Abstract
Increased insulin is associated with obesity-related airway hyperreactivity and asthma. We tested whether the use of metformin, an antidiabetic drug used to reduce insulin resistance, can reduce circulating insulin, thereby preventing airway hyperreactivity in rats with dietary obesity. Male and female rats were fed a high- or low-fat diet for 5 wk. Some male rats were simultaneously treated with metformin (100 mg/kg orally). In separate experiments, after 5 wk of a high-fat diet, some rats were switched to a low-fat diet, whereas others continued a high-fat diet for an additional 5 wk. Bronchoconstriction and bradycardia in response to bilateral electrical vagus nerve stimulation or to inhaled methacholine were measured in anesthetized and vagotomized rats. Body weight, body fat, caloric intake, fasting glucose, and insulin were measured. Vagally induced bronchoconstriction was potentiated only in male rats on a high-fat diet. Males gained more body weight, body fat, and had increased levels of fasting insulin compared with females. Metformin prevented development of vagally induced airway hyperreactivity in male rats on high-fat diet, in addition to inhibiting weight gain, fat gain, and increased insulin. In contrast, switching rats to a low-fat diet for 5 wk reduced body weight and body fat, but it did not reverse fasting glucose, fasting insulin, or potentiation of vagally induced airway hyperreactivity. These data suggest that medications that target insulin may be effective treatment for obesity-related asthma.
Keywords: airway hyperreactivity, asthma, insulin, metformin, obesity
INTRODUCTION
Asthma affects more than 350 million people worldwide (1). One characteristic of asthma is airway hyperreactivity, or excessive bronchoconstriction, in response to specific and nonspecific stimuli. Asthma is a heterogeneous disease, divided into multiple phenotypes based on clinical presentation and pathobiological biomarkers (2, 3). Recent studies suggest there are two distinct phenotypes of asthma in obesity (4): obesity-related late-onset asthma and obesity-related early-onset asthma. The pathophysiology of obesity-related early-onset asthma may be consistent with early-onset allergic asthma, which is characterized by TH2-driven lymphocytic inflammation, increased eosinophils and mast cells, and airway remodeling. Obesity can complicate the development of asthma in obesity-related early-onset asthma. Obesity can also lead to development of late-onset asthma, which is not characterized by airway eosinophilia or TH2 inflammation while having more severe symptoms. Obesity-related asthma is largely refractory to typical asthma medications, including anti-inflammatory drugs such as steroids (5–12), which merits an urgent need to develop novel and effective treatments for this asthma subtype.
The mechanisms underlying obesity-related, late-onset asthma are unknown. Although it has been proposed that gut microbiome or adipose tissue-related cytokines, such as leptin and adiponectin, are contributing factors (13), none of these hold up in clinical studies (14–20). Another hypothesis proposes that hyperinsulinemia, common in individuals with obesity, may contribute to airway hyperreactivity. We have shown that increased insulin acutely potentiates airway contraction induced by parasympathetic nerve stimulation in obese rats and that increased insulin correlates with airway hyperreactivity in rats on a high-fat diet (21, 22). In lungs, parasympathetic nerves running through the vagus nerves normally provide the dominant autonomic control of airway tone (23). These nerves release acetylcholine onto M3 muscarinic receptors on airway smooth muscle, causing contraction and bronchoconstriction. Release of acetylcholine is normally limited by inhibitory M2 muscarinic receptors on parasympathetic nerves (24). Loss of M2 receptor function is associated with increased acetylcholine release and increased airway hyperreactivity in animals that are antigen challenged (25), virus infected (26), or exposed to ozone (27) or organophosphorus pesticides (28). Decreased neuronal M2 receptor function and airway hyperreactivity are also characteristics of rats on a high-fat diet and appear to be linked to increased insulin (21). Depleting insulin reduces airway constriction to parasympathetic nerve stimulation in obese rats on a high-fat diet (21). Thus, targeting insulin may be a strategy to improve asthma control in obese asthmatics.
The biological effects of insulin are mediated by cell surface insulin receptors. Insulin receptors exist in two isoforms, due to alternative splicing. The difference lies in the absence (IR-A) or presence (IR-B) of exon 11. IR-A exerts mitogenic actions, whereas IR-B mainly plays a role in insulin metabolism. Changes in expression of IR isoforms are associated with development of cancer, insulin resistance, diabetes, and obesity (29). Insulin receptors on macrophages also mediate macrophage immune responses (30, 31). Alveolar macrophages may contribute to airway hyperreactivity via release of inflammatory cytokines including TNFα, IL-1β, IL-6, and TGFβ (32, 33). Thus, we measured expression of IR isoforms and cytokines in alveolar macrophages isolated from rats on a high- or low-fat diet.
Metformin, a biguanide analog, is widely prescribed for type 2 diabetes (34, 35). Metformin reduces glucose production by liver and lowers sugar absorption in the intestine (36). In addition, metformin can also reduce insulin resistance caused by obesity or diabetes, leading to a lower insulin demand (37). Because reducing insulin can inhibit vagally induced airway hyperreactivity in rats with dietary obesity (21), we tested whether metformin prevents airway hyperreactivity in a model of obesity-induced asthma. We also tested whether switching from a high to a low-fat diet reduces circulating insulin and airway hyperreactivity.
MATERIALS AND METHODS
Animals
Obese-prone rats were originally developed by selectively breeding Sprague-Dawley rat pairs that gained the most weight with each other – with their offspring becoming obese when fed a high-fat diet (38, 39). Obese-prone rats were outbred with a heterogenetic background, similar to humans (39, 40). Rats were originally purchased from Charles River Labs (Wilmington, MA) and subsequently bred in-house. They were housed in pairs on a 12:12-h light-dark cycle with food and water available ad libitum. Animals were handled in accordance with standards established by the US Animal Welfare Acts set forth in National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University. Three different protocols were used (Fig. 1). Initial studies used both male and female obese-prone Sprague-Dawley rats (Fig. 1, protocol A) as it has been suggested that sex has an effect on obesity-related asthma (41–46). Subsequent studies used male rats only (Fig. 1, protocols B and C).
Figure 1.
Experiment protocols. Male and female rats were fed either a high-fat or low-fat diet for 5 wk. Some male rats on a high- or low-fat diet were treated with metformin (100 mg/kg) or PBS (control) by daily gavage for 5 wk. Some male rats were fed a high-fat diet for 5 wk then switched to a low-fat diet, whereas other males continued the high-fat diet for an additional 5 wk.
Four-wk-old male and female rats were given access to either a pelleted high-fat diet (61.6% fat, 18.1% protein, and 20.3% carbohydrate, TestDiet 58Y1, St. Louis, MO) or a pelleted low-fat diet (10.2% fat, 18% protein, and 71.2% carbohydrate, TestDiet 58Y2) for 5 wk (Fig. 1A). In separate experiments, some male rats on the high- or low-fat diet were additionally gavaged daily with metformin (100 mg/kg, PHR1084-500MG, Sigma-Aldrich, St. Louis, MO) or PBS (300 µL) (Fig. 1B). In separate experiments, after 5 wk on a high-fat diet, some male rats were switched to a low-fat diet, whereas others remained on the high-fat diet for an additional 5 wk (Fig. 1C). It was not possible to extend experiments past 10 wk, because the weight of rats on a high-fat diet exceeded 700 g, which complicated physiological experiments. During feeding a high- or low-fat diet, rats’ food intake and body weight were measured twice per week. Food intake (g) was calculated by subtracting remaining food (g) from food administered (g). Consumption of calories was calculated by multiplying the amount of food consumed (g) by calorie content per gram of food. Because eating increases blood glucose and insulin, all rats were fasted for 16 h before testing airway physiology to get accurate measurement of fasting glucose, fasting insulin, and airway responses. In separate experiments, some female rats on a low-fat diet were acutely treated with exogenous insulin [1 IU/kg sc (47)] 16 h before recording airway physiology. These animals were not fasted to avoid hypoglycemia. Rats on a high-fat diet did not receive exogenous insulin because, as previously reported (21), their circulating insulin increased after 5 wk of a high-fat diet.
After 5 and 10 wk on a high- or low-fat diet, body fat was measured using a nuclear magnetic resonance (NMR)-based body composition analyzer (EchoMRI, Houston, TX) as previously described (48). Briefly, awake rats were placed into a holding tube and placed into a calibrated EchoMRI system. This system uses nuclear magnetic resonance relaxometry to detect and calculate grams of body water, fat, and lean mass based on the different spin relaxation rates in variable tissues. Body fat was presented as a percentage of body weight. Blood samples were withdrawn from the left carotid artery to measure blood glucose (OneTouch Ultra2, LifeScan, Inc., Milpitas, CA) and plasma insulin (rat insulin ELISA, 10–1250-01, Mercodia, Winston Salem NC).
Anesthesia and Measurements of Pulmonary Inflation Pressure, Heart Rate, and Blood Pressure
Bronchoconstriction in response to electrical stimulation of both vagus nerves was measured as previously described (21). Briefly, rats were anesthetized with urethane (1.4 g/kg ip for female rats and 1.6 g/kg ip for male rats; Sigma-Aldrich) to induce deep anesthesia, evidenced by loss of foot withdrawal reflex, and ventilated via a tracheal cannula using a Harvard Apparatus ventilator (South Natick, MA, tidal volume 2.5 mL, 100 breaths/min). Body temperature was maintained at 37°C using a heating blanket. Heart rate and blood pressure were measured by a pressure transducer attached to a cannula inserted into the internal carotid artery. All animals were chemically sympathectomized with guanethidine (5 mg/kg iv; Sigma-Aldrich, 30 min before physiological data collection) and paralyzed with succinylcholine (1.155 mL/min iv bolus then 0.155 mL/min continuous infusions into jugular vein; Sigma-Aldrich). Pulmonary inflation pressure (Ppi) was measured from a pressure transducer attached to a sidearm of the tracheal cannula. Bronchoconstriction was measured as the increase in pulmonary inflation pressure (in mmH2O) above baseline pressure induced by the ventilator. Bradycardia was measured as a fall in heart rate in beats/min. Heart rate, blood pressure, and pulmonary inflation pressure were recorded using the Lab Chart software (AD Instruments, Colorado Springs, CO).
Vagally Induced Bronchoconstriction and Bradycardia
Anesthetized, paralyzed, and ventilated rats were vagotomized by cutting both vagus nerves to remove input from the central nervous system and eliminate reflex responses. Distal ends of both vagus nerves were placed on platinum electrodes and immersed in warm mineral oil. Electrical stimulation of both vagus nerves (2–30 Hz, 20 V, 0.4 ms pulse duration, for 6 s at 40 s intervals) produced frequency-dependent bronchoconstriction and bradycardia that recovered upon cessation of electrical stimulation. At the end of experiments, a muscarinic antagonist (atropine, 1 mg/kg iv) was used to verify that vagally induced bronchoconstriction was mediated by effects of acetylcholine on muscarinic receptors.
Methacholine-Induced Bronchoconstriction and Bradycardia
Increasing doses of methacholine (MCh, 1–10 mM, in 20 µL, Sigma-Aldrich) were delivered to vagotomized rats via a nebulizer (AG-AL1100, Kent Scientific Corporation) for 20 s. In the absence of intact vagi, inhaled methacholine-induced bronchoconstriction is mediated only by M3 muscarinic receptors on smooth muscle (49–52) and bradycardia is mediated by M2 muscarinic receptors on cardiac muscle (53, 54).
Bronchoalveolar Lavage
Bronchoalveolar lavage was collected from deeply anesthetized rats by installing 25 mL of PBS via the tracheal cannula in 5 aliquots. Returned cells were washed, resuspended in PBS, and counted on a hemocytometer or were spun onto slides and stained with Hemacolor (Hemacolor Stain Set, EMD Millipore 65044) to obtain a differential cell count. To isolate alveolar macrophages, bronchoalveolar lavage cells were resuspended in media consisting of RPMI (Cellgro, Corning, NY), 10% heat-inactivated fetal bovine serum (Hyclone), 2 µM l-glutamine (Sigma-Aldrich), and penicillin-streptomycin. The cells were incubated in a 100-mm2 tissue culture dish at 37°C and 5% CO2-95% O2 for 2 h to allow macrophages to adhere to the dish. Before harvesting alveolar macrophages for RNA isolation, cells were rinsed three times with sterile PBS to remove nonadherent cells.
RNA Isolation and PCR
RNA from bronchoalveolar lavage macrophages were isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using random hexamers and Superscript III (Invitrogen, Carlsbad, CA). Cytokine and total insulin receptor mRNA expression was quantified using QuantiTect SYBR Green (Qiagen) and real-time RT-PCR (7500 Fast Real-Time PCR System; Applied Biosystems, Foster City, CA). Data were analyzed using the ΔΔCT method and normalized to 18S rRNA (55). Primers used were as following: insulin receptor (IR) 5′- ACATTGCCCTGAAGACCAAC and 3′- AACAGCATGAATCCCAGGAG; insulin receptor isoform A (IR-A) 5′- GTTTTTGTTCCCAGGCCATC and 3′- GATGGTGGAGGAGATGTTGG; insulin receptor isoform B (IR-B) 5′- GTCCCCACCTTTTGAGTCTG and 3′- CACCATTGCCTGAAGAGGTT; TNFα 5′- CCCAGACCCTCACACTCAGAT and 3′- TTGTCCCTTGAAGAGAACCTG; TGFβ 5′- GAGAGCCCTGGATACCAACTA and 3′- GTGTGTCCAGGCTCCAAATGT; IL-1β 5′- CACCTTCTTTTCCTTCATCTT and 3′- GTCGTTGCTTGTCTCTCCTTG; IL-6 5′- ACTTCACAAGTCGGAGGCTT and 3′- TTCTGACAGTGCATCATCGCT; and 18S 5′- GTAACCCGTTGAACCCCATT and 3′- CCATCCAATCGGTAGTAGCG.
Statistical Analysis
Bronchoconstriction and bradycardia in response to vagal nerve electrical stimulation and inhaled nebulized methacholine were analyzed using two-way repeated-measures analysis of variance (ANOVA). Physiological baselines, fasting blood glucose and fasting insulin, bronchoalveolar lavage cell count, and inflammatory cytokine mRNA expression were analyzed by one-way ANOVA with Bonferroni correction or Student’s t test. All data were analyzed with the Prism 8.0 software (GraphPad, La Jolla, CA). A P value < 0.05 was considered significant.
RESULTS
High-Fat Diet Induced Obesity and Airway Hyperreactivity in Male Rats
Resting inflation pressure, heart rate, and blood pressure were not different among male and female rats on either high- or low-fat diets for 5 wk (Fig. 1, protocol A; and Table 1). Male rats weighed more than age-matched females before (Fig. 2A) being starting a high- or low-fat diet. Five wk later, male rats still weighed more than age-matched females independent of diet (Fig. 2B). Body weight in male and female rats on high-fat diet was increased by 15% and 9%, respectively, compared with sex-matched rats on a low-fat diet. However, only males on a high-fat diet had a significant increase in body weight compared with low-fat diet (Fig. 2B). Females had significantly higher overall body fat than males (Fig. 2C), but body fat was not significantly increased in females by a high-fat diet. In contrast, body fat was significantly increased in males on a high-fat diet compared with males on a low-fat diet (Fig. 2C). Body fat was increased by 23% and 9% in male and female rats, respectively, by a high-fat diet versus a low-fat diet. Calorie intake was higher overall in males independent of diet (Fig. 2D). Fasting glucose was not different between males and females and was not changed by diet (Fig. 2E). In contrast, a high-fat diet significantly increased fasting insulin in males (3.25 ± 0.24 ng/mL compared with 1.81 ± 0.27 ng/mL in those on a low-fat diet) but not in females (1.13 ± 0.25 ng/mL compared with 0.72 ± 0.12 ng/mL on a low-fat diet) (Fig. 2F).
Table 1.
Ppi and cardiovascular parameters at baseline in rats on a high- or low-fat diet
| Diet and TreatmentGroup | Ppi, mmH2O | Heart Rate, beats/min | Systolic BP, mmHg | Diastolic BP, mmHg | Animals, n |
|---|---|---|---|---|---|
| Low-fat diet, 5 wk, female | 93 ± 3 | 427 ± 17 | 92 ± 4 | 31 ± 2 | 10 |
| High-fat diet, 5 wk, female | 90 ± 4 | 437 ± 8 | 77 ± 6 | 41 ± 3 | 12 |
| Low-fat diet+ PBS, 5 wk, male | 77 ± 3 | 403 ± 14 | 95 ± 5 | 36 ± 1 | 8 |
| Low-fat diet + 100 mg/kg metformin, 5 wk, male | 78 ± 8 | 391 ± 10 | 55 ± 2 | 45 ± 2 | 4 |
| High-fat diet + PBS, 5 wk, male | 80 ± 2 | 376 ± 15 | 89 ± 5 | 49 ± 3 | 22 |
| High-fat diet + 100 mg/kg metformin, 5 wk, male | 78 ± 4 | 434 ± 12* | 105 ± 6 | 49 ± 3 | 19 |
| High-fat diet, 10 wk, male | 80 ± 3 | 397 ± 23 | 103 ± 8 | 50 ± 4 | 13 |
| High-fat diet (5 wk) + low-fat diet, 5 wk, male | 85 ± 4 | 389 ± 27 | 110 ± 11 | 50 ± 2 | 10 |
Values are means ± SE. BP, blood pressure; PBS, phosphate-buffered saline; Ppi, pulmonary inflation pressure. Data were analyzed by one-way ANOVA *P = 0.0111, compared with male rats on a 5-wk high-fat diet + PBS.
Figure 2.
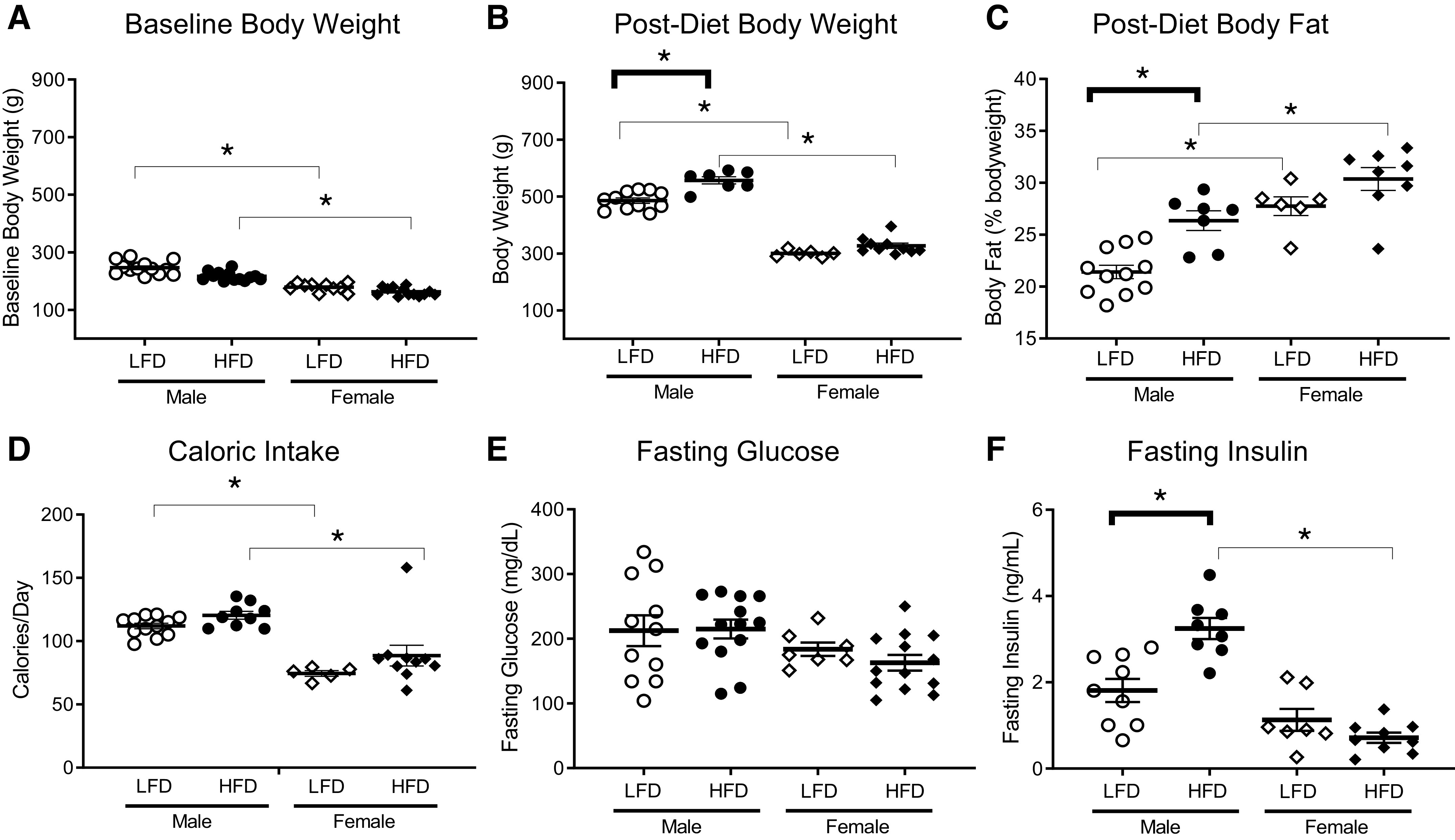
Male rats on a high-fat diet (HFD) gained more body fat and had higher fasting insulin than female rats. Male rats weighed slightly, but significantly, more than female rats at baseline before diet treatment (A). Male rats remained heavier than females after 5 wk and weighed significantly more on a high-fat diet than females (B). Male rats on the high-fat diet weighed significantly more (B) and had greater body fat (C) than male rats on the low-fat diet (LFD). Female rats had a higher percent body fat than males independent of diet, and this did not significantly change with diet (C). Although male rats ate more calories per day than females, diet fat content did not change caloric intake (D). Fasting glucose was similar in all groups (E). Fasting insulin was increased in males on a high-fat diet compared with males on a low-fat diet and females on a high-fat diet (F). Data are presented as means ± SE. *P < 0.05.
Electrical stimulation of both vagal nerves produced frequency-dependent bronchoconstriction (Fig. 3, A and B) in all groups, that was blocked by atropine (data not shown), confirming these responses were mediated by release of acetylcholine onto muscarinic receptors. In males, vagally induced bronchoconstriction was significantly greater in rats on a high-fat diet, than in rats on a low-fat diet (Fig. 3A). In contrast, high-fat diet did not potentiate vagally induced bronchoconstriction in female rats (Fig. 3B). In females, there was a trend towards reduced bronchoconstriction in animals on a high-fat diet. These animals are still responsive to heightened insulin levels since exogenous insulin increased vagally induced bronchoconstriction (data not shown). Methacholine caused dose-dependent bronchoconstriction in vagotomized rats that was not different between rats on a high- or low-fat diet, regardless of sex (Fig. 3, C and D). Methacholine-induced bronchoconstriction was blocked by atropine (data not shown), confirming it was mediated by muscarinic receptors on airway smooth muscle.
Figure 3.
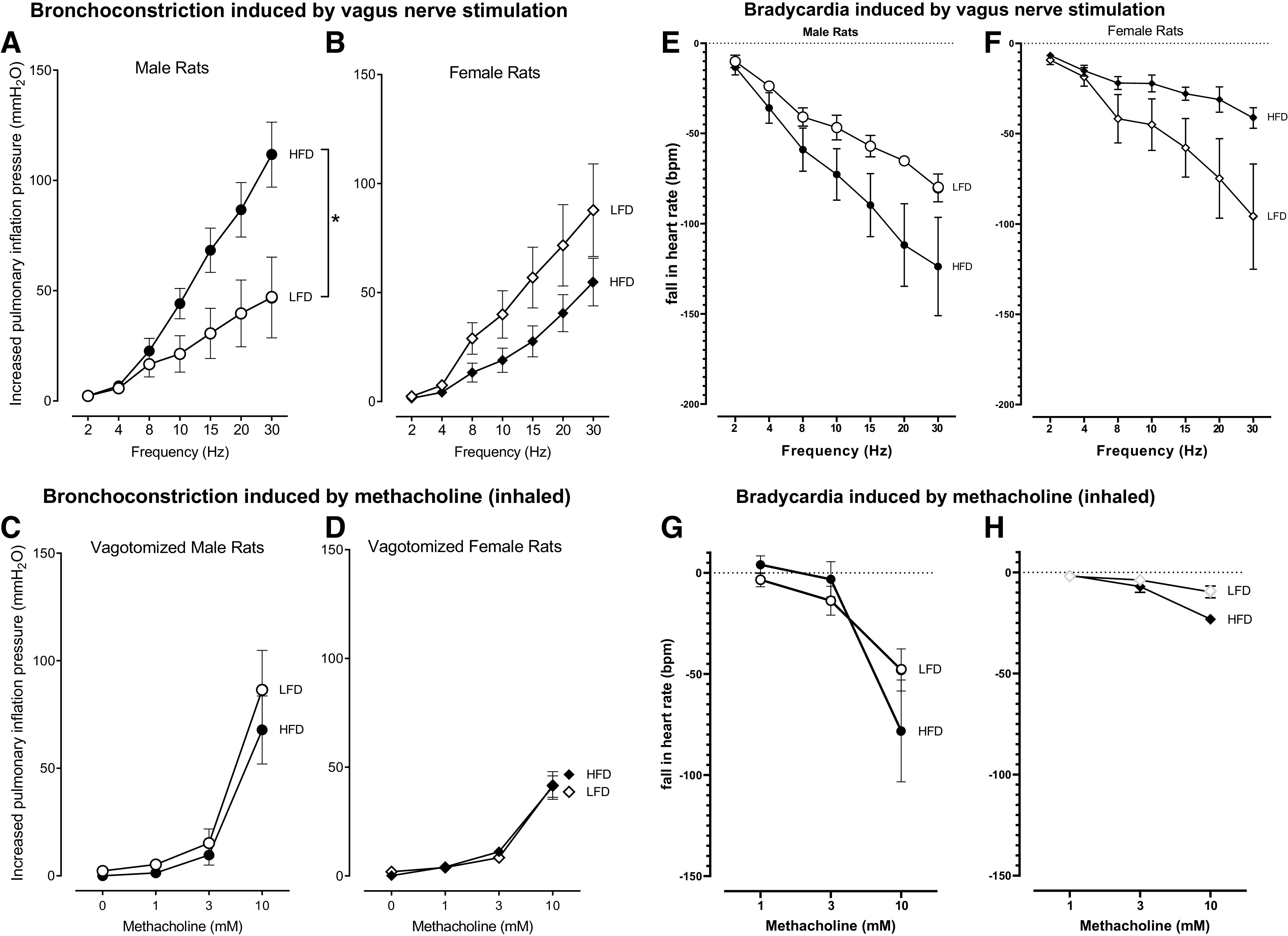
High-fat diet (HFD) induced airway hyperreactivity in male rats. Bronchoconstriction induced by electrical stimulation of both vagus nerves (2–30 Hz, 20 V, 0.4-ms pulse duration for 6 s with 40 s pause) was significantly potentiated in high-fat diet-fed male rats (HFD; filled circles) vs. low-fat diet-fed male rats (LFD; empty circles) (A). In contrast, there was a trend of reduced vagally induced bronchoconstriction in female rats fed high-fat diet (filled diamonds) vs. female rats on a low-fat diet (empty diamonds) that was not significant (B). Bronchoconstriction in response to inhaled methacholine was not affected by sex or by a high- or low-fat diet (C and D). High-fat diet had a trend of increased vagus nerve stimulation-induced bradycardia in male rats (E) but reduced vagus nerve stimulation-induced bradycardia in female rats (F). Bradycardia induced by inhaled methacholine was not affected by sex or by a high- or low-fat diet (G and H). n = 6–8 rats/group. Data are presented as means ± SE. *P < 0.05.
In the heart, electrical stimulation of both vagus nerves or inhaled methacholine induced frequency-dependent or dose-dependent falls in heart rate (Fig. 3, E–H). In male rats, vagally induced bradycardia was greater in rats on a high-fat diet than in rats on a low-fat diet (Fig. 3E). Conversely, vagally induced bradycardia in female rats was greatest in those on a low-fat diet (Fig. 3F). Thus, similar to vagally induced bronchoconstriction, the effect of a high-fat diet on vagally induced bradycardia in females was opposite to that in males. Although none of the differences in females were statistically significant, identical trends in female lung and heart function that are opposite of males are important to acknowledge. Inhaled methacholine also induced dose-dependent falls in heart rates that were not significantly different among groups (Fig. 3, G and H).
Metformin, but Not Diet Reversal, Prevented Hyperinsulinemia and Airway Hyperreactivity in Male Rats with Dietary Obesity
In separate experiments (Fig. 1, protocol B), male rats on a high- or low-fat diet were treated with either metformin or PBS for 5 wk. In addition, some rats already on a high-fat diet for 5 wk were switched to a low-fat diet, whereas other rats continued to receive the high-fat diet for an additional 5 wk (Fig. 1, protocol C). In rats that received metformin or had their diet reversed, resting pulmonary inflation pressure, heart rate, and blood pressure were not different among the groups (Table 1).
Metformin (100 mg/kg) prevented increased body weight (Fig. 4A), increased body fat (Fig. 4B), and increased fasting insulin (Fig. 4C) in rats fed a high-fat diet. Fasting glucose (Fig. 4D) and calorie intake/day (Fig. 4E) were similar across all groups of animals. Rats that were switched from high- to low-fat diet had significantly lower body weight (Fig. 4F) and body fat (Fig. 4G) than those remaining on the high-fat diet. However, changing diets did not significantly reduce fasting glucose (Fig. 4H) or fasting insulin (Fig. 4I).
Figure 4.
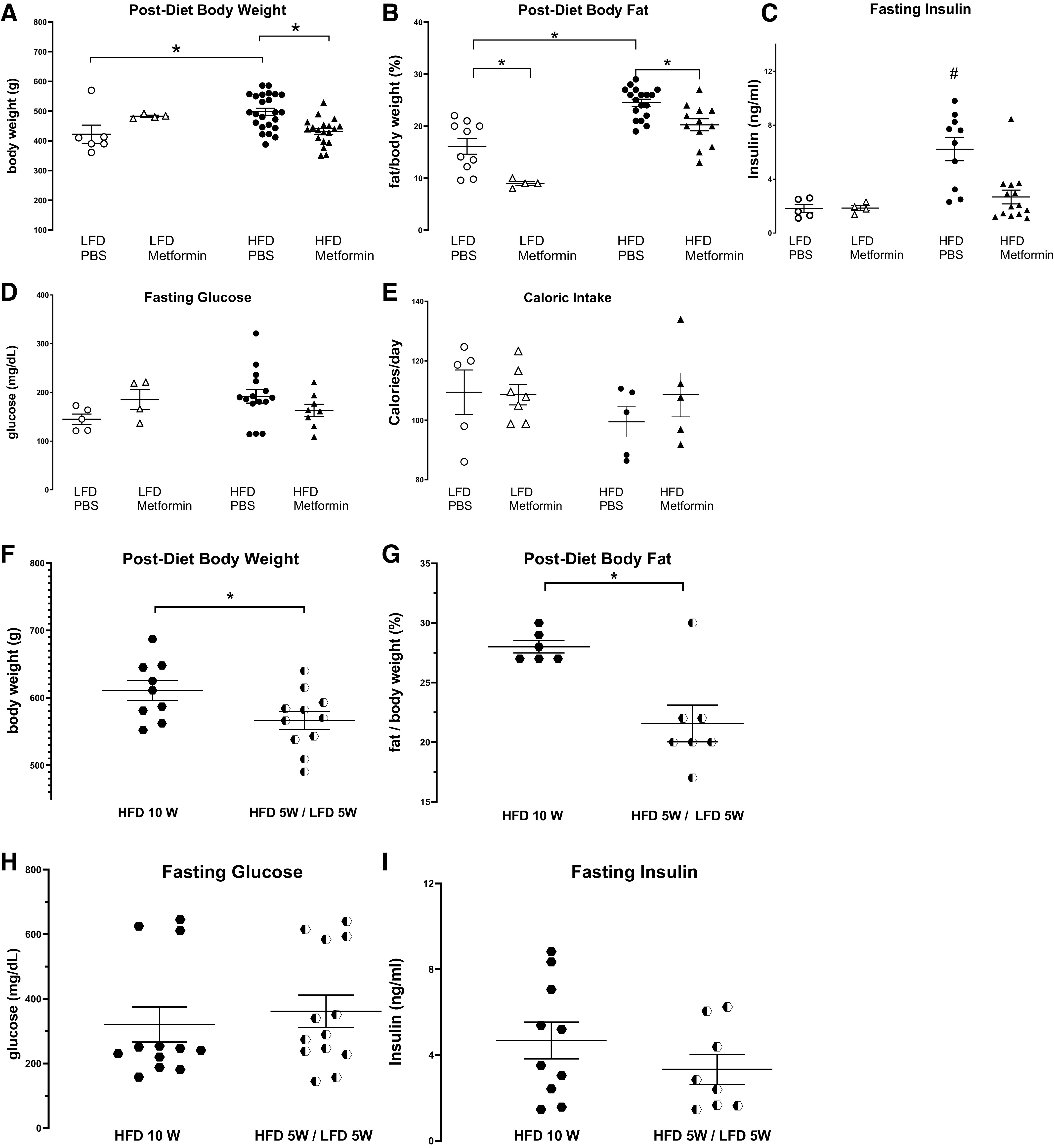
Rats on a high-fat diet (HFD) gained more body weight (A) and body fat (B) and had significantly higher fasting insulin (C) than rats on a low-fat diet (LFD). Metformin prevented high-fat diet induced gain in body weight (A), body fat (B), and hyperinsulinemia (C). Fasting glucose (D) and daily food intake (E) were not changed by diet or metformin. Rats that were switched from a high-fat diet to a low-fat diet (HFD 5 wk/LFD 5 wk) had significantly decreased body weight (F) and body fat (G), but similar fasting glucose (H) and fasting insulin (I) compared with rats remained on high-fat diet for 10 wk (HFD 10 wk). Each symbol represents an individual animal. Data are presented as means ± SE. *P < 0.05 is indicated by a bracket. #P < 0.05 compared with all other groups.
In rats on a low-fat diet, metformin had no effect on vagally induced bronchoconstriction (Fig. 5A). Similar to our previous data (Fig. 2A), rats on a high-fat diet for 5 wk and treated with PBS (orally) had significantly greater vagally induced bronchoconstriction compared with male rats on a low-fat diet for 5 wk and treated with PBS (orally), (Fig. 5B). Treatment with metformin completely prevented potentiation of vagally induced bronchoconstriction (Fig. 5B). Maintaining the rats on a high-fat diet for a longer time (10 wk) did not further increase vagally induced bronchoconstriction above the response measured at 5 wk (Fig. 5, B and C). Switching from a high-fat to a low-fat diet halfway through the 10 wk also did not reverse airway hyperactivity (Fig. 5C).
Figure 5.
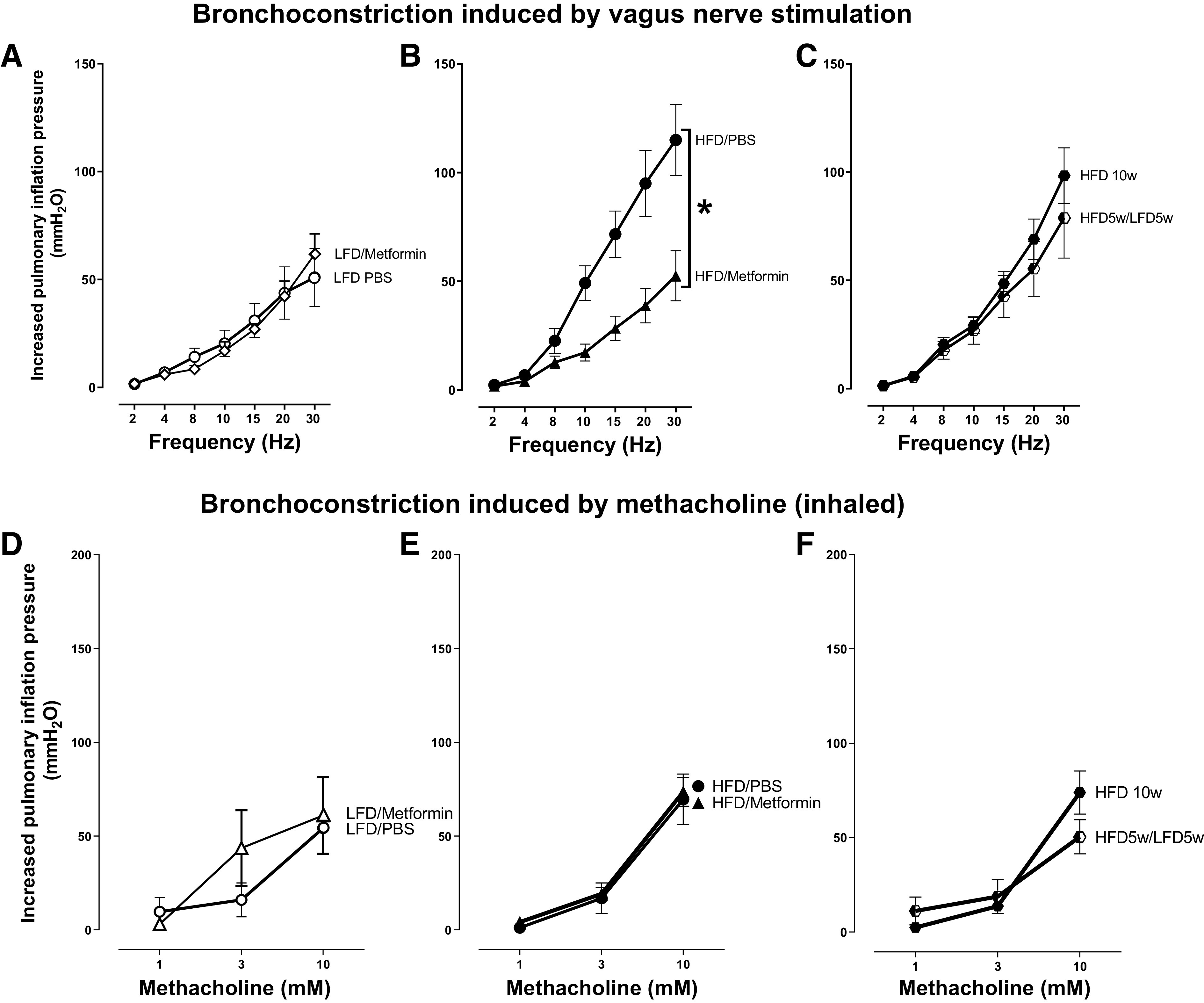
In the lung, high-fat diet (HFD)-induced airway hyperresponsiveness was reduced by metformin, but not by switching to a low-fat diet (LFD). Bronchoconstriction induced by electrical stimulation of both vagus nerves was potentiated by a high-fat diet (A).Metformin blocked the HFD-induced potentiation (B). However, switching the diet from high-fat to low-fat (HFD 5wk/LFD 5wk) did not reverse potentiation of vagally induced bronchoconstriction in rats on a high-fat diet (HFD 10wk; C). Bronchoconstriction induced by inhaled methacholine in vagotomized rats (D–F) was dose-dependent but was not significantly different among groups of rats, regardless of diet or treatment. n = 5–8 rats/group. Data are presented as means ± SE. *P < 0.05.
Bronchoconstriction induced by inhaled methacholine was not significantly different among vagotomized male rats on a high-fat diet or low-fat diet (Fig. 5, D and E), regardless of treatment with metformin (Fig. 5E) or switching the diet from a high-fat to a low-fat (Fig. 5F) after 5 wk.
In rats on a low-fat diet, metformin did not significantly affect bradycardia induced by electrical stimulation of both vagus nerves (Fig. 6A). Like Fig. 3E, bradycardia was slightly, but not significantly, greater in rats fed a high-fat diet compared with those that received low-fat diet for 5 wk and were given PBS (orally) (Fig. 6, A and B). Metformin partially prevented this increase in vagally induced bradycardia in animals that received the high-fat diet (Fig. 6B). Maintaining rats on a high-fat diet for an additional 5 wk did not further increase vagally induced bradycardia, and switching from a high-fat diet to a low-fat diet had no effect (Fig. 6C). None of the frequency-dependent falls in heart rate differed significantly from each other.
Figure 6.
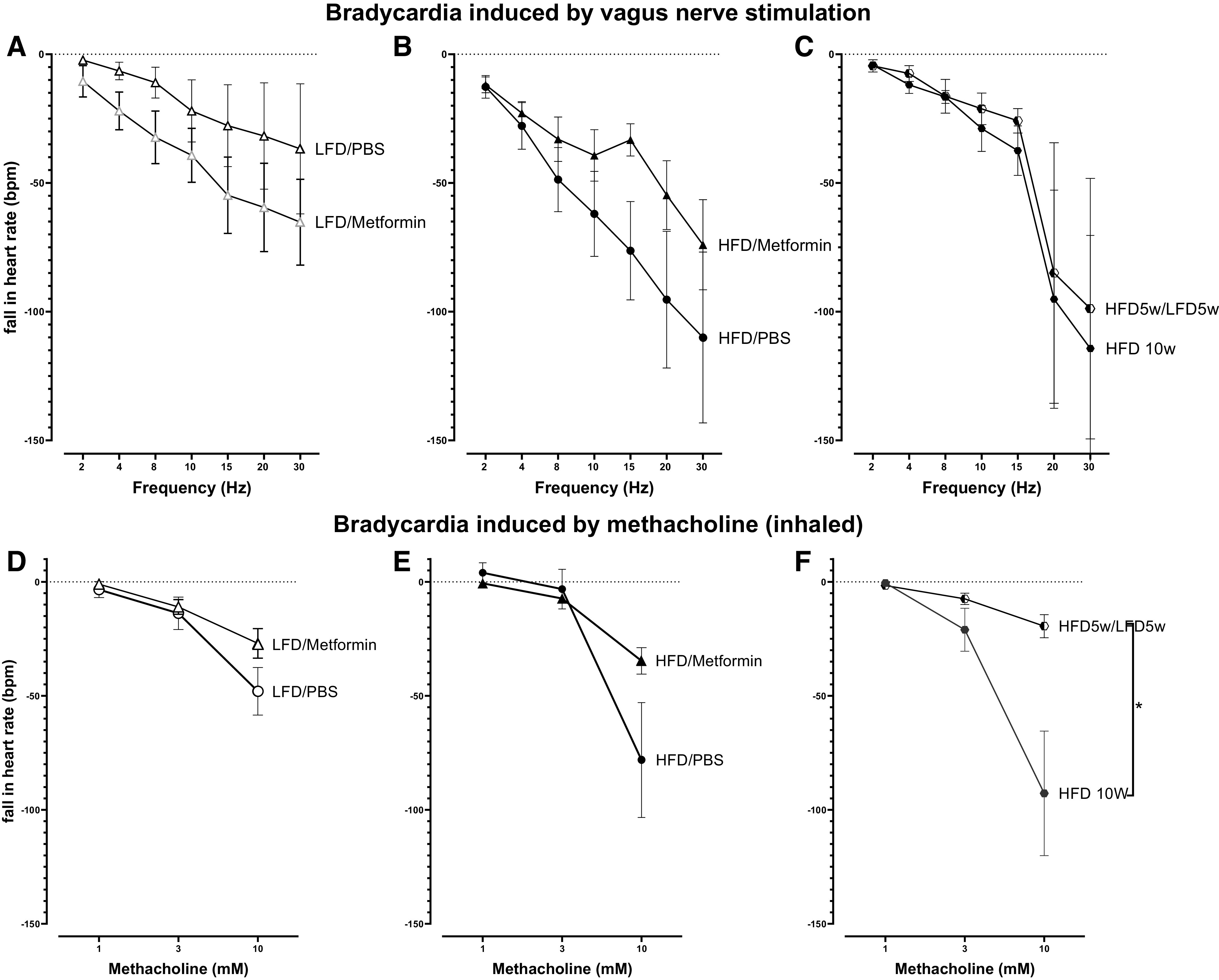
In the heart, vagus nerve electrical stimulation (2–30 Hz) induced bradycardia (measured as a fall in heart rate) that was increased in rats fed a high-fat diet (HFD, B and C) compared with rats fed a low-fat diet (LFD, A), although none of the differences were significant. Bradycardia induced by inhaled methacholine (1–10 mM, delivered in 20 μL of saline) in vagotomized rats was not significantly changed by diet or by metformin (D and E). However, methacholine induced bradycardia was significantly reduced in rats switched from a high-fat diet to a low-fat diet (HFD 5 wk/LFD 5wk) compared with rats on a high-fat diet for 10 wk (HFD 10 wk). n = 6–13. Data are presented as means ± SE. *P < 0.05.
Inhaled methacholine induced a dose-dependent fall in heart rate in all vagotomized rats (Fig. 6, D–F) that was not significantly different among rats on a low- or high-fat diet, regardless of the number of weeks on the diet (Fig. 6, D–F). Methacholine-induced bradycardia was slightly reduced by metformin (Fig. 6E) but significantly reduced by switching the diet from high fat to low fat (Fig. 6F).
The total number of inflammatory cells in bronchoalveolar lavage was not different between groups. The majority of inflammatory cells recovered in bronchoalveolar lavage were macrophages (Table 2). Neither diet nor metformin altered the number or types of inflammatory cells recovered in bronchoalveolar lavage (Table 2).
Table 2.
Bronchoalveolar lavage inflammatory cells isolated from male rats
| Diet Treatment | Total Cells(1 × 106 cells) | Macrophages(1 × 106 cells) | Lymphocytes(1 × 106 cells) | Neutrophils(1 × 106 cells) | Eosinophils(1 × 106 cells) |
|---|---|---|---|---|---|
| Low-fat diet | 1.000 ± 0.063 | 1.000 ± 0.052 | 0.002 ± 0.002 | 0.002 ± 0.002 | 0 |
| High-fat diet | 2.392 ± 0.731 | 2.383 ± 0.733 | 0.002 ± 0.002 | 0.007 ± 0.005 | 0 |
| High-fat diet + metformin | 1.285 ± 0.111 | 1.270 ± 0.112 | 0.008 ± 0.003 | 0.003 ± 0.003 | 0 |
Values are means ± SE.
Insulin receptors, including both isoform A (IR-A) and isoform B (IR-B), are expressed on inflammatory cells. As the majority of alveolar inflammatory cells are macrophages (Table 2), we measured expression of insulin receptor isoforms on alveolar macrophages. Neither diet nor metformin changed insulin receptor mRNA expression, measured as fold increase in mRNA (Fig. 7A). In contrast, TNFα and TGFβ mRNA expression were significantly decreased in alveolar macrophages isolated from rats on a high-fat diet compared with those collected from rats on a low-fat diet (Fig. 7B). Metformin did not prevent this reduction (Fig. 7B). IL-1β and IL-6 mRNA expression in alveolar macrophages were similar in high-fat and low-fat diet-fed rats; however, metformin treatment increased IL-6 mRNA, but not IL-1β mRNA, expression in alveolar macrophages from rats on a high-fat diet (Fig. 7B).
Figure 7.
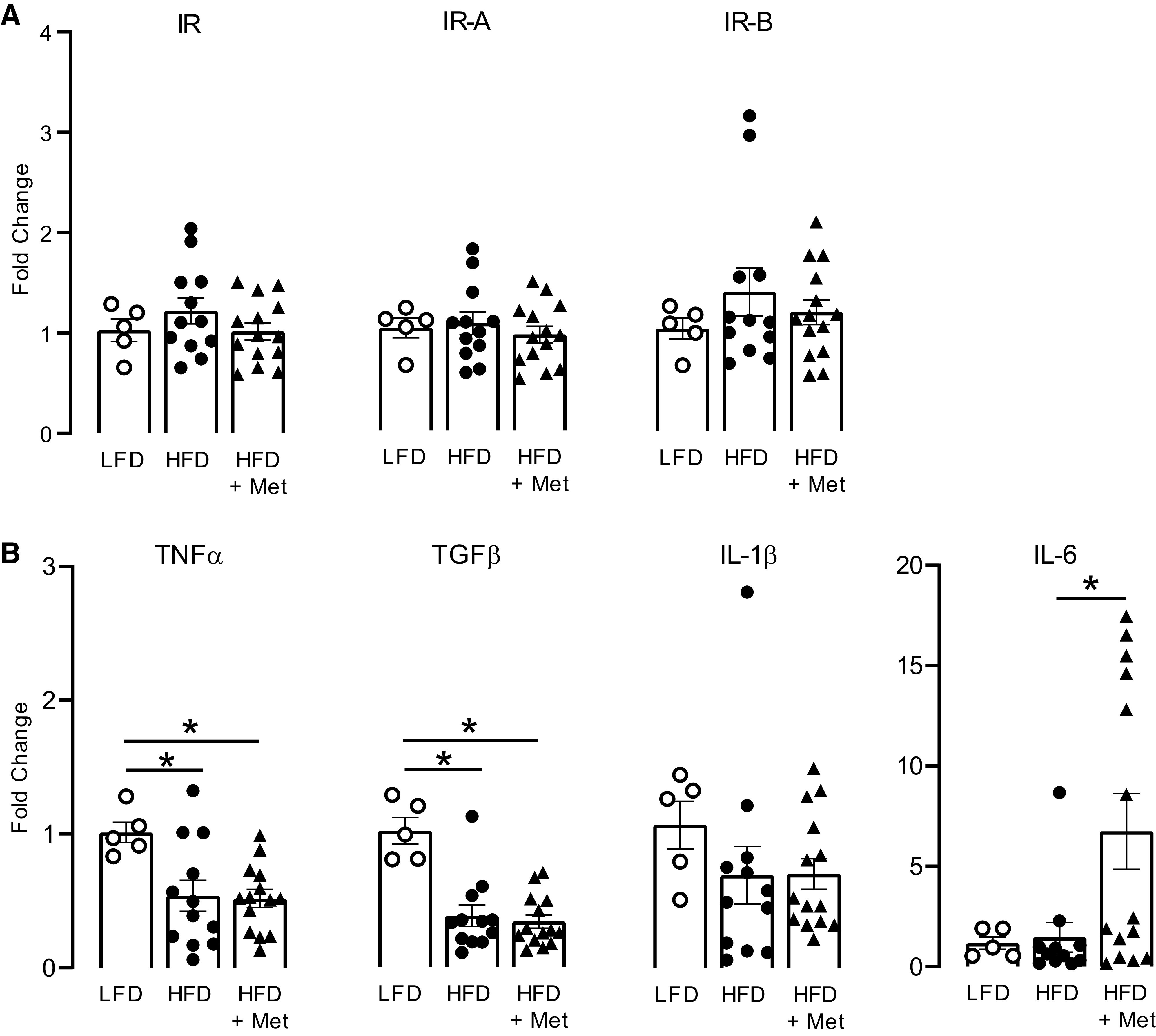
Effect of metformin on insulin receptor and cytokine mRNA expression in alveolar macrophages. Alveolar macrophages were isolated from bronchoalveolar lavage fluid collected from rats fed a low-fat diet (LFD) or a high-fat diet (HFD) and treated with metformin (Met; 100 mg/kg) or PBS by daily gavage for 5 wk. The expression of insulin receptor, insulin receptor isoform A (IR-A), and isoform B (IR-B) was all similar among groups of animals, regardless of diet or treatment of metformin (A). High-fat diet significantly reduced TNFα and TGFβ, but not IL-1β or IL-6 expression (B). Metformin did not change TNFα, TGFβ, or IL-1β expression in macrophage cells isolated from high-fat diet-fed rats, but significantly potentiated IL-6 mRNA expression (B). Data are presented as means ± SE. *P < 0.05.
DISCUSSION
Parasympathetic nerves running in the airway vagus nerves provide the dominant autonomic control of airway smooth muscle (23). They control airway tone, induce bronchoconstriction, and mediate airway hyperreactivity following antigen challenge, viral infection, and exposure to either ozone or organophosphorus pesticides in animals (26, 27, 56, 57) and in humans with asthma (58–60). The effectiveness of anticholinergic drugs demonstrates a significant role for parasympathetic nerves in asthma. We have shown that vagus nerves mediate airway hyperreactivity in diet-induced obese rats with high insulin, consistent with previous results (21). Metformin prevents high-fat diet-induced hyperinsulinemia, in addition to inhibiting weight gain and fat gain induced by a high-fat diet. Most importantly, metformin prevented hyperinsulinemia-induced airway hyperreactivity in obese animals, which may explain the beneficial effects of metformin in patients with obesity and asthma.
The molecular mechanisms behind the actions of metformin on insulin are still unknown. Homeostatic levels of insulin in systemic circulation are determined by pancreatic secretion and liver clearance. Upon its secretion from pancreatic β-cells, 80% of insulin is cleared by liver hepatocytes before it reaches the systemic circulation (61). Clinical data suggest metformin does not increase insulin clearance (62). Insulin secretion is a highly regulated process by pancreatic β-cells and is governed by interactions of nutrients (glucose, amino acids, fatty acids), hormones, and the autonomic nervous system. Metformin can inhibit hepatic gluconeogenesis (63–65) and sugar absorption (36), reduce plasma free fatty acid (64, 66), and obesity-induced insulin resistance (37). Furthermore, our data shows that metformin significantly reduced body fat (Fig. 4B) and slightly reduced fasting glucose (Fig. 4D) in rats on a high-fat diet, both of which may reduce insulin release. Therefore, metformin may reduce circulating insulin levels in rats with dietary obesity by reducing stimulators for insulin release or by improve insulin sensitivity.
Vagally induced bronchoconstriction was significantly increased by high-fat diet only in male rats (Fig. 3, A and B). Studies in both humans and animals have described sexual dimorphism as a potential factor in respiratory physiology and asthma (44). Asthma is more common in prepubescent males and boys with obesity are significantly more likely than girls with obesity to exhibit airway reactivity and develop asthma (67, 68). In contrast, following puberty, the trend reverses and adult females more commonly have asthma (69). Male/female differences in asthma are further complicated by obesity and metabolic syndrome. The incidence of insulin resistance is lower in premenopausal women than in men of a similar age (70). In mice, 17β-estradiol protects neurons from developing insulin resistance (71). Conflicting data make it difficult to ascribe a role for sex in adult obesity-related asthma (41–43, 45, 46). Our data show that female rats on a high-fat diet did not have significantly higher body weight (Fig. 2B) and body fat (Fig. 2C) compared with that in female rats on a low-fat diet, which is consistent with a previous study showing female rats were slower to gain weight when fed a high-fat diet compared with their male counterparts (39). The underlying mechanisms of sex differences on high-fat diet response may be related to age and sex hormones (72). It has been shown that juvenile male mice on a high-fat diet gain more weight than females mice (72), but if the diet is initiated in middle-aged mice, the sex difference is reversed, and females gain more weight than males (72). We are using male and female rats between 5 and 10 wk in protocol A (Fig. 1); therefore, all of them were around periadolescence (73). Our data also show that female rats on a high-fat diet have lower circulating insulin levels (Fig. 2F) than males independent of diet. When females on low-fat diet were administered exogenous insulin, these female rats are capable of developing increased airway response to vagus nerve stimulation. Because only the males on a high-fat diet developed hyperinsulinemia and airway hyperreactivity, only these animals were used to test the effects of metformin or dietary changes on obesity-related airway hyperreactivity.
Bradycardia caused by vagal nerve stimulation depends on sensitivity of cardiac M2 muscarinic receptors and neurotransmitter, acetylcholine, release from parasympathetic nerves. Although both M2 and M3 muscarinic receptors are expressed in heart, pharmacologic evidence shows M2 muscarinic receptors are the dominant muscarinic receptor responsible for bradycardia (53, 54). Thus, in vagotomized rats, bradycardia induced by inhalation of methacholine is mediated by cardiac M2 muscarinic receptors. In both male and female vagotomized rats, bradycardia induced by inhalation of methacholine is slightly increased in rats on a high-fat diet compared with a low-fat diet, although the difference is not significant. However, extending the feeding time of high-fat diet to 10 wk further increases methacholine-induced bradycardia, suggesting that a longer high-fat diet may make the heart more sensitive to muscarinic receptor agonists. Both metformin and switching diets from high-fat to a low-fat reduces methacholine-induced bradycardia, making it similar to rats on a low-fat diet. Thus, both metformin and dietary alterations may have a protective effect on cardiac M2 muscarinic receptor function.
Human cohort studies have shown metformin reduces both symptoms and exacerbation of asthma (74–76). Airway inflammation has been widely demonstrated in patients with asthma by the presence of an array of activated inflammatory cells and their mediators. Alveolar macrophages are the front line of inflammatory cell defense against respiratory pathogens (77) and are the dominant inflammatory cells recovered in rat bronchoalveolar lavage (Table 2). Insulin regulates macrophage function by binding to cell surface insulin receptors to modulate their response to pathogens or danger signals (78). High levels of insulin can promote an M2-like phenotype of macrophages and reduce their response to LPS in vivo and in vitro (31). Alveolar macrophages are a major source of inflammatory cytokines, including TNFα, IL-1β, IL-6, and TGFβ, which are all increased in patients with obesity (79, 80) and related to airway hyperreactivity (32, 33). Our data show that metformin and diet did not significantly change the number of macrophages recovered in bronchoalveolar lavage nor the insulin receptor mRNA expression in macrophages. These data suggest that rats’ alveolar macrophages have similar binding ability to insulin, regardless of diet or metformin treatment. Our data also show that a high-fat diet reduced TNFα and TGFβ mRNA expression in alveolar macrophages without reducing IL-1β and IL-6 mRNA expression, which was not changed by metformin treatment, indicating that metformin is unlikely to alter airway hyperreactivity by changing airway inflammatory response of airway macrophages.
Our data show that metformin can prevent hyperinsulinemia caused by a high-fat diet, which indicates a mechanism by which metformin can reduce airway hyperreactivity. We also show that metformin reduced body weight and body fat gain, consistent with other animal (81, 82) and human studies (83–87). Although weight loss is associated with reduced airway hyperreactivity (88, 89), weight loss alone does not always suppress asthma symptoms (90). Consistent with findings in those clinical trials (90), our data show that switching from a high-fat to a low-fat diet after 5 wk reduced rats’ body weight (Fig. 4F) and body fat (Fig. 4G), but did not reverse vagally induced airway hyperreactivity (Fig. 5C). In addition to reducing body weight gain and body fat gain by a high-fat diet, metformin prevented high-fat diet-induced hyperinsulinemia. In contrast, fasting insulin was still high in those rats that switching from a high-fat diet to a low-fat diet halfway through the 10 wk. Because high levels of insulin potentiate vagally induced bronchoconstriction (21, 22, 91) and reducing insulin inhibits airway hyperreactivity (21), it is likely that metformin prevents airway hyperactivity in male rats with dietary obesity by reducing circulating insulin.
Our data demonstrate that metformin can inhibit vagally induced airway hyperreactivity in obese rats, suggesting a possible mechanism for metformin to reduce asthma exacerbation in patients with obesity and asthma. Airway vagus nerves contain parasympathetic nerves, which release neurotransmitter acetylcholine that activates M3 muscarinic receptors on airway smooth muscle, causing smooth muscle contraction and bronchoconstriction. Airway vagus nerves also contain sensory nerves that sends signal to brain and back to lungs via parasympathetic nerves leading to acetylcholine release and bronchoconstriction. We (92) and others (93) have previously demonstrated that inhaled methacholine induces bronchoconstriction that is a combination of this reflex response plus direct stimulation of M3 muscarinic receptors on airway smooth muscle when the vagus nerves are intact. Thus, we only used vagotomized rats in our current study. Although both M2 and M3 muscarinic receptors are present on airway smooth muscle (50, 52, 94–96), M3 receptors play a dominant role in airway smooth muscle contraction in all species including humans, (50–52, 96), whereas M2 receptors only indirectly promote smooth muscle contraction by functionally antagonizing relaxation (94). Since bronchoconstriction in response to inhaled methacholine within the tested concentration was not altered by metformin, it indicates that inhibition effect of metformin on vagally induced airway hyperreactivity is unlikely due to reducing contractile response of airway smooth muscle to muscarinic receptor agonist. Vagally induced airway hyperreactivity can also be mediated by dysfunctional neuronal M2 muscarinic receptors on parasympathetic nerves. Neuronal M2 muscarinic receptors limit acetylcholine release (97–99), reducing vagally induced bronchoconstriction (24, 100). Loss of neuronal M2 muscarinic receptor function is a major mechanism of airway hyperreactivity in asthma (58–60) and in animal models of asthma (26, 27, 56, 57). As a partial M2 muscarinic receptor agonist, pilocarpine can reduce vagus nerve-induced bronchoconstriction when used in a specific dose range (24). However, all available muscarinic agonists lack good pharmacology and clinical selectivity, and also stimulate M3 muscarinic receptors, leading to increased bronchoconstriction. Therefore, the use of selective neuronal M2 muscarinic receptor agonists to treat asthma is limited. As shown before, high insulin inhibits neuronal M2 muscarinic receptor function and reducing insulin protects M2 muscarinic receptor function (21, 91, 101). Here, our data show that metformin prevented high-fat diet-induced hyperinsulinemia in obese rats. Thus, it is possible that metformin protects neuronal M2 muscarinic receptors on parasympathetic nerves via reducing insulin, thus inhibiting vagally induced airway hyperreactivity.
In 1999, Camargo et al. (102) first described the relationship between obesity and asthma. Since then, a significant number of studies have demonstrated increased risk of asthma in both children and adults with obesity (103). Patients with obesity and asthma account for the most severe asthmatics (7, 41), and their symptoms remain largely uncontrolled as they respond poorly to common asthma therapeutics (8, 104–106). Thus, there is a need for specific and effective treatments for obesity-related asthma. Obesity-associated metabolic dysfunction is a major risk factor in the development of asthma (21, 107). Metformin, a commonly used first-line antidiabetic medication, has been prescribed to more than 100 million people worldwide. In two recent retrospective cohort studies, metformin lowered the risk for asthma-related hospitalization and exacerbations in patients with asthma and diabetes (75, 76). However, the mechanisms remain unclear and have not been fully explored. Our studies suggest that metformin may be effective in treating asthma in patients with obesity or type 2 diabetes.
GRANTS
This work was supported by National Institutes of Health Grants R01HL131525, R01HL113023, R01AR061567, and R01HL124165.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.J.P., D.B.J., A.D.F., and Z.N. conceived and designed research; G.N.C., B.J.P., and Z.N. performed experiments; G.N.C., B.J.P., and Z.N. analyzed data; G.N.C., B.J.P., A.D.F., and Z.N. interpreted results of experiments; G.N.C., B.J.P., and Z.N. prepared figures; G.N.C., B.J.P., and Z.N. drafted manuscript; G.N.C., B.J.P., D.B.J., A.D.F., and Z.N. edited and revised manuscript; G.N.C., B.J.P., D.B.J., A.D.F., and Z.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Lauren Hales Beck and Jessica Maung for technical assistance in breeding rats and measuring body weight and food intake and Drs. Daniel Marks and Xinxia Zhu for technical assistance to measure rat body composition.
REFERENCES
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390: 1211–1259, 2017. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amelink M, de Nijs SB, de Groot JC, van Tilburg PM, van Spiegel PI, Krouwels FH, Lutter R, Zwinderman AH, Weersink EJ, ten Brinke A, Sterk PJ, Bel EH. Three phenotypes of adult-onset asthma. Allergy 68: 674–680, 2013. doi: 10.1111/all.12136. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther 26: 710–715, 2013. doi: 10.1016/j.pupt.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Mohanan S, Tapp H, McWilliams A, Dulin M. Obesity and asthma: pathophysiology and implications for diagnosis and management in primary care. Exp Biol Med (Maywood) 239: 1531–1540, 2014. doi: 10.1177/1535370214525302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J Asthma 41: 521–526, 2004. doi: 10.1081/JAS-120037651. [DOI] [PubMed] [Google Scholar]
- 6.Lessard A, Maltais F, Boulet LP. Clinical management of chronic obstructive pulmonary disease and asthma in an obese patient. Expert Opin Pharmacother 9: 83–93, 2008. doi: 10.1517/14656566.9.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 141: 1169–1179, 2018. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, Haselkorn T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol 133: 1549–1556, 2014. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Sharif M, Khan BT, Ajmal K, Anwar MA. Acute effect of insulin on guinea pig airways and its amelioration by pre-treatment with salbutamol. J Pak Med Assoc 64: 932–935, 2014. [PubMed] [Google Scholar]
- 10.Tashiro H, Shore SA. Obesity and severe asthma. Allergol Int 68: 135–142, 2019. doi: 10.1016/j.alit.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tosetti C, Corinaldesi R, Stanghellini V, Pasquali R, Corbelli C, Zoccoli G, Di Febo G, Monetti N, Barbara L. Gastric emptying of solids in morbid obesity. Int J Obes Relat Metab Disord 20: 200–205, 1996. [PubMed] [Google Scholar]
- 12.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18: 716–725, 2012. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Llorente MA, Romero R, Chueca N, Martinez-Cañavate A, Gomez-Llorente C. Obesity and asthma: a missing link. Int J Mol Sci 18: 1490, 2017. doi: 10.3390/ijms18071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holguin F, Rojas M, Brown LA, Fitzpatrick AM. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J Asthma 48: 217–223, 2011. doi: 10.3109/02770903.2011.555033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang AS, Kim TH, Park JS, Kim KU, Uh ST, Seo KH, Kim YH, Lim GI, Park CS. Association of serum leptin and adiponectin with obesity in asthmatics. J Asthma 46: 59–63, 2009. doi: 10.1080/02770900802444203. [DOI] [PubMed] [Google Scholar]
- 16.Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R, Viikari J, Raitakari OT. Obesity, adipokines and asthma. Allergy 64: 770–777, 2009. doi: 10.1111/j.1398-9995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol 19: 535–540, 2008. doi: 10.1111/j.1399-3038.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 18.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, Smith LJ, Jacobs DR Jr.. Association between asthma and serum adiponectin concentration in women. Thorax 63: 877–882, 2008. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sood A, Ford ES, Camargo CA Jr.. Association between leptin and asthma in adults. Thorax 61: 300–305, 2006. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland TJ, Sears MR, McLachlan CR, Poulton R, Hancox RJ. Leptin, adiponectin, and asthma: findings from a population-based cohort study. Ann Allergy Asthma Immunol 103: 101–107, 2009. doi: 10.1016/S1081-1206(10)60161-5. [DOI] [PubMed] [Google Scholar]
- 21.Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol 51: 251–261, 2014. doi: 10.1165/rcmb.2013-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proskocil BJ, Calco GN, Nie Z. Insulin acutely increases agonist-induced airway smooth muscle contraction in human and rat. Am J Physiol Lung Cell Mol Physiol 320: L545–L556, 2021. doi: 10.1152/ajplung.00232.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol (1985) 101: 971–985, 2006. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- 24.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 83: 973–978, 1984. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol Lung Cell Mol Physiol 273: L93–L103, 1997. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- 26.Fryer AD, Jacoby DB. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 102: 267–271, 1991. doi: 10.1111/j.1476-5381.1991.tb12164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultheis AH, Bassett DJ, Fryer AD. Ozone-induced airway hyperresponsiveness and loss of neuronal M2 muscarinic receptor function. J Appl Physiol (1985) 76: 1088–1097, 1994. doi: 10.1152/jappl.1994.76.3.1088. [DOI] [PubMed] [Google Scholar]
- 28.Proskocil BJ, Bruun DA, Thompson CM, Fryer AD, Lein PJ. Organophosphorus pesticides decrease M2 muscarinic receptor function in guinea pig airway nerves via indirect mechanisms. PLoS One 5: e10562, 2010. doi: 10.1371/journal.pone.0010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escribano O, Beneit N, Rubio-Longás LC, López-Pastor AR, Gómez-Hernández A. The role of insulin receptor isoforms in diabetes and its metabolic and vascular complications. J Diabetes Res 2017: 1403206, 2017. doi: 10.1155/2017/1403206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghanim H, Korzeniewski K, Sia CL, Abuaysheh S, Lohano T, Chaudhuri A, Dandona P. Suppressive effect of insulin infusion on chemokines and chemokine receptors. Diabetes Care 33: 1103–1108, 2010. doi: 10.2337/dc09-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ieronymaki E, Theodorakis EM, Lyroni K, Vergadi E, Lagoudaki E, Al-Qahtani A, Aznaourova M, Neofotistou-Themeli E, Eliopoulos AG, Vaporidi K, Tsatsanis C. Insulin resistance in macrophages alters their metabolism and promotes an M2-like phenotype. J Immunol 202: 1786–1797, 2019. doi: 10.4049/jimmunol.1800065. [DOI] [PubMed] [Google Scholar]
- 32.Haddad EB, Rousell J, Lindsay MA, Barnes PJ. Synergy between tumor necrosis factor α and interleukin 1β in inducing transcriptional down-regulation of muscarinic M2 receptor gene expression. Involvement of protein kinase A and ceramide pathways. J Biol Chem 271: 32586–32592, 1996. doi: 10.1074/jbc.271.51.32586. [DOI] [PubMed] [Google Scholar]
- 33.Haddad EB, Rousell J, Mak JC, Barnes PJ. Transforming growth factor-β 1 induces transcriptional down-regulation of m2 muscarinic receptor gene expression. Mol Pharmacol 49: 781–787, 1996. [PubMed] [Google Scholar]
- 34.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract 104: 1–52, 2014. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence. National Institute for Health and Care Excellence: clinical guidelines. In: Type 2 Diabetes in Adults: Management. London, UK: National Institute for Health and Care Excellence, 2015. [Google Scholar]
- 36.He L. Metformin and systemic metabolism. Trends Pharmacol Sci 41: 868–881, 2020. doi: 10.1016/j.tips.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab 29: 6s28–6s35, 2003. doi: 10.1016/s1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- 38.Giles ED, Jackman MR, MacLean PS. Modeling diet-induced obesity with obesity-prone rats: implications for studies in females. Front Nutri 3: 50, 2016. doi: 10.3389/fnut.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. doi: 10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins A, Campbell L. Future management of human obesity: understanding the meaning of genetic susceptibility. Adv Genom Genet 4: 219–232, 2014. doi: 10.2147/AGG.S53594. [DOI] [Google Scholar]
- 41.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 175: 661–666, 2007. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckett WS, Jacobs DR Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med 164: 2045–2050, 2001. doi: 10.1164/ajrccm.164.11.2004235. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Rennie D, Cormier Y, Dosman J. Sex specificity of asthma associated with objectively measured body mass index and waist circumference: the Humboldt study. Chest 128: 3048–3054, 2005. doi: 10.1378/chest.128.4.3048. [DOI] [PubMed] [Google Scholar]
- 44.Fuentes N, Silveyra P. Endocrine regulation of lung disease and inflammation. Exp Biol Med (Maywood) 243: 1313–1322, 2018. doi: 10.1177/1535370218816653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huovinen E, Kaprio J, Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med 97: 273–280, 2003. doi: 10.1053/rmed.2003.1419. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Wang K, Gao X, Paul TK, Cai J, Wang Y. Sex difference in the association between obesity and asthma in U.S. adults: findings from a national study. Respir Med 109: 955–962, 2015. doi: 10.1016/j.rmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Grant CW, Duclos SK, Moran-Paul CM, Yahalom B, Tirabassi RS, Arreaza-Rubin G, Spain LM, Guberski DL. Development of standardized insulin treatment protocols for spontaneous rodent models of type 1 diabetes. Comp Med 62: 381–390, 2012. [PMC free article] [PubMed] [Google Scholar]
- 48.Proskocil BJ, Fryer AD, Jacoby DB, Nie Z. Pioglitazone prevents obesity-related airway hyperreactivity and neuronal M(2) receptor dysfunction. Am J Physiol Lung Cell Mol Physiol 321: L236–L247, 2021. doi: 10.1152/ajplung.00567.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J 18: 711–713, 2004. doi: 10.1096/fj.03-0648fje. [DOI] [PubMed] [Google Scholar]
- 50.Haddad EB, Landry Y, Gies JP. Muscarinic receptor subtypes in guinea pig airways. Am J Physiol Lung Cell Mol Physiol 261: L327–L333, 1991. doi: 10.1152/ajplung.1991.261.4.L327. [DOI] [PubMed] [Google Scholar]
- 51.Roffel AF, Elzinga CR, Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm Pharmacol 3: 47–51, 1990. doi: 10.1016/0952-0600(90)90009-8. [DOI] [PubMed] [Google Scholar]
- 52.Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, Wess J, Haberberger RV. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol 64: 1444–1451, 2003. doi: 10.1124/mol.64.6.1444. [DOI] [PubMed] [Google Scholar]
- 53.Harvey RD, Belevych AE. Muscarinic regulation of cardiac ion channels. Br J Pharmacol 139: 1074–1084, 2003. doi: 10.1038/sj.bjp.0705338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stengel PW, Gomeza J, Wess J, Cohen ML. M(2) and M(4) receptor knockout mice: muscarinic receptor function in cardiac and smooth muscle in vitro. J Pharmacol Exp Ther 292: 877–885, 2000. [PubMed] [Google Scholar]
- 55.Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol 15: Unit 15.8, 2006. doi: 10.1002/0471142727.mb1508s73. [DOI] [PubMed] [Google Scholar]
- 56.Canning BJ, Woo A, Mazzone SB. Neuronal modulation of airway and vascular tone and their influence on nonspecific airways responsiveness in asthma. J Allergy (Cairo) 2012: 108149, 2012. doi: 10.1155/2012/108149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fryer AD, Wills-Karp M. Dysfunction of M2-muscarinic receptors in pulmonary parasympathetic nerves after antigen challenge. J Appl Physiol (1985) 71: 2255–2261, 1991. doi: 10.1152/jappl.1991.71.6.2255. [DOI] [PubMed] [Google Scholar]
- 58.Ayala LE, Ahmed T. Is there loss of protective muscarinic receptor mechanism in asthma? Chest 96: 1285–1291, 1989. doi: 10.1378/chest.96.6.1285. [DOI] [PubMed] [Google Scholar]
- 59.Minette PA, Barnes PJ. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J Appl Physiol (1985) 64: 2532–2537, 1988. doi: 10.1152/jappl.1988.64.6.2532. [DOI] [PubMed] [Google Scholar]
- 60.Minette PA, Lammers JW, Dixon CM, McCusker MT, Barnes PJ. A muscarinic agonist inhibits reflex bronchoconstriction in normal but not in asthmatic subjects. J Appl Physiol (1985) 67: 2461–2465, 1989. doi: 10.1152/jappl.1989.67.6.2461. [DOI] [PubMed] [Google Scholar]
- 61.Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda) 34: 198–215, 2019. doi: 10.1152/physiol.00048.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes 53: 2169–2176, 2004. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 63.DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab 73: 1294–1301, 1991. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- 64.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49: 2063–2069, 2000. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 60: 1577–1585, 2017. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perriello G, Misericordia P, Volpi E, Santucci A, Santucci C, Ferrannini E, Ventura MM, Santeusanio F, Brunetti P, Bolli GB. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes 43: 920–928, 1994. doi: 10.2337/diabetes.43.7.920. [DOI] [PubMed] [Google Scholar]
- 67.Menezes AM, Hallal PC, Muiño A, Chatkin M, Araújo CL, Barros FC. Risk factors for wheezing in early adolescence: a prospective birth cohort study in Brazil. Ann Allergy Asthma Immunol 98: 427–431, 2007. doi: 10.1016/S1081-1206(10)60756-9. [DOI] [PubMed] [Google Scholar]
- 68.Rose D, Mannino DM, Leaderer BP. Asthma prevalence among US adults, 1998–2000: role of Puerto Rican ethnicity and behavioral and geographic factors. Am J Public Health 96: 880–888, 2006. doi: 10.2105/AJPH.2004.050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA 268: 3437–3440, 1992. [PubMed] [Google Scholar]
- 70.Greenhill C. Obesity: sex differences in insulin resistance. Nat Rev Endocrinol 14: 65, 2018. doi: 10.1038/nrendo.2017.168. [DOI] [PubMed] [Google Scholar]
- 71.Qiu J, Bosch MA, Zhang C, Rønnekleiv OK, Kelly MJ. Estradiol protects neuropeptide Y/agouti-related peptide neurons against insulin resistance in females. Neuroendocrinology 110: 105–118, 2020. doi: 10.1159/000501560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salinero AE, Anderson BM, Zuloaga KL. Sex differences in the metabolic effects of diet-induced obesity vary by age of onset. Int J Obes (Lond) 42: 1088–1091, 2018. doi: 10.1038/s41366-018-0023-3. [DOI] [PubMed] [Google Scholar]
- 73.Sengupta P. The laboratory rat: relating its age with human's. Int J Prev Med 4: 624–630, 2013. [PMC free article] [PubMed] [Google Scholar]
- 74.Chen CZ, Hsu CH, Li CY, Hsiue TR. Insulin use increases risk of asthma but metformin use reduces the risk in patients with diabetes in a Taiwanese population cohort. J Asthma 54: 1019–1025, 2017. doi: 10.1080/02770903.2017.1283698. [DOI] [PubMed] [Google Scholar]
- 75.Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology 21: 1210–1218, 2016. doi: 10.1111/resp.12818. [DOI] [PubMed] [Google Scholar]
- 76.Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of metformin initiation and risk of asthma exacerbation. a claims-based cohort study. Ann Am Thorac Soc 16: 1527–1533, 2019. doi: 10.1513/AnnalsATS.201812-897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 141: 471–501, 1990. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 78.Rom WN, Pääkkö P. Activated alveolar macrophages express the insulin-like growth factor-I receptor. Am J Respir Cell Mol Biol 4: 432–439, 1991. doi: 10.1165/ajrcmb/4.5.432. [DOI] [PubMed] [Google Scholar]
- 79.Fain JN, Tichansky DS, Madan AK. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 54: 1546–1551, 2005. doi: 10.1016/j.metabol.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 80.Gosset P, Tsicopoulos A, Wallaert B, Vannimenus C, Joseph M, Tonnel AB, Capron A. Increased secretion of tumor necrosis factor α and interleukin-6 by alveolar macrophages consecutive to the development of the late asthmatic reaction. J Allergy Clin Immunol 88: 561–571, 1991. doi: 10.1016/0091-6749(91)90149-i. [DOI] [PubMed] [Google Scholar]
- 81.Chen D, Jia D, Wu X, Shi K, Ren C, Dou Y, Guo M, Wang J, Ma M, Wu Z, Shi HY, Li W, Feng Y, Wu F. A novel metformin derivative showed improvement of lipid metabolism in obese rats with type 2 diabetes. Clin Exp Pharmacol Physiol 47: 1382–1392, 2020. doi: 10.1111/1440-1681.13302. [DOI] [PubMed] [Google Scholar]
- 82.Dias MD, Goulart M, Dalécio C, Enes-Marques S, Salles ÉD, Venâncio M, Pereira EM, Paffaro VA Jr, Incerpi EK, Soncini R. Metformin influences on respiratory system in obese mice induced by postnatal overnutrition. Respir Physiol Neurobiol 247: 96–102, 2018. doi: 10.1016/j.resp.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 35: 731–737, 2012. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 22, Suppl 3: 1–203, 2016. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 85.Pu R, Shi D, Gan T, Ren X, Ba Y, Huo Y, Bai Y, Zheng T, Cheng N. Effects of metformin in obesity treatment in different populations: a meta-analysis. Ther Adv Endocrinol Metab 11: 2042018820926000, 2020. doi: 10.1177/2042018820926000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Solymár M, Ivic I, Pótó L, Hegyi P, Garami A, Hartmann P, Pétervári E, Czopf L, Hussain A, Gyöngyi Z, Sarlós P, Simon M, Mátrai P, Bérczi B, Balaskó M. Metformin induces significant reduction of body weight, total cholesterol and LDL levels in the elderly – a meta-analysis. PLoS One 13: e0207947, 2018. doi: 10.1371/journal.pone.0207947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang FF, Wu Y, Zhu YH, Ding T, Batterham RL, Qu F, Hardiman PJ. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: a systematic review and network meta-analysis. Obes Rev 19: 1424–1445, 2018. doi: 10.1111/obr.12720. [DOI] [PubMed] [Google Scholar]
- 88.Özbey Ü, Balaban S, Sözener Z, Uçar A, Mungan D, Mısırlıgil Z. The effects of diet-induced weight loss on asthma control and quality of life in obese adults with asthma: a randomized controlled trial. J Asthma 57: 618–626, 2020. doi: 10.1080/02770903.2019.1590594. [DOI] [PubMed] [Google Scholar]
- 89.Younas H, Vieira M, Gu C, Lee R, Shin MK, Berger S, Loube J, Nelson A, Bevans-Fonti S, Zhong Q, D'Alessio FR, McCormack MC, Hansel NN, Mitzner W, Polotsky VY. Caloric restriction prevents the development of airway hyperresponsiveness in mice on a high fat diet. Sci Rep 9: 279, 2019. doi: 10.1038/s41598-018-36651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okoniewski W, Lu KD, Forno E. Weight loss for children and adults with obesity and asthma. A systematic review of randomized controlled trials. Ann Am Thorac Soc 16: 613–625, 2019. doi: 10.1513/AnnalsATS.201810-651SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Belmonte KE, Jacoby DB, Fryer AD. Increased function of inhibitory neuronal M2 muscarinic receptors in diabetic rat lungs. Br J Pharmacol 121: 1287–1294, 1997. doi: 10.1038/sj.bjp.0701274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wagner EM, Jacoby DB. Methacholine causes reflex bronchoconstriction. J Appl Physiol (1985) 86: 294–297, 1999. doi: 10.1152/jappl.1999.86.1.294. [DOI] [PubMed] [Google Scholar]
- 93.McAlexander MA, Gavett SH, Kollarik M, Undem BJ. Vagotomy reverses established allergen-induced airway hyperreactivity to methacholine in the mouse. Respir Physiol Neurobiol 212–214: 20–24, 2015. doi: 10.1016/j.resp.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fernandes LB, Fryer AD, Hirshman CA. M2 muscarinic receptors inhibit isoproterenol-induced relaxation of canine airway smooth muscle. J Pharmacol Exp Ther 262: 119–126, 1992. [PubMed] [Google Scholar]
- 95.Mak JC, Barnes PJ. Autoradiographic visualization of muscarinic receptor subtypes in human and guinea pig lung. Am Rev Respir Dis 141: 1559–1568, 1990. doi: 10.1164/ajrccm/141.6.1559. [DOI] [PubMed] [Google Scholar]
- 96.Roffel AF, in't Hout WG, de Zeeuw RA, Zaagsma J. The M2 selective antagonist AF-DX 116 shows high affinity for muscarine receptors in bovine tracheal membranes. Naunyn Schmiedebergs Arch Pharmacol 335: 593–595, 1987. doi: 10.1007/BF00169130. [DOI] [PubMed] [Google Scholar]
- 97.Baker DG, Don HF, Brown JK. Direct measurement of acetylcholine release in guinea pig trachea. Am J Physiol Lung Cell Mol Physiol 263: L142–L147, 1992. doi: 10.1152/ajplung.1992.263.1.L142. [DOI] [PubMed] [Google Scholar]
- 98.Jacoby DB, Xiao HQ, Lee NH, Chan-Li Y, Fryer AD. Virus- and interferon-induced loss of inhibitory M2 muscarinic receptor function and gene expression in cultured airway parasympathetic neurons. J Clin Invest 102: 242–248, 1998. doi: 10.1172/JCI1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacoby DB, Yost BL, Kumaravel B, Chan-Li Y, Xiao HQ, Kawashima K, Fryer AD. Glucocorticoid treatment increases inhibitory m(2) muscarinic receptor expression and function in the airways. Am J Respir Cell Mol Biol 24: 485–491, 2001. doi: 10.1165/ajrcmb.24.4.4379. [DOI] [PubMed] [Google Scholar]
- 100.Blaber LC, Fryer AD, Maclagan J. Neuronal muscarinic receptors attenuate vagally-induced contraction of feline bronchial smooth muscle. Br J Pharmacol 86: 723–728, 1985. doi: 10.1111/j.1476-5381.1985.tb08951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Belmonte KE, Fryer AD, Costello RW. Role of insulin in antigen-induced airway eosinophilia and neuronal M2 muscarinic receptor dysfunction. J Appl Physiol (1985) 85: 1708–1718, 1998. doi: 10.1152/jappl.1998.85.5.1708. [DOI] [PubMed] [Google Scholar]
- 102.Camargo CA Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 159: 2582–2588, 1999. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 103.Ali Z, Ulrik CS. Obesity and asthma: a coincidence or a causal relationship? A systematic review. Respir Med 107: 1287–1300, 2013. doi: 10.1016/j.rmed.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 104.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med 101: 2240–2247, 2007. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 105.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest 134: 317–323, 2008. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 106.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM, Williamson A, Brassett K, Herbison GP, Taylor DR. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med 178: 469–475, 2008. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 107.Suratt BT, Ubags NDJ, Rastogi D, Tantisira KG, Marsland BJ, Petrache I, Allen JB, Bates JHT, Holguin F, McCormack MC, Michelakis ED, Black SM, Jain M, Mora AL, Natarajan V, Miller YI, Fessler MB, Birukov KG, Summer RS, Shore SA, Dixon AE; Allergy, Immunology, and Inflammation Assembly. An official American Thoracic Society workshop report: obesity and metabolism: obesity and metabolism. An emerging frontier in lung health and disease. Ann Am Thorac Soc 14: 1050–1059, 2017. doi: 10.1513/AnnalsATS.201703-263WS. [DOI] [PMC free article] [PubMed] [Google Scholar]