Keywords: beetroot juice, blood flow, dietary nitrate, neuromuscular fatigue, nitric oxide
Abstract
This study investigated the impact of dietary nitrate supplementation on peripheral hemodynamics, the development of neuromuscular fatigue, and time to task failure during cycling exercise. Eleven recreationally active male participants (27 ± 5 yr, V̇o2max: 42 ± 2 mL/kg/min) performed two experimental trials following 3 days of either dietary nitrate-rich beetroot juice (4.1 mmol NO3−/day; DNS) or placebo (PLA) supplementation in a blinded, counterbalanced order. Exercise consisted of constant-load cycling at 50, 75, and 100 W (4 min each) and, at ∼80% of peak power output (218 ± 12 W), to task-failure. All participants returned to repeat the shorter of the two trials performed to task failure, but with the opposite supplementation regime (iso-time comparison; ISO). Mean arterial pressure (MAP), leg blood flow (QL; Doppler ultrasound), leg vascular conductance (LVC), and pulmonary gas exchange were continuously assessed during exercise. Locomotor muscle fatigue was determined by the change in pre to postexercise quadriceps twitch-torque (ΔQtw) and voluntary activation (ΔVA; electrical femoral nerve stimulation). Following DNS, plasma [nitrite] (∼670 vs. ∼180 nmol) and [nitrate] (∼775 vs. ∼11 μmol) were significantly elevated compared with PLA. Unlike PLA, DNS lowered both QL and MAP by ∼8% (P < 0.05), but did not alter LVC (P = 0.31). V̇O2 across work rates, as well as cycling time to task-failure (∼7 min) and locomotor muscle fatigue following the ISO-time comparison were not different between the two conditions (ΔQtw ∼42%, ΔVA ∼4%). Thus, despite significant hemodynamic changes, DNS did not alter the development of locomotor muscle fatigue and, ultimately, cycling time to task failure.
NEW & NOTEWORTHY This study sought to characterize the impact of dietary nitrate supplementation on the hemodynamic response, locomotor muscle fatigue, and time to task failure during cycling exercise. Although nitrate supplementation lowered mean arterial pressure and exercising leg blood flow, leg vascular conductance and oxygen utilization were unaffected. Despite significant hemodynamic changes, there was no effect of dietary nitrate on neuromuscular fatigue development and, ultimately, cycling time to task failure.
INTRODUCTION
Despite some controversy (1, 2), dietary nitrate supplementation (DNS) has been suggested to enhance endurance performance during both single joint (3, 4) and locomotor (5, 6) exercise. The formation of nitric oxide (NO) from nitrate (NO3−) and nitrite (NO2−) (7) is thought to contribute to this potential ergogenic effect by enhancing peripheral hemodynamics and, therefore, oxygen (O2) transport to muscle (7, 8), a key determinant of athletic performance (9). Indeed, there is clear evidence of a DNS-induced improvement in resting vascular reactivity (10–12) and a decrease in arterial blood pressure during both single joint [i.e., small muscle mass (10, 12, 13)] and locomotor (14–16) exercise. However, the impact of DNS on skeletal muscle blood flow is less clear, with studies in humans, limited to small muscle mass exercise, documenting either an increase (10) or no change (11–13, 17) in limb blood flow (QL) and limb vascular conductance (LVC). Therefore, although the effect of DNS on the peripheral hemodynamic response to exercise recruiting a small muscle mass remains equivocal, the impact of DNS on limb blood flow during locomotor exercise has yet to be assessed.
Neuromuscular fatigue is a reversible reduction in force/power generating capacity of a muscle and is attributed to both a peripheral and central component (18). Peripheral fatigue comprises of intramuscular biochemical changes which lead to an attenuated response to neural stimulation. Central fatigue refers to a reduction in the output of spinal motoneurons and the resulting decrease in muscle activation by the central nervous system (CNS). Importantly, DNS has been documented to improve intramuscular calcium handling (4, 19), excitation-contraction coupling (20), and mitochondrial efficiency (21). These mechanisms likely contribute, individually or in concert, to the nitrate-induced decrease in exercise-driven intramuscular metabolic perturbation and, specifically, to the attenuated accumulation of inorganic phosphate (Pi) (4, 19, 22), a key determinant of peripheral fatigue (23). Furthermore, Baily et al. (4) documented that DNS does not affect the relationship between the O2 cost of exercise (V̇o2) and intramuscular [Pi]. These authors also determined that dietary nitrate lowers V̇o2 at a given workload (due to a reduced ATP cost of force production) and, as a consequence, reduces the accumulation of Pi (4, 19), indirectly supporting the tenet that DNS might attenuate the development of peripheral fatigue during exercise. Indeed, although DNS has been documented to reduce the development of peripheral, but not central, fatigue during small muscle mass knee-extensor exercise (3), these findings are not universal (24). The reason for these conflicting results remains unclear, but may potentially be related to differing DNS protocols. Specifically, the study employing a multiday DNS regime revealed a positive effect (3), whereas the study utilizing only a single dose reported no effect (24). Of note, a comprehensive investigation of the effects of prolonged DNS on the development of neuromuscular fatigue during locomotor exercise has yet to be performed.
It was therefore the intention of this study to investigate the impact of DNS on peripheral hemodynamics, the development of neuromuscular fatigue, and the resulting effect on cycling exercise performance in young, recreational active men. We tested the hypotheses that DNS increases QL and attenuates the development of neuromuscular fatigue, leading to an improvement in cycling time to task failure.
METHODS
Ethical Approval
Written informed consent was obtained from each participant before involvement in this study. All experimental procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center and conducted according to the Declaration of Helsinki for human experimentation.
Subjects
Eleven recreationally active, healthy men participated in this study. Subjects were 27 ± 1 yr of age, noncompetitive, not involved in any systematic endurance exercise training regime, and exhibited the following physical characteristics: height: 182 ± 2 cm; body mass: 79 ± 2 kg. At maximal cycle exercise (Wpeak: 277 ± 14 W), subjects reached a V̇o2max of 42 ± 2 mL/kg/min, qualifying their cardiovascular fitness level as “fair” to “average” (25). All subjects were nonsmokers, not medicated, asymptomatic for cardiovascular and respiratory disease, and refrained from vigorous exercise, caffeine, and alcohol consumption for 24 h before each study visit. Due to the sensitive nature of the commensal bacteria, necessary for dietary nitrate breakdown and utilization, participants were instructed to refrain from using mouthwash, oral hygiene products containing triclosan, or swimming in chlorinated pools for the 24 h before ingestion of the nitrate supplement. To avoid the extraneous influence of normal dietary nitrate ingestion during the 3-day supplementation (both DNS and PLA) period, participants were instructed to avoid leafy green and root vegetables that have high nitrate concentrations.
Experimental Protocol
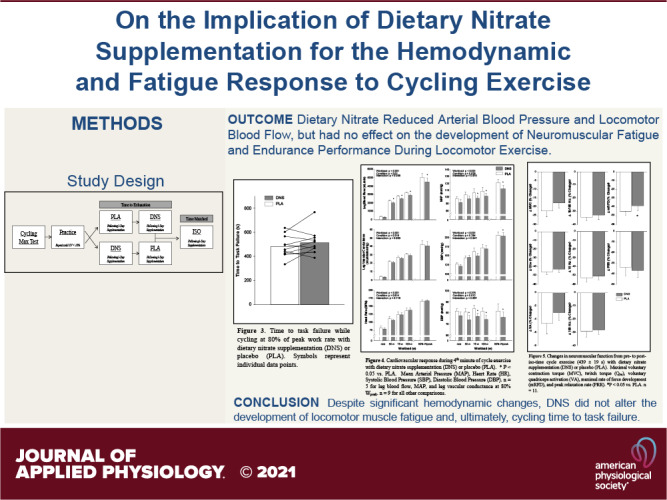
Figure 1 illustrates the flow of laboratory visits included in this study. During the first visit, subjects performed a maximal incremental exercise test (20 W + 25 W/min) (26) on a cycle ergometer (Velotron, Elite Model, Racer Mate, Seattle, WA) to determine peak workload (Wpeak) and maximal oxygen consumption (V̇o2max). During the next two to four visits, subjects practiced performing three bouts of submaximal intensity (50, 75, and 100 W) cycling exercise, each of 4 min duration. Then, following a 5-min recovery period and to quantify each participants endurance performance, subjects performed constant-load cycling exercise at 80% of Wpeak to task failure (i.e., pedal frequency dropped below 65 revolutions per minute (RPM) for >5 s; Tlim). These practice visits were repeated until the coefficient of variation for time to task failure was <10% (27). The purpose of the three stages (50/75/100 W) was a) warm-up for the subsequent performance test and b) to quantify cardiovascular and hemodynamic responses over a range of submaximal exercise intensities. During the next two visits, in randomized order and counterbalanced, subjects repeated the same exercise task following a 3-day supplementation of either a nitrate-rich beetroot concentrate (DNS), or a nitrate stripped placebo (PLA) (double-blind, separated by ≥3days). The final study visit was identical to the prior two visits with the exception that exercise time was matched to the shorter of the two performance times, but with the alternate supplemental condition (iso-time; ISO). To quantify exercise-induced locomotor muscle fatigue, neuromuscular quadriceps function were assessed before and again 30 s after the end of exercise. During all visits, which were separated by 48–72 h and performed ≥4 h post prandium, subjects were provided with verbal encouragement and asked to maintain a constant cadence of 75 RPM.
Figure 1.
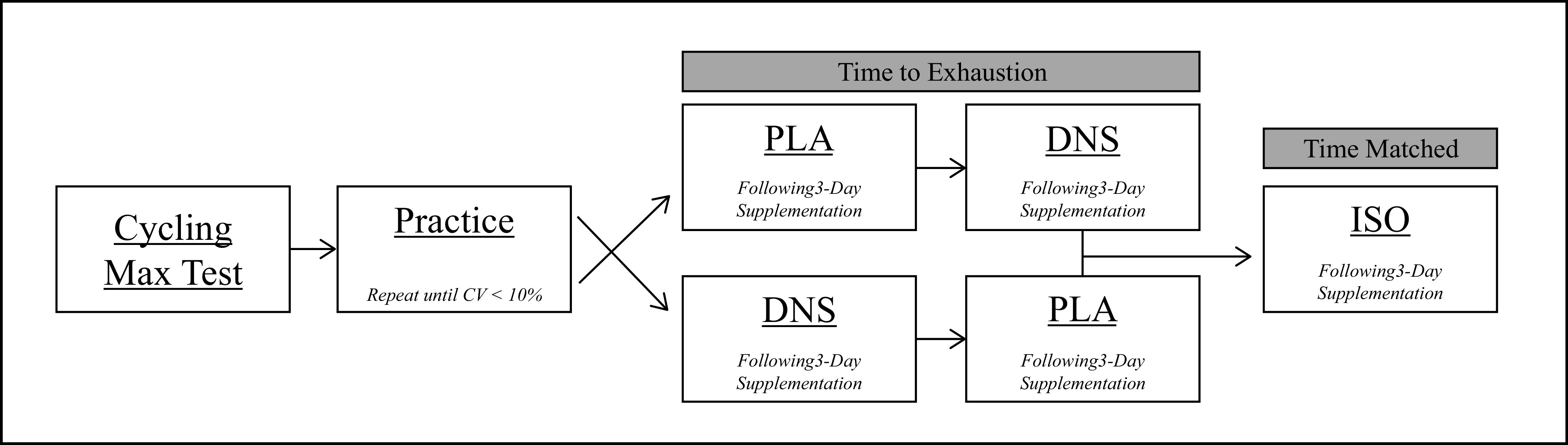
Study design outline. DNS, dietary nitrate supplementation; PLA, placebo; ISO, iso-time. n = 11.
Supplementation
On each of the two days before an experimental trial, subjects were supplemented with either a single dose of DNS (4.1 mmol of NO3−), administered as 70 mL of a commercially available (Beet-it, James White Drinks, Ipswich, UK) beetroot juice concentrate, or a nitrate stripped (0.03 mmol of NO3−) PLA, identical in taste, texture, and packaging (Beet-it, James White Drinks, Ipswich, UK). On the day of an experimental trial and 2 h before arrival at the laboratory, subjects consumed a double-dose of DNS or PLA (28).
Measurements
Cardiovascular responses and rating of perceived exertion.
Cardiovascular responses at rest and during exercise were continuously recorded and averaged over the 4th minute of each bout of exercise. Systolic (SBP) and diastolic (DBP) blood pressure were collected using an automated blood pressure device (Tango M2, SunTech Medical, Inc., NC). Mean arterial pressure (MAP) was calculated as DBP + 1/3[SBP-DBP]. HR was recorded using a 12-lead electrocardiogram (CardioCard; Nasiff Associates, Central Square, NY). A rating of perceived exertion (RPE) was obtained at the end of the 80% Wpeak bout using Borg’s Category Ratio 10 scale (29).
Ventilatory and metabolic responses.
Pulmonary ventilation (minute ventilation, V̇e; tidal volume, Vt; breathing frequency, ƒB) and gas exchange (oxygen uptake, V̇o2; carbon dioxide production, V̇co2) were continuously measured at rest and during exercise using an open-circuit calorimetry system (Innocor; Innovision, Glamsbjerg, Denmark) and averaged during the 4th minute of each exercise bout. Cycling delta efficiency was calculated as (30):
Neuromuscular quadriceps function.
Neuromuscular function was assessed using a custom-made bench on which participants sat in an upright position, arms folded across the chest, with a trunk-thigh angle of 120° and a right knee-joint angle of 90°. A noncompliant cuff attached to a calibrated linear strain gauge (MLP 300; Transducer Techniques, Inc., Temecula, CA) was fixed to the subjects’ right ankle, just superior to the malleoli. Self-adhesive electrodes were placed at the stimulation site, with the cathode on the femoral triangle, and the anode on the anterior portion of the greater trochanter. The positions of these electrodes were marked with indelible ink to ensure a similar stimulation site across visits. A constant current stimulator (Model DS7AH; Digitimer Ltd, Welwyn Garden City, UK) delivered a square wave stimulus (200 μs). To ensure supramaximality during these tests, the stimulation intensity was set to 130% of the intensity eliciting the maximal spatial recruitment of the quadriceps (i.e., 228 ± 9 mA). For the evaluation of quadriceps neuromuscular function, participants performed 3 s maximal voluntary contractions (MVCs) three times, separated by 30 s, before and again 30 s following Tlim and ISO exercise. Superimposed twitches (SITs) and potentiated quadriceps twitches (Qtw) were evoked by a single electrical stimulation of the femoral nerve during the peak torque of each MVC and 2 s after each MVC, respectively. Potentiated electrical doublet stimulation at low (10 Hz) and high (100 Hz) frequencies were performed following Qtw to quantify the low/high frequency fatigue ratio (10/100 Hz); the time between stimulations was ∼2 s. The calculation of voluntary activation (VA) of the quadriceps is as follows: VA (%) = (1 – SIT/Qtw) × 100 (31). Furthermore, the maximal rate of force development (mRFD, calculated as the highest positive derivative of the torque during a 10-ms interval) and maximal relaxation rate (mRR, the highest negative derivative of the torque during a 10-ms interval) were analyzed for each quadriceps twitch. The results of the three sets of assessments, before and after exercise, were averaged and neuromuscular fatigue was quantified as a pre to postexercise change in neuromuscular function.
Leg blood flow (QL) and leg vascular conductance.
Femoral artery blood velocity and vessel diameter in the right leg were continuously recorded (distal to the inguinal ligament and proximal to the deep, superficial femoral bifurcation) at rest, during each submaximal exercise stage, and during the 80% Wpeak trial using a Doppler ultrasound system (LOGIQ 7, GE Medical Systems, Milwaukee, WI). The Doppler ultrasound system was equipped with 12–14 MHz linear array transducers. Artery diameter was determined at a 90° angle along the central axis of the scanned area, with the depth of the measured vessels falling between 2.5 and 4.5 cm. Velocity was measured using the same probe at a frequency of 5 MHz, with the probe positioned to maintain an insonation angle of 60° or less. Blood velocities and vessel diameters were quantified as previously described (32) and averaged during the 4th minute of each exercise bout. Leg blood flow was calculated as QL (mL/min) = [Vmean × π (vessel diameter/2)2 × 60] and leg vascular conductance (LVC) was calculated as LVC (mL/min/mmHg) = QL/MAP.
Plasma Nitrate-Nitrite levels.
Upon arrival at the laboratory following DNS or PLA supplementation, 10 mL of blood was drawn from the antecubital vein. Following centrifugation (5,000 g for 6 min at 4°C), the plasma was extracted, and the samples were immediately frozen in liquid nitrogen and stored at −80°C (33) for later analysis of plasma NO3− and NO2−. At a later date, and within 15 min of thawing, a chemiluminescence detector (Ionic/Sievers NO analyzer (NOA 280i; Sievers Instruments, Boulder, CO) was used to assay all samples for plasma NO3− and NO2−. Potassium iodide in acetic acid was used to reduce NO2− to NO3−. This reductant is able to reduce NO2− to NO, but is not capable of reducing higher oxides of nitrogen (i.e., NO3−). Plasma NO3− concentrations were determined using the reductant vanadium chloride in hydrochloric acid at 95°C, which reduces all nitrogen oxides with an oxidation state of +2 or higher, including NO3− (µmol/L) and NO2− (nmol/L) (34).
Statistical Analysis
Two-way repeated-measures ANOVAs were used to compare cardiovascular and pulmonary responses both at rest and during the 4th minute of each submaximal exercise bout (i.e., 50, 75, 100 W) with DNS and PLA. Tukey’s post hoc tests were performed to identify differences if identified by the ANOVA. Paired samples t tests were employed to compare plasma nitrate-nitrite concentrations, cardiovascular, and pulmonary responses to exercise at 80% Wpeak, and pre to postchanges in neuromuscular function with DNS and PLA. Coefficients of variation (CVs) were calculated to assess the reproducibility of the exercise time to task failure during the Tlim trials performed during the practice sessions. All data are expressed as means ± SE Significance was set at <0.05.
RESULTS
Plasma Nitrate-Nitrite Levels
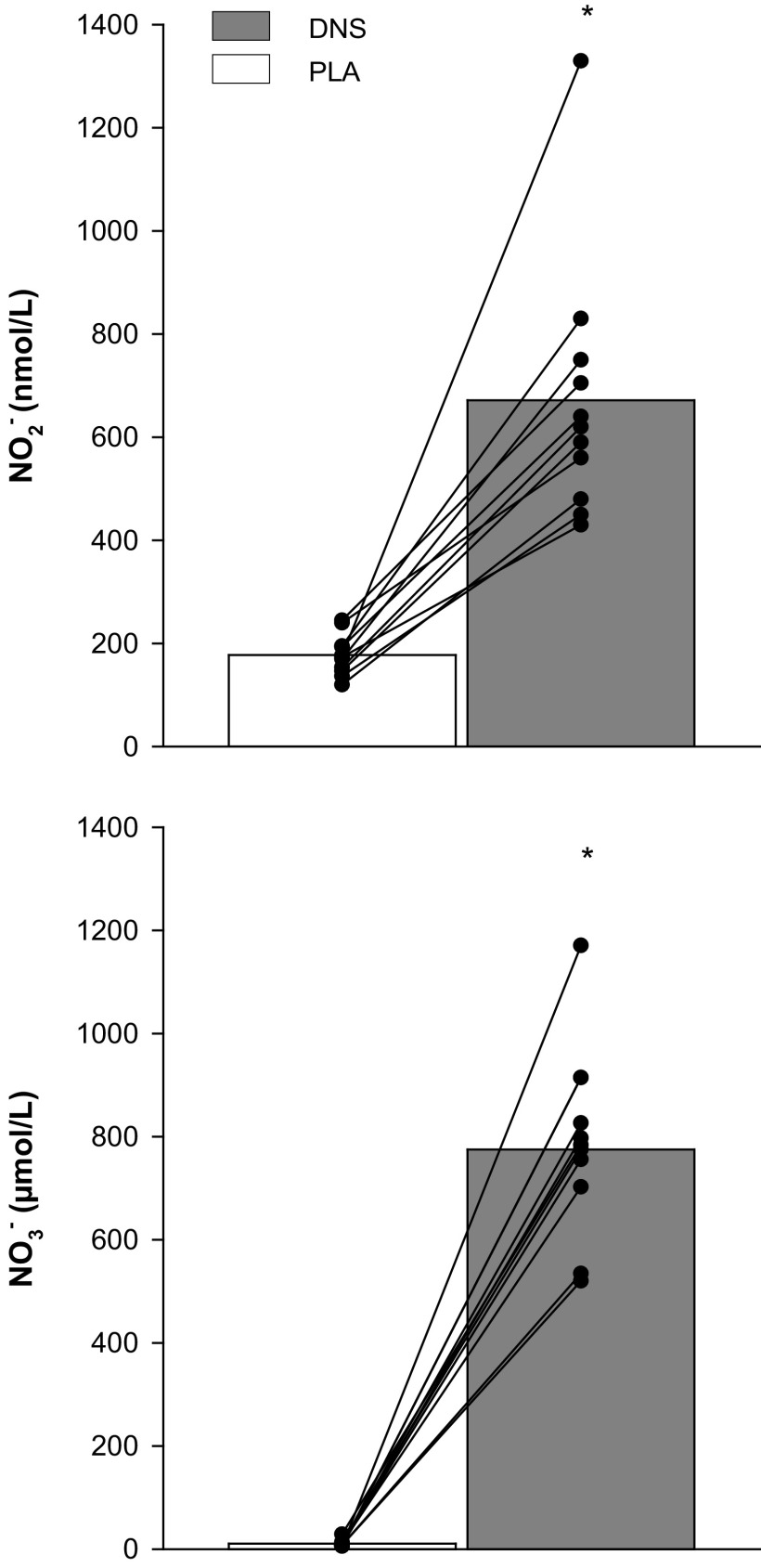
As illustrated in Fig. 2, DNS significantly increased both [NO2−] and [NO3−] compared with PLA. Of note, this increase in plasma [NO2−] and [NO3−] was clearly apparent in all subjects.
Figure 2.
Plasma nitrate and nitrite concentration with dietary nitrate supplementation (DNS) or placebo (PLA). Nitrate, NO3−; Nitrite, NO2−. *P < 0.05 vs. PLA. Other symbols represent individual data points.
Time to Task Failure
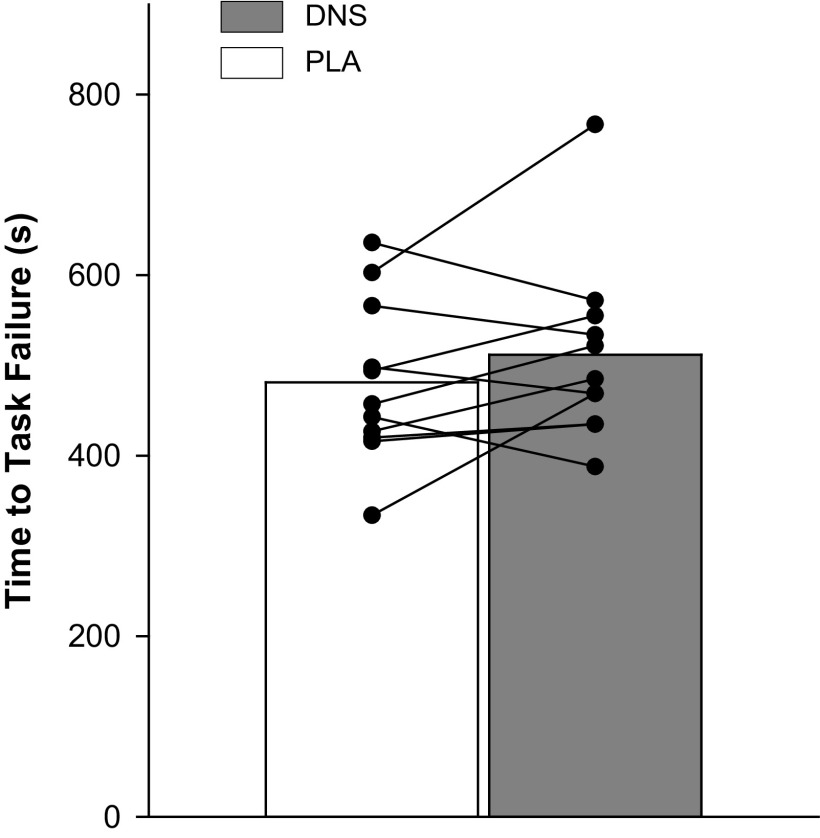
Based upon maximal cycling exercise work rate, 80% Wpeak was determined to be 223 ± 12 W. As illustrated in Fig. 3, at 80% Wpeak, there was no difference in cycling time to task failure between the DNS and PL conditions (PLA: 488 ± 23 s vs. DNS: 503 ± 27 s, P = 0.49).
Figure 3.
Time to task failure while cycling at 80% of peak work rate with dietary nitrate supplementation (DNS) or placebo (PLA). Symbols represent individual data points.
Cardiovascular, Pulmonary, and Perceptual Responses to Exercise
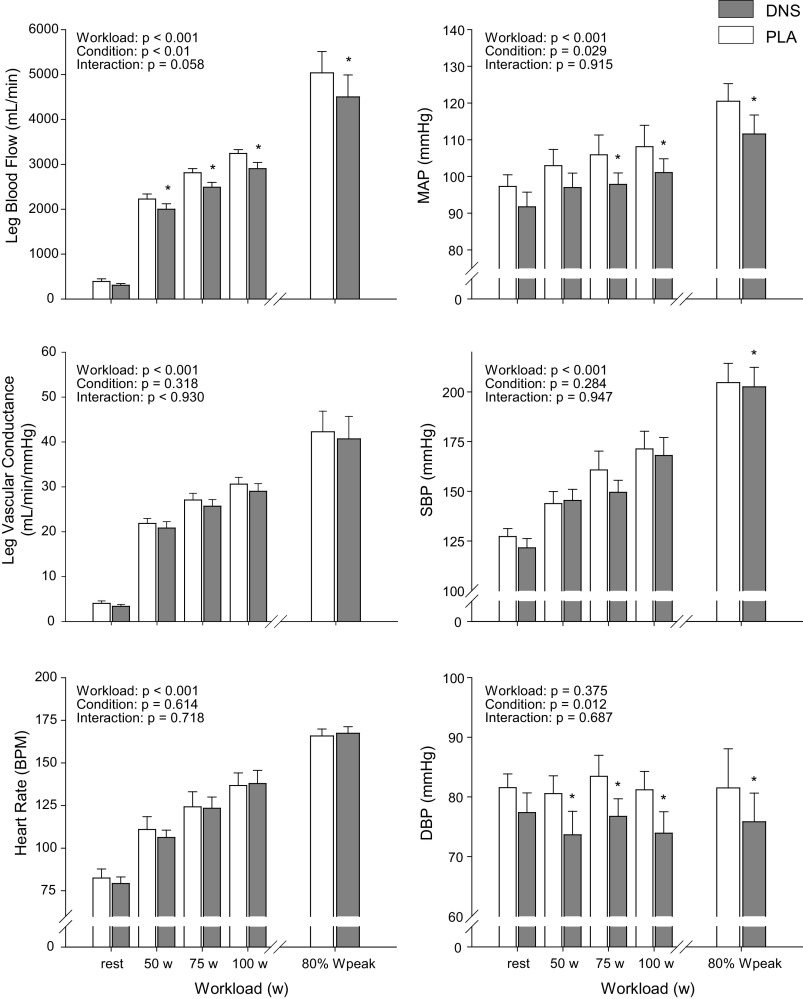
The cardiovascular responses to exercise with and without DNS are illustrated in Fig. 4. Femoral artery diameter did not change from rest to exercise in either condition (PLA: 0.94 ± 0.01 cm, DNS: 0.93 ± 0.02 cm) and was not affected by DNS (P > 0.4). With DNS, QL was significantly attenuated during all absolute, submaximal work rates (n = 9), and at 80% Wpeak (n = 5). Technical difficulties with quantifying QL during high intensity cycling exercise only allowed us to report data from five subjects. DNS reduced MAP between 6% and 8% (corresponding to 6–8 mmHg), which achieved statistical significance at 75 (PLA: 106 ± 5 vs. DNS: 98 ± 3 mmHg) and 100 W (PLA: 108 ± 6 vs. DNS: 101 ± 4 mmHg) as well as at 80% Wpeak (PLA: 119 ± 7 vs. DNS: 113 ± 3 mmHg). With no changes in SBP (P = 0.28) at the absolute, submaximal work rates, the effect of DNS on MAP during the lower intensities was mainly driven by the significant reduction in DBP. During high intensity exercise (80% Wpeak), DNS resulted in a significant reduction in both SBP (∼5%; PLA: 157 ± 5 vs. DNS: 153 ± 5 mmHg) and DBP (∼10%; PLA: 82 ± 2 vs. DNS: 76 ± 2 mmHg), which, in combination, resulted in a significant decrease in MAP. As a consequence of these blood pressure changes, coupled with the reduction in QL, there was no significant effect of DNS on LVC (P = 0.32). DNS had no effect on HR (Fig. 4), pulmonary ventilation, and gas exchange during exercise (Table 1) and did not alter RPE at the 80% Wpeak bout (PLA: 9 ± 1 vs. DNS: 9 ± 1). Delta efficiency (V̇o2/W) was also not altered following DNS (75 W: PLA: 27 ± 1% vs. DNS: 27 ± 1%; 100 W: PLA: 26 ± 1% vs. DNS: 25 ± 1%; 80% Wpeak: PLA: 24 ± 1% vs. DNS: 25 ± 1%; P = 0.83).
Figure 4.
Cardiovascular response during 4th minute of cycle exercise with dietary nitrate supplementation (DNS) or placebo (PLA). *P < 0.05 vs. PLA. MAP, mean arterial pressure; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure. n = 5 for leg blood flow, MAP, and leg vascular conductance at 80% Wpeak. n = 9 for all other comparisons.
Table 1.
Pulmonary ventilation and gas exchange at rest and during the 4th minute of cycle exercise
| Rest |
50 W |
75 W |
100 W |
Intensity |
Nitrate |
Interaction |
80% Wpeak |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA | DNS | PLA | DNS | PLA | DNS | PLA | DNS | P | P | P | PLA | DNS | |
| V̇e, L/min | 14.8 ± 0.6 | 15.0 ± 0.5 | 35.5 ± 0.9 | 35.2 ± 1.2 | 35.5 ± 0.9 | 35.2 ± 1.2 | 44.4 ± 1.3 | 45.3 ± 1.4 | < 0.001 | 0.801 | 0.473 | 146.1 ± 5.3 | 143.7 ± 5.2 |
| ƒB, breaths/min | 16.9 ± 1.9 | 16.7 ± 1.1 | 24.3 ± 1.1 | 25.1 ± 1.2 | 26.5 ± 1.3 | 27.3 ± 1.2 | 28.8 ± 1.5 | 28.9 ± 1.2 | < 0.001 | 0.291 | 0.599 | 53.3 ± 1.9 | 54.4 ± 1.9 |
| V̇t, L/min | 1.04 ± 0.07 | 1.06 ± 0.08 | 1.51 ± 0.06 | 1.46 ± 0.05 | 1.74 ± 0.06 | 1.71 ± 0.06 | 1.97 ± 0.07 | 1.96 ± 0.07 | < 0.001 | 0.332 | 0.592 | 2.76 ± 0.10 | 2.67 ± 0.12 |
| V̇o2, L/min | 0.37 ± 0.01 | 0.38 ± 0.01 | 1.18 ± 0.02 | 1.13 ± 0.03 | 1.45 ± 0.03 | 1.42 ± 0.03 | 1.70 ± 0.04 | 1.71 ± 0.03 | < 0.001 | 0.19 | 0.226 | 3.15 ± 0.15 | 3.14 ± 0.16 |
| V̇o2, % of max | 12 ± 1 | 12 ± 1 | 37 ± 2 | 35 ± 2 | 45 ± 2 | 45 ± 2 | 53 ± 3 | 55 ± 3 | 97 ± 2 | 96 ± 2 | |||
| V̇co2, L/min | 0.27 ± 0.01 | 0.27 ± 0.01 | 0.91 ± 0.03 | 0.89 ± 0.03 | 1.19 ± 0.03 | 1.16 ± 0.03 | 1.48 ± 0.04 | 1.45 ± 0.04 | < 0.001 | 0.153 | 0.706 | 3.05 ± 0.12 | 2.93 ± 0.13* |
| RER | 0.74 ± 0.02 | 0.74 ± 0.03 | 0.77 ± 0.02 | 0.77 ± 0.01 | 0.83 ± 0.02 | 0.81 ± 0.01 | 0.86 ± 0.02 | 0.85 ± 0.02 | < 0.001 | 0.335 | 0.787 | 0.98 ± 0.02 | 0.94 ± 0.02 |
| V̇e/ V̇o2, L/min | 41.4 ± 3.4 | 40.3 ± 3.3 | 27.6 ± 0.8 | 28.5 ± 0.7 | 27.9 ± 0.6 | 29.2 ± 0.9 | 29.5 ± 1.0 | 29.9 ± 0.9 | 0.013 | 0.12 | 0.604 | 43.7 ± 1.7 | 43.2 ± 1.6 |
| V̇e/ V̇co2, L/min | 51.4 ± 3.7 | 51.2 ± 3.4 | 35.8 ± 1.2 | 37.4 ± 1.1 | 33.9 ± 0.6 | 35.9 ± 0.9 | 34.4 ± 0.8 | 35.3 ± 0.9 | 0.027 | 0.042 | 0.474 | 45.0 ± 1.3 | 45.9 ± 1.7 |
DNS, dietary nitrate supplementation; ƒB, breathing frequency; PLA, placebo; V̇E, minute ventilation; V̇t, tidal volume; V̇co2, carbon dioxide production; V̇o2, oxygen consumption. *P < 0.05 vs. PLA. n = 11.
Neuromuscular Fatigue and Exercise Performance
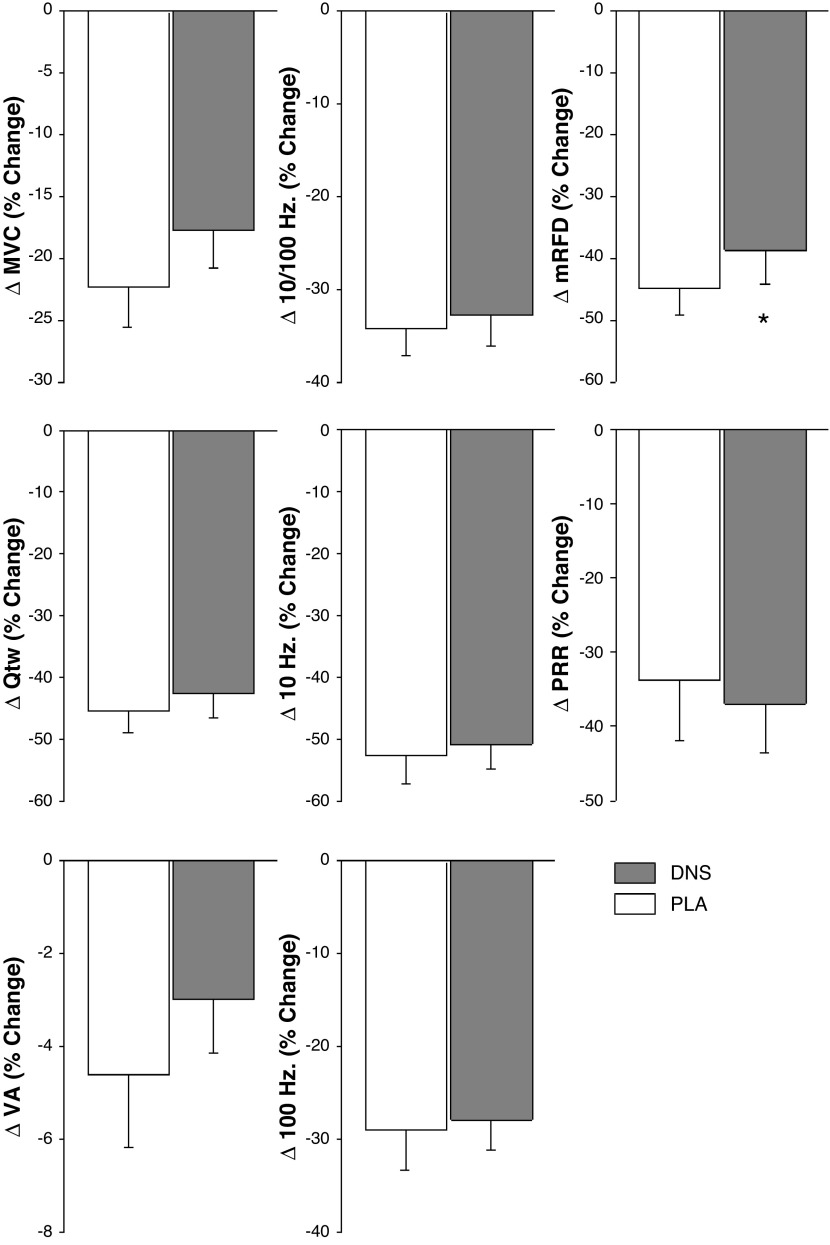
Preexercise, resting, and neuromuscular function were not affected by DNS (MVC: 259 ± 9 Nm, P = 0.79; Qtw: 76 ± 3 Nm, P = 0.67; VA: 91 ± 1%, P = 0.40; mRFD: 1,552 ± 52 N/s, P = 0.20; mRR: 638 ± 22 N/s, P = 0.36). Pre to postexercise changes in neuromuscular function were not different between the DNS and PLA conditions (PLA vs. DNS, P > 0.10): ΔMVC: −51 ± 5 Nm, ΔQtw: −33 ± 2 Nm, ΔVA: −4 ± 1%, ΔmRFD: −669 ± 68 Nm/ms, ΔmRR: 238 ± 36 Nm/ms). The exercise-induced changes in neuromuscular function following PLA-ISO and DNS-ISO are illustrated in Fig. 5.
Figure 5.
Changes in neuromuscular function from pre to post iso-time cycle exercise (439 ± 19 s) with dietary nitrate supplementation (DNS) or placebo (PLA). MVC; maximal voluntary contraction torque; Qtw, twitch torque; VA, voluntary quadriceps activation; mRFD, maximal rate of force development; PRR, peak relaxation rate. *P < 0.05 vs. PLA. n = 11.
DISCUSSION
This study investigated the impact of DNS on the peripheral hemodynamics, the development of neuromuscular fatigue, and the time to task failure during cycling exercise. DNS successfully elevated both plasma [NO2−] and [NO3−] and lowered both QL and MAP, but did not alter LVC. In contrast, V̇o2 across work rates and cycling time to task failure and neuromuscular fatigue following the ISO comparison were not affected by DNS. Thus, despite significant hemodynamic changes, DNS did not alter the rate of development of locomotor muscle fatigue and the time to task failure during high-intensity cycling exercise.
Hemodynamic and Metabolic Responses to Cycle Exercise
Following DNS, MAP and QL during cycling exercise were significantly reduced (up to 10%) compared with the same level of exercise performed after PLA supplementation (Fig. 4). Although the decrease in MAP is in line with earlier investigations utilizing cycling exercise (15, 35), the concomitant reduction in QL, and subsequently no change in LVC, contrasts with other human studies that have documented increased (10) or unchanged (11–13, 17) arm blood flow and vascular conductance during handgrip exercise following DNS. The current finding of attenuated QL and unchanged LVC also differs from a study in rats that revealed that DNS increased hind limb blood flow and vascular conductance during treadmill exercise (33), but is in agreement with another study in rats with heart failure, in which DNS diminished diaphragm blood flow (36). Interestingly, it has previously been suggested that DNS may not only attenuate peripheral vascular resistance, but can also result in vasodilation in other visceral tissue beds (14, 37). Therefore, in the current study, this systemic vasodilation could, at the expense of QL, have altered the distribution of cardiac output, facilitating blood flow to other tissues/organs. Thus, although not quantified as in this investigation, it is possible that there was a nitrate-induced increase in systemic vascular conductance without a change in LVC (Fig. 4). This could, at least in part, account for the observed fall in MAP and the subsequent fall in QL during cycling exercise following DNS. An alternative explanation for the decrease in QL following DNS could be that the effect of DNS on augmenting the microvascular diffusive surface area within locomotor muscles (13, 38) might have been more prominent than its effect of augmenting systemic vascular conductance. Specifically, the facilitating effect of dietary nitrate on locomotor muscle O2 extraction could have diminished the need for O2 delivery to maintain a consistent O2 consumption, and QL might have fallen proportionally without compromising V̇o2.
In terms of metabolism, the current findings are in agreement with previous human studies utilizing cycling (38) and running (39), and suggest that the metabolic cost of cycling, as measured by pulmonary V̇o2, is not altered by DNS (Table 1). However, the unchanged V̇o2 and delta efficiency during exercise following DNS are also at odds with earlier cycling studies documenting a decrease in V̇o2 and thus an improved cycling efficiency during submaximal exercise following DNS (8, 40). Of note, despite the decrease in QL in the current study, V̇o2 remained similar during PLA and DNS. To interpret this finding, it is important to recognize that the V̇o2 of working limb muscle is, at least in normoxia, tightly regulated (41) and the main determinant of pulmonary V̇o2 during cycling exercise (42). Furthermore, as DNS has no effect on hemoglobin saturation (11), QL can be considered a surrogate for locomotor muscle O2 delivery. Based on these assumptions, the most likely explanation for the unchanged V̇o2 occurring in the face of a decreased QL is an increase in locomotor muscle O2 extraction, a compensatory response assuring the maintenance of V̇o2 in the face of decreased locomotor muscle O2 delivery. Indeed, such a supposition is in line with the already noted observation that nitrate supplementation appears to improve the distribution of blood within the microvasculature of the working muscle (13, 38), and thereby facilitates O2 extraction by increasing the microvascular diffusive surface area in regions that were previously under perfused.
Neuromuscular Fatigue
DNS did not affect the development of neuromuscular fatigue during high-intensity cycling exercise (Fig. 5). Given a) the similar V̇o2 in PLA and DNS (Table 1), b) the previously documented lack of an effect of DNS on the linear relationship between pulmonary (and muscle) V̇o2 and intramuscular [Pi] (4), and c) the strong relationship between intramuscular [Pi] and peripheral fatigue (43), the similar level of fatigue revealed in the iso-time comparison was not unexpected. The observed lack of an impact of DNS on the development of fatigue is supported by 31P-MRS-based assessments that document the increase in intramuscular fatigue metabolites (Pi and H+) from the start of exercise to task failure (the time to which was not different in the current study, Fig. 5) to be similar following placebo and dietary nitrate supplementation (4). However, it should be acknowledged that in this previous MRS-based work, the kinetics leading to the similar intramuscular metabolic milieu at the end of exercise might have differed from the current study. Specifically, based on the previously identified linear relationship between pulmonary V̇O2 and muscle [Pi] (4), the similar V̇o2 during exercise following DNS and PLA (Table 1) suggests that dietary nitrate did not affect intramuscular metabolism during exercise in the current study. This is different from a previous knee-extensor study where DNS lowered V̇o2 and intramuscular [Pi] during a large portion of the trial, but did not affect the values at end-exercise (4). However, insight from this comparison is limited as fatigue was not quantified in this study.
Data on the impact of DNS on the development of neuromuscular fatigue in exercising humans are scarce. Although the current investigation is the first to document the effect during locomotor exercise, others have previously focused on single joint, small muscle mass exercise. However, the findings of these prior single joint exercise studies are contradictory, with one, utilizing dynamic knee-extension exercise, revealing a decrease in end-exercise peripheral fatigue following 5 days of DNS (3), and the other, utilizing intermittent isometric knee-extensions, documenting no effect after a single dose (24). The exact reason for this discrepancy in findings remains unknown, but might be related to the quite different supplementation regimes and/or potential between-subject differences in elevating plasma nitrite and nitrate levels in the response to nitrate supplementation (2). Regardless, more work is needed to gain a better understanding of the impact of dietary nitrate on the development of neuromuscular fatigue during both small muscle mass and locomotor exercise. Taken together, the similar level of end-exercise fatigue in the current investigation is likely explained by compensatory mechanisms maintaining V̇o2 in the face of significant DNS-related decreases in locomotor muscle O2 delivery.
Exercise Performance
DNS did not affect the time to task failure during high-intensity cycling exercise (Fig. 3). Although this lack of an effect confirms several earlier studies utilizing locomotor (44, 45) and single joint (24) exercise, it contradicts other investigations documenting a positive effect of DNS on endurance performance during various events, including cycling (5, 40), running (46), and knee-extensor (3) exercise. The reasons for the discrepancy between the performance-related outcomes in these investigations remain unclear. The absence/presence of sufficient prior practice of the task utilized to quantify performance, differences in exercise modality and duration (47, 48), environmental conditions (22), participant training status (49), and supplemental dosing/timing (28, 50) have all been considered as factors contributing the between-studies differences in performance-related outcomes. In fact, it could be argued that, compared with other studies using 5+ days of dietary nitrate in well-trained endurance athletes, the current supplementation protocol (3 days, 4.1 mmol NO3−/day; untrained individuals) could potentially have been too short to alter the cardiopulmonary and neuromuscular fatigue response to exercise and, consequently, cycling performance. However, according to previously established dose-response curves, plasma [NO2−] and [NO3−] usually peaks between 2 and 4 days of consistent DNS (28), and significant performance enhancements have been reported as early as 2.5 h after acute oral nitrate supplementation (5). Thus, despite efforts to ensure a supplementation regime consistent with successful earlier protocols (28, 50) and investigations documenting enhanced endurance performance following DNS (7, 16, 44, 51), the current group mean time to task failure was very similar following DNS and PLA (Fig. 3). As fatigue is a key determinant of locomotor endurance exercise performance (52), the current findings, in agreement with a recent knee-extensor study (24), suggest that that the unchanged exercise performance following DNS may largely be explained by its lack of an effect on the development of neuromuscular fatigue.
Finally, the 15 s longer group mean time to task failure following DNS (P = 0.49) resulted from the 4%–40% improvement in endurance time in 7 of the 11 subjects combined with the 4%–12% curtailed performance in 4 subjects. To address the concern that learning effects (53) and the often large error of measurement in Tlim trials (coefficient of variation up to 30%) could mask treatment-induced changes in endurance performance (54), the current study design included 3–5 Tlim practice sessions. This strategy resulted in a coefficient of variation of 6% (range: 2–9%) before the first experimental session and assured optimal sensitivity of the Tlim tests (27) to detect the impact of DNS on locomotor exercise performance. Although this methodological rigor offers confidence in the current outcome, the varying effects of DNS on endurance performance warrant further investigations focusing on the possibility of a DNS-related response heterogeneity.
One limitation of the current study relates to the QL measures obtained during the 80% Wpeak bout. Technical difficulties associated with the quantification of QL during high-intensity cycling exercise limited the sample size to 5, compromising generalizability of these findings. Furthermore, although the manuscript offers potential causes, the available data are insufficient to identify the exact mechanism(s) underlying the peripheral hemodynamic changes associated with DNS. Future studies should therefore include femoral arterial and venous blood sampling and determine the impact of DNS on leg V̇o2 and O2 extraction during locomotor exercise. Also, the observation that all participants nearly reached their V̇o2max during the 80% Wpeak trial in both conditions (Table 1) suggests that the DNS-induced decreases in blood flow and pressure did not considerably limit their maximal aerobic capacity. However, as these measures were taken at minute 4 of the 80% Wpeak bout and ∼3 min before task failure, the potential for a difference in V̇o2 closer to the end of the performance task (i.e., at exhaustion) cannot be excluded. Finally, given the effect of training status on the influence of nitrate supplementation on exercise performance (49), it is important to emphasize that the participants in the current study were recreationally active, but not competitive at any level and not involved in habitual endurance or resistance exercise training. This homogeneity limits the overall generalizability of our findings, and the potential for different effects of DNS on, for example, endurance trained athletes cannot be excluded.
Summary and Conclusion
DNS successfully elevated plasma nitrate and nitrite and lowered QL and MAP, without affecting LVC, during locomotor exercise in recreationally active, young men. Despite these significant hemodynamic changes, DNS did not alter V̇o2, the development of locomotor muscle fatigue, or cycling endurance performance. Nitrate-induced improvements in muscle O2 extraction and the previously suggested lack of an effect of dietary nitrate on the accumulation of intramuscular fatigue metabolites at a given V̇o2 are likely key elements of this outcome.
GRANTS
This study was supported by the National Heart, Lung, and Blood Institute Grants HL457 116579 and HL-103786 as well as Ruth L. Kirschstein National Research Service Award T32 458 HL 139451, and by the Veterans Affairs Rehabilitation Research and Development (E6910-R, 459 E1697-R, E1572-P, and E3207-R).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S.T. and M.A. conceived and designed research; T.S.T., J.C.W., T.J.H., J.R.G., V.P.G., H-Y.W., D.T.L., and M.A. performed experiments; T.S.T. analyzed data; T.S.T. and M.A. interpreted results of experiments; T.S.T. prepared figures; T.S.T. drafted manuscript; T.S.T., J.C.W., T.J.H., J.R.G., V.P.G., H-Y.W., D.T.L., R.S.R., and M.A. edited and revised manuscript; T.S.T., J.C.W., T.J.H., J.R.G., V.P.G., H-Y.W., D.T.L., R.S.R., and M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate our participants’ enthusiasm and willingness to participate in this study.
REFERENCES
- 1.Lee S, Abel MG, Thomas T, Symons TB, Yates JW. Acute beetroot juice supplementation does not attenuate knee extensor exercise muscle fatigue in a healthy young population. J Exerc Nutrition Biochem 23: 55–62, 2019. doi: 10.20463/jenb.2019.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur J Appl Physiol 112: 4127–4134, 2012. doi: 10.1007/s00421-012-2397-6. [DOI] [PubMed] [Google Scholar]
- 3.Husmann F, Bruhn S, Mittlmeier T, Zschorlich V, Behrens M. Dietary nitrate supplementation improves exercise tolerance by reducing muscle fatigue and perceptual responses. Front Physiol 10: 404, 2019. doi: 10.3389/fphys.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 109: 135–148, 2010. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 5.Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc 43: 1125–1131, 2011. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 6.Cermak NM, Gibala MJ, van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab 22: 64–71, 2012. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- 7.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med 44, Suppl 1: S35–45, 2014. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 9.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol (1985) 104: 861–870, 2008. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- 10.Richards JC, Racine ML, Hearon CM Jr, Kunkel M, Luckasen GJ, Larson DG, Allen JD, Dinenno FA. Acute ingestion of dietary nitrate increases muscle blood flow via local vasodilation during handgrip exercise in young adults. Physiol Rep 6: e13572, 2018. doi: 10.14814/phy2.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentley RF, Walsh JJ, Drouin PJ, Velickovic A, Kitner SJ, Fenuta AM, Tschakovsky ME. Dietary nitrate restores compensatory vasodilation and exercise capacity in response to a compromise in oxygen delivery in the noncompensator phenotype. J Appl Physiol (1985) 123: 594–605, 2017. doi: 10.1152/japplphysiol.00953.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey DP, Treichler DP, Ganger CT 4th, Schneider AC, Ueda K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J Appl Physiol (1985) 118: 178–186, 2015. doi: 10.1152/japplphysiol.00662.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig JC, Broxterman RM, Smith JR, Allen JD, Barstow TJ. Effect of dietary nitrate supplementation on conduit artery blood flow, muscle oxygenation, and metabolic rate during handgrip exercise. J Appl Physiol (1985) 125: 254–262, 2018. doi: 10.1152/japplphysiol.00772.2017. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Stebbins CL, Jung E, Nho H, Kim JK, Chang MJ, Choi HM. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. Am J Physiol Regul Integr Comp Physiol 309: R459–R466, 2015. doi: 10.1152/ajpregu.00099.2015. [DOI] [PubMed] [Google Scholar]
- 15.Bond V Jr, Curry BH, Adams RG, Millis RM, Haddad GE. Cardiorespiratory function associated with dietary nitrate supplementation. Appl Physiol Nutr Metab 39: 168–172, 2014. doi: 10.1139/apnm-2013-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey SJ, Varnham RL, DiMenna FJ, Breese BC, Wylie LJ, Jones AM. Inorganic nitrate supplementation improves muscle oxygenation, O2 uptake kinetics, and exercise tolerance at high but not low pedal rates. J Appl Physiol (1985) 118: 1396–1405, 2015. doi: 10.1152/japplphysiol.01141.2014. [DOI] [PubMed] [Google Scholar]
- 17.Kim JK, Moore DJ, Maurer DG, Kim-Shapiro DB, Basu S, Flanagan MP, Skulas-Ray AC, Kris-Etherton P, Proctor DN. Acute dietary nitrate supplementation does not augment submaximal forearm exercise hyperemia in healthy young men. Appl Physiol Nutr Metab 40: 122–128, 2015. doi: 10.1139/apnm-2014-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48: 2294–2306, 2016. doi: 10.1249/MSS.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol 590: 3575–3583, 2012. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haider G, Folland JP. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med Sci Sports Exerc 46: 2234–2243, 2014. doi: 10.1249/MSS.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 21.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab 13: 149–159, 2011. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 589: 5517–5528, 2011. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 24.Le Roux-Mallouf T, Laurent J, Besset D, Marillier M, Larribaut J, Belaidi E, Corne C, Doutreleau S, Verges S. Effects of acute nitric oxide precursor intake on peripheral and central fatigue during knee extensions in healthy men. Exp Physiol 104: 1100–1114, 2019. doi: 10.1113/EP087493. [DOI] [PubMed] [Google Scholar]
- 25.Shvartz E, Reibold RC. Aerobic fitness norms for males and females aged 6 to 75 years: a review. Aviat Space Environ Med 61: 3–11, 1990. [PubMed] [Google Scholar]
- 26.Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc 36: 613–622, 2004. doi: 10.1249/01.mss.0000122076.21804.10. [DOI] [PubMed] [Google Scholar]
- 27.Amann M, Hopkins WG, Marcora SM. Similar sensitivity of time to exhaustion and time-trial time to changes in endurance. Med Sci Sports Exerc 40: 574–578, 2008. doi: 10.1249/MSS.0b013e31815e728f. [DOI] [PubMed] [Google Scholar]
- 28.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 29.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 30.Katch VL, McArdle WD, Katch FI. Essentials of Exercise Physiology. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health, 2011. [Google Scholar]
- 31.Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hureau TJ, Weavil JC, Thurston TS, Wan HY, Gifford JR, Jessop JE, Buys MJ, Richardson RS, Amann M. Pharmacological attenuation of group III/IV muscle afferents improves endurance performance when oxygen delivery to locomotor muscles is preserved. J Appl Physiol (1985) 127: 1257–1266, 2019. doi: 10.1152/japplphysiol.00490.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591: 547–557, 2013. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen JD, Miller EM, Schwark E, Robbins JL, Duscha BD, Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide 20: 231–237, 2009. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HM, Kim BH, Nho H, Kim KA, Park J, Chang MJ, Kim JK. Dietary nitrate supplementation attenuates blood pressure in young prehypertensive men during exercise. J Mens Health 12: 25–33, 2016. doi: 10.31083/jomh.v12i1.22. [DOI] [Google Scholar]
- 36.Smith JR, Ferguson SK, Hageman KS, Harms CA, Poole DC, Musch TI. Dietary nitrate supplementation opposes the elevated diaphragm blood flow in chronic heart failure during submaximal exercise. Respir Physiol Neurobiol 247: 140–145, 2018. doi: 10.1016/j.resp.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bond V, Curry BH, Adams RG, Asadi MS, Stancil KA, Millis RM, Haddad GE. Effects of nitrate supplementation on cardiovascular and autonomic reactivity in African-American females. ISRN Physiol 2014: 676235, 2014. doi: 10.1155/2014/676235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breese BC, Poole DC, Okushima D, Bailey SJ, Jones AM, Kondo N, Amano T, Koga S. The effect of dietary nitrate supplementation on the spatial heterogeneity of quadriceps deoxygenation during heavy-intensity cycling. Physiol Rep 5: e13340, 2017. doi: 10.14814/phy2.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasconcellos J, Henrique Silvestre D, Dos Santos Baião D, Werneck-de-Castro JP, Silveira Alvares T, Paschoalin VM. A single dose of beetroot gel rich in nitrate does not improve performance but lowers blood glucose in physically active individuals. J Nutr Metab 2017: 7853034, 2017. doi: 10.1155/2017/7853034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 107: 1144–1155, 2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 41.Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol (1985) 75: 2586–2594, 1993. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- 42.Poole DC, Gaesser GA, Hogan MC, Knight DR, Wagner PD. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol (1985) 72: 805–810, 1992.doi: 10.1152/jappl.1992.72.2.805. [DOI] [PubMed] [Google Scholar]
- 43.Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315, 2016. doi: 10.1113/JP272283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 45.Bescós R, Ferrer-Roca V, Galilea PA, Roig A, Drobnic F, Sureda A, Martorell M, Cordova A, Tur JA, Pons A. Sodium nitrate supplementation does not enhance performance of endurance athletes. Med Sci Sports Exerc 44: 2400–2409, 2012. doi: 10.1249/MSS.0b013e3182687e5c. [DOI] [PubMed] [Google Scholar]
- 46.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol (1985) 110: 591–600, 2011. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 47.Wylie LJ, Bailey SJ, Kelly J, Blackwell JR, Vanhatalo A, Jones AM. Influence of beetroot juice supplementation on intermittent exercise performance. Eur J Appl Physiol 116: 415–425, 2016. doi: 10.1007/s00421-015-3296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelly J, Vanhatalo A, Wilkerson DP, Wylie LJ, Jones AM. Effects of nitrate on the power-duration relationship for severe-intensity exercise. Med Sci Sports Exerc 45: 1798–1806, 2013. doi: 10.1249/MSS.0b013e31828e885c. [DOI] [PubMed] [Google Scholar]
- 49.Hultström M, Amorim de Paula C, Antônio Peliky Fontes M, Porcelli S, Bellistri G, Pugliese L, et al. Commentaries on viewpoint: can elite athletes benefit from dietary nitrate supplementation? J Appl Physiol (1985) 119: 762–769, 2015. doi: 10.1152/japplphysiol.00640.2015. [DOI] [PubMed] [Google Scholar]
- 50.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Masschelein E, Van Thienen R, Wang X, Van Schepdael A, Thomis M, Hespel P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J Appl Physiol (1985) 113: 736–745, 2012. doi: 10.1152/japplphysiol.01253.2011. [DOI] [PubMed] [Google Scholar]
- 52.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol 586: 161–173, 2008. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 30: 1–15, 2000. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 54.McLellan TM, Cheung SS, Jacobs I. Variability of time to exhaustion during submaximal exercise. Can J Appl Physiol 20: 39–51, 1995. doi: 10.1139/h95-003. [DOI] [PubMed] [Google Scholar]