Keywords: glomerular filtration rate, macula densa, mTOR, protein synthesis, renin
Abstract
Macula densa (MD) cells, a chief sensory cell type in the nephron, are endowed with unique microanatomic features including a high density of protein synthetic organelles and secretory vesicles in basal cell processes (“maculapodia”) that suggest a so far unknown high rate of MD protein synthesis. This study aimed to explore the rate and regulation of MD protein synthesis and their effects on glomerular function using novel transgenic mouse models, newly established fluorescence cell biology techniques, and intravital microscopy. Sox2-tdTomato kidney tissue sections and an O-propargyl puromycin incorporation-based fluorescence imaging assay showed that MD cells have the highest level of protein synthesis within the kidney cortex followed by intercalated cells and podocytes. Genetic gain of function of mammalian target of rapamycin (mTOR) signaling specifically in MD cells (in MD-mTORgof mice) or their physiological activation by low-salt diet resulted in further significant increases in the synthesis of MD proteins. Specifically, these included both classic and recently identified MD-specific proteins such as cyclooxygenase 2, microsomal prostaglandin E2 synthase 1, and pappalysin 2. Intravital imaging of the kidney using multiphoton microscopy showed significant increases in afferent and efferent arteriole and glomerular capillary diameters and blood flow in MD-mTORgof mice coupled with an elevated glomerular filtration rate. The presently identified high rate of MD protein synthesis that is regulated by mTOR signaling is a novel component of the physiological activation and glomerular hemodynamic regulatory functions of MD cells that remains to be fully characterized.
NEW & NOTEWORTHY This study discovered the high rate of protein synthesis in macula densa (MD) cells by applying direct imaging techniques with single cell resolution. Physiological activation and mammalian target of rapamycin signaling played important regulatory roles in this process. This new feature is a novel component of the tubuloglomerular cross talk and glomerular hemodynamic regulatory functions of MD cells. Future work is needed to elucidate the nature and (patho)physiological role of the specific proteins synthesized by MD cells.
INTRODUCTION
Macula densa (MD) cells, the tubular component of the juxtaglomerular (JG) apparatus (JGA), are crucial for the regulation of renal and glomerular hemodynamics via the tubuloglomerular feedback loop as well as renin release from the neighboring JG cells. Strategically positioned at the glomerular vascular pole, the MD cell plaque consists of approximately 20–25 highly specialized epithelial cells, present at the end of the thick ascending limb of the nephron (1, 2). MD cells have a distinctive polarized morphology: the apical surface includes a prominent primary cilium (3, 4) and a large nucleus (1), whereas the basolateral surface is densely packed with secretory organelles (1) and endowed with a newly identified cell processes network called “maculapodia” (5). These unique microanatomic features play a critical role in mediating the traditional function of MD cells as sensors of tubular salt, flow (6), and other local tissue environmental factors including metabolites (7–9). A decrease in tubular salt concentration, detected by apical Na+-K+-2Cl− cotransporter 2 (NKCC2), initiates an intracellular signaling cascade of MAPKs (p38 and ERK1/2) (2, 6, 10) along with the enzymes cyclooxygenase 2 (COX2) and microsomal prostaglandin E2 (PGE2) synthase 1 (mPGES1) (11, 12), resulting in the release of the autocoid PGE2, which, in turn, triggers renin release from JG cells (13).
The various renal cell populations synthesize cell-specific structural proteins, peptides, and hormones to maintain their structural integrity and mediate their functions. For example, podocytes are potent sources of vascular endothelial growth factor-A (14, 15), interstitial fibroblasts produce erythropoietin (16), and JG cells secrete renin (17). Cellular protein synthesis is tightly regulated at several checkpoints (transcription, translation, and degradation) and involves various organelles including the endoplasmic reticulum (ER), Golgi apparatus, and mitochondria to maintain proteostasis (18, 19). A central regulator of cellular protein synthesis and metabolism, the mammalian target of rapamycin (mTOR) signaling cascade is activated by amino acids and growth factors, whereas it is impaired during oxygen and nutrient deficiency. Activated mTOR complex 1 (mTORC1) acts on several downstream targets including ribosomal protein S6 (RPS6) kinase (RPS6K) and mRNA translation proteins like eukaryotic translation initiation factor 4 (eIF4) and eukaryotic translation elongation factor 2, thereby resulting in increased mRNA translation, ribosome biogenesis, and protein synthesis. In addition to protein synthesis and cellular bioenergetics, the mTOR pathway also plays a crucial role in regulating cellular proliferation and hypertrophy (20, 21). Regulated protein synthesis is fundamental to the proper functioning of a specific cell type. Dysregulation of ER function and protein biosynthesis due to stress factors, hypoxia, accumulation of misfolded proteins, and other pathogenic insults is known to be involved in the pathogenesis of several diseases including diabetic nephropathy, nephrotic syndrome, cardiorenal syndrome, and Alzheimer’s disease (22–24).
Although the morphological features of MD cells suggest the presence of robust protein synthesis machinery at the basal surface (1), the relative inaccessibility of MD cells and complex architecture of the JGA has limited our understanding of the protein synthesis and secretion capacity of MD cells using conventional histology techniques. The recent development of novel fluorescence techniques including an imaging-based, single cell-level global protein synthesis assay, and transgenic mouse models (25–28) has made it possible to extensively characterize and quantify specific aspects of renal cell biology.
This study aimed to characterize the rate and regulatory mechanisms of MD protein synthesis using newly developed fluorescence imaging tools and MD-specific genetic mouse models. In addition, our study also addressed the autocrine and paracrine effects of enhanced MD cell protein synthesis on renin expression and glomerular hemodynamics.
MATERIALS AND METHODS
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Southern California. Tamoxifen-inducible, conditional MD cell-specific mTOR gain-of-function (MD-mTORgof) mice on a C57BL6/J background were generated by intercrossing neuronal nitric oxide synthase (nNOS)/CreERT2 and TSC2/fl mice [exons 2–4 of tuberous sclerosis complex (TSC2) are flanked by loxP sites] (Jackson Laboratory, Bar Harbor, ME) (29). MD-mTORgof mice were further backcrossed with two-color fluorescent mTmG/fl reporter mice (Jackson Laboratory) for the expression of the cell membrane-targeted tandem dimer Tomato (tdTomato; mT) and enhanced green fluorescent protein (eGFP; mG) such that MD cells specifically express eGFP, whereas all other cells express tdTomato (30). Tamoxifen-inducible, conditional MD-GFP mice on a C57BL6/J background were generated by intercrossing nNOS/CreERT2 and mTmG/fl reporter mice (Jackson Laboratory) as previously described (5, 30). Constitutive Sox2-tdTomato mice on a C57BL6/J background were generated by intercrossing Sox2/Cre (31) and tdTomato/fl mice (Jackson Laboratory) for ubiquitous expression of tdTomato in all renal cell types.
Treatments
Tamoxifen administration (75 mg/kg body wt, Alfa Aesar, Harverhill, MA) was via oral gavage for a total of three times every alternate day. Low-salt (LS) diet (TD 90228, Harlan Teklad, Madison, WI) treatment was carried out for 2 wk ad libitum for a subset of mice. For rapamycin (Rapa) treatment, mice were injected intraperitoneally with Rapa (8 mg/kg body wt, Alfa Aesar) every alternate day for 2 wk by dissolving Rapa in ethanol followed by dilution in filter-sterilized vehicle (0.9% NaCl, 5% polyethylene glycol, and 5% Tween 80) as previously described (32). Cycloheximide (CHX; Sigma-Aldrich, St. Louis, MO) treatment was carried out by injecting mice intraperitoneally with 300 µL of 16 mg/mL CHX as previously described (33).
Tissue Processing, Immunofluorescence, and Histology
Mice were anesthetized using a combination of ketamine (100 mg/kg body wt) and xylazine (10 mg/kg body wt) followed by perfusion with ice-cold 1× PBS and 4% paraformaldehyde for 2 min using a peristaltic pump. Kidney tissue was harvested and fixed in 4% paraformaldehyde for 2 h at room temperature. For frozen tissue blocks, tissue was kept overnight in 30% sucrose at 4°C for cryoprotection and then embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and flash frozen. Cryosections were cut at 25 µm, washed with 1× PBS, and mounted using DAPI-containing VectaShield mounting media (Vector Laboratories, Burlingame, CA). For paraffin tissue blocks, tissue was dehydrated, embedded in paraffin wax, and then sectioned to 8 µm thick. For immunofluorescence, tissue sections were treated with 0.5% Triton X-100 for 15 min followed by heat-induced antigen retrieval by boiling sections in sodium citrate (pH 6.0) or Tris-EDTA (pH 9.0) buffer for 12 min. To reduce nonspecific binding of antibodies, sections were blocked for 30 min with normal donkey serum (1:20). Primary and secondary antibodies were applied sequentially overnight at 4°C and for 1 h at room temperature, respectively, and mounted using DAPI-containing VectaShield mounting media. Primary antibodies and dilutions were as follows: nNOS (sc-648, Santa Cruz Biotechnology, 1:100), renin (AS-54371, Anaspec, 1:100), H+-ATPase [a generous gift from Dr. Mark Knepper (34), 1:50], p57 (sc-8298, Santa Cruz Biotechnology, 1:100), tdTomato (A8181-200, SICGEN Antibodies, 1:100), total eIF4E-binding protein 1 (EIF4EBP1; CST-9644, Cell Signaling Technology, 1:100), RPS6 (CST-2217, Cell Signaling Technology, 1:100), and TSC2 (CST-3612, Cell Signaling Technology, 1:50). Alexa Fluor 488- and 5947-conjugated secondary antibodies (Thermo Fisher Scientific, Waltham, MA) were all used at 1:500 dilution. Sections were examined with the Leica TCS SP8 DIVE (Leica Microsystems, Wetzlar, Germany) confocal/multiphoton laser scanning microscope system as previously described (5). For histological staining, tissue was sectioned 4 µm thick and periodic acid-Schiff (PAS) staining was carried out using a PAS Stain kit (Polysciences, Warrington, PA) following the manufacturer’s instructions.
Global Protein Synthesis Assay Using O-Propargyl-Puromycin Fluorescence Imaging and Quantification
For O-propargyl-puromycin (OPP) labeling (Thermo Fisher Scientific), mice were injected intraperitoneally with 25 µL of 20 mM OPP dissolved in DMSO for 1 h before tissue harvest and staining as previously described (28, 35). For OPP labeling, sections were developed using the Alexa Fluor 594 Click-iT OPP Protein Synthesis kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. OPP fluorescence intensity was quantified by imaging all tissue sections using identical laser power and confocal microscopy settings. Fluorescence intensity was measured by placing 10 regions of interest across the entire MD plaque (FOPP) and normalized to the fluorescence intensity of red blood cells (RBCs) (FRBC). The average FOPP of 5–10 different representative MD cell plaques was quantified in each tissue section.
Intravital Multiphoton Microscopy
For intravital imaging of control MD-GFP and MD-mTORgof mice, animals were anesthetized with 1–4% isoflurane and the SomnoSuite low-flow anesthesia system (Kent Scientific, Torrington, CT) and the left kidney was exteriorized via a small incision. Mice were injected with Alexa Fluor 680-conjugated BSA (Thermo Fisher Scientific) retroorbitally to label the circulating plasma and placed on the stage of an inverted microscope, with the exteriorized kidney bathed in a coverslip-bottomed chamber containing normal saline as previously described (28). Body temperature was maintained with a homeothermic blanket system (Harvard Apparatus, Holliston, MA) throughout the imaging session. Intravital imaging was performed using a Leica SP8 DIVE multiphoton microscope (Leica Microsystems) with a ×40 Leica water-immersion objective (numerical aperture 1.1) and a Chameleon Discovery laser system (Coherent, Santa Clara, CA) at 960 nm and external Leica 4Tune spectral hybrid detectors (emission at 530 ± 20 nm for mG and 590 ± 20 nm for mT). Changes in glomerular hemodynamics were measured based on Alexa Fluor 680-conjugated BSA to label the circulating plasma, whereas RBCs were identified based on their albumin-excluding negative label. The velocity of RBCs (RBCV) was detected based on xt (line) scans performed in the central axis of the blood vessels as previously described (28, 36). Using the Leica LAS X software analysis tools (v. 3.7.4.23463), glomerular diameter as well as afferent arteriole (AA) and efferent arteriole (EA) internal diameters (d) were measured. Single AA and EA blood flow (Q) were calculated based on the following formula: Q = RBCV × [π × (d/2)2] and expressed in fL/ms.
Immunoblot Analysis
Mouse kidney cortical tissue homogenates were prepared by manually dissecting the kidney cortex and homogenizing for 2 min using an Ultra-Turrax homogenizer (IKA Works, Wilmington, NC) in buffer containing 5% sorbitol, 25 mM histidine-imidazole (pH 7.5), 100 mM Na2EDTA (pH 7.0), and 1× protease/phosphatase inhibitor cocktail (BD Bioscience, San Jose, CA). Forty micrograms of protein were loaded in Any Kd precast TGX gels (Bio-Rad, Hercules, CA) and stained with Coomassie blue dye (Bio-Rad), and multiple bands were quantified to ensure uniform loading of samples as previously described (37, 38). For semiquantitative immunoblot analysis, 30–50 µg of protein were loaded in wells of either 7.5% or Any Kd precast TGX gels and then transferred onto a polyvinylidene difluoride membrane (MilliporeSigma, Burlington, MA). Blots were blocked for 30 min at room temperature using Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) followed by incubation in primary and secondary antibodies suspended in a 1% BSA (Research Products, Prospect, IL) in 1× Tris-buffered saline with 0.1% Tween 20 buffer (Bioland Scientific, Paramount, CA) sequentially overnight at 4°C and for 1 h at room temperature on a shaker, respectively. Primary antibodies and dilutions were as follows: renin (AS-54371, Anaspec, 1:1,000), COX2 (sc-1745, Santa Cruz Biotechnology, 1:500), mPGES1 (no. 160140, Cayman Chemical, 1:500), Pappa2 (PA5-21046, Thermo Fisher Scientific, 1:500), phosphorylated (p)EIF4EBP1 (CST-2855, Cell Signaling Technology, 1:500), total EIF4EBP1 (CST-9644, Cell Signaling Technology, 1:500), pERK1/2 (CST-4376, Cell Signaling Technology, 1:500), total ERK1/2 (CST-4696, Cell Signaling Technology, 1:1,000), pp38 (CST-9216, Cell Signaling Technology, 1:1,000), total p38 (CST-8690, Cell Signaling Technology, 1:1,000), pRPS6K (CST-9205, Cell Signaling Technology, 1:500), and total RPS6K (CST-9202, Cell Signaling Technology, 1:500). IRDye 680RD and IRDye 800CW secondary antibodies (LI-COR Biosciences) were all used at 1:5,000 dilution. Blots were then visualized using the Odyssey Infrared Imaging System (LI-COR Biosciences) and quantified using Image Studio Software (LI-COR Biosciences).
Glomerular Filtration Rate Measurements
For glomerular filtration rate (GFR) measurements, mice were injected retroorbitally with FITC-sinistrin (7.5 mg/100 g body wt, MediBeacon, St. Louis, MO). GFR measurements were performed using the MediBeacon Transdermal GFR Measurement System (MediBeacon) by placing the sensor on the shaved dorsal skin for 90 min to detect fluorescence. GFR was then calculated based on the decay kinetics of FITC-sinistrin using MediBeacon Data Studio software (MediBeacon).
Blood Pressure Measurements
Blood pressure was measured by tail-cuff plethysmography using a Visitech BP-2000 system (Visitech Systems, Apex, NC) as previously described (30). All animals underwent a training period of 5 days before the start of experimental measurements.
Statistical Methods
Data are expressed as means ± SE and were analyzed using a Student’s t test (between two groups) or one-way or two-way ANOVA (between multiple groups) with post hoc comparison by Tukey’s multiple comparisons test. Sample numbers (n) for each group are as indicated in the figures. P < 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA).
RESULTS
Characterization of Global Protein Synthesis in Renal Cell Populations
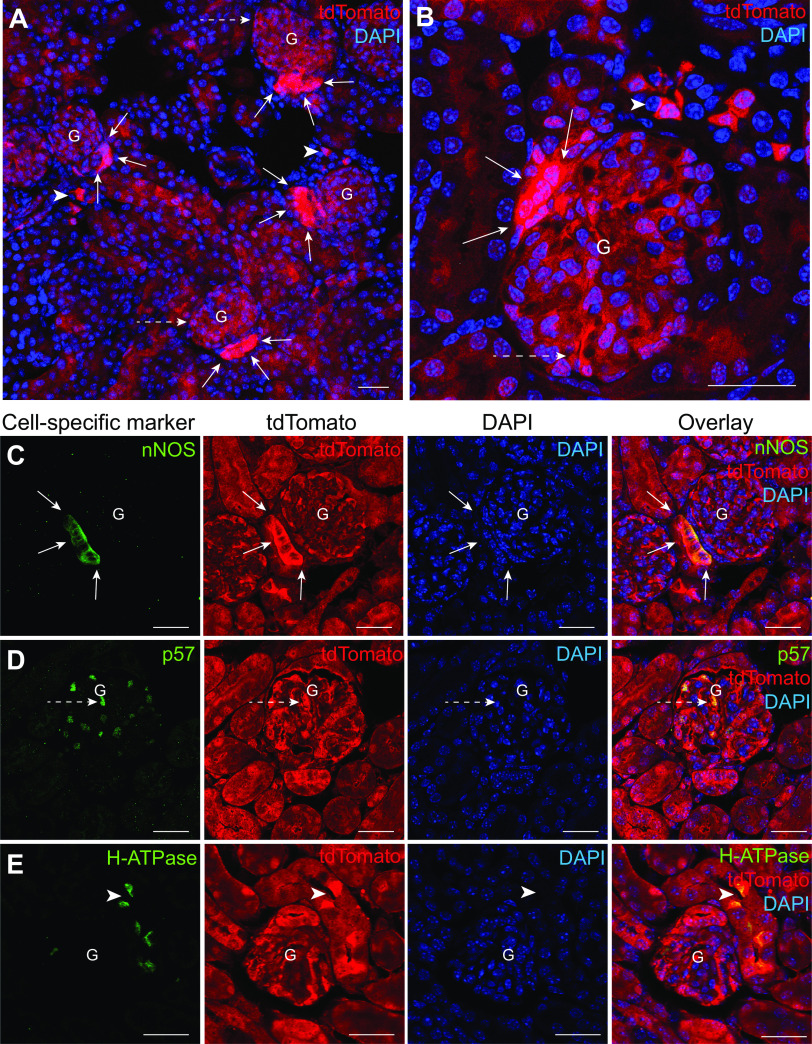
Protein synthesis activity in renal cell populations is dynamic, reflecting functional and structural differences between the cell types. To gain visual insights into the basal rate of protein synthesis in the different cell types of the kidney, we developed Sox2-tdTomato mice that feature tdTomato expression in all renal cell populations (31). As shown in Fig. 1A, fluorescence imaging of frozen kidney tissue sections of Sox2-tdTomato mice showed constitutive and ubiquitous cytosolic expression of tdTomato (in red) across the kidney cortex. A magnified fluorescence image of the kidney tissue highlighted the differential expression of tdTomato protein in specific cell types with the highest intensity found in MD cells followed by intercalated cells (ICs) and podocytes as identified based on tissue morphology (Fig. 1B). To validate the identity of these cell types, we performed immunofluorescence labeling with cell-specific markers on formalin-fixed, paraffin-embedded (FFPE) kidney tissue sections. Colocalization of tdTomato immunolabeling (in red) with immunolabeling of nNOS for MD cells (in green) (Fig. 1C), p57 for podocytes (in green) (Fig. 1D), and H+-ATPase for ICs (in green) (Fig. 1E) confirmed these findings. Among these three cell types, tdTomato expression appeared to be the highest in MD cells.
Figure 1.
Histological features of the Sox2-tdTomato mouse model. A: representative fluorescence image of a Sox2-tdTomato mouse kidney tissue section confirming the expression of tandem dimer Tomato (tdTomato) in all kidney cell types (in red). Nuclei are labeled blue with DAPI. The specific cell types highlighted are macula densa (MD) cells (solid white arrows), podocytes (dashed white arrows), and intercalated cells (ICs) (white arrowheads). B: magnified fluorescence image of a Sox2-tdTomato mouse kidney section focusing on a single glomerulus (G) with its MD cell plaque (solid white arrows) and podocytes (dashed white arrows). Neighboring ICs are also shown (white arrowheads). Nuclei are labeled blue with DAPI. Representative immunofluorescence colocalization images for MD cell marker neuronal nitric oxide synthase (nNOS; in green; C), the podocyte marker p57 (in green; D), and the IC marker H+-ATPase (in green; E) with endogenous tdTomato expression (in red). Note the intense tdTomato expression in these specific cell types compared with the other cell populations. Nuclei are labeled blue with DAPI. Scale bars = 30 µm.
Next, to specifically determine the rate of overall protein synthesis in renal cell populations, an OPP incorporation-based fluorescence imaging approach was implemented. As previously described (28, 35), this method relies on the development of OPP incorporated into newly synthesized peptide strands using a fluorescent azide. Wild-type (WT) mice were given an intraperitoneal injection of either 20 mM OPP or DMSO as a vehicle control, and labeling was then developed on FFPE kidney tissue sections. To confirm the specificity of OPP in labeling nascent protein strands, a subset of WT mice was also pretreated with 16 mg/mL CHX [a potent inhibitor of protein translation (33)] for 1 h followed by OPP injection, and this served as a negative control. We did not observe any OPP labeling (FOPP) on FFPE kidney tissue sections of mice injected with DMSO (0.47 ± 0.04, n = 4; Fig. 2, A and E), whereas strong OPP labeling (in red) was observed in mice injected with OPP (0.94 ± 0.05, n = 6, P = 0.0002; Fig. 2, B and E). Fluorescence imaging of kidney sections demonstrated a high level of protein synthesis in MD cells, podocytes, and ICs with strong OPP labeling in nuclear as well as cytoplasmic compartments. Moreover, in mice pretreated with CHX, OPP labeling was absent in almost all renal cell populations, including MD cells (0.53 ± 0.03, n = 3, P = 0.0015) and podocytes (Fig. 2, C and E). However, faint OPP labeling was observed in the cytoplasm of a few scattered cells in distal tubular segments (Fig. 2C). Finally, we treated WT mice with Rapa to pharmacologically inhibit mTORC1, a central regulator of cellular protein synthesis (21). Short-term Rapa treatment (8 mg/kg body wt) for a period of 2 wk (32) followed by OPP injection showed a marked reduction but not a complete loss of OPP staining in MD cells (0.79 ± 0.08, n = 5; Fig. 2, D and E) compared with mice injected with OPP.
Figure 2.
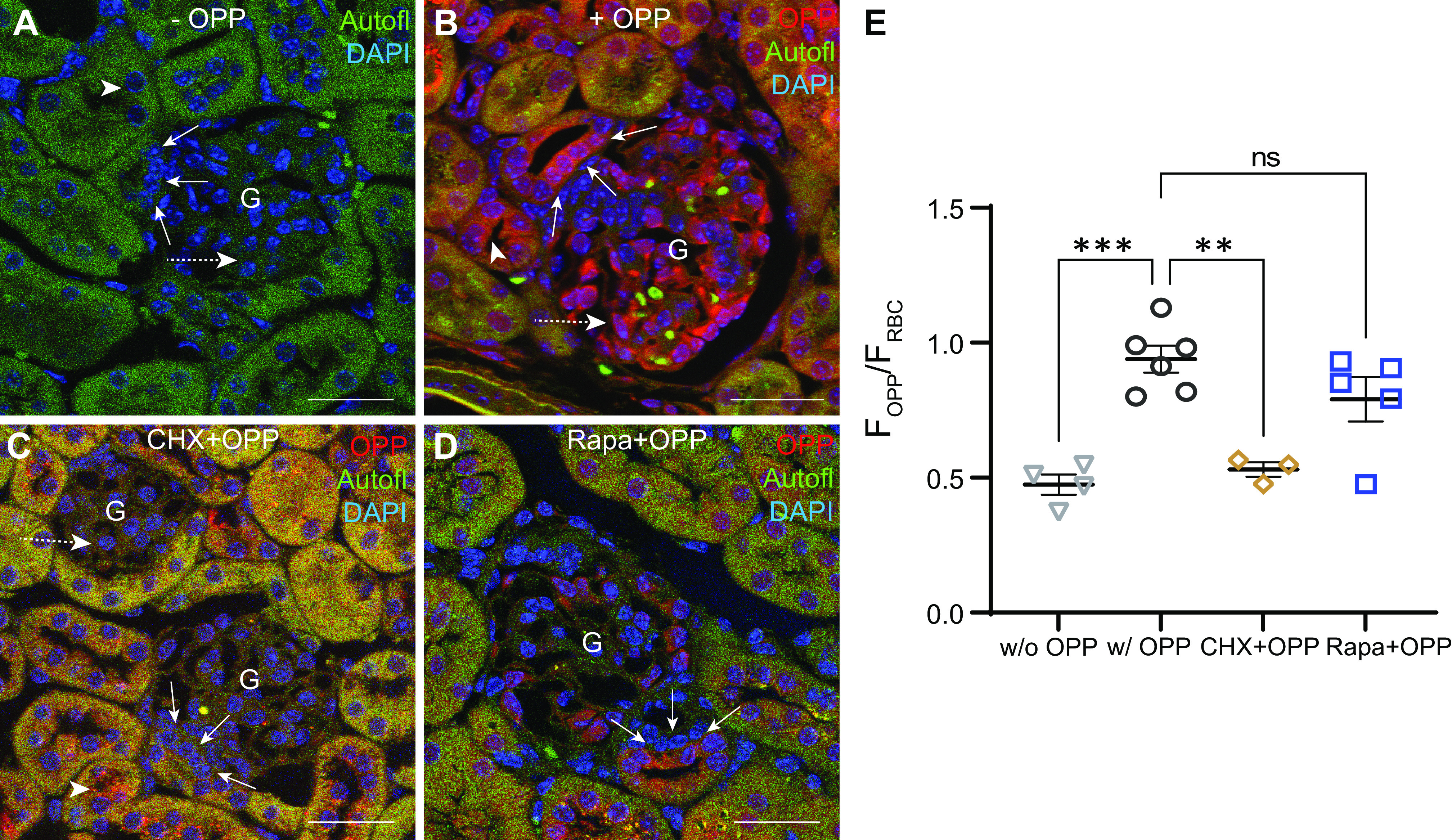
Quantitative visualization of protein synthesis activity in the kidney at the single cell level using O-propargyl puromycin (OPP) incorporation-based fluorescence imaging. Representative fluorescence images of wild-type mouse kidney sections without (−) OPP (A), with (+) OPP (in red; B), with cycloheximide (CHX) pretreatment and OPP (in red; C), and with rapamycin (Rapa) treatment and OPP (in red; D) with tissue autofluorescence (Autofl; in green) for morphological details. Nuclei are labeled blue with DAPI. Note the strong OPP labeling (in red) in the macula densa (MD) cell plaque (solid white arrows), podocytes (dashed white arrows), and intercalated cells (white arrowheads). E: statistical summary of average OPP fluorescence intensity in the MD cell plaque (FOPP) normalized to red blood cell (RBC) fluorescence intensity (FRBC) in WT mice without (w/o) OPP (n = 4), with (w/) OPP (n = 6), with CHX pretreatment and OPP (n = 3), and with Rapa treatment and OPP (n = 5). In each kidney tissue section, OPP intensity in 5–10 MD plaques was quantified by placing 10 circular regions of interest across the MD plaque (FOPP) and normalized to FRBC in the same imaging area. Each data point in the graph corresponds to an average of FOPP/FRBC values from 5 to 10 MD plaques per animal. Data are expressed as means ± SE. **P <0.01 and ***P < 0.001 by one-way ANOVA with Dunnett’s multiple comparisons test. G, glomerulus; ns, not significant. Scale bars = 30 µm.
Generation, Validation, and Characterization of the MD-mTORgof Mouse Model
The central regulator of cellular protein synthesis is the mTOR signaling pathway via mTORC1 (21). We first verified the expression of several mTOR signaling proteins in the human MD using immunohistochemistry data from the Human Protein Atlas (39). As shown in Fig. 3, A–D, the human MD plaque has a high level of expression of TSC2 (Fig. 3A) and DEP domain containing mTOR interacting protein (Deptor; Fig. 3B) along with RPS6K (Fig. 3C) and eukaryotic translation initiation factor 3 C (eIF3C; Fig. 3D), respectively. In addition, we corroborated the expression of these mTOR signaling proteins on mouse FFPE kidney tissue via immunofluorescence labeling. As shown in Fig. 3, E–G, mouse MD cells had high expression of RPS6 (in red) (Fig. 3E), total EIF4EBP1 (in red) (Fig. 3F), and TSC2 (in red) (Fig. 3G). Of these proteins, expression of EIF4EBP1 was highly specific for MD cells, whereas RPS6 and TSC2 had high expression in MD cells and in a few other cell types (Fig. 3, E–G).
Figure 3.

Validation of expression of mammalian target of rapamycin (mTOR) signaling elements in human and control and MD-mTORgof mouse kidneys. Human protein atlas (HPA) validation of immunohistochemistry labeling for tuberous sclerosis complex 2 (TSC2; A), DEP domain containing mTOR interacting protein (Deptor; B), ribosomal protein S6 (RPS6) kinase (RPS6K; C), and eukaryotic translation initiation factor 3 C (eIF3C; D) in the human kidney. Magnified insets show labeling in the macula densa (MD; solid black arrows). Immunohistochemistry images for the above are available as follows: TSC2 (https://images.proteinatlas.org/30409/62646_A_9_5.jpg), Deptor (https://images.proteinatlas.org/23945/53320_A_7_5.jpg); RPS6K (https://images.proteinatlas.org/2852/7862_A_8_5.jpg); and eIF3C (https://images.proteinatlas.org/50112/117399_A_8_5.jpg. Representative immunofluorescence images of wild-type mouse kidney tissue sections with RPS6 labeling (in red; E) and eukaryotic translation initiation factor 4E-binding protein 1 (EIF4EBP1) labeling (in red; F) and tissue autofluorescence (Autofl; in green) for morphological details. Nuclei are labeled blue with DAPI. Note the strong labeling in MD cells (solid white arrows). Representative immunofluorescence images of mouse kidney tissue sections of control mice (G) and MD-mTORgof mice (H) with TSC2 labeling (in red) and tissue autofluorescence (in green) for morphological details. Nuclei are labeled blue with DAPI. Note the absence of TSC2 labeling in MD cells of the MD-mTORgof mouse kidney (solid white arrows) in H compared with control in G. The boundaries of the MD plaque are shown by dashed lines. I: immunoblots for total (t)RPS6K and phosphorylated (p)RPS6K) in control and MD-mTORgof kidney cortex homogenates (n = 7) with the [pRPS6K]/[tRPS6] statistical summary. J: immunoblots for tEIF4EBP1 and pEIF4EBP1 in control and MD-mTORgof kidney cortex homogenates (n = 7) with the [pEIF4EBP1]/[tEIF4EBP1] statistical summary. Representative periodic acid-Schiff staining of histological images of kidney tissue sections of control mice (K) and MD-mTORgof mice (L) depicting the entire kidney cross-sectional area along with a specific cortical region. Magnified insets show the cortical collecting duct (CD). M: statistical summary of kidney weight (KW) normalized to body weight in control and MD-mTORgof mice (n = 6). Data are expressed as means ± SE. *P < 0.05 and **P < 0.01 by an unpaired Student’s t test. G, glomerulus; MW, molecular weight; ns, not significant. Scale bars = 30 µm.
To specifically test the role of the mTOR signaling pathway in regulating MD protein synthesis, we developed a MD-specific gain of function of mTOR signaling mouse model using a Cre/lox-driven approach. Using the classic MD-specific marker nNOS (5), tamoxifen-inducible MD-mTORgofmice were developed by intercrossing nNOS/CreERT2 and TSC2/fl mice (29) on a C57BL6/J background. Furthermore, these mice were backcrossed with mTmG/fl mice to ensure MD-specific membrane eGFP expression (mG), whereas all other cells in the kidney express membrane-targeted tdTomato (mT). Upon full tamoxifen induction, MD cells had targeted disruption of TSC2 pa negative regulator of the mTORC1 complex (21) along with membrane eGFP expression. Control MD-GFP mice were generated by intercrossing nNOS/CreERT2 and mTmG/fl mice as previously described (5), in which tamoxifen induction resulted in MD-specific expression of membrane eGFP. To validate the MD cell specificity of altered TSC2 expression in MD-mTORgof mice, immunofluorescence labeling for TSC2 was performed on FFPE kidney tissue sections of control and MD-mTORgof mice after tamoxifen induction. Immunofluorescence imaging of kidney tissue sections confirmed TSC2 expression (in red) in the MD of control mice (Fig. 3G), which was specifically absent in the MD cell plaque in MD-mTORgof mice (Fig. 3H). To confirm the upregulation of mTOR signaling in MD-mTORgof mice, immunoblot analysis for RPS6K [an important downstream target of mTORC1 (21)] in kidney cortex homogenates showed significantly higher phosphorylated/total RPS6K in MD-mTORgof mice (4.60 ± 0.47) compared with control mice (2.79 ± 0.33, n = 7 each, P = 0.0080; Fig. 3I) and higher phosphorylated/total EIF4EBP1 [an important downstream negative regulator of mRNA translation (19)] in MD-mTORgof mice (0.52 ± 0.02) compared with control mice (0.43 ± 0.03, n = 7 each, P = 0.0304; Fig. 3J).
Finally, histological analysis of PAS-stained kidney tissue sections of control (Fig. 3K) and MD-mTORgof (Fig. 3L) mice found no discernible gross renal morphological variations or alterations in the microanatomy of tubular compartments (including collecting ducts) between control and MD-mTORgof mice. Moreover, kidney tissue weight was not significantly different between MD-mTORgof (0.0060 ± 0.0001) and control mice (0.0059 ± 0.0002, n = 6 each, P = 0.5559; Fig. 3M).
Quantification of MD Cell Global Protein Synthesis in Control and MD-mTORgof Mice
The OPP incorporation-based fluorescence imaging method was used to measure changes in MD protein synthesis activity in response to upregulated mTOR signaling and physiological activation of MD cells using a LS diet (5). Control MD-GFP and MD-mTORgof mice were induced using tamoxifen at 14 wk of age, and, after 4 wk of induction, mice were treated with either a normal-salt (NS) diet or LS diet for a period of 2 wk. Mice were then injected with OPP, and labeling was developed on FFPE sections using a fluorescent azide. All kidney tissue sections were imaged using the same confocal microscopy imaging settings, and MD cell plaques were identified based on their clearly discernible morphology (Fig. 4, A–D). First, we aimed to quantify changes in the rate of overall protein synthesis in response to upregulation of mTOR signaling in MD cells. Compared with control mice on the NS diet (0.94 ± 0.05, n = 6; Fig. 4, A and E), MD-mTORgof mice on the NS diet (1.30 ± 0.06, n = 8, P = 0.0004; Fig. 4, C and E) had significantly increased protein synthesis activity, as reflected by an increase in red fluorescence intensity (FOPP) in the MD cell plaque. Furthermore, stimulation of the salt sensing activity of MD cells using the LS diet increased the rate of protein synthesis by nearly 40% in control mice (1.40 ± 0.05, n = 6, P < 0.0001; Fig. 4, B and E) and by nearly 20% in MD-mTORgof mice (1.48 ± 0.05, n = 8, P = 0.09; Fig. 4, D and E) compared with respective mice on the NS diet. In addition, to confirm the mTOR specificity of these responses, we treated control and MD-mTORgof mice on the LS diet with Rapa (8 mg/kg body wt) for 2 wk as previously described (32) for pharmacological inhibition of mTORC1. Following Rapa treatment, we observed a significant reduction in MD OPP labeling in both control mice on the LS diet (LS diet: 1.40 ± 0.05, n = 6, and LS diet + Rapa: 1.17 ± 0.04, n = 4, P = 0.0153) and MD-mTORgof mice on the LS diet (LS diet: 1.48 ± 0.05, n = 8, and LS diet + Rapa: 1.20 ± 0.02, n = 4, P = 0.0038; data not shown).
Figure 4.
Quantification of macula densa (MD) cell global protein synthesis in control and MD-mTORgof mice using O-propargyl puromycin (OPP) incorporation-based fluorescence imaging. Representative fluorescence images of mouse kidney tissue sections of control mice on a normal-salt (NS) diet (A) or low-salt (LS) diet (B) as well as MD-mTORgof mice on a NS diet (C) or a LS diet (D) OPP labeling (in red) and tissue autofluorescence (Autofl; in green) for morphological details. Nuclei are labeled blue with DAPI. Note the strong OPP labeling (in red) in the MD cell plaque (solid white arrows). E: statistical summary of average OPP fluorescence intensity in the MD cell plaque (FOPP) normalized to red blood cell (RBC) fluorescence intensity (FRBC) in control mice on the NS or LS diet (n = 6) and in MD-mTORgof mice on the NS or LS diet (n = 8). In each kidney tissue section, OPP intensity in 5–10 MD plaques was quantified by placing 10 circular regions of interest across the MD plaque (FOPP) and normalized to FRBC in the same imaging area. Each data point in the graph corresponds to an average of FOPP/FRBC values from 5 to 10 MD plaques per animal. Data are expressed as means ± SE. ***P < 0.001 and ****P <0.0001 by one-way ANOVA with Tukey’s multiple comparisons test. G, glomerulus; mTOR, mammalian target of rapamycin; ns, not significant. Scale bars = 25 µm.
Characterization of the Autocrine Effects of MD-mTOR Signaling
In addition to playing a central role in protein synthesis and secretion, the mTOR signaling pathway is known to regulate cell growth, proliferation, and autophagy (21). A recent publication from our laboratory identified a unique feature of MD cell microanatomy: the presence of a dense network of major and minor processes named “maculapodia,” which are associated with secretory function and vesicular transport (5). To investigate the autocrine effect of upregulated MD mTOR signaling, we quantified changes in the number of MD cells per plaque and the length of the maculapodia (using mG expression to identify MD cells) in control MD-GFP and MD-mTORgof mice after tamoxifen induction. To assess both parameters, 25-µm-thick frozen kidney tissue sections were used to image the entire volume of the glomerulus via high resolution z-stacks. Quantification of the length of maculapodia at the basal surface of MD cells showed a significant increase in the length of the processes in MD-mTORgof mice (5.91 ± 0.31 µm, n = 5; Fig. 5B) compared with control mice (4.26 ± 0.23 µm, n = 8, P = 0.0011; Fig. 5, A and C). With respect to the change in MD cell number, MD-mTORgof mice had a significantly higher number of MD cells per JGA area (12.00 ± 0.97 cells/area, n = 5; Fig. 5, E and F) compared with control mice (9.20 ± 0.48 cells/area, n = 6, P = 0.0229; Fig. 5, D and F).
Figure 5.

Autocrine and paracrine effects of upregulated macula densa (MD) mammalian target of rapamycin (mTOR) signaling. Representative fluorescence images of control (A) and MD-mTORgof (B) mice with MD cells expressing enhanced green fluorescent protein (eGFP; in green) and all other cells expressing tandem dimer Tomato (tdTomato; in red). Nuclei are labeled blue with DAPI. Note the change in the length of maculapodia at the base of MD cells (solid white arrow). C: statistical summary of the length of maculapodia in control (n = 8) and MD-mTORgof (n = 5) mice. In each kidney tissue section, the length of maculapodia of multiple MD cells in 5–10 MD plaques was measured using high-resolution z-stacks of the entire MD volume. Each data point in the graph corresponds to an average of the length of maculapodia in 5–10 MD plaques per animal. Representative fluorescence images of control (D) and MD-mTORgof (E) mice with the MD cell plaque expressing eGFP (in green) and other cells expressing tdTomato (in red). Nuclei are labeled blue with DAPI. Note the change in the number of MD cells per area (solid white arrows). F: statistical summary of the number of MD cells per juxtaglomerular apparatus area in control (n = 6) and MD-mTORgof (n = 5) mice. In each kidney tissue section, the number of MD cells per area was counted using high-resolution z-stacks of the entire volume of the MD plaque in which single MD cells could be clearly visualized based on their mG expression and DAPI labeling. Each data point in the graph corresponds to an average of the number of MD cells in 5–10 MD plaques per animal. G: statistical summary of the systolic blood pressure (BP) of control and MD-mTORgof mice on either a normal-salt (NS) diet or a low-salt (LS) diet at baseline, 4 wk postinduction, and 4 wk postinduction and 2 wk of dietary treatment (n = 3–6). All animals underwent a training period of 5 days for acclimatization, and each data point in the graph corresponds to an average of systolic BP over 2 days per animal after acclimatization. H: statistical summary of the glomerular filtration rate (GFR) in control (n = 10) and MD-mTORgof (n = 14) mice 4 wk postinduction. Data are expressed as means ± SE. *P < 0.05 and **P < 0.01 by two-way ANOVA (between multiple groups) with Tukey’s multiple comparisons test and an unpaired Student’s t test (between two groups). BW, body weight; G, glomerulus; ns, not significant. Scale bars = 15 µm.
Blood Pressure and Kidney Function in MD-mTORgof Mice
MD cells are chief regulators of GFR, renal blood flow (RBF), and blood pressure via their effects on hemodynamics and the renin-angiotensin system (2). To elucidate the effect of upregulated MD mTOR signaling on kidney function, the blood pressure and GFR of control and MD-mTORgof mice were measured. As shown in Fig. 5G, no significant difference was observed in the systolic blood pressure of control and MD-mTORgof mice at baseline and after induction as well as after dietary salt treatment. Regarding kidney function, MD-mTORgof mice had a significantly elevated GFR after 4 wk of induction (1,981 ± 121 μL/min/100 g body wt, n = 14) compared with control mice (1,444 ± 99 μL/min/100 g body wt, n = 10, P = 0.0039; Fig. 5H). No significant change was observed in GFR after LS dietary treatment in both mouse strains (data not shown). In addition, no change was observed in the urinary albumin-to-creatinine ratio in control and MD-mTORgof mice (data not shown).
Intravital Imaging of Glomerular Hemodynamics in MD-mTORgof Mice
Using intravital multiphoton microscopy, changes in various structural and functional parameters of glomerular hemodynamics at a single nephron level were quantified in control MD-GFP and MD-mTORgof mice after 7 days of tamoxifen induction. In both mouse strains, MD cells expressed mG, whereas all other cells in the kidney expressed mT. Intravital imaging showed that MD-mTORgof mice had a significantly enlarged glomerular tuft area (3,021 ± 103 μm2) compared with control mice (2,656 ± 87 μm2, 4 glomeruli in n = 4 mice each in both groups, P = 0.0198; Fig. 6A). Next, we visualized the glomerular vascular pole region at the base of the MD to quantify changes in AA and EA diameter and blood flow. MD-mTORgof mice (Fig. 6, F and H) had significantly larger AA diameter (8.8 ± 0.4 μm) compared with control (7.6 ± 0.3 μm, 4 glomeruli in n = 4 mice each in both groups, P = 0.0345; Fig. 6, D and H). EA diameter measurements followed a similar trend in glomeruli of MD-mTORgof mice (6.6 ± 0.3 μm; Fig. 6, G and J) and control mice (5.3 ± 0.2 μm, 4 glomeruli in n = 4 mice each in both groups, P = 0.0023; Fig. 6, E and J). Finally, xt line scans of the lumen of blood vessels were used to measure blood flow in single AAs and EAs. AA blood flow was significantly higher in MD-mTORgof mice (299 ± 76 fL/ms) compared with control mice (131 ± 9 fL/ms, 1 glomerulus from n = 4 mice each in both groups, P = 0.0089; Fig. 6I). Similar observations were made with respect to EA blood flow in control mice (89 ± 10 fL/ms) and MD-mTORgof mice (163 ± 27 fL/ms, 2 glomeruli from n = 4 mice each in both groups, P = 0.0074; Fig. 6K). In addition, we also quantified changes in the diameter and blood flow within glomerular capillaries. Similar to observations made in the AA and EA, glomerular capillary diameter was significantly elevated in MD-mTORgof mice (7.0 ± 0.2 μm) compared with control mice (5.0 ± 0.1 μm, 4 glomeruli in n = 4 mice each in both groups, P < 0.0001; Fig. 6B) along with a significant increase in glomerular capillary RBCV in MD-mTORgof mice (3.7 ± 0.4 μm/s) compared with control mice (2.2 ± 0.2 μm/s, 4 glomeruli in n = 4 mice each in both groups, P = 0.0013; Fig. 6C and Supplemental Video S1; see https://doi.org/10.6084/m9.figshare.14740815).
Figure 6.

Intravital imaging of glomerular hemodynamics in MD-mTORgof mice. Statistical summary of the glomerular tuft area (A), glomerular capillary (GC) diameter (B), and GC red blood cell velocity (RBCV; C) in control and MD-mTORgof mice. Each data point in the graph corresponds to an average of five different measurements in four glomeruli in n = 4 mice each in both groups. Representative intravital multiphoton microscopy images of glomeruli with their afferent arteriole (AA) and efferent arteriole (EA) of control (D and E) and MD-mTORgof (F and G) mice. Note the specificity of the membrane-targeted enhanced green fluorescent protein (eGFP) expression (in green) to macula densa (MD) cells and expression of membrane-targeted tandem dimer Tomato (tdTomato; in red) in all other renal cells. The circulating plasma is labeled with Alexa Fluor 680-conjugated BSA (in gray). Statistical summary of AA diameter (H), AA blood flow (I), EA diameter (J), and EA blood flow (K) in control and MD-mTORgof mice. Each data point in the graph corresponds to different measurements in four AA/EA (H and J) or one AA and two EA (I and K) in n = 4 mice each in both groups. Data are expressed as means ± SE. *P < 0.05, **P < 0.01, and ****P < 0.0001 by an unpaired Student’s t test. G, glomerulus; mTOR, mammalian target of rapamycin. Scale bars = 30 µm.
Effects of MD mTOR Signaling on Renin Expression
To determine the role of mTOR signaling in LS-driven physiological activation of MD cells and renin expression, immunofluorescence labeling for renin was performed on FFPE kidney tissue sections of control and MD-mTORgof mice treated with a NS diet or LS diet for 2 wk. On the NS diet, MD-mTORgof mice displayed a significant increase in the number of renin-positive cells at the vascular pole of the glomerulus (5.0 ± 0.3 cells/area, n = 8; Fig. 7, C and E) compared with control mice (3.3 ± 0.2 cells/area, n = 6, P = 0.0018; Fig. 7, A and E). As expected, LS dietary treatment for a period of 2 wk resulted in a significant increase in the number of renin-positive cells in both control mice (5.2 ± 0.2 cells/area, n = 6, P = 0.0018; Fig. 7, B and E) and MD-mTORgof mice (6.3 ± 0.3 cells/area, n = 8, P = 0.0130; Fig. 7, D and E). However, the LS-induced increase in renin was significantly higher in MD-mTORgof mice compared with control mice (P = 0.0445; Fig. 7E).
Figure 7.

Changes in renin expression in MD-mTORgof mice. Representative maximum projection immunofluorescence images of mouse kidney tissue sections of control mice on a normal salt (NS) diet (A) or a low-salt (LS) diet (B) as well as MD-mTORgof mice on a NS diet (C) or a LS diet (D) with renin labeling (in red) and tissue autofluorescence (Autofl; in green) for morphological details. Nuclei are labeled blue with DAPI. The macula densa (MD) cell plaque is highlighted (solid white arrows). E: statistical summary of the average number of renin-positive cells/juxtaglomerular area in control (n = 6) and MD-mTORgof mice (n = 8) on either the NS or LS diet. In each kidney tissue section, the number of renin-positive cells in 10 juxtaglomerular areas was counted using high-resolution z-stacks of the entire volume of the glomerulus in which single renin-positive cells could be clearly visualized based on immunofluorescence and DAPI labeling. Each data point in the graph corresponds to an average of the number of renin-positive cells per juxtaglomerular area from 10 glomeruli per animal. Data are expressed as means ± SE. *P < 0.05 and **P < 0.01 by one-way ANOVA with Tukey’s multiple comparisons test. G, glomerulus; mTOR, mammalian target of rapamycin. Scale bars = 35 µm.
Changes in MD Signaling in MD-mTORgof Mice
To address the effects of upregulated mTOR signaling on the elements of the classic MD signaling pathway of renin secretion (COX2, mPGES1, p38, ERK1/2, and renin), immunoblots for these proteins were quantified in kidney cortex homogenates of control and MD-mTORgof mice (Fig. 8A). Mice were treated with tamoxifen at ∼4 wk of age, and tissue was harvested after 20 wk of induction. Immunoblots for renin in kidney cortex homogenates showed a trend toward a higher level of expression in MD-mTORgof mice (616 ± 57, n = 7) compared with control mice (497 ± 17, n = 7, P = 0.0694), although it did not reach statistical significance (Fig. 8, A and B). COX2 expression was significantly higher in MD-mTORgof mice (3,322 ± 203, n = 7) compared with control mice (2,689 ± 199, n = 7, P = 0.0228; Fig. 8, A and C). Furthermore, immunoblots for ERK1/2 (Fig. 8, A and D) and p38 (Fig. 8, A and E) MAPKs showed no significant change in the ratio of phosphorylated to total protein in control and MD-mTORgof mice. Similar to COX2, expression of mPGES1 was significantly higher in MD-mTORgof mice (76 ± 4, n = 7) compared with control mice (56 ± 8, n = 7, P = 0.0379; Fig. 8, A and F). Finally, since the overall rate of protein synthesis was significantly higher in MD-mTORgof mice compared with control mice (Fig. 4E), we specifically probed for changes in Pappa2, a recently identified secreted protease highly expressed in MD cells (5, 40, 41). We observed a significant increase in Pappa2 expression in MD-mTORgof mice (700 ± 45, n = 7) compared with control mice (584 ± 21, n = 7, P = 0.0372; Fig. 8, A and G).
Figure 8.

Changes in macula densa (MD) signaling in MD-mTORgof mice. A: immunoblots for renin, cyclooxygenase 2 (COX2), total ERK1/2 (tERK1/2), phosphorylated ERK1/2 (pERK1/2), total p38 (tp38), phosphorylated p38 (pp38), microsomal prostaglandin E2 synthase 1 (mPGES1), and pappalysin 2 (Pappa2) in kidney cortex homogenates from control and MD-mTORgof mice (n = 7). Statistical summary of immunoblot density for renin (B), COX2 (C), [pERK1/2]/[tERK1/2] (D), [pp38]/[tp38] (E), mPGES1 (F), and Pappa2 (G). Data are expressed as means ± SE. *P < 0.05 by an unpaired Student’s t test. MW, molecular weight; ns, not significant.
DISCUSSION
This study provided the first direct view of the high protein synthesis capacity of the specialized cells of the MD and the autocrine and paracrine effects of mTOR-mediated upregulation of MD protein synthesis on glomerular hemodynamics. This study implemented a comprehensive experimental approach using novel in vivo transgenic mouse models, intravital imaging techniques, and fluorescence assays along with tissue, single cell, and molecular tools including specific genetic and pharmacological manipulation. Dual methodologies to visualize the basal rate of global protein synthesis at the single cell level confirmed the high rate of protein synthesis activity in MD cells. In the MD-mTORgof mouse model, we quantified further elevations in the rate of overall MD protein synthesis in response to both upregulated mTOR signaling and LS diet-induced physiological activation of MD cells. Pharmacological inhibition with Rapa further confirmed the important regulatory role of mTOR signaling in MD cell protein synthesis under both control and physiological activation conditions. Finally, quantification of the traditional functions of MD cells in MD-mTORgof mice versus control mice showed significant elevations in glomerular hemodynamics, RBF, and GFR along with an increase in the expression of renin and both classic (COX2) and novel (Pappa2) molecular players in MD cell signaling. This study provided valuable new insights regarding MD protein synthesis capacity and its novel role in mediating cell-to-cell communications between the MD and surrounding cell populations.
Since the identification of the MD cell plaque by Zimmerman and later works by Goormaghtigh, the unique features of the polarized MD cell microanatomy—a prominent primary cilium and nucleus at the apical surface and numerous mitochondria, secretory organelles, and cytoplasmic processes, at the basal surface—have long been established using transmission electron microscopy and conventional histology techniques (1, 17). These microanatomic features suggested that MD cells are endowed with extensive protein synthesis and secretory machinery primarily located toward the basal surface of these cells. However, the complexity of the three-dimensional space of the JGA and the relative inaccessibility of the MD cell plaque have limited our ability to clarify the details of MD cell biological functions including the rate of protein synthesis and the functional implications of these unique features of MD cells. Recent advances in imaging techniques, especially intravital multiphoton microscopy (26, 28), and the development of MD-specific genetic mouse models have made it possible to interrogate various aspects of MD cell biology in the intact living kidney (5).
Cellular protein synthesis is a tightly regulated process to maintain proteostasis; disruption of the proteostatic networks has been linked to the development of various pathophysiological conditions including acute kidney injury, Fabry’s disease, and Alzheimer’s disease (18, 24). To visualize the basal level of global protein synthesis in the kidney in an unbiased fashion at the single cell level, we used two distinct approaches: the Sox2-tdTomato mouse model and the OPP fluorescence assay. In the newly established Sox2-tdTomato genetic mouse model (Fig. 1, A and B), cytoplasmic expression of the red fluorescent protein tdTomato driven by the CAG promoter inserted in the R26 locus served as a surrogate marker for the direct qualitative visualization of the overall rate of cellular protein synthesis. The differential expression of tdTomato within the renal cell populations allowed for the identification of purported high protein-synthesizing cells in the kidney cortex. Among these cell types, MD cells interestingly appeared to have by far the highest level of tdTomato followed by that in collecting duct ICs and glomerular podocytes (Fig. 1, A–E). Although CAG promoter activity can be regulated to increase cellular expression of genetic reporters (42), it is unclear if this mechanism was responsible for the observed high MD-specific tdTomato expression in Sox2-tdTomato mice. Therefore, to confirm these observations using a specific method, we next injected WT mice with OPP to fluorescently label and specifically visualize nascent protein synthesis (28, 35). In this histological imaging-based assay, the metabolic labeling of newly synthesized proteins made it possible to quantify the rate of protein synthesis across different cell types. OPP labeling was observed in both the nucleus and cytoplasm in all cells in the kidney, corresponding to the earliest synthesized proteins. Importantly, the labeling was stronger in MD cells, podocytes, and ICs, confirming the observations from the Sox2-tdTomato mouse model (Fig. 2). Thus, both of these techniques provided an unbiased spatial map of protein synthesis activity within the kidney cortex. As expected, OPP labeling was completely abolished in MD cells in WT mice that were pretreated with CHX, a potent inhibitor of protein translation (Fig. 2C) (33). Interestingly, we observed that certain epithelial cells in the distal tubule collecting duct, most likely ICs based on their anatomy, retained a low level of OPP labeling even after CHX pretreatment, which was not observed in either MD cells or podocytes (Fig. 2C). This observation suggests that in addition to having a high level of protein synthesis, there may be a rapid rate of protein turnover or secretion within MD cells in contrast to more protein storage in ICs that could be blocked with a short-term pretreatment with CHX. Finally, treatment with the mTORC1 inhibitor Rapa resulted in an observable decrease in OPP labeling within the MD cell plaque. Interestingly, Rapa appeared less effective under control conditions (Fig. 2D) compared with its significant inhibitory effects on MD protein synthesis in the stimulated state (in MD-mTORgof mice and with a LS diet), suggesting relatively lower mTOR activity at baseline. Nevertheless, these results underscore the important role of the mTOR signaling pathway in MD cells.
Immunohistochemistry labeling on human kidney tissue sections for several proteins in the mTOR signaling cascade, including TSC2, Deptor, RPS6K, and eIF3C (Fig. 3, A–D), from the Human Protein Atlas database clearly suggested the high level of expression of these proteins in MD cells, although the specificity of these immunolabeling results was not confirmed in this study. In addition, immunofluorescence labeling for mTOR signaling proteins on WT mouse kidney tissue sections confirmed the high level of expression of EIF4EBP1, RPS6, and TSC2 in the MD plaque (Fig. 3, E–G). Interestingly, expression of EIF4EBP1 was highly specific for the MD plaque (Fig. 3F). Since TSC2 is a key negative regulator of mTORC1 (21), we used a nNOS/Cre-driven approach (5) to disrupt TSC2 expression and upregulate mTOR signaling specifically in MD cells and establish a new MD-mTORgof mouse model (29). It should be noted that two recent studies have demonstrated the entirely MD cell-specific expression of fluorescent reporters and successful MD-specific genetic manipulations in the renal cortex using the applied nNOS/Cre mouse model (5, 30). In addition, no gross morphological changes in the kidney or alterations in the microanatomy of renal tubule segments (including collecting ducts) were found in MD-mTORgof mice (Fig. 3, K–M) arguing against indirect or nonspecific off-target effects. The validation of the successful MD cell-specific genetic manipulation was performed using immunofluorescence labeling for TSC2 on kidney tissue sections of control and MD-mTORgof mice, which confirmed the presence or absence of TSC2 expression in MD cells, respectively (Fig. 3, G and H). In addition, we also confirmed the functional upregulation of mTOR signaling activity using several classic mTORC1 downstream targets. Expression of phosphorylated/total RPS6K in the kidney cortex homogenate of MD-mTORgof mice was significantly higher compared with control mice (Fig. 3I). In addition, we observed a significant increase in phosphorylated/total EIF4EBP1 expression, which was confirmed to be highly MD cell specific (Fig. 3, F and J), in MD-mTORgof mice, indicating increased activity of EIF4E in MD cells. These multiple layers of evidence confirmed the upregulation of mTOR signaling in MD cells in MD-mTORgof mice.
Comparison of the rate of overall MD cell protein synthesis using the OPP assay in control and MD-mTORgof mice showed that the upregulation of MD mTOR signaling resulted in a significant increase in global MD protein synthesis (Fig. 4, A and C). This increase in MD protein synthesis was most likely due to the phosphorylation and activation of RPS6K and RPS6 and the release of the inhibitory effect of EIF4EBP1 after genetic disruption of TSC2 in MD cells (21). In addition, it has been well established that a LS diet results in increased activity of p38 and ERK1/2 MAPKs and physiological activation of MD cells (2, 5). Reports from various eukaryotic model systems have demonstrated the extensive cross talk between ERK1/2 and p38 MAPKs and the mTOR signaling pathway. For example, growth factor-derived activation of the MAPK pathway triggers the activation of mTORC1 via its inhibition of the TSC1/TSC2 complex as well as by independently activating RPS6K (43). WT mice treated with a LS diet showed a discernible increase in OPP labeling compared with WT mice on a NS diet (Fig. 4, A and B). There was no further increase in MD protein synthesis activity with LS diet in MD-mTORgof mice, suggesting that mTOR signaling in this model is at its highest level under control conditions and cannot be increased further with the LS challenge (Fig. 4, D and E). Although the results suggest that the LS diet may activate mTOR in MD cells, it is possible that other signaling pathways (e.g., Ras-ERK) could be involved that activate mTOR downstream elements like RPS6K independent of the mTORC1 complex.
Recent reports from our group (5) and others (3) have shown that basal maculapodia are likely associated with secretory function due to the presence of dense-core vesicles that contain diverse cargo. Upregulated MD mTOR signaling resulted in a significant increase in the length of these basal processes (Fig. 5, A–C) and in MD cell numbers (Fig. 5, D–F), supporting the proposed role of maculapodia and MD cells in general in the regulation of paracrine communication to JGA effector cells (5). Although mTOR signaling is known to regulate cell proliferation and differentiation, the exact mechanism responsible for the increased length of the maculapodia and the identification of the diverse cargo in secretory vesicles needs further study.
The traditional role of MD cells as the tubular component of the JGA involves the regulation of tubuloglomerular feedback control of renal and glomerular hemodynamics and renin release from JG cells in response to alterations in tubular fluid salt and flow rate (2, 6). To determine the overall functional consequences of altered MD cell protein synthesis with respect to kidney function, we quantified changes in both GFR and RBF in response to upregulated MD mTOR signaling. Using the MediBeacon transdermal sensor technique, we measured a significantly higher GFR in MD-mTORgof mice. No changes in systolic blood pressure were observed (Fig. 5, G and H), although the less accurate tail-cuff method (rather than radio telemetry) was used in this study, which required animal training. In addition, the use of intravital multiphoton microscopy provided an unparalleled in-depth view of the otherwise inaccessible but critical vascular pole region of the glomerulus along with high-resolution visualization of the AA and EA that feed into the glomerulus (1, 26). The combination of using genetic fluorescent reporters (eGFP) for easy identification of MD cells and quantitative intravital multiphoton microscopy allowed us to uncover the robust elevation in RBF (including the first-time measurement of single AA and EA blood flow in vivo) and vascular diameters in MD-mTORgof versus control mice (Fig. 6 and Supplemental Video S1). Since the difference between the AA and EA RBF rate was greater than the 20% filtration fraction (Fig. 6, I and K) (36), these results further support the globally measured elevated GFR in MD-mTORgof mice (Fig. 5H). Regarding the mechanistic connection between increased MD protein synthesis and increased GFR and RBF, the increased expression of the classic MD-specific proteins COX2 and mPGES1 (Fig. 8A) likely resulted in increased synthesis and release of the vasodilator PGE2 from MD cells, which could explain, at least in part, these hemodynamic changes found in MD-mTORgof mice. However, one limitation of this study is that the role of MD-derived PGE2 in this response was not addressed experimentally. Therefore, it is possible that other targets of mTOR signaling independent of increased protein synthesis were involved, at least in part.
Since MD cells play a critical role in the regulation of renin release from JG cells via the release of PGE2 from the basal surface, we analyzed the changes in JG renin cell number (and indirectly the JGA renin granule content) in response to upregulated mTOR signaling and LS diet. Treatment of control and MD-mTORgof mice with the LS diet for 2 wk predictably resulted in a significant increase in renin expression at the glomerular vascular pole (Fig. 7, B, D, and E). Interestingly, upregulation of MD mTOR signaling also resulted in a significant elevation in renin expression (Fig. 7, C and E), and this effect was even more pronounced in MD-mTORgof mice on a LS diet (Fig. 7, D and E). These results suggest that the mTOR signaling pathway has a combinatorial effect with the LS diet in regulating renin expression in the kidney cortex via the MD mechanism of renin expression. Moreover, the classic MD enzymes COX2 and mPGES1, which generate PGE2, the classic paracrine mediator of renin expression (2), also had a significantly higher level of expression in MD-mTORgof mice compared with control mice (Fig. 8). Traditionally, these enzymes act downstream of p38 and ERK1/2 MAPKs (2), and the MAPK pathway is known to feed into the mTOR signaling cascade (43). However, since we did not observe any changes in the expression or activity (phosphorylation) of p38 and ERK1/2 MAPKs, we propose that the MD mTOR pathway acts on COX2 and mPGES1 downstream of MAPK signaling likely via selective regulation of protein synthesis.
Finally, our observation of increased protein synthesis in the MD led us to quantify changes in specific MD-expressed proteins, both classic and novel. The increased expression of the classic MD-specific proteins COX2 and mPGES1 (Fig. 8A) likely resulted in elevated PGE2 synthesis and release from MD cells, which could explain, at least partially, the increased GFR, RBF, and renin content found in MD-mTORgof mice (Figs. 5H, 6, and 7). However, it is highly likely that the increased expression of other MD cell proteins also contributed to the observed alterations in glomerular hemodynamics and renin. Another limitation of the present study is that the increased expression of other MD proteins was not examined systematically, and this requires future study. In a recent report from our laboratory, we have shown expression of Pappa2 in both human and mouse MD cells and its localization in likely secretory-type vesicles (5). In this study, we observed a significant increase in Pappa2 expression in response to upregulated MD mTOR signaling (Fig. 8G). Future studies are needed to determine the functional significance of MD Pappa2 expression and its regulation.
Perspectives and Significance
The present study discovered the high rate of MD protein synthesis by applying direct imaging techniques with single cell resolution and established the important regulatory role of physiological activation and mTOR signaling in this process. This new feature of MD cells is a novel component of the tubuloglomerular cross talk and glomerular hemodynamic regulatory functions of MD cells. Future work is needed to elucidate the nature and (patho)physiological role of the specific proteins synthesized and likely secreted by MD cells, which will bring us closer to the more complete understanding of this mysterious renal cell type.
SUPPLEMENTAL DATA
Supplemental Video S1: https://doi.org/10.6084/m9.figshare.14740815.
GRANTS
This work was supported by National Institutes of Health Grants DK064324, DK123564, and S10OD021833 (to J.P-P). U.N.S. was funded by predoctoral research fellowship 19PRE34380886 of the American Heart Association.
DISCLOSURES
J.P-P. and G.G. are cofounders of Macula Densa Cell LLC, a biotechnology company that develops therapeutics to target macula densa cells for a regenerative treatment for chronic kidney disease. Macula Densa Cell LLC has a patent entitled “Targeting macula densa cells as a new therapeutic approach for kidney disease.” J.P-P. received consulting fees from Travere Therapeutics and Eli Lilly & Co. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
U.N.S., G.G., and J.P-P. conceived and designed research; U.N.S., G.G., A.I., and S.D. performed experiments; U.N.S., G.G., A.I., and S.D. analyzed data; U.N.S., G.G., and J.P-P. interpreted results of experiments; U.N.S. and G.G. prepared figures; U.N.S. drafted manuscript; U.N.S. and J.P-P. edited and revised manuscript; U.N.S., G.G., A.I., S.D., and J.P-P. approved final version of manuscript.
REFERENCES
- 1.Yu A, Chertow G, Luyckx V, Marsden P, Skorecki K, Taal M (Editors). Anatomy of the kidney. In: Brenner and Rector's The Kidney E-Book. Amsterdam, The Netherlands: Elsevier Health Sciences, 2015, p. 42–82. [Google Scholar]
- 2.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cangiotti AM, Lorenzi T, Zingaretti MC, Fabri M, Morroni M. Polarized ends of human macula densa cells: ultrastructural investigation and morphofunctional correlations. Anat Rec (Hoboken) 301: 922–931, 2018. doi: 10.1002/ar.23759. [DOI] [PubMed] [Google Scholar]
- 4.Sipos A, Vargas S, Peti-Peterdi J. Direct demonstration of tubular fluid flow sensing by macula densa cells. Am J Physiol Renal Physiol 299: F1087–F1093, 2010. doi: 10.1152/ajprenal.00469.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gyarmati G, Shroff UN, Riquier-Brison A, Kriz W, Kaissling B, Neal CR, Arkill KP, Ahmadi N, Gill IS, Moon JY, Desposito D, Peti-Peterdi J. A new view of macula densa cell microanatomy. Am J Physiol Renal Physiol 320: F492–F504, 2021. doi: 10.1152/ajprenal.00546.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol 65: 481–500, 2003. doi: 10.1146/annurev.physiol.65.050102.085730. [DOI] [PubMed] [Google Scholar]
- 7.Peti-Peterdi J. High glucose and renin release: the role of succinate and GPR91. Kidney Int 78: 1214–1217, 2010. doi: 10.1038/ki.2010.333. [DOI] [PubMed] [Google Scholar]
- 8.Peti-Peterdi J, Kishore BK, Pluznick JL. Regulation of vascular and renal function by metabolite receptors. Annu Rev Physiol 78: 391–414, 2016. doi: 10.1146/annurev-physiol-021115-105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol 20: 1002–1011, 2009. doi: 10.1681/ASN.2008070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng HF, Wang JL, Zhang MZ, McKanna JA, Harris RC. Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride. J Clin Invest 106: 681–688, 2000. doi: 10.1172/JCI10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuson AL, Komlosi P, Unlap TM, Bell PD, Peti-Peterdi J. Immunolocalization of a microsomal prostaglandin E synthase in rabbit kidney. Am J Physiol Renal Physiol 285: F558–564, 2003. doi: 10.1152/ajprenal.00433.2002. [DOI] [PubMed] [Google Scholar]
- 12.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest 112: 76–82, 2003. doi: 10.1172/JCI200318018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweda F, Klar J, Narumiya S, Nüsing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287: F427–433, 2004. doi: 10.1152/ajprenal.00072.2004. [DOI] [PubMed] [Google Scholar]
- 14.Simon M, Gröne HJ, Jöhren O, Kullmer J, Plate KH, Risau W, Fuchs E. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol 268: F240–250, 1995. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- 15.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol 10: 2125–2134, 1999. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, Doe BG, Ferguson DJ, Johnson MH, Ratcliffe PJ. Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44: 1149–1162, 1993. doi: 10.1038/ki.1993.362. [DOI] [PubMed] [Google Scholar]
- 17.Barajas L. Anatomy of the juxtaglomerular apparatus. Am J Physiol 237: F333–F343, 1979. doi: 10.1152/ajprenal.1979.237.5.F333. [DOI] [PubMed] [Google Scholar]
- 18.Inagi R, Ishimoto Y, Nangaku M. Proteostasis in endoplasmic reticulum–new mechanisms in kidney disease. Nat Rev Nephrol 10: 369–378, 2014. doi: 10.1038/nrneph.2014.67. [DOI] [PubMed] [Google Scholar]
- 19.Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D. mRNA translation: unexplored territory in renal science. J Am Soc Nephrol 17: 3281–3292, 2006. doi: 10.1681/ASN.2006050488. [DOI] [PubMed] [Google Scholar]
- 20.Huber TB, Walz G, Kuehn EW. mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int 79: 502–511, 2011. doi: 10.1038/ki.2010.457. [DOI] [PubMed] [Google Scholar]
- 21.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 122: 3589–3594, 2009. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y, Lee K, Wang N, He JC. The role of endoplasmic reticulum stress in diabetic nephropathy. Curr Diab Rep 17: 17, 2017. doi: 10.1007/s11892-017-0842-y. [DOI] [PubMed] [Google Scholar]
- 23.Inagi R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol 10: 156–165, 2010. doi: 10.1016/j.coph.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol 112: e1–e9, 2009. doi: 10.1159/000210573. [DOI] [PubMed] [Google Scholar]
- 25.Gyarmati G, Kadoya H, Moon JY, Burford JL, Ahmadi N, Gill IS, Hong Y-K, Dér B, Peti-Peterdi J. Advances in renal cell imaging. Semin Nephrol 38: 52–62, 2018. doi: 10.1016/j.semnephrol.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peti-Peterdi J, Kidokoro K, Riquier-Brison A. Intravital imaging in the kidney. Curr Opin Nephrol Hypertens 25: 168–173, 2016. doi: 10.1097/MNH.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peti-Peterdi J, Kidokoro K, Riquier-Brison A. Novel in vivo techniques to visualize kidney anatomy and function. Kidney Int 88: 44–51, 2015. doi: 10.1038/ki.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shroff UN, Schiessl IM, Gyarmati G, Riquier-Brison A, Peti-Peterdi J. Novel fluorescence techniques to quantitate renal cell biology. Methods Cell Biol 154: 85–107, 2019. doi: 10.1016/bs.mcb.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez O, Way S, McKenna J 3rd, Gambello MJ. Generation of a conditional disruption of the Tsc2 gene. Genesis 45: 101–106, 2007. doi: 10.1002/dvg.20271. [DOI] [PubMed] [Google Scholar]
- 30.Riquier-Brison ADM, Sipos A, Prókai Á, Vargas SL, Toma L, Meer EJ, Villanueva KG, Chen JCM, Gyarmati G, Yih C, Tang E, Nadim B, Pendekanti S, Garrelds IM, Nguyen G, Danser AHJ, Peti-Peterdi J. The macula densa prorenin receptor is essential in renin release and blood pressure control. Am J Physiol Renal Physiol 315: F521–F534, 2018. doi: 10.1152/ajprenal.00029.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev 119, Suppl 1: S97–S101, 2002. doi: 10.1016/S0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber KH, Arriola Apelo SI, Yu D, Brinkman JA, Velarde MC, Syed FA, Liao CY, Baar EL, Carbajal KA, Sherman DS, Ortiz D, Brunauer R, Yang SE, Tzannis ST, Kennedy BK, Lamming DW. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun 10: 3194, 2019. doi: 10.1038/s41467-019-11174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morales-Corraliza J, Mazzella MJ, Berger JD, Diaz NS, Choi JH, Levy E, Matsuoka Y, Planel E, Mathews PM. In vivo turnover of tau and APP metabolites in the brains of wild-type and Tg2576 mice: greater stability of sAPP in the beta-amyloid depositing mice. PLoS One 4: e7134, 2009. doi: 10.1371/journal.pone.0007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen BM, Marples D, Kim YH, Wang W, Frøkiær J, Nielsen S. Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004. doi: 10.1152/ajpcell.00266.2003. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Xu Y, Stoleru D, Salic A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc Natl Acad Sci USA 109: 413–418, 2012. doi: 10.1073/pnas.1111561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang JJ, Toma I, Sipos A, Peti-Peterdi J. From in vitro to in vivo: imaging from the single cell to the whole organism. Curr Protoc Cytom 44: 12.12.1–12.12.26, 2008. [DOI] [PubMed] [Google Scholar]
- 37.McDonough AA, Veiras LC, Minas JN, Ralph DL. Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol 308: C426–C433, 2015. doi: 10.1152/ajpcell.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 40.Cowley AW Jr, Yang C, Kumar V, Lazar J, Jacob H, Geurts AM, Liu P, Dayton A, Kurth T, Liang M. Pappa2 is linked to salt-sensitive hypertension in Dahl S rats. Physiol Genomics 48: 62–72, 2016. doi: 10.1152/physiolgenomics.00097.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindström NO, De Sena Brandine G, Tran T, Ransick A, Suh G, Guo J, Kim AD, Parvez RK, Ruffins SW, Rutledge EA, Thornton ME, Grubbs B, McMahon JA, Smith AD, McMahon AP. Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev Cell 45: 651–660.e4, 2018. doi: 10.1016/j.devcel.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dou Y, Lin Y, Wang TY, Wang XY, Jia YL, Zhao CP. The CAG promoter maintains high-level transgene expression in HEK293 cells. FEBS Open Bio 11: 95–104, 2021. doi: 10.1002/2211-5463.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36: 320–328, 2011. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]