wei W., Liu Y., Liu S. (2021b). Multifunctional Plasmonic Core-Satellites Nanoprobe for Cancer Diagnosis and Therapy Based on a Cascade Reaction Induced by MicroRNA. Anal. Chem. 93 (27), 9521–9530. 10.1021/acs.analchem.1c01539 [DOI] [PubMed] [Google Scholar]
- Zhang F. e., Lammi M. J., Tan S., Meng P., Wu C., Guo X. (2020a). Cell Cycle-Related lncRNAs and mRNAs in Osteoarthritis Chondrocytes in a Northwest Chinese Han Population. Medicine 99 (24), e19905. 10.1097/MD.0000000000019905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. Q., Wang Z., Zhang H., Liu L., Luo X. L., Liu W. W. (2019c). MiR-27a Alleviates Osteoarthritis in Rabbits via Inhibiting Inflammation. Eur. Rev. Med. Pharmacol. Sci. 23 (3 Suppl. l), 89–95. 10.26355/eurrev_201908_18634 [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhang R., Zhang X., Wu Y., Li X., Zhang S., et al. (2018a). Comprehensive Analysis of circRNA Expression Pattern and circRNA-miRNA-mRNA Network in the Pathogenesis of Atherosclerosis in Rabbits. Aging 10 (9), 2266–2283. 10.18632/aging.101541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Sun Y., Wang Y., Liu R., Bao Y., Li Q. (2016b). MiR-502-5p Inhibits IL-1β-induced Chondrocyte Injury by Targeting TRAF2. Cell Immunol. 302, 50–57. 10.1016/j.cellimm.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Zhang G., Wu Y., Xu D., Yan X. (2016c). Long Noncoding RNA UFC1 Promotes Proliferation of Chondrocyte in Osteoarthritis by Acting as a Sponge for miR-34a. DNA Cel. Biol. 35 (11), 691–695. 10.1089/dna.2016.3397 [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang Q., Zhu J., Tang J., Nie M. (2020b). LncRNA ARFRP1 Knockdown Inhibits LPS-Induced the Injury of Chondrocytes by Regulation of NF-Κb Pathway through Modulating miR-15a-5p/TLR4 axis. Life Sci. 261, 118429. 10.1016/j.lfs.2020.118429 [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhou Y., Su M., Yang X., Zeng B. (2020c). Inhibition of microRNA ‐27b‐3p Relieves Osteoarthritis Pain via Regulation of KDM4B ‐dependent DLX5. BioFactors 46 (5), 788–802. 10.1002/biof.1670 [DOI] [PubMed] [Google Scholar]
- Zhang H., Chen C., Cui Y., Li Y., Wang Z., Mao X., et al. (2019d). lnc-SAMD14-4 Can Regulate Expression of the COL1A1 and COL1A2 in Human Chondrocytes. PeerJ 7, e7491. 10.7717/peerj.7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Li J., Shao W., Shen N. (2020d). LncRNA CTBP1-AS2 Is Upregulated in Osteoarthritis and Increases the Methylation of miR-130a Gene to Inhibit Chondrocyte Proliferation. Clin. Rheumatol. 39 (11), 3473–3478. 10.1007/s10067-020-05113-4 [DOI] [PubMed] [Google Scholar]
- Zhang H., Li J., Shao W., Shen N. (2020e). LncRNA SNHG9 Is Downregulated in Osteoarthritis and Inhibits Chondrocyte Apoptosis by Downregulating miR-34a through Methylation. BMC Musculoskelet. Disord. 21 (1), 511. 10.1186/s12891-020-03497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cheng F., Rong G., Tang Z., Gui B. (2020f). Hsa_circ_0005567 Activates Autophagy and Suppresses IL-1β-Induced Chondrocyte Apoptosis by Regulating miR-495. Front. Mol. Biosci. 7, 216. 10.3389/fmolb.2020.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. L. (2020). CircRNA-PTPRA Promoted the Progression of Atherosclerosis through Sponging with miR-636 and Upregulating the Transcription Factor SP1. Eur. Rev. Med. Pharmacol. Sci. 24 (23), 12437–12449. 10.26355/eurrev_202012_24039 [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang P., Sun X., Zhou L., Zhao J. (2018b). Long Non-coding RNA DANCR Regulates Proliferation and Apoptosis of Chondrocytes in Osteoarthritis via miR-216a-5p-JAK2-STAT3 axis. Biosci. Rep. 38 (6), BSR20181228. 10.1042/BSR20181228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Gao G., Zhou Z., He X. (2021c). microRNA-130b Downregulation Potentiates Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Targeting SOX9. Braz. J. Med. Biol. Res. 54 (4), e10345. 10.1590/1414-431X202010345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Sun J., Liang C., Gu B., Xu Y., Lu H., et al. (2020g). lncRNA IGHCγ1 Acts as a ceRNA to Regulate Macrophage Inflammation via the miR-6891-3p/TLR4 Axis in Osteoarthritis. Mediators Inflamm. 2020, 1–11. 10.1155/2020/9743037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Qiao X., Xia W. (2020h). CircSERPINE2 Weakens IL-1β-caused Apoptosis and Extracellular Matrix Degradation of Chondrocytes by Regulating miR-495/TGFBR2 axis. Biosci. Rep. 40 (11), BSR20201601. 10.1042/BSR20201601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang Y., Zhang M., Ying H. (2019e). Retracted : Green tea Polyphenols Attenuate LPS‐induced Inflammation through Upregulating microRNA‐9 in Murine Chondrogenic ATDC5 Cells. J. Cel Physiol 234 (12), 22604–22612. 10.1002/jcp.28826 [DOI] [PubMed] [Google Scholar]
- Zhang W., Cheng P., Hu W., Yin W., Guo F., Chen A., et al. (2020i). Correction: Inhibition of microRNA-384-5p Alleviates Osteoarthritis through its Effects on Inhibiting Apoptosis of Cartilage Cells via the NF-Κb Signaling Pathway by Targeting SOX9. Cancer Gene Ther. 27 (10-11), 836–837. 10.1038/s41417-020-0202-y [DOI] [PubMed] [Google Scholar]
- Zhang W., Cheng P., Hu W., Yin W., Guo F., Chen A., et al. (2018c). Downregulated microRNA‐340‐5p Promotes Proliferation and Inhibits Apoptosis of Chondrocytes in Osteoarthritis Mice through Inhibiting the Extracellular Signal‐regulated Kinase Signaling Pathway by Negatively Targeting the FMOD Gene. J. Cel Physiol 234 (1), 927–939. 10.1002/jcp.26921 [DOI] [PubMed] [Google Scholar]
- Zhang W., Hsu P., Zhong B., Guo S., Zhang C., Wang Y., et al. (2018d). MiR-34a Enhances Chondrocyte Apoptosis, Senescence and Facilitates Development of Osteoarthritis by Targeting DLL1 and Regulating PI3K/AKT Pathway. Cel Physiol Biochem 48 (3), 1304–1316. 10.1159/000492090 [DOI] [PubMed] [Google Scholar]
- Zhang W., Hu C., Zhang C., Luo C., Zhong B., Yu X. (2021d). MiRNA-132 Regulates the Development of Osteoarthritis in Correlation with the Modulation of PTEN/PI3K/AKT Signaling. BMC Geriatr. 21 (1), 175. 10.1186/s12877-021-02046-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Qi L., Chen R., He J., Liu Z., Wang W., et al. (2021e). Circular RNAs in Osteoarthritis: Indispensable Regulators and Novel Strategies in Clinical Implications. Arthritis Res. Ther. 23 (1), 23. 10.1186/s13075-021-02420-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang C., Hu C., Luo C., Zhong B., Yu X. (2020j). Circular RNA-CDR1as Acts as the Sponge of microRNA-641 to Promote Osteoarthritis Progression. J. Inflamm. 17, 8. 10.1186/s12950-020-0234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhong B., Zhang C., Luo C., Zhan Y. (2018e). miR-373 Regulates Inflammatory Cytokine-Mediated Chondrocyte Proliferation in Osteoarthritis by Targeting the P2X7 Receptor. FEBS open bio 8 (3), 325–331. 10.1002/2211-5463.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huang C.-R., Pan S., Pang Y., Chen Y.-S., Zha G.-C., et al. (2020k). Long Non-coding RNA SNHG15 Is a Competing Endogenous RNA of miR-141-3p that Prevents Osteoarthritis Progression by Upregulating BCL2L13 Expression. Int. immunopharmacology 83, 106425. 10.1016/j.intimp.2020.106425 [DOI] [PubMed] [Google Scholar]
- Zhang X., Liang H., Kourkoumelis N., Wu Z., Li G., Shang X. (2020l). Comprehensive Analysis of lncRNA and miRNA Expression Profiles and ceRNA Network Construction in Osteoporosis. Calcif Tissue Int. 106 (4), 343–354. 10.1007/s00223-019-00643-9 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang C., Zhao J., Xu J., Geng Y., Dai L., et al. (2017). miR-146a Facilitates Osteoarthritis by Regulating Cartilage Homeostasis via Targeting Camk2d and Ppp3r2. Cel Death Dis 8 (4), e2734. 10.1038/cddis.2017.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhu X.-L., Ji B.-Y., Cao X., Yu L.-J., Zhang Y., et al. (2019f). LncRNA-1810034E14Rik Reduces Microglia Activation in Experimental Ischemic Stroke. J. Neuroinflammation 16 (1), 75. 10.1186/s12974-019-1464-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Dong Q., Sun X. (2020m). Positive Feedback Loop LINC00511/miR-150-5p/SP1 Modulates Chondrocyte Apoptosis and Proliferation in Osteoarthritis. DNA Cel. Biol. 39 (9), 1506–1512. 10.1089/dna.2020.5718 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Jia J., Yang S., Liu X., Ye S., Tian H. (2014). MicroRNA-21 Controls the Development of Osteoarthritis by Targeting GDF-5 in Chondrocytes. Exp. Mol. Med. 46, e79. 10.1038/emm.2013.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ma L., Wang C., Wang L., Guo Y., Wang G. (2020n). Long Noncoding RNA LINC00461 Induced Osteoarthritis Progression by Inhibiting miR-30a-5p. Aging 12 (5), 4111–4123. 10.18632/aging.102839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang F., Chen G., He R., Yang L. (2019g). LncRNA MALAT1 Promotes Osteoarthritis by Modulating miR-150-5p/AKT3 axis. Cel Biosci 9, 54. 10.1186/s13578-019-0302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-Y., Gao X.-H., Ma M.-Y., Zhao C.-L., Zhang Y.-L., Guo S.-S. (2020o). CircRNA_101237 Promotes NSCLC Progression via the miRNA-490-3p/MAPK1 axis. Sci. Rep. 10 (1), 9024. 10.1038/s41598-020-65920-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Wang Y., Jin H., Yu T. (2017). Knockdown of microRNA-203 Alleviates LPS-Induced Injury by Targeting MCL-1 in C28/I2 Chondrocytes. Exp. Cel. Res. 359 (1), 171–178. 10.1016/j.yexcr.2017.07.034 [DOI] [PubMed] [Google Scholar]
- Zhao G., Gu W. (2020). Effects of miR-146a-5p on Chondrocyte Interleukin-1β-Induced Inflammation and Apoptosis Involving Thioredoxin Interacting Protein Regulation. J. Int. Med. Res. 48 (11), 030006052096955. 10.1177/0300060520969550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Gong N. (2019). miR-20a Regulates Inflammatory in Osteoarthritis by Targeting the IκBβ and Regulates NK-Κb Signaling Pathway Activation. Biochem. biophysical Res. Commun. 518 (4), 632–637. 10.1016/j.bbrc.2019.08.109 [DOI] [PubMed] [Google Scholar]
- Zhao J., Li T., Luo W. (2021). Silencing of Circ-PRKCH Protects against Lipopolysaccharide (LPS)-evoked Chondrocyte Damage and Extracellular Matrix Loss by the miR-140-3p/ADAM10 axis. Gen. Physiol. Biophys. 40 (2), 89–101. 10.4149/gpb_2021001 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Yang Y., Ren G., Ge E., Fan C. (2019a). Integrating Bipartite Network Projection and KATZ Measure to Identify Novel CircRNA-Disease Associations. IEEE Trans.on Nanobioscience 18 (4), 578–584. 10.1109/TNB.2019.2922214 [DOI] [PubMed] [Google Scholar]
- Zhao X., Li H., Wang L. (2019b). MicroRNA-107 Regulates Autophagy and Apoptosis of Osteoarthritis Chondrocytes by Targeting TRAF3. Int. immunopharmacology 71, 181–187. 10.1016/j.intimp.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao J., Guo X., She J., Liu Y. (2018). Long Non-coding RNA PVT1, a Molecular Sponge for miR-149, Contributes Aberrant Metabolic Dysfunction and Inflammation in IL-1β-simulated Osteoarthritic Chondrocytes. Biosci. Rep. 38 (5), BSR20180576. 10.1042/BSR20180576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Dai X.-S., Wang Z.-Y., Bao Z.-Q., Guan J.-Z. (2019c). MicroRNA-26a Reduces Synovial Inflammation and Cartilage Injury in Osteoarthritis of Knee Joints through Impairing the NF-Κb Signaling Pathway. Biosci. Rep. 39 (4), BSR20182025. 10.1042/BSR20182025 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zheng W., Hou G., Li Y. (2021). Circ_0116061 Regulated the Proliferation, Apoptosis, and Inflammation of Osteoarthritis Chondrocytes through Regulating the miR-200b-3p/SMURF2 axis. J. Orthop. Surg. Res. 16 (1), 253. 10.1186/s13018-021-02391-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zhao F.-C., Pang Y., Li D.-Y., Yao S.-C., Sun S.-S., et al. (2017). Downregulation of miR-221-3p Contributes to IL-1β-induced Cartilage Degradation by Directly Targeting the SDF1/CXCR4 Signaling Pathway. J. Mol. Med. 95 (6), 615–627. 10.1007/s00109-017-1516-6 [DOI] [PubMed] [Google Scholar]
- Zhi L., Zhao J., Zhao H., Qing Z., Liu H., Ma J. (2020). Downregulation of LncRNA OIP5-AS1 Induced by IL-1β Aggravates Osteoarthritis via Regulating miR-29b-3p/PGRN. Cartilage 2020, 194760351990080. 10.1177/1947603519900801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.-H., Li J., Liu C.-F., Liu N., Bian R.-X., Zhao S.-M., et al. (2017). Effects of microRNA-146a on the Proliferation and Apoptosis of Human Osteoarthritis Chondrocytes by Targeting TRAF6 through the NF-Κb Signalling Pathway. Biosci. Rep. 37 (2), BSR20160578. 10.1042/BSR20160578 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhou B., Li H., Shi J. (2017). miR-27 Inhibits the NF-Κb Signaling Pathway by Targeting Leptin in Osteoarthritic Chondrocytes. Int. J. Mol. Med. 40 (2), 523–530. 10.3892/ijmm.2017.3021 [DOI] [PubMed] [Google Scholar]
- Zhou C., He T., Chen L. (2021a). LncRNA CASC19 Accelerates Chondrocytes Apoptosis and Proinflammatory Cytokine Production to Exacerbate Osteoarthritis Development through Regulating the miR-152-3p/DDX6 axis. J. Orthop. Surg. Res. 16 (1), 399. 10.1186/s13018-021-02543-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. L., Deng S., Fang H. S., Du X. j., Peng H., Hu Q. j. (2021b). Circular RNA circANKRD36 Regulates Casz1 by Targeting miR‐599 to Prevent Osteoarthritis Chondrocyte Apoptosis and Inflammation. J. Cel. Mol. Med. 25 (1), 120–131. 10.1111/jcmm.15884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. X., Tian Z. G., Zhu L. F., Wu W. D., Zhou S. L., Zhao Y. T., et al. (2018a). MicroRNA-615-3p Promotes the Osteoarthritis Progression by Inhibiting Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 22 (19), 6212–6220. 10.26355/eurrev_201810_16027 [DOI] [PubMed] [Google Scholar]
- Zhou L., Gu M., Ma X., Wen L., Zhang B., Lin Y., et al. (2021c). Long Non-coding RNA PCAT-1 Regulates Apoptosis of Chondrocytes in Osteoarthritis by Sponging miR-27b-3p. J. Bone Miner Metab. 39 (2), 139–147. 10.1007/s00774-020-01128-8 [DOI] [PubMed] [Google Scholar]
- Zhou M., Zhang Z., Zhao H., Bao S., Cheng L., Sun J. (2018b). An Immune-Related Six-lncRNA Signature to Improve Prognosis Prediction of Glioblastoma Multiforme. Mol. Neurobiol. 55 (5), 3684–3697. 10.1007/s12035-017-0572-9 [DOI] [PubMed] [Google Scholar]
- Zhou X., Jiang L., Fan G., Yang H., Wu L., Huang Y., et al. (2019a). Role of the ciRS-7/miR-7 axis in the Regulation of Proliferation, Apoptosis and Inflammation of Chondrocytes Induced by IL-1β. Int. immunopharmacology 71, 233–240. 10.1016/j.intimp.2019.03.037 [DOI] [PubMed] [Google Scholar]
- Zhou X., Li J., Zhou Y., Yang Z., Yang H., Li D., et al. (2020a). Down-regulated ciRS-7/up-Regulated miR-7 axis Aggravated Cartilage Degradation and Autophagy Defection by PI3K/AKT/mTOR Activation Mediated by IL-17A in Osteoarthritis. Aging 12 (20), 20163–20183. 10.18632/aging.103731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Luo D., Sun H., Qi Y., Xu W., Jin X., et al. (2018c). MiR‐132‐3p Regulates ADAMTS‐5 Expression and Promotes Chondrogenic Differentiation of Rat Mesenchymal Stem Cells. J. Cel. Biochem. 119 (3), 2579–2587. 10.1002/jcb.26421 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Ming J., Li Y., Li B., Deng M., Ma Y., et al. (2021d). Exosomes Derived from miR-126-3p-Overexpressing Synovial Fibroblasts Suppress Chondrocyte Inflammation and Cartilage Degradation in a Rat Model of Osteoarthritis. Cell Death Discov. 7 (1), 37. 10.1038/s41420-021-00418-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang Z., Chen X., Zhang J., Yang L., Liu S., et al. (2020b). Identification of Differentially Expressed miRNAs and mRNAs in Synovial of Osteoarthritis via RNA-Sequencing. BMC Med. Genet. 21 (1), 46. 10.1186/s12881-020-0978-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.-B., Du D., Huang G.-X., Chen A., Zhu L. (2018d). Circular RNA Atp9b, a Competing Endogenous RNA, Regulates the Progression of Osteoarthritis by Targeting miR-138-5p. Gene 646, 203–209. 10.1016/j.gene.2017.12.064 [DOI] [PubMed] [Google Scholar]
- Zhou Z.-B., Huang G.-X., Fu Q., Han B., Lu J.-J., Chen A.-M., et al. (2019b). circRNA.33186 Contributes to the Pathogenesis of Osteoarthritis by Sponging miR-127-5p. Mol. Ther. 27 (3), 531–541. 10.1016/j.ymthe.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Du D., Chen A., Zhu L. (2018e). Circular RNA Expression Profile of Articular Chondrocytes in an IL-1β-induced Mouse Model of Osteoarthritis. Gene 644, 20–26. 10.1016/j.gene.2017.12.020 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Ma J., Lu J., Chen A., Zhu L. (2021e). Circular RNA CircCDH13 Contributes to the Pathogenesis of Osteoarthritis via CircCDH13/miR‐296‐3p/PTEN axis. J. Cel Physiol 236 (5), 3521–3535. 10.1002/jcp.30091 [DOI] [PubMed] [Google Scholar]
- Zhu H., Hu Y., Wang C., Zhang X., He D. (2020). CircGCN1L1 Promotes Synoviocyte Proliferation and Chondrocyte Apoptosis by Targeting miR-330-3p and TNF-α in TMJ Osteoarthritis. Cel Death Dis 11 (4), 284. 10.1038/s41419-020-2447-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Fu H., Wu Y., Zheng X. (2013). Function of lncRNAs and Approaches to lncRNA-Protein Interactions. Sci. China Life Sci. 56 (10), 876–885. 10.1007/s11427-013-4553-6 [DOI] [PubMed] [Google Scholar]
- Zhu J. K., He T. D., Wei Z. X., Wang Y. M. (2018). LncRNA FAS-AS1 Promotes the Degradation of Extracellular Matrix of Cartilage in Osteoarthritis. Eur. Rev. Med. Pharmacol. Sci. 22 (10), 2966–2972. 10.26355/eurrev_201805_15051 [DOI] [PubMed] [Google Scholar]
- Zhu Y. J., Jiang D. M. (2019). LncRNA PART1 Modulates Chondrocyte Proliferation, Apoptosis, and Extracellular Matrix Degradation in Osteoarthritis via Regulating miR-373-3p/SOX4 axis. Eur. Rev. Med. Pharmacol. Sci. 23 (19), 8175–8185. 10.26355/eurrev_201910_19124 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li R., Wen L.-M. (2021). Long Non-coding RNA XIST Regulates Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells from Temporomandibular Joint via miR-27b-3p/ADAMTS-5 axis. Cytokine 137, 155352. 10.1016/j.cyto.2020.155352 [DOI] [PubMed] [Google Scholar]
- Zou L.-X., Yu L., Zhao X.-M., Liu J., Lu H.-G., Liu G.-W., et al. (2019). MiR-375 Mediates Chondrocyte Metabolism and Oxidative Stress in Osteoarthritis Mouse Models through the JAK2/STAT3 Signaling Pathway. Cells Tissues Organs 208 (1-2), 13–24. 10.1159/000504959 [DOI] [PubMed] [Google Scholar]