Abstract
Over 40 million people use e-cigarettes worldwide, but the impact of chronic e-cigarette use on health has not been adequately defined. In particular, effects of e-cigarette aerosol inhalation on inflammation and host defenses across the body are not fully understood. We conducted a longitudinal cohort pilot study to explore changes in the inflammatory state and monocyte function of e-cigarette users (n = 20) versus healthy controls (n = 13) and to evaluate effects of e-cigarette use reduction on the same. Saliva, sputum, and blood were obtained from e-cigarette users at baseline and after a 2-wk intervention of decreased e-cigarette use. Overall, across 38 proteins quantified by multiplex, airway samples from e-cigarette users tended to have decreased levels of immunomodulatory proteins relative to healthy controls, whereas levels of cytokines, chemokines, and growth factors in the circulation tended to be elevated. Specifically, e-cigarette users had lower levels of IL-1 receptor antagonist (IL-1Ra) in saliva (P < 0.0001), with higher IL-1Ra and growth-regulated oncogene (GRO) levels in sputum (P < 0.01 and P < 0.05, respectively), and higher levels of both TNFβ (P < 0.0001) and VEGF (P < 0.0001) in plasma. Circulating monocytes from e-cigarette users had alterations in their inflammatory phenotype in response to reduced e-cigarette use, with blunted IL-8 and IL-6 release upon challenge with bacterial lipopolysaccharide (P < 0.001 and P < 0.05, respectively), suggesting a decreased ability to appropriately respond to bacterial infection. Based on these findings, chronic inhalation of e-cigarette aerosols alters the inflammatory state of the airways and systemic circulation, raising concern for the development of both inflammatory and infectious diseases in chronic users of e-cigarettes.
Keywords: cytokine, e-cigarette, host defense, inflammation, monocyte
INTRODUCTION
Electronic cigarettes (e-cigarettes) were introduced into the US market in the late 2000s (1). Advertised as an attractive alternative to conventional tobacco, and as an appealing recreational inhalant, the use of e-cigarettes has grown worldwide (1, 2). Despite its origin as a proxy to aid tobacco cessation, data suggest that e-cigarettes may promote continued nicotine dependence with many e-cigarette users continuing to smoke cigarettes (3, 4). Studies have additionally shown that e-cigarette use may lead to initiation of tobacco smoking in nonsmokers (5) and relapse of smoking in ex-smokers (5, 6). But most ominously, many teenage and young adult never-smokers have taken up vaping of e-cigarettes, becoming yet another population to succumb to nicotine addiction via these drug delivery devices.
E-cigarettes work by heating and aerosolizing a liquid mixture of propylene glycol, glycerin, flavoring chemicals, and nicotine. Most e-cigarette liquids (e-liquids) contain numerous toxicants (7, 8), the effects of which are poorly understood. A key area of interest is the potential impact of e-cigarette use on inflammatory responses locally and systemically. Although we previously showed that e-cigarette inhalation alters innate immunity and increases the virulence of colonizing bacteria via ex vivo exposures of human cells and bacteria (9, 10), we lacked corroborating studies in human subjects. Similarly, studies from other groups have primarily been performed in mice or cells via in vitro approaches (11), and data about inflammation have been, at times, contradictory, possibly related to the disparate experimental methods and models used (1). However, reviewing all data available to date yields compelling evidence that e-cigarettes impact the inflammatory status and responsiveness in different organs (1, 12–15).
We hypothesized that daily, chronic use of e-cigarettes induces immunomodulation of both the airways and systemically, which fundamentally alters host responses to inflammatory and infectious challenges. In this pilot study, we explored the inflammatory state of the upper and lower airways and systemic circulation in young healthy e-cigarette users versus nonsmoking nonvaping controls. Furthermore, we assessed the phenotype and function of circulating monocytes, to determine whether e-cigarette use impacts the inflammatory state and antimicrobial function of this host defense cell. In addition, we conducted a 2-wk intervention with decreased e-cigarette use, to evaluate the effects of short-term reduction of e-cigarette use on the same. These data bring us one step closer to understanding the broad impact of e-cigarette use on inflammation and the immune system. Moreover, this pilot study provides a useful model to study e-cigarette toxicity in humans.
MATERIALS AND METHODS
Participants and Procedure
A longitudinal cohort study was conducted on subjects who exclusively used e-cigarettes (n = 20). Inclusion criteria were active e-cigarette use without known lung disease and with normal lung function, between the ages of 18 and 30 yr. Inhalant use, including details regarding e-cigarette device and e-liquid types, flavors, nicotine concentrations, frequency of use, as well as detailed information about both tobacco and marijuana inhalants, was quantified by the University of California San Diego (UCSD) Inhalant Survey (16, 17), which is freely available on RedCap. Inhalant use was further confirmed with an in-person interview and cotinine levels. For this study, active vaping was defined as use of ≥0.5–1 mL e-liquid/day or 3.5–7 mL/wk for >6 mo. Exclusion criteria included concomitant use of conventional tobacco. Twenty active e-cigarette users and 13 nonsmoking nonvaping controls were enrolled. Seven e-cigarette subjects completed only the initial visit, giving 13 vapers who completed both initial and follow-up visits. Subjects were asked to stop vaping for 2 wk at the time of the initial visit. Only 1 of the 13 e-cigarette users successfully ceased vaping, whereas the rest of the vapers reported reduced usage by Likert scale between baseline and follow-up visits, which was confirmed by lower cotinine levels in blood. This study was approved by the University of California San Diego Institutional Review Board (project 160204) in accordance with the provisions of the Declaration of Helsinki. All subjects provided written informed consent.
Saliva, Sputum, and Plasma Collection
Saliva, sputum, and plasma were obtained at the initial visit and after 2 wk of decreased e-cigarette use. For saliva, patients were instructed not to brush their teeth and not to eat or drink anything but water the morning of collection. Subjects rinsed their mouths with water, assumed a tripod position, and allowed saliva to drip into a sterile collection cup for 5 min. The volume of saliva was recorded (was >3 mL in all cases), and the sample was centrifuged at 5,000 rpm for 5 min at 4°C. Protease inhibitors were added to the supernatant before storage at −70°C for quantification of cytokines. For sputum collection, all samples were collected by a team member with over 10 yr of experience in the procedure. Patients inhaled nebulized hypertonic 3% saline for 5 min (with escalation to 4% and 5% saline, as needed) and expectorated sputum was collected and placed on ice. Sputum was weighed, liquefied via treatment with sputolysin and agitation, and the supernatant aliquoted and stored at −70°C for cytokine quantification. The cellular fraction underwent cytospin and Giemsa Wright staining. For plasma, 8 mL of whole blood was collected in BD plasma separator tubes and centrifuged at 13,000 g. Plasma was aliquoted and stored at −70°C for measurement of inflammatory cytokines and cotinine.
Monocyte Isolation by Plastic Adherence
Twenty-four milliliters of blood was drawn into EDTA tubes from each subject. Whole blood was mixed with an equal volume of PBS and layered over PolymorphPrep before centrifugation at 800 g for 30 min at room temperature, without braking. The buffy coat layer containing the peripheral blood mononuclear cells (PBMC) was collected and washed two times with PBS containing 2% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific) and centrifuged at 100 g for 20 min at room temperature to pellet the PBMCs. The monocytes were isolated from the PBMCs using the plastic adhesion method, as described before (19–21). Briefly, PBMC pellets were resuspended in RPMI-1640 medium containing 10% FBS and seeded in T‐75 cell culture flasks (Thermo Fisher Scientific) at 37°C with 5% CO2 for 2 h. Monocytes adhere to the plastic surfaces (adherent cells), whereas lymphocytes are nonadherent and remain in the culture media suspension. Nonadherent cells were removed by suctioning off the supernatant and adherent cells were gently washed twice. Adherent monocytes were collected by incubation with 0.5% EDTA followed by cell scraper.
Monocyte Seeding and Stimulation with LPS
Monocytes were quantified using trypan blue exclusion, a hemocytometer, and light microscopy. Monocytes (1 × 106 cells in 2 mL of RPMI-1640) were seeded in six-well plates and incubated at 37°C and 5% CO2 overnight. Supernatant was collected from the monocytes (unstimulated) for cytokine evaluation by ELISA. Lipopolysaccharide (LPS) stimulation was performed by adding Escherichia coli LPS (O111:B4, Sigma-Aldrich) 1 ug/mL before overnight incubation at 37°C and 5% CO2. Supernatant was then collected (stimulated) for cytokine quantification.
Measurement of Inflammatory Markers
Because e-cigarette aerosols and e-liquids have been found via in vitro and in vivo studies in animals to impact inflammation and host defense, as well as impacting and inducing cardiovascular, neurological, renal, and metabolic diseases, a multiplex array was chosen based on its ability to assess the primary cytokines, chemokines, and growth factors involved in these processes. Broad profiling and quantification of inflammatory cytokines, chemokines, and growth factors from saliva, sputum, and plasma were performed using Millipore Multiplex HCYTA-60K-PX38 (Burlington, MA), according to the manufacturer’s instructions. For ex vivo monocyte cell cultures, supernatants were collected from unstimulated and LPS-stimulated monocytes at 16 to 18 h, and IL-8, IL-6, and TNF-α were quantified by ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 9.0 (San Diego, CA). χ2 Test was used to assess for demographic differences. Inflammatory marker data from each compartment from healthy controls were compared to that from e-cigarette subjects at the initial baseline visit. To determine the most significant changes in each compartment with high rigor, two-way ANOVA with correction for multiple comparisons by controlling the false discovery rate, using the two-stage step-up method of Benjamini, Krieger, and Yekutieli, was used. Because of the exploratory as well as the longitudinal nature of this pilot study, individual analytes were further assessed by Student’s t test, with unpaired t tests in controls versus e-cigarette users and paired t tests of e-cigarette users at each visit. Inflammatory marker data were expressed as individual data points with the mean and 95% confidence interval (CI). For ex vivo monocyte cell culture studies, individual responsiveness was assessed by dividing cytokine levels in LPS-stimulated monocytes by that released from unstimulated cells of the same subject.
RESULTS
Demographic Characteristics of E-Cigarette Users
Twenty e-cigarette users completed the baseline visit and 13 completed the follow-up visit. The mean age of subjects was 21. Race, ethnicity, and age were not significantly different between the two groups (Table 1). There was a male predominance in the e-cigarette subset with 95% of users being male, compared to 25% of controls (P < 0.01; Table 1). The majority of subjects used pod-based devices, with JUUL being the most prevalent (Table 2). On average, subjects vaped 23 ± 21 puffs/day for 6 ± 1 days/wk. Subjects used their e-devices for a mean of 1.3 ± 1 yr. On follow-up visit, all e-cigarette subjects reported decreased usage of e-cigarettes in the prior 2 wk by Likert scale, with one subject successfully achieving complete cessation. Decreased e-cigarette use was confirmed by cotinine analysis (means ± SD of 62.15 ± 57.09 ng/mL vs. 34.26 ± 42.55 ng/mL).
Table 1.
Demographics by group category
| Control |
E-cigarette Users |
Overall |
P Value | |
|---|---|---|---|---|
| n = 13 (%) | n = 20 (%) | n = 33 (%) | ||
| Gender | ||||
| Female | 10 (77) | 1 (5) | 11 (33) | <0.01 |
| Male | 3 (23) | 19 (95) | 22 (67) | |
| Race | ||||
| African American | 1 (8) | 0 (0) | 1 (3) | 0.41 |
| Asian | 7 (54) | 11 (55) | 18 (55) | |
| Caucasian | 5 (38) | 7 (35) | 12 (36) | |
| Mixed | 0 (0) | 2 (10) | 2 (6) | |
| Ethnicity | ||||
| Caucasian, Non-Hispanic | 1 (20) | 5 (71) | 6 (50) | 0.08 |
| Caucasian, Hispanic | 4 (80) | 2 (29) | 6 (50) | |
| Total | 5 | 7 | 12 | |
| Age | ||||
| Mean (range) | 21 (18–28) | 21 (18–26) | 21 (18–28) | 0.41 |
A χ2 test was used to test the statistical differences between both controls and e-cigarette users. Caucasian, Hispanic includes those that self-identify as Hispanic. Mixed race includes a variety of mixed racial composition. The difference in age was calculated using an unpaired t test between the two groups.
Table 2.
Inhalant use
| E-cigarette Users | |
|---|---|
| n = 20 | |
| Duration | |
| Average (range) | |
| Device use length, yr | 1.3 (0.1–3.5) |
| Days per week of use | 6 (4–7) |
| Times per day, max puffs/day | 23 (1–60) |
| Type of user | Count (%) |
| Single user, only e-cig | 8 (40) |
| Dual user, e-cigarettes and cigarettes | 2 (10) |
| Dual user, e-cigarettes and marijuana | 7 (35) |
| Triple user | 3 (15) |
| Device information | |
| Count (%) | |
| E-device type | |
| Pod-device | 10 (50) |
| Box mod/ vape pen | 10 (50) |
| E-device brand | |
| Juul | 6 (30) |
| Sourin | 3 (15) |
| Smok | 5 (25) |
| Wismec | 2 (10) |
| Jaybo | 1 (5) |
| E-Leaf | 1 (5) |
| Joyetech | 1 (5) |
| Sigelei | 1 (5) |
Device use length calculated by averaging years of use. If duration was given in months, the number of months was divided by 12 to get a portion of a year of usage. Days of e-cigarette use per week were calculated averaging between all users, if range of use was given, the median was used to average. Times per day was calculated using the median of the range of puffs per day.
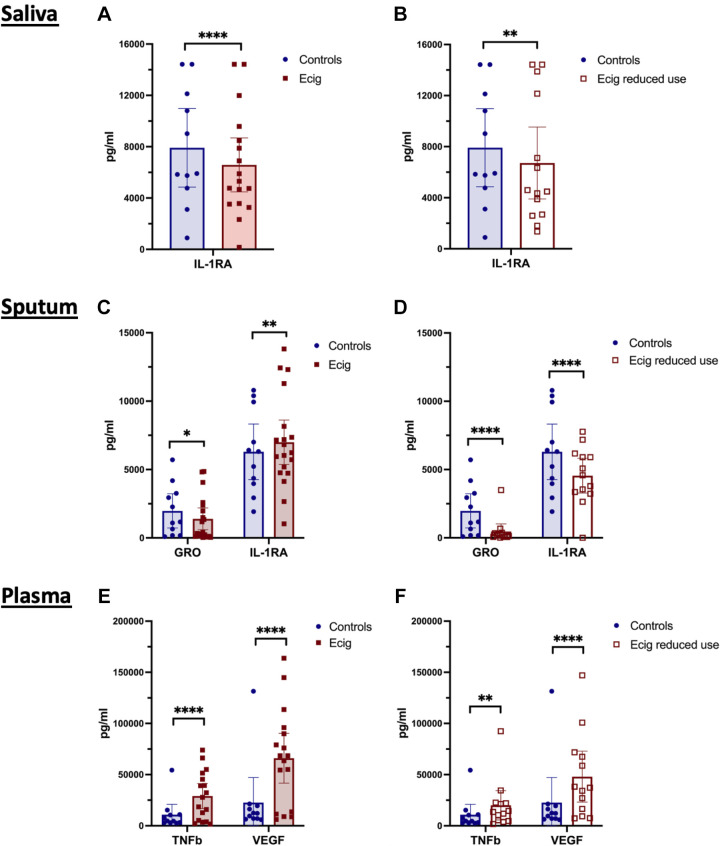
E-Cigarette Use Leads to Decreased Levels of IL-1Ra in Saliva
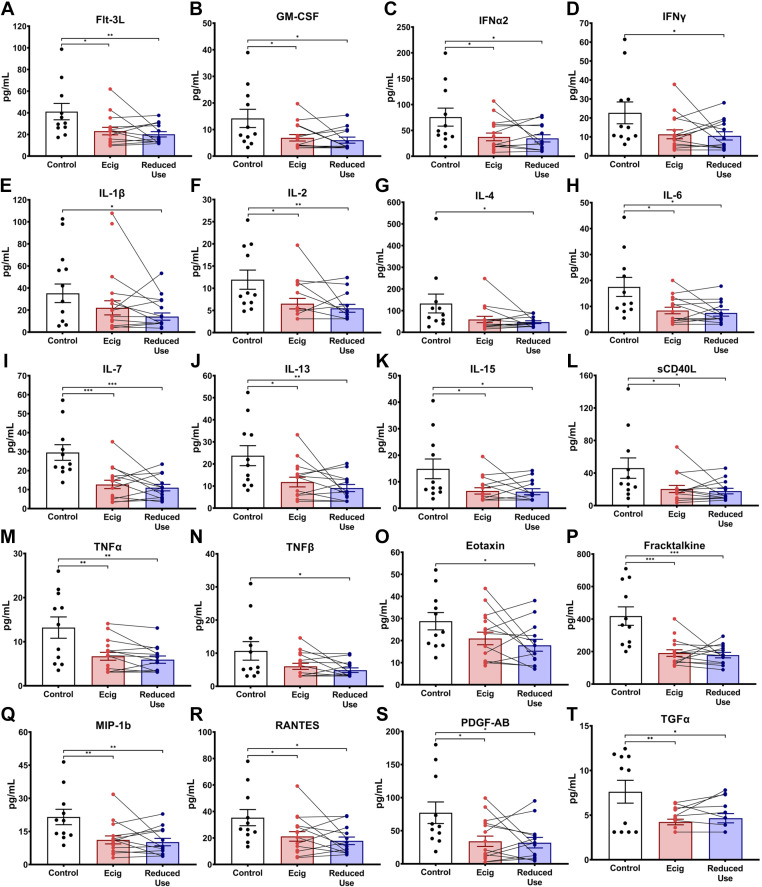
Evaluation of the inflammatory cytokine, chemokine, and growth factor milieu in the saliva via multiplex array yielded identification of decreased salivary levels of IL-1Ra in vapers relative to controls (P < 0.0001; Fig. 1A). This reduction persisted after short-term reduction in vaping (P < 0.0001; Fig. 1B). An overall trend of decreased immunomodulatory and inflammatory proteins was observed in the saliva of e-cigarette vapers, although statistical significance was not maintained after correction for multiple comparisons (Table 3). Namely, salivary cytokines (Flt-3L, Fractalkine, IFNα2, IL-2, IL-6, IL-7, IL-13, IL-15, sCD40L, and TNFα), chemokines [macrophage inflammatory protein-1beta (MIP-1b) and regulated upon activation, normal T cell expressed and secreted (RANTES)] and growth factors [granulocyte-macrophage colony stimulating factor (GM-CSF), platelet-derived growth factor-AB (PDGF-AB), and TGFα] had lower levels in the saliva of e-cigarette users by individual t tests (Fig. 2). The majority of these alterations persisted in the setting of diminished e-cigarette use (Fig. 2).
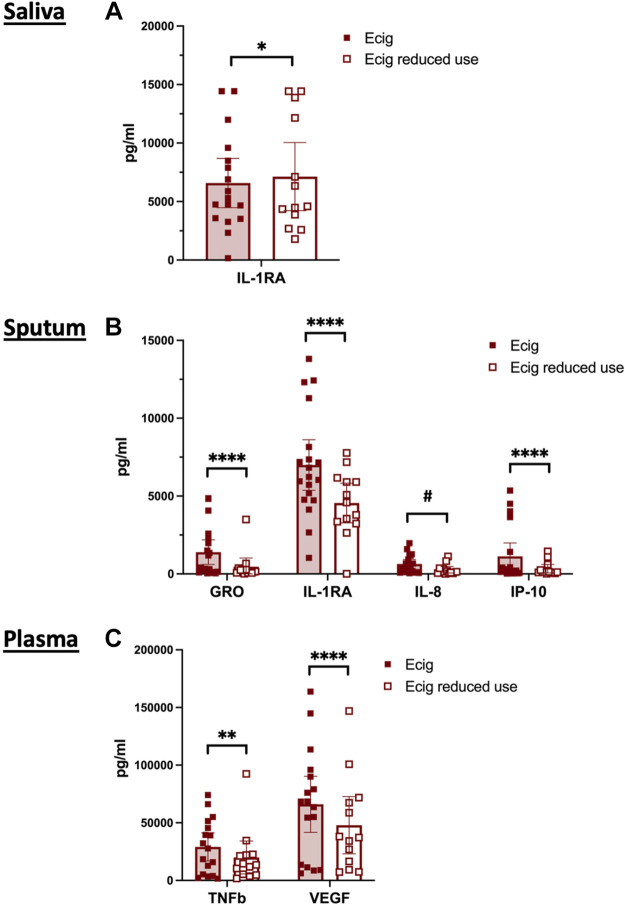
Figure 1.
E-cigarette use associated alterations in immunomodulatory protein levels in saliva, sputum, and plasma. A: inflammatory markers were assessed in the saliva of nonsmoking nonvaping healthy controls (n = 11; blue circles) and e-cigarette users at baseline (n = 17; solid red squares), with lower levels of IL-1RA identified in the oral airways of e-cigarette users. B: IL-1RA remained low in e-cigarette users after 2 wk of reduced e-cigarette usage (n = 14; hollow red squares). C: sputum demonstrated reductions in both GRO and IL-1RA in the lower airways of e-cigarette users vs. healthy controls. D: GRO and IL-1RA remained low in lower airway samples after 2 wk of reduced e-cigarette usage. E: plasma samples demonstrated elevations in TNFβ and VEGF in the circulation of e-cigarette users vs. controls. F: after 2 wk of reduced e-cigarette use, plasma levels of TNFβ and VEGF were persistently elevated. Data are represented as individual data points with the geometric mean. Statistical analysis was conducted with two-way ANOVA with correction for multiple comparisons by controlling the false discovery rate, using the two-stage step-up method of Benjamini, Krieger, and Yekutieli. *P < 0.05, **P < 0.01, and ****P < 0.0001.
Table 3.
Cytokines, chemokines, and growth factors in saliva
| Control vs. E-cigarette |
Control vs. Reduced Use |
E-cigarette vs. Reduced Use |
|
|---|---|---|---|
| Mean/Mean (P Value) | Mean/Mean (P Value) | Mean → Mean (P Value) | |
| Cytokines | |||
| Flt-3L | 41/23 (0.02) | 41/20 (0.01) | 25 → 21 (0.28) |
| IFNa2 | 76/38 (0.03) | 76/36 (0.03) | 45 → 37 (0.47) |
| IFNγ | 23/12 (0.05) | 23/11 (0.05) | 13 → 11 (0.58) |
| IL-1β | 45/28 (0.22) | 45/18 (0.02) | 27 → 19 (0.30) |
| IL-2 | 12/7 (0.03) | 12/5 (0.01) | 7 → 6 (0.24) |
| IL-4 | 133/59 (0.08) | 133/17 (0.05) | 68 → 47 (0.29) |
| IL-6 | 18/8 (0.01) | 18/7 (0.01) | 9 → 8 (0.41) |
| IL-7 | 30/13 (<0.01) | 30/11 (<0.01) | 14 → 11 (0.29) |
| IL-13 | 24/12 (0.02) | 24/9 (<0.01) | 13 → 9 (0.18) |
| IL-15 | 15/7 (0.02) | 15/6 (0.03) | 7 → 7 (0.55) |
| sCD40L | 46/20 (0.04) | 46/18 (0.03) | 23 → 18 (0.39) |
| TNFα | 13/7 (0.01) | 13/6 (0.01) | 7 → 6 (0.11) |
| TNFβ | 11/6 (0.08) | 11/5 (0.04) | 7 → 5 (0.14) |
| Chemokines | |||
| Eotaxin | 29/21 (0.11) | 29/18 (0.03) | 23 → 18 (0.21) |
| Fractalkine | 418/191 (<0.01) | 418/178 (<0.01) | 192 → 182 (0.72) |
| MIP-1b | 22/11 (0.01) | 22/10 (0.01) | 13 → 10 (0.38) |
| RANTES | 35/21 (0.04) | 35/18 (0.01) | 24 → 18 (0.27) |
| Growth factors | |||
| GM-CSF | 14/7 (0.03) | 14/6 (0.02) | 8 → 6 (0.37) |
| PDGF-AB | 77/34 (0.01) | 77/32 (0.02) | 40 → 34 (0.53) |
| TGFα | 8/4 (0.01) | 8/5 (0.03) | 4 → 5 (0.32) |
Columns represent individual Student’s t test comparisons between controls (n = 11) and e-cigarette users (n = 16), control and reduced use samples (n = 13), and e-cigarette users with and without reduced use, respectively. Not all e-cigarette users returned for a follow-up visit; therefore, the comparison of levels between e-cigarette users and reduced use may have differing means. First two columns included comparison using an unpaired t test between the controls vs. the e-cigarette users; however, the third column was compared using a paired t test between samples from the same subject at a 2-wk follow-up. Bold P value indicates a significant difference by t test (without controlling for multiple comparisons) between the groups mentioned in the heading column.
Figure 2.
Exploratory assessment of inflammatory modulatory proteins, chemokines, and growth factors in saliva samples. Inflammatory markers were assessed in the saliva of nonsmoking healthy controls (black), e-cigarette users at baseline (red) and after 2 wk of reduced e-cigarette usage (blue). Only the analytes with statistically significant differences by Student’s t test (without controlling for multiple comparisons) were included in this figure and are further separated into cytokines (A–N), chemokines (O–R), and growth factors (S–T). Paired lines between e-cigarette and reduced dose represent the change in the levels in the same individual after reducing the e-cigarette dose. Error bars represent the means ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by Student’s t test.
E-Cigarette Use Is Associated with Decreased GRO and Increased IL-1Ra in Sputum
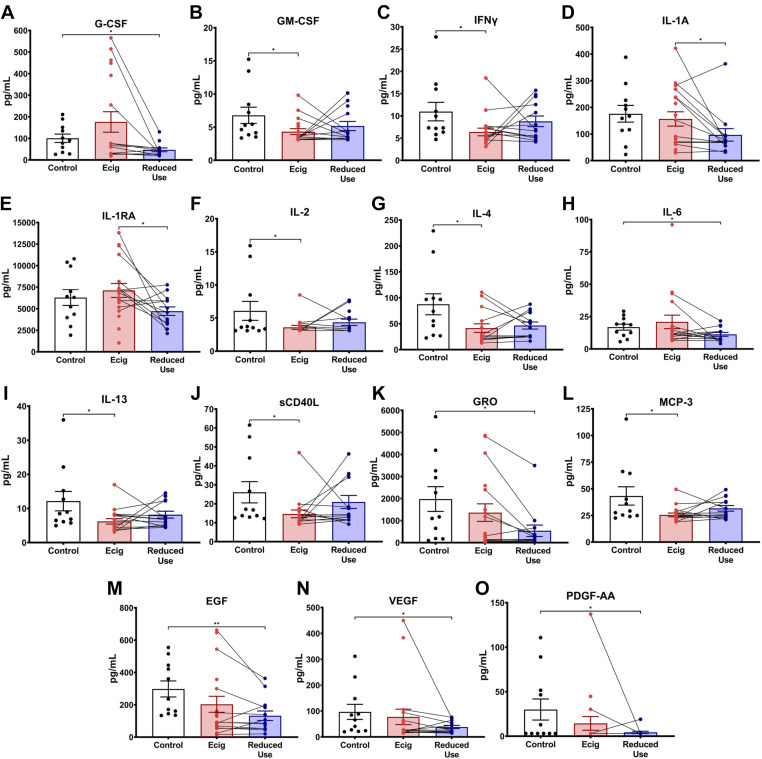
Similar to the oral airway compartment, lower airways from e-cigarette users, assessed by sputum, had alterations in the IL-1 axis. Increased levels of IL-1Ra (Fig. 1, C and D) demonstrated increased anti-inflammatory signaling. The chemokine growth-regulated oncogene (GRO) was significantly lower in the sputum of e-cigarette vapers (Fig. 1, C and D). Overall, the lower airway compartment had a pattern similar to that seen in the upper airways with diminished levels of pro-inflammatory cytokines, chemokines, and growth factors in the sputum of e-cigarette users relative to healthy controls (Table 4). Namely, cytokines GM-CSF, IFNγ, IL-2, IL-4, IL-13, and sCD40L, chemokine monocyte chemoattractant protein-1 (MCP-3), and growth factors EGF, VEGF, and PDGF-AA were modestly lower in e-cigarette users relative to nonsmoking nonvaping controls by individual t tests (Fig. 3).
Table 4.
Cytokines, chemokines, and growth factors in sputum
| Control vs. E-cigarette |
Control vs. Reduced Use |
E-cigarette vs. Reduced Use |
|
|---|---|---|---|
| Mean/Mean (P Value) | Mean/Mean (P Value) | Mean → Mean (P Value) | |
| Cytokines | |||
| GM-CSF | 7/4 (0.03) | 7/5 (0.25) | 5 → 4.91 (0.73) |
| G-CSF | 101/176 (0.24) | 101/47 (0.01) | 147 → 43 (0.07) |
| IFNγ | 11/6 (0.03) | 11/9 (0.35) | 7 → 8 (0.62) |
| IL-1A | 176/156 (0.65) | 176/97 (0.05) | 184 → 97 (0.03) |
| IL-1RA | 6,297/7,121 (0.52) | 6,297/4,715 (0.13) | 7,998 → 4,812 (0.02) |
| IL-2 | 6/4 (0.04) | 6/4 (0.23) | 4 → 4 (0.57) |
| IL-4 | 88/42 (0.02) | 88/47 (0.05) | 44 → 44 (0.94) |
| IL-6 | 17/21 (0.56) | 17/11 (0.05) | 16 → 11 (0.16) |
| IL-13 | 12/6 (0.02) | 12/8 (0.18) | 7 → 8 (0.68) |
| sCD40L | 26/15 (0.03) | 26/21 (0.43) | 16 → 20 (0.47) |
| Chemokines | |||
| GRO | 1,977/1,363 (0.37) | 1,977/539 (0.02) | 1,217 → 555 (0.05) |
| MCP-3 | 43/26 (0.02) | 43/32 (0.18) | 27 → 31 (0.35) |
| Growth factors | |||
| EGF | 298/203 (0.21) | 298/132 (0.01) | 179 → 137 (0.27) |
| PDGF-AA | 30/14 (0.26) | 30/4 (0.03) | 17 → 4 (0.31) |
| VEGF | 97/77 (0.67) | 97/38 (0.05) | 74 → 38 (0.28) |
Columns represent comparison between the controls (n = 10) and e-cigarette users (n = 18), control and reduced use samples (n = 13), and e-cigarette users with and without reduced use, respectively. Not all e-cigarette users returned for a follow-up visit; therefore, the comparison of levels between e-cigarette users and their reduced use follow-up may have differing means. First two columns included comparison using an unpaired t test between the controls vs. the e-cigarette users; however, the third column was compared using a paired t test between samples from the same subject at a 2-wk follow-up. Bold P value indicates a significant difference by t test (without controlling for multiple comparisons) between the groups mentioned in the heading column. IL-1RA, IL-1 receptor antagonist; MCP, monocyte chemoattractant protein.
Figure 3.
Exploratory assessment of inflammatory modulatory proteins, chemokines, and growth factors in sputum samples. Inflammatory markers were assessed in the sputum of nonsmoking healthy controls (black), e-cigarette users at baseline (red) and after 2 wk of reduced e-cigarette usage (blue). Only the analytes with statistically significant differences by Student’s t test (without controlling for multiple comparisons) were included in this figure and are further separated into cytokines (A–J), chemokines (K–L), and growth factors (M–O). Paired lines between e-cigarette and reduced dose represent the change in the levels in the same individual after reducing the e-cigarette dose. Error bars represent the means ± SD. *P < 0.05, and **P < 0.01, as determined by Student’s t test.
E-Cigarette Use Is Associated with Increased Plasma Levels of TNFβ and VEGF
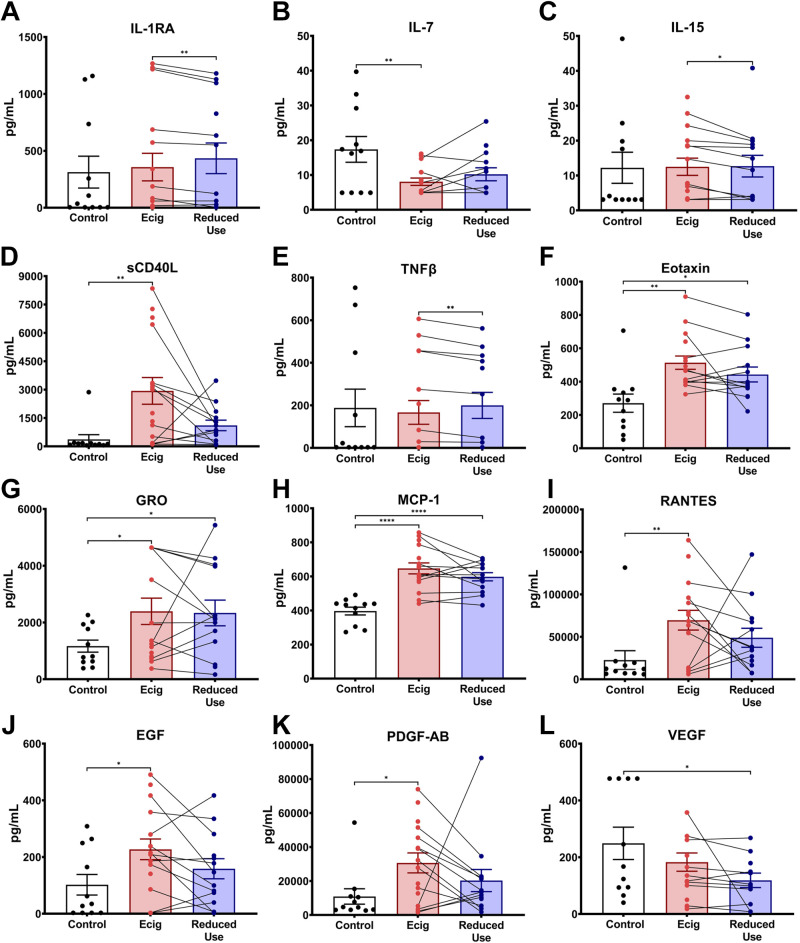
Within the circulation, e-cigarette users had higher levels of both TNFβ and VEGF (Fig. 1, E and F). Because of the exploratory nature of this pilot study, plasma concentrations of inflammatory cytokines, chemokines, and growth factors were also assessed by individual t tests and were broadly found to be higher in the plasma of e-cigarette users relative to healthy controls (Table 5). Specifically, chemokines Eotaxin, GRO, and monocyte chemoattractant protein-1 (MCP-1) were higher in the circulation of in e-cigarette users (Table 5; Fig. 4). All three of these chemokines trended down after 2 wk of decreased e-cigarette use but did not reach levels seen in controls. Similar patterns were seen in plasma levels of cytokine sCD-40L, chemokine RANTES, and growth factors EGF and PDGF-AB, with each of these inflammatory biomarkers tending to be higher in e-cigarette users at the initial visit and decreased with reduced use (Table 5; Fig. 4, D, I, J, and K). Interestingly, IL-7 was the only cytokine in the circulation that tended to be lower in e-cigarette users, compared to controls (Table 5; Fig. 4B).
Table 5.
Cytokines, chemokines, and growth factors in plasma
| Control vs. E-cigarette |
Control vs. Reduced Use |
E-cigarette vs. Reduced Use |
|
|---|---|---|---|
| Mean/Mean (P Value) | Mean/Mean (P Value) | Mean → Mean (P Value) | |
| Cytokines | |||
| IL-1RA | 313/357 (0.82) | 313/434 (0.54) | 445 → 401 (0.01) |
| IL-7 | 17/8 (0.01) | 17/10 (0.08) | 8 → 10 (0.32) |
| IL-15 | 12/12 (0.95) | 12/13 (0.93) | 13 → 10 (0.01) |
| sCD40L | 367/2,931 (0.01) | 367/1,108 (0.06) | 2479 → 1,149 (0.11) |
| TNFβ | 188/167 (0.83) | 188/200 (0.91) | 204 → 185 (0.01) |
| Chemokines | |||
| Eotaxin | 271/514 (0.001) | 271/443 (0.02) | 513 → 454 (0.20) |
| GRO | 1,166/2,392 (0.04) | 1,166/2,335 (0.04) | 2,459 → 2,493 (0.95) |
| MCP-1 | 397/647 (<0.01) | 397/598 (<0.01) | 634 → 594 (0.22) |
| RANTES | 22,649/69,591 (0.01) | 22,649/48,891 (0.11) | 64,249 → 51,132 (0.49) |
| Growth factors | |||
| EGF | 102/227 (0.03) | 102/159 (0.28) | 221.6 → 155 (0.09) |
| PDGF-AB | 10,887/30,652 (0.02) | 10,887/20,287 (0.27) | 28,168 → 21,189 (0.49) |
Columns represent comparison between the controls (n = 11) and e-cigarette users (n = 16), control and reduced use samples (n = 13), and e-cigarette users with and without reduced use, respectively. Not all e-cigarette users returned for a follow-up visit; therefore, the comparison of levels between e-cigarette users and their reduced use follow-up may have differing means. First two columns included comparison using an unpaired t test between the controls vs. the e-cigarette users; however, the third column was compared using a paired t test between samples from the same subject at a 2-wk follow-up. Bold P value indicates a significant difference by t test (without controlling for multiple comparisons) between the groups mentioned in the heading column. IL-1RA, IL-1 receptor antagonist; MCP, monocyte chemoattractant protein.
Figure 4.
Exploratory assessment of inflammatory modulatory proteins, chemokines, and growth factors in plasma samples. Inflammatory markers were assessed from the plasma in nonsmoking healthy controls (black), e-cigarette users at baseline (red) and after 2 wk of reduced e-cigarette usage (blue). Only the analytes with statistically significant differences by Student’s t test (without controlling for multiple comparisons) were included in this figure and are further separated into cytokines (A–E), chemokines (F–I), and growth factors (J–L). Paired lines between e-cigarette and reduced dose represent the change in the levels in the same individual after reducing the e-cigarette dose. Error bars represent the means ± SD. *P < 0.05, **P < 0.01, and ****P < 0.0001, as determined by Student’s t test.
Reduction in E-Cigarette Use Alters the Immunophenotype across Compartments
After evaluating all 38 proteins in saliva, sputum, and plasma across time in e-cigarette users, we found that although changes in the airways were persistent with short-term decreases in e-cigarette use (Tables 3 and 4), the levels in the blood compartment decreased, suggesting movement toward a normal, nonvaping state (Table 5 and Fig. 5). This finding may be due to the nature of cellular turnover in these compartments, where airway cells remain for weeks to months, whereas circulating neutrophils and monocytes turn over in hours to days. Thus, a short-term partial change in e-cigarette use may be detectable by measurements of plasma inflammatory biomarkers, whereas assessments within the airway compartments may be less sensitive to acute changes and may require longer periods of vaping cessation before the immunophenotype changes.
Figure 5.
Reductions in sputum and plasma immunomodulatory proteins in response to short-term reduction in e-cigarette use. A: IL-1RA in saliva modestly increased after 2 wk reduction in vaping. B: in sputum, four proteins had diminished levels in the setting of short-term reduced vaping, GRO, IL-1RA, IL-8, and IP-10. C: plasma samples demonstrated reduced levels of TNFβ and VEGF in the circulation of e-cigarette users in response to 2 wk of reduced e-cigarette use. Individual data points are shown with the mean and 95% CI. Statistical analysis was conducted with two-way ANOVA with correction for multiple comparisons by controlling the false discovery rate, using the two-stage step-up method of Benjamini, Krieger, and Yekutieli. *P = 0.02, #P = 0.03, **P < 0.01, and ****P < 0.0001. CI, confidence interval.
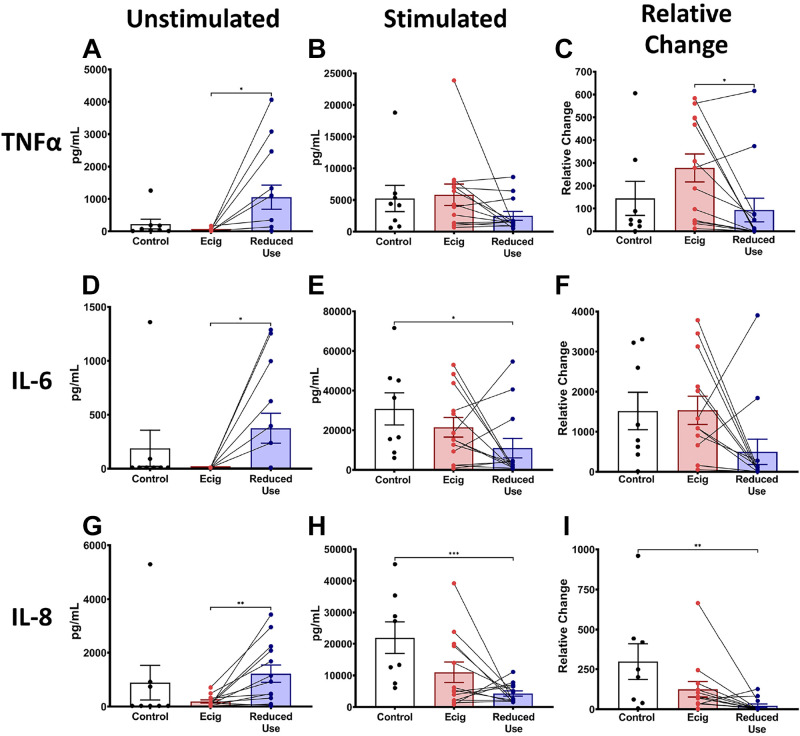
Reduction in E-Cigarette Use Alters the Functional State of Circulating Monocytes
To assess the impact of e-cigarette use on the inflammatory and functional state of one of the primary cells of inflammation and host defense, monocytes were isolated from the blood of e-cigarette users and controls. To particularly assess for alterations in immediate responses to infectious and inflammatory challenges, we quantified the levels of cytokines TNFα, IL-6, and IL-8 released from monocytes with and without LPS stimulation. Unstimulated monocytes from e-cigarette users tended to release cytokines at levels lower than those released from controls (Fig. 6, A, D, and G). Interestingly, the level of cytokines released from unstimulated monocytes after 2 wk of reduced e-cigarette use was significantly increased relative to the initial visit, suggesting a return to normal function (Fig. 6, A, D, and G). Evaluation of the effect of e-cigarette use on monocyte function, via quantification of the response to bacterial LPS challenge, found that monocytes stimulated with LPS released similar levels of cytokines across controls and e-cigarette users at the initial visit (Fig. 6, B, E, and H), but cytokine release was diminished in the setting of 2 wk of reduced e-cigarette use (Fig. 6, B, E, and H). To evaluate individual’s responses to LPS challenge, the cytokines released after LPS stimulation were normalized to the cytokine levels from the same donor cells without stimulation (baseline, unstimulated). After 2 wk of reduced e-cigarette use, circulating monocytes secreted less cytokines than at the initial visit (Fig. 6, C, F, and I).
Figure 6.
E-cigarette alters the inflammatory response of circulating human monocytes. Inflammatory markers TNFα (A–C), IL-6 (D–F), and IL-8 (G–I) were obtained from circulating monocytes in nonsmoking nonvaping healthy controls (black) and from e-cigarette users at baseline (red) and after 2 wk of reduced e-cigarette usage (blue). This figure is further separated into cytokines secreted under unstimulated conditions (A, D, and G) and LPS-stimulated conditions (B, E, and H). Normalized TNFα (C), IL-6 (F), and IL-8 (I) were calculated by dividing cytokine levels from LPS-stimulated monocytes by cytokine levels from nonstimulated monocytes from each subject. Paired lines between e-cigarette and reduced use represent paired changes in cytokine levels from the same subject in the setting of reducing use of e-cigarettes. Error bars represent the means ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001, as determined by Student’s t test. LPS, lipopolysaccharide.
DISCUSSION
Although e-cigarettes are used by some as a harm reduction tool, there is mounting evidence from in vivo and in vitro studies that e-cigarettes can impact the inflammatory status and responsiveness in different organs (11, 15, 22). In this novel pilot study, we present further evidence that e-cigarette use modifies the inflammatory state of the oral cavity, airways, and systemic circulation. By quantifying the inflammatory cytokines, chemokines, and growth factors in these three compartments, our data demonstrate the significant impact of chronic e-cigarette aerosol inhalation on the immune profile not just in the airways but throughout the body as well. Specifically, our findings demonstrate an overall pattern of reduction in inflammatory markers in the airways but an increase in these analytes in the systemic circulation. We hypothesize that although the cells of the upper and lower airways have slow turnover, such that they are exposed multiple times a day to e-cigarette aerosols, for weeks to months, the rapid turnover of cells in the circulation is such that neutrophils and monocytes may only be exposed for a few hours, up to 2–3 days. This finding may reflect the underlying pathology for the pattern of lower levels of immunomodulatory proteins in the airways, where the exposed cells have time to set-up feedback loops to drive suppression of inflammation, whereas cells in the bloodstream become activated by exposure to the chemicals contained in the e-cigarette aerosols (84%–93% of which crossover into the bloodstream; 23) and release inflammatory proteins into their surroundings.
The oral cavity is the first line of host defense against e-cigarette aerosols. As it is a gateway for microbes, alterations in the oral cavity can lead to negative local and systemic effects. Although the relevance of decreased cytokines and growth factors in saliva is unclear, this reduction in the airways raises concern for diseases that are known to occur in the states of immunomodulation (24). IL-1Ra, a naturally occurring antagonist of IL-1 cell surface receptors, had a significant reduction in our e-cigarette cohort. Decreased salivary IL-1Ra may be indicative of early-stage gingival inflammation (25). As IL-1Ra has an inhibitory effect on IL-1, the reduced levels may play a role in the pathogenesis of periodontitis and pulpitis (26–28). We recently found that e-cigarette use leads to alterations in the oral microbiome (29), which complements these data on the alteration in immune proteins, and again supports the hypothesis that through modulation of both microbes and host cells, e-cigarette use adversely affects oral health. More studies are needed to define further the oral microbiome and immune changes caused by e-cigarette use.
Similar to saliva, sputum samples showed altered levels of cytokines and chemokines, namely, increased IL-1Ra and decreased GRO. GRO, a member of the CXC chemokine family, induces neutrophil chemotaxis (30). Reduction in the levels of GRO in sputum may be suggestive of increased vulnerability to respiratory infection owing to the immunosuppressed state. Increase in airway IL-1 expression has been seen in obstructive airway disease with higher risk of exacerbation (31, 32). Increased levels of IL-1Ra, an inhibitor of IL-1, may indicate a response to an induced pro-inflammatory state and may lead to an imbalanced immune response against a respiratory pathogen making them prone to a disease state. As sputum cytokines levels represent the inflammatory state of the airways, the similar illustration of decreased levels of analytes in saliva and sputum samples may indicate a dampened immune state in the oral cavity and airways of e-cigarette users, making them more susceptible to invasion with pathogens.
In contrast to the saliva and sputum findings, subjects who used e-cigarettes chronically tended to have higher circulating levels of inflammatory cytokines, chemokines, and growth factors. In particular, TNFβ and VEGF were elevated in the plasma of e-cigarette users. VEGF is implicated in vascular remodeling and angiogenesis particularly in asthma and chronic obstructive pulmonary disease (COPD; 33). TNFβ, also known as lymphotoxin α, is a member of the TNF family, which is implicated in the pathogenesis of atopic asthma (34). Given their different roles in the inflammatory response, it suggests that chronic inhalation of e-cigarette aerosols may drive multiple types of pathology. Although the exact mechanisms are not yet understood, these data suggest that inhalation of e-cigarette aerosols fundamentally changes the inflammatory milieu in systemic circulation. A recent study by Davis et al. (35) demonstrated changes in alveolar macrophages from e-cigarette users, most consistent with altered lung host defenses in the lungs of vapers, suggesting that vaping establishes an immunosuppressed state, which will predispose e-cigarette users to abnormal immune responses. Another recent study of ex vivo exposures of macrophages to e-liquids demonstrated flavor-specific effects on both cytotoxic and inflammatory modulatory effects (36). Further studies are needed to investigate the risk of dysregulated inflammatory response in e-cigarette users.
In nonstimulated monocytes, we found that lower levels of inflammatory cytokines (IL-8, IL-6, and TNF-α) in e-cigarette users significantly increased after reduced e-cigarette use, suggesting an increase in the potentially protective inflammatory responses after reduction in e-cigarette use. In LPS-stimulated monocytes, the level of inflammatory cytokines was lower in subjects with reduced e-cigarette use compared to healthy controls, suggesting that e-cigarettes could alter the functional state of monocytes and increase the susceptibility to infections especially by gram negative bacteria that include LPS. E-cigarette aerosols and flavoring chemicals have been shown to increase the secretion of inflammatory cytokines such as IL-6, IL-8, and MCP-1 from human bronchial epithelial cells, human mucoepidermoid carcinoma epithelial cells (H292), human lung fibroblasts, and in vivo mouse model (22, 37). E-cigarette vapor condensate increased the secretion of IL-6, TNF-α, CXCL-8, MCP-1, and matrix metalloproteinase 9 in human alveolar macrophages (38). Similarly, exposure of monocytic cells to flavoring chemicals and flavored e-liquid increased the secretion of IL-8 and reactive oxygen species (ROS) (39). Also, e-cigarette flavored pods stimulate inflammation and DNA damage in lung epithelial cells and a monocytic cell line, although the inflammatory effect seems to be related to flavoring (40). Moreover, e-cigarette triggers human neutrophil inflammatory responses such as neutrophil elastase (NE), matrix metallopeptidase 9 (MMP-9), and the activation of inflammatory signaling (41). Thus, our data are aligned with previous findings in cell lines and isolated leukocytes. Collectively, our findings suggest that e-cigarette use can alter the functional and inflammatory responses of circulating immune cells.
Our study has several limitations. Our study consisted of a small subset of subjects with its absolute number limiting definitive statistical analyses. Larger studies will allow for more detailed analyses of the impact of particular flavors or e-devices on inflammatory changes and immune function. Another limitation is that our UCSD Inhalant Survey was in its infancy and was not yet accurately quantifying e-cigarette use and defining e-device and e-liquid characteristics. We are now in the sixth iteration of the UCSD Inhalant Survey, such that future studies will be able to accurately discern between heavy and light users, tobacco, mint, and fruit flavor users, base nicotine versus nicotinic salt users, and levels of nicotine within e-liquids used. Unfortunately, gender characteristics of e-cigarette users and controls were statistically different at baseline. Further studies to assess for gender and sex-specific differences in inflammatory and immune responses to e-cigarette use are needed. Investigational analyses may have been strengthened if complete e-cigarette cessation was possible after 2 wk, rather than simply a decrease in e-cigarette use. However, the fact that only one of the subjects could truly quit vaping in those 2 wk, even though all subjects expressed confidence that they could stop vaping for 2 wk during enrollment, confirms the addictive nature of these nicotine delivery devices. This is a common limitation in tobacco studies, and we confirmed that it is a limitation in e-cigarette studies as well.
In conclusion, daily inhalation of e-cigarette vapor may change the immunophenotype of the lungs and systemic circulation, as detected via alterations in levels of inflammatory proteins and alterations in immune cell phenotypes and functional states. In this study, we found that e-cigarette use was associated with a modified inflammatory state of the oral cavity, airways, and circulation, with changes in IL-1Ra and GRO in the airways most consistent with a dampened inflammatory state, but a proinflammatory state in systemic circulation, with elevated levels of TNFβ and VEGF. This dysregulation of the immune system may ultimately play a role in susceptibility to infections and development of inflammatory disease in users with chronic aerosol inhalation. Moreover, our pilot study may provide an approach for future studies to assess the toxicity of e-cigarettes and perhaps to motivate cessation among those afflicted.
GRANTS
This work was funded by an American Heart Association, Beginning Grant-in-Aid, #16BGIA27790079 (to L.E.C.A.), VA Merit Award 1I01BX004767 (to L.E.C.A.), and National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant R01-HL13705201 (to L.E.C.A.). In addition, this work was supported in part by a Tobacco-Related Disease Research Program (TRDRP) award T30IP0965 (to L.E.C.A.) and NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant DK107585 (to S.D.).
DISCLOSURES
P.A. has received research funding from AstraZeneca, GlaxoSmithKline, and Regeneron and has received consultancy fees from AstraZeneca and GlaxoSmithKline. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
I.M.S., A.M., J.S., C.M.B., S.D., and L.E.C.A. conceived and designed research; I.M.S., J.A.M.-S., A.P., A.M., J.S., C.M.B., S.D., P.A., and L.E.C.A., performed experiments; I.M.S, J.A.M.-S., A.M., A.P., E.L., A.M., J.S., C.M.B., S.D., P.A., and L.E.C.A. analyzed data; I.M.S., J.A.M.-S., A.M., A.P., E.L., A.M., J.S., C.M.B., S.D., P.A., and L.E.C.A. interpreted results of experiments; I.M.S., J.A.M.-S., A.M., A.P., E.L., A.M., J.S., S.D., and L.E.C.A. prepared figures; I.M.S., J.A.M.-S., A.M., A.P., E.L., A.M., J.S., S.D., and L.E.C.A. drafted manuscript; I.M.S., J.A.M.-S., A.M., A.P., E.L., A.M., J.S., C.M.B., S.D. , P.A., and L.E.C.A. edited and revised manuscript; I.M.S., J.A.M.-S., A.M., A.P., E.L., A.M., J.S., C.M.B., S.D., P.A., and L.E.C.A. approved final version of manuscript.
REFERENCES
- 1.Bozier J, Chivers EK, Chapman DG, Larcombe AN, Bastian N, Masso-Silva JA, Byun MK, McDonald CF, Alexander Crotty LE, Ween MP. The evolving landscape of electronic cigarettes: a systematic review of recent evidence. Chest 157: 1362–1390, 2020. doi: 10.1016/j.chest.2019.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014–2018. Jama 322: 1824–1827, 2019. doi: 10.1001/jama.2019.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 380: 629–637, 2019. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 4.Martinez U, Martinez-Loredo V, Simmons VN, Meltzer LR, Drobes DJ, Brandon KO, Palmer AM, Eissenberg T, Bullen CR, Harrell PT, Brandon TH. How does smoking and nicotine dependence change after onset of vaping? A retrospective analysis of dual users. Nicotine Tob Res 22: 764–770, 2020. doi: 10.1093/ntr/ntz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillen R, Klein JD, Wilson K, Winickoff JP, Tanski S. E-cigarette use and future cigarette initiation among never smokers and relapse among former smokers in the PATH study. Public Health Rep 134: 528–536, 2019. doi: 10.1177/0033354919864369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomajee R, El-Khoury F, Goldberg M, Zins M, Lemogne C, Wiernik E, Lequy-Flahault E, Romanello L, Kousignian I, Melchior M. Association between electronic cigarette use and smoking reduction in France. JAMA Intern Med 179: 1193–1200, 2019. doi: 10.1001/jamainternmed.2019.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur G, Pinkston R, McLemore B, Dorsey WC, Batra S. Immunological and toxicological risk assessment of e-cigarettes. Eur Respir Rev 27: 170119, 2018. doi: 10.1183/16000617.0119-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girvalaki C, Tzatzarakis M, Kyriakos CN, Vardavas AI, Stivaktakis PD, Kavvalakis M, Tsatsakis A, Vardavas C. Composition and chemical health hazards of the most common electronic cigarette liquids in nine European countries. Inhal Toxicol 30: 361–369, 2018. doi: 10.1080/08958378.2018.1527879. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, Crotty Alexander LE. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 94: 667–679, 2016. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 10.Corriden R, Moshensky A, Bojanowski CM, Meier A, Chien J, Nelson RK, Crotty Alexander LE. E-cigarette use increases susceptibility to bacterial infection by impairment of human neutrophil chemotaxis, phagocytosis, and NET formation. Am J Physiol Cell Physiol 318: C205–C214, 2020. doi: 10.1152/ajpcell.00045.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Lee J, Fonseca AG, Moshensky A, Kothari T, Sayed IM, Ibeawuchi SR, Pranadinata RF, Ear J, Sahoo D, Crotty-Alexander LE, Ghosh P, Das S. E-cigarettes compromise the gut barrier and trigger inflammation. iScience 24: 102035, 2021. doi: 10.1016/j.isci.2021.102035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh KP, Lawyer G, Muthumalage T, Maremanda KP, Khan NA, McDonough SR, Ye D, McIntosh S, Rahman I. Systemic biomarkers in electronic cigarette users: implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res 5: 00182-2019, 2019. doi: 10.1183/23120541.00182-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, Fry RC, Jaspers I. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 311: L135–L144, 2016. doi: 10.1152/ajplung.00170.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Münzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J 41: 4057–4070, 2020. doi: 10.1093/eurheartj/ehaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotty Alexander LE, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, Breen EC. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol Regul Integr Comp Physiol 314: R834–R847, 2018. doi: 10.1152/ajpregu.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Advani I, Gunge D, Banks S, Mehta S, Park K, Patel M, Malhotra A, Crotty Alexander LE. Is increased sleep responsible for reductions in myocardial infarction during the COVID-19 pandemic? Am J Cardiol 131: 128–130, 2020. doi: 10.1016/j.amjcard.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boddu SA, Bojanowski CM, Lam MT, Advani IN, Scholten EL, Sun X, Montgrain P, Malhotra A, Jain S, Alexander LEC. Use of electronic cigarettes with conventional tobacco is associated with decreased sleep quality in women. Am J Respir Crit Care Med 200: 1431–1434, 2019. doi: 10.1164/rccm.201904-0890LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delirezh N, Shojaeefar E, Parvin P, Asadi B. Comparison the effects of two monocyte isolation methods, plastic adherence and magnetic activated cell sorting methods, on phagocytic activity of generated dendritic cells. Cell J 15: 218–223, 2013. [PMC free article] [PubMed] [Google Scholar]
- 20.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology 114: 204–212, 2005. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen MC, Andersen MN, Moller HJ. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology 159: 63–74, 2020. doi: 10.1111/imm.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Helen G, Havel C, Dempsey DA, Jacob P 3rd, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111: 535–544, 2016. doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javed F, Kellesarian SV, Sundar IK, Romanos GE, Rahman I. Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Dis 23: 1052–1057, 2017. doi: 10.1111/odi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belstrøm D, Damgaard C, Könönen E, Gürsoy M, Holmstrup P, Gürsoy UK. Salivary cytokine levels in early gingival inflammation. J Oral Microbiol 9: 1364101, 2017. doi: 10.1080/20002297.2017.1364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu HX, Xiao MZ, Niu ZY, Guo XM, Zhao SL, Wang HG, Guo HY. Effect of IL-1ra on human dental pulp cells and pulpal inflammation. Int Endod J 35: 807–811, 2002. doi: 10.1046/j.1365-2591.2002.00542.x. [DOI] [PubMed] [Google Scholar]
- 27.Wu YF, Tan C, Zhang JY, Meng S, Guo YH. Interleukin-1beta and IL-1 receptor antagonist levels in gingival crevicular fluid and their relationship to clinical indices of periodontitis. Sichuan Da Xue Bao Yi Xue Ban 35: 683–686, 2004. [PubMed] [Google Scholar]
- 28.Rawlinson A, Dalati MH, Rahman S, Walsh TF, Fairclough AL. Interleukin-1 and IL-1 receptor antagonist in gingival crevicular fluid. J Clin Periodontol 27: 738–743, 2000. doi: 10.1034/j.1600-051x.2000.027010738.x. [DOI] [PubMed] [Google Scholar]
- 29.Chopyk J, Bojanowski CM, Shin J, Moshensky A, Fuentes AL, Bonde SS, Chuki D, Pride DT, Crotty Alexander LE. Compositional differences in the oral microbiome of e-cigarette users. Front Microbiol 12: 599664, 2021. doi: 10.3389/fmicb.2021.599664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer-Hoffert U, Lezcano-Meza D, Bartels J, Montes-Vizuet AR, Schröder JM, Teran LM. Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol 131: 264–271, 2003. doi: 10.1159/000072138. [DOI] [PubMed] [Google Scholar]
- 31.Baines KJ, Fu JJ, McDonald VM, Gibson PG. Airway gene expression of IL-1 pathway mediators predicts exacerbation risk in obstructive airway disease. Int J Chron Obstruct Pulmon Dis 12: 541–550, 2017. doi: 10.2147/COPD.S119443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, Lindblad K, Patel H, Rugman P, Dodson P, Jenkins M, Saunders M, Newbold P, Green RH, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 184: 662–671, 2011. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 33.Zanini A, Chetta A, Imperatori AS, Spanevello A, Olivieri D. The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res 11: 132, 2010. doi: 10.1186/1465-9921-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Sharma A, Kumar S, Sharma SK, Ghosh B. Association of TNF haplotypes with asthma, serum IgE levels, and correlation with serum TNF-alpha levels. Am J Respir Cell Mol Biol 35: 488–495, 2006. doi: 10.1165/rcmb.2006-0084OC. [DOI] [PubMed] [Google Scholar]
- 35.Davis ES, Ghosh A, Coakley RD, Wrennall JA, Lubamba BA, Rowell TR, Dang H, Pawlak EA, Li Q, Alexis NE, Ribeiro CMP, Tarran R. Chronic e-cigarette exposure alters human alveolar macrophage morphology and gene expression. Nicotine Tob Res. In Press. doi: 10.1093/ntr/ntab186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ween MP, Moshensky A, Thredgold L, Bastian NA, Hamon R, Badiei A, Nguyen PT, Herewane K, Jersmann H, Bojanowski CM, Shin J, Reynolds PN, Crotty Alexander LE, Hodge SJ. E-cigarettes and health risks: more to the flavor than just the name. Am J Physiol Lung Cell Mol Physiol 320: L600–L614, 2021. doi: 10.1152/ajplung.00370.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerloff J, Sundar IK, Freter R, Sekera ER, Friedman AE, Robinson R, Pagano T, Rahman I. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol 3: 28–40, 2017. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott A, Lugg ST, Aldridge K, Lewis KE, Bowden A, Mahida RY, Grudzinska FS, Dosanjh D, Parekh D, Foronjy R, Sapey E, Naidu B, Thickett DR. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73: 1161–1169, 2018. doi: 10.1136/thoraxjnl-2018-211663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol 8: 1130, 2017. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthumalage T, Lamb T, Friedman MR, Rahman I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep 9: 19035, 2019. doi: 10.1038/s41598-019-51643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higham A, Rattray NJ, Dewhurst JA, Trivedi DK, Fowler SJ, Goodacre R, Singh D. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir Res 17: 56, 2016. doi: 10.1186/s12931-016-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]